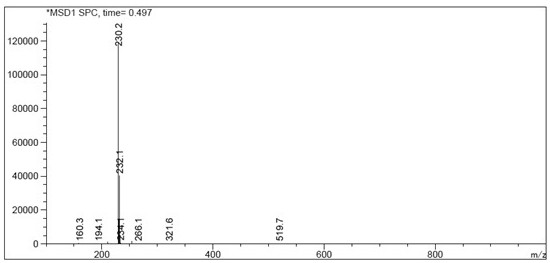
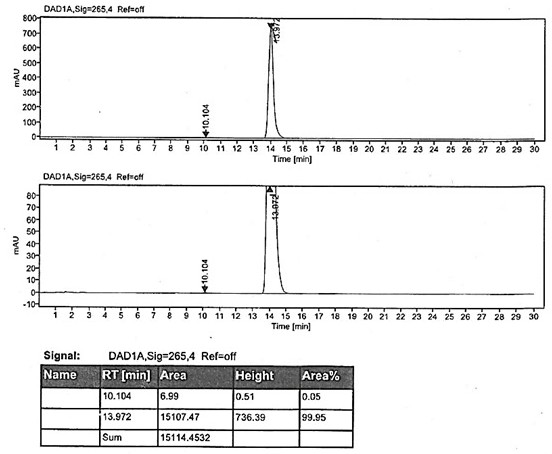
Preparation method of rabeprazole chloride and intermediate thereof
A technology of esterification and acylation reagents, applied in the field of drug synthesis, can solve the problems of high reaction temperature, long time, and long cycle, and achieve the effect of mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0048] Preparation of embodiment 13-methyl-2-hydroxymethyl-4-(3-methoxypropoxy)pyridine
[0049]Add 50g of 2,3-dimethyl-4-(3-methoxypropoxy)pyridine nitrogen oxide, 48.5g of acetic anhydride, and 4g of p-toluenesulfonic acid into the reaction flask, stir evenly and then raise the temperature, and control the temperature at 50±5 After reacting at ℃ for 3 hours, TLC showed that the reaction of the raw materials was complete (the reaction solution was detected by HPLC, and the purity of the target product was 93.6%), and the excess acetic anhydride was concentrated under negative pressure. Add 10% sodium hydroxide aqueous solution, adjust the pH to 14, then raise the temperature to 80°C for 1 hour, TLC confirms that the reaction is complete, then lowers to room temperature, extracts with toluene and concentrates to dryness to obtain the compound shown in the title, with a molar yield of 90.1 %, purity 94.71%.
Embodiment 23
[0050] The preparation of embodiment 23-methyl-2-hydroxymethyl-4-(3-methoxypropoxy)pyridine
[0051] Add 50g of 2,3-dimethyl-4-(3-methoxypropoxy)pyridine nitrogen oxide, 48.5g of acetic anhydride, and 8g of p-toluenesulfonic acid into the reaction flask, stir evenly and then raise the temperature, and control the temperature at 60±5 After reacting at ℃ for 3 hours, TLC showed that the reaction of the raw materials was complete (the reaction solution was detected by HPLC, and the purity of the target product was 95.1%), and the excess acetic anhydride was concentrated under negative pressure. Add 10% sodium hydroxide aqueous solution, adjust the pH to 14, then raise the temperature to 80 ° C for 1 hour, TLC confirms that the reaction is complete, then lowers to room temperature, extracts with toluene and concentrates to dryness to obtain the compound shown in the title, with a molar yield of 91.6 %, purity 95.35%.
Embodiment 33
[0052] Preparation of Example 33-methyl-2-hydroxymethyl-4-(3-methoxypropoxy)pyridine
[0053] Add 50g of 2,3-dimethyl-4-(3-methoxypropoxy)pyridine nitrogen oxide, 97g of acetic anhydride, and 4g of p-toluenesulfonic acid into the reaction flask, stir well and then raise the temperature, and control the temperature at 50±5°C After 3 hours of reaction, TLC showed that the reaction of the raw materials was complete (the reaction solution was detected by HPLC, and the purity of the target product was 94.9%), and the excess acetic anhydride was concentrated under negative pressure. Add 10% sodium hydroxide aqueous solution, adjust the pH to 14, then raise the temperature to 80°C for 1 hour, TLC confirms that the reaction is complete, then lowers to room temperature, extracts with toluene, and concentrates to dryness to obtain the compound shown in the title, with a molar yield of 92.1 %, purity 95.52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com