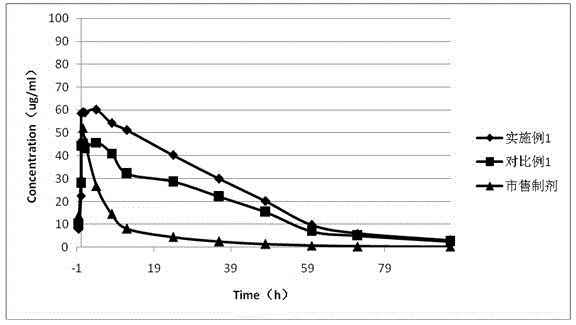
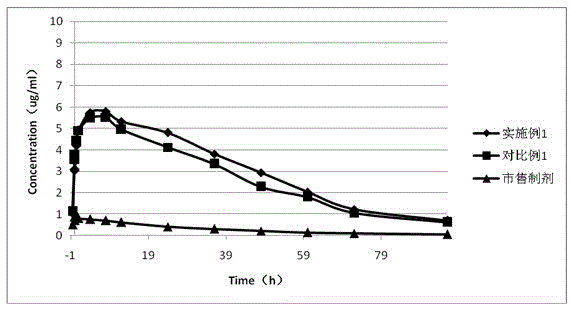
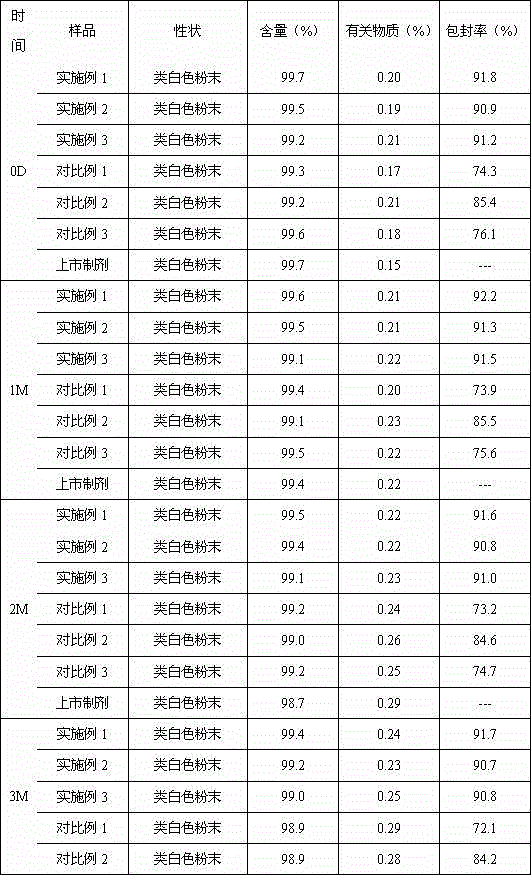
Long-circulating rabeprazole liposome composition and preparation method and application thereof
A technology of rabeprazole fat and rabeprazole, which is applied in the field of long-circulation rabeprazole liposome composition and its preparation, can solve the instability of rabeprazole oral preparations and the leakage of drug phospholipid bimolecules , particle size and distribution increase, etc., to achieve good product stability, solve thermal discoloration, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of long-circulation rabeprazole liposome freeze-dried powder injection
[0062] Prescription: (1000 bottles)
[0063] Component Dosage
[0064] Rabeprazole 20g
[0065] Hydrogenated Soy Phosphatidylcholine 120g
[0066] Cholesterol 60g
[0067] Cultured Phosphatidylethanolamine 10g
[0068] α-Tocopherol 0.4g
[0069] Edetate Calcium Sodium 0.4g
[0070] Trehalose 60g
[0071] Preparation Process:
[0072] (1) Add 20g of rabeprazole, 120g of hydrogenated soybean phosphatidylcholine, 60g of cholesterol and 0.4g of α-tocopherol into 2000ml of ethanol, heat to 45°C, stir and disperse evenly, and filter through a 0.22μm organic membrane;
[0073] (2) Add 60g of trehalose and 0.4g of calcium sodium edetate to 2000ml of 10mmol / L phosphate buffer solution (pH = 7.0), shake and stir to dissolve, filter through a 0.22μm water membrane, and store at 45°C Lower heat preservation;
[0074] (3) Slowly add the solution prepared in step (1) into the ...
Embodiment 2
[0079] Example 2 Preparation of long-circulation rabeprazole liposome freeze-dried powder injection
[0080] Prescription: (1000 bottles)
[0081] Component Dosage
[0082] Rabeprazole 20g
[0083] Distearoylphosphatidylcholine 80g
[0084] Cholesterol 20g
[0085] Cultured Phosphatidylethanolamine 20g
[0086] α-Tocopherol 0.6g
[0087] Edetate Disodium 0.6g
[0088] Trehalose 50g
[0089] Preparation Process:
[0090] (1) Add 20g of rabeprazole, 100g of distearoylphosphatidylcholine, 40g of cholesterol and 0.6g of α-tocopherol into 1600ml of ethanol and tert-butanol solution with a volume ratio of 3:1, heat to 45°C and stir Uniform dispersion, 0.22μm organic membrane filtration;
[0091] (2) Add 50g trehalose and 0.6g edetate disodium into 1600ml citrate buffer solution (pH = 7.0) with a concentration of 10mmol / L, shake and stir to dissolve, filter through a 0.22μm water membrane, and filter at 45 Keep warm at ℃;
[0092] (3) Slowly add the solution item obtai...
Embodiment 3
[0097] Example 3 Preparation of long-circulation rabeprazole liposome freeze-dried powder injection
[0098] Prescription: (1000 bottles)
[0099] Component Dosage
[0100] Rabeprazole 20g
[0101] Dipalmitoyl Phosphatidylcholine 160g
[0102] Cholesterol 50g
[0103] Cultured Phosphatidylethanolamine 20g
[0104] α-Tocopherol 1.0g
[0105] Edetate Calcium Sodium 1g
[0106] Sucrose 20g
[0107] Preparation Process:
[0108] (1) Add 20g of rabeprazole, 130g of hydrogenated soybean phosphatidylcholine, 50g of cholesterol, 20g of cultured phosphatidylethanolamine and 0.7g of α-tocopherol into 2000ml of ethanol, heat to 45°C and stir to disperse evenly. membrane filtration;
[0109] (2) Add 60g of sucrose and 1g of calcium sodium edetate to 2000ml of 10mmol / L phosphate buffer solution (pH = 7.0), shake and stir to dissolve, filter through a 0.22μm water film, and keep warm at 45°C ;
[0110] (3) Slowly add the solution item obtained in step (1) into the solution item...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com