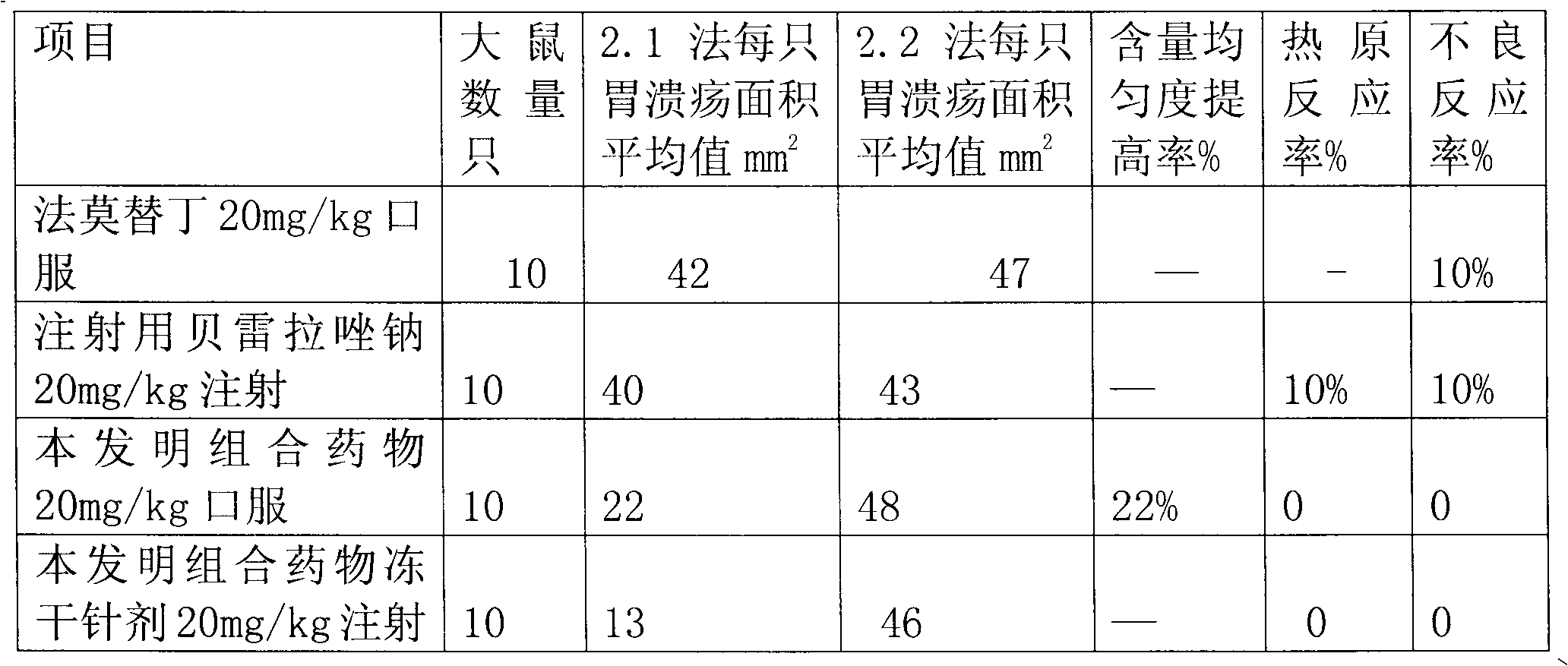
Rabeprazole sodium combined medicament and preparation process thereof
The technology of bereprazole sodium and composition is applied in the field of combination medicine of bereprazole sodium and preparation technology thereof, which can solve the problems of drug oxidation harm, allergic effect, physical damage and the like, and achieves the promotion of drug allergy effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Bereprazole sodium combination medicine provided by the invention is combined by the raw material composition of following weight ratio:
[0025] Bereprazole sodium: 5
[0026] Weight ratio of reduced glutathione to hepatocyte growth-stimulating hormone
[0027] 1:10 composition 20
[0028] Diammonium Glycyrrhizinate 50
[0029] Preparation Process:
[0030] 1. Dissolve bereprazole sodium, reduced glutathione, hepatocyte growth-stimulating hormone, and diammonium glycyrrhizinate completely with water for injection that is 100-120 times the weight of bereprazole sodium under normal speed stirring;
[0031] 2. Add the activated carbon that has been removed by dry heat at 180°C to the combined drug liquid prepared in the first step, and the ratio of the weight of activated carbon to the volume of the liquid is 0.1%;
[0032] 3. The combined drug liquid is sterilized by damp heat at 121°C for 20 minutes, and when the temperature of the liquid is 60-70°C, it is filtered ...
Embodiment 2
[0039] Bereprazole sodium combination medicine provided by the invention is combined by the raw material composition of following weight ratio:
[0040] Bereprazole Sodium: 10
[0041] Weight ratio of reduced glutathione to hepatocyte growth-stimulating hormone
[0042] 1:10 composition 30
[0043] Diammonium Glycyrrhizinate 60
[0044] Preparation Process:
[0045] 1. Dissolve bereprazole sodium, reduced glutathione, hepatocyte growth-stimulating hormone, and diammonium glycyrrhizinate completely with water for injection that is 100-120 times the weight of bereprazole sodium under normal speed stirring;
[0046] 2. Add activated carbon that has been dry-heated at 180°C to the combined drug liquid prepared in the first step, and the ratio of the weight of activated carbon to the volume of the liquid is 0.1%;
[0047] 3. The combined drug liquid is sterilized by damp heat at 121°C for 20 minutes, and when the temperature of the liquid is 60-70°C, it is filtered with a two-l...
Embodiment 3
[0054] Bereprazole sodium combination medicine provided by the invention is combined by the raw material composition of following weight ratio:
[0055] Bereprazole sodium: 5
[0056] Weight ratio of reduced glutathione to hepatocyte growth-stimulating hormone
[0057] 1:10 composition 30
[0058] Diammonium Glycyrrhizinate 50
[0059] Preparation Process:
[0060] 1. Dissolve bereprazole sodium, reduced glutathione, hepatocyte growth-stimulating hormone, and diammonium glycyrrhizinate completely with water for injection that is 100-120 times the weight of bereprazole sodium under normal speed stirring;
[0061] 2. Add the activated carbon that has been removed by dry heat at 180°C to the combined drug liquid prepared in the first step, and the ratio of the weight of activated carbon to the volume of the liquid is 0.1%;
[0062] 3. The combined drug liquid is sterilized by damp heat at 121°C for 20 minutes, and when the temperature of the liquid is 60-70°C, it is filtered ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com