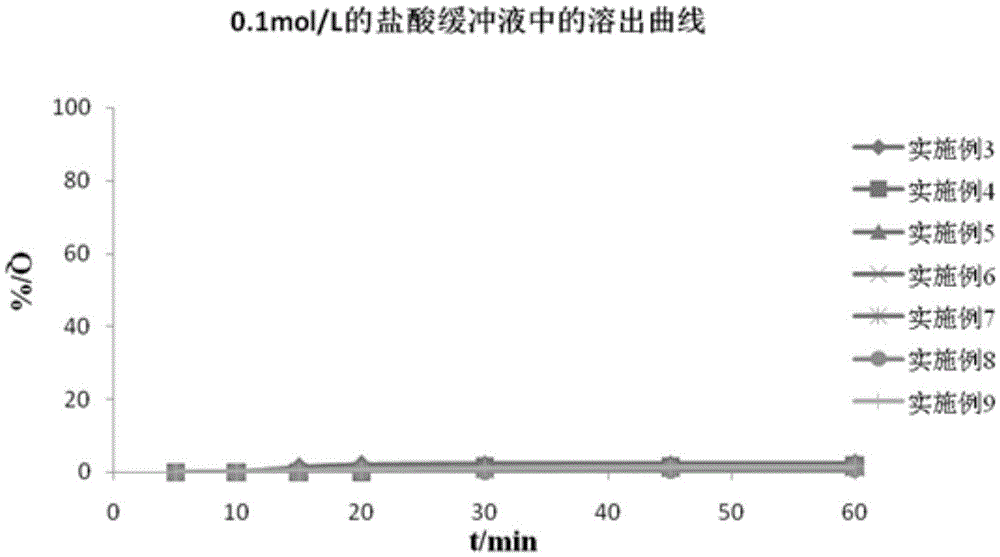
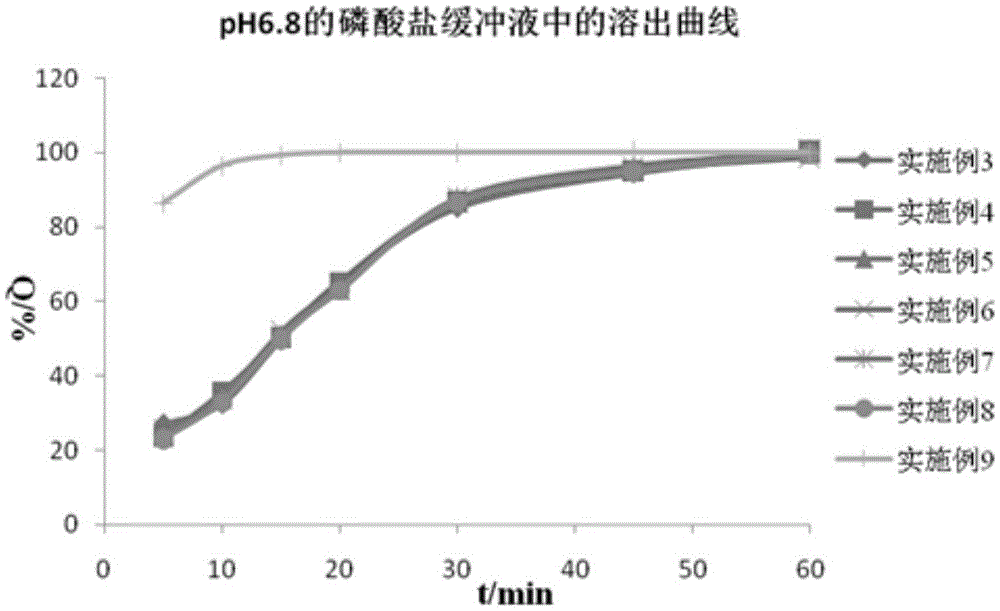
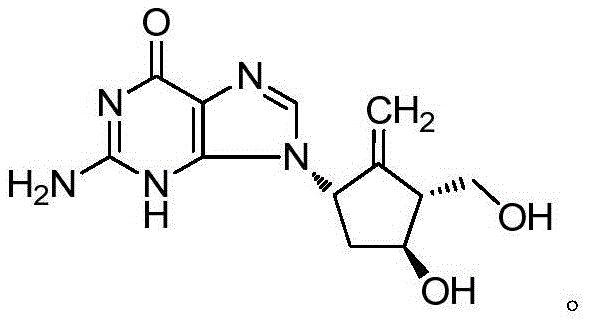
Compound enteric-coated tablets of entecavir phospholipid complex and diammonium glycyrrhizinate
A technology of diammonium glycyrrhizinate and phosphatidylcholine, which is applied in the field of medicine, can solve the problems of lower drug efficacy, low bioavailability, and damage to the injection site, and achieve improved safety and effectiveness, improved bioavailability, and improved The effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of entecavir phospholipid complex
[0033] Dissolve entecavir and lecithin (the ratio of the two is 1:5~80, w / w) in a mixed solvent of isopropanol and ethanol (the volume ratio of the two solvents is 1:1), and the drug concentration of entecavir is 0.2~2.0mg / mL, magnetically stirred at 120r / min in a constant temperature water bath at 55°C for 30 minutes, recovered the solvent under reduced pressure, and dried in vacuum to obtain the phospholipid complex.
Embodiment 2
[0034] Embodiment 2: the preparation of enteric coating liquid
[0035] prescription:
[0036] components
Component weight (g)
Udage L100-55
16.0
polyethylene glycol 4000
2.0
NaOH
0.2
6.8
75.0
total
100.0
[0037] Preparation:
[0038] Weigh the prescribed amount of polyethylene glycol 4000 and NaOH, dissolve them in the prescribed amount of purified water, add talcum powder and Eudragit L100-55 to it under stirring to make a suspension, stir well and pass through 80 mesh Sieve to obtain an aqueous coating solution containing 16% of the enteric coating material Eudragit L100-55.
Embodiment 3~5
[0039] Embodiments 3-5: Preparation of Entecavir Phospholipid Complex and Diammonium Glycyrrhizinate Compound Enteric-Coated Tablets (made into 1000 tablets)
[0040] prescription:
[0041]
[0042] Preparation:
[0043] 1. Preparation of Entecavir Phospholipid Complex
[0044] The entecavir phospholipid complex was prepared according to the method in Example 1, and it was ground through an 80-mesh sieve for subsequent use.
[0045] 2. Tablet core preparation
[0046] 1) Diammonium Glycyrrhizinate, Sodium Lauryl Sulfate, and micropowder silica gel of prescription quantity are taken by weighing and pulverized through 80 mesh sieves respectively, then in a V-type mixer, add entecavir phospholipid complex and mix homogeneously by equal addition method, Intermediate 1 was obtained;
[0047]2) Pregelatinized starch, mannitol, hypromellose, and cross-linked carmellose sodium were weighed and pulverized through an 80-mesh sieve, and then mixed uniformly in a tank mixer to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com