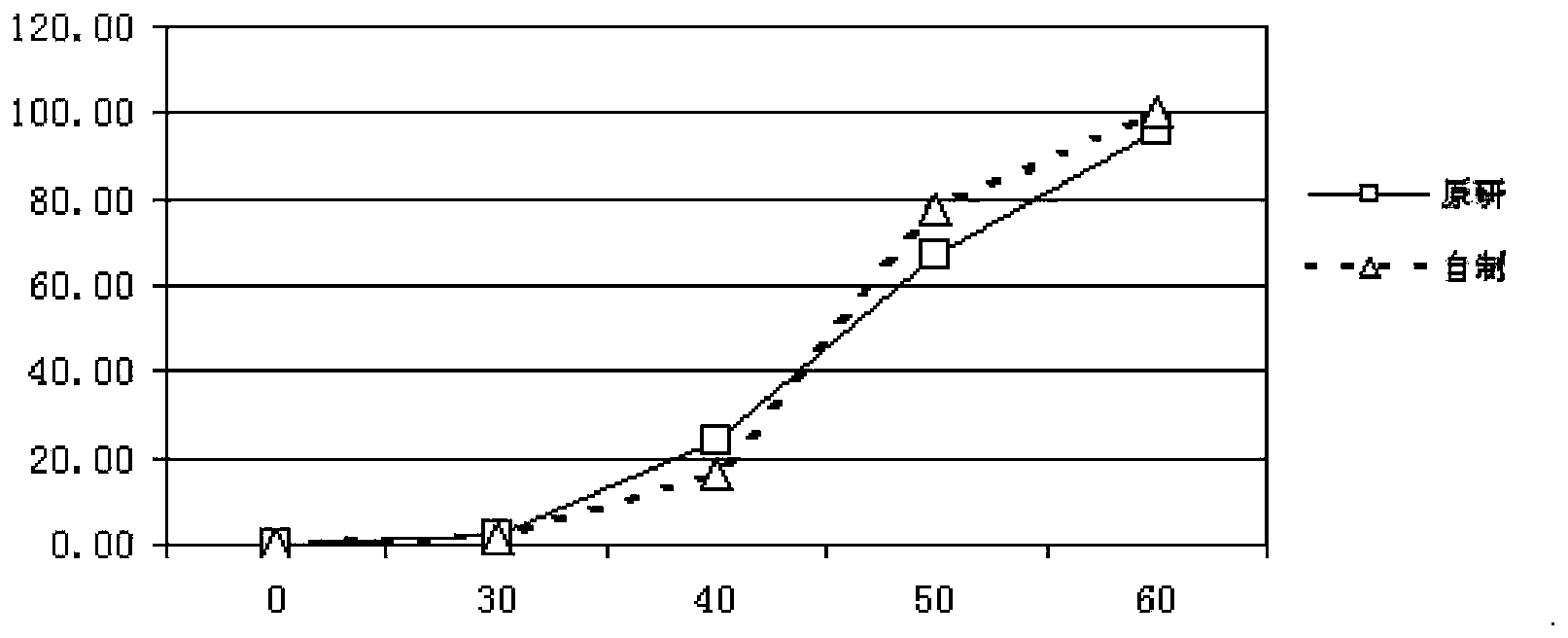
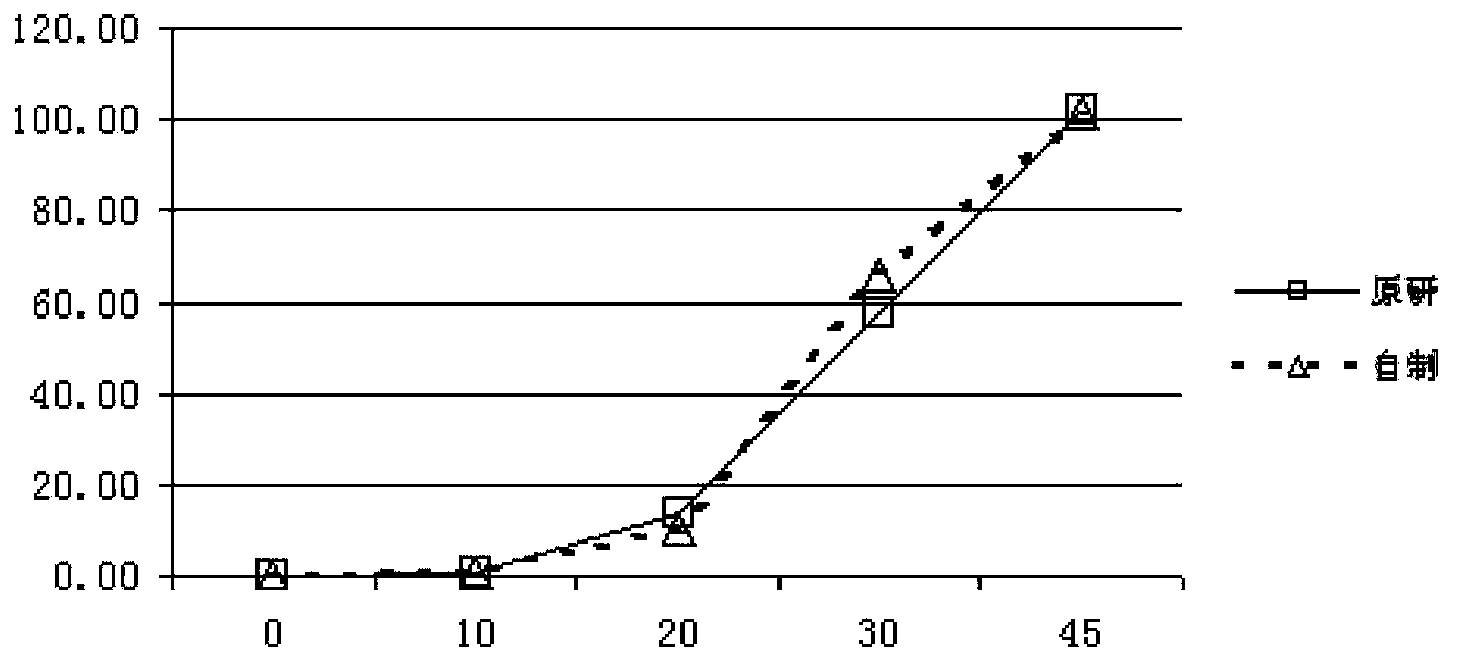
Pantoprazole sodium enteric-coated tablet and preparation method thereof
A technology for pantoprazole sodium tablet and pantoprazole sodium is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc., and can solve problems such as non-compliance with quality control requirements and the like , to ensure the coating effect, improve product quality, and facilitate the effect of coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 pantoprazole sodium enteric-coated tablet
[0044] 1. Components and contents of pantoprazole sodium tablets
[0045] Name of raw material 1000 tablets (g) Proportion (%) effect Pantoprazole Sodium 44.3 35.00 Main drug Mannitol 44.00 34.76 filler microcrystalline cellulose 11.00 8.70 filler Sodium carboxymethyl starch (additional) 4.85 3.83 disintegrant Anhydrous Sodium Carbonate 9.56 7.55 stabilizer Crospovidone (additional) 9.71 7.67 disintegrant 16% povidone K30 50% ethanol solution 15.75 1.99 Adhesive Magnesium Stearate (additional) 0.63 0.50 lubricant
[0046] 2. The composition and content of the isolation layer
[0047] Excipient name Proportion (%) effect Hydroxypropylmethylcellulose 31.25 Gown material Titanium dioxide 18.75 Sunscreen talcum powder 50 Antisticking agent 50% ethanol Prepare 8% HP...
Embodiment 2
[0056] The preparation of embodiment 2 pantoprazole sodium enteric-coated tablets
[0057] (1) Components and content of pantoprazole sodium tablet
[0058] Name of raw material 1000 tablets (g) Proportion (%) effect Pantoprazole Sodium 44.3 45 Main drug Mannitol 14.77 15 filler microcrystalline cellulose 14.77 15 filler Sodium carboxymethyl starch (additional) 8.86 9 disintegrant Anhydrous Sodium Carbonate 4.92 5 stabilizer Crospovidone (additional) 8.86 9 disintegrant 16% povidone K30 50% ethanol solution 9.23 1.5 Adhesive Magnesium Stearate (additional) 0.49 0.5 lubricant
[0059] (2) The composition and content of the isolation layer
[0060]
[0061] The coating weight gain of the isolation layer is 6% of the plain tablet.
[0062] (3) Components and content of enteric-coated layer
[0063] Excipient name effect Udage L30D-55 Polymer solids are 18% of the...
Embodiment 3
[0067] The preparation of embodiment 3 pantoprazole sodium enteric-coated tablets
[0068] (1) Components and content of pantoprazole sodium tablet
[0069] Name of raw material 1000 pieces Proportion (%) effect Pantoprazole Sodium 44.3 35 Main drug Mannitol 52.14 41.2 filler microcrystalline cellulose 13.04 10.3 filler Sodium carboxymethyl starch (additional) 3.42 2.7 disintegrant magnesium oxide 6.33 5 stabilizer Crospovidone (internal addition) 6.83 5.4 disintegrant 2% povidone K30 50% ethanol solution 12.66 0.2 Adhesive Magnesium Stearate (additional) 0.25 0.2 lubricant
[0070] (2) The composition and content of the isolation layer
[0071] Excipient name Proportion (%) effect Hydroxypropylmethylcellulose 31.25 Gown material Titanium dioxide 18.75 Sunscreen Magnesium stearate 50 Antisticking agent 50% ethanol Prepare 6% HPMC50% ethanol s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com