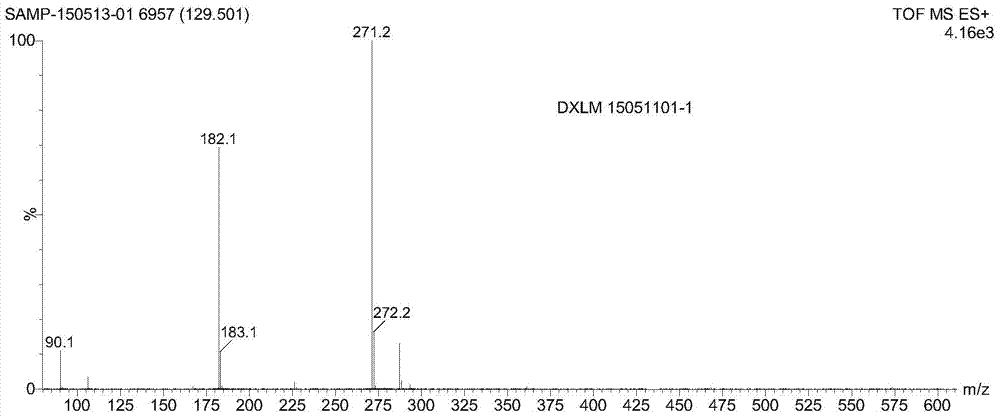
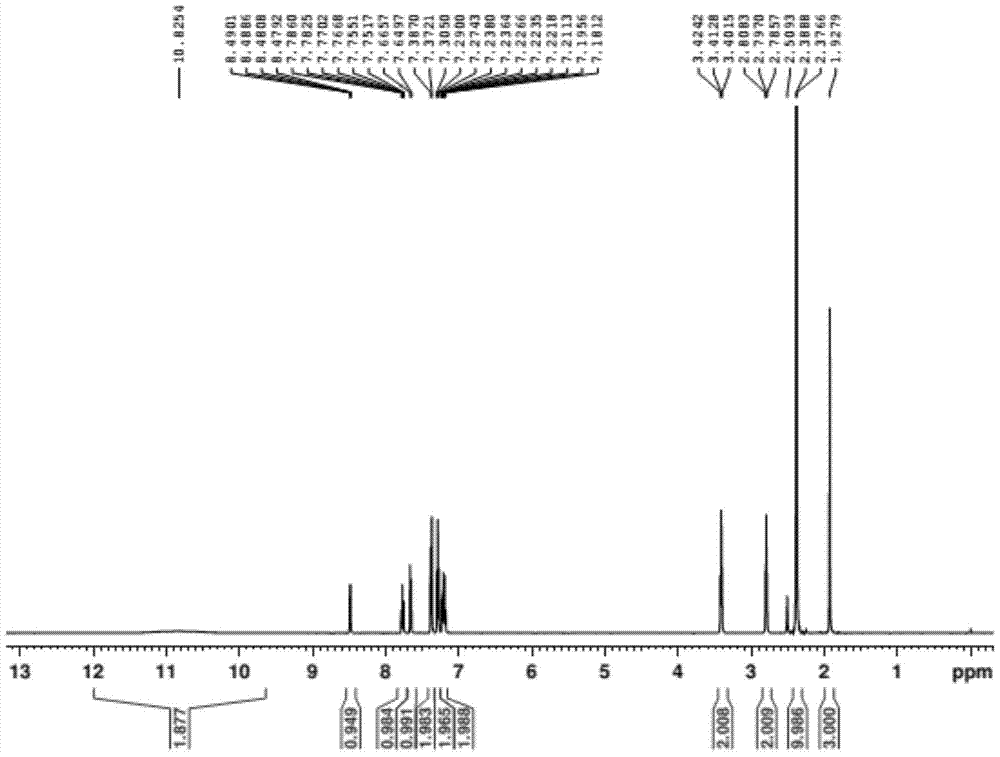
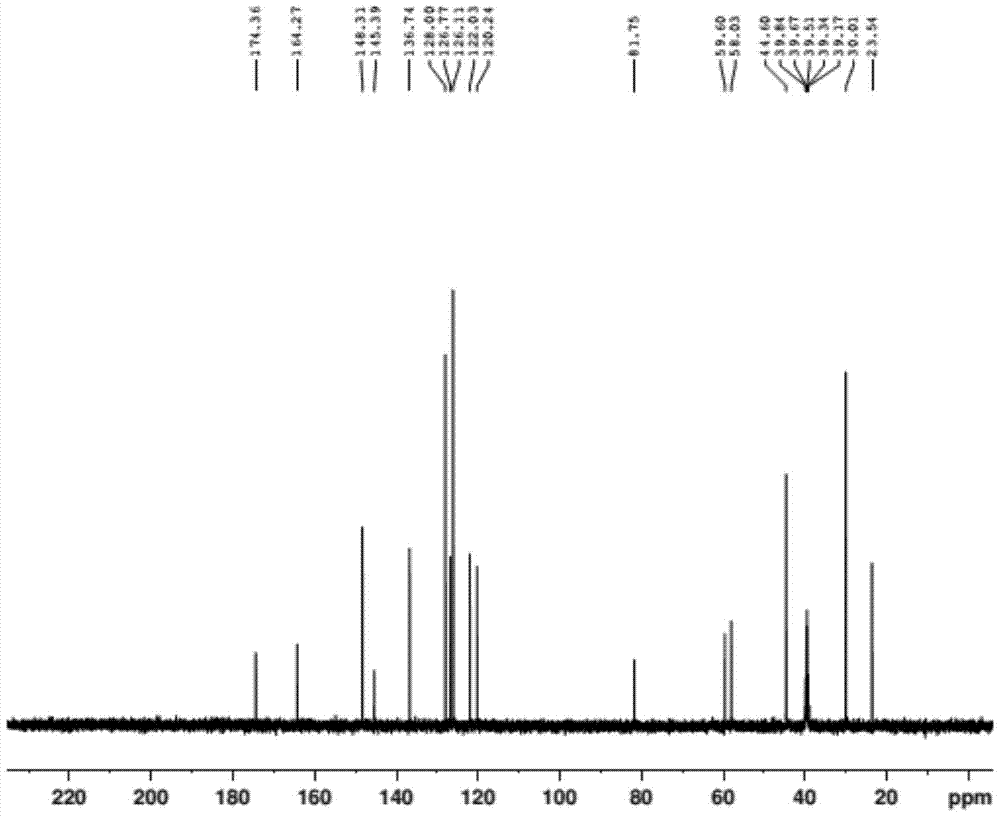
A kind of preparation method of doxylamine succinate
A technology of doxylamine succinate and hydrochloride, which is applied in the field of medicine, can solve the problems of low purity, multiple crystallization, and large solubility of finished products, and avoid cumbersome operations, less reaction and side effects, and simple post-processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The reaction equation is as follows:
[0045] S1: Synthesis of N,N-dimethyl-2-[1-phenyl-1-(2-pyridine)ethoxy]ethylamine
[0046]
[0047]Dissolve 200g of 2-pyridylphenylmethylmethanol in 1L of xylene, pass through nitrogen protection, add 222g of sodium amide at 0-5°C, stir for 15-30 minutes, add 1.07kg of dimethylaminoethyl chloride salt Acid salt, stir at 0-5°C for 15-30 minutes, slowly heat up to 140°C, reflux and stir for 1 hour, cool down to 0-5°C after the reaction, add 500ml of 20-30% ammonium chloride dropwise, stir for 30 minutes, and let stand Separate the layers, discard the water layer, add 300ml of 3-5% dilute hydrochloric acid, mix thoroughly for 15 minutes, let the layers stand, discard the organic phase, add 300ml of the water phase, 20-30% sodium carbonate aqueous solution and 1L ethyl acetate, fully After mixing, let stand to separate the layers, dry the ethyl acetate layer with anhydrous sodium sulfate, filter and distill off the ethyl acetate und...
Embodiment 2
[0052] The reaction equation is as follows:
[0053] S1: Synthesis of N,N-dimethyl-2-[1-phenyl-1-(2-pyridine)ethoxy]ethylamine
[0054] Dissolve 200g of 2-pyridylphenylmethylmethanol in 1L of xylene, pass through nitrogen protection, add 254g of sodium amide at 0-5°C, stir for 15-30 minutes, add 1.39kg of dimethylaminoethyl chloride salt Acid salt, stir at 0-5°C for 15-30 minutes, slowly heat up to 140°C, reflux and stir for 1 hour, cool down to 0-5°C after the reaction, add 500ml of 20-30% ammonium chloride dropwise, stir for 30 minutes, and let stand Separate the layers, discard the water layer, add 300ml of 3-5% dilute hydrochloric acid, mix thoroughly for 15 minutes, let stand to separate the layers, discard the organic phase, add 300ml of the water phase, 20-30% aqueous sodium bicarbonate and 1L ethyl acetate, Mix well and let stand to separate layers, dry the ethyl acetate layer with anhydrous sodium sulfate, filter and distill off the ethyl acetate under reduced pressu...
Embodiment 3
[0058] S1: Synthesis of N,N-dimethyl-2-[1-phenyl-1-(2-pyridine)ethoxy]ethylamine
[0059] Dissolve 200g of 2-pyridylphenylmethylmethanol in 1L of xylene, pass through nitrogen protection, add 190g of sodium amide at 0-5°C, stir for 15-30 minutes, add 0.89kg of dimethylaminoethyl chloride salt Acid acid salt, stir at 0-5°C for 15-30 minutes, slowly heat up to 150°C, reflux and stir for 1 hour, cool down to 0-5°C after the reaction, add 500ml of 20-30% ammonium chloride dropwise, stir for 30 minutes, and let stand Separate layers, discard the water layer, add 300ml of 3-5% dilute hydrochloric acid, mix thoroughly for 30 minutes, let stand to separate layers, discard the organic phase, add 300ml, 20-30% aqueous sodium carbonate solution and 1L dichloromethane to the water phase, and fully After mixing, let it stand and separate layers, dry the dichloromethane layer with anhydrous sodium sulfate, filter and distill off the dichloromethane under reduced pressure to obtain N,N-dimet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com