Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2393results about How to "Large caliber" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
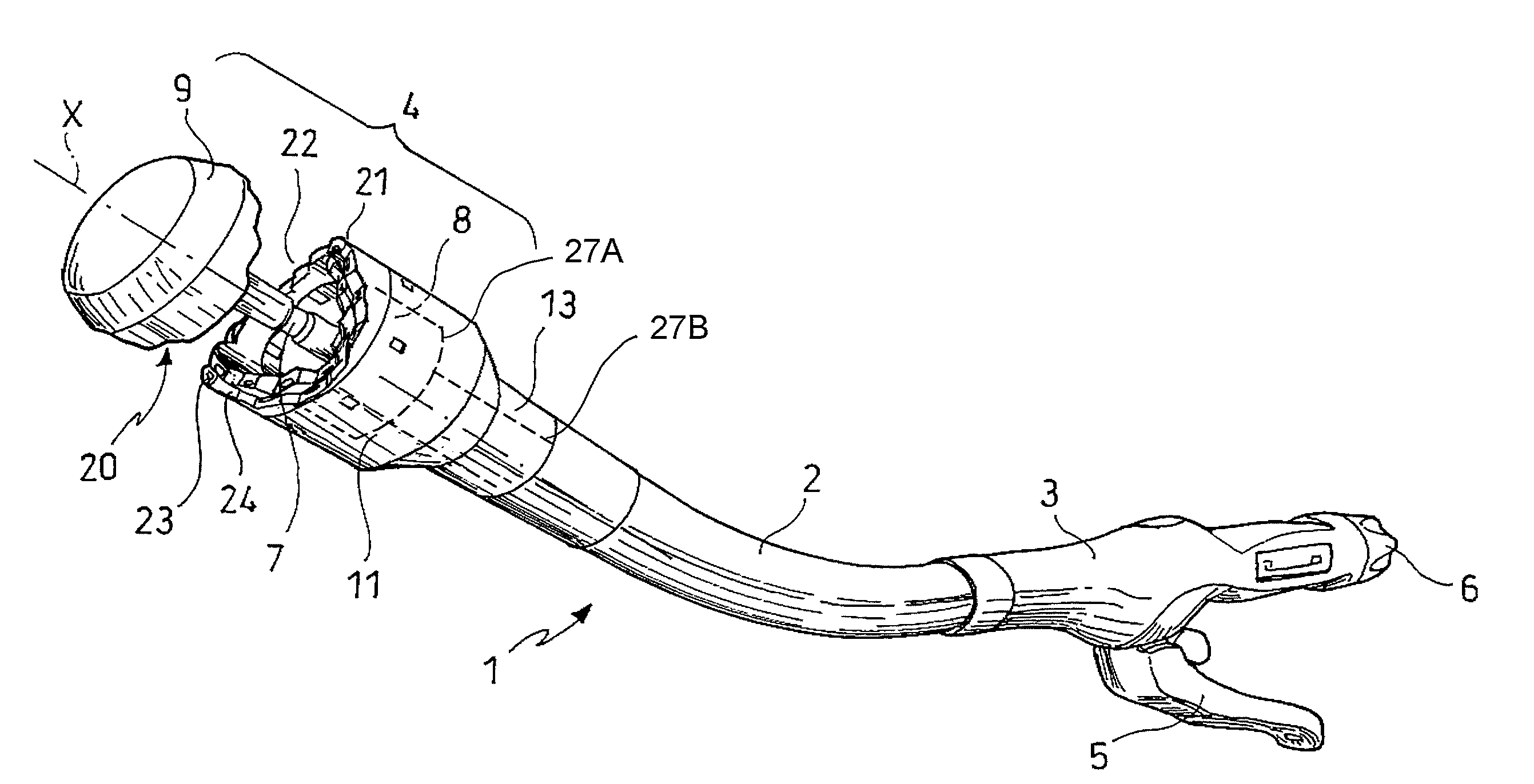
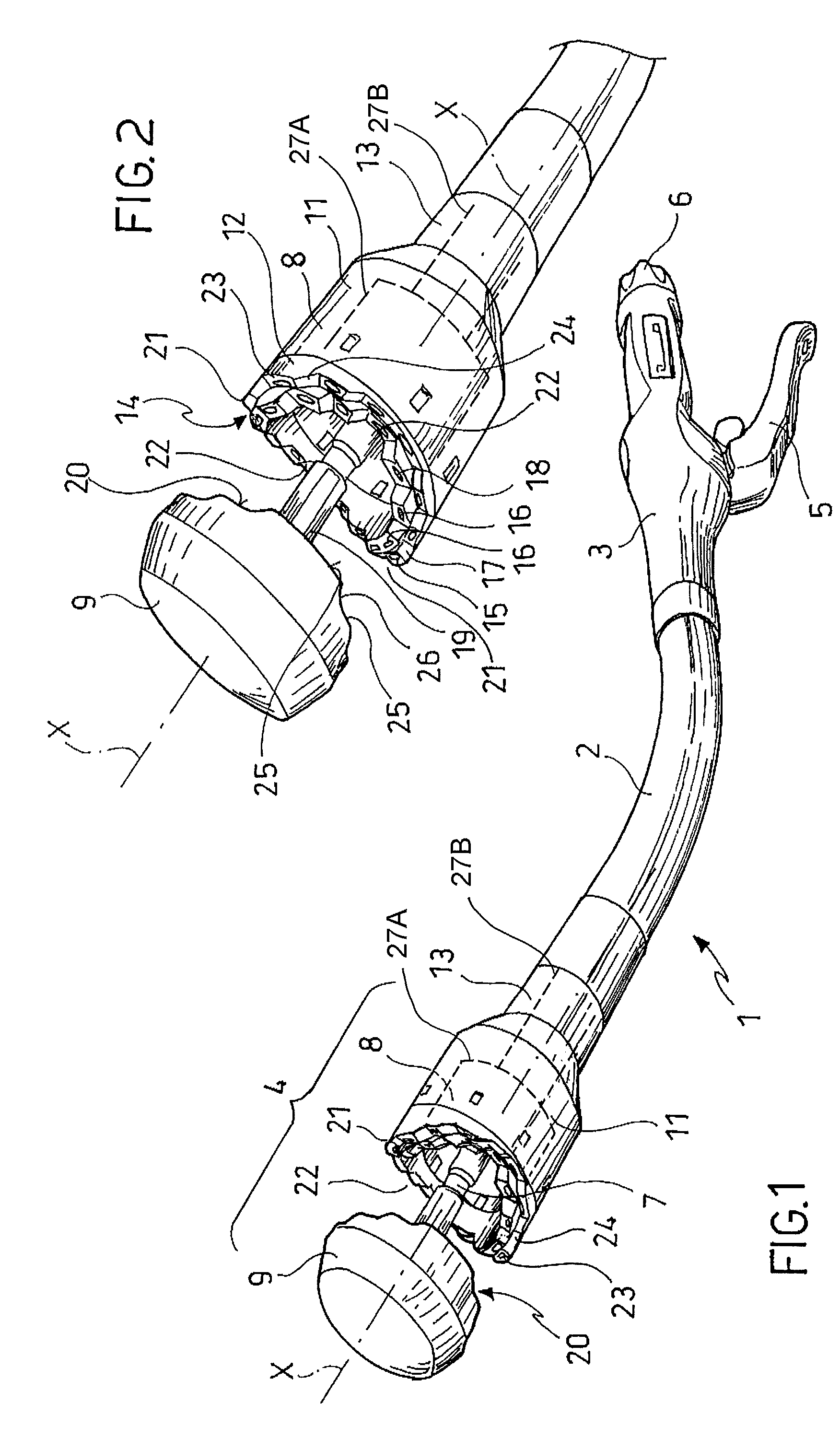
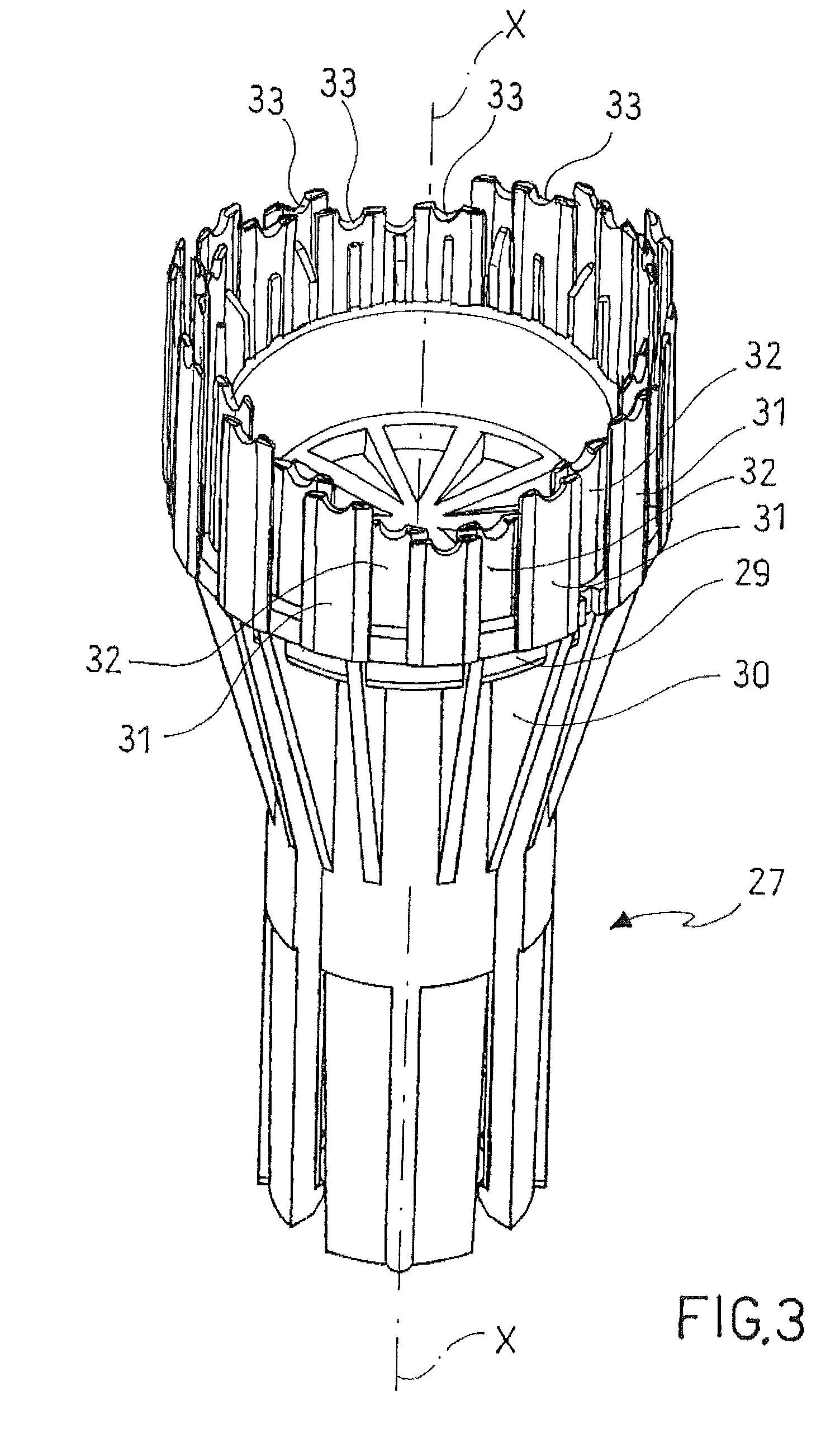
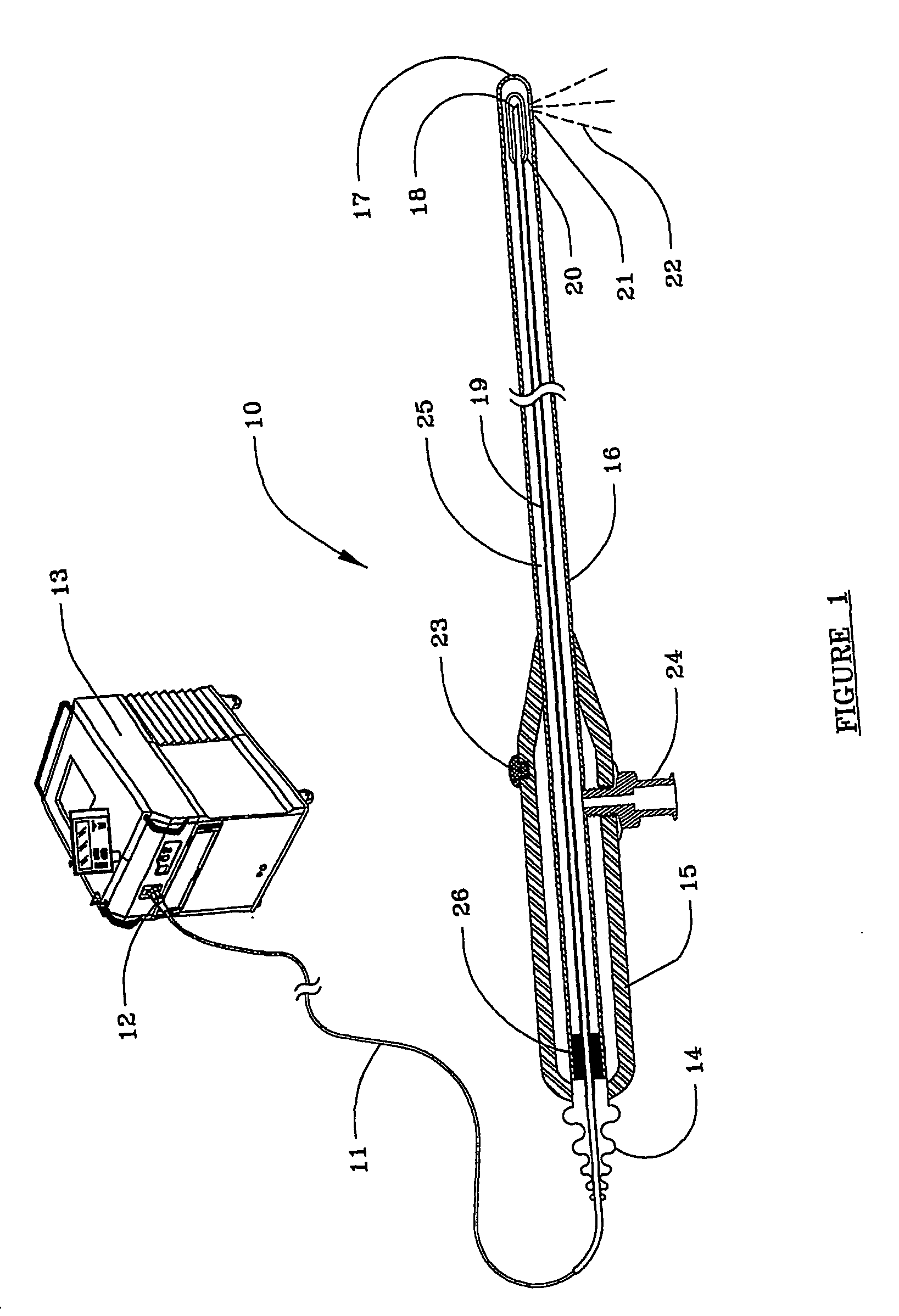
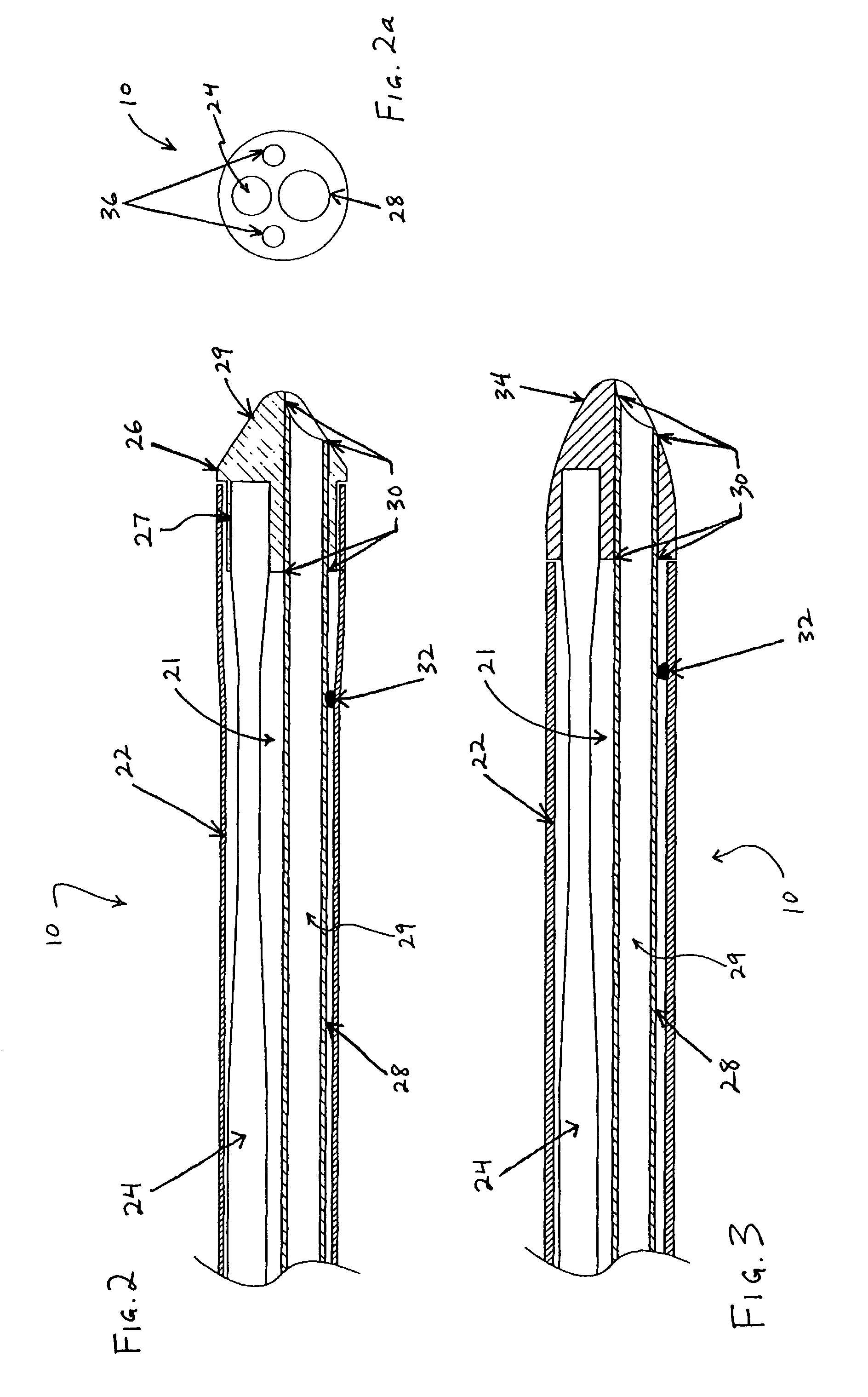
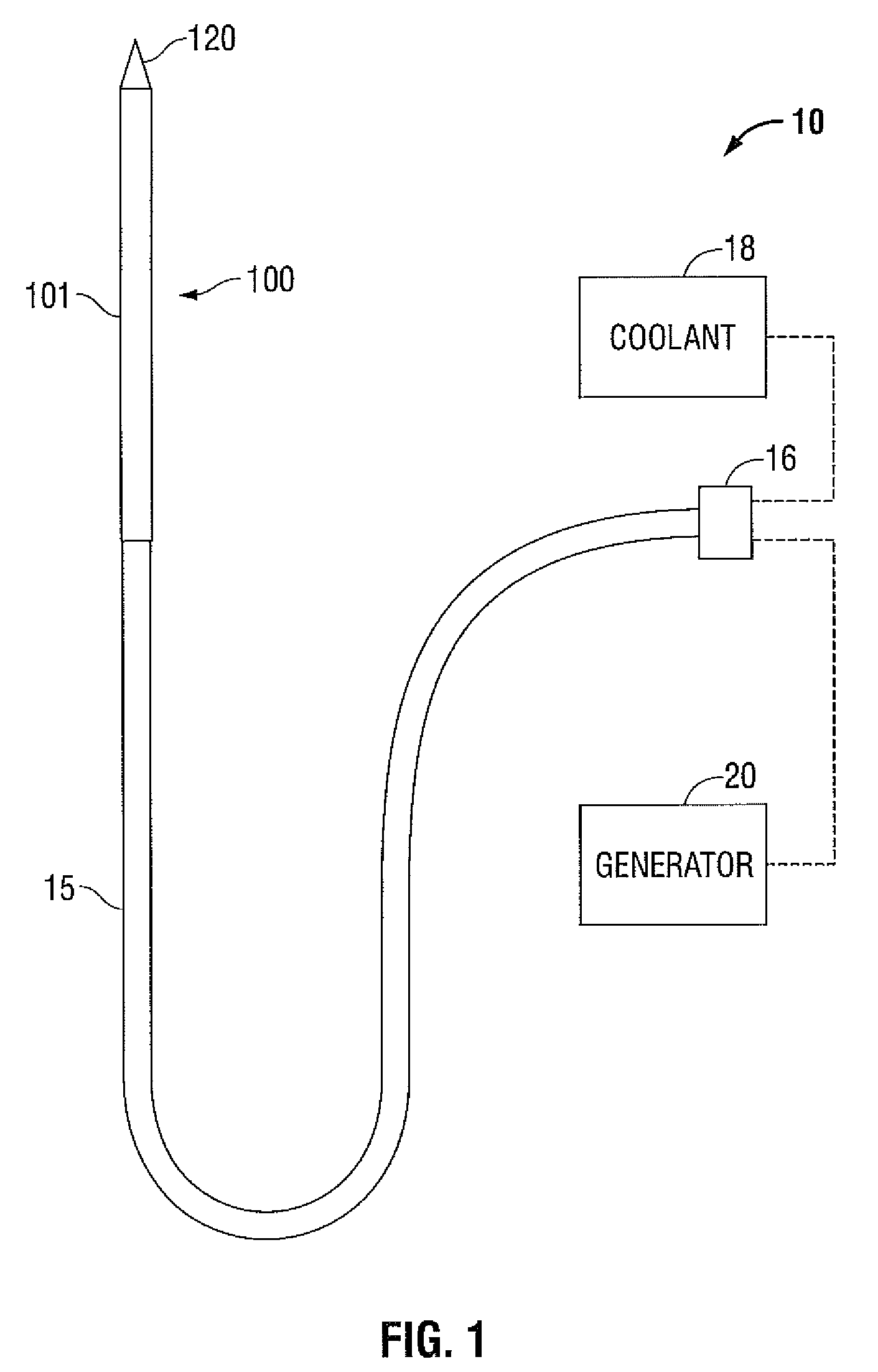
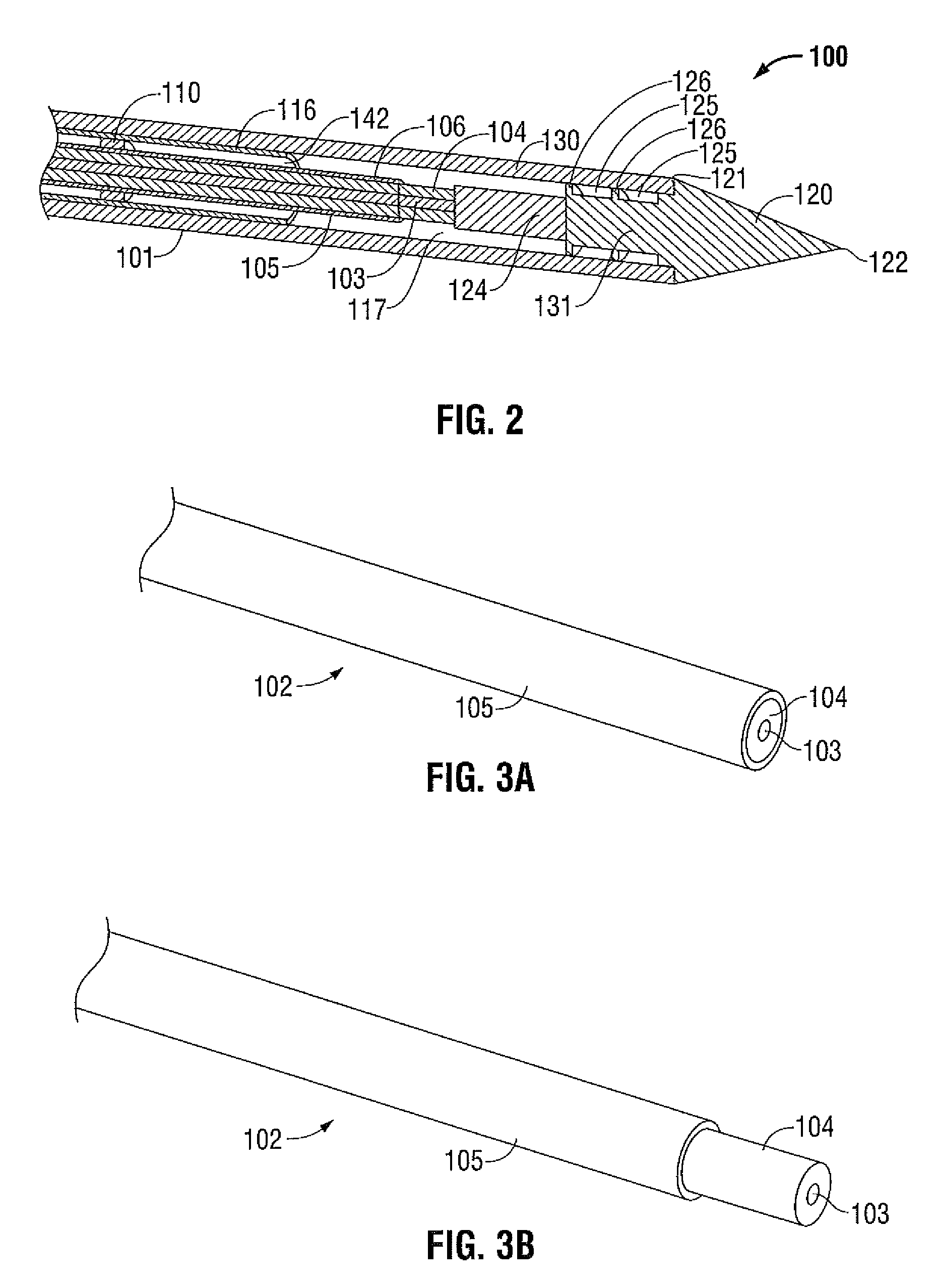
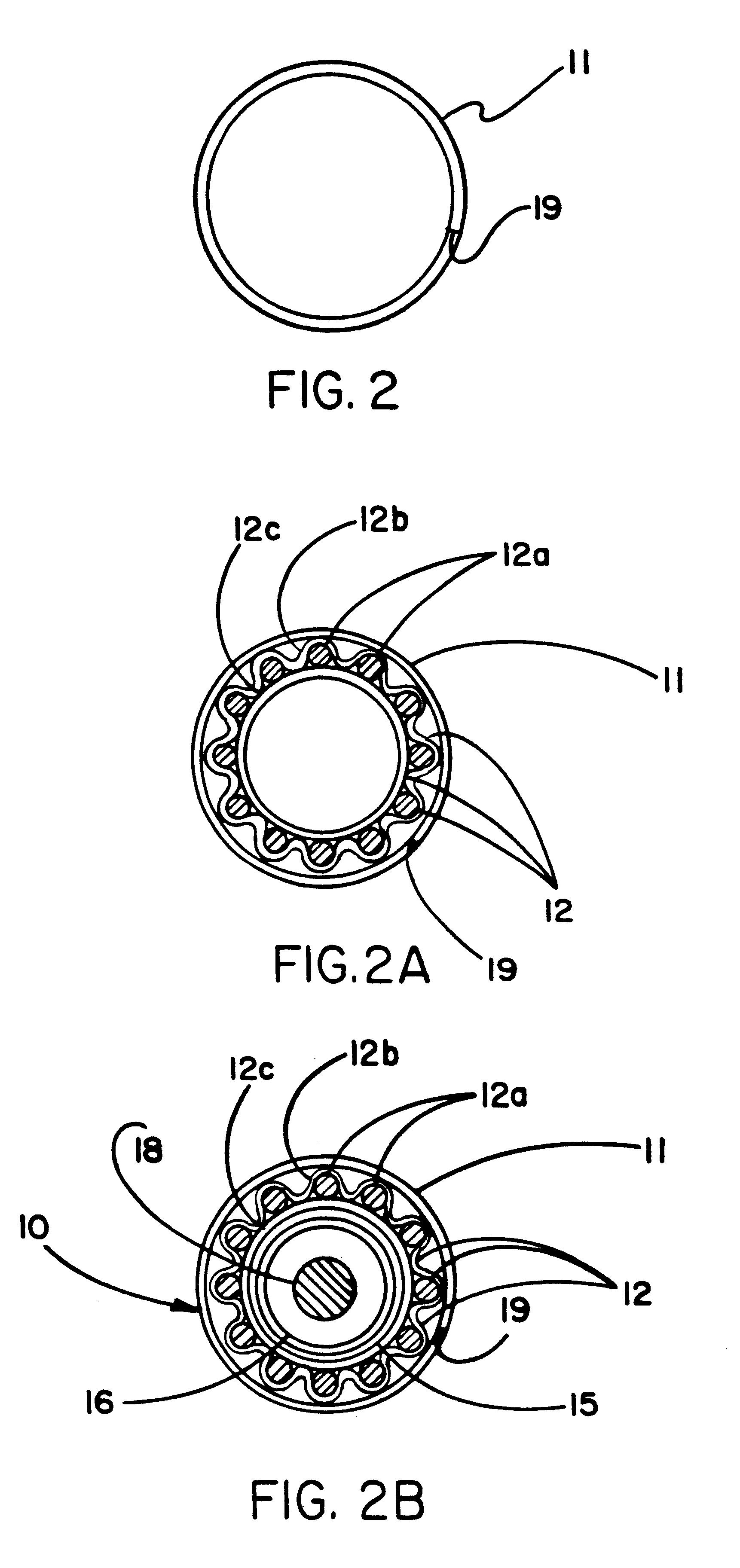
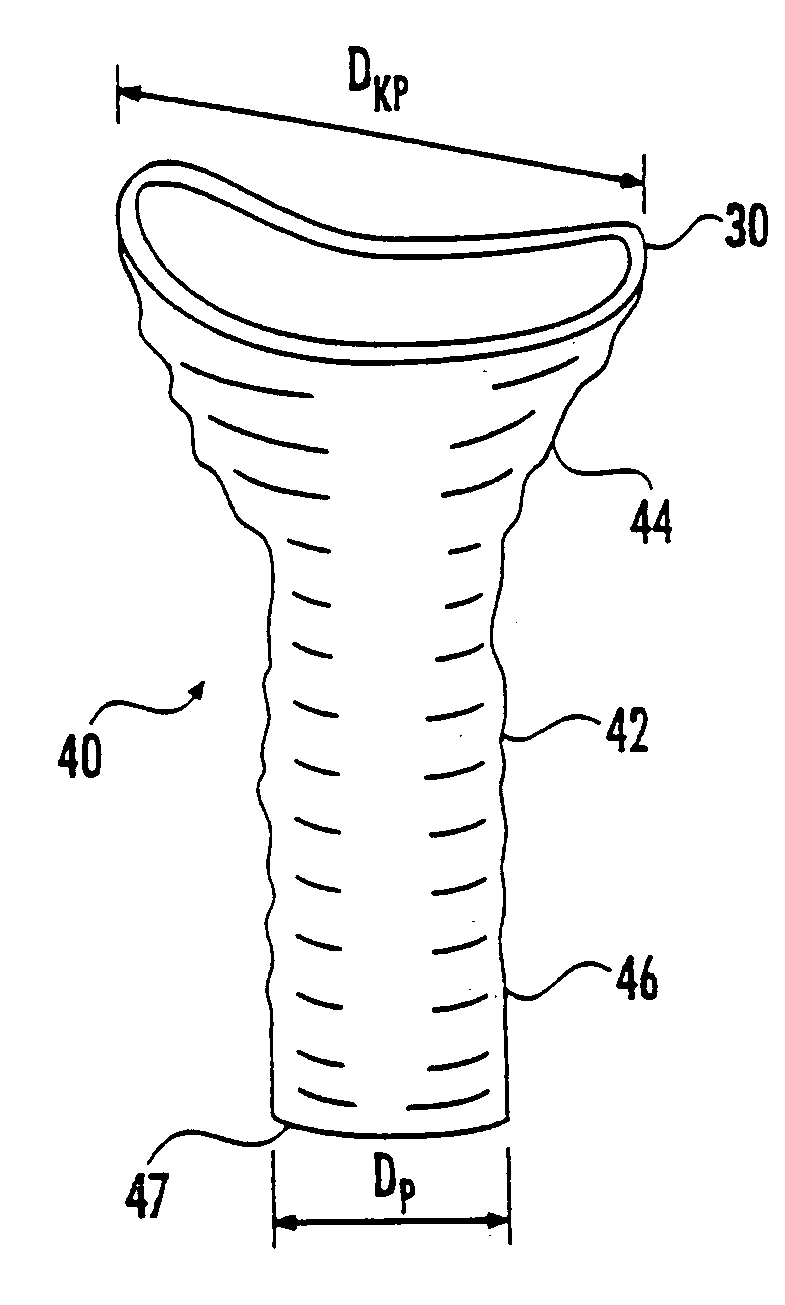
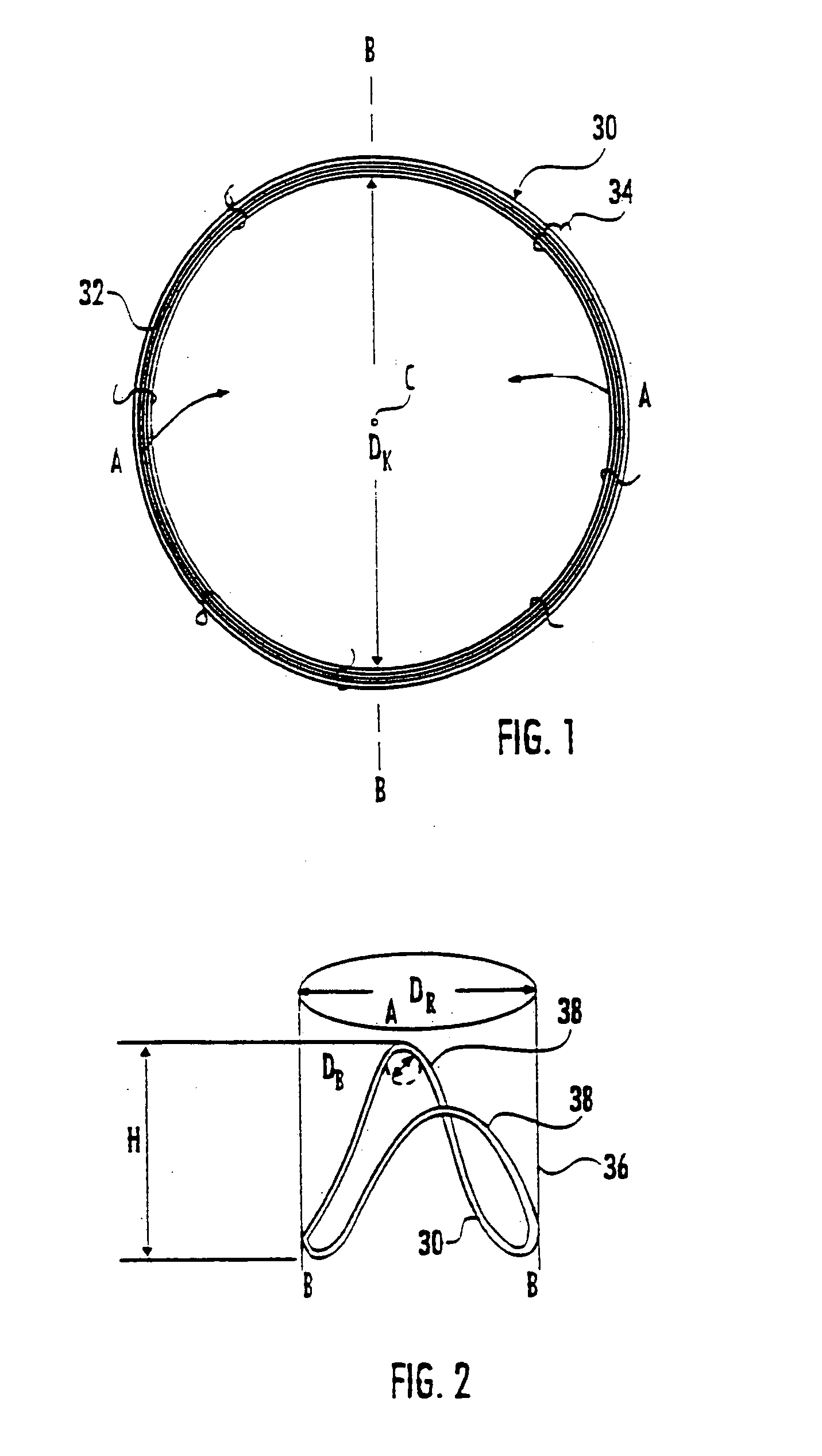
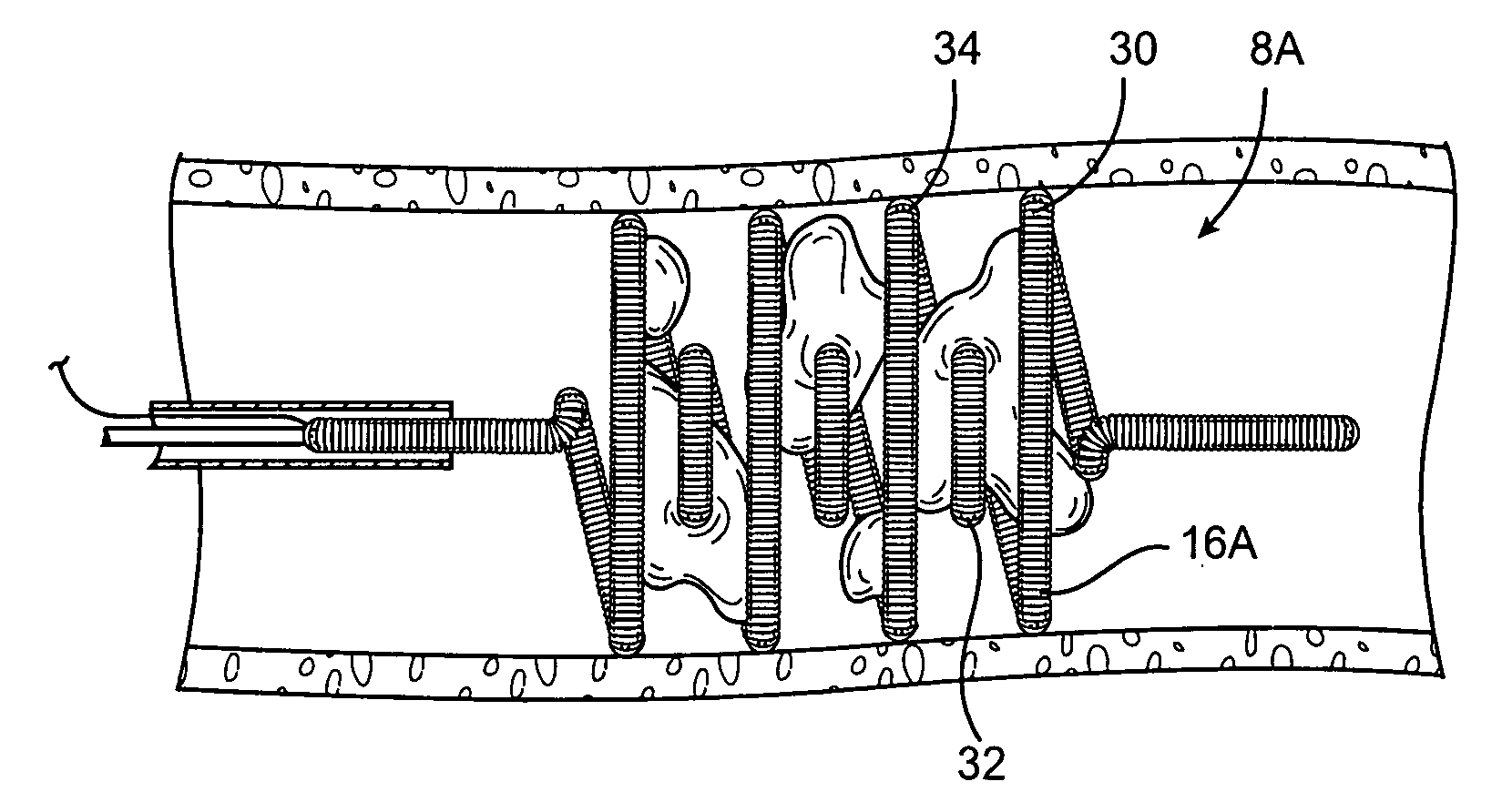
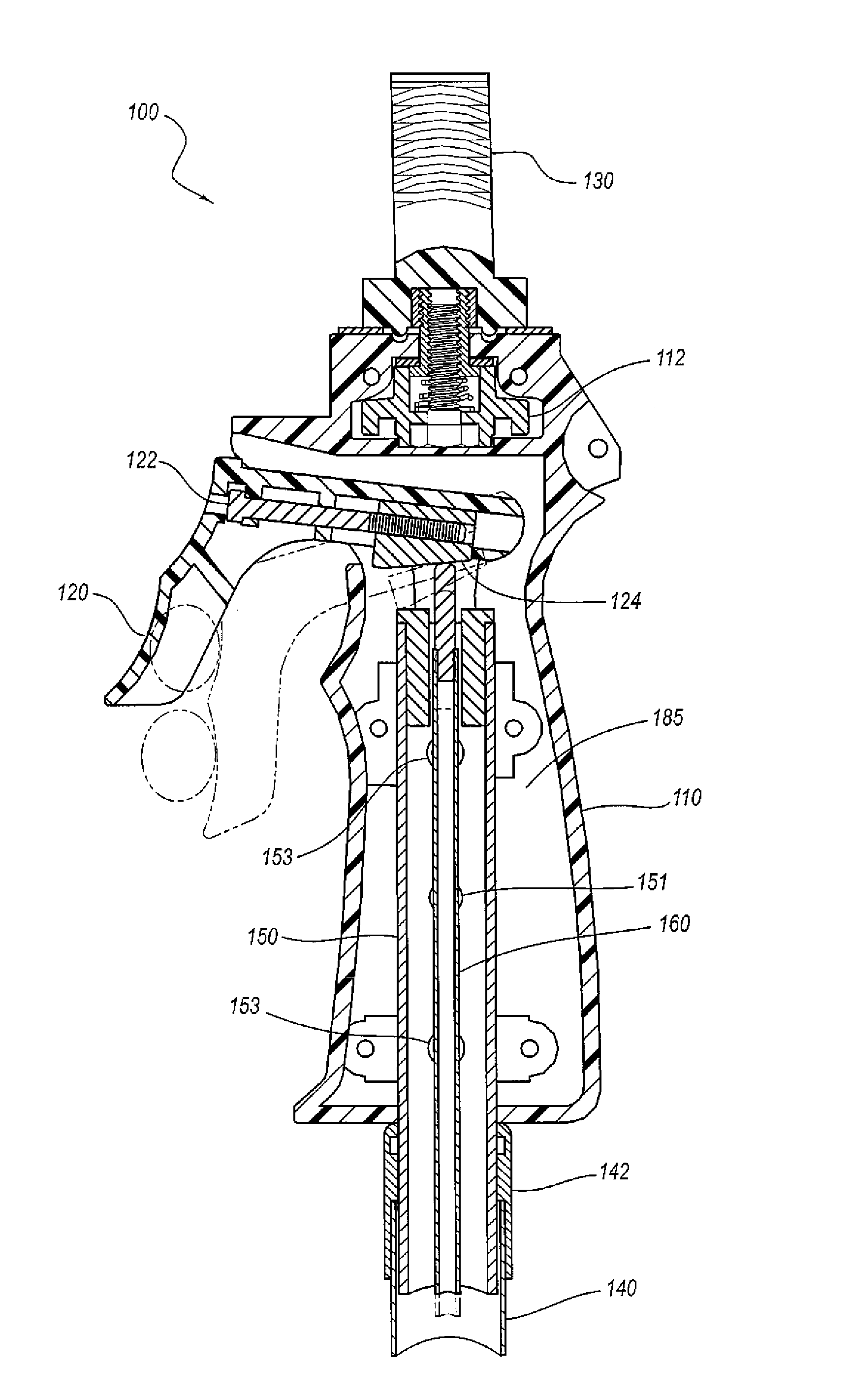
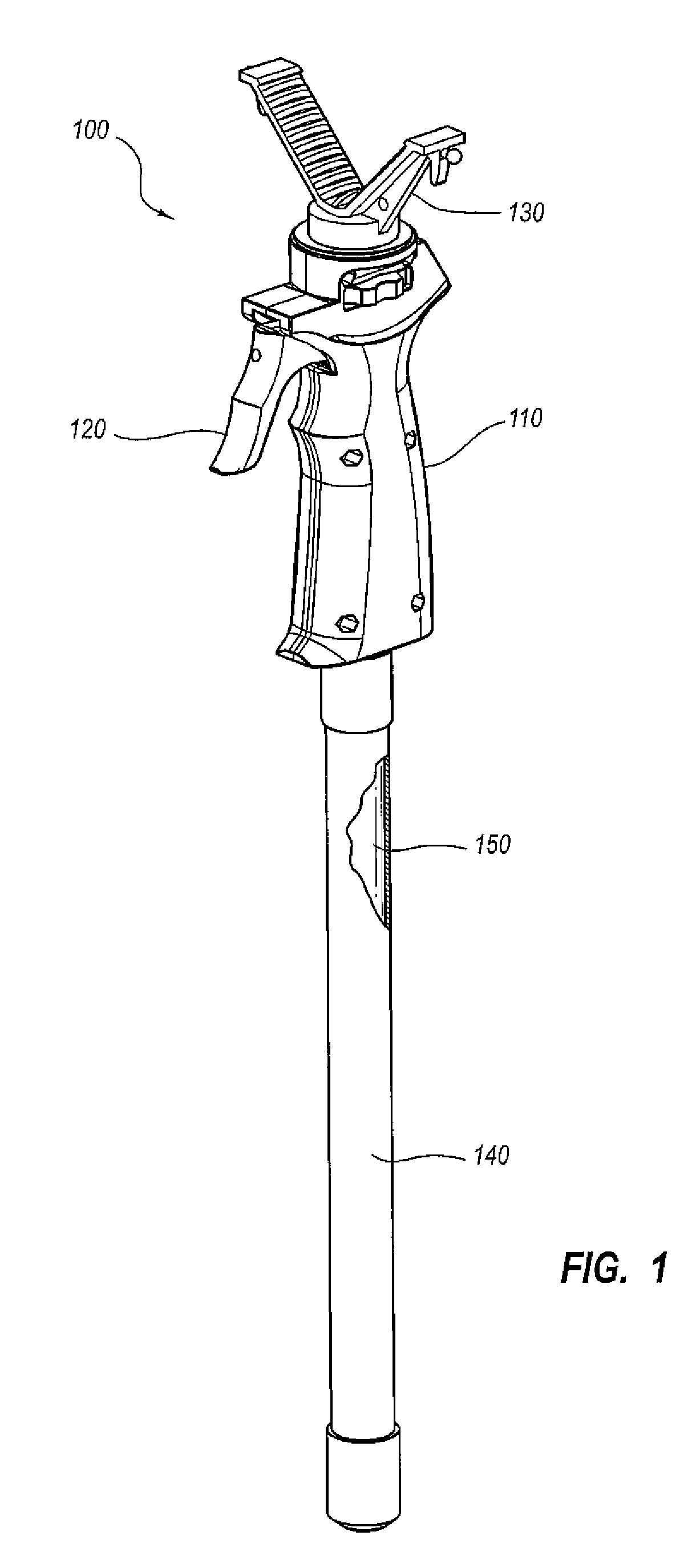
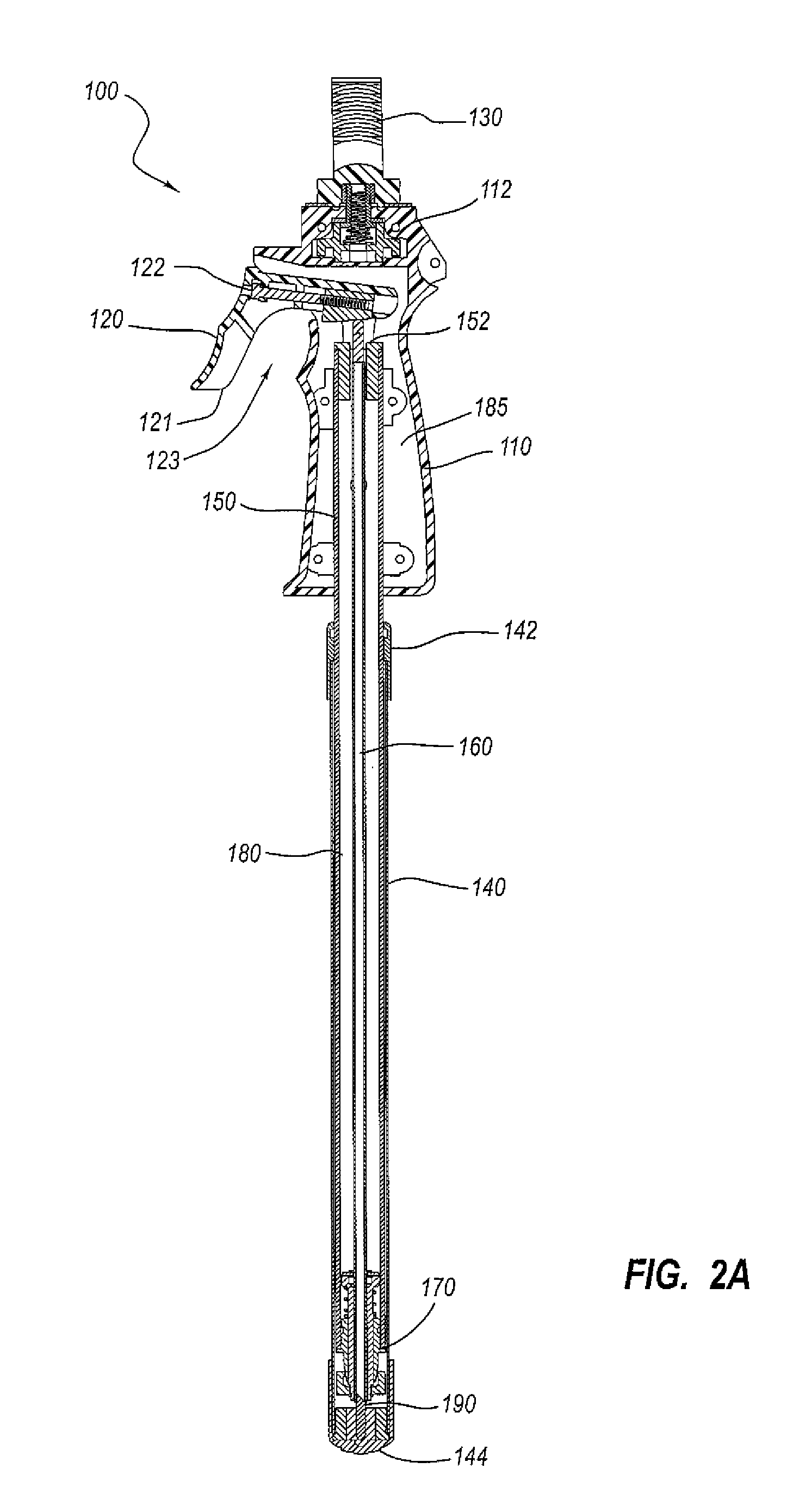
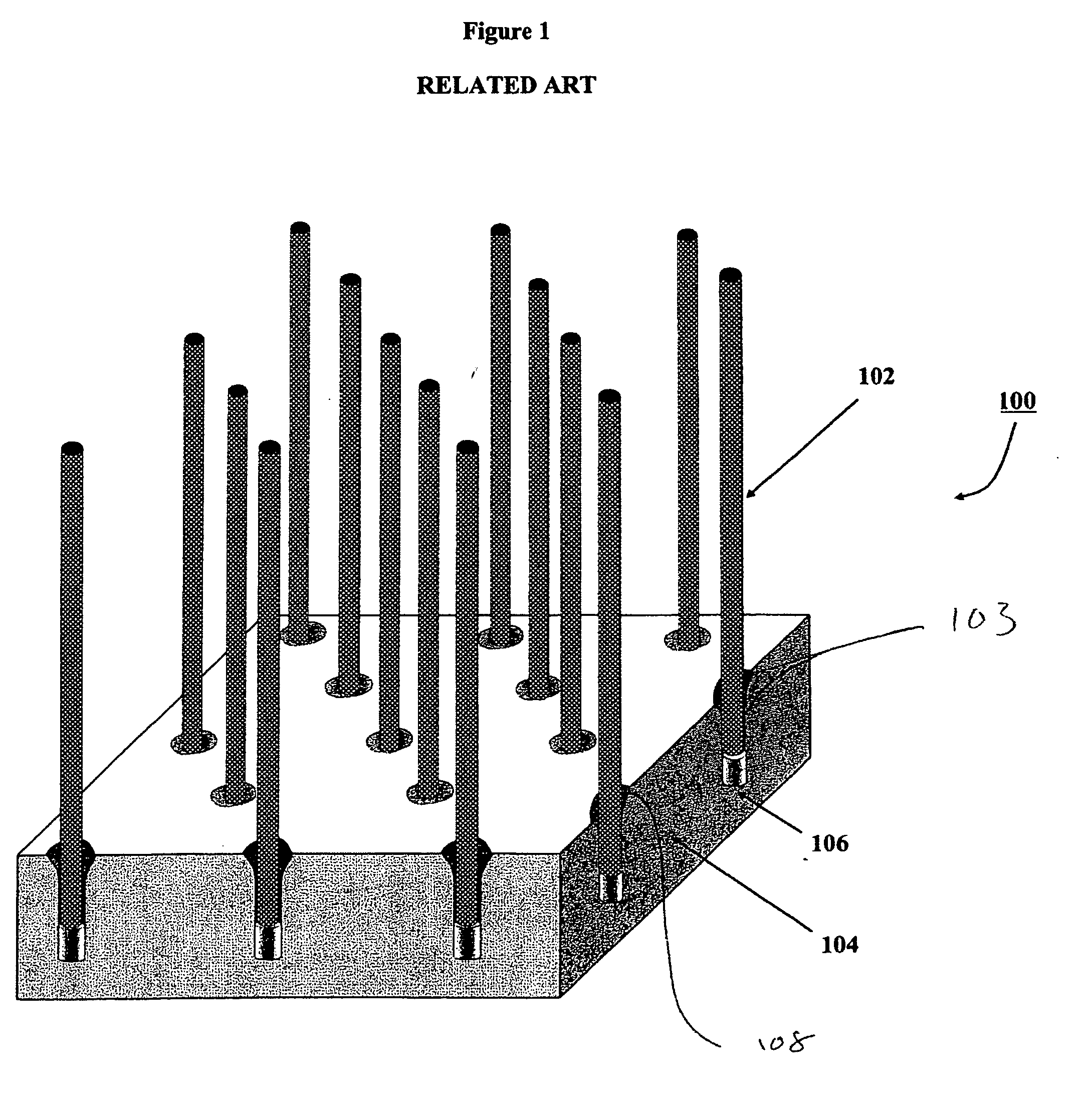
Surgical stapling instrument
InactiveUS8413870B2Improve scalabilityHigh gradientSuture equipmentsStapling toolsSurgical stapleBiomedical engineering
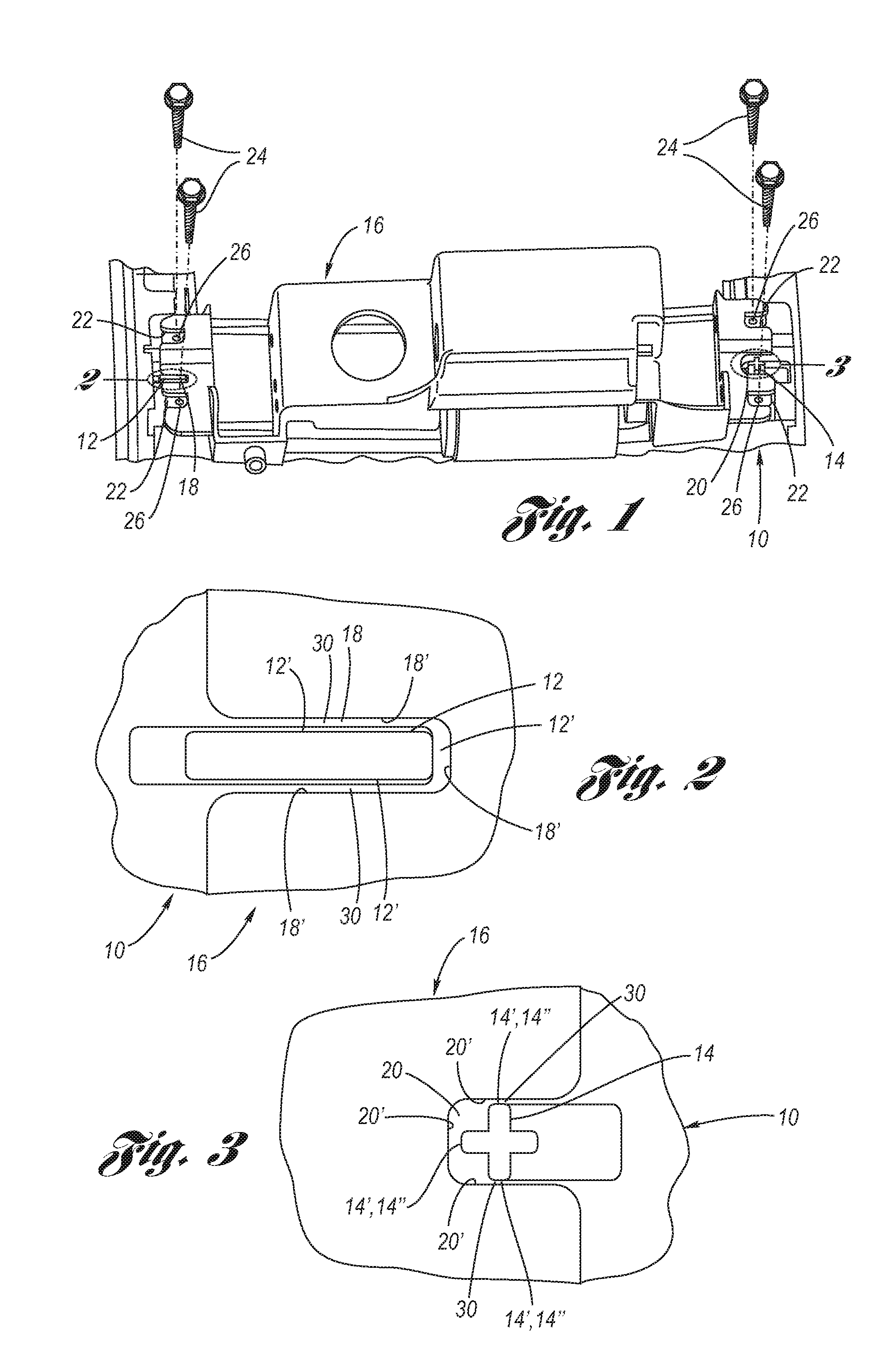
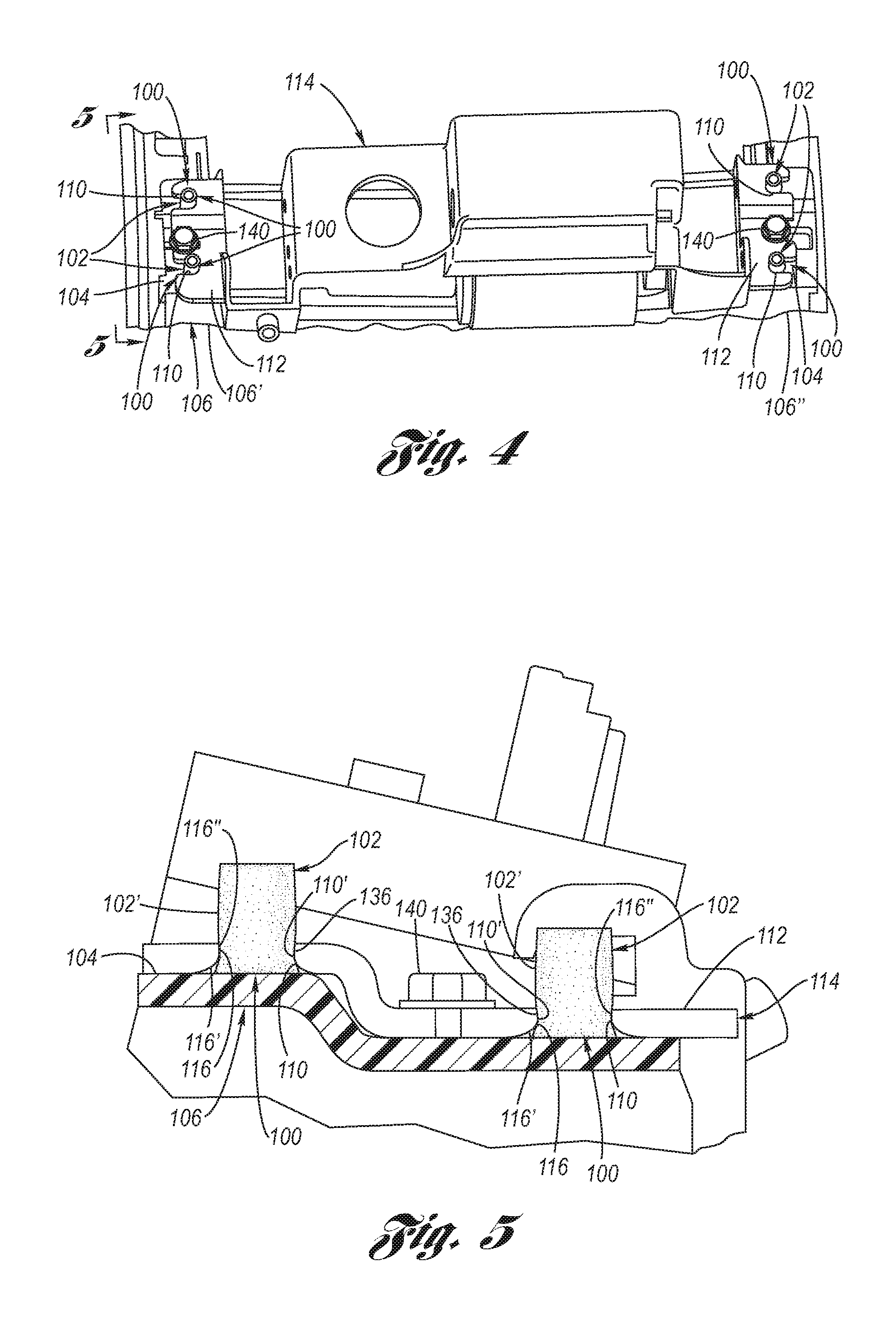
A surgical stapling instrument (1) comprises a staple fastening assembly (4) in the distal region of said instrument, the staple fastening assembly (4) including a cartridge device (8) which comprises at least one closed row (17, 18) of staples and defines a wavy distal end surface (14), and an anvil (9) which defines a wavy proximal staple forming surface (20) substantially matching the distal end surface (14) and which is adapted to cooperate with the cartridge device (8) for forming the ends of the staples exiting from the cartridge device (8), wherein the distal end surface (14) and the staple forming surface (20) have a globally wavy shape including two opposite peaks (21) and two opposite valleys (22) angularly spaced at about 90° from the adjacent peaks (21) and in that said two-peaks-two-valleys wavy shape is formed by a stepped configuration of said surfaces (14, 20).
Owner:ETHICON ENDO SURGERY INC
Devices and methods for minimally invasive treatment of degenerated spinal discs
InactiveUS20050222681A1Accurate spacingBone debris is eliminatedBone implantJoint implantsExpandable cageRadio frequency
Spinal stabilization devices and their methods of insertion and use to treat degenerated lumbar, thoracic or cervical spinal discs in minimally invasive, outpatient procedures are described. In one embodiment, the spinal stabilization device is an expandable cage made of a coil or perforated cylindrical tube with a bulbous or bullet-shaped distal end and a flat or rounded proximal end. In a preferred embodiment, the spinal stabilization device is mechanically expanded to a larger diameter or is made of a superelastic nickel-titanium alloy which is thermally programmed to expand to a relatively larger diameter when a pre-determined transition temperature below body temperature is reached. To treat a degenerated disc, a guide wire is inserted into the disc and an endoscope is inserted through a posterolateral puncture in the back and advanced up to the facet of the spine. Mechanical tools or laser energy, under endoscopic visualization, are used to remove or vaporize a portion of the facet bone, creating an opening into the foraminal space in the spine for insertion of an endoscope, which enables the disc, vertebra and nerves to be seen. The passageway is expanded, mechanical tools or laser of RF energy are used to make a tunnel into the disc, and a delivery cannula is inserted up to the opening of the tunnel. An insertion tool is used to insert one or more spinal stabilization devices into the tunnel in the disc, preserving the mobility of the spine, while maintaining the proper space between the vertebra. Laser or radio frequency (RF) energy is used to coagulate bleeding, vaporize or remove debris and shrink the annulus of the disc to close, at least partially, the tunnel made in the disc.
Owner:TRIMEDYNE
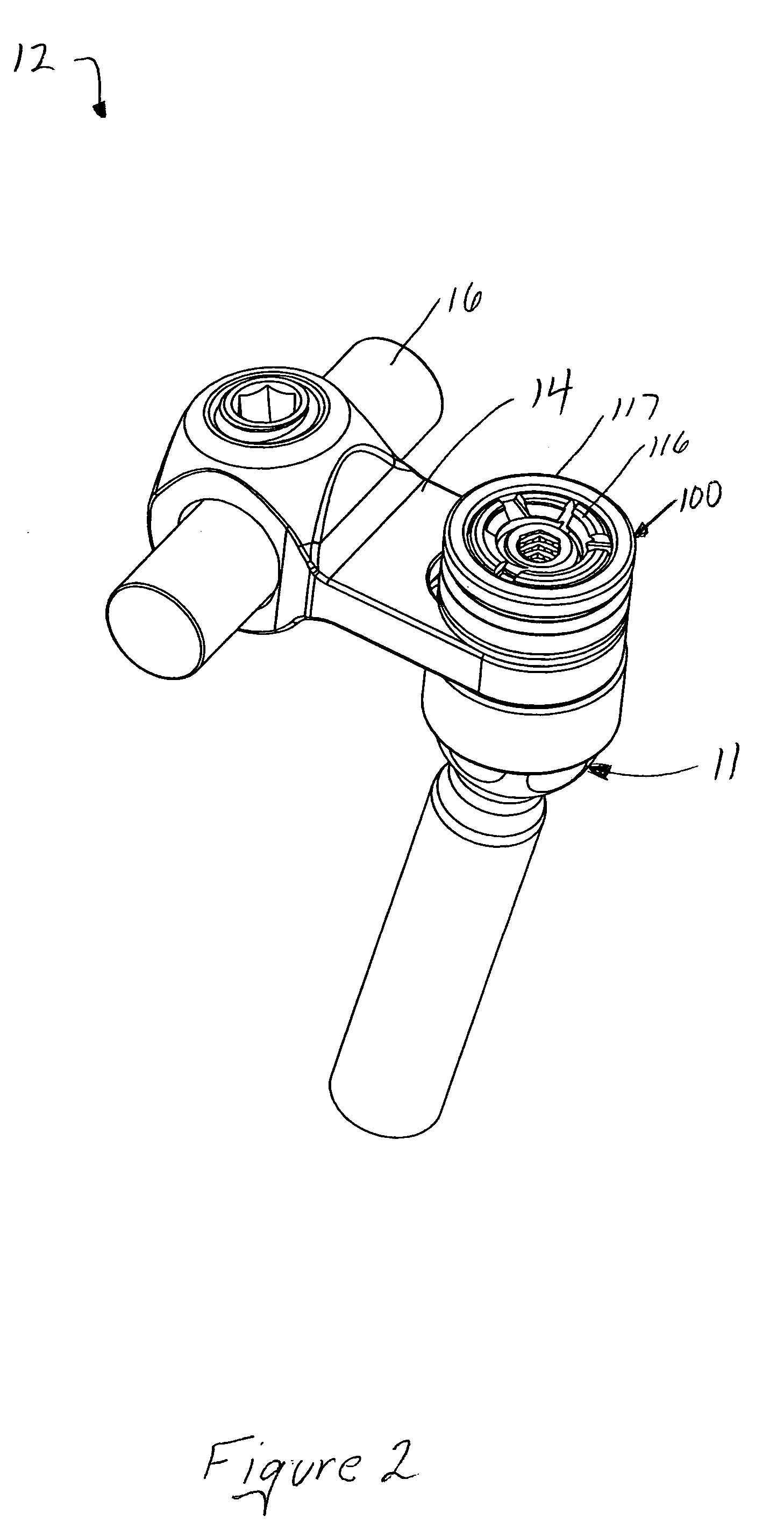
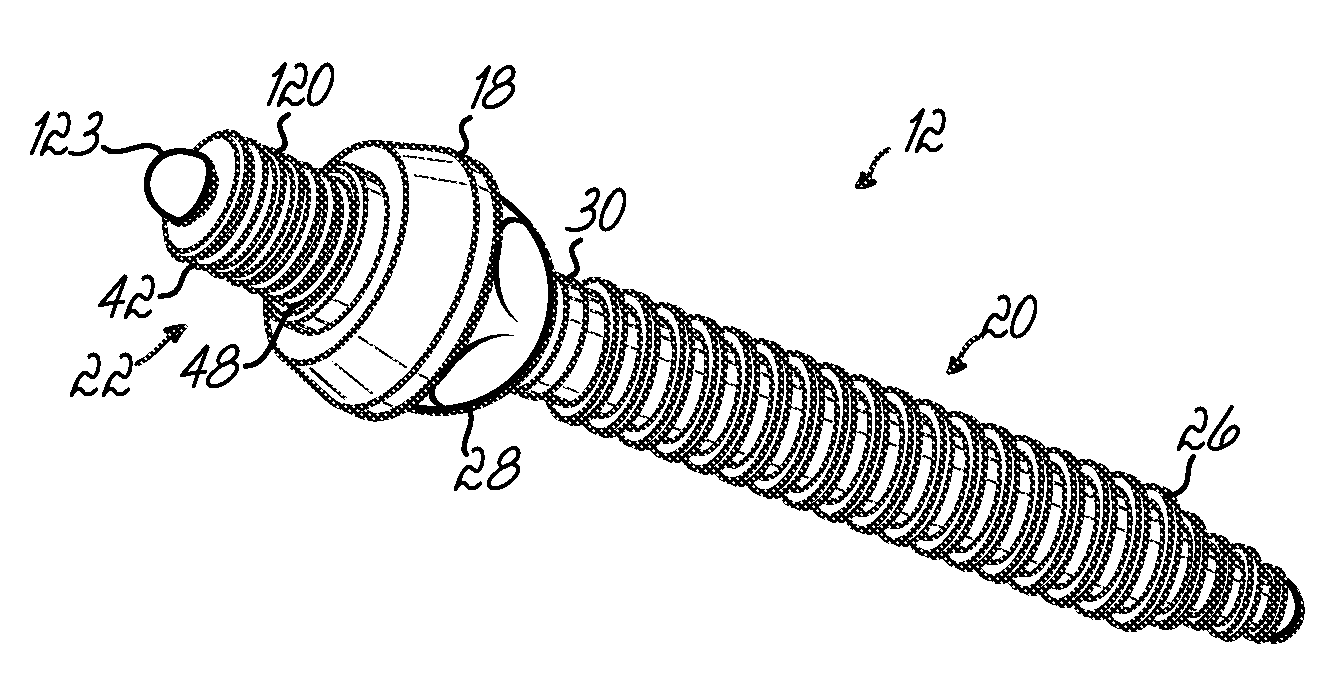
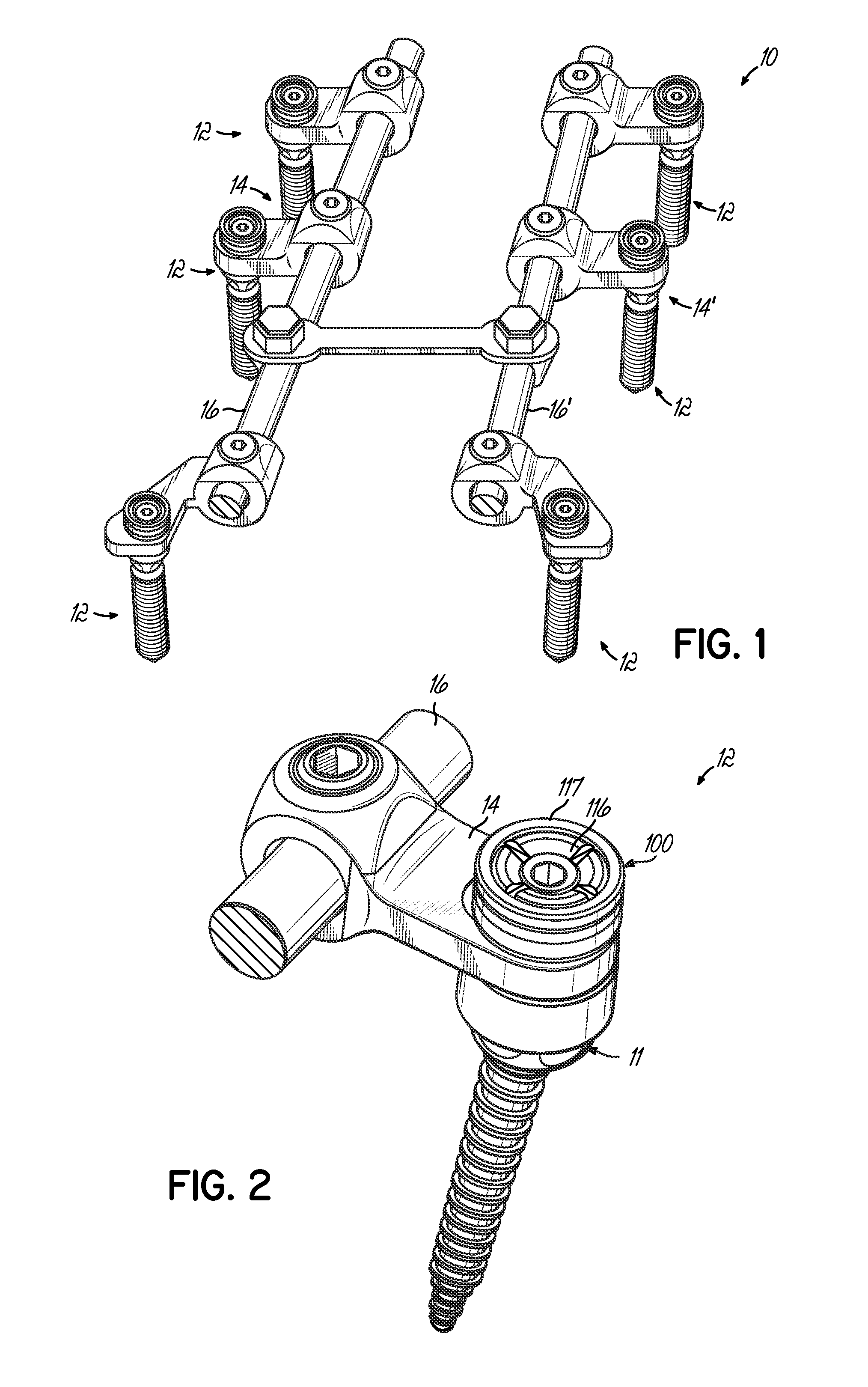
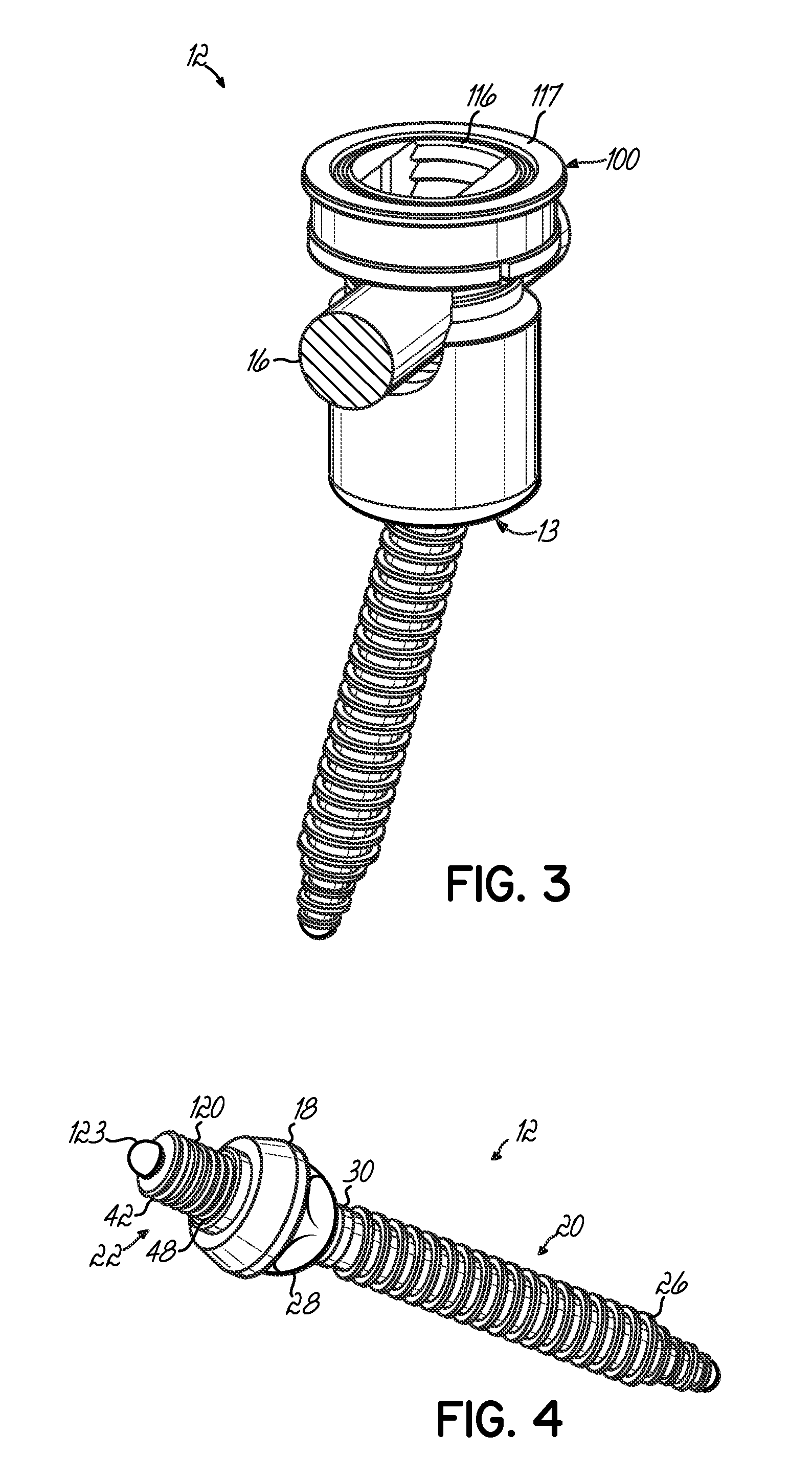
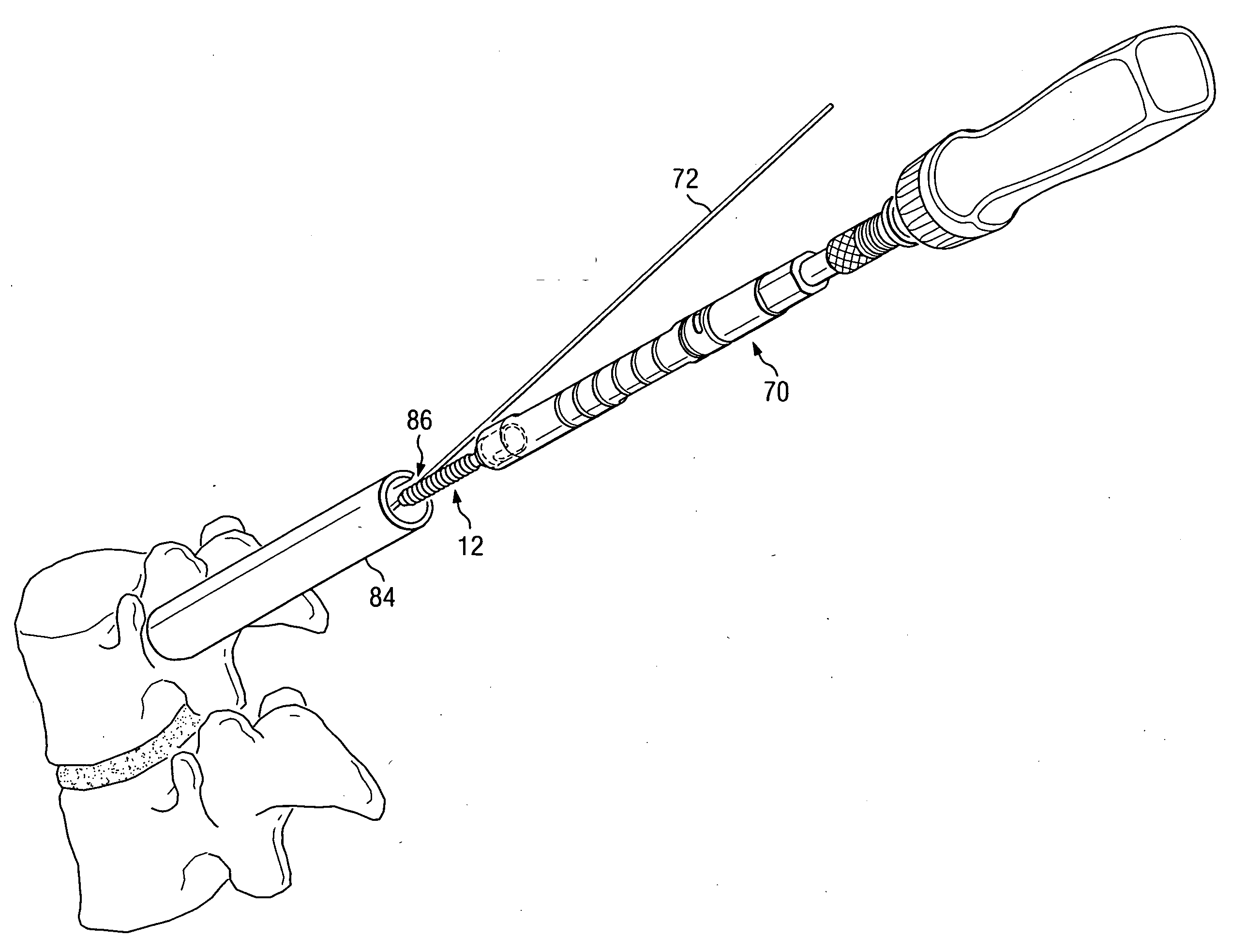
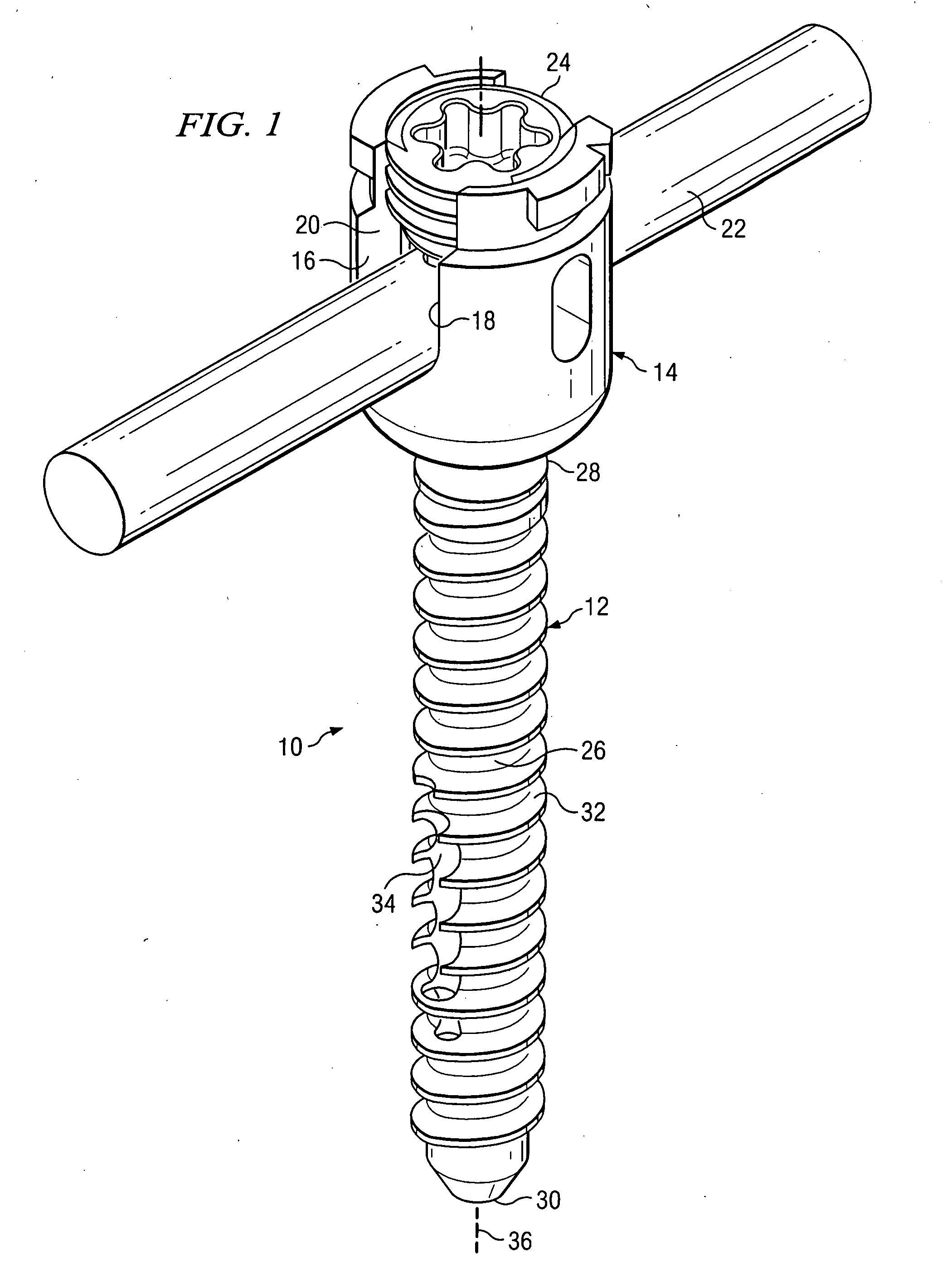
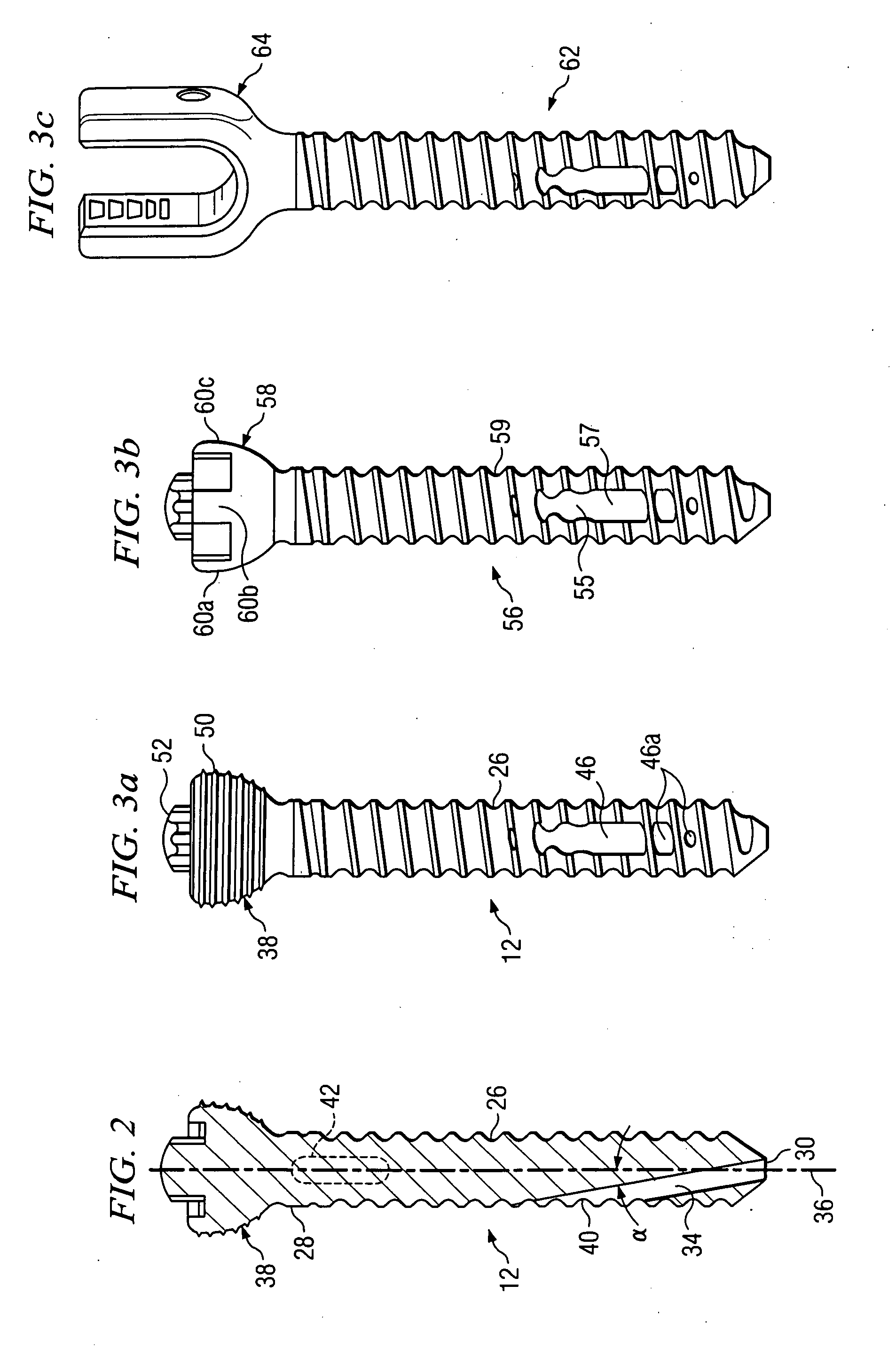
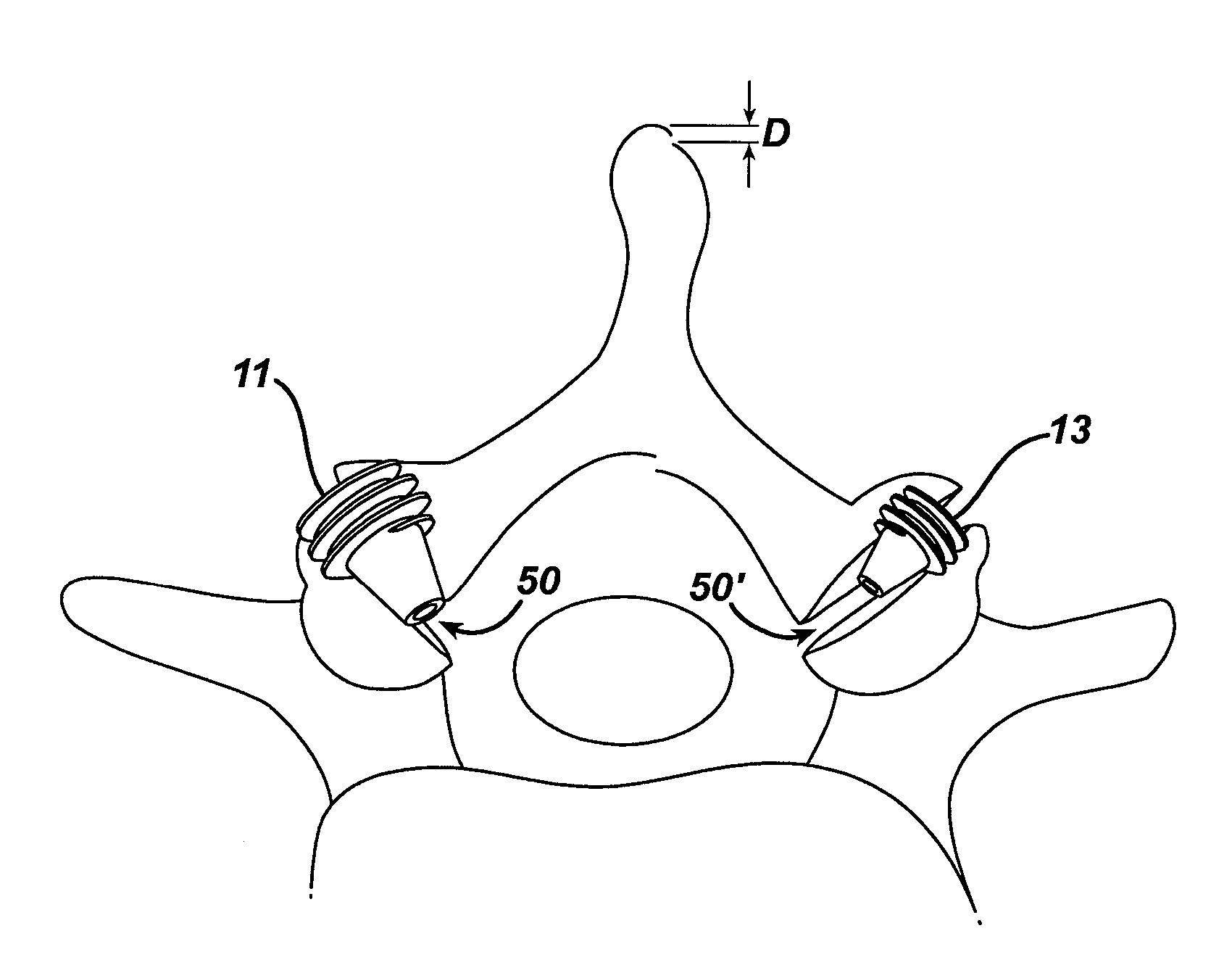
Polyaxial bone screw with torqueless fastening
ActiveUS20050070899A1Avoid disassemblyLarge caliberSuture equipmentsInternal osteosythesisBiomedical engineeringBone screws
An adjustable spinal fixation system is composed of a collection of anchoring assemblies attached, via a variety of connectors, to spine-stabilizing rods. The anchoring assemblies include a linking member attached in a ball-and-socket fashion to a bone-engaging member that is adapted to engage a spinal bone of a patient. The linking member joins one of the included connectors to an associated bone-engaging member. The connectors are selectively attached to one of the stabilizing rods. The anchoring assemblies each include a support collar and a split retention ring that cooperate to allow adjustment of the bone-engaging member and corresponding connector during surgery. When surgery is complete, a linear engaging fastener cooperates with the support collar and split retention ring to maintain the relative position of the entire fixation system, preventing unwanted movement between the system components.
Owner:SPINAL
Polyaxial bone screw with torqueless fastening
ActiveUS7335201B2Avoid disassemblyReduce the overall diameterSuture equipmentsInternal osteosythesisBiomedical engineeringBone screws
Owner:SPINAL
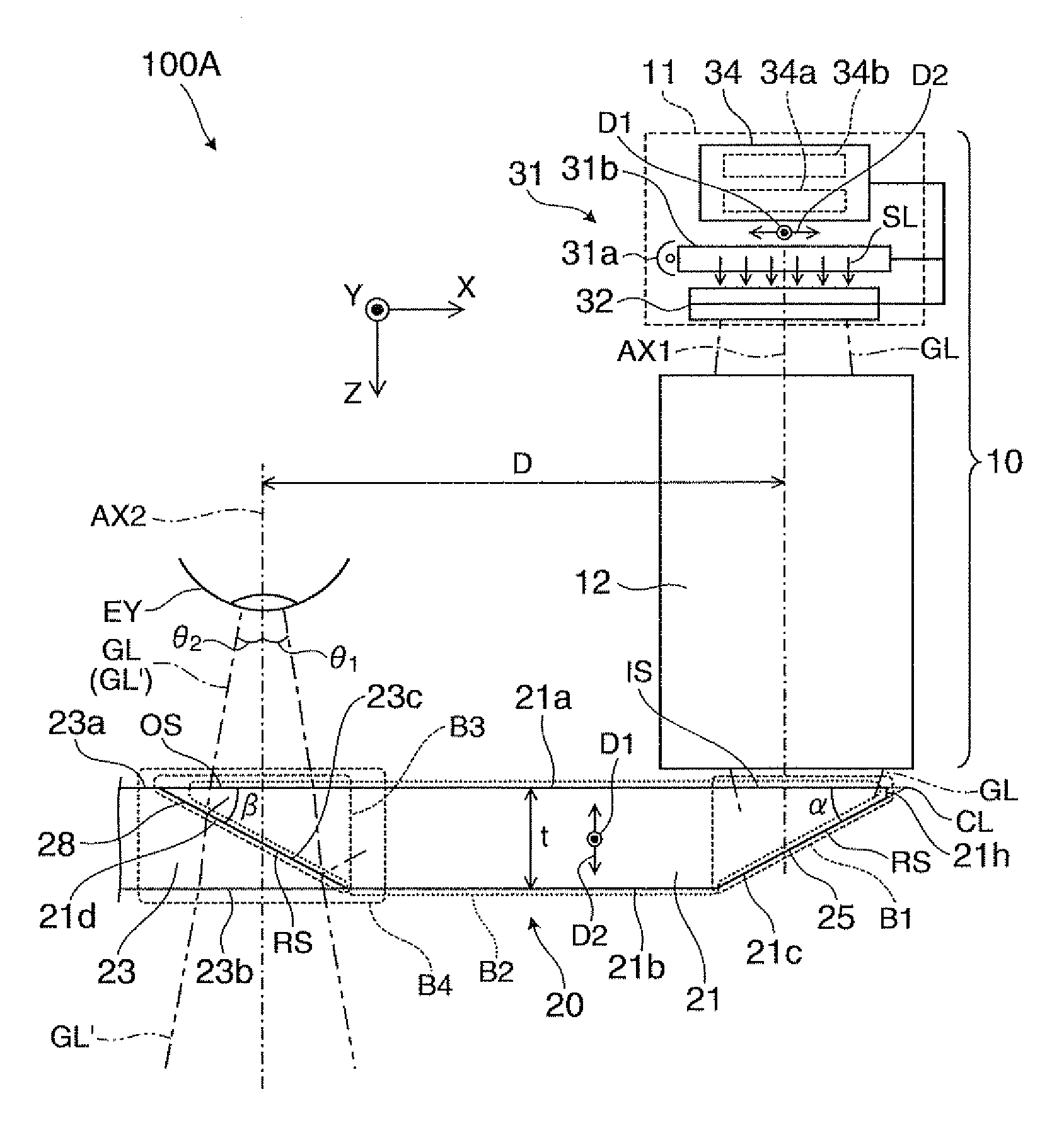
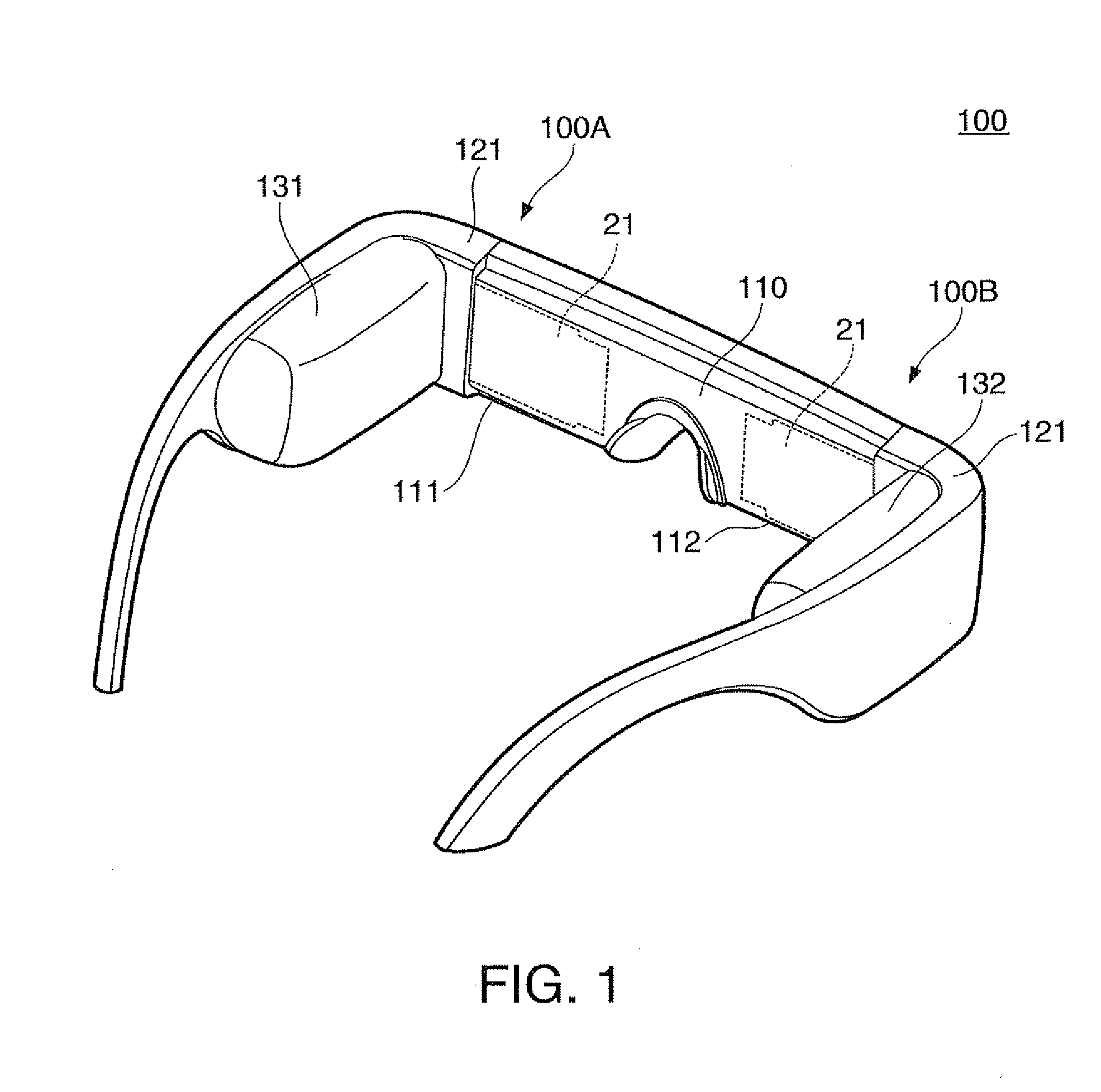
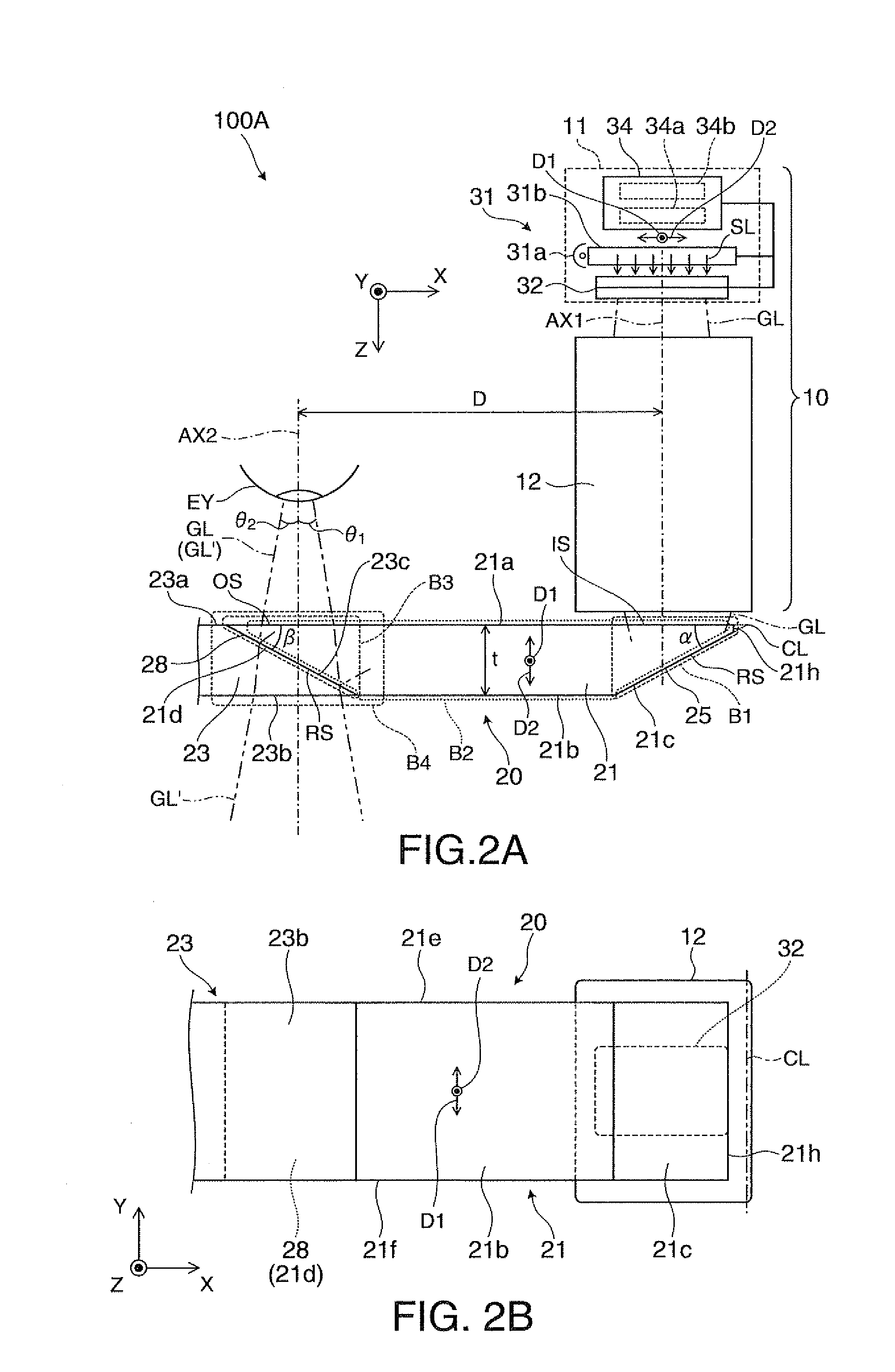
Virtual image display device
ActiveUS8564883B2Simple configurationMake up bulkyCathode-ray tube indicatorsOptical light guidesDisplay deviceLight beam
Owner:SEIKO EPSON CORP
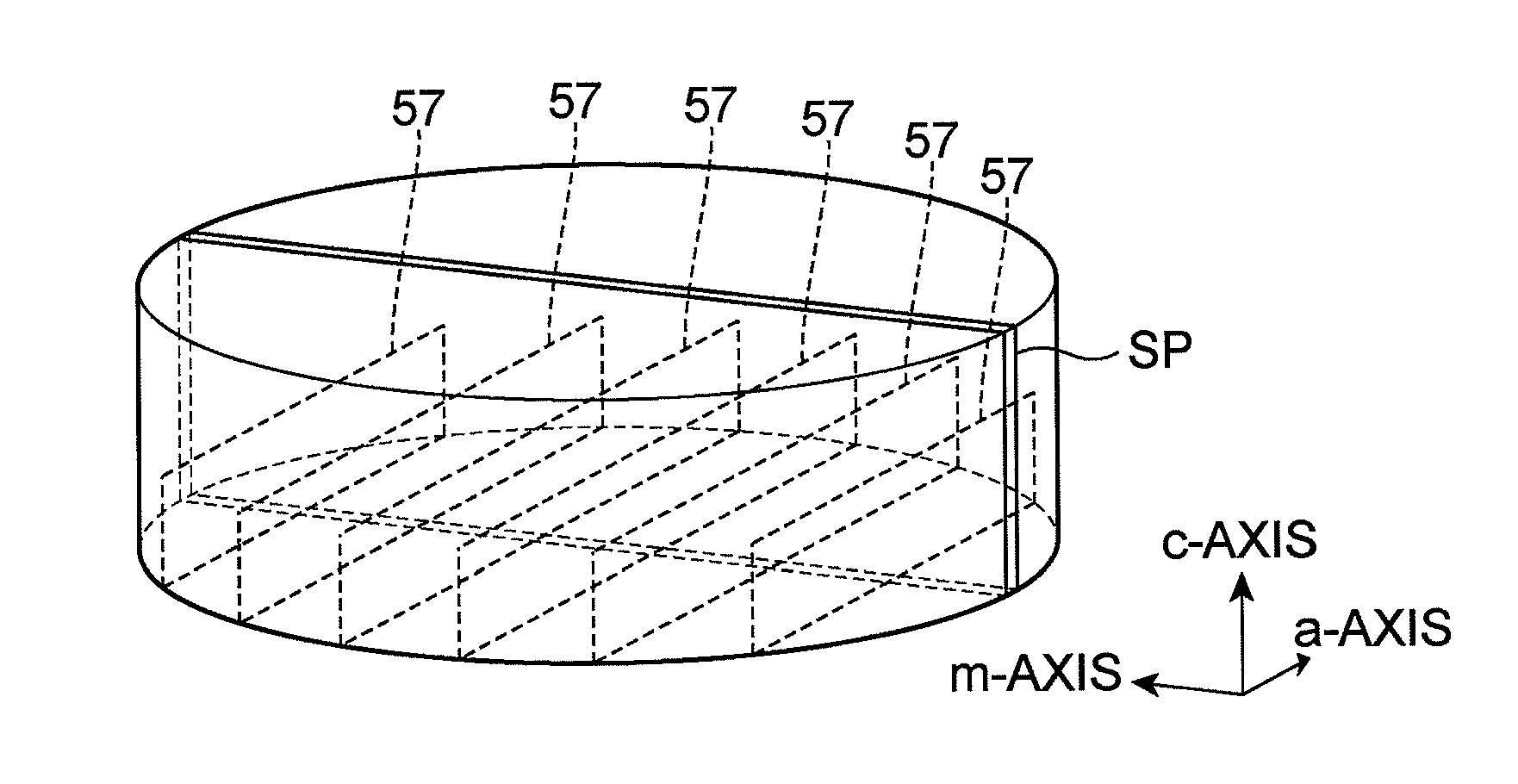
Method of fabricating nitride semiconductor laser
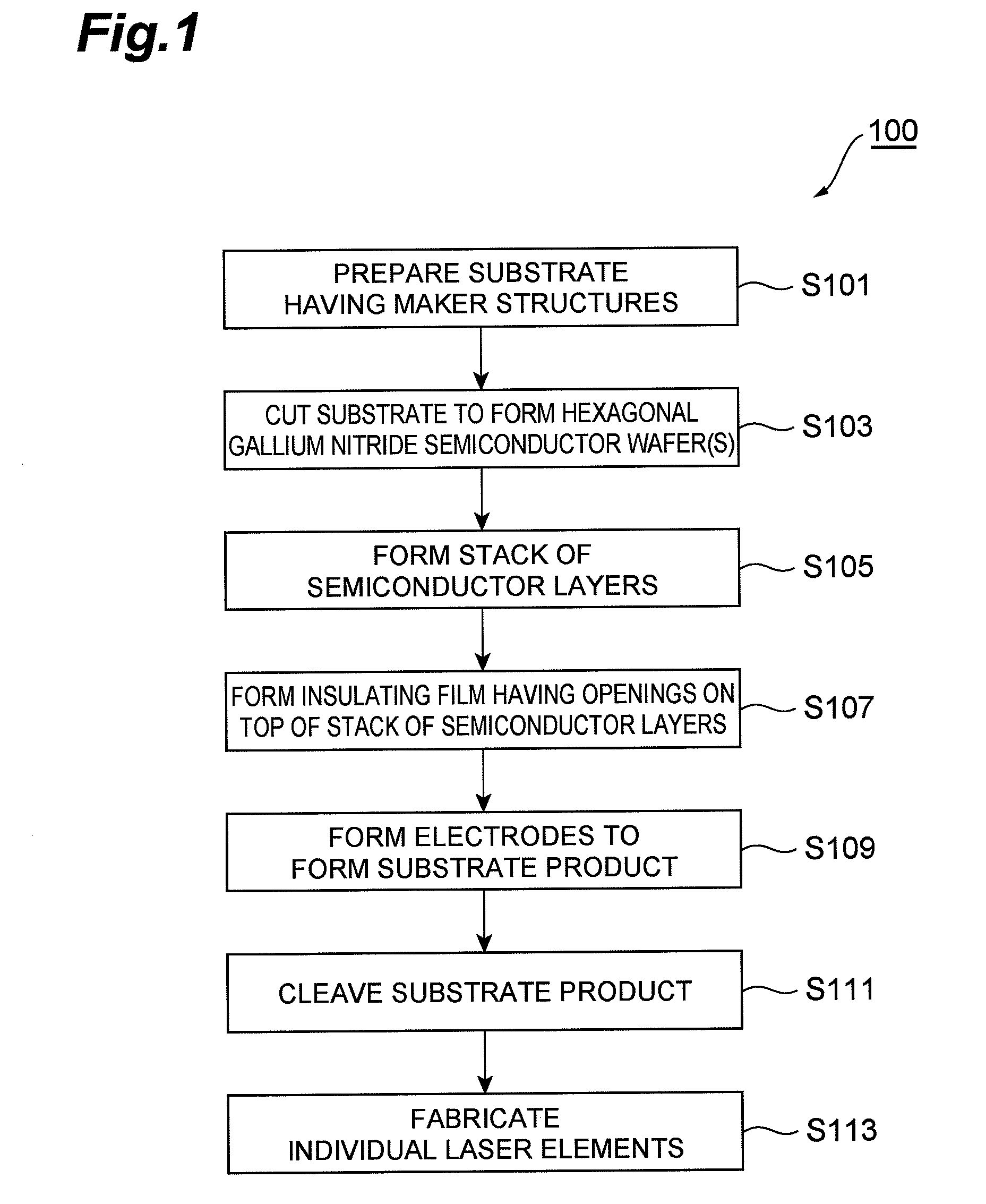
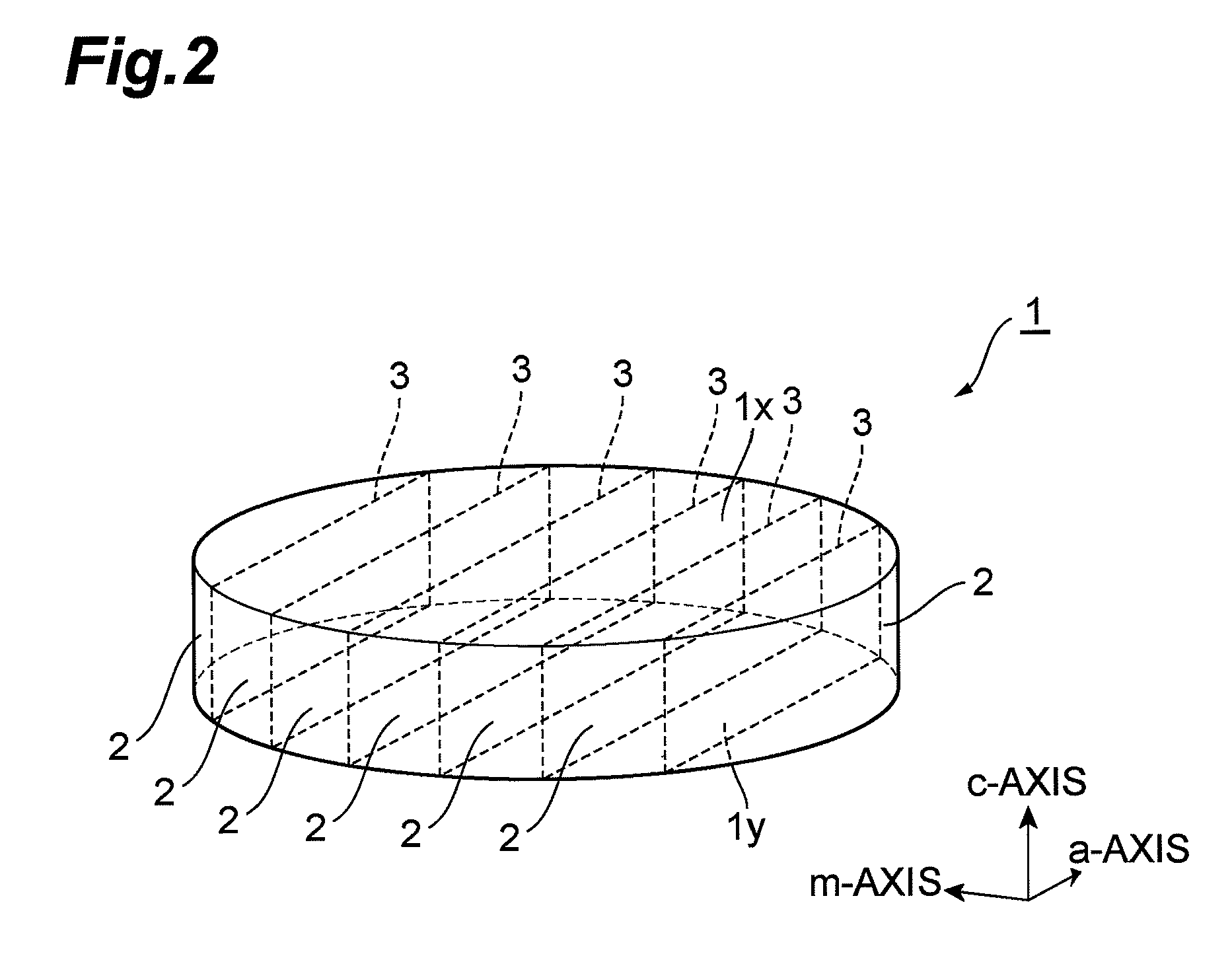
InactiveUS7939354B2Good orientationLarge caliberSemiconductor/solid-state device detailsSemiconductor laser structural detailsGallium nitrideNitride semiconductors
A method of fabricating a nitride semiconductor laser comprises preparing a substrate having a plurality of marker structures and a crystalline mass made of a hexagonal gallium nitride semiconductor. The primary and back surfaces of the substrate intersect with a predetermined axis extending in the direction of a c-axis of the hexagonal gallium nitride semiconductor. Each marker structure extends along a reference plane defined by the c-axis and an m-axis of the hexagonal gallium nitride semiconductor. The method comprises cutting the substrate along a cutting plane to form a wafer of hexagonal gallium nitride semiconductor, and the cutting plane intersects with the plurality of the marker structures. The wafer has a plurality of first markers, each of which extends from the primary surface to the back surface of the wafer, and each of the first markers comprises part of each of the marker structures. The primary surface of the wafer is semipolar or nonpolar. The method comprises growing a number of gallium nitride based semiconductor layers for a semiconductor laser. The method comprises cleaving the substrate product at a cleavage plane of the hexagonal gallium nitride semiconductor, after forming a substrate product in an electrode forming step.
Owner:SUMITOMO ELECTRIC IND LTD
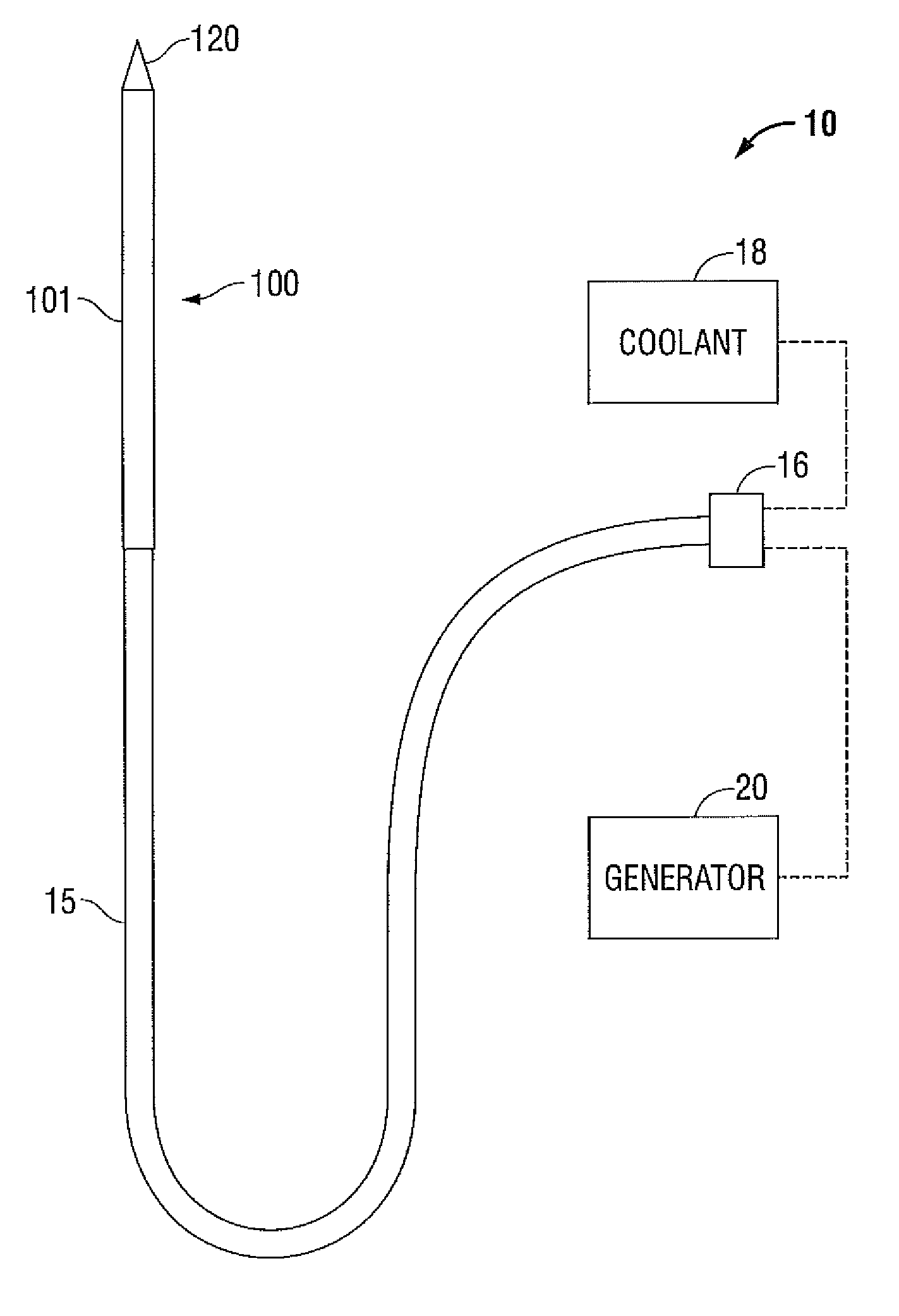
Ultrasound catheter for disrupting blood vessel obstructions
InactiveUS7137963B2Accelerated destructionImprove abilitiesChiropractic devicesMedical devicesBlood vessel occlusionGuide wires
Ultrasound catheter devices and methods provide enhanced disruption of blood vessel obstructions. Generally, an ultrasound catheter includes an elongate flexible catheter body with one or more lumens. An ultrasound transmission member or wire extends longitudinally through the catheter body lumen and, in many embodiments, a guide wire tube also extends through the same lumen. A distal head is fixed to or otherwise mechanically coupled with the distal end of the ultrasound transmission member or wire and is positioned adjacent the distal end of the catheter body. Although the distal end of the catheter body overlaps the distal head, the distal head is not directly affixed to the distal end of the catheter body. Thus, the distal tip may move freely, relative to the distal end of the catheter body when ultrasonic energy is applied through the ultrasound transmission member. Such a freely floating distal head enhances the efficiency of an ultrasound catheter, enabling the catheter to ablate calcific occlusions and increasing the useful life of the ultrasound transmission member and catheter.
Owner:FLOWCARDIA
Vascular sealing device with locking system
InactiveUS20060058844A1Large caliberPrevent retractionSuture equipmentsVessel sealingCollagen sponge
An internal tissue puncture closure method and apparatus provides a locking device for compressing and holding an external component such as a collagen sponge at a puncture situs. The locking device facilitates compression of the external component in a first direction, but prevents or locks against retraction.
Owner:KENSEY NASH HLDG
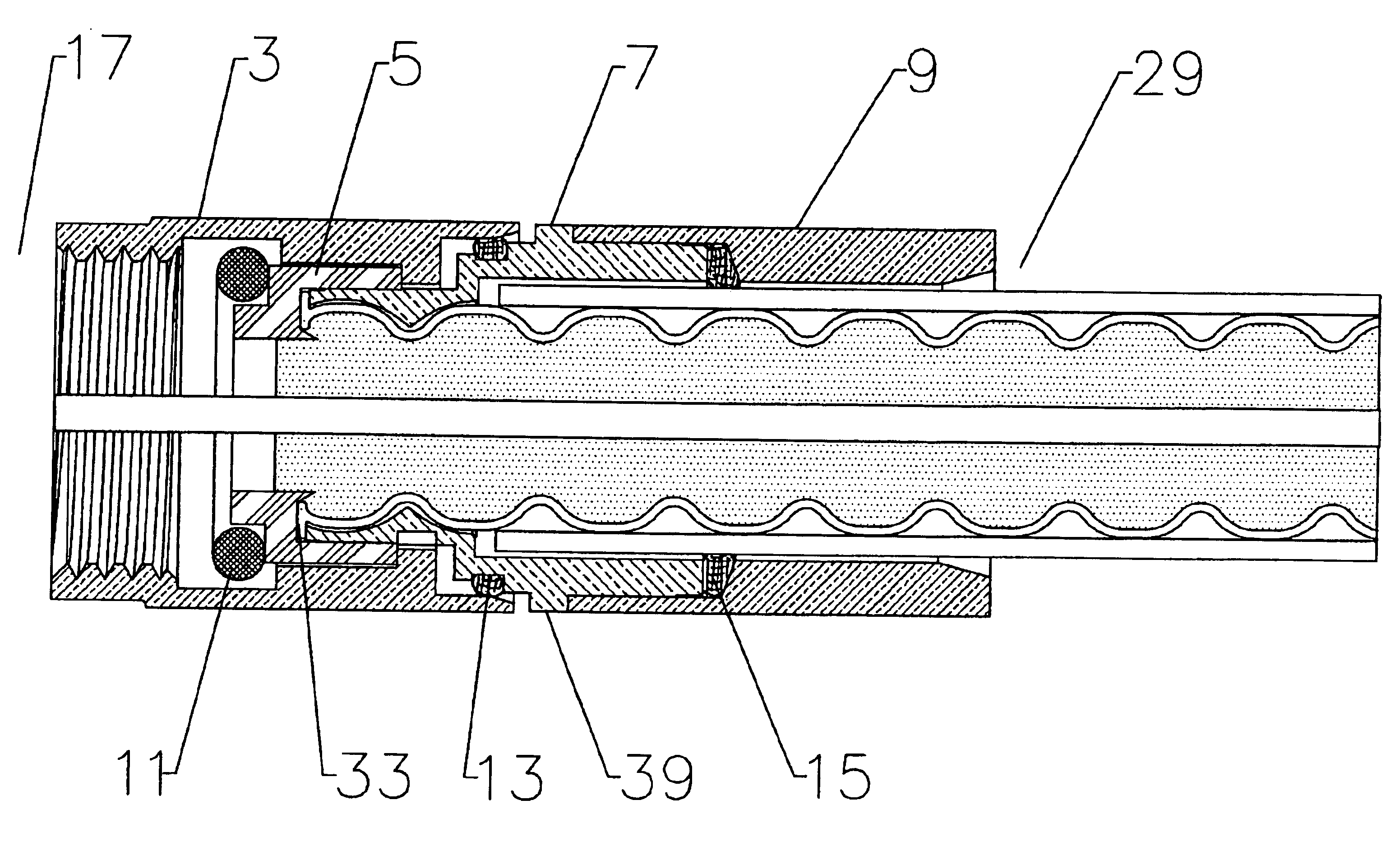
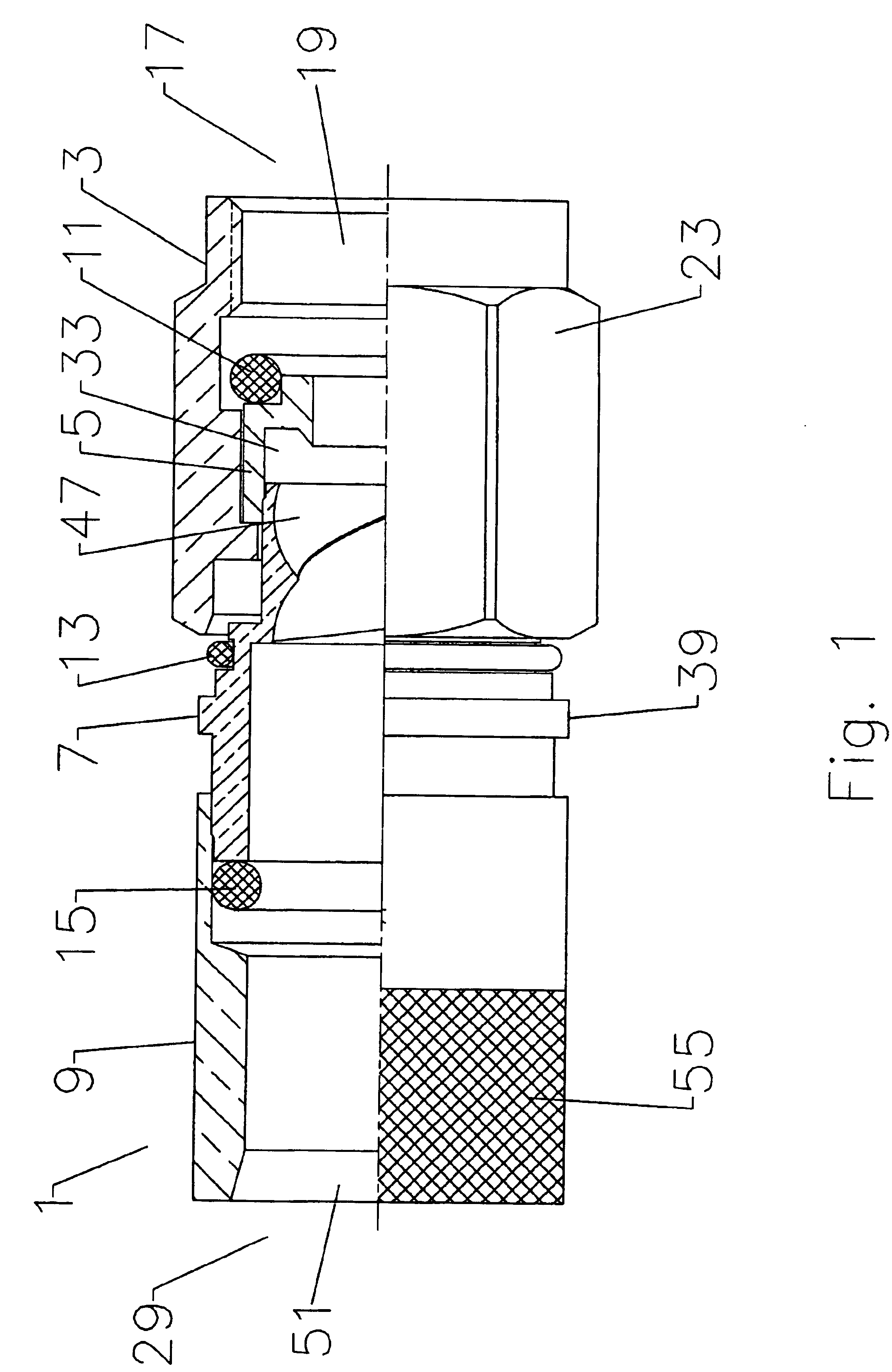
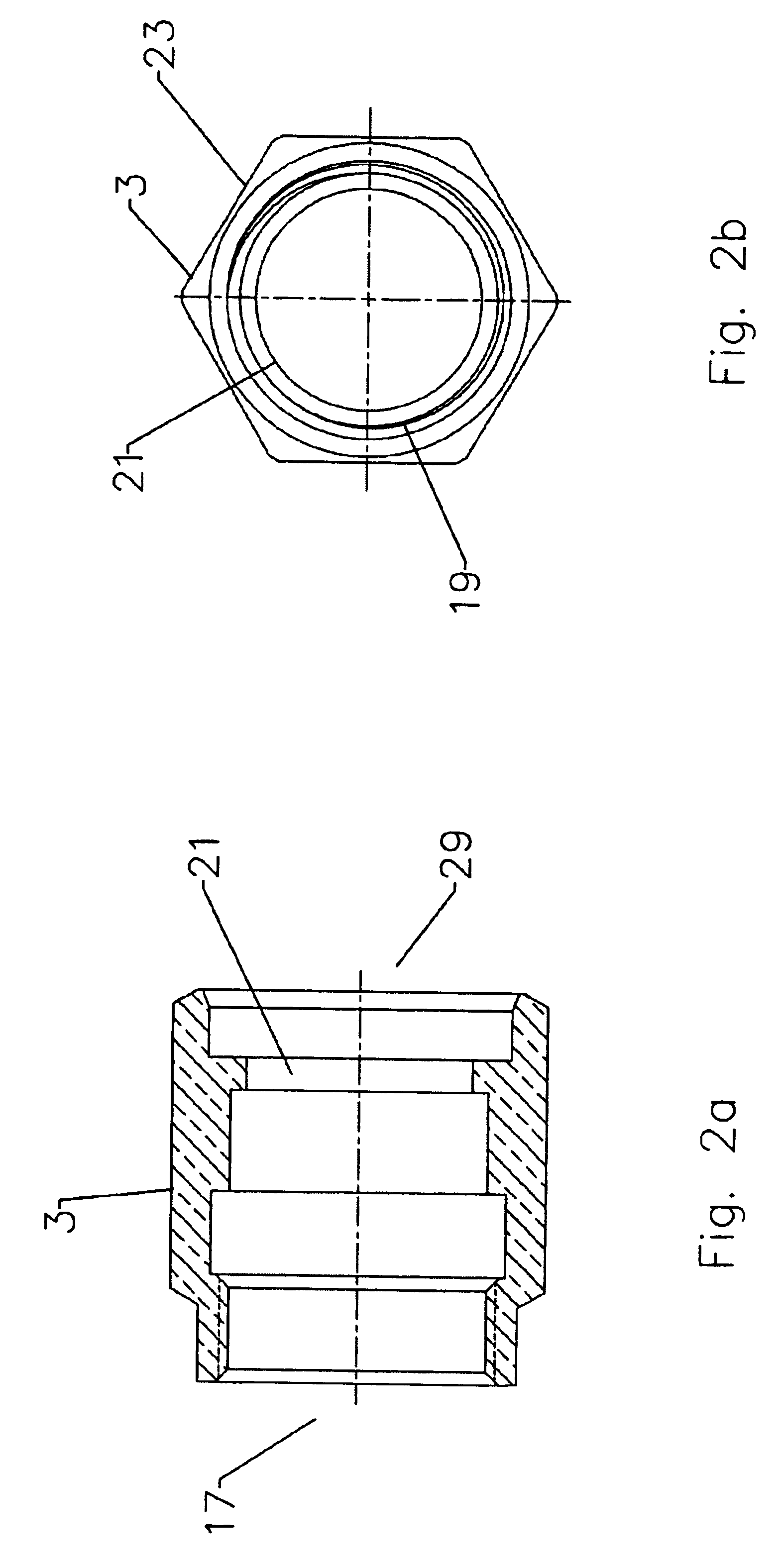
Axial compression electrical connector
InactiveUS6939169B2Improve gripLarge caliberElectrically conductive connectionsTwo pole connectionsInterference fitLeading edge
An electrical connector adapted for interconnection with a helically corrugated outer conductor coaxial cable via axial compression. Threads formed in an interior bore of the connector body threadably engage helical corrugations of the outer conductor. Upon axial compression of an interface into an interference fit with the body, a leading edge of the outer conductor is deformed, creating a high quality uniform electrical interconnection and preventing unthreading of the cable from the connector. Gaskets environmentally sealing the various entry paths into the connector are also sealably compressed by the axial movement of the various connector components during axial compression.
Owner:ANDREW LLC
Narrow gauge high strength choked wet tip microwave ablation antenna
ActiveUS8292881B2Improve performanceIncrease powerSurgical needlesSurgical instruments for heatingCooling chamberDipole antenna
An electromagnetic surgical ablation probe having a coaxial feedline and cooling chamber is disclosed. The disclosed probe includes a dipole antenna arrangement having a radiating section, a distal tip coupled to a distal end of the radiating section, and a ring-like balun short) or choke, which may control a radiation pattern of the probe. A conductive tube disposed coaxially around the balun short includes at least one fluid conduit which provides coolant, such as dionized water, to a cooling chamber defined within the probe. A radiofrequency transparent catheter forms an outer surface of the probe and may include a lubricious coating.
Owner:TYCO HEALTHCARE GRP LP
Deployment system for intraluminal devices
InactiveUS6899727B2Facilitate removalFacilitate fluoroscopic visualizationStentsBlood vesselsProsthesisBiomedical engineering
A constraining sheath for use around an endoprosthesis (e.g., a stent device, with or without a graft covering), which may be a balloon expandable endoprosthesis but more preferably is a self-expanding prosthesis. The endoprosthesis is coaxially enclosed within the constraining sheath, which is an outer, disruptable, preferably implantable tubular sheath, preferably made of ePTFE. The constraining sheath and endoprosthesis are preferably mounted together as an assembly on an angioplasty balloon for delivery. Deployment of the endoprosthesis entails inflating the angioplasty balloon to a pressure sufficient to disrupt or break the constraining sheath in a prescribed fashion, thereby allowing a self-expanding endoprosthesis to spontaneously deploy. The constraining sheath of ePTFE may be attached to the endoprosthesis and implanted along with the device, or alternatively attached to the balloon catheter shaft and removed with the balloon catheter.
Owner:WL GORE & ASSOC INC
Combination sensor guidewire and methods of use
ActiveUS20060074318A1Improve accuracyImprove consistencyBlood flow measurement devicesCatheterUltrasonic sensorTransducer
The present invention provides for an improved combination sensor tip that includes an ultrasound transducer and a pressure sensor both disposed at or in close proximity to the distal end of the combination sensor tip. The present invention also provides for an improved connector to couple a guide wire to a physiology monitor that reduces torsional resistance when maneuvering the guide wire.
Owner:VOLCANO CORP
Digital electrode for cardiac rhythm management
InactiveUS20050165456A1Large caliberLess-flexibleTransvascular endocardial electrodesHeart stimulatorsElectricityEnergy storage
A cardiac rhythm management apparatus includes a proximal housing, a distal housing and a lead. The proximal housing includes a first energy storage device. The distal module is implantable within a patient's heart, and includes a second energy storage device, at least one electrode, and a control module. The control module controls the delivery of at least one electrical stimulus from the second energy storage device to a location in communication with the patient's heart. The lead connects the proximal housing to the distal module and is configured to communicate one or more digital signals between the proximal housing and the distal module.
Owner:PACESETTER INC
Elastic Tube Alignment System For Precisely Locating Components
ActiveUS20130019455A1Smoothly and easily performedFacilitate their initial entryDeformable pinsPinsEngineering
An elastic tube alignment system for the mating of components utilizing the principle of elastic averaging. A plurality of geometrically separated elastic tube (male) alignment features are disposed on a first component, while a plurality of one-to-one corresponding aperture (female) alignment features are provided on a second component. During the mating of the components, each elastic tube and its respective aperture provide elastic deformation, which, on average, precisely aligns the components.
Owner:GM GLOBAL TECH OPERATIONS LLC
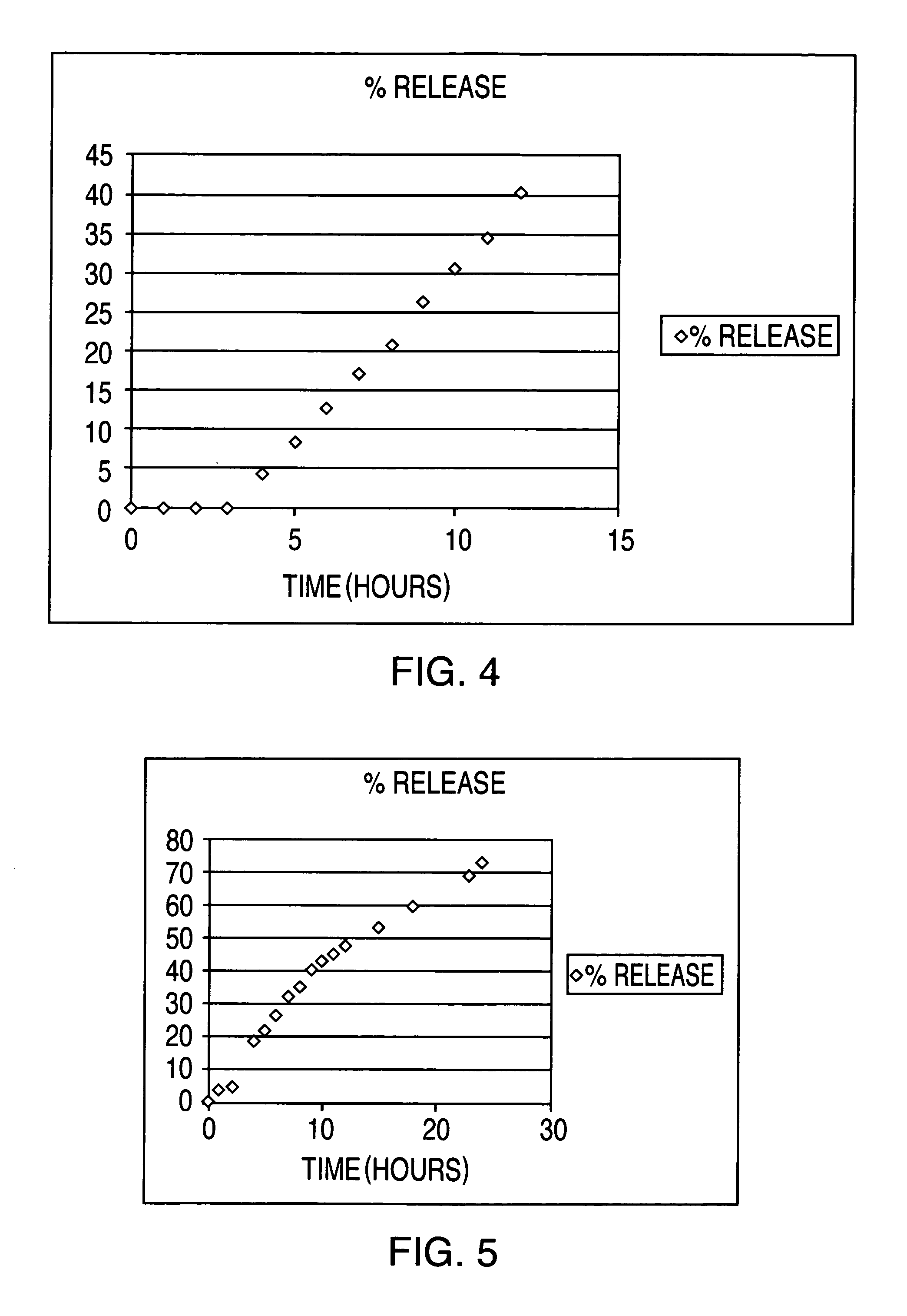
Drug delivery system for zero order, zero order-biphasic, ascending or descending drug delivery
InactiveUS7195778B2Delay swellingSlow drug releaseOrganic active ingredientsNervous disorderDrug deliveryPharmaceutical preservatives
The invention is directed to a drug delivery device for controlled release of a drug, comprising a core that has a cylindrical plug embedded therein; and a coating that at least partially surrounds the core. The core is comprised of a drug and excipients. The coating surrounding the core is essentially impermeable to the drug. The cylindrical plug, which is embedded in the core, may be hollow or solid. The drug delivery device enables zero-order drug release profiles as well as more complicated release profiles to be obtained. The invention is also directed to a method of making the drug delivery device.
Owner:TEVA PHARM USA INC
Prosthetic repair of body passages
ActiveUS20060041305A1Large caliberReduce the cross-sectional areaStentsHeart valvesProsthesisVascular stent
A prosthesis that resiliently engages a body passage includes an annular clamping ring which may be folded along a diametric axis for insertion into the body passage. The clamping ring is adapted to resiliently spring outwardly, once in position inside the body passage, and to be continually resiliently biased against the interior surface of the body passage. One or more of the clamping rings may be attached to opposed ends of a tubular graft. The rings and connected graft may be positioned in the body passage using a applicator which selectively permits expansion and / or in some embodiments contraction of the annular ring in position within a body passage. Alternatively a retaining member may be used to retain the annular ring in a compressed condition until it is in a desired position within a body passage. Among other potential uses, the present invention may be useful as a vascular stent for treating abdominal aortic aneurysms.
Owner:VASCUTEK
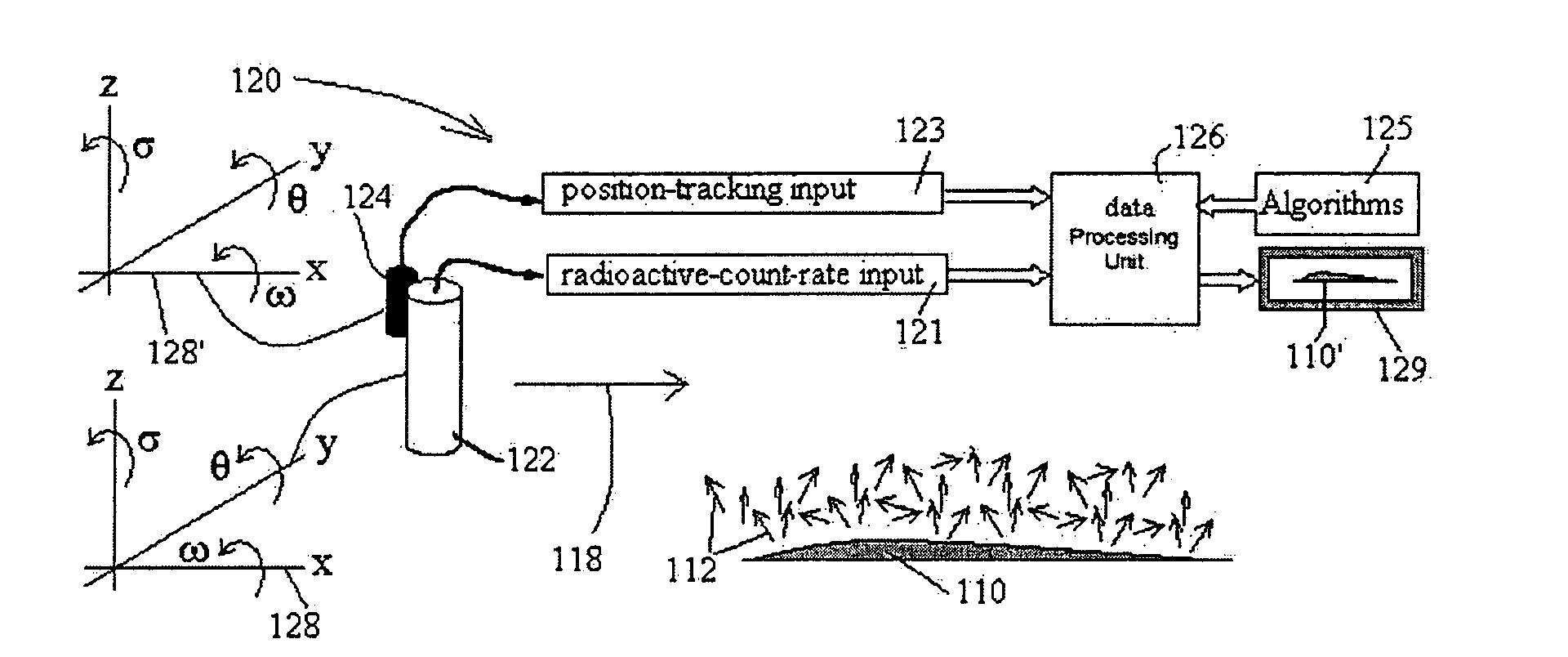
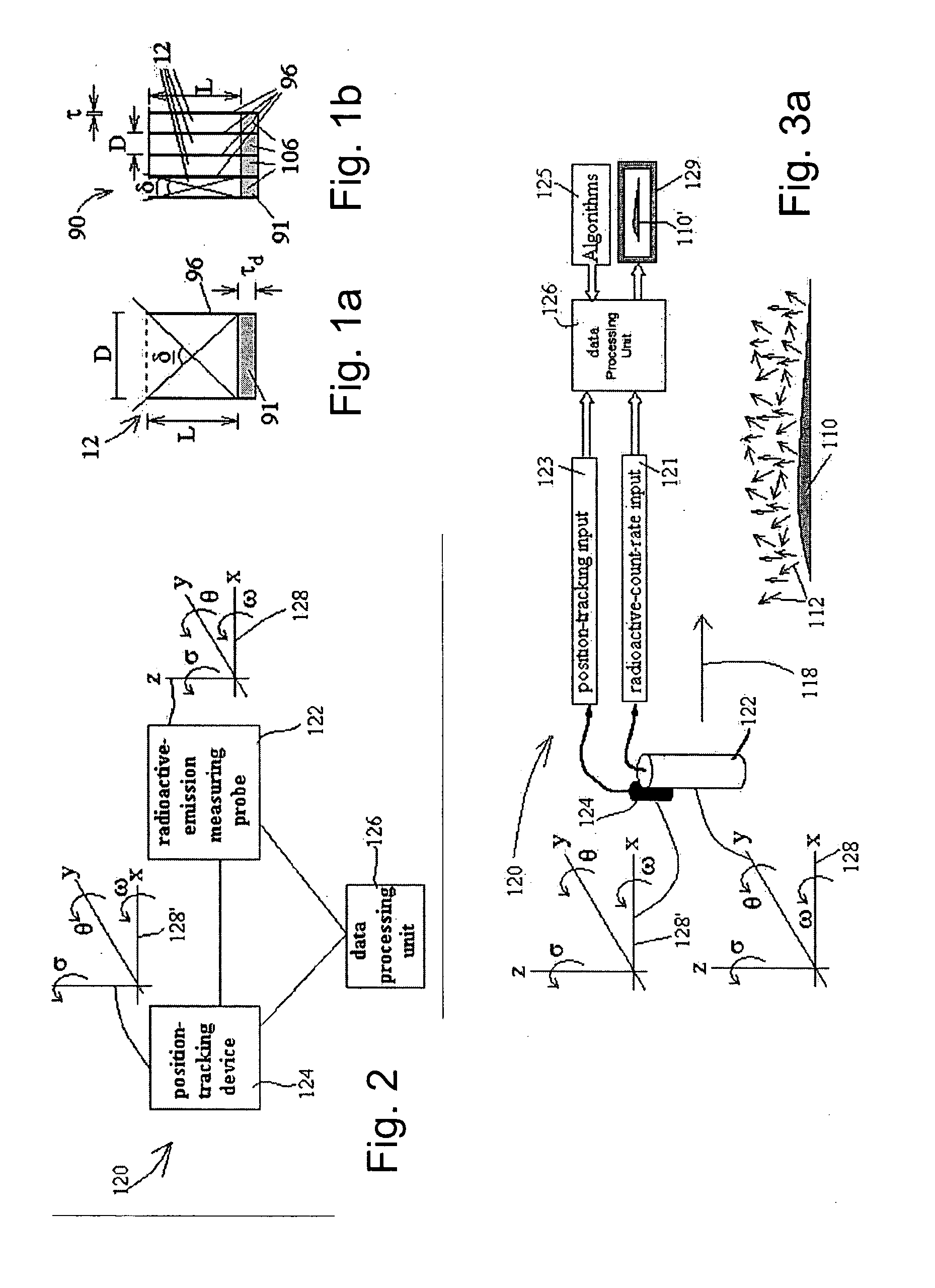
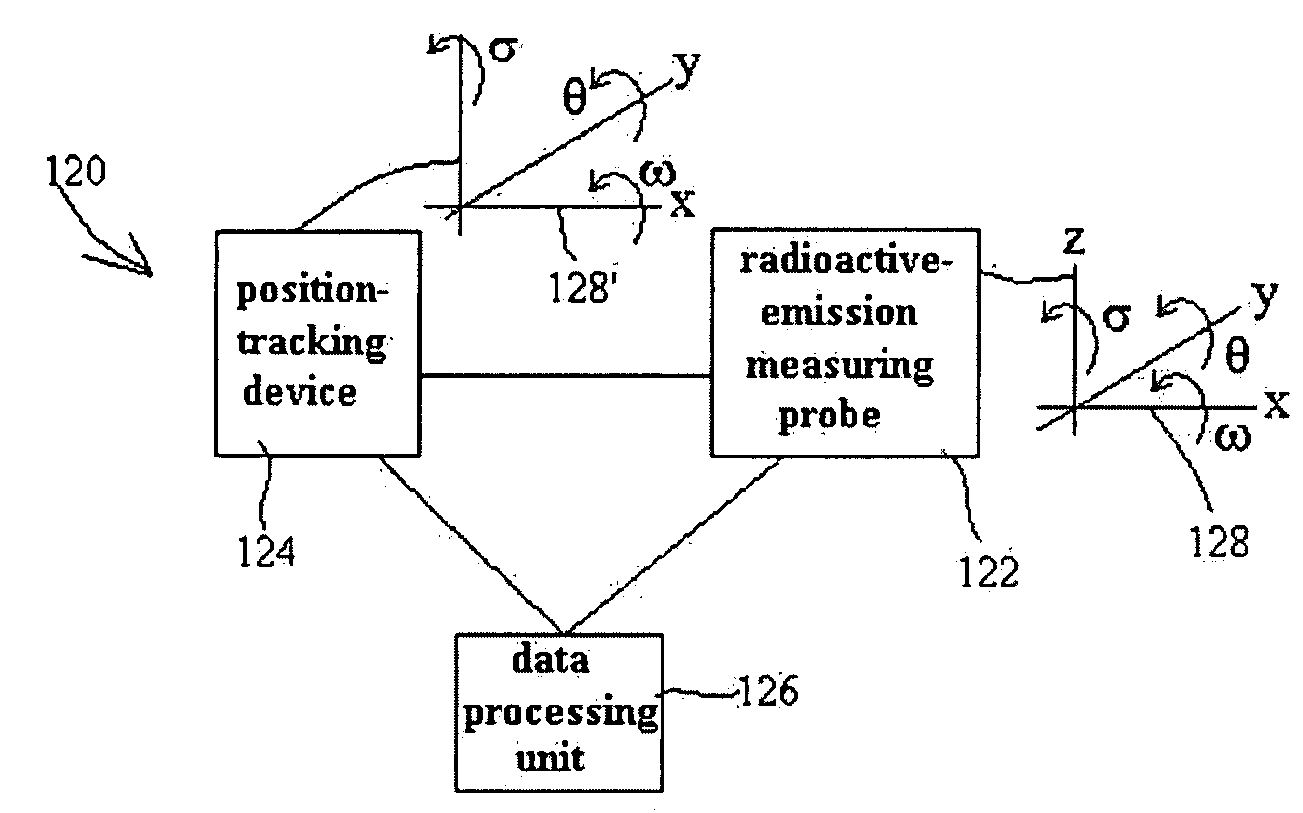
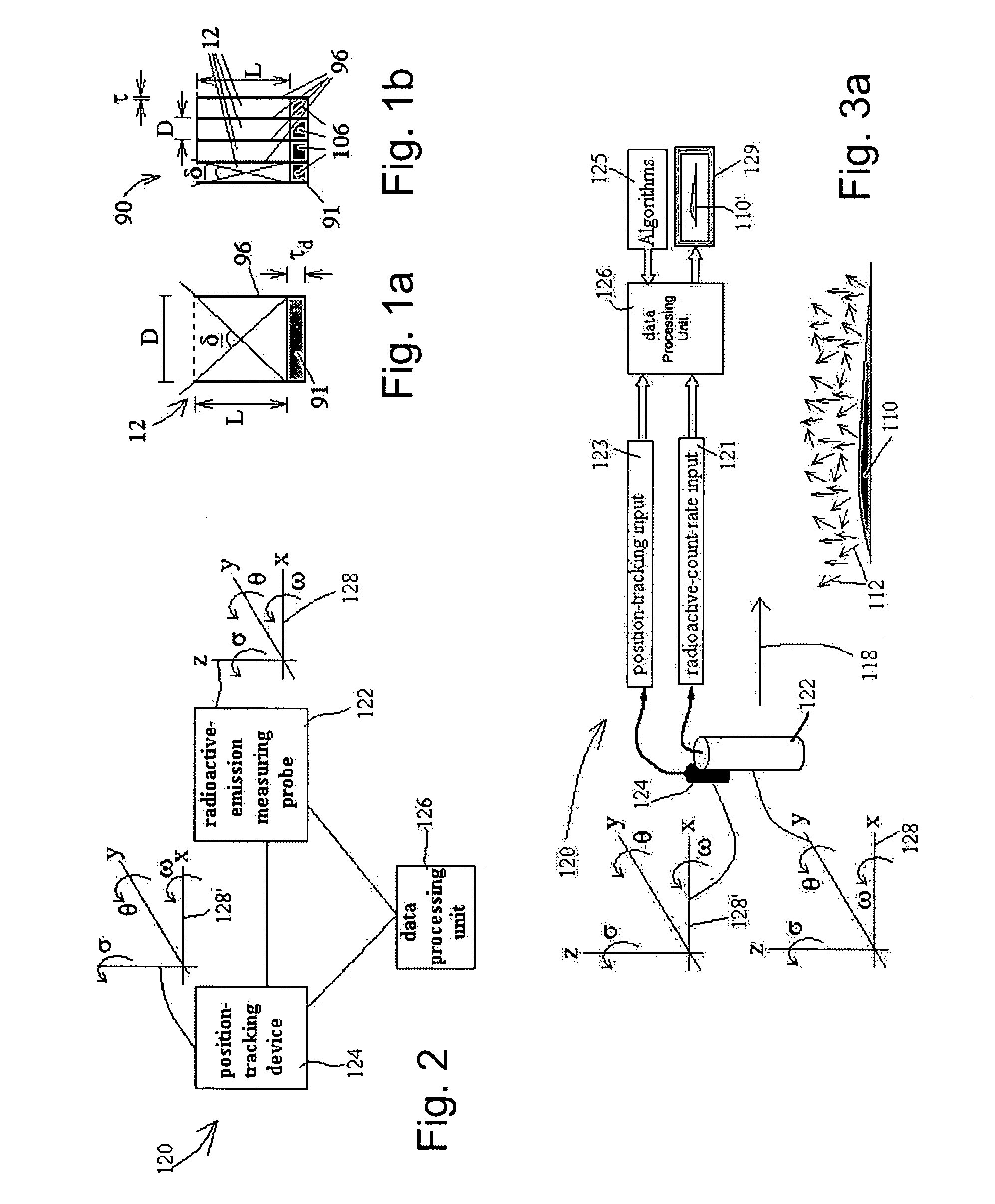
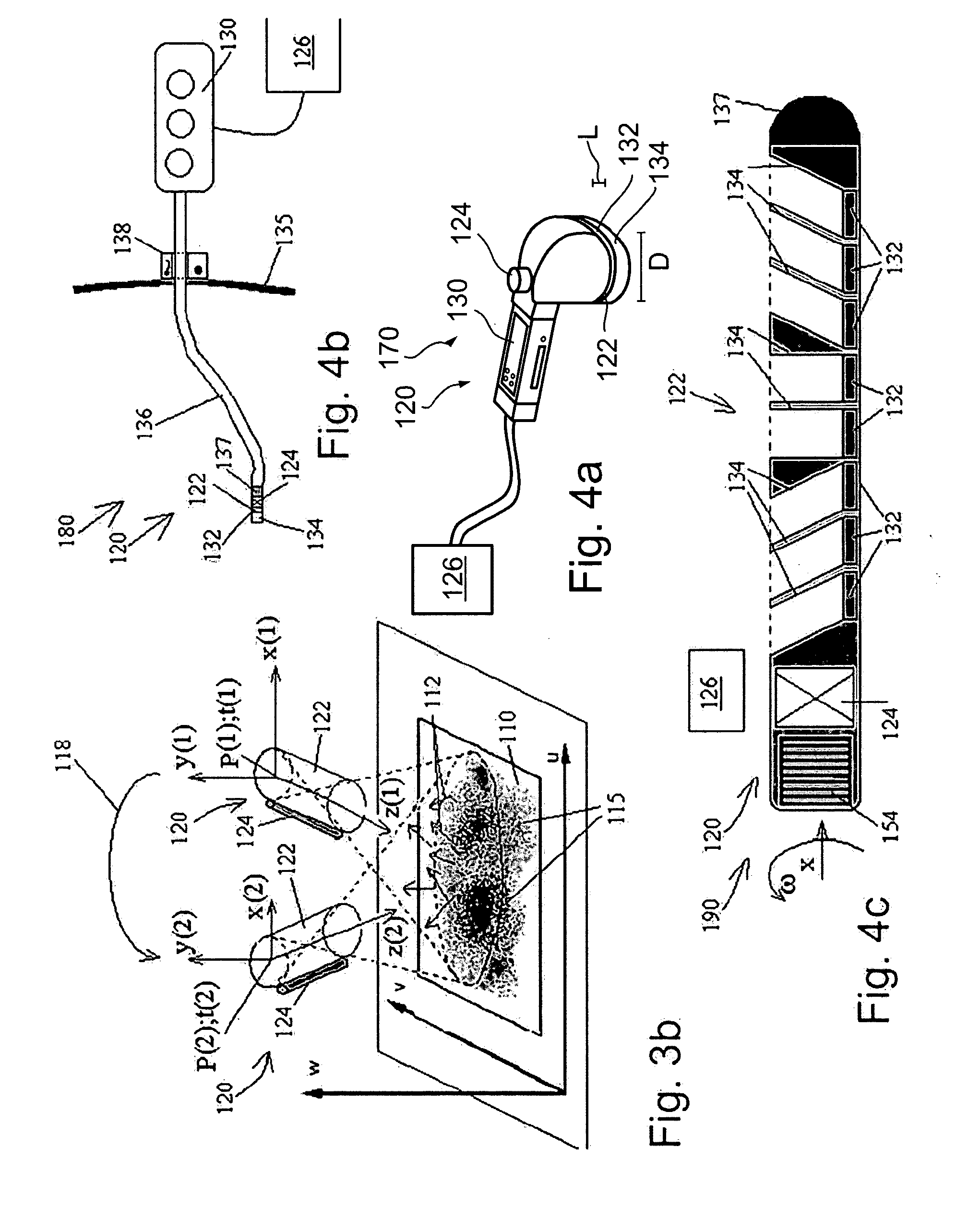
Radioimaging
ActiveUS20080042067A1Unprecedented sensitivityAvoid saturationHandling using diaphragms/collimetersMaterial analysis by optical meansNuclear medicineRadioactive drug
Owner:SPECTRUM DYNAMICS MEDICAL LTD
Radioimaging
ActiveUS20080230705A1Prevent saturationUnprecedented sensitivityMaterial analysis by optical meansHandling using diaphragms/collimetersNuclear medicine
Owner:SPECTRUM DYNAMICS MEDICAL LTD
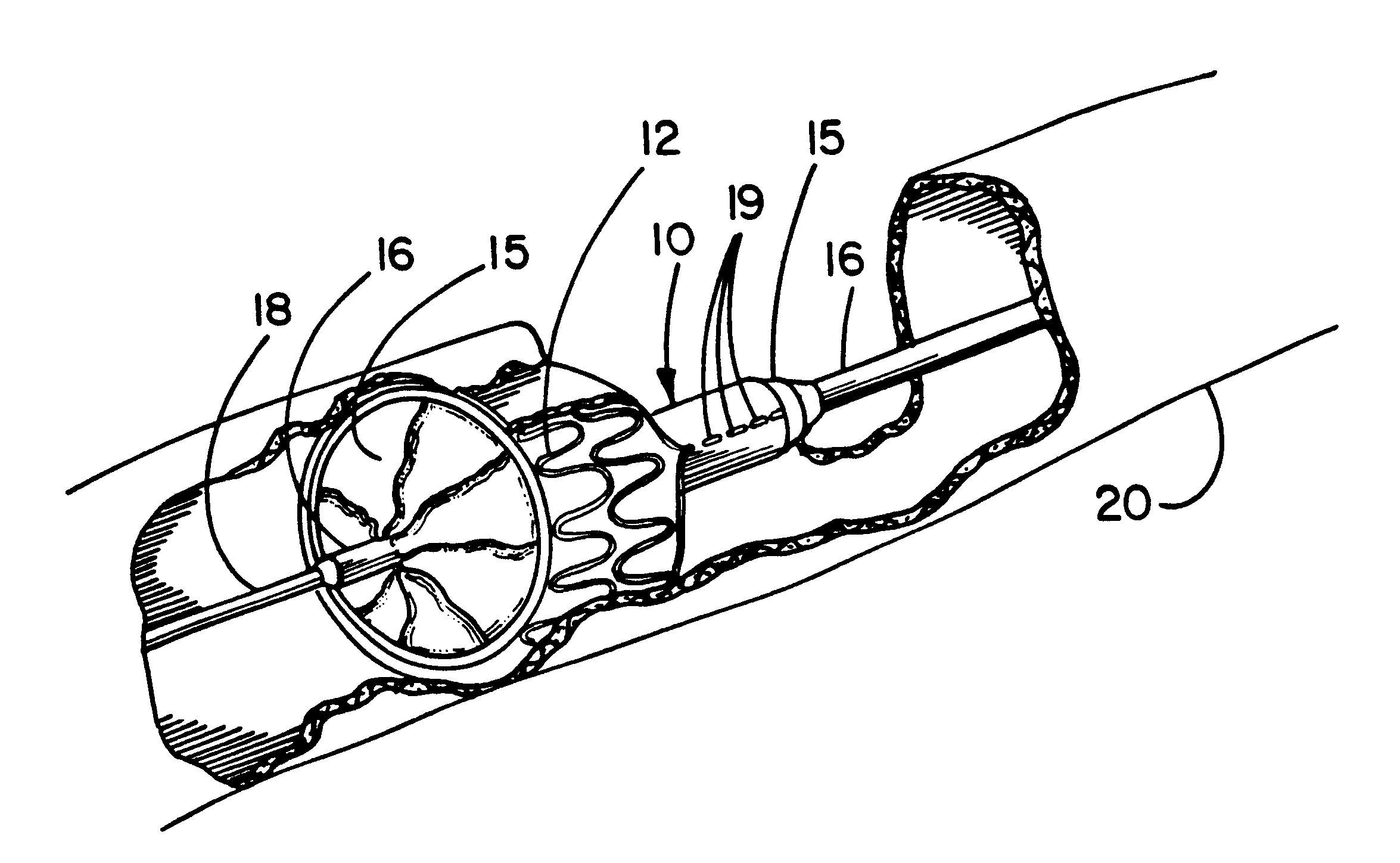
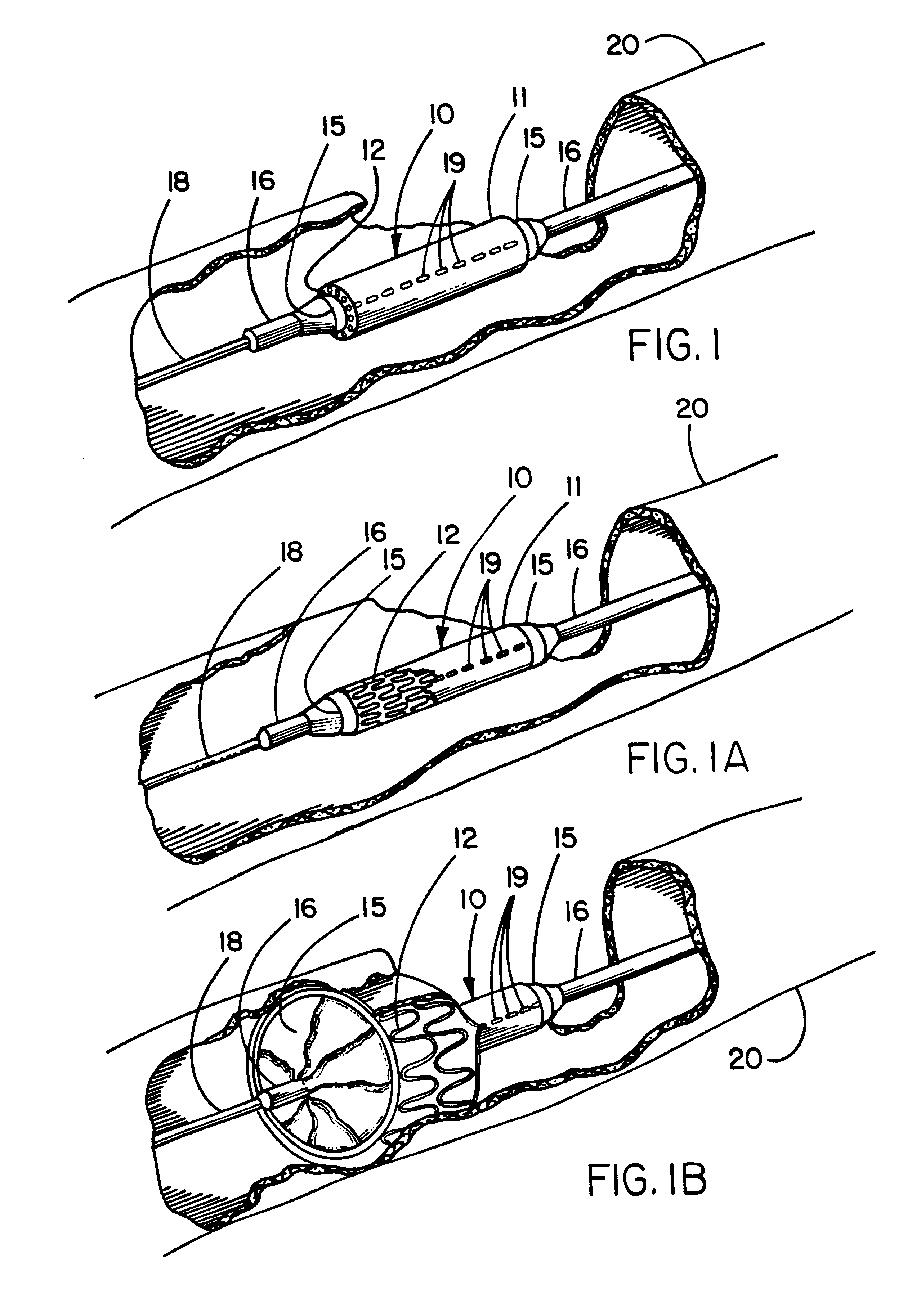
Systems, methods and devices for removing obstructions from a blood vessel
A system for removing an obstruction from a blood vessel includes an obstruction engaging element and an expandable capture element. The capture element preferably has a flexible cover and an expandable support structure. The engaging element engages the obstruction and moves the obstruction into the capture element. The capture element protects the obstruction when the obstruction is moved into the catheter.
Owner:STRYKER CORP
Sound beam loudspeaker system
InactiveUS20060204022A1Large directivityLow costTelevision system detailsMicrophonesTransducerEngineering
A loudspeaker system is described including an array of electro-acoustic transducers capable of generating steerable beams of sound and additional transducers adapted to reproduce low frequency sound being placed at the perimeter of said array.
Owner:1 LIMITED ST JOHN S INNOVATION CENT
Non-expandable transluminal access sheath
InactiveUS20060253102A1Improved radiopaque characteristicMinimize potential for damageCannulasInfusion syringesFluid transportVisibility
A transluminal sheath is disclosed that permits instrumentation to be passed therethrough. The transluminal sheath comprises a composite structure with an inner layer, an outer layer, and a reinforcing layer. The materials comprising the inner and outer layer are plastically deformable and maintain their shape, once bent into a specific configuration. The reinforcing layer further has radiopacity enhancing coatings to improve visibility under fluoroscopy and a system of flutes running longitudinally, to enhance fluid transport and reduce friction.
Owner:ONSET MEDICAL CORP
Off-axis anchor guidance system
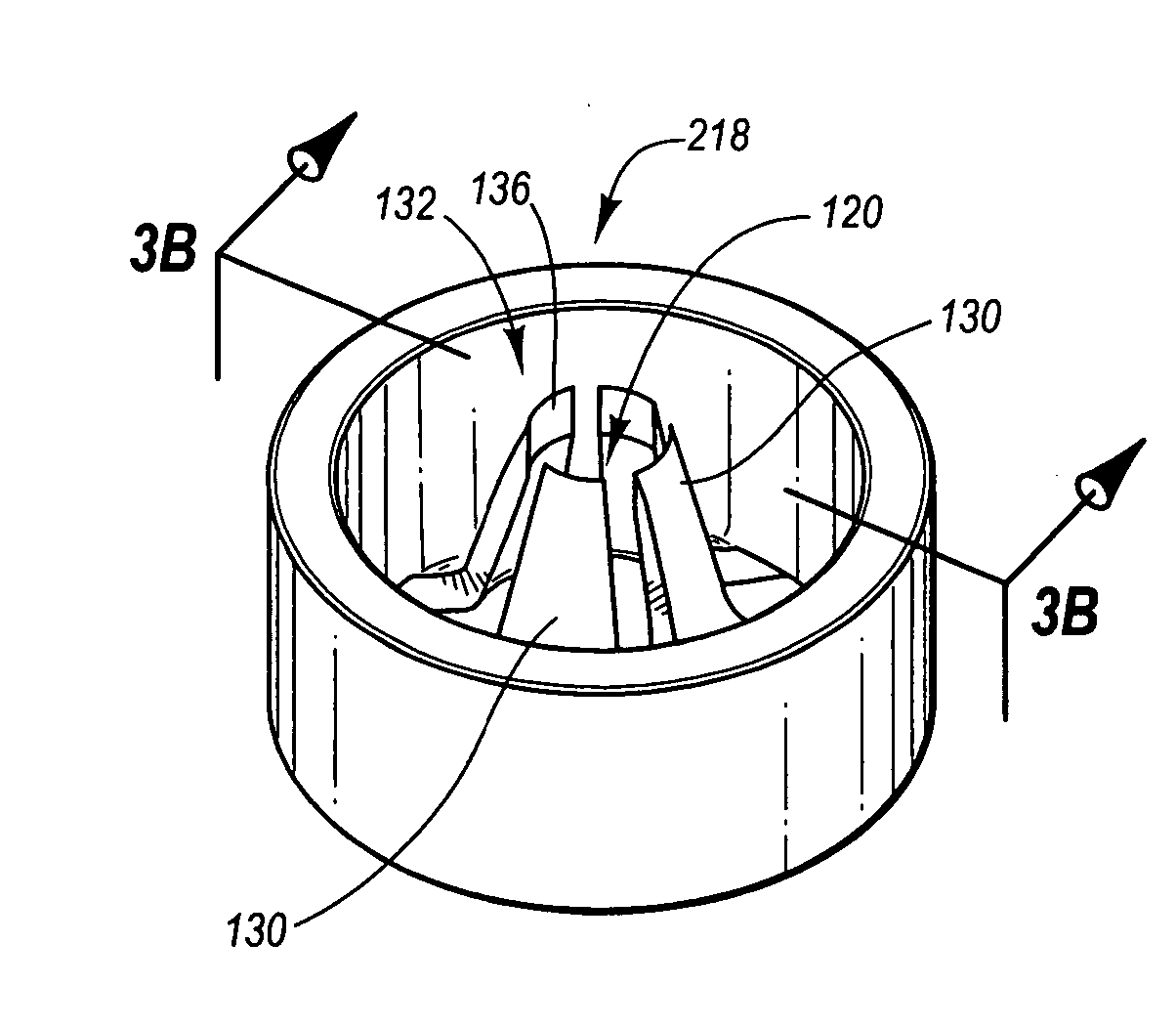
ActiveUS20060106394A1Large caliberHigh strengthInternal osteosythesisJoint implantsGuidance systemDilator
Disclosed is a guided bone anchoring device comprising a head, a shank and a passage for receiving a guide wire, the passage having a longitudinal center axis which is not concentric to the longitudinal center axis of the shank. Also disclosed is a system for guiding bone anchors, the system comprising: a guide wire, an anchor comprised of a shank wherein the shank has an offset bore at the distal end which is adapted to allow the guide wire to slide through the bore, a dilator having a longitudinal slot wherein the longitudinal slot allows the guide wire to extend through the longitudinal slot when the anchor is positioned within the dilator, and a driving device which is adapted to engage the proximal end of the anchor.
Owner:THEKEN SURGICAL LLC
Intra-facet fixation device and method of use
ActiveUS20080234758A1Avoid relative motionRelieve stenosisSuture equipmentsInternal osteosythesisTarsal JointBiomedical engineering
A implantable interference device configured for intra-facet placement within a facet joint is provided. The device includes a shank capable of engaging opposing faces of the facet joint. The shank can further include a head extending from a proximal end of the shank wherein the head is configured to engage and / or buttress opposing faces of the joint, and also configured for preventing over-insertion of the device. Optionally, at least a portion of the shank can include or be formed of a fusion-promoting bioactive material. Further, a method for providing fixation of a facet joint by intra-facet placement of an interference device within the facet joint is provided.
Owner:DEPUY SPINE INC (US)
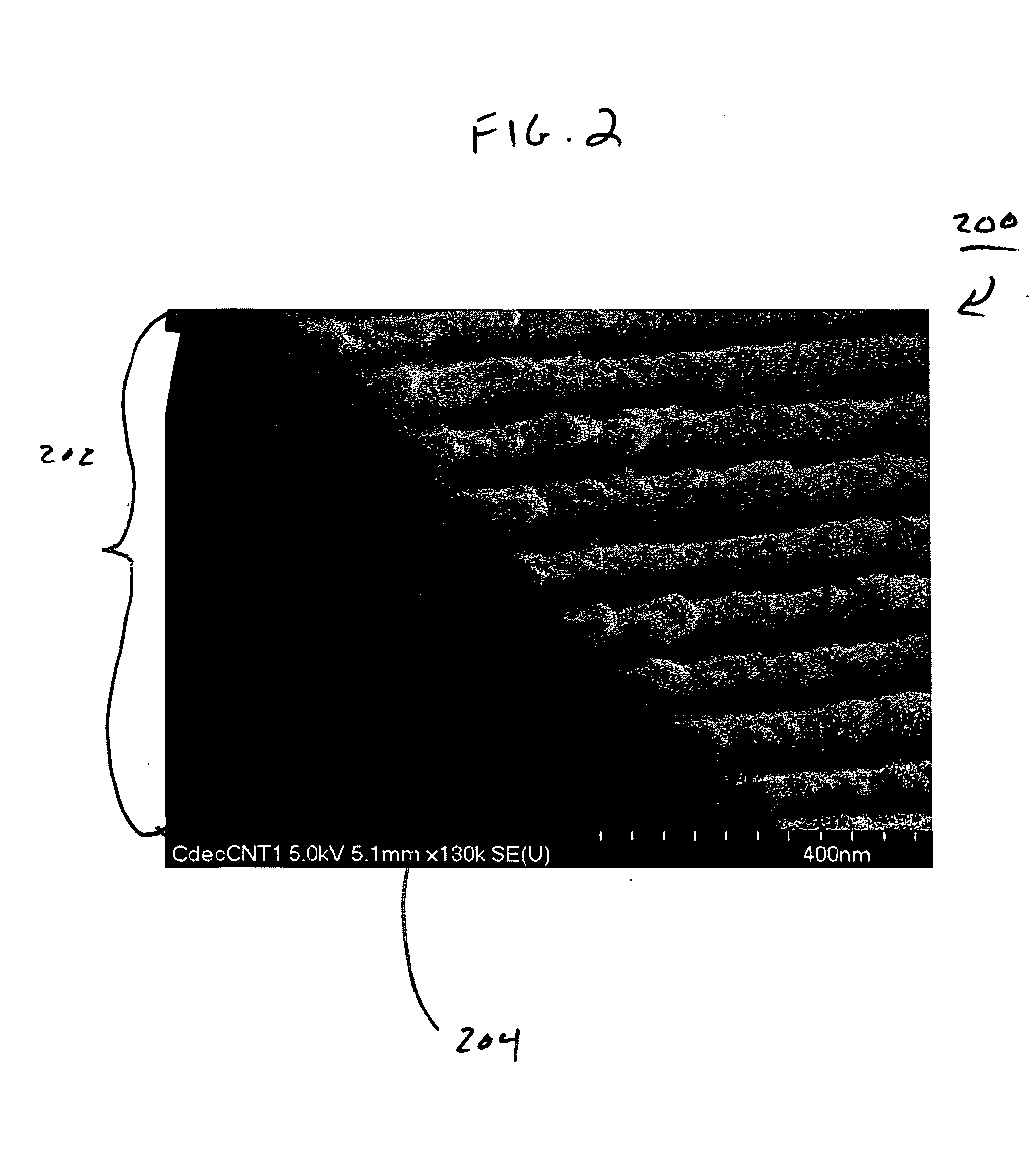
High performance ultracapacitors with carbon nanomaterials and ionic liquids
InactiveUS20080192407A1Excellent electrolyte accessibilityImprove performanceHybrid capacitor electrolytesElectrolytic capacitorsSupercapacitorCarbon nanomaterials
The present invention is directed to the use of carbon nanotubes and / or electrolyte structures comprising ionic liquids in various electrochemical devices, such as ultracapacitors.
Owner:ADA TECH
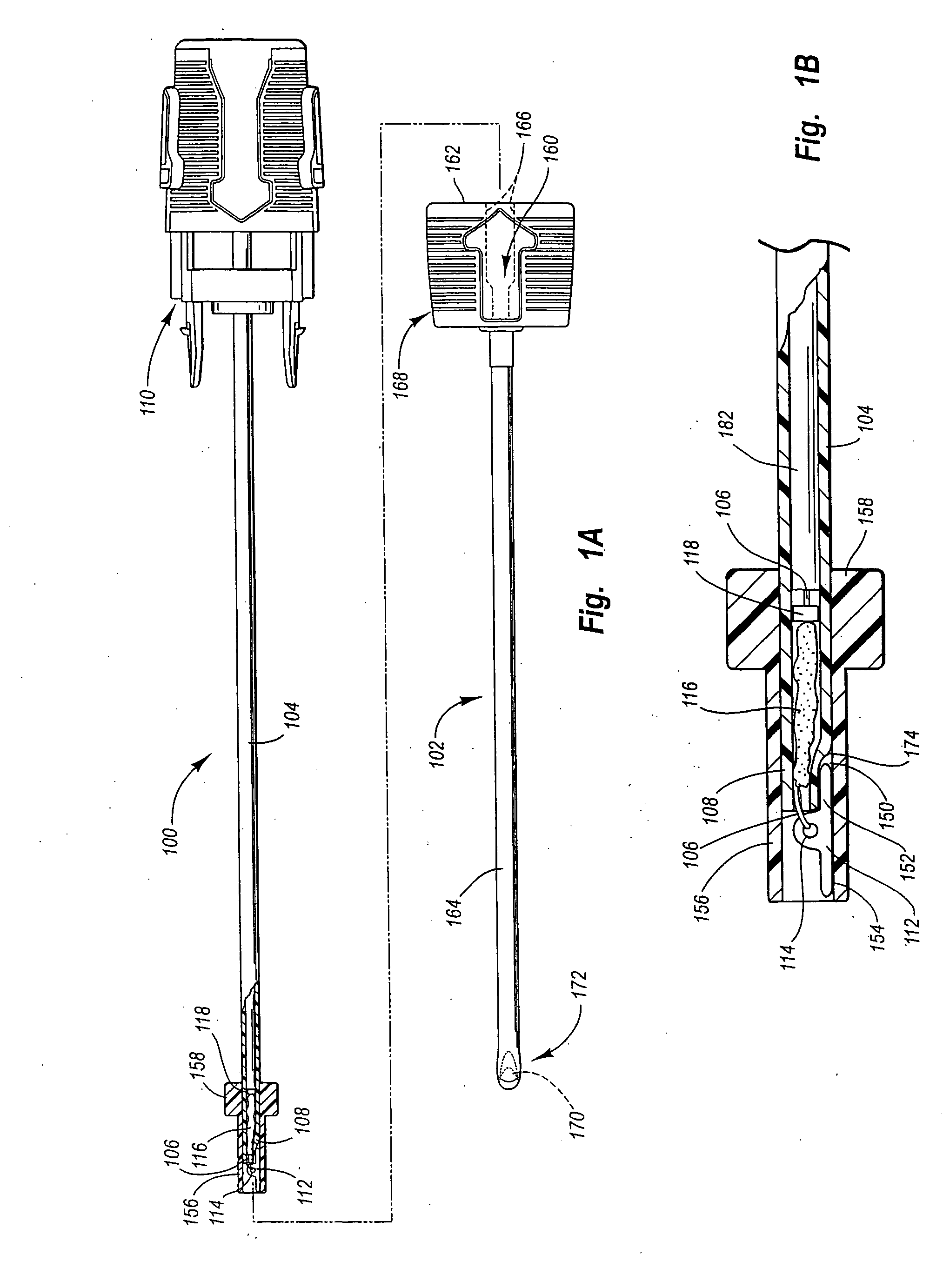
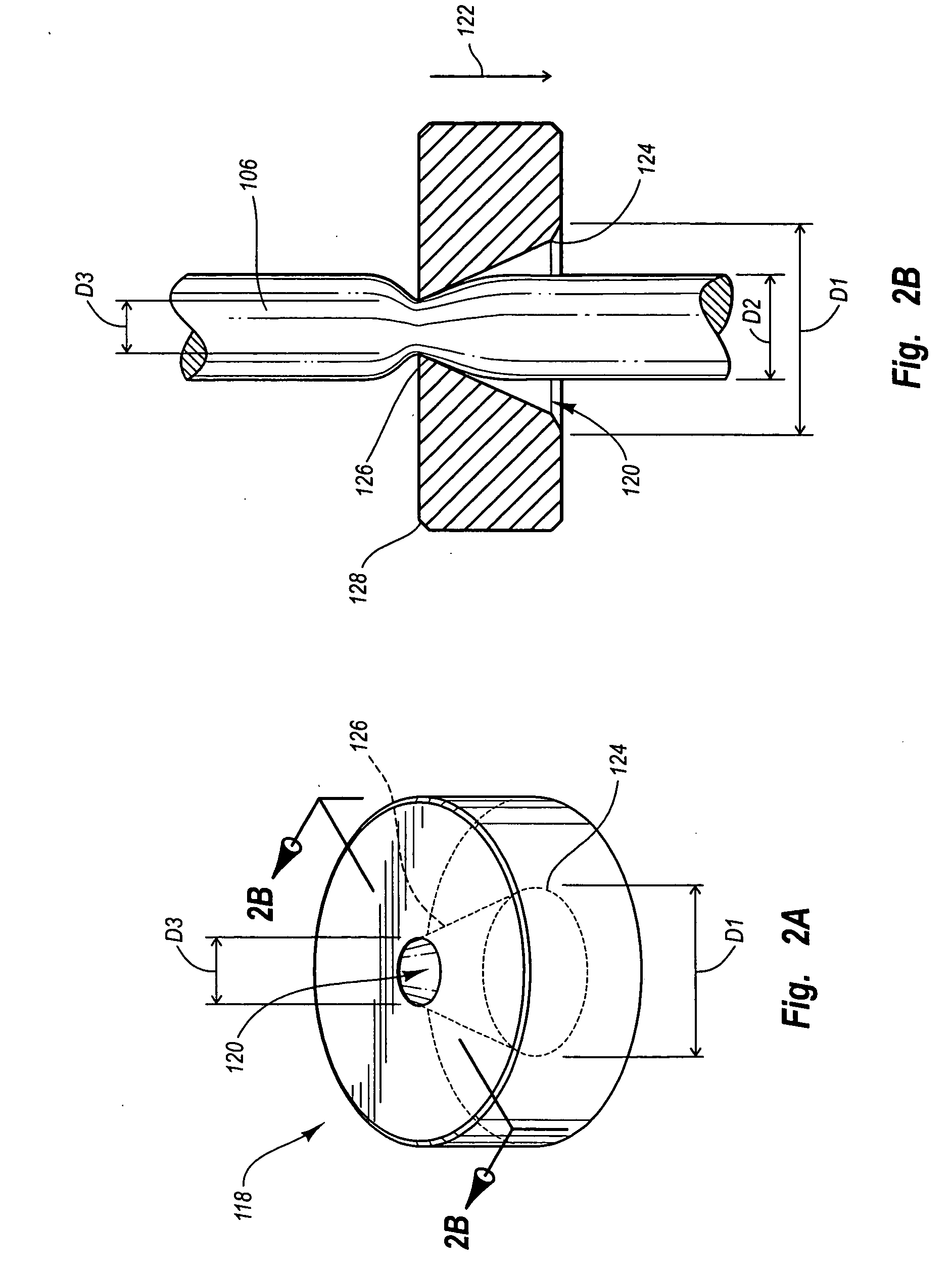
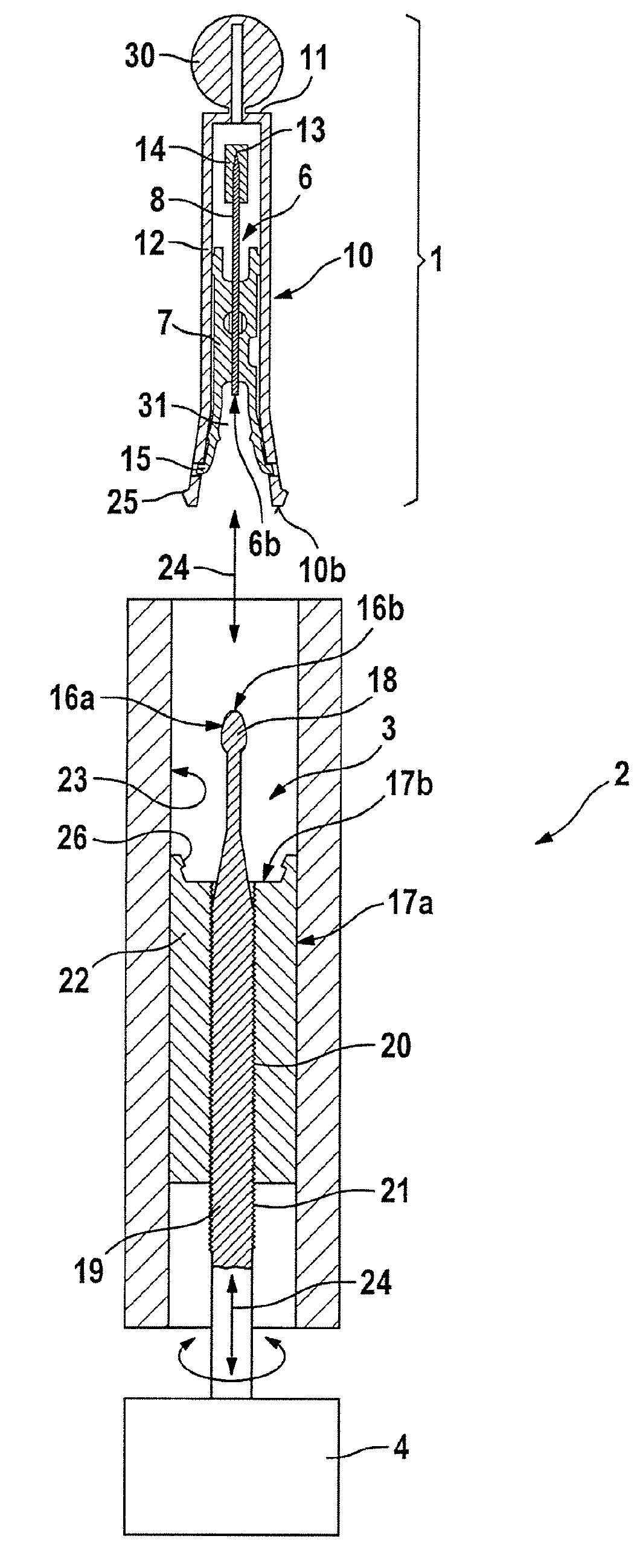
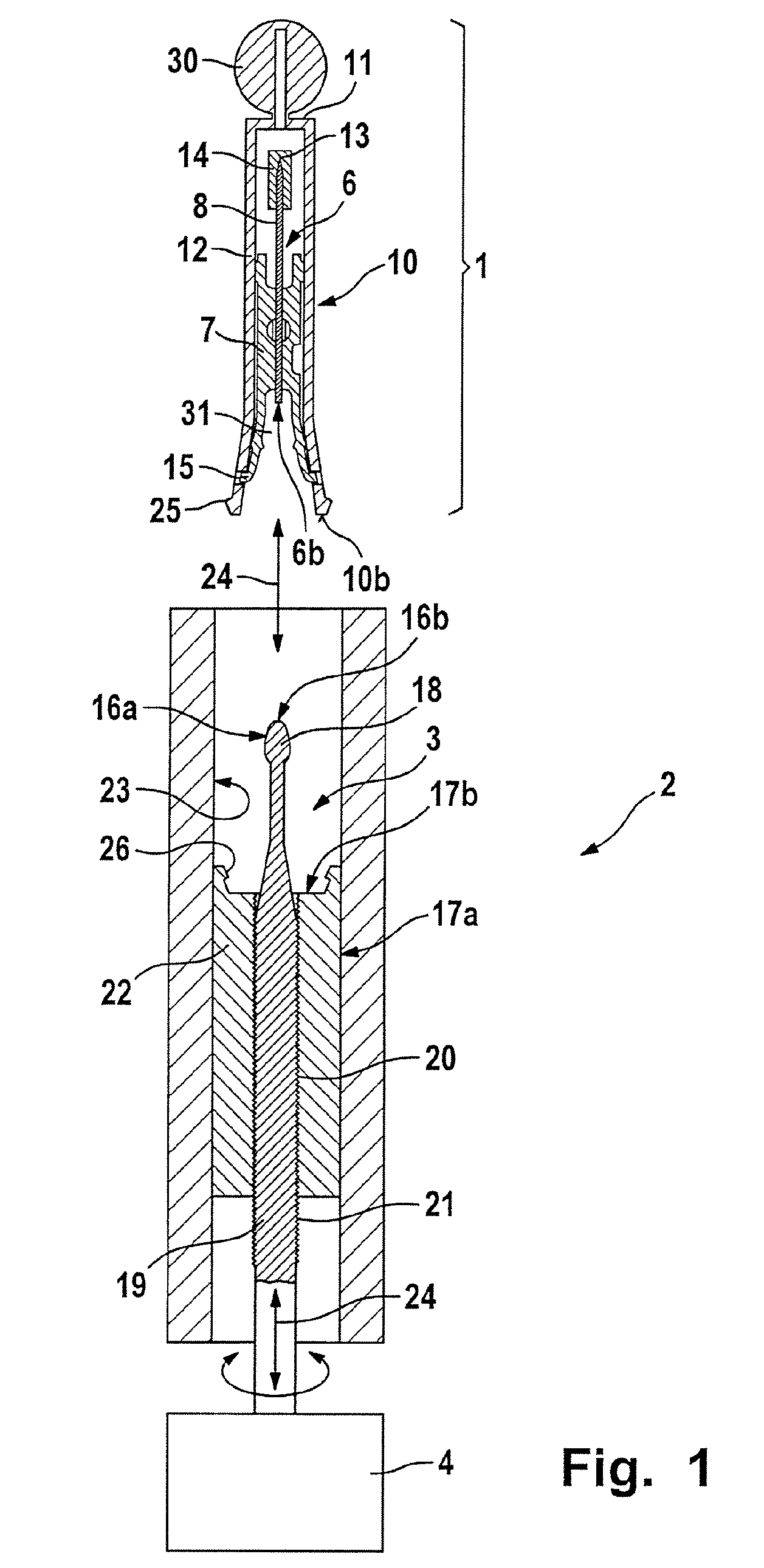
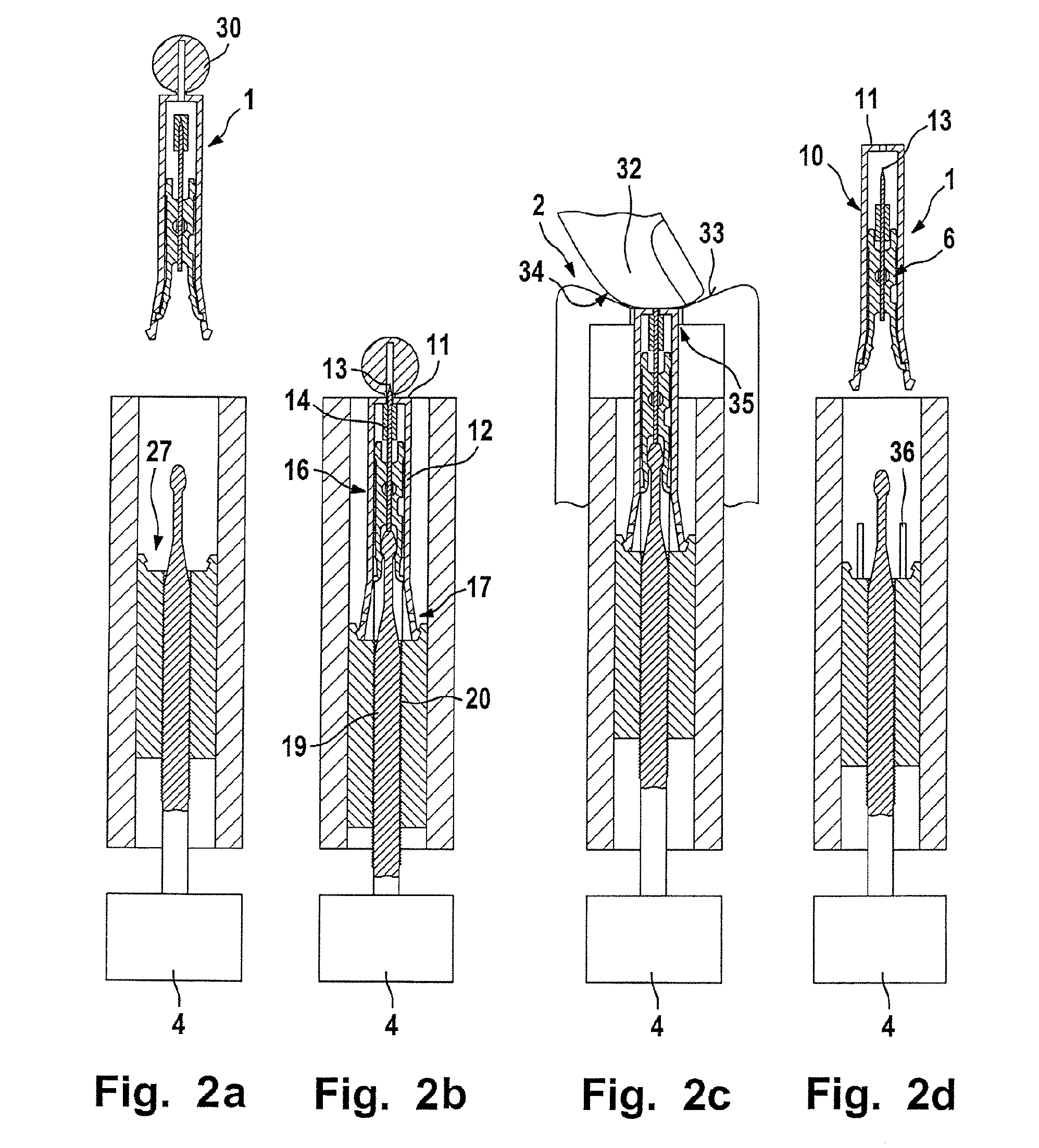
Puncturing system for withdrawing a body fluid
ActiveUS20080082023A1Cost advantageEasy constructionCatheterDiagnostic recording/measuringSkin contactBody fluid
The present invention provides a puncturing system for withdrawing body fluid. The puncturing system comprises a disposable puncturing unit, which includes a needle element for piercing into skin and a puncturing depth reference element with a skin contact area. The system includes a puncturing instrument which has a puncturing drive, wherein the puncturing drive is coupled by a coupling mechanism to the puncturing unit for driving the needle element to a puncturing depth, the puncturing depth being determined by the distance in the piercing direction between the skin contact area and the position of the tip of the needle at a reversal point of the puncturing movement. The puncturing depth reference element is connected to the needle element such that the puncturing depth reference element moves with the needle element during at least part of the puncturing movement. The puncturing depth reference element has a defined longitudinal position in the piercing direction relative to the needle element at least at the reversal point of the puncturing movement.
Owner:ROCHE DIABETES CARE INC
Telescoping support stand apparatus
ActiveUS7845602B1Avoid vertical movementPrevent crashRod connectionsStands/trestlesLocking mechanismEngineering
A telescoping support stand comprising a first tube partially defining a first enclosed area and a second tube partially defining a second enclosed area. A first end of the second tube may be telescopically slidable within the first tube. The telescoping support stand may also comprise a first locking mechanism attached to the second tube. The first locking mechanism may releasably secure the first tube to the second tube to prevent longitudinal movement of the first tube relative to the second tube. The telescoping support stand may also comprise a first air exchange aperture dimensioned to allow air to flow between the first and second enclosed areas. A corresponding method of assembly is also disclosed.
Owner:BUSHNELL HLDG
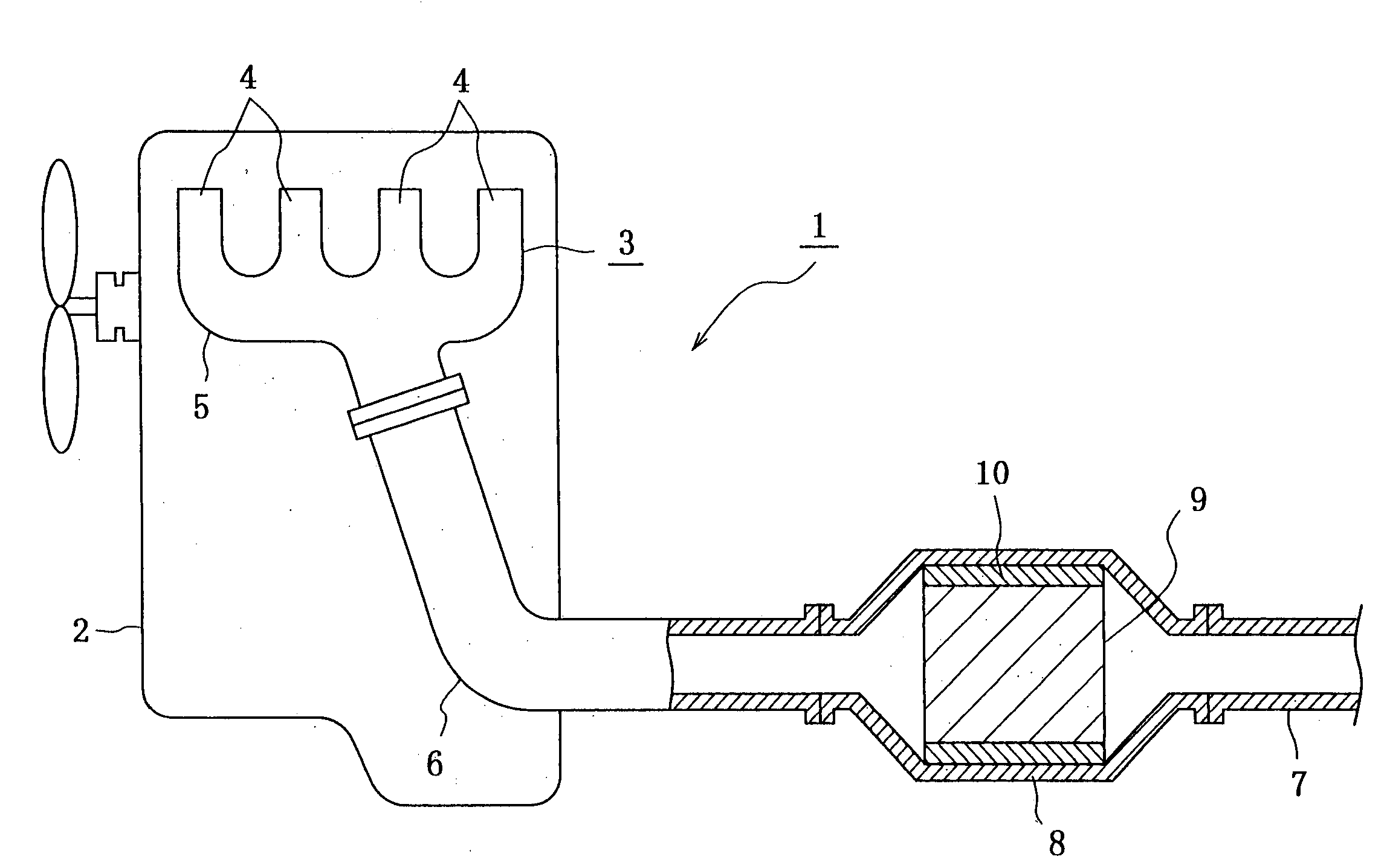
Ceramic filter and exhaust gas decontamination unit
ActiveUS20050102987A1Improve filtering efficiencyExtended service lifeCombination devicesInternal combustion piston enginesMetallurgyActive component
It is to provide a filter for an exhaust gas having a high thermal conductivity irrespective of a relatively high porosity or showing characteristics that the whole of the filter containing a high refractive index substance or pigment is easily warmed but hardly cooled while making low the thermal conductivity of the filter as a whole. This filter is provided with a catalyst coat layer formed by carrying a catalyst active component on a surface of a porous ceramic carrier, in which a porosity of the porous ceramic carrier is 40-80% and a substance or a pigment indicating a thermal conductivity as the filter of 3-60 W / mk or having a large refractive index at a thermal conductivity of 0.3-3 W / mk.
Owner:IBIDEN CO LTD
Catheter and method of manufacture
ActiveUS20100217235A1Increase flexibilityExcellent kink resistanceLaminationLamination apparatusMedical deviceBiomedical engineering
The instant invention relates generally to introducer catheters used to help deliver catheters or other medical devices to locations within the human body. In particular, the instant invention relates to large diameter introducer catheters (introducer catheters with lumens greater than about 6 French) having increased strength, flexibility, and kink resistance. Introducer catheters according to the teachings herein may also include curved distal ends and flared (that is, funnel-like) transition sections within their lumens.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
Method for fabrication of porous metal templates and growth of carbon nanotubes and utilization thereof
InactiveUS20050276743A1Large caliberSimple methodAnodisationMaterial nanotechnologyChemical speciesFuel cells
The present invention relates to controlled growth of carbon nanotube (CNT) arrays via chemical vapor deposition (CVD) using novel porous anodic aluminum oxide (AAO) templates, which have been seeded with transition metal catalysts. The resulting CNT bundles may be dense and long and can be used for numerous applications. Further, the porous AAO templates and the CNTs grown thereby, can be functionalized and used for separation of chemical species, hydrogen storage, fuel cell electrocatalyst and gas flow membranes, other catalytic applications, and as a bulk structural material.
Owner:BOARD OF RGT NEVADA SYST OF HIGHER EDUCATION ON BEHALF OF THE UNIV OF NEVADA RENO
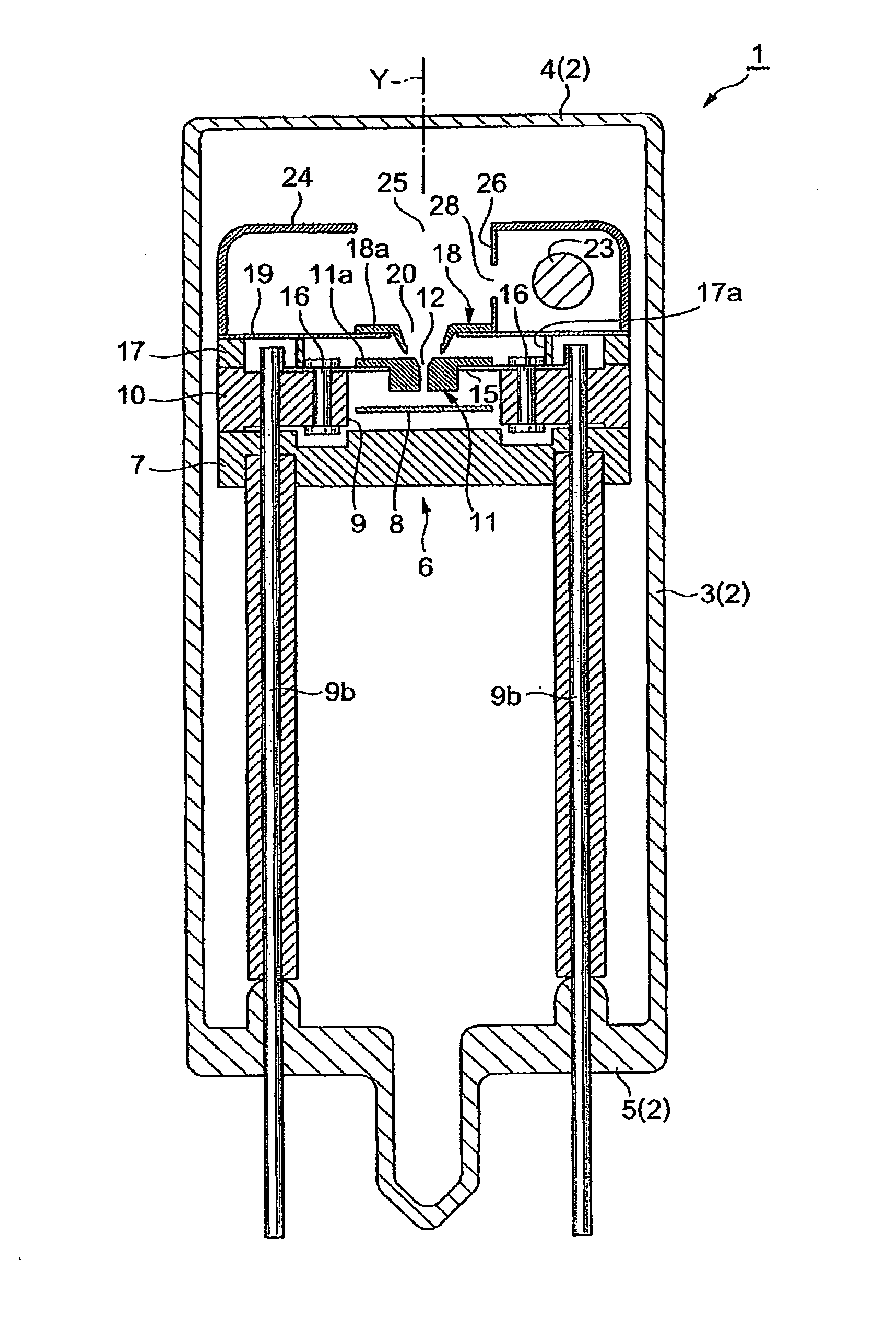
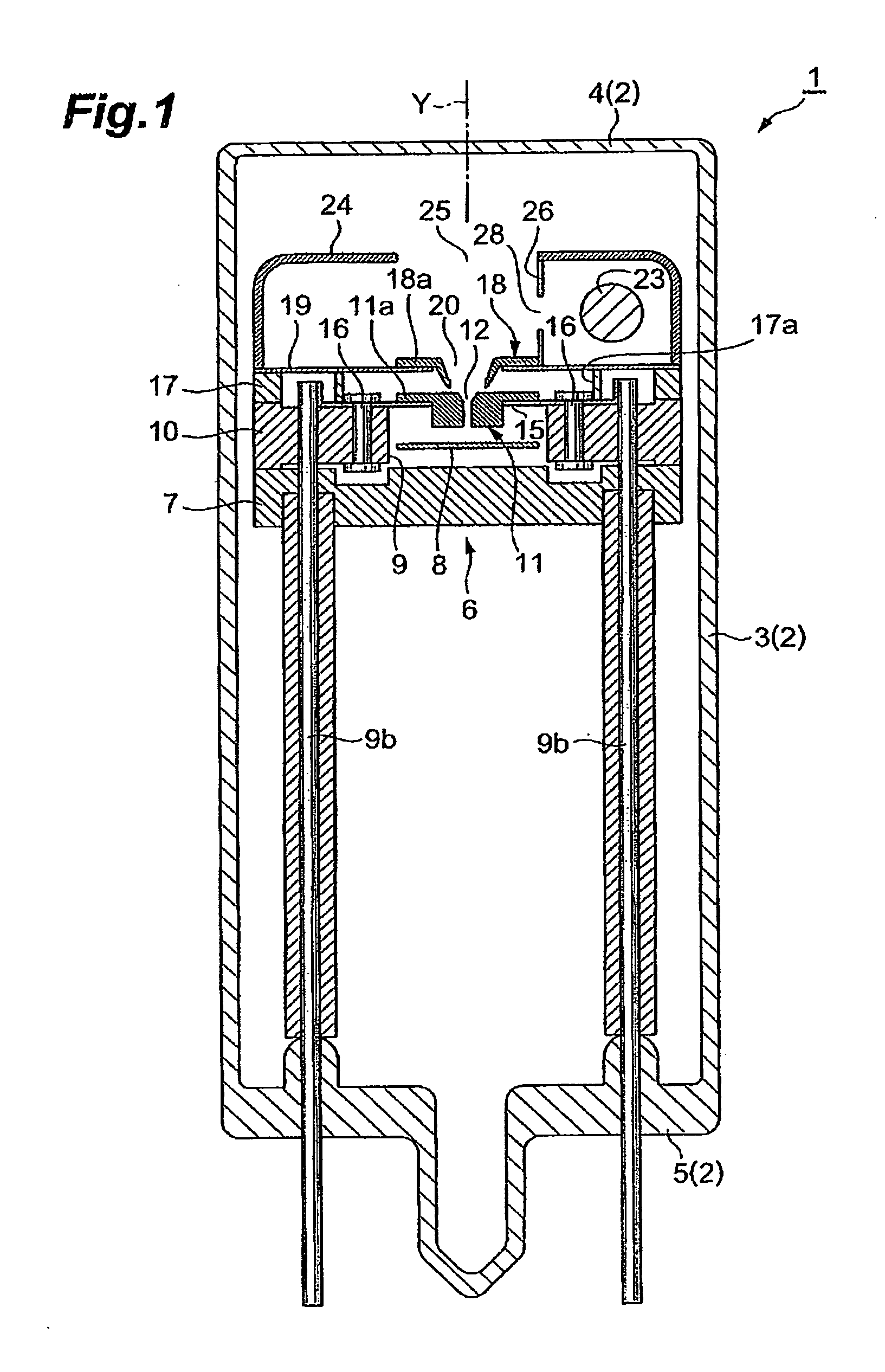
Gas discharge tube
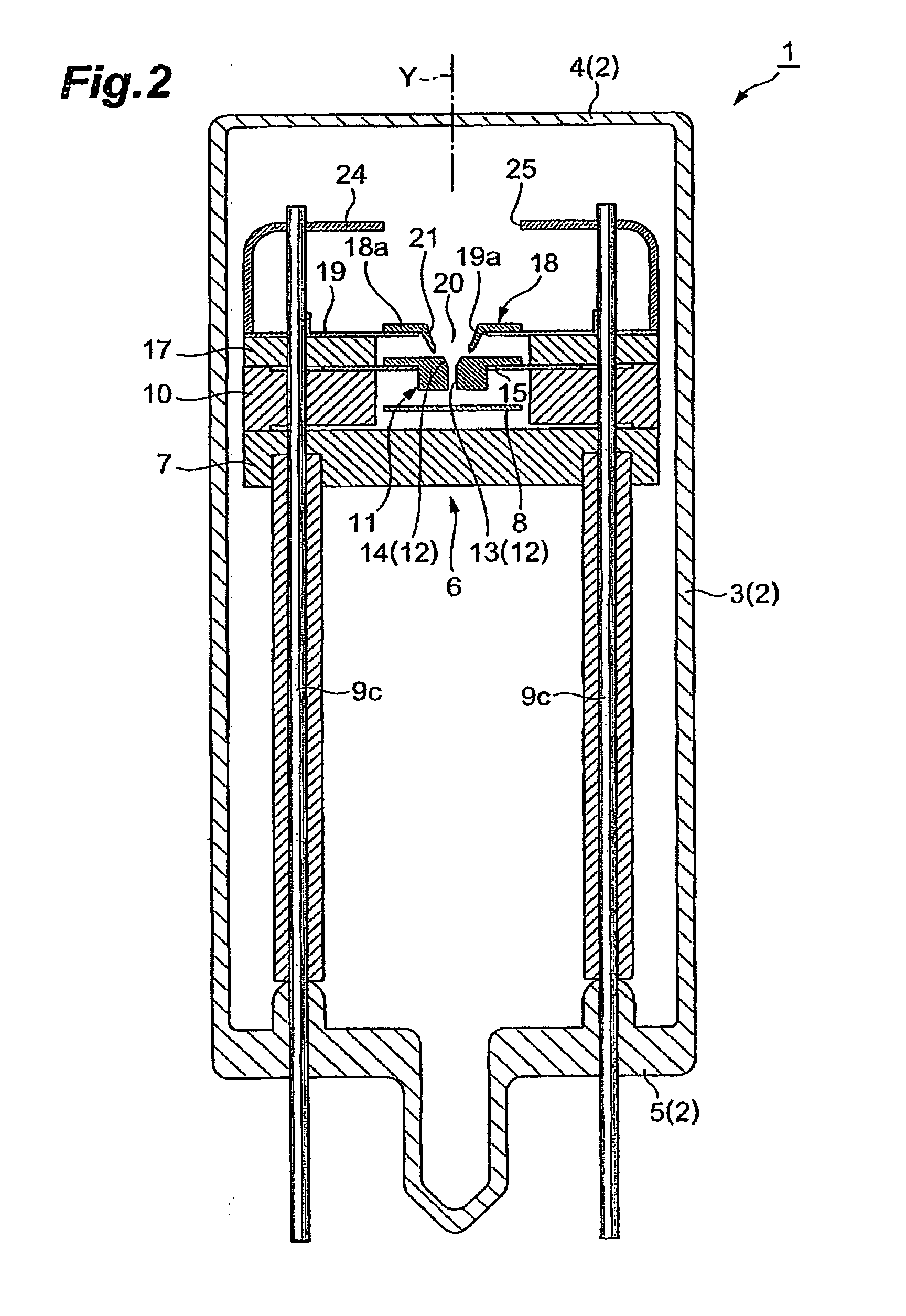
ActiveUS20050231119A1Improvement of start propertyIncrease plasma densitySolid cathode detailsGas discharge lamp detailsStart timeHigh luminance
In the gas discharge tube according to the present invention, there is carried out narrowing of the discharge path with cooperation of the first opening 20 and the second opening 12 in order to obtain higher luminance of light. Further, in order to maintain excellent starting-properties of a lamp even if the discharge path is narrowed, a predetermined voltage is applied to a second discharge path limit portion 11 externally. Thereby, a positive or active starting discharge is produced in such a manner as to pass through the first opening 20. Further, the second opening 12 is comprised of not only a straight section 13 extending in a direction of an optical axis Y, but also a spread section 14 extending from an end portion of the straight section 13 toward the first opening 20. The spread section 14 has a function of improving the starting properties of the lamp and forms an arc ball, and the straight section has a function of improving a plasma density. Thereby, discharge at a starting time is made easy to pass through the second discharge path limit portion 11. As a result, a rapid start of discharge between a cathode 23 and an anode portion 8 is achieved in the second discharge path limit portion 11, and that contributes to proper generation of an arc ball after lighting.
Owner:HAMAMATSU PHOTONICS KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com