Multiple-effect azole microcapsule suspension formulation with slow-release function and preparation method thereof
A technology of azole microcapsules and paclobutrazol, which is applied in the field of paclobutrazol microcapsule suspension preparations and its preparation, can solve the problems of paclobutrazol with too fast and too strong drug effects, drug damage, etc., and achieve protection of the natural ecological environment, strong penetration, and improved stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
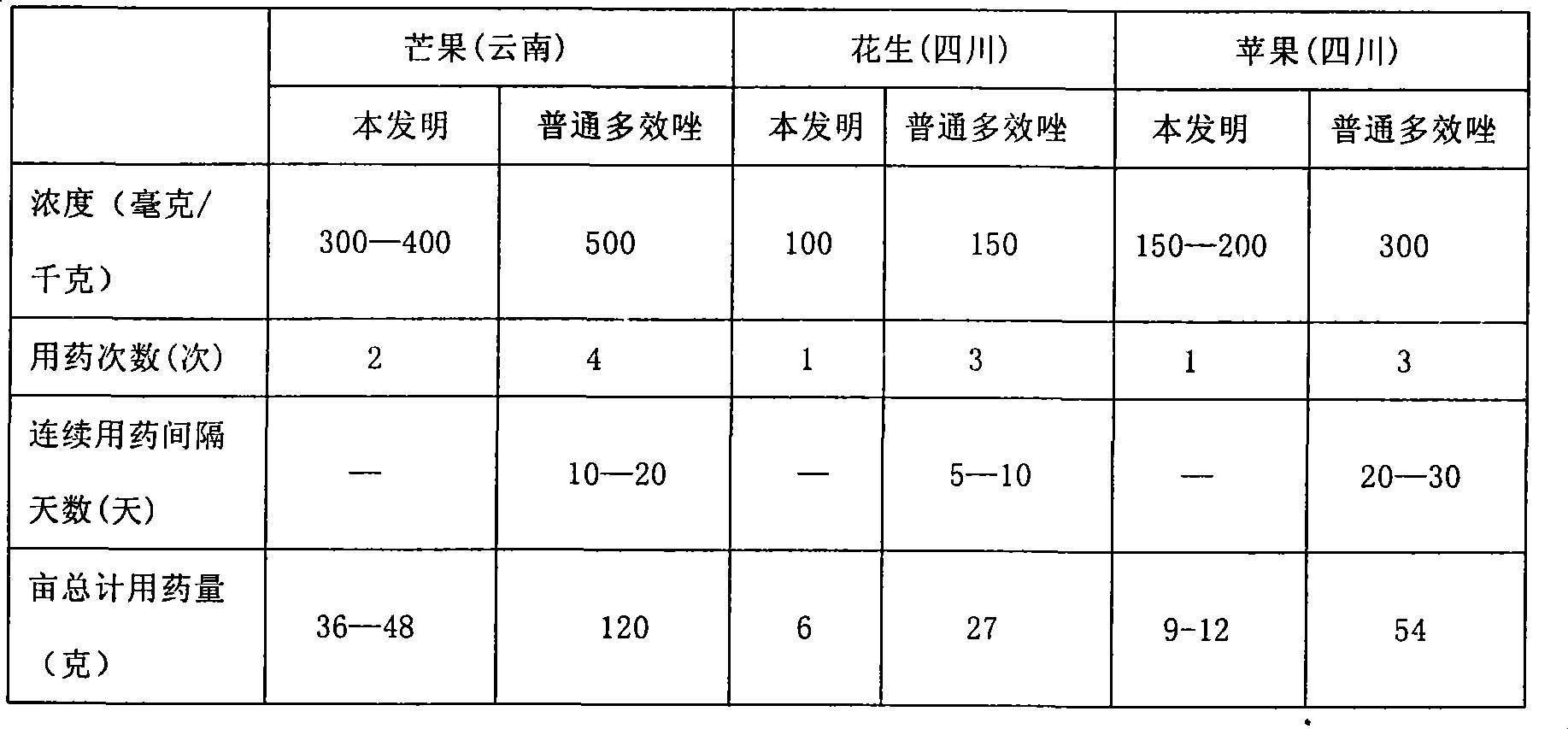
Image
Examples
Embodiment 1
[0022] The paclobutrazol microcapsule suspension preparation with sustained-release function in this embodiment 1 consists of the following components in 100 parts by weight:
[0023] Polytoluene-2,4-diisocyanate 10 parts
[0024] Ethylenediamine 4 parts
[0025] Paclobutrazol 5 parts
[0026] Alkylphenol polyoxyethylene ether 11 parts
[0027] 70 parts of water,
[0028] The preparation method of the preparation of the present embodiment 1 is proportioning, polytoluene-2,4-diisocyanate and ethylenediamine are stirred at a speed of 150r / min for 1 hour, and paclobutrazol is put into the stirred polytoluene-2,4 —Diisocyanate, in ethylenediamine, under the condition of 35°C±5°C and normal pressure, stirring and reacting at 200r / min for 1 hour to prepare the capsule membrane-capsule core complex, and then add the alkyl group Phenol polyoxyethylene ether multi-dimensional emulsified for 0.5 hours, finally added to water, continued to micro-emulsify in the water phase with 200r / ...
Embodiment 2
[0030] The paclobutrazol microcapsule suspension preparation with sustained-release function in this embodiment 2 is composed of the following components in 100 parts by weight:
[0031] Carboxymethylcellulose 15 parts
[0032] Diethylenetriamine 7 parts
[0033] Paclobutrazol 20 parts
[0034] Alkyl polyglucoside 11 parts
[0035] 47 parts of water
[0036] The preparation method of the present embodiment 2 preparation is proportioning, carboxymethylcellulose, diethylenetriamine are stirred at 100r / min rotating speed for 1.5 hours, paclobutrazol is put into the stirred hydroxymethylcellulose, In diethylenetriamine, under the condition of 45°C±5°C and normal pressure, stirring and reacting at 1200r / min for 1 hour, the capsule membrane-capsule core complex was prepared, and then the alkyl polyglycoside was added for multi-dimensional emulsification for 0.5 hour, Finally add water, continue to emulsify for 2 hours in the water phase with 500r / min stirring condition, 150 time...
Embodiment 3
[0038] The paclobutrazol microcapsule suspension formulation with sustained-release function in Example 3 is composed of the following components in 100 parts by weight:
[0039] Polyvinylpyrrolidone 20 parts
[0040] Polyethylene glycol 10 parts
[0041] Paclobutrazol 40 parts
[0042] 8 parts of quaternary ammonium glucoside
[0043] 22 parts of water
[0044] The preparation method of the preparation of the present embodiment 3 is to proportion the ingredients, stir polyethylene glycol and polyvinylpyrrolidone at 200r / min for 1 hour, put paclobutrazol into the stirred polyethylene glycol, polyvinylpyrrolidone In the medium, under the condition of 65℃±5℃ and normal pressure, stirring and reacting at 1200r / min for 2 hours, the membrane-capsule-core complex was prepared, and after adding quaternary ammonium glucoside for multi-dimensional emulsification for 1 hour, finally adding water, in the water phase The emulsification was continued for 3 hours under the stirring cond...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com