In-situ phase change gel slow release system taking phospholipid as substrate and preparation method thereof
A technology of sustained-release preparations and phospholipids, which is applied in the field of in-situ injection phase-change gel sustained-release systems, which can solve problems such as drug concentration differences, small molecule drug leakage, and small injection volumes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
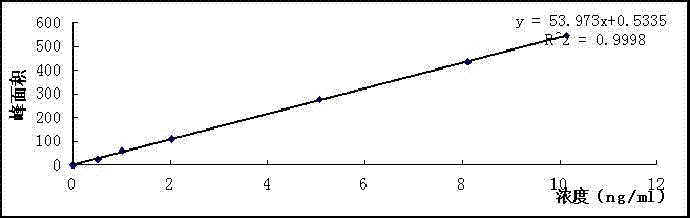
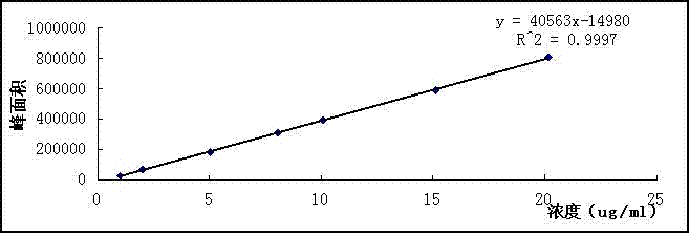
Image
Examples
Embodiment 1
[0074] Take 50 mg of octreotide acetate, dissolve it in 1.5 g of 85% (v / v) ethanol-pH4.0 lactic acid buffer solution to obtain a drug solution, filter it through a 0.22 μm microporous membrane, add 7.0 g of egg yolk lecithin E80, and then add After filtering 1.5g of soybean oil through a 0.22μm microporous membrane, stir it magnetically for about 0.5h in a sterile environment until the E80 is completely dissolved to obtain a milky yellow opaque liquid containing a large number of air bubbles. Let it stand until the air bubbles completely disappear. Seal and serve.
[0075]
Embodiment 2
[0077] Take 20 mg of thymopentin, dissolve it in 2.0 g of absolute ethanol to obtain a drug solution, filter it through a 0.22 μm microporous membrane, add 6.0 g of egg yolk lecithin E80, and then add medium-chain fatty acid glycerides filtered through a 0.22 μm microporous membrane 2.0g, magnetically stirred for about 0.5h, until E80 was completely dissolved, and a milky yellow opaque liquid containing a large number of bubbles was obtained, which was left to stand until the bubbles completely disappeared, then packaged and sealed to obtain a high-concentration thymopentin sustained-release preparation of phospholipids.
[0078]
Embodiment 3
[0080] Take 100 mg of insulin, dissolve it in 2.0 g of 90% (v / v) ethanol-physiological saline solution to obtain a drug solution, filter it through a 0.22 μm microporous membrane, add 5.5 g of soybean lecithin, and then add 0.22 μm microporous membrane to filter 2.5g of soybean oil, stirred by magnetic force for about 0.5h, until the soybean lecithin was completely dissolved to obtain a milky yellow opaque liquid containing a large number of air bubbles, left to stand until the air bubbles completely disappeared, subpackaged and sealed to obtain a high-concentration insulin phospholipid sustained-release preparation.
[0081]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com