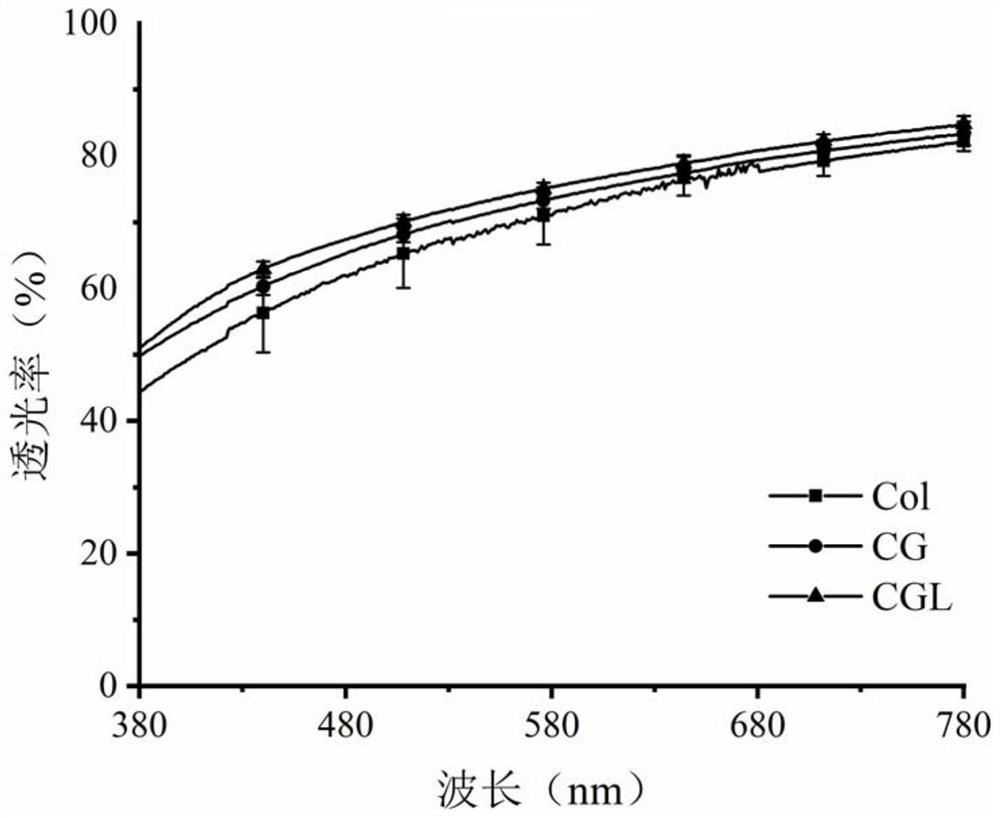
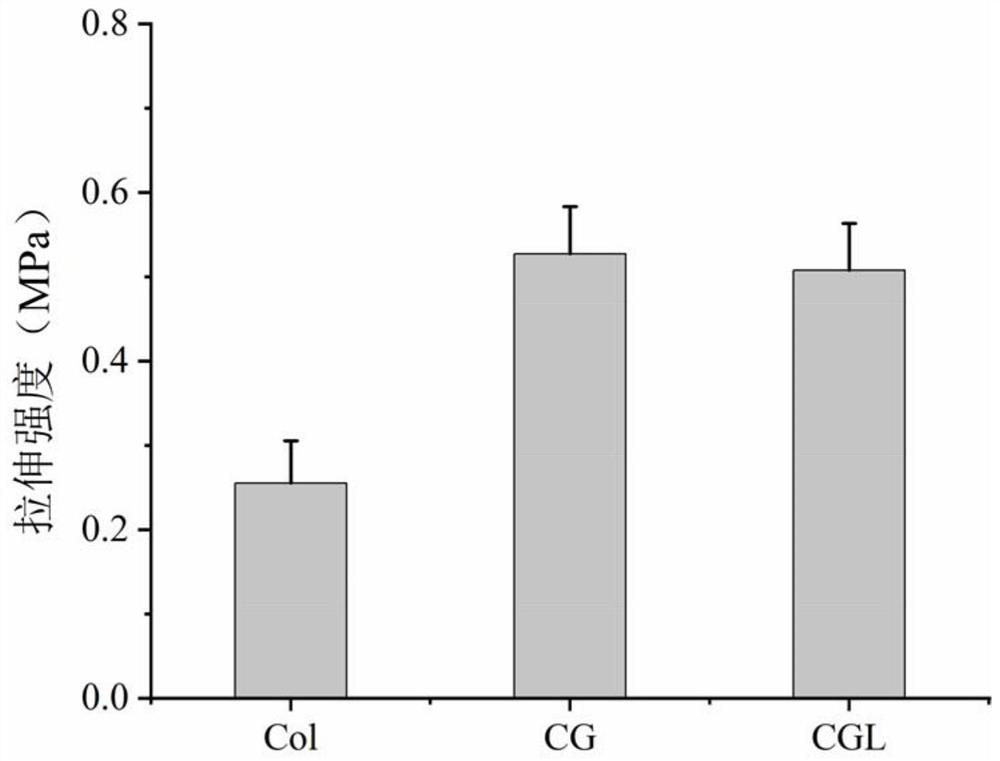
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Cornea repair" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
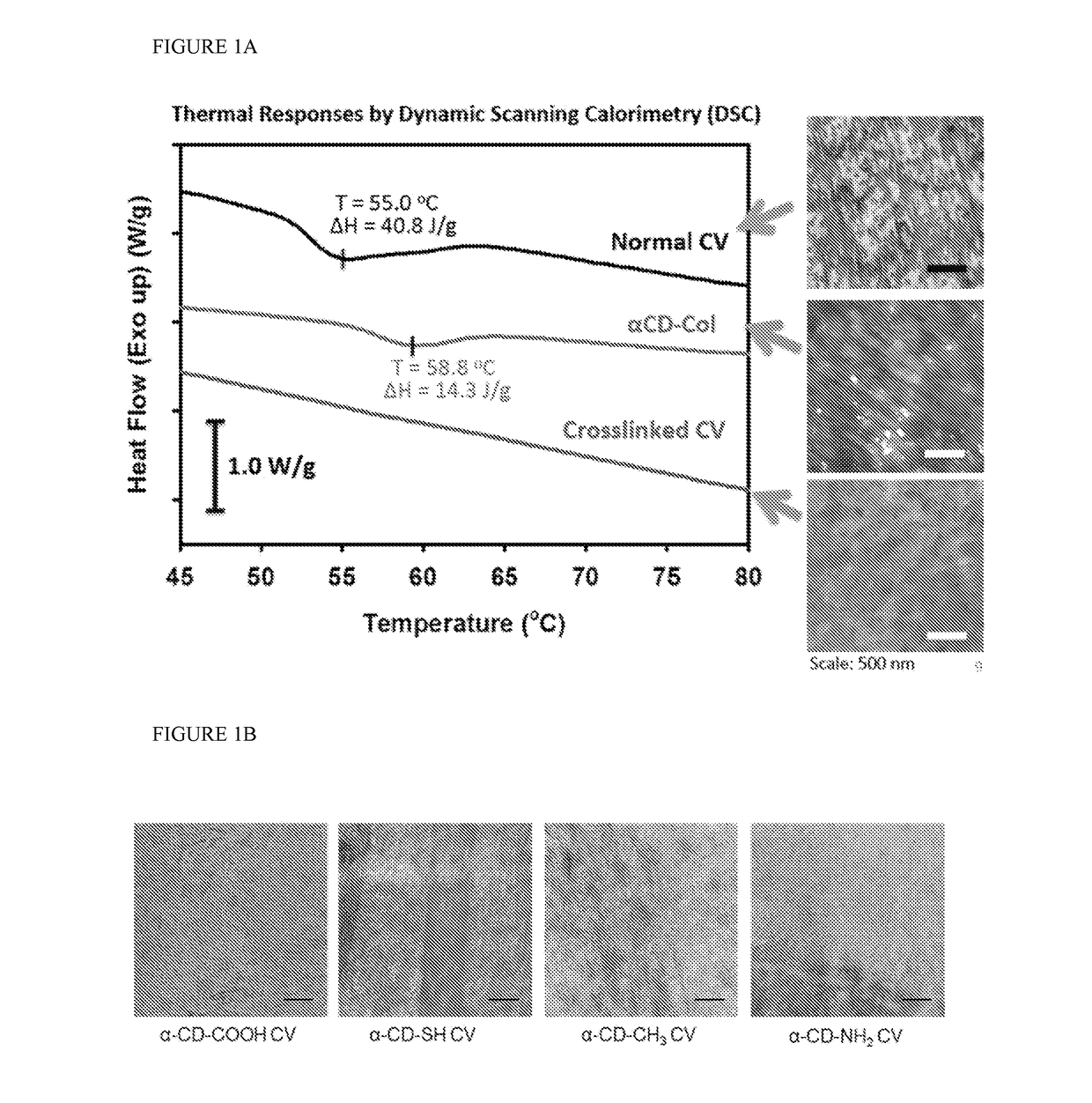
Remend® Corneal Repair Gel can be utilized in the treatment of acute injuries to the cornea. The corneal repair gel drops are composed of cross-linked, modified hyaluronic acid, which provides a suitable environment to facilitate cell migration, thereby enhancing healing and leaving less room for scarring.
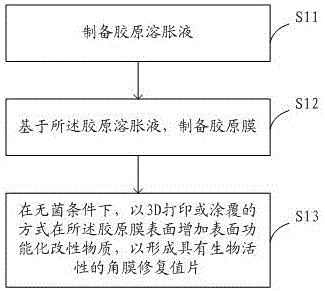
Preparation method of cornea repair graft with biological activity and cornea repair graft
ActiveCN106039403AHigh strengthImprove water absorptionPharmaceutical delivery mechanismTissue regenerationActive cellDrug biological activity
The invention discloses a preparation method of a cornea repair graft with biological activity and the cornea repair graft, and solves the technical problem of deficiency of cornea donors. The preparation method comprises the following steps: preparing collagen dispersion; preparing a collagen membrane on the basis of the collagen dispersion; adding surface functionalization modified substances on the surface of the collagen membrane in a 3D printing or coating mode under the sterile condition to form the cornea repair graft with biological activity. The surface functionalization modified substances are fine particles which are prepared from a cornea stroma, which does not contain a lesion part and contains active cells, of a patient, the biology and the biological stability of the high-strength collagen membrane can be improved, the physiological function of cornea is improved, after the modified collagen membrane is implanted into an eye of the patient, transparency and moisture content are good, the transparency can be recovered quickly clinically, the biological compatibility is good, and a good stent is provided for cornea cell culture of a cornea transplantation receptor.
Owner:广州尤尼智康生物科技有限公司
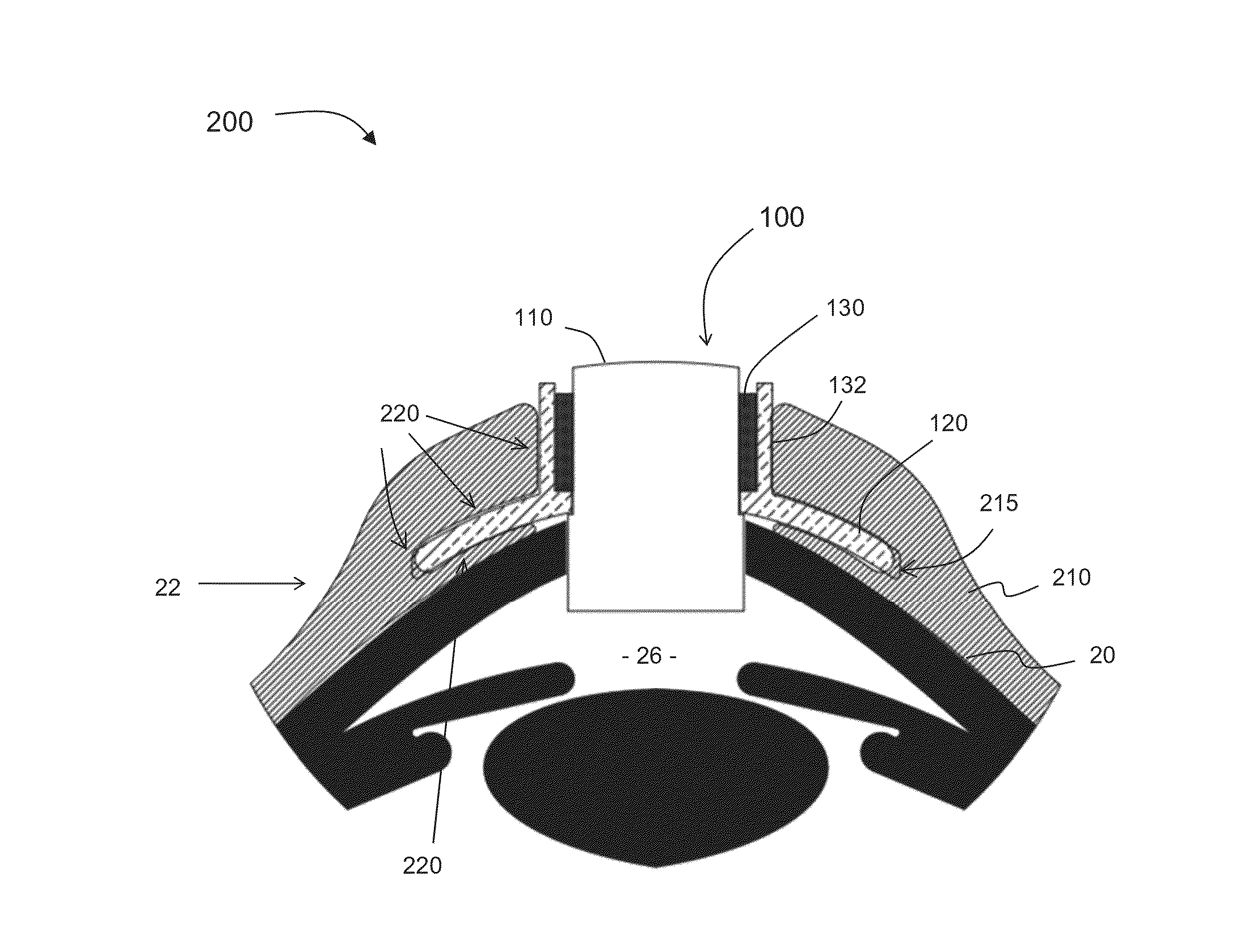
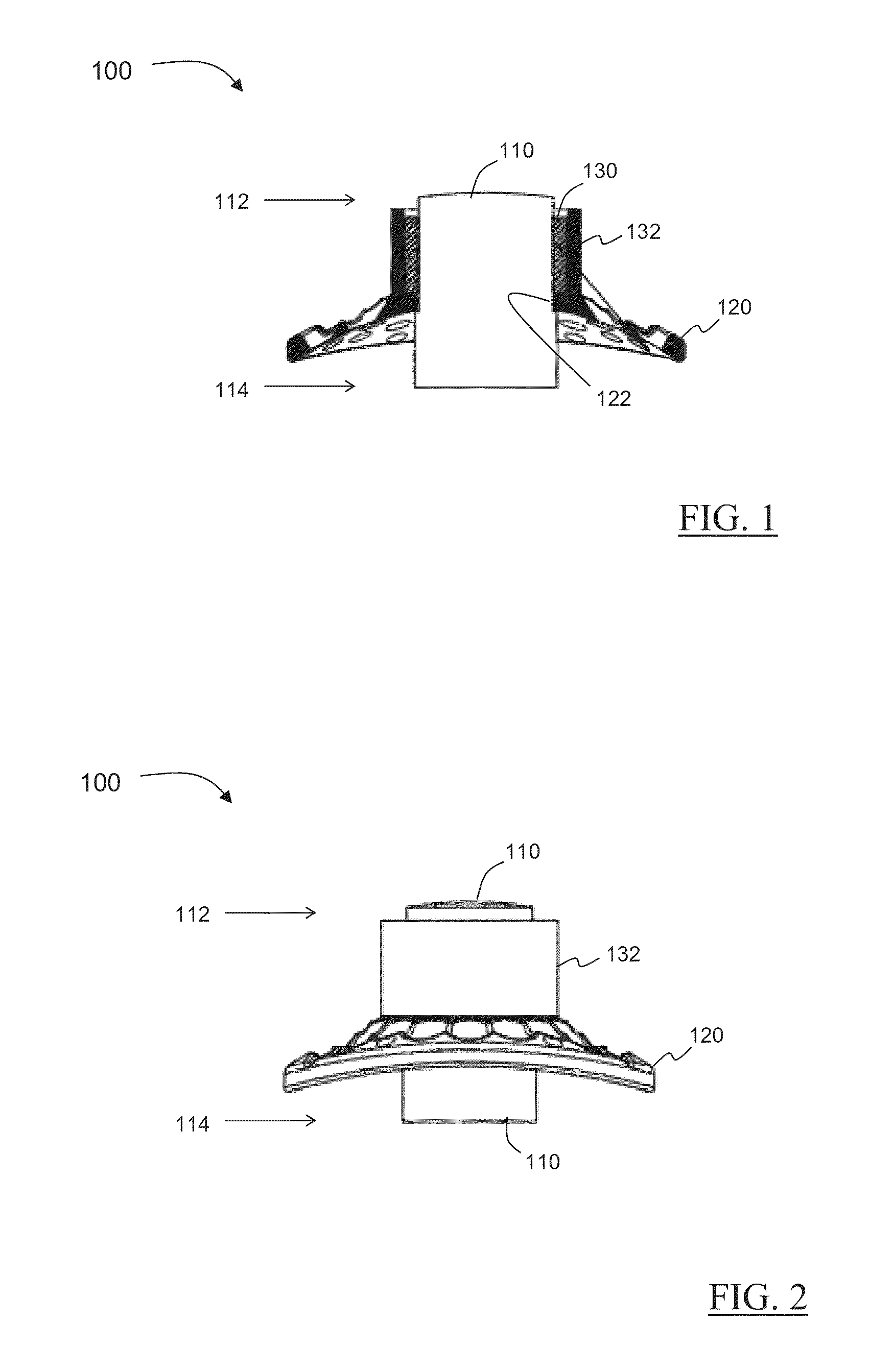
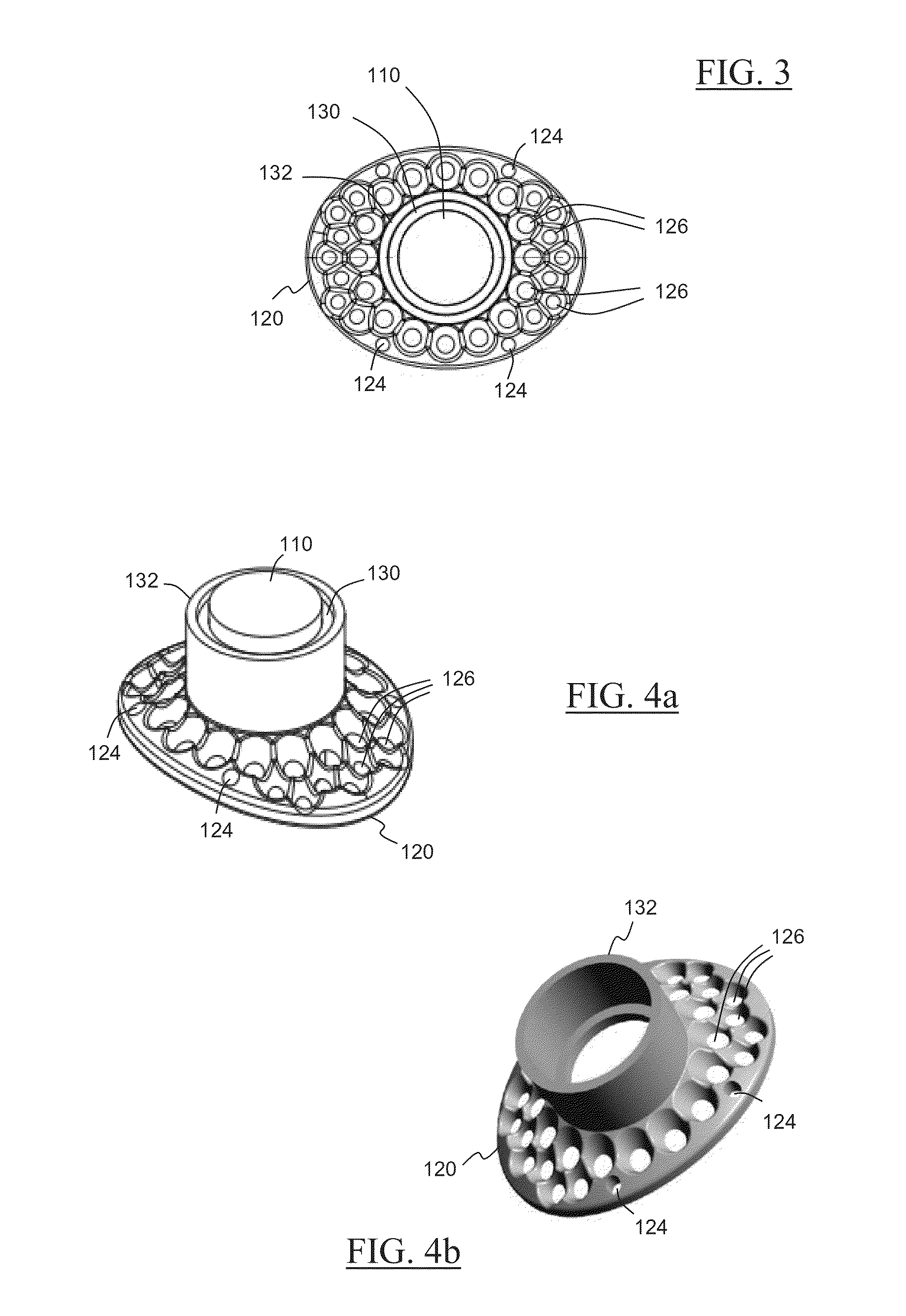
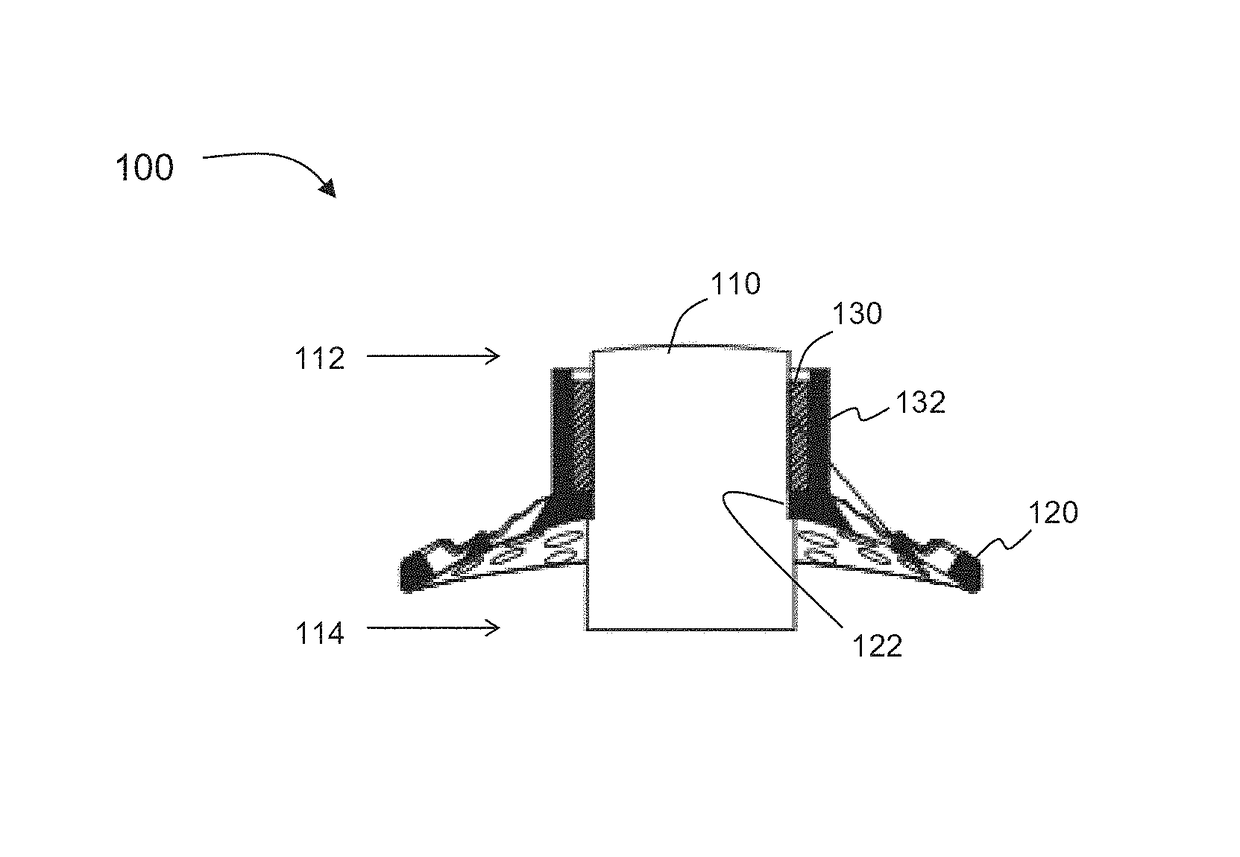
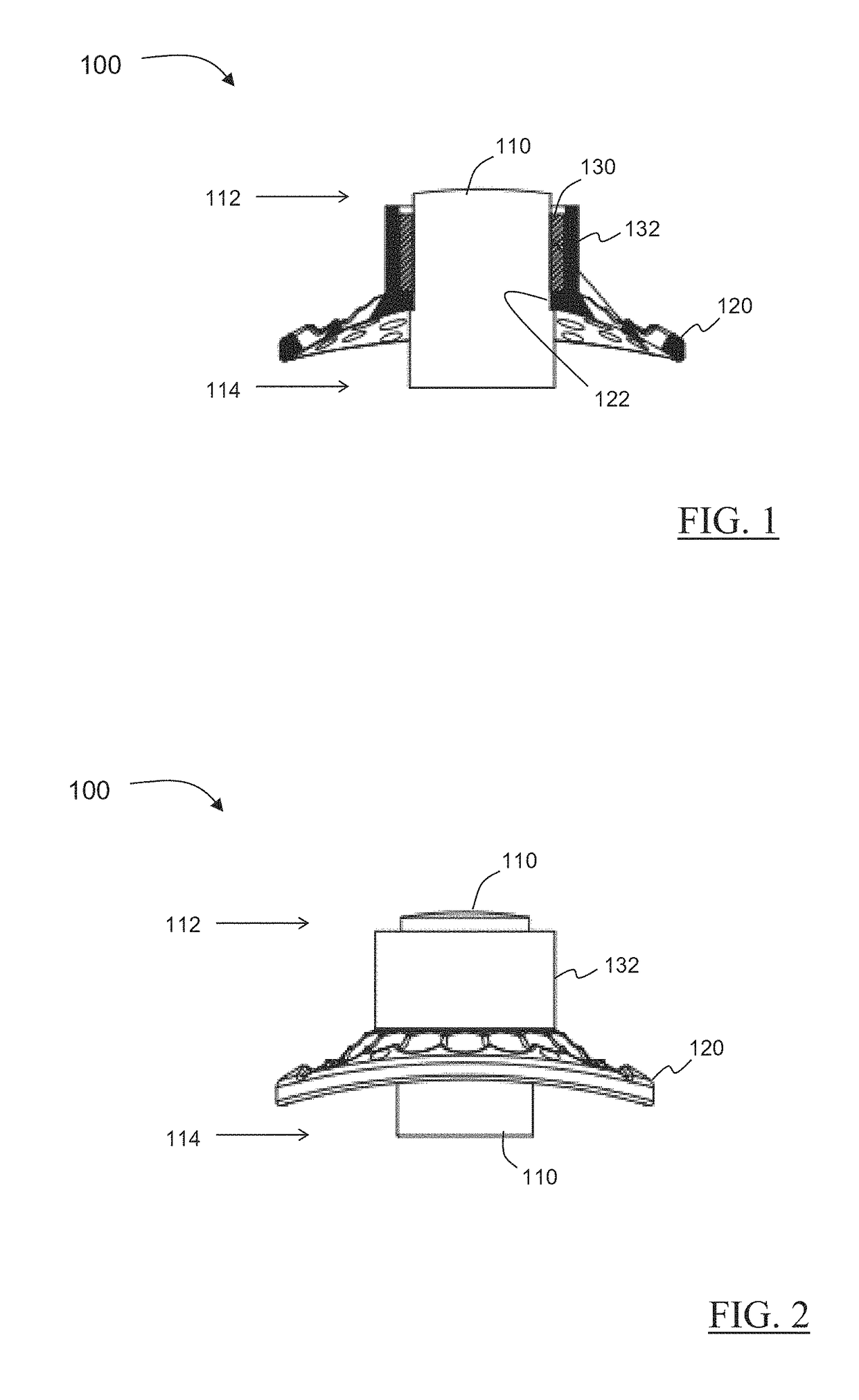
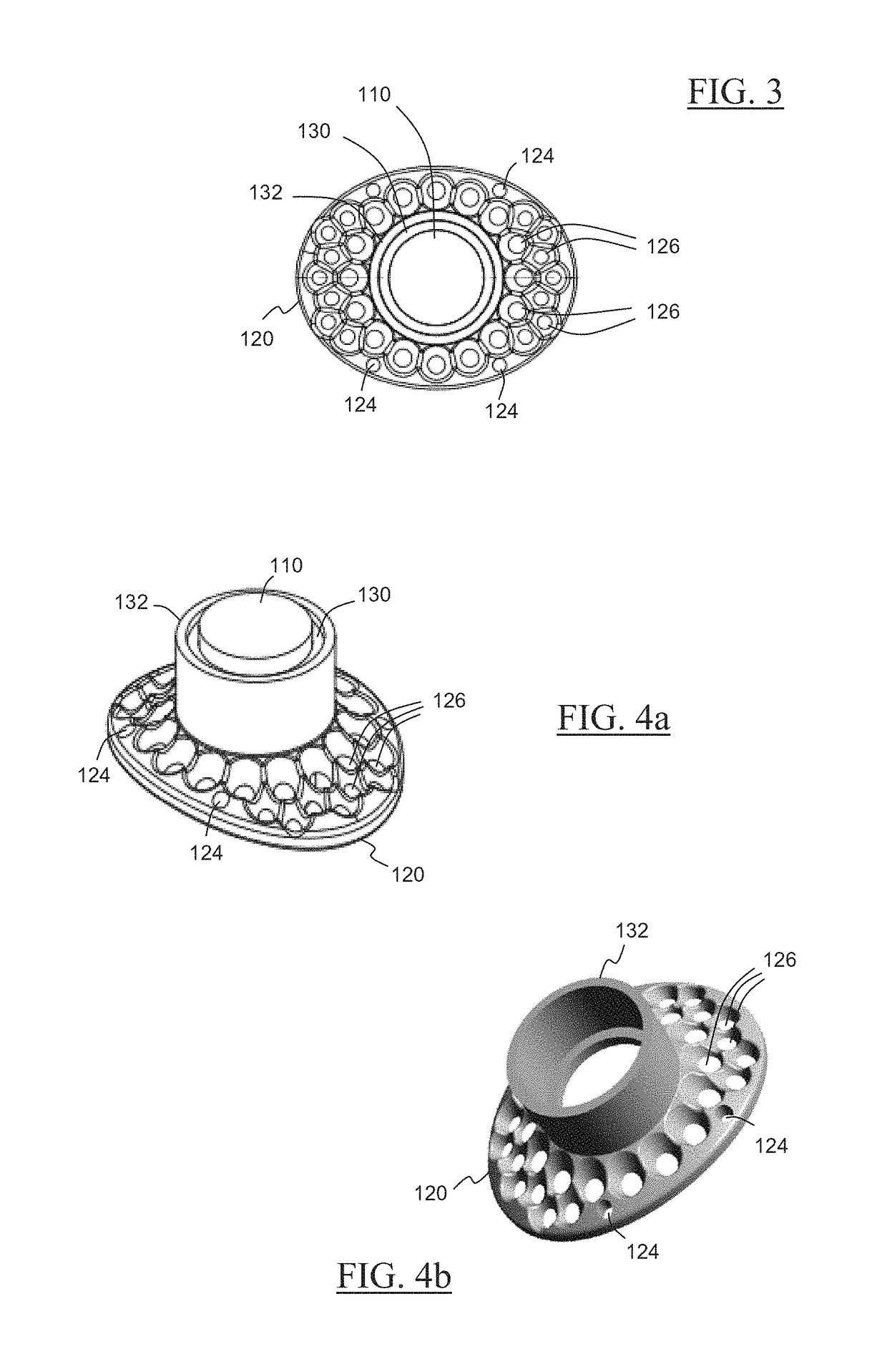
Novel keratoprosthesis, and system and method of corneal repair using same
ActiveUS20150216651A1Improved soft tissue adhesionLess squeezeEye implantsAcid etchingAnterior cornea
A keratoprosthesis and system and method of using same for corneal repair. The keratoprosthesis comprises a biocompatible support and an optic member disposed through a channel within the support. The support includes metal, preferably titanium, and treated, such as by sandblasting and / or acid etching, to create textured surfaces that promote soft tissue adhesion. A locking member interconnects the optic member and support. An outer surface of the locking member a collar extending from the support and disposed around the optic member is also metal, preferably titanium, and is similarly treated to promote soft tissue adhesion. A locking member interconnects the optic member and support. The system includes the keratoprosthesis positioned within an isolated soft tissue segment of a non-ocular tissue, such as buccal mucosa, placed on the anterior cornea. The method includes removing corneal epithelium, isolating and transplanting a segment of soft tissue to the de-epithelialized cornea, creating a receiving area in the soft tissue, positioning a keratoprosthesis relative to the receiving area anterior to the cornea, and securing the keratoprosthesis.
Owner:UNIV OF MIAMI
Surface immobilized modified cornea repair material and preparation method thereof
InactiveCN107185038AGood physical and chemical propertiesImprove repair effectTissue regenerationProsthesisCross-linkPolymer science
The invention discloses a method for preparing a surface immobilized modified cornea repair material. The method comprises the following specific steps: (1) adding a high polymer material a into a solvent for mechanically stirring, preparing a natural high polymer solution I with the weight ratio of 0.1-15wt%, and injecting the high polymer solution I into a mold so as to prepare a high polymer film; (2) preparing a high polymer solution II from a high polymer material b and a cross-linking agent according to the method in the step (1), wherein a and b are different high polymer materials; (3) drying the high polymer film in the step (1), soaking the film in the solution II prepared in the step (2), soaking for 0.5-36 hours, taking out and drying the film, thereby obtaining the surface immobilized modified cornea repair material. The preparation method is simple in process, high in repeatability and capable of realizing large-scale volume production, and the prepared material has excellent physical and chemical properties and is excellent in repair effect.
Owner:GUANGZHOU PUDAO LIANXIN BIOTECH CO LTD
Adipose-derived stem cell preparation eye drops for treating corneal injury and preparation method thereof
InactiveCN107320492AImprove repair effectGood effectSenses disorderPharmaceutical delivery mechanismSerum free mediaTissue repair
The invention discloses adipose-derived stem cell preparation eye drops for treating corneal injury and a preparation method thereof and solves the problem that in the prior art, the tissue repair condition is less than satisfactory, cicatrization is obvious and visual function recovery is not ideal. The adipose-derived stem cell preparation eye drops comprise a cell culture liquid which is a concentrated liquid prepared by the following steps: culturing adipose-derived stem cells through a mesenchymal stem cell serum-free medium; when the cell density in the mesenchymal stem cell serum-free medium reaches 90% or above, performing separation to obtain a culture supernatant fluid; and finally concentrating the culture supernatant fluid to obtain the concentrated liquid. The adipose-derived stem cell preparation eye drops disclosed by the invention have the advantages of remarkably improving the cornea repair effect and the like.
Owner:成都远山博桥生物科技有限公司
Antibacterial cornea repair material and preparation method thereof
ActiveUS20160082151A1Reduce riskLow costConnective tissue peptidesEye implantsBiocompatibility TestingMechanical property
Disclosed in the invention is a preparation method of an antibacterial cornea repair material, including the following steps: (1) purifying type [Iota] collagen extracted from beef tendons, and using an acetic acid or hydrochloric acid solution to prepare a collagen solution with a concentration of 6.0-10.0 mg / ml; (2) casting the collagen solution into the forming mould of the cornea repair material, and then naturally air drying at room temperature to form a membrane; (3) soaking the obtained collagen membrane in an antibiotic solution, stirring until the membrane is fully in contact with the solution; and then adding a crosslinking agent and a catalyst to the above-mentioned solution, and stirring to perform a crosslinking reaction; and (4) taking out the crosslinked membrane material obtained in step (3) and washing 3-5 times with deionized water, and then naturally air drying at room temperature to obtain the antibacterial cornea repair material. The material has relatively good mechanical properties, optical properties and biocompatibility, and has a good antibacterial effect. It can be used for repairing and replacing damaged cornea tissue in the field of medicine.
Owner:SOUTH CHINA UNIV OF TECH
Pharmaceutical composition for corneal tissue repair of insulin nano system and application of pharmaceutical composition
PendingCN114099645AReduce dosing frequencyImprove complianceSenses disorderPeptide/protein ingredientsTissue repairOphthalmology
The invention discloses a pharmaceutical composition for corneal tissue repair by an insulin nano system and application of the pharmaceutical composition, and belongs to the technical field of pharmaceutical compositions, and the pharmaceutical composition is characterized by comprising nano materials such as lipidosome and insulin wrapped in the nano materials such as lipidosome; the pharmaceutical composition for corneal tissue repair by the insulin nano system is applied to corneal repair. The invention is mainly used for providing a novel preparation ophthalmic pharmaceutical composition which has the advantages of safety, effectiveness, side effect and low administration frequency, and can effectively improve the compliance of patients; meanwhile, the ophthalmic pharmaceutical composition has important biological effects of resisting inflammation, resisting apoptosis and promoting corneal cell and nerve repair, and has more advantages in the treatment effects of resisting inflammation, resisting infection, promoting corneal repair and strengthening amniotic transplantation compared with a traditional treatment method.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV
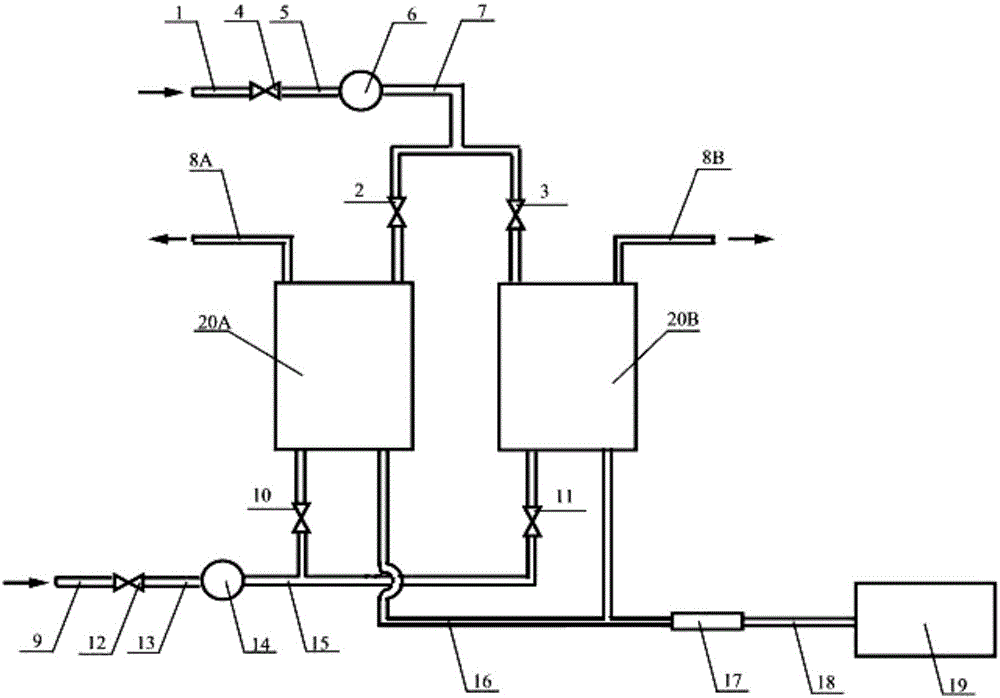
Method and device for testing oxygen permeation of cornea repair material
InactiveCN101832982AAchieving Oxygen Permeability TestLow priceComponent separationCornea repairOxygen
The invention relates to a method and a device for testing the oxygen permeation of a cornea repair material. A sample is placed in a sample clamp; oxygen and carrier gas are introduced to two sides of the sample; and the oxygen passes through the sample and is taken to a detection device by the carrier gas for detecting the concentration of the oxygen. The test device comprises the sample clamp which consists of an oxygen cavity and a carrier gas cavity; an inlet end of the oxygen cavity is communicated with the oxygen through an inlet pipeline of the oxygen cavity; an outlet end of the oxygen cavity is evacuated through an outlet pipeline of the oxygen cavity; and an inlet end of the carrier gas cavity is communicated with the carrier gas through an inlet pipeline of the carrier gas cavity, and an outlet end of the carrier gas cavity is communicated with the detection device through an outlet pipeline of the carrier gas cavity. Compared with a coulomb sensor, a gas chromatograph used by the invention has lower price, lower repair and maintenance frequency and longer service cycle, and the operation cost is obviously reduced.
Owner:SOUTH CHINA UNIV OF TECH
Medium, corneal stromal slice prepared by medium and preparation method
ActiveCN111235109AGood biocompatibilityGood cell affinityEye implantsCulture processFibrosisBiocompatibility
The invention relates to the technical field of medical biomaterials, in particular to a culture medium, a corneal stromal slice prepared by the culture medium and a preparation method. The medium provided by the invention is a Dulbecco's modified eagle medium / F12 (DMEM / F12) medium containing a porcine corneal extract, Y-27632, insulin-transferrin-selenium (ITS), fibroblast growth factors (FGF), ascorbic acid and fetal bovine serum. According to the medium, by selecting suitable active components and proportion, a corneal stromal cell and corneal stromal slice prepared by employing the mediumhave the functions of maintaining the corneal stromal cell phenotype, promoting proliferation, improving vitality, resisting fibrosis and resisting apoptosis, and the like in comparison with a cornealstromal cell and corneal stromal slice prepared by a traditional medium. The corneal stromal slice disclosed by the invention is prepared by taking a corneal lens as a raw material and culturing thecorneal lens by employing the medium; and compared with animal-derived corneal repair material, the corneal lens has good biocompatibility and no antigenicity, can be used as substitutes of various donor materials for corneal transplantation, and can be accepted by the majority of patients and is clinically applied for long.
Owner:AIER EYE HOSPITAL GRP CO LTD
Keratoprosthesis, and system and method of corneal repair using same
A keratoprosthesis and system and method of using same for corneal repair. The keratoprosthesis comprises a biocompatible support and an optic member disposed through a channel within the support. The support includes metal, preferably titanium, and treated, such as by sandblasting and / or acid etching, to create textured surfaces that promote soft tissue adhesion. A locking member interconnects the optic member and support. An outer surface of the locking member a collar extending from the support and disposed around the optic member is also metal, preferably titanium, and is similarly treated to promote soft tissue adhesion. A locking member interconnects the optic member and support. The system includes the keratoprosthesis positioned within an isolated soft tissue segment of a non-ocular tissue, such as buccal mucosa, placed on the anterior cornea. The method includes removing corneal epithelium, isolating and transplanting a segment of soft tissue to the de-epithelialized cornea, creating a receiving area in the soft tissue, positioning a keratoprosthesis relative to the receiving area anterior to the cornea, and securing the keratoprosthesis.
Owner:UNIV OF MIAMI
Oxygen permeability test device and oxygen permeability test method
InactiveCN105929078AAchieving Oxygen Permeability TestLow priceComponent separationVapor phase chromatographyCornea repair
The invention discloses an oxygen permeability test device and an oxygen permeability test method. The device comprises two sample clamps, an oxygen feeding device, a carrier gas feeding device and a detection device, wherein the sample clamps consist of oxygen cavities and carrier gas cavities; the oxygen cavities are connected with the oxygen feeding device through oxygen cavity feeding pipelines and are further provided with oxygen cavity discharging pipelines; the carrier gas cavities are connected with the carrier gas feeding device through carrier gas cavity feeding pipelines and are connected with the detection device through carrier gas cavity discharging pipelines; the detection device comprise a quantitative sample injection device and a gas chromatograph. The method disclosed by the invention comprises the following steps: placing a sample into the sample clamps, and feeding oxygen and carrier gas respectively to two sides of the sample. The oxygen enters the carrier gas through the sample, and the carrier gas is detected to obtain oxygen permeability data of the material. The device and the method can be used for testing the oxygen permeability of a cornea repair material; the device is long in service life, low in operation cost, small in system error and high in test precision.
Owner:GUANGZHOU PUDAO LIANXIN BIOTECH CO LTD
3D printing fibroin annular stent reinforced collagen-based corneal repair material and preparation method thereof
InactiveCN114732950AImprove mechanical propertiesGood biocompatibilityAdditive manufacturing apparatusTissue regenerationPolymer scienceTissue defect
The preparation method comprises the following steps: 1) preparing a silk fibroin solution, designing and optimizing 3D printing parameters of a silk fibroin annular stent, and constructing a plurality of silk fibroin annular stent materials with regular three-dimensional space structures; 2) carrying out surface chemical modification on the obtained silk fibroin annular scaffold material through a citric acid molecular solution; (3) preparing a collagen solution, introducing the carboxylated fibroin annular scaffold obtained in the step (2) into a collagen solution system, and enabling fibroin molecules and collagen molecules to form amido bond combination by taking EDC and NHS as a cross-linking agent and a catalyst respectively, so as to obtain a collagen solution; and finally, the silk fibroin reinforced collagen-based cornea repair material which is doubly compounded on a macroscopic physical structure and a microscopic chemical assembly level is formed. The material obtained by the invention has better physical and chemical properties and biocompatibility, is controllable in molding, and can be used for clinical repair and treatment of corneal tissue defects.
Owner:CHANGZHOU UNIV
Recombinant human truncated-type keratinocyte growth factor-1 eye drops and preparing method and application thereof
ActiveCN110339345AImprove stabilityImprove biological activitySenses disorderPeptide/protein ingredientsFiltrationCornea repair
The invention discloses recombinant human truncated-type keratinocyte growth factor-1 eye drops. The recombinant human truncated-type keratinocyte growth factor-1 eye drops are composed of recombinanthuman truncated-type keratinocyte growth factors-1 and auxiliary materials. The preparing method of the eye drops comprises the steps of preparing a thickener stock solution, preparing a recombinanthuman truncated-type keratinocyte growth factor-1 mixed solution, mixing the two solutions, and conducting aseptic filtration and volume metering. The eye drops can be used for promoting cornea repair, does not promote the growth of blood vessels, and have the advantage of being capable of improving the stability and biological activity of the recombinant human truncated-type keratinocyte growth factors-1 in the eye drops. Besides, the method is used for obtaining the aseptic recombinant human truncated-type keratinocyte growth factor-1 eye drops. The eye drops are applied to treating cornealinjuries, and have a good industrial application prospect.
Owner:CHONGQING PEG BIO BIOTECH CO LTD
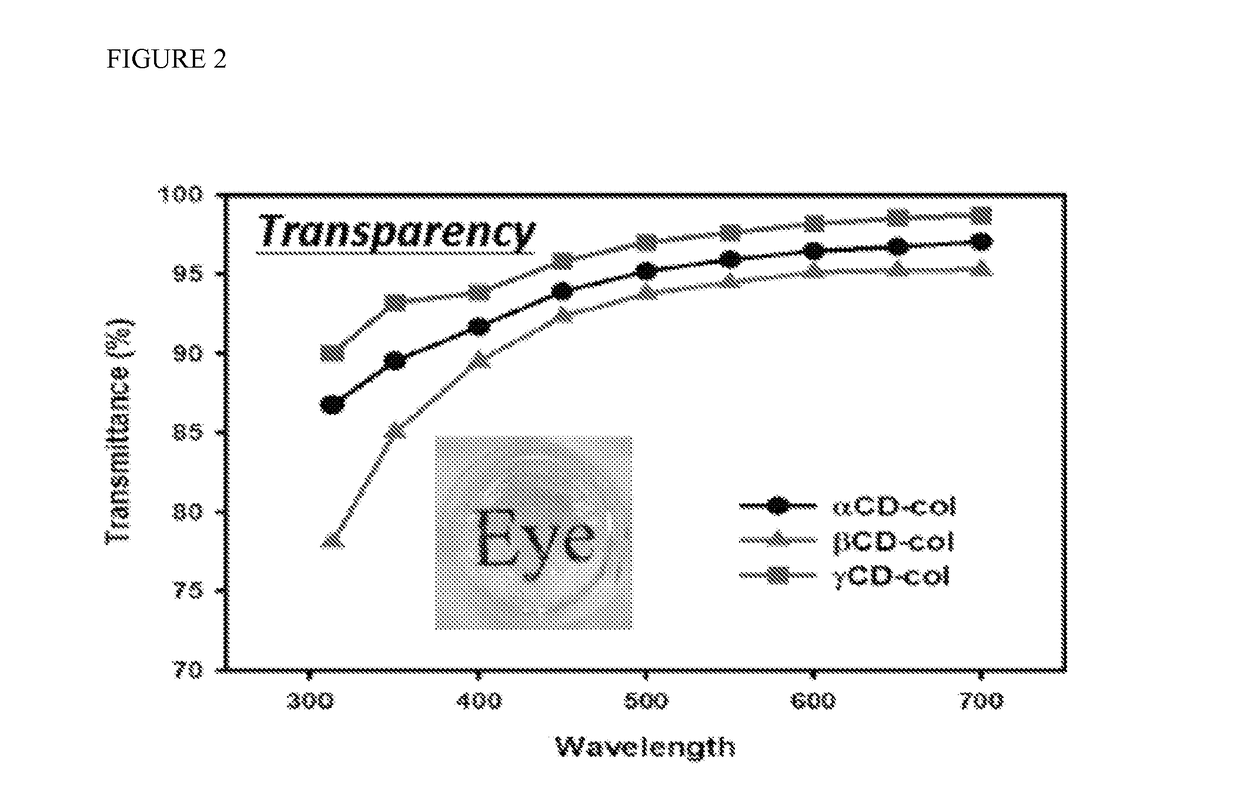
Cornea mimetic biomaterials: vitrified collagen-cyclodextrin implants
PendingUS20170182213A1Better mimicOptimization propertiesEye implantsTissue regenerationFiberBiological property
The present inventors employed cyclodextrins for use as a proteoglycan substitute to engineer a biomimetic collagen-based matrix composition. The resulting incorporation of cyclodextrin in the inventive collagen compositions increased collagen thermal stability and reduced collagen fibrogenesis. As a result, a thick, transparent and mechanically strong collagen-based composition was formed. This cyclodextrin-collagen composition holds a great potential to be used as a therapeutic eye patch for corneal repair. Additionally, the composition can support development of multi-layered structures, with different layers promoting different biological properties. Methods for making these inventive compositions and their use are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for preparing surface-functionalized chitosan cornea repair material
ActiveCN101480505BImprove mechanical propertiesGood optical performanceProsthesisRepair materialCornea repair
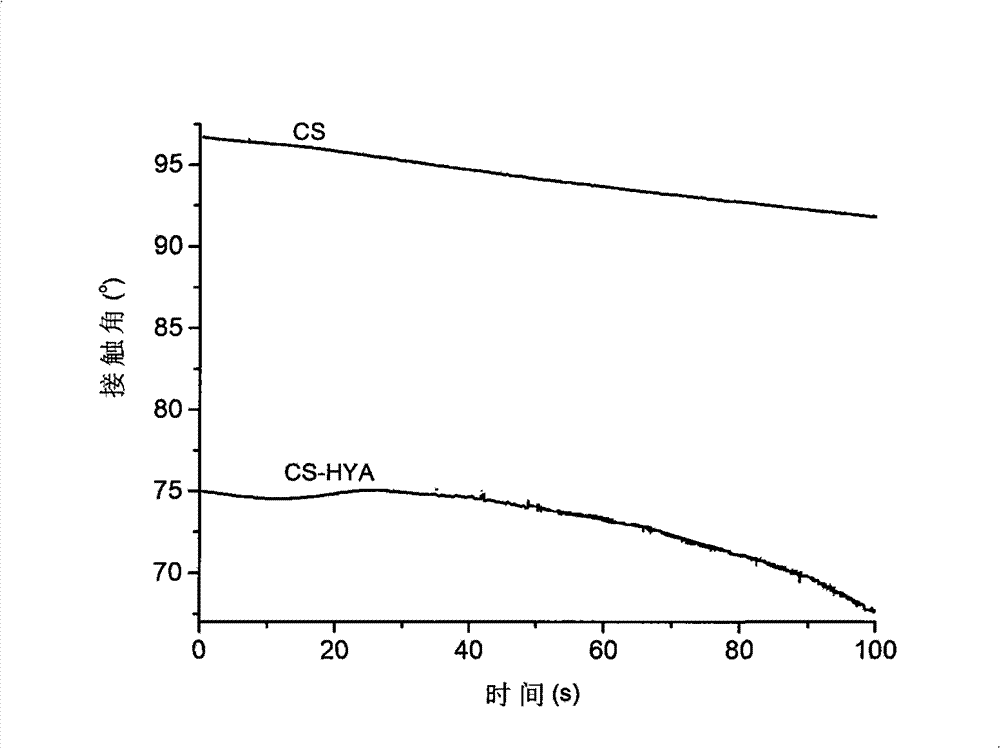
The invention discloses a surface functionalized chitosan cornea rehabilitating material and a preparation method thereof. The method comprises the following steps: dissolving chitosan into acid solution; injecting the chitosan solution into a mould to be dried; and drying the chitosan solution after being processed in alkali solution to obtain chitosan cornea. Biomacromolecule and short peptide are fixed on the surface of the chitosan cornea by adopting the chemical crosslinking method. The contact angle of the surface functionalized chitosan cornea declines obviously and the hydrophilic property is improved. The prepared surface functionalized chitosan cornea rehabilitating material has over 95 percent of light transmission, good cell compatibility and biologic compatibility, can be used for rehabilitating corneal damage in medical fields, and has the advantages of simple preparation process, simple equipment, cheap and accessible raw materials, good application prospect and scientific value.
Owner:GUANGZHOU PUDAO LIANXIN BIOTECH CO LTD
Antibacterial cornea repair material and preparation method thereof
ActiveUS9585984B2Reduce riskLow costConnective tissue peptidesEye implantsBiocompatibility TestingMechanical property
A preparation method of an antibacterial cornea repair material includes (1) purifying type I collagen extracted from beef tendons, and preparing a collagen solution; (2) casting the collagen solution into a mold to form a membrane by air drying at room temperature; (3) soaking the membrane in an antibiotic solution, stirring until the membrane is in contact with the antibiotic solution, adding a crosslinking agent and a catalyst to the antibiotic solution contacted with the membrane, and stirring the antibiotic solution contacted with the membrane with the crosslinking agent and the catalyst added therein to perform a crosslinking reaction to obtain a crosslinked membrane; and (4) washing the crosslinked membrane material with deionized water, and air drying at room temperature. The antibacterial cornea repair material has relatively good mechanical properties, optical properties, biocompatibility, and antibacterial effect, and can be used for repairing and replacing damaged cornea tissue.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method for antifungal cornea repairing material
ActiveCN107670116AHigh transparencyImprove mechanical propertiesCapsule deliveryTissue regenerationCross-linkAcetic acid
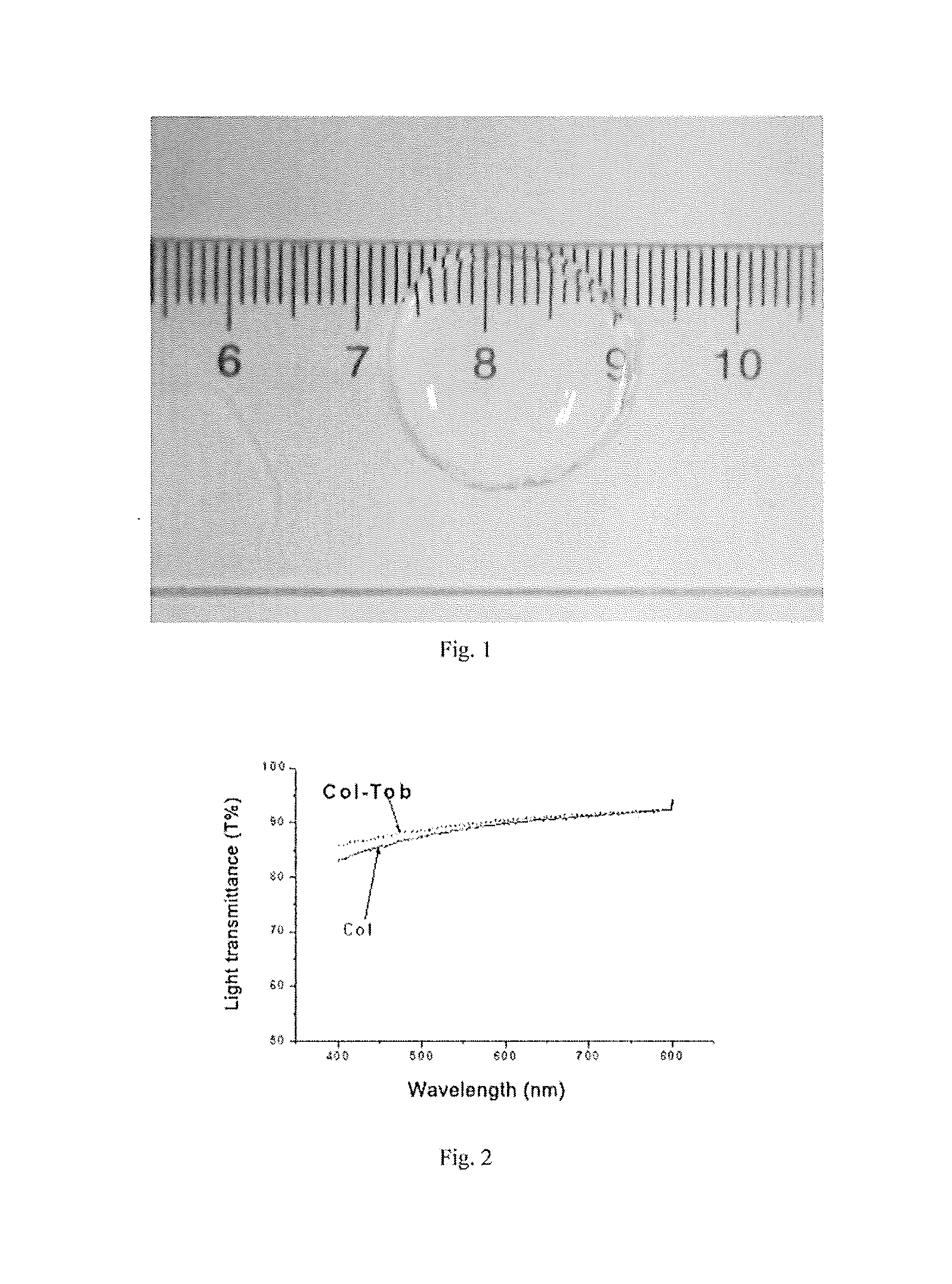
The invention discloses a preparation method for an antifungal cornea repairing material. The preparation method comprises the following steps: (1) preparing a saturated solution of dialdehyde beta-cyclodextrin, adding a medicine and carrying out a full reaction so as to obtain a dialdehyde beta-cyclodextrin / medicine inclusion complex; (2) extracting I type collagen, subjecting the extracted I type collagen to freeze-drying and dissolving the freeze-dried I type collagen in an acetic acid or hydrochloric acid solution to prepare a collagen solution; (3) adding the inclusion complex obtained inthe step (1) as a cross-linking agent into the collagen solution to remove bubbles in the solution; and (4) casting the cross-linked collagen solution to a cornea-repairing-material forming die for film formation. The material prepared by using the method has high transparency, good mechanical properties, and temperature-sensitive release properties, is obviously different in releasing effect atdifferent temperatures, and can be used for different medical fields.
Owner:GUANGZHOU PUDAO LIANXIN BIOTECH CO LTD
Stem cell preparation for cornea repair and preparation method and application thereof
InactiveCN105797138AHigh transparencyPrevent excessive fibrosisSenses disorderPeptide/protein ingredientsFibrosisMesenchymal stem cell
The invention relates to a stem cell preparation for cornea repair.The stem cell preparation comprises polysaccharide, KGF-2 and mesenchymal stem cells.By combining the polysaccharide, the KGF-2 and the mesenchymal stem cells, the anti-inflammatory effect is achieved, cornea surface cell proliferation can be promoted, cornea epithelial cell proliferation is stimulated, cornea epithelium repair ability and cornea transparency are improved, cornea excessive fibrosis can be prevented, and therefore healing of a damaged cornea is promoted.
Owner:SHEN ZHEN ISTEM REGENERATIVE MEDICINE SCI TECH CO LTD
A kind of culture medium, corneal stroma sheet prepared therefrom and preparation method
The invention relates to the technical field of medical biomaterials, in particular to a culture medium, a corneal stroma sheet prepared by it and a preparation method. The medium provided by the invention is a DMEM / F12 medium containing porcine corneal extract, Y-27632, ITS, FGF, ascorbic acid and fetal calf serum. Compared with the corneal stromal cells and corneal stromal sheets prepared by the traditional culture medium, the present invention has the ability to maintain keratocytes by selecting appropriate active components and proportioning ratios. Phenotype, promote proliferation, improve vitality, anti-fibrosis and anti-apoptosis and other functions. The corneal stroma sheet of the present invention is prepared by using corneal lens as a raw material and cultured in the medium. Compared with animal-derived corneal repair materials, it has good biocompatibility and no antigenicity, and can be used for various supplies of corneal transplantation. It is an alternative to body materials, which can be accepted by a large number of patients and used in clinical practice for a long time.
Owner:AIER EYE HOSPITAL GRP CO LTD
Preparation method of hierarchical drug-loaded cornea repairing material
InactiveCN107261208ALoad balancingUniform and sustained release effectProsthesisDrugs solutionMedicine
The invention discloses a preparation method of a hierarchical drug-loaded cornea repairing material. The preparation method comprises the following steps: firstly, adding a polymer material into a solvent to prepare a polymer solution; secondly, drying the polymer solution to form a film; thirdly, dropwise adding a drug solution on the surface of a polymer film and then carrying out uniform tiling on the drug solution. According to the preparation process disclosed by the invention, the hierarchical drug-loaded cornea repairing material has the advantages of simple and feasible technology and good repeatability, and is suitable for mass production of novel functional cornea repairing materials.
Owner:GUANGZHOU PUDAO LIANXIN BIOTECH CO LTD
Preparation method of bioactive corneal repair graft and corneal repair graft
ActiveCN106039403BHigh strengthImprove water absorptionPharmaceutical delivery mechanismTissue regenerationActive cellDrug biological activity
The invention discloses a preparation method of a cornea repair graft with biological activity and the cornea repair graft, and solves the technical problem of deficiency of cornea donors. The preparation method comprises the following steps: preparing collagen dispersion; preparing a collagen membrane on the basis of the collagen dispersion; adding surface functionalization modified substances on the surface of the collagen membrane in a 3D printing or coating mode under the sterile condition to form the cornea repair graft with biological activity. The surface functionalization modified substances are fine particles which are prepared from a cornea stroma, which does not contain a lesion part and contains active cells, of a patient, the biology and the biological stability of the high-strength collagen membrane can be improved, the physiological function of cornea is improved, after the modified collagen membrane is implanted into an eye of the patient, transparency and moisture content are good, the transparency can be recovered quickly clinically, the biological compatibility is good, and a good stent is provided for cornea cell culture of a cornea transplantation receptor.
Owner:广州尤尼智康生物科技有限公司
A kind of corneal repair material with stroma repair ability and preparation method thereof
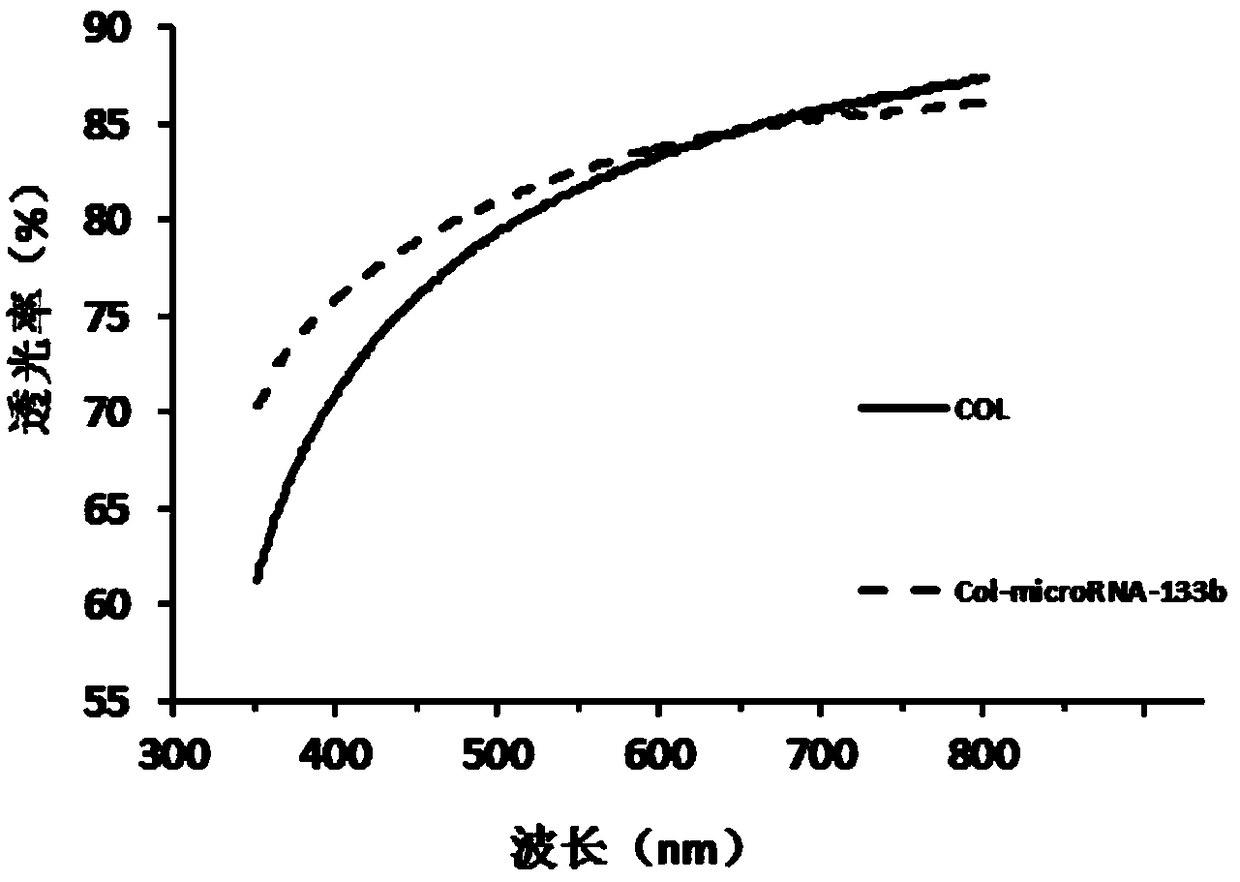
ActiveCN105854087BGood optical transmissionIncrease moisture contentTissue regenerationProsthesisNanoparticleCornea repair
The invention discloses a cornea repairing material with matrix repairing capability and a preparation method thereof. The method comprises the following steps: (1) preparing a microRNA solution; (2) preparing a collagen solution; (3) preparing a collagen membrane; (4) preparing a gold nano-particle solution; (5) preparing a microRNA and gold nano-particle mixed solution; and (6) dropwise adding the mixed solution onto the collagen membrane to obtain the cornea repairing material with matrix repairing capability. The cornea repairing material has a laminar structure, has the advantages of good transparency, high moisture content and excellent ion permeation performance, and can be used for effectively reducing and retarding occurrence of cicatrix repairing. The cornea repairing material with matrix repairing capability can be used for repairing a damaged cornea matrix in the medical field, is prepared from low-price and easily available raw materials, is simple in preparation process and simple in equipment, and has excellent application prospects and scientific values.
Owner:GUANGZHOU PUDAO LIANXIN BIOTECH CO LTD
Application of short-chain peptide composition in the prevention or treatment of dry eye
ActiveCN111632128BPromote repairIncrease humiditySenses disorderPeptide/protein ingredientsCornea repairAmino acid composition
The present invention relates to the application of a short-chain peptide composition in preventing or treating dry eye. The short-chain peptide composition of the present invention comprises a short-chain peptide composed of 5-10 amino acids, and has the effects of promoting corneal repair, increasing eye humidity and improving dry eye syndrome.
Owner:PRO SUNFUN BIOTECH RES & DEV CO LTD
A kind of preparation method of antifungal corneal repair material
ActiveCN107670116BHigh transparencyImprove mechanical propertiesCapsule deliveryTissue regenerationAntifungalCross linker
The invention discloses a preparation method for an antifungal cornea repairing material. The preparation method comprises the following steps: (1) preparing a saturated solution of dialdehyde beta-cyclodextrin, adding a medicine and carrying out a full reaction so as to obtain a dialdehyde beta-cyclodextrin / medicine inclusion complex; (2) extracting I type collagen, subjecting the extracted I type collagen to freeze-drying and dissolving the freeze-dried I type collagen in an acetic acid or hydrochloric acid solution to prepare a collagen solution; (3) adding the inclusion complex obtained inthe step (1) as a cross-linking agent into the collagen solution to remove bubbles in the solution; and (4) casting the cross-linked collagen solution to a cornea-repairing-material forming die for film formation. The material prepared by using the method has high transparency, good mechanical properties, and temperature-sensitive release properties, is obviously different in releasing effect atdifferent temperatures, and can be used for different medical fields.
Owner:GUANGZHOU PUDAO LIANXIN BIOTECH CO LTD
GelMA-collagen dual-network antibacterial cornea repair material as well as preparation method and application thereof
ActiveCN113456895AGood optical transmissionIncrease moisture contentTissue regenerationProsthesisEngineeringBiocompatibility
The invention discloses a GelMA-collagen dual-network antibacterial cornea repair material as well as a preparation method and application thereof. The preparation method of the cornea repair material comprises the following steps: S1, uniformly mixing a lysozyme solution and a methacrylated gelatin solution, and adding a cross-linking agent for cross-linking to obtain a cross-linked product; S2, blending the cross-linked product with a collagen solution, adding a cross-linking agent for cross-linking, then adding a photoinitiator, and conducting reacting in a dark place at room temperature to obtain a uniform and bubble-free mixed solution; and S3, performing ultraviolet crosslinking on the mixed solution in S2, and performing air drying to form a film, thereby obtaining the GelMA-collagen dual-network antibacterial cornea repair material. The GelMA-collagen dual-network antibacterial cornea repair material has good biocompatibility and biodegradability, has a layered structure, is good in optical permeability and water-containing performance and has certain mechanical properties, tearing is not caused when an operation line is used for suturing, and the cornea repair material also has an inhibition effect on gram-negative bacteria and gram-positive bacteria, and has excellent antibacterial performance.
Owner:GUANGZHOU PUDAO LIANXIN BIOTECH CO LTD
Bio-ink for 3D printing of cornea repair material, preparation method of bio-ink and preparation method of cornea repair material
ActiveCN113750292AImprove defects that are difficult to print at low densityImprove printing accuracyAdditive manufacturing apparatusTissue regenerationCornea repairCorneal epithelial cell
The invention discloses bio-ink for 3D printing of a cornea repair material. The bio-ink comprises methacrylamide gelatin, a photoinitiator, collagen and a solvent. The concentration of the methacrylamide gelatin in the bio-ink is 50-150 mg / mL; the concentration of the photoinitiator in the bio-ink is 1-5 mg / mL; and the concentration of the collagen solution in the bio-ink is 0.5-6.0 mg / mL. The invention further discloses a preparation method of the bio-ink for 3D printing of the cornea repair material and a preparation method of the cornea repair material based on the bio-ink for 3D printing of the cornea repair material. The printing precision of the cornea repair material is improved, the mechanical property of the printing stent is improved, and the printing stent has a complete curvature structure, is good in optical permeability and water-containing performance and can fully meet the requirements of growth and proliferation of cornea epithelial cells.
Owner:SOUTH CHINA UNIV OF TECH
Ophthalmic product with cornea repair function
InactiveUS20200405751A1Stay healthy and comfortableAvoid injuryPowder deliverySenses disorderOphthalmologyCornea repair
An ophthalmic product having a cornea repair function includes an ophthalmic composition. The ophthalmic composition includes gold nanoparticles serving as the main repairing ingredient and at least one auxiliary repairing ingredient. The effective concentration of the gold nanoparticles is from 0.01 ppm to 3000 ppm. The content of the at least one auxiliary repairing ingredient is greater than 0 wt % and less than 20 wt % based on 100 wt % of the ophthalmic composition.
Owner:PEGAVISION CORP
Cornea repair hydrogel and preparation method and application thereof
InactiveCN113082287AMaintain biological activityGuaranteed functionTissue regenerationProsthesisDrug biological activityMaterials science
The invention belongs to the field of biological medicine, and discloses cornea repair hydrogel and a preparation method and application thereof. The cornea repair hydrogel is prepared through a cross-linking reaction of an IKK-beta specific small-molecule inhibitor and silk fibroin, and the preparation method of the cornea repair hydrogel specifically comprises the following steps of 1, using mulberry silk as a raw material, performing degumming, dissolving, dialysis and centrifugation, and obtaining a silk fibroin solution; (2) adding a TPCA-1 solution into the silk fibroin solution obtained in the step (1), and uniformly mixing; and (3) carrying out ultrasonic treatment on the mixed solution of the silk fibroin and the TPCA-1 obtained in the step (2), and carrying out cross-linking reaction at 37 DEG C to obtain the silk fibroin hydrogel compounded with the TPCA-1. The cornea repair hydrogel prepared by the invention is hydrophilic gel with a three-dimensional network structure, has certain adhesion to a cornea injury surface, is suitable for cornea injury repair, is simple in preparation process, does not use additives such as a denaturing agent, a coagulant and a cross-linking agent in the preparation process, and ensures the biological activity of TPCA-1 to the greatest extent.
Owner:SOUTHEAST UNIV
Ophthalmic product with cornea repair function
PendingCN112138022AAvoid damageRelieve symptomsPowder deliverySenses disorderGold particlesNanoparticle
The present invention relates to an ophthalmic product with cornea repair function. The ophthalmic product having a cornea repair function includes an ophthalmic composition. The ophthalmic composition includes gold nanoparticles serving as the main repairing ingredient and at least one auxiliary repairing ingredient. An effective concentration of the gold nanoparticles is from 0.01 ppm to 3000 ppm. The content of the at least one auxiliary repairing ingredient is greater than 0 wt % and less than 20 wt % based on 100 wt % of the ophthalmic composition. The ophthalmic product can enhance the protection and repairing capacity of cells in each layer of tissues of the eyes, and can improve the wearing comfort of the contact lenses.
Owner:PEGAVISION CORP
Preparation method of cornea repair material of double-layer structure
ActiveCN105412974AWith specific performancePromote epithelializationCosmetic preparationsToilet preparationsMedicinePolyvinyl alcohol
The invention discloses a preparation method of a cornea repair material of a double-layer structure, and relates to the related fields of biomedical materials, medical apparatuses and instruments, cosmetics and the like. The method includes the following steps of dissolving collagen in weak acid to obtain a collagen solution of a certain concentration, preparing polyvinyl alcohol (PVA) into a solution, pouring the solution into a mold, conducting drying forming to obtain a polyvinyl alcohol film, laying the collagen solution on the surface of the polyvinyl alcohol membrane flat, and conducting drying to obtain a collagen compound membrane. The obtained compound membrane solves the problem that an existing collagen material is not enough in mechanical strength, and can promote adherency and proliferation of cells, and a new way is provided for application of collagen. The preparation method is simple in preparation process, high in repeatability and suitable for industrialized large-scale production of modified collagen compound films with different requirements.
Owner:GUANGZHOU PUDAO LIANXIN BIOTECH CO LTD
Application of short-chain peptide composition in prevention or treatment of xerophthalmia
ActiveCN111632128AImprove acuteImprove long-term dry eye syndromeSenses disorderPeptide/protein ingredientsXerophthalmiaMedicine
The invention relates to an application of a short-chain peptide composition in prevention or treatment of xerophthalmia. The short-chain peptide composition disclosed by the invention comprises a short-chain peptide consisting of 5-10 amino acids, and has the effects of promoting corneal repair, increasing eye humidity and improving xerophthalmia.
Owner:PRO SUNFUN BIOTECH RES & DEV CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com