Application of neutrophil elastase in preparation of product for diagnosing biliary atresia
A technology of elastase and neutrophils, applied in the application field of the product, can solve the problems of poor prognosis of children after surgery, unable to meet the needs of children with BA, bile duct damage and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Detection of the expression of neutrophil elastase in biliary atresia
[0071] (1) Clinical sample collection
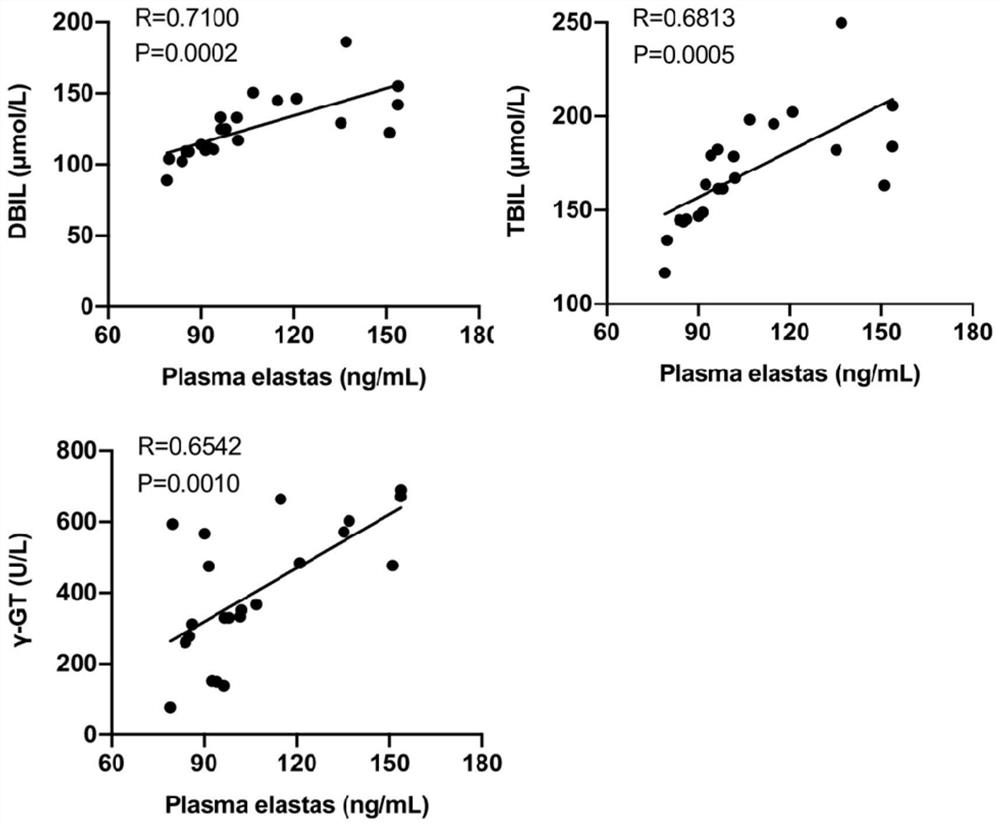
[0072] All healthy control children (infants without evidence of liver disease, NC, n=16), children in the cholestatic group (defined as serum direct bilirubin above 17 μmol / L, IHC, n=32) and children in the biliary atresia group (Bile duct atresia was confirmed by intraoperative cholangiography and postoperative histopathological examination of extrahepatic bile ducts, BA, n=39) from the Department of Surgery of Guangzhou Women and Children's Medical Center (Guangzhou, China) between March 2019 and March 2020 )recruit. The diagnosis of children with IHC and BA is based on the clinical characteristics, biochemical indicators of liver and gallbladder, intraoperative cholangiography, and histopathological examination of extrahepatic bile ducts after operation. EDTA anticoagulated blood samples (2ml) were collected from children in each group before o...
Embodiment 2
[0080] Example 2 Demonstrates the importance of neutrophil elastase in BA in biliary atresia mice
[0081](1) Pregnant BALB / c mice (10-12 weeks old, body weight 35-40 g) were purchased from Guangdong Provincial Animal Experiment Center, and CD177- / -Balb / c mice were purchased from Cyagen (USA) Biological Co., Ltd. Mice were reared in air-filtered, specific pathogen-free individual micro-isolation cages (temperature 25°C, dark and light intermittently produced for 12 h), and had free access to sterile water and autoclaved food. Newborn mice within 24 hours of birth (average body weight 1.5-1.6 g) were used to establish the BA mouse model and conduct downstream experiments. The experimental protocol was approved by the Animal Care and Use Committee of the Experimental Animal Center of Guangzhou Medical University (IACUC-DB-16-0602), and all animal experiments were carried out in the center;
[0082] (2) Rhesus monkey rotavirus (RRV) strain MMU 18006 was purchased from ATCC (ATCC...
Embodiment 3
[0087] Example 3 In vitro experiments further confirm that neutrophil elastase plays an important role in BA
[0088] Human neutrophil culture medium and normal fetal bile duct epithelial cells (BEC) were co-cultured in vitro:
[0089] (1) Separation of neutrophils in children with BA and hemangioma: According to the manufacturer's instructions, the blood samples of the BA group were lysed into a single-cell suspension with red blood cell lysate, added with Fc Block and incubated at room temperature for 10 minutes, and then added with anti -human PEconjugated CD177 antibody was incubated at 4°C for 30min, after washing, anti-PE Microbeads were added and incubated at 4°C for 15min, after washing, neutrophils were sorted on a magnetic sorter (Miltenyi Biotec, Germany) using an LD separation column , the sorted neutrophils were tested for cell purity on BD FACS Aria II (BD Biosciences, USA), and cells with cell purity>95% were used for culturing;
[0090] (2) Human biliary epith...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com