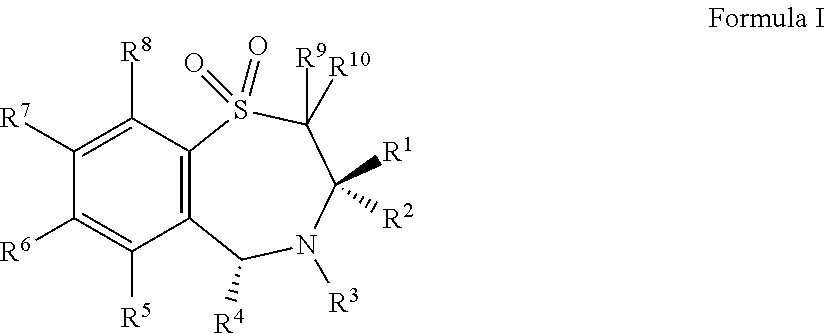
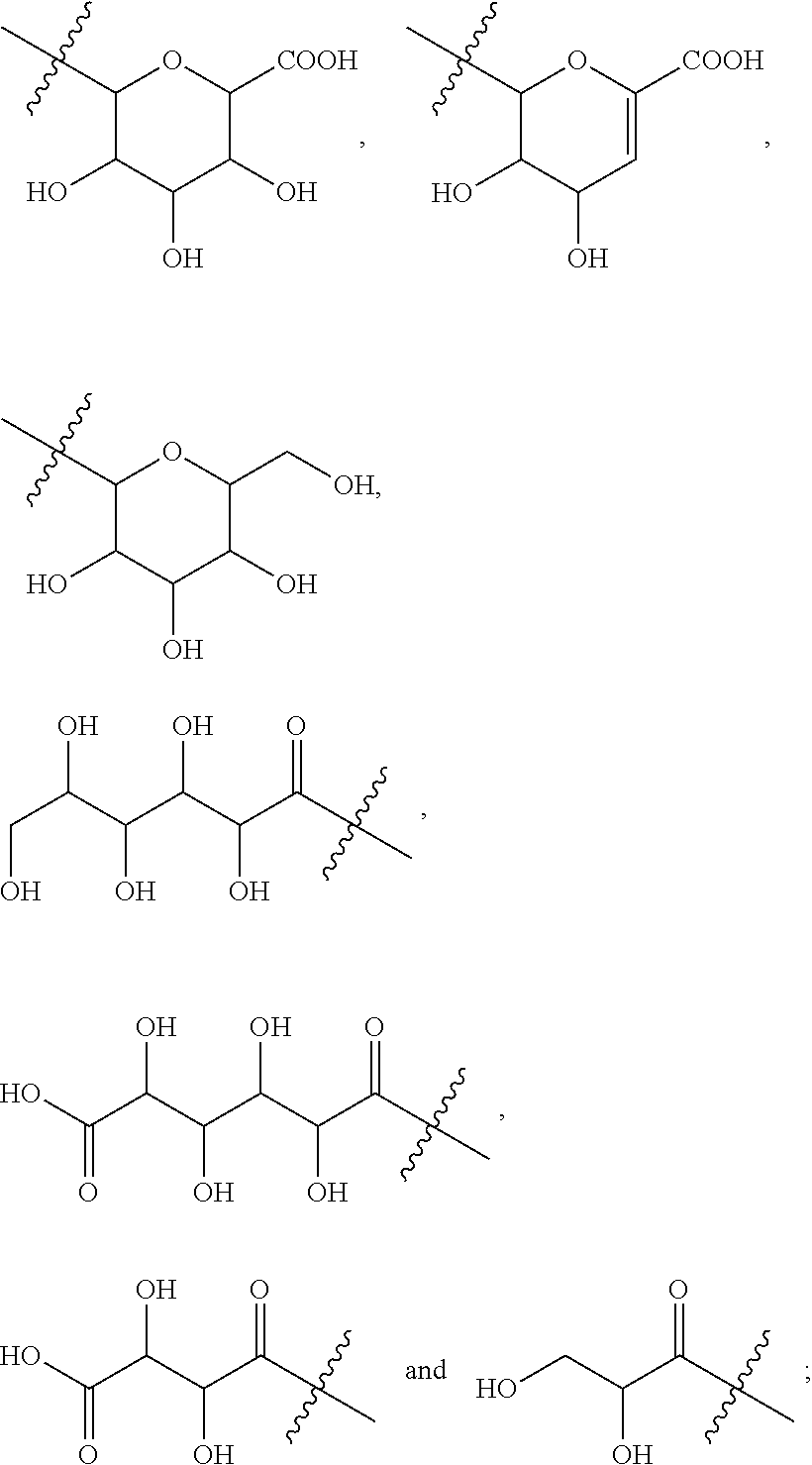
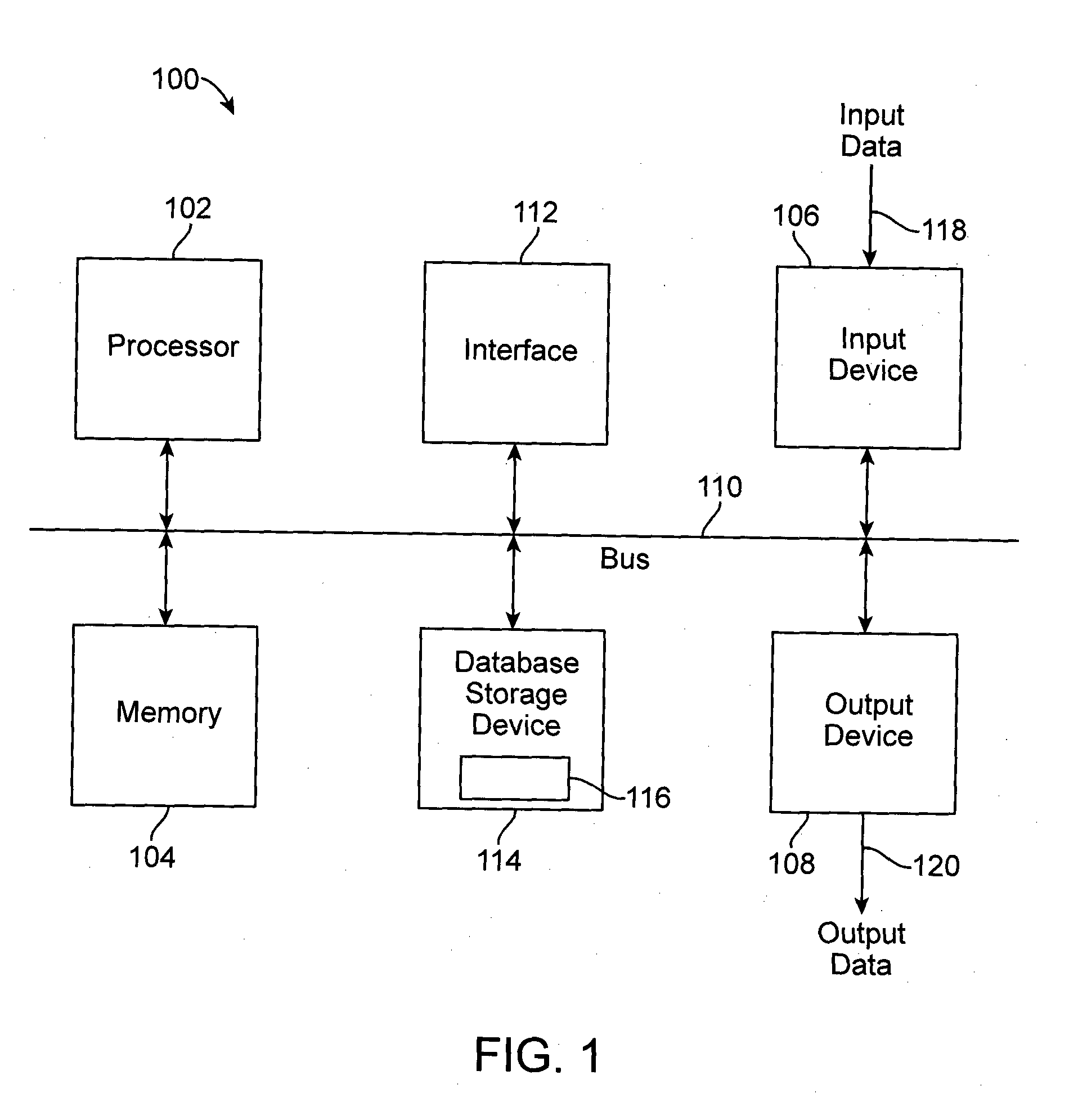
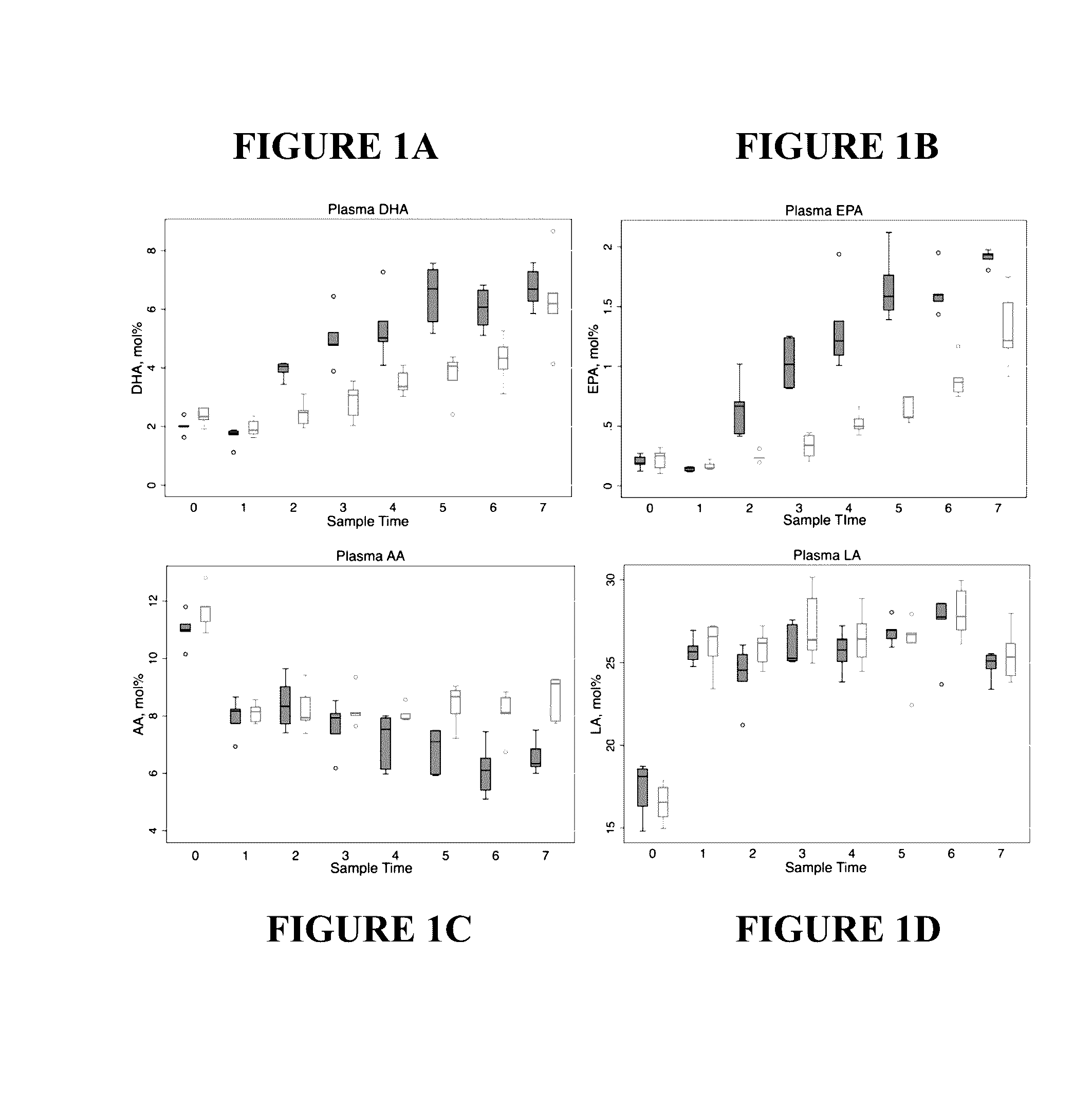
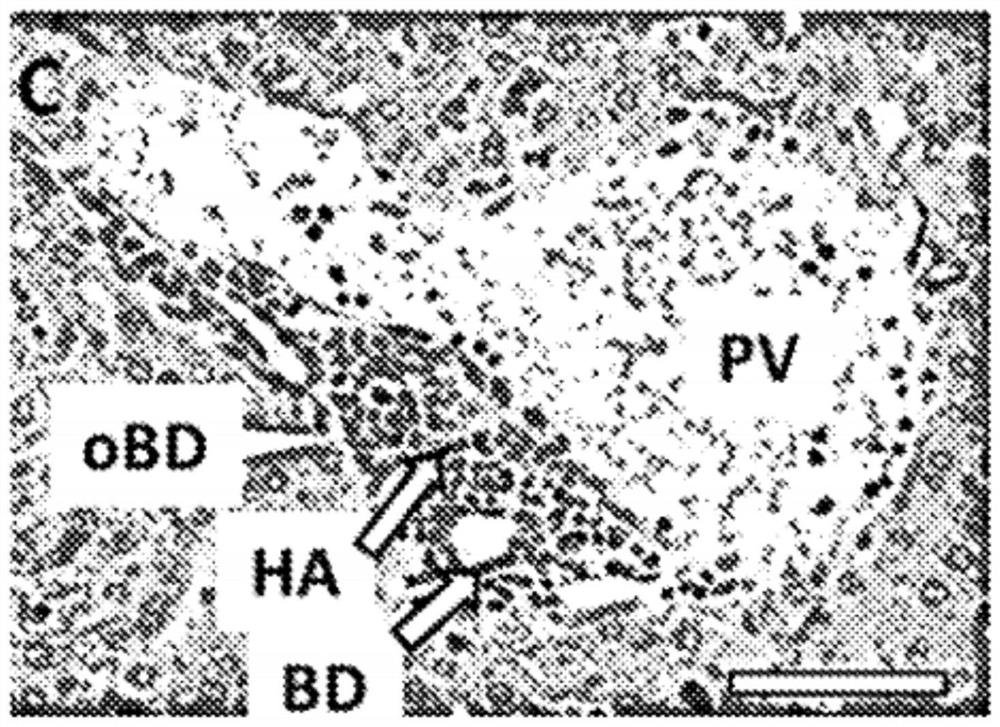
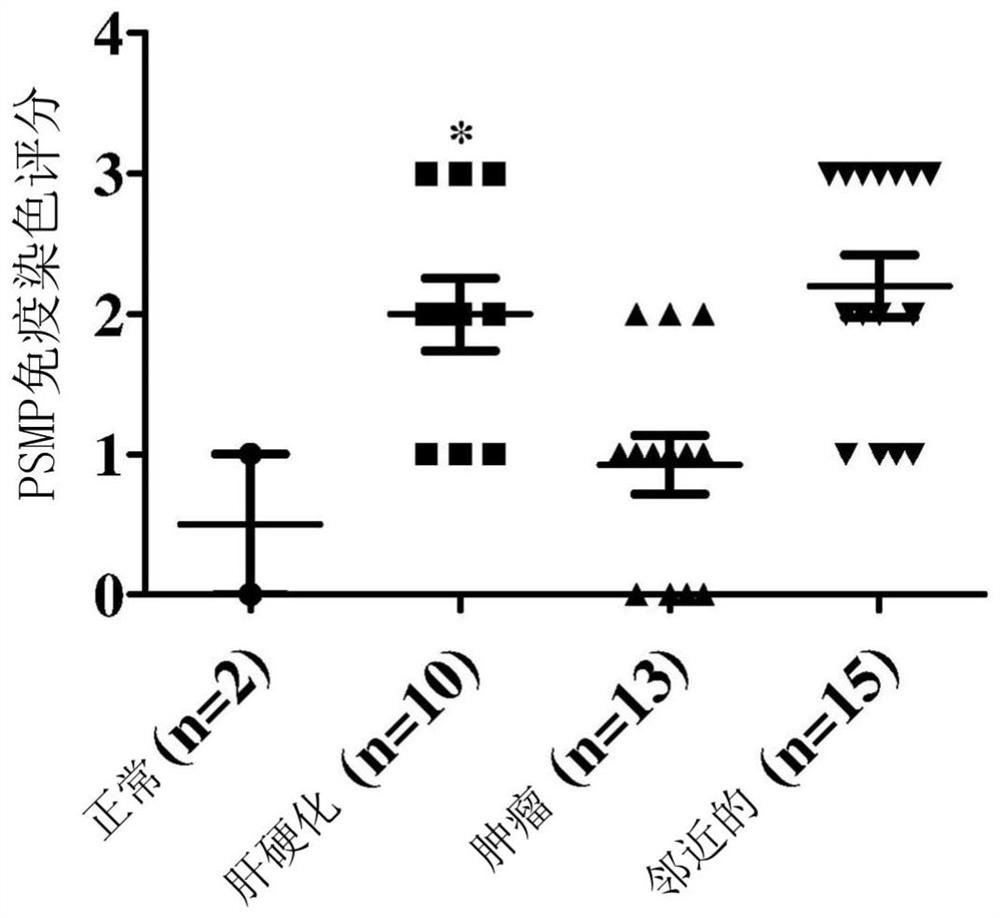
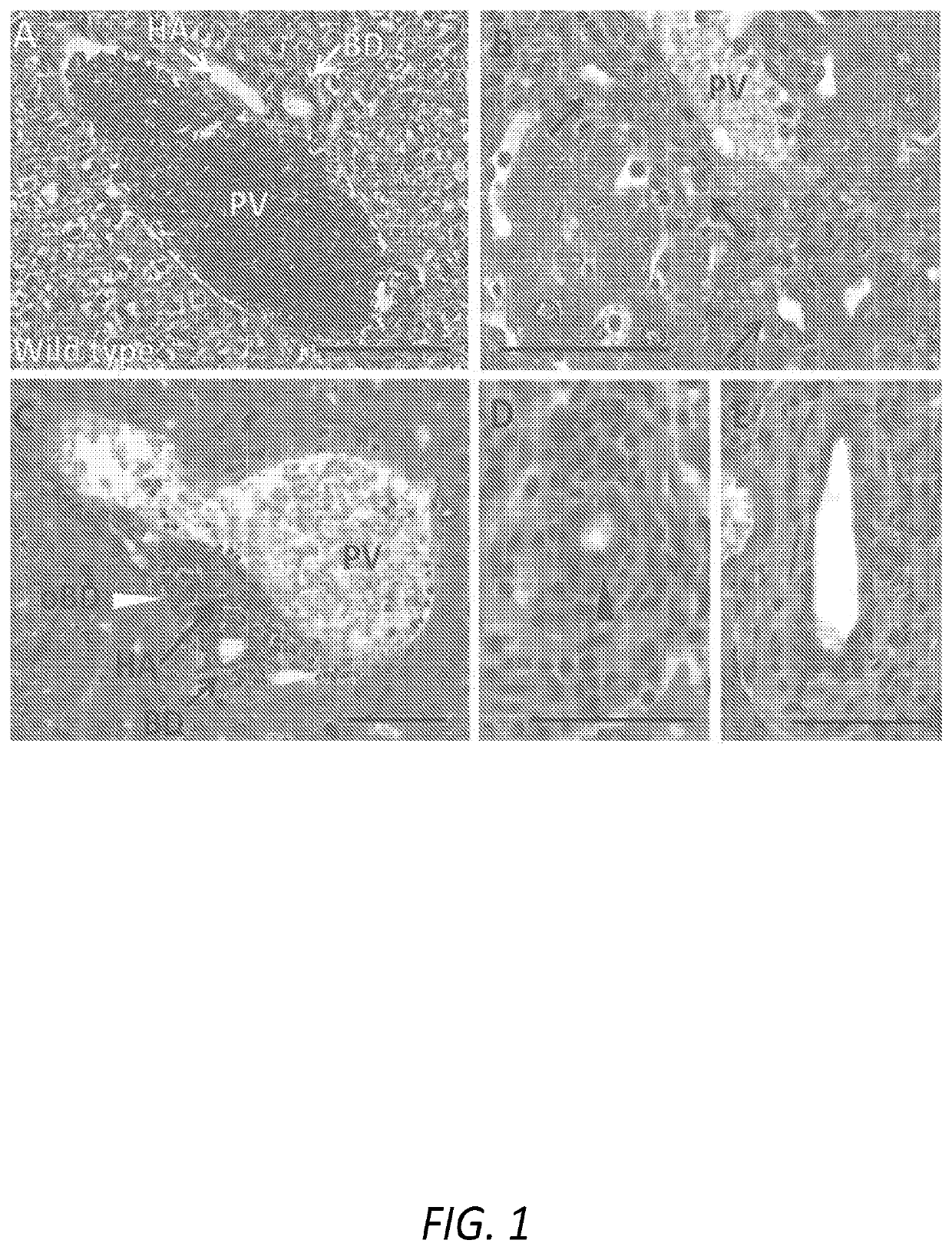
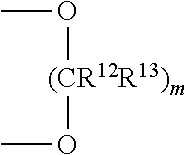Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Primary sclerosing cholangitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chronic liver disease involving inflammation, scarring and narrowing of bile ducts.
Bile acid recycling inhibitors for treatment of primary sclerosing cholangitis and inflammatory bowel disease
InactiveUS20140275090A1Reduce erosionExtension of timeBiocideOrganic chemistryApical sodium-dependent bile acid transporterInflammatory bowel disease
Provided herein are methods of treating or ameliorating primary sclerosing cholangitis and inflammatory bowel disease by administering to an individual in need thereof a therapeutically effective amount of an Apical Sodium-dependent Bile Acid Transporter Inhibitor (ASBTI) or a pharmaceutically acceptable salt thereof. Also provided are methods for treating or ameliorating primary sclerosing cholangitis comprising administering to an individual in need thereof a therapeutically effective amount of ASBTI or a pharmaceutically acceptable salt thereof.
Owner:LUMENA PHARMA INC
Novel long-term three-dimensional tissue culture system
InactiveUS20030096411A1Restore liver functionRelieve symptomsBiocideHepatocytesArtificial liverCell adhesion
The present invention relates to a novel tissue culture system that provides for the long term culture of proliferating hepatocytes that retain hepatic function. Disclosed are methods and compositions for ex vivo culturing of hepatocytes and nonparenchymal cells on a matrix coated with a molecule that promotes cell adhesion, proliferation or survival, in the presence of growth factors, resulting in a long-term culture of proliferating hepatocytes that retain hepatic function. The co-culturing method results in the formation of matrix / hepatic cell clusters that may be mixed with a second structured or scaffold matrix that provides a three-dimensional structural support to form structures analogous to liver tissue counterparts. The hepatic cell culture system can be used to form bio-artificial livers through which a subjects blood is perfused. Alternatively, the novel hepatic cell culture system may be implanted into the body of a recipient host having a hepatic disorder. Such hepatic disorders, include, for example, cirrhosis of the liver, induced hepatitis, chronic hepatitis, primary sclerosing cholangitis and alpha1 antitrypsin deficiency.
Owner:PITTSBURGH UNIV OF
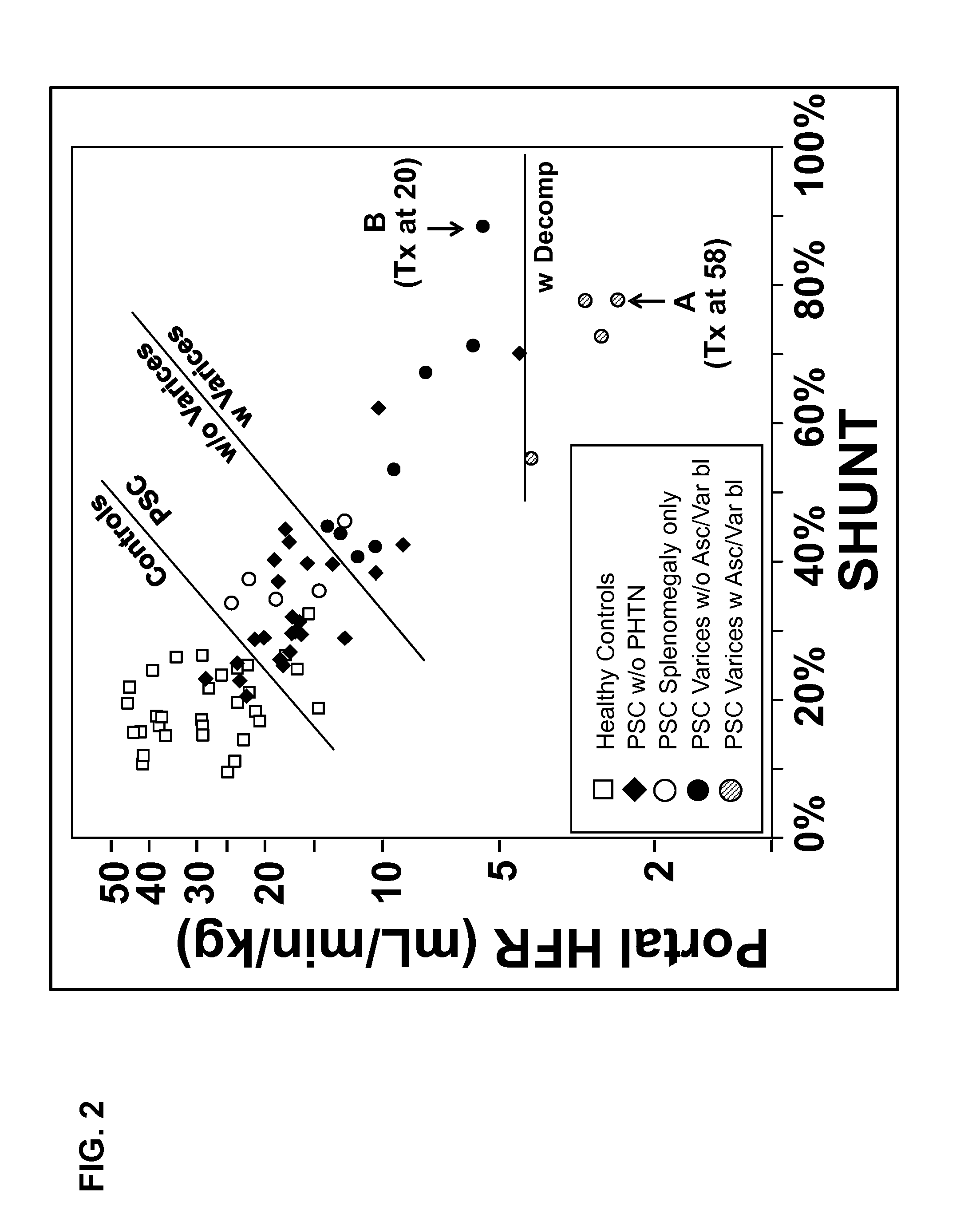
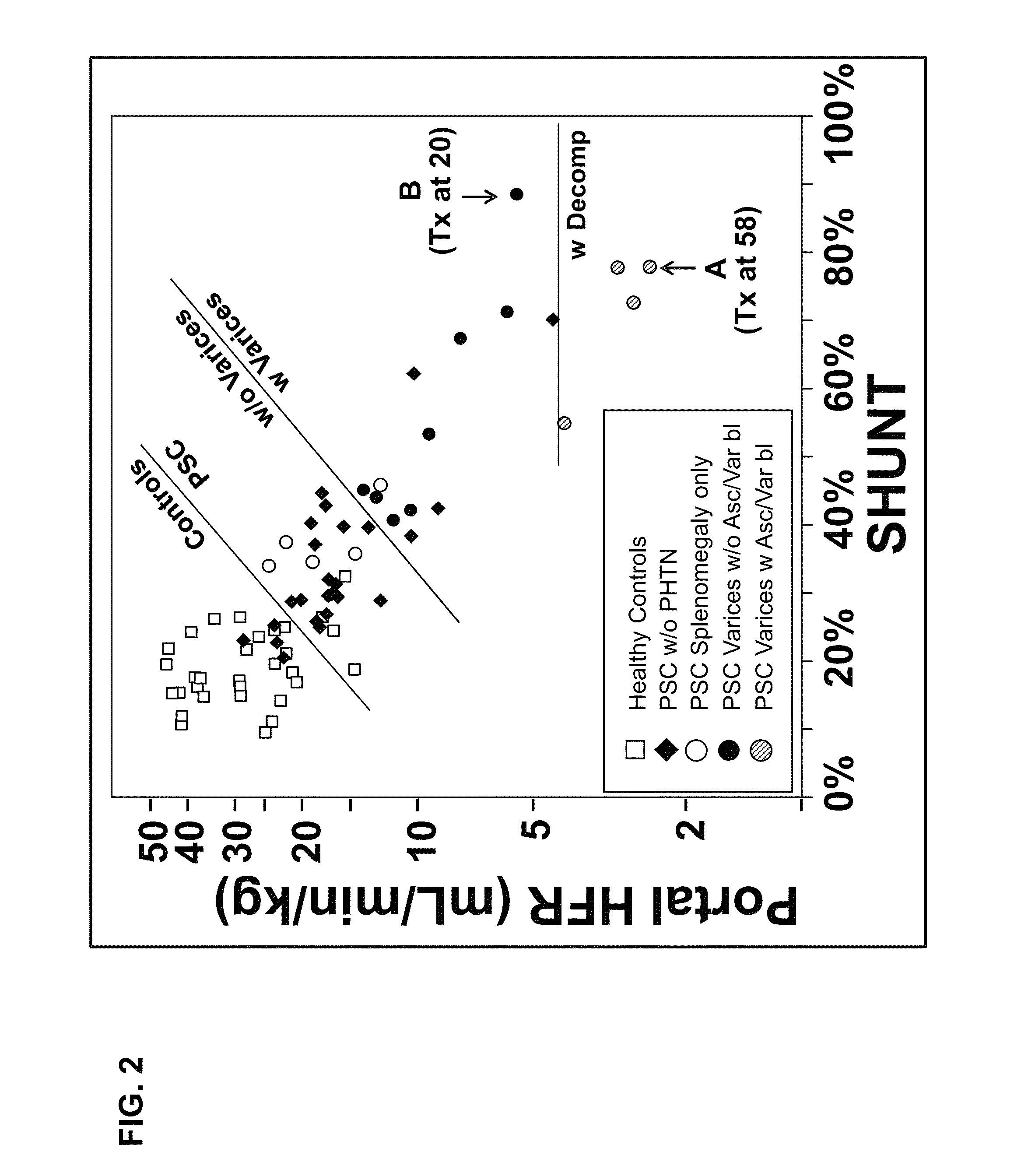
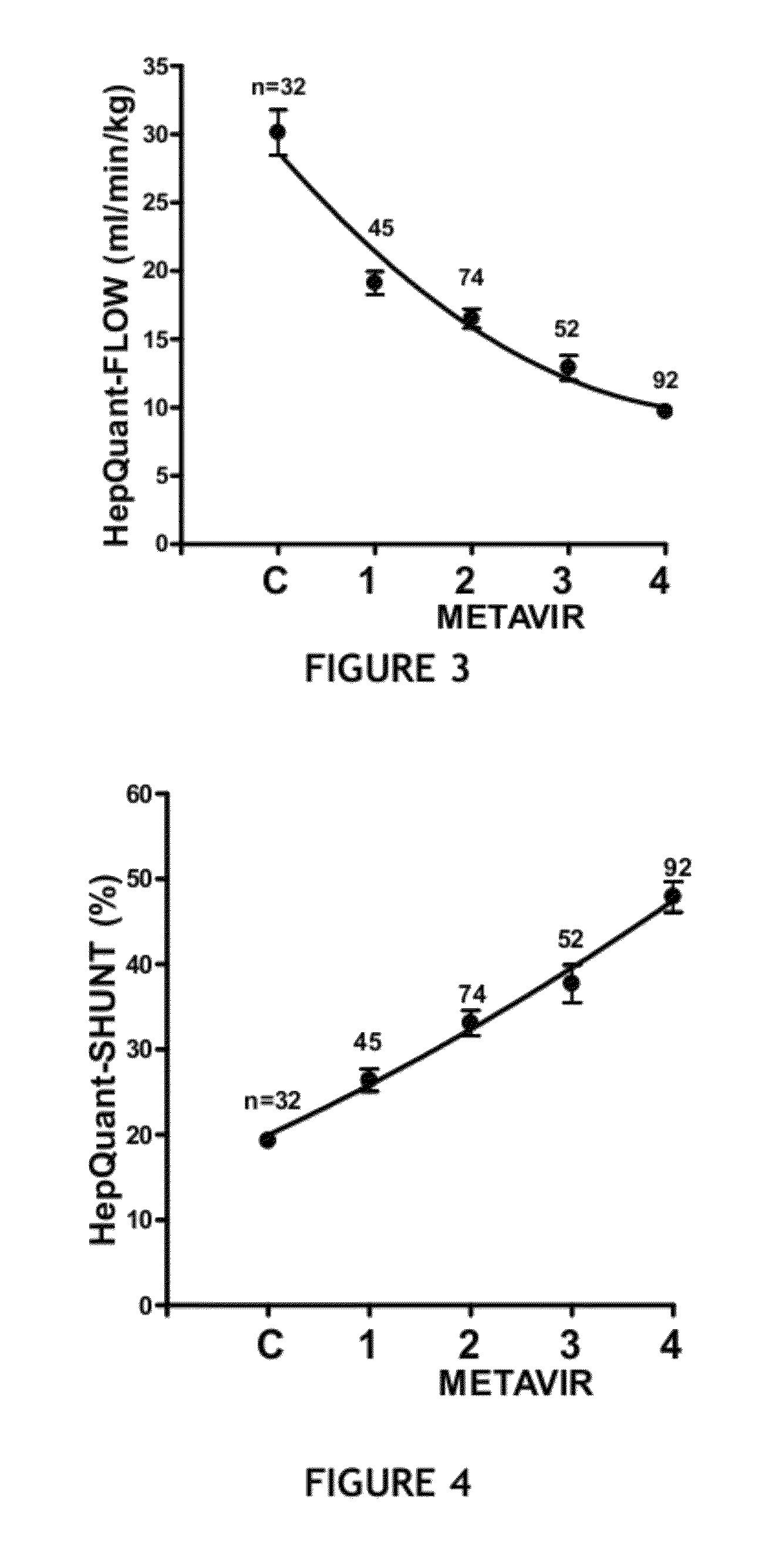
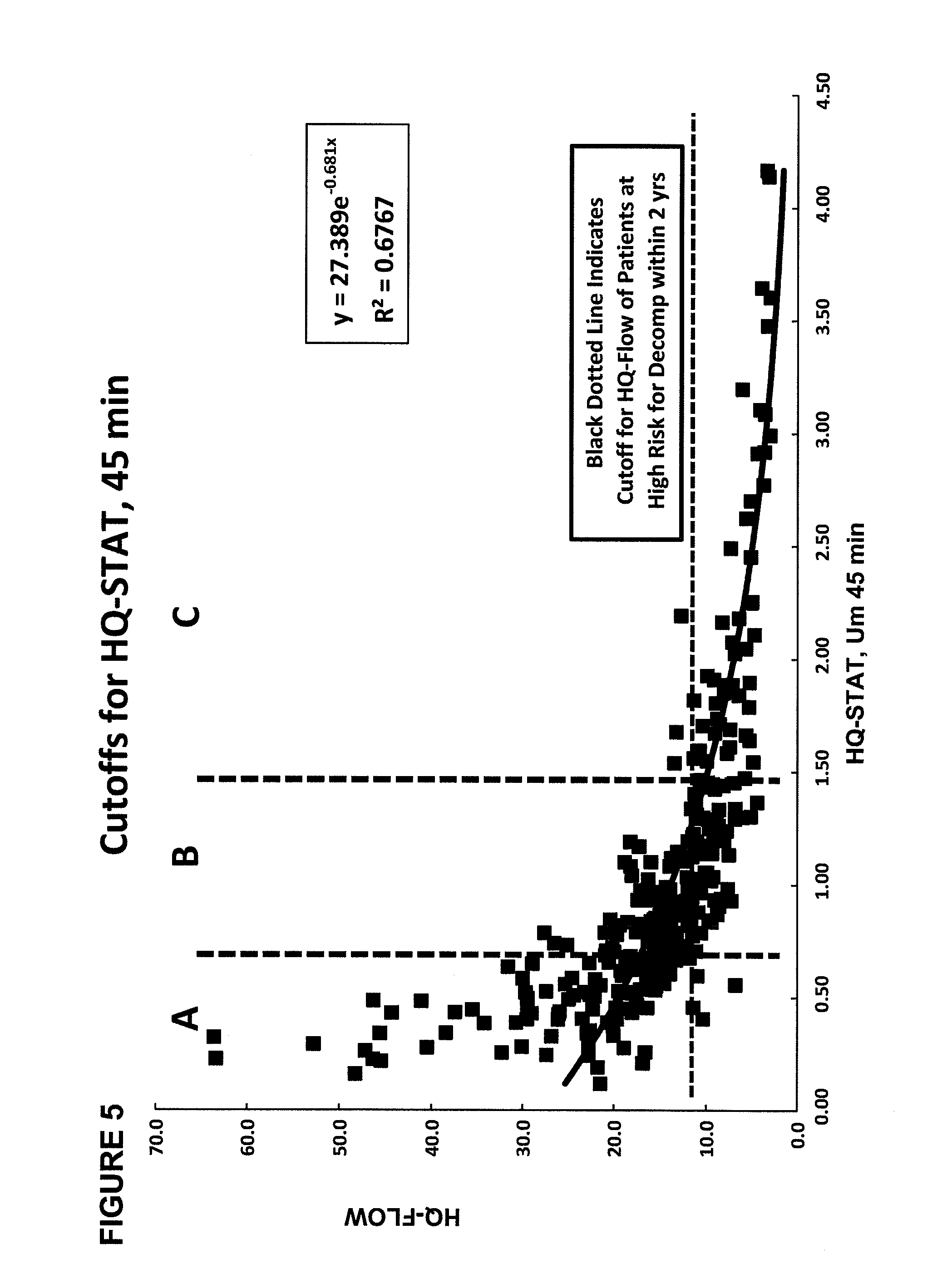
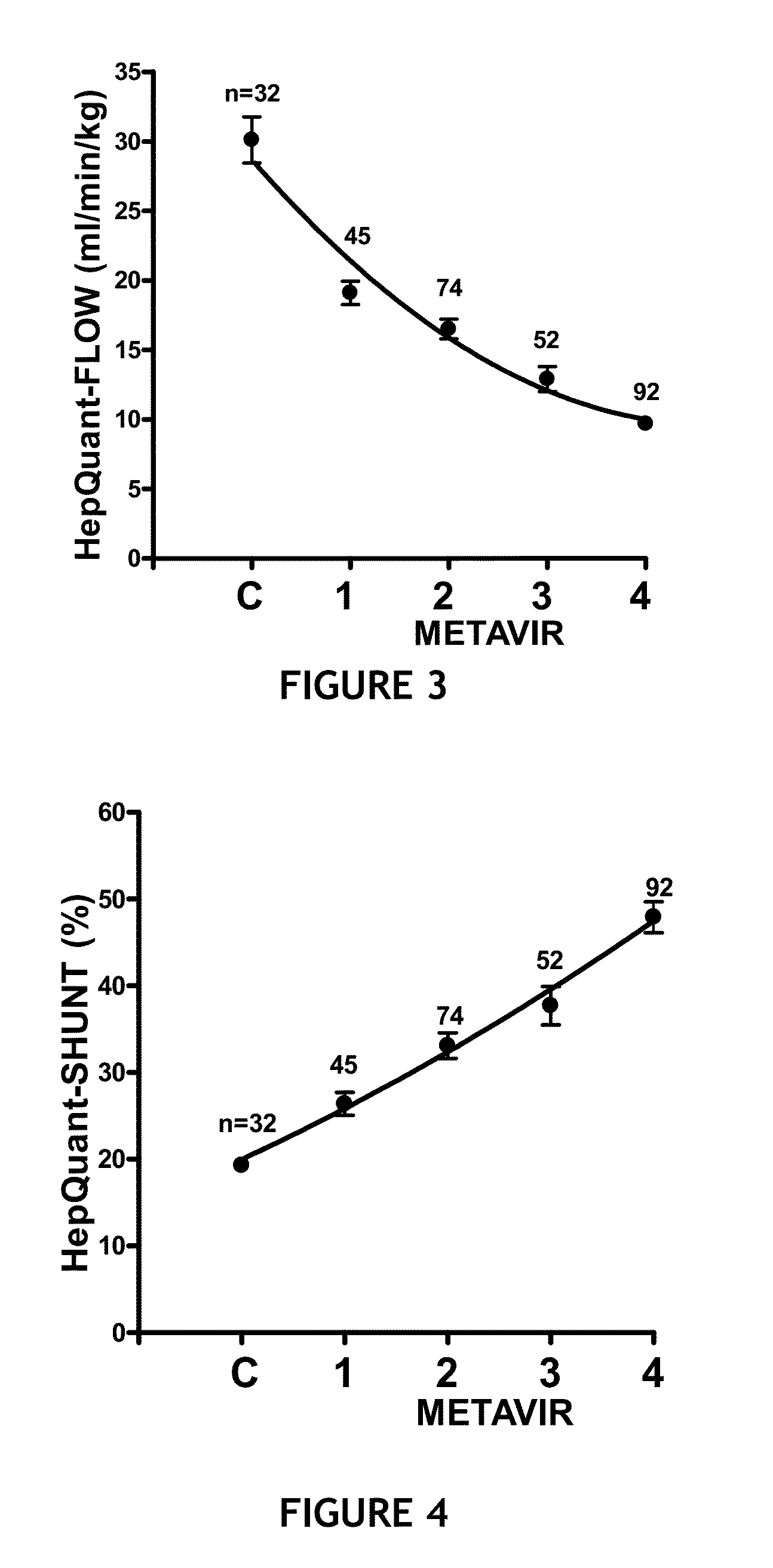
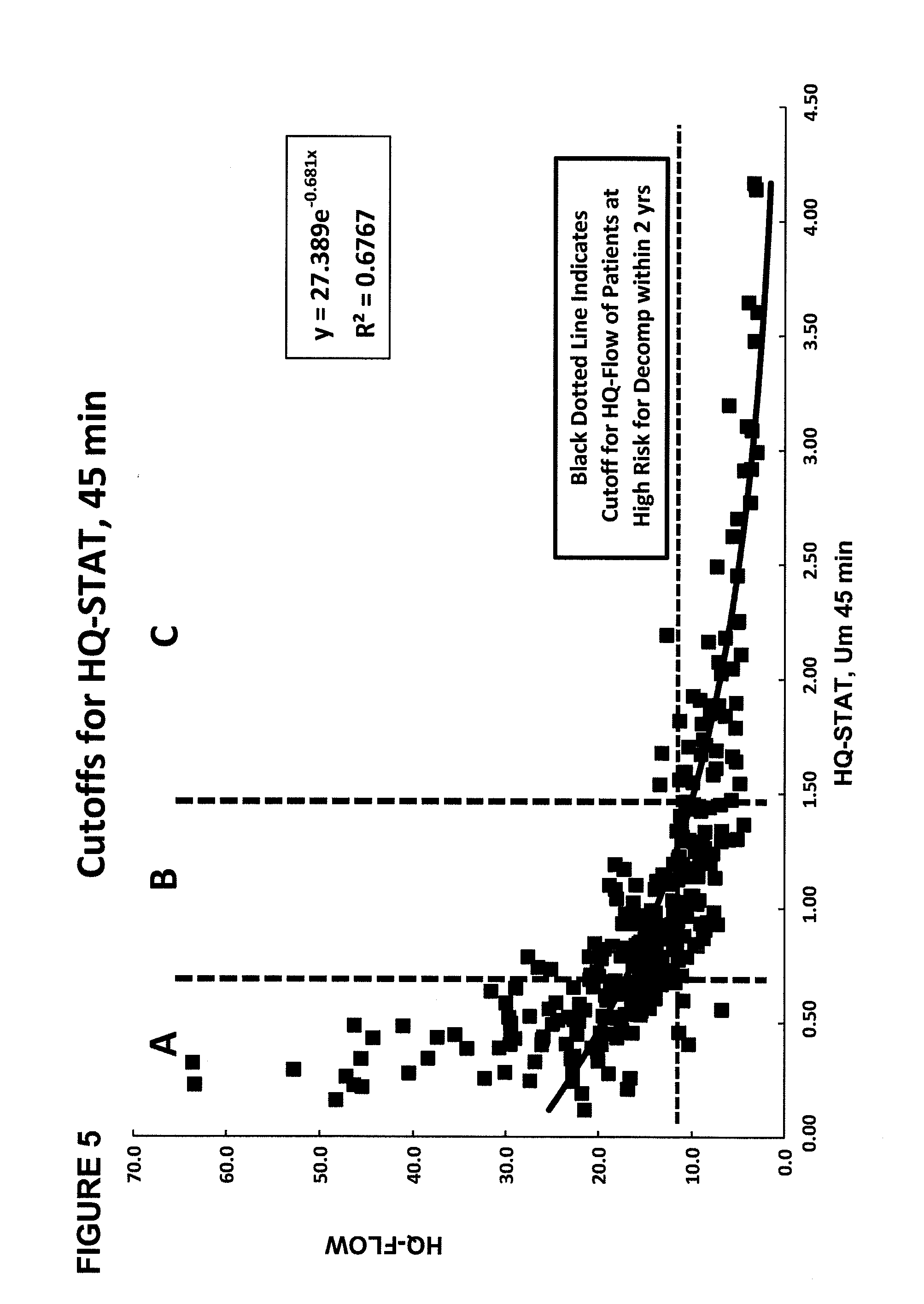
Disease severity index for assessment of chronic liver disease and method for diagnosis of three distinct subtypes of primary sclerosing cholangitis
ActiveUS20140147875A1Low indexOrganic active ingredientsHealth-index calculationDisease severityNon invasive
A Disease Severity Index (DSI) is provided for assessment of chronic liver disease in a patient using non-invasive liver function test results. A DSI was derived from non-invasive liver function test results based on hepatic blood flow. The DSI is used in methods for prediction of clinical outcomes, prediction of response to antiviral treatment, and assessment of progression of chronic liver diseases. Non-invasive methods to diagnose three distinct categories of patients with Primary Sclerosing Cholangitis (PSC) are provided. The methods can be used to diagnose PSC patients as Slow Progressors, Moderate Progressors and Rapid Progressors.
Owner:UNIV OF COLORADO THE REGENTS OF
Application of colchicin in preparing cholestatic liver disease drug
InactiveCN101822659AEffective treatmentLessen liver damageOrganic active ingredientsDigestive systemGestationPhospholipid
The invention relates to application of colchicin in preparing drugs, in particular to application of colchicin in pharmacotherapy of cholestatic liver disease, the application of colchicin in preparing a cholestatic liver disease drug, and the application of colchicin in preparing the drug for treating gestation intrahepatic cholestasis syndrome, inner primary cirrhose biliaire, primary sclerosing cholangitis severe jaundice or immunity jaundice cholestatic liver disease. The colchicin can effectively lower liver injury and cholestasis caused by cholestatic liver disease, i.e. the colchicin can effectively treat cholestatic liver disease. The colchicin can effectively lighten hepatic cell necrosis, inflammatory cell infiltration and bile capillary hyperplasia caused by cholestasis. The colchicin can effectively increase bile acid, bilirubin and expression of phospholipid transporters BSEP, MRP2 and MDR3 in a bile duct, accelerates excretion of bile acid, bilirubin and phospholipid deposited in the body and reduces liver damage.
Owner:CHINA PHARM UNIV
Disease severity index for assessment of chronic liver disease and method for diagnosis of three distinct subtypes of primary sclerosing cholangitis
ActiveUS9091701B2Low indexOrganic active ingredientsHealth-index calculationDisease severityChronic liver disease
A Disease Severity Index (DSI) is provided for assessment of chronic liver disease in a patient using non-invasive liver function test results. A DSI was derived from non-invasive liver function test results based on hepatic blood flow. The DSI is used in methods for prediction of clinical outcomes, prediction of response to antiviral treatment, and assessment of progression of chronic liver diseases. Non-invasive methods to diagnose three distinct categories of patients with Primary Sclerosing Cholangitis (PSC) are provided. The methods can be used to diagnose PSC patients as Slow Progressors, Moderate Progressors and Rapid Progressors.
Owner:UNIV OF COLORADO THE REGENTS OF
Pharmaceutical compositions for combination therapy
The present invention relates to a pharmaceutical composition comprising a combination of an FXR agonist and at least one lipid lowering agent (e.g., PPAR-alpha agonist, PPAR-delta agonist, PPAR-alpha and delta dual agonist, and / or statin). Also disclosed is use of the combination for the treatment or prevention of a FXR mediated disease or condition, such as primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), portal hypertension, bile acid diarrhea, NAFLD (nonalcoholic fatty liver disease), NASH (non-alcohol-induced steatohepatitis), and other chronic liver diseases. The combination of the present invention is useful for the treatment or prevention of conditions related to elevated lipid and liver enzyme levels. The present invention also relates to packs or kits including the pharmaceutical combination.
Owner:INTERCEPT PHARMA INC
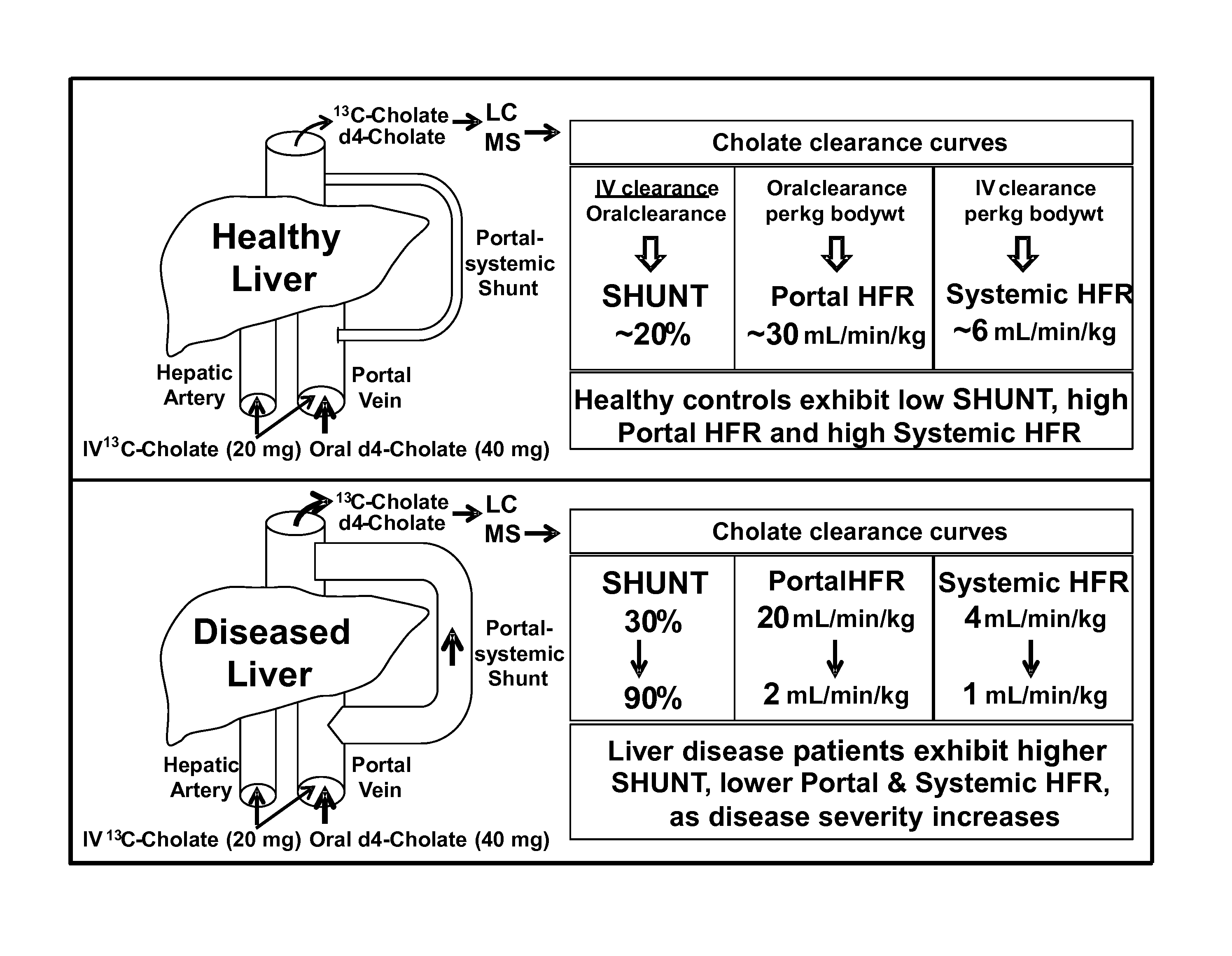
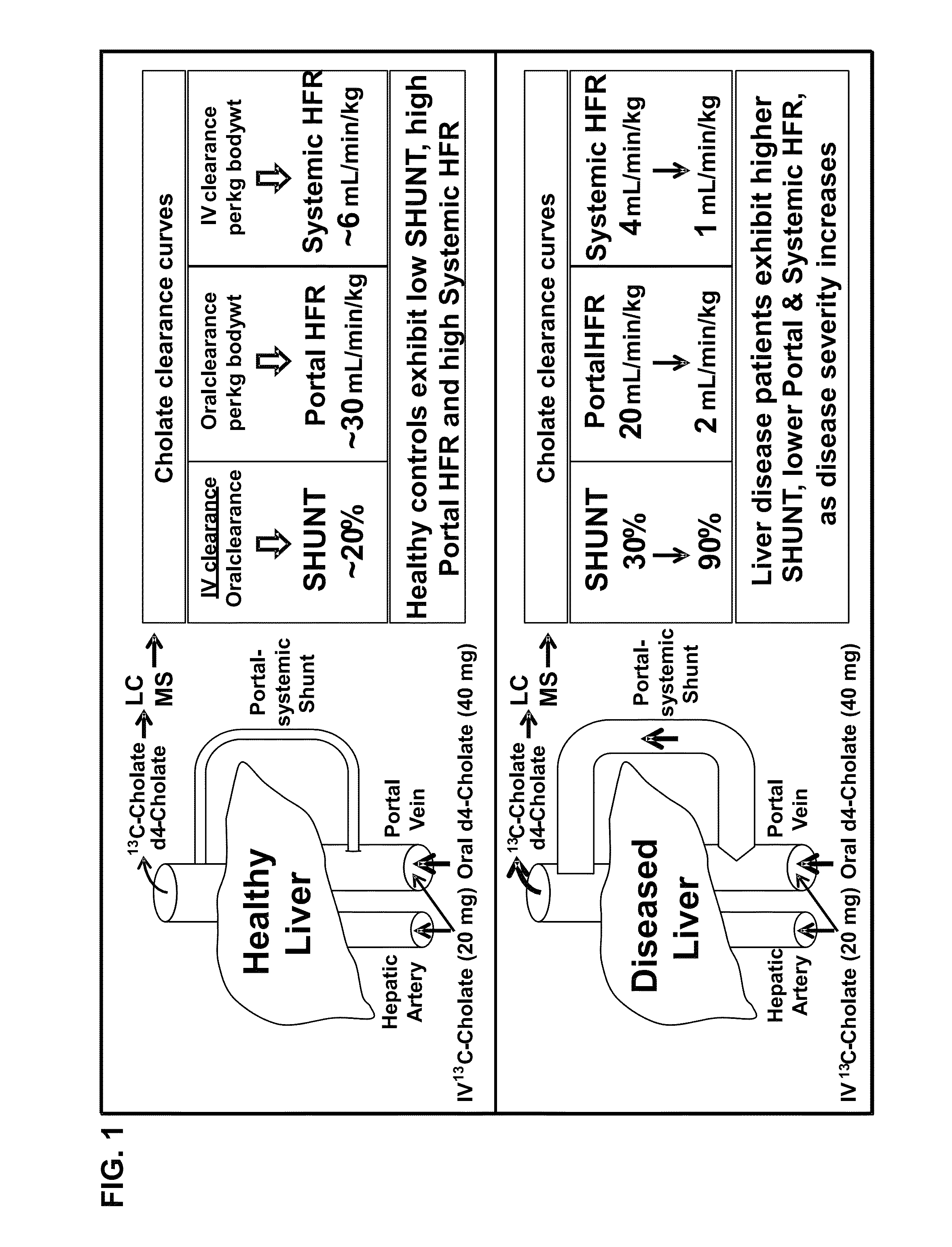
Method for assessment of hepatic function and portal blood flow
ActiveUS8961925B2In-vivo radioactive preparationsPharmaceutical containersChronic viral hepatitis CPortal vein flow
A method for estimating portal blood flow and hepatic function in a subject is provided. In one example, the STAT test is an in vitro simplified, convenient test intended for screening purposes that can reasonably estimate the portal blood flow from a single blood sample taken 60 minutes after orally administered deuterated-cholate. The test can be administered to a patient having, or suspected of having, Chronic Hepatitis C, Primary Sclerosing Cholangitis (PSC), Non-Alcoholic Fatty Liver Disease (NAFLD), or any chronic liver disease.
Owner:UNIV OF COLORADO THE REGENTS OF
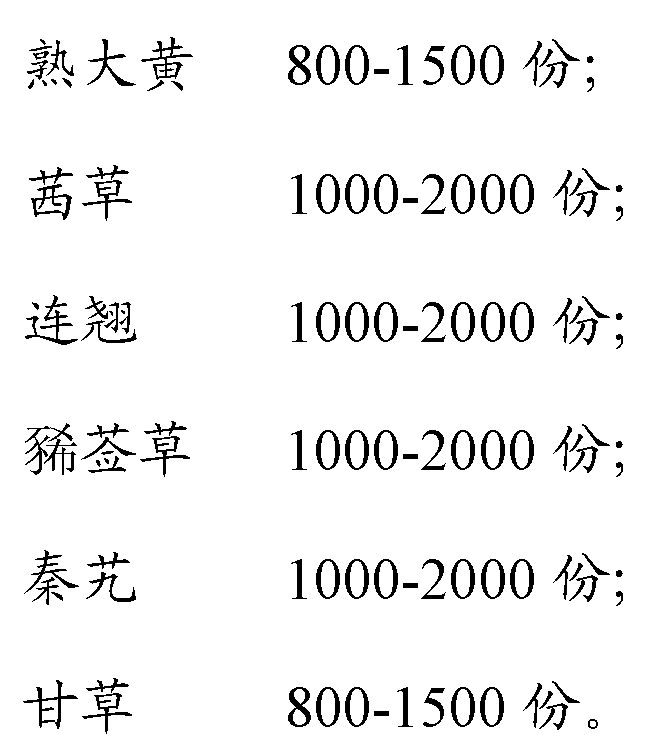
Chinese medicine composition for treating autoimmune liver disease, and preparation method thereof
The invention discloses a Chinese medicine composition for treating an autoimmune liver disease. The Chinese medicine composition is prepared from the Chinese medicinal materials, namely rheum officinale, radix rubiae, fructus forsythia, herba siegesbeckiae, large-leaved gentian and liquorice. The invention also discloses a preparation method of the Chinese medicine composition and application of the Chinese medicine composition. The composition has the effects of clearing away heat and toxic materials, smoothing liver-gallbladder, and promoting blood circulation to remove blood stasis, and is mainly used for treating autoimmune hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Method for assessment of hepatic function and portal blood flow
InactiveUS20150204842A1Pharmaceutical containersMedical packagingChronic viral hepatitis CPortal vein flow
A method for estimating portal blood flow and hepatic function in a subject is provided. In one example, the STAT test is an in vitro simplified, convenient test intended for screening purposes that can reasonably estimate the portal blood flow from a single blood sample taken 60 minutes after orally administered deuterated-cholate. The test can be administered to a patient having, or suspected of having, Chronic Hepatitis C, Primary Sclerosing Cholangitis (PSC), Non-Alcoholic Fatty Liver Disease (NAFLD), or any chronic liver disease.
Owner:UNIV OF COLORADO THE REGENTS OF
Methods and compositions for treating psc (primary sclerosing cholangitis) or pbc (primary biliary cirrhosis) with Anti-cd3 immune molecule therapy
InactiveUS20150132289A1Easy to doImprove complianceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPrimary biliary cirrhosisAnti cd3
A method or composition comprising an anti-CD3 immune molecule for treatment of PSC (primary sclerosing cholangitis) or PBC (primary biliary cirrhosis) in a subject.
Owner:THERAPIX BIOSCI
Novel compositions and methods useful for treating or preventing liver diseases or disorders, and promoting weight loss
ActiveUS20170232027A1Reduces fat-induced obesityGood for weight lossOrganic active ingredientsDigestive systemToxic liver diseaseHepatic fibrosis
The invention includes a method of preventing or treating a toxic liver disease or disorder, such as but not limited to non-alcoholic steatohepatitis (NASH), liver injury associated with or caused by alcohol consumption in a mammal afflicted with NASH, alcoholic hepatitis, drug induced liver injury, primary sclerosing cholangitis, viral hepatitis, liver fibrosis, liver cirrhosis, and other toxic liver conditions, in a subject, such as a mammal. The invention also includes a method of promoting weight loss in a subject, such as a mammal. The invention comprises administering to the subject a therapeutically effective dose of at least one cardiac glycoside or a solvate, salt, prodrug or derivative thereof.
Owner:YALE UNIV
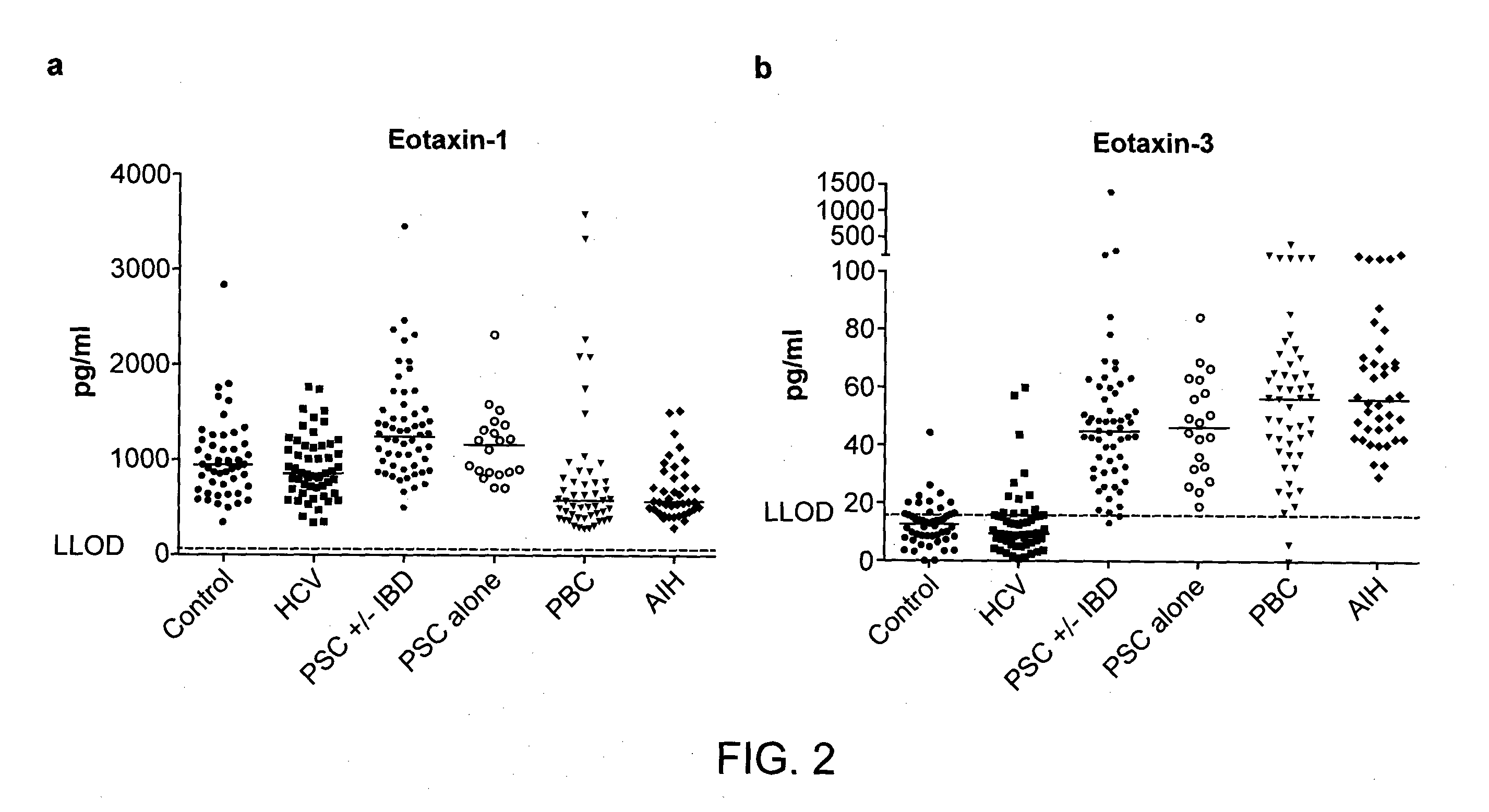
Methods and Compositions for Diagnosis of Inflammatory Liver Disease
ActiveUS20150219664A1Easy diagnosisGood differential diagnosisOrganic active ingredientsHealth-index calculationPrimary biliary cirrhosisLiver disease
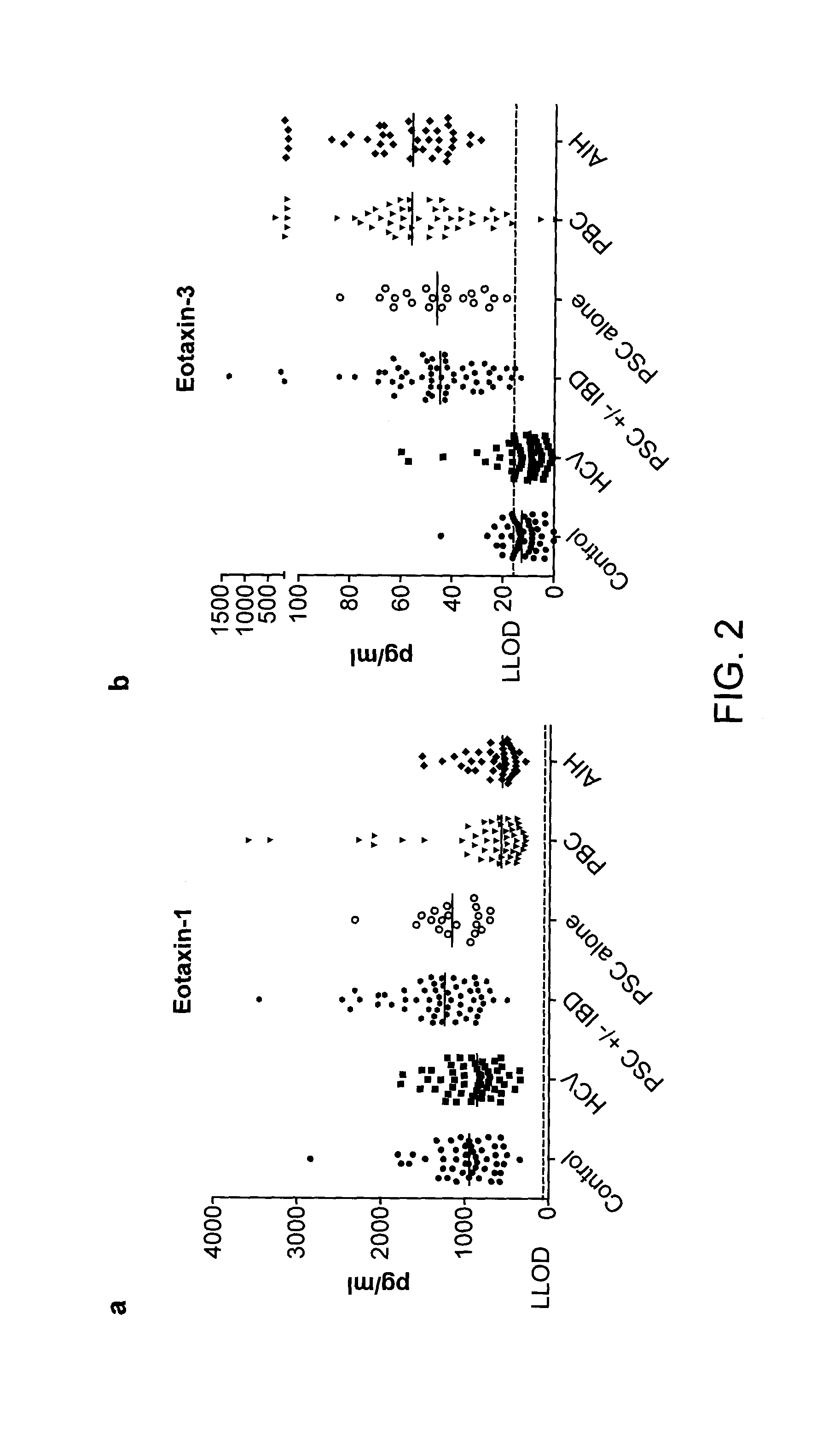
The present disclosure provides methods and compositions that find use in facilitating a diagnosis of inflammatory liver disease in a subject. The methods and compositions generally involve detection of eotaxin-3 (E3) levels, either alone or with levels of eotaxin-1 (E1), and optionally, with levels of CCL22 and, further optionally, with levels of IL15. These levels can be used to facilitate a diagnosis of a liver disease of at least one of autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC), and / or to facilitate a differential diagnosis between AIH, PBC, and PSC. The methods and compositions of the present disclosure also find use in facilitating treatment decisions for a subject.
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER +1
Non human animal model for ulcerative colitis and its main complications
InactiveUS20140373186A1Compounds screening/testingMicrobiological testing/measurementTargeted disruptionHuman animal
The present invention relates to a non human model animal for ulcerative colitis and its main complications such as primary sclerosing cholangitis and colorectal cancer. More particularly, the present invention relates to a transgenic non human animal model for ulcerative colitis and its main complications such as primary sclerosing cholangitis, and colorectal cancer comprising a targeted disruption in the IL10 and NOX1 genes so that IL10 and NOX1 are not expressed in said animal.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
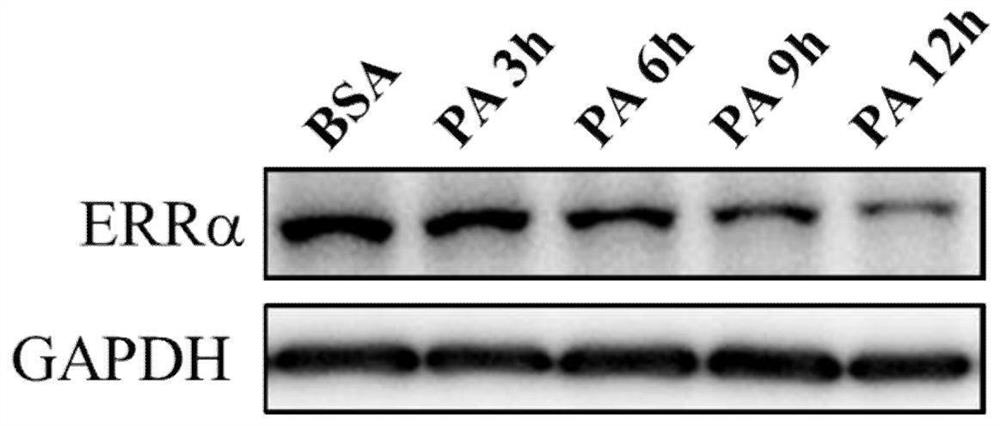
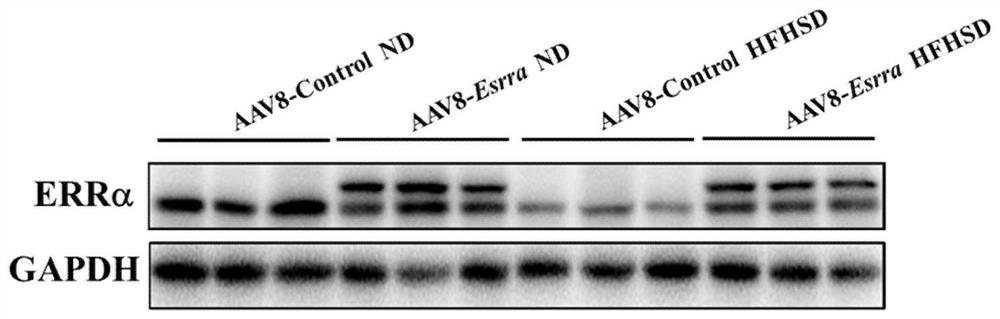
Medical application of encoding gene ESRRA of estrogen-related receptor alpha (ERR alpha)
PendingCN113117097AEasily damagedReduce lipid accumulationPeptide/protein ingredientsDigestive systemAdenoassociated virusBile Juice
The invention relates to an application of an encoding gene ESRRA of an estrogen-related receptor alpha (ERR alpha) in preparation of a medicine for preventing or treating liver diseases. The liver diseases include non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), alcoholic fatty liver disease (AFLD), hepatic fibrosis, or cirrhosis. The adeno-associated virus is utilized to construct an ERRalpha overexpression vector system, and the fact that the ERR alpha gene and a delivery system of the ERR alpha gene can be used for preventing or treating the liver diseases is proved on various disease models.
Owner:CHINA PHARM UNIV
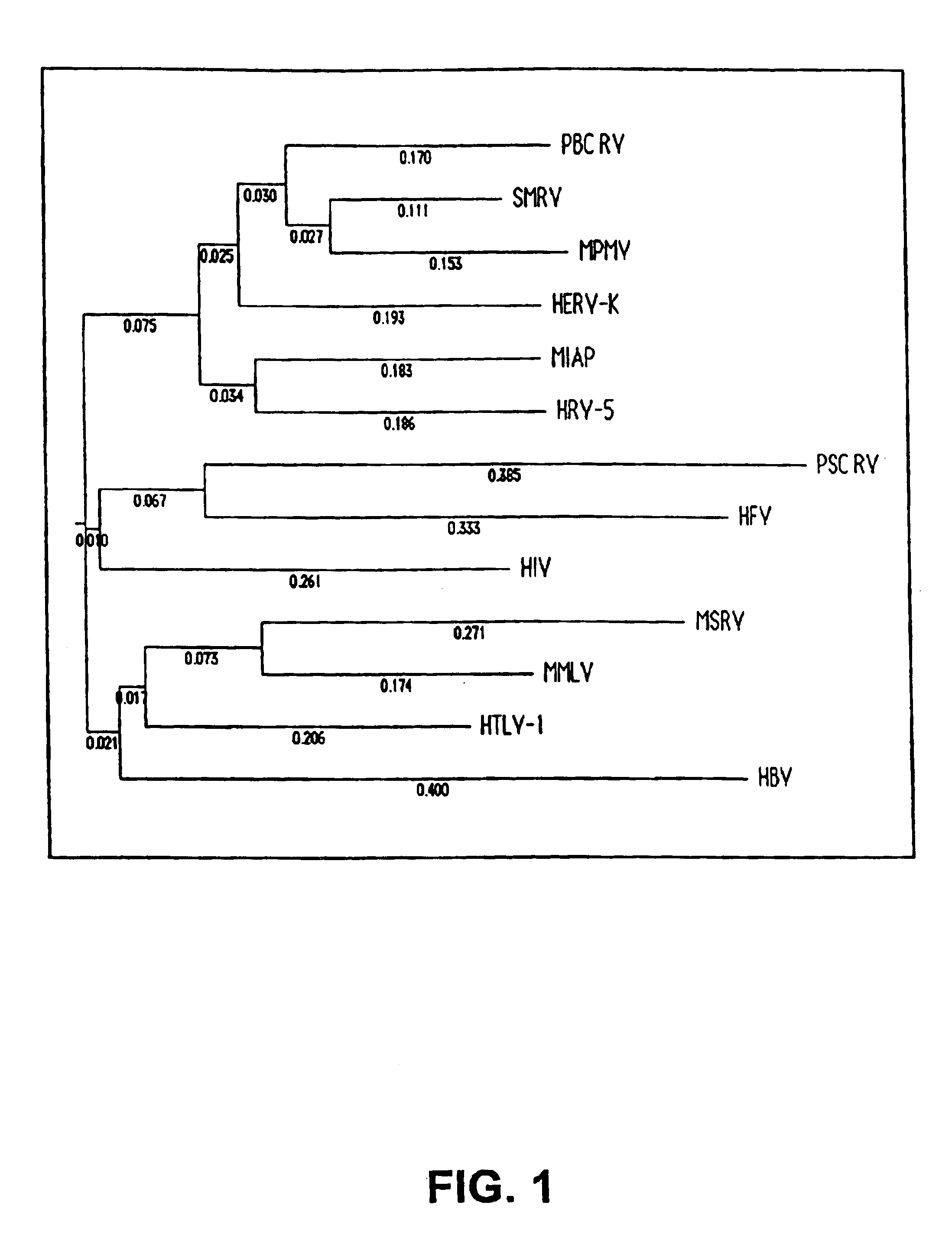
Identification of a novel retrovirus associated with primary sclerosing cholangitis and autoimmune hepatitis
InactiveUS6787303B1Improve bindingReduce degradationSugar derivativesMicrobiological testing/measurementCrohn's diseaseUlcerative colitis
The present invention relates first, to the identification of a retrovirus and the novel nucleotide sequences encoding a retroviral polymerase gene (POL nucleotides) associated with the existence of primary sclerosing cholangitis (PSC), autoimmune hepatitis (AIH) Crohn's disease and ulcerative colitis. The present invention further relates to methods for using the PSC associated retroviral nucleotides for the detection of PSC, AIH, Crohn's disease and ulcerative colitis in patient samples. The present invention also relates to methods for using and targeting the PSC associated retroviral POL nucleotides in gene therapy protocols for the treatment of PSC, AIH, Crohn's disease or ulcerative disease in patients in need of such treatment. The present invention further relates to diagnostic protocols and kits for the detection of PSC, AIH, Crohn's disease and ulcerative colitis in tissue samples.
Owner:ALTON OCHSNER MEDICAL FOUND
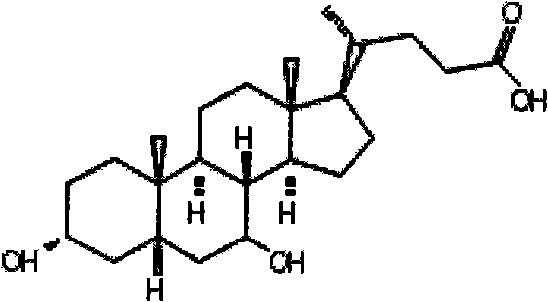
Preparation method of high-purity natural ursodesoxycholic acid and application thereof in medicament
The invention belongs to the technical field of medical biology, in particular to a preparation method of high-purity natural ursodesoxycholic acid. The high-purity natural ursodesoxycholic acid is obtained by separating and purifying bear gall powder prepared from a manual drained bear gall, and the high performance liquid chromatography purity is not lower than 98 percent. The preparation method comprises the following steps of: firstly, separating and purifying by applying a cation exchange medium to prepare tauroursodeoxycholic acid with purity not lower than 90 percent; and then thoroughly hydrolyzing the tauroursodeoxycholic acid into ursodesoxycholic acid and taurine in the presence of alkali and separating and purifying by applying a silica gel adsorption chromatography technology to prepare the ursodesoxycholic acid with purity not lower than 98 percent. The natural ursodesoxycholic acid prepared by the invention has the effects on treating primary biliary cirrhosis and primary sclerosing cholangitis.
Owner:重庆寰瑞生物技术有限公司
Methods and compositions for diagnosis of inflammatory liver disease
ActiveUS9535071B2Good differential diagnosisDiagnosis can be complexHealth-index calculationDigestive systemPrimary biliary cirrhosisInflammatory bowel disease
The present disclosure provides methods and compositions that find use in facilitating a diagnosis of inflammatory liver disease in a subject. The methods and compositions generally involve detection of eotaxin-3 (E3) levels, either alone or with levels of eotaxin-1 (E1), and optionally, with levels of CCL22 and, further optionally, with levels of IL15. These levels can be used to facilitate a diagnosis of a liver disease of at least one of autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC), and / or to facilitate a differential diagnosis between AIH, PBC, and PSC. The methods and compositions of the present disclosure also find use in facilitating treatment decisions for a subject.
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER +1
Diagnosis of gluten sensitive enteropathy and other autoimmunopathies
InactiveUS7781169B1Increased riskReduce the overall heightDisease diagnosisBiological testingInsulin dependentRheumatism
Method for diagnosis of autoimmune diseases of the GSE-type or associated with gluten sensitive enteropathy comprising taking a sample and testing the sample for antibodies against human tissue transglutaminase, tissue-specific transglutaminases, or other transglutaminases. It was found that autoimmune diseases other than celiac disease can be diagnosed and distinguished in this way, notably, dermatitis herpetiformis Duhring, Crohn's disease, Addison's disease, AI hemolytic anemia, AI thrombocytopenic purpura, AI thyroid diseases, atrophic gastritis—pernicious anemia, IgA nephropathy or IgA glomerulonephritis, myasthenia gravis, partial lipodystrophy, polymyositis, primary biliary cirrhosis, primary sclerosing cholangitis, recurrent pericarditis, relapsing polychondritis, rheumatoid arthritis, rheumatism, sarcoidosis, Sjögren's syndrome, SLE, splenic atrophy, type I (insulin-dependent) diabetes mellitus, diabetes mellitus of other types, ulcerative colitis, vasculitis (both systemic and cutaneous), vitiligo as well as autoimmune diseases associated with infertility, increased risk of abortion, or reduced fetal growth.
Owner:PAULSSON MATS +5
Keratin 8 mutations are risk factors for developing liver disease of multiple etiologies
ActiveUS7838217B1High incidenceModulate expressionSugar derivativesPeptide/protein ingredientsEtiologyHepatobiliary disease
Keratin 8 and 18 (K8 / K18) mutations are shown to be associated with a predisposition to liver or biliary tract disease, particularly noncryptogenic hepatobiliary disease. Unique K8 / K18 mutations are shown in patients with diseases including but without limitation to viral hepatitis, biliary atresia, alcoholic cirrhosis and other acute or chronic toxic liver injury, cryptogenic cirrhosis, acute fulminant hepatitis, autoimmune liver disease, cystic fibrosis, primary biliary cirrhosis, primary sclerosing cholangitis, diseases that are linked with cryptogenic cirrhosis, such as nonalcoholic steatohepatitis, and the like. Livers with keratin mutations had increased incidence of cytoplasmic filamentous deposits. Therefore, K8 / K18 are susceptibility genes for developing cryptogenic and noncryptogenic forms of liver disease. Mutant alleles are associated with disease susceptibility, and their detection is used in the diagnosis of a predisposition to these conditions.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Application of prevotella in preparation of medicine for treating cholestatic diseases
PendingCN113509494AReduce sedimentationImprove fibrosisBacteria material medical ingredientsDigestive systemEnterohepatic circulationBile Juice
The invention discloses application of prevotella in preparation of a medicine for treating cholestatic diseases. The cholestatic diseases include, but is not limited to, primary sclerosing cholangitis. After the inventor constructs a PSC animal model, it is determined that the abundance of P.copri in mice is consistently reduced in the same population, then intervention effects of P.copri on inflammation, fibrosis, cholestasis and the like of the PSC model are observed and evaluated through intragastric administration for one week and daily supplementation of 1*10<8> CFU of P.copri bacterial liquid, bile acid metabolism changes are determined through total bile acid determination and targeted bile acid detection analysis, and the action mechanism is analyzed. Experiments prove that the P.copri can obviously relieve cholestasis in hepatointestinal circulation of PSC mice and obviously improve liver fibrosis, which is of great significance to prevention or treatment of cholestatic diseases.
Owner:NANJING MEDICAL UNIV
Compositions and methods for treating neonatal biliary atresia
ActiveUS20180147267A1Peptide/protein ingredientsDigestive systemPrimary biliary cirrhosisSerine Protease Inhibitors
Disclosed are methods and compositions for treatment of a subject having a biliary disorder. The methods include administering a therapeutically effective amount of a serine protease inhibitor to a subject in need thereof. The biliary disorder include biliary atresia, a biliopathy, Primary Biliary Cirrhosis (PBC), Primary Sclerosing Cholangitis (PSC), and combinations thereof. In certain aspects, the serine protease inhibitor may be a protease inhibitor rC1 Inhibitor.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Compositions and methods for treating neonatal biliary atresia
Disclosed are methods and compositions for treatment of a subject having a biliary disorder. The methods include administering a therapeutically effective amount of a serine protease inhibitor to a subject in need thereof. The biliary disorder include biliary atresia, a biliopathy, Primary Biliary Cirrhosis (PBC), Primary Sclerosing Cholangitis (PSC), and combinations thereof. In certain aspects, the serine protease inhibitor may be a protease inhibitor rC1 Inhibitor.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Self-micellizing fatty acids and fatty acid ester compositions and their use in the treatment of disease states
InactiveUS20160296492A1Increase surface areaImprove bioavailabilityHydroxy compound active ingredientsPharmaceutical non-active ingredientsTG - TriglycerideBrain traumas
Described herein are compositions including at least one omega-3 fatty acid (either in the triglyceride, ester or free fatty acid ester form) and at least one surface active agent; wherein the compositions form micelles when in contact with an aqueous medium. Also provided are methods of administering to a subject a composition including at least one omega-3 fatty acid (either in the triglyceride, ester or free fatty acid ester form) and at least one surface active agent, wherein the compositions form micelles when in contact with an aqueous medium, and the bioavailability of the omega-3 fatty acid is substantially independent of a food effect. The compositions are useful for treating certain disease states which may include (1) malabsorption syndromes, (2) primary sclerosing cholangitis (PSC), (3) non-alcoholic fatty liver disease (NAFLD), (4) sickle cell disease (SCD), (5) age-related macular degeneration (AMD), and (6) neurodegenerative disease, including, Parkinson's Disease (PD), Alzheimer's Disease (AD), Amyotrophic Lateral Sclerosis (ALS or Lou Gehrig's Disease), Epilepsy, Bi-polar Syndrome, traumatic brain injury, peripheral neuropathy, and Multiple Sclerosis (MS). Described are also various dosage forms for administering the compositions and use of the compositions in functional foods. Provided herein are also kits with instructions for their administration.
Owner:SANCILIO
Fluorinated bile acid derivatives
ActiveCN112771063AHas FXR agonist activityIncrease valueSulfonylurea active ingredientsDigestive systemBile JuiceBiliary tract
Compounds of general formula (I): wherein R2a, R2b, R3a, R3b, R5, Y and R7 are as defined herein are selective agonists at the FXR receptor and are useful for the treatment or prevention of diseases and conditions including nonalcoholic steatohepatitis (NASH); primary biliary cirrhosis; primary sclerosing cholangitis; biliary atresia; cholestatic liver disease; hepatitis C infection; alcoholic liver disease; fibrosis; or liver damage arising from fibrosis.
Owner:NZP UK LTD +1
LANCL ligands
Provided are compounds of Formula (I):The compounds target the lanthionine synthetase C-like (LANCL) family of proteins, including LANCL2 and LANCL3. The compounds can be used to treat conditions such as inflammatory diseases, metabolic diseases, autoimmune diseases, cancers, and infectious diseases. Exemplary conditions include inflammatory conditions of the liver, such as nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and cirrhosis; inflammatory conditions of the bile duct, such as primary biliary cholangitis, primary sclerosing cholangitis; inflammatory bowel disease, such as Crohn's disease and ulcerative colitis; lupus, such as systemic lupus erythematosus, lupus nephritis, and cutaneous lupus; arthritis, such as rheumatoid arthritis; hyperglycemia, such as type 1 diabetes, type 2 diabetes, and prediabetes and associated conditions such as atherosclerosis and diabetic kidney disease; psoriasis; and multiple sclerosis.
Owner:NIMMUNE BIOPHARMA INC
Method for treating primary sclerosing cholangitis
The invention relates to the use of pharmaceutical compositions of the SGLT2 inhibitor, remogliflozin etabonate, to treat primary sclerosing cholangitis (PSC). Methods and compositions associated with the invention can improve or maintain clinical outcomes of PSC symptoms, such as ascites accumulation, hepatic encephalopathy, development of varices, jaundice, variceal bleeding, cholangiocarcinoma, hepatocellular carcinoma, evidence of cirrhosis, and colorectal cancer.
Owner:アヴォリント
Psmp antagonists for use in treatment of fibrotic disease of the lung, kidney or liver
PendingCN113348178AEliminate or reduce riskSmall doseDigestive systemImmunoglobulins against cytokines/lymphokines/interferonsAntiendomysial antibodiesBile Juice
Disclosed are antagonists of PC3-secreted microprotein (PSMP) and use of the antagonists for treatment of liver, lung, or kidney fibrosis, including various diseases or disorders associated with liver, lung, or kidney fibrosis such as, e.g., non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), drug-induced lung injury, acute kidney injury (AKI), chronic kidney disease (CKD), lupus nephritis, IgA nephropathy, and membranous glomerulonephritis. Also disclosed are PSMP antagonists and their use for treatment of graft-versus-host disease (GVHD) and systemic lupus erythematosus (SLE). Suitable PSMP antagonists for use in disease treatment include PSMP-binding proteins such as, for example, neutralizing anti-PSMP antibodies.
Owner:MAPLE BIOTECH LLC
Application of Babaodan in preparation of medicine for treating primary sclerosing cholangitis
The invention discloses eight-jewel elixir and novel application of preparations of the eight-jewel elixir, and particularly relates to application of eight-jewel elixir to preparing medicine for treating primary sclerosing cholangitis. The application has the advantages that the eight-jewel elixir and preparations of the eight-jewel elixir can be applied to wide fields, and a novel traditional Chinese medicine treatment method is developed for treating the primary sclerosing cholangitis.
Owner:XIAMEN TRADITIONAL CHINESE MEDICINE
Use of sglt2 inhibitors to treat primary sclerosing cholangitis
PendingUS20210393662A1Improve and maintain clinical outcomeOrganic active ingredientsDispersion deliveryOncologyCancer research
The invention relates to the use of pharmaceutical compositions of the SGLT2 inhibitor, remogliflozin etabonate, to treat primary sclerosing cholangitis (PSC). Methods and compositions associated with the invention can improve or maintain clinical outcomes of PSC symptoms, such as ascites accumulation, hepatic encephalopathy, development of varices, jaundice, variceal bleeding, cholangiocarcinoma, hepatocellular carcinoma, evidence of cirrhosis, and colorectal cancer.
Owner:AVOLYNT
Sickle Cell Disease Treatment Utilizing Omega-3 Fatty Acids
ActiveUS20200093779A1Increase surface areaImprove bioavailabilityHydroxy compound active ingredientsPharmaceutical non-active ingredientsActive agentIngested food
Described herein are compositions including at least one omega-3 fatty acid (either in the triglyceride, ester or free fatty acid ester form) and at least one surface active agent; wherein the compositions form micelles when in contact with an aqueous medium. Also provided are methods of administering to a subject a composition including at least one omega-3 fatty acid (either in the triglyceride, ester or free fatty acid ester form) and at least one surface active agent, wherein the compositions form micelles when in contact with an aqueous medium, and the bioavailability of the omega-3 fatty acid is substantially independent of a food effect. The compositions are useful for treating certain disease states which may include (1) malabsorption syndromes, (2) primary sclerosing cholangitis (PSC), (3) non-alcoholic fatty liver disease (NAFLD), (4) sickle cell disease (SCD), (5) age-related macular degeneration (AMD), and (6) neurodegenerative disease, including, Parkinson's Disease (PD), Alzheimer's Disease (AD), Amyotrophic Lateral Sclerosis (ALS or Lou Gehrig's Disease), Epilepsy, Bi-polar Syndrome, traumatic brain injury, peripheral neuropathy, and Multiple Sclerosis (MS). Described are also various dosage forms for administering the compositions and use of the compositions in functional foods. Provided herein are also kits with instructions for their administration.
Owner:GENERX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com