Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48 results about "Adenoassociated virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method to generate mirrored adenoassociated viral vectors
The present invention describes mirrored adenoassociated virus genomes that can spontaneously fold to form double-stranded DNA structures capable of directing efficient RNA transcription in mammalian cell nuclei. Also described are mirrored adenoassociated viral particles that incorporate the mirrored vector genome and a suitable adenoassociated viral capsid. Further described are DNA templates and methods for producing the mirrored adenoassociated vector genomes and mirrored adenoassociated viral particles. Methods of administering these reagents to mammals are also described as are specific in vitro and in vivo applications where the mirrored adenoassociated virus has unique utility.
Owner:PETRAS OGNJEN
Adenoassociated virus vectors for the treatment of lysosomal storage disorders
The present invention provides new adenoassociated virus vectors and pharmaceutical compositions containing the same for the treatment of lysosomal storage disorders and specially, for the treatment of mucopolysaccharidoses Type IIIB.
Owner:AUTONOMOUS UNIVERSITY OF BARCELONA +1
Gene therapy medicament for Leber congenital amaurosis
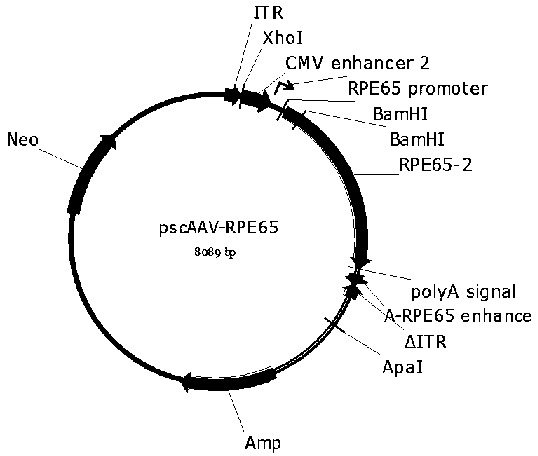
The invention provides a gene therapy medicament for specific expression of retinal pigment epithelium 65 (RPE65) of eyes in order to treat Leber congenital amaurosis. A vector contains a CMV enhancer, a promoter of a human RPE65 gene, a human RPE65 gene coding region and an intron composed sequence, and a poly A sequence and an artificially spliced RPE65 gene enhancer. The recombinant vector is mediated by a single chain and / or double chain adeno-associated virus (AAV), including but not limited to 9 type AAV type 9 (AAV9).
Owner:BEIJING GENECRADLE PHARM CO LTD
Antibody capable of binding to AAV1-13
ActiveCN111925438AEasy to separateEasy to identifyBiological material analysisSolid sorbent liquid separationAdenoassociated virusEngineering
The invention relates to the field of immunology, in particular to an antibody capable of binding to AV1-13. The antibody can recognize VP1, VP2 and VP3 proteins of all serotypes of AV1-13, is high inaffinity and good in specificity; and the antibody can be more conveniently used for separation and identification of adeno-associated viruses due to the fact that the VP proteins are located on thesurfaces of the adeno-associated viruses.
Owner:OBIO TECH SHANGHAI CORP LTD
Avian adenoassociated virus and uses thereof
The present invention provides an Avian adeno-associated virus (AAAV) virus and vectors and particles derived therefrom. In addition, the present invention provides methods of delivering a nucleic acid to a cell using the AAAV vectors and particles. Methods of isolating the AAAV are provided.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH OFFICE OF TECH TRANSFER +1
Adenoassociated virus vectors for the treatment of mucopolysaccharidoses
ActiveUS20180169272A1HydrolasesPharmaceutical delivery mechanismAdenoassociated virusAdeno associate virus
The present invention provides new Adeno-associated virus-derived vectors and pharmaceutical compositions containing the same for the treatment of lysosomal storage disorders and specially, for the treatment of mucopolysaccharidoses Type II.
Owner:ESTEVE PHARMA SA +1
Adenoassociated virus vectors for the treatment of mucopolysaccharidoses
The present invention provides new adeno-associated virus-derived vectors and pharmaceutical compositions containing the same for the treatment of lysosomal storage disorders and specially, for the treatment of mucopolysaccharidoses Type IIID.
Owner:ESTEVE PHARMA SA +1
Avian adenoassociated virus and uses thereof
ActiveUS20070092866A1Genetic therapy composition manufactureVirus peptidesAdenoassociated virusVirus
The present invention provides an Avian adeno-associated virus (AAAV) virus and vectors and particles derived therefrom. In addition, the present invention provides methods of delivering a nucleic acid to a cell using the AAAV vectors and particles. Methods of isolating the AAAV are provided.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH OFFICE OF TECH TRANSFER +1
Use of the CYP46A1 Gene for the Treatment of Alzheimer's Disease
The invention relates to a viral vector for treating Alzheimers disease, which vector comprises a cholesterol 24-hydroxylase (CYP46A1) encoding nucleic acid. In a preferred embodiment, the viral vector may be an Adeno-Associated-Virus (AAV) vector, preferably an AVV5 vector. The vector may be useful for the manufacture of a pharmaceutical composition for the treatment of Alzheimers disease in a subject, wherein the vector is to be administered directly into the brain of the subject or by intravenous or intrathecal injection.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
siRNA for inhibiting expression of human PCSK9 gene and application thereof
PendingCN111154760ALower levelEnhanced LDL-C intake functionOrganic active ingredientsMetabolism disorderDiseaseAdenoassociated virus
The invention discloses an siRNA sequence for specifically inhibiting the expression of a human protein convertase subtilisin 9 (PCSK9) target gene and an application thereof. The siRNA can be chemically synthesized, and can also be constructed into corresponding recombinant lentivirus or adeno-associated virus and the like according to the sequence of the siRNA. After human hepatoma carcinoma cell HepG2 and human normal hepatoma carcinoma cell LO2 are transfected by the siRNA or the virus, the expression of PCSK9 can be effectively inhibited; the protein level of a low-density lipoprotein receptor (LDLR) is increased; and the activity of the hepatoma carcinoma cell for taking in low-density lipoprotein (LDL) is remarkably enhanced. After the recombinant lentivirus is subjected to high-pressure caudal vein injection administration, the levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) in plasma of a hypercholesterolemia model mouse can be remarkably reduced. The siRNA or the recombinant virus or a modifier thereof can be used for preventing and / or treating diseases like hyperlipidemia, atherosclerosis, cardiovascular and cerebrovascular diseases, obesity, diabetes and nephropathy.
Owner:CHINA PHARM UNIV
Method for improving expression level of human coagulation factor IX
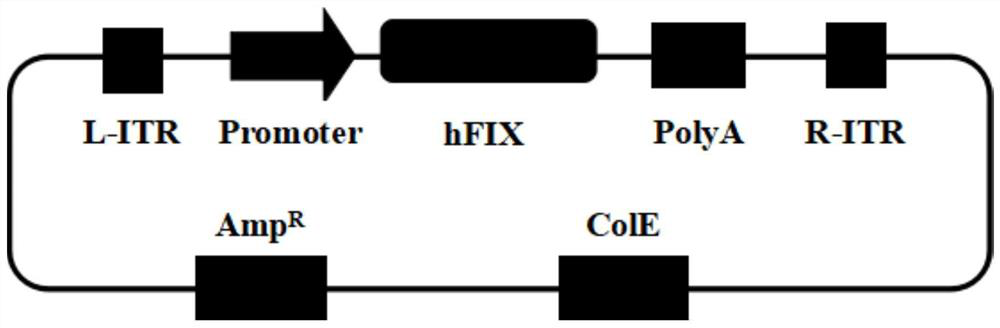
PendingCN111647625AImprove expression levelReduce manufacturing costFermentationVector-based foreign material introductionAdenoassociated virusTherapeutic effect
The invention discloses a method for improving the expression level of human coagulation factor IX. The method comprises the following steps: optimizing human coagulation factor IX gene; replacing Kozak sequence; and constructing AAV (adeno-associated virus) expression plasmid by the optimized human FIX gene and Kozak sequence, and packaging the recombinant AAV. The expression level of the human coagulation factor FIX gene is remarkably improved by the optimized gene coding sequence and Kozak sequence. The optimized human FIX gene and Kozak sequence can be used for efficiently producing FIX protein for treating hemophilia B, and the production cost of the FIX protein is reduced; and the optimized human FIX gene and Kozak sequence can also be used in AAV-mediated gene replacement therapy totreat hemophilia B, the expression level of the FIX gene in human bodies is increased, so that the administration dosage is reduced, and the therapeutic effect is improved.
Owner:SHENZHEN CHANGENE MEDICAL TECH CO LTD
Novel transgenic medaka, gene fragments
InactiveCN1664099AImprove viewing valueFermentationAnimals/human peptidesAdenoassociated virusTransgene
The invention also relates to a novel nucleic acid fragment including: (1)medaka beta-actin promotor; (2) a fluoyescent gene; and (3) inverted terminal repeats (ITR) of adeno-associated virus. The invention further relates to a plasmid comprising the nucleic acid fragment of the invention.
Owner:TAIKONG +1
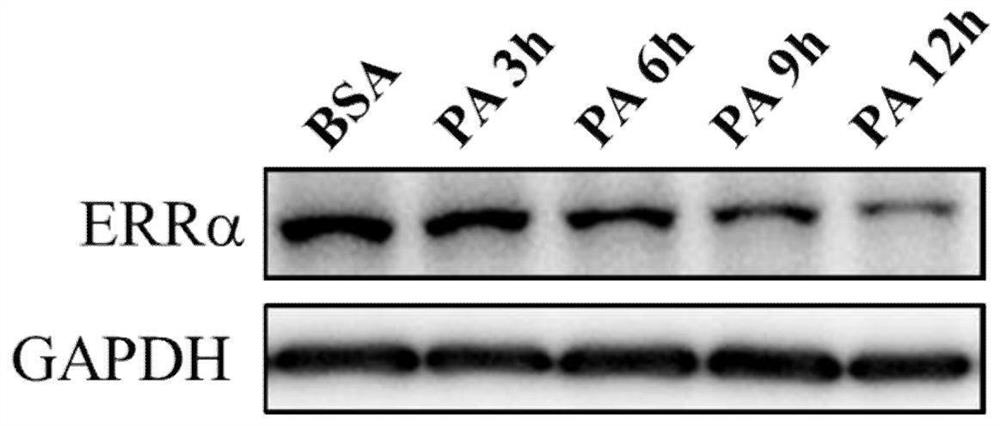
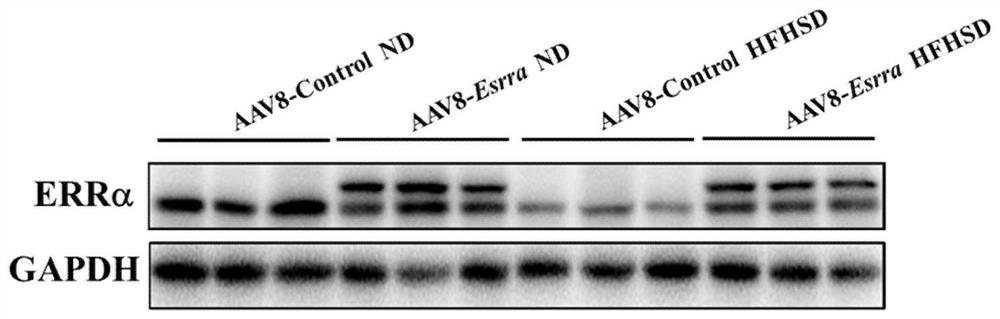
Medical application of encoding gene ESRRA of estrogen-related receptor alpha (ERR alpha)
PendingCN113117097AEasily damagedReduce lipid accumulationPeptide/protein ingredientsDigestive systemAdenoassociated virusBile Juice
The invention relates to an application of an encoding gene ESRRA of an estrogen-related receptor alpha (ERR alpha) in preparation of a medicine for preventing or treating liver diseases. The liver diseases include non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), alcoholic fatty liver disease (AFLD), hepatic fibrosis, or cirrhosis. The adeno-associated virus is utilized to construct an ERRalpha overexpression vector system, and the fact that the ERR alpha gene and a delivery system of the ERR alpha gene can be used for preventing or treating the liver diseases is proved on various disease models.
Owner:CHINA PHARM UNIV
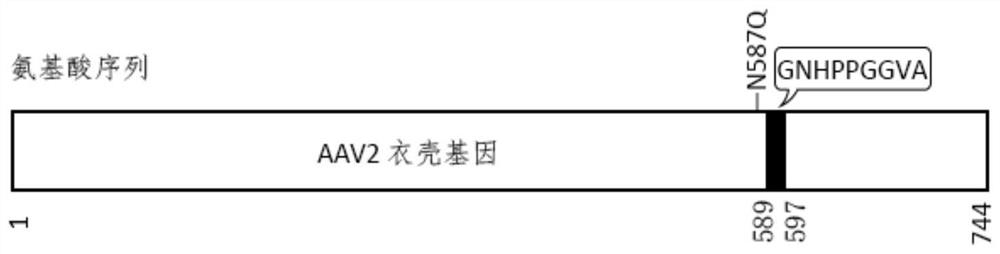
Adeno-associated virus vector targeting cardiac vascular endothelium and application thereof
ActiveCN111850045AEnhanced eNOS activityVirus peptidesDepsipeptidesAdenoassociated virusEnzyme digestion
The invention belongs to the technical field of biology, and particularly relates to an adeno-associated virus vector for targeting cardiac vascular endothelium and an application of the adeno-associated virus vector. The invention aims to solve the problem that natural AAV has poor cardiovascular endothelial transduction capacity in vivo. The invention provides an adeno-associated virus vector targeting heart vascular endothelium. The library is constructed by inserting an R588 site of an AAV2 capsid protein gene into a coding sequence of random heptapeptide, and inserting the coding sequenceinto a plasmid skeleton containing an AAV2 rep gene and ITR through HindIII / NotI double enzyme digestion. After two rounds of in-vivo directed evolution screening, the two AAV variants EC71 and EC73are obtained, the transduction capacity of AAV to the heart vascular endothelium in vivo is improved, meanwhile, transduction to the liver is greatly reduced, and transgenic expression is maintained for at least four months in the mouse heart vascular endothelium. The EC71 vector is used for conveying the eNOS gene into a myocardial infarction mouse body, so that the activity of the eNOS protein of the heart and the lung is effectively improved, and the EC71 vector has a certain application prospect in gene therapy of cardiovascular endothelial related diseases.
Owner:SICHUAN UNIV
Preparation method of adeno-associated virus with epigenetic modification function and application thereof
InactiveCN110863012AImprove efficiencySignificant epigenetic modificationNervous disorderPeptide/protein ingredientsHeterologousAdenoassociated virus
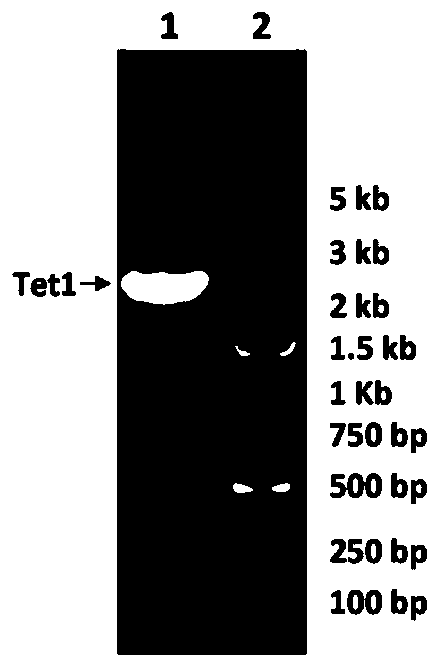
The invention relates to an application of a recombinant single-stranded DNA virus vector with a heterologous nucleic acid sequence in preparation of a medicine for treating stress cognitive impairment, which comprises a pharmaceutical composition comprising the virus vector, a method for preparing the virus vector, and an application of the virus vector in research on an epigenetic regulation mechanism and treatment on cognitive disorder caused by stress. Wherein the recombinant single-stranded DNA virus vector is an adeno-associated virus vector, and the heterologous nucleic acid encodes therapeutic protein; wherein the therapeutic protein is TET1 (1418 to 2136aa). The adeno-associated virus with the epigenetic modification function is artificially prepared, expression of neurotrophic factors such as BDNF can be induced through epigenetic modification after the adeno-associated virus infects an organism, neuron survival and neurogenesis are maintained, dendritic spine maturation is promoted, and therefore the effect of resisting and treating stress cognitive injuries is achieved.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Methods for improved therapeutic use of recombinant aav
PendingUS20220347298A1Peptide/protein ingredientsBoron compound active ingredientsAdenoassociated virusHost immunity
Provided herein are methods for managing host immune responses to improve therapeutic outcomes in adeno-associated virus (AAV)-mediated gene therapy. Such methods may include administering a recombinant adeno-associated vims (rAAV) to a subject following administration of a CD 19 inhibitor, e.g., an anti-CD 19 antibody. The methods described herein can facilitate improved transgene expression, help overcome pre-existing NAbs, and / or permit redosing with the same or substantially similar rAAV or transgene.
Owner:ULTRAGENYX PHARMA
Liver-specific viral promoters and methods of using the same
The present invention relates to promoters that function specifically or preferentially in the liver. These promoters are capable of enhancing liver-specific expression of genes.The invention also relates to expression constructs, vectors and cells comprising such liver- specific promoters, and to methods of their use.The present invention future relates to adeno-associated virus (AAV) gene therapy vectors comprising the liver-specific promoters, therapeutic agents comprising the liver-specific promoters, and methods using the same.
Owner:优尼科IP有限公司
Optogenetic visual restoration using chrimson
InactiveUS20190269755A1Improve expression levelIncreases cellular traffickingSenses disorderNervous disorderRetinal ganglionVisual acuity
Disclosed are, among other methods, methods for reactivating retinal ganglion cells in mammals by administering an effective amount of channelrhodopsins (such as ChrimsonR), or an effective amount of such channelrhodopsins (such as ChrimsonR) fused to a fluorescent protein, in the form of protein or nucleic acids, and compositions thereof. The methods may include a light stimuli level inducing RGCs response that is below radiation safety limit. The methods may include delivery by an adenoassociated virus vector. The methods may include use of a CAG promoter. The methods may result in a long term expression of an effective amount of the channelrhodopsins (such as ChrimsonR protein).
Owner:GENSIGHT BIOLOGICS SA +2
Adenoassociated virus vectors for the treatment of mucopolysaccharidoses type iv a
The present invention provides new polynucleotide sequences, adeno-associated virus-derived vectors and pharmaceutical compositions containing the same for the treatment of lysosomal storage disorders and specially, for the treatment of mucopolysaccharidosis type IVA or Morquio A syndrome.
Owner:ESTEVE PHARMA SA +1
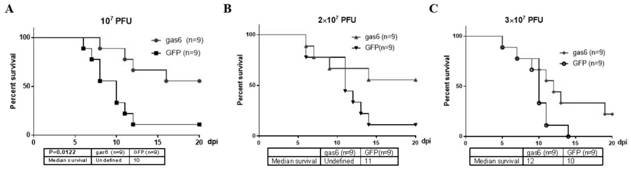
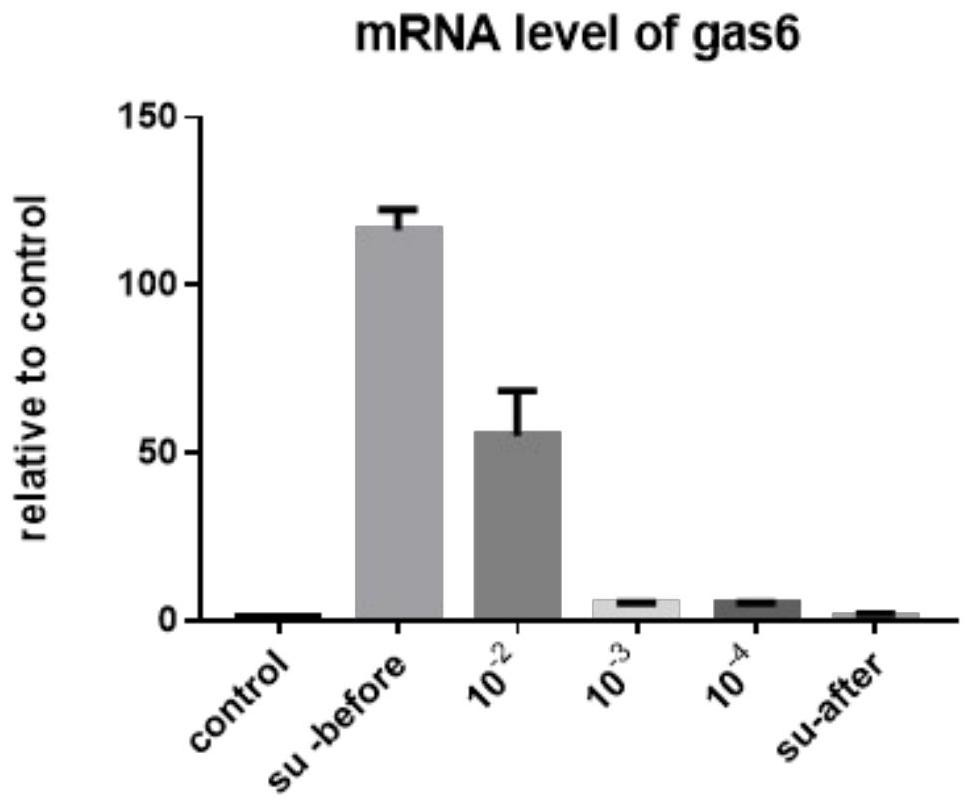
Recombinant gland-related virus AAV-gas6 and application
PendingCN112522216AInhibits inflammatory damageImprove integrityMicroorganism based processesViral/bacteriophage medical ingredientsAdenoassociated virusNucleic acid sequencing
The invention relates to the technical field of recombinant gland-related viruses and particularly relates to a recombinant gland-related virus AAV-gas6, a preparation method therefor and applicationof the recombinant gland-related virus AAV-gas6. The invention provides the recombinant gland-related virus AAV-gas6 containing a nucleic acid sequence represented by SEQ ID NO: 1. The invention further provides the preparation method for the recombinant gland-related virus AAV-gas6. The recombinant gland-related virus AAV-gas6 provided by the invention is advantageously applied to preparation ofdrugs for treating or relieving JEV-induced viral encephalitis.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Adenoassociated viral mediated persistant anti-VEGF therapy for ovarian cancer
ActiveUS10946094B2Organic active ingredientsGenetic material ingredientsAdenoassociated virusAntigen Binding Fragment
Owner:CORNELL UNIVERSITY
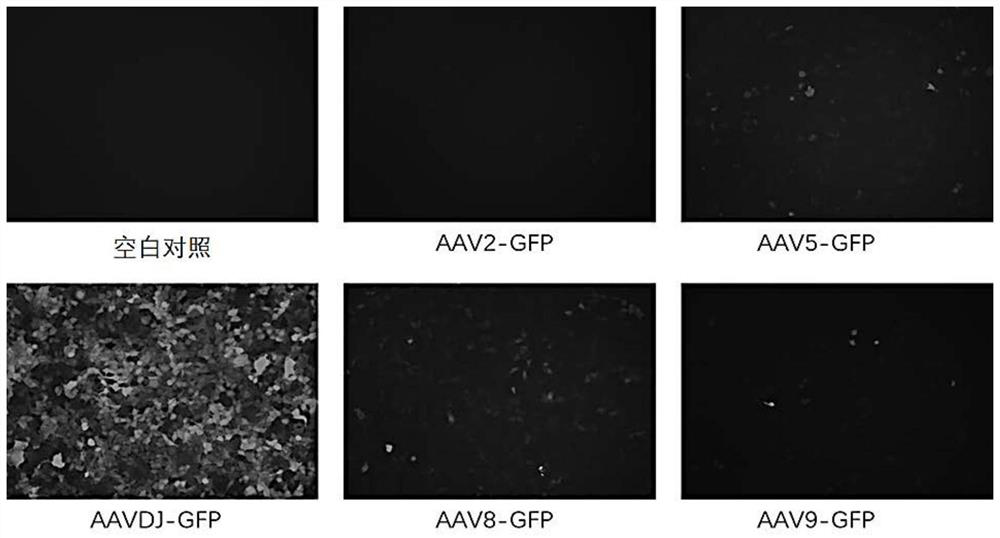
Method for improving infection efficiency of adeno-associated virus to infect cells
PendingCN112980884ANucleic acid vectorPharmaceutical non-active ingredientsAdenoassociated virusPharmaceutical drug
The invention relates to a method for improving infection efficiency of AAV (adeno-associated virus) to infect cells. The method comprises the following steps: making cells to-be-infected contacted with DNA replication inhibitors, wherein the DNA replication inhibitors comprise cell cycle non-specific drugs and / or CDK inhibitors. According to the method disclosed by the invention, the infection efficiency of the AAV to infect cells can be remarkably improved.
Owner:CHIGENOVO CO LTD
shRNA for hepatitis b virus and recombinant adeno-associated virus vector treating vector carrying same
ActiveCN101906417BInhibition of replicationInhibit expressionDNA/RNA fragmentationAdenoassociated virusCytotoxicity
The invention belongs to the field of biomedicine, and relates to a recombinant adeno-associated virus carrying shRNA (shorthairpin RNA, shRNA) with the effect of inhibiting hepatitis B virus, a preparation method thereof and application thereof in preparing medicaments for treating and / or preventing viral diseases. By adopting gene recombination technology, the shRNA with the effect of inhibiting the hepatitis B virus is cloned into skeleton plasmid of an adeno-associated virus vector, and the skeleton plasmid, together with helper plasmid, performs cotransfection on packaging cells to obtain the recombinant adeno-associated virus. The recombinant adeno-associated virus can effectively inhibit the copy and expression of the hepatitis B virus, and has obvious in-vivo inhibition effect, long lasting time and smaller cytotoxicity.
Owner:BEIJING TRI PRIME GENE PHARMA CO LTD
Antibodies capable of binding to aav1-13
ActiveCN111925438BEasy to separateEasy to identifyBiological material analysisSolid sorbent liquid separationAdenoassociated virusEngineering
The invention relates to the field of immunology, in particular to an antibody capable of binding to AV1-13. The antibody can recognize VP1, VP2 and VP3 proteins of all serotypes of AV1-13, is high inaffinity and good in specificity; and the antibody can be more conveniently used for separation and identification of adeno-associated viruses due to the fact that the VP proteins are located on thesurfaces of the adeno-associated viruses.
Owner:OBIO TECH SHANGHAI CORP LTD
Optogenetic visual restoration using chrimson
ActiveCN110267673APrevent retinal degenerationAvoid sex changeSenses disorderNervous disorderAdenoassociated virusRetinal ganglion
Disclosed are, among other methods, methods for reactivating retinal ganglion cells in mammals by administering an effective amount of channelrhodopsins (such as ChrimsonR), or an effecti ve amount of such channelrhodopsins (such as ChrimsonR) fused to a fluorescent protein, in the form of protein or nucleic acids, and compositions thereof. The methods may include a light stimuli level inducing RGCs response that is below radiation safety limit. The methods may include delivery by an adenoassociated virus vector. The methods may include use of a CAG promoter. The methods may result in a long term expression of an effective amount of the channelrhodopsins (such as ChrimsonR protein).
Owner:GENSIGHT BIOLOGICS SA +3
Method for treating ischemic tissue
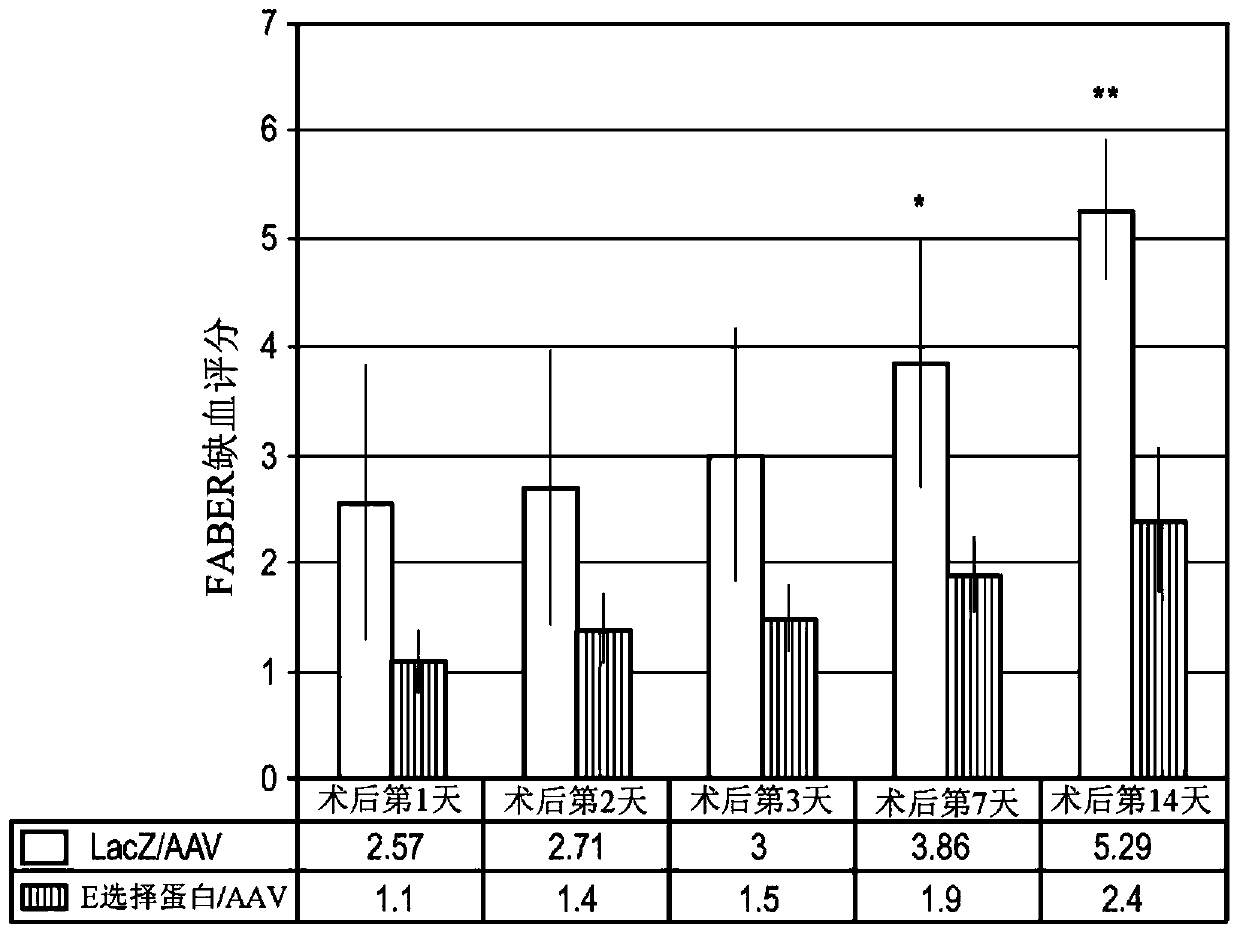
The invention provides a method of increasing blood flow or perfusion in an ischemic tissue. The method comprises the steps: inducing angiogenesis, neovascularization or revascularization; increasingskeletal muscle viability; promoting ischemic skin wound healing; treating or preventing gangrene; and / or treating CLI. In various aspects, the method comprises administering to a subject a hybrid adenoassociated virus (AAV) comprising a nucleotide sequence encoding an E-selectin, AAV serotype 2 (AAV2) inverted terminal repeats (ITRs), and a capsid from an AAV other than serotype 2. In various aspects, the method comprises the step of administering to the subject a cell comprising an AAV comprising a nucleotide sequence encoding an E-selectin, AAV2 ITRs, and an AAV2 capsid.
Owner:UNIV OF MIAMI
Adenoassociated virus vectors for the treatment of mucopolysaccharidoses
Owner:ESTEVE PHARMA SA +1
Recombinant adeno-associated virus vector, gene composition and application for treating atherosclerosis
ActiveCN113265424BClear specificityIdentify security issuesCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsAdenoassociated virusA lipoprotein
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Adenoassociated virus vectors for the treatment of mucopolysaccharidoses
The present invention provides new adeno-associated virus-derived vectors and pharmaceutical compositions containing the same for the treatment of lysosomal storage disorders and specially, for the treatment of mucopolysaccharidoses Type IIID.
Owner:ESTEVE PHARMA SA +1
Application of poloxamer P338 in improving virus infection efficiency of cells and method thereof
PendingCN109880852AHigh infection efficiencyInfection effect is goodGenetic engineeringFermentationLentivirusViral vector
The invention discloses a method for utilizing poloxamer P338 to improve the virus infection efficiency of cells and application thereof. The use of the poloxamer P338 can increase the infection efficiency of lentivirus at low MOI to 293T cells, LLC-MK2 cells and the like. The use of the poloxamer P338 can also promote the infection of adeno-associated virus at the same MOI value to the 293T cellsto enhance the expression of foreign genes carried by viral vectors.
Owner:OBIO TECH SHANGHAI CORP LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com