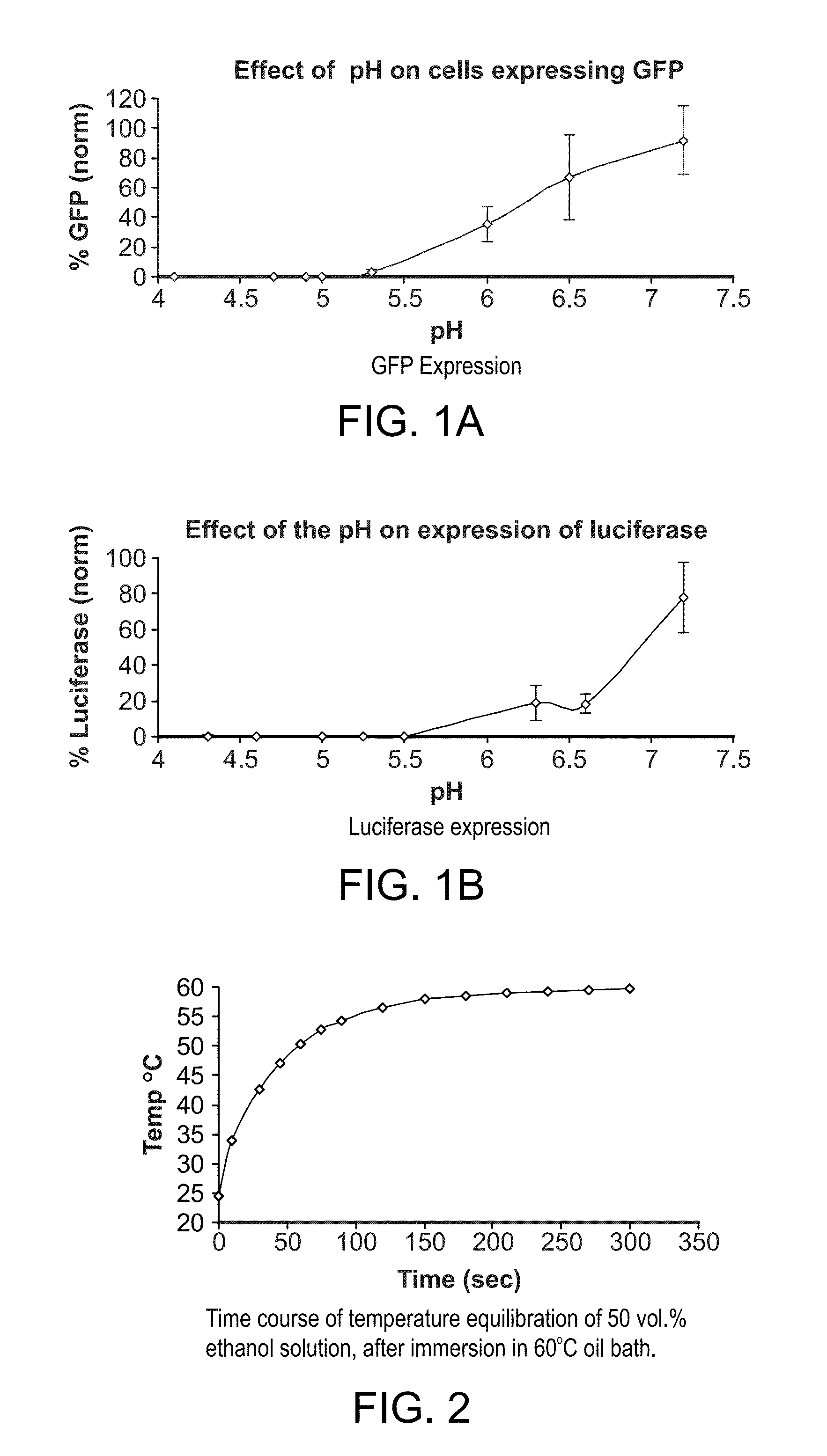
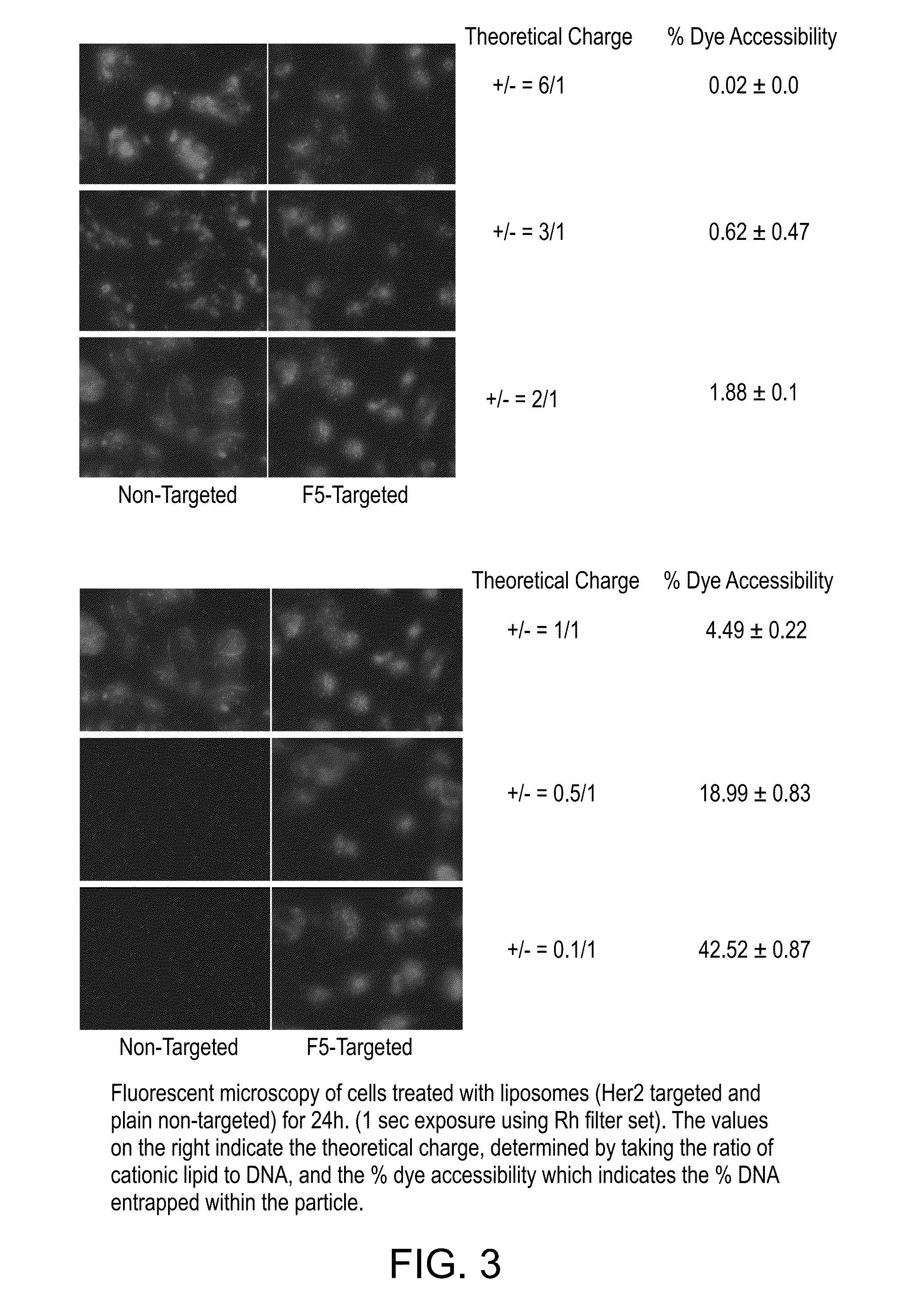
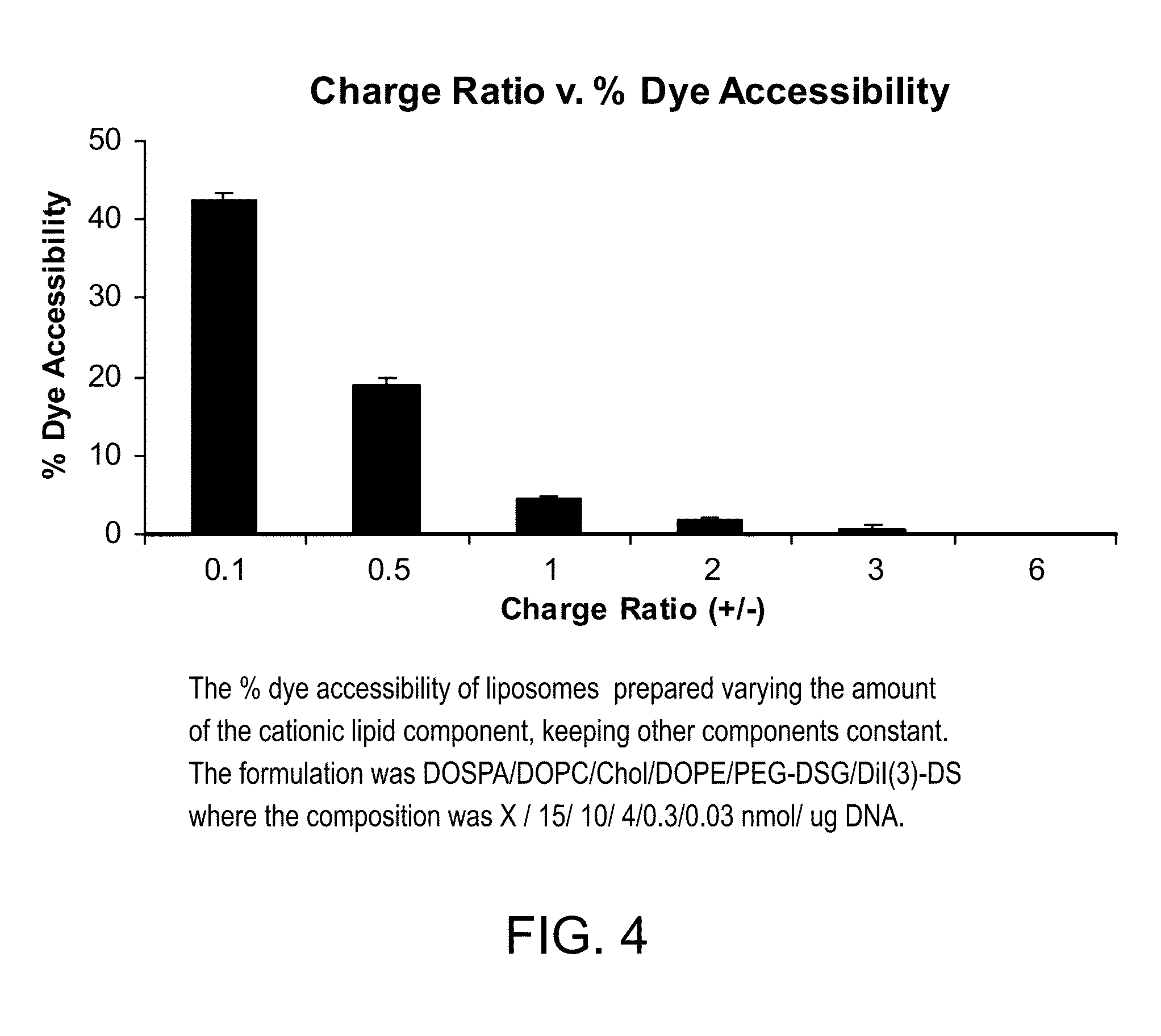
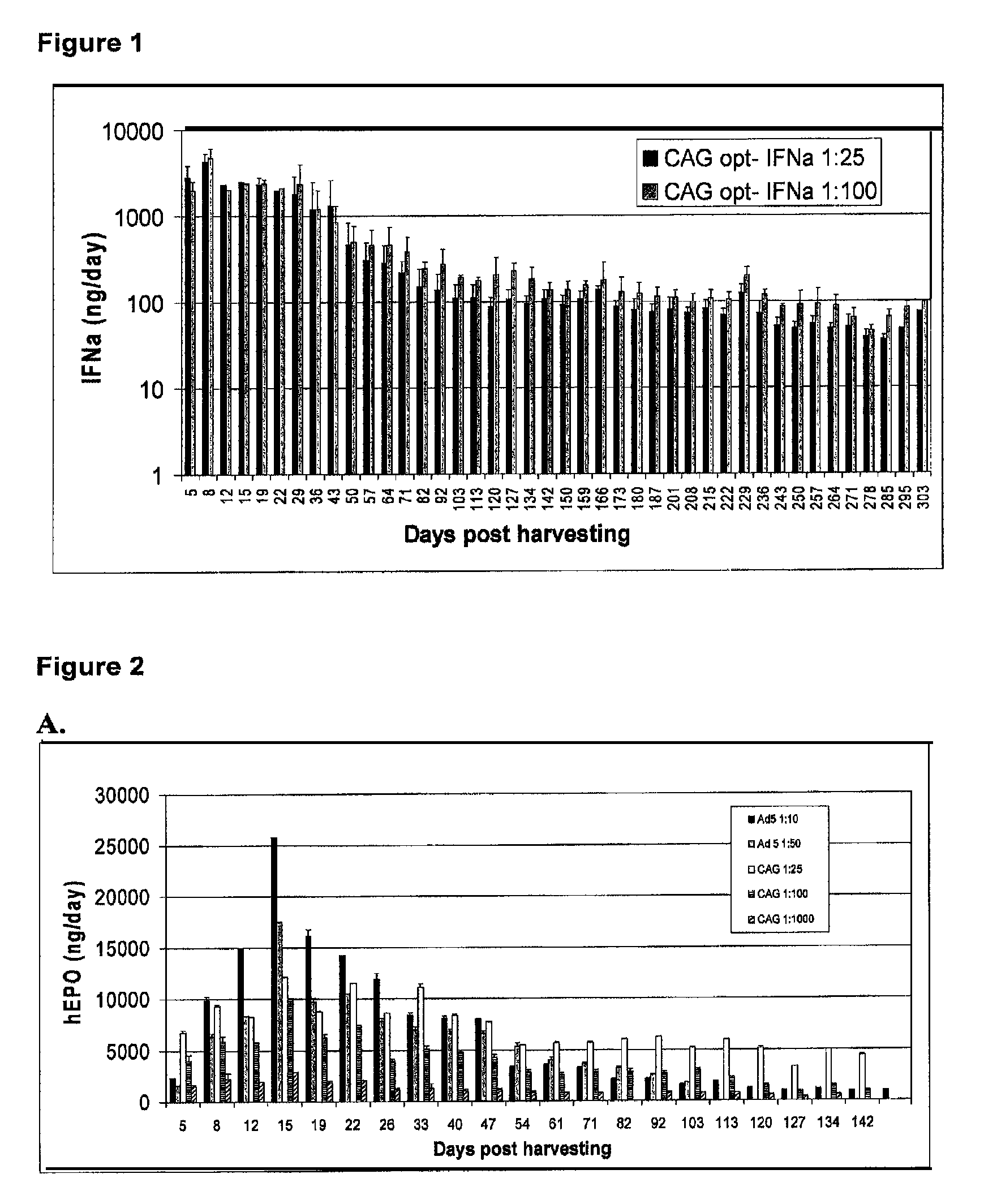
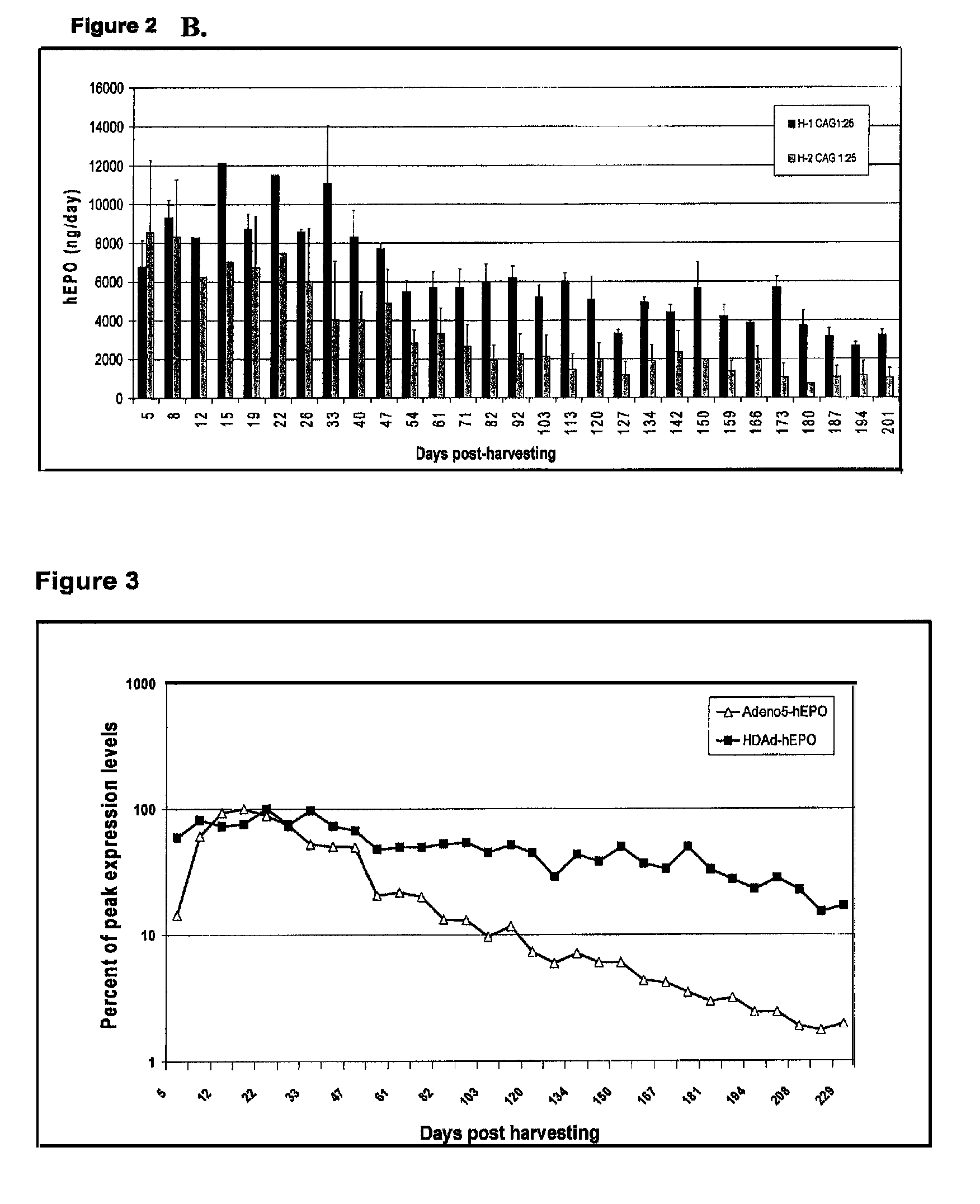
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
276results about "Genetic therapy composition manufacture" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
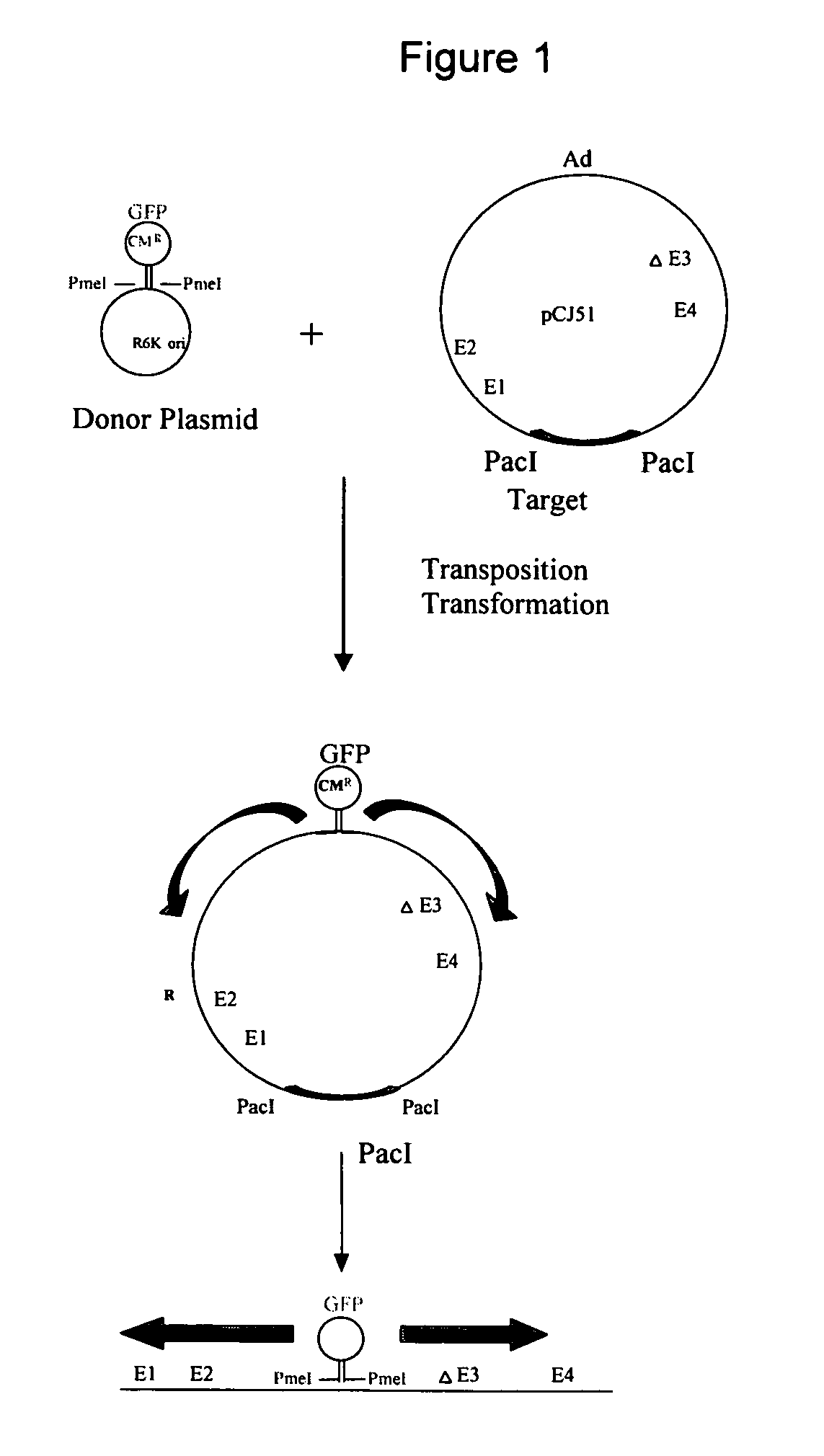
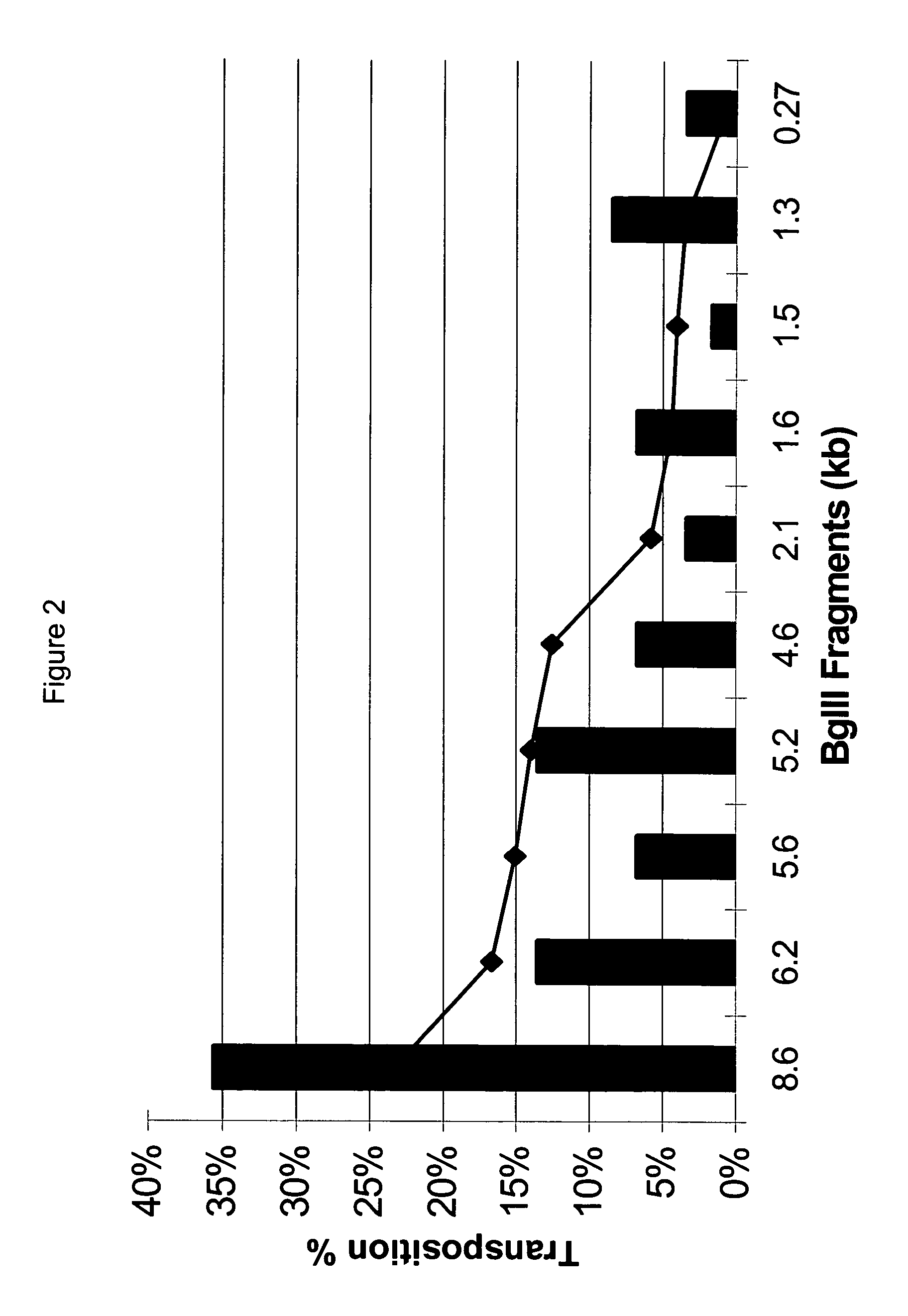
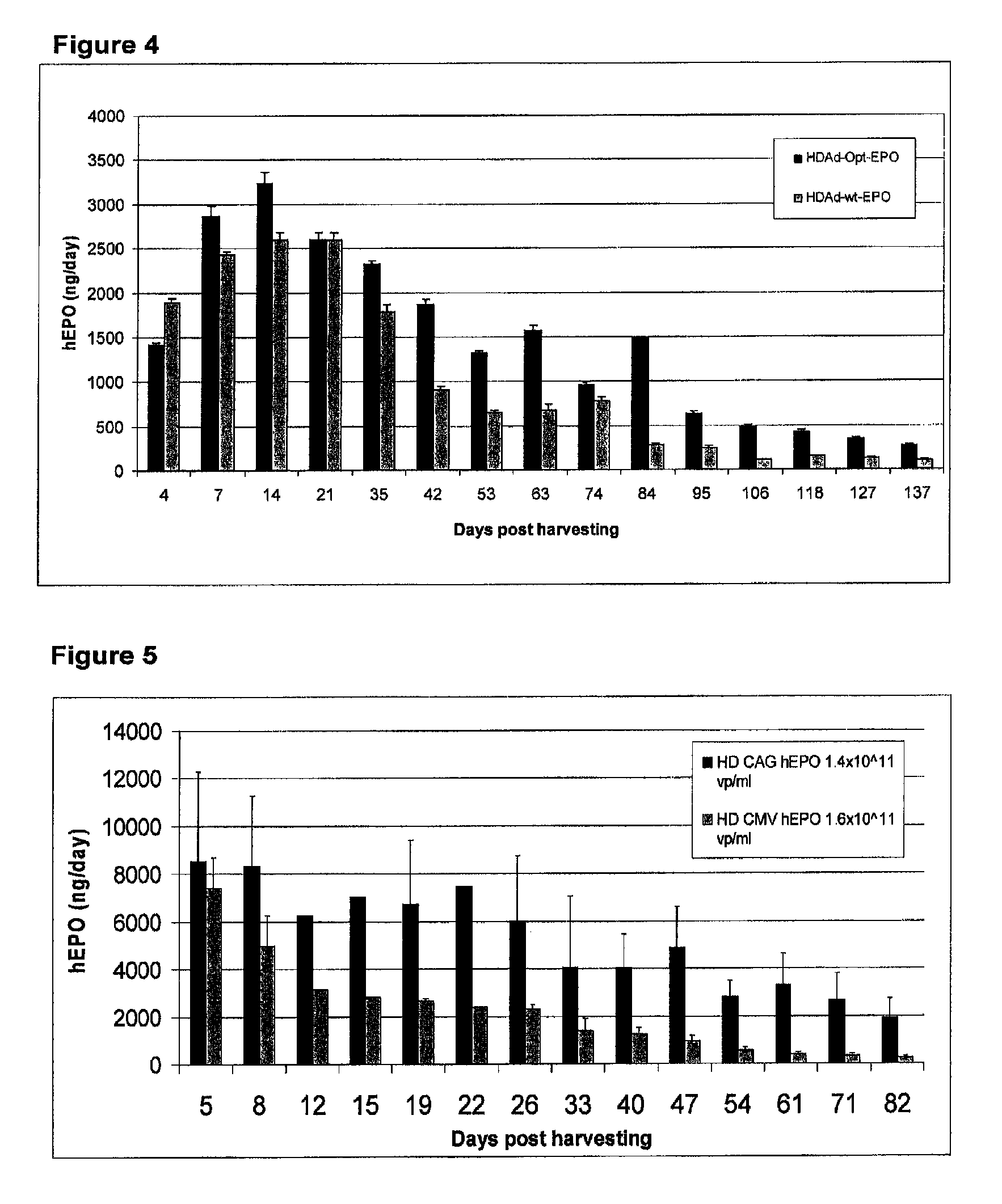
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
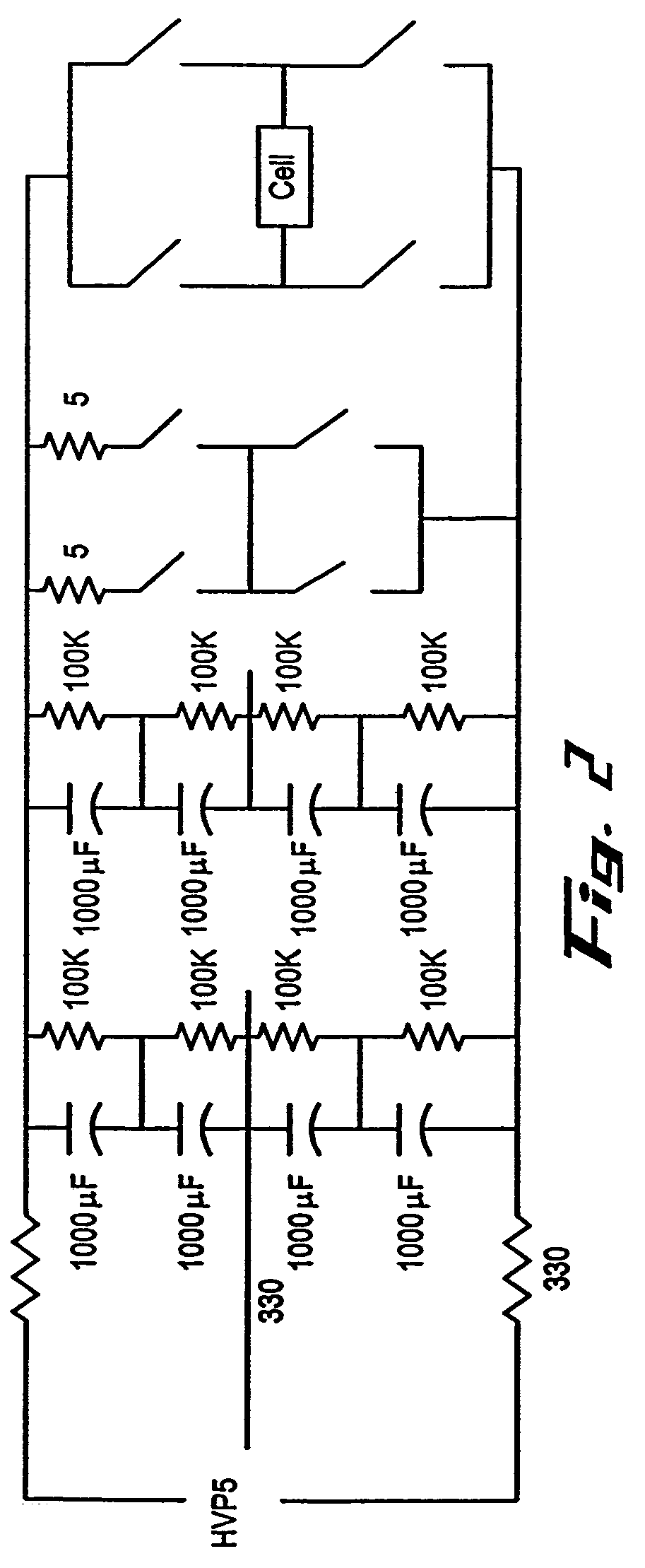
InactiveUS6989264B2Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
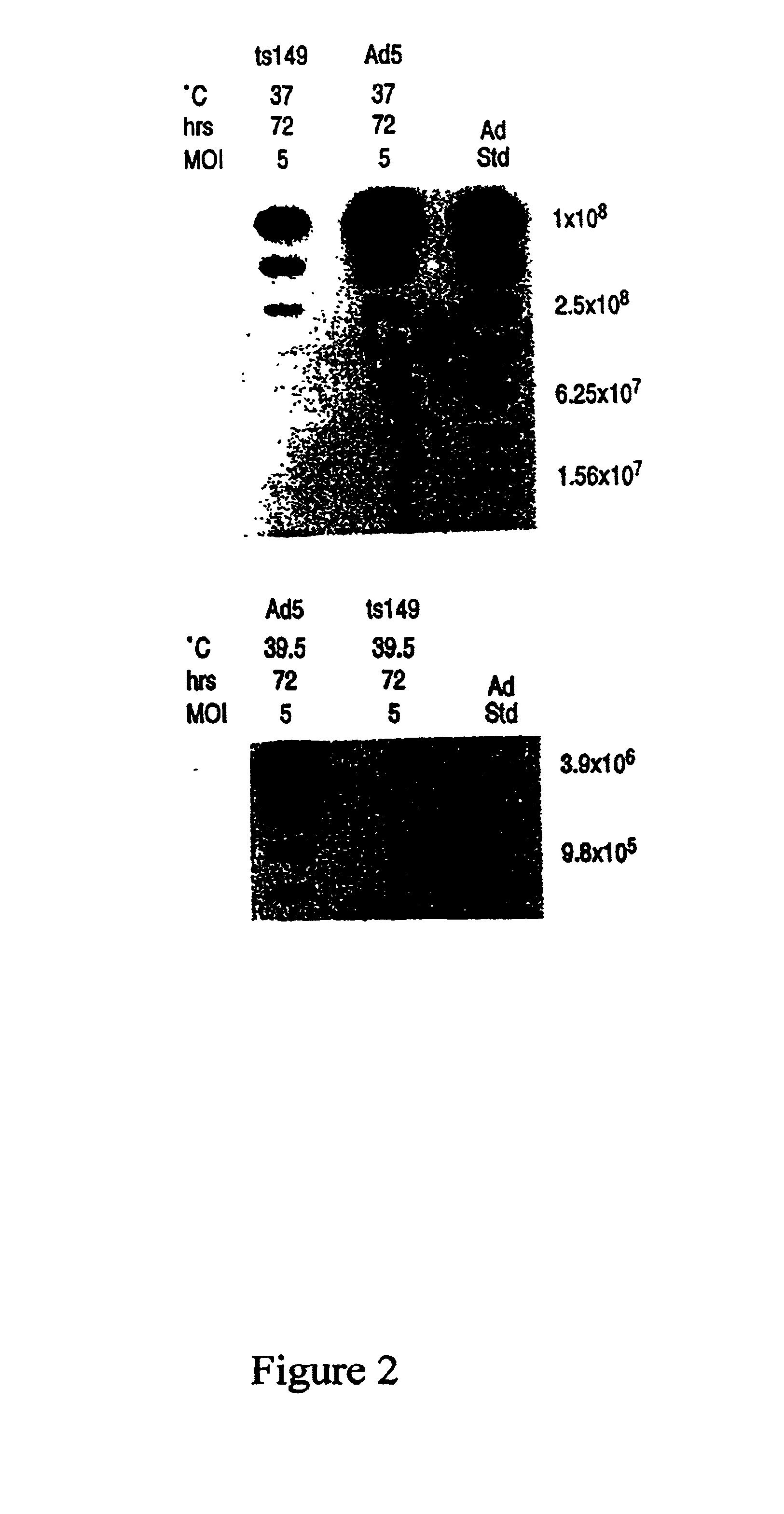
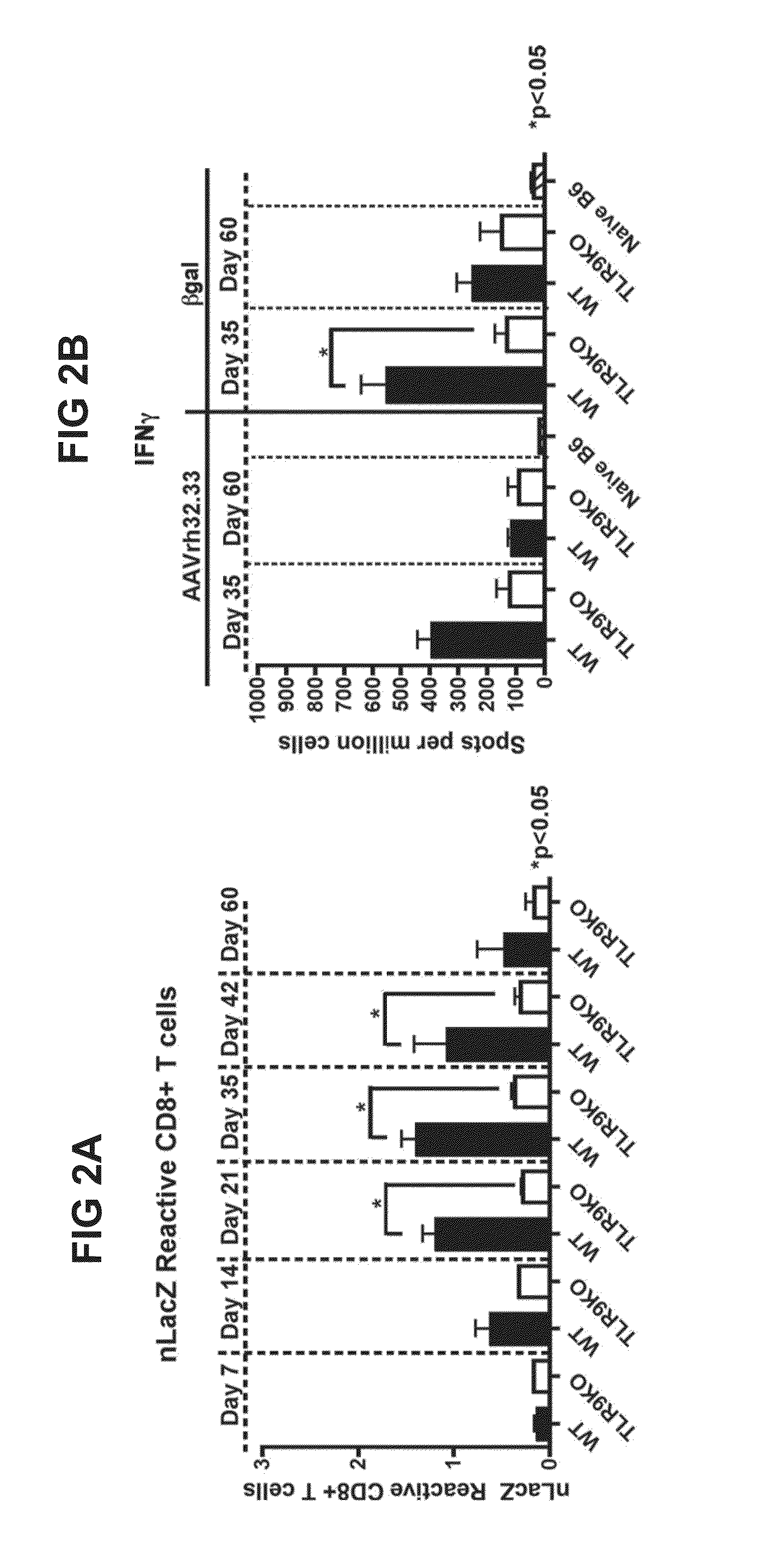
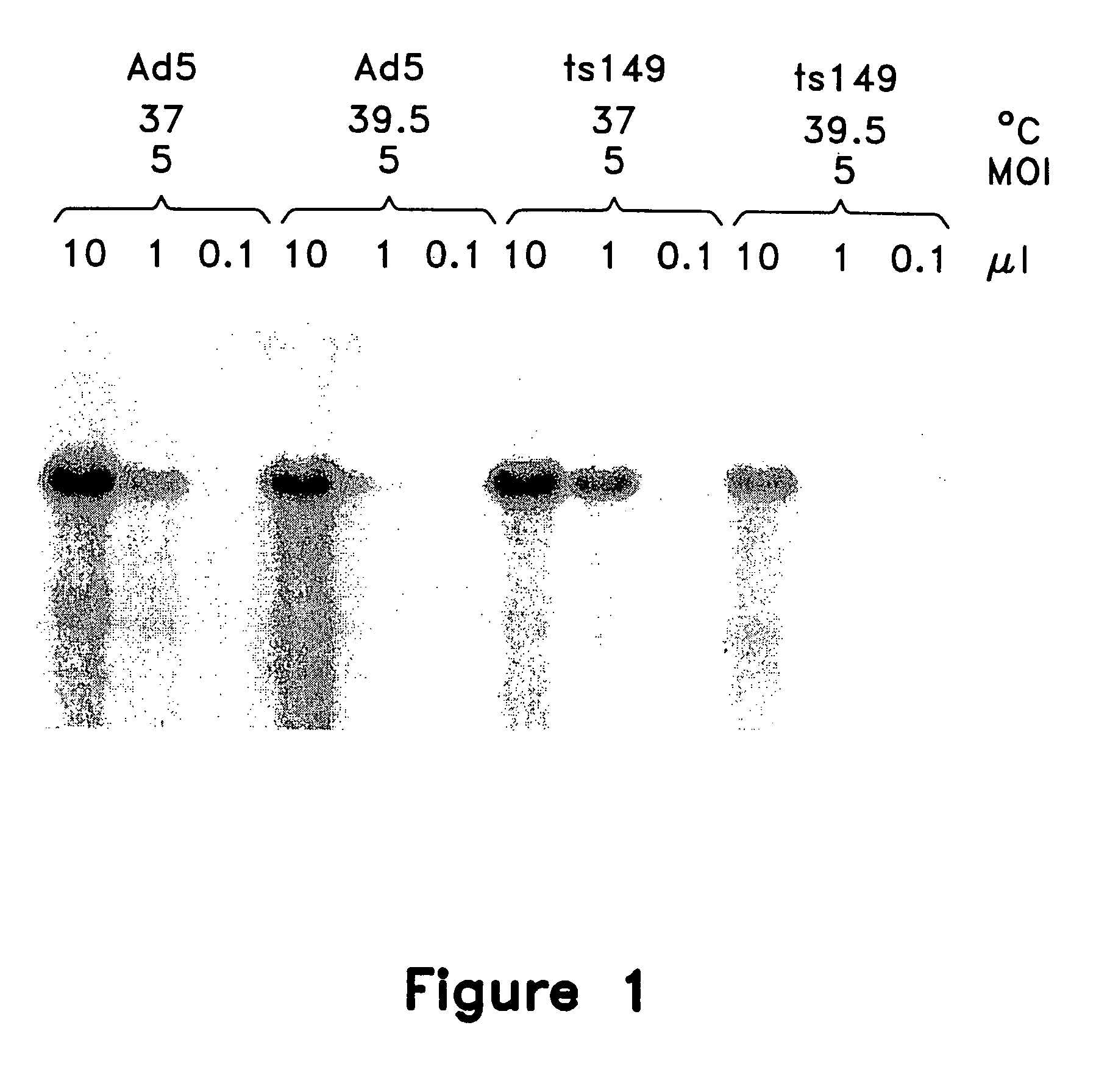
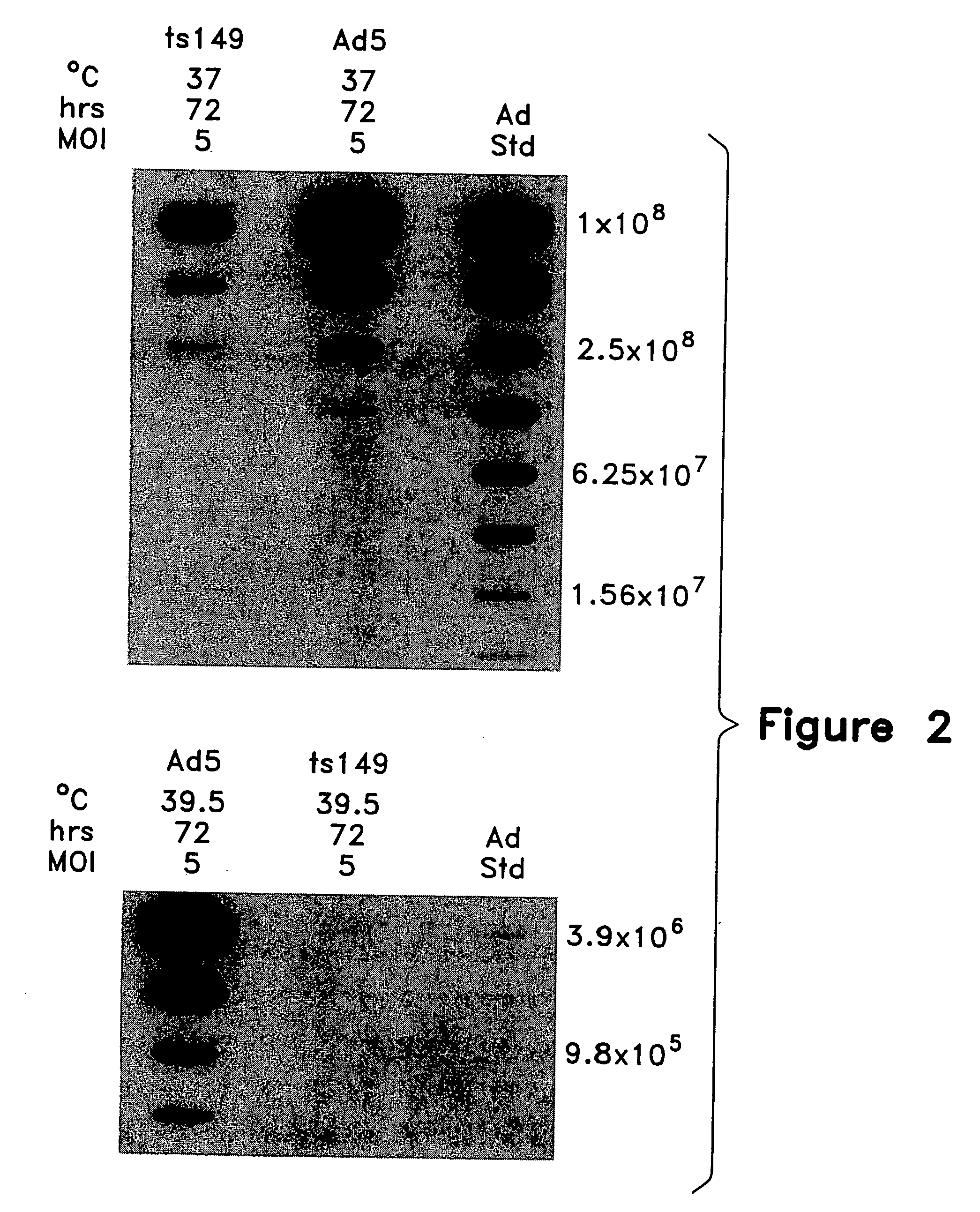
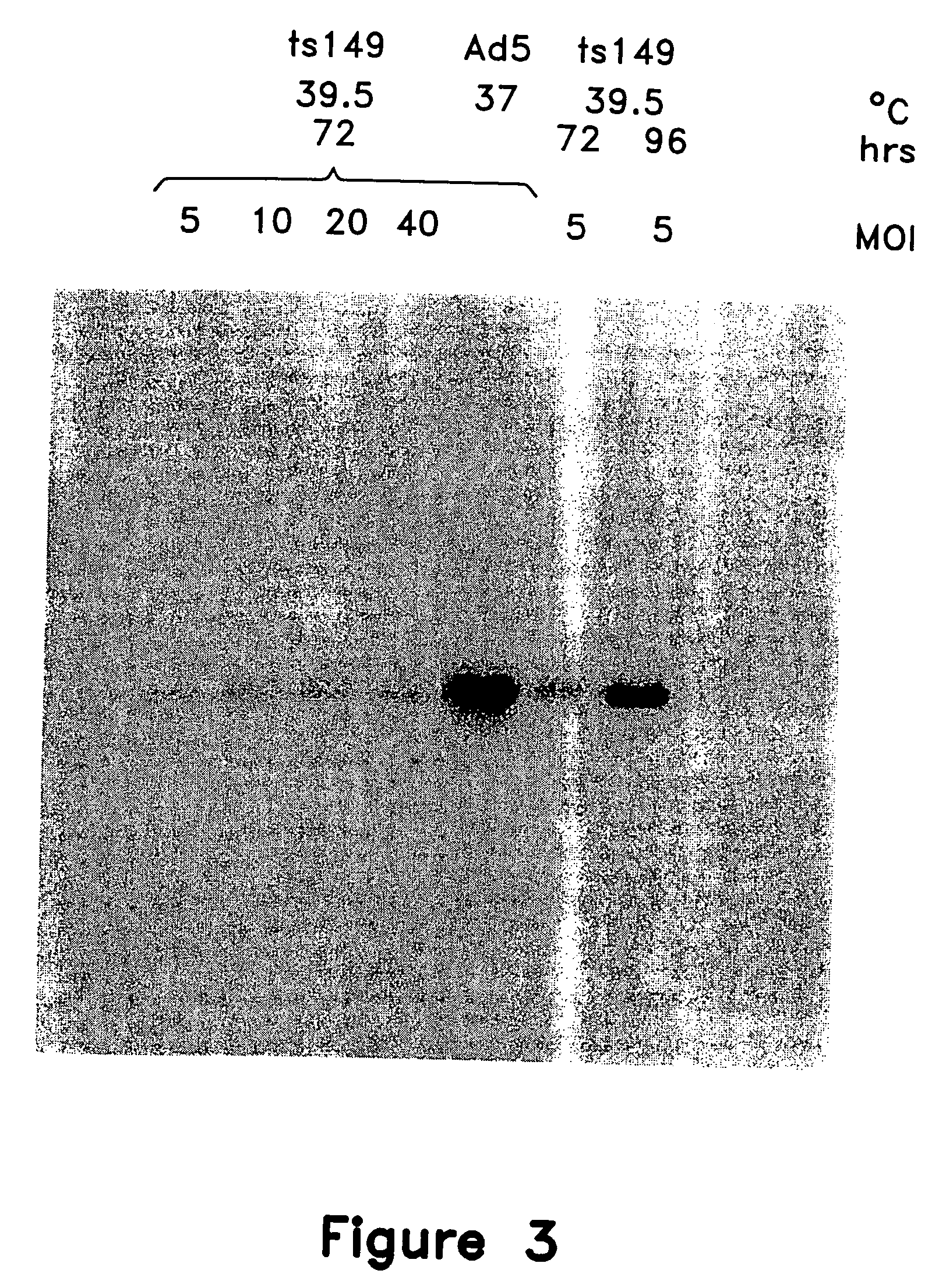
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:TARGETED GENETICS CORPORTION
Compositions and methods for helper-free production of recombinant adeno-associated viruses
InactiveUS6953690B1Efficient productionIncrease the number ofBiocideGenetic therapy composition manufactureMammalWild type
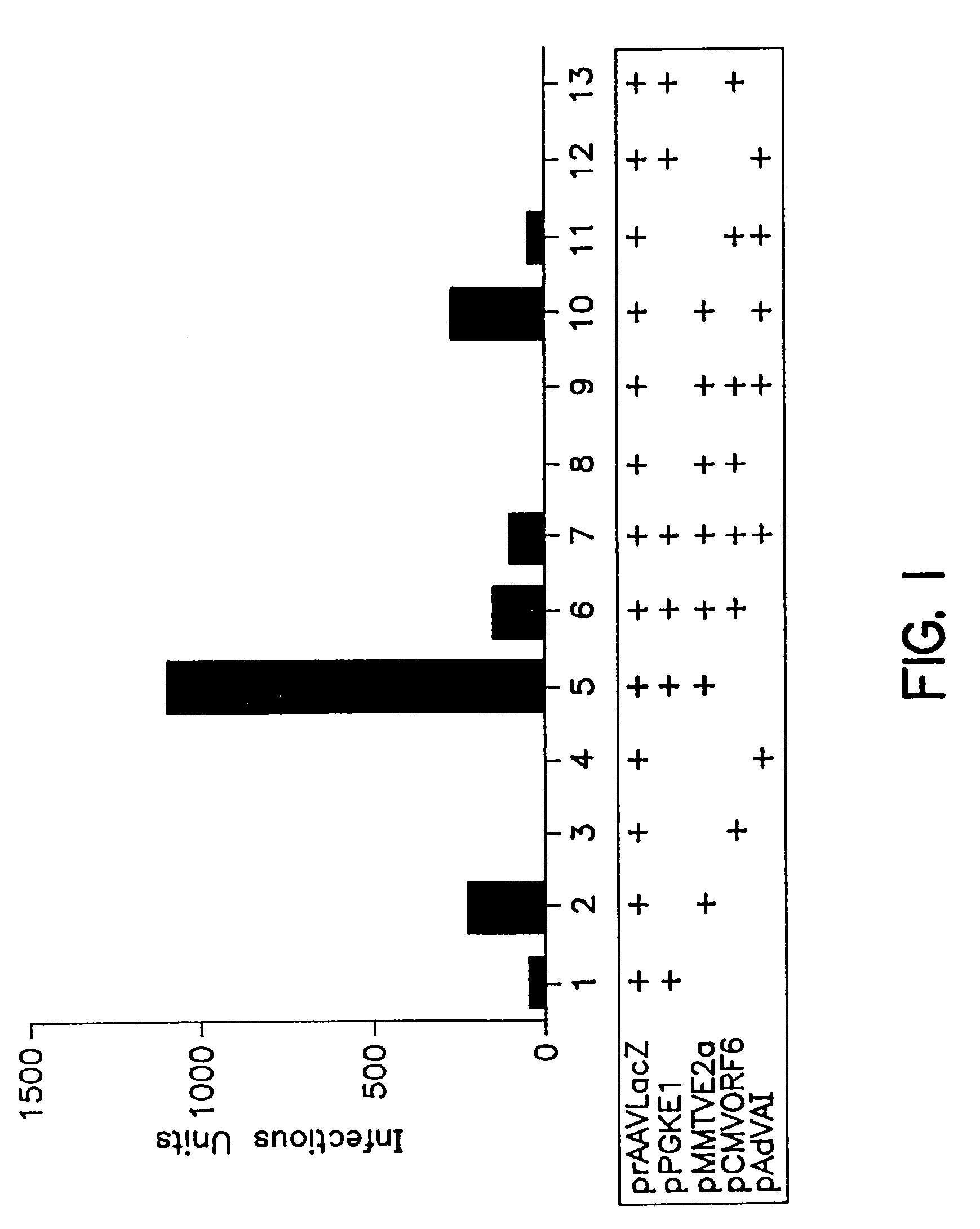
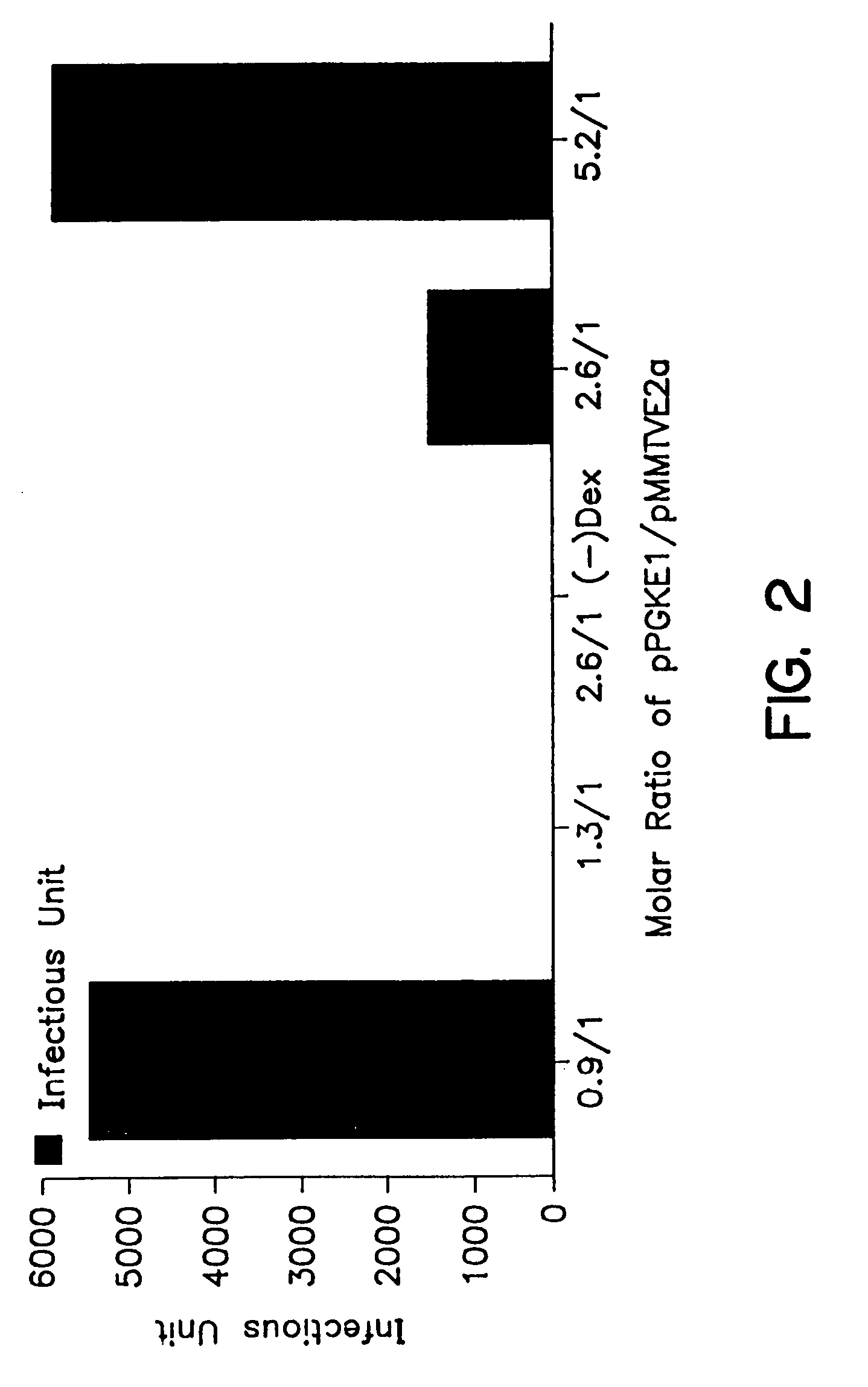
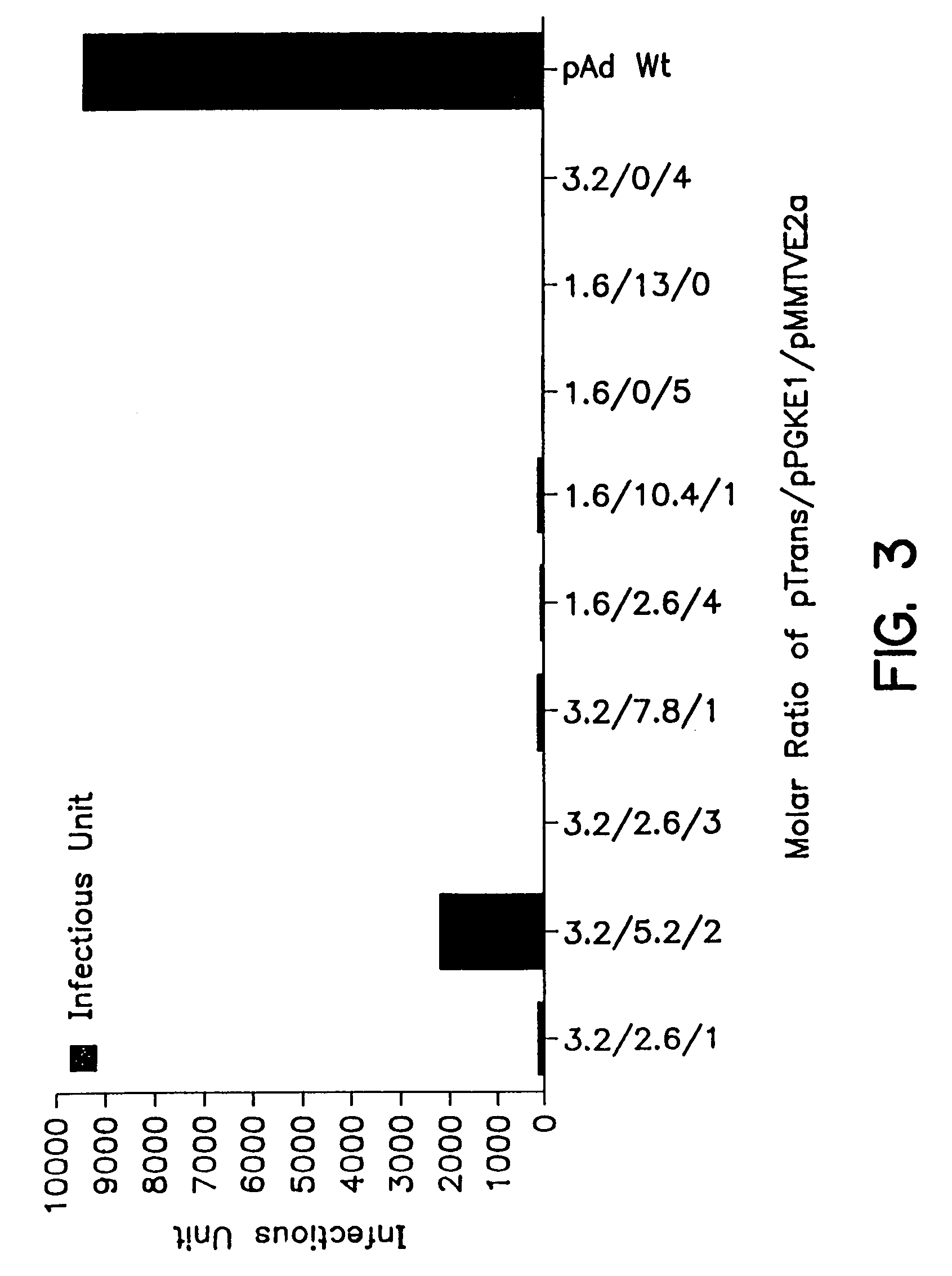
A method for producing recombinant adeno-associated virus in the absence of contaminating helper virus or wild-type virus involves culturing a mammalian host cell containing a transgene flanked by adeno-associated virus (AAV) inverse terminal repeats and under the control of regulatory sequences directing expression thereof, an AAV rep sequence and an AAV cap sequence under the control of regulatory sequences directing expression thereof, and the minimum adenovirus DNA required to express an E1a gene product, an E1b gene product and an E2a gene product, and isolating therefrom a recombinant AAV which expresses the transgene in the absence of contaminating helper virus or wildtype AAV. This method obviates a subsequent purification step to purify rAAV from contaminating virus. Also provided are various embodiments of the host cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Apparatus and method for electroporation of biological samples
InactiveUS7141425B2Simple and efficientBioreactor/fermenter combinationsElectrotherapyElectroporationBiochemistry
The present invention relates to methods and apparatus for the encapsulation of biologically-active substances in various cell populations. More particularly, the present invention relates to a method and apparatus for the encapsulation of biologically-active substances in various cell populations in blood by electroporation to achieve therapeutically desirable changes in the physical characteristics of the various cell populations in blood.
Owner:MAXCYTE
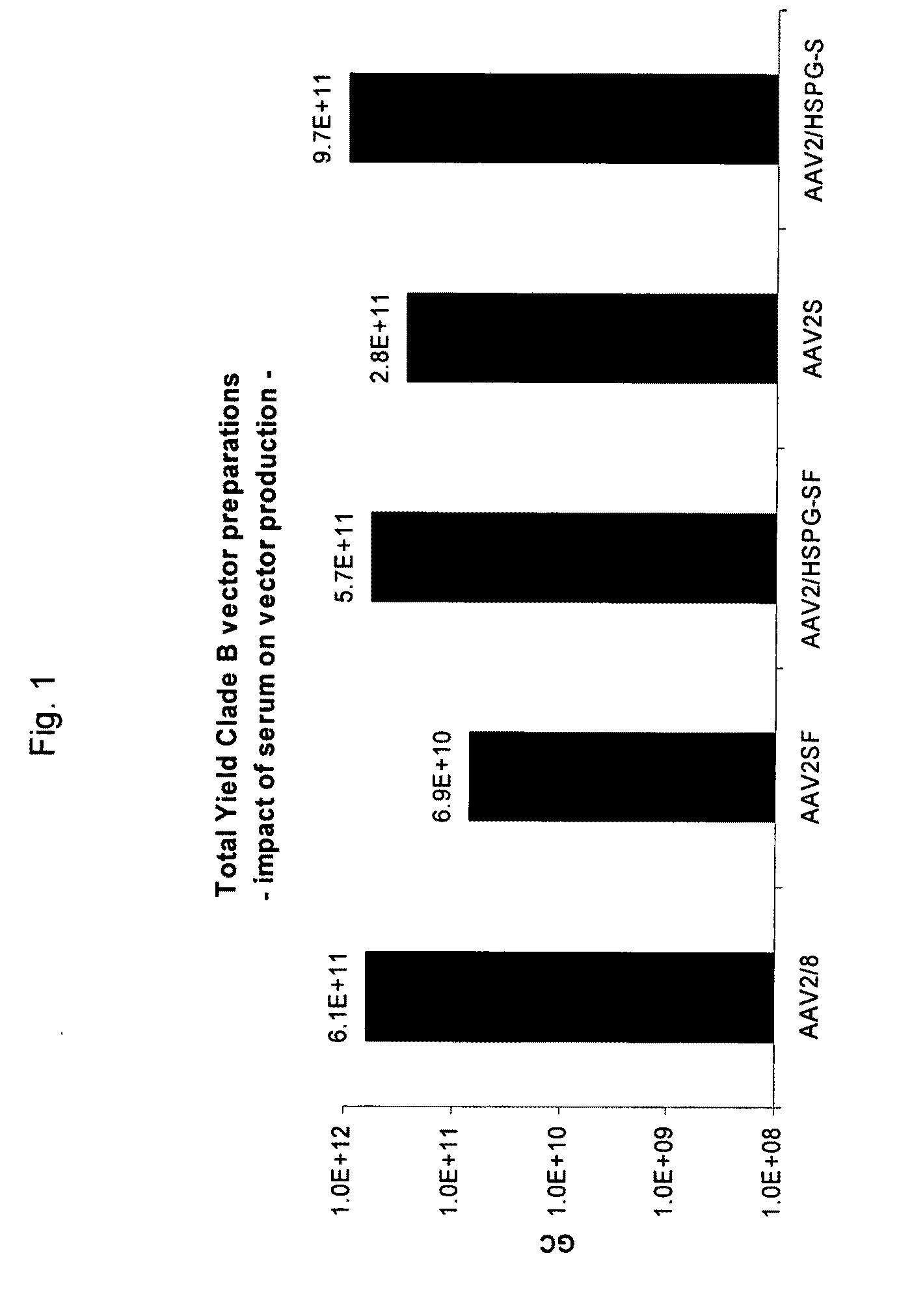
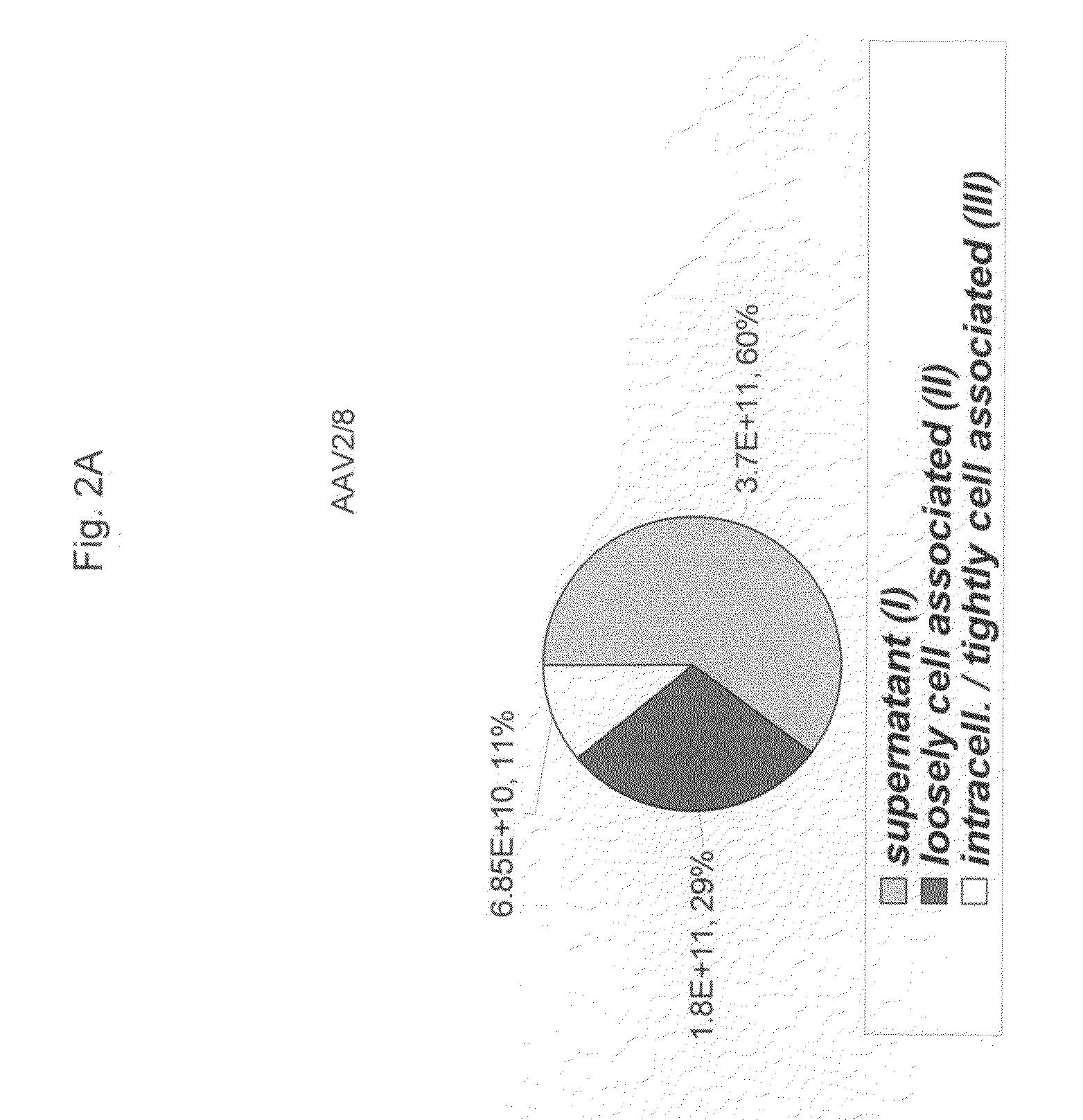
Scalable Production Method for AAV
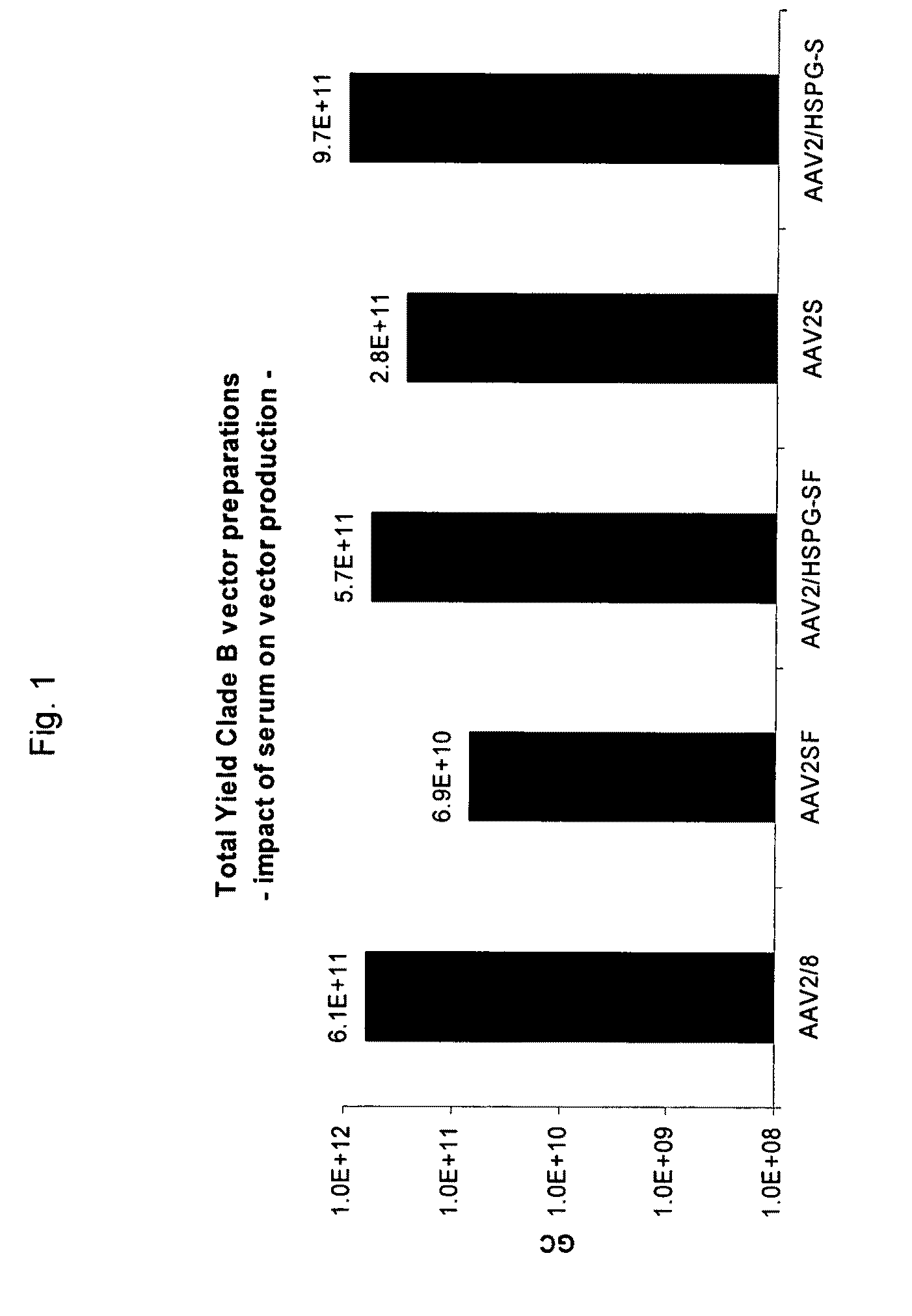
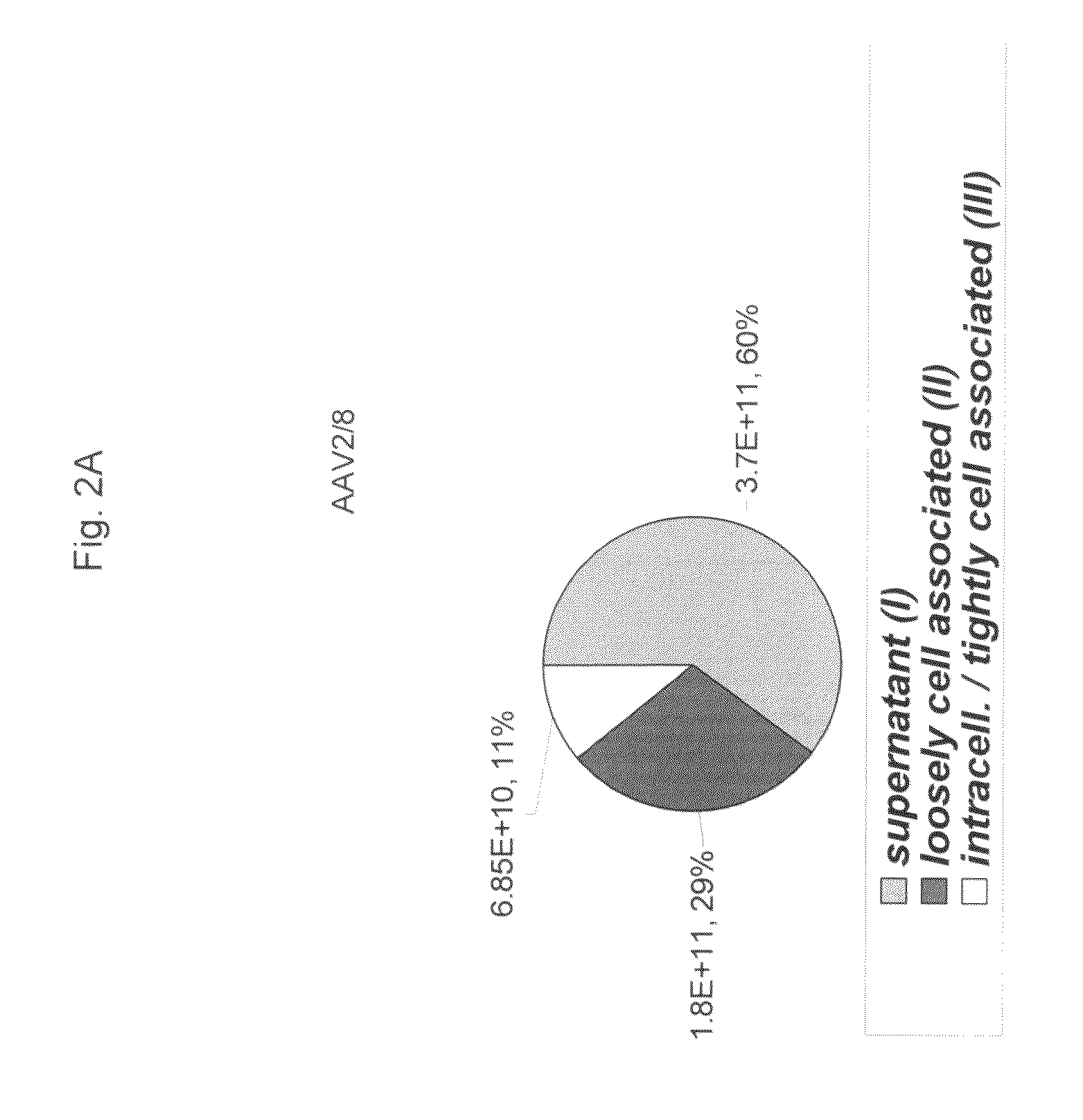
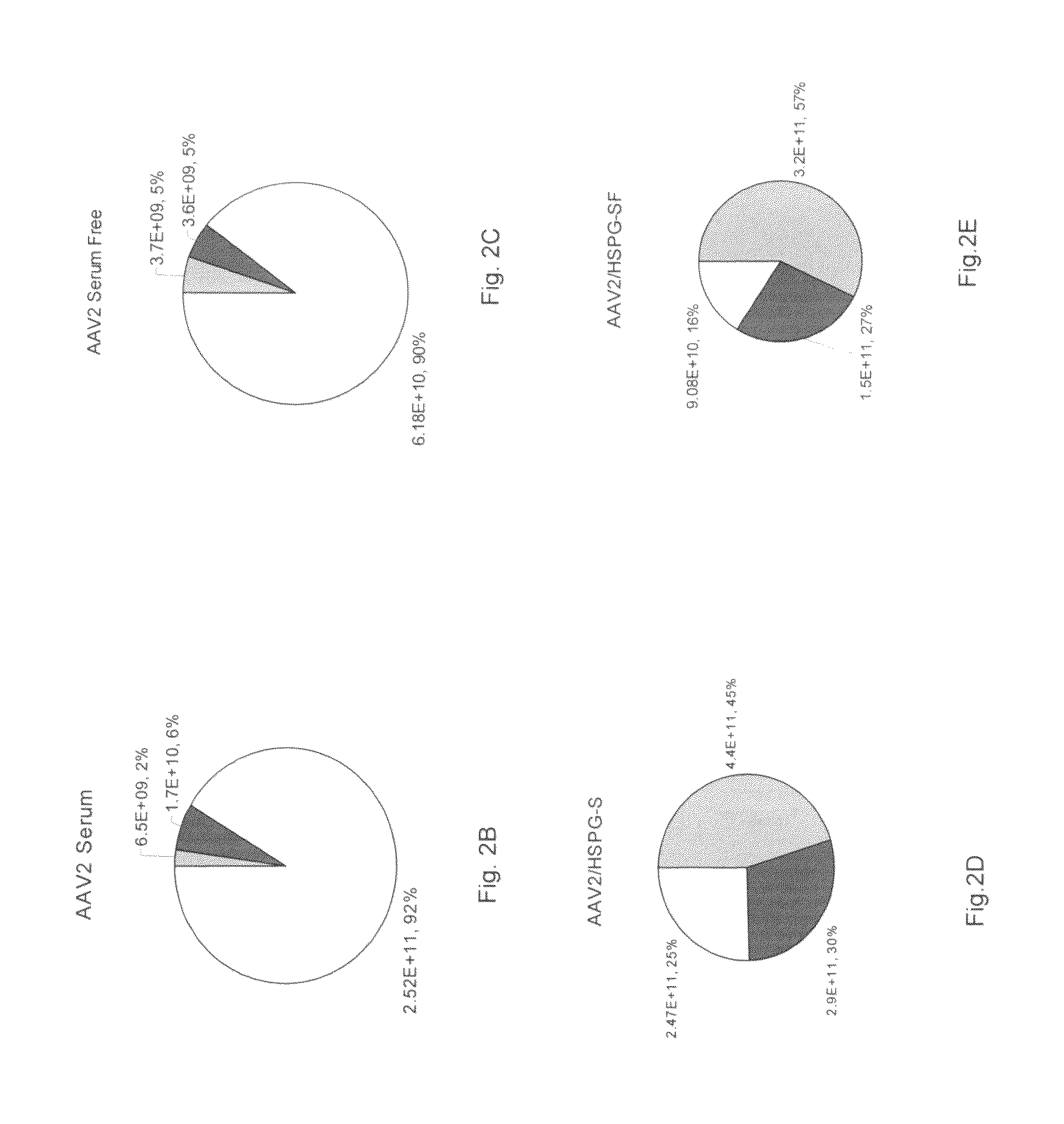
ActiveUS20090275107A1Reduce and eliminate bindingHigh yieldGenetic therapy composition manufactureVirus peptidesLysisBinding site
A method for producing AAV, without requiring cell lysis, is described. The method involves harvesting AAV from the supernatant. For AAV having capsids with a heparin binding site, the method involves modifying the AAV capsids and / or the culture conditions to ablate the binding between the AAV heparin binding site and the cells, thereby allowing the AAV to pass into the supernatant, i.e., media. Thus, the method of the invention provides supernatant containing high yields of AAV which have a higher degree of purity from cell membranes and intracellular materials, as compared to AAV produced using methods using a cell lysis step.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
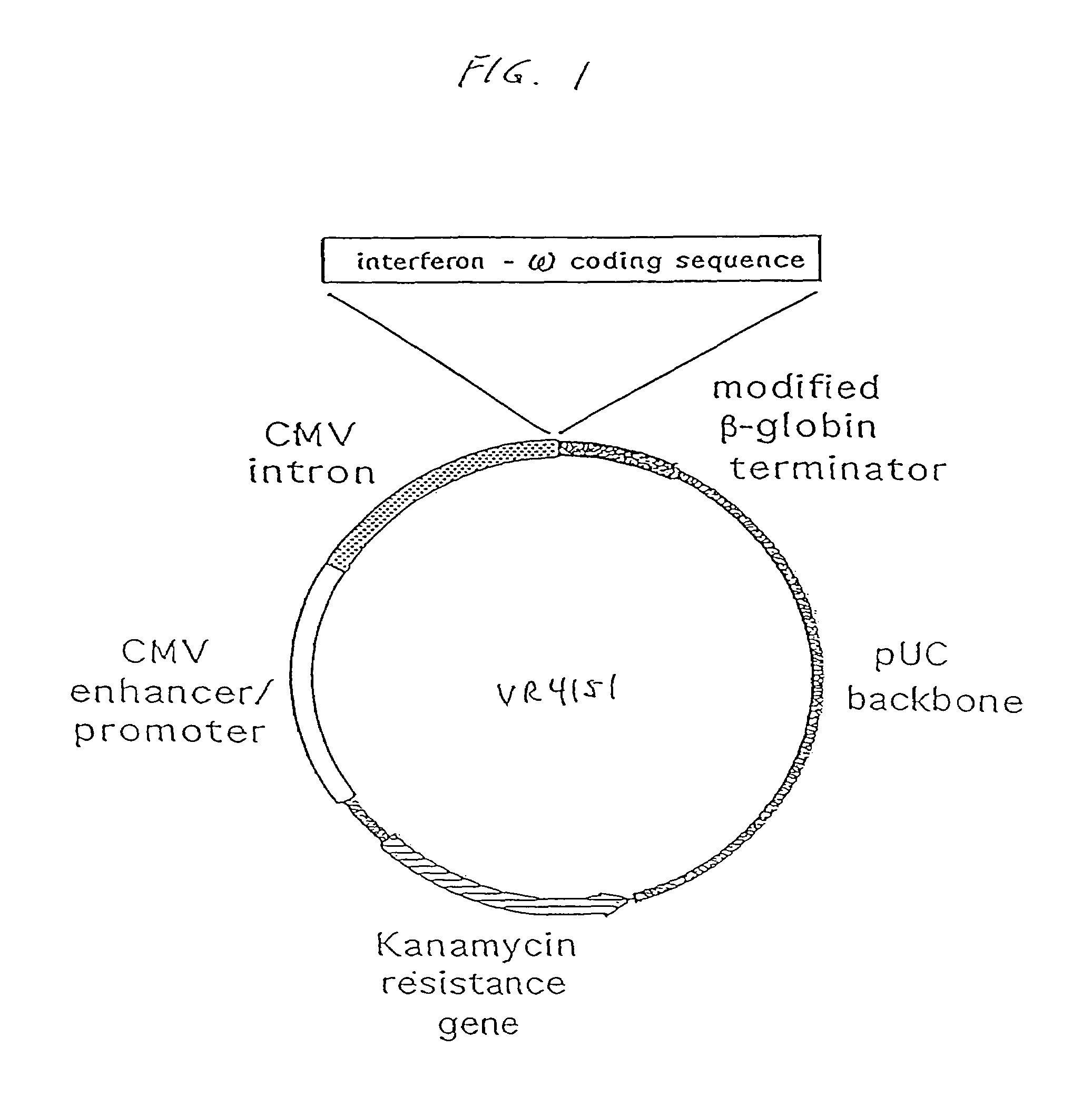
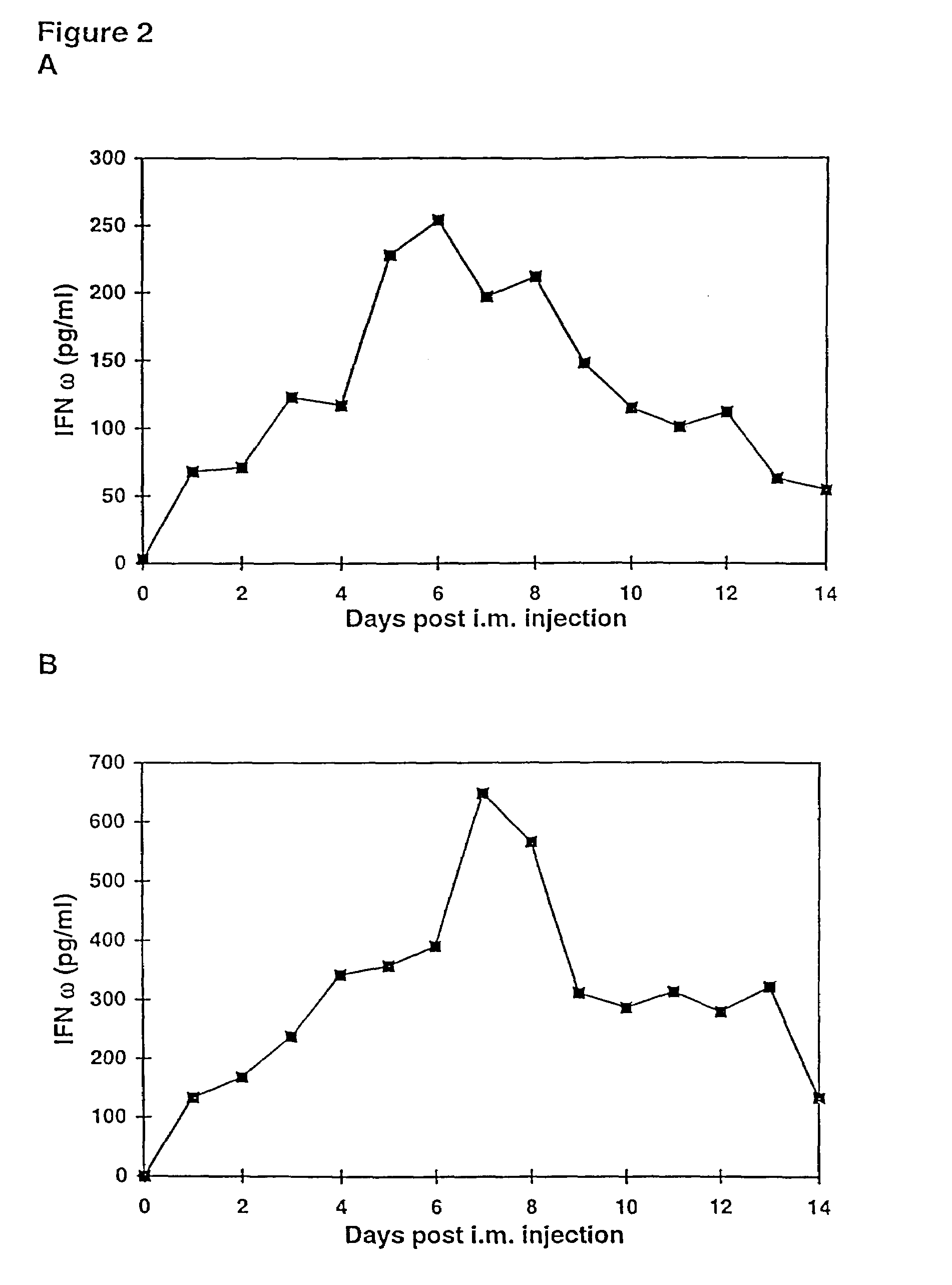
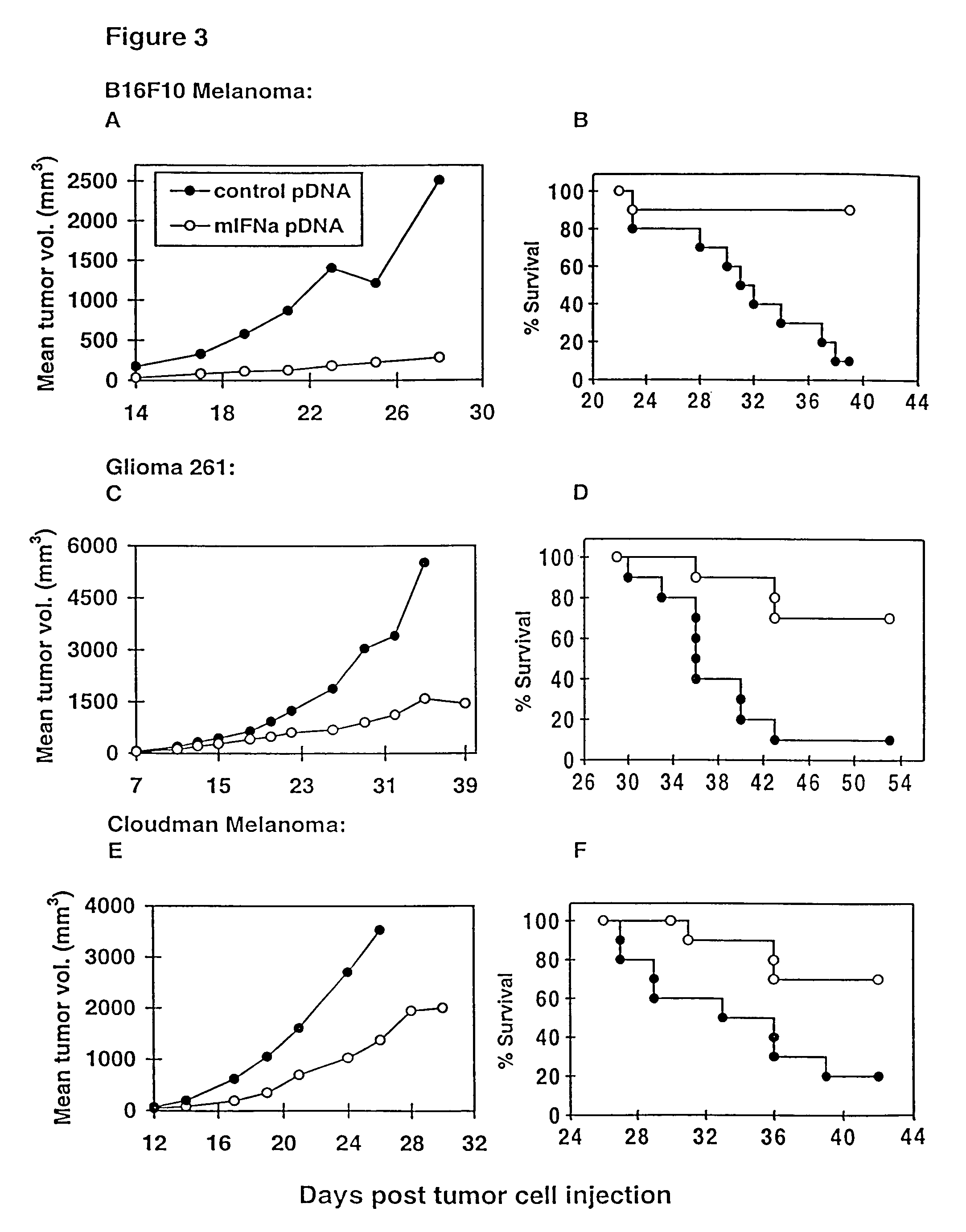
Methods for treating cancer using cytokine-expressing polynucleotides
InactiveUS7268120B1Improved in vivo polypeptide expressionMinimizing adverse side effectBiocideOrganic active ingredientsMammalSodium phosphates
The present invention provides a pharmaceutical composition, comprising a non-infectious, non-integrating polynucleotide construct comprising a polynucleotide encoding an interferon ω and one or more cationic compounds. The present invention also provides methods of treating cancer in a mammal, comprising administering into a muscle of the mammal a non-infectious, non-integrating DNA polynucleotide construct comprising a polynucleotide encoding a cytokine. In addition, the present invention also relates to the methodology for selective transfection of malignant cells with polynucleotides expressing therapeutic or prophylactic molecules in intra-cavity tumor bearing mammals. More specifically, the present invention provides a methodology for the suppression of an intra-cavity dissemination of malignant cells, such as intraperitoneal dissemination. Furthermore, the invention relates to compositions and methods to deliver polynucleotides encoding polypeptides to vertebrate cells in vivo, where the composition comprises an aqueous solution of sodium phosphate.
Owner:VICAL INC
Generation of replication competent viruses for therapeutic use
InactiveUS7550296B2Reduced viral genomeImprove effectivenessSsRNA viruses positive-sensePeptide/protein ingredientsDiseaseReplication competent virus
The present invention relates to the generation of replication-competent viruses having therapeutic utility. The replication-competent viruses of the invention can express proteins useful in the treatment of disease.
Owner:PSIOXUS THERAPEUTICS LTD
Compositions and methods for reprogramming eukaryotic cells
The present invention relates to methods for changing the state of differentiation of a eukaryotic cell, the methods comprising introducing mRNA encoding one or more reprogramming factors into a cell and maintaining the cell under conditions wherein the cell is viable and the mRNA that is introduced into the cell is expressed in sufficient amount and for sufficient time to generate a cell that exhibits a changed state of differentiation compared to the cell into which the mRNA was introduced, and compositions therefor. For example, the present invention provides mRNA molecules and methods for their use to reprogram human somatic cells into pluripotent stem cells.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and methods for delivering nucleic acid to a cell
InactiveUS20140065204A1Efficient transfectionSpecial deliveryGenetic therapy composition manufactureCell biologyNucleic acid
Owner:MERRIMACK PHARMACEUTICALS INC
Long lasting drug formulations
The present invention is directed to long-lasting erythropoietin therapeutic formulations and their methods of use wherein the formulation comprises a genetically modified micro-organ that comprises a vector which comprises a nucleic acid sequence operably linked to one or more regulatory sequences, wherein the nucleic acid sequence encodes erythropoietin.
Owner:MEDGENICS MEDICAL ISRAEL
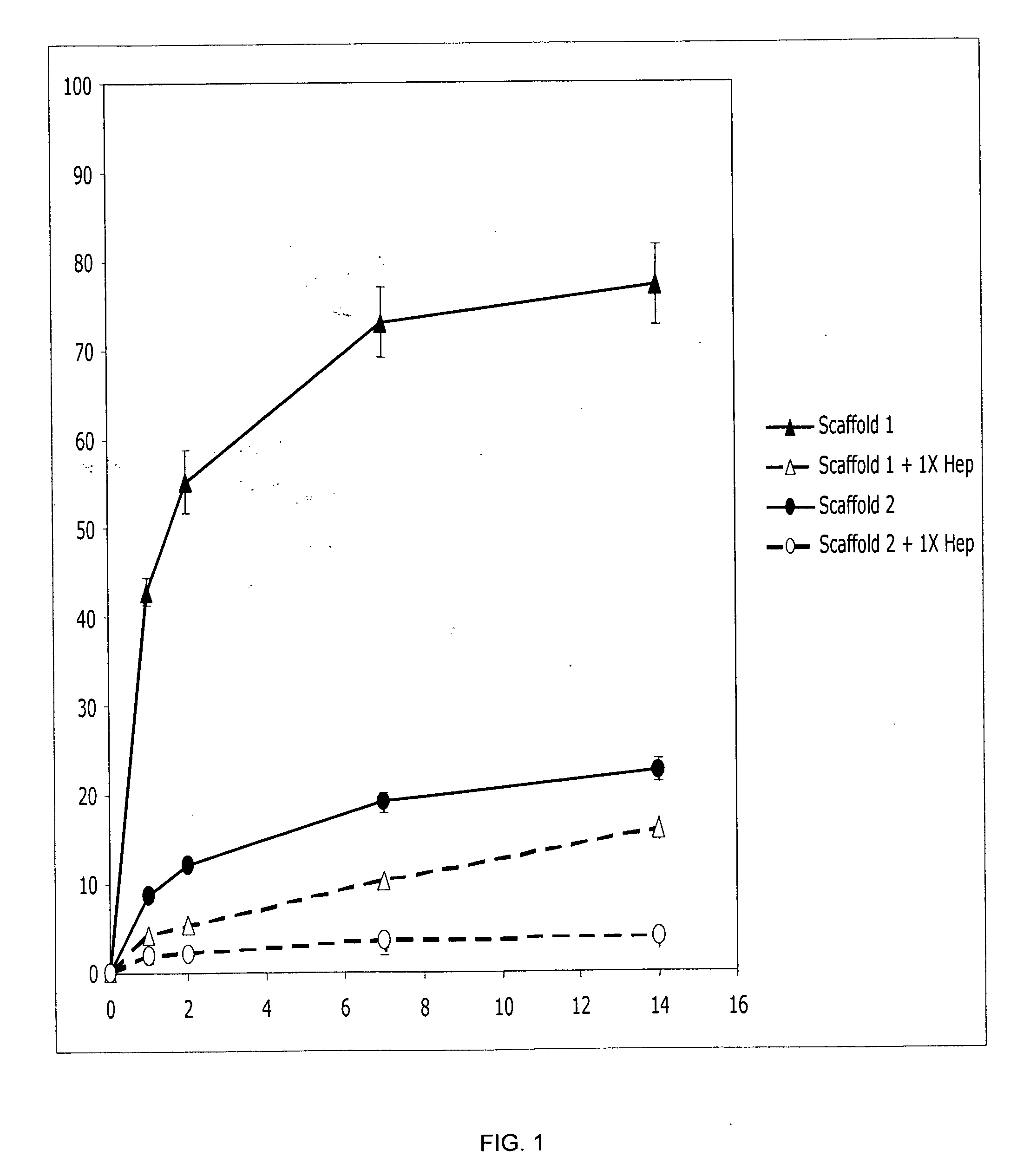
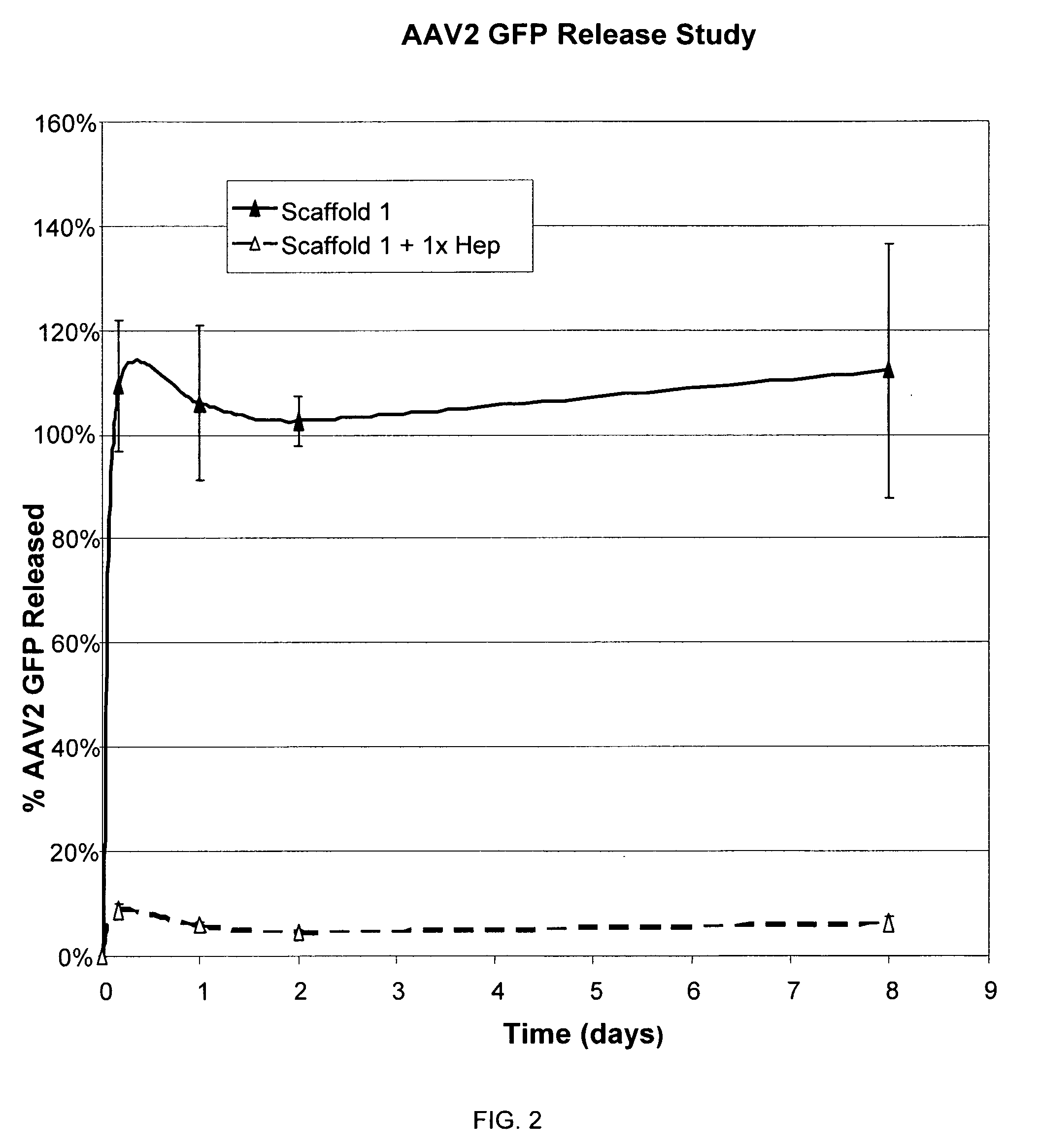
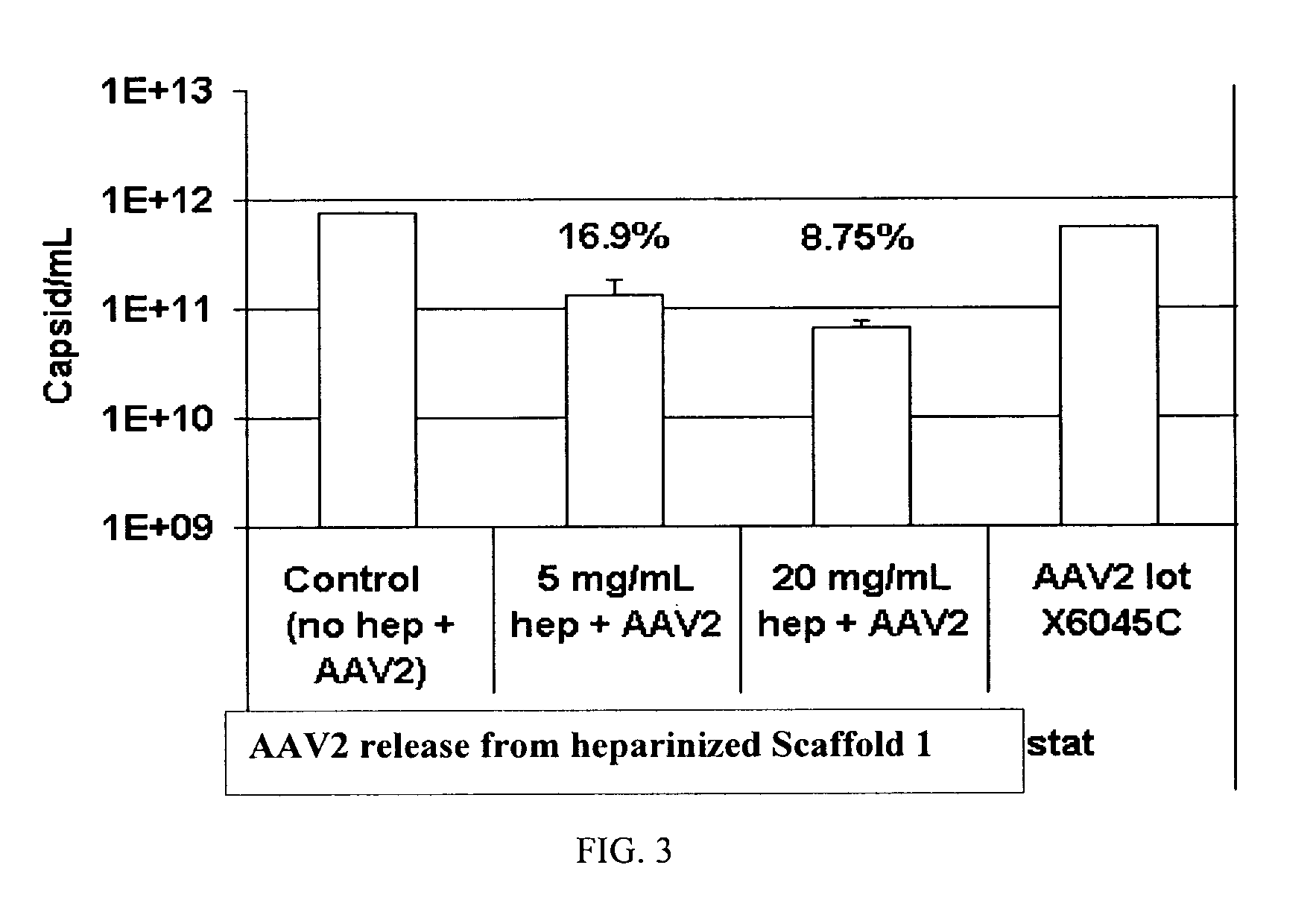
Prolonged delivery of heparin-binding growth factors from heparin-derivatized collagen
InactiveUS20090192079A1Improve biological activityPeptide/protein ingredientsGenetic therapy composition manufactureMuscle tissueCollagen scaffold
The present invention relates to a heparin-derivatized collagen matrix comprising a fragment of heparin covalently linked to a collagen scaffold, wherein the fragment of heparin has molecular weight of less than about 15 kDa, and at least one heparin-binding growth factor (HBGF) or heparin-binding adeno-associated virus (HB-AAV) or a combination thereof and methods for promoting bone growth, bone repair, cartilage repair, bone development, neo-angiogensis, wound healing, tissue engraftment and muscle tissue regeneration and / or tissue augmentation comprising administering a heparin-derivatized collagen matrix that includes at least one heparin-binding growth factor or heparin-binding adeno-associated virus or a combination thereof.
Owner:GENZYME CORP
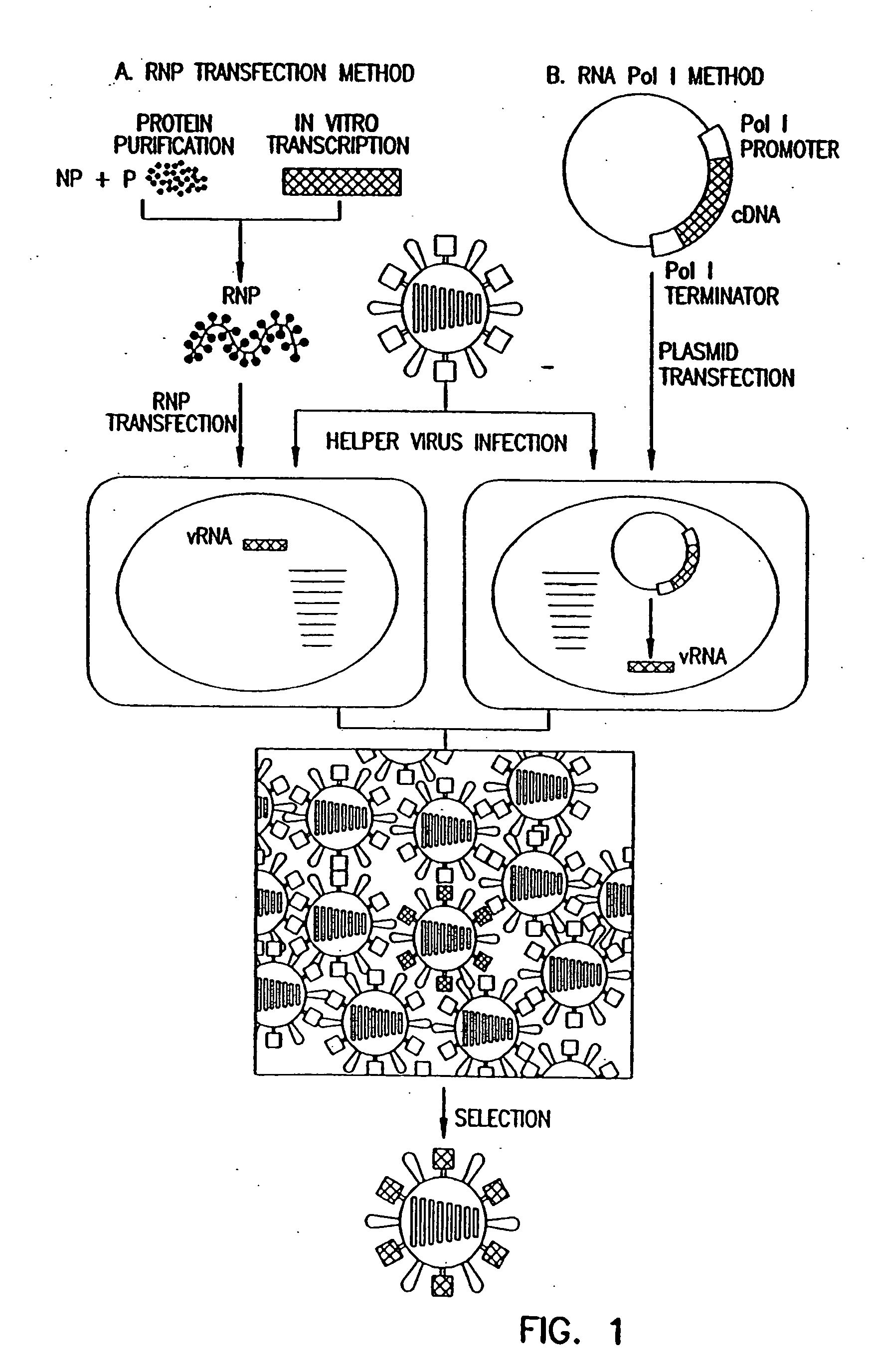
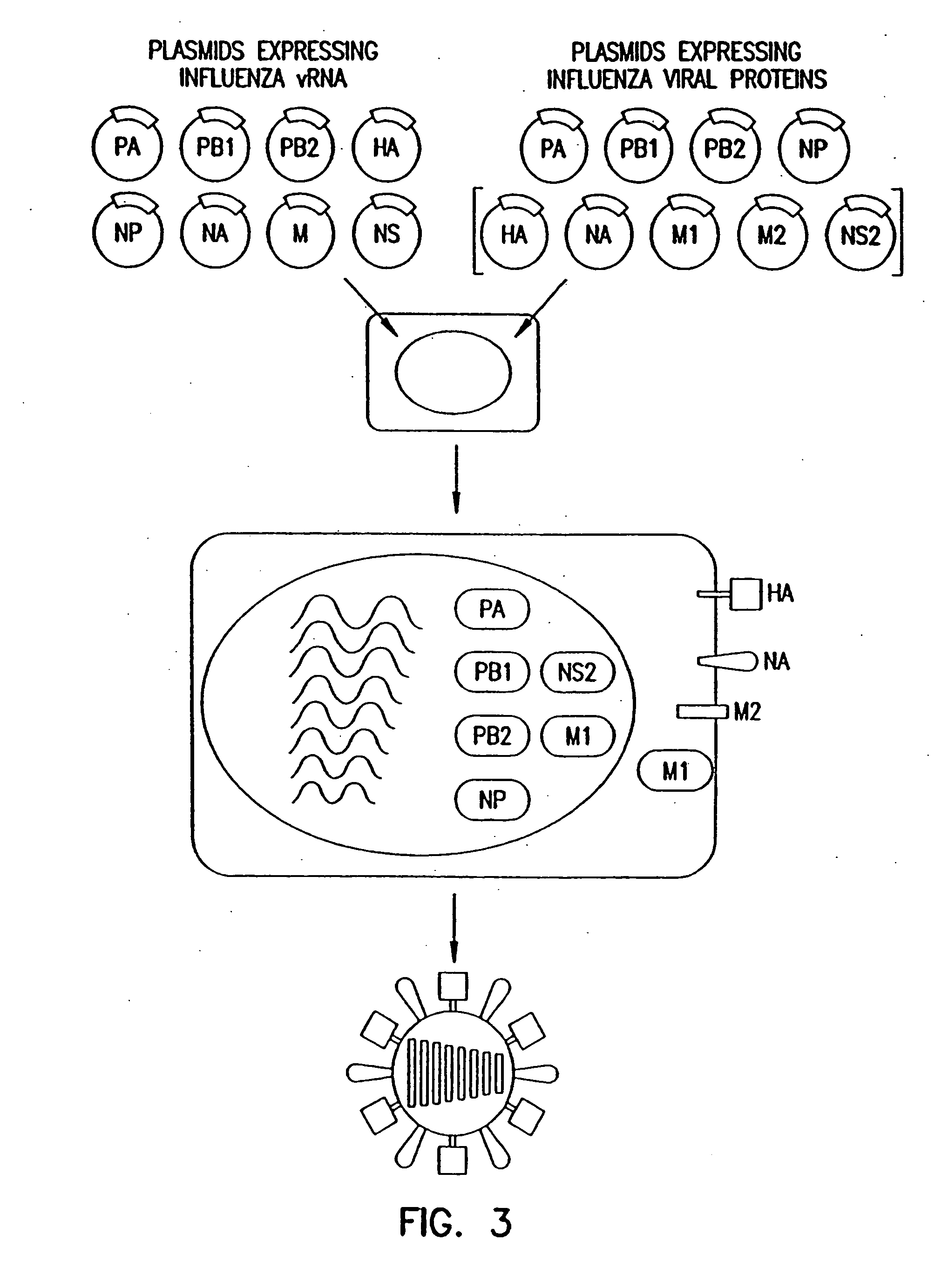
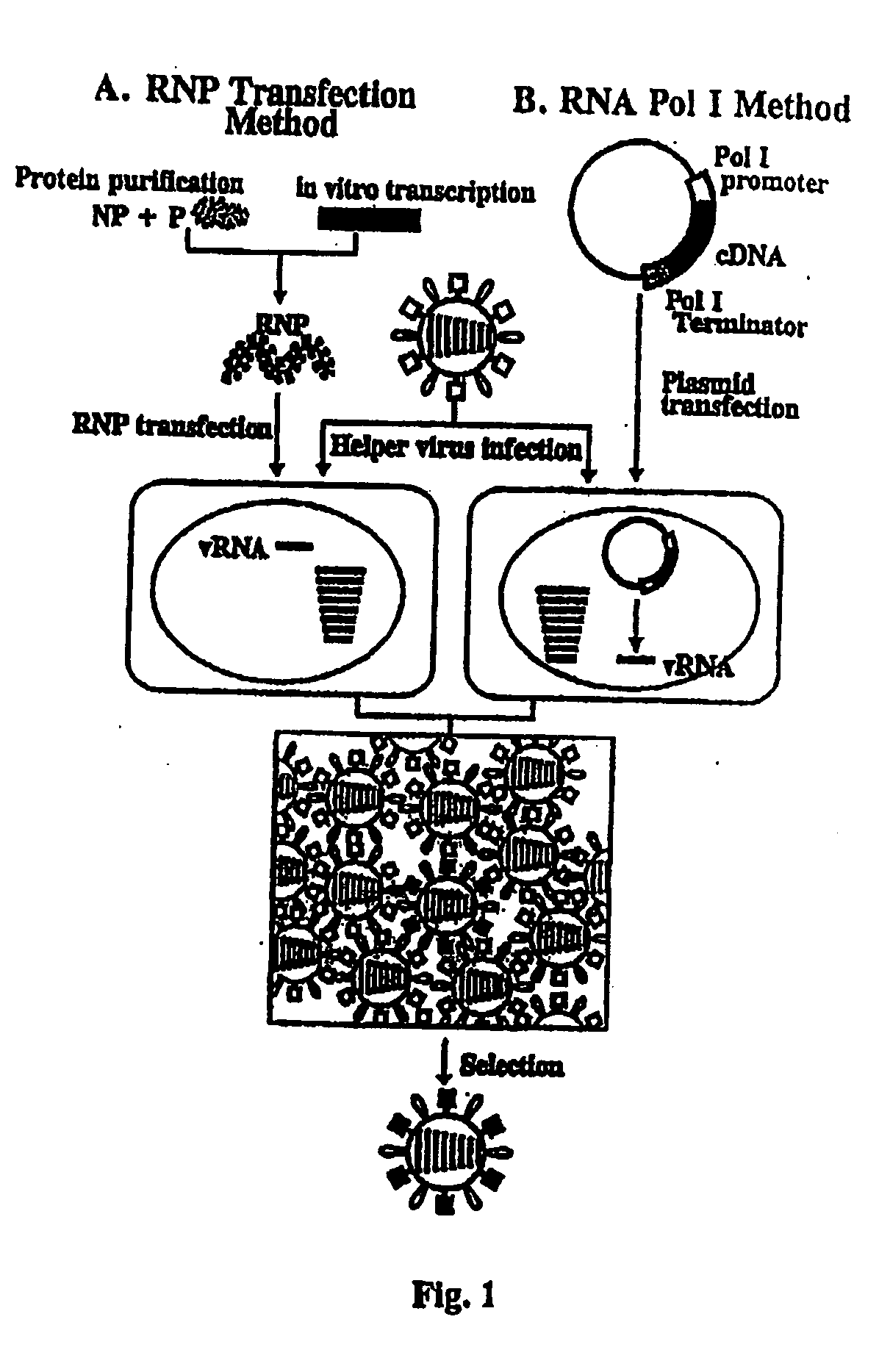
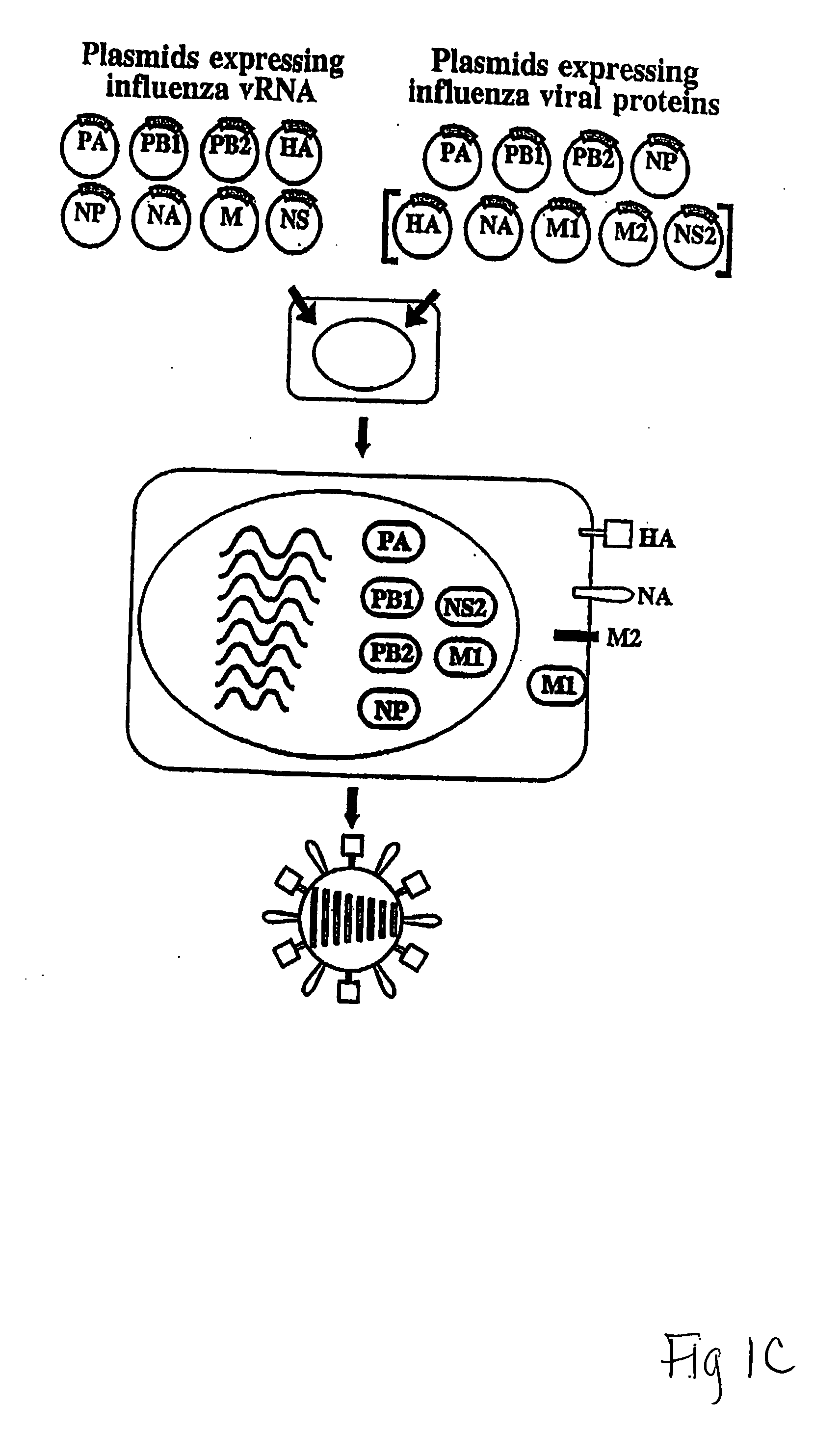
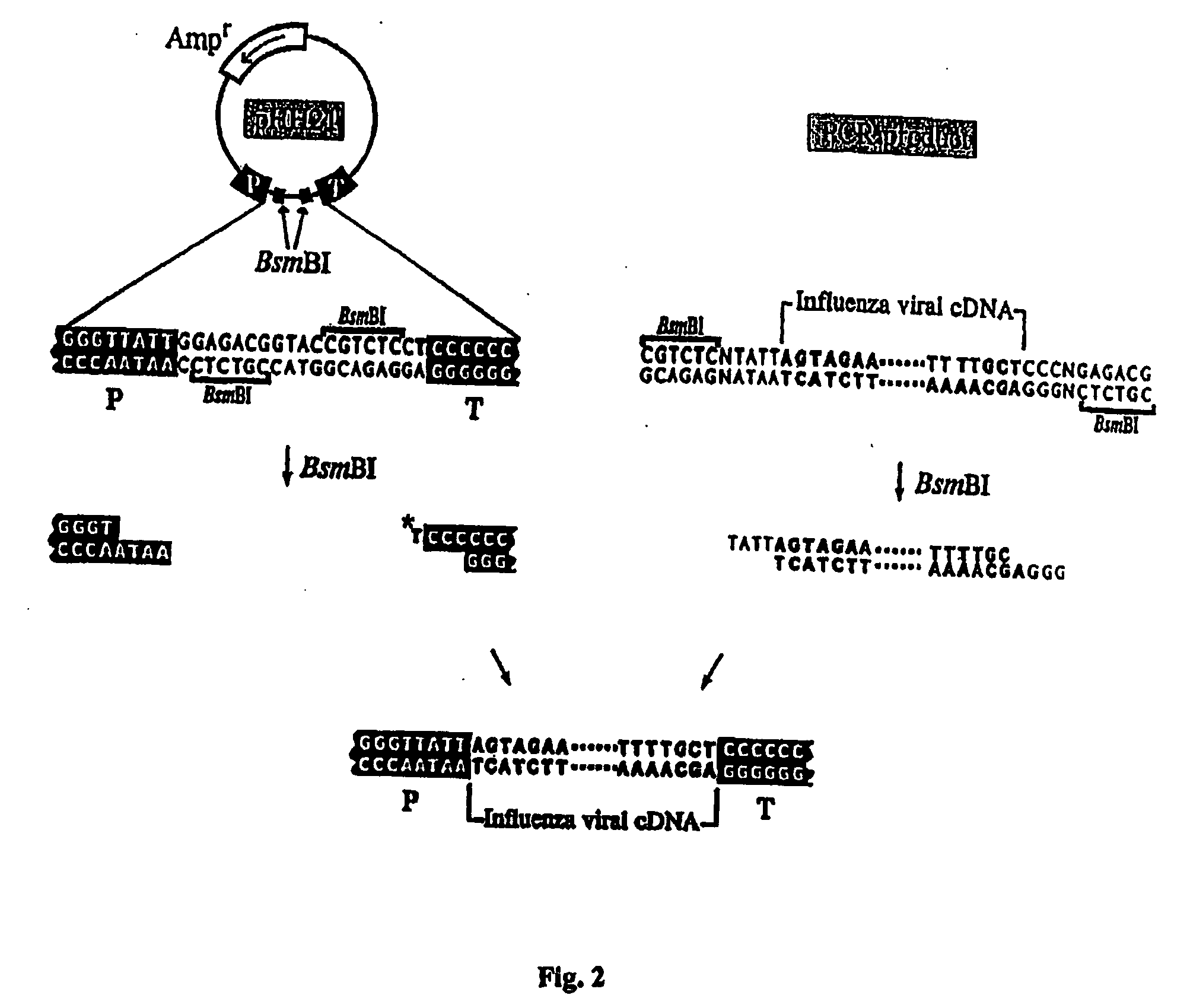
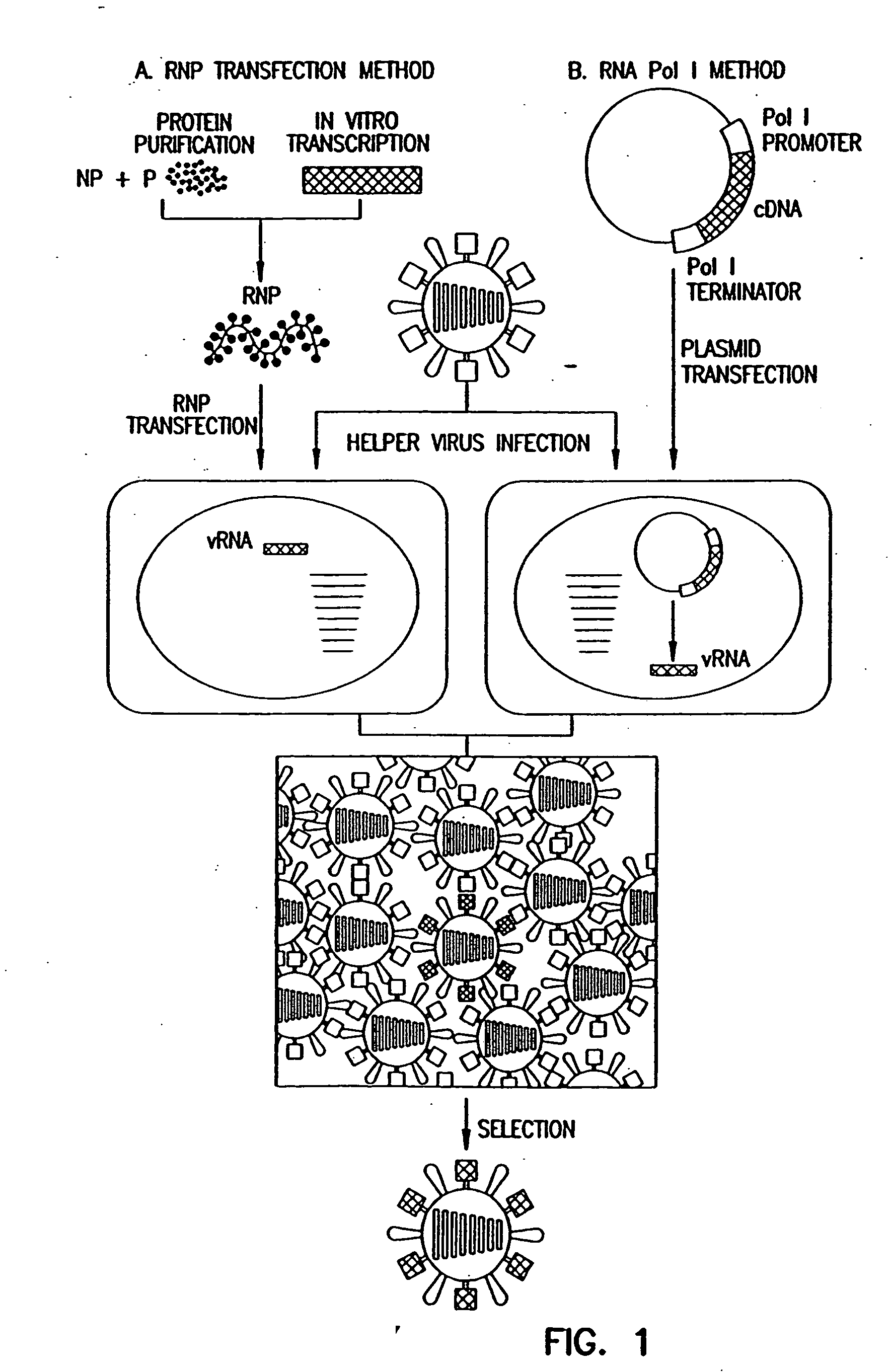
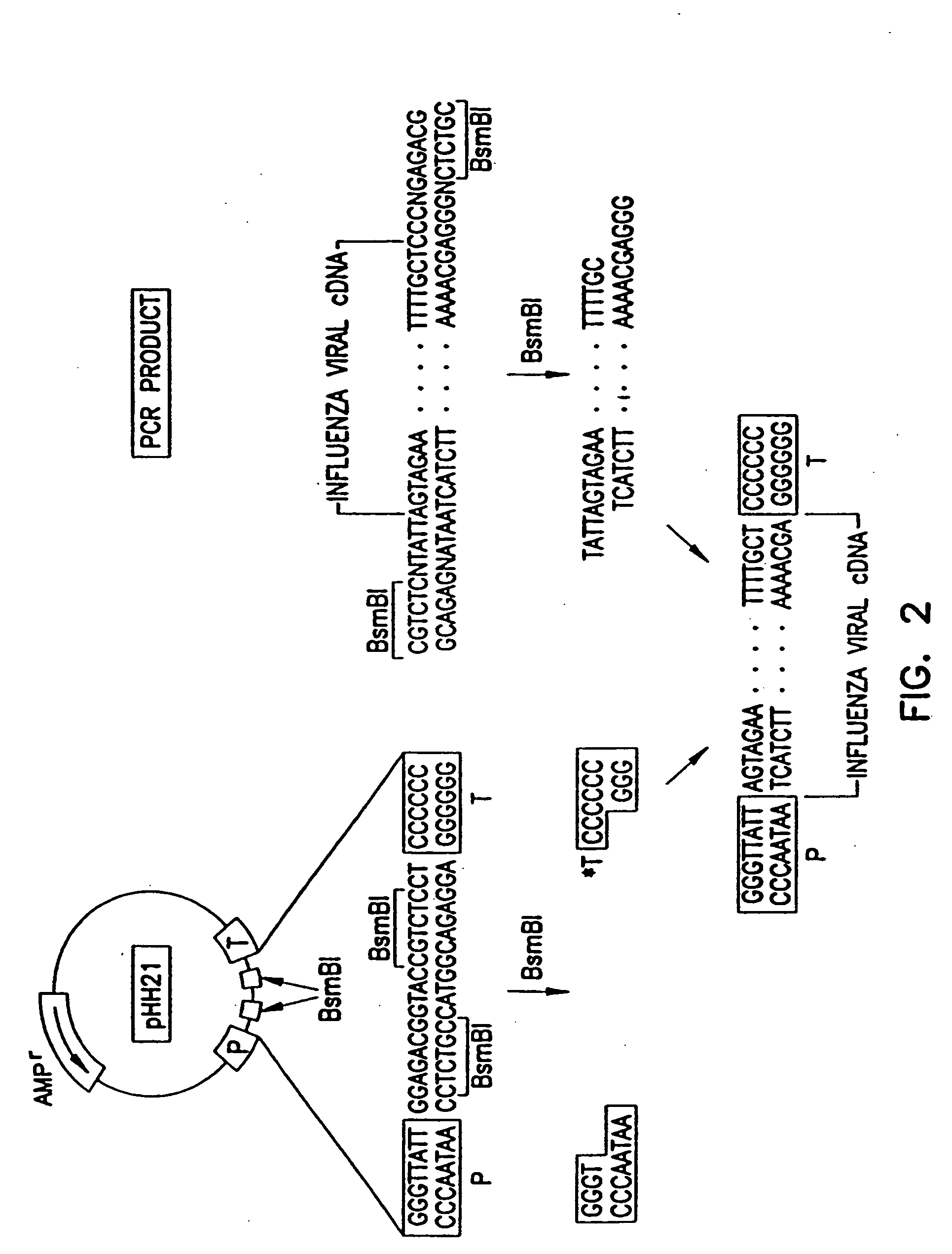
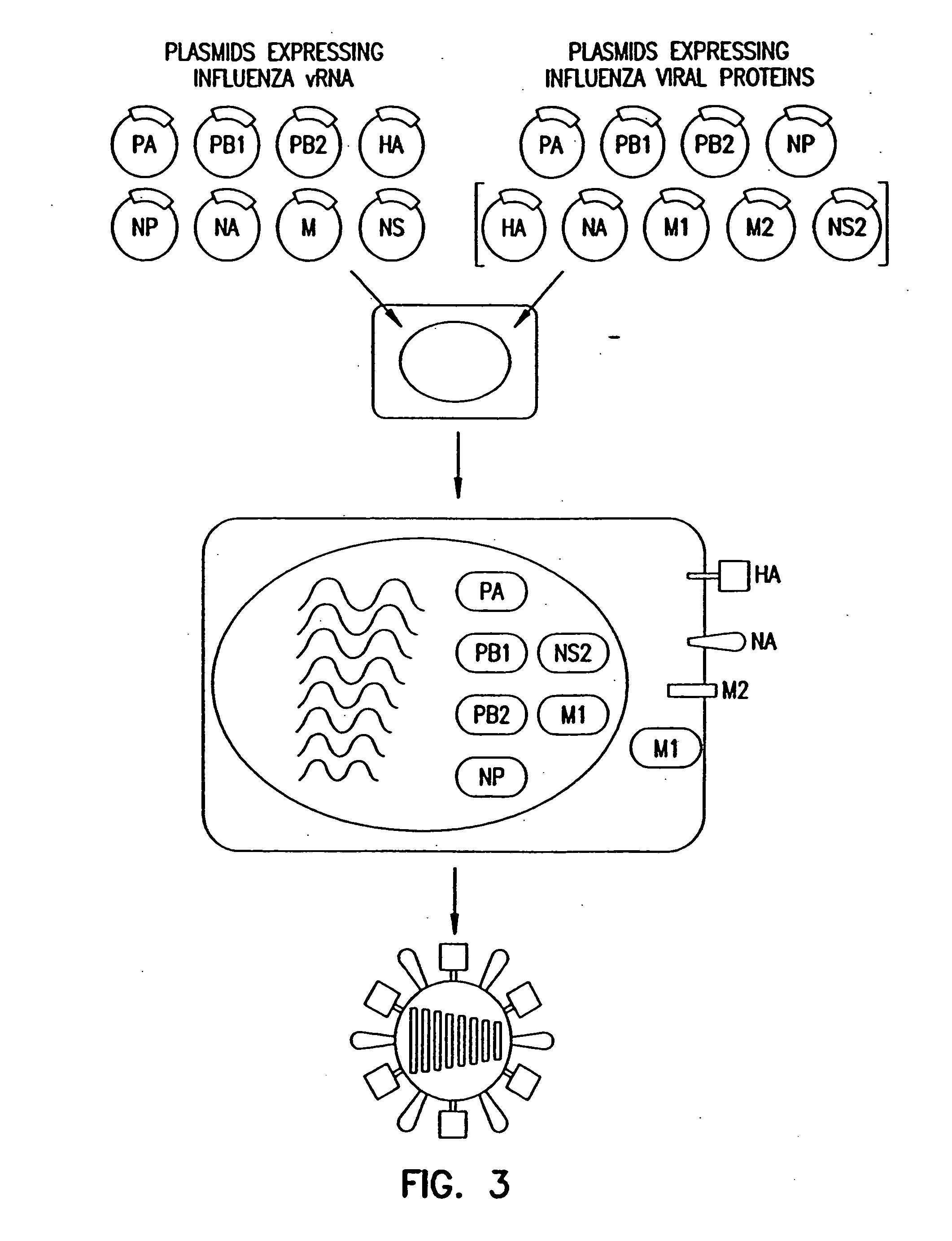
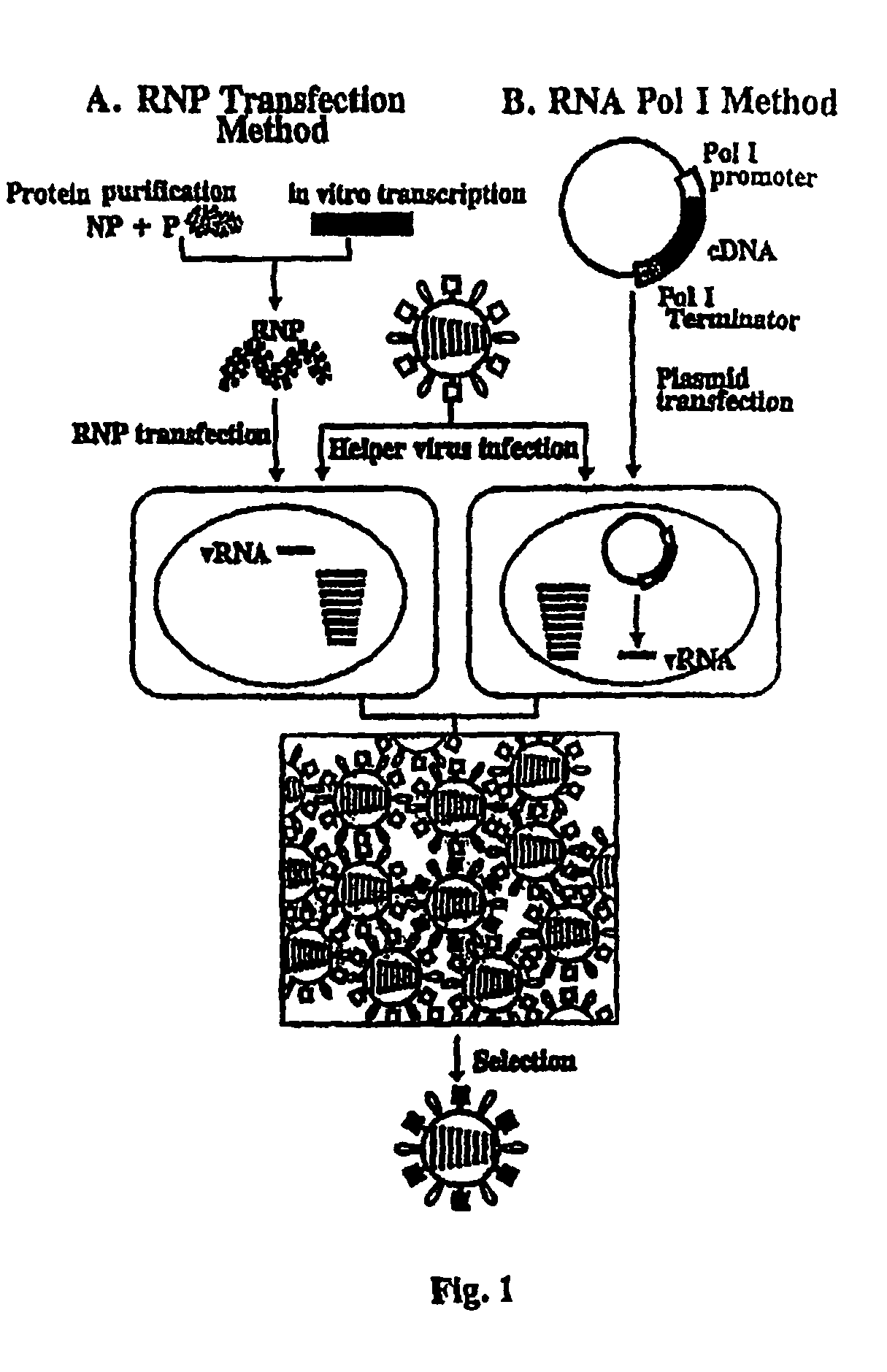
Recombinant influenza viruses for vaccines and gene therapy
InactiveUS20060057116A1Improve efficiencyIncrease productionSsRNA viruses negative-senseBiocideHelper virusGene
The invention provides a composition useful to prepare influenza A viruses, e.g., in the absence of helper virus.
Owner:KAWAOKA YOSHIHIRO +1
Apparatus and method for electroporation of biological samples
The present invention relates to methods and apparatus for the encapsulation of biologically-active substances in various cell populations. More particularly, the present invention relates to a method and apparatus for the encapsulation of biologically-active substances in various cell populations in blood by electroporation to achieve therapeutically desirable changes in the physical characteristics of the various cell populations in blood.
Owner:MAXCYTE
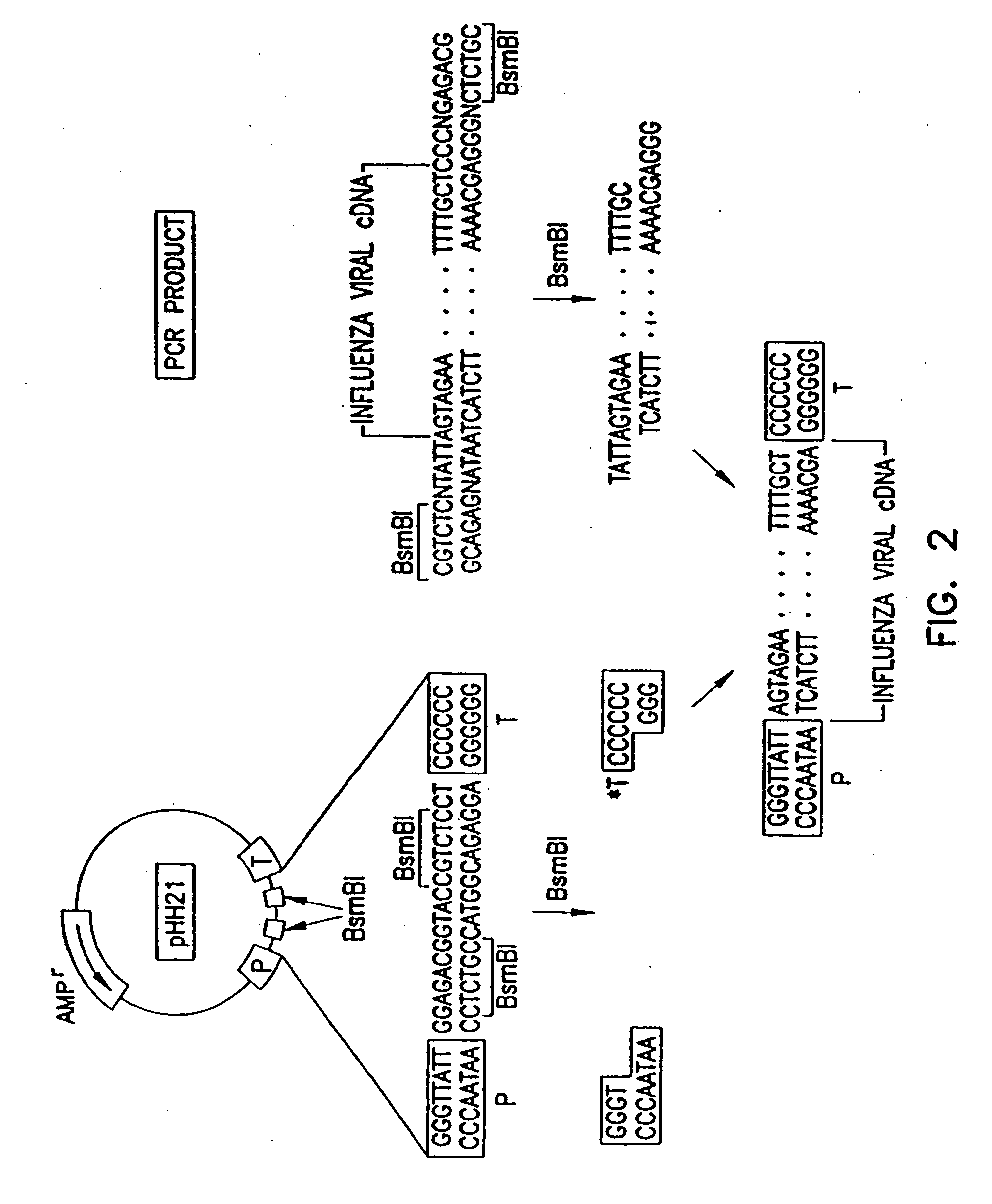
Recombinant influenza vectors with tandem transcription units
InactiveUS20060166321A1Efficient and robust generationReduce in quantitySsRNA viruses negative-senseBiocideViral vectorHelper virus
The invention provides a composition useful to prepare influenza viruses, e.g., in the absence of helper virus, using vectors which include tandem transcription cassettes containing PolI and / or PolII promoters.
Owner:WISCONSIN ALUMNI RES FOUND
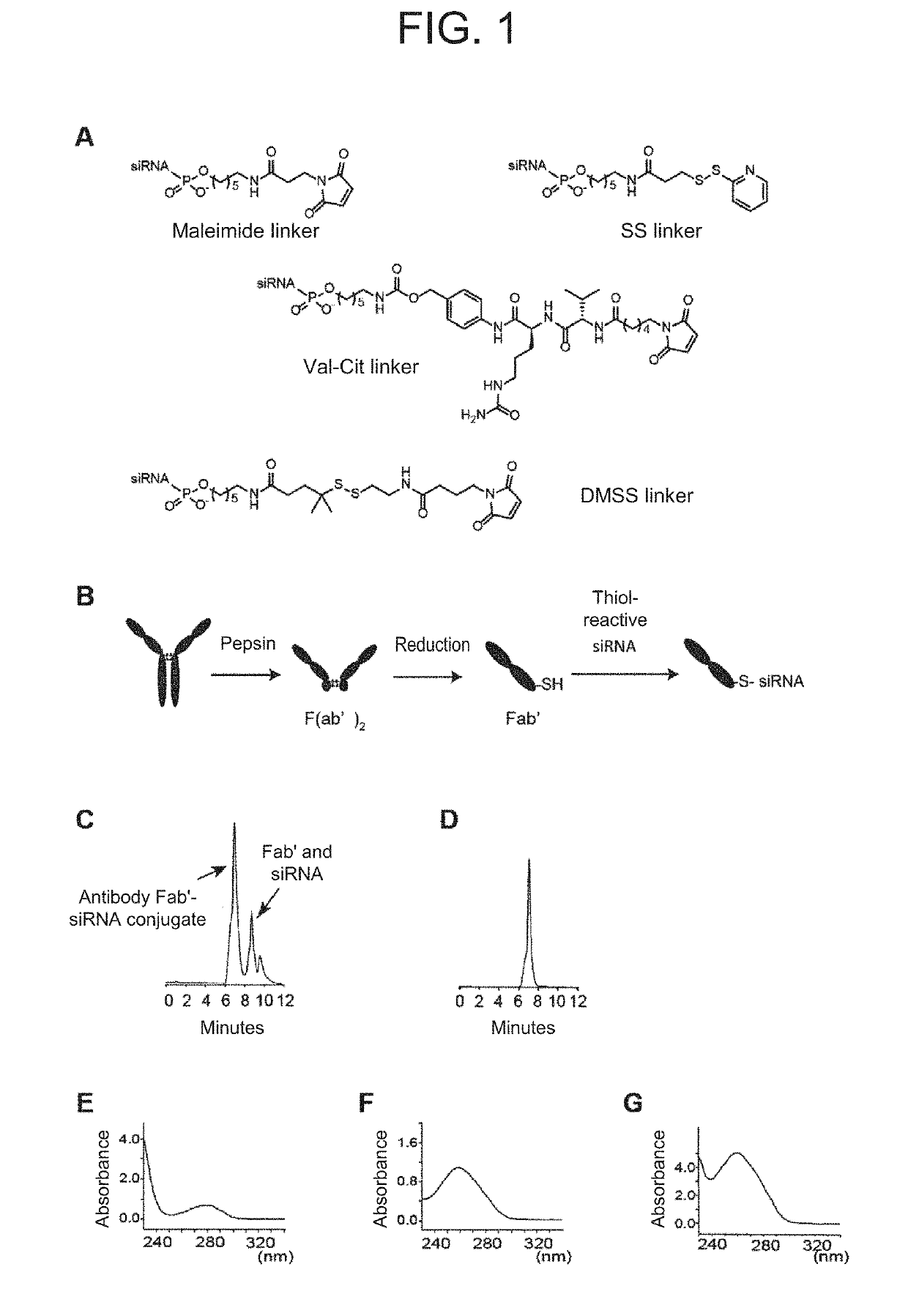
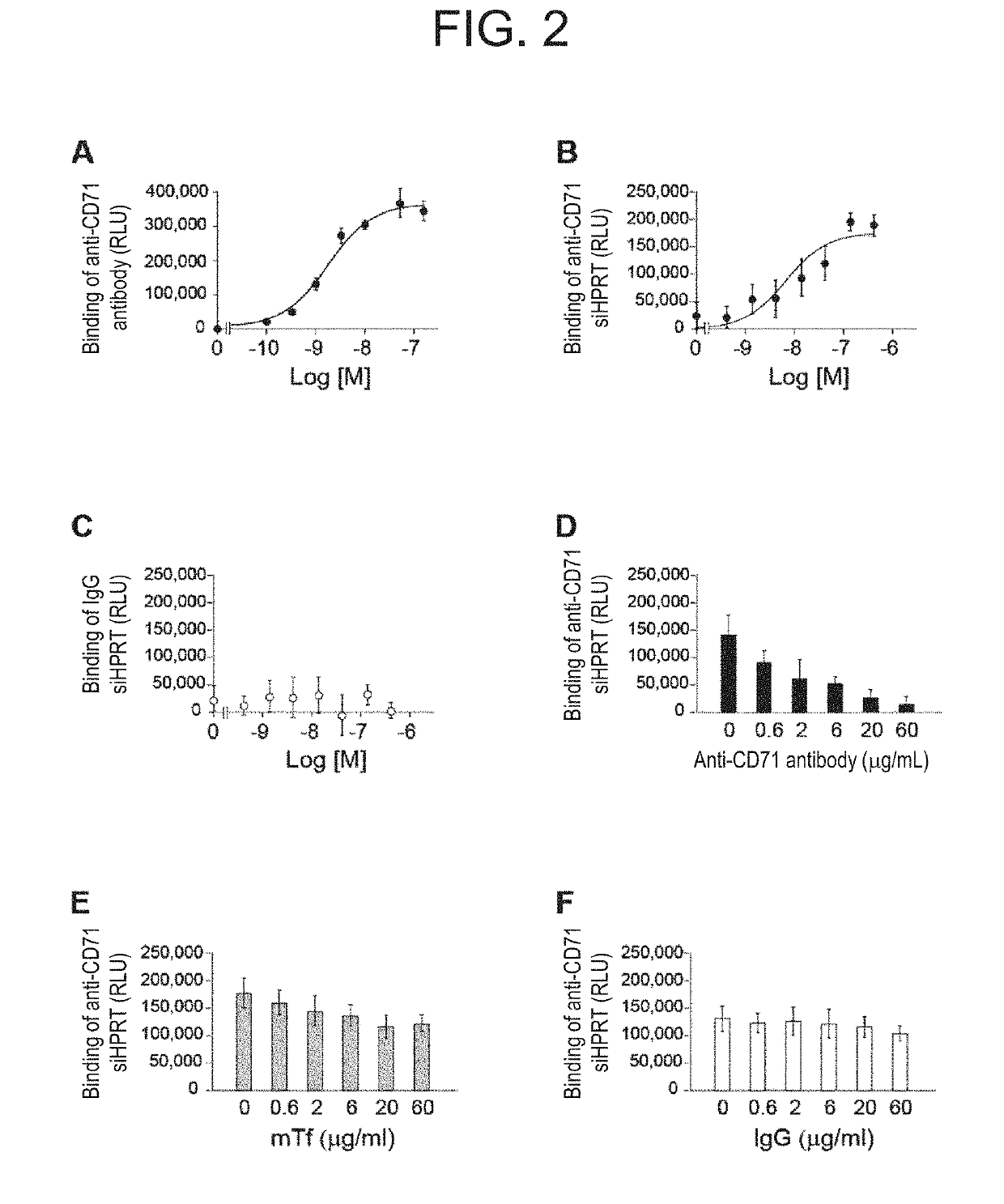
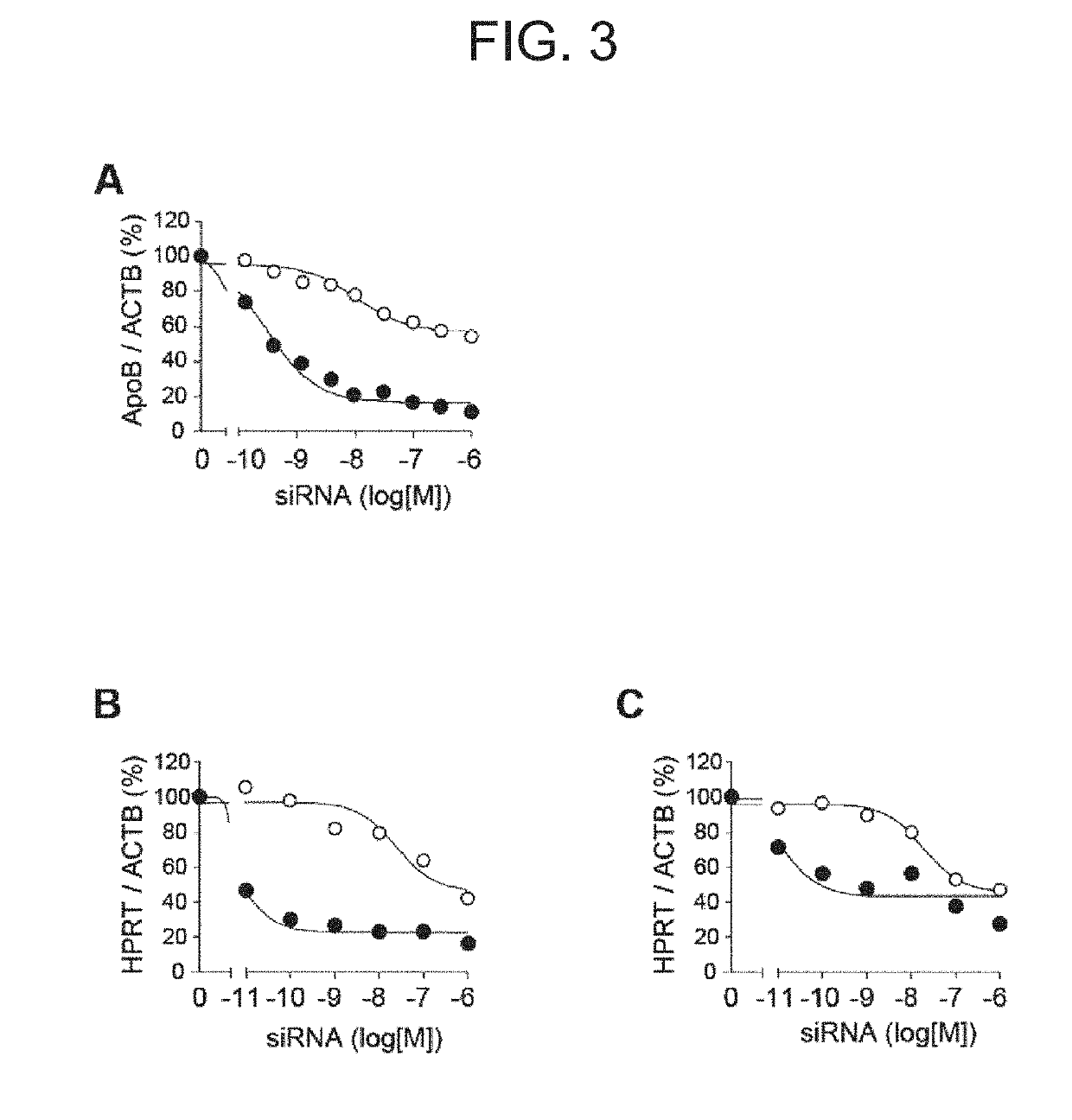
Antibody-drug conjugate
PendingUS20190240346A1Efficient deliveryPrevent and therapeutically treatOrganic active ingredientsGenetic therapy composition manufactureAntigenAntigen Binding Fragment
[Problem to be solved] The present invention provides an antibody-drug conjugate (ADC). The present invention also provides a conjugate of an anti-CD71 antibody or an antigen-binding fragment thereof with a drug. The present invention further provides a conjugate of Fab′ with a drug. The present invention further provides a composition comprising the conjugate for delivering the drug to a tissue or a cell of muscle or the like.[Solution] A conjugate of an anti-CD71 antibody or an antigen-binding fragment thereof with a drug, and a conjugate of Fab′ of an antibody with a drug.
Owner:GENAHEAD BIO INC
Recombinant influenza viruses for vaccines and gene therapy
InactiveUS20060134138A1Improve efficiencyEnhances these viruses as vaccine vectorsSsRNA viruses negative-senseAnimal cellsHelper virusGene
The invention provides a composition useful to prepare influenza A viruses, e.g., in the absence of helper virus.
Owner:WISCONSIN ALUMNI RES FOUND
Use of flexible bag containers for viral production
InactiveUS20060166364A1Reduce the amount requiredOpportunities decreaseBioreactor/fermenter combinationsBiological substance pretreatmentsVirus
Owner:INTROGEN THERAPEUTICS INC
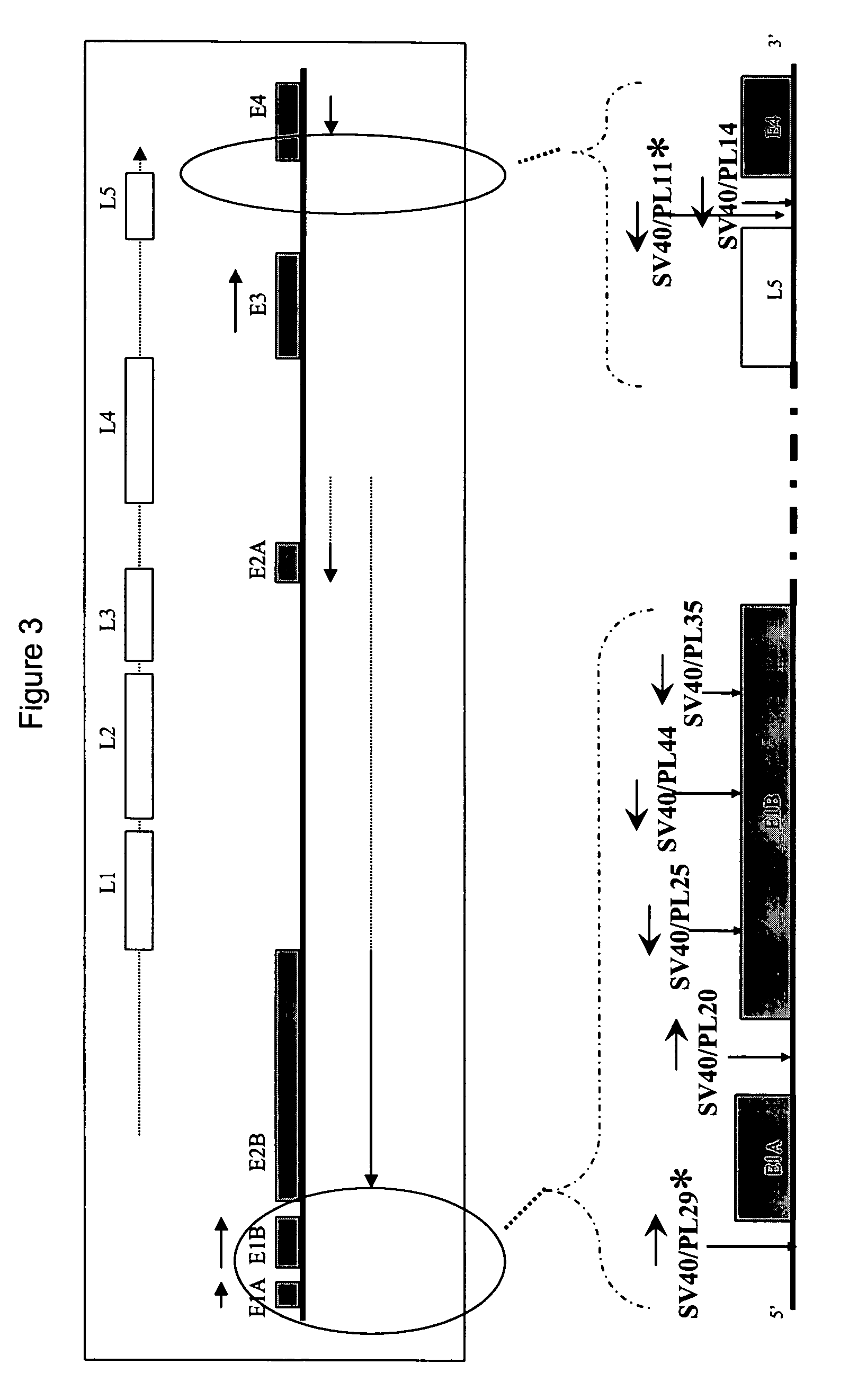
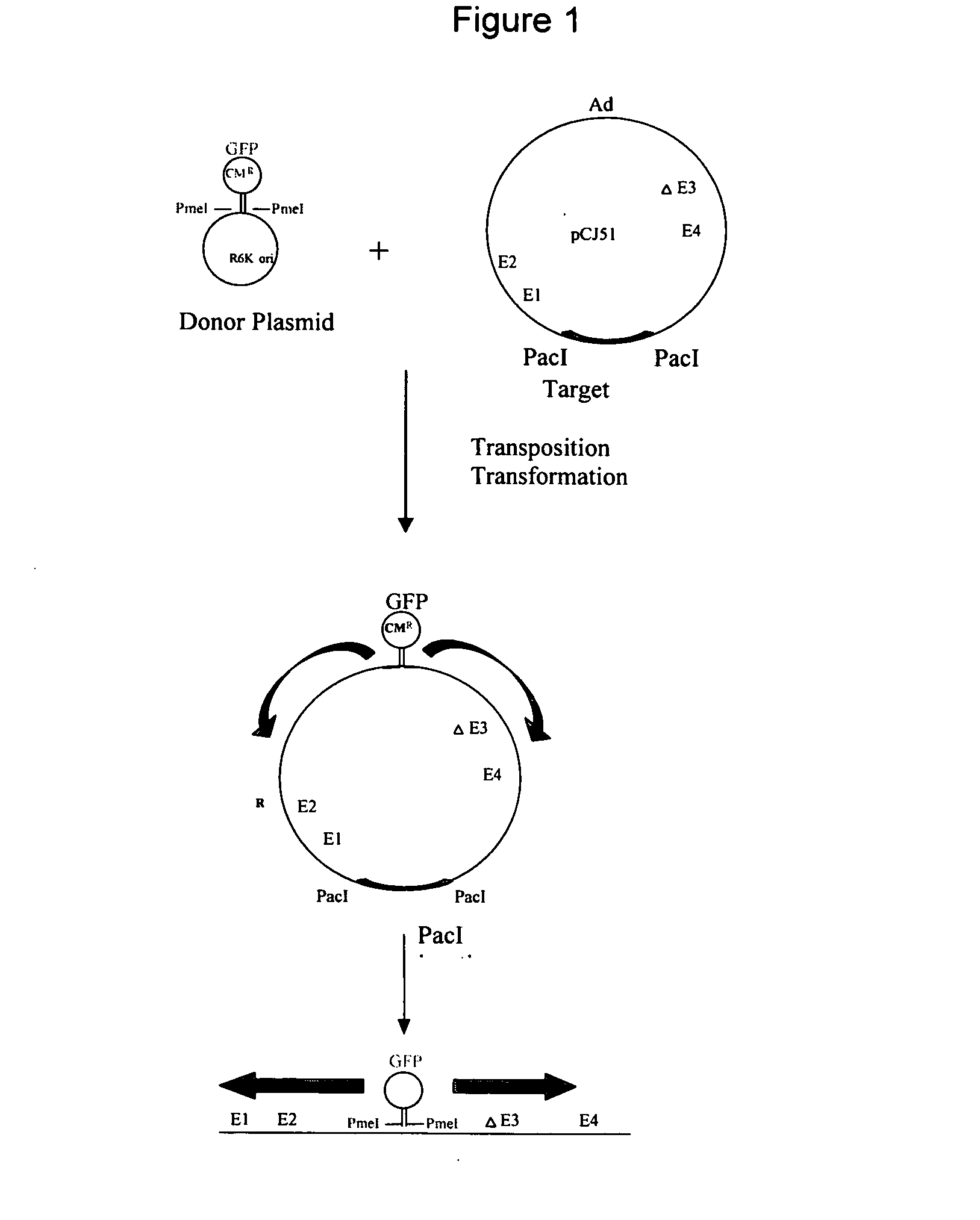
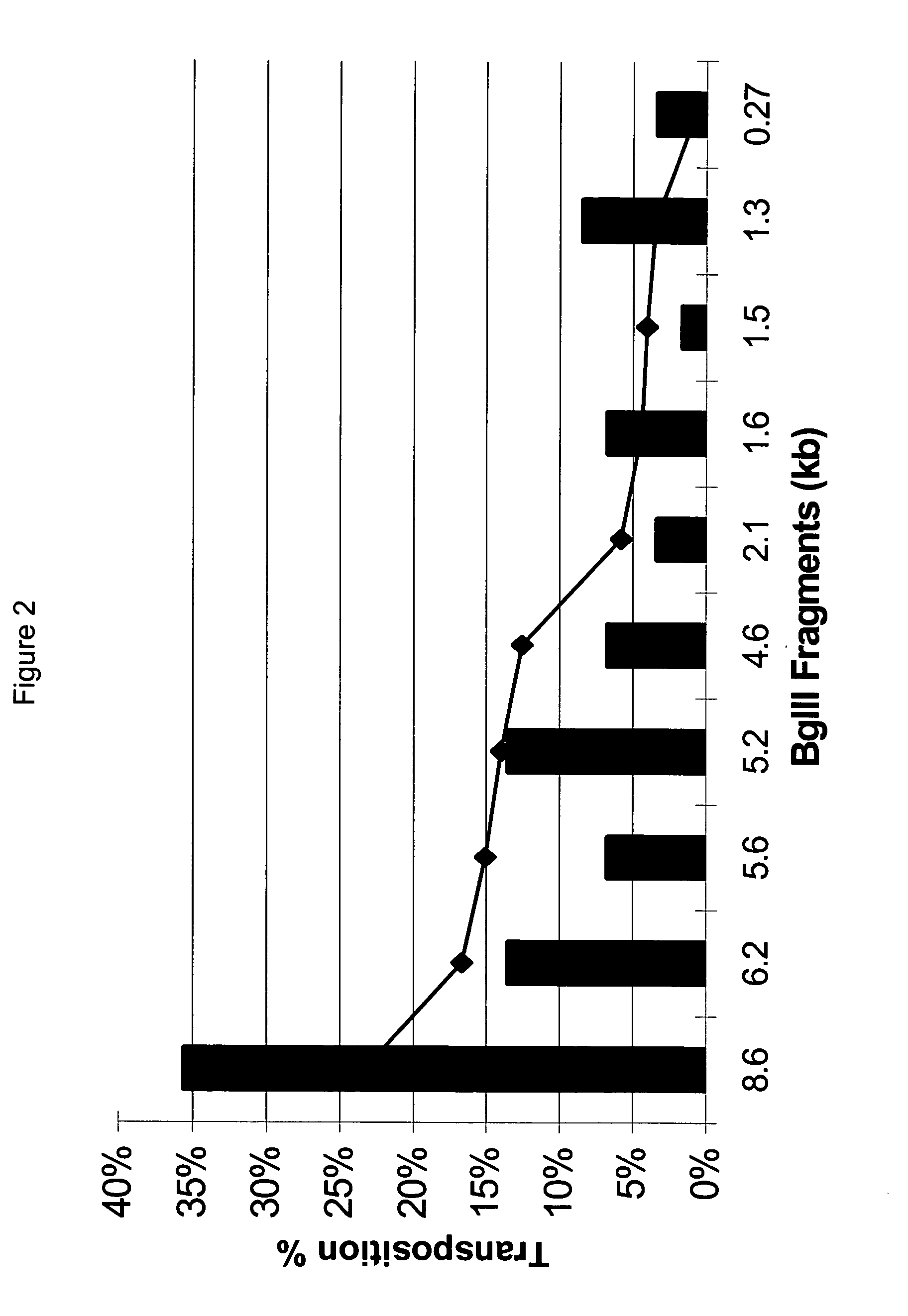
System for rapid production of high-titer and replication-competent adenovirus-free recombinant adenovirus vectors
InactiveUS20090175897A1Shorten production timeLow costSsRNA viruses negative-senseBiocideHeterologousImmunologic function
The present invention relates generally to the fields of gene therapy, immunology, and vaccine technology. More specifically, the invention relates to a novel system that can rapidly generate high titers of adenovirus vectors that are free of replication-competent adenovirus (RCA). Also provided are methods of generating these RCA-free adenoviral vectors, immunogenic or vaccine compositions comprising these RCA-free adenovirus vectors, methods of expressing a heterologous nucleic acid of interest in these adenovirus vectors and methods of eliciting immunogenic responses using these adenovirus vectors.
Owner:TANG DE CHU C +2
Generation of replication competent viruses for therapeutic use
InactiveUS20060121509A1Reduced viral genomeImprove effectivenessSsRNA viruses positive-sensePeptide/protein ingredientsReplication competent virusDisease cause
The present invention relates to the generation of replication-competent viruses having therapeutic utility. The replication-competent viruses of the invention can express proteins useful in the treatment of disease
Owner:PSIOXUS THERAPEUTICS LTD
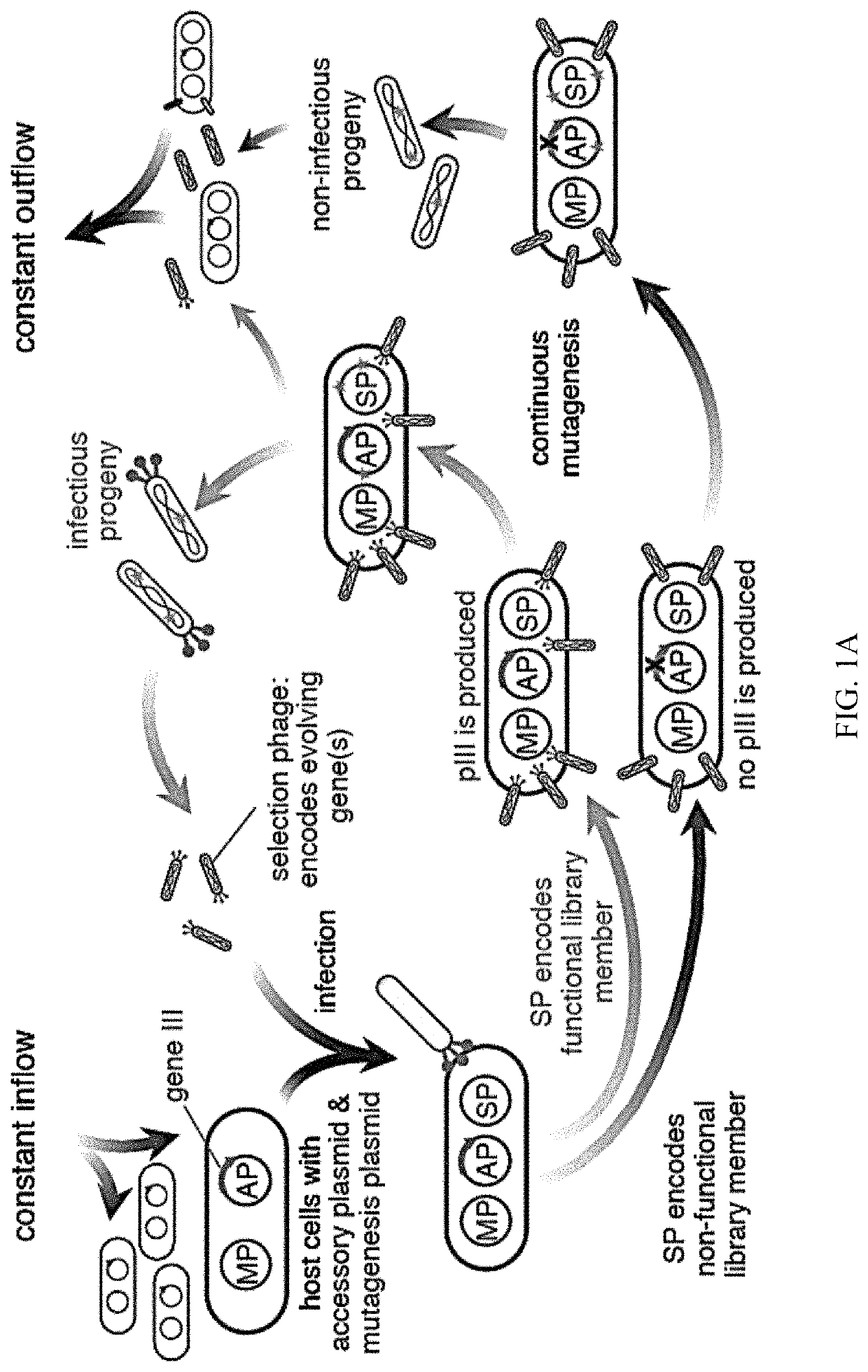
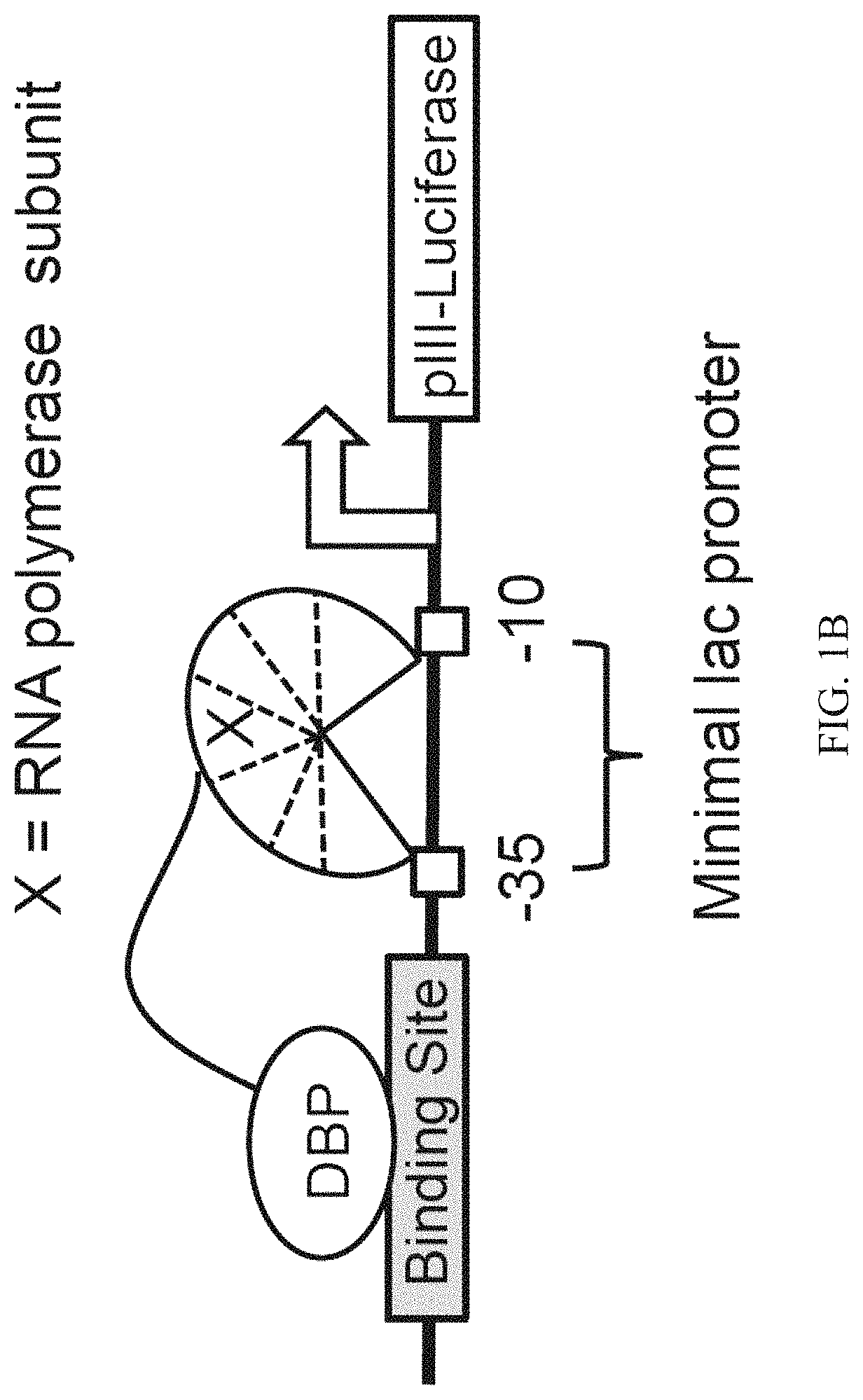
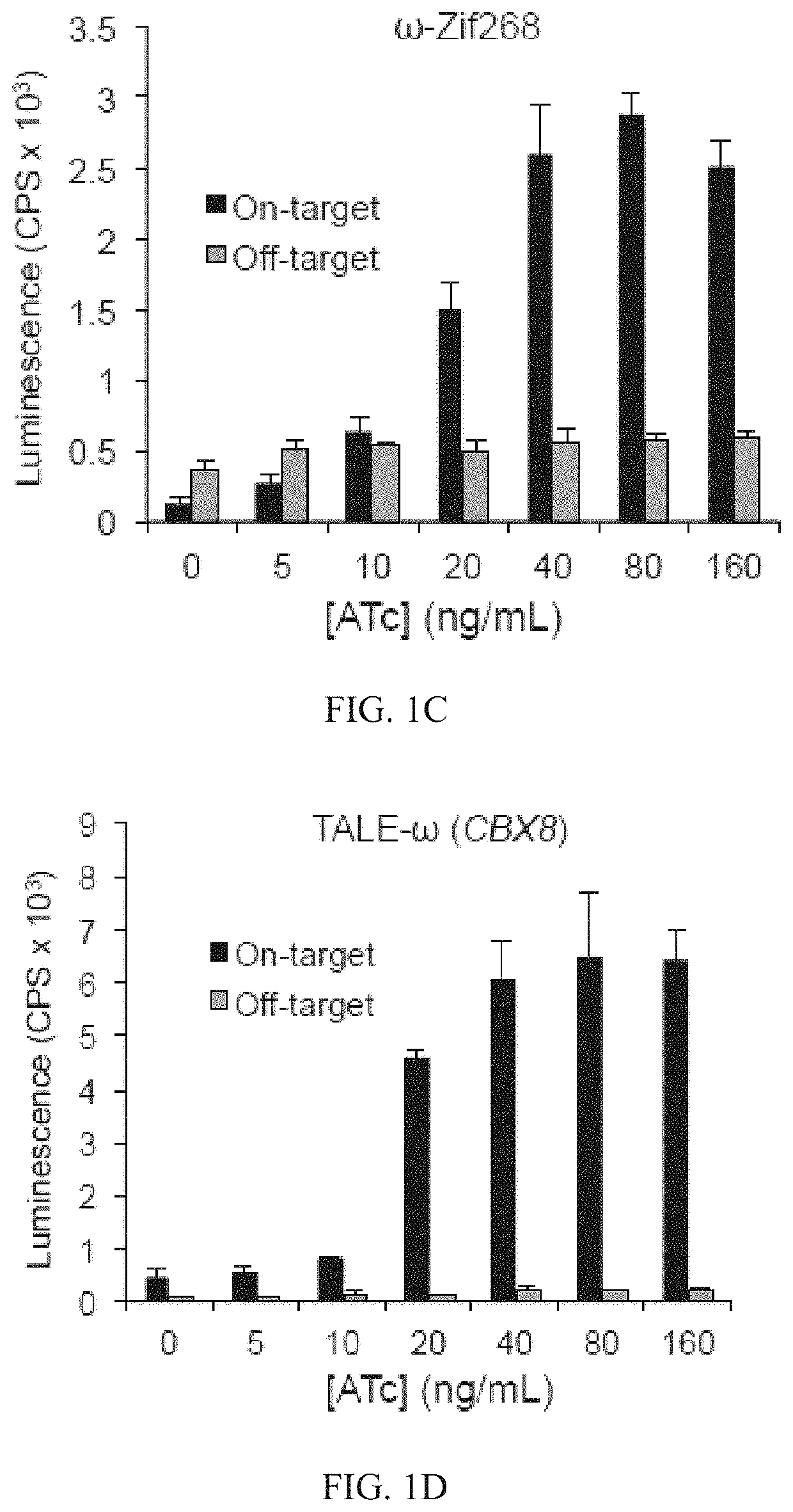
Evolution of talens
ActiveUS20200277587A1Strong specificityHydrolasesGenetic therapy composition manufactureNucleotideEpigenetic Profile
Engineered transcriptional activator-like effectors (TALEs) are versatile tools for genome manipulation with applications in research and clinical contexts. One current drawback of TALEs is that the 5′ nucleotide of the target is specific for thymine (T). TALE domains with alternative 5′ nucleotide specificities could expand the scope of DNA target sequences that can be bound by TALEs. Another drawback of TALEs is their tendency to bind and cleave off-target sequence, which hampers their clinical application and renders applications requiring high-fidelity binding unfeasible. This disclosure provides methods and strategies for the continuous evolution of proteins comprising DNA-binding domains, e.g., TALE domains. In some aspects, this disclosure provides methods and strategies for evolving such proteins under positive selection for a desired DNA-binding activity and / or under negative selection against one or more undesired (e.g., off-target) DNA-binding activities. Some aspects of this disclosure provide engineered TALE domains and TALEs comprising such engineered domains, e.g., TALE nucleases (TALENs), TALE transcriptional activators, TALE transcriptional repressors, and TALE epigenetic modification enzymes, with altered 5′ nucleotide specificities of target sequences. Engineered TALEs that target ATM with greater specificity are also provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Vectors comprising stuffer/filler polynucleotide sequences and methods of use
Owner:COLGATE PALMOLIVE CO
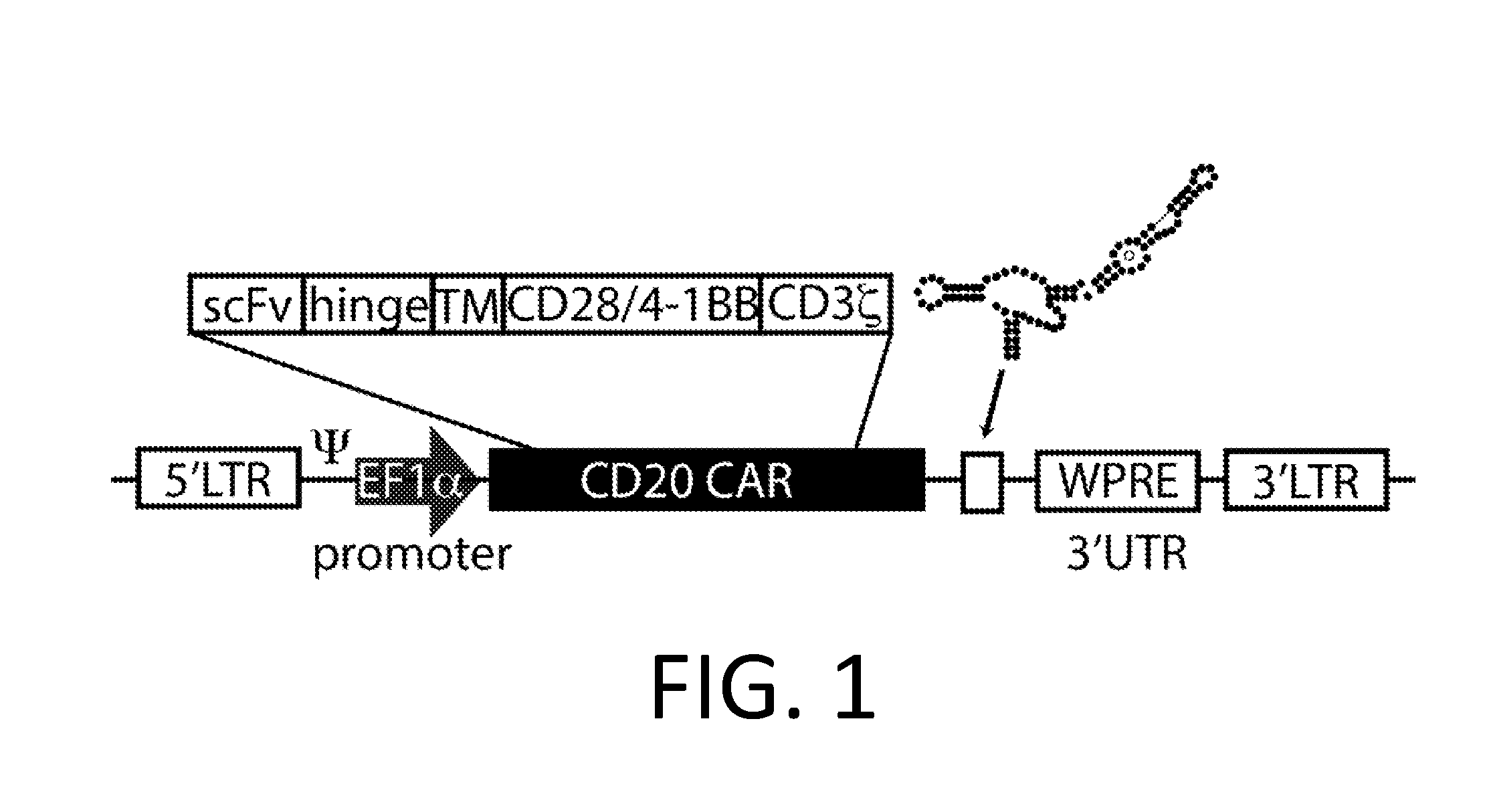
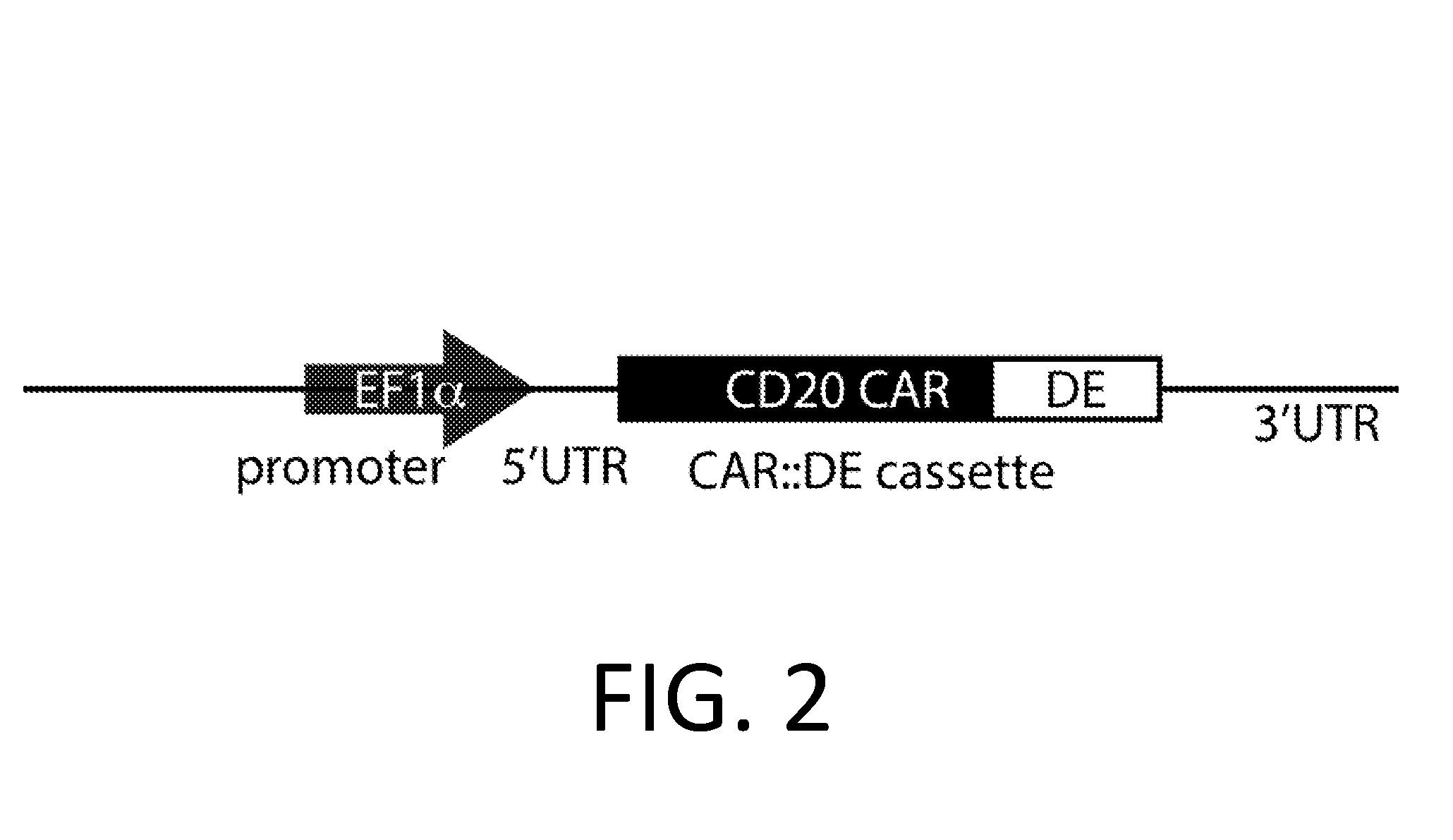
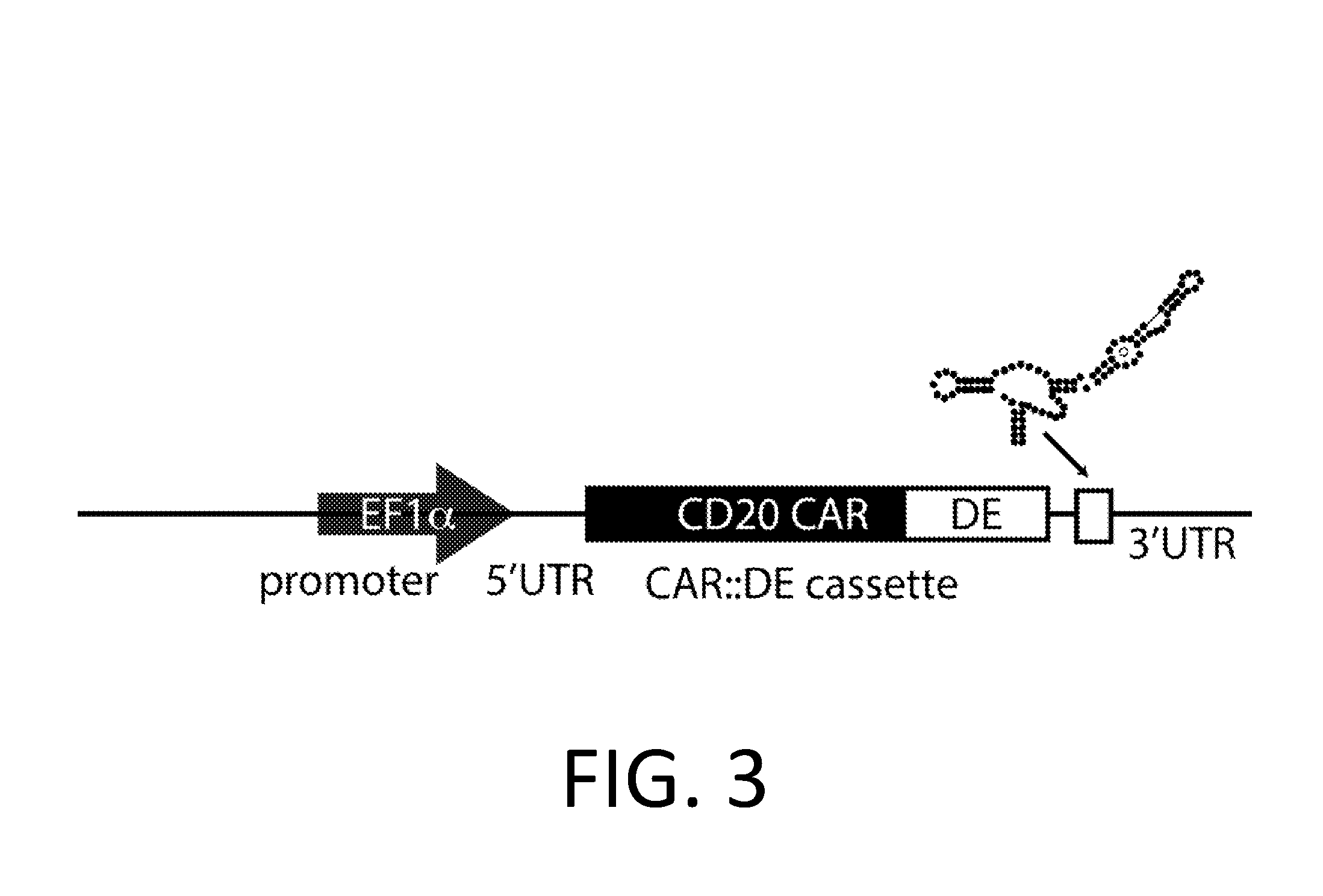
Smart CAR Devices, DE CAR Polypeptides, Side CARs and Uses Thereof
ActiveUS20160272718A1Strong specificityHigh selectivityPolypeptide with localisation/targeting motifGenetic therapy composition manufactureDiseaseAntigen receptors
The present invention relates generally to the field of RNA Control Devices and / or destabilizing elements (DE) combined with Chimeric Antigen Receptors (CARs) in eukaryotic cells. The present invention also relates to split CARs (Side-CARs) in eukaryotic cells. More specifically, the present invention relates to DEs, RNA Control Devices, and / or side-CARs combined with Chimeric Antigen Receptors to make small molecule actuatable CAR polypeptides. The present invention also relates to DE-CARs, Smart CARs (Smart=small molecule actuatable RNA trigger), Smart-DE-CARs, and / or Side-CARs for use in the treatment of disease.
Owner:CHIMERA BIOENG INC
Evolution of TALENs
ActiveUS10612011B2Strong specificityHydrolasesGenetic therapy composition manufactureNucleotideEpigenetic Profile
Engineered transcriptional activator-like effectors (TALEs) are versatile tools for genome manipulation with applications in research and clinical contexts. One current drawback of TALEs is that the 5′ nucleotide of the target is specific for thymine (T). TALE domains with alternative 5′ nucleotide specificities could expand the scope of DNA target sequences that can be bound by TALEs. This disclosure provides methods and strategies for the continuous evolution of proteins comprising DNA-binding domains, e.g., TALE domains. In some aspects, this disclosure provides methods and strategies for evolving such proteins under positive selection for a desired DNA-binding activity and / or under negative selection against one or more undesired (e.g., off-target) DNA-binding activities. Some aspects of this disclosure provide engineered TALE domains and TALEs comprising such engineered domains, e.g., TALE nucleases (TALENs), TALE transcriptional activators, TALE transcriptional repressors, and TALE epigenetic modification enzymes, with altered 5′ nucleotide specificities of target sequences. Engineered TALEs that target ATM with greater specificity are also provided.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
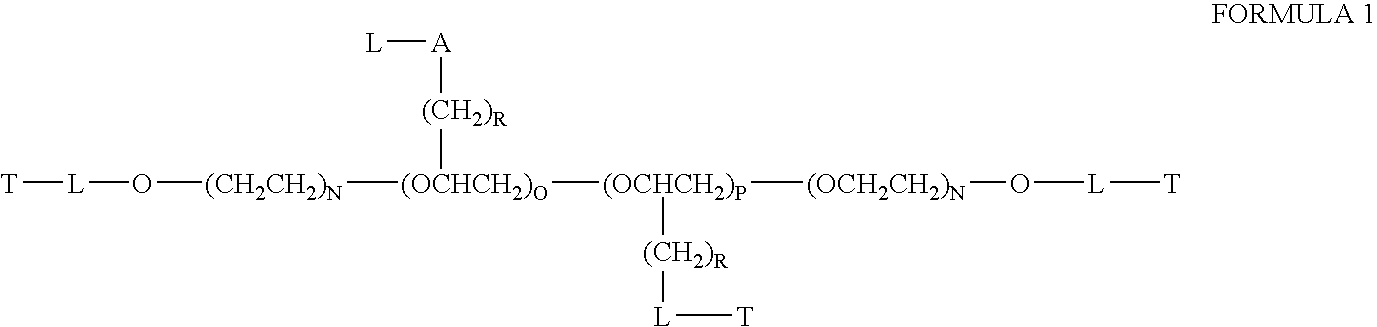
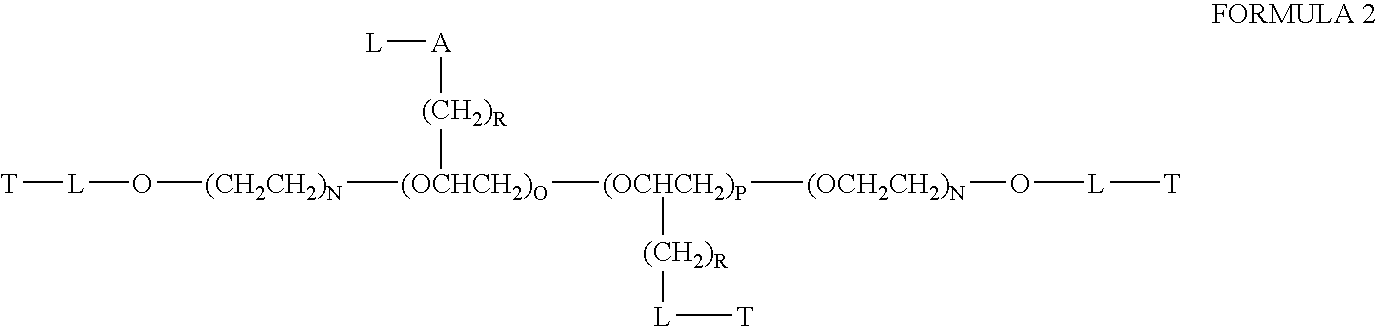
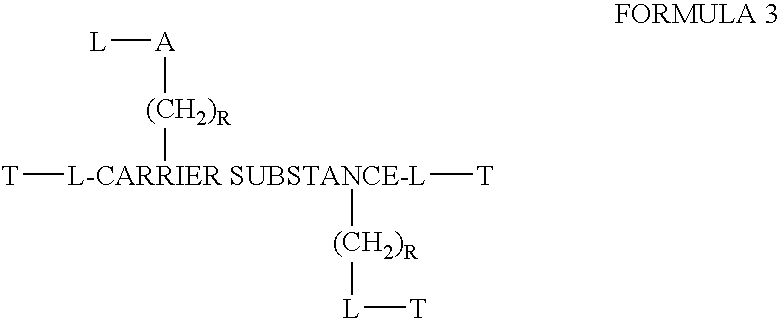
Chloroquine coupled nucleic acids and methods for their synthesis
InactiveUS20060040879A1Overcome limitationsFacilitated releaseSugar derivativesGenetic therapy composition manufactureTherapeutic effectPharmaceutical Substances
This invention discloses compositions and methods for preparing chloroquine-coupled nucleic acid compositions. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the nucleic acid needs to be released, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a nucleic acid directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing nucleic acids for therapeutic or other medical uses. The invention also discloses nucleic acid carrier compositions that are coupled to targeting molecules for targeting the delivery of nucleic acids to their site of action.
Owner:KOSAK KENNETH
Recombinant influenza vectors with tandem transcription units
InactiveUS7968101B2Efficient and robust generationReduce in quantitySsRNA viruses negative-senseBiocideHelper virusViral vector
The invention provides a composition useful to prepare influenza viruses, e.g., in the absence of helper virus, using vectors which include tandem transcription cassettes containing PolI and / or PolII promoters.
Owner:WISCONSIN ALUMNI RES FOUND
Compostions comprising viruses and methods for concentrating virus preparations
InactiveUS6544769B1Maintain their viabilityMaintain abilityBiocidePeptide/protein ingredientsVirusHydrocarbon
A composition is disclosed comprising virus in a formulation comprising a polyhydroxy hydrocarbon buffered to maintain a pH in a range from about 7 to about 8.5 at a temperature in the range from about 2° C. to 27° C. Methods for concentrating and purifying virus preparations are also disclosed.
Owner:MERCK SHARP & DOHME CORP
Method for introducing and expressing genes in animal cells, and live invasive bacterial vectors for use in the same
A method for introducing and expressing genes in animal cells is disclosed comprising infecting said the animal cells with live invasive bacteria, wherein bacteria contain a eukaryotic expression cassette encoding said gene. The gene may encode, e.g., a vaccine antigen, an therapeutic agent, an immunoregulatory agent or a anti-sense RNA or a catalytic RNA.
Owner:UNIV OF MARYLAND
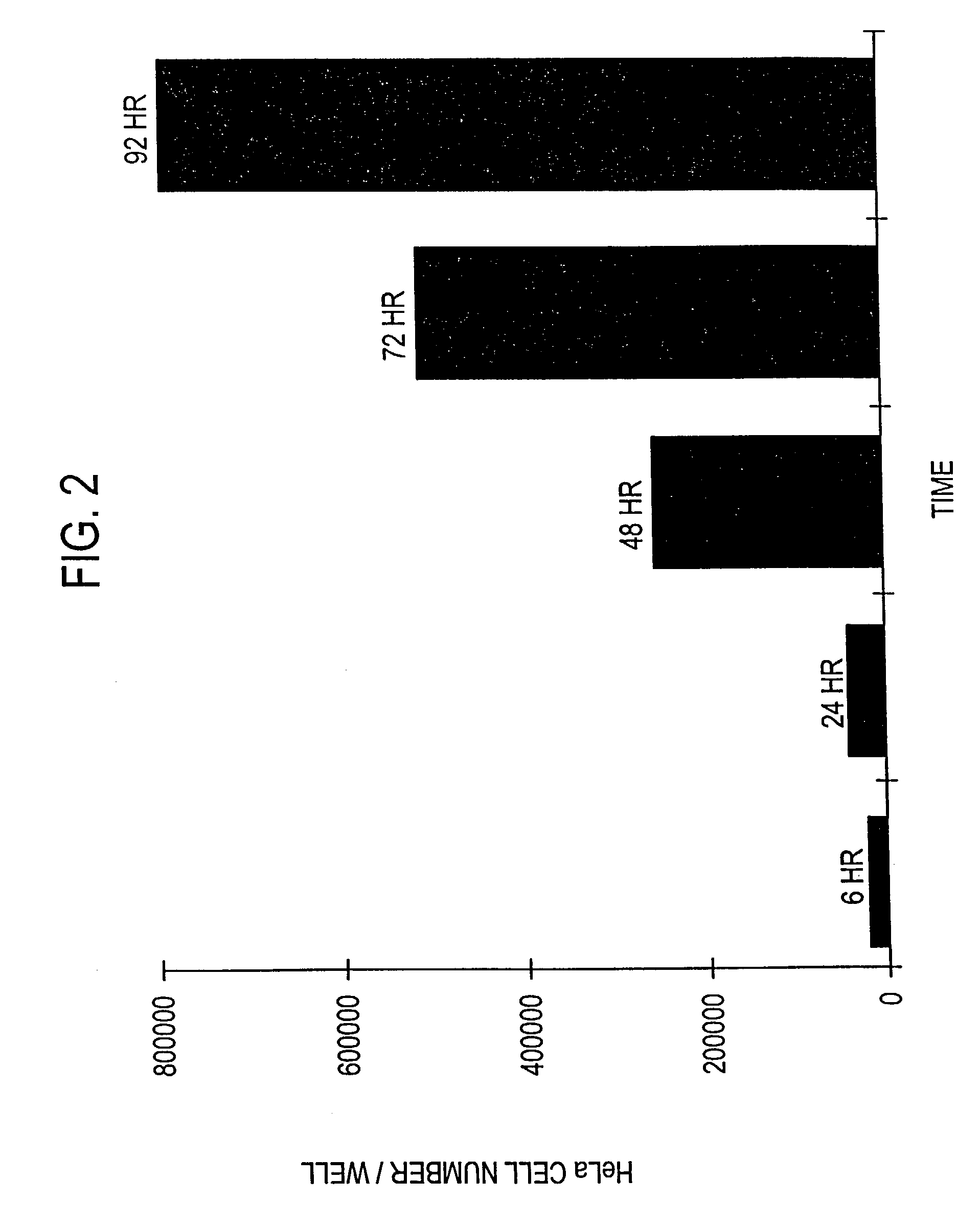
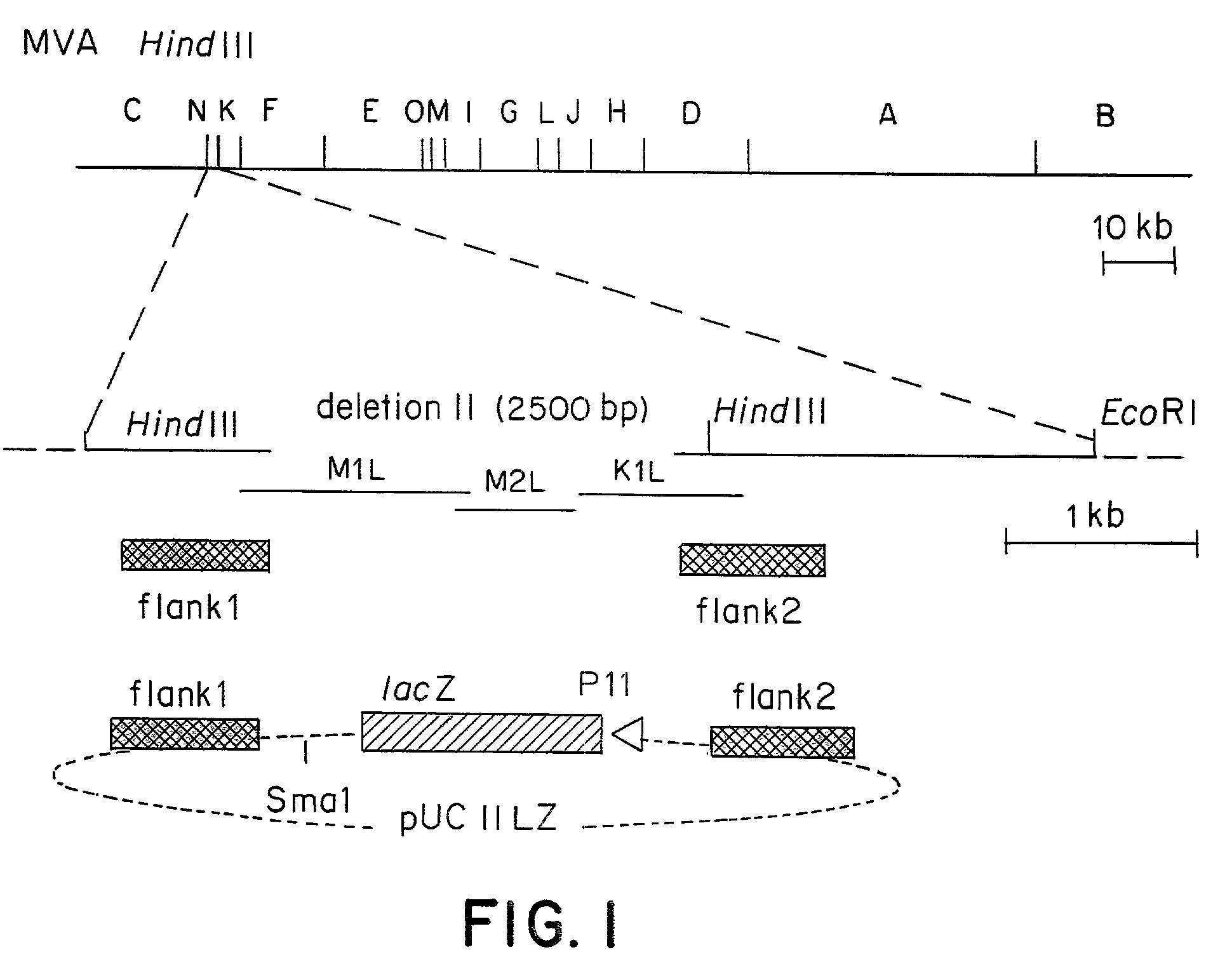
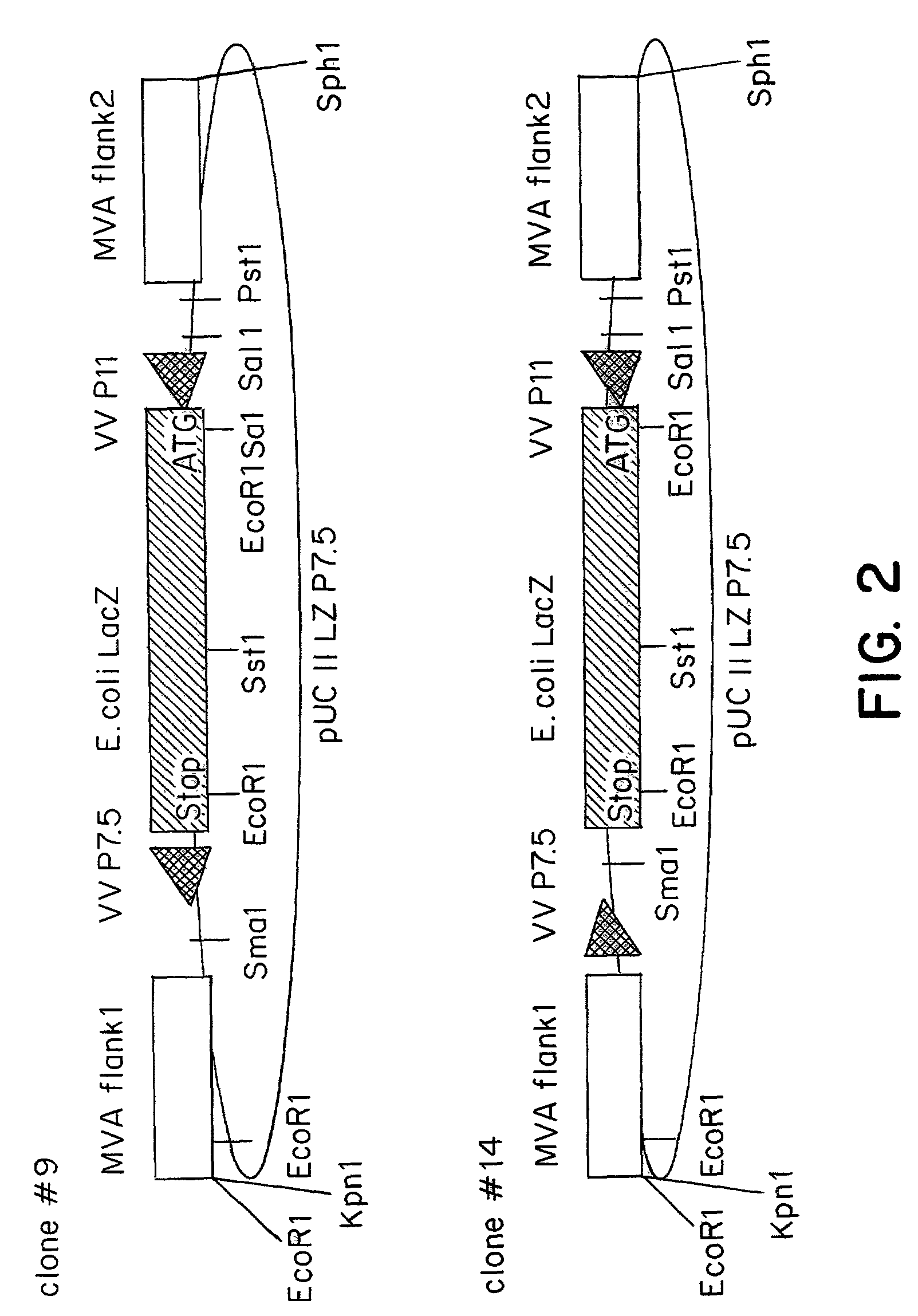
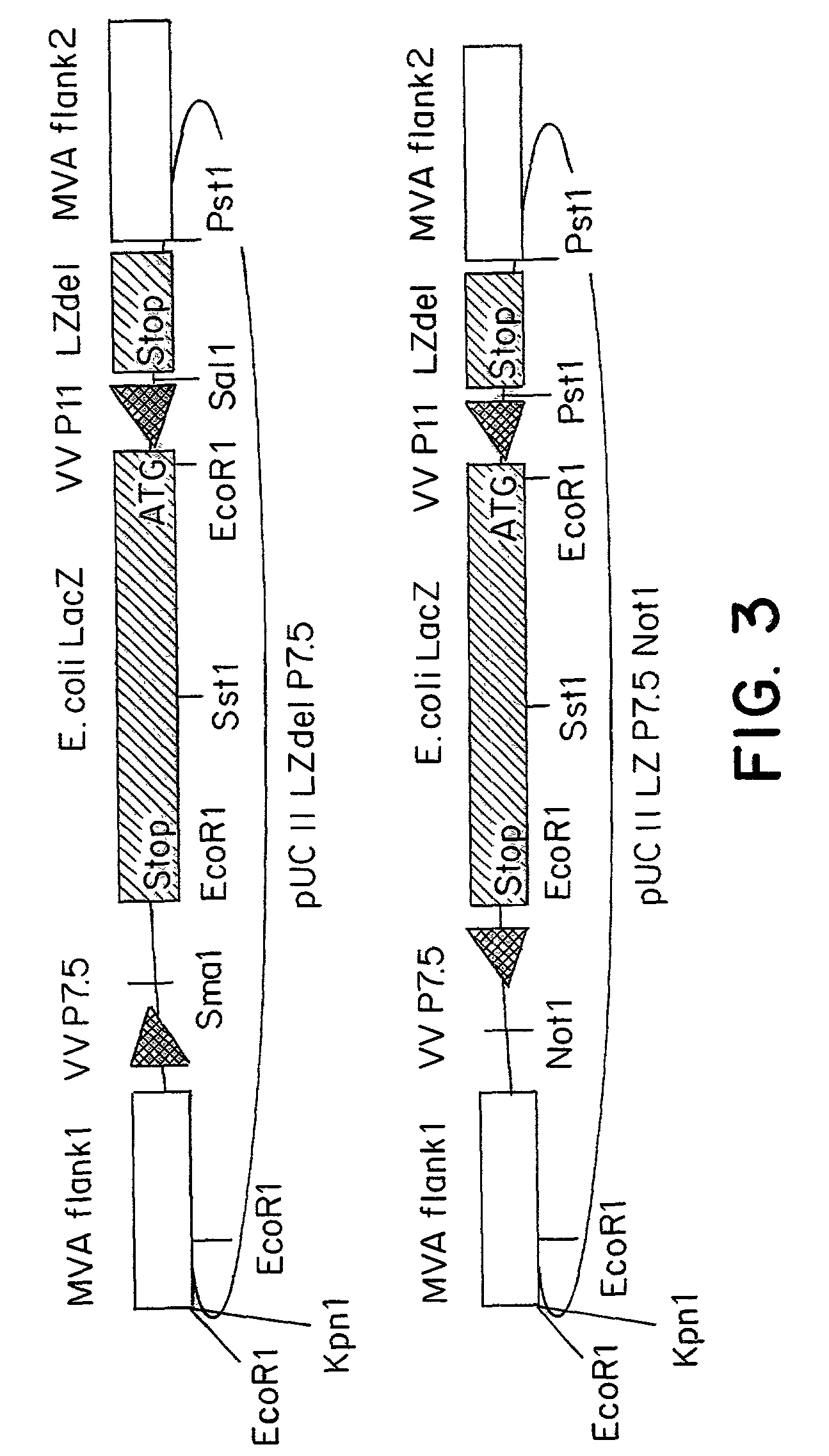
Recombinant MVA virus, and the use thereof
InactiveUS7198934B2Improve efficiencyReduce the amount of solutionOrganic active ingredientsVirusesAntigenViral vector
The present invention relates to recombinant vaccinia viruses derived from the modified vaccinia virus Ankara (MVA) and containing and capable of expressing foreign genes which are inserted at the site of a naturally occurring deletion in the MVA genome, and the use of such recombinant MVA viruses for the production of polypeptides, e.g. antigens or therapeutic agents, or viral vectors for gene therapy, and the use of such recombinant MVA viruses encoding antigens as vaccines.
Owner:GSF FORSCHUNGSZENT FUR UMWELT & GESUNDHEIT
Scalable production method for AAV
ActiveUS9198984B2High yieldHigh purityPeptide/protein ingredientsGenetic therapy composition manufactureLysisBinding site
A method for producing AAV, without requiring cell lysis, is described. The method involves harvesting AAV from the supernatant. For AAV having capsids with a heparin binding site, the method involves modifying the AAV capsids and / or the culture conditions to ablate the binding between the AAV heparin binding site and the cells, thereby allowing the AAV to pass into the supernatant, i.e., media. Thus, the method of the invention provides supernatant containing high yields of AAV which have a higher degree of purity from cell membranes and intracellular materials, as compared to AAV produced using methods using a cell lysis step.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Constructs and Methods for Delivering Molecules via Viral Vectors with Blunted Innate Immune Responses
InactiveUS20140271550A1Increase gene expressionLower immune responseBiocideSugar derivativesNucleotideGene product
A CpG-modified recombinant adeno-associated viral (AAV) vector is described. The vector carries a nucleic acid molecule comprising AAV inverted terminal repeat (ITR) sequences and an exogenous gene sequence under the control of regulatory sequences which control expression of the gene product, in which the nucleic acid sequences carried by the vector are modified to significantly reduce CpG di-nucleotides such that an immune response to the vector is reduced as compared to the unmodified AAV vector. Also provided are methods and regimens for delivering transgenes using these AAV viral vectors, in which the innate immune response to the vector and / or transgene is significantly modulated.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +2
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
InactiveUS20050266567A1Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:ATKINSON EDWARD M +5
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com