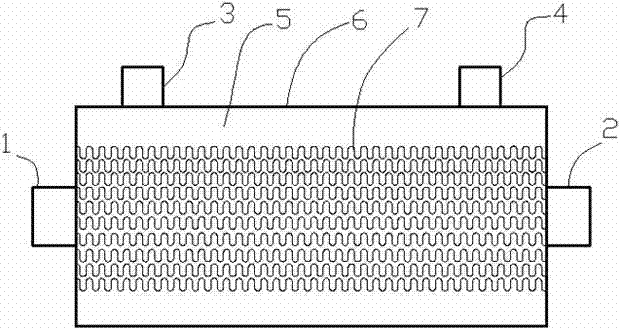
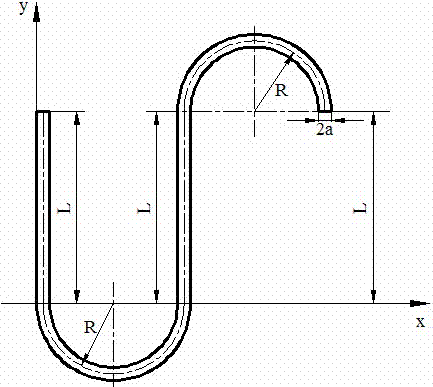
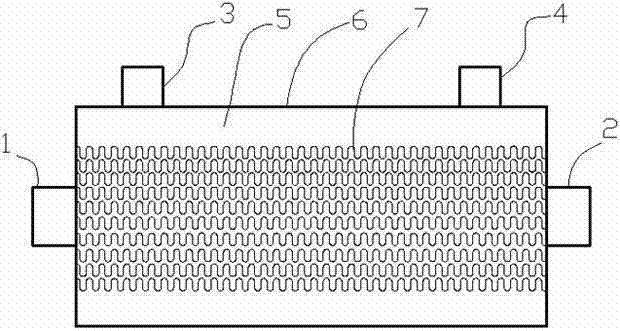
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
79 results about "Artificial livers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An artificial liver is a device which is generally made from real human liver cells. Most times it is used to help patients who suffer from acute or chronic liver failure.
Induced hepatocytes
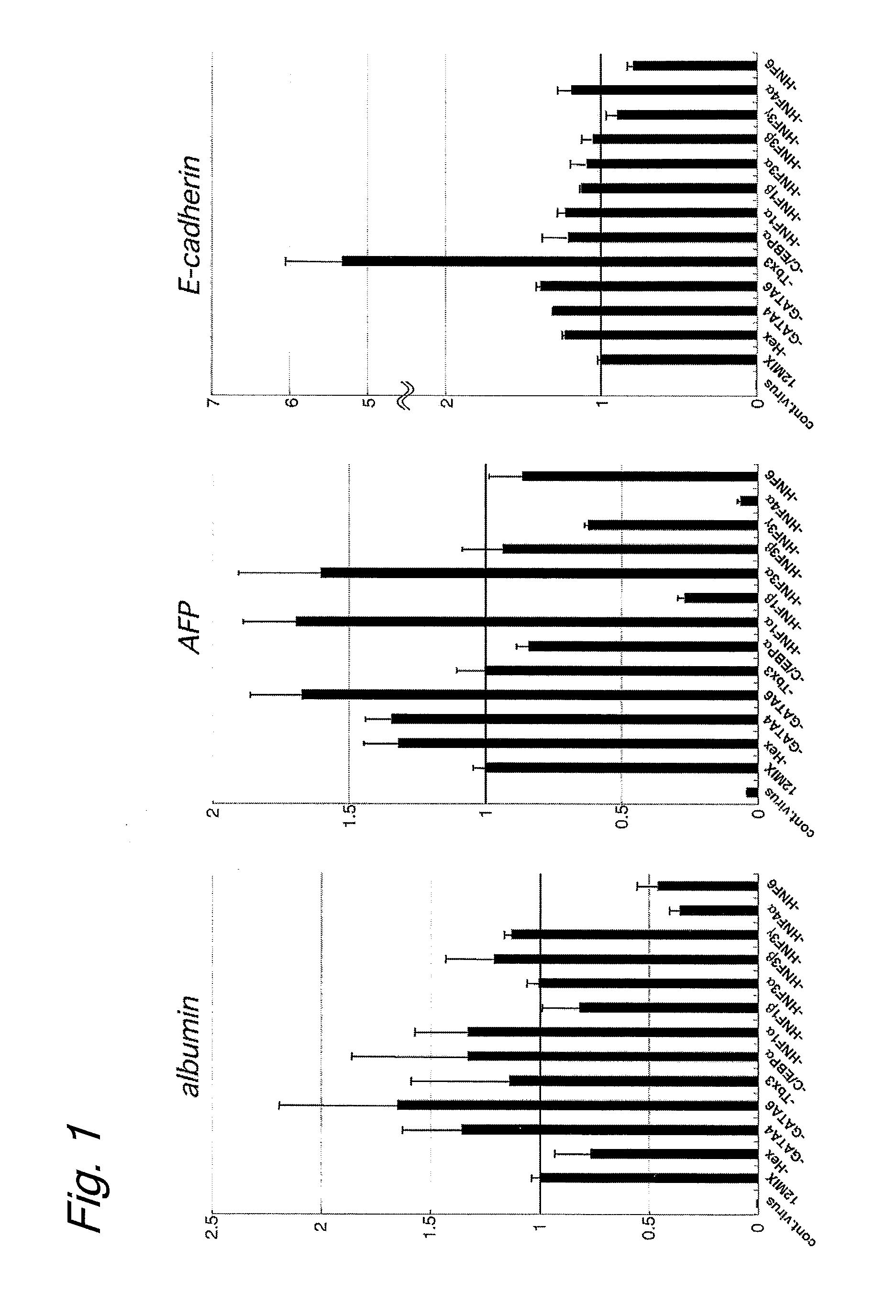
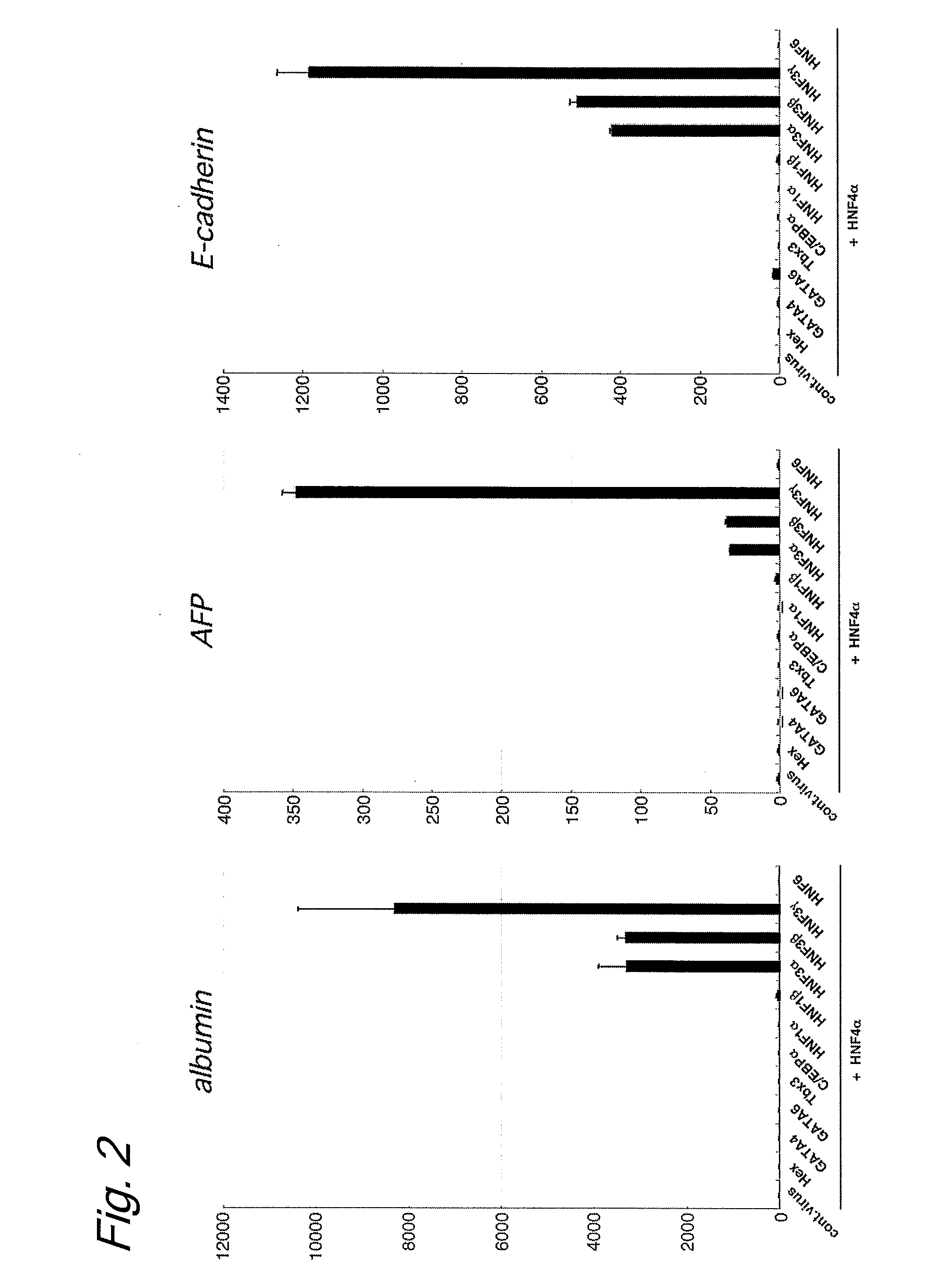
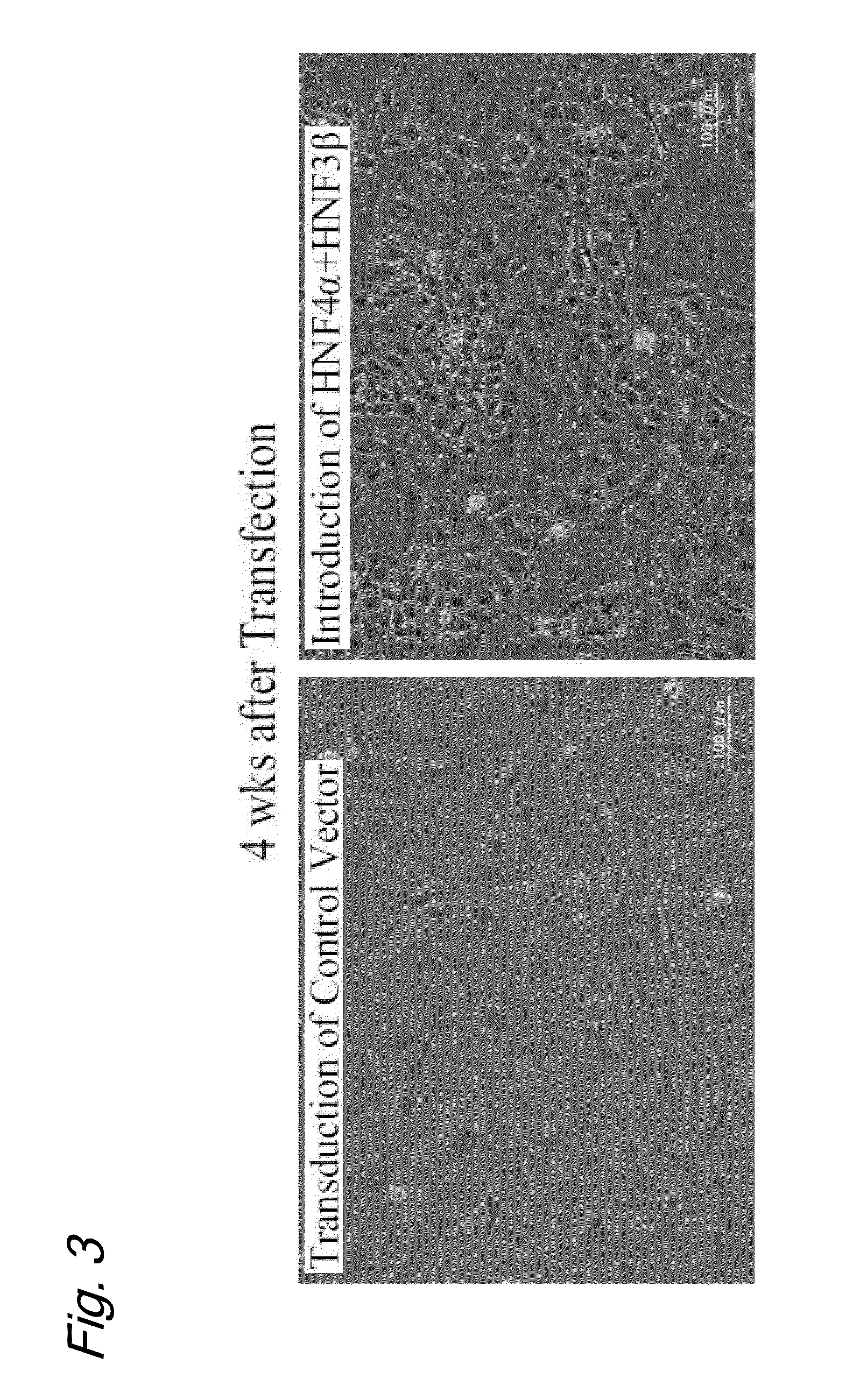
An object of the present invention is to produce hepatic cells from non-hepatic cells that can be obtained less invasively and at low cost. The gene of HNF3α, HNF3β, or HNF3γ and the gene of HNF4α are transduced into non-hepatic cells. The present invention enables the production of induced hepatocytes from somatic cells that are non-hepatic cells, such as skin cells. The use of the induced hepatocytes obtained by the present invention can establish and develop, as general medical treatment, cell transplantation into the liver, artificial livers, and drug response tests, which are the areas that have not been generalized because of the difficulty of procuring hepatocytes.
Owner:JAPAN SCI & TECH CORP
Dialysis device
A dialysis apparatus for treating a patient with liver and / or kidney disease. In one embodiment a liver dialysis apparatus comprises an artificial liver. The artificial liver comprises a blood compartment, a vegetable protein compartment, and a clear dialysis compartment. In another embodiment the liver dialysis apparatus comprises cartridge made up of at least one layer of vegetable protein. In a further embodiment of the invention a dialysate regeneration device is provided which comprises at least one layer of vegetable protein. The vegetable protein is preferably a soy protein. The soy protein may be unmodified soy protein with urease enzyme activity, modified soy protein with or without urease activity, alone or in combination.
Owner:DE PAOLIS POTITO +1
Implantation type artificial hepar
ActiveCN101428154AOvercoming longstanding vascularization challengesHigh activityProsthesisBiological materialsBlood vessel
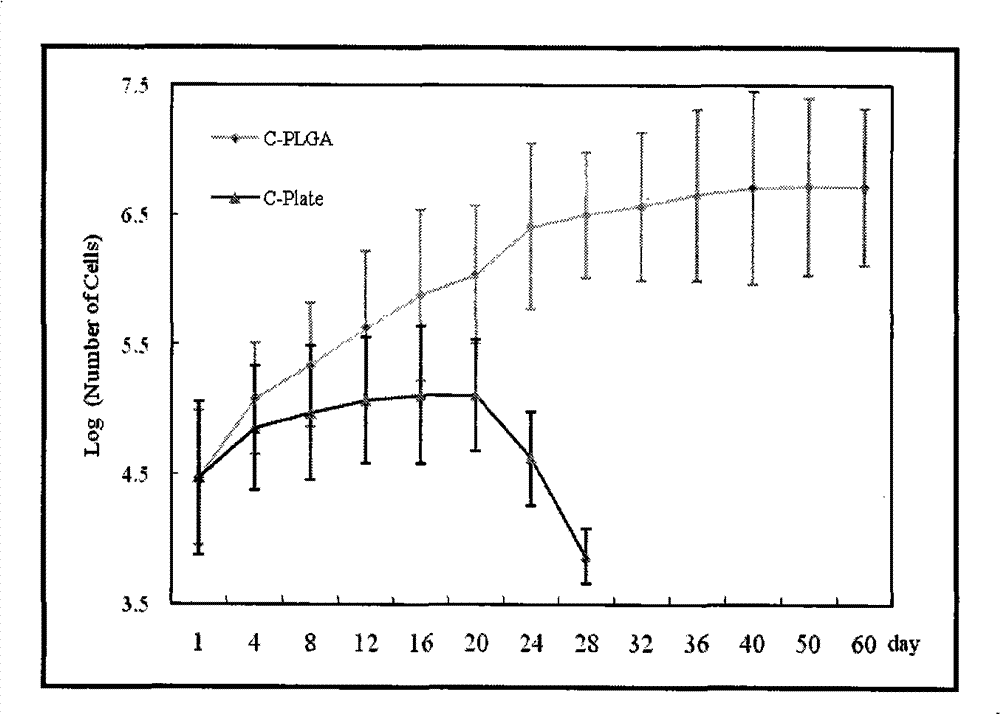
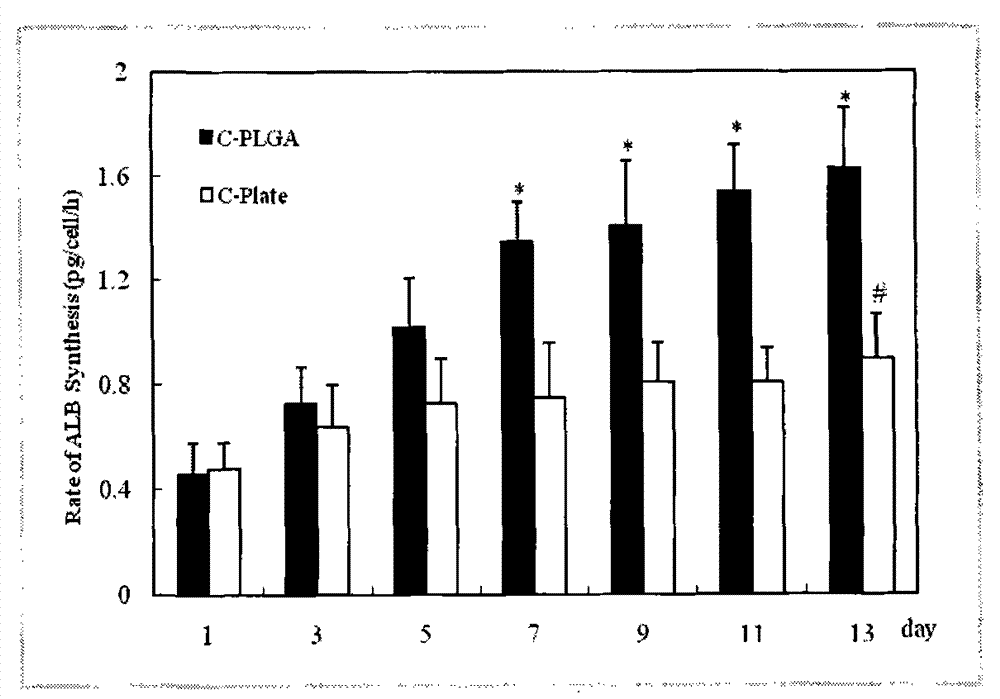
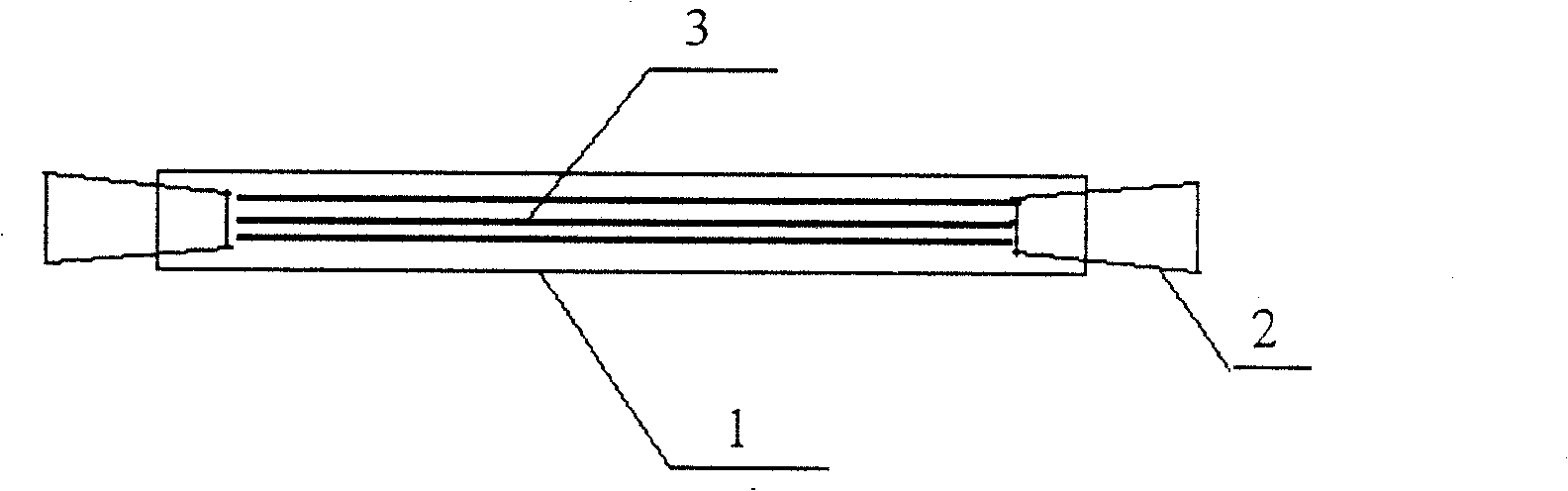
The invention discloses an implanted artificial liver comprising a polyglycolic acid or polylactic-co-glycolic acid sleeve, more than one three-dimensional porous scaffold, and polyglycolic acid or polylactic-co-glycolic acid tube seals; the three-dimensional porous scaffolds are mounted inside the polyglycolic acid or polylactic-co-glycolic acid sleeve; and polyglycolic acid or polylactic-co-glycolic acid tubes are used for sealing two ends of the polyglycolic acid or polylactic-co-glycolic acid sleeve. The invention has the beneficial effects of maximally simulating the structure of a hepatic lobule, developing implanted stem cells into liver cells through directional differentiation, and inducing the regeneration of microvessels of a tissue engineering liver. Meanwhile, the exterior of the three-dimensional porous scaffolds is wrapped in the highly biocompatible biomaterial sleeve which serves to simulate the structure of a liver capsule; and small lacunas are formed between the sleeve and the scaffolds to allow blood to circulate through tissue engineering brackets, thereby facilitating the regeneration of vessels of the engineering liver in an envelope, and ultimately forming an artificial liver to replace the damaged liver of a parasitifer.
Owner:ZHEJIANG UNIV
Dialysis device
A dialysis apparatus for treating a patient with liver and / or kidney disease. In one embodiment a liver dialysis apparatus comprises an artificial liver. The artificial liver comprises a blood compartment, a vegetable protein compartment, and a clear dialysis compartment. In another embodiment the liver dialysis apparatus comprises cartridge made up of at least one layer of vegetable protein. In a further embodiment of the invention a dialysate regeneration device is provided which comprises at least one layer of vegetable protein. The vegetable protein is preferably a soy protein. The soy protein may be unmodified soy protein with urease enzyme activity, modified soy protein with or without urease activity, alone or in combination.
Owner:DE PAOLIS POTITO +1
Functional hepatocyte induction method and special three-dimensional induction culture medium and application thereof
The invention discloses a functional hepatocyte induction method as well as a special three-dimensional induction culture medium and an application thereof, which belongs to the technical field of bioengineering. The functional hepatocyte three-dimensional induction culture medium provided by the invention contains a plurality of small molecules and cell factors, can be effectively applied to themethod for obtaining functional hepatocytes through induction of liver organs with stem / progenitor cell characteristics. The functional hepatocytes obtained by induction culture can be used for artificial liver production and in-vitro liver function simulation, and can also be used as seed cells for establishing a liver disease treatment drug screening model and an in-vitro liver disease simulation model.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Immortal unipotent porcine PICM-19H and PICM-19B stem cell lines
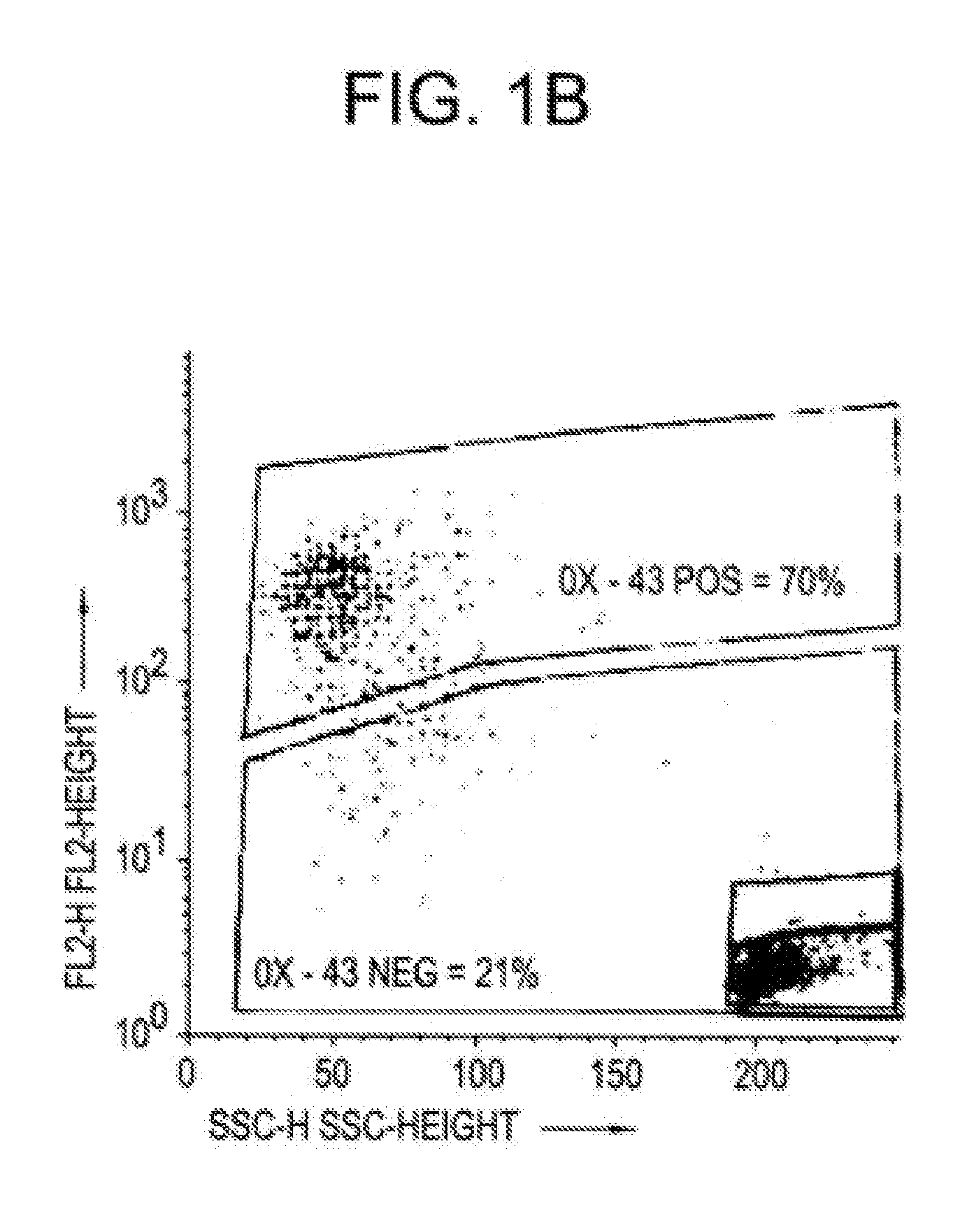
InactiveUS20090291064A1Confirmed its differentiationLow levelBiocideHepatocytesLipid formationArtificial liver
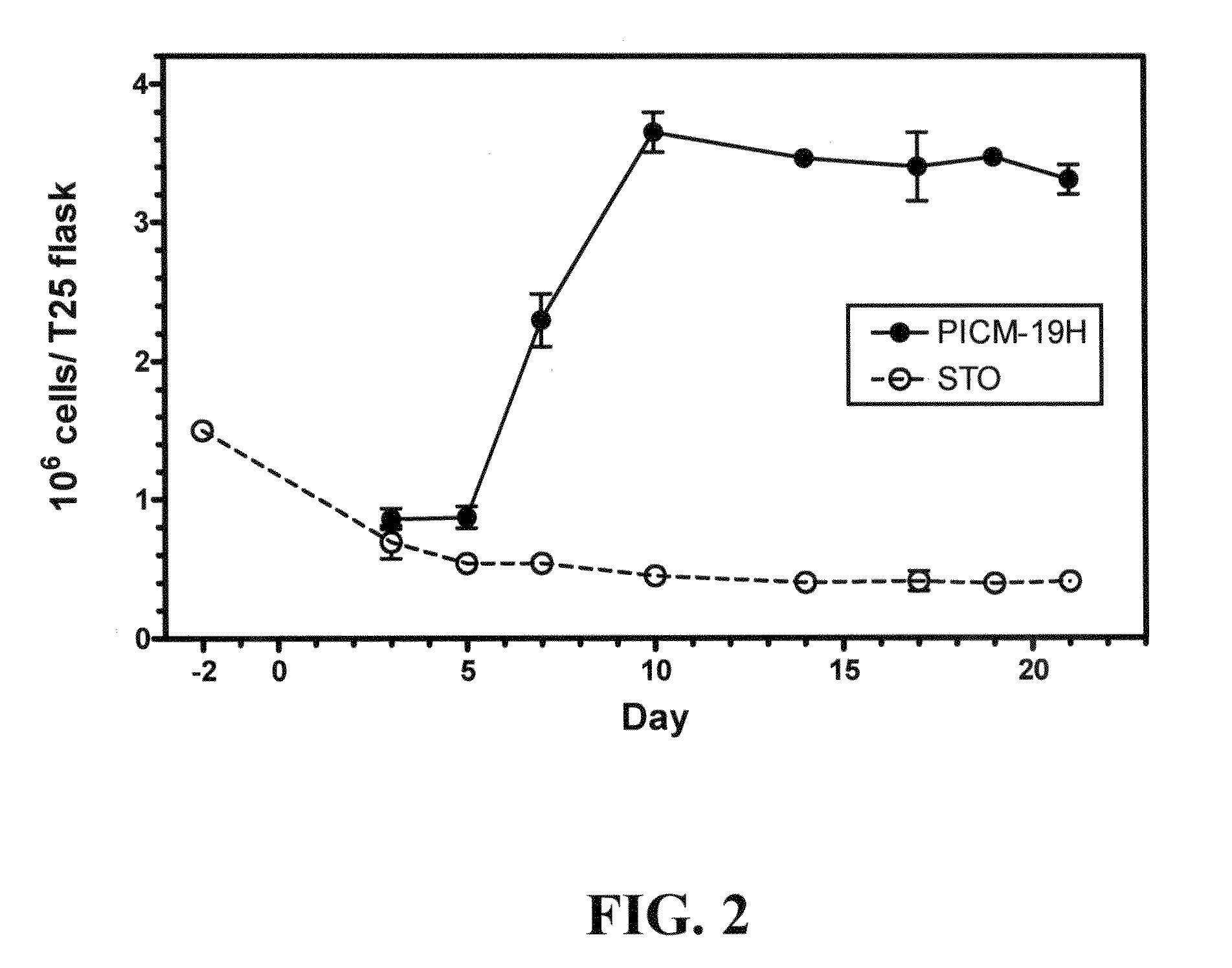
Two cell lines, PICM-19H and PICM-19B, were derived from the bipotent ARS-PICM-19 pig liver stem cell line and assessed for their potential application in artificial liver devices. The study included assessments of growth rate and cell density in culture, morphological features, and hepatocyte detoxification functions, i.e., inducible CYP450 activity, ammonia clearance, and urea production. The PICM-19H cells contain numerous mitochondria, Golgi apparatus, smooth and rough endoplasmic reticulum, vesicular bodies and occasional lipid vacuoles. PICM-19H cells display inducible CYP450 activity, clear ammonia, and produce urea in a glutamine-free medium. Ultrastructural analysis of the PICM-19B monolayers show that the roughly cuboidal cells display basal-apical polarization and are joined by tight junction-like complexes. Other ultrastructure features are similar to those of PICM-19H cells except that they possess numerous cell bodies resembling mucus vacuoles. The PICM-19B cells possess relatively high levels of GGT activity, but retain some inducible CYP450 activity, and some ammonia clearance and urea synthesis ability. These data indicate that both cell lines, either together or alone, may be useful as the cellular substrate for an artificial liver device. In vitro models of the liver are needed to replace animal models for the rapid assessment of drug biotransformation and toxicity. A unipotent porcine stem cell line PICM-19H differentiates exclusively into hepatocytes and can be induced to express CYP450 enzymes. These cells have many activities associated with xenobiotic phase I and phase II metabolism lacking in other liver cell lines. The PICM-19H cell line was also compared to the tumor-derived human HepG2 C3A cell line and to primary cultures of adult porcine hepatocytes. The results demonstrate the potential for the use of PICM-19H cells in drug biotransformation and toxicity testing and further support their use in artificial liver device technology.
Owner:UNITED STATES OF AMERICA
Micro cell hollow fiber reactor
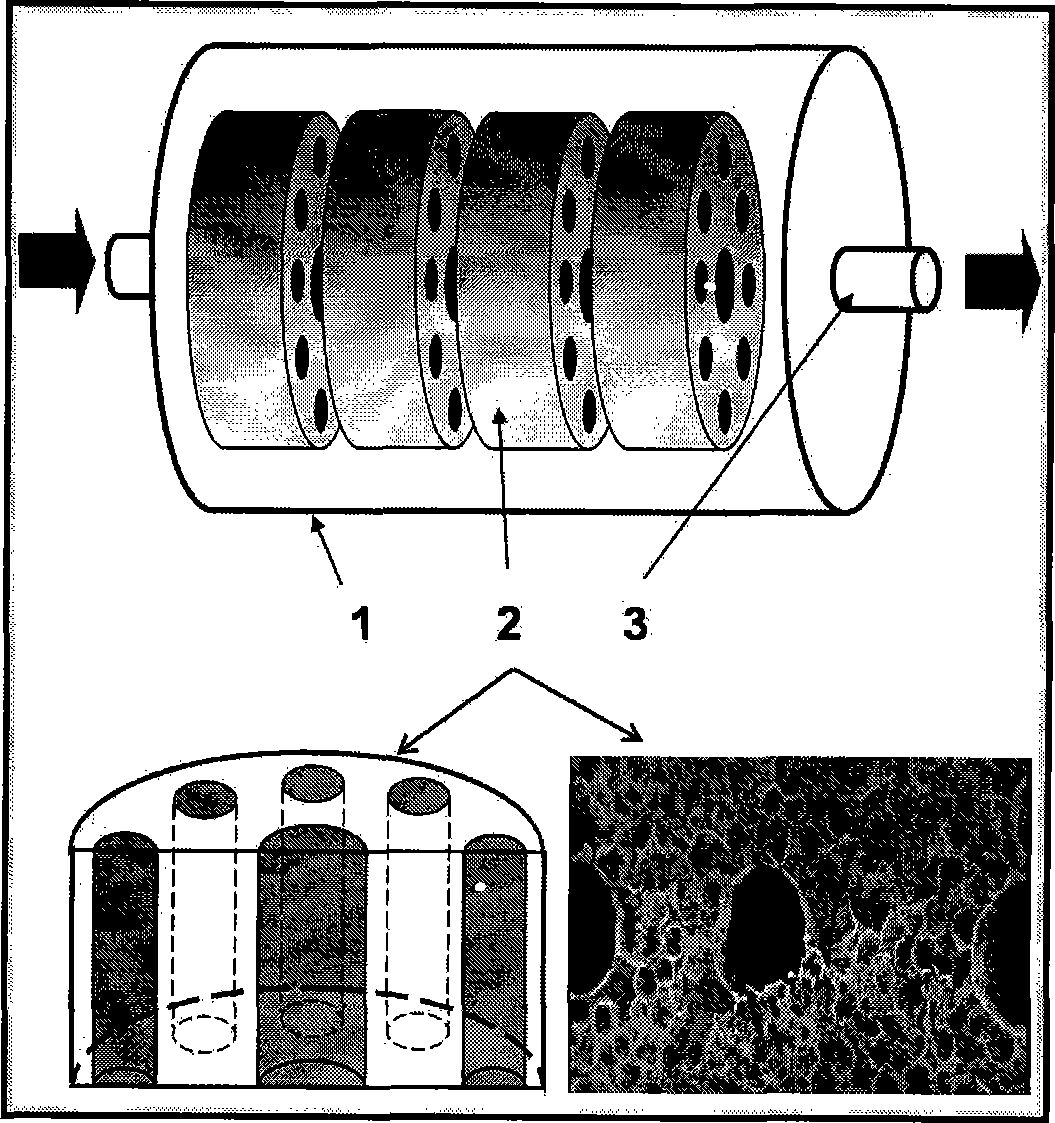
The micro hollow fiber cell reactor consists of silica gel tube of permeable silica gel material, permeable silica gel stopper and hollow fiber filament. Cell is embedded inside the hollow fiber filament with collagen gel, and the micro hollow fiber cell reactor has oxygen transferring and mass transferring capacity suitable for cell to survive and maintains the medicine metabolizing and other functions of cell. The micro hollow fiber cell reactor combines the advantages of miniature orifice plate and 3D bionic artificial liver reactor, maintains the bioactivity of animal cell. It may be used as in vitro mold of liver cell for the research of medicine metabolism and pharmacological and toxicological research and may be used in the lab research of cell culturing condition and cell product. It may be also used as the carrier for the research of animal cell culturing condition and lays the foundation for industrial production.
Owner:ZHEJIANG UNIV
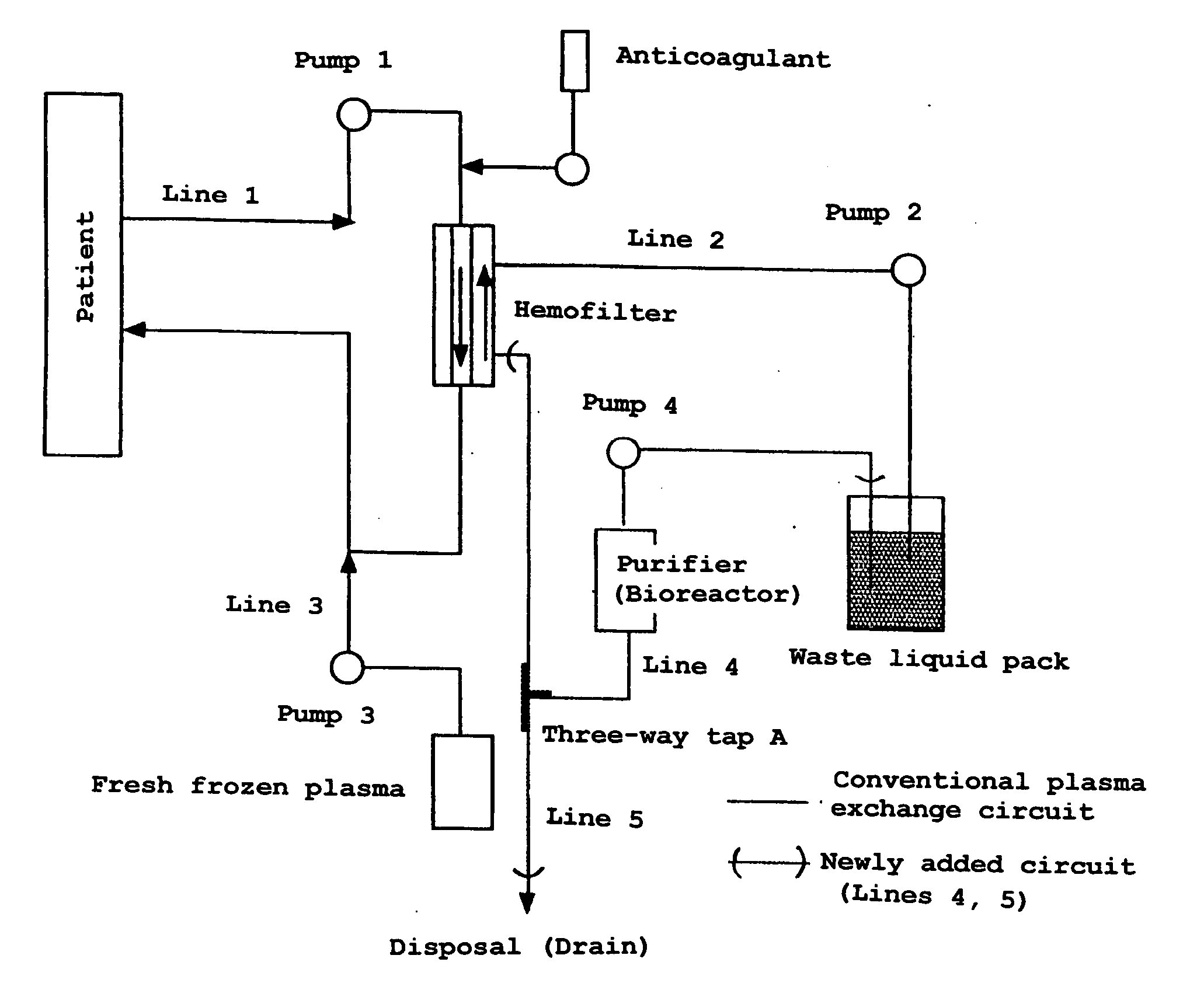
Plasma Exchange Waste Liquid Purification And Circulation Dialysis Apparatus
InactiveUS20070289928A1Safe to useEffectively removeLiquid separation auxillary apparatusOther blood circulation devicesUrologyBlood plasma
The object of the invention is to provide an apparatus used in a plasma exchange therapy, which is operable with only a minor modification to a conventional plasma exchange apparatus and wherein valuable plasma is not disposed but recycled as a dialysis solution. The present invention is related to an apparatus used in a plasma exchange therapy wherein at least a portion of separated plasma is purified and circulated as a dialysis solution to dialyze patient's blood to remove harmful substances contained in the plasma; a plasma exchange apparatus incorporating said apparatus; and an artificial liver equipped with these apparatuses.
Owner:JAPAN SCI & TECH CORP
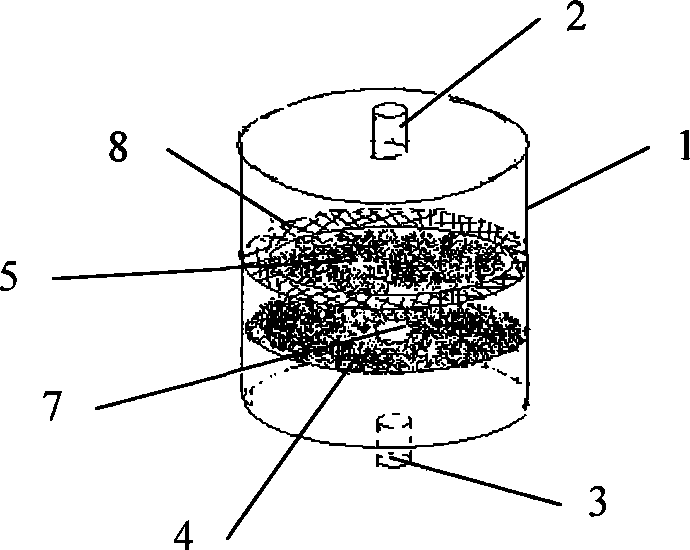
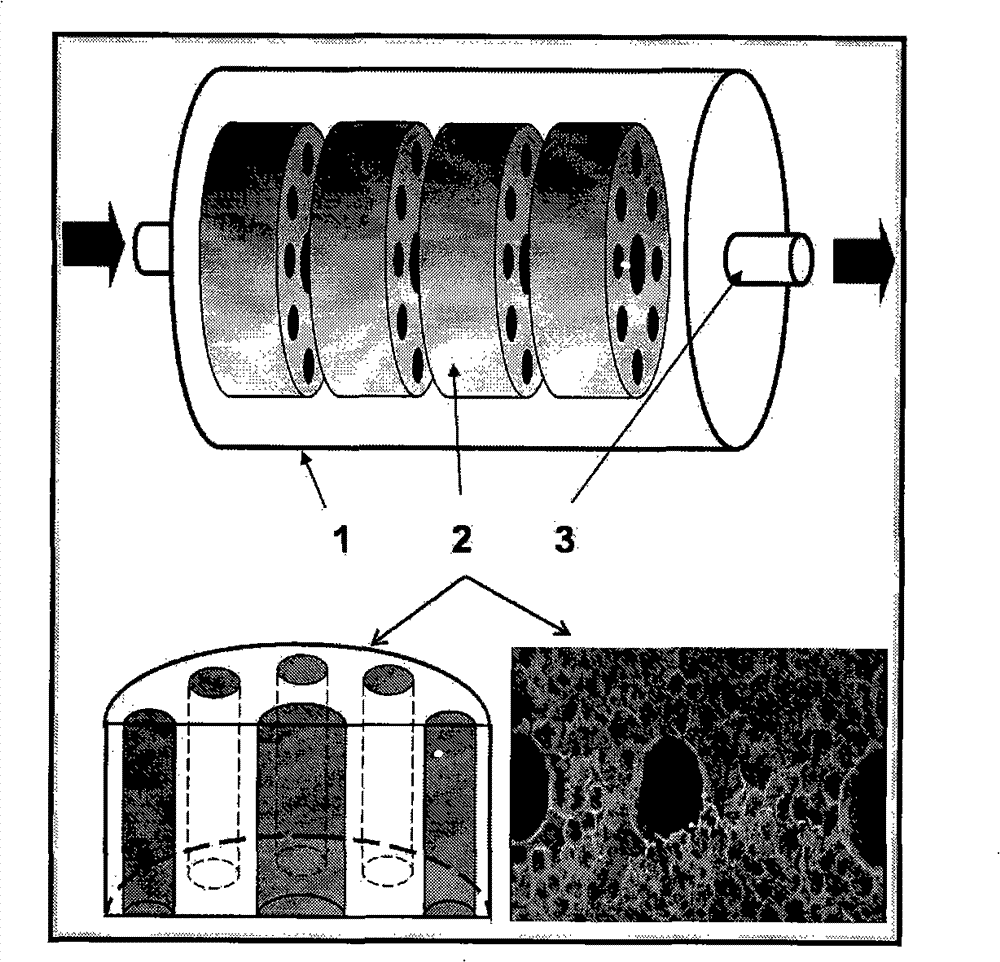
Multi-layer diaphragm structure perfusion bioreactor and application
ActiveCN101381678AMaintain active functionEvenly distributedTissue/virus culture apparatusMultilayer membranePerfusion bioreactor
The invention provides a perfusion type bioreactor with a multilayer membrane structure, which comprises a cylindrical case body, a liquid inlet, a liquid outlet, a concentric membrane and a solid circular membrane, wherein a groove is arranged on the inner wall of the case body; a channel is arranged in the middle of the concentric membrane; a latticed hole is arranged on the edge of the solid circular membrane; the membranes are embedded in the case body through the groove; and the liquid inlet and the liquid outlet are in threaded connection with a spiral cover. The perfusion type bioreactor provides a three-dimensional growing environment for cells, and allows the cells to be distributed evenly, so that the cells can maintain active function for a long time; flood flows through the multilayer membrane structure in the reactor and is directly contacted with the cells for sufficient exchange of substances. The perfusion type bioreactor has the advantages of reasonable design, compact arrangement of the structure, easy amplification and convenient transportation, and can be applied to a biologic artificial liver hepatic perfusion system.
Owner:ZHEJIANG UNIV
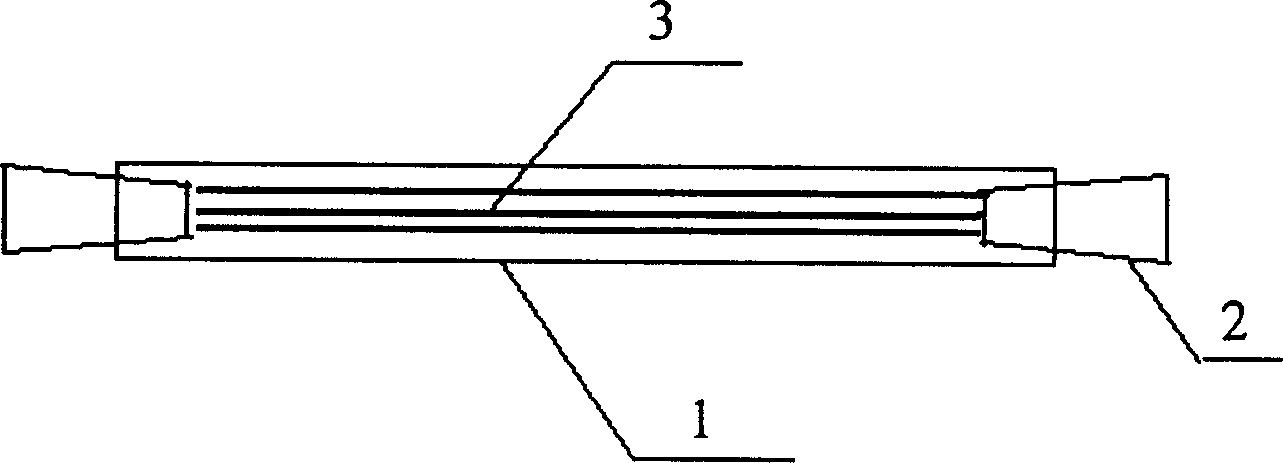
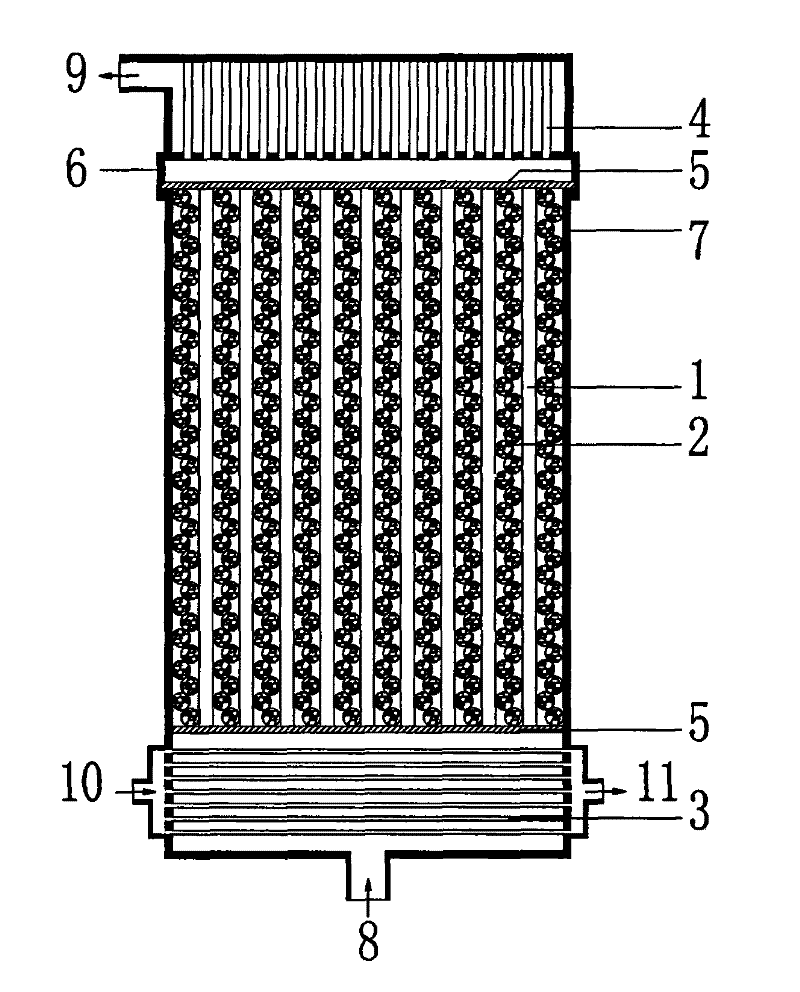
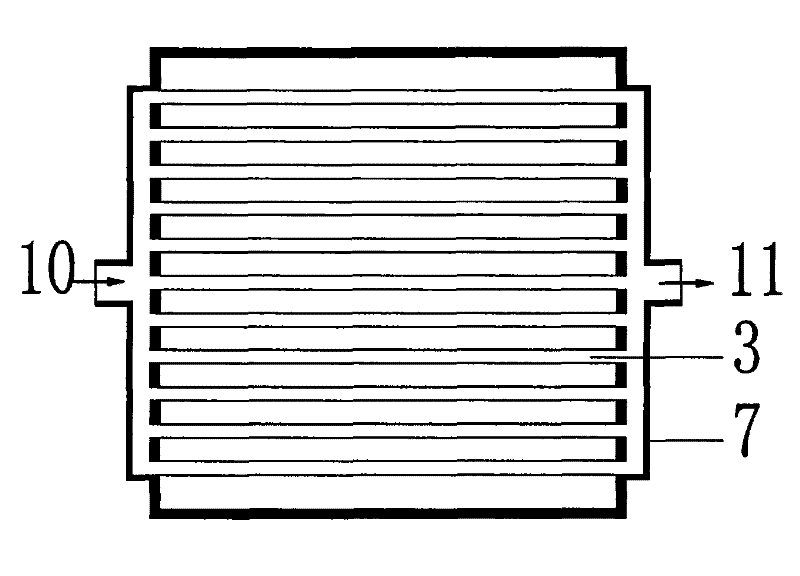
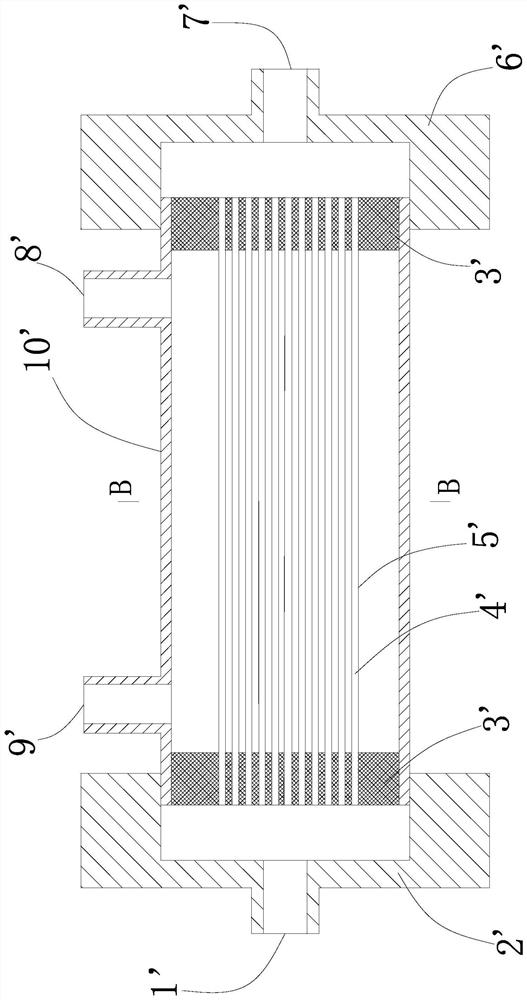
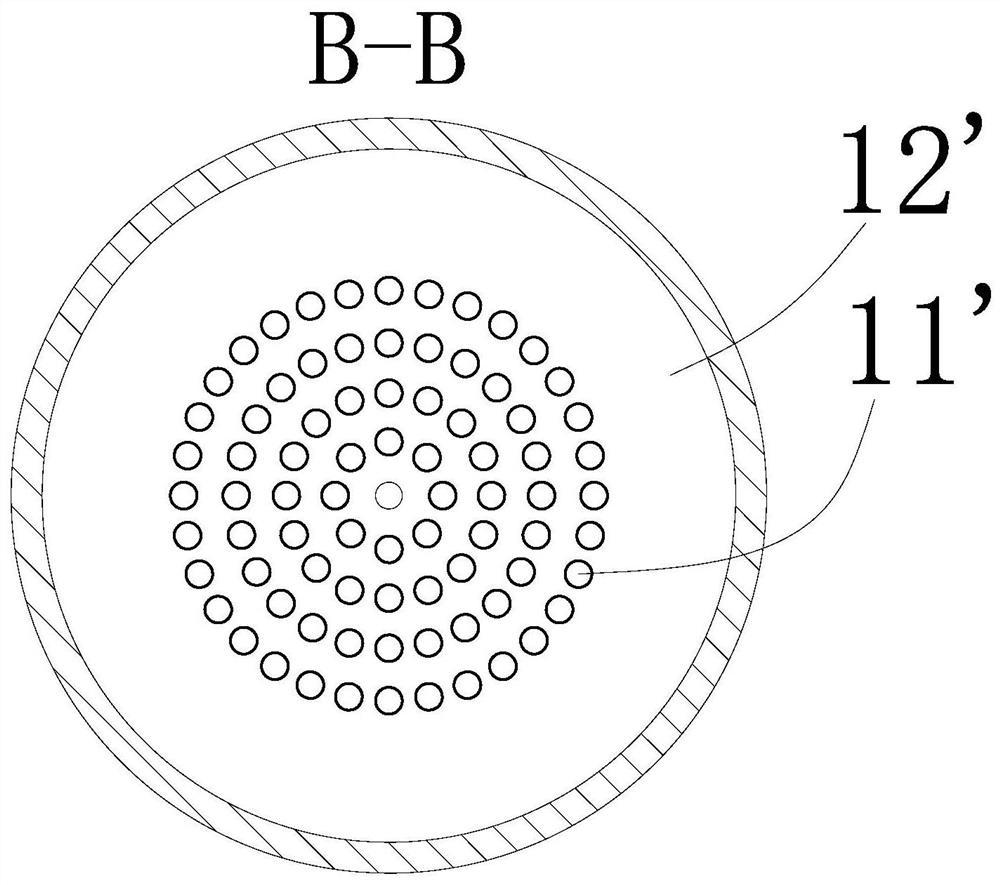
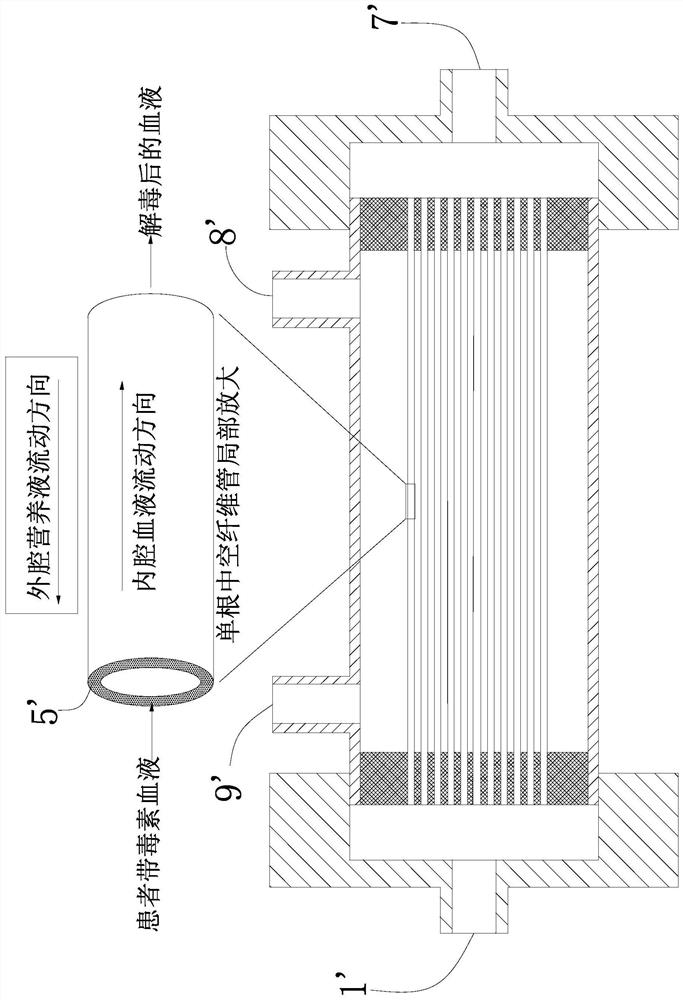
U-shaped hollow fiber pipe artificial liver bioreactor
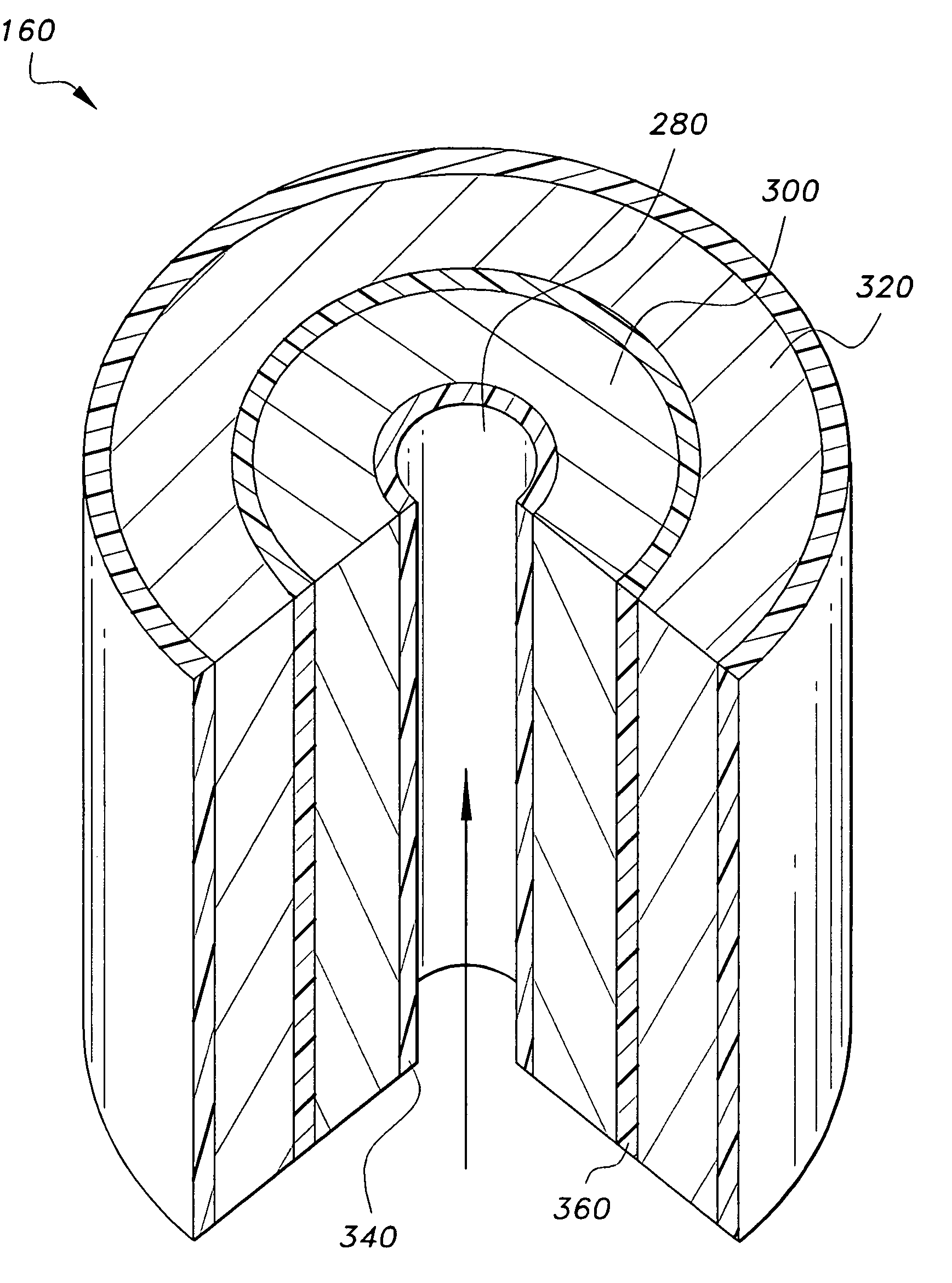
The invention discloses a U-shaped hollow fiber pipe artificial liver bioreactor. A plurality of hollow fiber pipes are uniformly encapsulated in parallel at the center in a transparent cylindrical vessel, the insides of the hollow fiber pipe from an inner cavity, an outer cavity is arranged outside the hollow fiber pipes, a patient plasma / blood inlet and a patient plasma / blood outlet of the inner cavity are respectively arranged at the two ends of the cylindrical vessel, a liver cell culture solution inlet and a liver cell culture solution outlet of the outer cavity are respectively arranged on the cylindrical surface of the cylindrical vessel in parallel; and the plurality of hollow fiber pipes are U-shaped hollow fiber pipes. The U-shaped hollow fiber pipe bioreactor disclosed by the invention can guarantee enough area so that liver cells are attached to the walls of the pipes to grow, and blood flow of the curved parts of the U-shaped pipes can generate secondary flow, thus the blood can be fully mixed; and the phenomena that plasma protein is adhered to the walls of the pipes in large amount to block the hollow fiber membrane holes can be avoided, thus being beneficial to detoxification and mass transfer of the hollow fiber, prolonging the supporting action time of an in vitro artificial liver and improving the treatment efficiency of an artificial liver.
Owner:ZHEJIANG UNIV
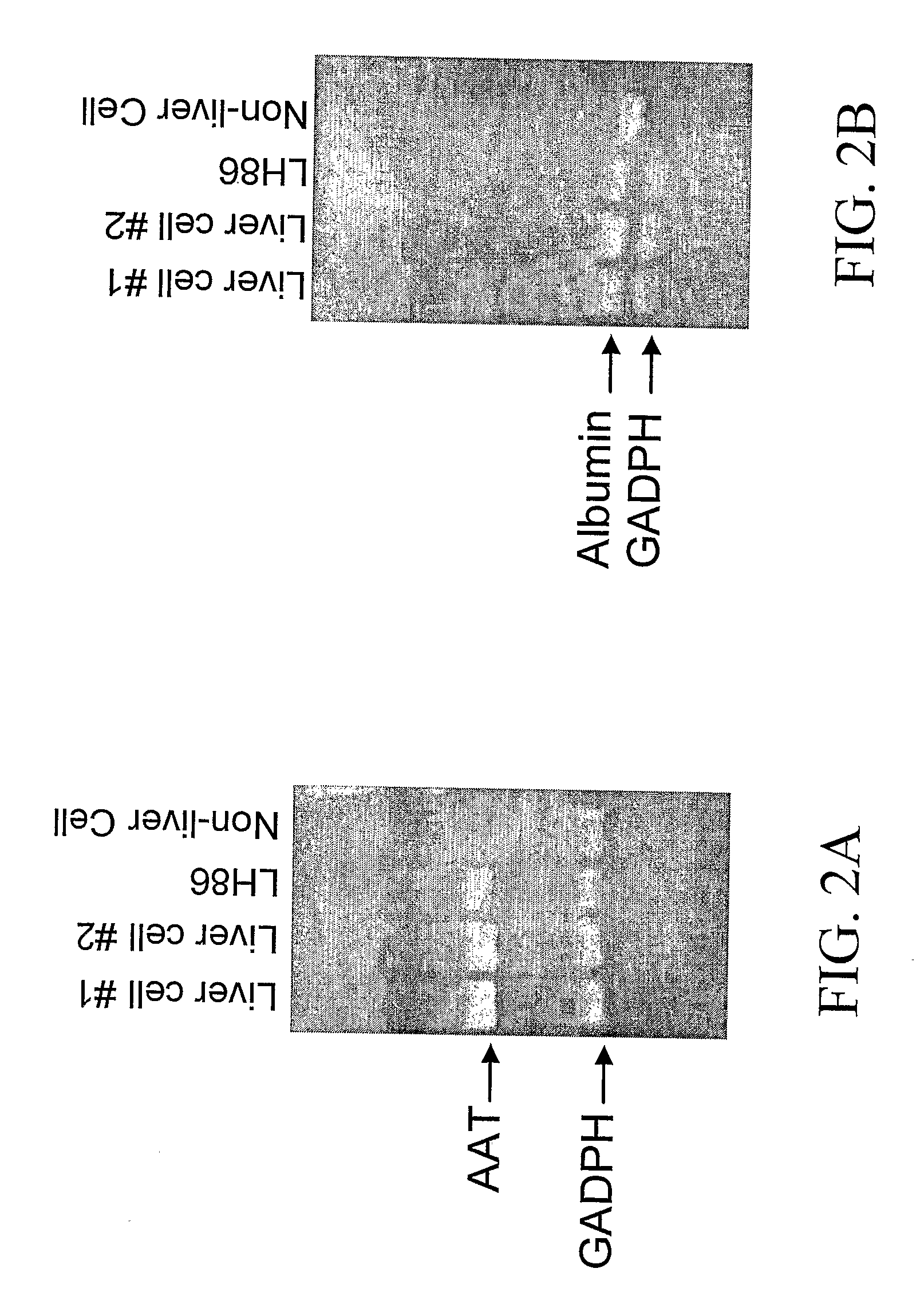
Human Liver Cancer Cell Line
InactiveUS20090222933A1Microbiological testing/measurementGenetically modified cellsHuman animalArtificial liver
The present invention relates to a human hepatoma-derived cell line, LH86, liver cell cultures, non-human animal models, artificial livers, liver assist devices, screening assays, and other applications of the cell line as an investigational tool.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
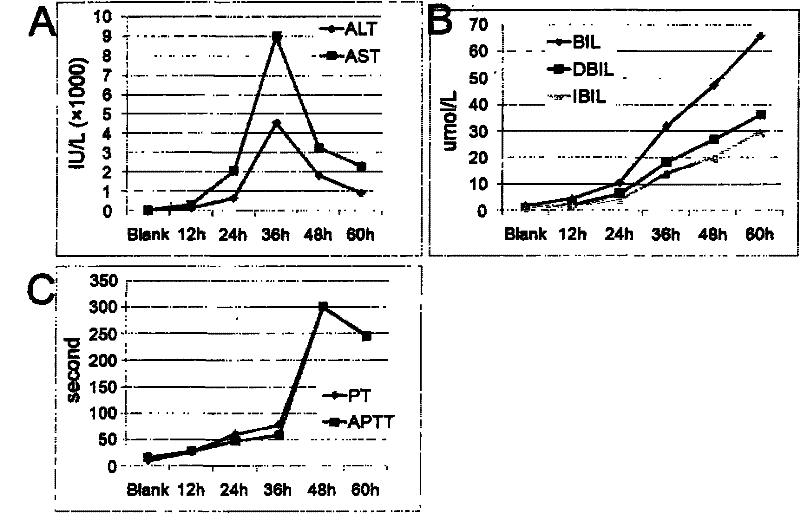
Establishment of fulminant liver function failure model of rhesus
The invention provides preparation of an animal model for screening hepatocyte growth-promoting regenerative medicines in fulminant liver function failure curing process and evaluating therapeutic effect of a tissue engineering liver supporting system or hepatic cell and stem cell transplantation by using amanitin and endotoxin lipopoly-saccharide, wherein an application method adopts single intraperitoneal instillation with dosage of 0.1mg / kg, and the dosage of the endotoxin is 1mug / kg. The invention further provides a method for preparing fulminant liver function failure and a fulminant liver function failure animal model prepared by the method. The animal model can be used for evaluation of biotechnological new drugs that a rodent animal model cannot accomplish, and can be used for the evaluation of tissue engineering liver, biological artificial liver, the hepatic cell and stem cell transplantation treatment technologies.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +2
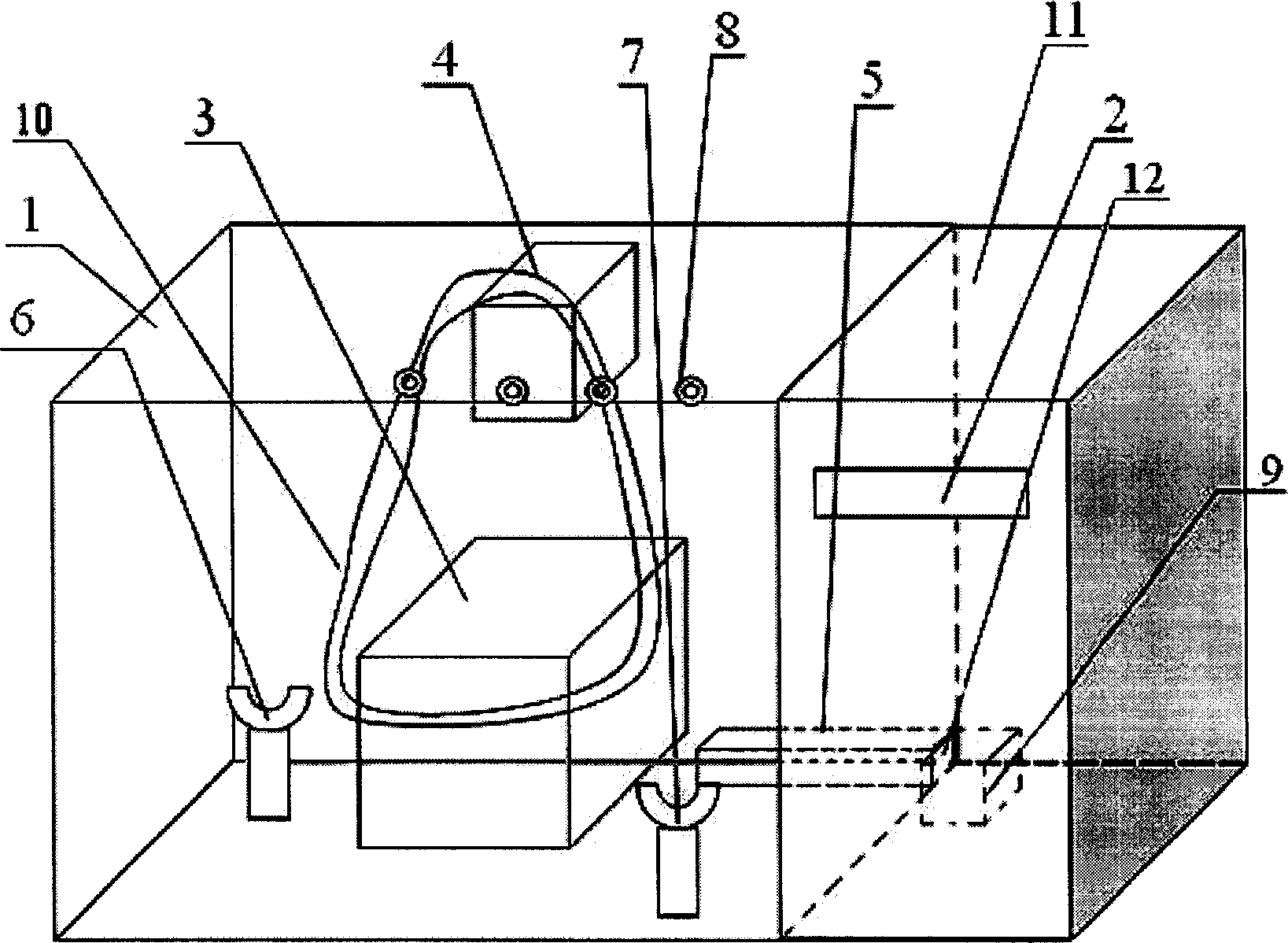
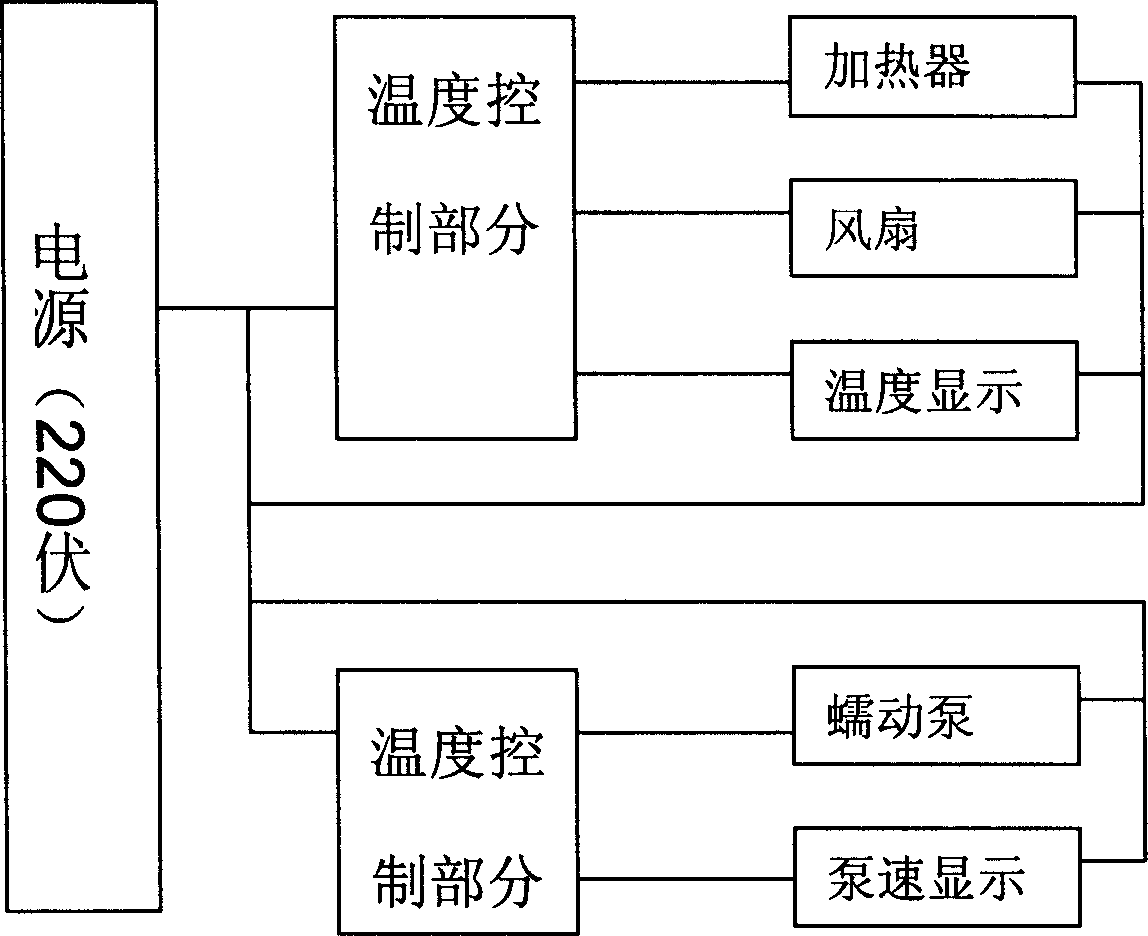
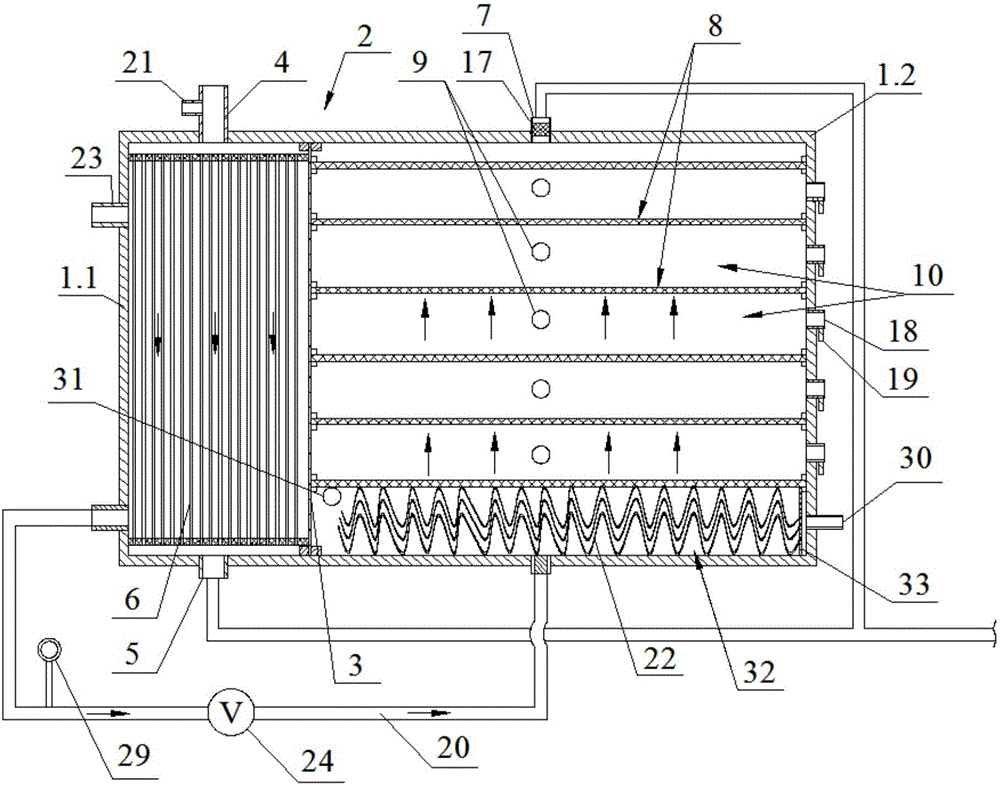
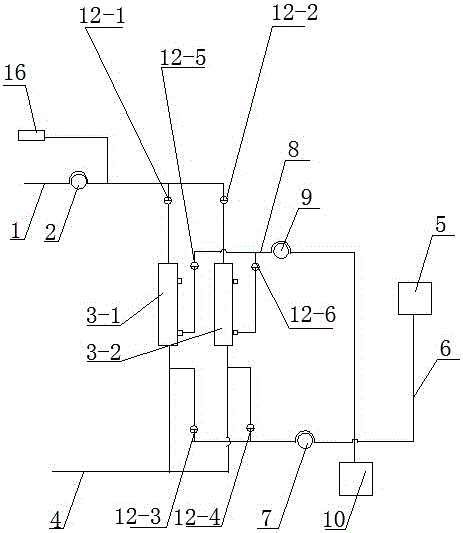
Apparatus adapted for artificial liver
The invention discloses an apparatus adapted for artificial liver, which comprises a holding portion and a control portion, wherein the holding portion is a transparent container made of heat-insulating material equipped with peristaltic pump, a clip holder and conduit support bracket, the control component comprises an electric heater, a heating arrangement, a temperature control system and a control display screen.
Owner:ZHEJIANG UNIV
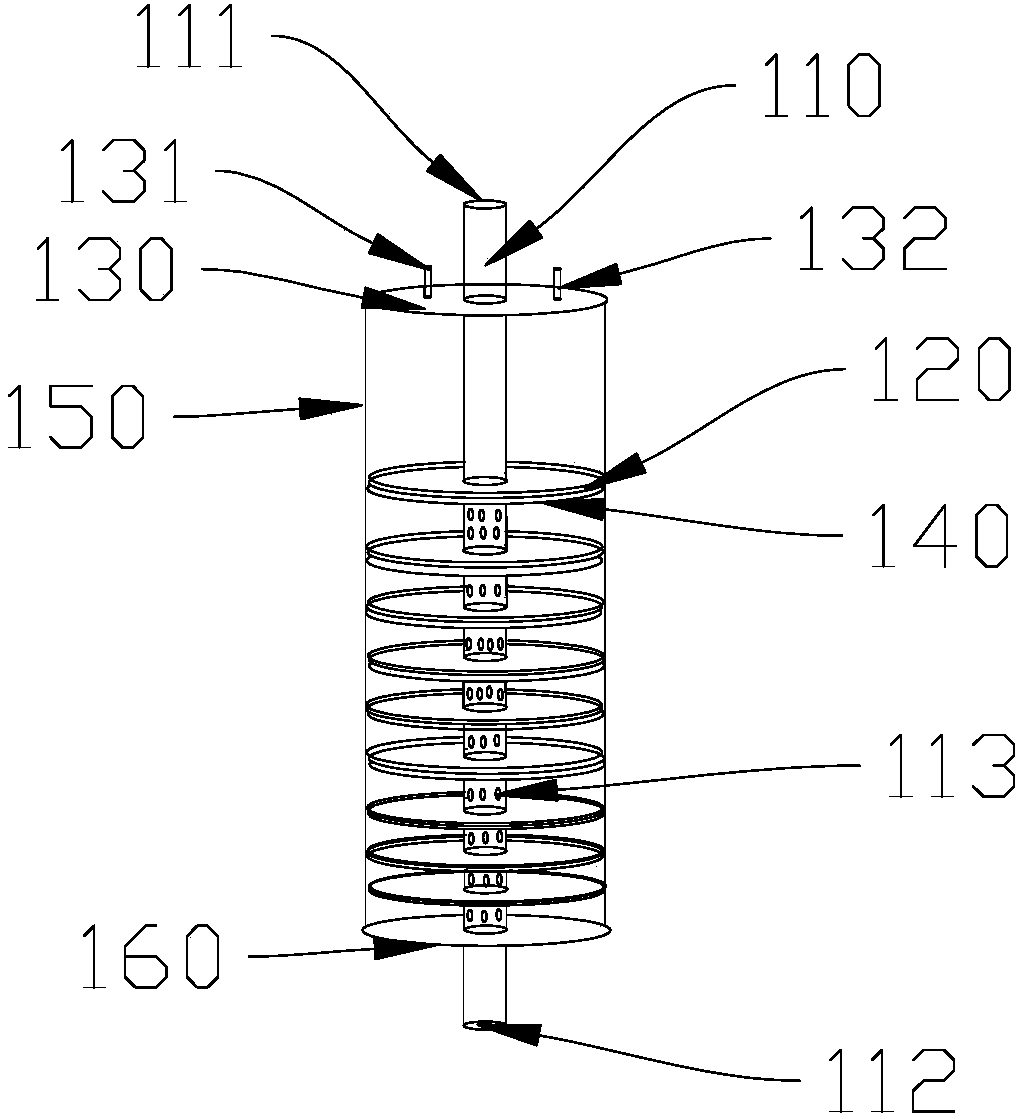
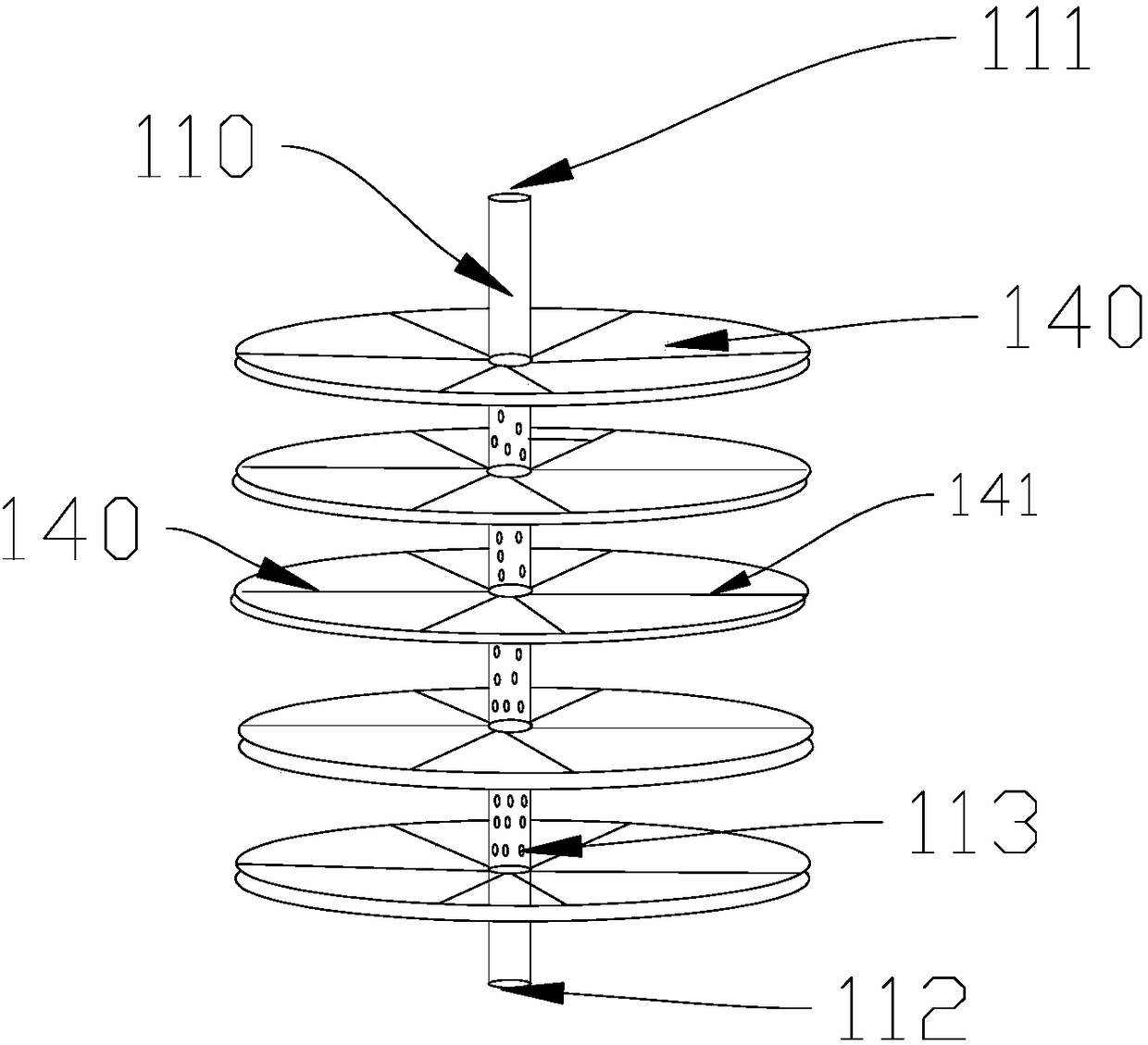
Membrane type platy biological artificial liver reactor
InactiveCN108485965ADensity controllablePromote growthBioreactor/fermenter combinationsBiological substance pretreatmentsSupporting systemLiver support systems
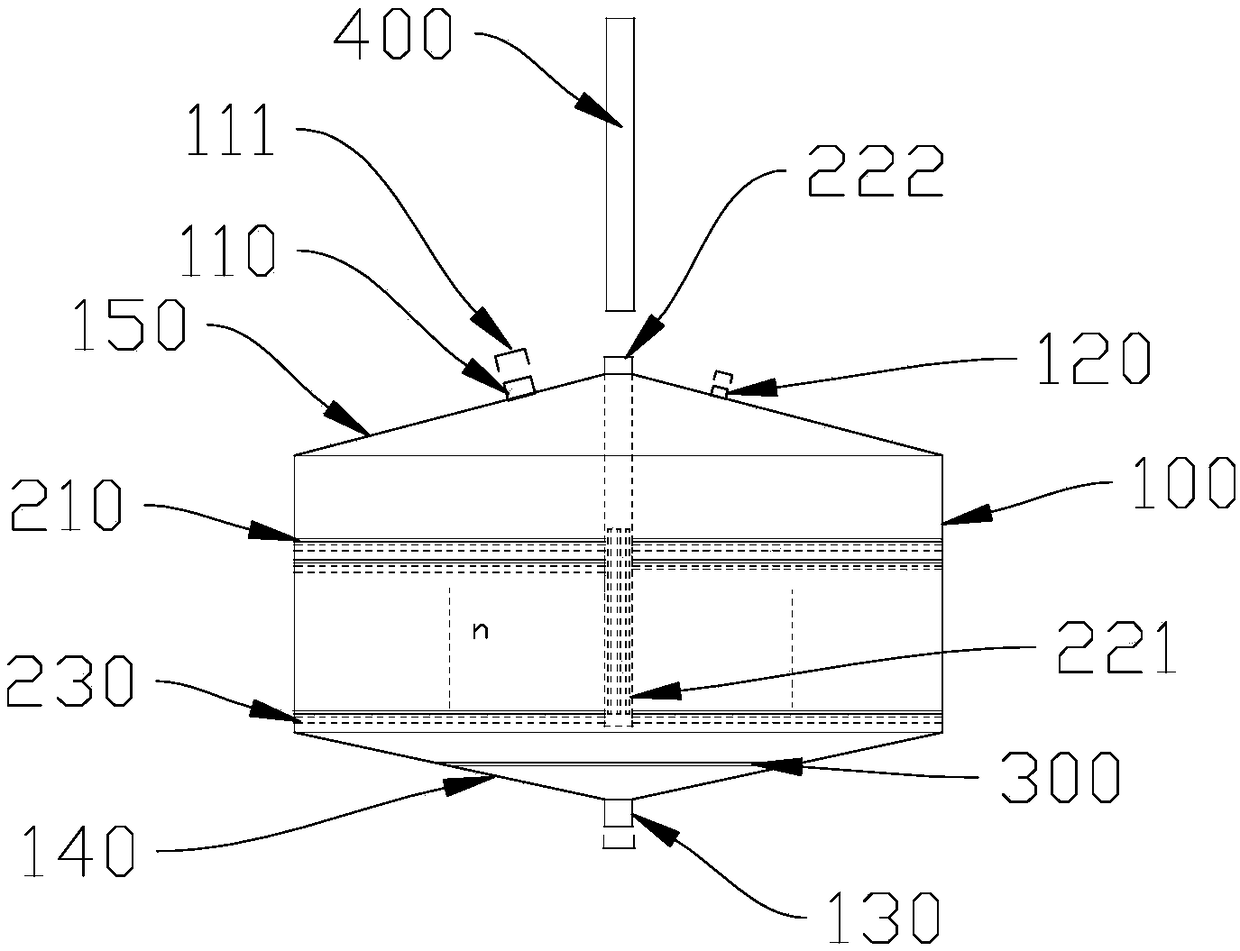
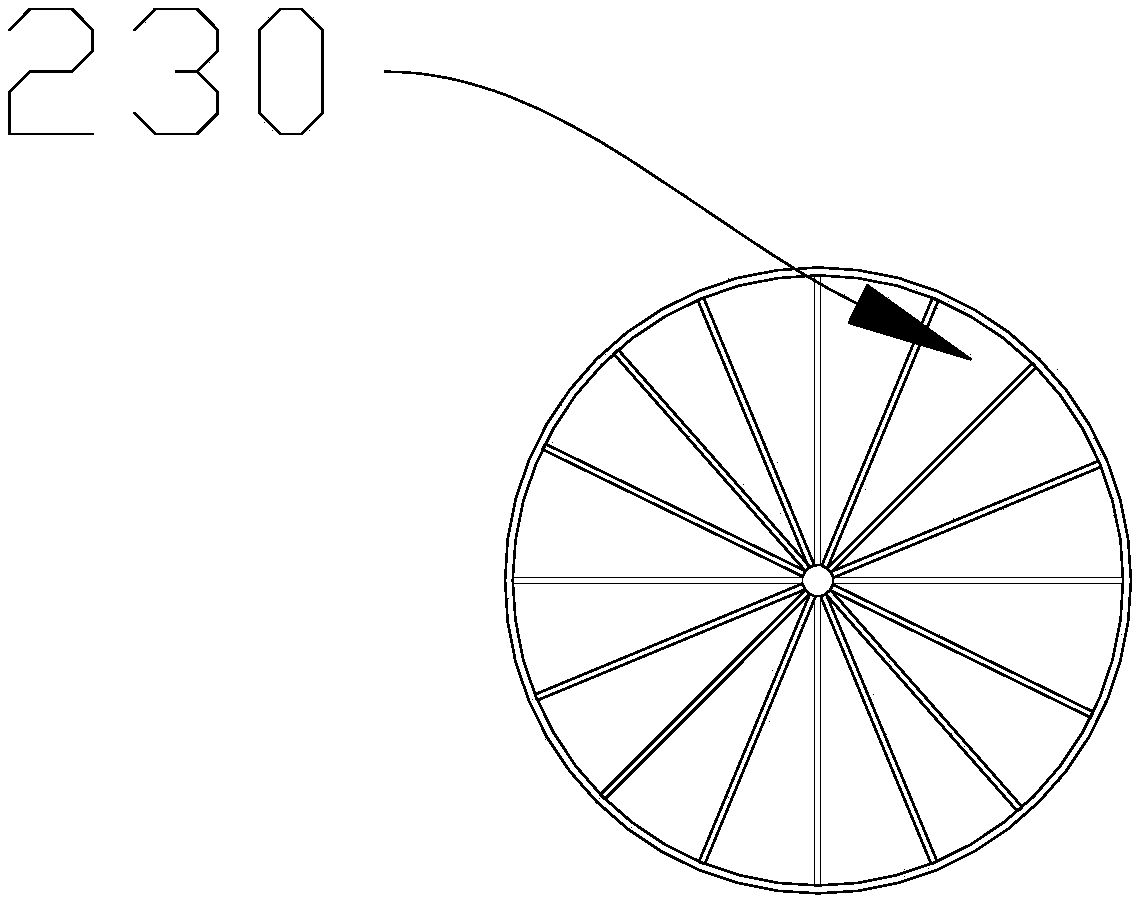
The invention discloses a membrane type platy biological artificial liver reactor. The reactor comprises a tank, and a multilayer cell culture unit and a central cell suspension injection tube which are arranged in the tank, wherein in the multilayer cell culture unit, a cell culture membrane is circularly horizontally arranged, a scaffold is spoke-shaped or screen-shaped and has a central hole, the cell culture membrane is arranged on the upper side, and the scaffold is arranged on the lower side; the total height of the multilayer unit is about 2 / 3 of a cylindrical part of the tank. According to the membrane type platy biological artificial liver reactor designed in the invention, the cell culture membrane is horizontally favorable for liver cell adherence, a flow direction of plasma isperpendicular to the cell culture membrane, the cells difficultly drop, and the plasma and the cells can be fully contacted and can perform exchange of substances; due to the cell suspension injectionamount, the occupancy of membrane cells on each layer can be controlled, the plasma permeability is ensured, enough liver cells can be obtained by virtue of a multilayer plate structure, and a bioreactor in the conventional artificial liver support system is replaced.
Owner:CHONGQING UNIV OF TECH
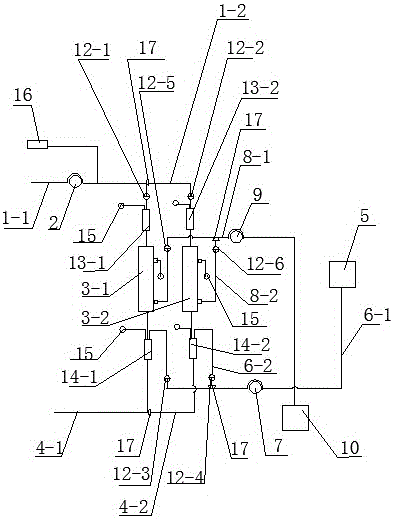
Bioartificial liver system based on human iHep cells and membrane type reactor slab
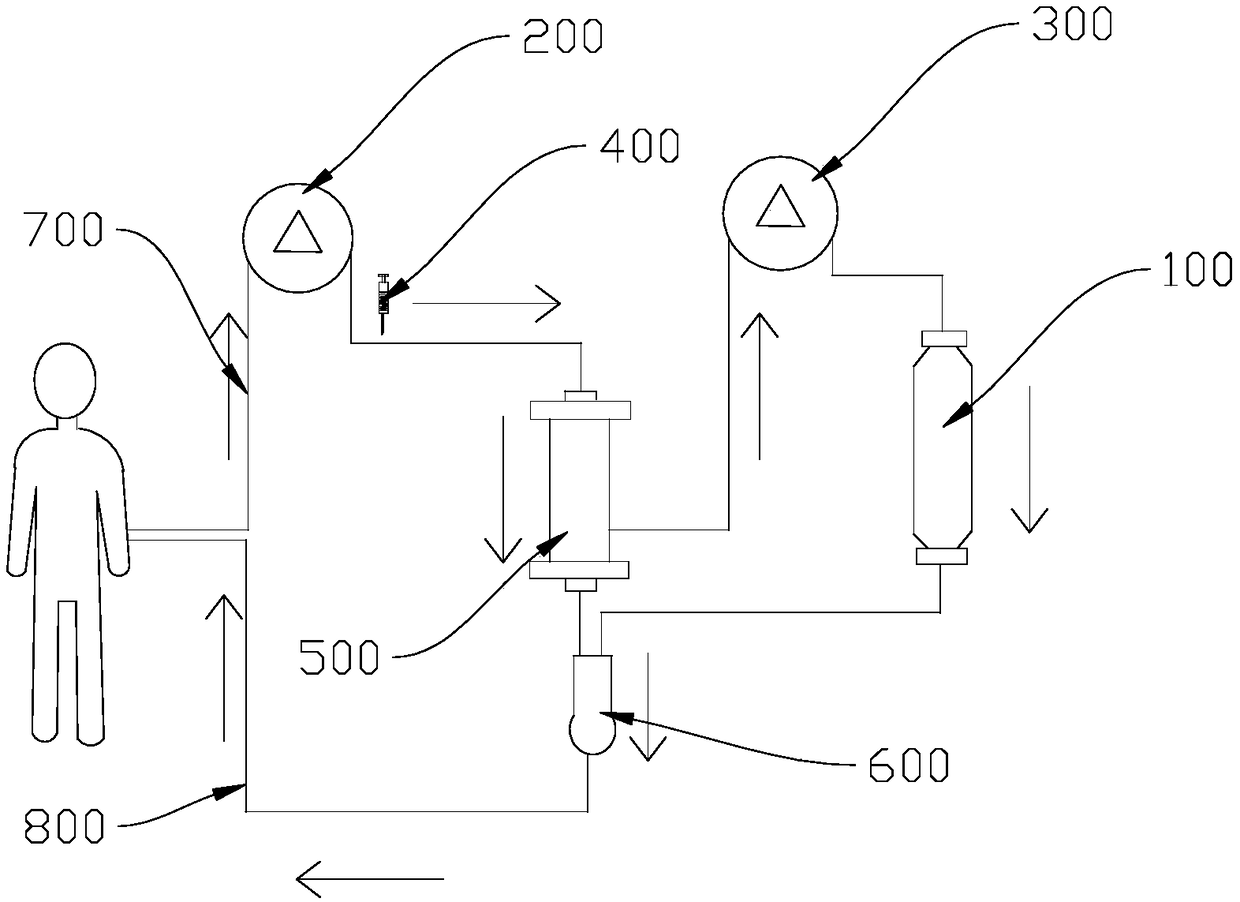
InactiveCN108421106AVersatileEnsure treatment safetyBioreactor/fermenter combinationsBiological substance pretreatmentsBioartificial liver deviceBlood circulating
The invention discloses a bioartificial liver system based on human iHep cells and a membrane type reactor slab. The bioartificial liver system comprises a first blood circulating pump, a heparin pump, a plasma separator, a second blood circulating pump and a bioreactor, wherein an inlet of the first blood circulating pump is connected with a blood input pipe, an outlet of the first blood circulating pump is connected with the heparin pump and the plasma separator, and an outlet of the plasma separator is connected with an inlet of the blood input pipe and an inlet of the second blood circulating pump. The bioartificial liver system uses an artificial liver bioreactor with a biological film, accordingly has enough large capacity for containing liver cells capable of maintaining human bodyfunctions. The bioreactor can be put in a conventional cell culture box for liver cell culture, is long in service life and perfect in function, ensures that cultured liver cells do not enter a humanbody blood circulating system and accordingly ensures treatment safety.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
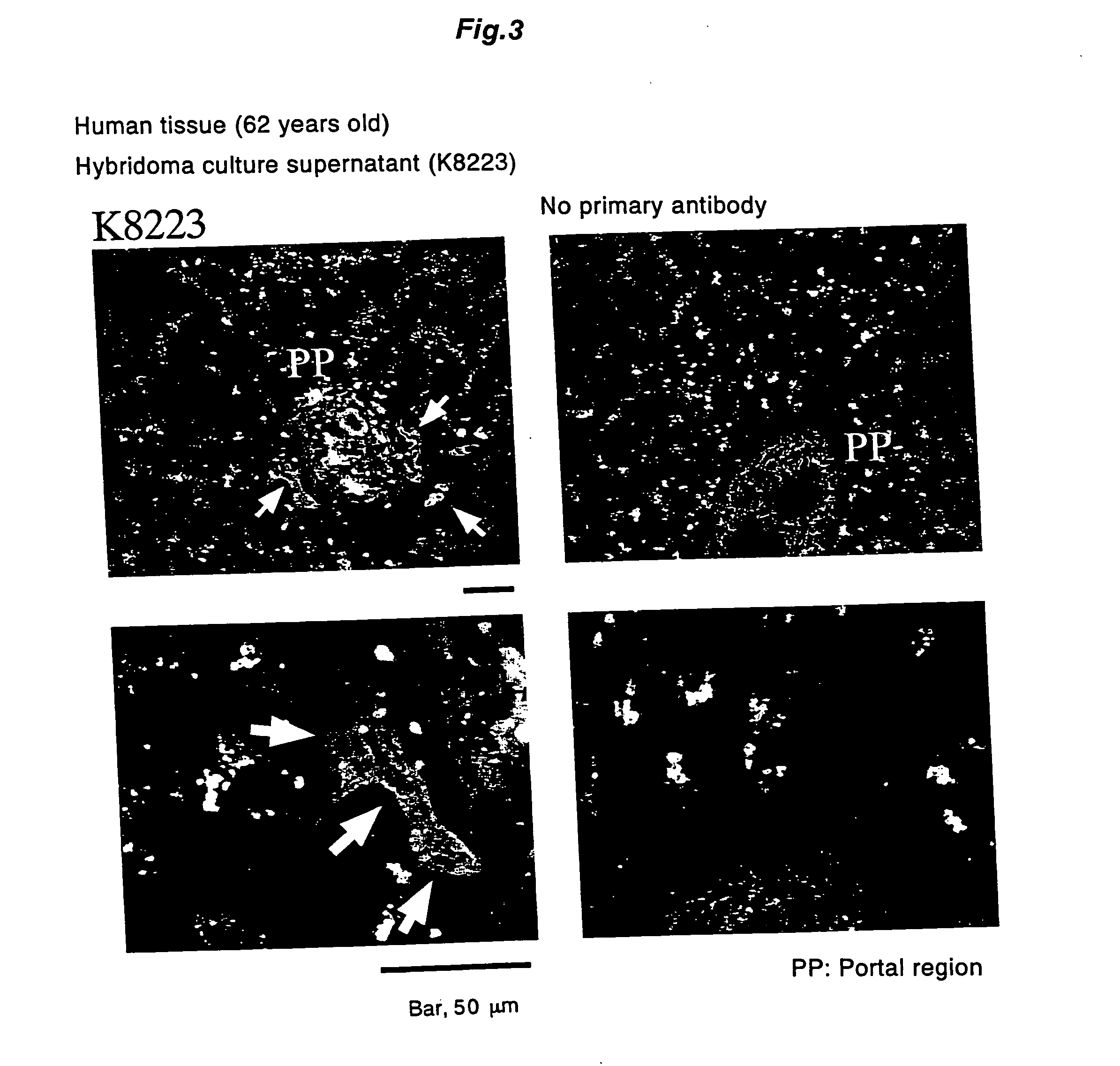
Antibody recognizing proliferative human liver cells proliferative human liver cells and functional human liver cells
InactiveUS20050119463A1Increase the number of cellsGenetically modified cellsArtificial cell constructsArtificial liverMonoclonal antibody
The invention provides a monoclonal antibody which specifically recognizes proliferative human hepatocytes, a method for isolating proliferative human hepatocytes by using the antibody, and a method for inducing to differentiate the proliferative human hepatocytes to functional human hepatocytes. Further, the invention provides the proliferative human hepatocytes and functional human hepatocytes obtained by the methods, as well as a cell kit and a hybrid artificial liver comprising those hepatocytes.
Owner:JAPAN SCI & TECH CORP +1
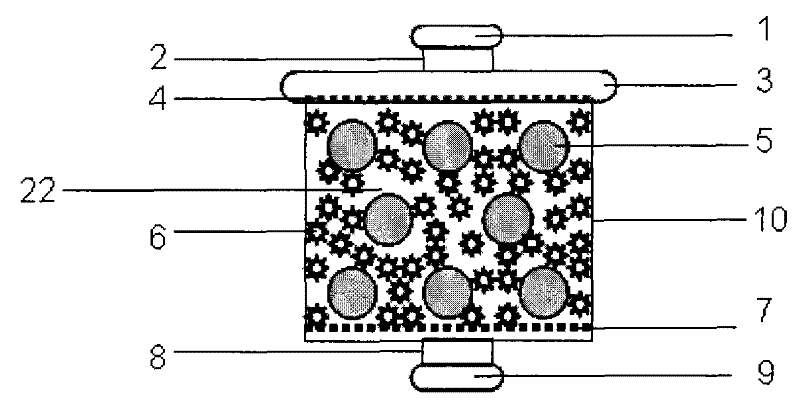
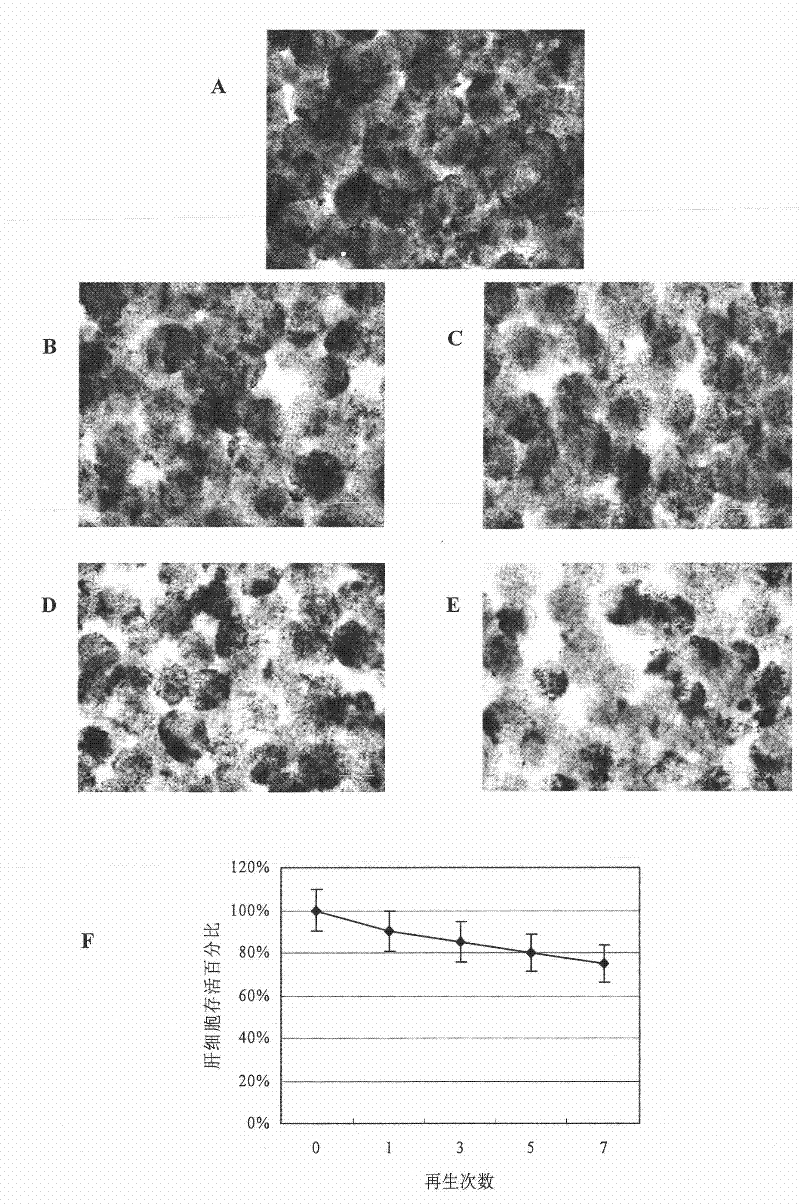
Cell reactor and artificial liver support system comprising same
ActiveCN101732771BOptimize the connection methodHigh biosecurityOther blood circulation devicesTissue/virus culture apparatusBiotechnologyProcess engineering
The invention provides a cell reactor and an artificial liver support system comprising the same. The cell reactor comprises a container, a liquid outlet is formed at the bottom of the container, a sealing cover is arranged at the top of the container, a liquid inlet is formed on the sealing cover, layers of filter meshes of 100-400 meshes are respectively arranged at the bottom of the container and in the sealing cover at the top of the container, covers which are in seal fit with the liquid outlet and the inlet are formed at the liquid outlet and the inlet, and a mixture of a micro-carrier growing liver cells and resin is accommodated between the two layers of the filter meshes in the container. The reactor is connected on a dialysate circulation path of a blood purifying device for constituting the artificial liver support system, thereby leading the liver cells not to be in direct contact with blood and realizing good biological safety; furthermore, the reactor can be used for a plurality of times through regeneration treatment, thereby greatly reducing the treatment cost of a biological artificial liver.
Owner:苏州瑞徕生物科技有限公司
Methods of producing in vitro liver constructs and uses thereof
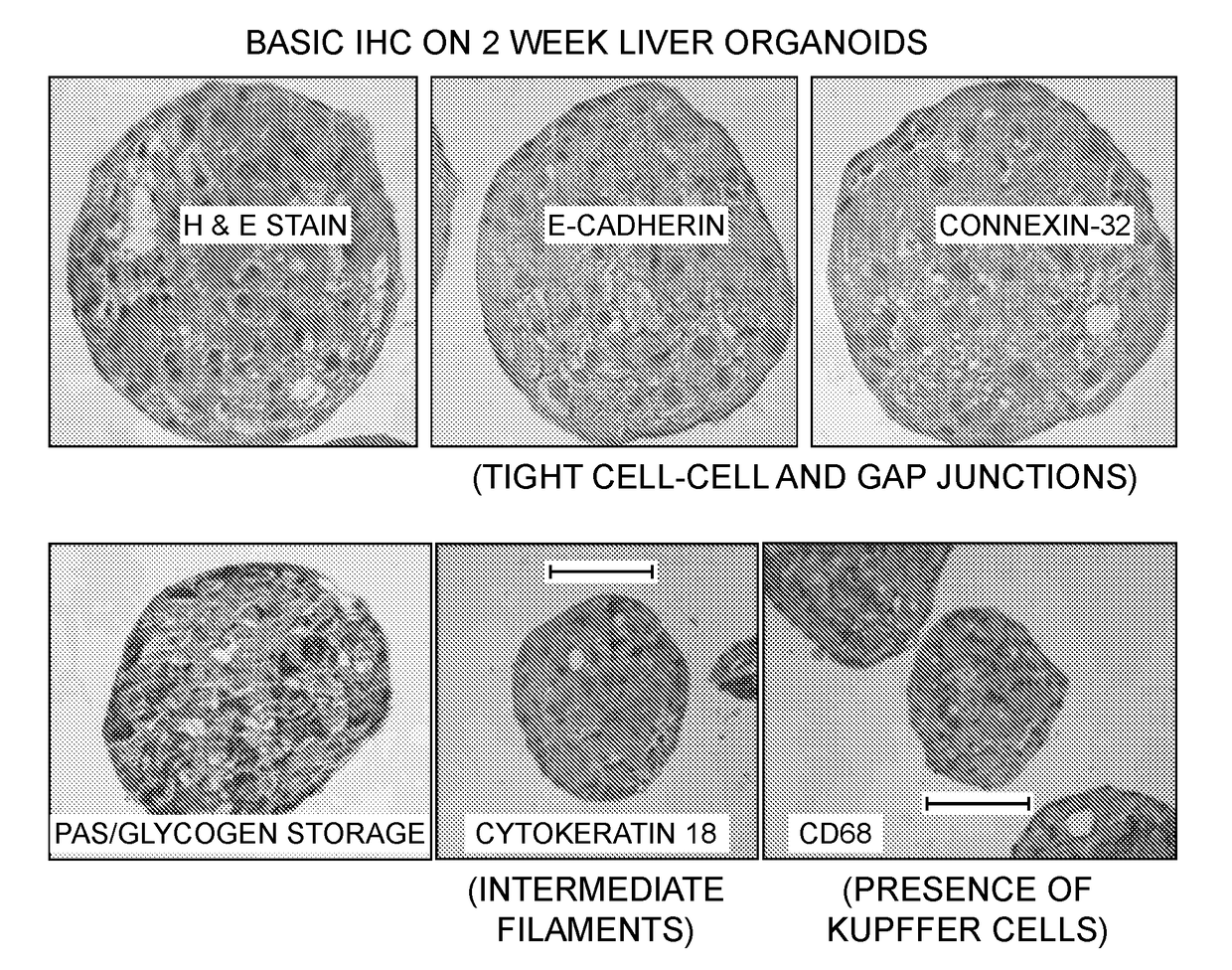
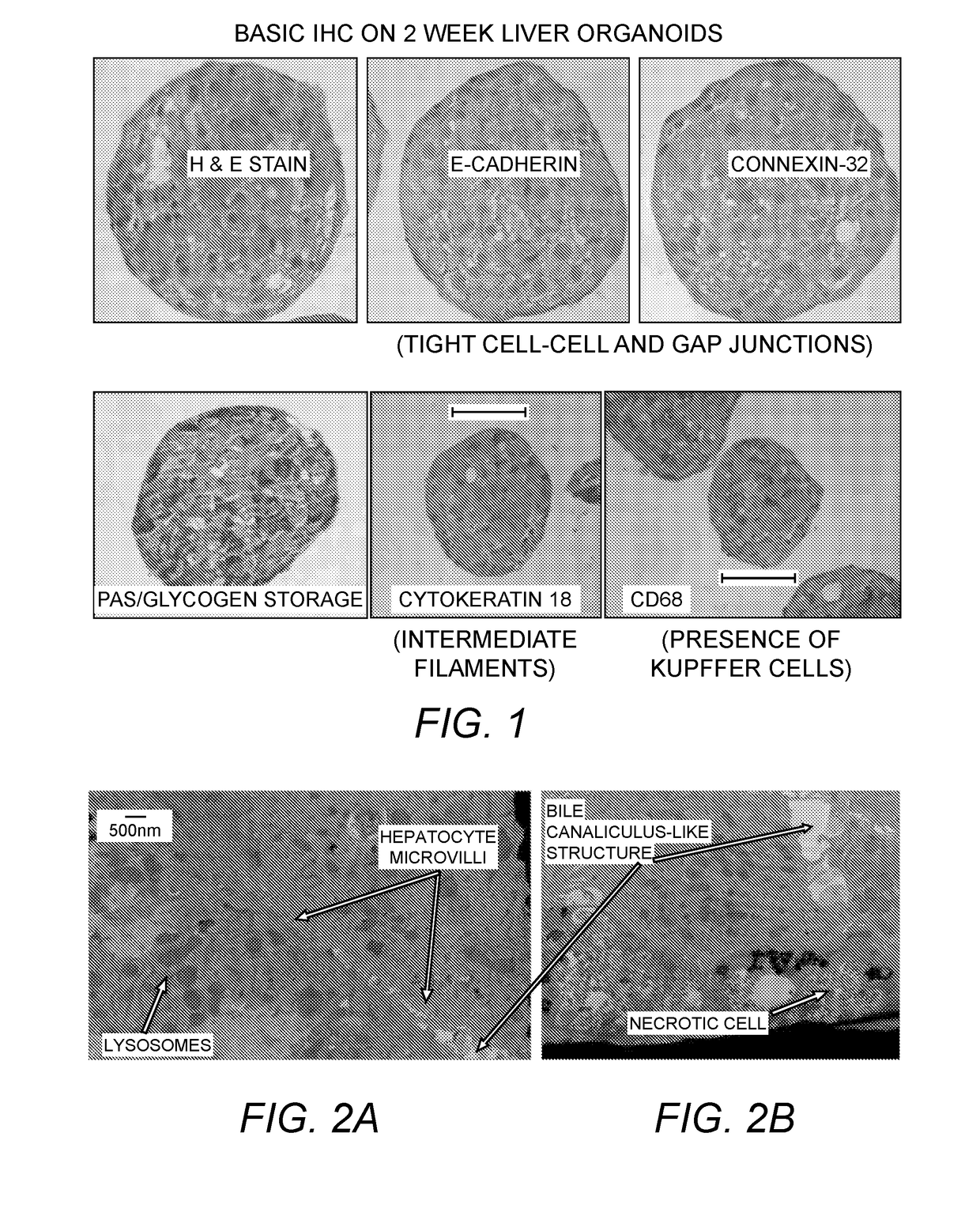
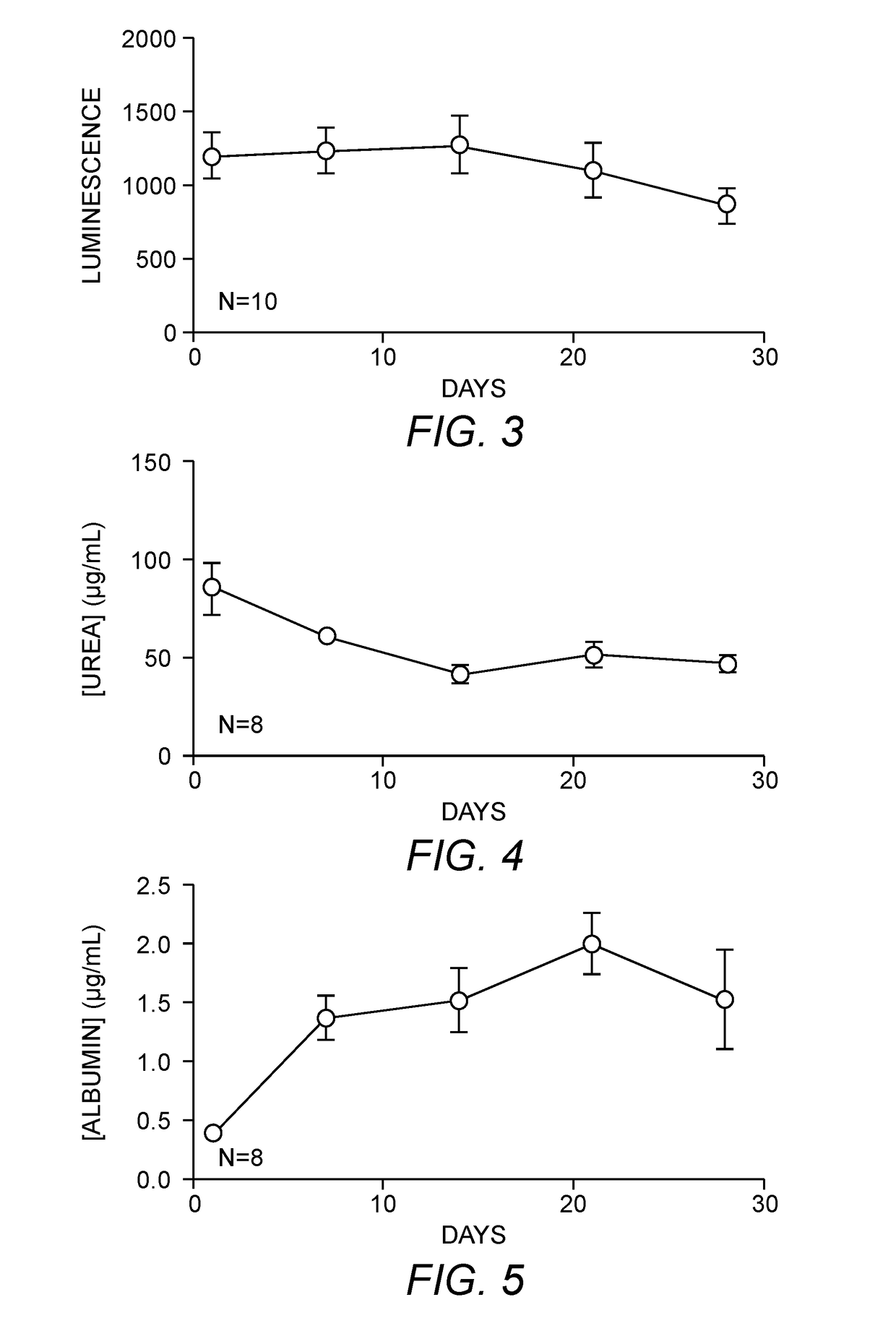
Provided herein are cell compositions useful for making artificial liver constructs. The cell composition my include, in combination, (a) hepatocyte cells, (b) Kuppfer cells, (c) hepatic stellate cells, (d) sinusoidal endothelial cells, and (e) cholangiocyte cells.
Owner:WAKE FOREST UNIV HEALTH SCI INC
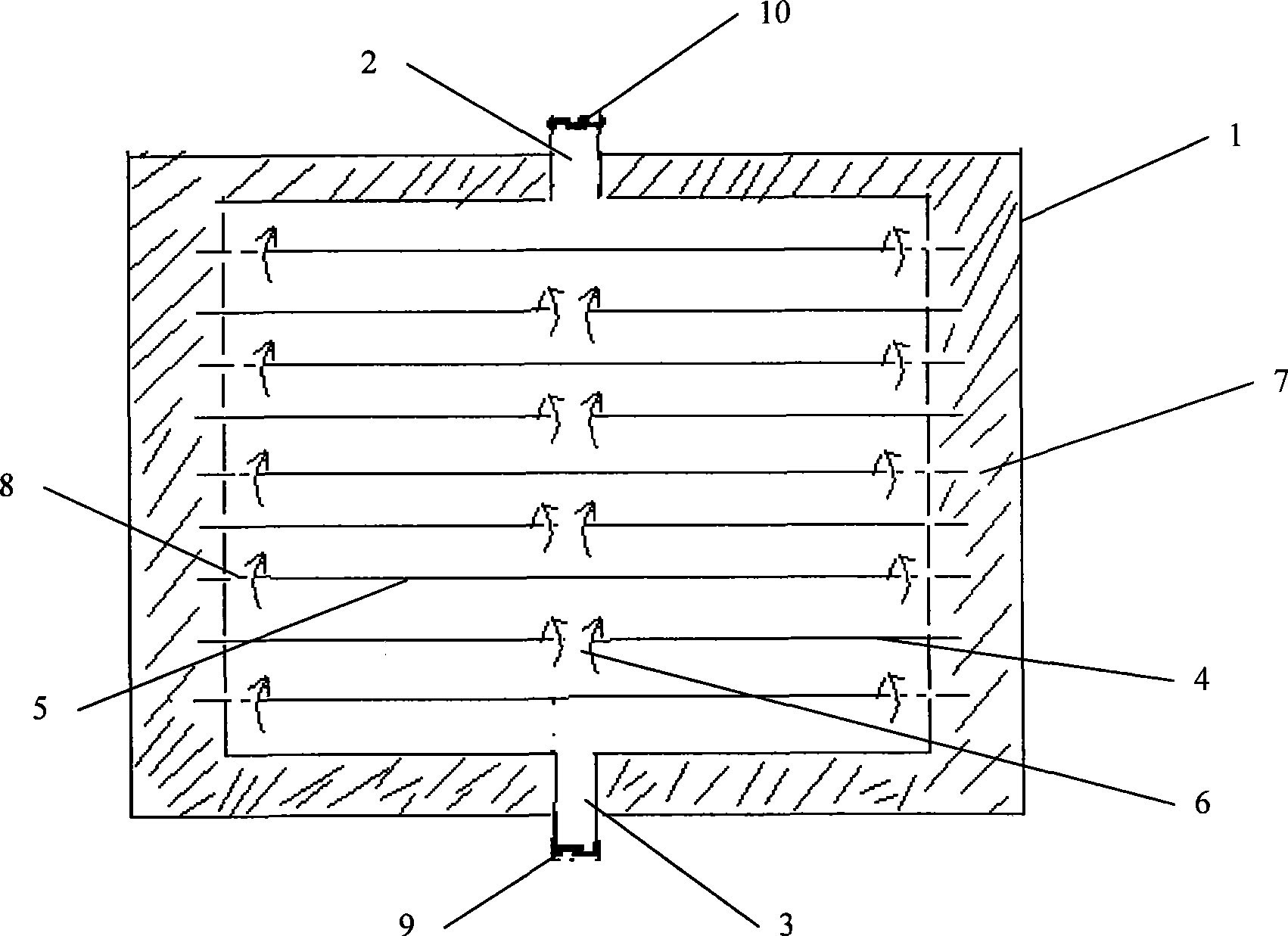
Spiral Hollow Fiber Tube Artificial Liver Bioreactor
InactiveCN102258817AImprove treatment efficiencyFull wall actionTissue/virus culture apparatusSuction devicesFiberHollow fibre membrane
The invention discloses a spiral hollow fiber tube artificial liver bioreactor. A plurality of hollow fiber tubes are evenly packed in parallel in the center of the transparent cylindrical container. The inside of the hollow fiber tube is the inner cavity, and the outside of the hollow fiber tube is the outer cavity. The patient plasma / blood inlet and patient plasma / blood outlet of the inner cavity are respectively set. At both ends of the cylindrical container, the inlet of the hepatic cell culture solution and the outlet of the hepatic cell culture solution in the outer cavity are arranged side by side on the cylindrical surface of the cylindrical container. The plurality of hollow fiber tubes are a plurality of helical hollow fiber tubes, and the helix angle is not more than 45° and not less than 10°. The invention ensures that there is enough area for the hepatocytes to adhere to the wall and grow, and at the same time, the blood flow in the spiral tube will generate a secondary flow, which is conducive to the full mixing of blood and prevents a large amount of plasma protein from adhering to the tube wall to block the holes of the hollow fiber membrane. It is helpful for the detoxification and mass transfer of the hollow fiber, prolongs the supporting time of the artificial liver in vitro, and improves the therapeutic efficiency of the artificial liver.
Owner:ZHEJIANG UNIV
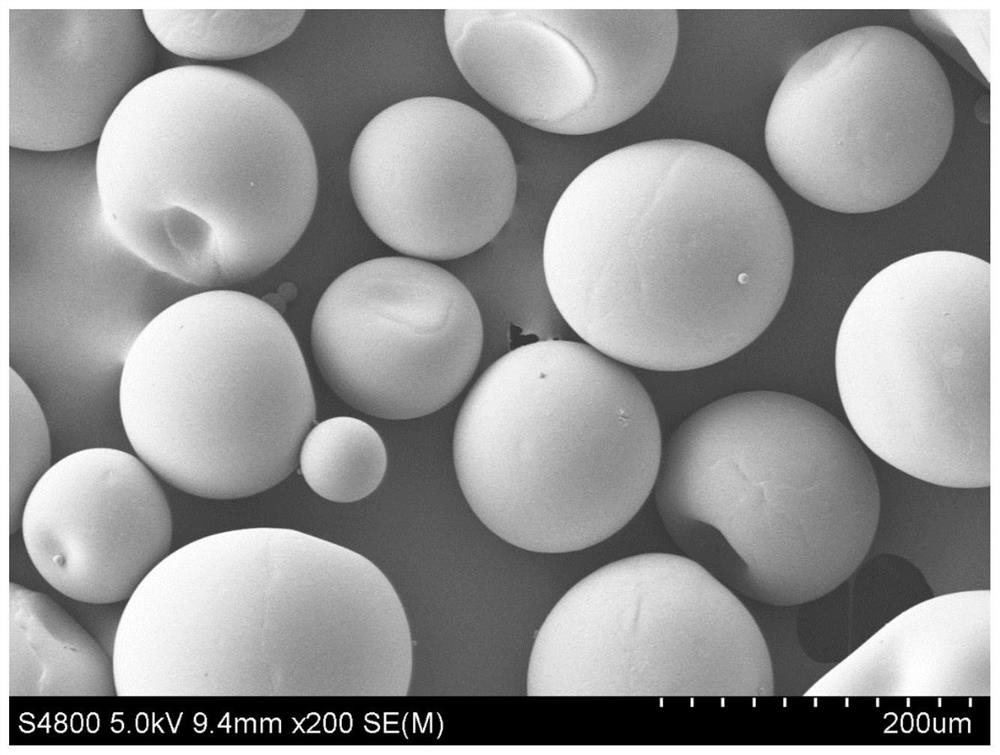
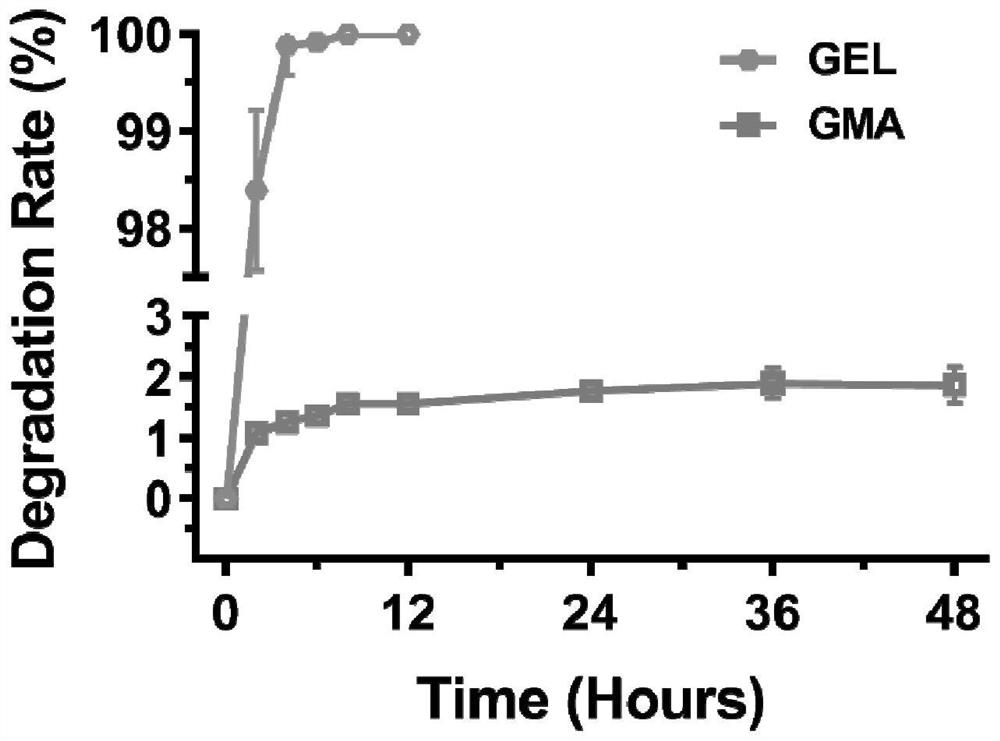
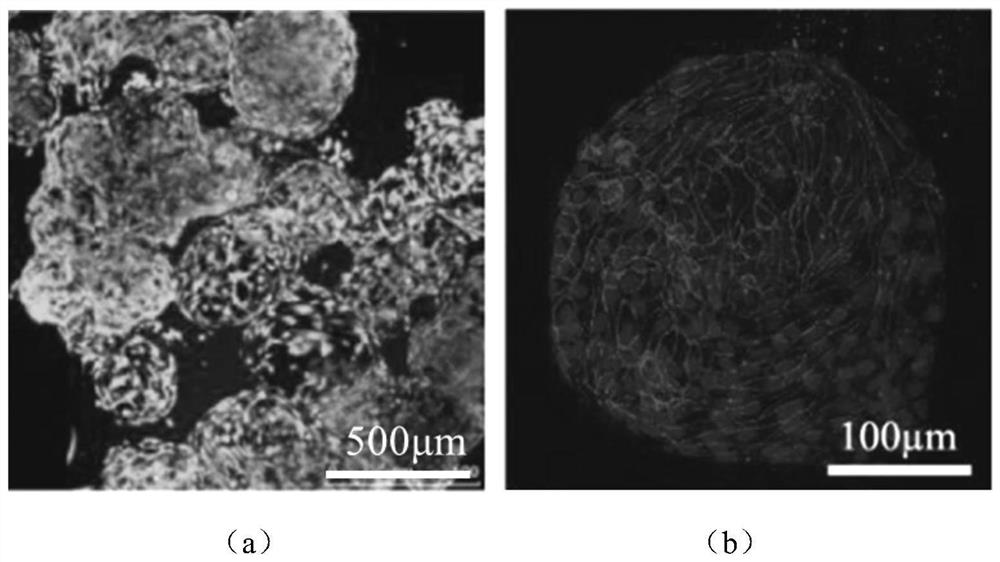
Anti-degradation gelatin microspheres, artificial liver model and construction method and application of artificial liver model
PendingCN113929934AResistant to degradationSimple and efficient regulation of degradation rateCompound screeningApoptosis detectionLiver functionsVascularizes
The invention discloses anti-degradation gelatin microspheres, an artificial liver model and a construction method and application of the artificial liver model. Methacrylamide modified gelatin is firstly synthesized, and then photo-crosslinking gelatin microspheres are further prepared through emulsification, so that the gelatin microspheres have the anti-degradation property in a physiological environment; and moreover, simple and efficient regulation and control of the degradation rate of the microspheres can be realized by adjusting the methacrylamide amidation degree and the illumination condition, and a cell carrier is provided for construction of an in-vitro tissue engineering artificial liver. The anti-degradation gelatin microspheres are used as cell carriers, vascular endothelial cells and hepatocyte electrodes are inoculated on the surfaces of the anti-degradation gelatin microspheres, and the artificial liver model is formed through spontaneous bonding and assembling, so that the anti-degradation characteristic of the artificial liver model is improved, the obtained artificial liver model is high in vascularization degree, liver function expression and long-term in-vitro application of the artificial liver model are facilitated, and the artificial liver model can be used for Baijiu quality identification and alcoholic liver disease treatment drug treatment effect evaluation.
Owner:LUZHOU GUOZHIRONGYAO LIQUOR IND CO LTD
Hybrid artificial liver supporting system equipped with perforated brick type filling bracket type reactor
The invention relates to a medical equipment and aims at providing a hybrid artificial liver supporting system equipped with a perforated brick type filling bracket type reactor. The system comprises a plasma separating part, a non-biological liver circulating part and a biological liver circulating part; the biological liver circulating part comprises a constant temperature air bath incubator which plays the role of heat preservation and an extracorporeal circulation pool, a plasma pump, a dissolved oxygen and temperature testing bottle and the perforated brick type filling bracket type reactor which are connected into a circulation system in sequence. The invention not only solves the problem that the bracket type reactor is lack of immune isolation and is insufficient for oxygen supply,but also makes up the defects that the hollow fiber type reactor has insufficient cell growing space and poor cell adhesion, is lack of three-dimensional growing environment and is easy to block semi-permeable membranes. The invention integrates the functions of oxygenation, immune isolation, three-dimensional culture and the like and has strong comprehensive performance, simple manufacturing technique, low cost and easy amplification. As the reactor of the system has the function of oxygenation, the system saves an oxygenator which generally needs to be equipped independently.
Owner:ZHEJIANG UNIV
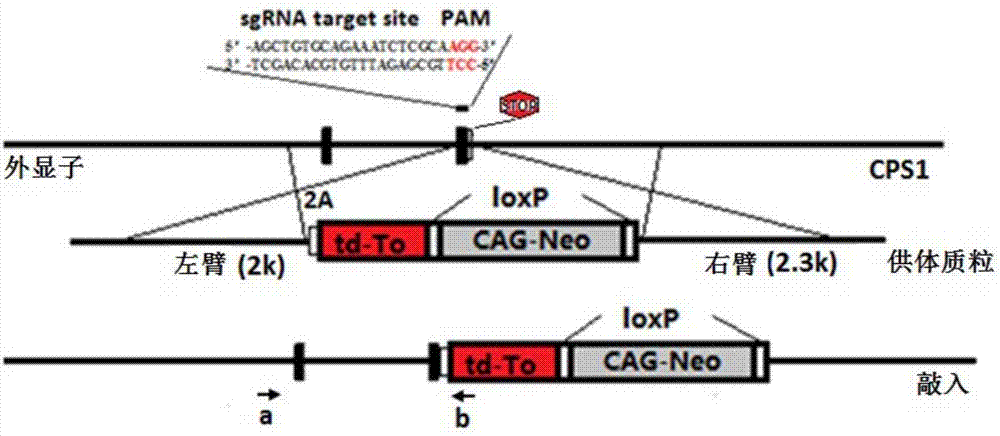
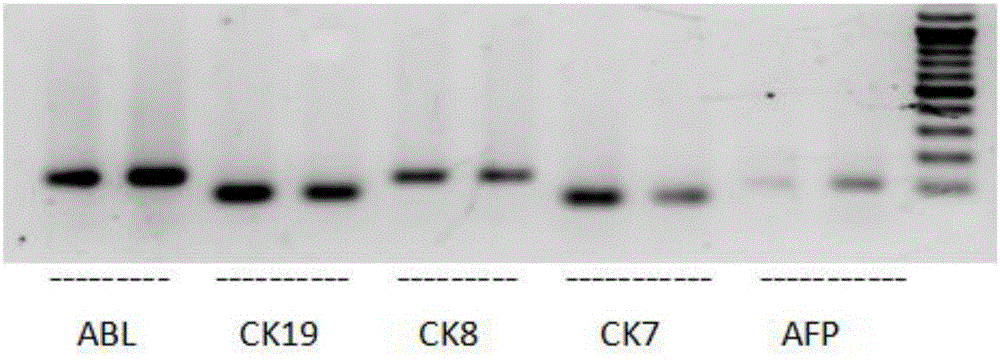
CPS1 reporter gene human liver cell line, construction method hereof and applications thereof
InactiveCN106947740AHigh purityHigh ammonia metabolism capacityMicrobiological testing/measurementMicroorganism based processesArtificial liverAdult liver
The invention discloses a CPS1 reporter gene human liver cell line and its construction method and application. Aminoacylphosphate synthase 1 (CPS1) and a reporter gene are used to construct a human liver cell line. The obtained CPS1 reporter gene human liver cell has the characteristics of human primary adult The functions of liver parenchymal cells, including albumin secretion, cytochrome P450 enzyme metabolism and ammonia metabolism, can be used in artificial livers, drug metabolism research, drug toxicity evaluation or drug screening, and have broad application prospects.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
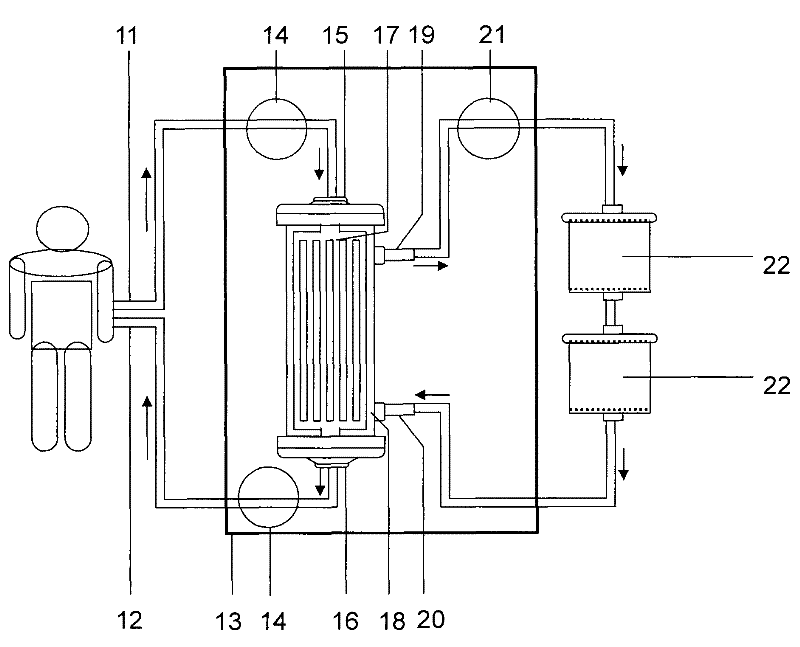
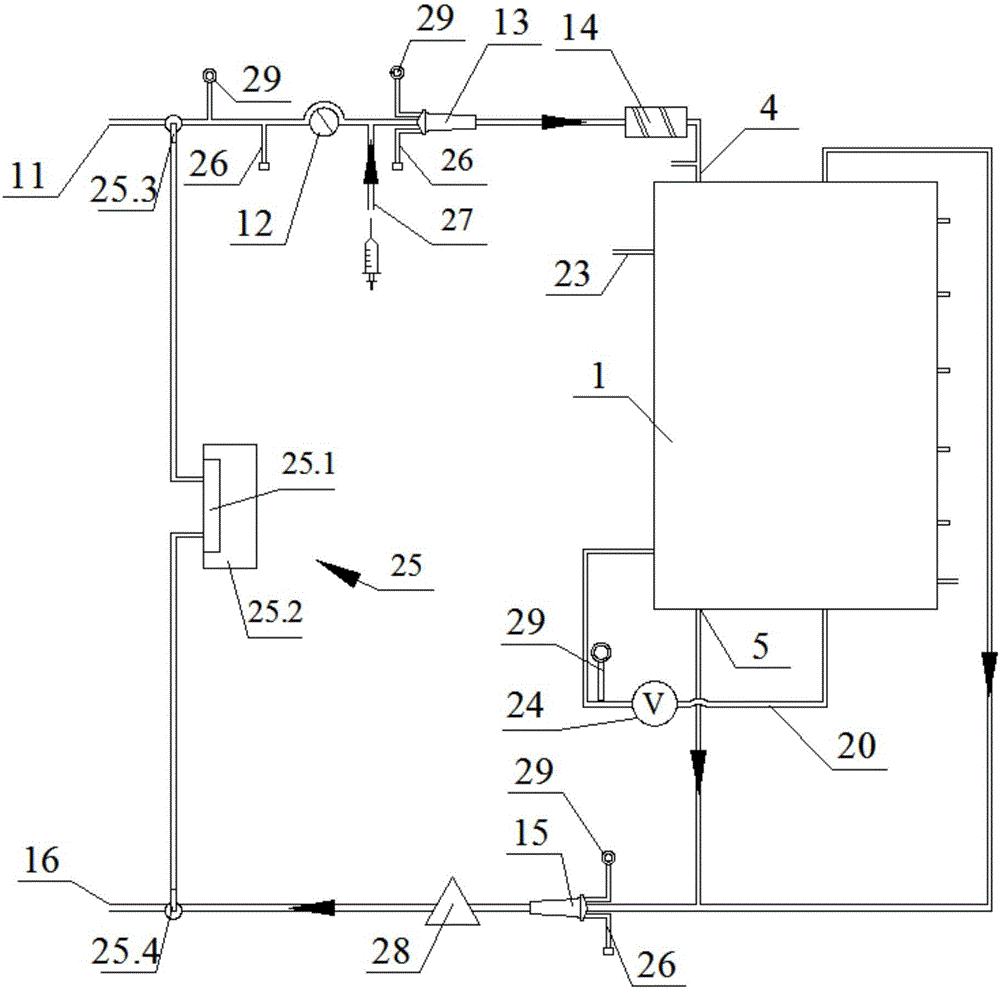
Semipermeable membrane layered bio-artificial liver online monitoring and constant-temperature heating integrated system
The invention discloses a semipermeable membrane layered bio-artificial liver online monitoring and constant-temperature heating integrated system, which comprises a blood input port, a blood pump, an arterial kettle, a semipermeable membrane layered integrated bioreactor, an intravenous kettle and a blood return port which are sequentially connected; the semipermeable membrane layered integrated bioreactor comprises a cavity, a plasma separator which is arranged at one side of the cavity and a bio-reactor body which is arranged at the other side of the cavity; the plasma separator and the bio-reactor body are spaced by virtue of a spacing semipermeable membrane; and multiple layers of filtering semipermeable membranes are horizontally arranged in the bio-reactor body. The integrated system disclosed by the invention has the beneficial effects that the integrated system is simpler and more practical in design; cells and plasma get into full contact, which is conducive to improving an exchange efficiency of the two types of substances, and a plasma degrading effect is enhanced; a cell storage space is enlarged and a loading capacity is increased.
Owner:WUHAN TOGO MEDITECH CO LTD
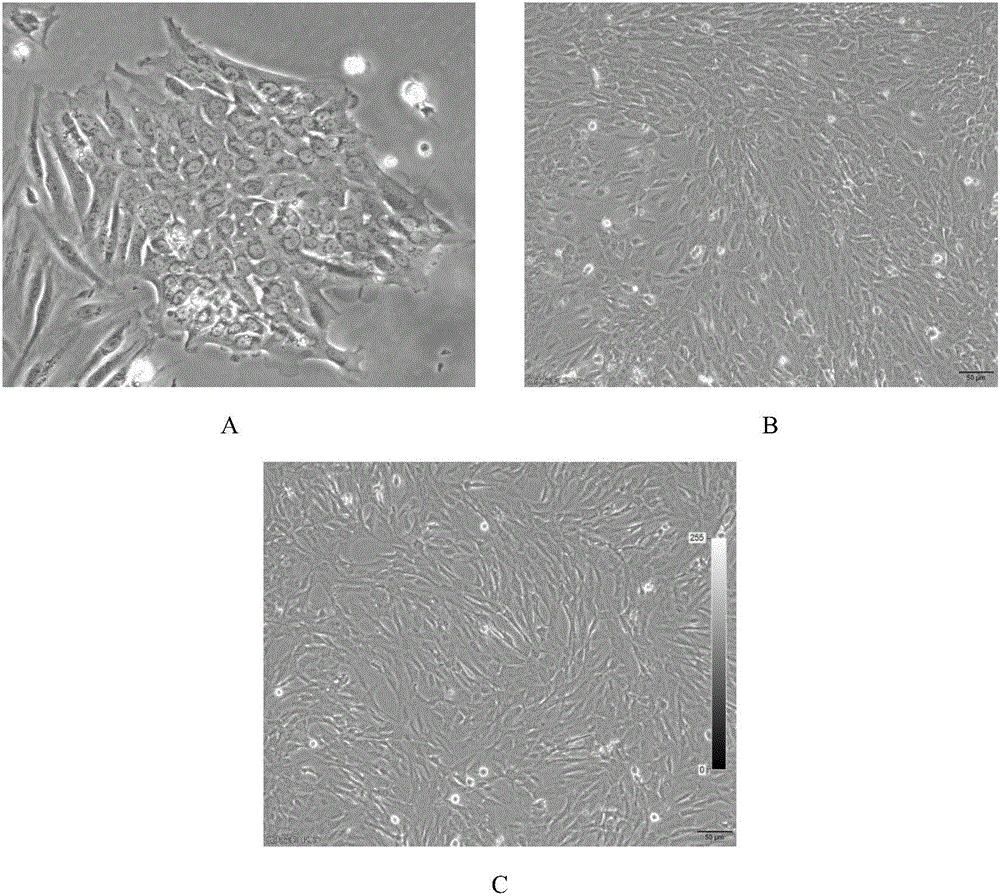
Adult human hepatogenic stem cell line HN, preparation method thereof and application
InactiveCN107523537AHigh purityImprove proliferative abilityCell dissociation methodsHepatocytesAbnormal tissue growthAcute hepatic failure
The invention relates to an adult human hepatogenic stem cell line HN, a preparation method thereof and an application. The human hepatogenic stem cell line is collected at the China General Microbiological Culture Collection Center, the collection address is No. 3, first yard, Beichen West Road, Chaoyang District, Beijing, the collection date is May 15, 2017, and the collection number is CGMCC No. 13844. The human hepatogenic stem cell line is used for in vitro and vivo directional induction of liver cells and hepatic duct epithelial cells, preparation of biological artificial livers, resistance of acute hepatic failure, chronic hepatic failure and alcoholic fatty livers, inhibition of liver fibrosis and anti-tumor effects. The cells have the biological characteristics that the cells have typical epithelial cell morphology and strong multiplication capacity, grow to achieve contact inhibition and density inhibition, bidirectionally express liver cell specific marker albumin, cytokeratin 8 and biliary epithelial cell specific marker cytokeratin 7 and 9, have various functions of the liver cells, have multi-directional differentiation potential and avoid tumorigenicity.
Owner:洪丰
Method of culturing proliferative hepatocytes
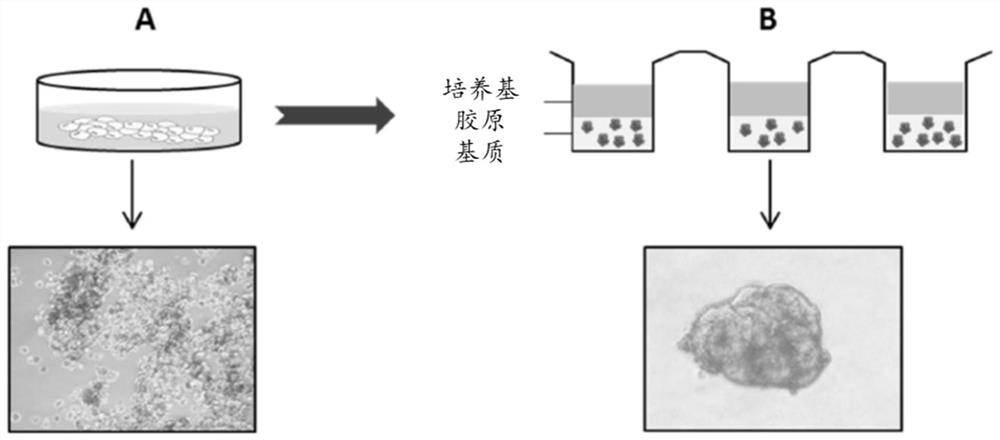
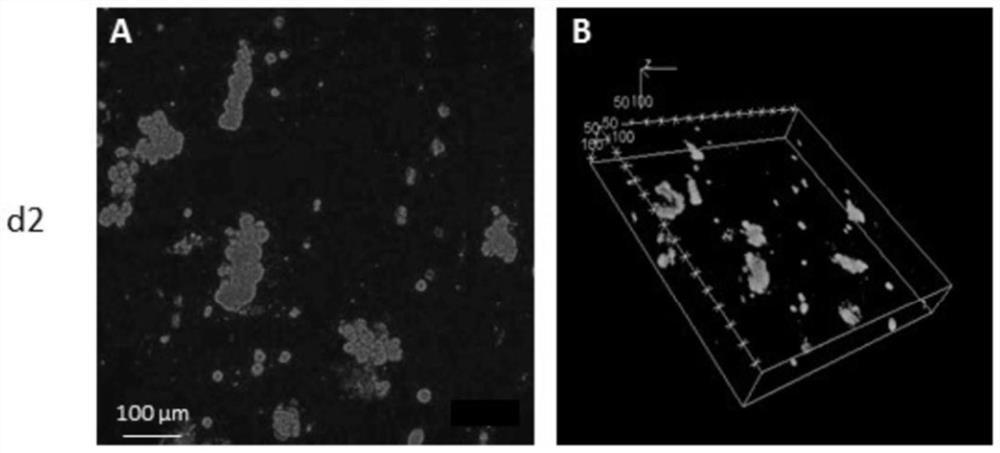
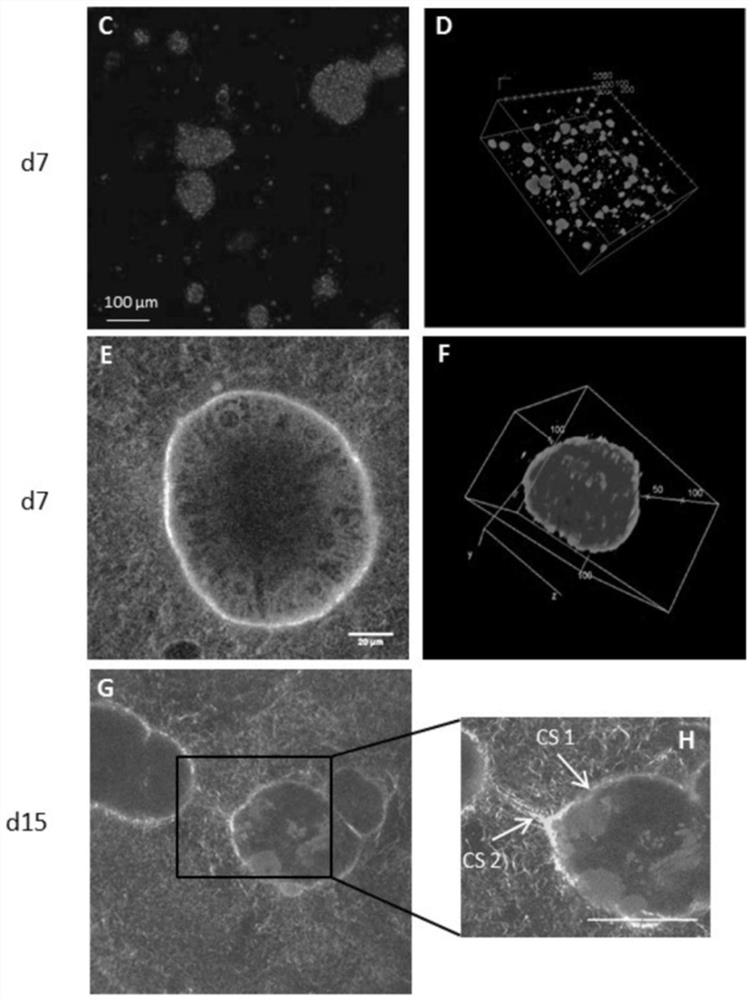
The present invention relates to a method of culturing animal cells, preferably primary hepatocytes, comprising a first step of culturing the animal cells in non-adherent culture vessel, preferably alow or ultra-low attachment culture vessel, a second step of embedding the animal cells in a collagen matrix or in a gelatin matrix, and a third step of culturing the animal cells embedded in the collagen matrix or in the gelatin matrix, thereby obtaining 3D animal cell structures comprising proliferative animal cells, preferably spheroids comprising proliferative primary hepatocytes. The invention also relates to a spheroid comprising proliferative primary hepatocytes and the uses thereof for engineering an artificial liver model or an artificial liver organ, and for assessing in vitro the liver toxicity, genotoxicity and / or the effects of a drug or a compound.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Hollow fiber bioreactor for artificial liver
PendingCN113679900AIncrease pressure differenceEfficient detoxificationDialysis systemsHollow fibreEngineering
The invention discloses a hollow fiber bioreactor of an artificial liver. The hollow fiber bioreactor comprises a round hose sleeving the outer sides of all hollow fiber tubes, and two ends of the round hose are fixedly connected with colloid sealing structures in a sealing manner; a circular cylinder is a reducing circular cylinder, the diameter of one end close to a blood distributor is small, the diameter of one end close to a blood outlet collector is large, a small-diameter cylinder and a large-diameter cylinder are in arc transition, and a nutrient solution inlet pipe penetrating through and fixedly sealed on the wall of the large-diameter cylinder communicates with the interior of the round hose; a nutrient solution outlet pipe which penetrates through and is fixedly sealed on the small-diameter cylinder wall communicates with the interior of the round hose; a fluid inlet is formed in the wall of the large-diameter cylinder, and a fluid outlet is formed in the wall of the small-diameter cylinder; and when fluid flows in from the fluid inlet and flows from the large-diameter cylinder to the small-diameter cylinder with a reduced cross section, the venturi effect occurs, toxins can penetrate through a hollow fiber membrane from the blood side to enter the nutrient solution side easily, the detoxification effect can be enhanced, efficient detoxification of the artificial liver is achieved, and the expected treatment effect is achieved.
Owner:浙大宁波理工学院
Hepatoblasts and method of isolating same
InactiveUS20070117205A1Reduce in quantityIncrease diversityCell dissociation methodsHepatocytesArtificial liverLiver function
This invention relates to methods of isolating hepatoblasts utilizing panning techniques and fluorescence activated cell sorting. This invention further relates to isolated hepatoblasts and to a method of treating liver dysfunction as well as to methods of forming artificial livers.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Extracorporeal circulation pipeline of plasma exchange device capable of switching blood separators
ActiveCN105879138ASolve the problem of failure of artificial liver technologyOther blood circulation devicesHaemofiltrationExtracorporeal circulationFluid replacement
The invention discloses an extracorporeal circulation pipeline of a plasma exchange device capable of switching blood separators in order to solve the problem that due to blocking of blood separators, artificial liver treatment is forced to interrupt in the artificial liver treatment technical process. The extracorporeal circulation pipeline comprises a blood circulation system, a liquid supplement system, a liquid discharge system, the standby blood separator and a pipeline body, wherein the blood circulation system is composed of a blood inlet pipeline, a blood pump, the blood separators and a blood return pipeline, the liquid supplement system comprises an exchange liquid container, a liquid inlet pipeline and a liquid pump, the liquid discharge system comprises a liquid discharge pipeline, a plasma pump and a waste liquid collection container, and the standby blood separator can be supported to cut into the extracorporeal circulation pipeline immediately through the pipeline body when the blood separators in use are blocked. Thus, the problem that due to blocking of the blood separators, artificial liver treatment is forced to interrupt in the artificial liver treatment technical process is solved.
Owner:赵晓玲
Implantation type artificial hepar
ActiveCN101428154BOvercoming longstanding vascularization challengesHigh activityProsthesisBiological materialsBlood vessel
The invention discloses an implanted artificial liver comprising a polyglycolic acid or polylactic-co-glycolic acid sleeve, more than one three-dimensional porous scaffold, and polyglycolic acid or polylactic-co-glycolic acid tube seals; the three-dimensional porous scaffolds are mounted inside the polyglycolic acid or polylactic-co-glycolic acid sleeve; and polyglycolic acid or polylactic-co-glycolic acid tubes are used for sealing two ends of the polyglycolic acid or polylactic-co-glycolic acid sleeve. The invention has the beneficial effects of maximally simulating the structure of a hepatic lobule, developing implanted stem cells into liver cells through directional differentiation, and inducing the regeneration of microvessels of a tissue engineering liver. Meanwhile, the exterior of the three-dimensional porous scaffolds is wrapped in the highly biocompatible biomaterial sleeve which serves to simulate the structure of a liver capsule; and small lacunas are formed between the sleeve and the scaffolds to allow blood to circulate through tissue engineering brackets, thereby facilitating the regeneration of vessels of the engineering liver in an envelope, and ultimately forming an artificial liver to replace the damaged liver of a parasitifer.
Owner:ZHEJIANG UNIV
Micro cell hollow fiber reactor
The micro hollow fiber cell reactor consists of silica gel tube of permeable silica gel material, permeable silica gel stopper and hollow fiber filament. Cell is embedded inside the hollow fiber filament with collagen gel, and the micro hollow fiber cell reactor has oxygen transferring and mass transferring capacity suitable for cell to survive and maintains the medicine metabolizing and other functions of cell. The micro hollow fiber cell reactor combines the advantages of miniature orifice plate and 3D bionic artificial liver reactor, maintains the bioactivity of animal cell. It may be used as in vitro mold of liver cell for the research of medicine metabolism and pharmacological and toxicological research and may be used in the lab research of cell culturing condition and cell product. It may be also used as the carrier for the research of animal cell culturing condition and lays the foundation for industrial production.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com