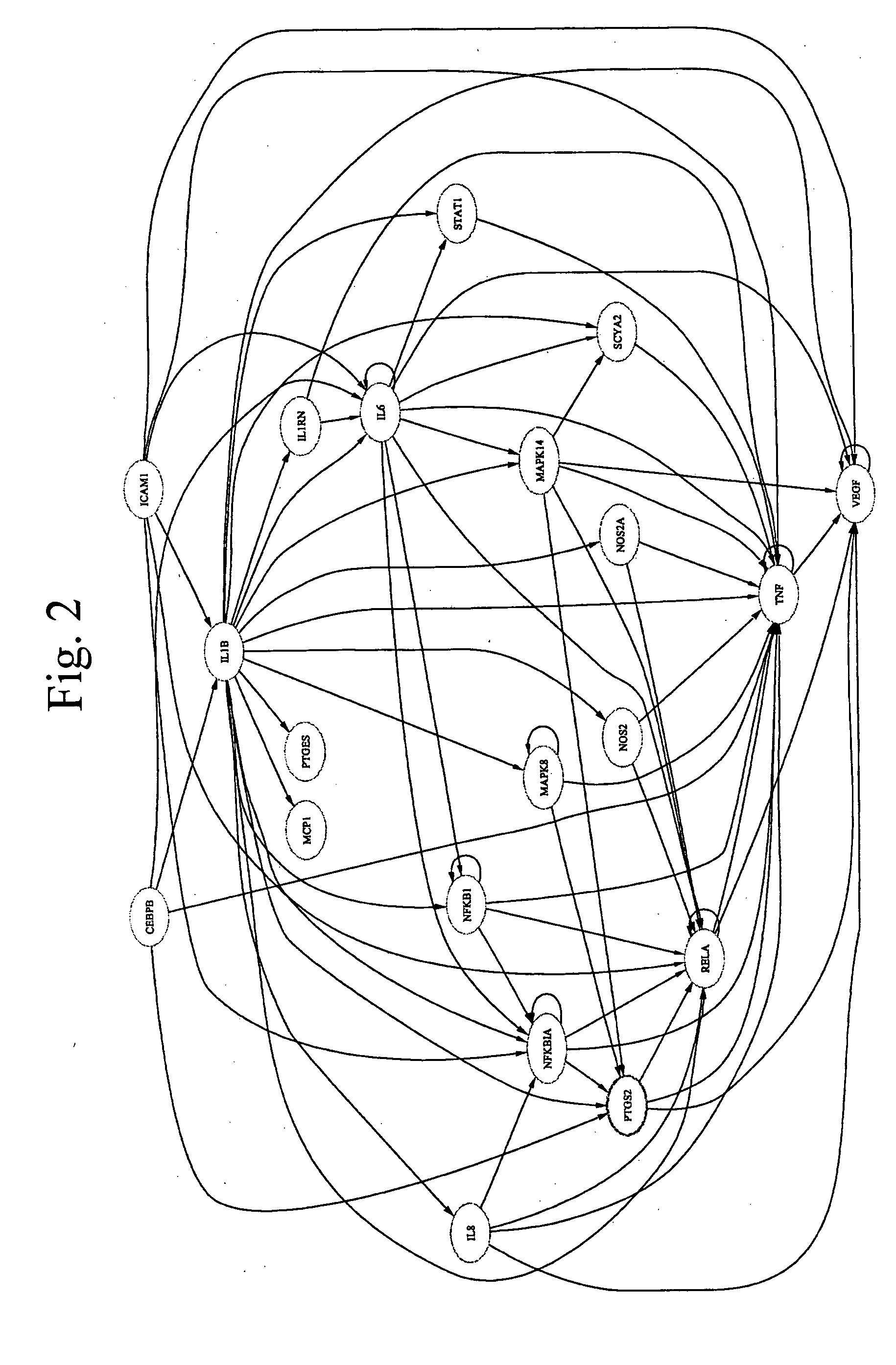
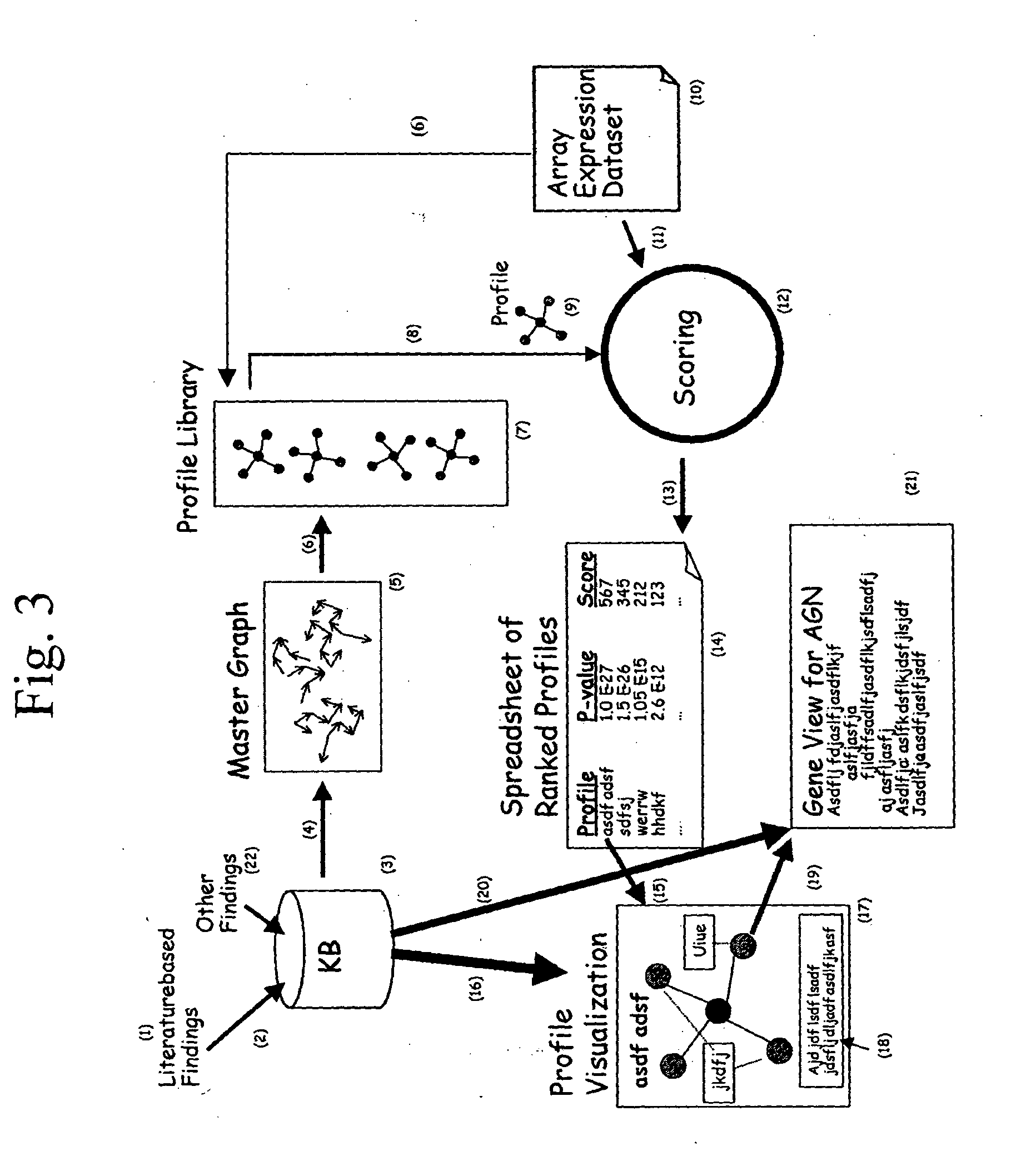
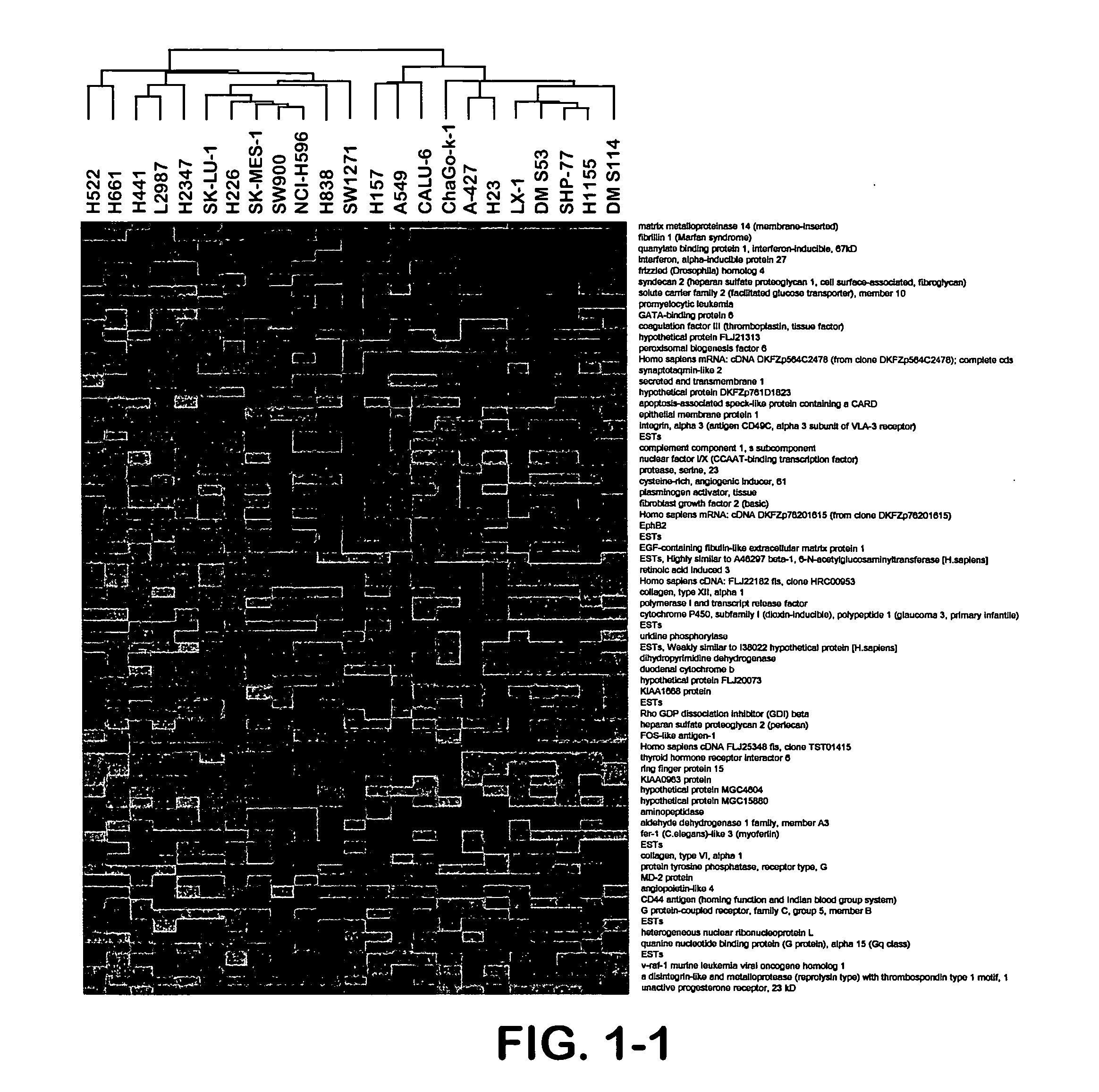
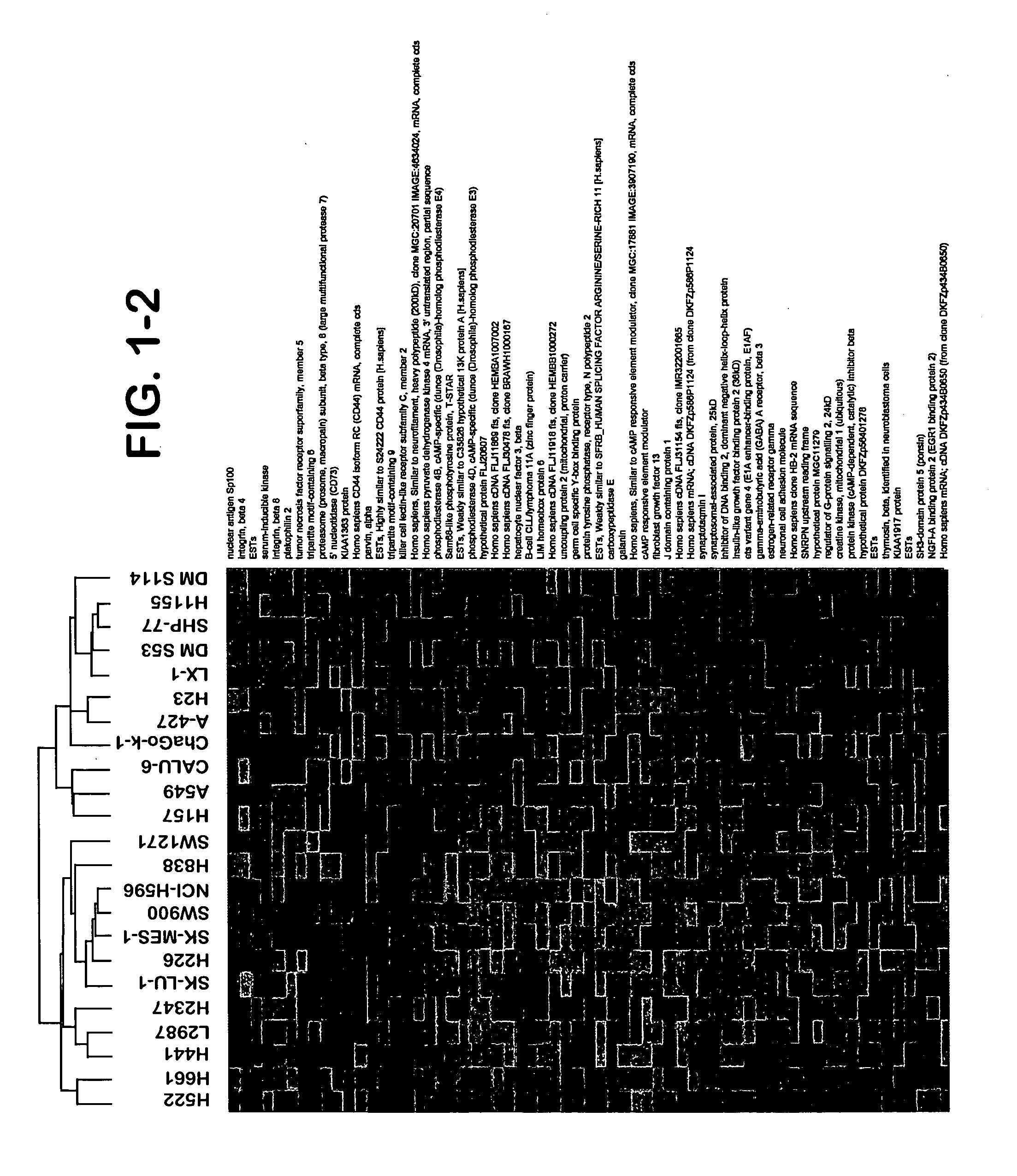
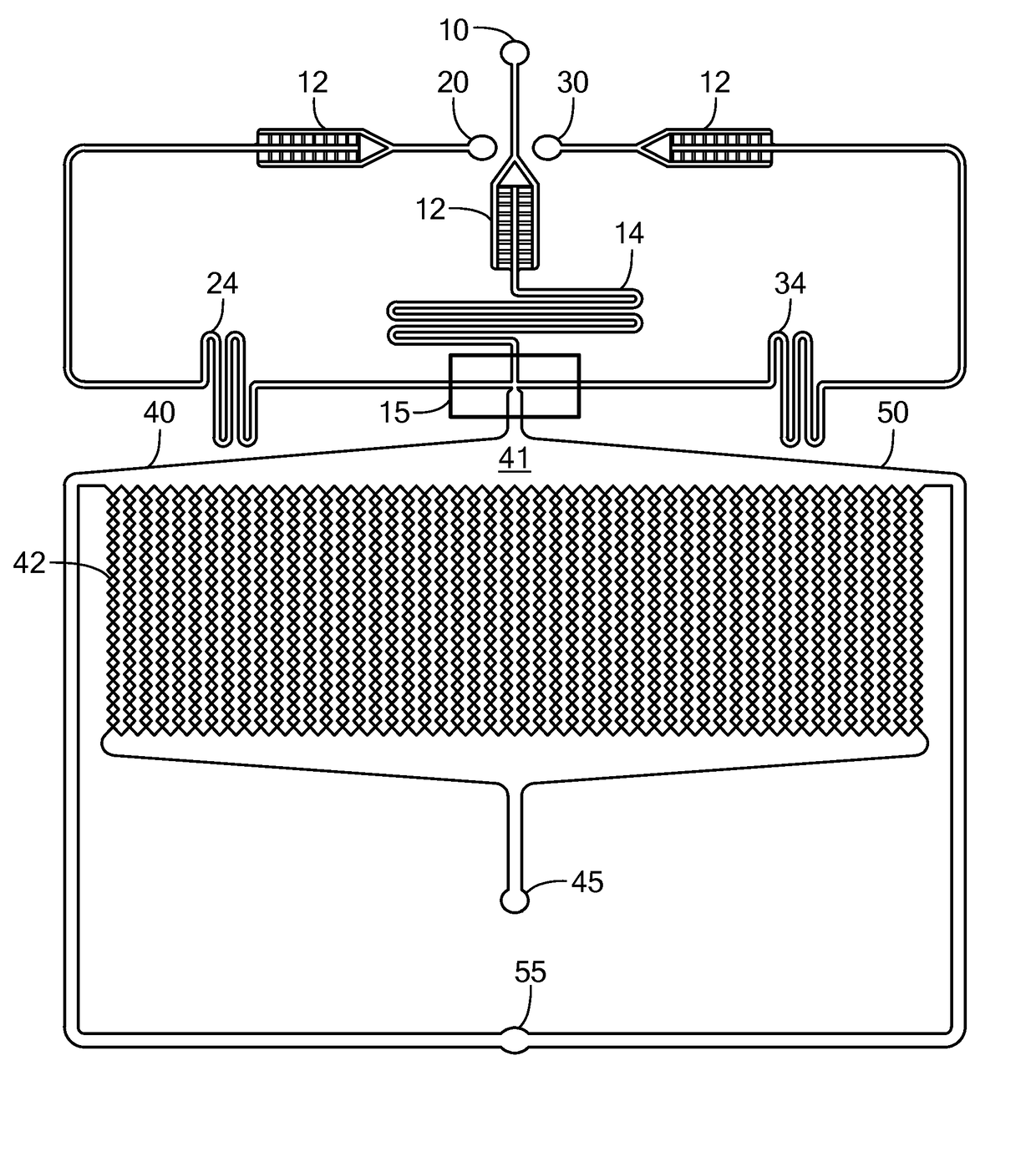
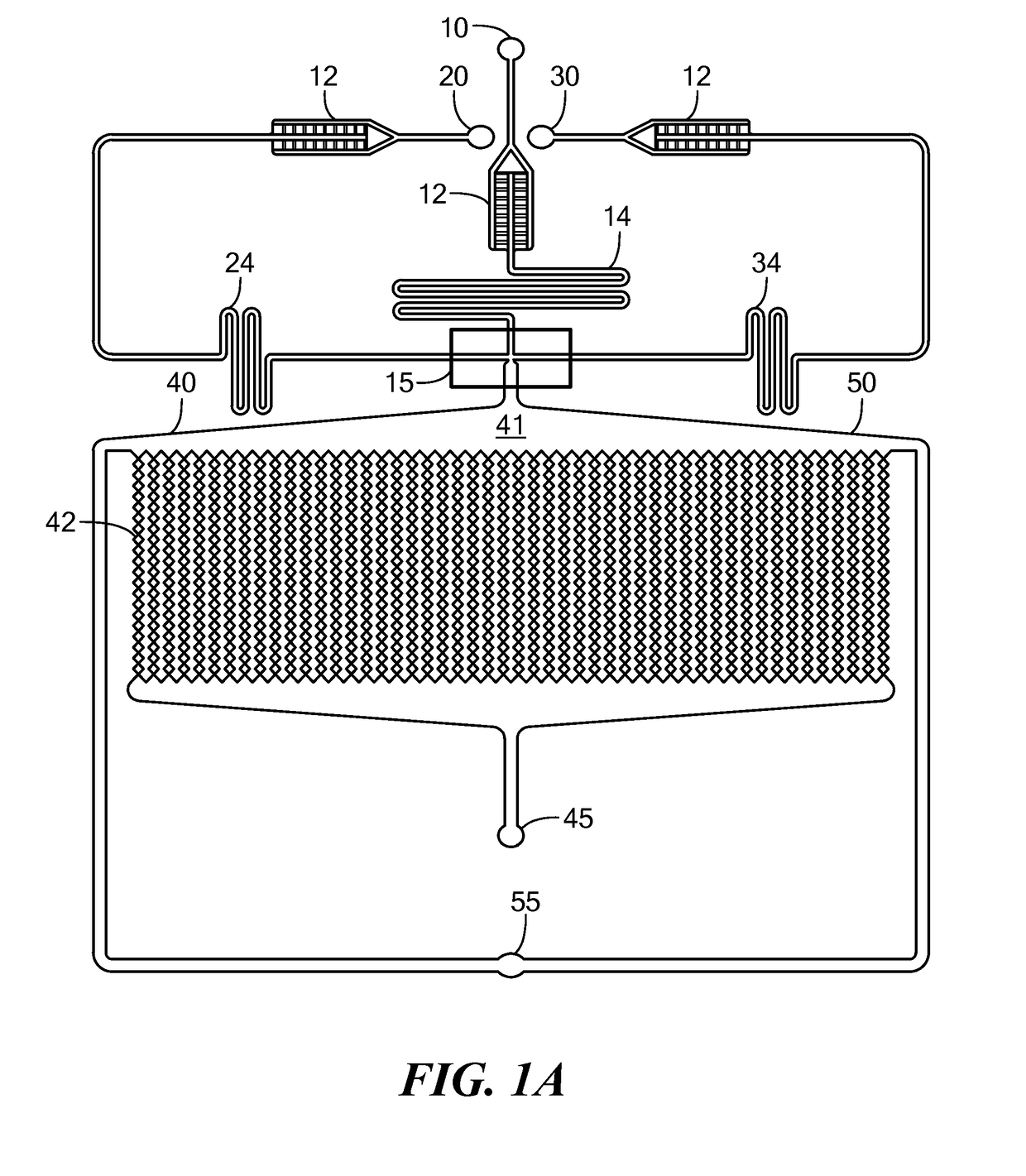
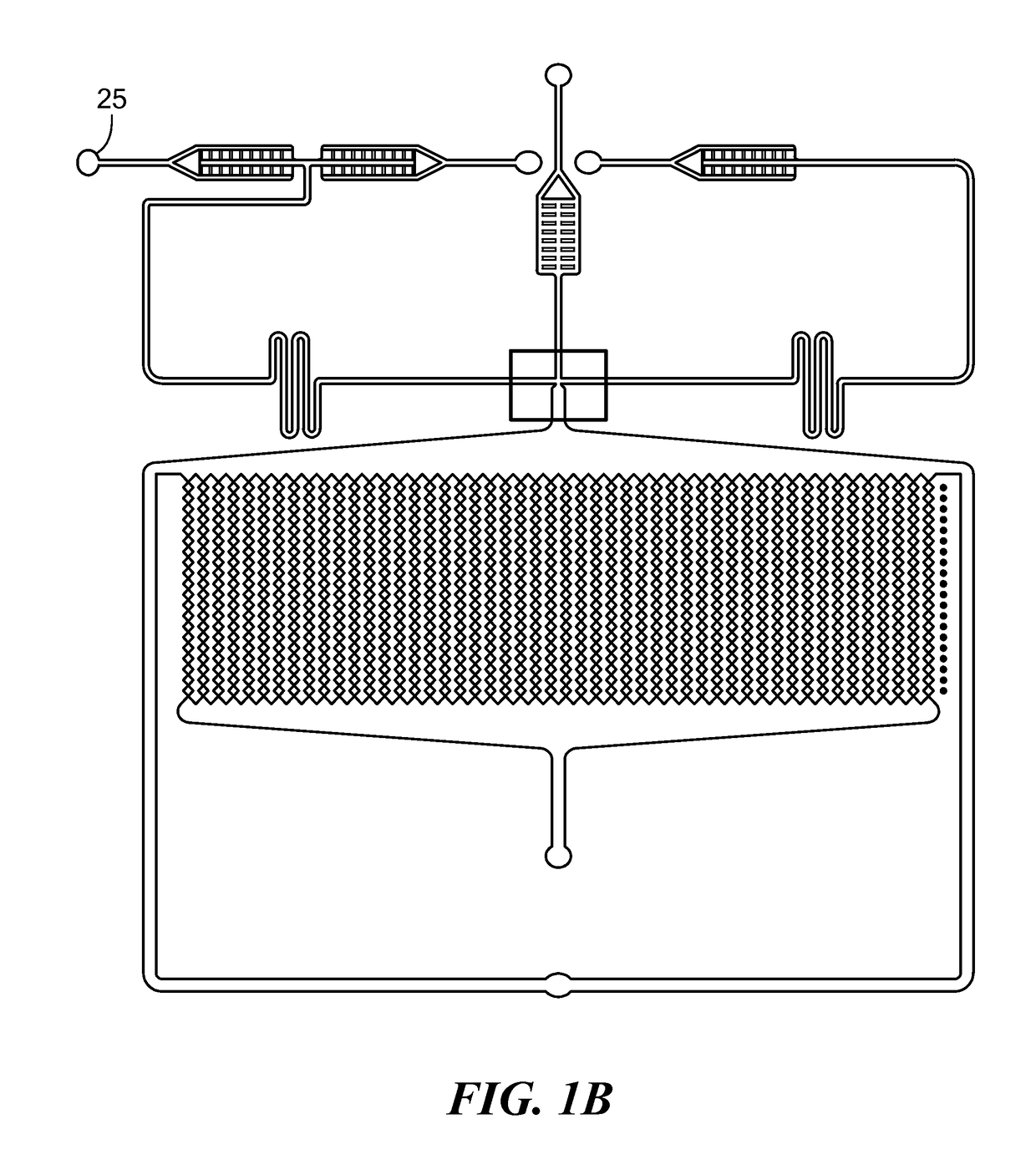
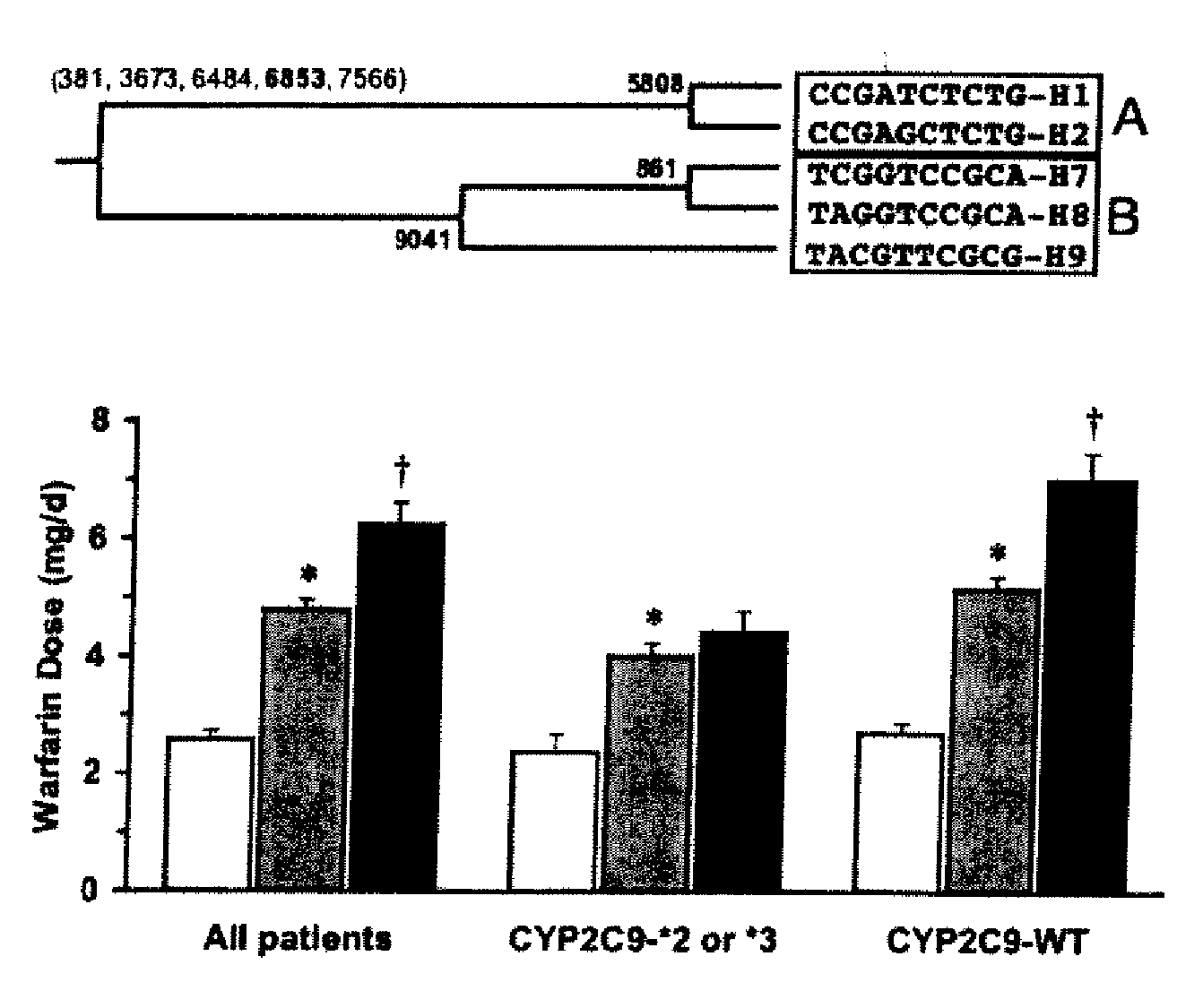
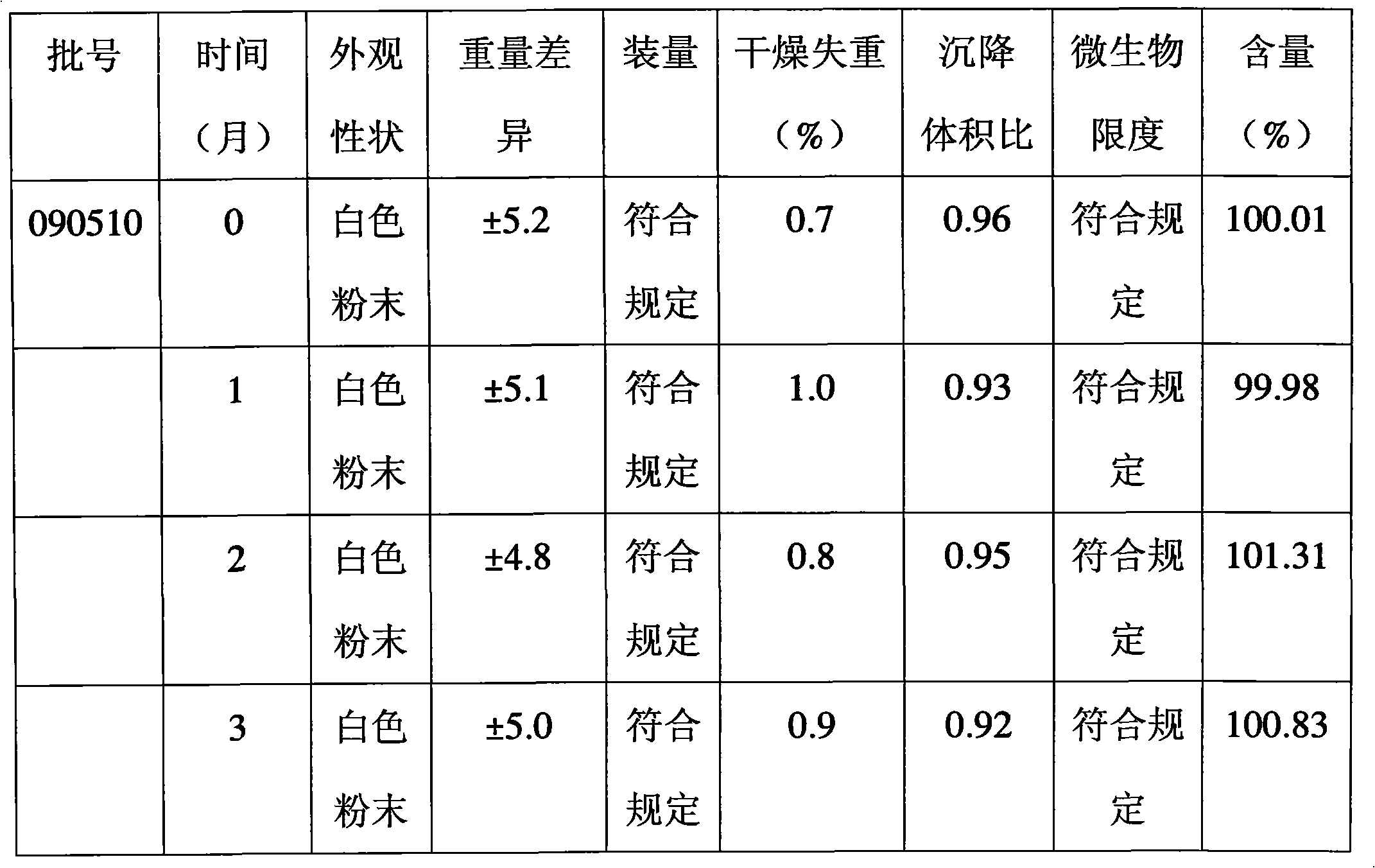
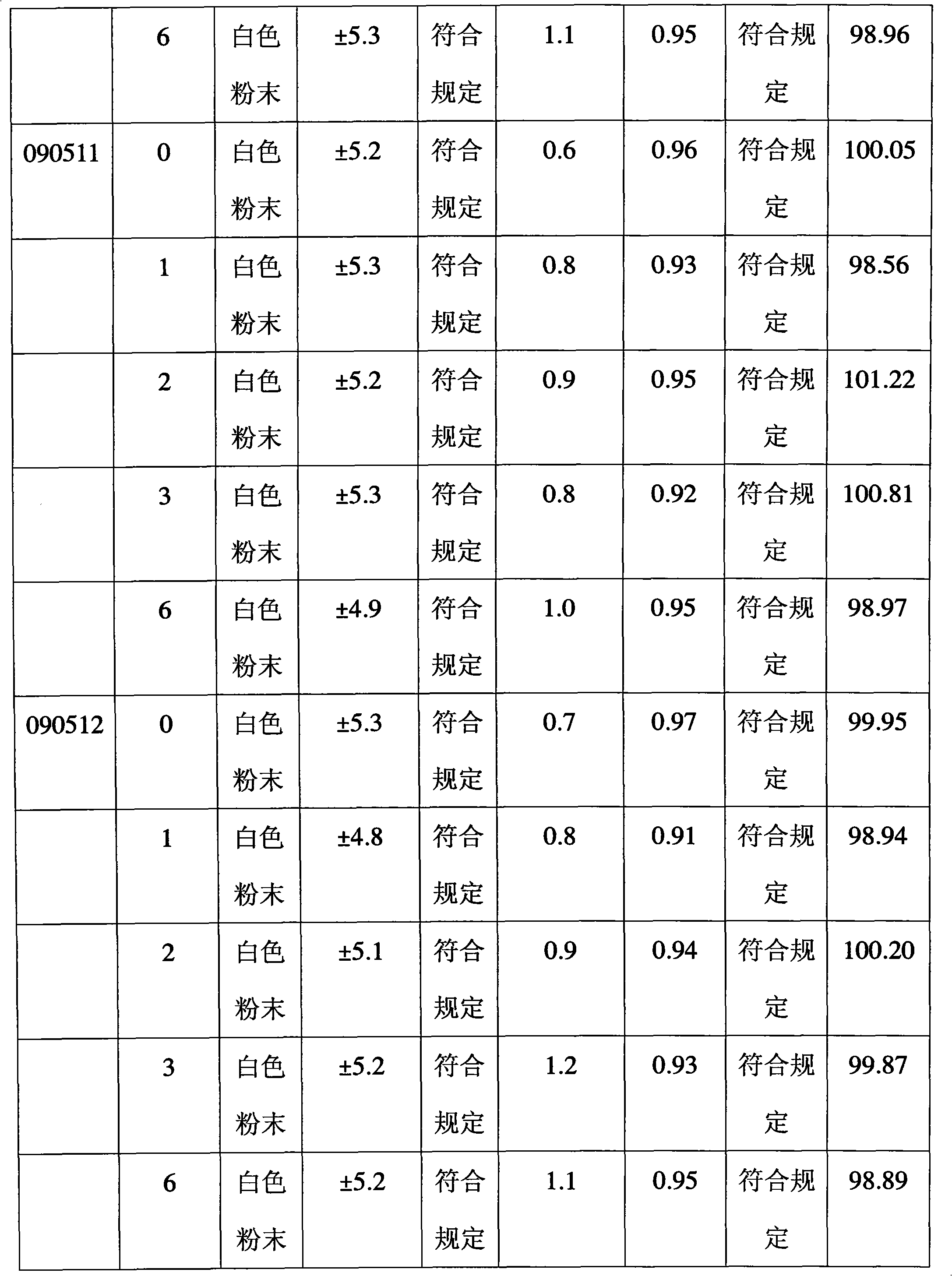
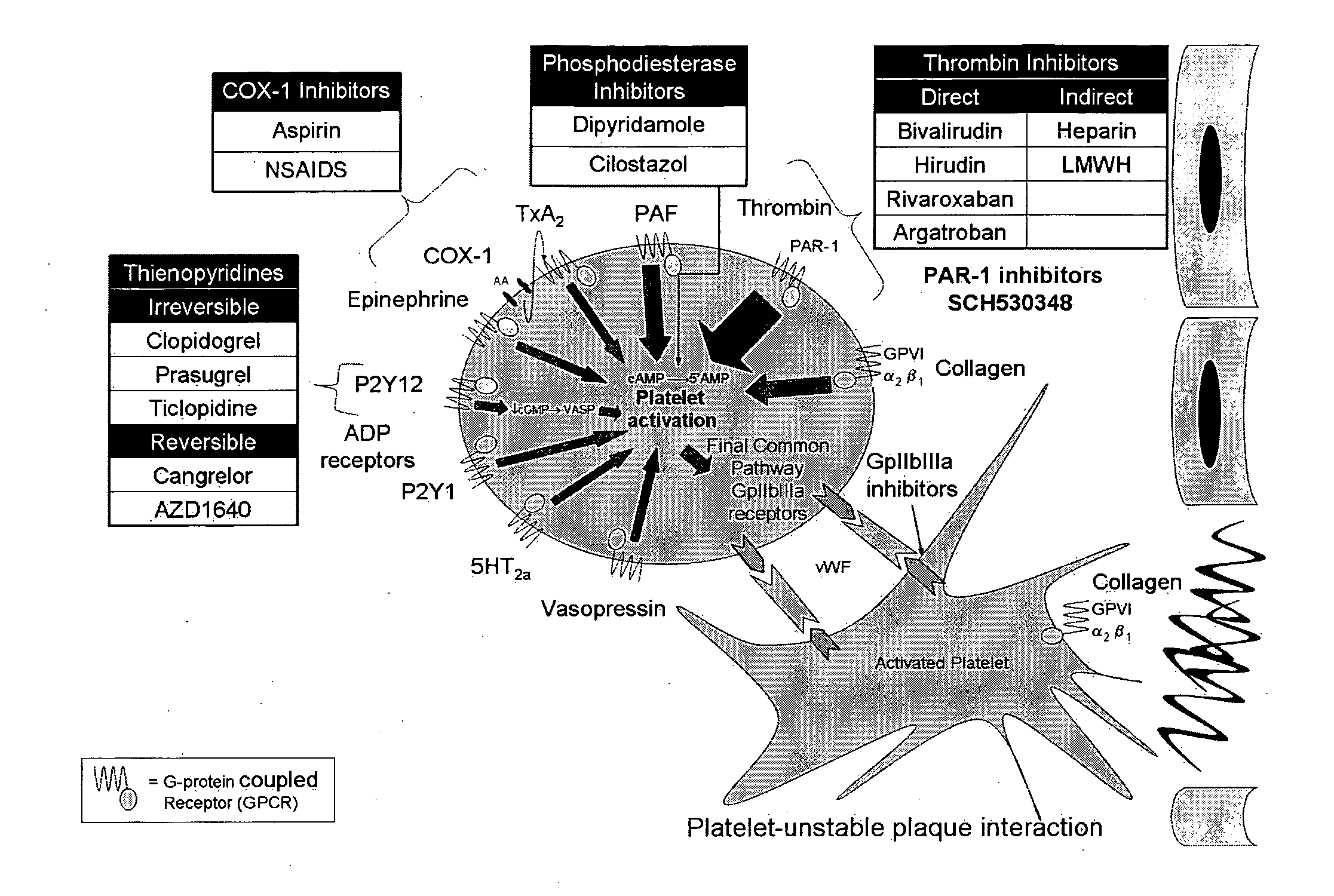
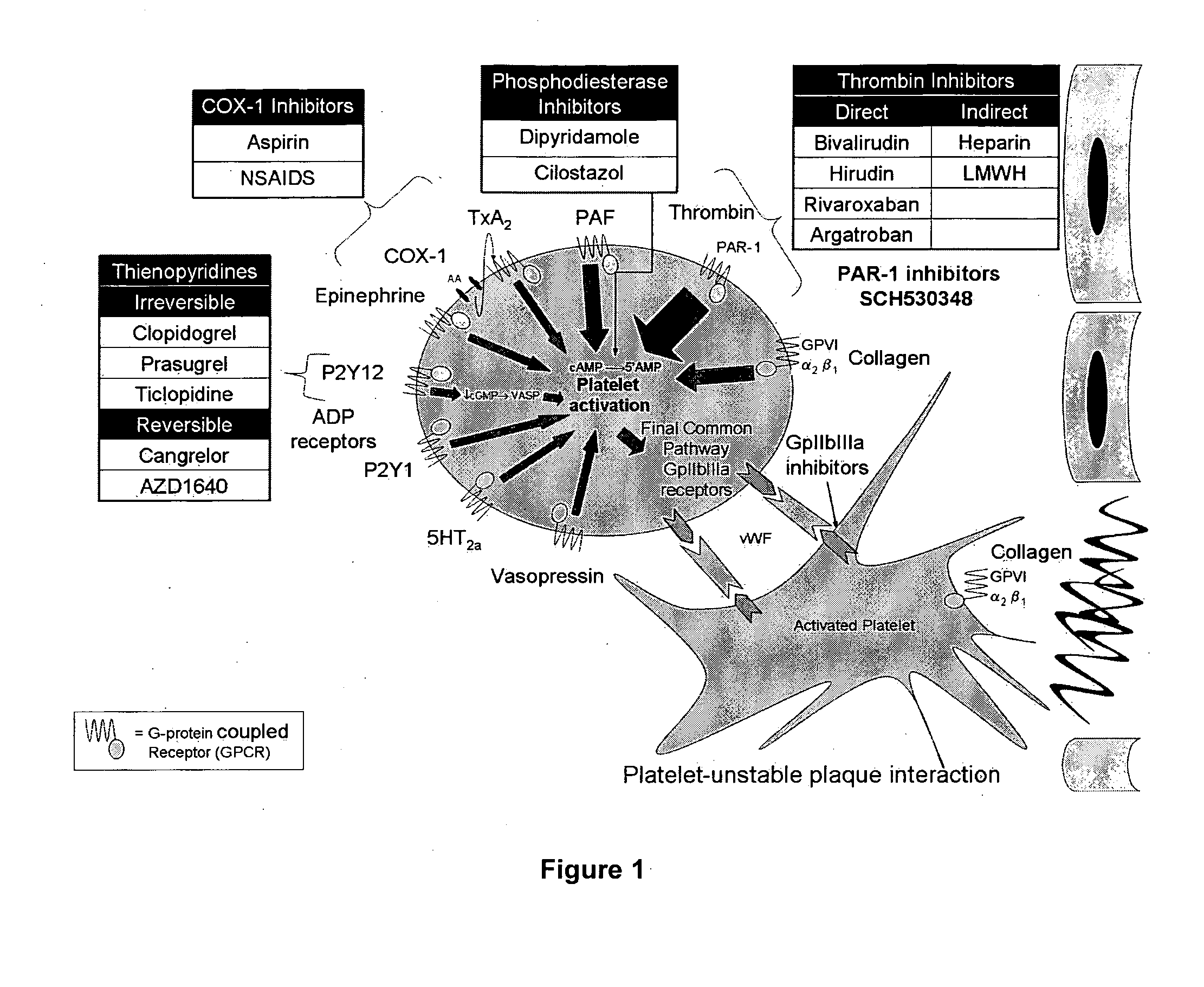
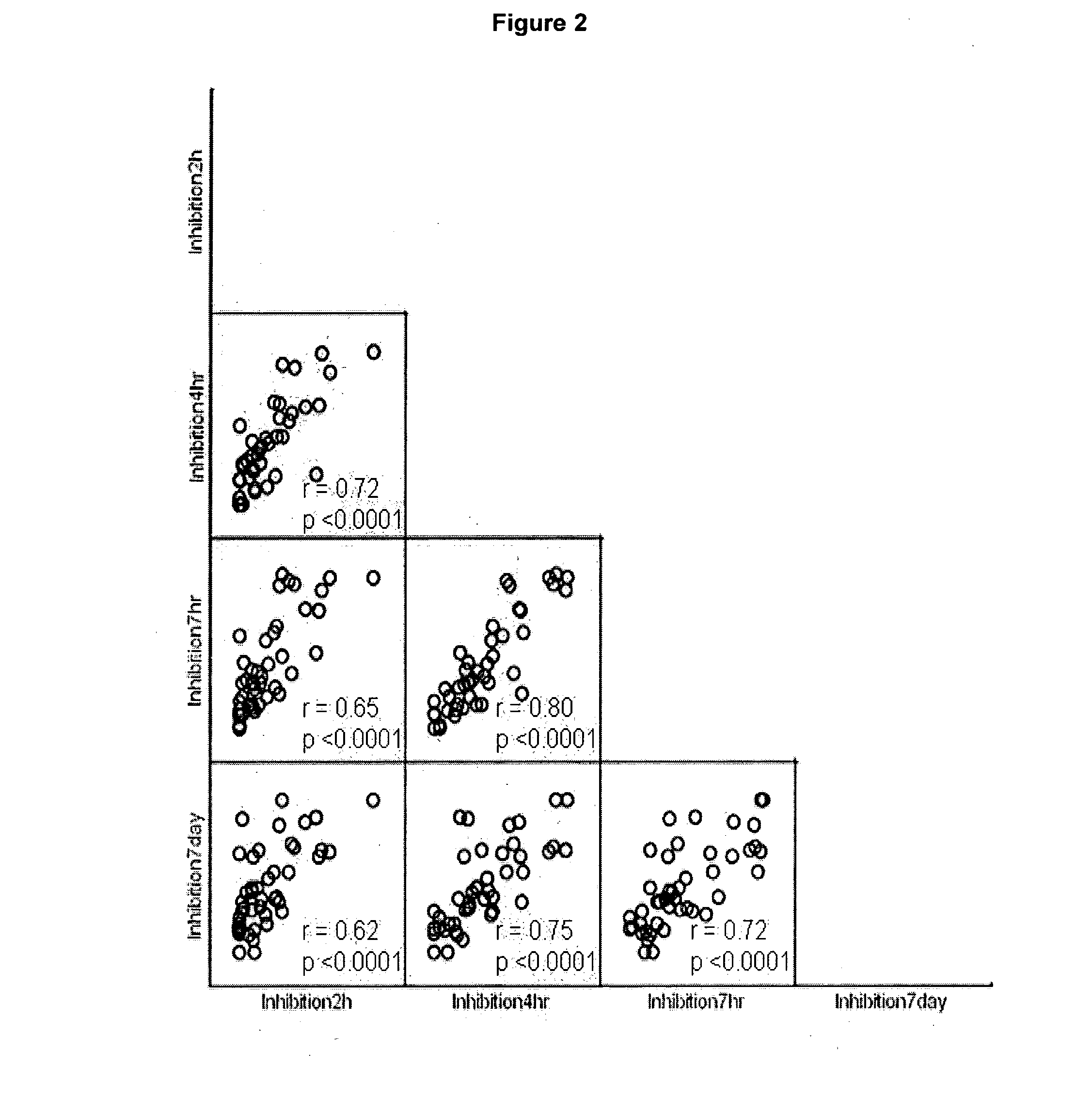
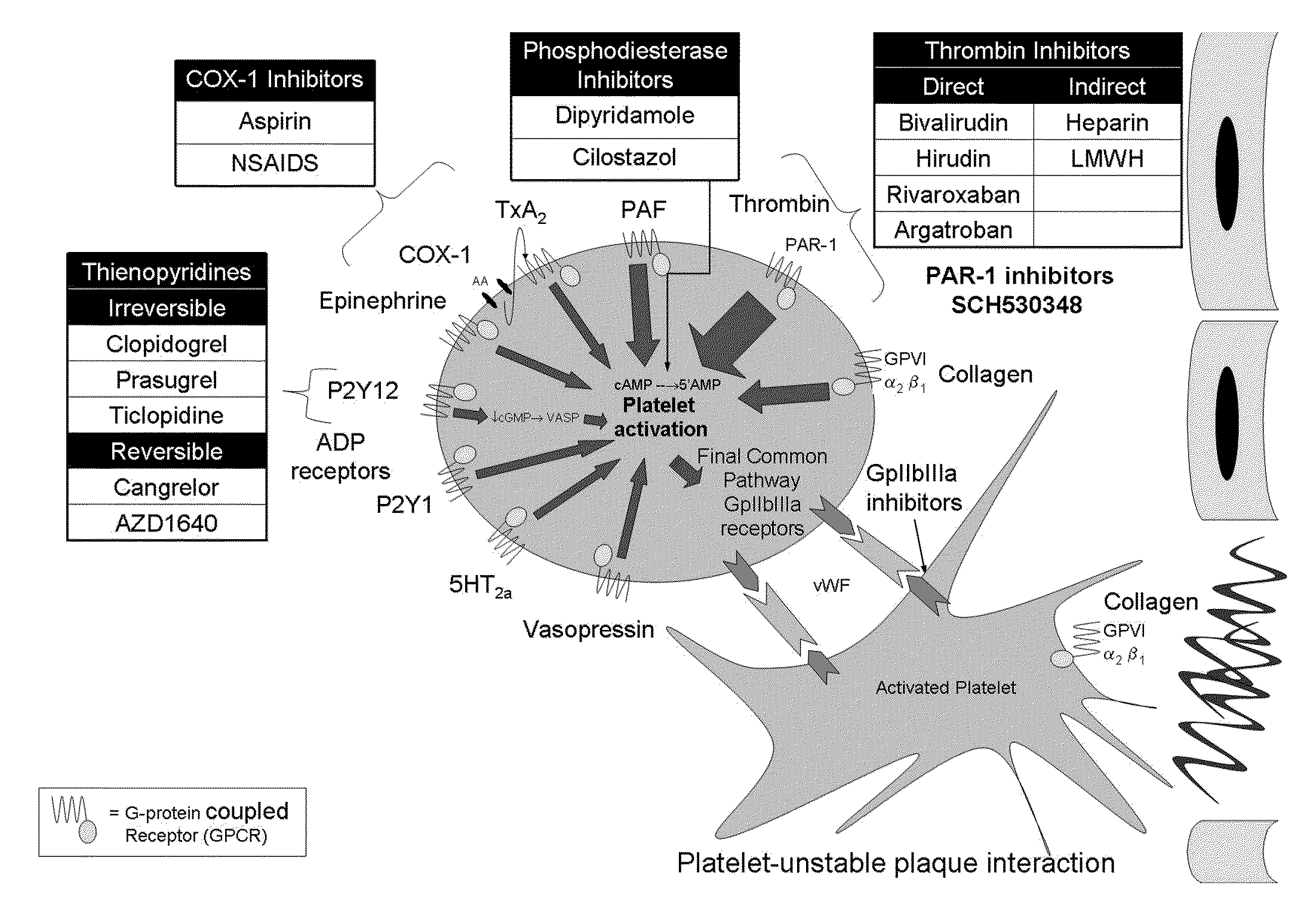
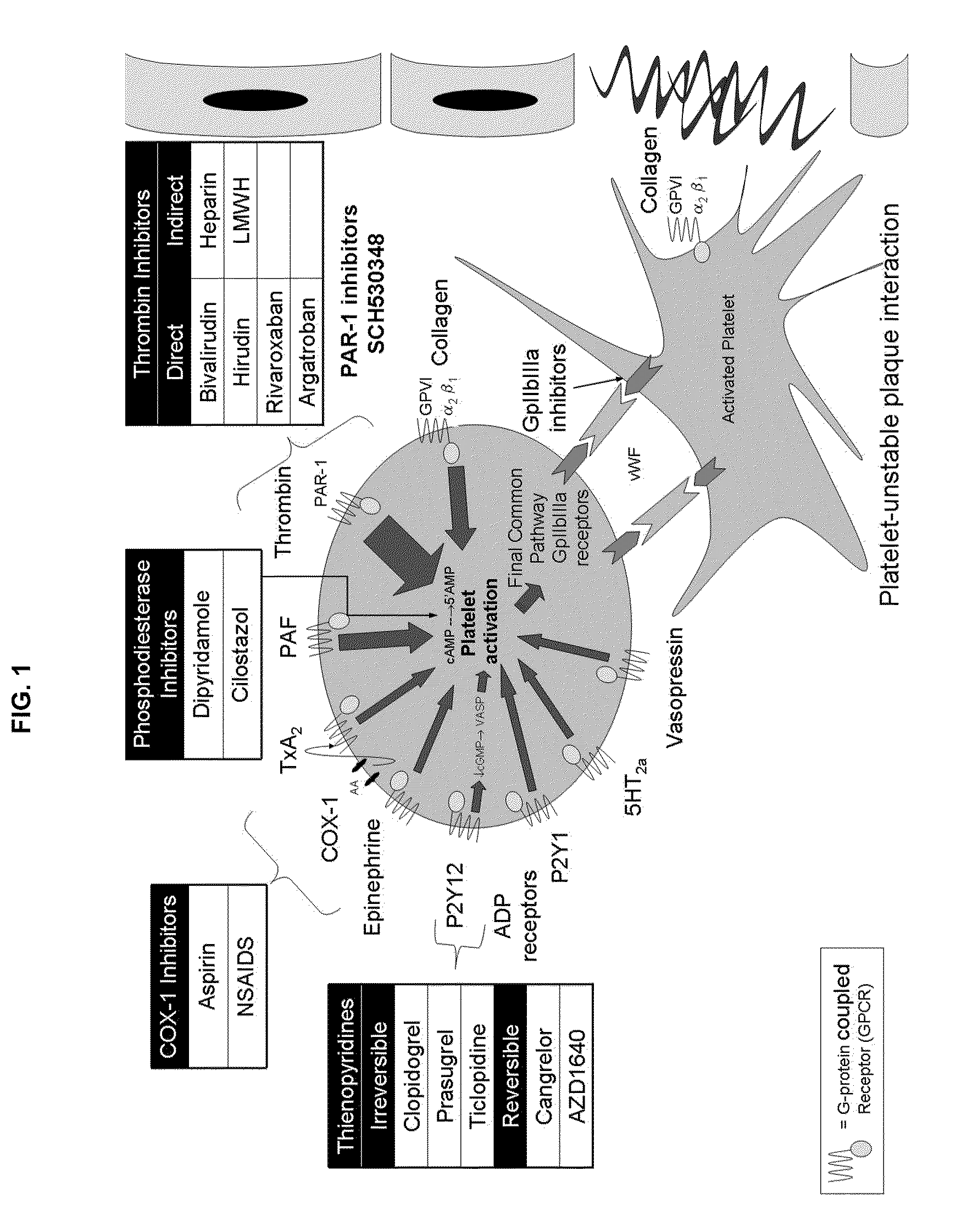
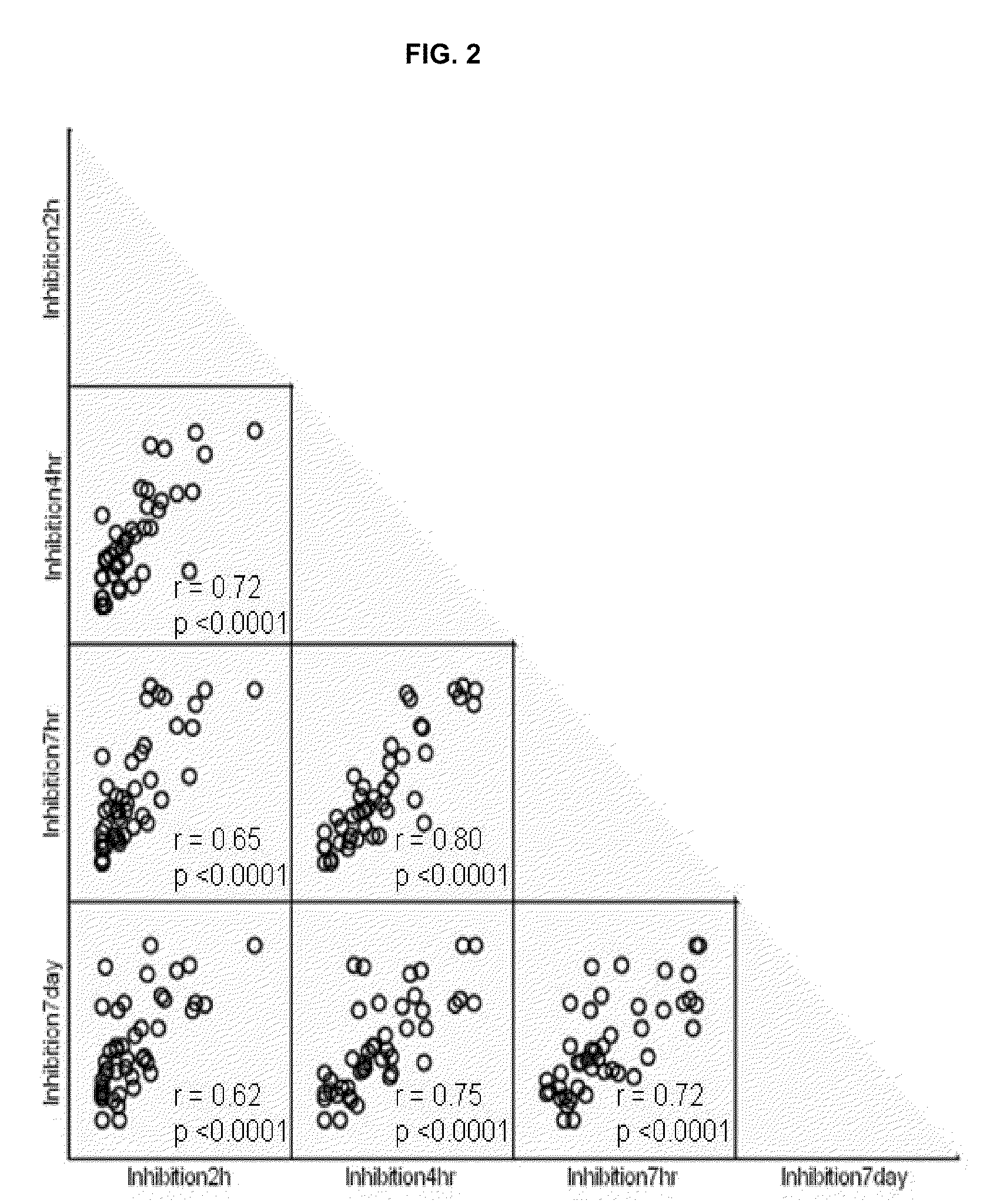
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
131 results about "Drugs response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for genomic analysis
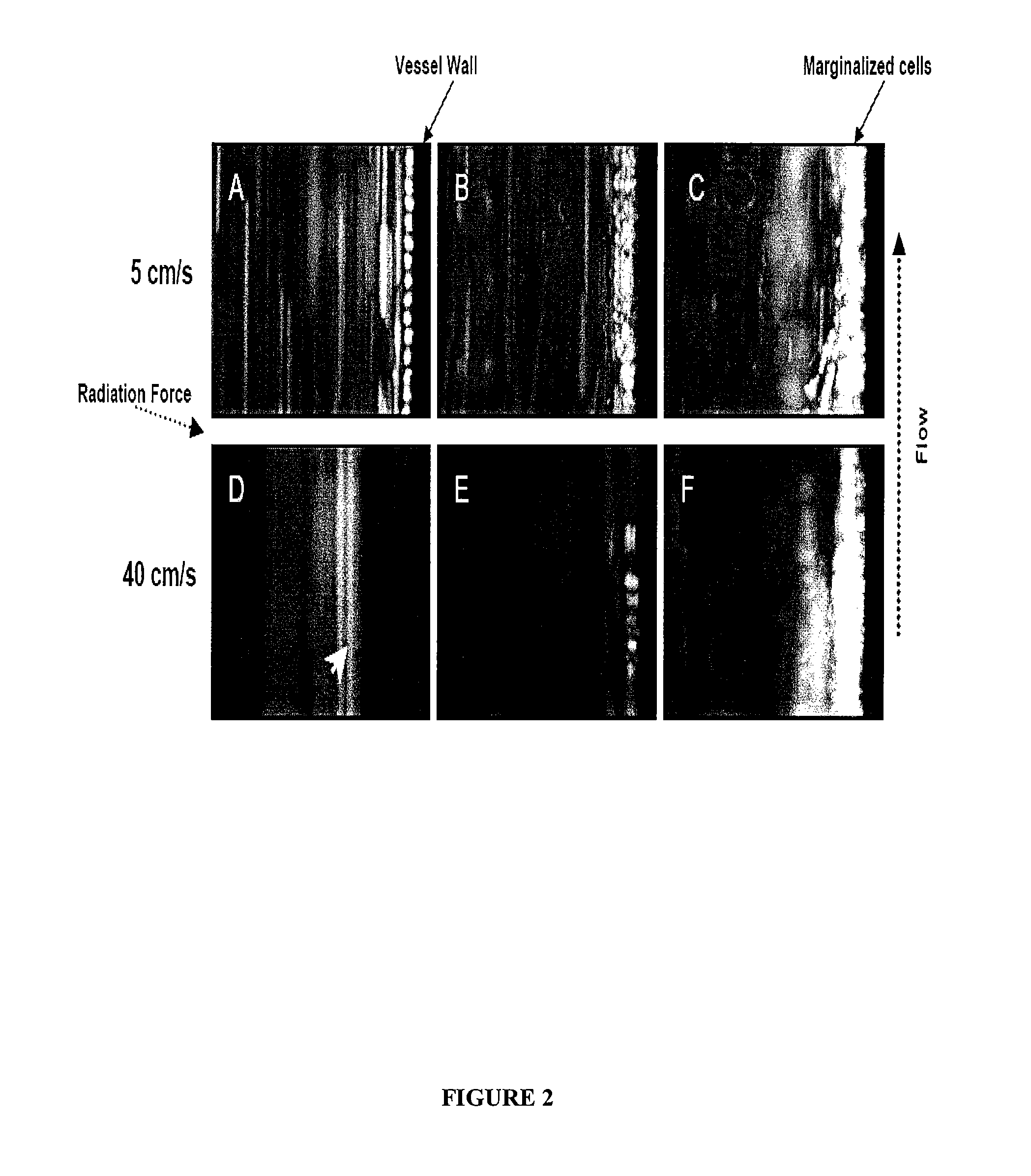
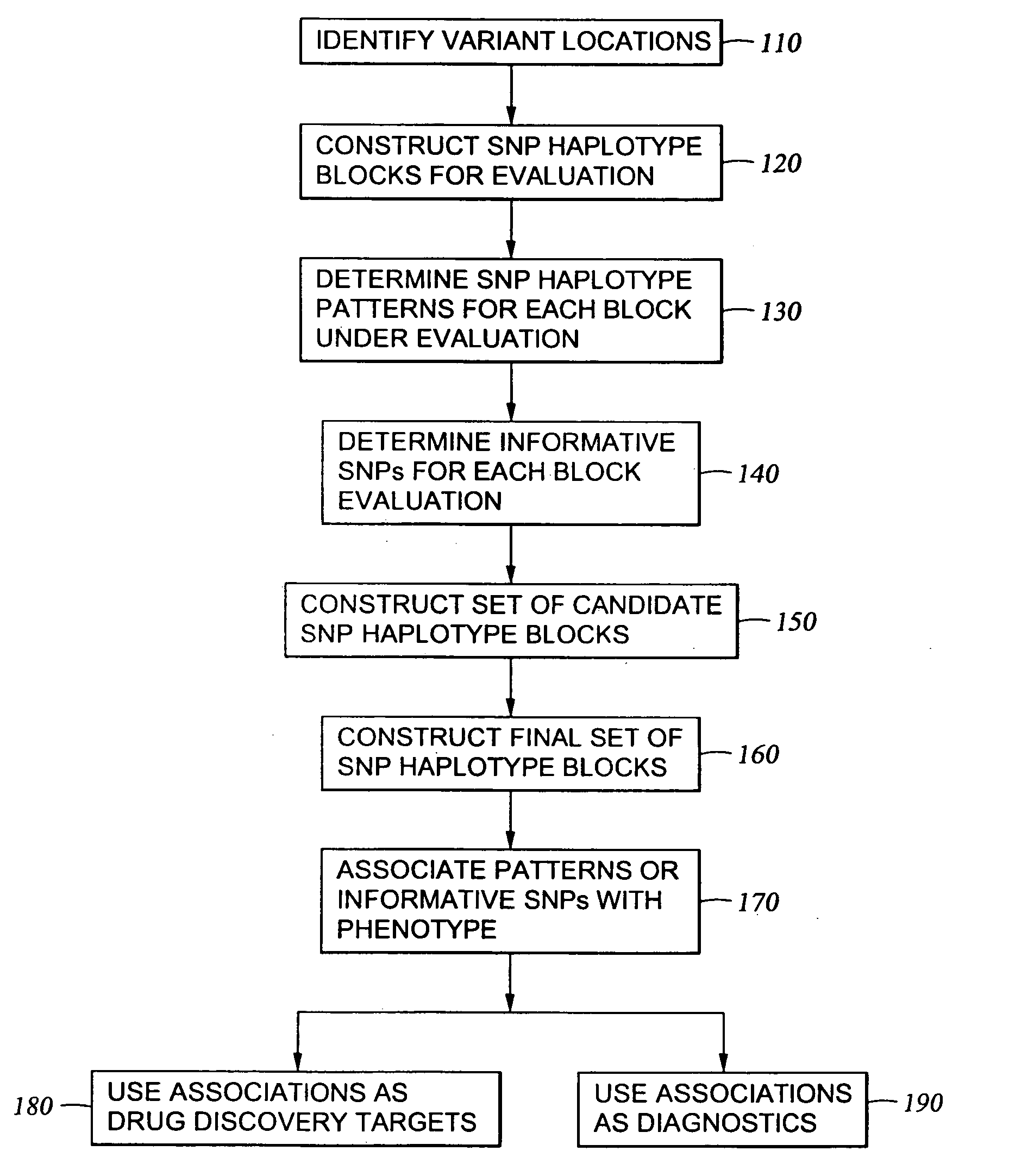
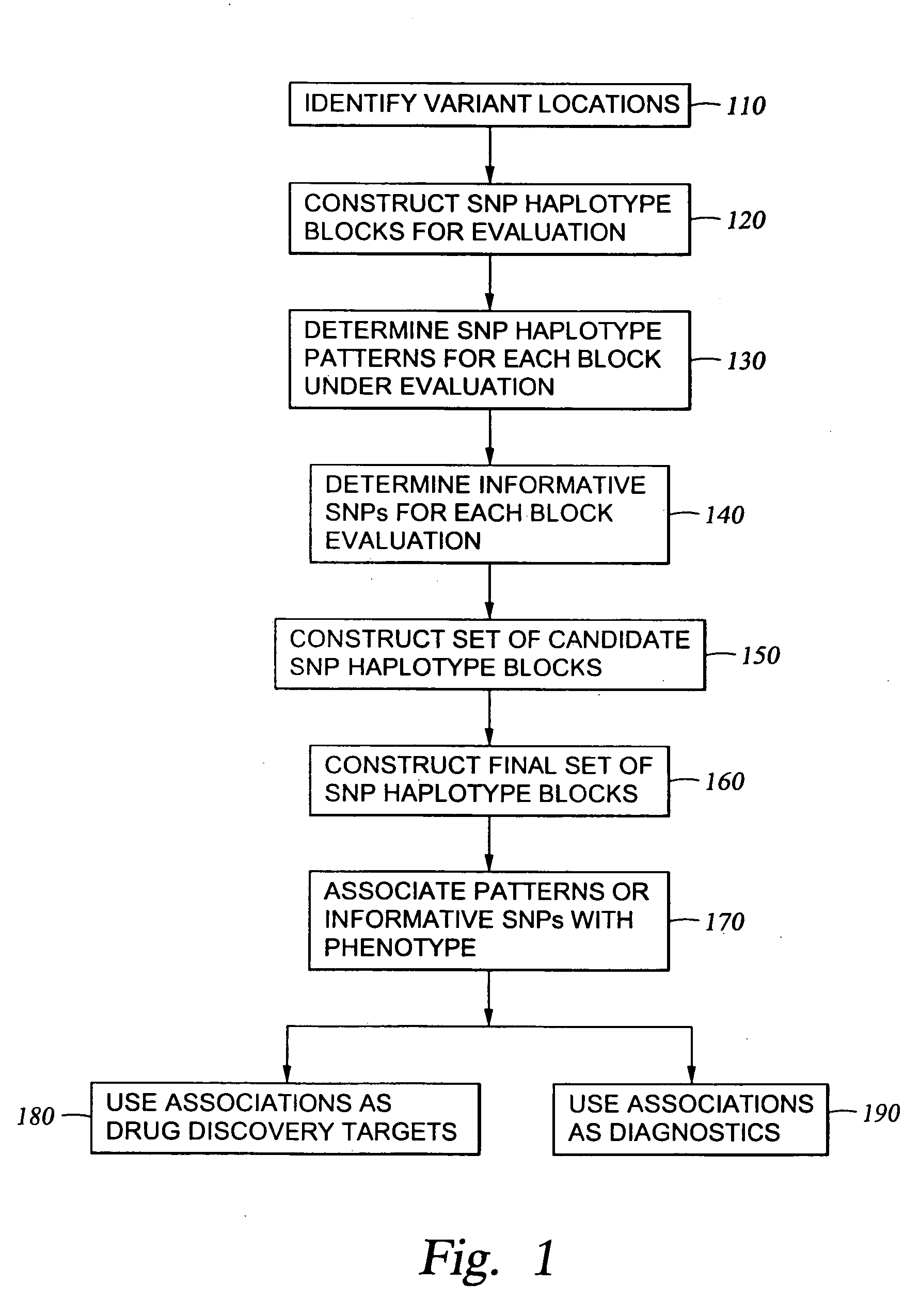
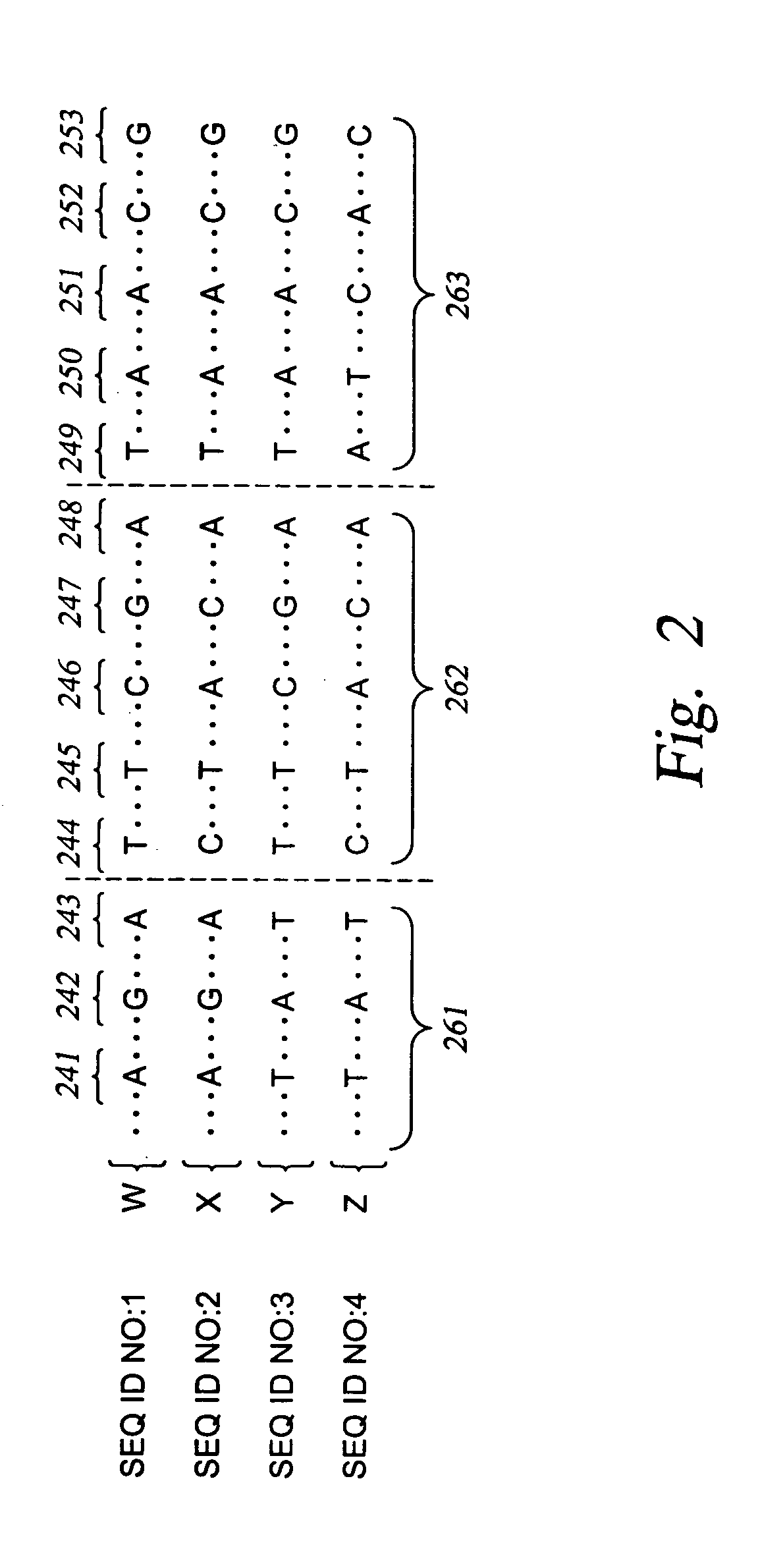
InactiveUS6969589B2Quick scanSugar derivativesMicrobiological testing/measurementHuman DNA sequencingGenetics
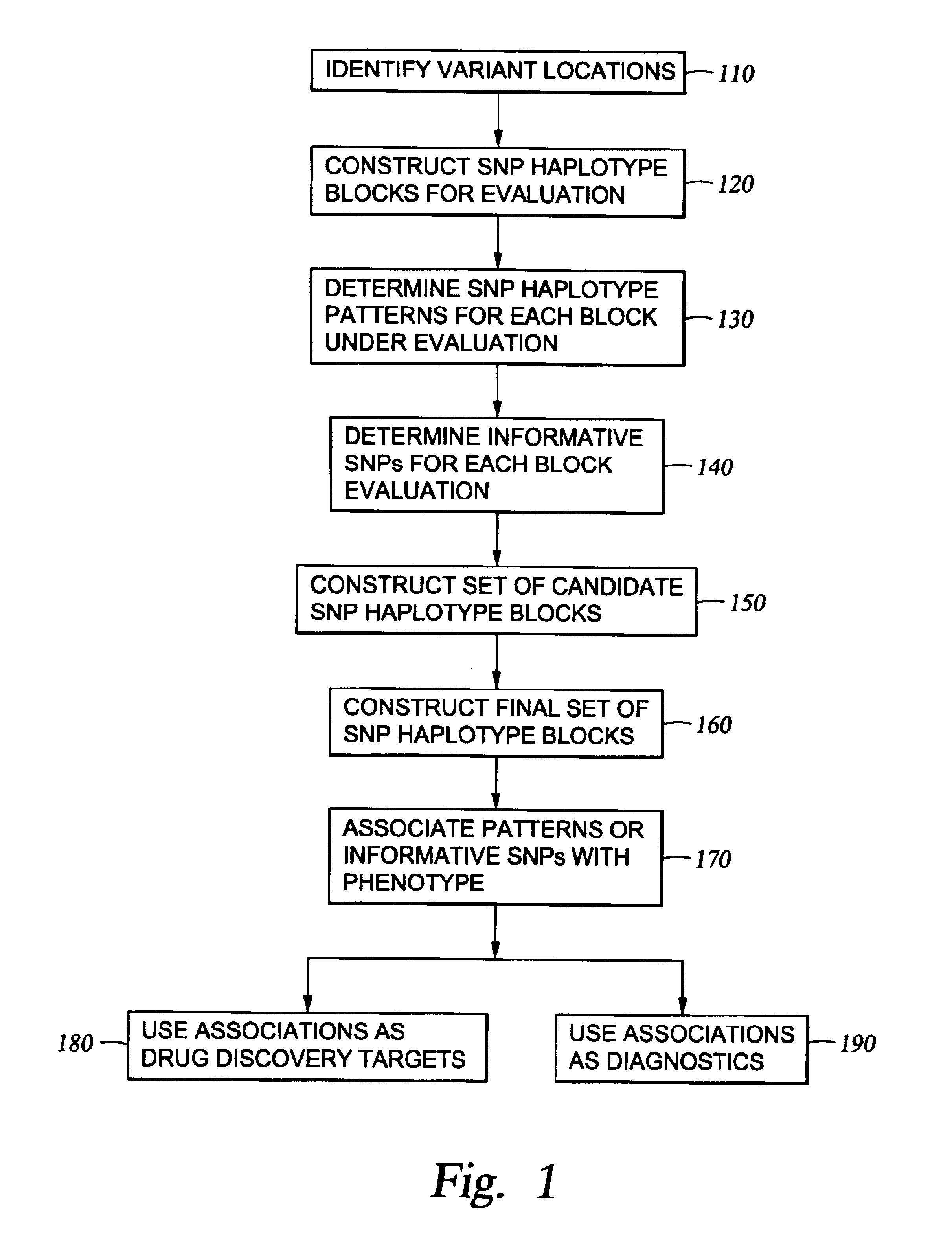
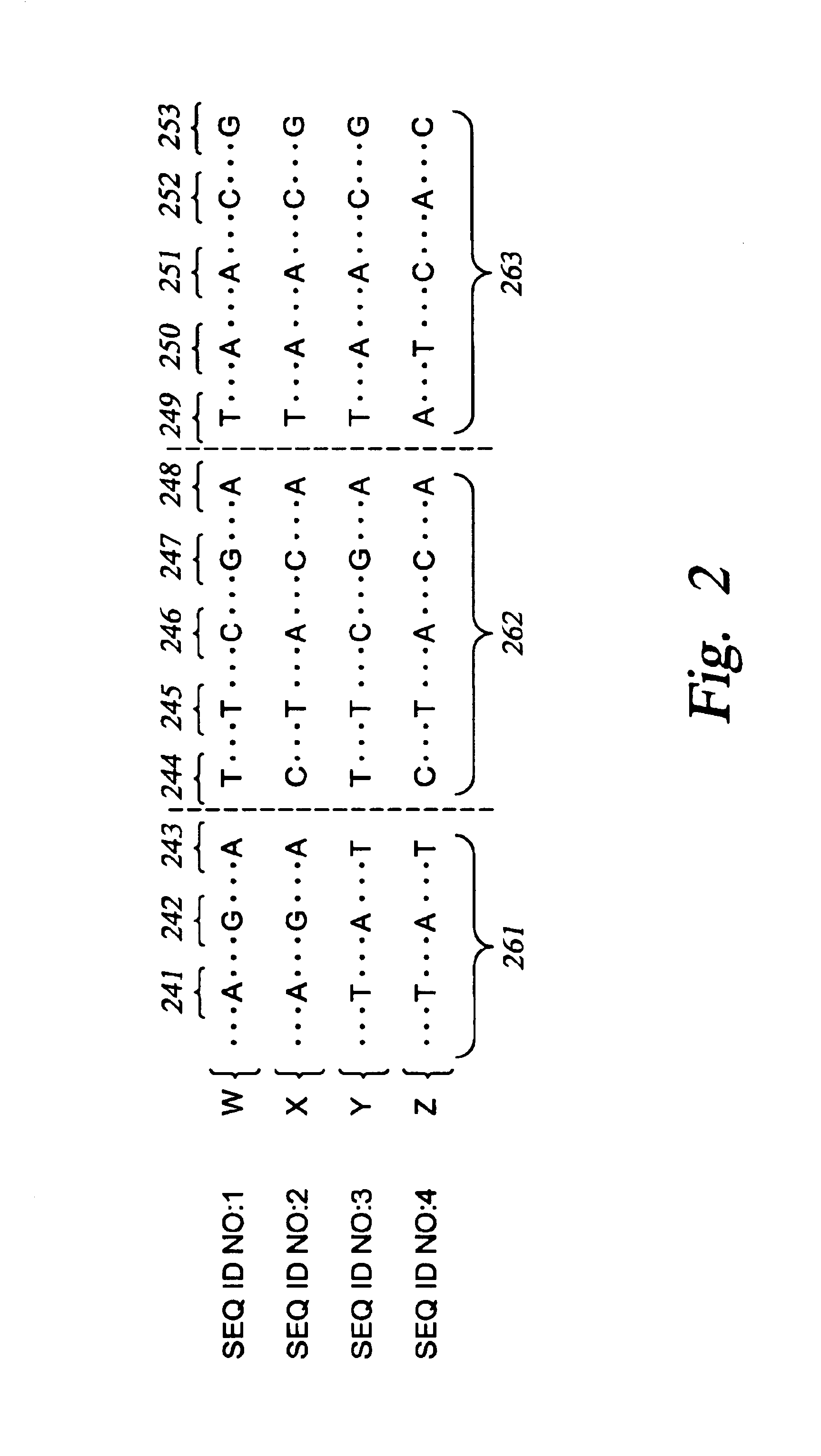
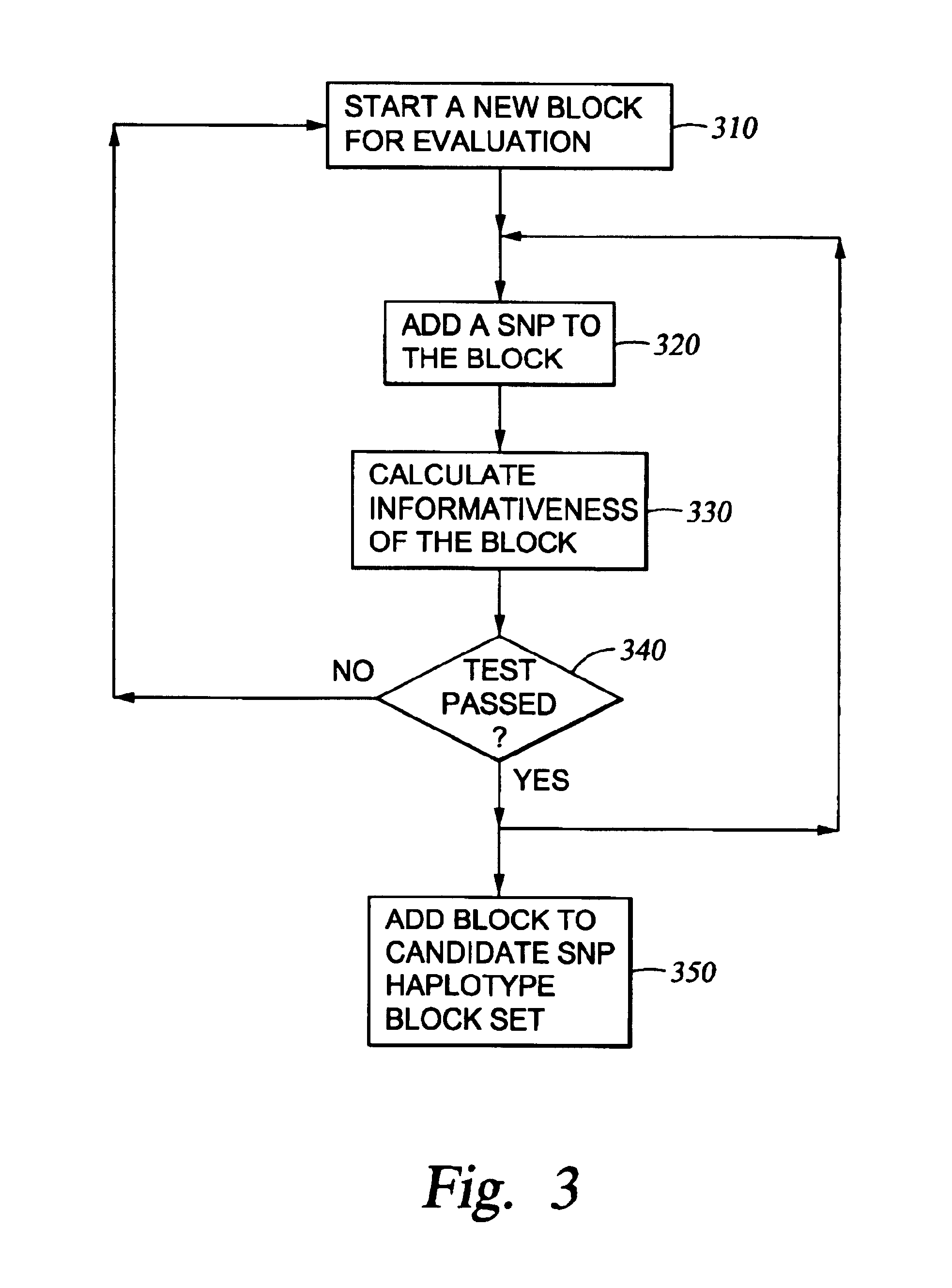
The present invention relates to methods for identifying variations that occur in the human genome and relating these variations to the genetic basis of disease and drug response. In particular, the present invention relates to identifying individual SNPs, determining SNP haplotype blocks and patterns, and, further, using the SNP haplotype blocks and patterns to dissect the genetic bases of disease and drug response. The methods of the present invention are useful in whole genome analysis.
Owner:AFFYMETRIX INC +1
Lipidomic Approaches to Determining Drug Response Phenotypes in Cardiovascular Disease
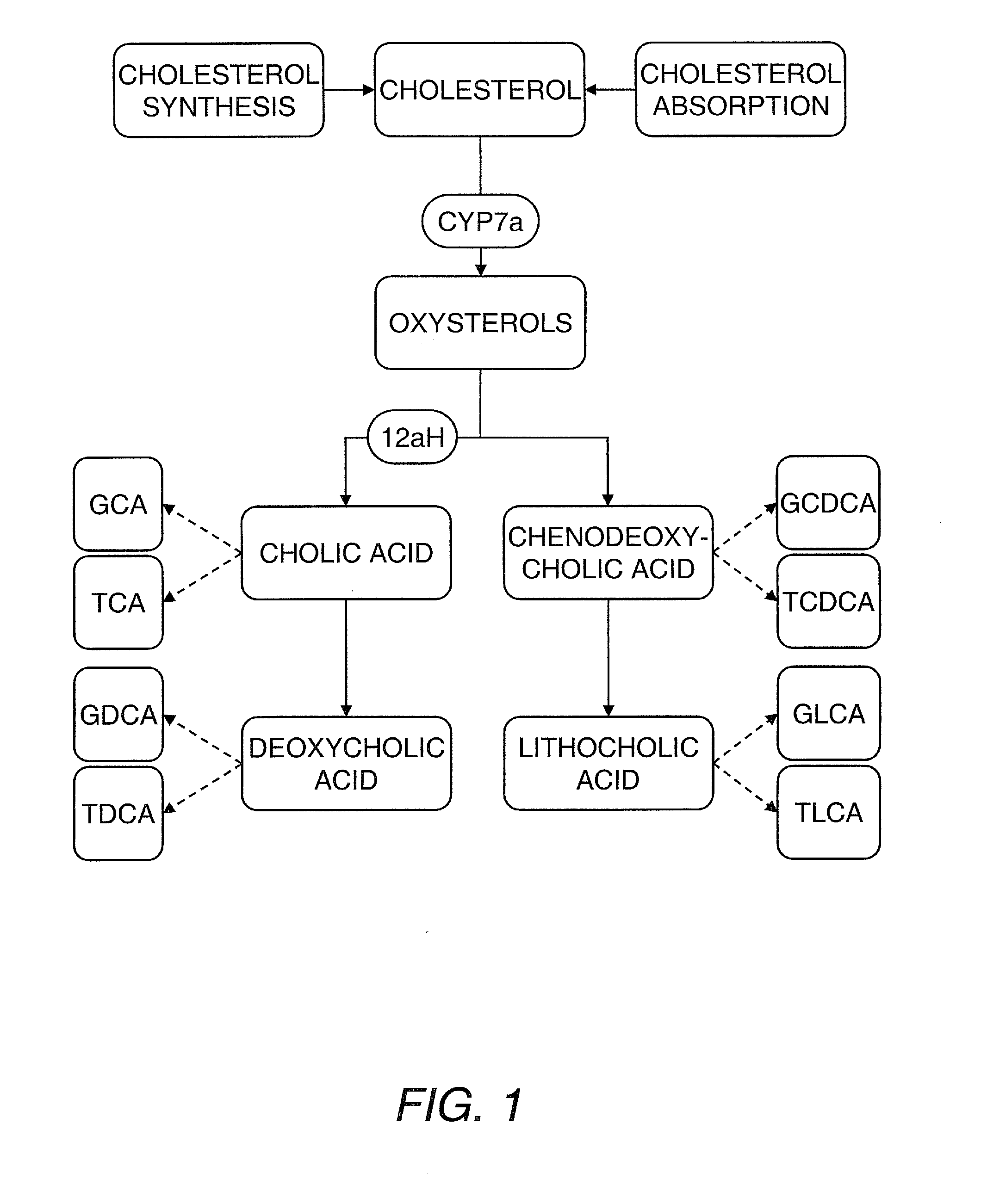
The present invention concerns the application of lipidomics to statin treatment for disorders such as cardiovascular disorders. Hence, the invention provides, among other things, a method of correlating a lipid profile with a positive or negative response to a statin treatment regimen by obtaining a lipid profile of a sample from a mammalian subject following commencement of the treatment regimen; and correlating the lipid profile in the sample with a positive or negative response to the treatment regimen. The invention further provides a method of correlating a lipid profile with a positive or negative response to a statin treatment regimen by obtaining a lipid profile of a sample from a mammalian subject before commencement of the treatment regimen; and correlating the lipid profile in the sample with a positive or negative response to the treatment regimen.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN +4
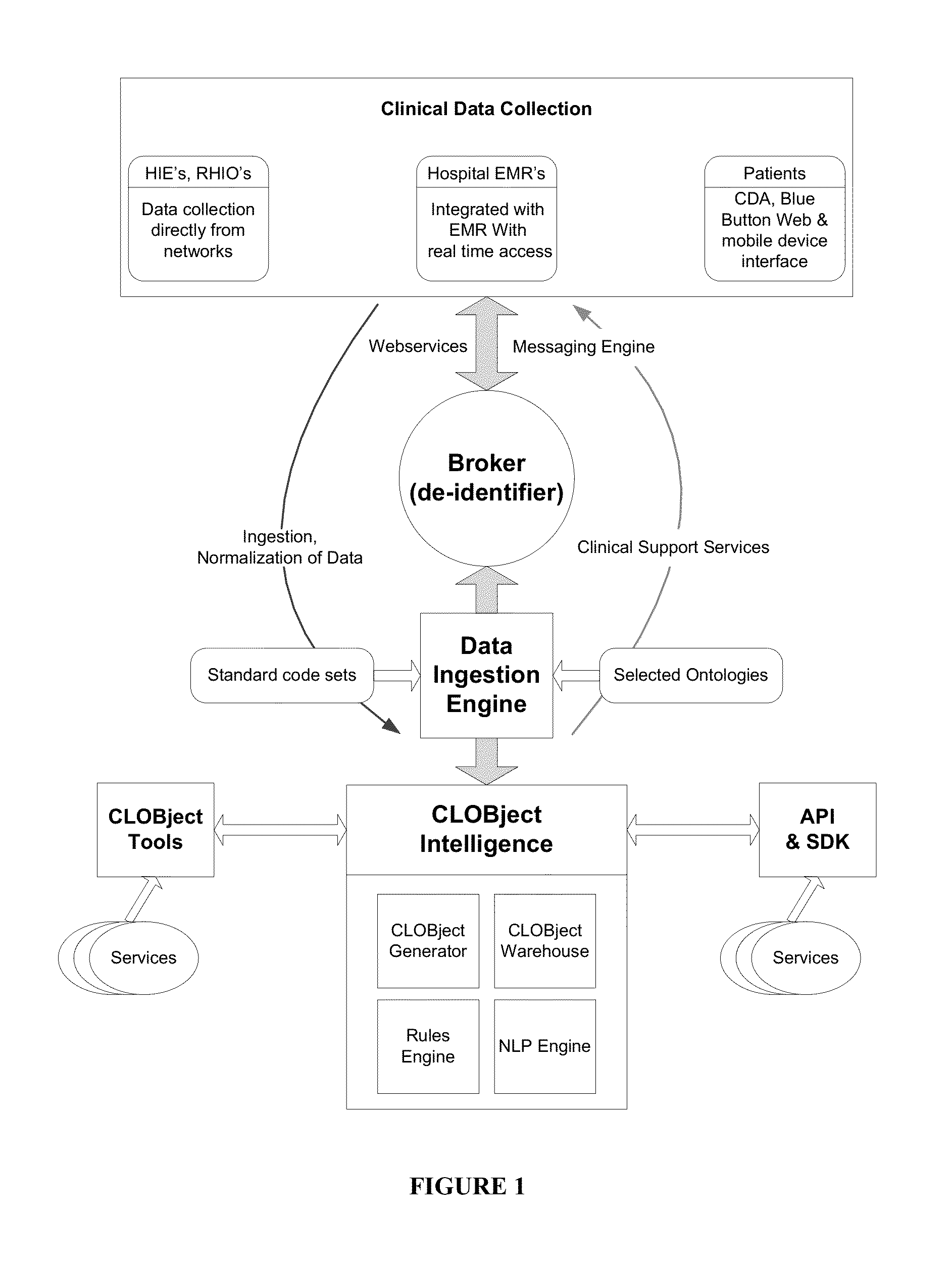
System and Methods for Personalized Clinical Decision Support Tools
InactiveUS20140350954A1Data processing applicationsMedical automated diagnosisPersonalizationDisease risk
The use of a medical software platform to collect, normalize, and aggregate clinical data. Supports clinical care and provides research and clinical tools for institutions, healthcare providers, researchers, and patients. The invention provides a graphical interface to customize searches in the database for specified subsets of conditions, treatments or outcomes. The invention also provides a system and method for searching through clinical databases for desired terms which may provide additional information to a physician regarding patient care. Furthermore, this invention facilitates personalized medicine-based practices as relationships between genetics, personal heath data from multiple sources, disease risk and drug response can be more easily visualized and utilized for patient care and research.
Owner:ONTOMICS
Compositions and methods for discovery of causative mutations in genetic disorders
Owner:POPULATION BIO INC
Drug discovery methods
ActiveUS20070178473A1Raise priorityMicrobiological testing/measurementLibrary screeningDrug responseDisease cause
Owner:INGENUITY SYST INC
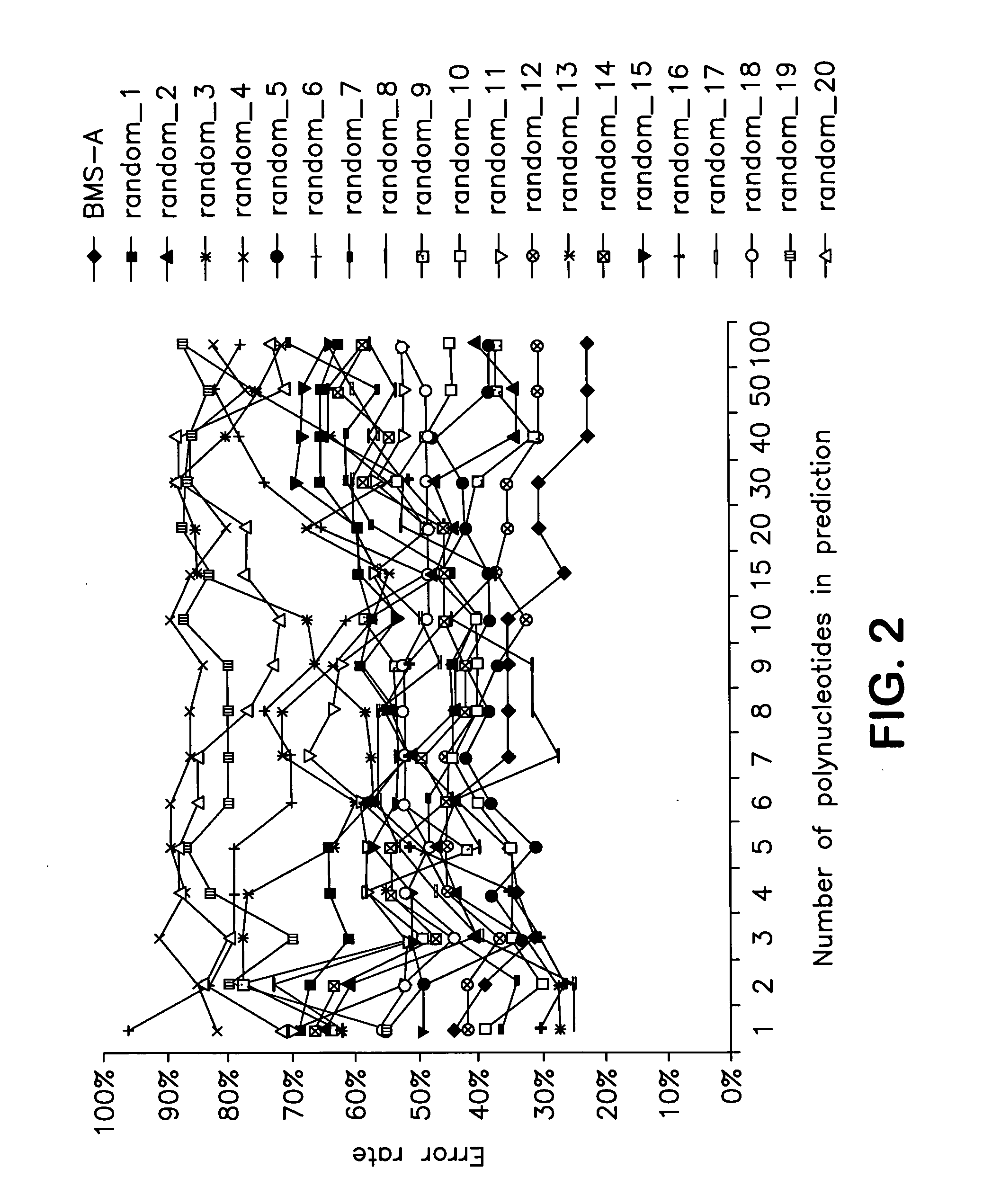
Identification of polynucleotides for predicting activity of compounds that interact with and/or modulate protein tyrosine kinases and/or protein tyrosine kinase pathways in lung cancer cells
InactiveUS20060019284A1Improved prognosisContinue treatmentMicrobiological testing/measurementTumor/cancer cellsDisease areaProtein-Tyrosine Kinases
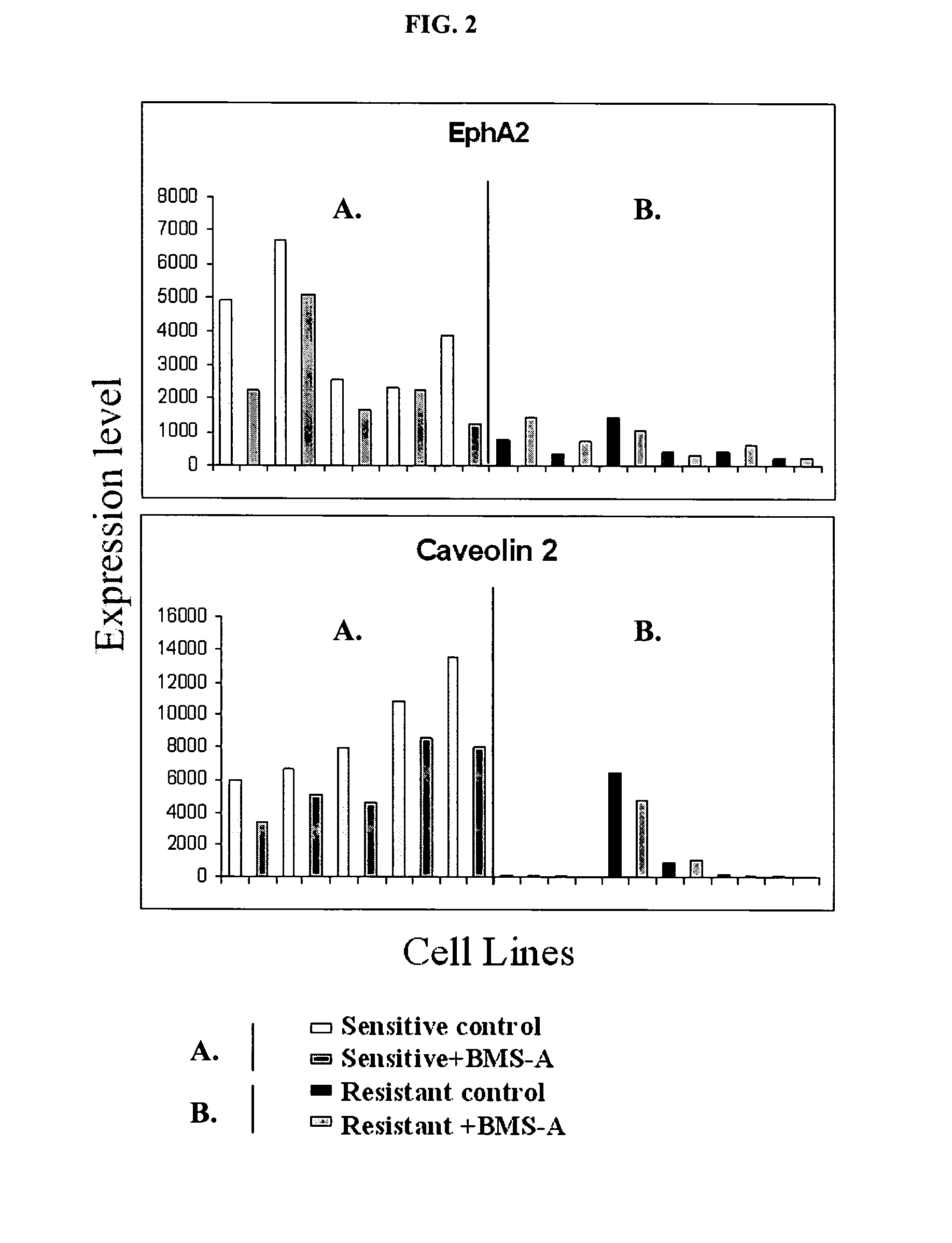
The present invention describes polynucleotides that have been discovered to correlate to the relative intrinsic sensitivity or resistance of cells, e.g., lung cell lines, to treatment with compounds that interact with and modulate, e.g., inhibit, protein tyrosine kinases, such as, for example, members of the Src family of tyrosine kinases, e.g., Src, Fgr, Fyn, Yes, Blk, Hck, Lck and Lyn, as well as other protein tyrosine kinases, including, Bcr-abl, Jak, PDGFR, c-kit and Ephr. These polynucleotides have been shown, through a weighted voting cross validation program, to have utility in predicting the resistance and sensitivity of lung cell lines to the compounds. The expression level of some polynucleotides is regulated by treatment with a particular protein tyrosine kinase inhibitor compound, thus indicating that these polynucleotides are involved in the protein tyrosine kinase signal transduction pathway, e.g., Src tyrosine kinase. Such polynucleotides, whose expression levels correlate highly with drug sensitivity or resistance and which are modulated by treatment with the compounds, comprise polynucleotide predictor or marker sets useful in methods of predicting drug response, and as prognostic or diagnostic indicators in disease management, particularly in those disease areas, e.g., lung cancer, in which signaling through the protein tyrosine kinase pathway, such as the Src tyrosine kinase pathway, is involved with the disease process.
Owner:BRISTOL MYERS SQUIBB CO
Compositions and methods for discovery of causative mutations in genetic disorders
Owner:POPULATION BIO INC
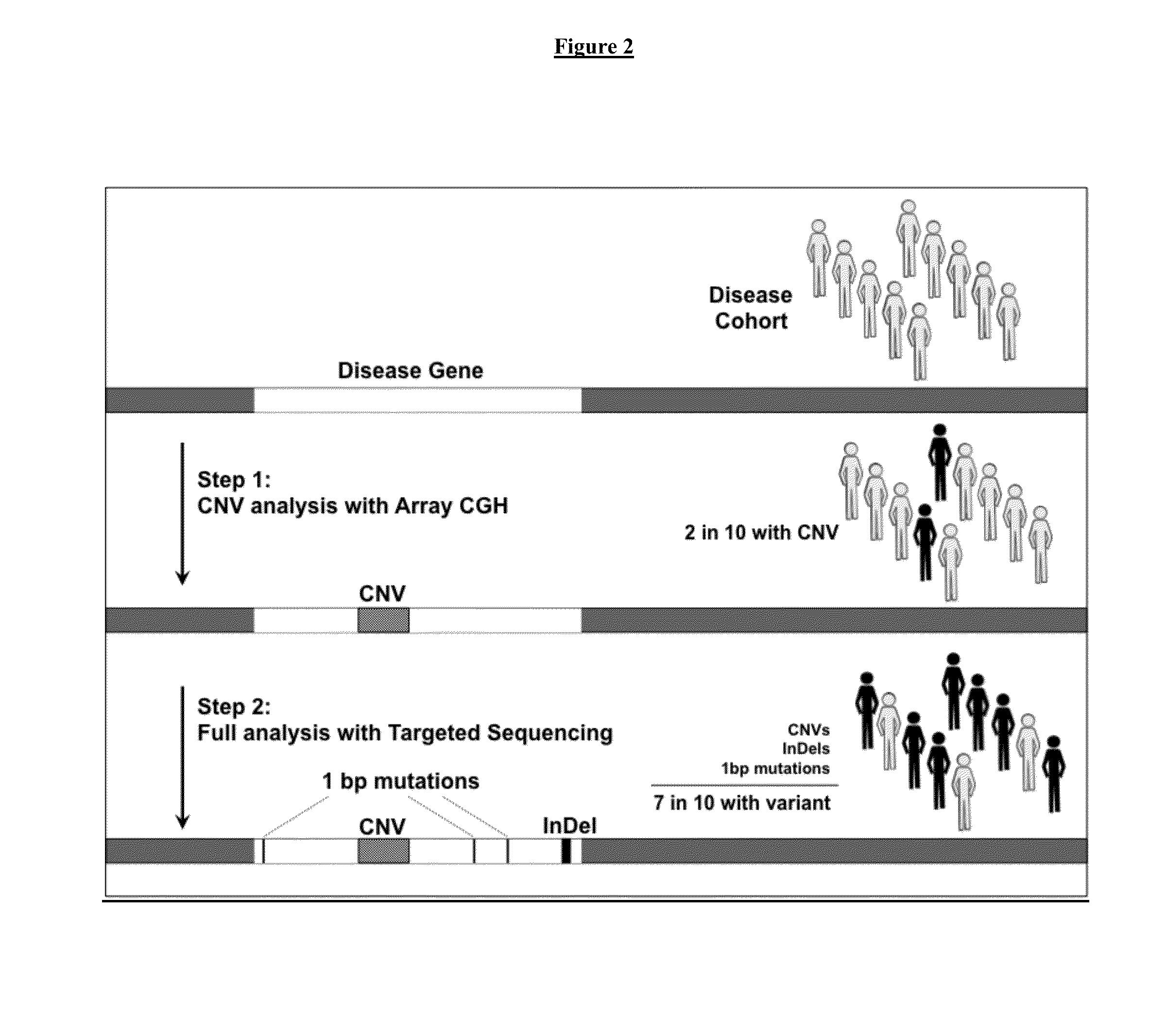
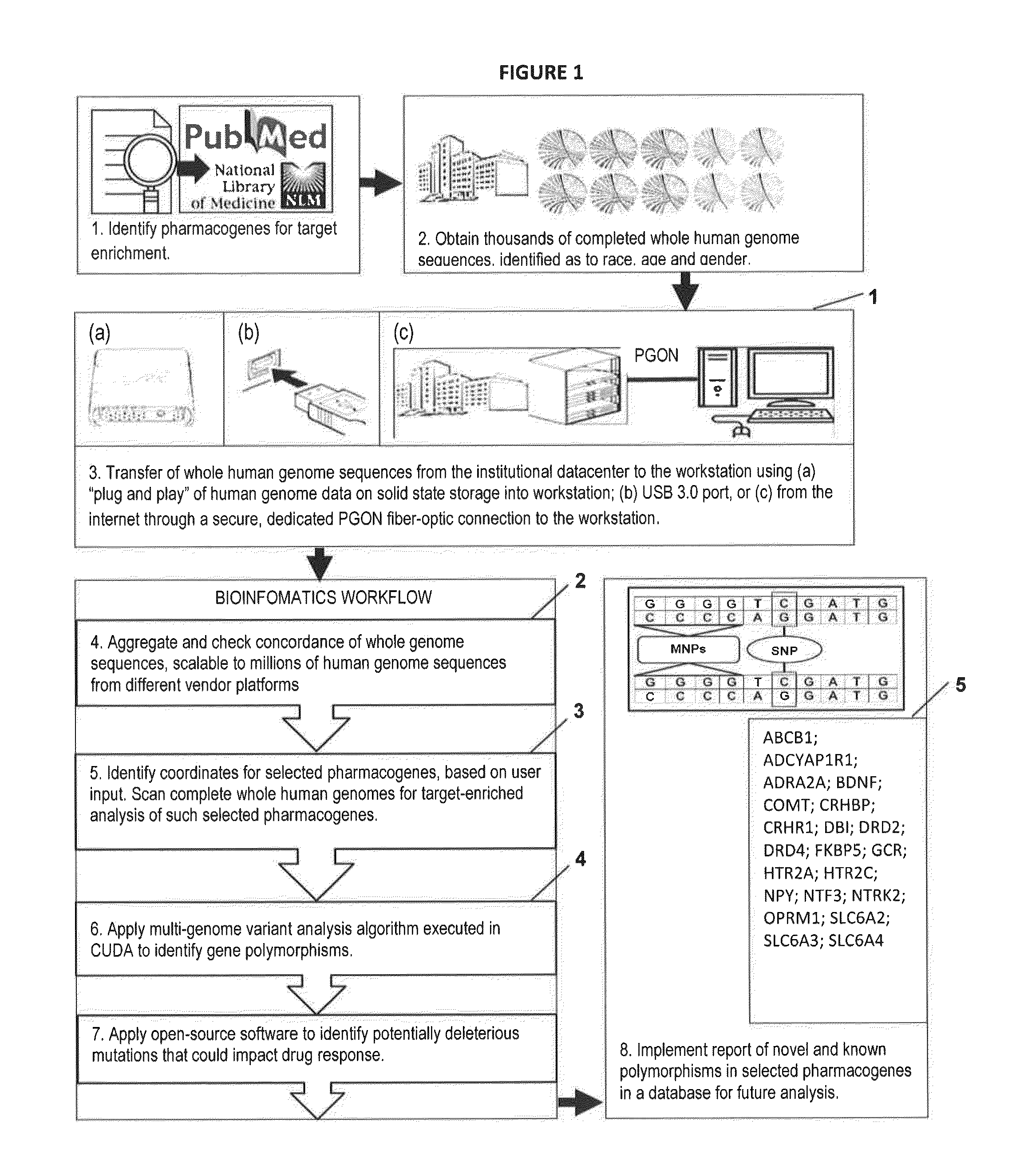
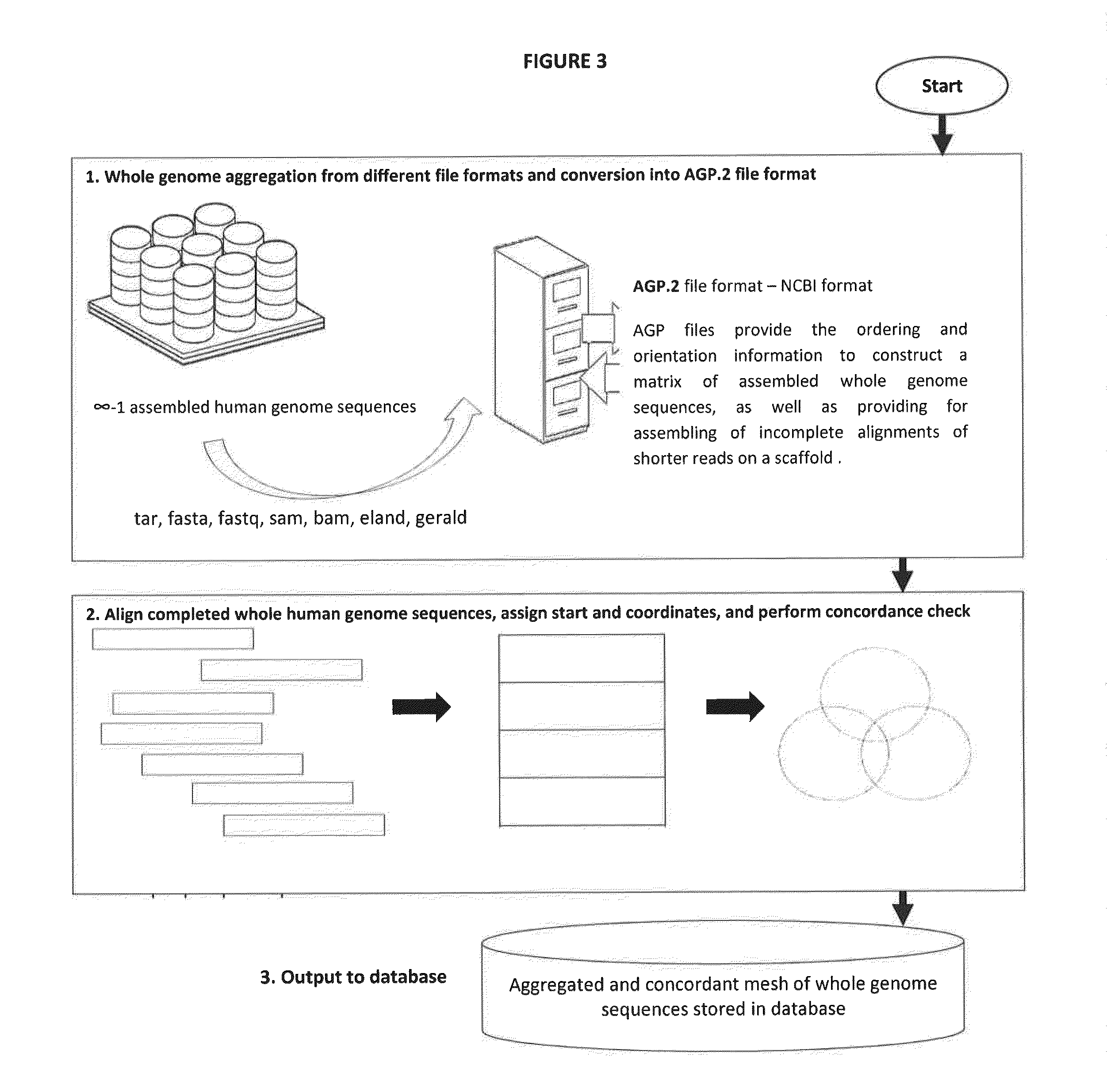
Novel Pharmacogene Single Nucleotide Polymorphisms and Methods of Detecting Same
InactiveUS20140038836A1Energy efficiencyInexpensive, energy-efficientSugar derivativesMicrobiological testing/measurementHuman DNA sequencingNucleotide
The present invention provides pharmacogene polymorphisms and their use in predicting therapeutic effectiveness. The present invention also provides methods comprising targeted analysis of selected pharmacogenes in thousands of compiled whole human genome sequences for identifying polymorphic sequences associated with drug response are described. The methods also provide confirmation and validation of these pharmacogene polymorphisms, based on concordance between different sequencing technologies, and statistical error-checking. Imputation of the deleterious consequences of novel variants is predicted by bioinformatics analysis.
Owner:ASSUREX HEALTH INC
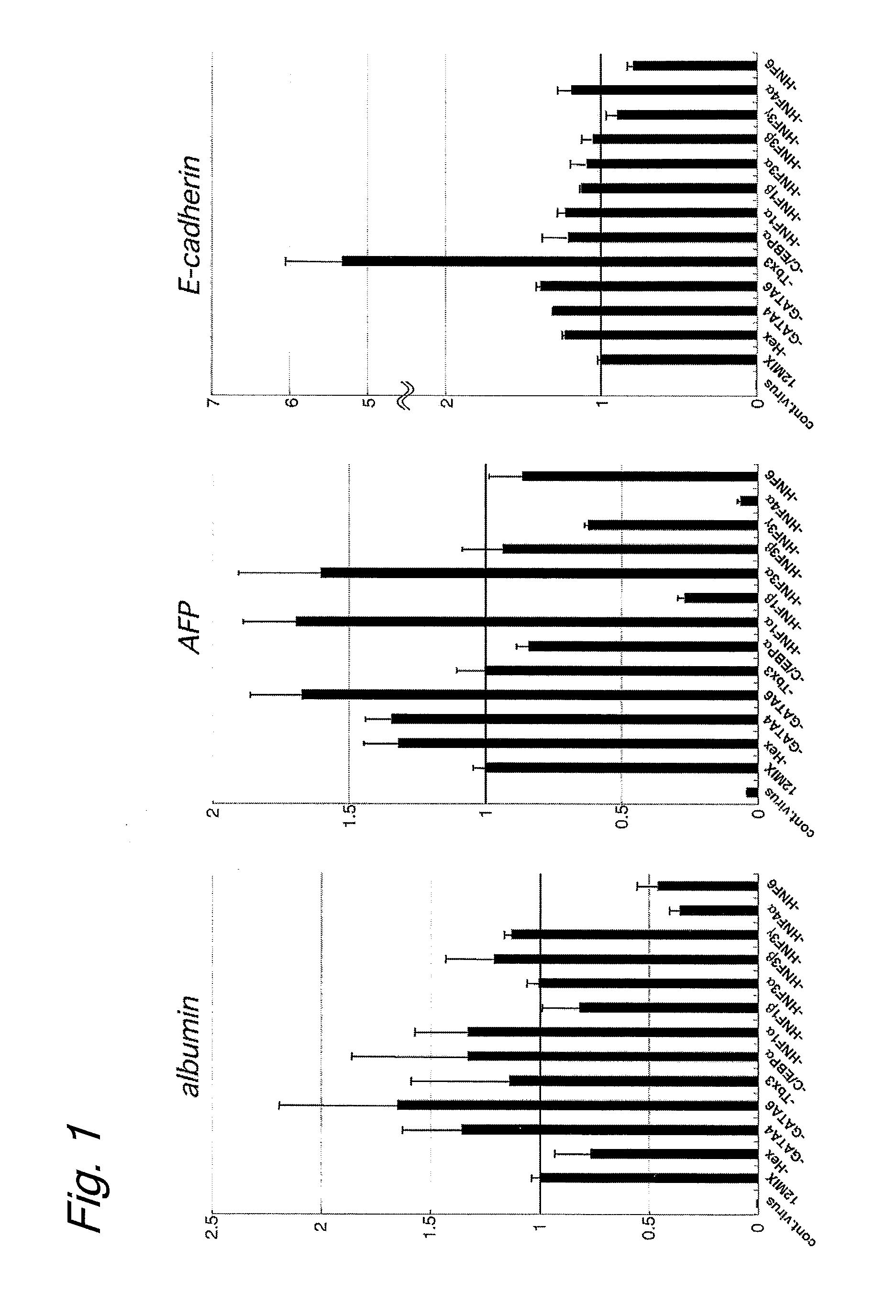
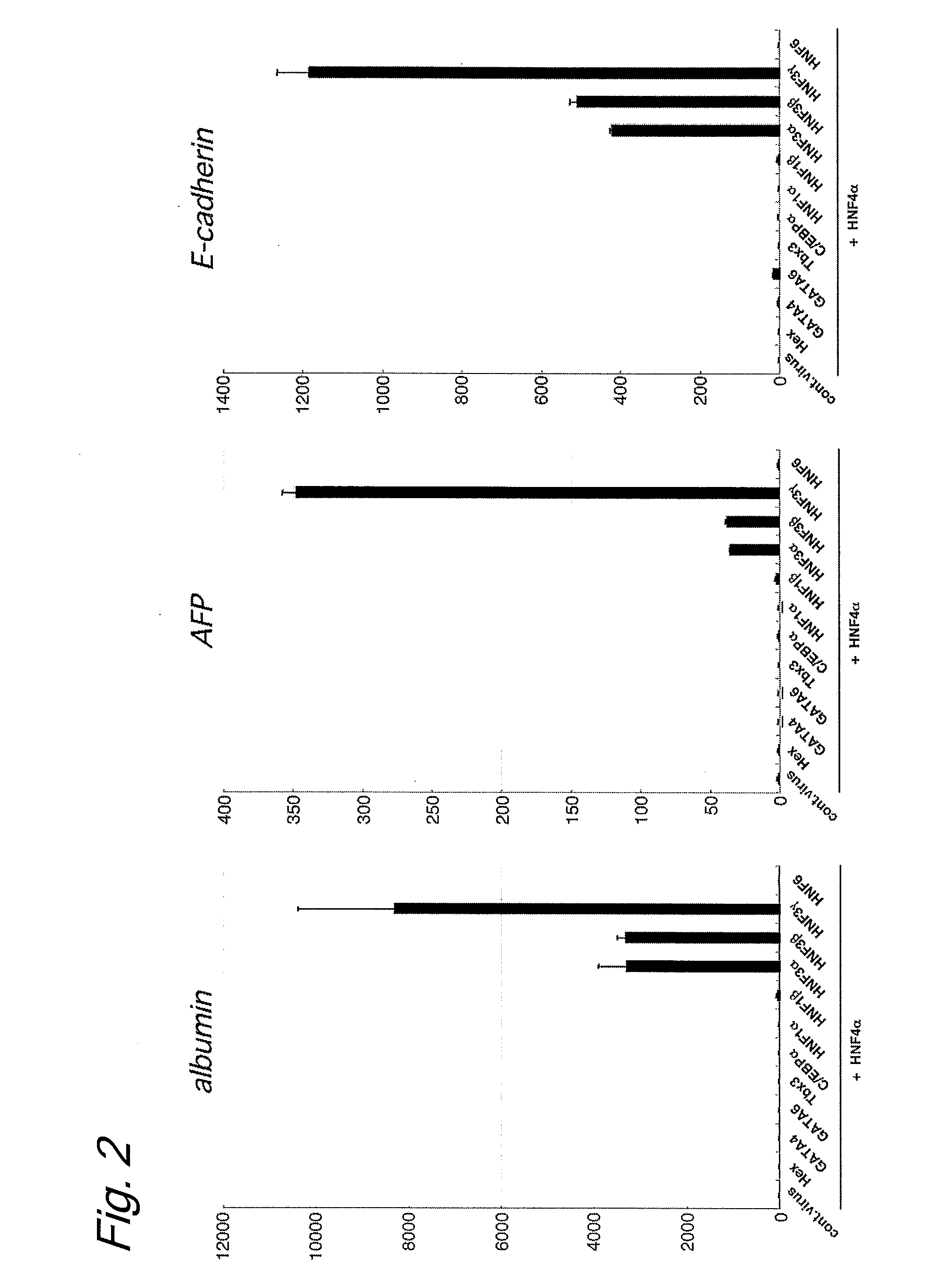
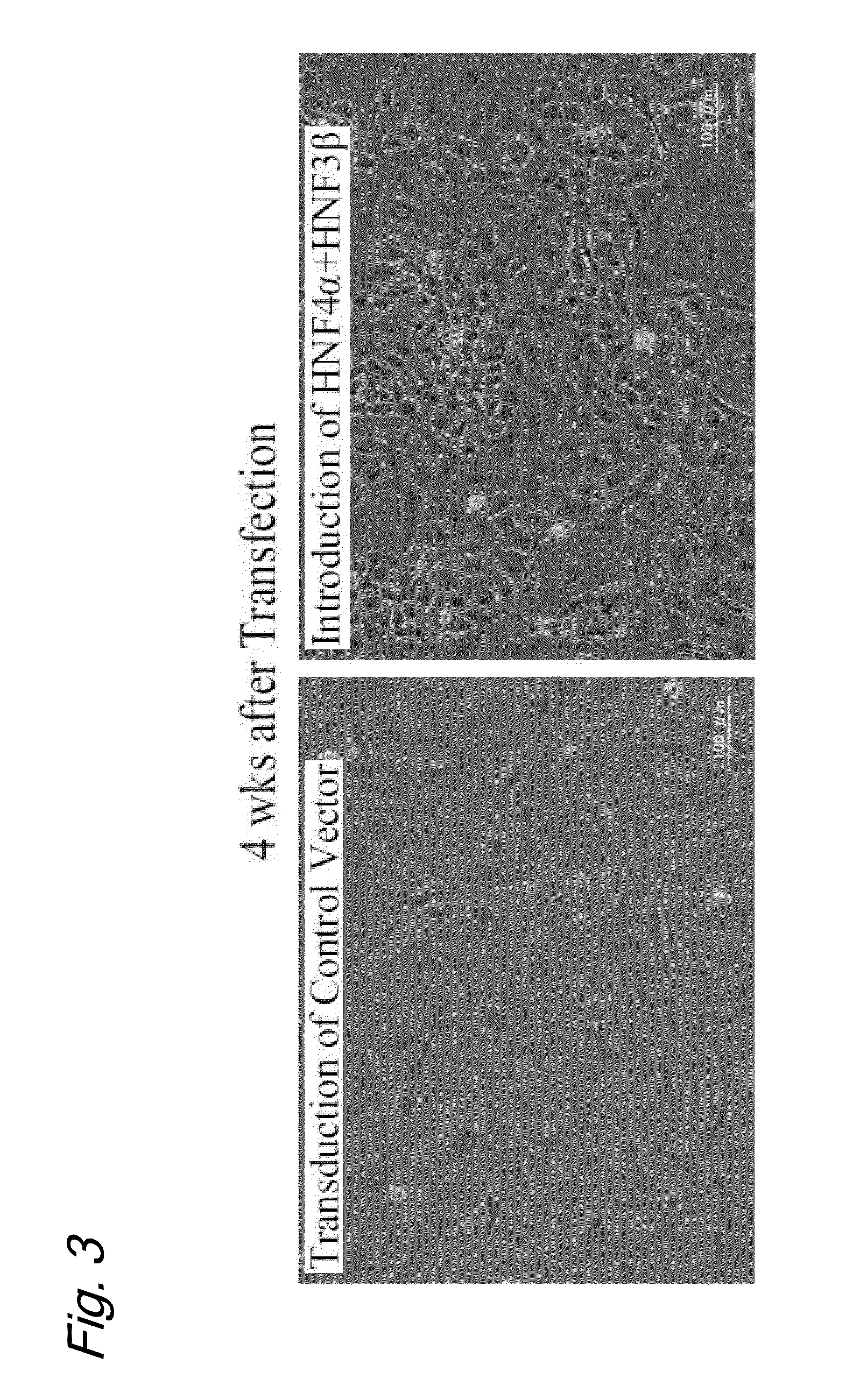
Induced hepatocytes
An object of the present invention is to produce hepatic cells from non-hepatic cells that can be obtained less invasively and at low cost. The gene of HNF3α, HNF3β, or HNF3γ and the gene of HNF4α are transduced into non-hepatic cells. The present invention enables the production of induced hepatocytes from somatic cells that are non-hepatic cells, such as skin cells. The use of the induced hepatocytes obtained by the present invention can establish and develop, as general medical treatment, cell transplantation into the liver, artificial livers, and drug response tests, which are the areas that have not been generalized because of the difficulty of procuring hepatocytes.
Owner:JAPAN SCI & TECH CORP
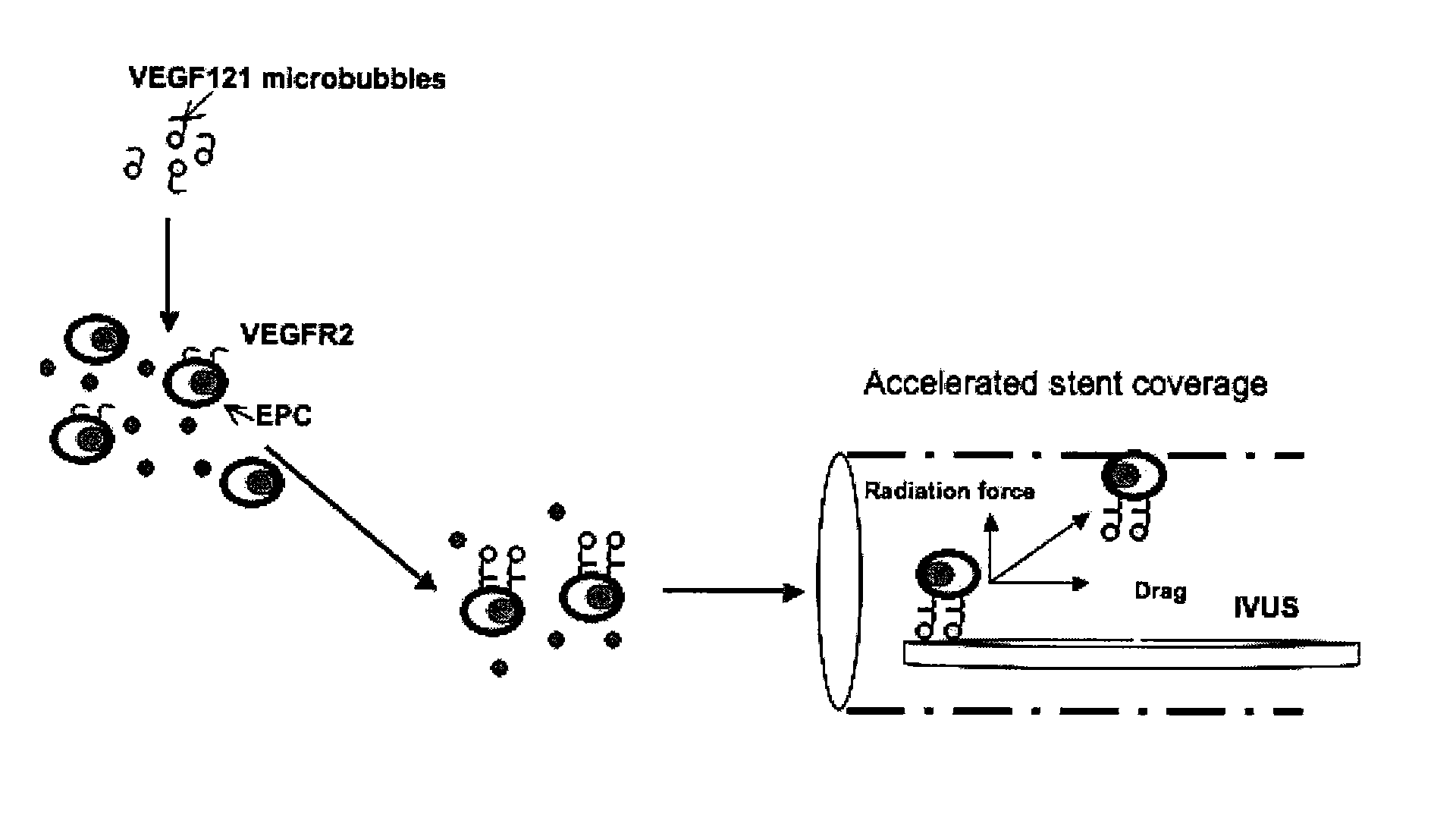
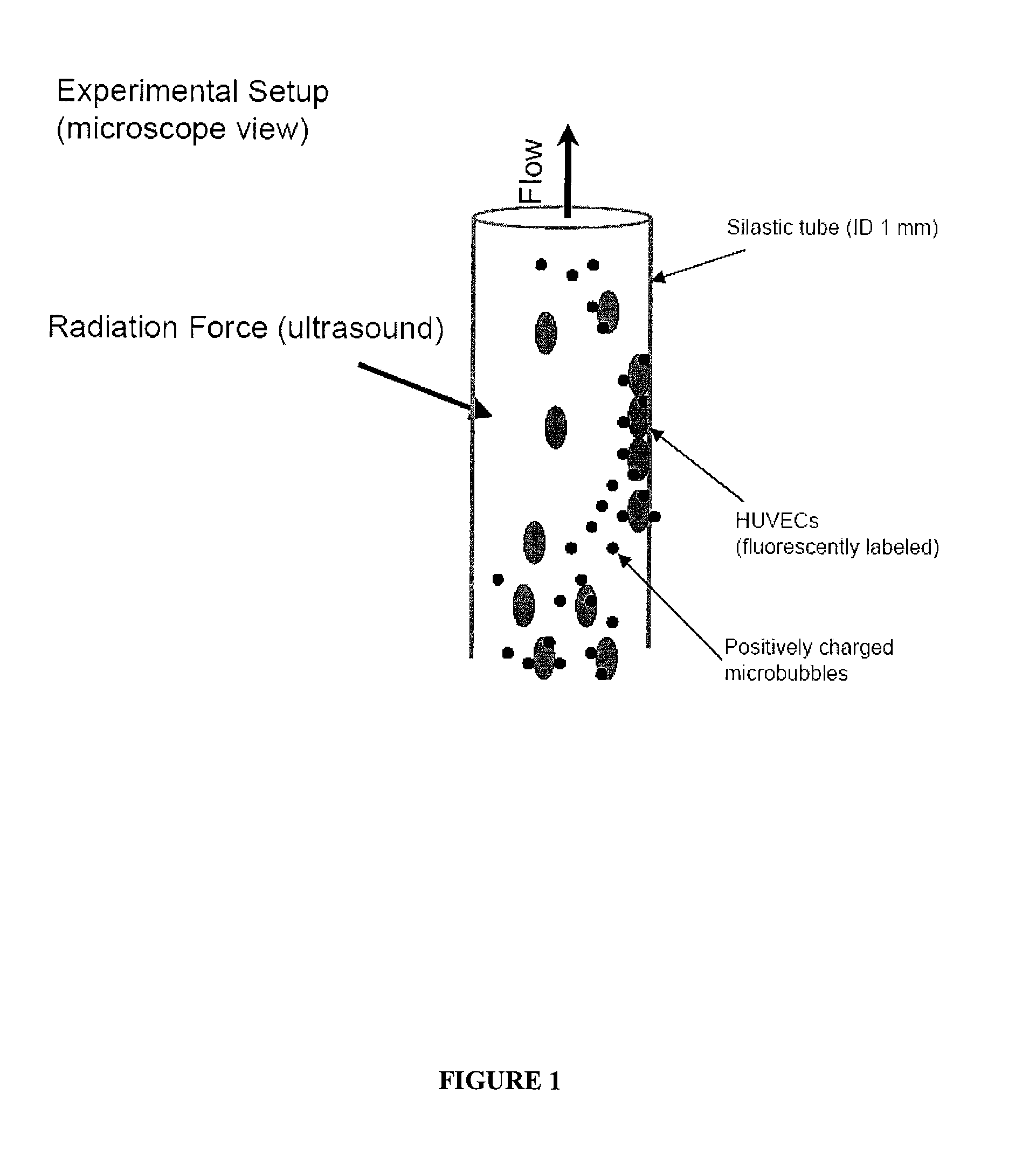
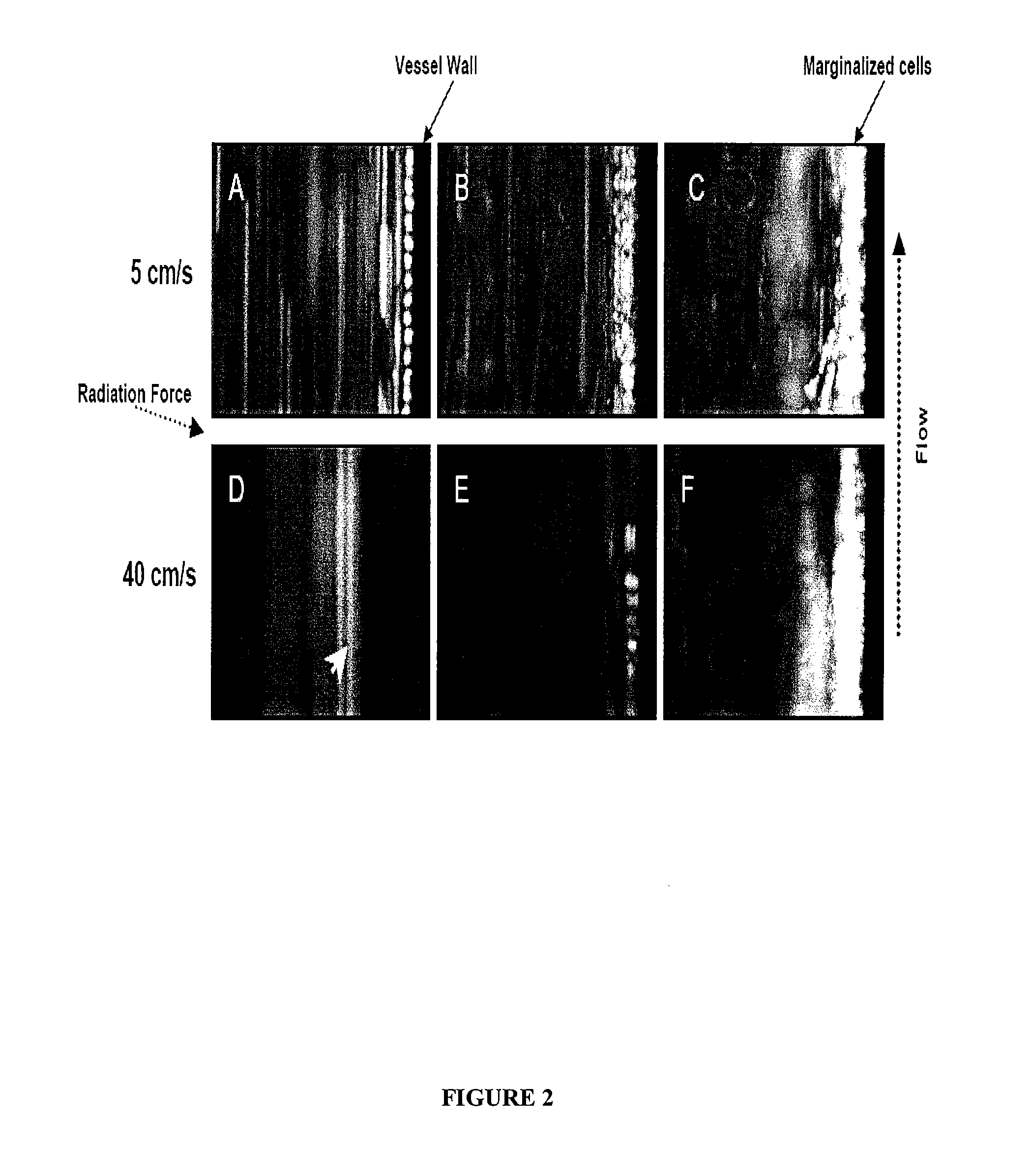
Directed Cell-Based Therapy Using Microbubble Tagged Cells
ActiveUS20110208113A1Promote tissue growthImprove satisfactionBiocideUltrasound therapyAuditory radiationProgenitor
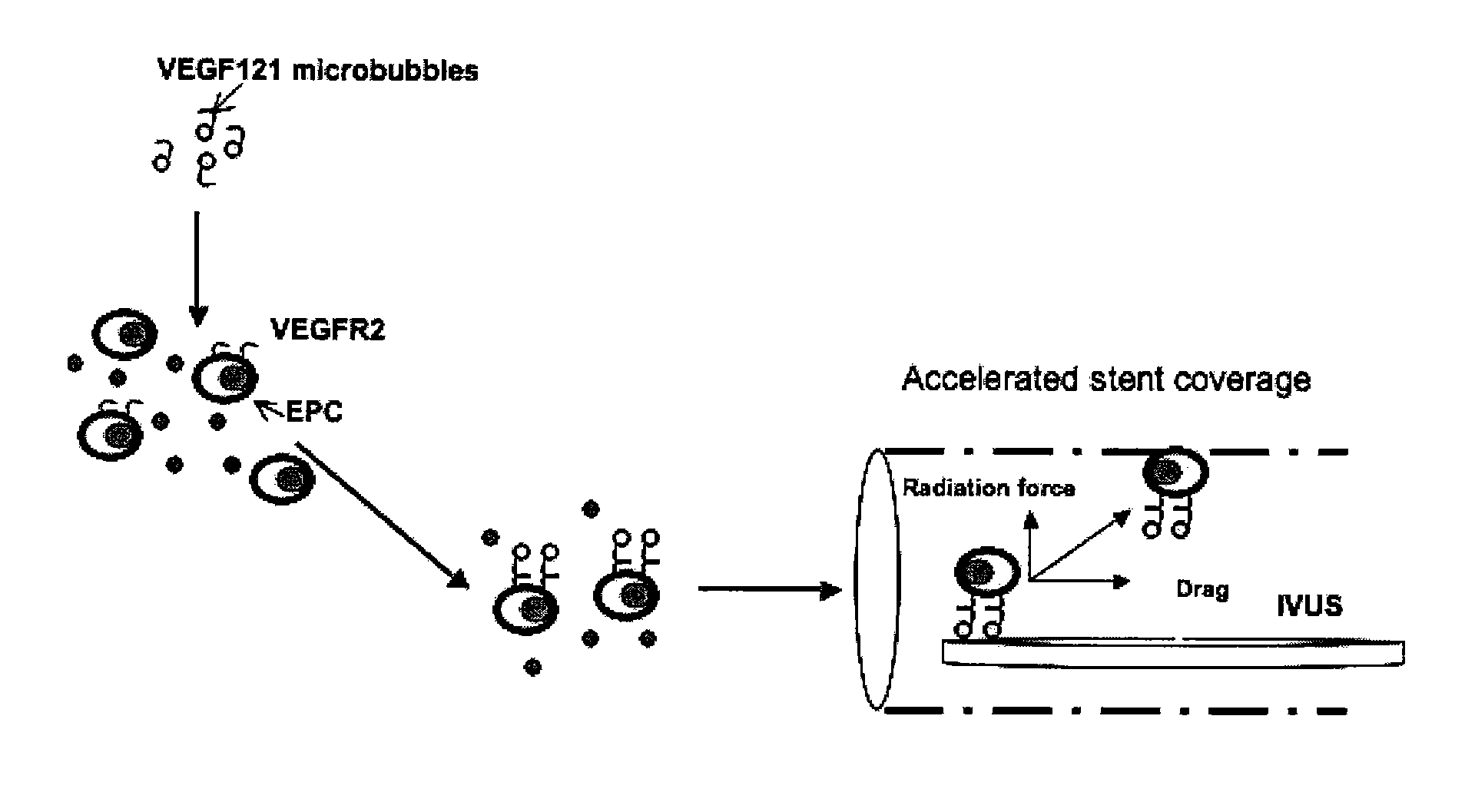
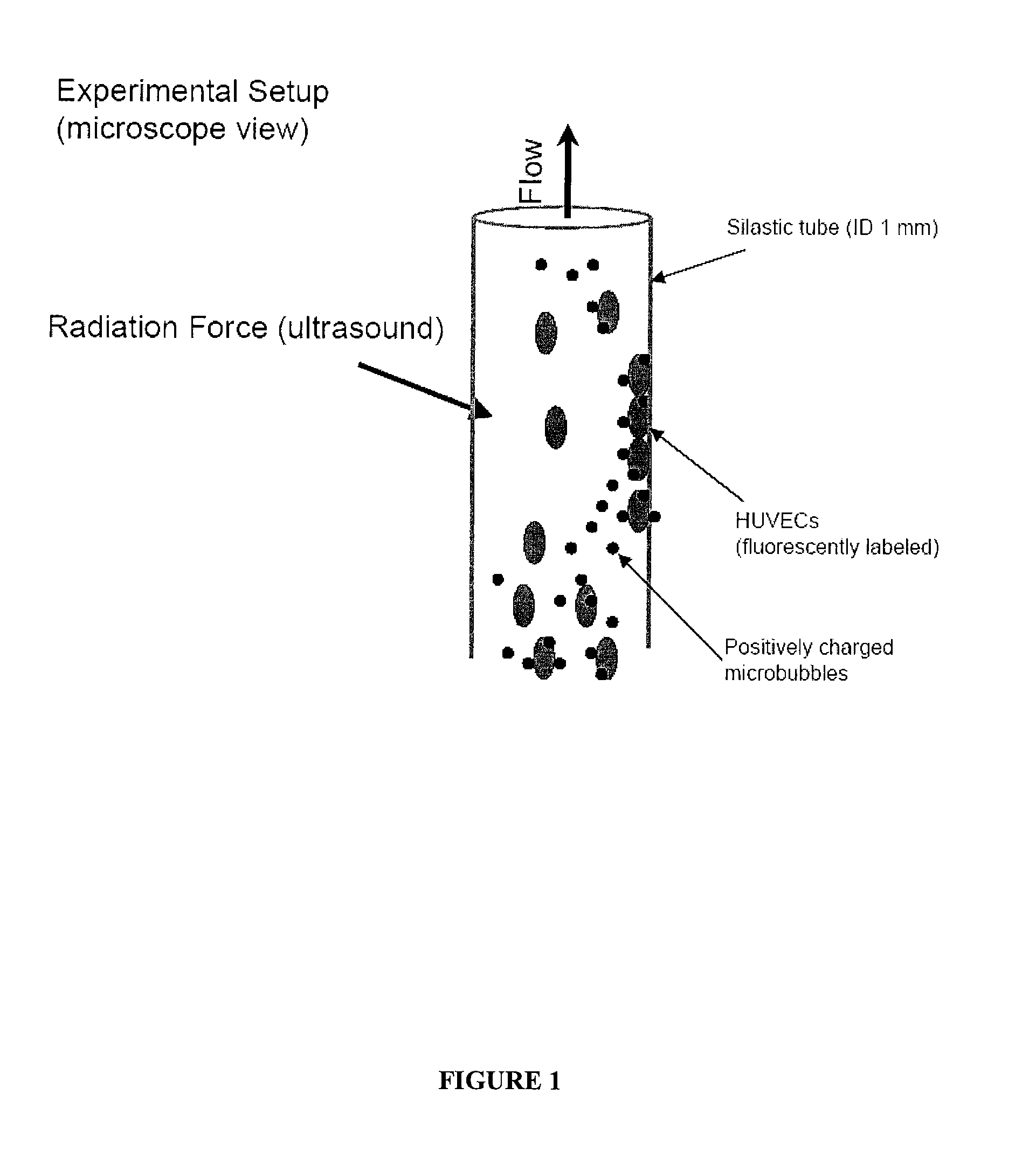
The disclosed technology describes compositions and methods useful for providing cell based therapy. For example, one embodiment of cell based therapy involves the regeneration of injured tissue and / or promoting wound healing. Certain embodiments provide improved therapeutic compositions using microbubbles by delivering biological progenitor cells to the injured tissues. The administration of the microbubbles is directed by acoustic radiation forces that interact with embodiments of microbubbles comprising an acoustically active gas. As such, a high efficiency of progenitor cell delivery to injured tissue is realized. One advantage of this technique over targeted delivery of pharmaceutical compounds, is that the delivered progenitors cells may be derived from the patient (i.e., personalized therapy), thereby avoiding side effects, allergic reactions, and overall problems associated with refractive drug responses.
Owner:UNIVERSITY OF PITTSBURGH
Methods and compositions for predicting drug responses
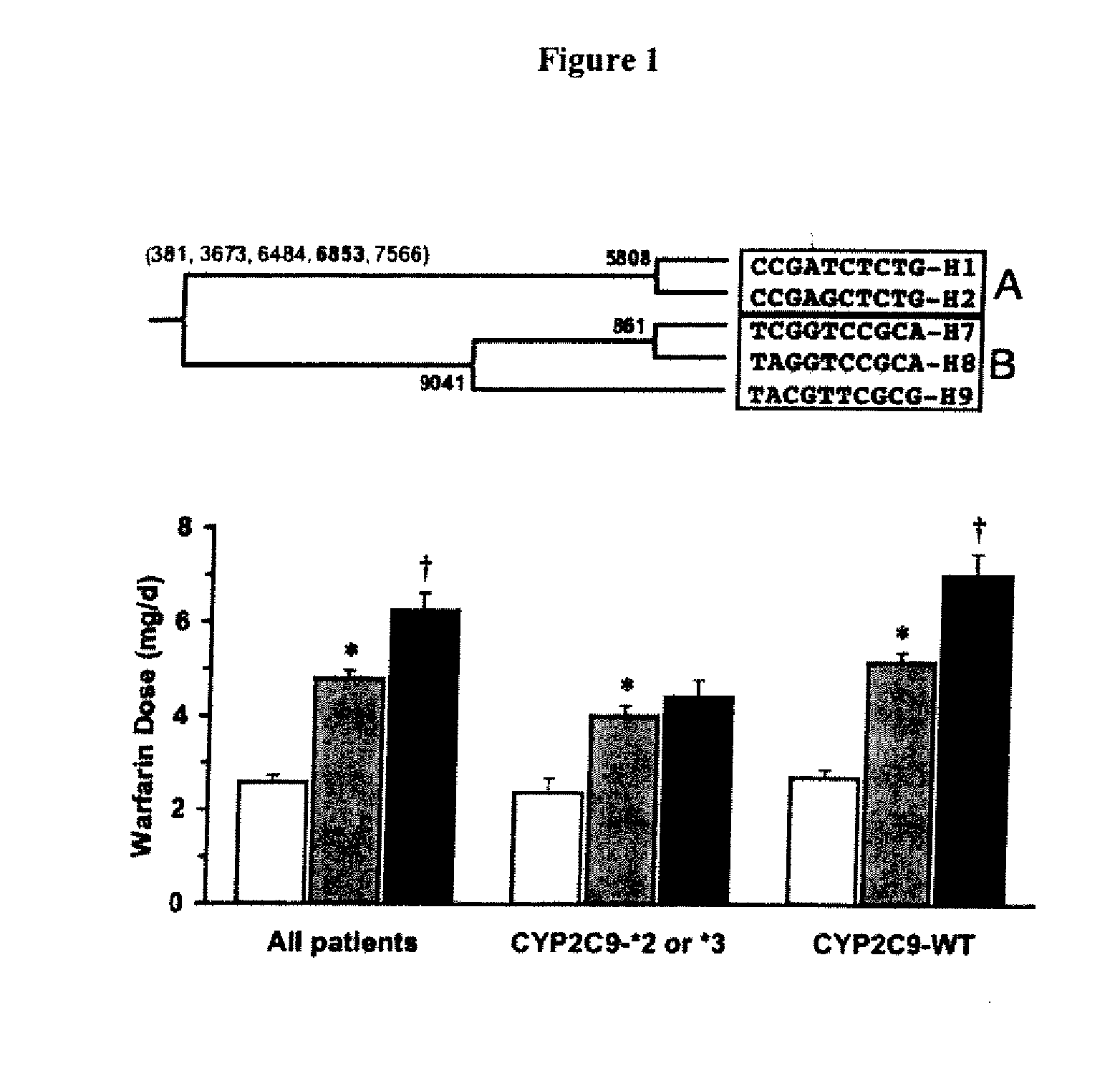
InactiveUS20060084081A1Determining responsivenessMicrobiological testing/measurementBiological testingWarfarinGenetics
Owner:UNIV OF WASHINGTON
Genes involved estrogen metabolism
ActiveUS7888019B2Bioreactor/fermenter combinationsBiological substance pretreatmentsEstrogen MetabolismPharmaceutical drug
Owner:GENOMIC HEALTH INC
Single nucleotide polymorphisms sensitively predicting adverse drug reactions (adr) and drug efficacy
InactiveUS20070128597A1Reducing primaryReducing secondary riskGenetic material ingredientsDisease diagnosisNucleotideEfficacy
Single Nucleotide Polymorphisms sensitively predicting Advserse Drug Reactions (ADR) and Drug Efficacy Abs tract. The invention provides diagnostic methods and kits including oligo and / or polynucleotides or derivatives, including as well antibodies determining whether a human subject is at risk of getting adverse drug reaction after statin therapy or whether the human subject is a high or low responder or a good a or bad metabolizer of statins. The invention provides further diagnostic methods and kits including antibodies determining whether a human subject is at risk for a cardiovascular disease. Still further the invention provides polymorphic sequences and other genes. The present invention further relates to isolated polynucleotides encoding a phenotype associated (PA) gene polypeptide useful in methods to identify therapeutic agents and useful for preparation of a medicament to treat cardiovascular disease or influence drug response, the polynucleotide is selected from the group comprising: SEQ ID 1-168 with allelic variation as indicated in the sequences section contained in a functional surrounding like full length cDNA for PA gene polypeptide and with or without the PA gene promoter sequence.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS GMBH
Microfluidic Device and Method for Analysis of Tumor Cell Microenvironments
ActiveUS20170199173A1High throughput generationRealistic drug responseLaboratory glasswaresCell culture supports/coatingCellular MicroenvironmentMicrofluidics
A microfluidic device provides high throughput generation and analysis of defined three-dimensional cell spheroids with controlled geometry, size, and cell composition. The cell spheroids of the invention mimic tumor microenvironments, including pathophysiological gradients, cell composition, and heterogeneity of the tumor mass mimicking the resistance to drug penetration providing more realistic drug response. The device is used to test the effects of antitumor agents.
Owner:NORTHEASTERN UNIV
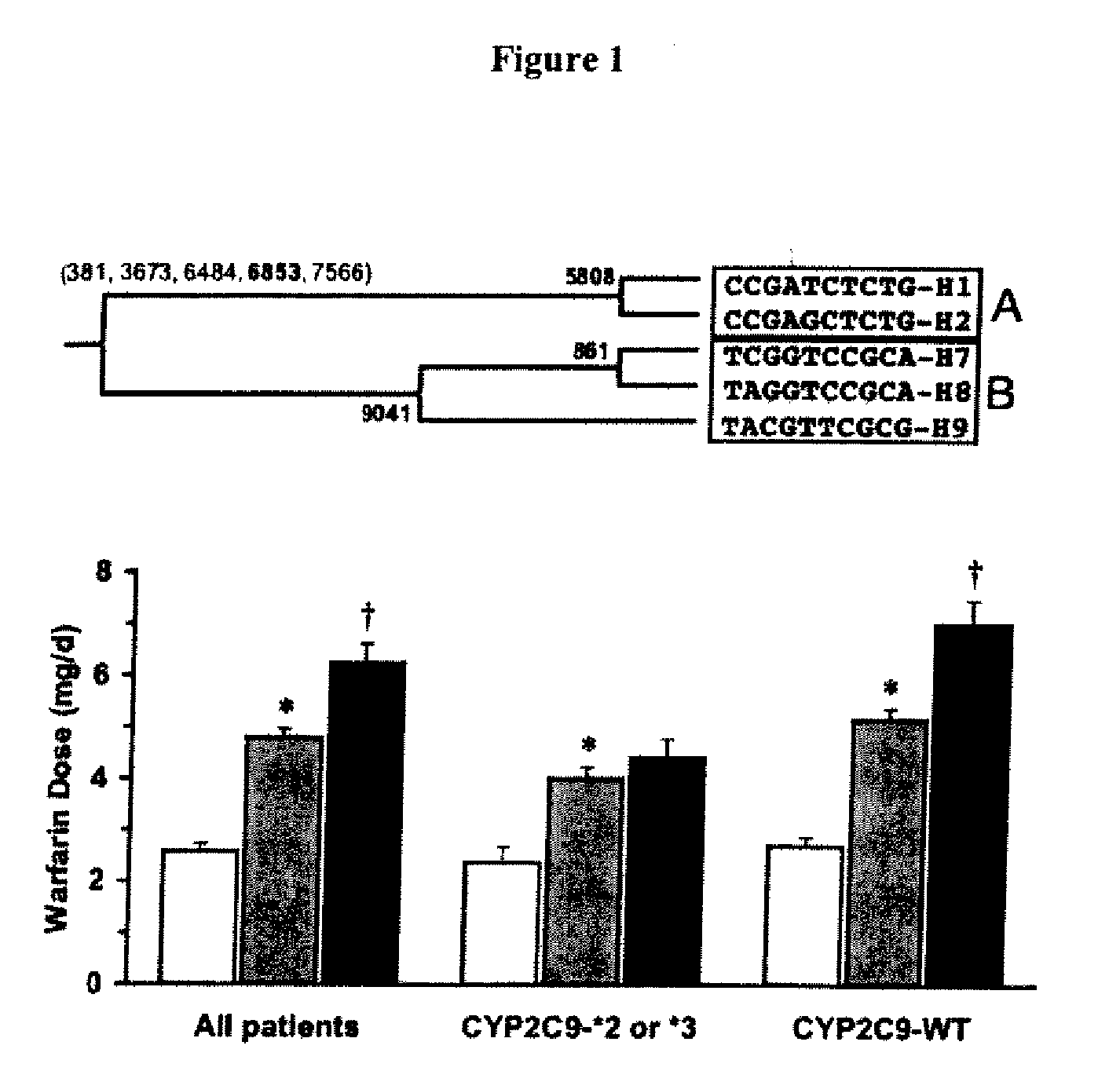
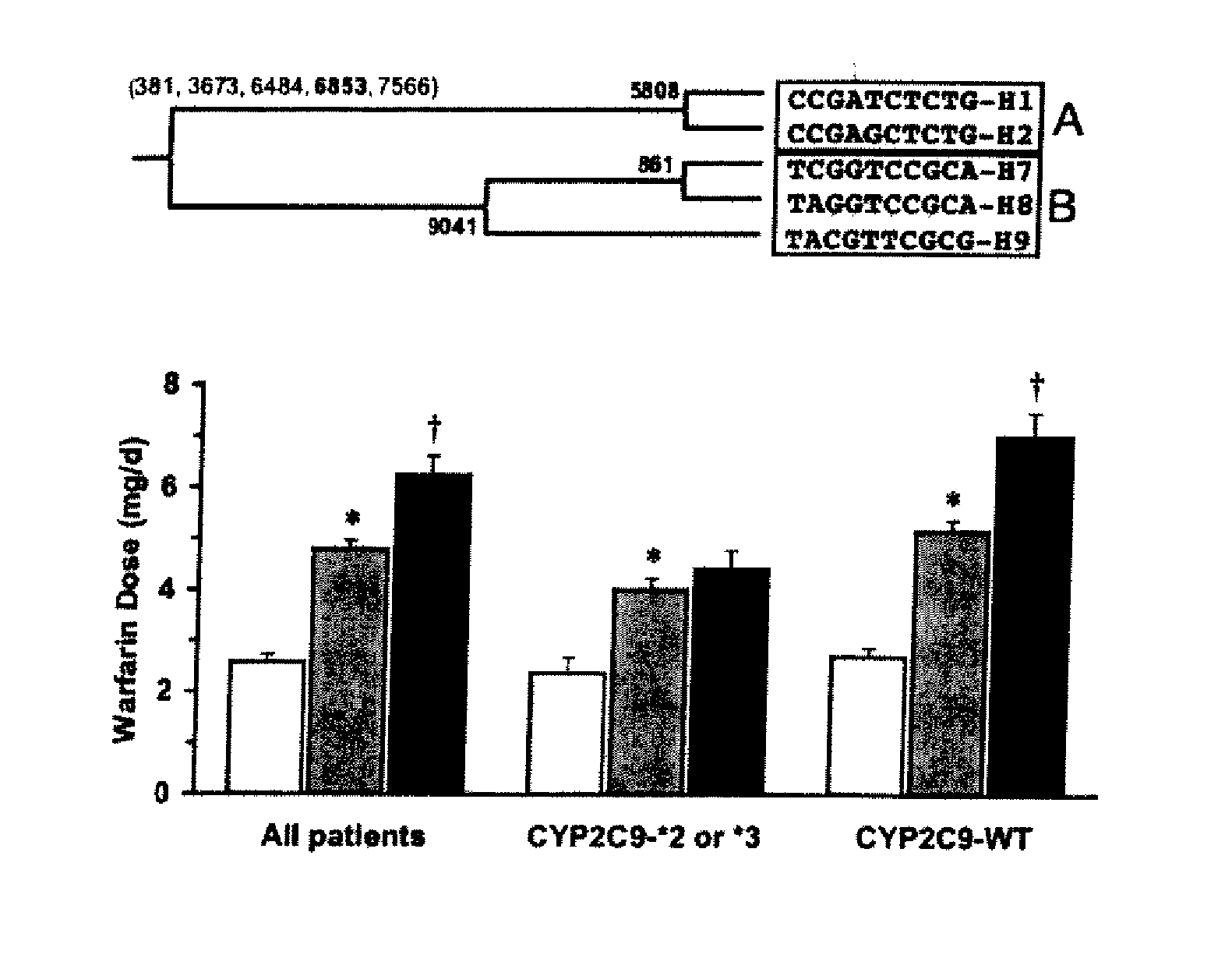
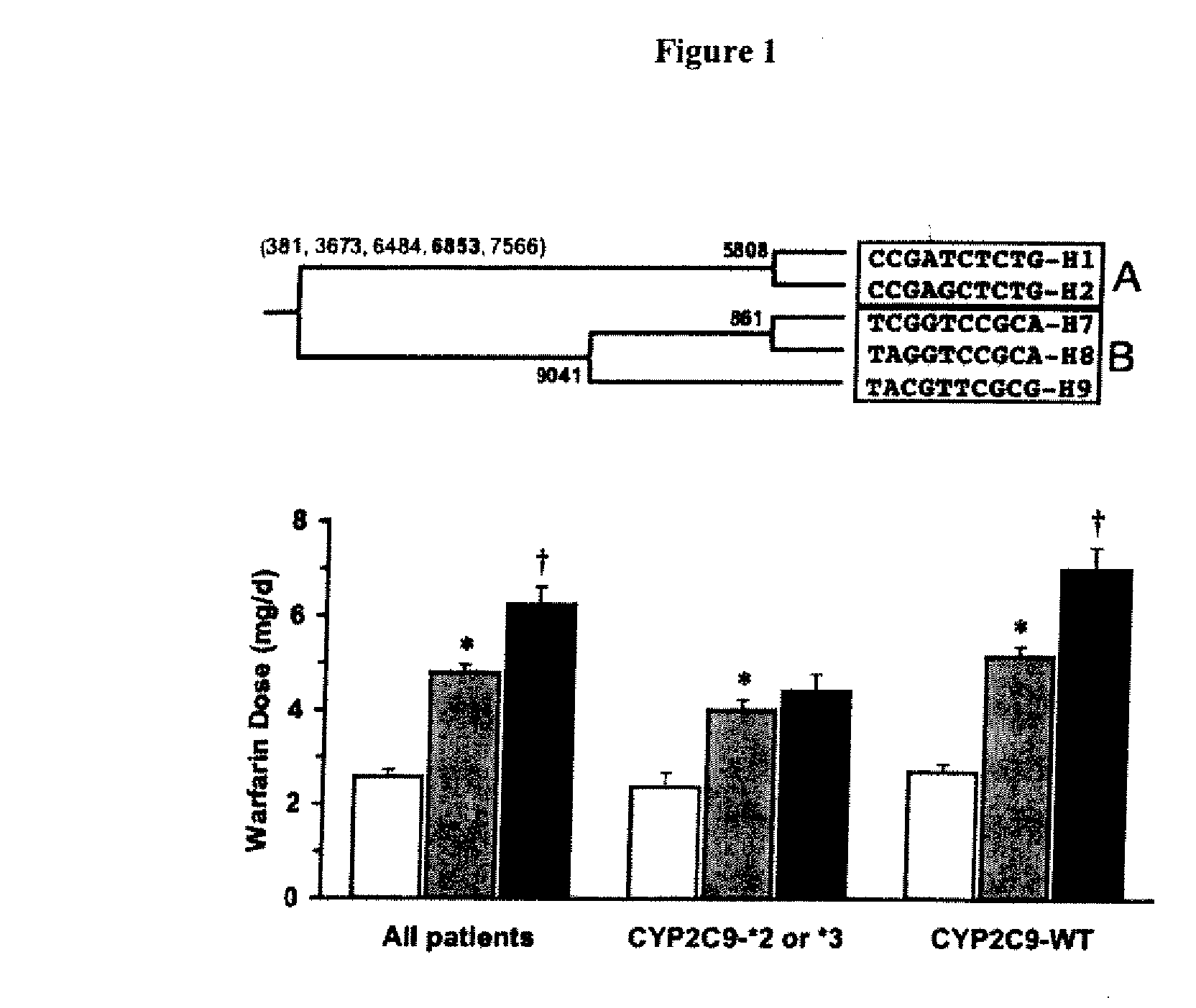
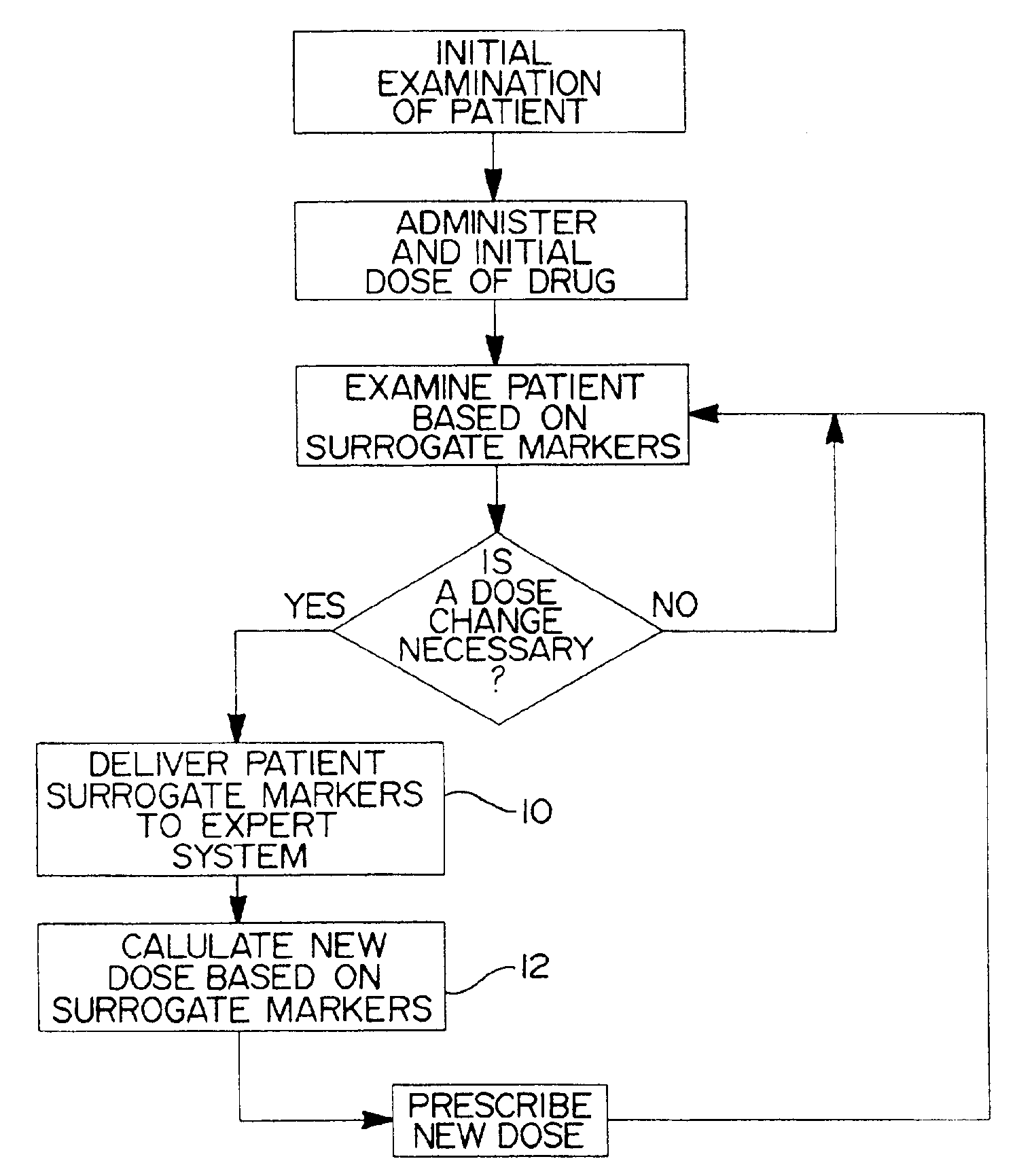
Method and system for use in treating a patient with an anticoagulant to optimize therapy and prevent an adverse drug response
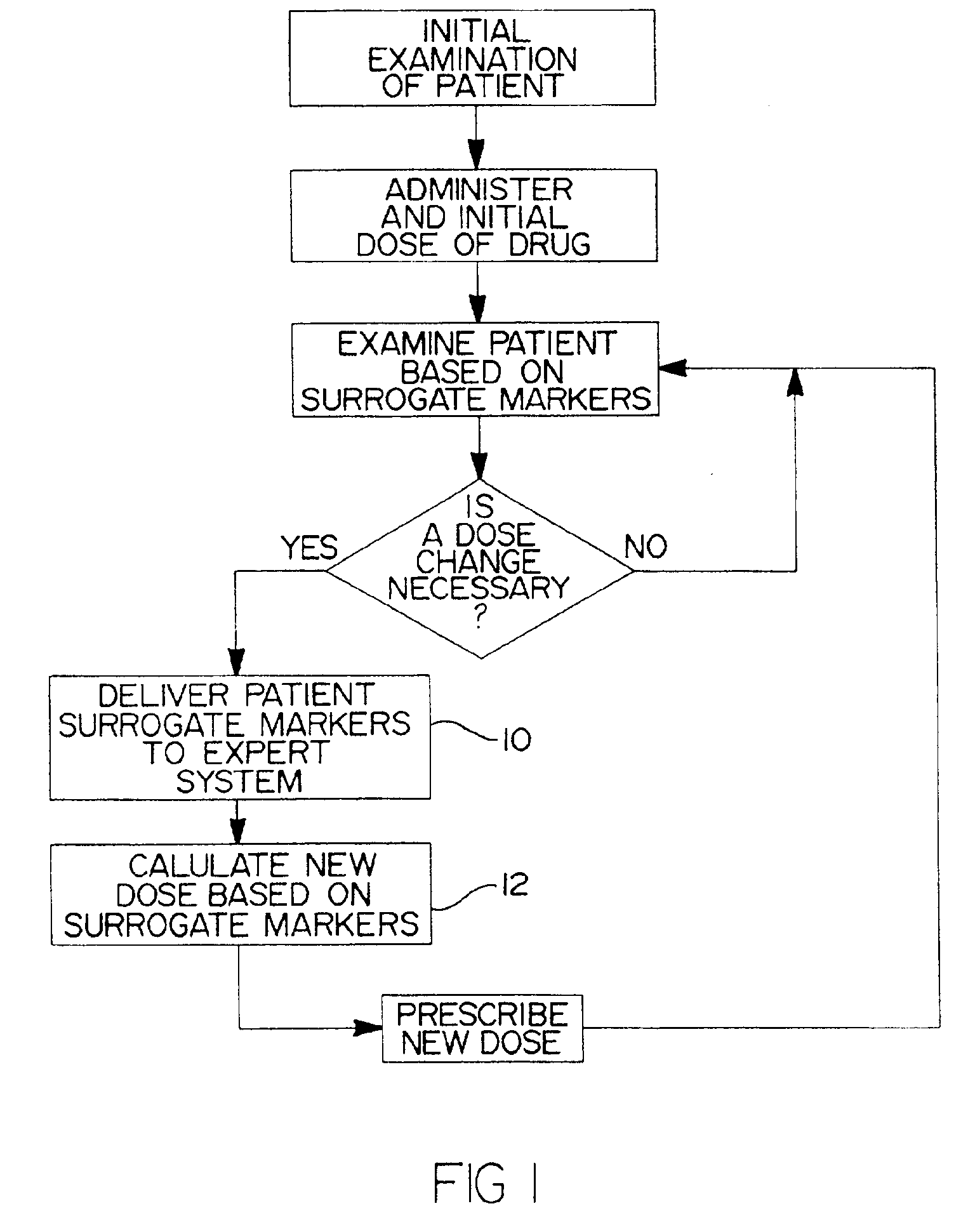
InactiveUS6942614B1Reduce doseMeet growth needsCompounds screening/testingSurgeryBlood levelWarfarin
A method and system for use in treating a patient receiving an anticoagulant or a substance containing warfarin to optimize therapy and prevent an adverse drug response. This system employs surrogate markers or indicators including blood levels of the anticoagulant to determine the next required dose for a patient. Since the surrogate markers are employed as a percent change in status, virtually any indicator can be used. Surrogate markers could include any measure of the effectiveness of the anticoagulant's action. Given the effectiveness of the anticoagulant's action relative to the surrogate markers, a change in anticoagulant dose is calculated by the system. Conversely, by employing this system, one could determine the expected result of the anticoagulant dose change on the surrogate markers.
Owner:THE RXFILES CORP
Genetic polymorphisms associated with cardiovascular disorders and drug response, methods of detection and uses thereof
InactiveUS20100190172A1Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsVascular diseaseCoronary event
The present invention is based on the discovery of genetic polymorphisms that are associated with cardiovascular disorders, particularly acute coronary events such as myocardial infarction and stroke, and genetic polymorphisms that are associated with responsiveness of an individual to treatment of cardiovascular disorders with statin. In particular, the present invention relates to nucleic acid molecules containing the polymorphisms, variant proteins encoded by such nucleic acid molecules, reagents for detecting the polymorphic nucleic acid molecules and proteins, and methods of using the nucleic acid and proteins as well as methods of using reagents for their detection.
Owner:CELERA CORPORATION
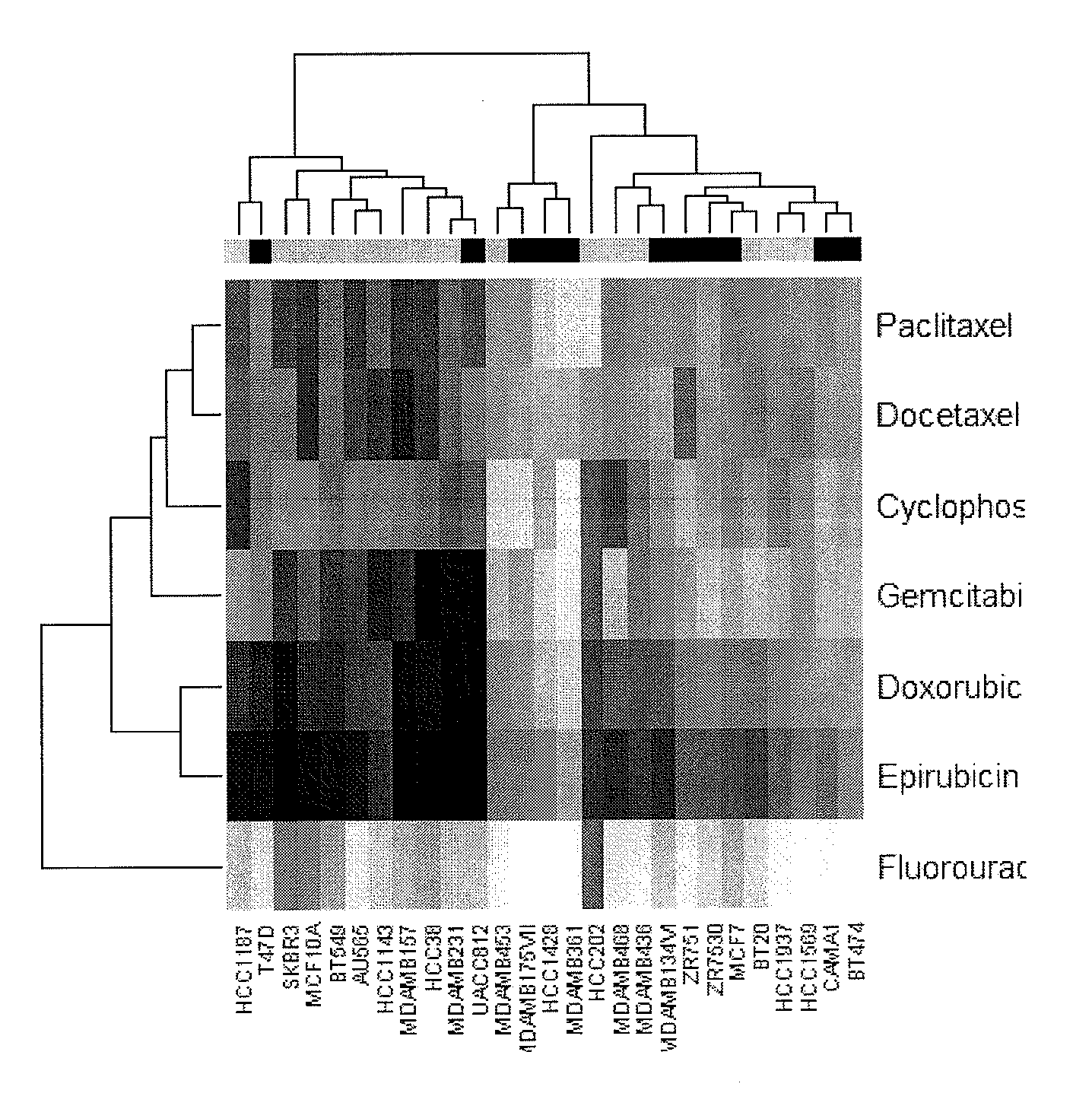
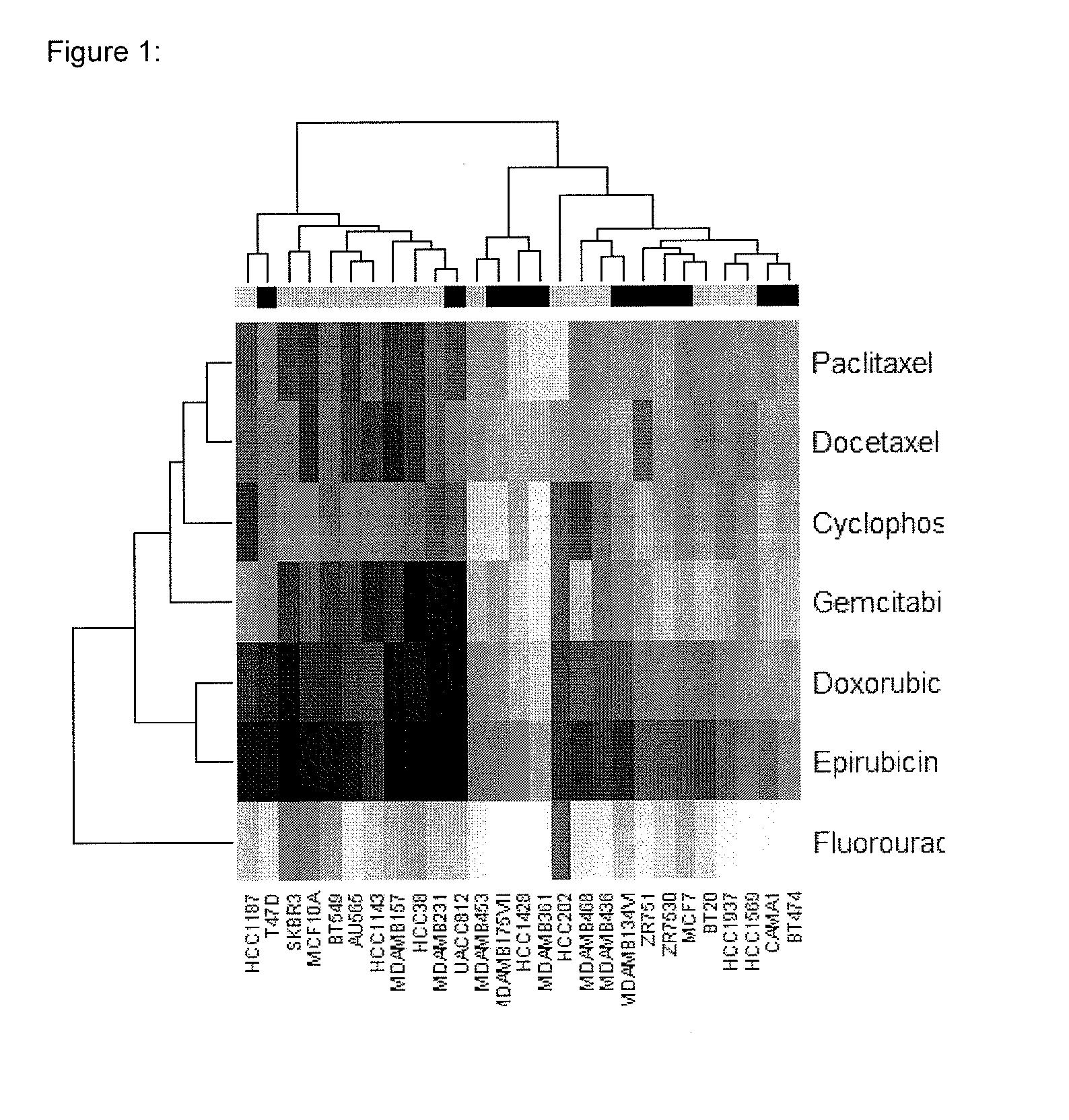
Multi drug response markers for breast cancer cells
The present invention provides methods for preparing a gene expression profile of a breast cancer cell, tumor, or cell line, where the gene expression profile may be evaluated for one or more gene expression signatures indicative of multidrug resistance. The signature may be indicative of resistance to one or more chemotherapeutic agents selected from a Taxol (e.g., Docetaxel or Paclitaxel), an antibiotic (e.g., Doxorubicin or Epirubicin), an antimetabolite (e.g., Fluorouracil and / or Gemcitabine), and an alkylating agent (e.g., Cyclophosphamide). Generally, the gene expression profile contains the level of expression for a plurality of genes listed in FIGS. 3, 4, and / or 5. Gene expression profiles for evaluating multidrug resistance for ER positive and ER negative breast cancers are also provided.
Owner:PRECISION THERAPEUTICS
Cut-point in PTEN protein expression that accurately identifies tumors and is predictive of drug response to a pan-ErbB inhibitor
ActiveUS8338456B2Accurate identificationBiocideMicrobiological testing/measurementTyrosine-kinase inhibitorErbB
A cut-point in the quantitative measurement of PTEN protein expression that accurately identifies tumors with two inactivated alleles of the PTEN gene. Patients with a normalized PTEN score of PTEN null will be treated with a pan-ErbB tyrosine kinase inhibitor. A normalized PTEN protein expression score is obtained by comparing the tumor PTEN OD expression value with the non-malignant PTEN OD expression value.
Owner:WYETH LLC
Salinomycin sodium suspension as well as preparation method and application thereof
ActiveCN101926776AUniform particle distributionEvenly distributedOrganic active ingredientsPharmaceutical non-active ingredientsSoft materialsSuspending Agents
The invention discloses a salinomycin sodium suspension as well as a preparation method and an application thereof. The salinomycin sodium suspension comprises the following components in parts by weight: 2-30 parts of salinomycin sodium, 20-90 parts of filler, 5-40 parts of flavoring agent, 2-20 parts of suspending agent and 1-30 parts of wetting agent. The preparation method of the salinomycin sodium suspension comprises the following steps: sieving and mixing all raw materials, adding the wetting agent to prepare soft material, drying and pelletizing to obtain the product in the invention. The salinomycin sodium suspension in the invention has evenly distributed particles, high bioavailability, rapid drug response, simple preparation process and wide application prospect, and can become a liquid preparation after water is added.
Owner:GUANGDONG RONGDA BIOENG CO LTD +1
Methods and compositions for the assessment of drug response
The present invention provides methods for predicting or determining a subject's response to an antiplatelet agent, and methods for determining a subject's suitability to a treatment regime or intervention for a disease associated with platelet aggregation, using analysis of genetic polymorphisms. The present invention also relates to the use of genetic polymorphisms in assessing a subject's response to an antiplatelet agent. Nucleotide probes and primers, kits, and microarrays suitable for such assessment are also provided.
Owner:THERANOSTICS LAB
Methods and compositions for the assessment of drug response
Owner:THERANOSTICS LAB
Identification of polynucleotides for predicting activity of compounds that interact with and/or modulate protein tyrosine kinases and/or protein tyrosine kinase pathways in breast cells
InactiveUS7537891B2Improved prognosisContinue treatmentSugar derivativesPeptide/protein ingredientsDisease areaProtein-Tyrosine Kinases
The present invention describes polynucleotides that have been discovered to correlate to the relative sensitivity or resistance of cells, e.g., breast cell lines, to treatment with compounds that interact with and modulate, e.g., inhibit, protein tyrosine kinases, such as, for example, members of the Src family of tyrosine kinases, e.g., Src, Fgr, Fyn, Yes, Blk, Hck, Lck and Lyn, as well as other protein tyrosine kinases, including, Bcr-abl, Jak, PDGFR, c-kit and Eph receptors. These polynucleotides have been shown to have utility in predicting the resistance and sensitivity of breast cell lines to the compounds. Such polynucleotides comprise polynucleotide predictor or marker sets useful in methods of predicting drug response, and as prognostic or diagnostic indicators in disease management, particularly in those disease areas, e.g., breast cancer, in which signaling through one or more of the aforementioned Src tyrosine and protein tyrosine kinases is involved with the disease process.
Owner:BRISTOL MYERS SQUIBB CO
Directed cell-based therapy using microbubble tagged cells
ActiveUS8460269B2Promote tissue growthImprove satisfactionBiocideUltrasound therapyProgenitorAuditory radiation
The disclosed technology describes compositions and methods useful for providing cell based therapy. For example, one embodiment of cell based therapy involves the regeneration of injured tissue and / or promoting wound healing. Certain embodiments provide improved therapeutic compositions using microbubbles by delivering biological progenitor cells to the injured tissues. The administration of the microbubbles is directed by acoustic radiation forces that interact with embodiments of microbubbles comprising an acoustically active gas. As such, a high efficiency of progenitor cell delivery to injured tissue is realized. One advantage of this technique over targeted delivery of pharmaceutical compounds, is that the delivered progenitors cells may be derived from the patient (i.e., personalized therapy), thereby avoiding side effects, allergic reactions, and overall problems associated with refractive drug responses.
Owner:UNIVERSITY OF PITTSBURGH
Methods for genomic analysis
InactiveUS20050272086A1Quick scanSugar derivativesMicrobiological testing/measurementHuman DNA sequencingGenetics
The present invention relates to methods for identifying variations that occur in the human genome and relating these variations to the genetic basis of disease and drug response. In particular, the present invention relates to identifying individual SNPs, determining SNP haplotype blocks and patterns, and, further, using the SNP haplotype blocks and patterns to dissect the genetic bases of disease and drug response. The methods of the present invention are useful in whole genome analysis.
Owner:GENETIC TECHNOLOGIES LIMTIED
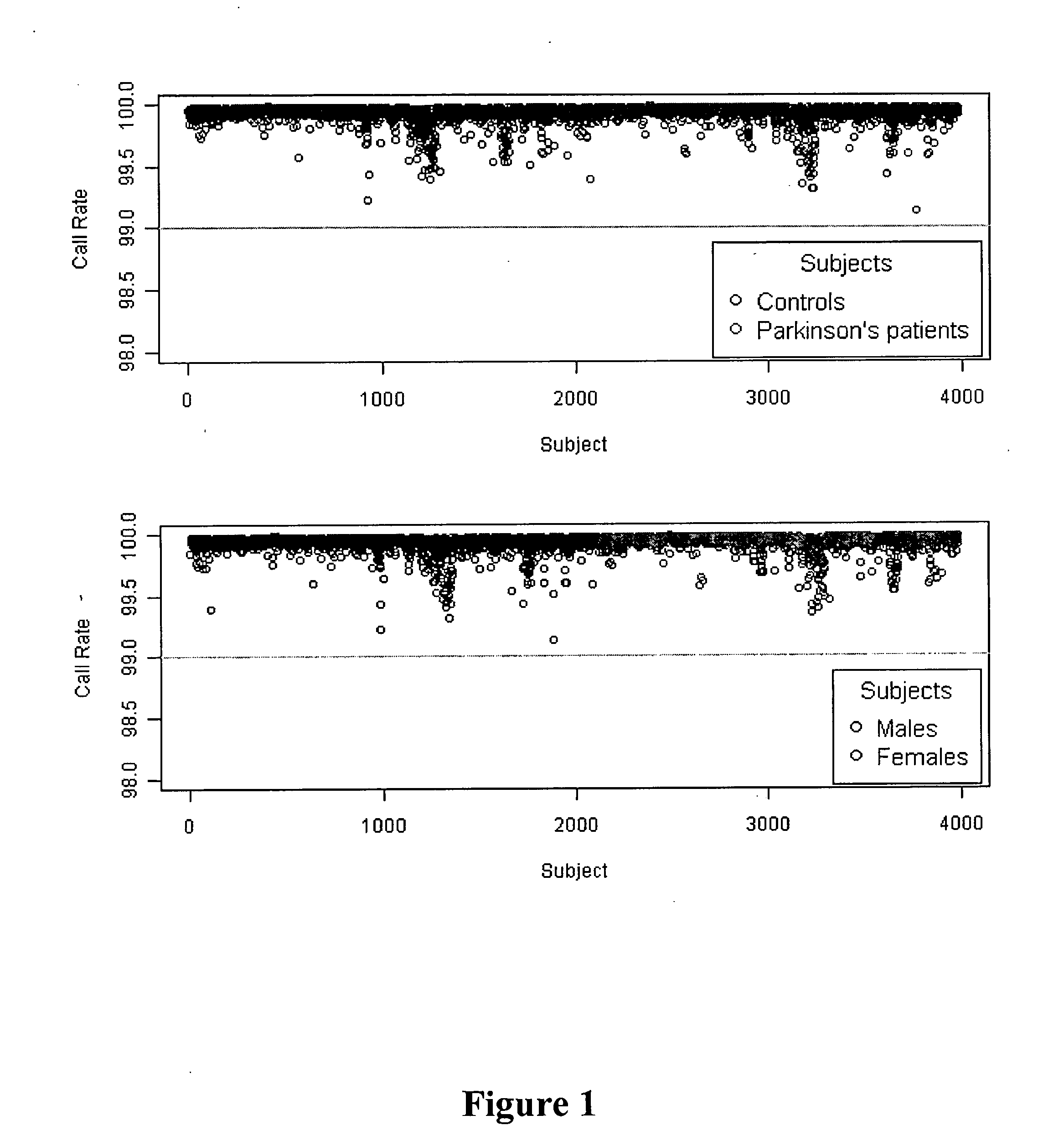
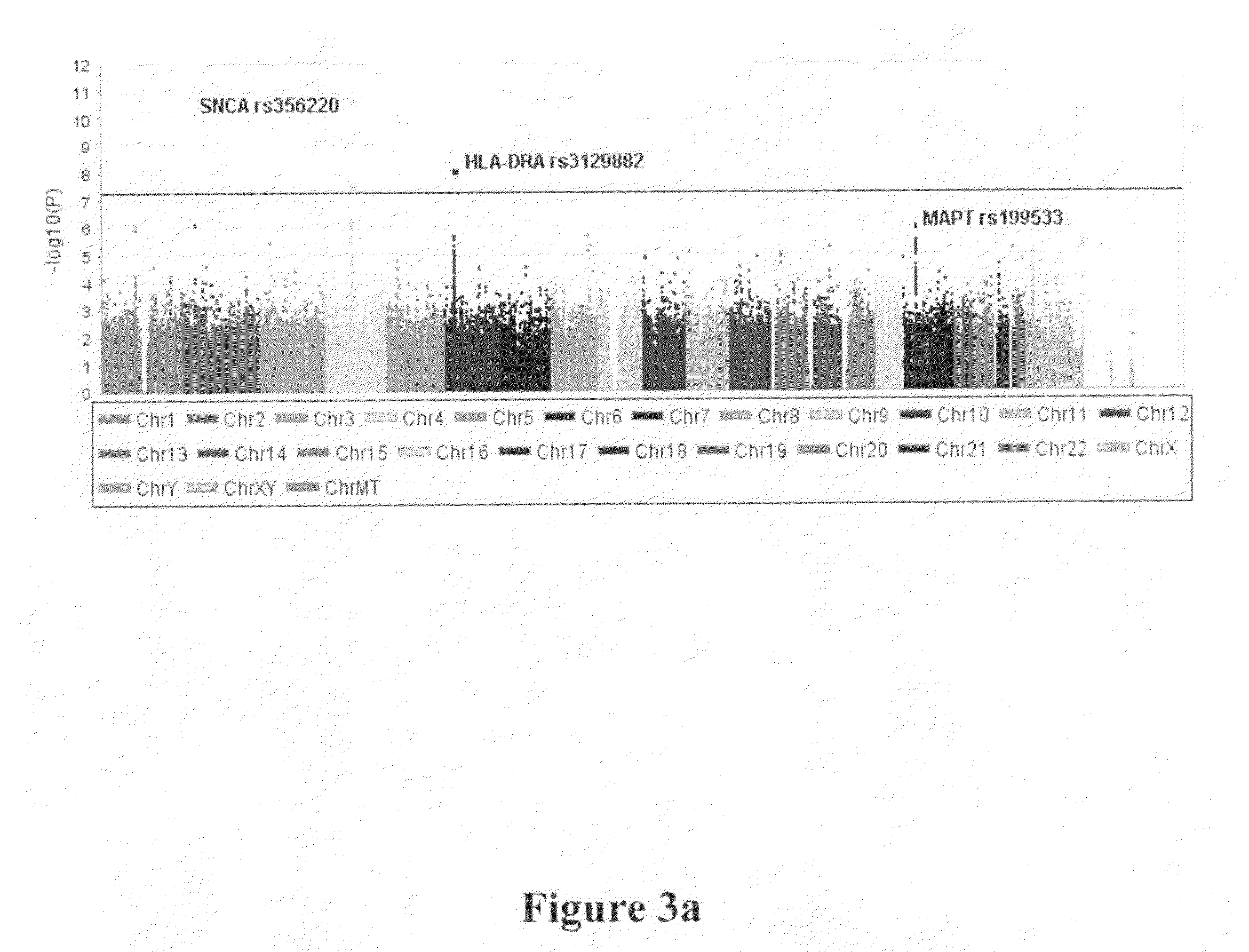
Method of identifying and treating a person having a predisposition to or afflicted with Parkinson disease
InactiveUS20130324503A1Significant neuroprotective effectAvoid developmentBiocideSalicyclic acid active ingredientsFAVORABLE RESPONSEIndividualized treatment
The present invention relates to methods of treatment for Parkinson Disease (PD) in a person by identifying gene variants which may indicate a more favorable response to specific medicaments, thereby allowing for personalized or individualized treatment. The present invention relates to a method of screening for a genetic predisposition to PD in a person. The present invention is also directed to a method of testing a person for the presence of particular gene variants, wherein the presence of a gene variant indicates a higher predisposition to PD, and the absence of a gene variant indicates a lower predisposition to PD, compared to a control sample. The present invention further relates to methods and kits for treating, or inhibiting the development of, PD in a person. The present invention is also directed to a method of identifying the heritage of an individual based on the genetic profile of the individual.
Owner:NAT INST OF HEALTH OFFICE OF TECHNILOGY TRANSFER +6
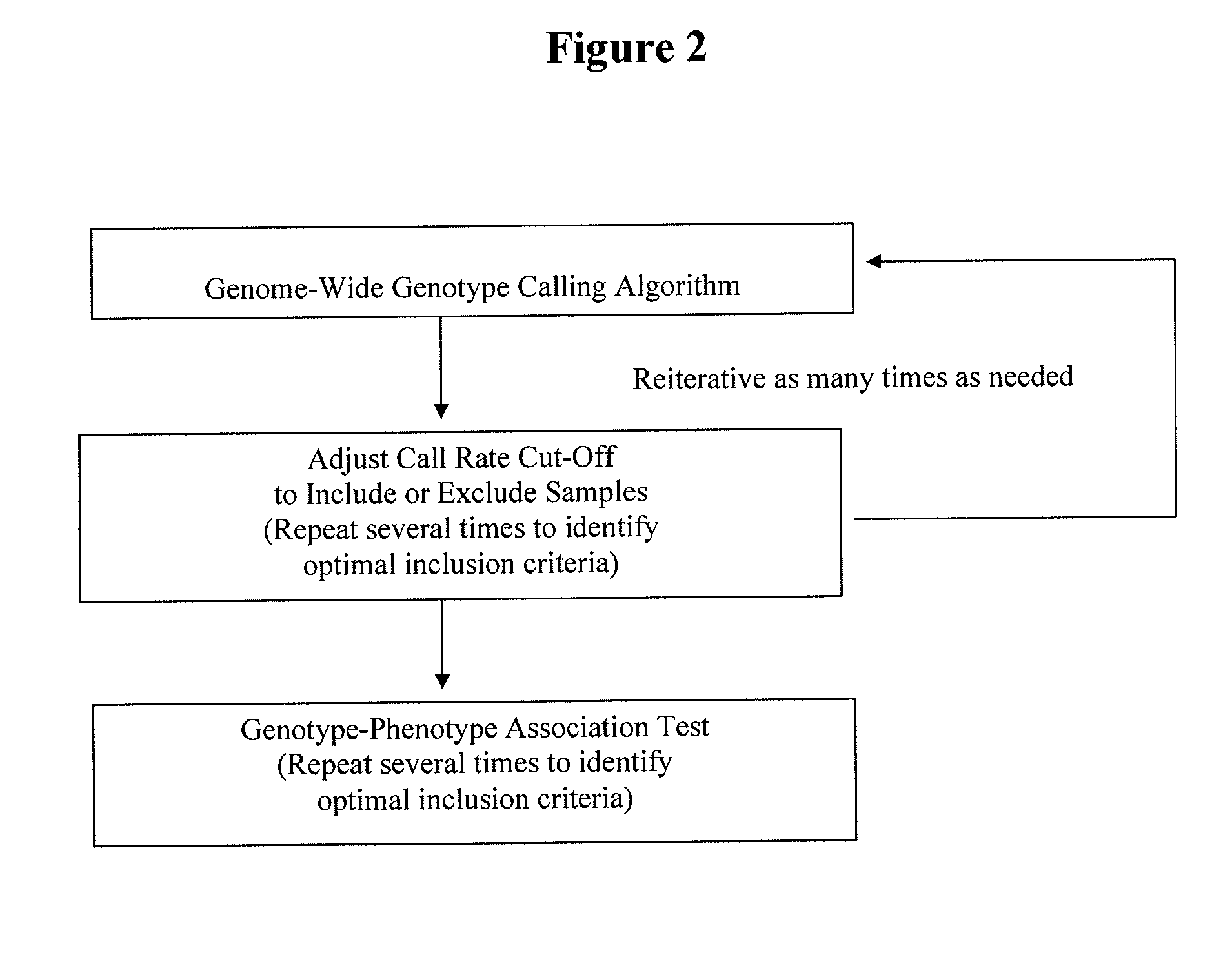
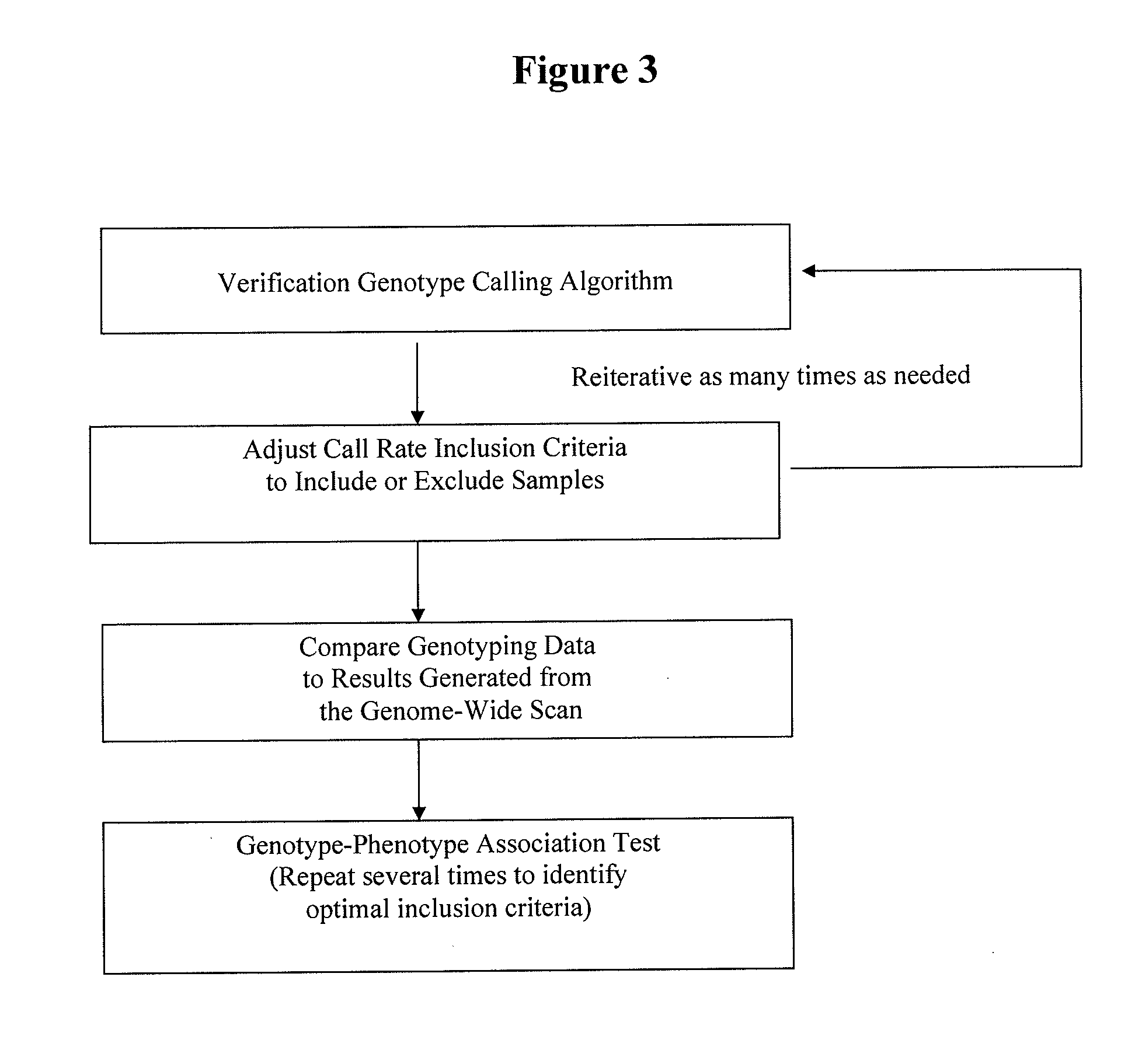
Method for discovering pharmacogenomic biomarkers
InactiveUS20140031242A1Quality improvementPredict likelihood of successSugar derivativesMicrobiological testing/measurementPharmacogenomicsDrugs response
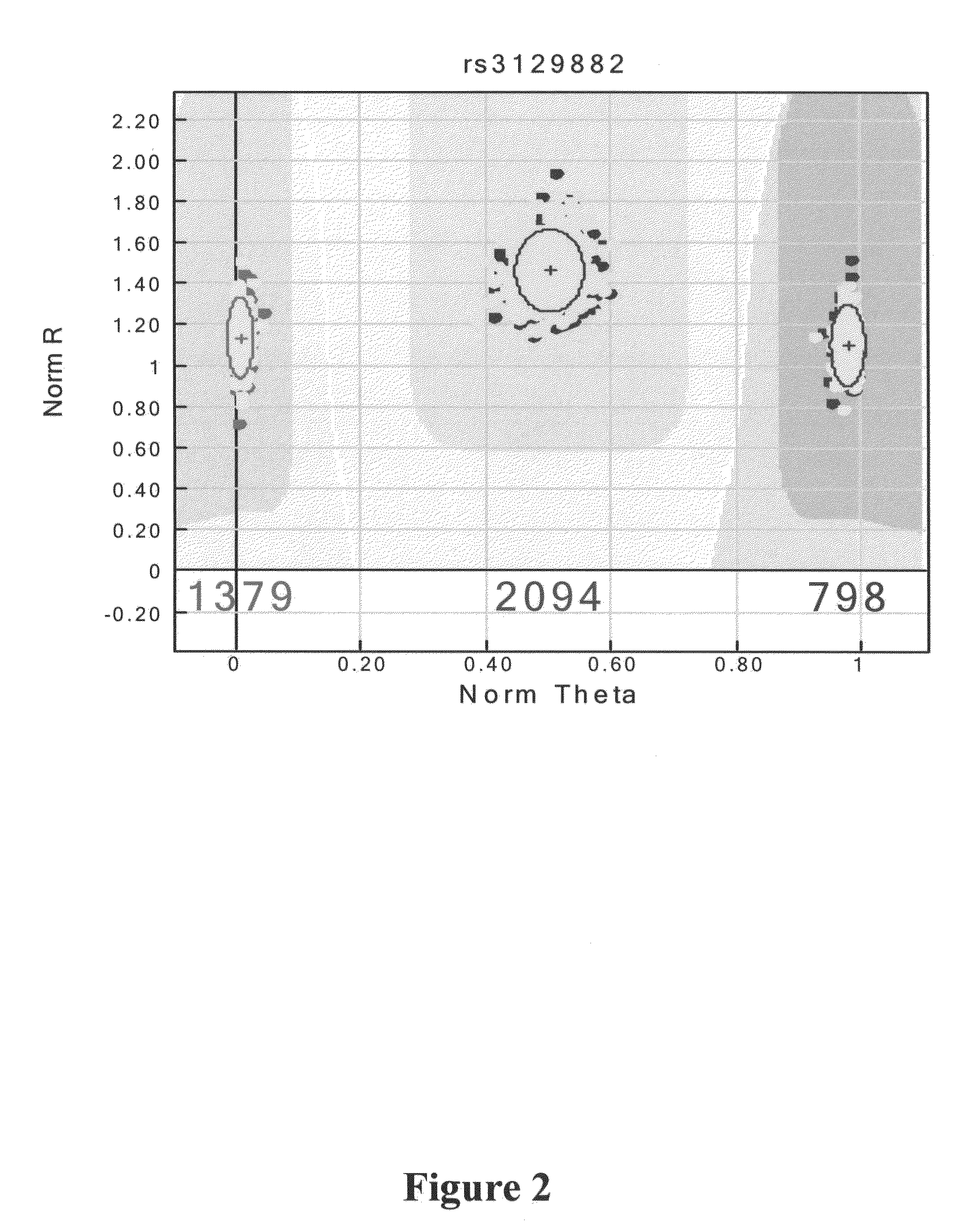
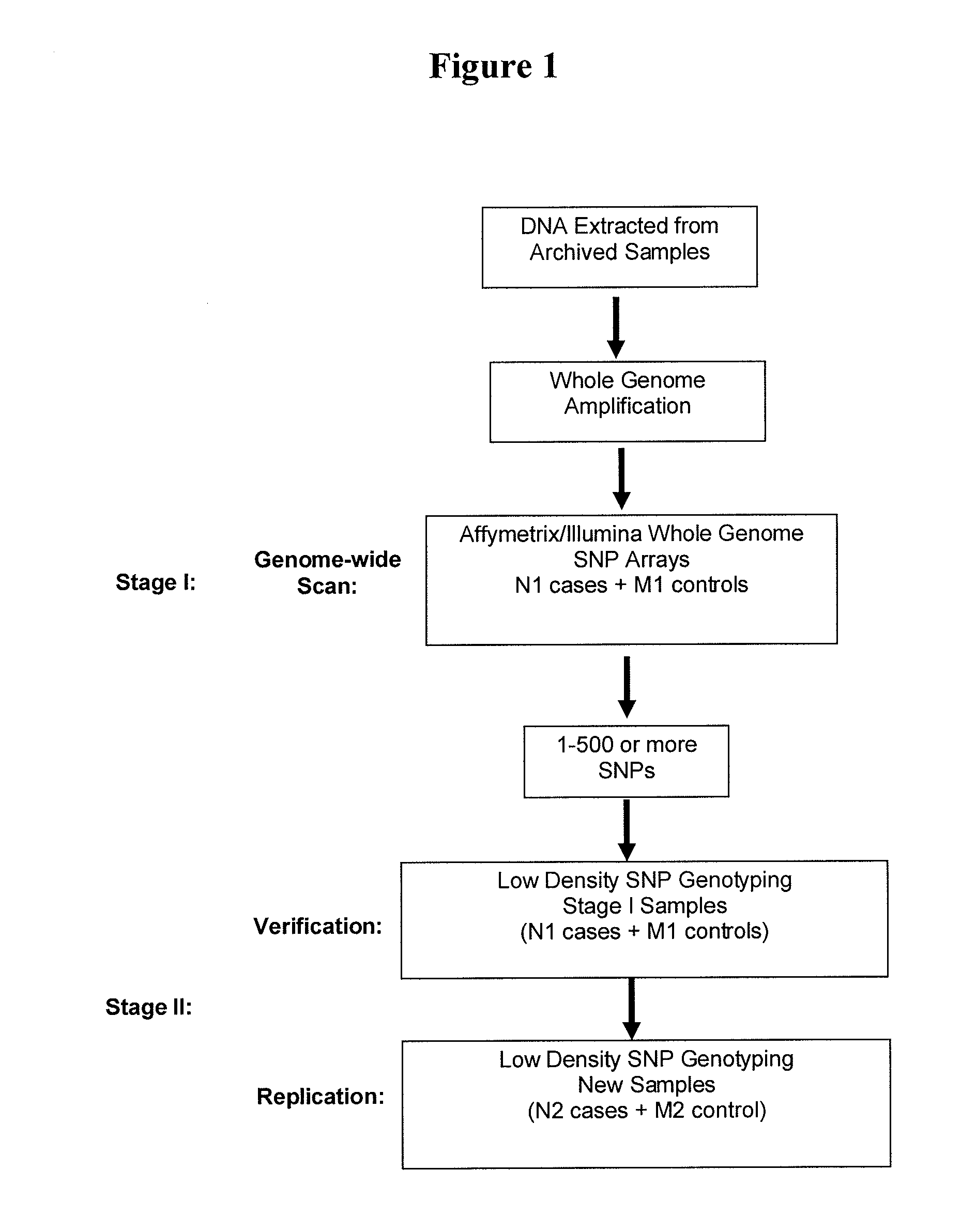
The present invention relates to a method of discovering pharmacogenomic biomarkers that are correlated with varied individual responses (efficacy, adverse effect, and other end points) to therapeutic agents. The present invention provides a mean to utilize archived clinical samples to perform genome-wide association study in order to identify novel pharmacogenomic biomarkers. The newly discovered biomarkers can then be developed into companion diagnostic tests which can help to predict drug responses and apply drugs only to those who will be benefited, or exclude those who might have adverse effects, by the treatment.
Owner:DENOVO BIOPHARMA HANGZHOU LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com