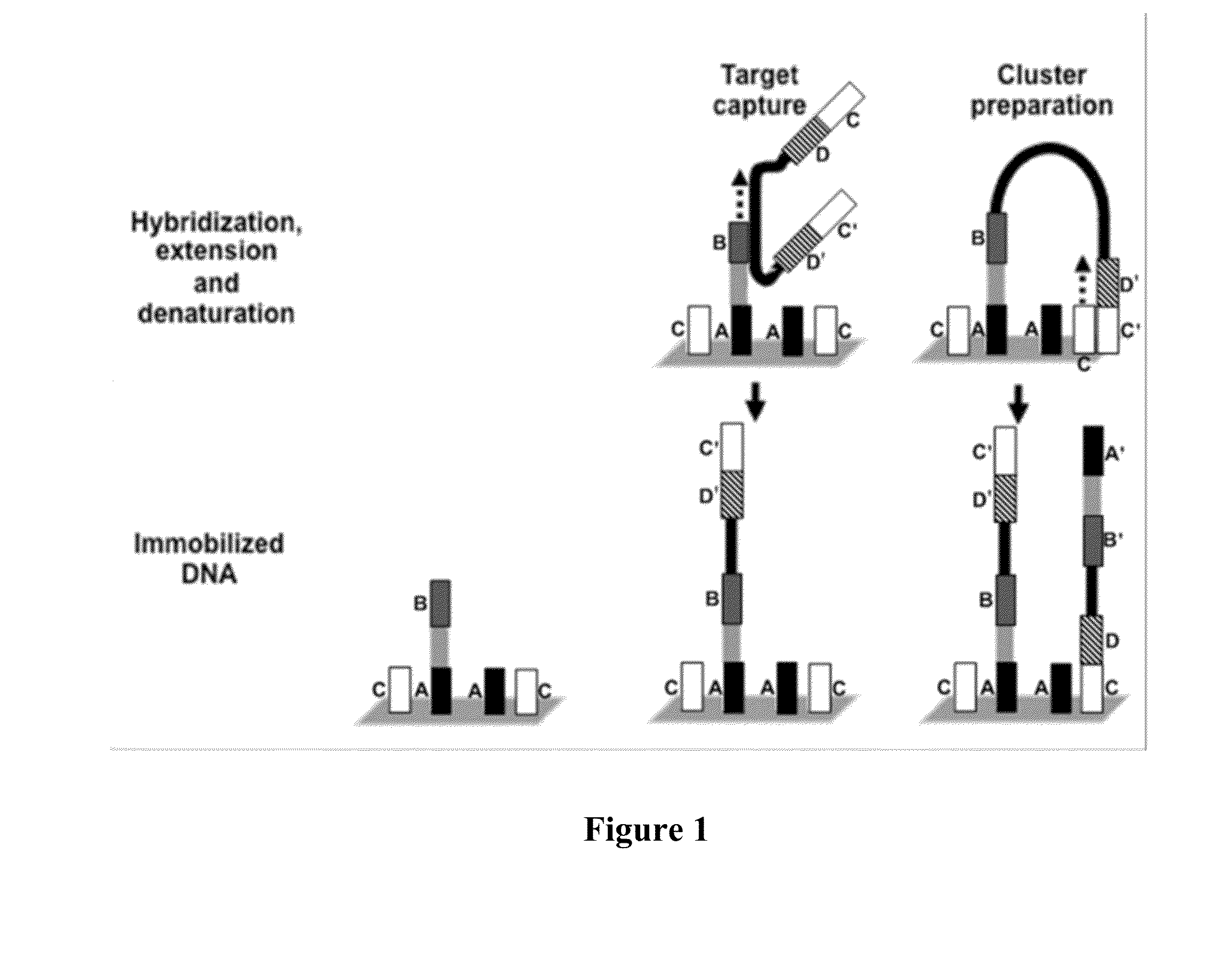
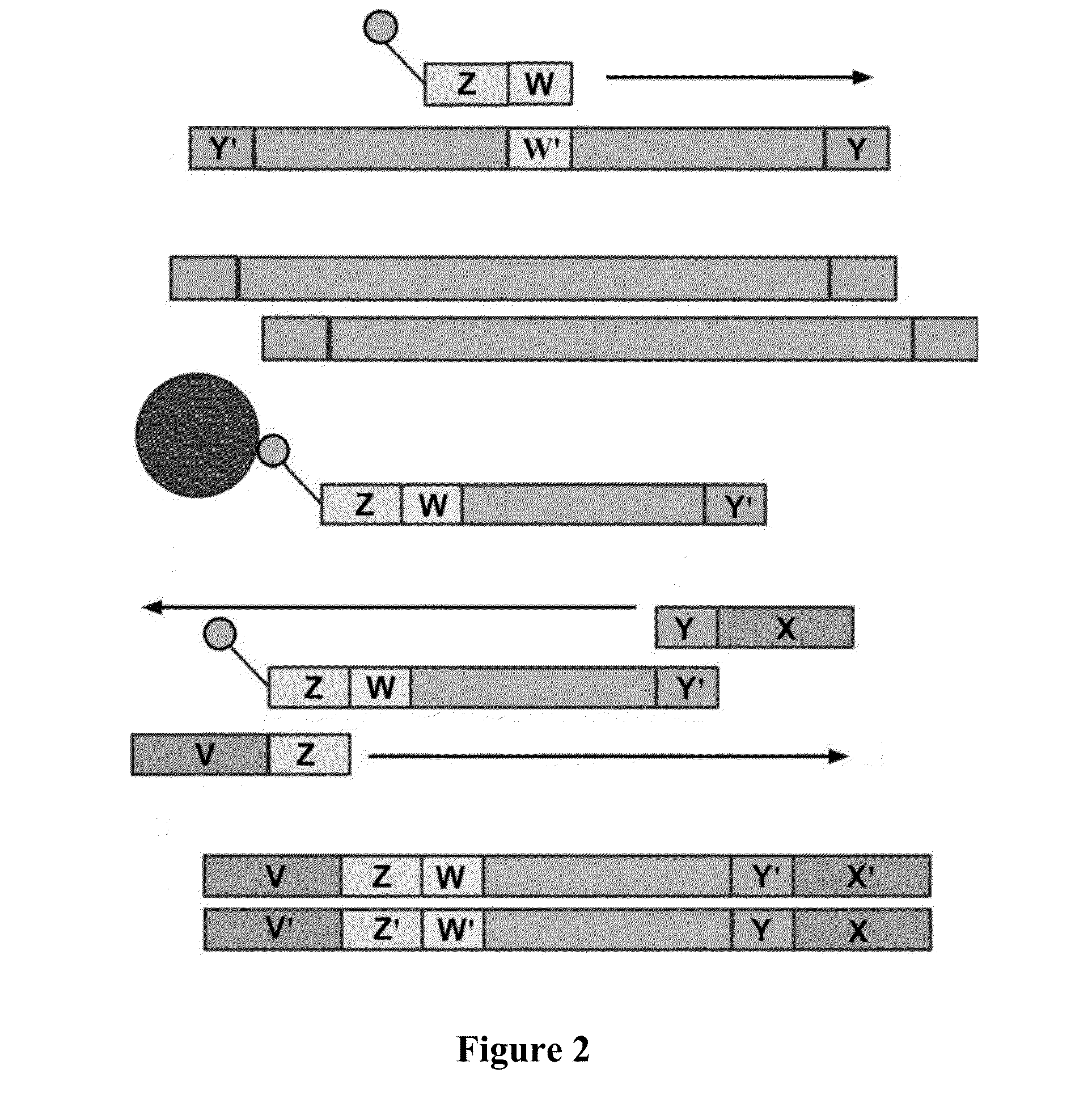
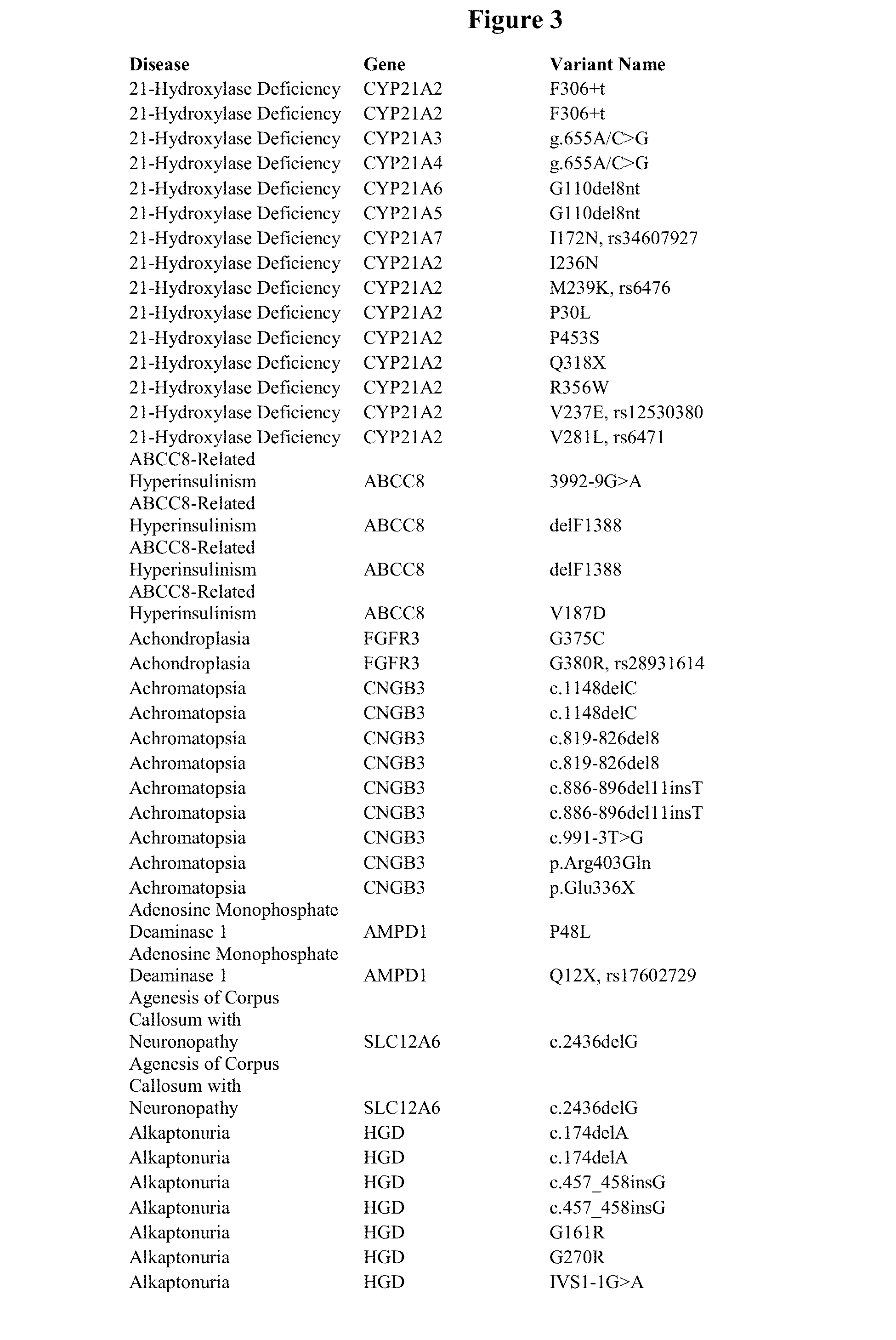
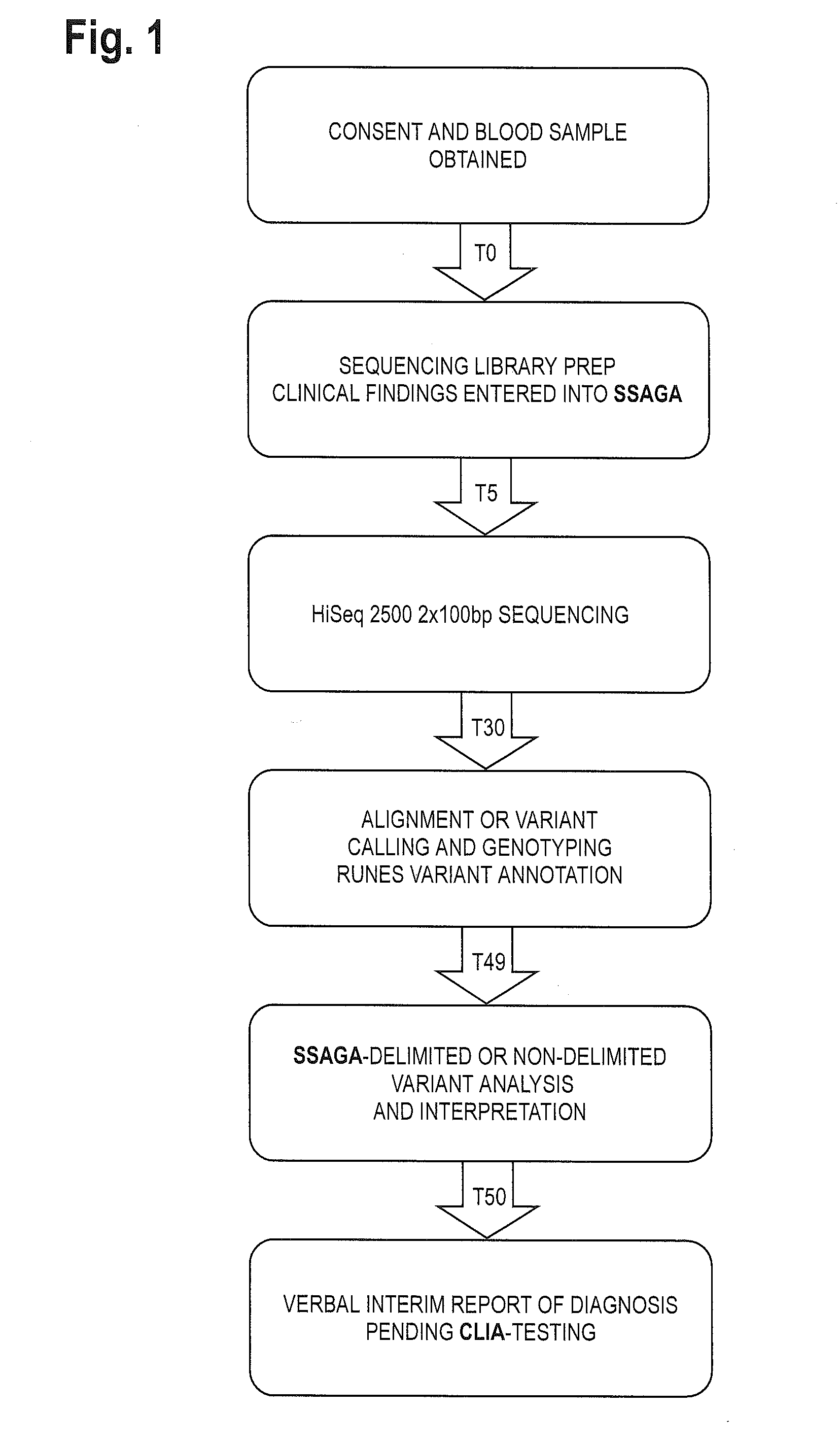
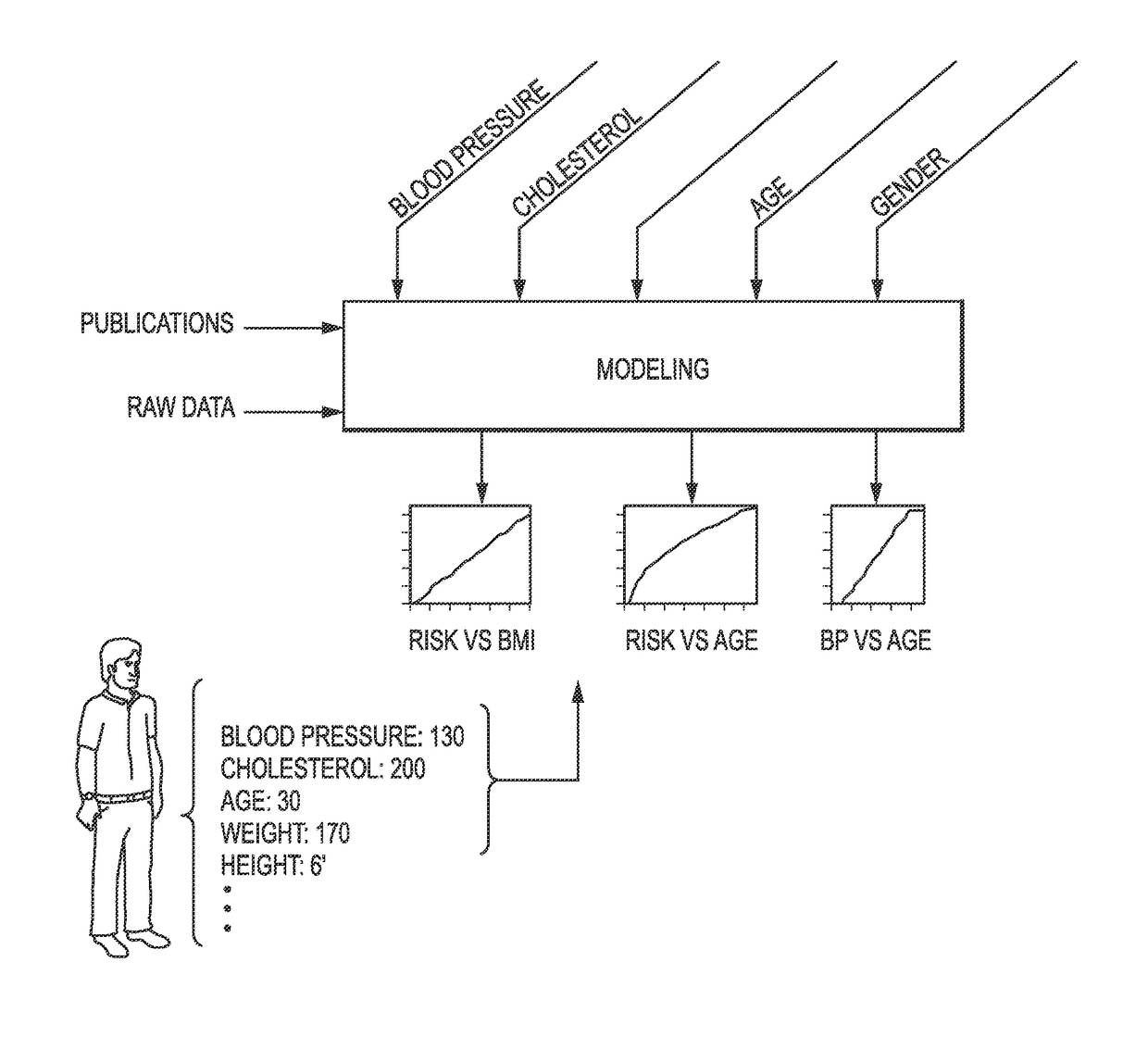
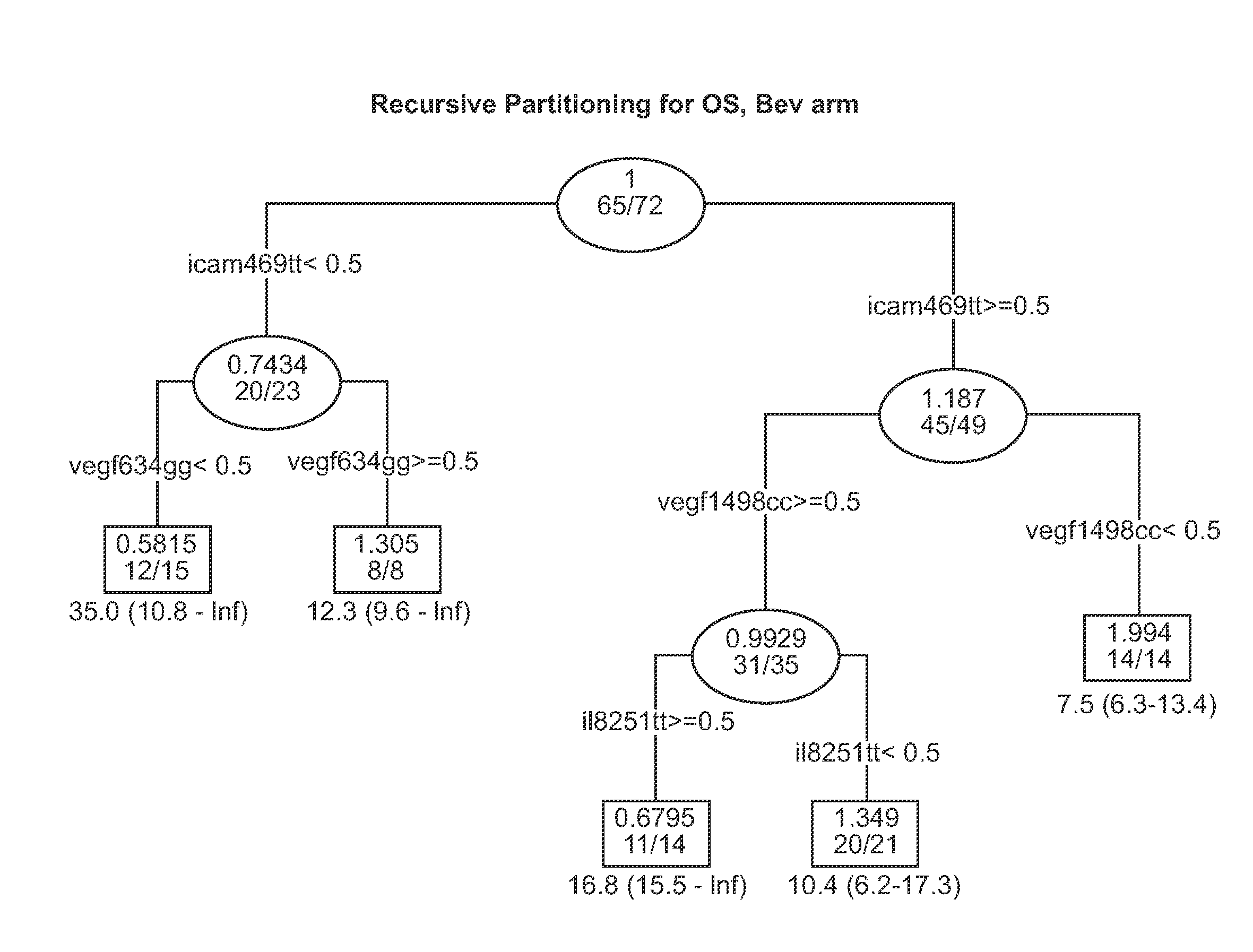
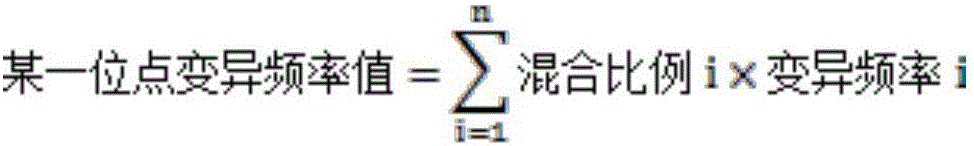Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
384 results about "Gene Variant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Genetic variant may refer to: A single-nucleotide polymorphism (SNP), in case it is a common genetic variant. A mutation, in a case where it is a rare genetic variant.
System and Methods for Detecting Genetic Variation
The invention provides methods, apparatuses, and compositions for high-throughput amplification sequencing of specific target sequences in one or more samples. In some aspects, barcode-tagged polynucleotides are sequenced simultaneously and sample sources are identified on the basis of barcode sequences. In some aspects, sequencing data are used to determine one or more genotypes at one or more loci comprising a causal genetic variant. In some aspects, systems and methods of detecting genetic variation are provided.
Owner:MYRIAD WOMENS HEALTH INC
System for genome analysis and genetic disease diagnosis
InactiveUS20150310163A1High sensitivityIncrease the number ofProteomicsBiological testingData setGenome wide analysis
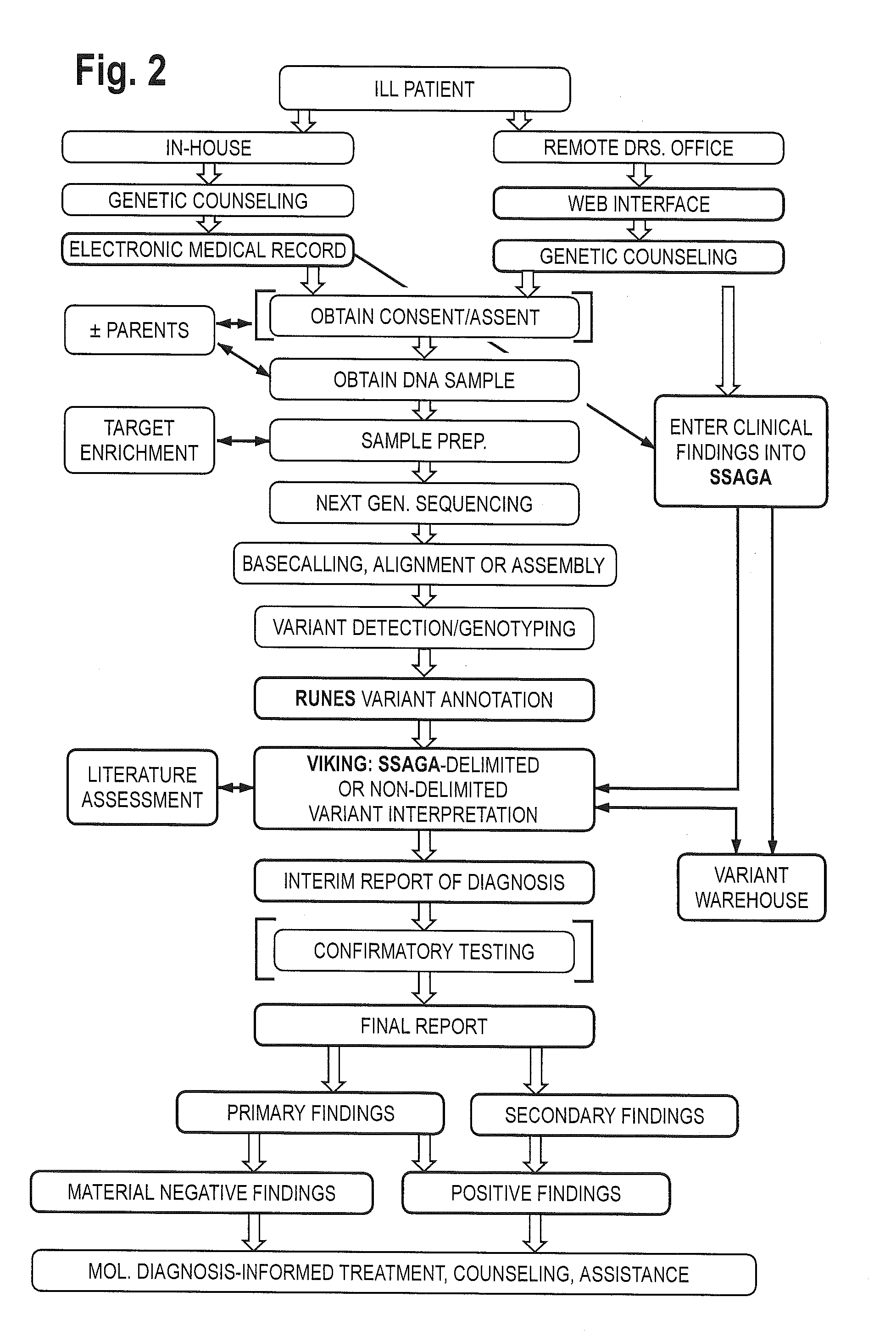
The method for genome analysis translates the clinical findings in the patient into a comprehensive test order for genes that can be causative of the patient's illness, delimits analysis of variants identified in the patient's genome to those that are “on target” for the patient's illness, and provides clinical annotation of the likely causative variants for inclusion in a variant warehouse that is updated as a result of each sample that is analyzed and that, in turn, provides a source of additional annotation for variants. The method uses a genome sequence having the steps of entering at least one clinical feature of a patient by an end-user, assigning a weighted value to the term based on the probability of the presence of the term, mapping the term to at least one disease by accessing a knowledge base containing a plurality of data sets, wherein the data sets are made up of associations between (i) clinical features and diseases, (ii) diseases and genes, (iii) genes and genetic variants, and (iv) diseases and gene variants, assigning a truth value to each of the mapped terms based on the associated data sets and the weighted value, to provide a list of results of possible diagnoses prioritized based on the truth values, with continuous adjustment of the weightings of associations in the knowledge base based on updating of each discovered diagnosis and attendant clinical features, genes and gene variants. This method can be performed in fifty hours or twenty-four hours or less.
Owner:CHILDRENS MERCY HOSPITAL
Physiogenomic method for predicting response to diet
InactiveUS20070196841A1Reduce weightReduce morbiditySugar derivativesMicrobiological testing/measurementGenomicsPersonalization
The present invention relates to the use of genetic variants of associated marker genes to predict an individual's response to diet. The present invention further relates to analytical assays and computational methods using the novel marker gene set. The present invention has utility for developing personalized diet regimens to optimize physiological response, including changes in body mass index (BMI) and blood lipid and triglyceride levels.
Owner:GENOMAS
Target-specific compomers and methods of use
InactiveUS20050287533A1Improve accuracyImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsLipid formationMass increment
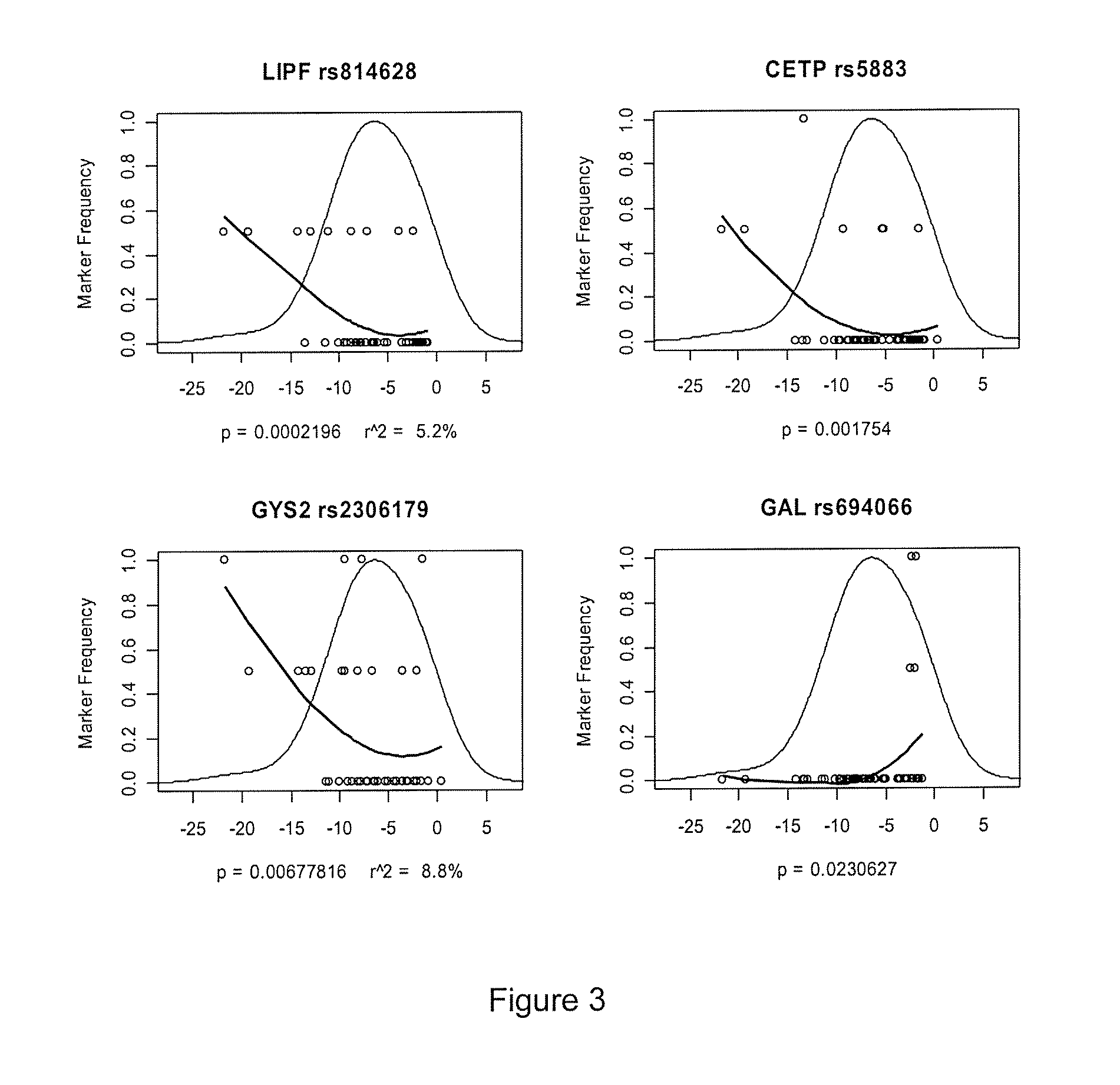
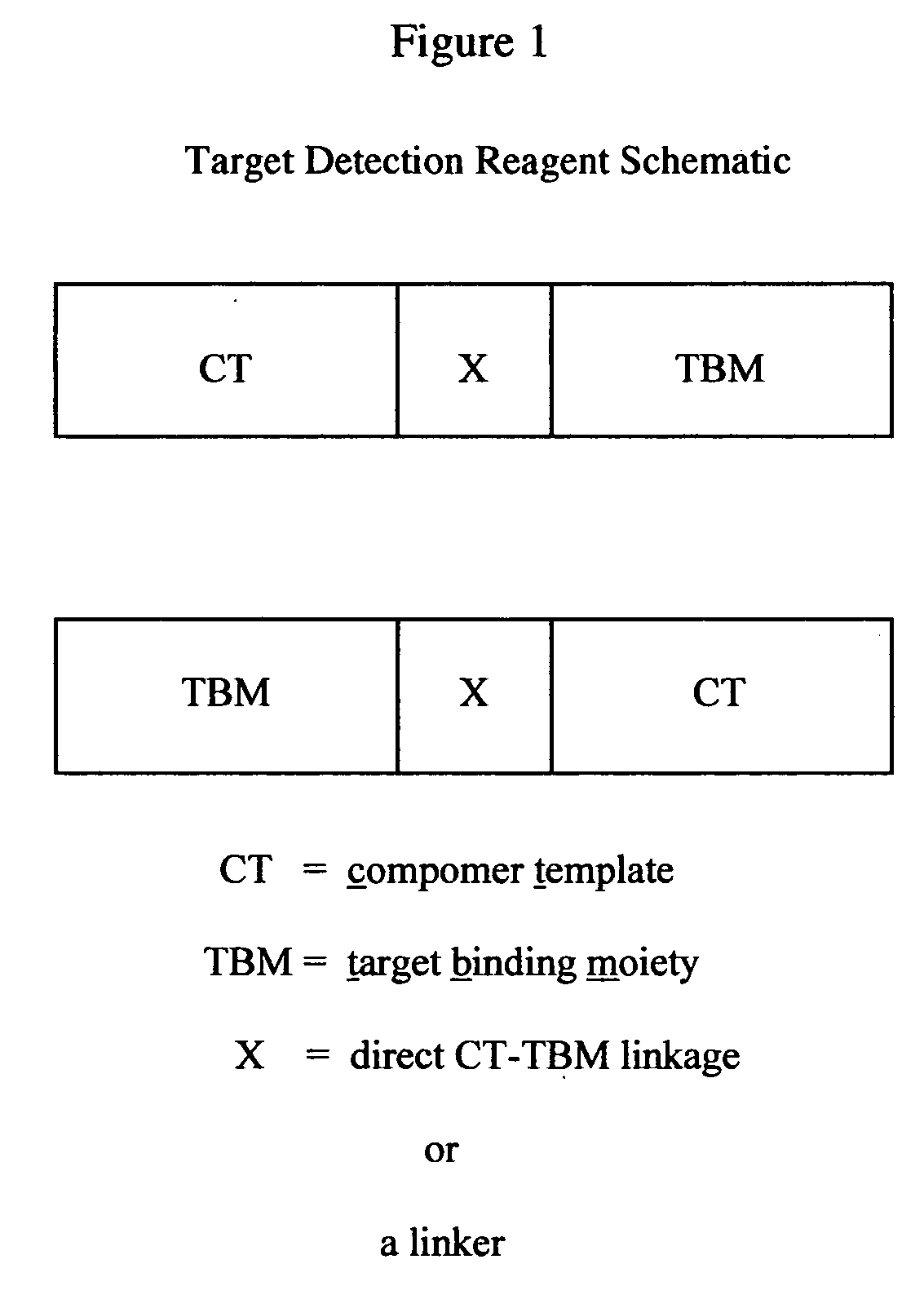
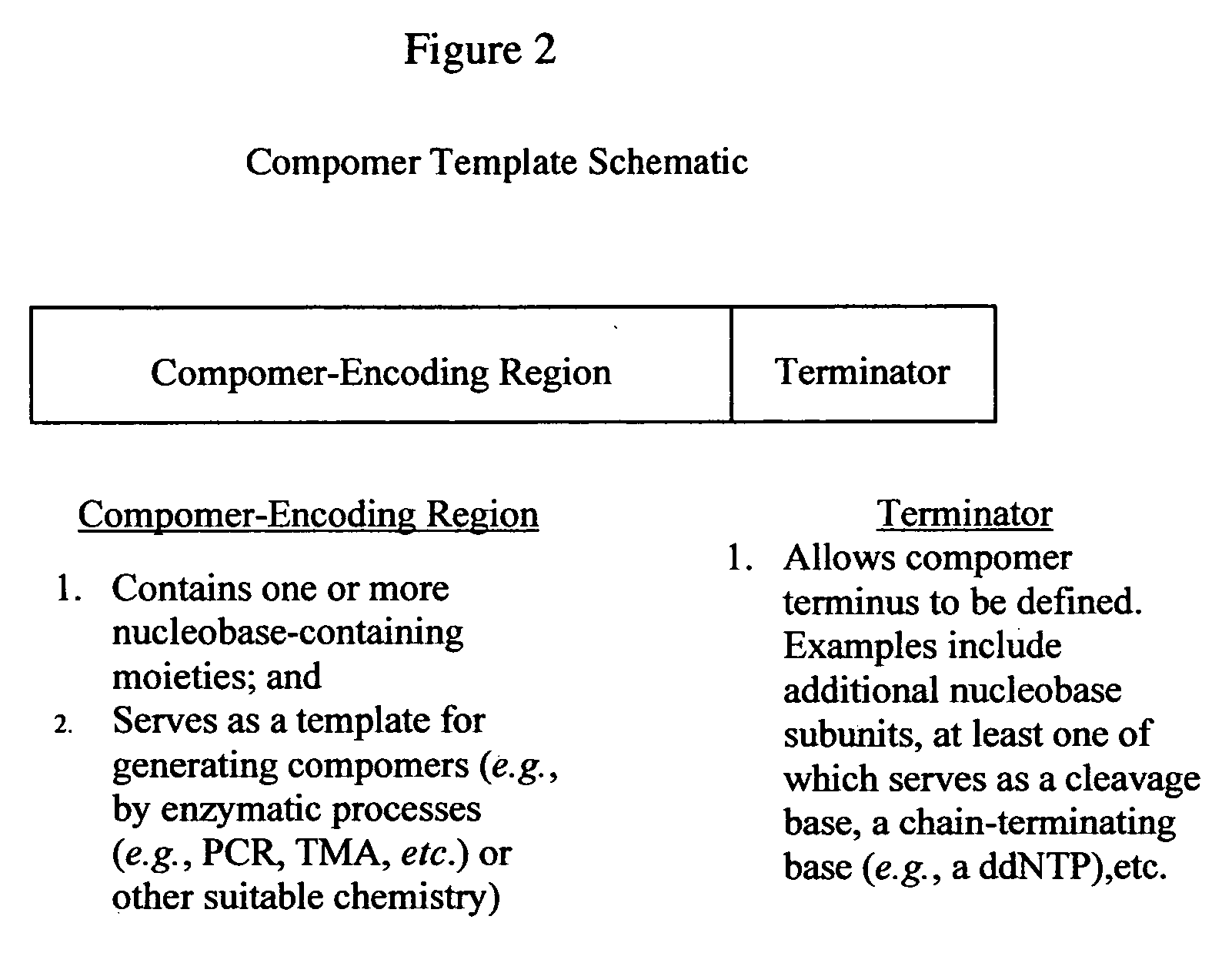
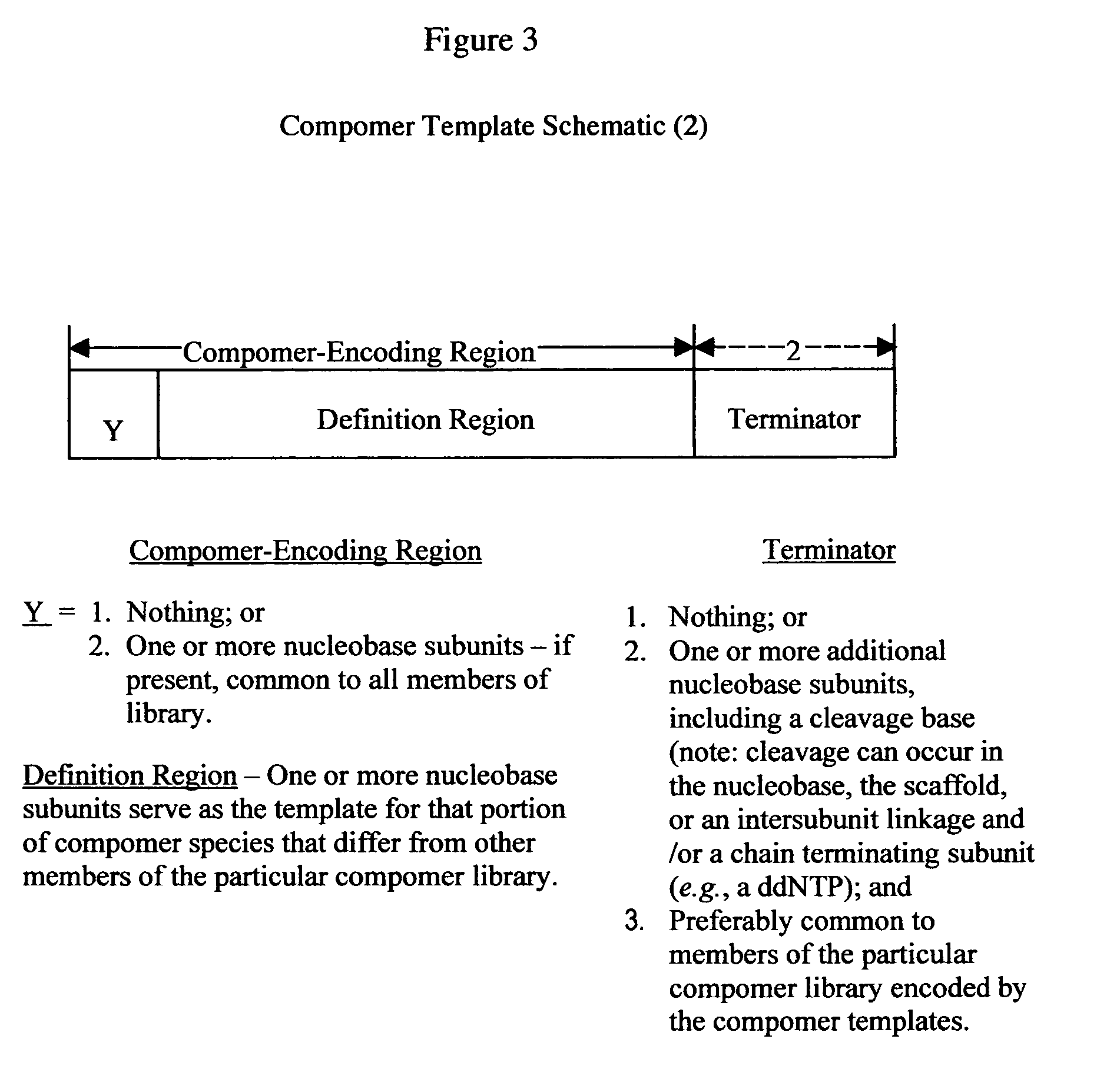
The present invention provides a novel class of molecules, termed “compomers,” that enable the indirect detection of target molecules, as well as novel target detection reagents and compomer templates that encode compomers. Compomers are linear polymers generated from the compomer template portion of a target detection reagent during the course of an assay. In a given assay, each compomer species is correlated with a different target molecule, e.g., a carbohydrate, lipid, polypeptide, or target nucleic acid, particularly a specific nucleotide sequence within a target nucleic acid molecule. When, for example, a target nucleic acid is present in an assay, a compomer species specifically and uniquely correlated with the particular target (e.g., a known SNP or other genetic variant) is generated directly from a target-specific detection reagent (or indirectly from a larger precursor encoded by the compomer template and from which it is subsequently released), after which it can readily be detected, even in an assay where tens, hundreds, or thousands of different compomer species may be generated, as each compomer species is engineered to differ from the others by a small, resolvable defined characteristic (e.g., a mass increment, a difference in subunit composition, sequence, size, length, etc.). When coupled with highly sensitive detection techniques (e.g., MALDI-TOF mass spectrometry, nucleic acid hybridization, nuclear magnetic resonance, etc.), a large number of different compomer species can be detected in a single reaction, thereby facilitating highly multiplexed analyses of complex samples.
Owner:AGENA BIOSCI
Genetic analysis systems and methods
InactiveUS6897025B2Bioreactor/fermenter combinationsBiological substance pretreatmentsGenomic DNAGenetics
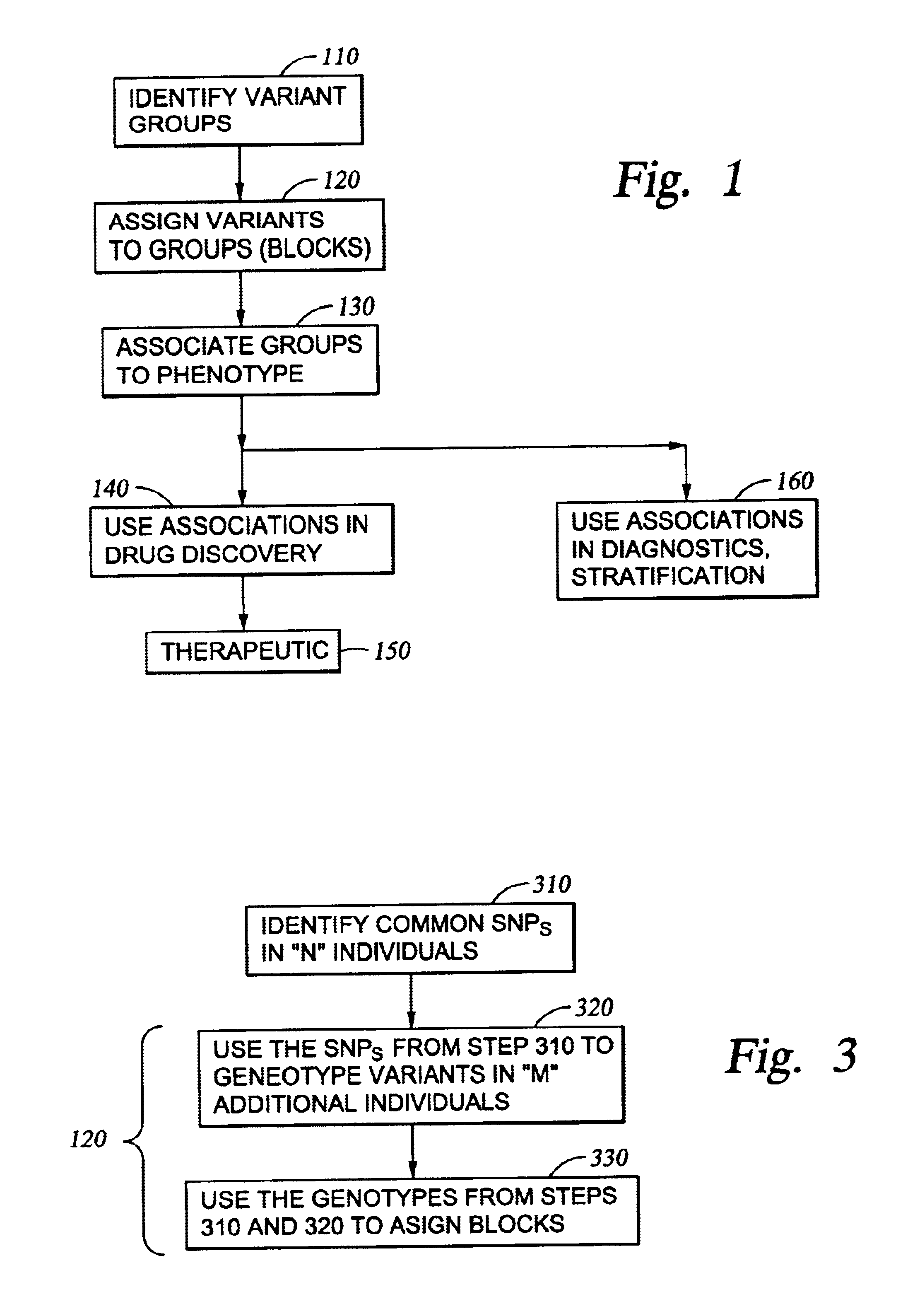
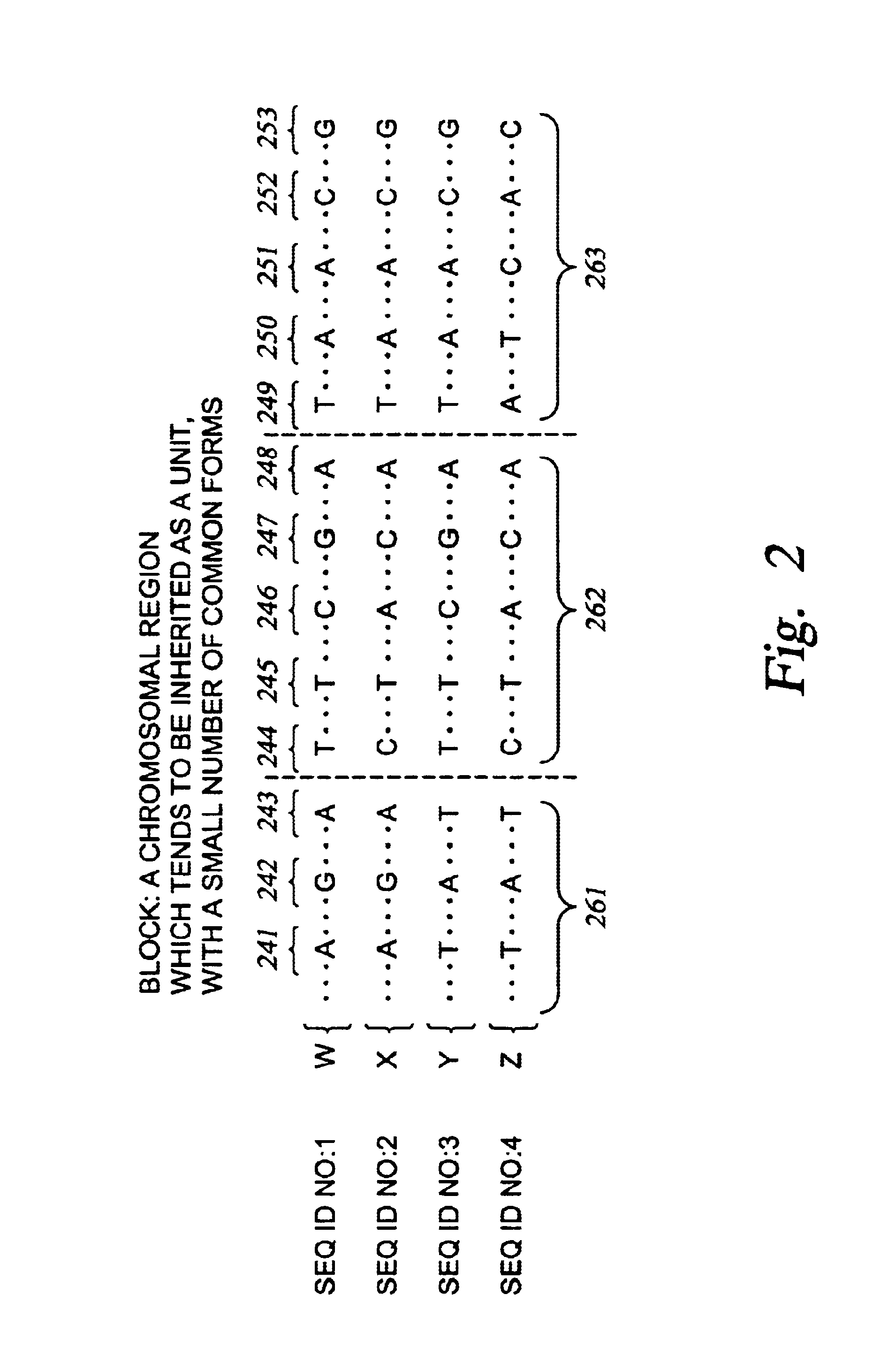
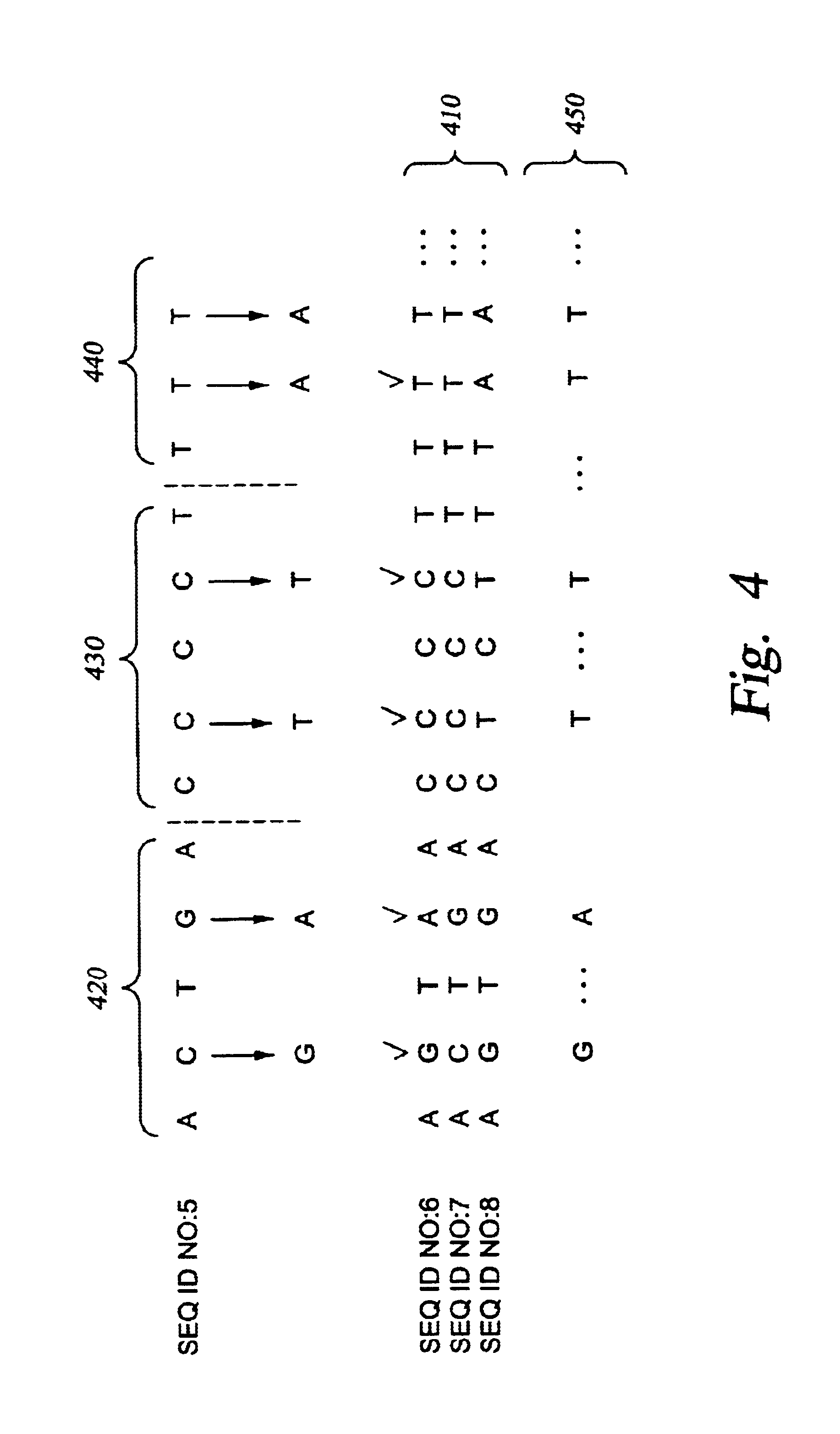
Improved systems and methods for performing genetic analyses. Full genomic DNA scans are performed on the genetic DNA from a plurality of individuals to identify genetic variants. For those variants, but not based on a full genetic DNA scan, the variants alone are scanned in additional individuals to identify blocks of the variants that tend to be inherited together.
Owner:GENETIC TECHNOLOGIES LIMTIED
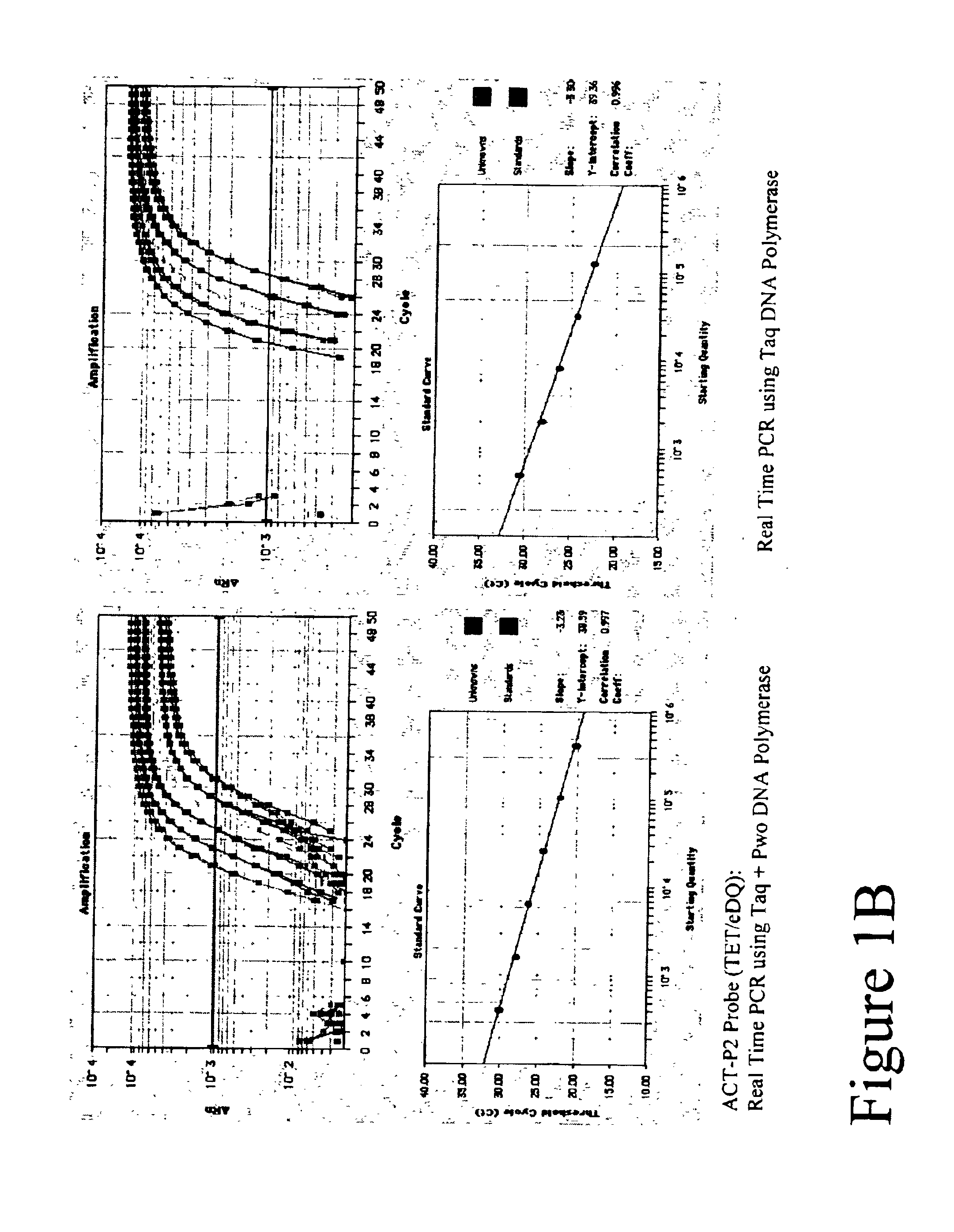
Methods of using FET labeled oligonucleotides that include a 3'->5' exonuclease resistant quencher domain and compositions for practicing the same
Methods and compositions are provided for detecting a primer extension product in a reaction mixture. In the subject methods, a primer extension reaction is conducted in the presence of a polymerase having 3′→5′ exonuclease activity and at least one FET labeled oligonucleotide probe that includes a 3′→5′ exonuclease resistant quencher domain. Also provided are systems and kits for practicing the subject methods. The subject invention finds use in a variety of different applications, and are particularly suited for use in high fidelity PCR based reactions, including SNP detection applications, allelic variation detection applications, and the like.
Owner:LIFE TECH CORP
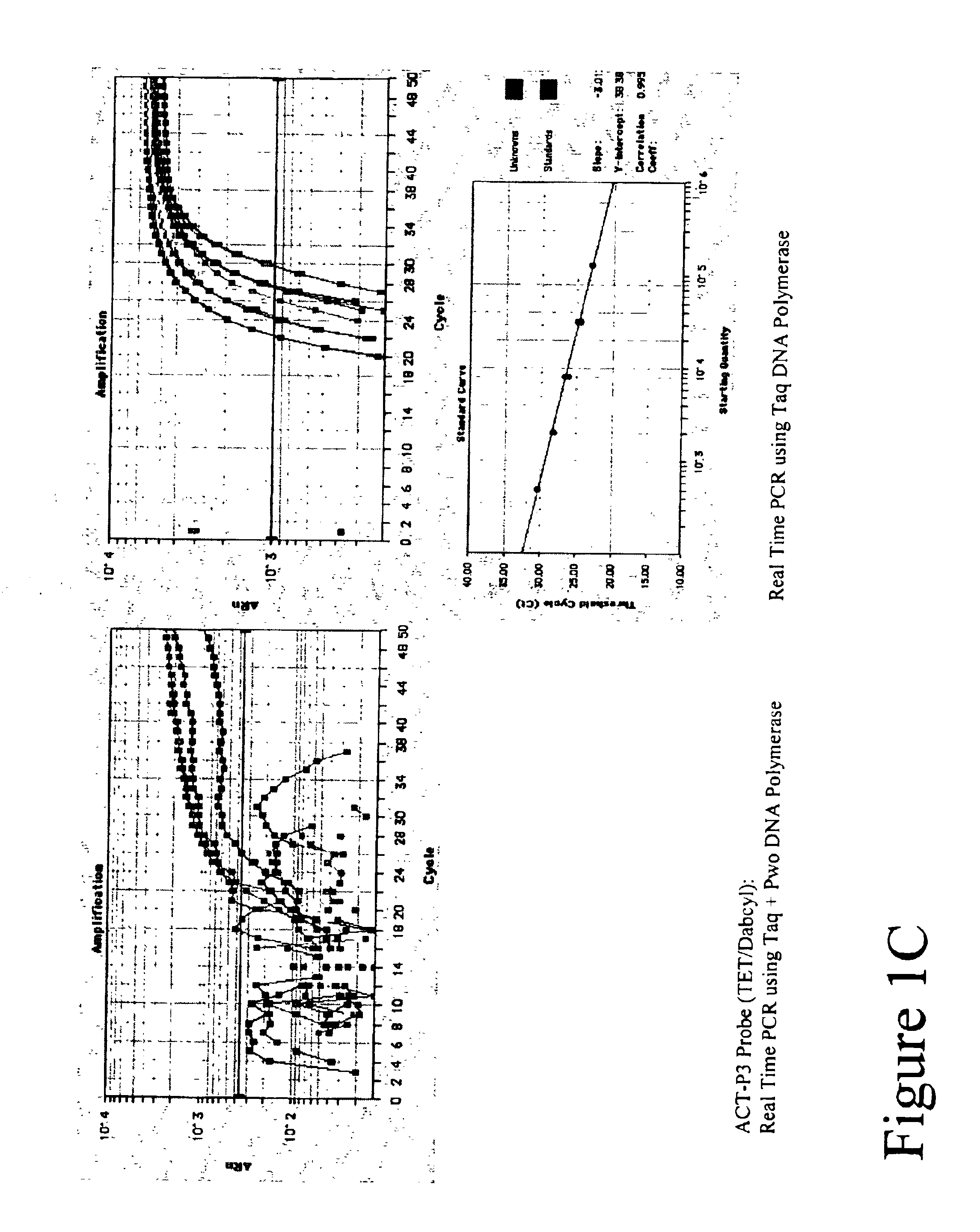
Gene variants and use thereof
InactiveUS20070082347A1Useful predictionBioreactor/fermenter combinationsBiological substance pretreatmentsFKBP1ATAP2
Variants in TLK1, WARS2, ARTS2, MSR, AKAP9, DNAJD1, GOLPH4, RABEP1, TAP2, NARG2, DDX58, CD39, FKBP1a, SRI, XRRA1, IRF5 and AMFR genes are disclosed which are useful as biomarkers for predicting the TLK1, WARS2, ARTS2, MSR, AKAP9, DNAJD1, GOLPH4, RABEP1, TAP2, NARG2, DDX58, CD39, FKBP1a, SRI, XRRA1, IRF5 or AMFR gene expression level and the biological functions associated thereof.
Owner:MYRIAD GENETICS
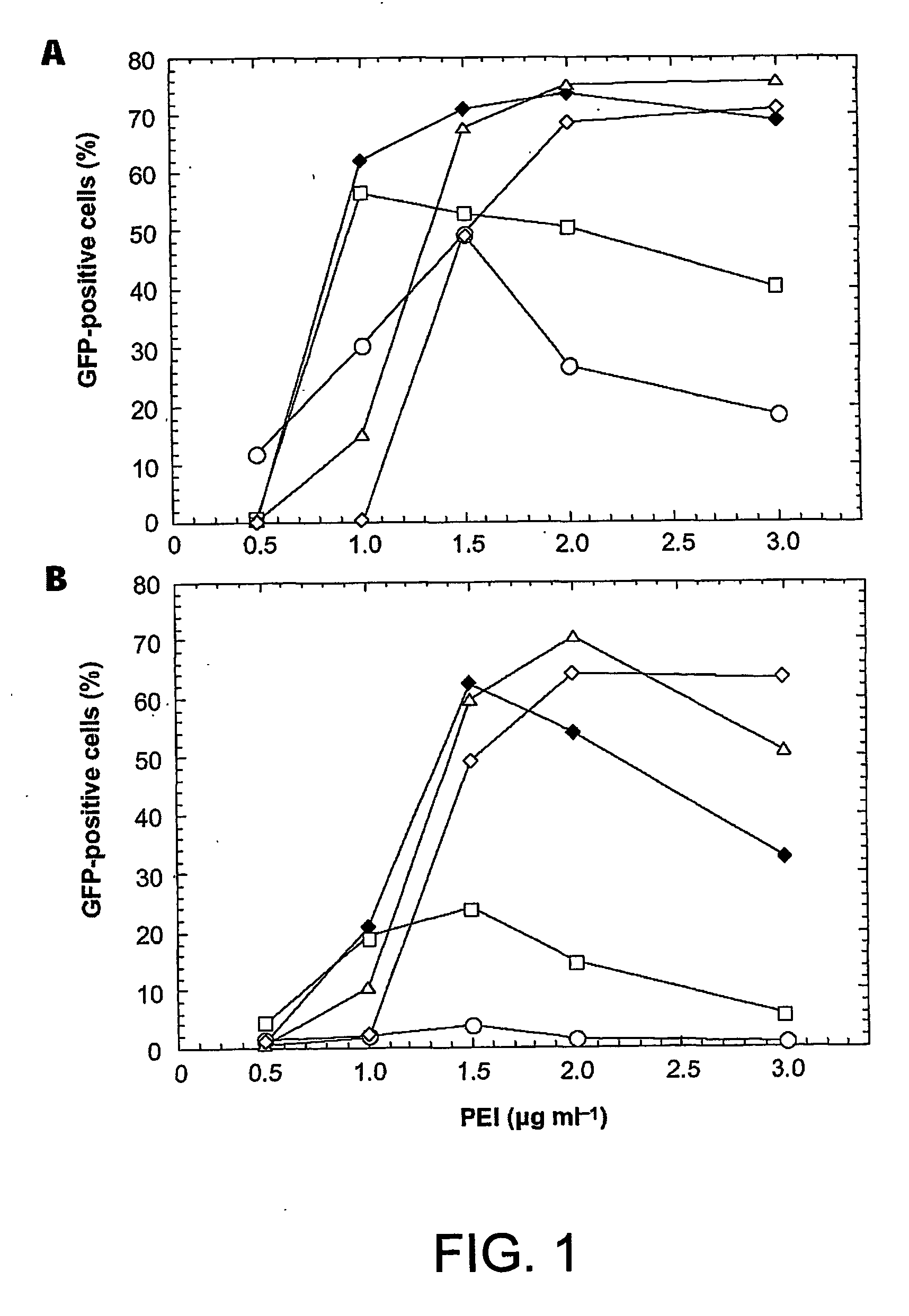
Enhanced production of recombinant proteins by transient transfection of suspension-growing mammalian cells
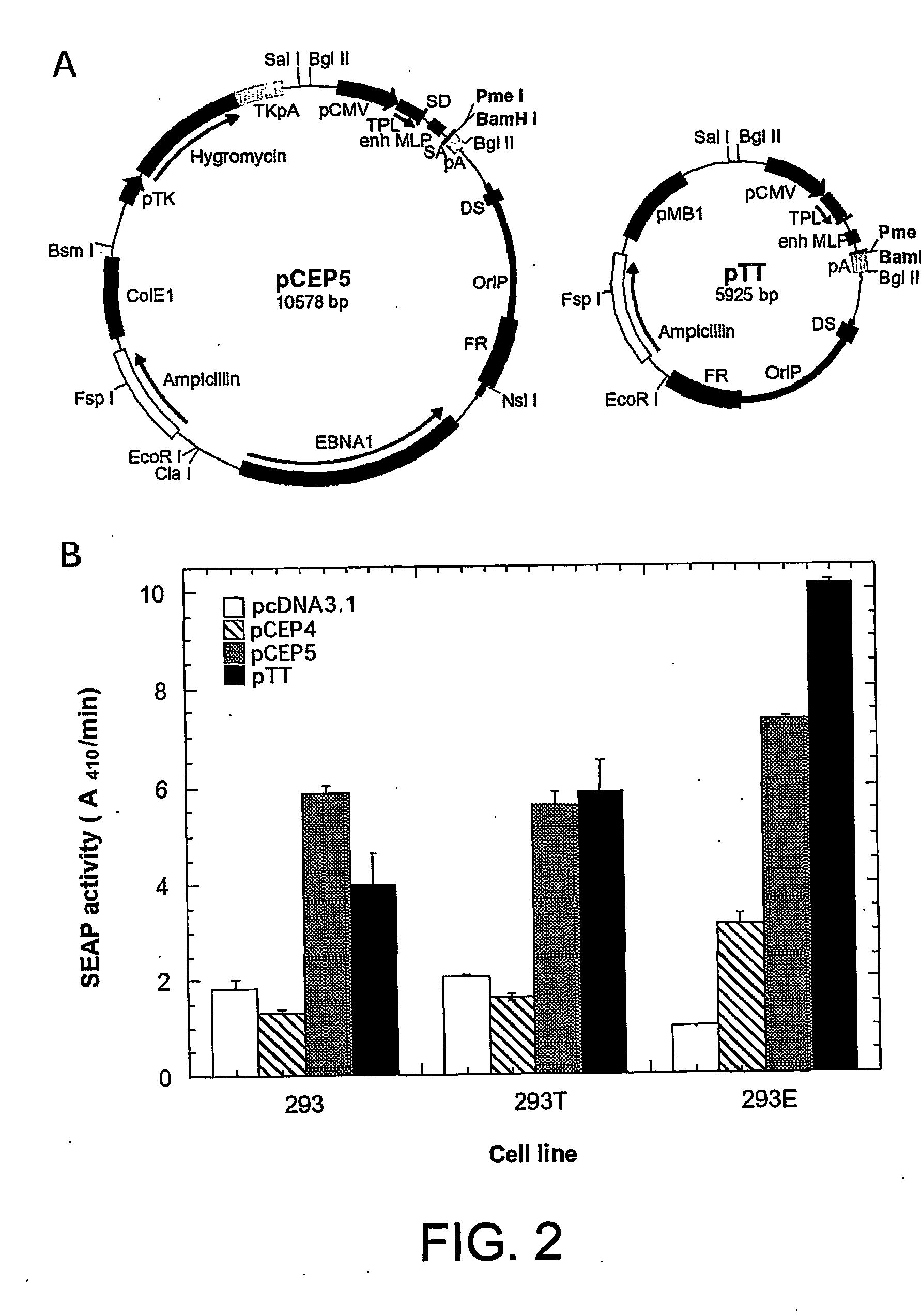
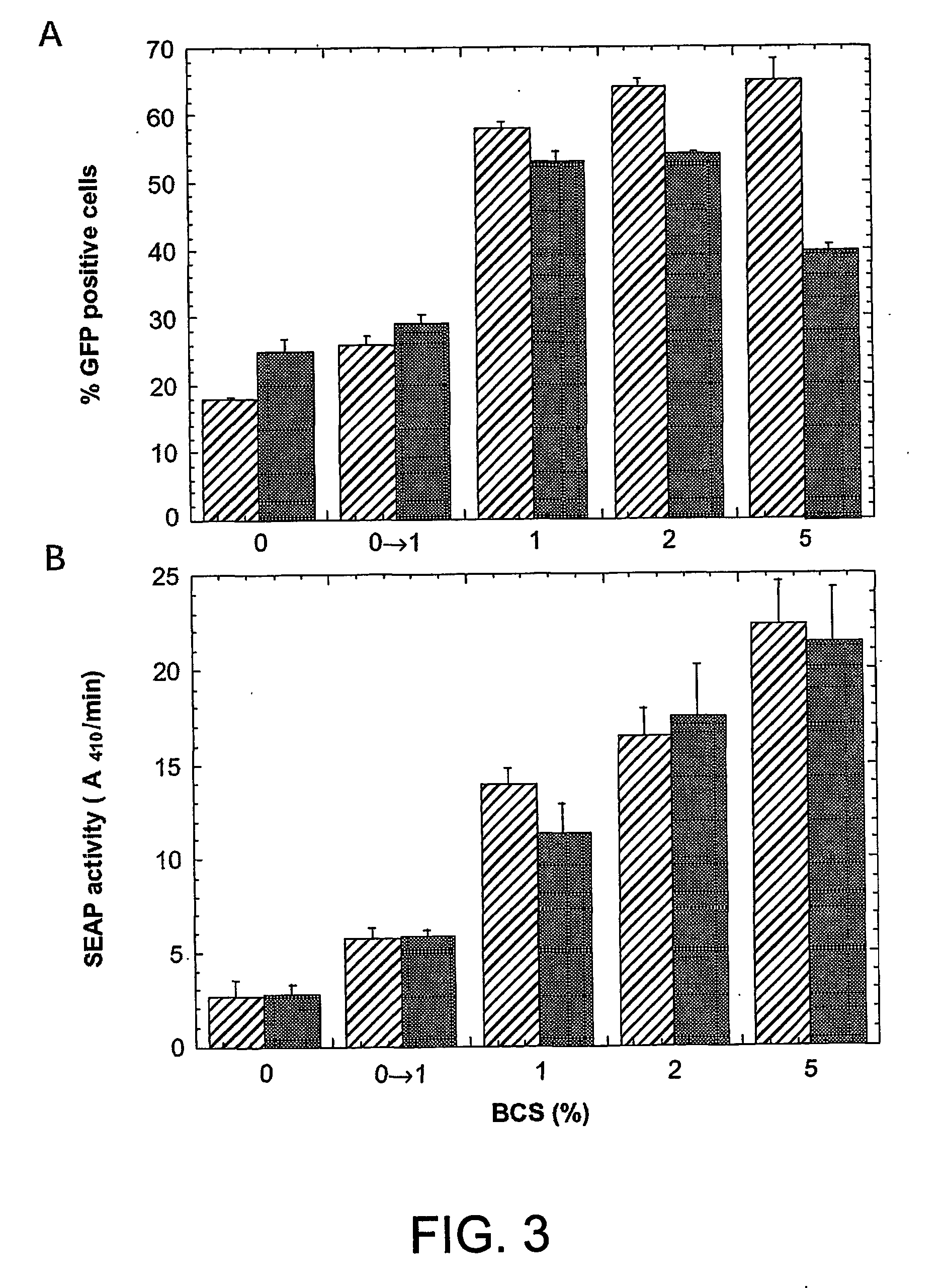
ActiveUS20050170450A1Increase transcriptional activityIncreasing nuclear importGenetically modified cellsVirus peptidesEpstein–Barr virusGene Variant
Disclosed is a new process for the production of recombinant proteins, by transient transfection of suspension-grown human embryonic kidney cells (293 cell line and its genetic variants) with an expression vector, using polyethylenimine (PEI) as a transfection reagent. In a preferred embodiment, the process uses 293E cells expressing the Epstein-Barr virus (EBV) EBNA 1 protein, in combination with an oriP-based episomal expression vector having an improved cytomegalovirus expression cassette comprising the CMV5 promoter. The process combines in a single step the cell growth, transfection and protein expression, is carried out without changing the culture medium, and allows to achieve high expression levels in a short period of time. The process may be carried out in a serum-free, low-protein culture medium, is easily scalable, compatible with continuous production processes, and fully adapted to high-throughput production of milligram quantities of recombinant proteins.
Owner:NAT RES COUNCIL OF CANADA
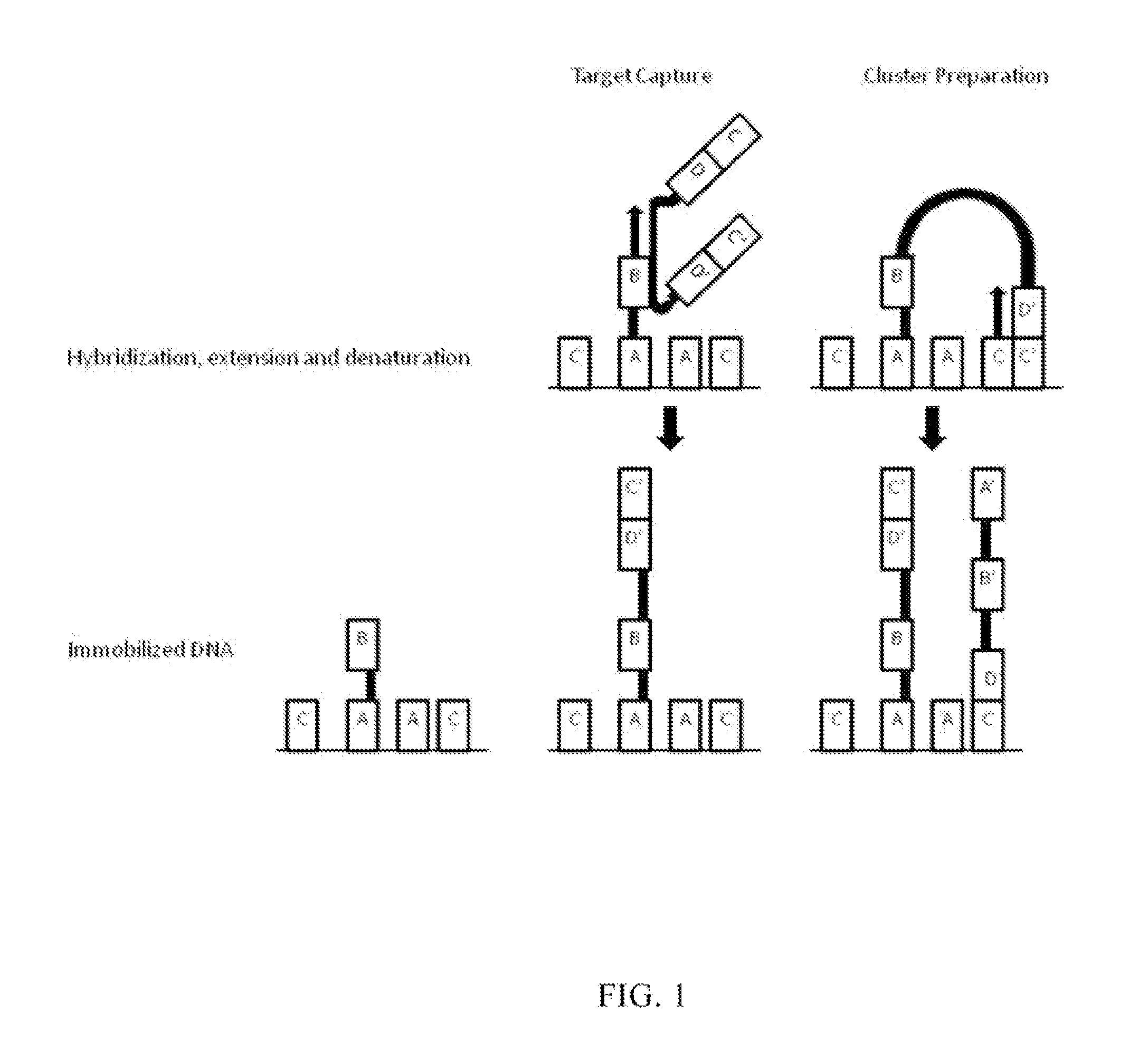
Methods and compositions for high-throughput sequencing
InactiveUS20140024541A1Microbiological testing/measurementLibrary screeningBarcodeHigh throughput sequence
The invention provides methods, apparatuses, and compositions for high-throughput amplification sequencing of specific target sequences in one or more samples. In some aspects, barcode-tagged polynucleotides are sequenced simultaneously and sample sources are identified on the basis of barcode sequences. In some aspects, sequencing data are used to determine one or more genotypes at one or more loci comprising a causal genetic variant.
Owner:COUNSYL INC
Genetic Variants Predictive of Cancer Risk
InactiveUS20110212855A1Increased susceptibilityHigh riskMicrobiological testing/measurementLibrary screeningDiseaseGenetic variants
The invention discloses genetic variants that have been determined to be susceptibility variants of cancer. Methods of disease management, including determining increased susceptibility to cancer, methods of predicting response to therapy and methods of predicting prognosis of cancer using such variants are described. The invention further relates to kits useful in the methods of the invention.
Owner:DECODE GENETICS EHF
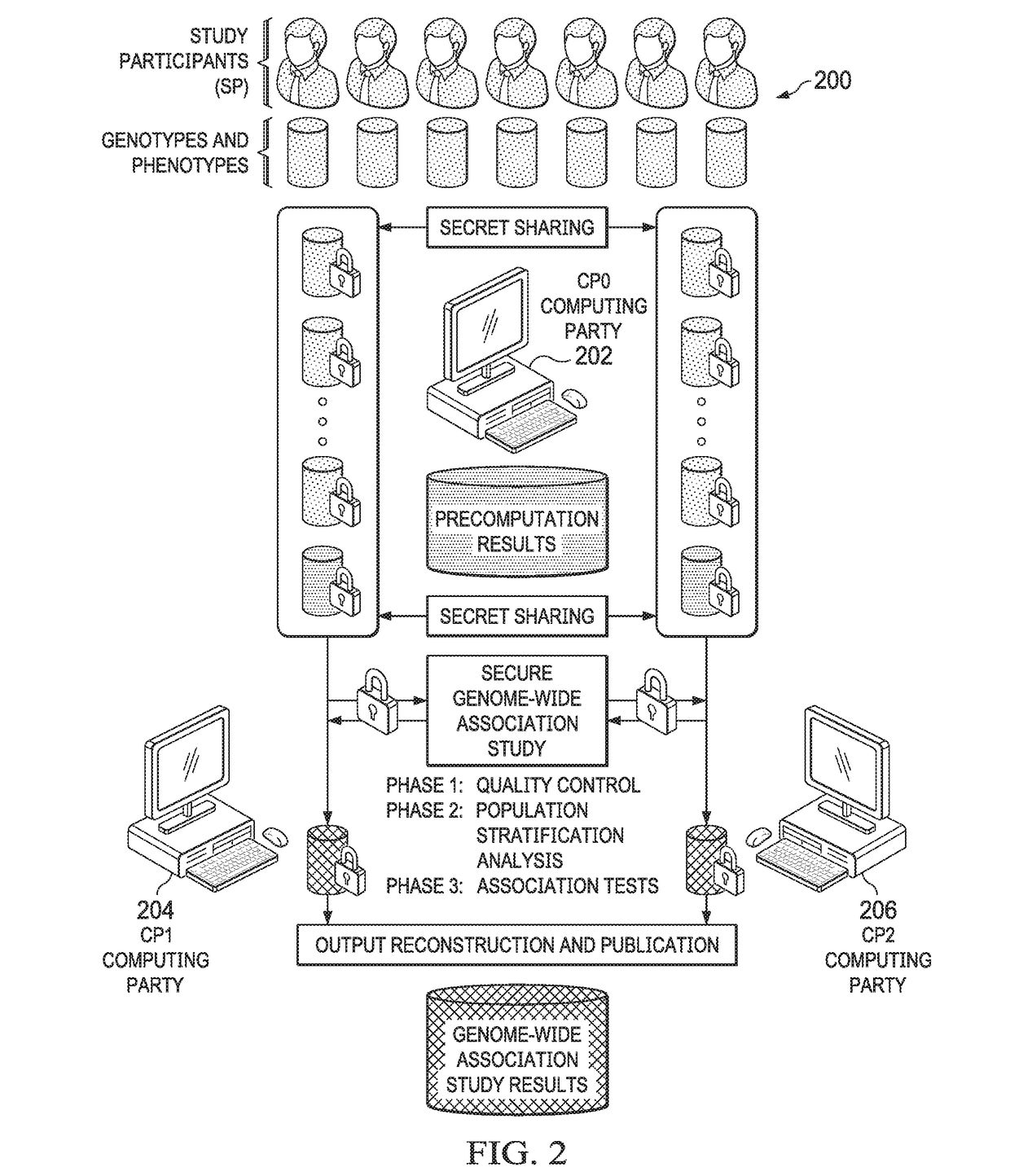
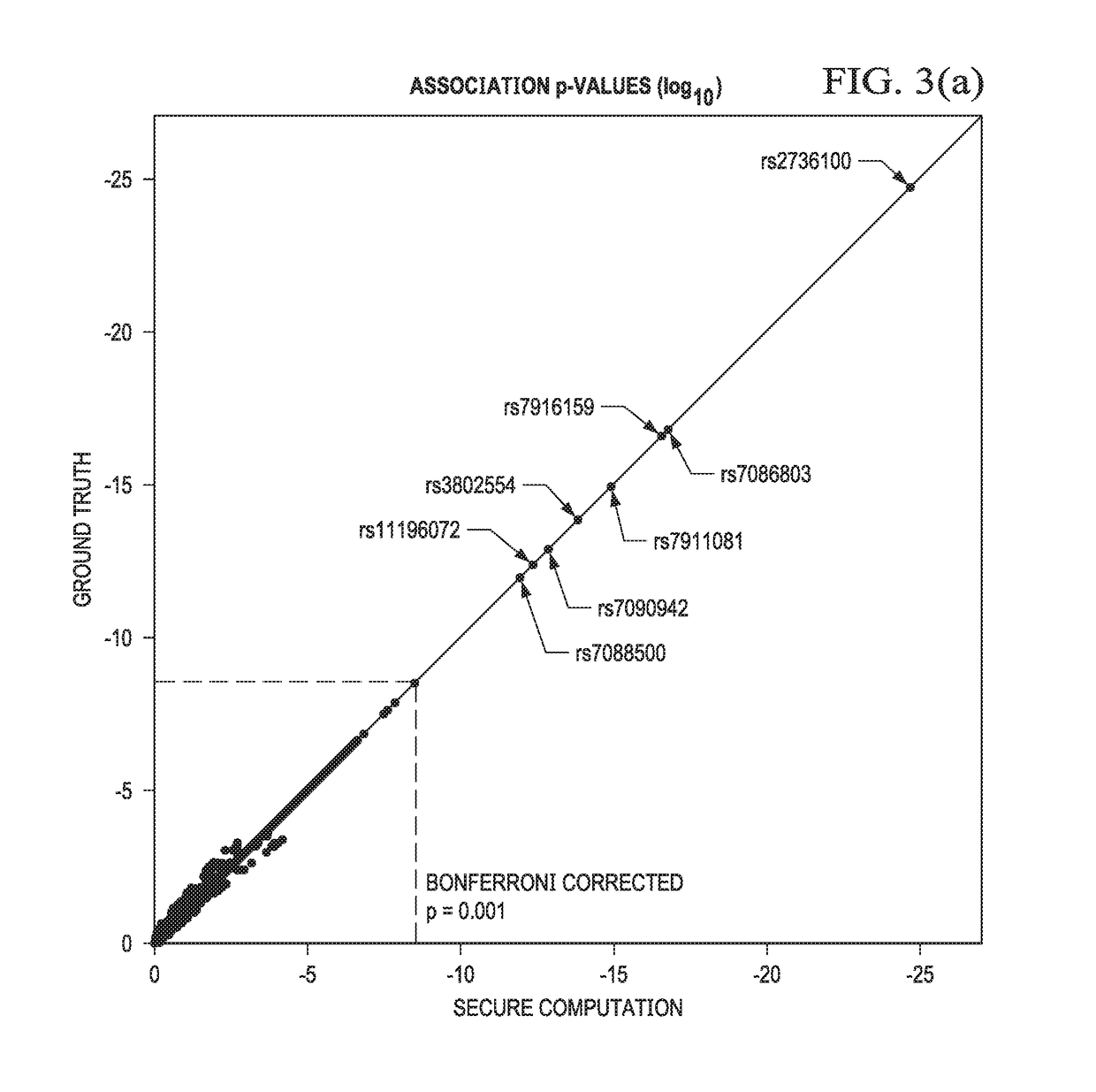
Secure genome crowdsourcing for large-scale association studies
ActiveUS20180373834A1Secure crowdsourcingPrivacy protectionProteomicsGenomicsData setIndividual study
Computationally-efficient techniques facilitate secure crowdsourcing of genomic and phenotypic data, e.g., for large-scale association studies. In one embodiment, a method begins by receiving, via a secret sharing protocol, genomic and phenotypic data of individual study participants. Another data set, comprising results of pre-computation over random number data, e.g., mutually independent and uniformly-distributed random numbers and results of calculations over those random numbers, is also received via secret sharing. A secure computation then is executed against the secretly-shared genomic and phenotypic data, using the secretly-shared results of the pre-computation over random number data, to generate a set of genome-wide association study (GWAS) statistics. For increased computational efficiency, at least a part of the computation is executed over dimensionality-reduced genomic data. The resulting GWAS statistics are then used to identify genetic variants that are statistically-correlated with a phenotype of interest.
Owner:CHO HYUNGHOON +2
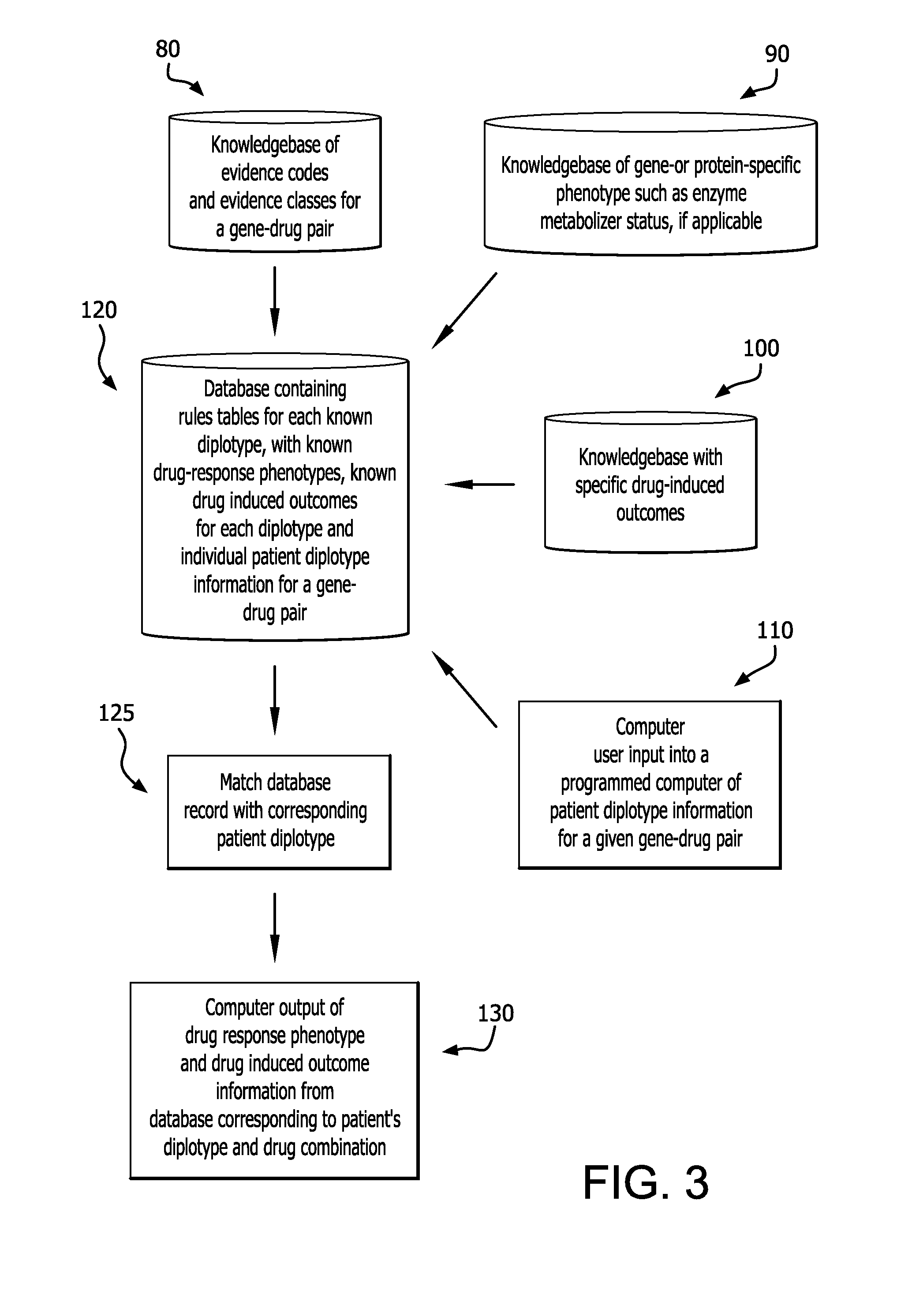
Method for translating genetic information for use in pharmacogenomic molecular diagnostics and personalized medicine research
A gene-drug specific system for classifying individual genetic variants based on strength-of-evidence of clinical utility from published scientific and clinical data that support their effect on modifying drug response and behavior. This allows categorization of the genetic variants into evidence classes that have a wide range of uses such as pharmacogenomic molecular diagnostics and personalized medicine research designed to guide the clinical implementation of PGx. Furthermore, this information can be combined with a knowledgebase of drug-response phenotypes, a knowlegebase of specific drug-induced outcomes and individual patient diplotype information for a gene-drug combination into a programmed computer to output corresponding patient-specific predicted drug responses.
Owner:CORIELL INST FOR MEDICAL RES
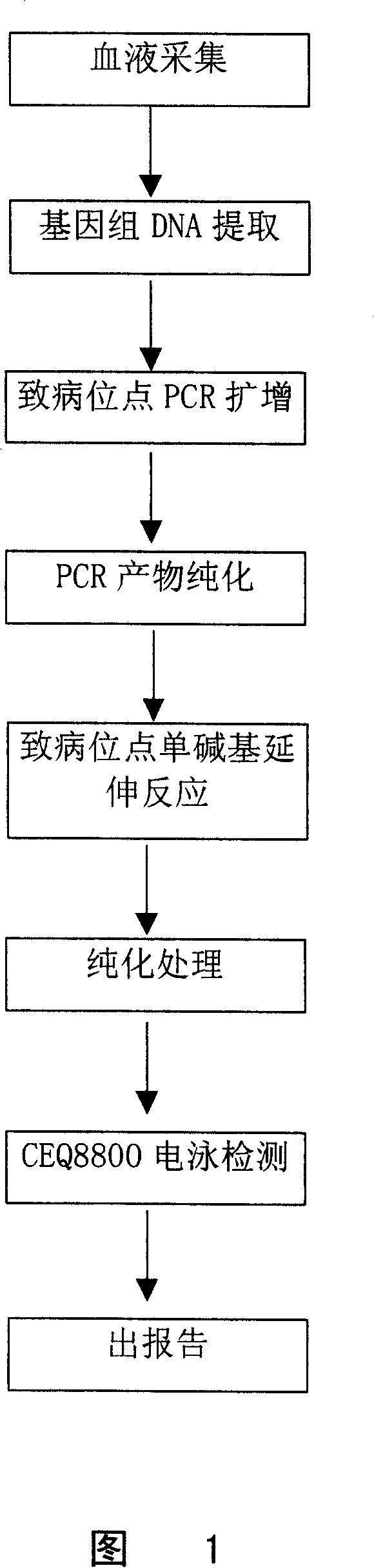
Method for detecting multiple endocrine adenoma II gene mutation
InactiveCN101148684AMicrobiological testing/measurementBiological testingRet geneMultiple endocrine adenomas
The present invention belongs to the field of biotechnology, and discloses one method of in vitro determining whether to have RET gene variation in the nucleic acid sample, one kit for determining RET gene variation and one process of obtaining RET gene amplifying product. The method of the present invention is accurate, simple, fast and stable.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
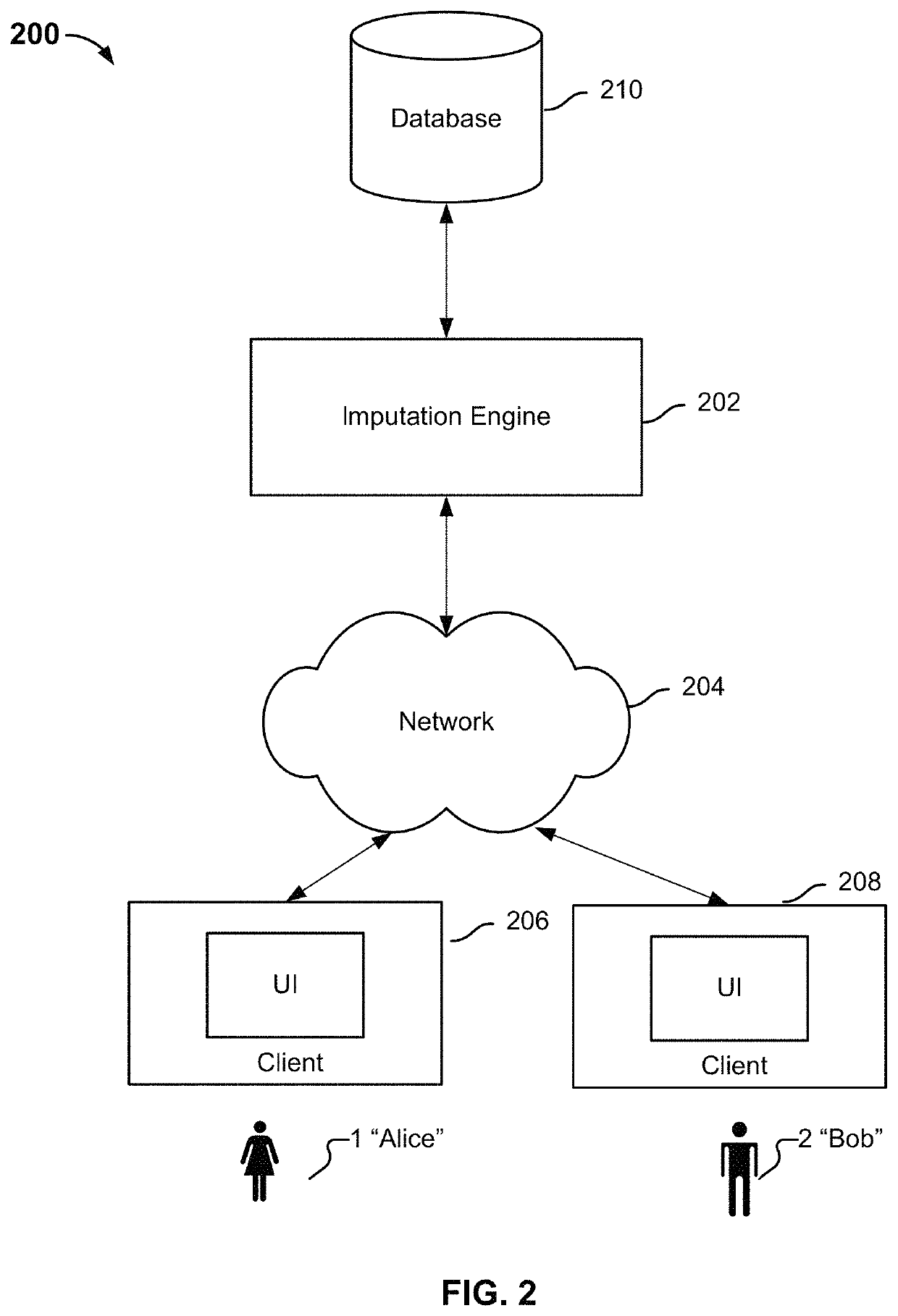
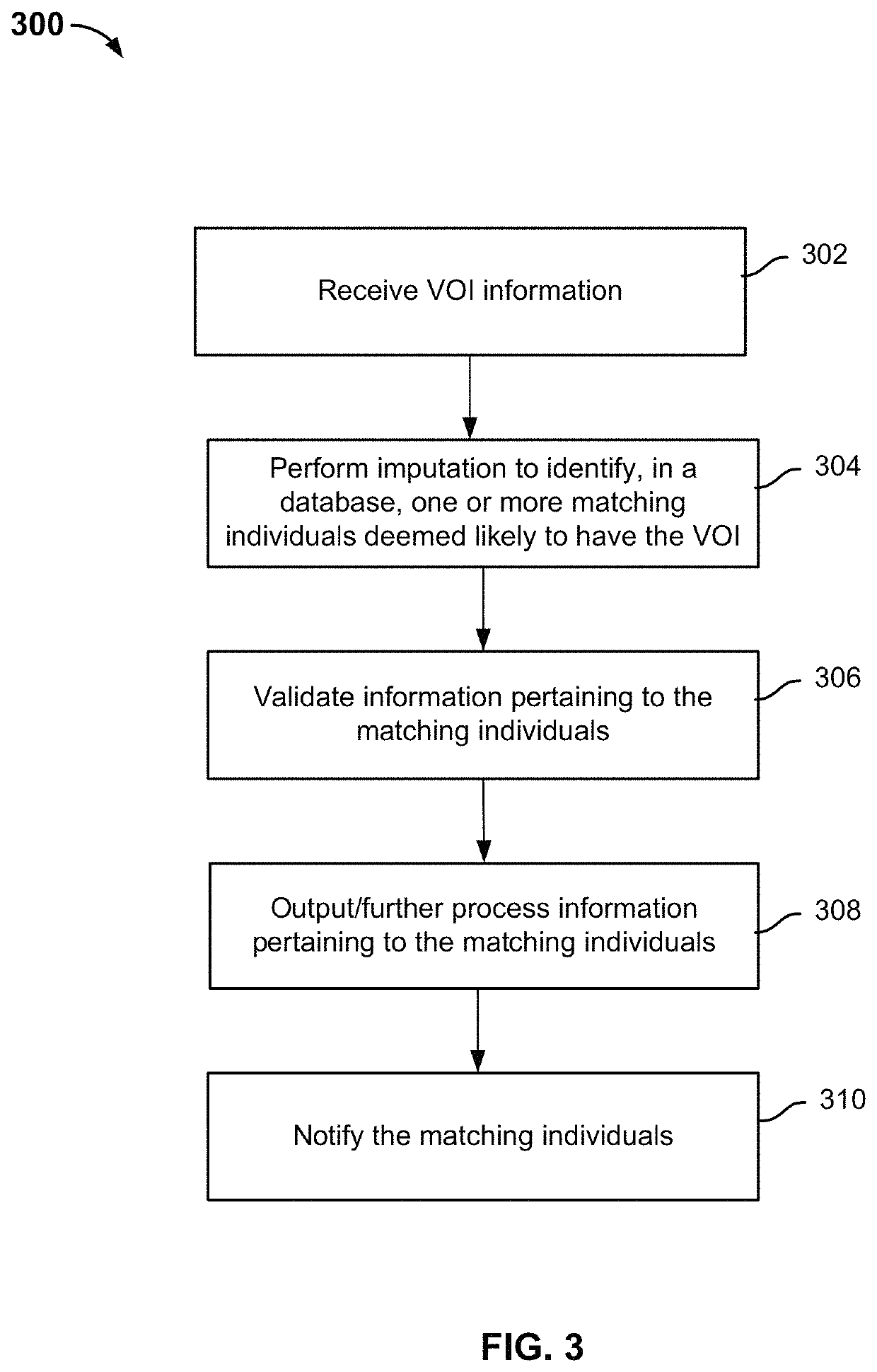
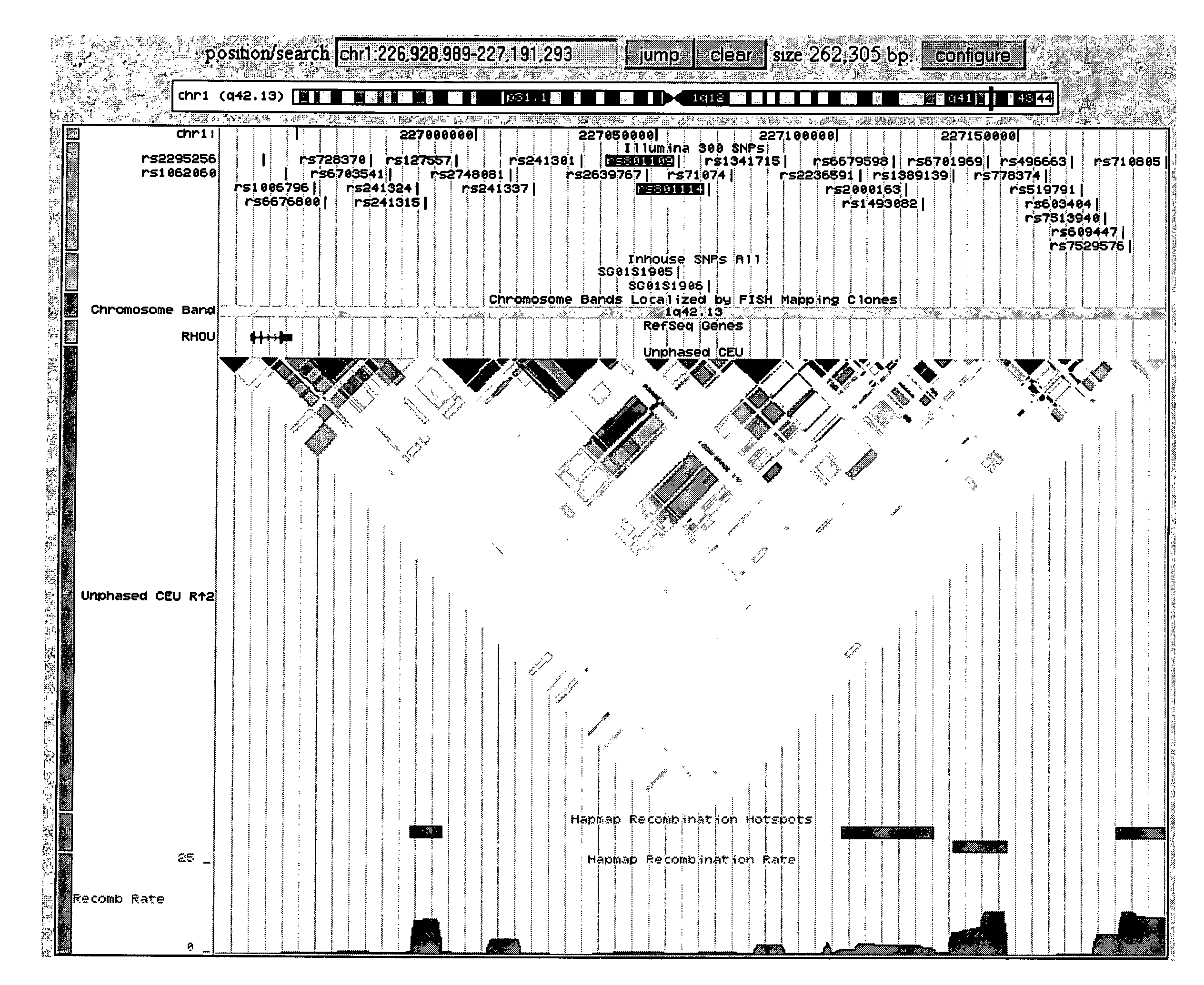
Identifying variants of interest by imputation
Processing genetic information comprises: receiving an input that includes information pertaining to a specific genetic variant; and identifying, in a database comprising genotype information of a plurality of candidate individuals, a matching individual imputed to have the specific genetic variant. The genotype information of the matching individual corresponding to the specific genetic variant is not directly assayed.
Owner:23ANDME
Method of detection of SP-A2 gene variants
The invention provides a method for predicting the susceptibility of an individual to pulmonary tuberculosis, the method comprising amplifying genomic DNA of pulmonary tuberculosis patients and normal control individuals using oligonuecleotide primers, sequencing the amplified PCR product and identifying the sequence variation computationally by comparing it with the already existing sequence of human SP-A2 gene.
Owner:COUNCIL OF SCI & IND RES
Genetic Variants Predictive of Cancer Risk in Humans
InactiveUS20120122698A1Reduce sensitivityMicrobiological testing/measurementLibrary member identificationComputerized systemBasal cell carcinoma
The present invention discloses genetic variants that have been found to be predictive of risk of particular forms of cancer, in particular basal cell carcinoma and cutaneous melanoma. The invention provides methods of predicting risk of developing such cancers, and other methods pertaining to risk management of cancer utilizing such risk variants. The invention furthermore provides kits and computer systems for use in such methods.
Owner:DECODE GENETICS EHF
Physiogenomic method for predicting diabetes and metabolic syndromes induced by psychotropic drugs
InactiveUS20090075254A1Microbiological testing/measurementFermentationSide effectPsychotropic medication
The invention is generally directed to a physiogenomic method for predicting diabetes and metabolic syndromes induced by psychotropic drugs. In one embodiment, the invention relates to the use of genetic variants of marker genes to predict the likelihood that an individual will experience undesirable metabolic side effects as a result of the use of a drug including, but not limited to, psychotropic drugs. The invention also relates to methods predicting the likelihood of diabetes and metabolic syndromes induced by the use of drugs with undesirable metabolic side effects.
Owner:GENOMAS
Disease risk factors and methods of use
ActiveUS20110166185A1Increased riskDecreased and increased riskBiocideNervous disorderDisease riskActive agent
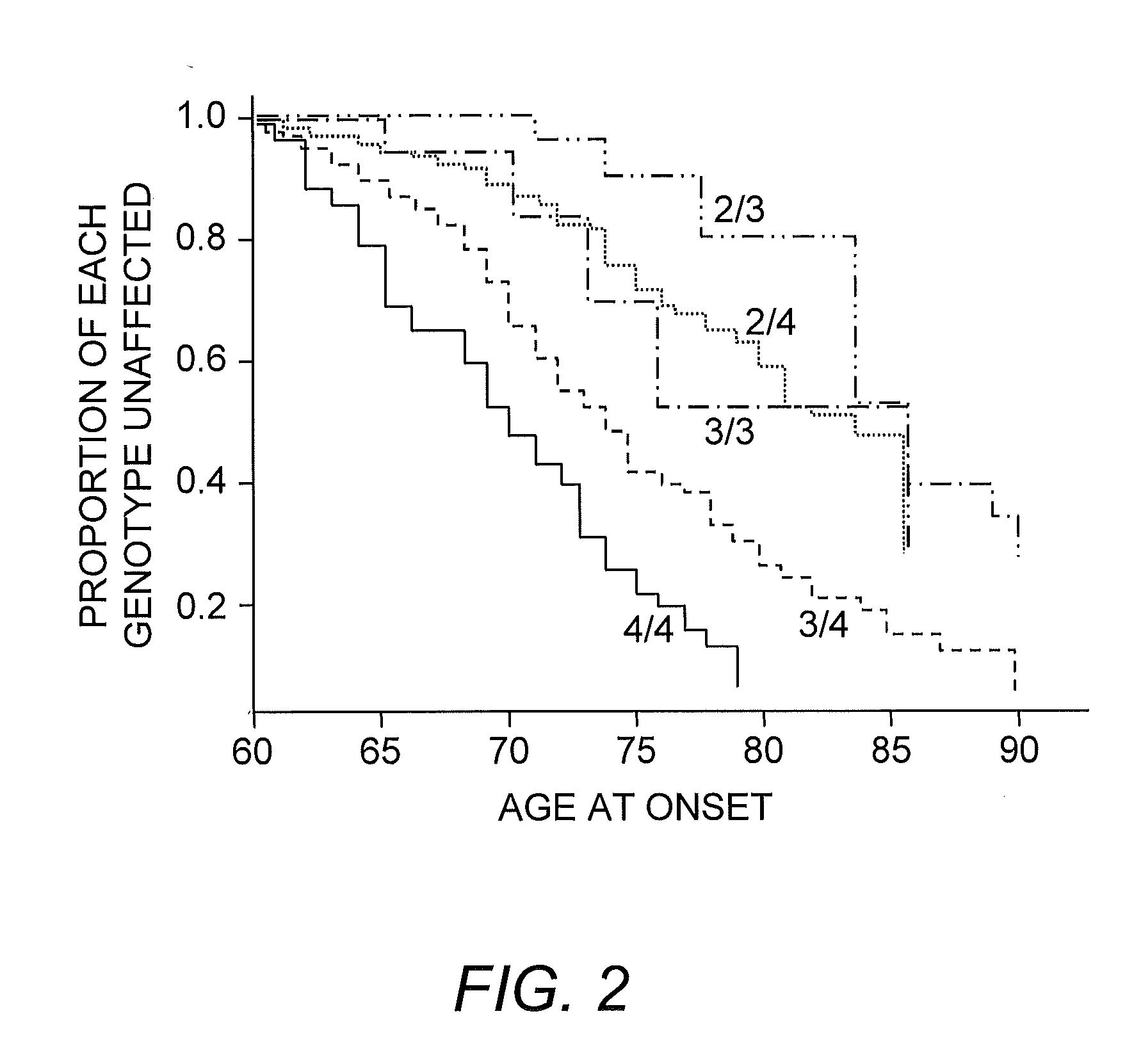
Provided herein are genetic variants associated with development of a condition of interest (e.g., Alzheimer's disease). Methods of treatment with an active agent (e.g., with a particular active agent and / or at an earlier age) is also provided, upon detecting a genetic variant described herein. In some embodiments, the genetic variant is a deletion / insertion polymorphism (DIP) of the TOMM40 gene.
Owner:ZINFANDEL PHARMA
Identifying variants of interest by imputation
Processing genetic information comprises: receiving an input that includes information pertaining to a specific genetic variant; and identifying, in a database comprising genotype information of a plurality of candidate individuals, a matching individual imputed to have the specific genetic variant. The genotype information of the matching individual corresponding to the specific genetic variant is not directly assayed.
Owner:23ANDME INC
Methods and compositions for enrichment of target polynucleotides
The invention provides methods, apparatuses, and compositions for high-throughput amplification sequencing of specific target sequences in one or more samples. In some aspects, barcode-tagged polynucleotides are sequenced simultaneously and sample sources are identified on the basis of barcode sequences. In some aspects, sequencing data are used to determine one or more genotypes at one or more loci comprising a causal genetic variant.
Owner:MYRIAD WOMENS HEALTH INC
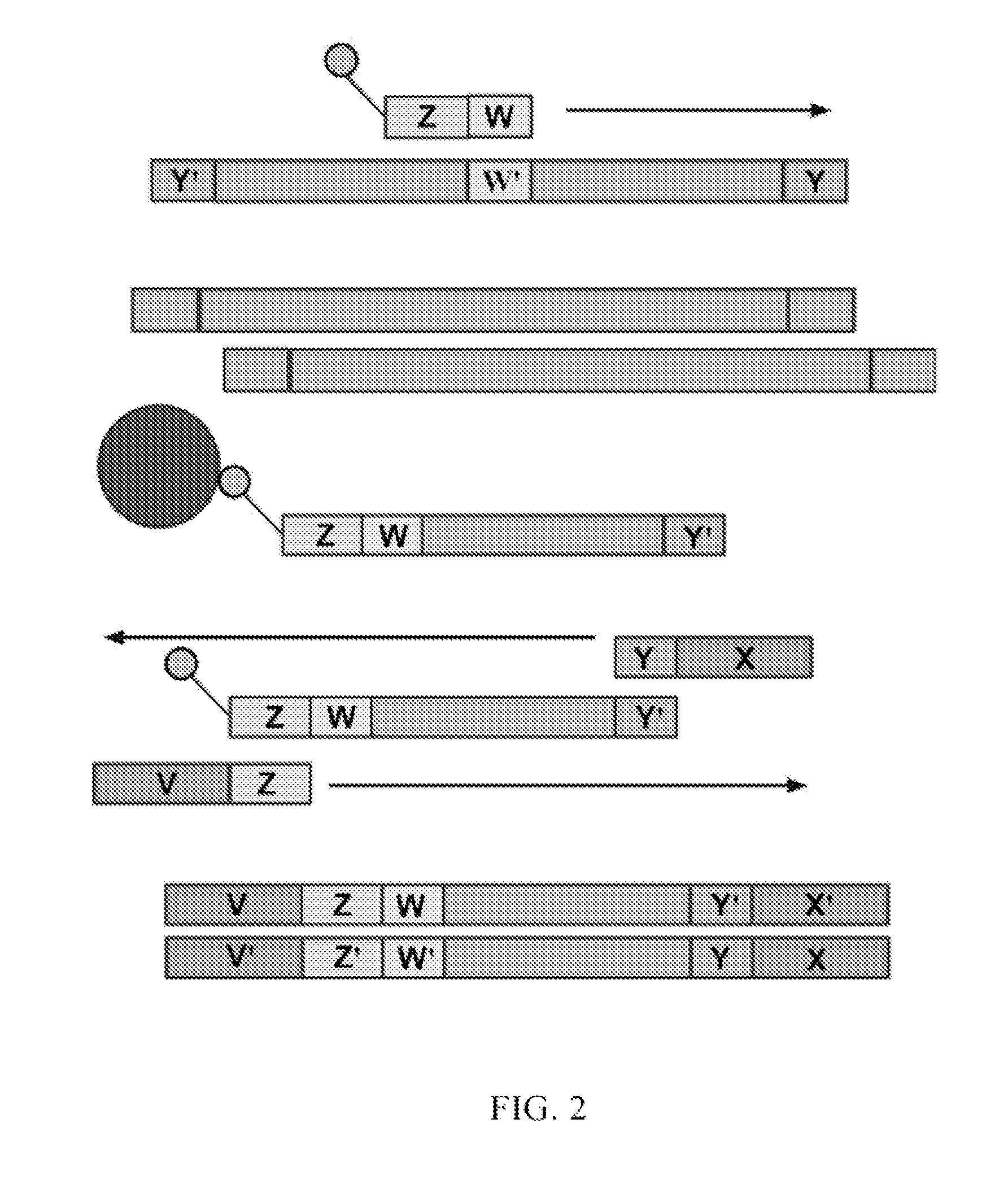
Multiplexed sequential ligation-based detection of genetic variants
ActiveUS20140024538A1Detected as abnormalityImprove throughputMicrobiological testing/measurementDNA preparationNucleotideGenetics
The present invention provides multiplexed sequential ligation-based analysis of genetic variants in a mixed sample, including copy number variations and single nucleotide polymorphisms. The invention employs the techniques of sequential ligation and amplification.
Owner:ROCHE MOLECULAR SYST INC
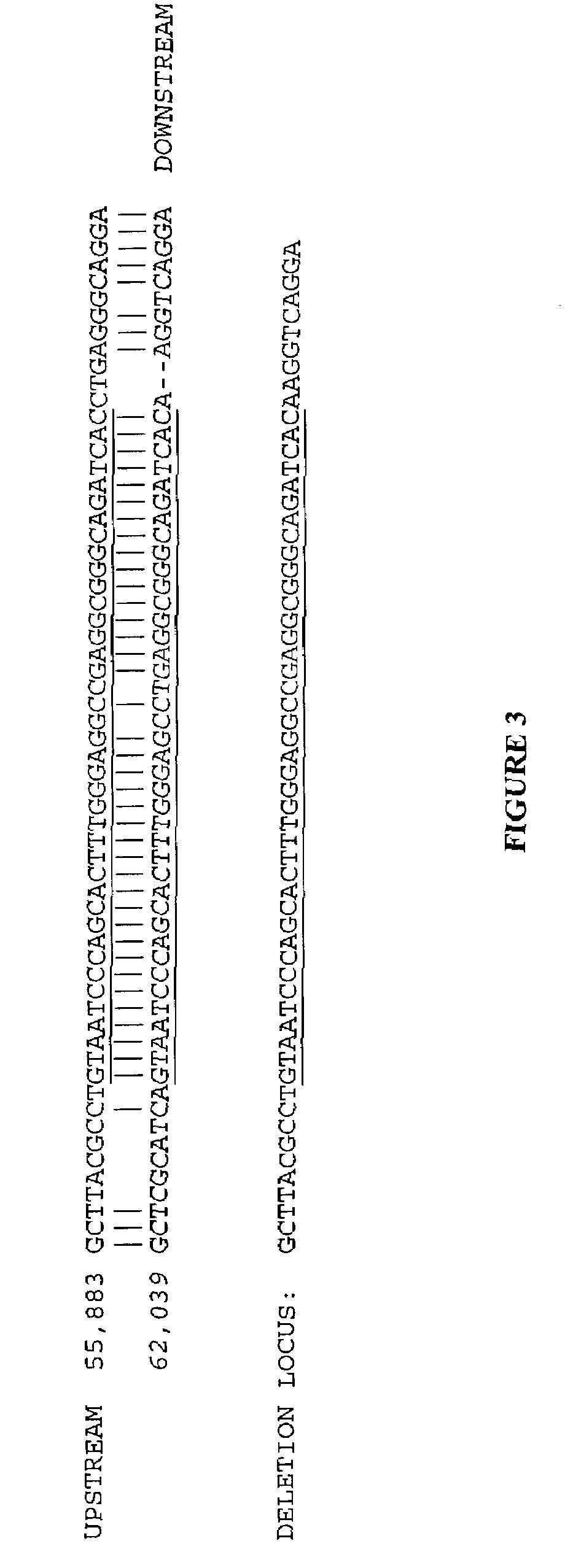
Large deletions in human BRCA1 gene and use thereof
Large deletions have been identified in the BRCA1 gene in patients. The large deletions predispose the patients to breast cancer and ovarian cancer. Thus, methods for detecting the genetic variants are provided which can be used in detecting a predisposition to cancer.
Owner:MYRIAD GENETICS
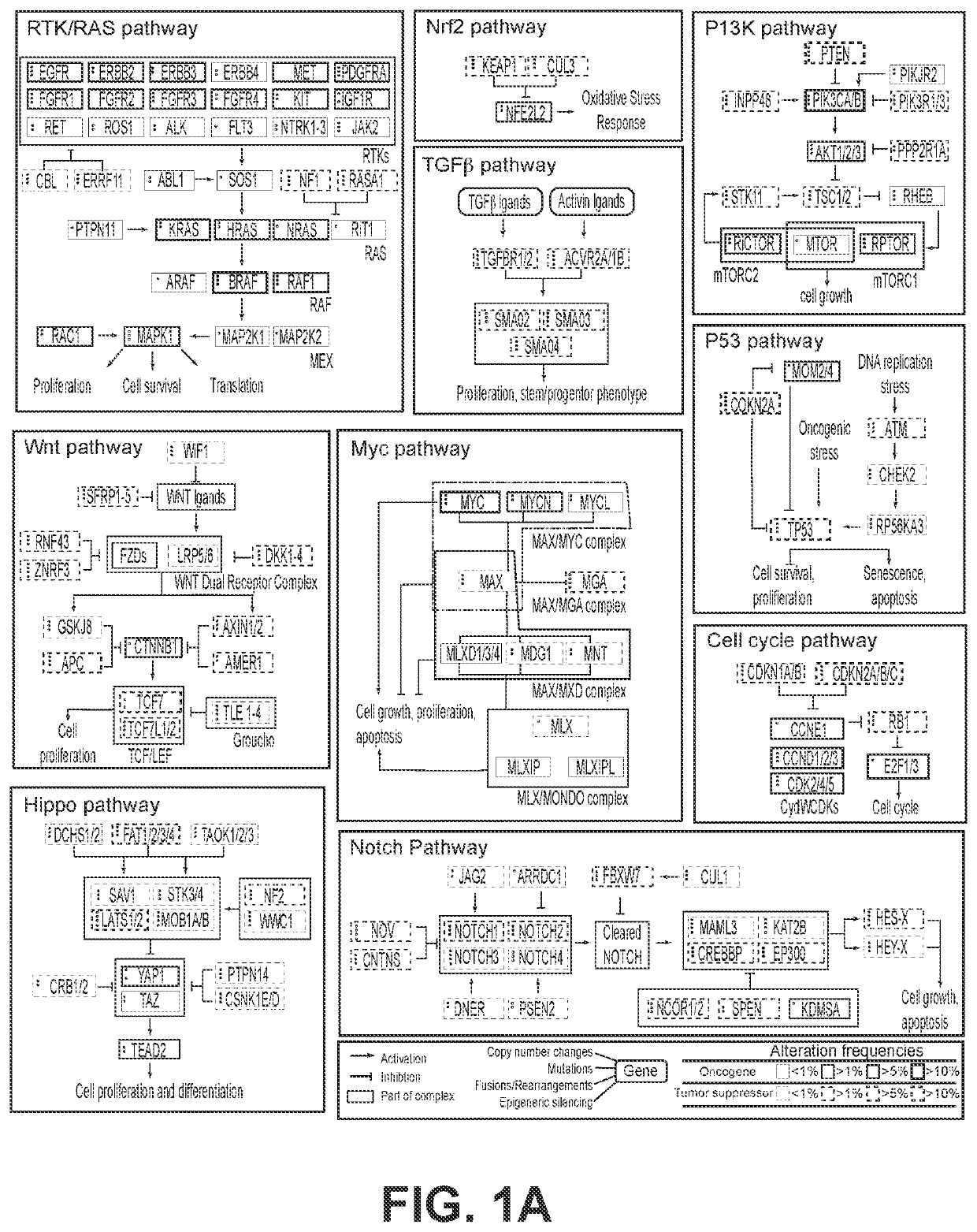
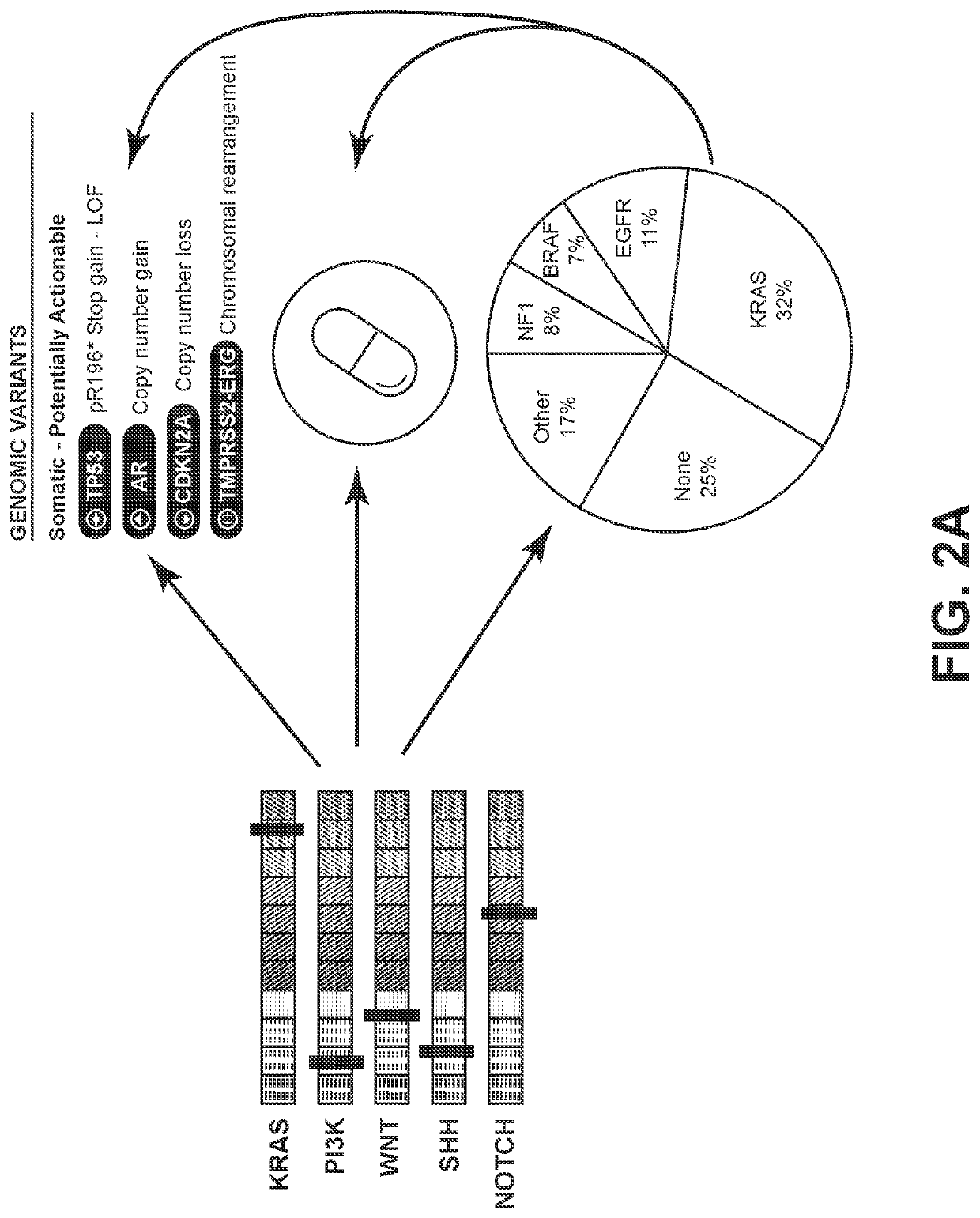
Systems and methods for detecting cellular pathway dysregulation in cancer specimens
Disclosed herein are systems, methods, and compositions useful for determining cellular pathway disruption comprising the use of RNA expression level information. This determined level of disruption can assist in the identification of genetic variants that alter pathway activity, to correlate these variants with disease state and disease progression, and to identify those therapeutics most likely to be effective and which should be avoided.
Owner:TEMPUS LABS
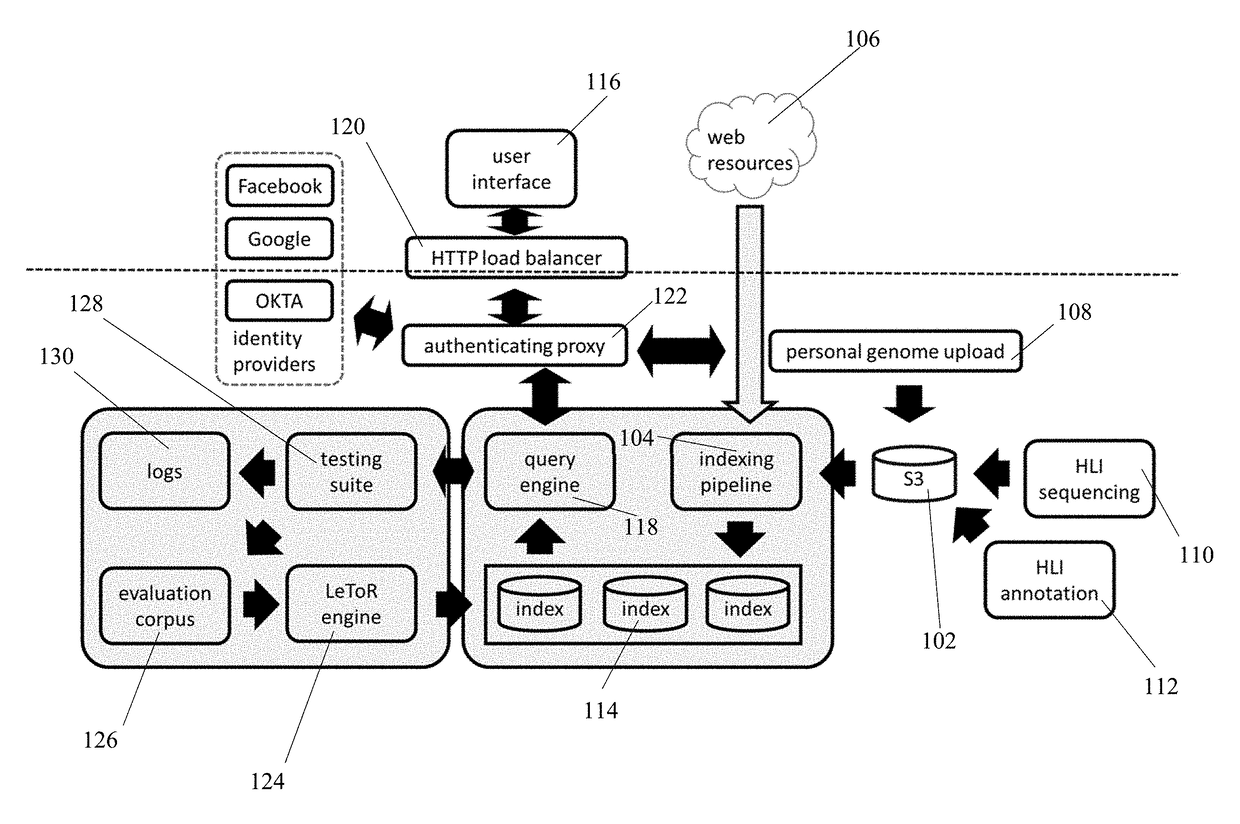
Genomic, metabolomic, and microbiomic search engine
InactiveUS20170270212A1Reduce utilizationImprovement in genomic queryingDigital data information retrievalBiostatisticsGenomicsGenomic data
Disclosed are systems, media, and methods for providing a genomic search engine application comprising: a plurality of indices, recorded in the computer storage, the indices comprising tokenized genomic data; a software module providing an indexing pipeline, the indexing pipeline ingesting genomic data and annotation associated with the genomic data, tokenizing the data while preserving gene names and gene variant names, and updating the indices with the tokenized data; and a software module presenting a user interface allowing a user to enter a user query; a software module providing a query engine, the query engine accepting the user query, selecting one or more relevant indices, and applying a ranking formula to the selected indices to return ranked results.
Owner:HUMAN LONGEVITY
Methods and compositions relating to the pharmacogenetics of different gene variants
InactiveUS20090017452A1Reduce Toxicity RiskAddress bad outcomesMicrobiological testing/measurementPharmacogeneticsGene product
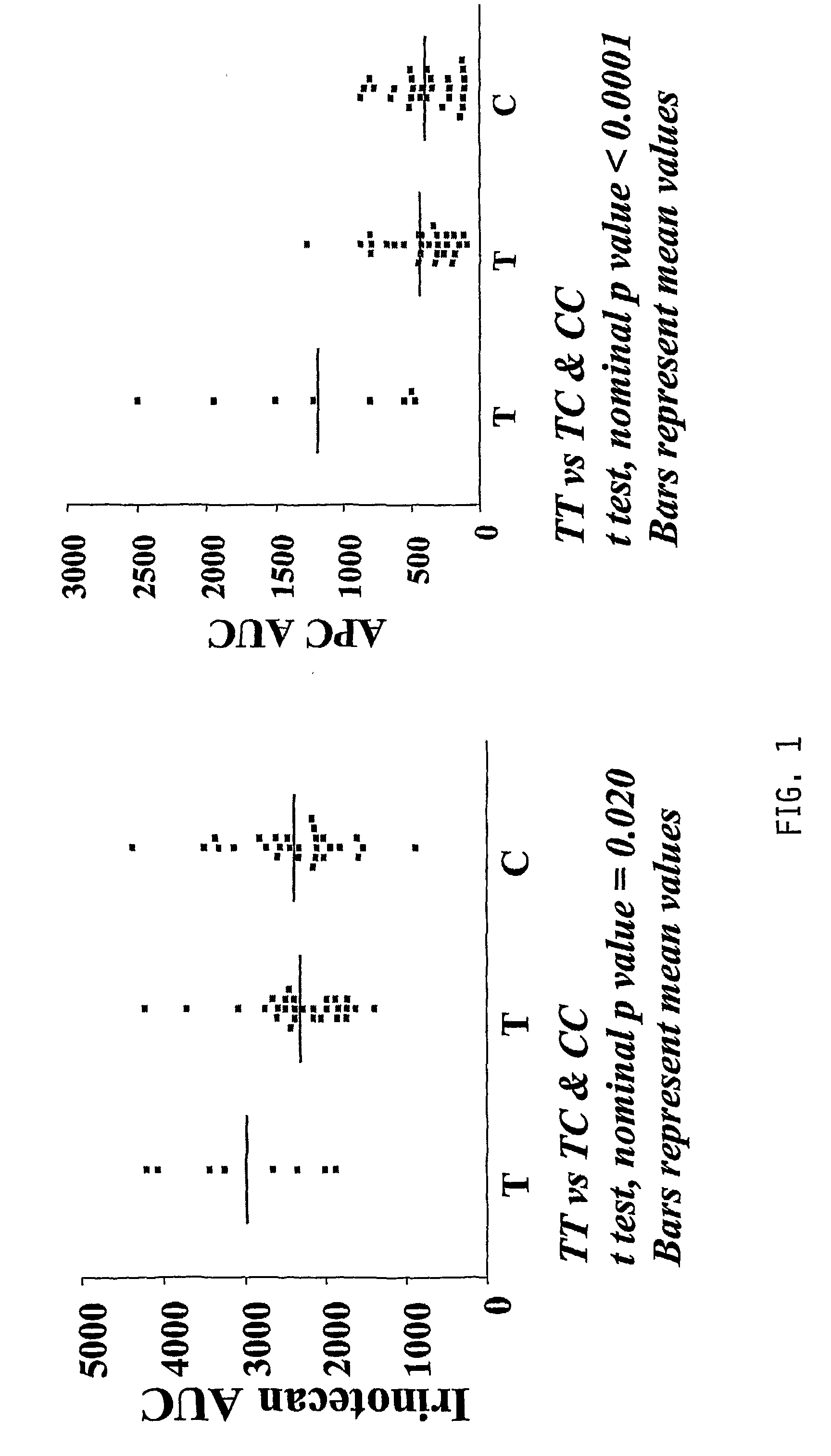
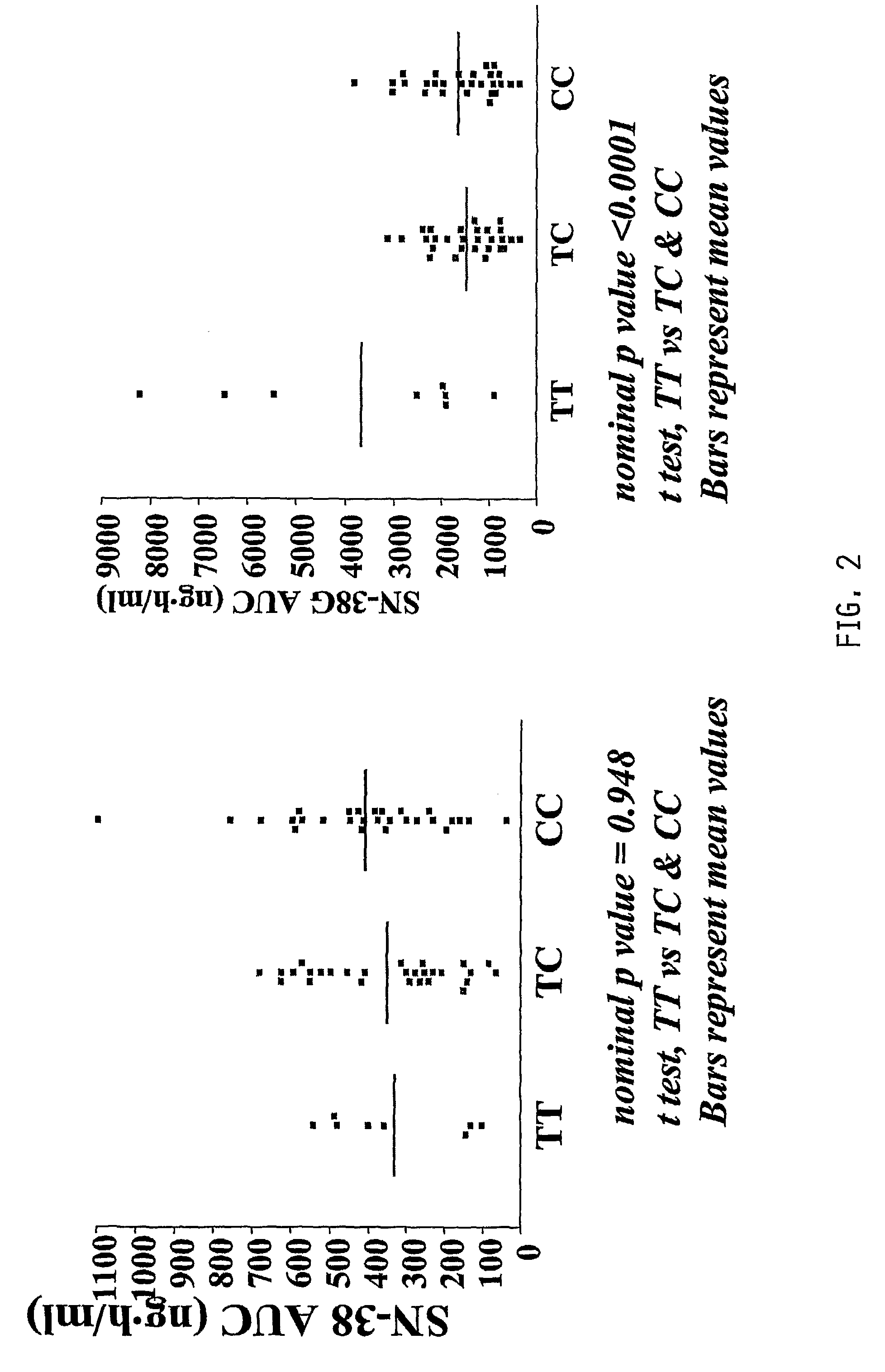
The present invention is directed to methods and compositions for determining the presence or absence of polymorphisms within an ABCC2, UGT1A1, and / or SLCO1B1 gene and correlating these polymorphisms with activity levels of their gene products and making evaluations regarding the effect on their substrates, particularly those substrates that are drugs. In addition, there are methods and compositions of evaluating the risk of an individual for developing toxicity or adverse event(s) to an ABCC2, UGT1A1, and / or SLCO1B1 substrate. In some embodiments, the invention concerns methods and compositions for determining the presence or absence of ABCC2 3972C>T variant and predicting or anticipating the level of activity of ABCC2 and determining dosages of an ABCC2 drug substrate, such as irinotecan, in a patient. Such methods and compositions can be used to evaluate whether irinotecan-based therapy, or therapy involving other ABCC2 substrates, may pose toxicity problems if given to a particular patient or predicting their efficacy. Alterations in suggested therapy may ensue based on genotyping results.
Owner:RGT UNIV OF CALIFORNIA +1
Classification and Actionability Indices for Lung Cancer
The disclosure provides compositions, kits, and methods for detecting a plurality of genes and associated variants in a sample from a subject with lung cancer. The compositions, kits, and methods include a set of oligonucleotides, typically primers and / or probes that can hybridize to identify a gene variant. The methods disclosed herein provide for a mutation status of a tumor to be determined and subsequently associated with an actionable treatment recommendation.
Owner:LIFE TECH CORP
Genetic and environmental risk engine and methods thereof
Risk engines and related methods may benefit patients and their physicians. For example, patients may benefit from being able to determine their personal genetic, environmental, and behavioral risks. Moreover, physicians may be able to provide statistically-driven individual recommendations based on a risk engine's determination of such risks. A method can include selecting one or more candidate genetic variants associated with a phenotype from the scientific literature. The method can also include scoring a genetic association between the one or more candidate genetic variants and the phenotype. The method can further include selecting one or more high-scoring genetic variants. Selecting a best genetic variant within each of at least one linkage disequilibrium (LD) block can also be included in the method. The method can additionally include calculating risk associated with the best genetic variant from the at least one LD block.
Owner:RR HEALTH INC
DNA polymerase with increased gene mutation specificity and PCR buffer composition for increasing activity thereof
ActiveCN109251907AIncreased efficiency of gene variant-specific amplificationHigh match extension selectivityMicrobiological testing/measurementTransferasesA-DNATransgene
The present invention relates to a DNA polymerase having increased gene mutation specificity and a PCR buffer composition for increasing activity of the DNA polymerase. More specifically, provided, inthe present invention, are a DNA polymerase in which a mutation is induced at a specific amino acid position to increase gene mutation specificity, a nucleic acid sequence encoding the polymerase, avector comprising the nucleic acid sequence, and a host cell transformed with the vector. In addition, provided is a method for in vitro detecting one or more gene mutations or SNPs in one or more templates by using a DNA polymerase having increased gene mutation specificity, a composition for detecting a gene mutation or SNP comprising the DNA polymerase, and a PCR kit comprising said composition. Furthermore, provided are a PCR buffer composition for increasing the activity of a DNA polymerase having increased gene mutation specificity, a PCR kit for detecting a gene mutation or SNP comprising the PCR buffer composition and / or the DNA polymerase having increased gene mutation specificity, and a method for in vitro detecting one or more gene mutations or SNPs in one or more templates by using the kit.
Owner:GENECAST CO LTD
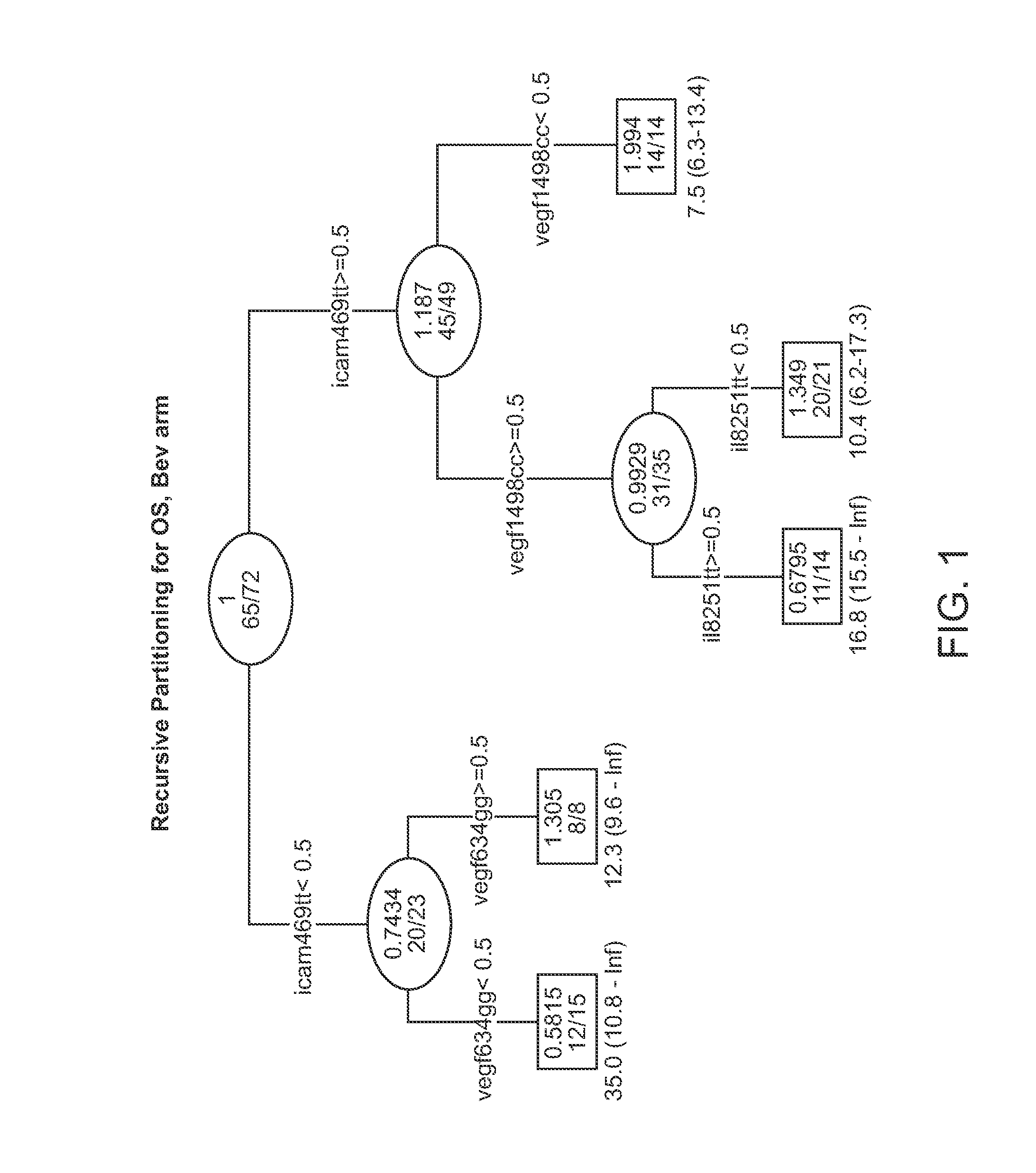
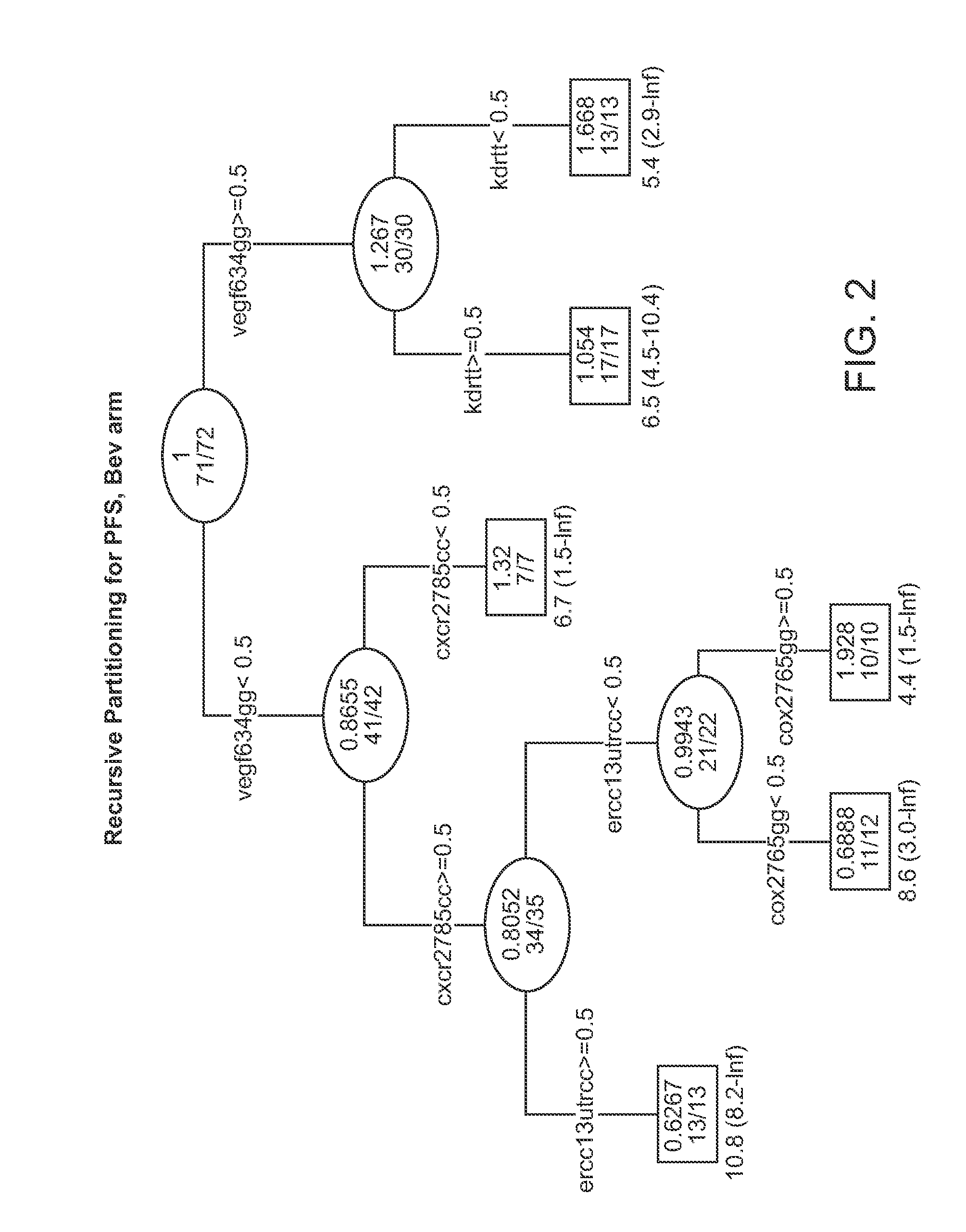
Genetic variants in angiogenesis pathway associated with clinical outcome
InactiveUS20120100134A1Heavy metal active ingredientsMicrobiological testing/measurementGenotype determinationMedicine
The invention provides methods for determining the clinical outcomes for treatment with various treatment regimens available to cancer patients based on genotypes of the patients for genetic polymorphism markers. The invention also provides kits for making the determination.
Owner:UNIV OF SOUTHERN CALIFORNIA
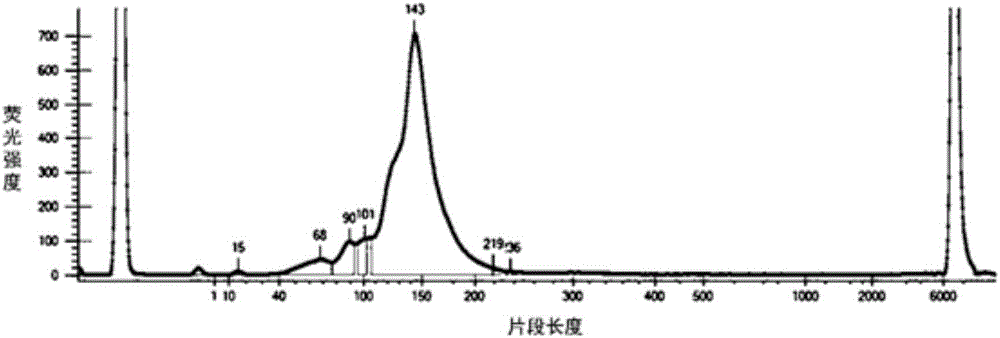
Quality control method for detecting human EGFR (Epidermal Growth Factor Receptor) gene variation based on high-throughput sequencing and kit
InactiveCN106636404AImprove efficiencyReduce testing quality control costsMicrobiological testing/measurementDNA preparationSanger sequencingPositive control
The invention discloses a quality control method for detecting human EGFR (Epidermal Growth Factor Receptor) gene variation based on high-throughput sequencing and a kit, and applications thereof. The quality control method comprises the following steps: extracting a plurality of genome DNAs of an EGFR gene variation-positive human tumor cell line; measuring variation positive sites as positive control sites through a Sanger sequencing method; fragmenting the genome DNAs and mixing according to a certain proportion to obtain a quality control product which can be applied high-throughput sequencing detection of human EGFR gene variation. The kit comprises the human EGFR gene variation detection quality control product.
Owner:3D BIOMEDICINE SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com