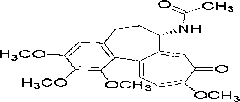
Application of colchicin in preparing cholestatic liver disease drug
A technology of cholestasis and colchicine, which is applied in the application field of colchicine in the preparation of drugs for the treatment of cholestatic liver disease, can solve the problems of no colchicine, etc., protect bile duct epithelial cells, reduce liver damage, alleviate The effect of liver cell necrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, to α-naphthalene isothiocyanate (ANIT) induced rat cholestasis effect
[0048] The cholestasis model induced by α-naphthalene isothiocyanate (ANIT) is a classic model for studying intrahepatic cholestasis. ANIT-induced cholestasis in rats occurred after 12 hours of intragastric administration of ANIT 50-100mg / kg, and liver damage and jaundice occurred 24 hours later. Experimental scheme: 30 220-250g male Wistar rats were randomly divided into 5 groups, 6 in each group: normal control group, model group (ANIT 50mg / kg gavage), melatonin 50mg / kg gavage treatment group, autumn Narcisine 50 and 100μg / kg intraperitoneal injection treatment group. The treatment group was given prophylactic administration for 5 days, fasted overnight after the 5th day, and was given ANIT for modeling, and then 0.5ml of orbital blood was collected 48 hours after the modeling to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP...
Embodiment 2
[0051] Embodiment 2, to 17α-ethinyl estradiol (EE)-induced rat cholestasis effect
[0052] The cholestasis induced by 17α-ethinylestradiol (EE) was used to establish the model. EE modeling is a commonly used animal model for studying cholestasis during pregnancy. Subcutaneous injection of EE 5mg / kg for 5 consecutive days will cause cholestasis. Experimental scheme: 30 male rats were randomly divided into the following 5 groups according to body weight, 6 rats in each group: normal control group, model group (EE 5mg / kg, sc), ursodeoxycholic acid 50mg / kg gavage treatment group, Colchicine 50 and 100μg / kg intraperitoneal injection treatment group. Among them, EE was administered for 5 days for modeling, colchicine was given 1 hour before modeling, fasted overnight after the last administration, and 1ml of orbital blood was collected to measure ALT, AST, ALP, GGT, TBIL and TBA. The livers were fixed in neutral formalin, embedded in paraffin to make tissue sections, and the expre...
Embodiment 3
[0055] Example 3. Effect on extrahepatic cholestasis caused by bile duct ligation
[0056] The extrahepatic cholestasis induced by bile duct ligation was used to establish the model. Cholestatic jaundice occurs on the second day after common bile duct ligation in rats. The rat bile duct ligation model is a commonly used animal model for studying extrahepatic stasis jaundice. Experimental scheme: 32 SD rats, half male and half female, were anesthetized by intraperitoneal injection of 400 mg / kg chloral hydrate. Incision was made along the abdominal linea alba, the common bile duct was separated, double ligated and cut from the middle, and 6 rats in the control group were sham-operated. Method, only the bile duct was separated without ligation, and sutured with surgical thread. Twenty-four survived two days later. Divided into: control group, model group, ursodeoxycholic acid 50mg / kg intragastric administration treatment group, colchicine 50 and 100μg / kg intraperitoneal injecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com