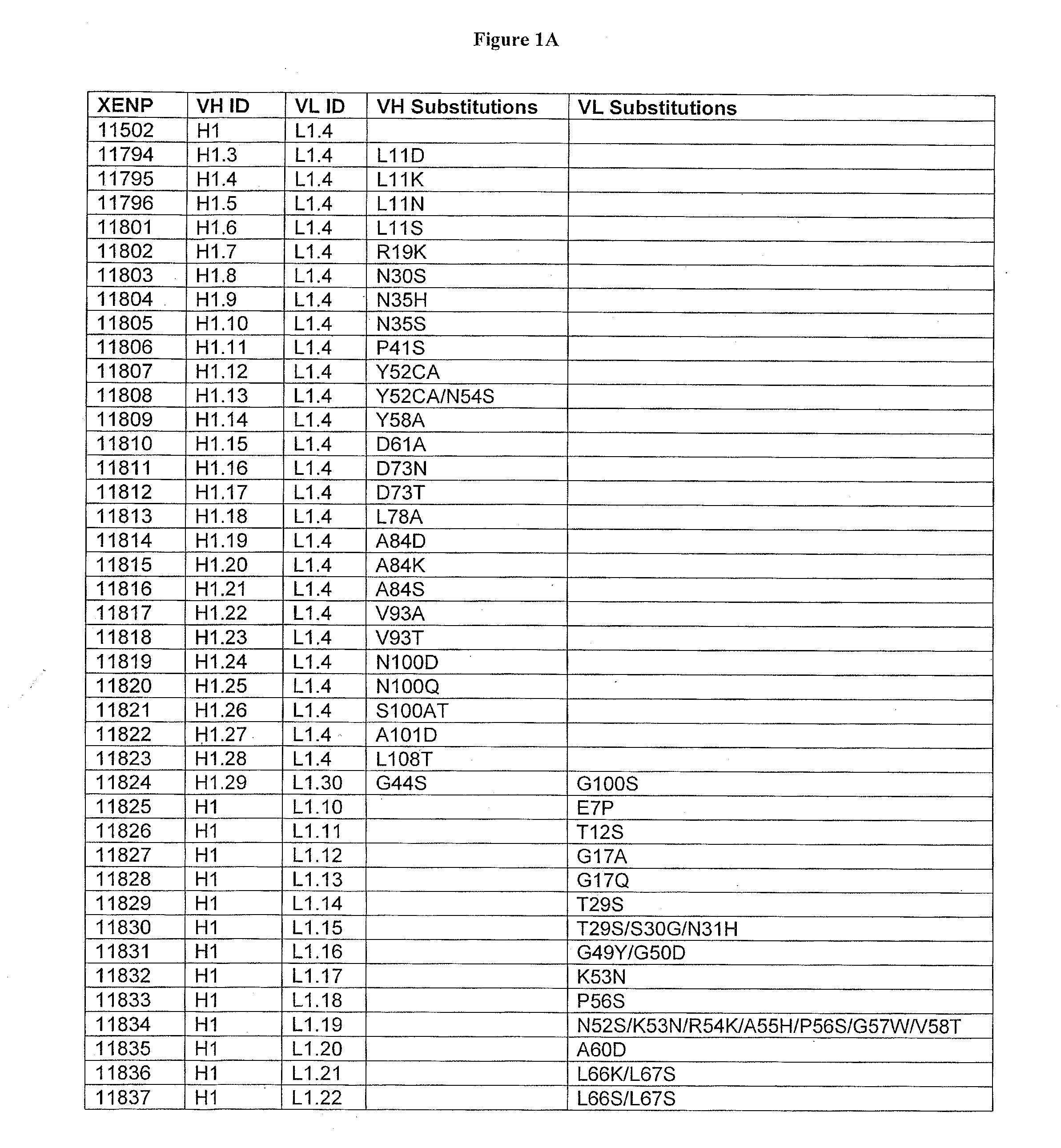
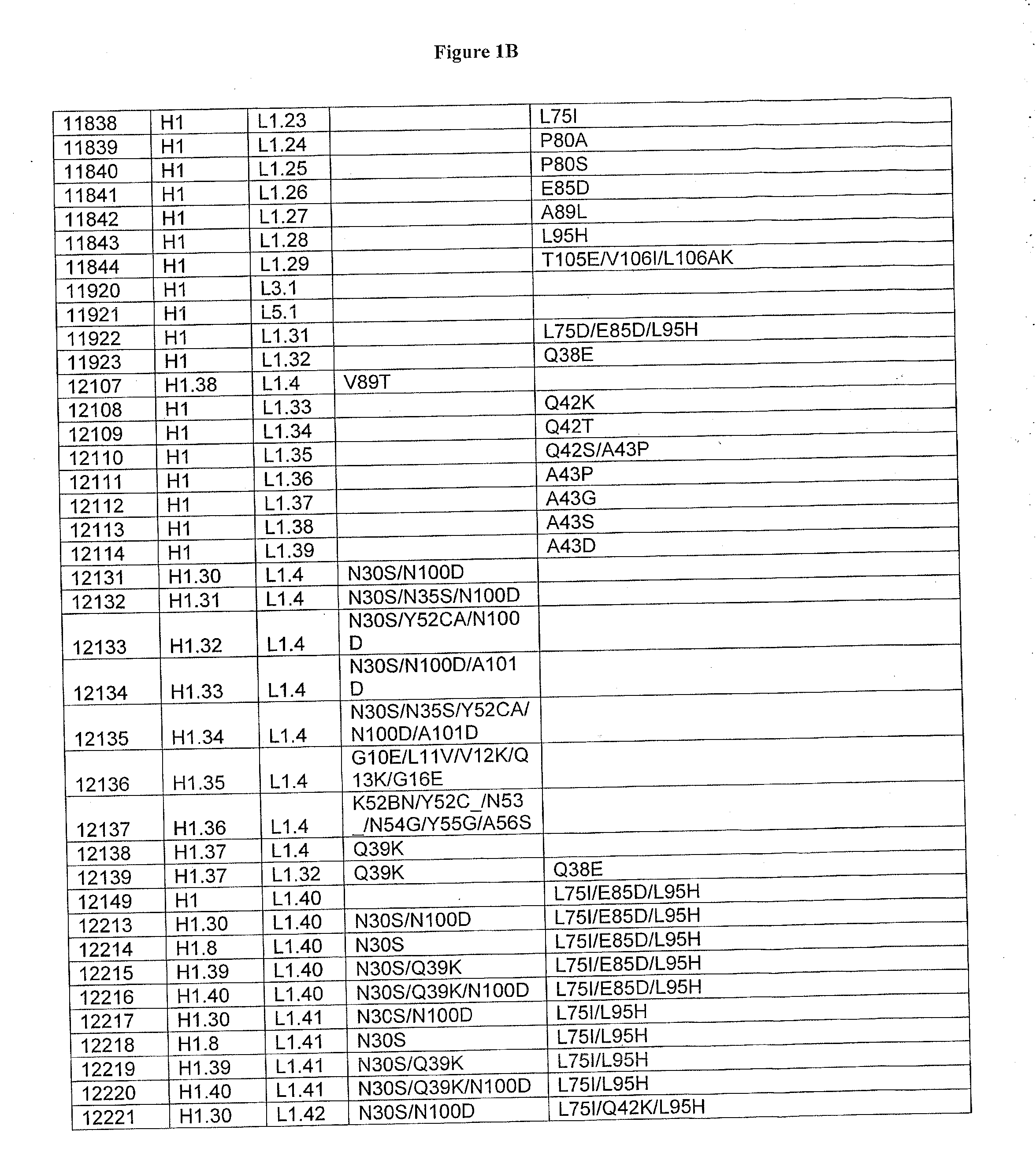
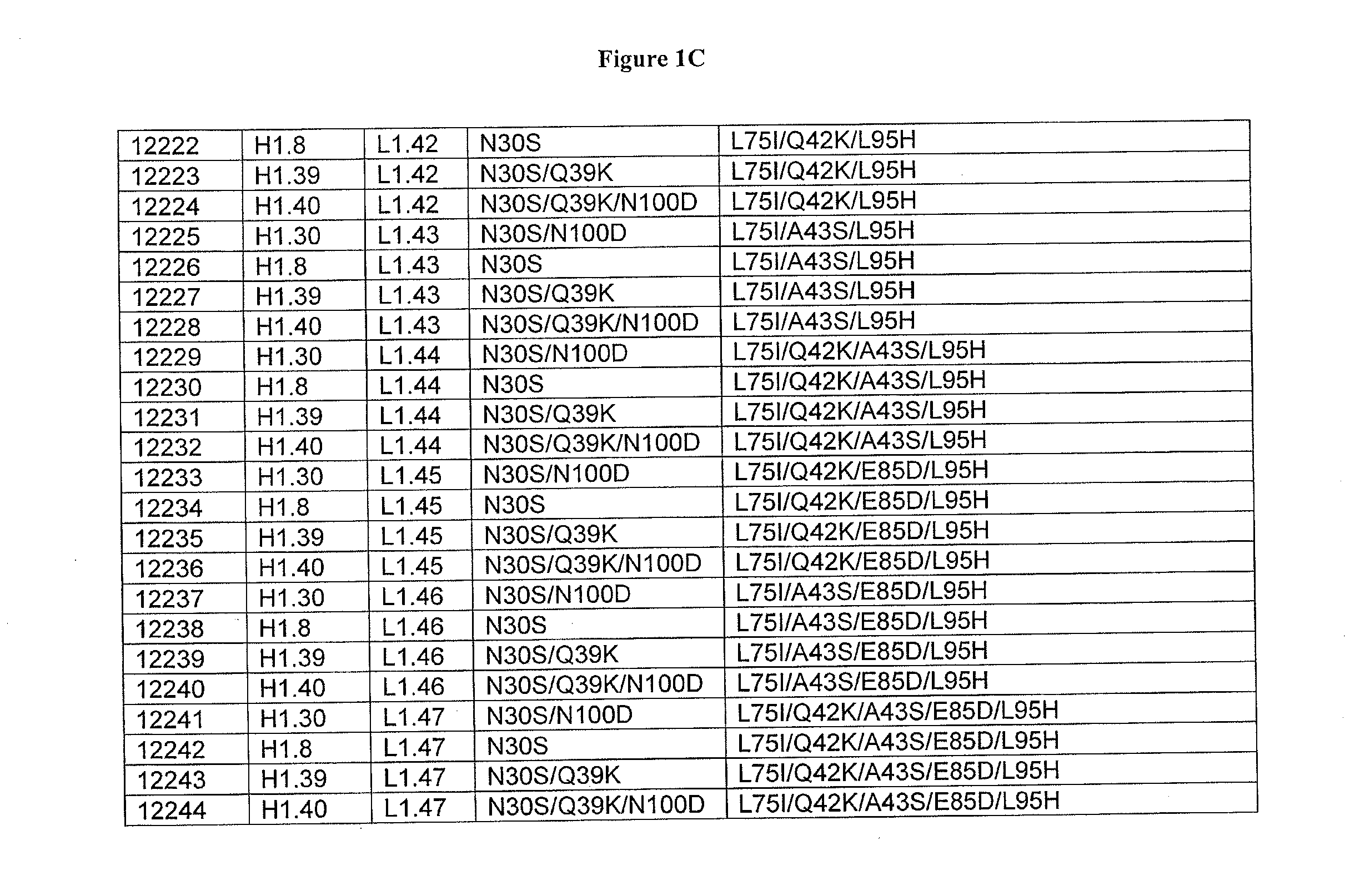
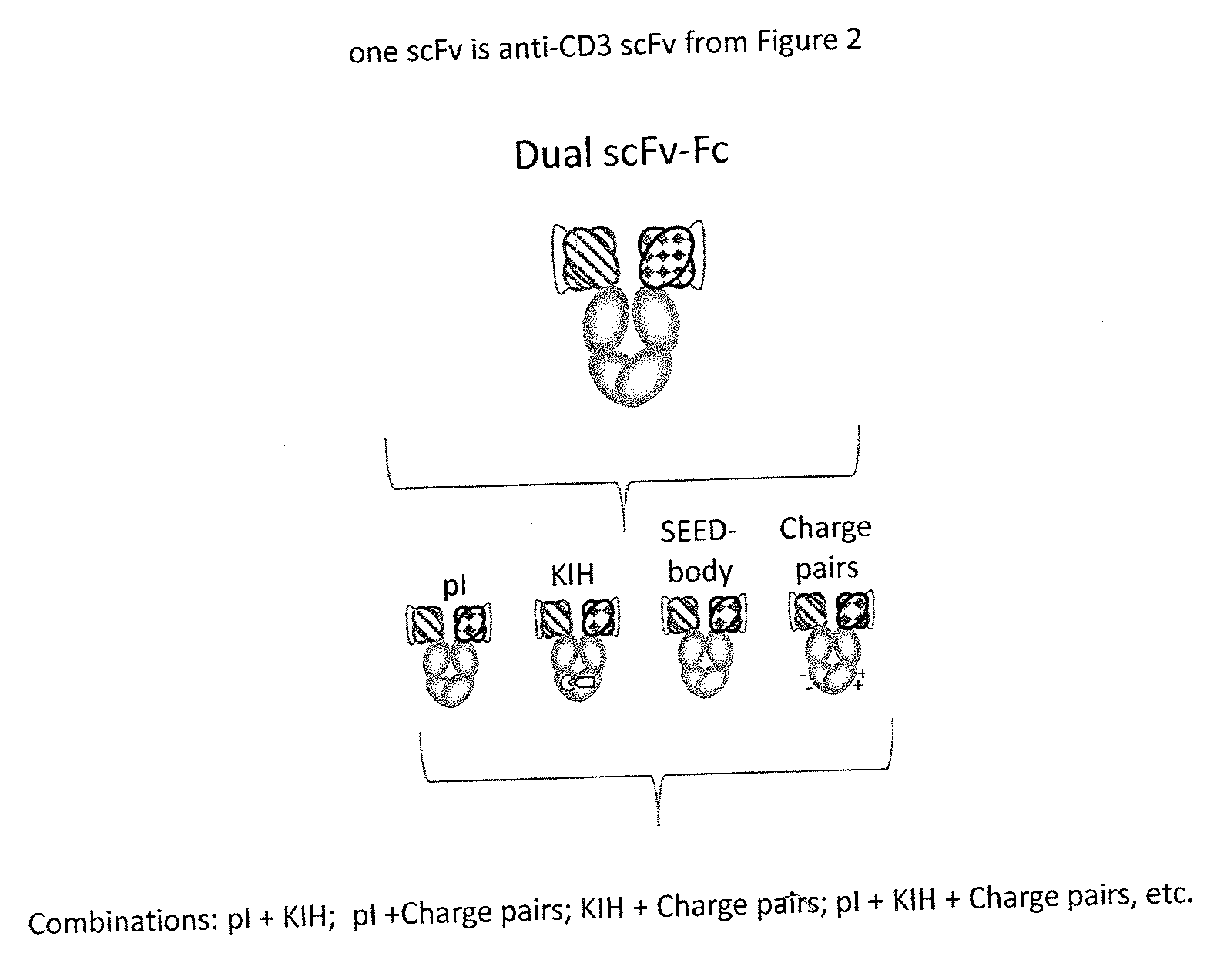
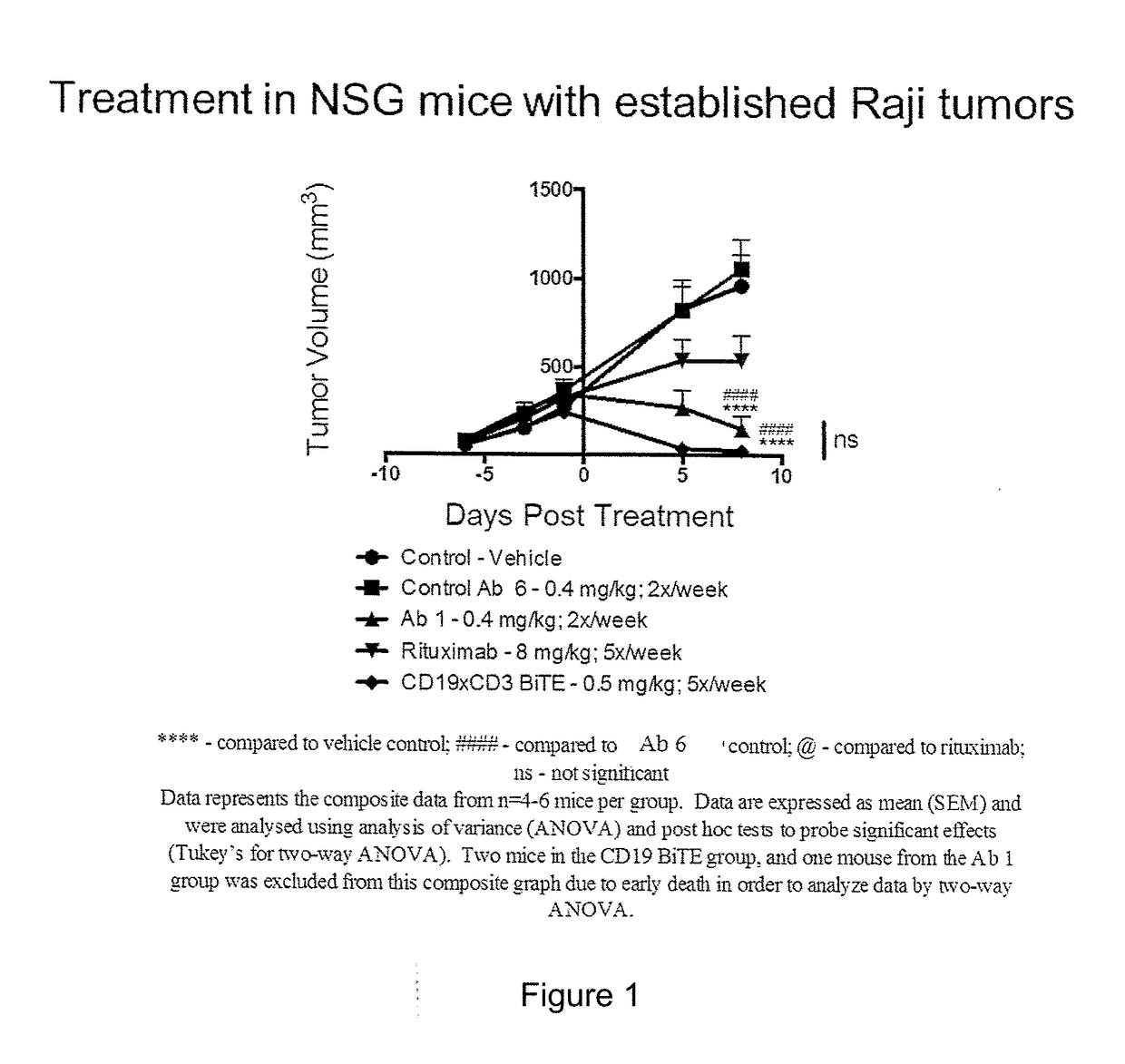
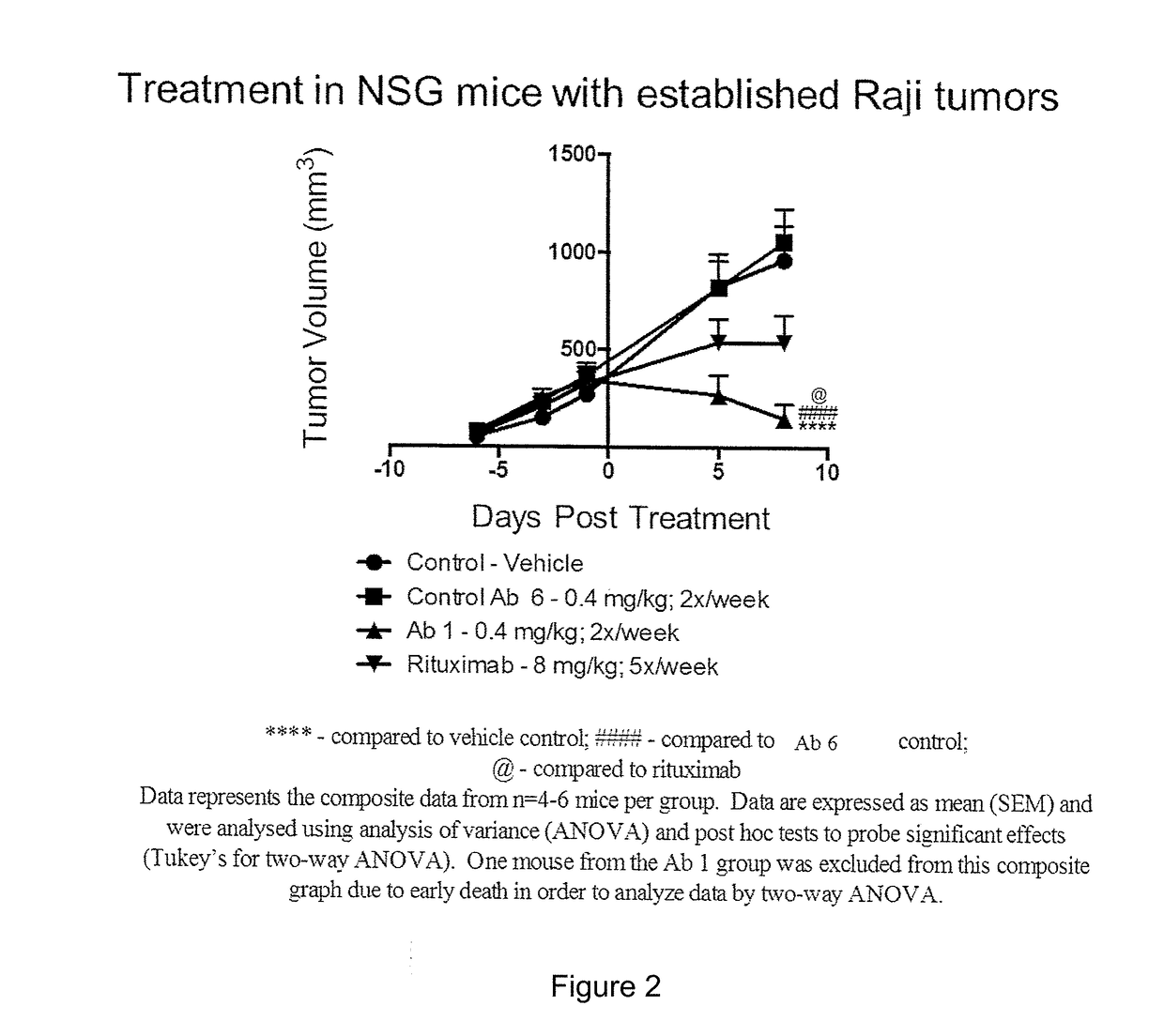
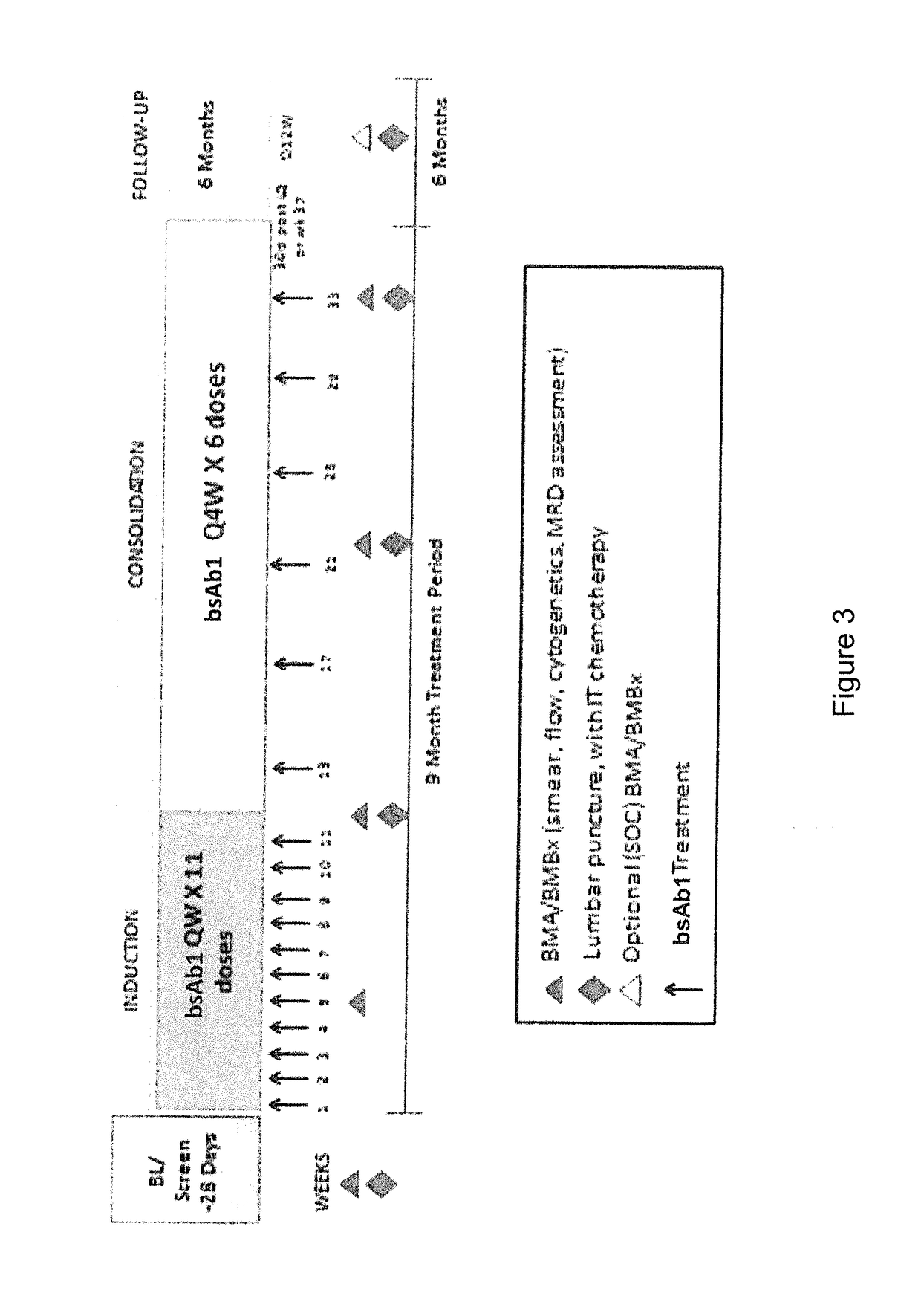
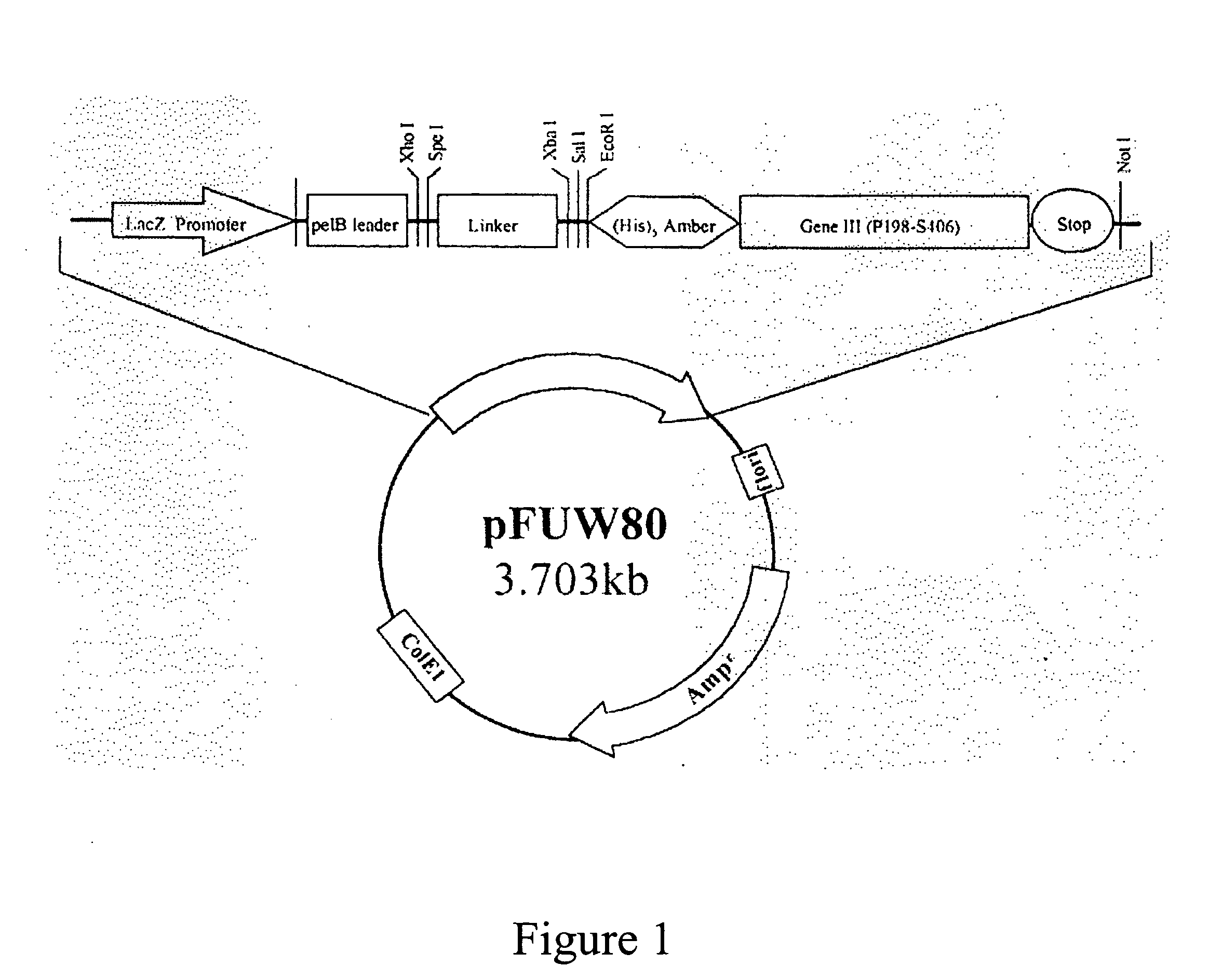
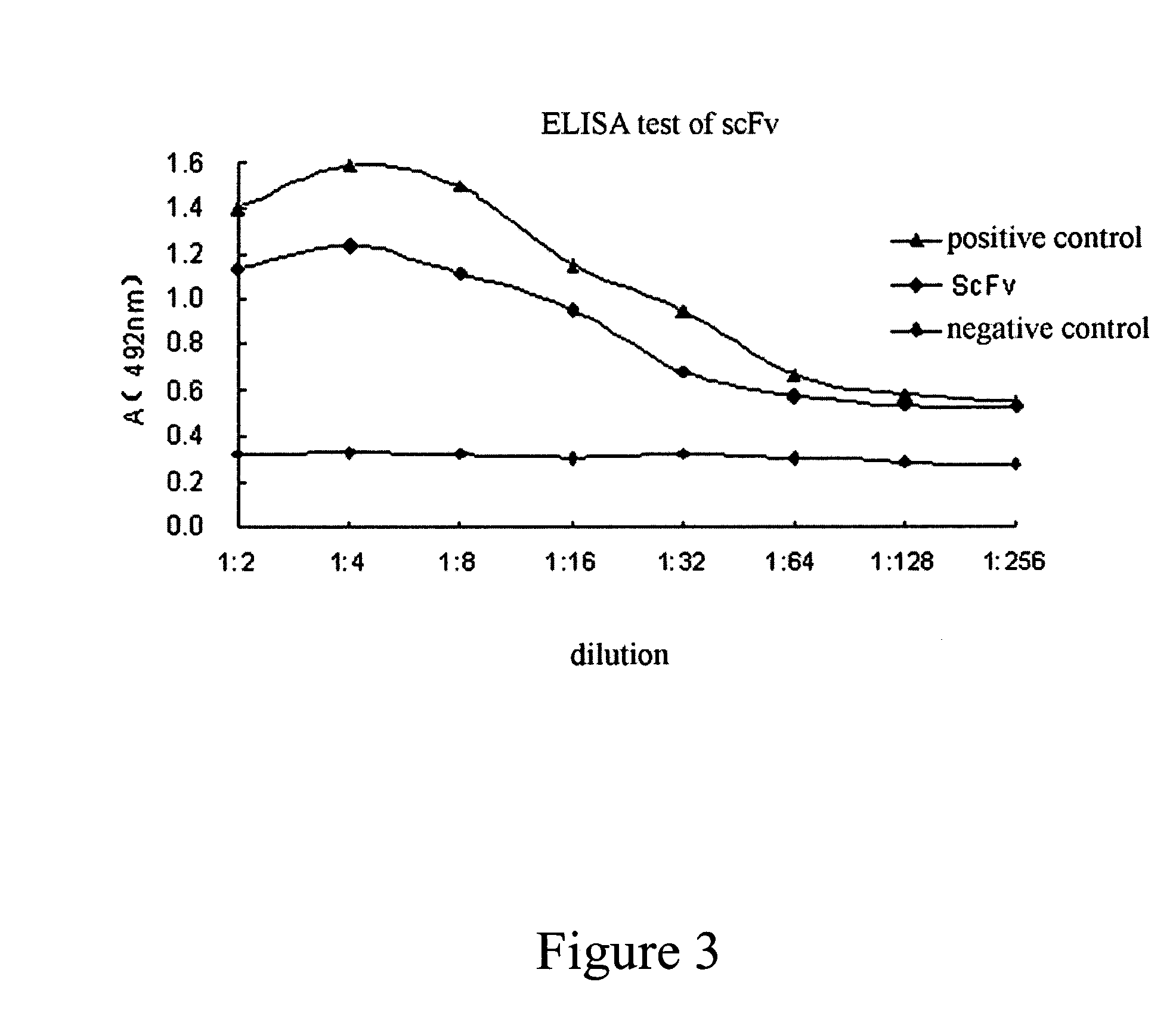
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "Anti cd3" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
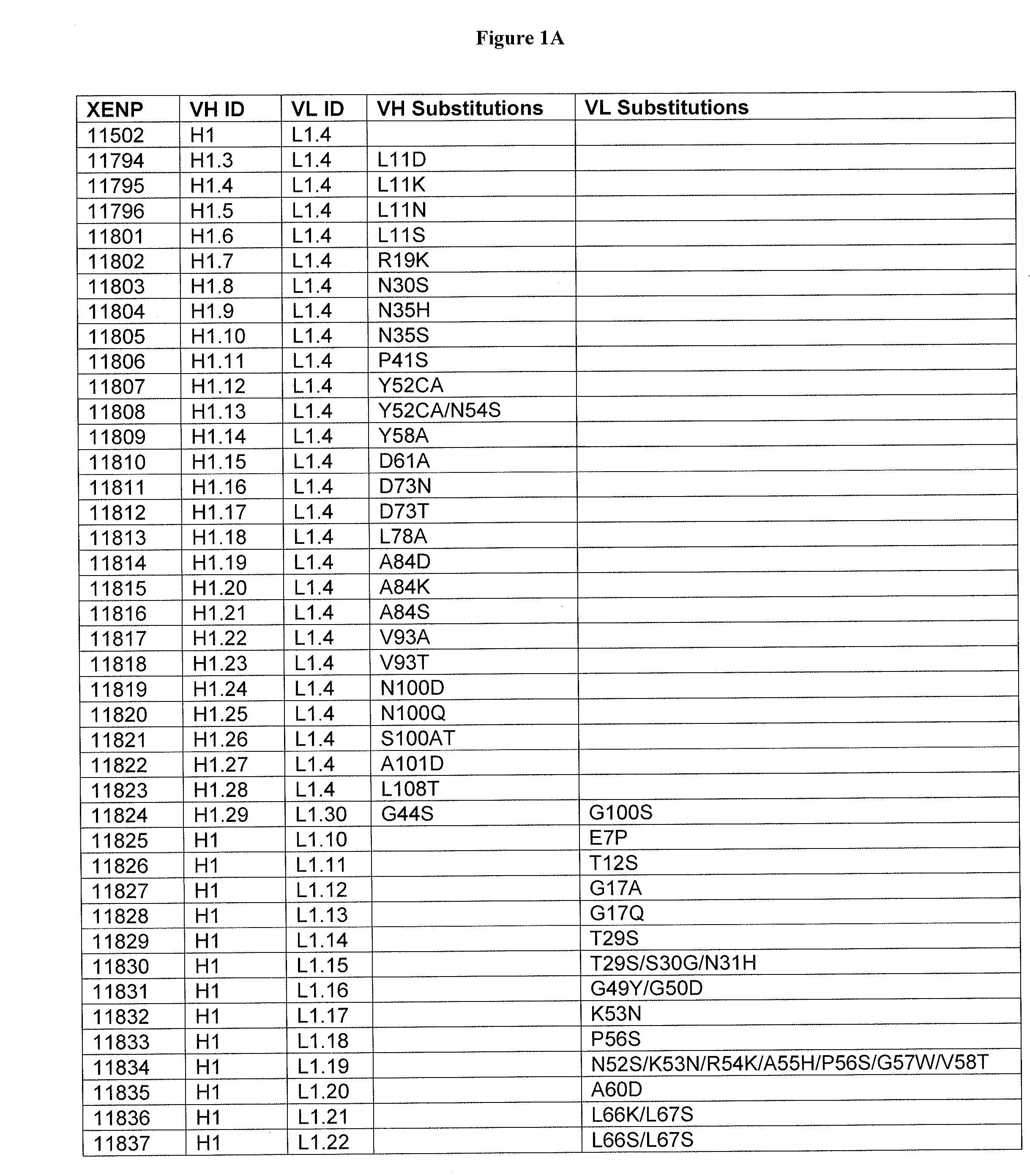
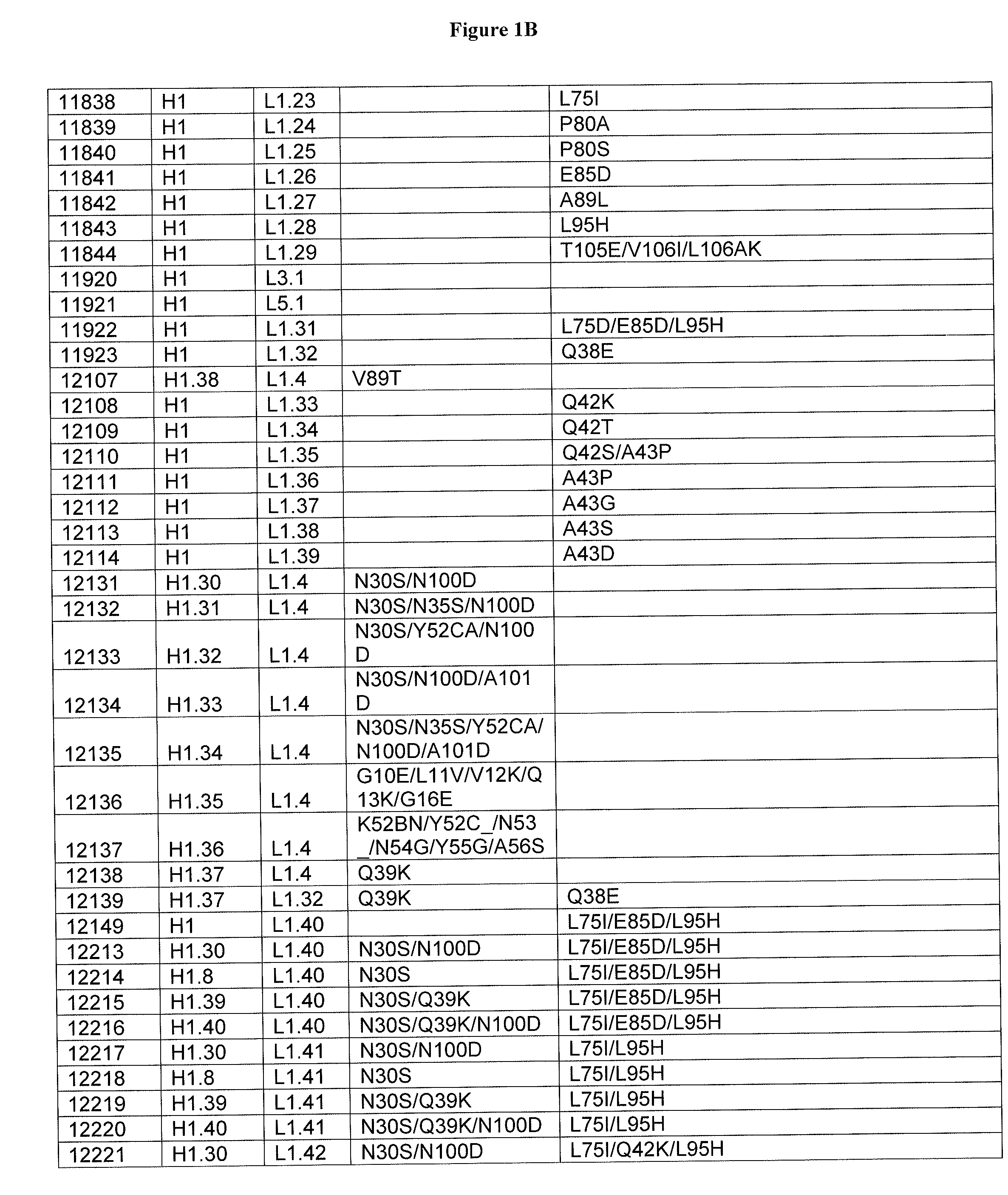
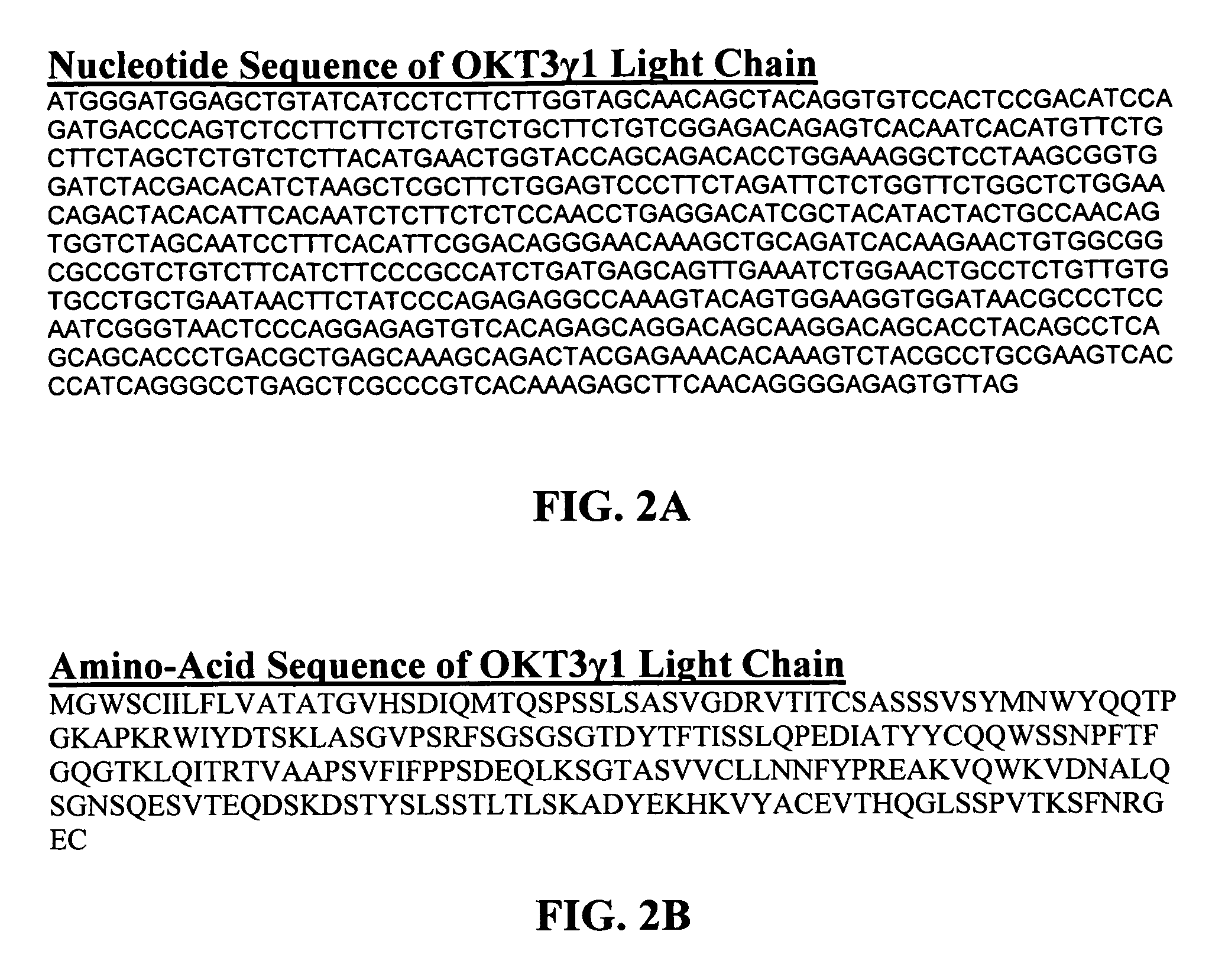
Optimized antibody variable regions
Owner:XENCOR INC
Optimized Anti-cd3 variable regions
Owner:XENCOR INC
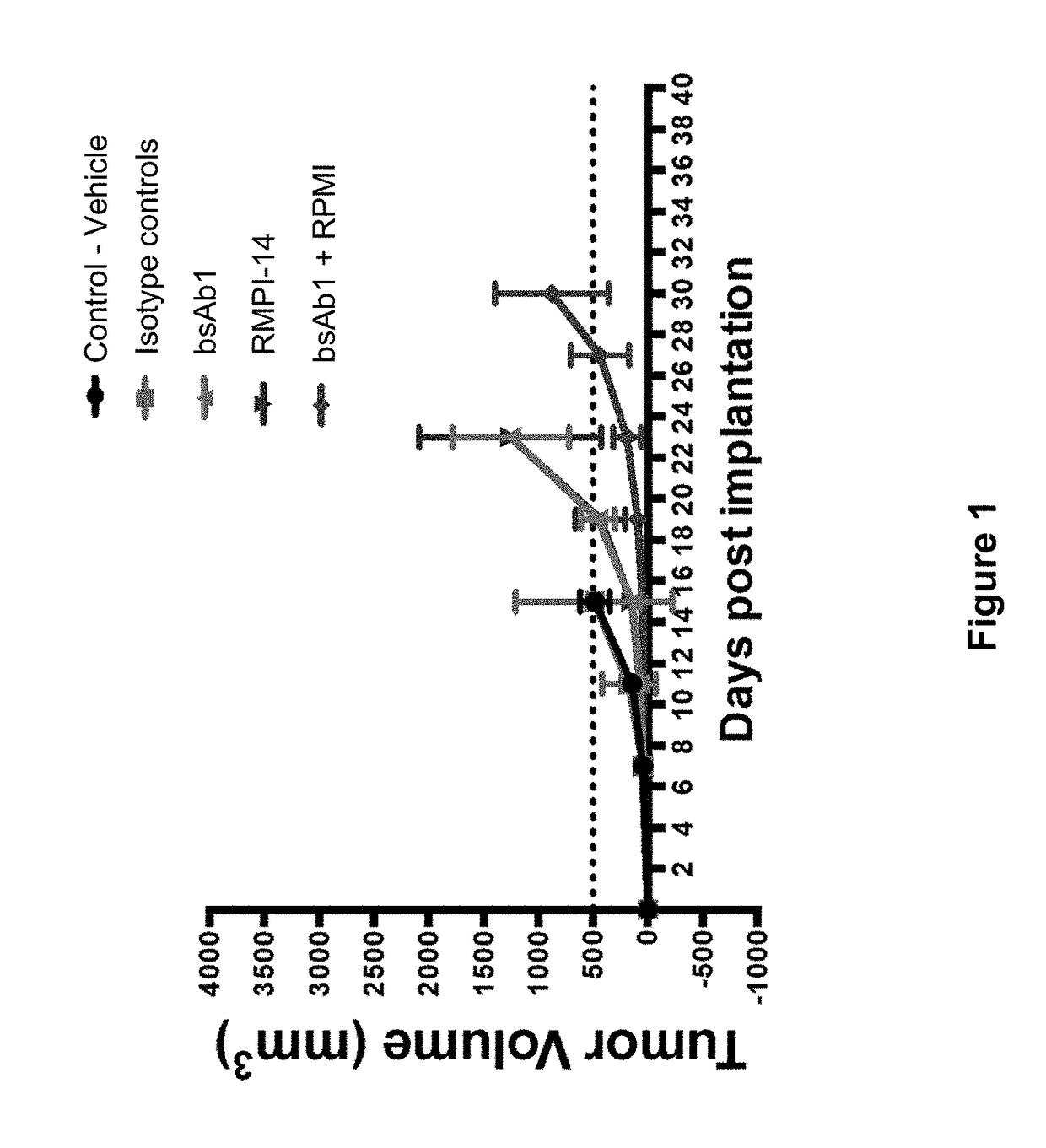
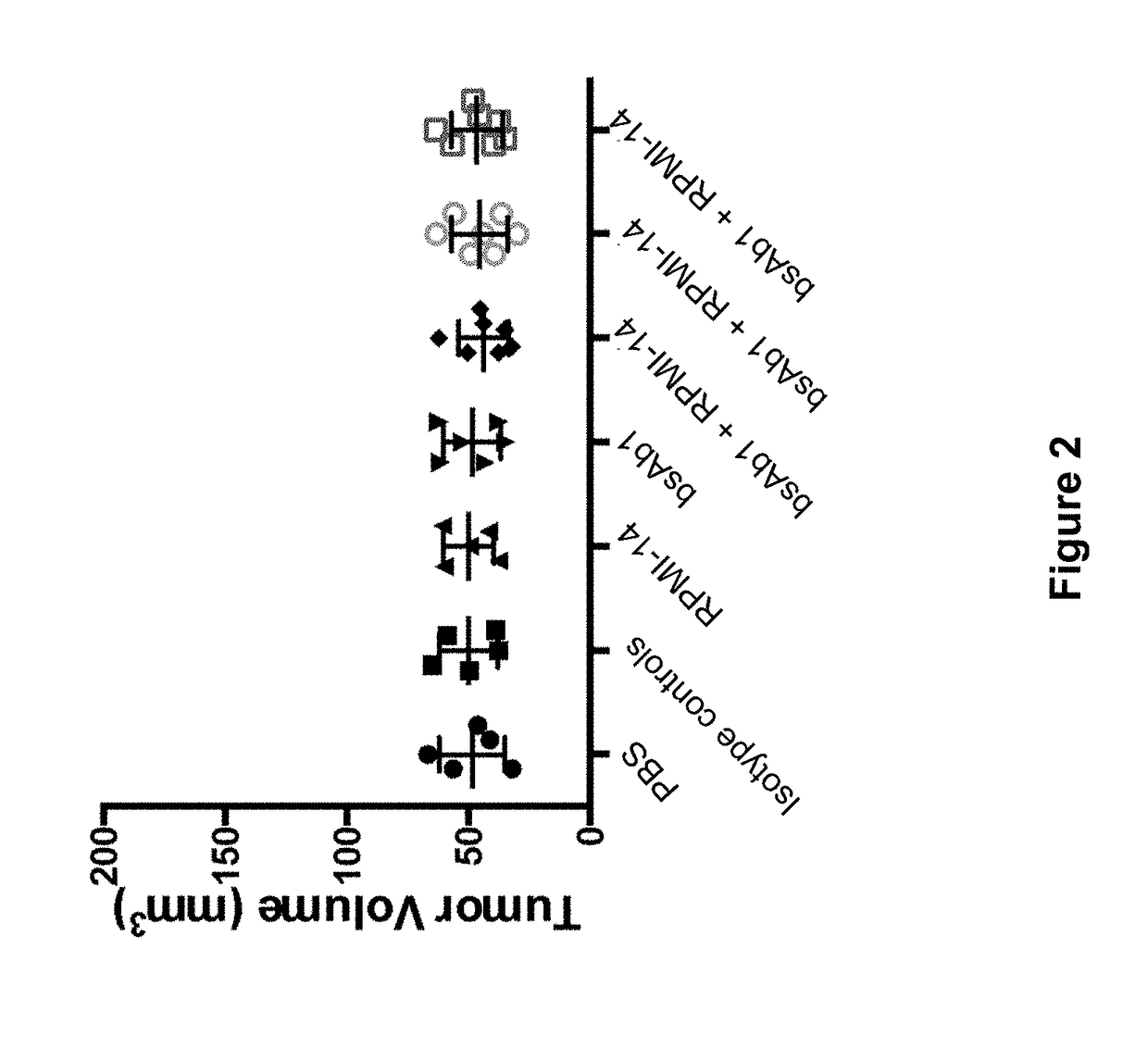
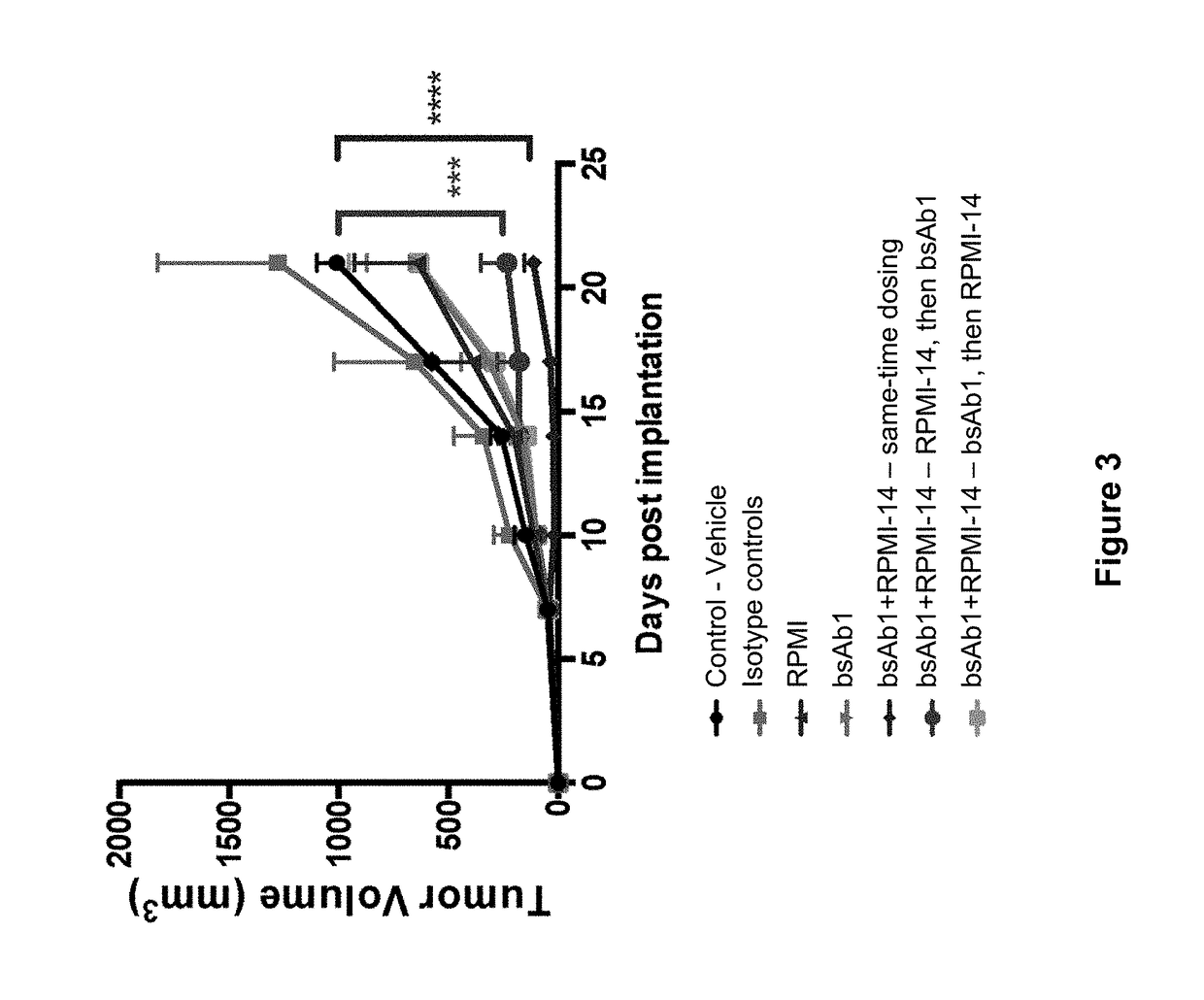
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS20070077246A1Treating and preventing and ameliorating symptomSlow and reduce damagePeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
The present invention provides methods of treating, preventing or ameliorating the symptoms of T cell-mediated immunological diseases, particularly autoimmune diseases, through the use of anti-CD3 antibodies. In particular, the methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-CD3 antibodies.
Owner:PROVENTION BIO INC
Combination of Anti-PD-1 Antibodies and Anti-CD20/Anti-CD3 Antibodies to Treat Cancer
InactiveUS20170174779A1Growth inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenCD20
The present invention provides methods for treating, reducing the severity, or inhibiting the growth of cancer (e.g., a B-cell cancer such as Hodgkin's lymphoma or acute lymphoblastic leukemia). The methods of the present invention comprise administering to a subject in need thereof a therapeutically effective amount of an antibody or antigen-binding fragment thereof that specifically binds to programmed death 1 (PD-1) receptor in combination with a therapeutically effective amount of a bispecific antibody that specifically binds to CD20 and CD3.
Owner:REGENERON PHARM INC
Methods of promoting immunopotentiation and preparing antibodies with anti-CD3 antibodies
Disclosed are immunopotentiating agents, and vaccines thereof, which enhance and / or otherwise modify immune responses, and method for their preparation and use in vivo. Immunopotentiating agents can be single agents that act directly, adjuvants added concurrently with the agents, or heteroconjugates wherein the immunopotentiating agent is chemically coupled to the compound against which an immune response is desired. Examples of immunopotentiating agents include monoclonal antibodies, such as anti-CD3, anti-CD2) and anti-CD5 antibodies, and proteins derived from microorganisms (e.g., enterotoxins) which activate T cells. The compounds against which an immune response can be generated, which may be the second component in a heteroconjugate, include compound from abnormal or diseased tissues such as tumors, or infectious agents, such as viruses, bacteria, fungi, protozoal or metozoal parasites, and can be obtained by natural or recombinant means. Methods of using the invention to prepare monoclonal antibodies are particularly disclosed.
Owner:MACROGENICS INC
Bispecific Anti-CD20/Anti-CD3 Antibodies to Treat Acute Lymphoblastic Leukemia
The present invention provides methods for treating, reducing the severity, or inhibiting the growth of acute lymphoblastic leukemia. The methods of the present invention comprise administering to a subject in need thereof a therapeutically effective amount of a bispecific antibody that specifically binds to CD20 and CD3.
Owner:REGENERON PHARM INC
Anti human ovarian cancer-anti CD3 bispecific antibody
InactiveUS7262276B2Low toxicityHigh efficiency and cost-effectivenessImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsComplementarity determining regionAntiendomysial antibodies
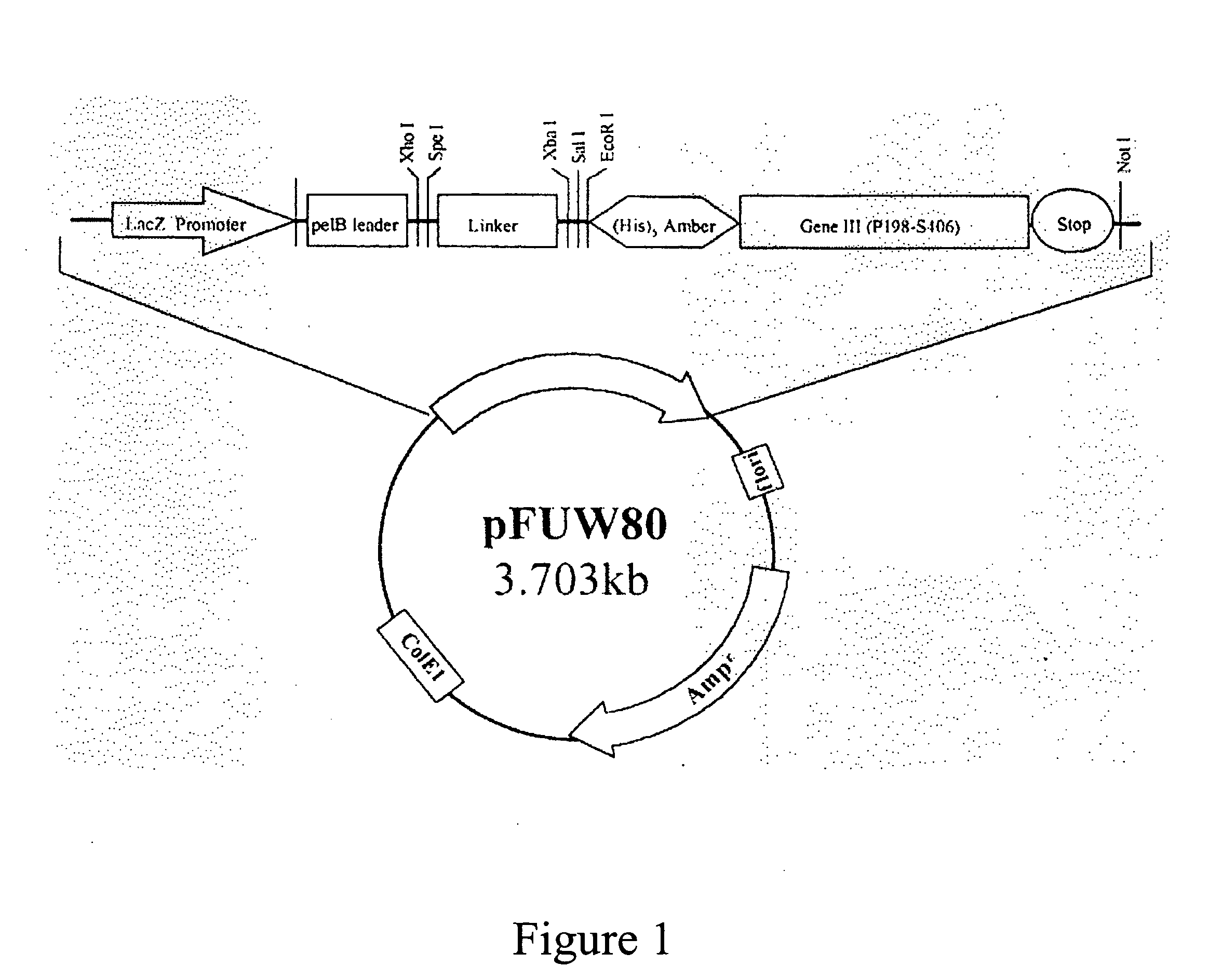
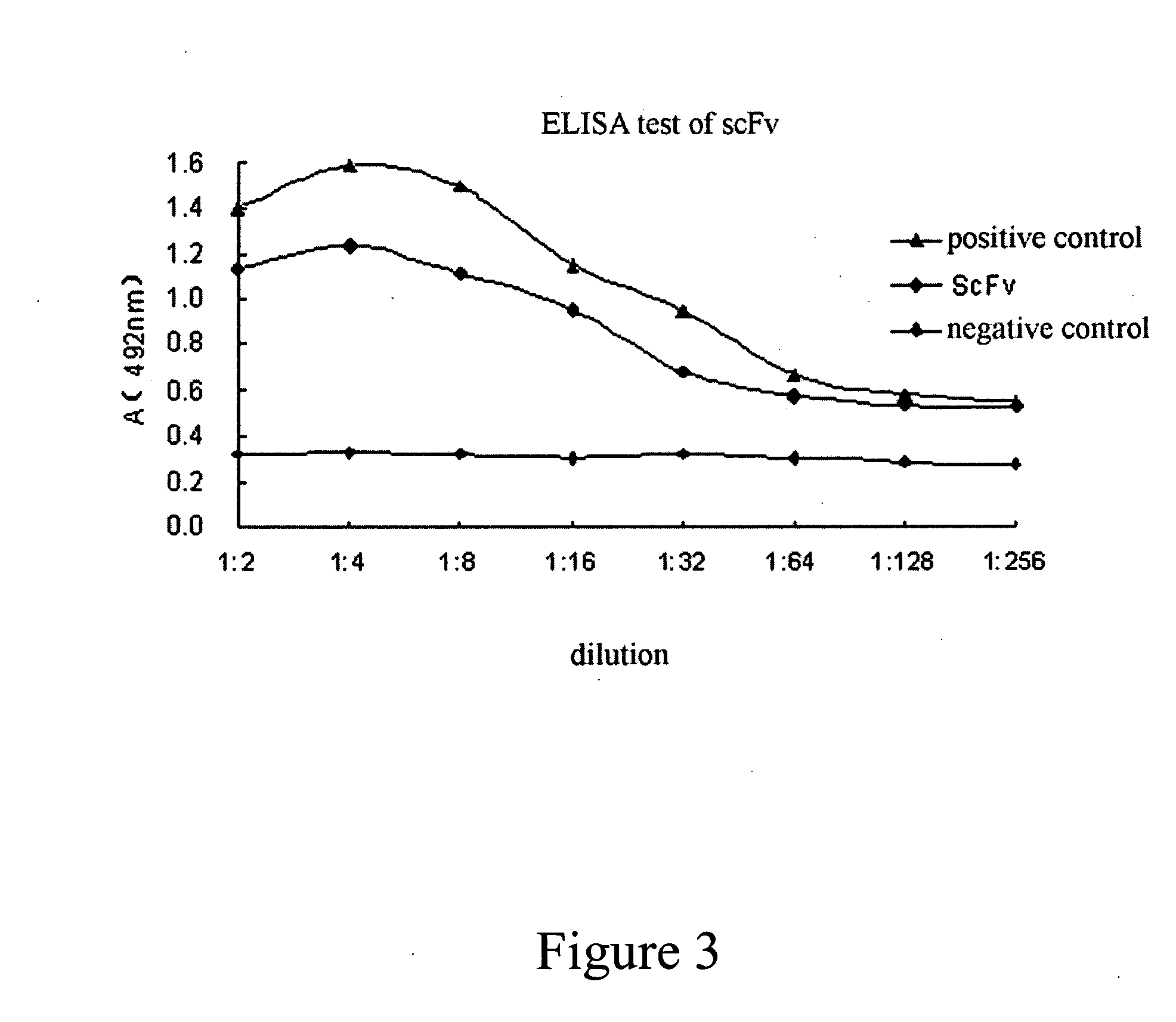
The present invention provides an anti-ovarian cancer bispecific antibody. Said antibody includes two polypeptide domains connected by a polypeptide linker, one is anti-ovarian cancer antibody, or its Fab fragment, single complementarity determining region (CDR) antibody or single chain Fv (scFv) and the other is anti-CD3 antibody, or its Fab fragment, single CDR antibody or scFv. The present invention also provides DNA sequences encoding said antibody, an expression vector containing said DNA sequence, and a host cell containing said expression vector.
Owner:DONGGUAN HAOFA BIOTECH DEVAL +2
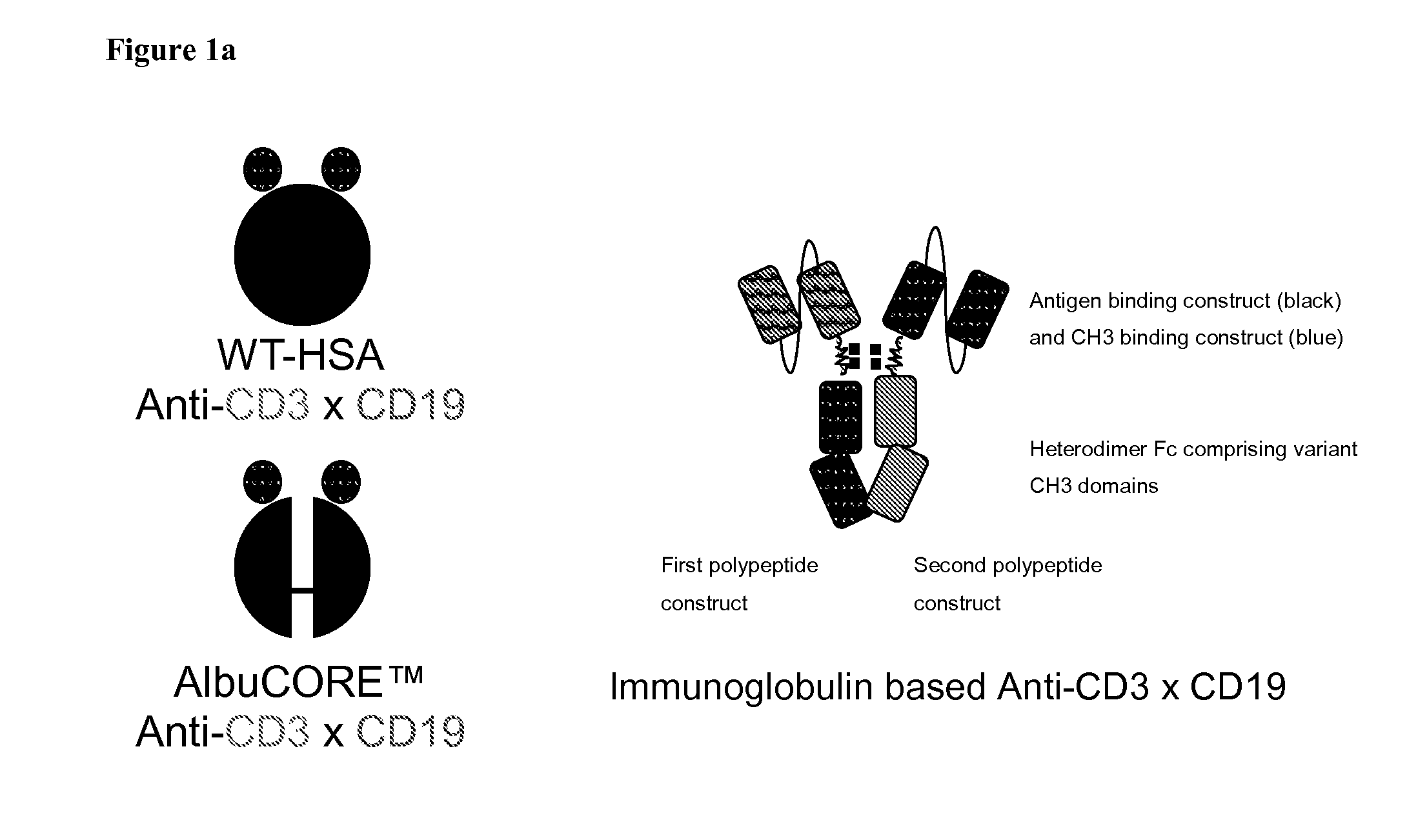
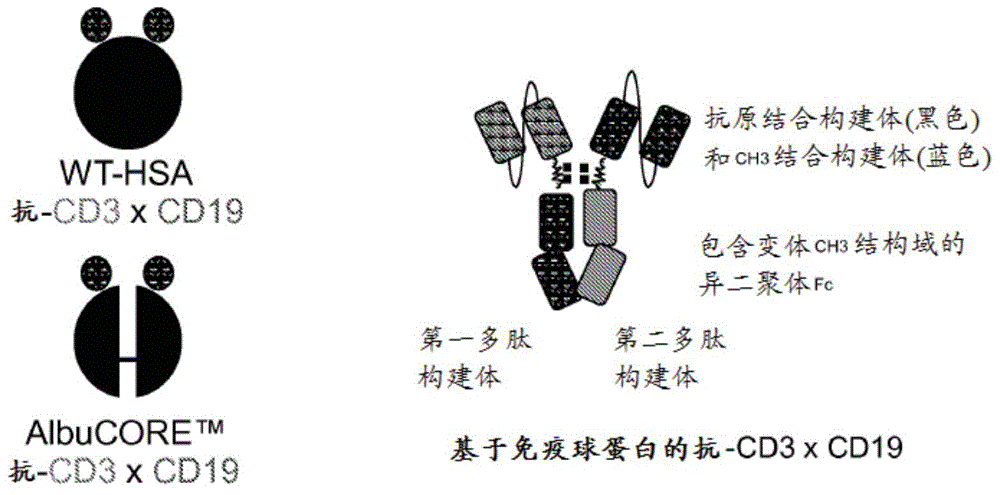
Bispecific Asymmetric Heterodimers Comprising Anti-CD3 Constructs
Disclosed herein are isolated multi-specific heteromultimer constructs that bind to CD3 expressed on T-cells and to an antigen expressed on B-cells. The multi-specific heteromultimer constructs are capable of bridging T- and B-cells and mediating killing of B-cells. The multi-specific heteromultimer constructs are based on a heterodimeric Fc scaffold or on a segmented albumin scaffold. Also disclosed herein are multi-specific heteromultimer constructs that bind to HER2 and HER3.
Owner:ZYMEWORKS INC
Pharmaceutical compositions comprising bispecific anti-cd3, anti-cd19 antibody constructs for the treatment of b-cell related disorders
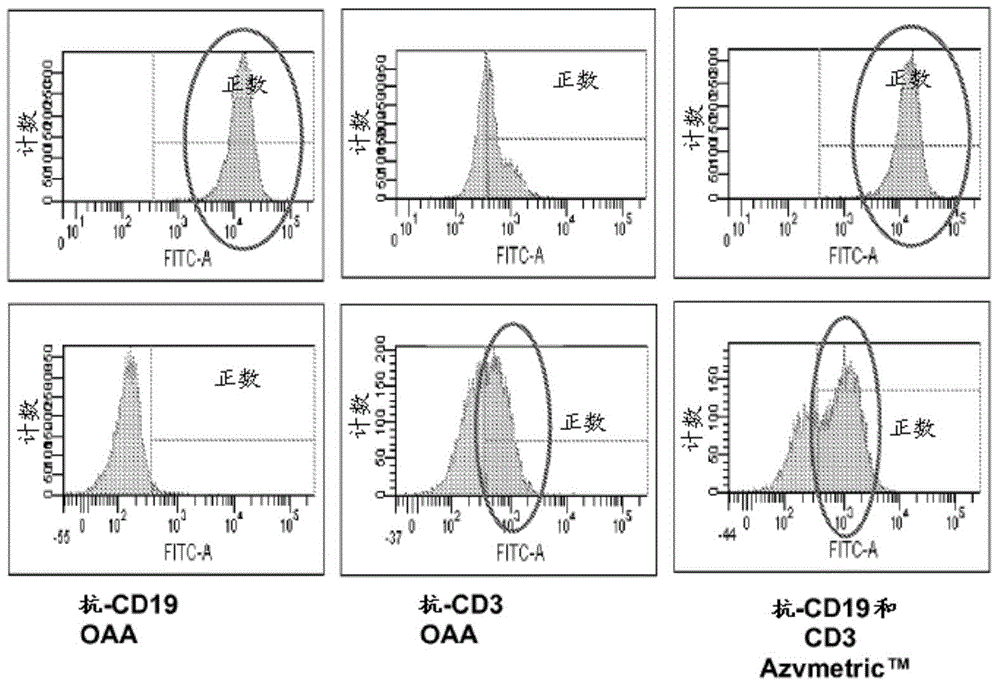
The present invention relates to a pharmaceutical composition comprising a bispecific single chain antibody construct comprising human CD3 and human CD19 specific binding domains, wherein the corresponding variable heavy chain regions (VH) and the corresponding variable light chain region (VL) from N-terminus to C-terminus in the following order: VH (CD19)-VL (CD19)-VH (CD3)-VL (CD3), VH (CD3)-VL (CD3)-VH (CD19)-VL (CD19) or VH (CD3)-VL (CD3)-VL (CD19)-VH (CD19). In addition, the present invention discloses the preparation method of the pharmaceutical composition and the medical / pharmaceutical use of the specific bispecific single chain antibody molecule specific to human CD3 antigen and human CD19 antigen.
Owner:AMGEN RES (MUNICH) GMBH
Gene Engineering Recombinant Anti-CEA, Anti-CD3, And Anti-CD28 Single-Chain Tri-Specific Antibody
InactiveUS20090117108A1Increase production costHigh expressionBacteriaSugar derivativesAntibody fragmentsSingle-domain antibody
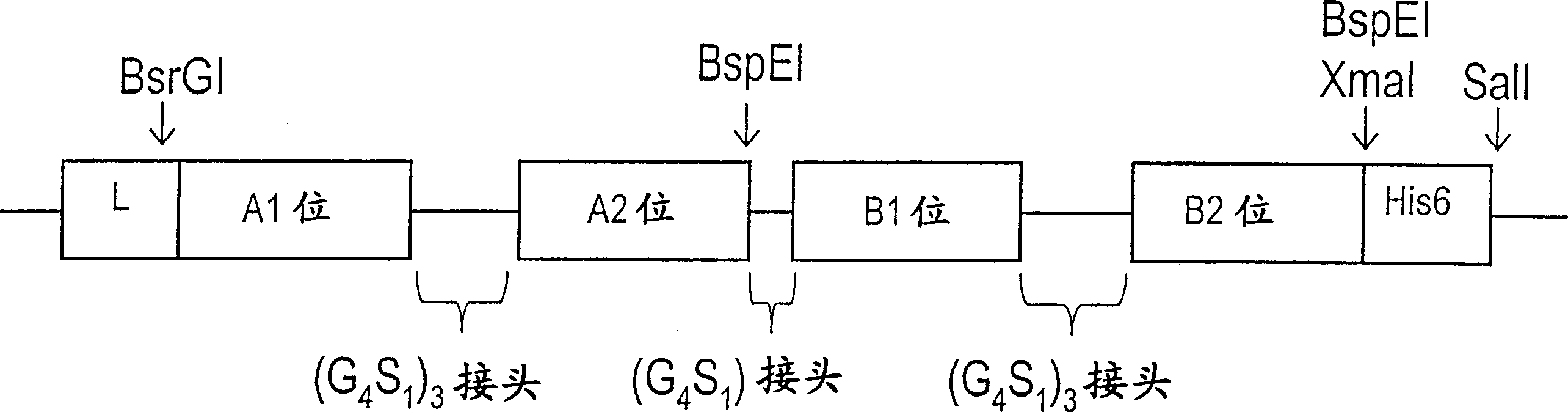
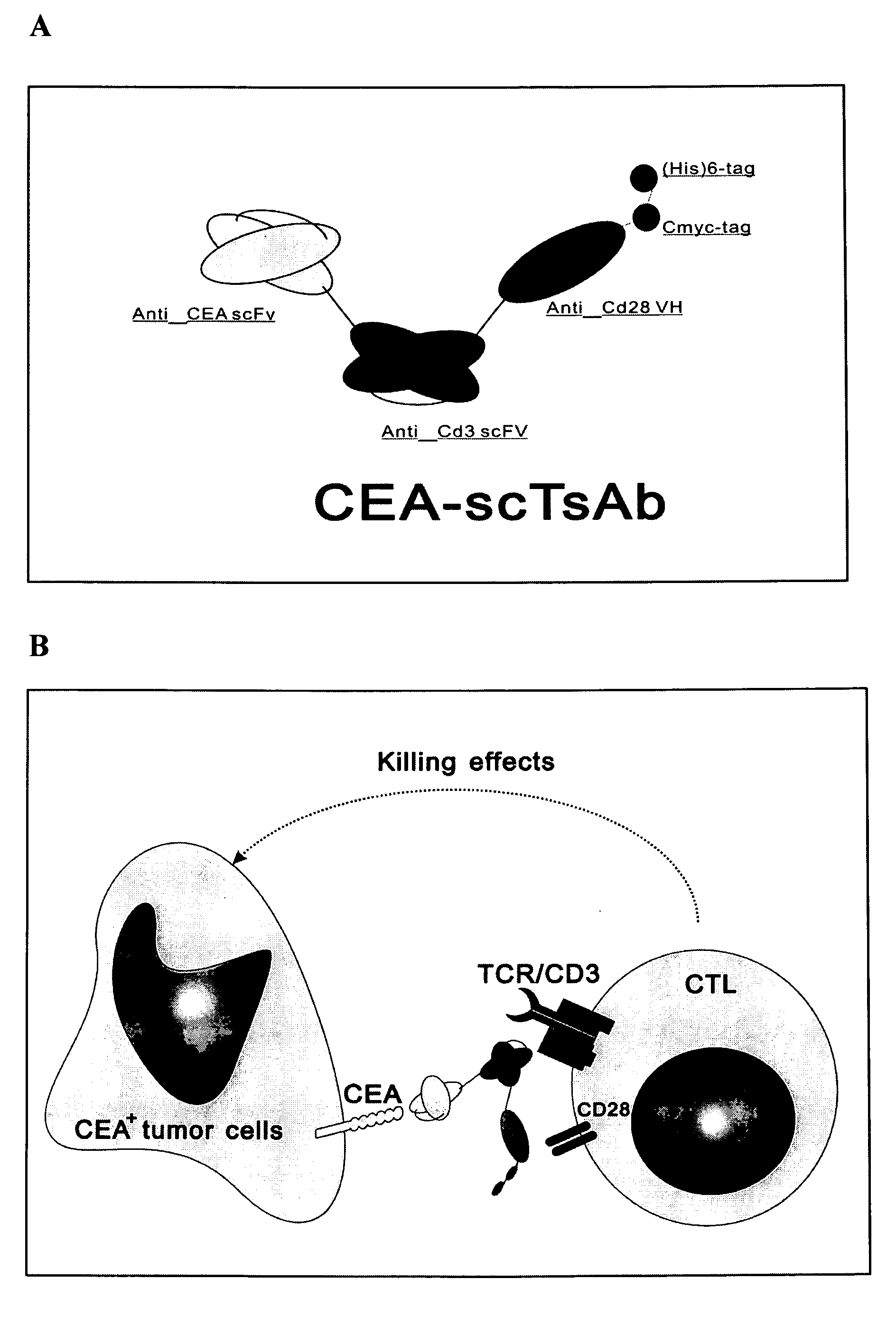
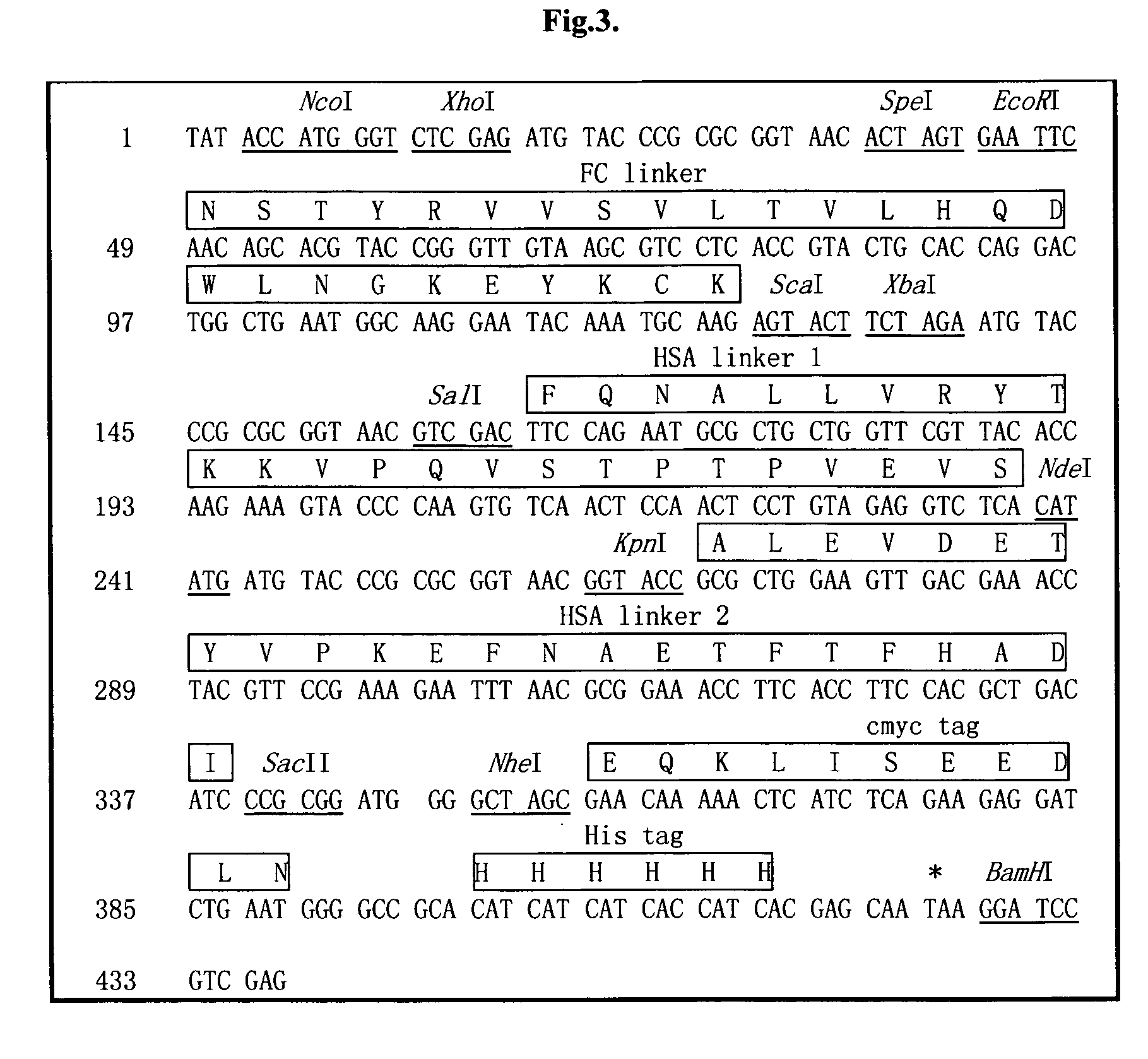
The invention is related to a recombinant single-chain tri-specific antibody made from anti-Tumor Associated Antigen (TAA) antibody, FC interlinker, anti-CD3 antibody, HSA interlinker and anti-CD28 antibody in turn. Particularly, the invention relates to an anti-CEA, anti-CD3, anti-CD28 recombinant single-chain tri-specific antibody, CEA-scTsAb, which was constructed with three tandem antibody fragments (anti-CEA scFv, anti-CD3 scFv and anti-CD28 single-domain antibody) linked by two interlinkers (FC interlinker, HSA interlinker), and could be appended by C myc tag or histidine tag ((His)6-tag) at the C terminal. It also concerns a method for construction, expression and purification of the antibody. It also offers the encoded DNA sequence of the antibody, expression vectors and host cells for the vectors.
Owner:WANG XIANGBIN +5
Anti-CD3 and antigen-specific immunotherapy to treat autoimmunity
InactiveUS20070190045A1Improve immune toleranceMaintain long-termNervous disorderPeptide/protein ingredientsDiseaseTolerability
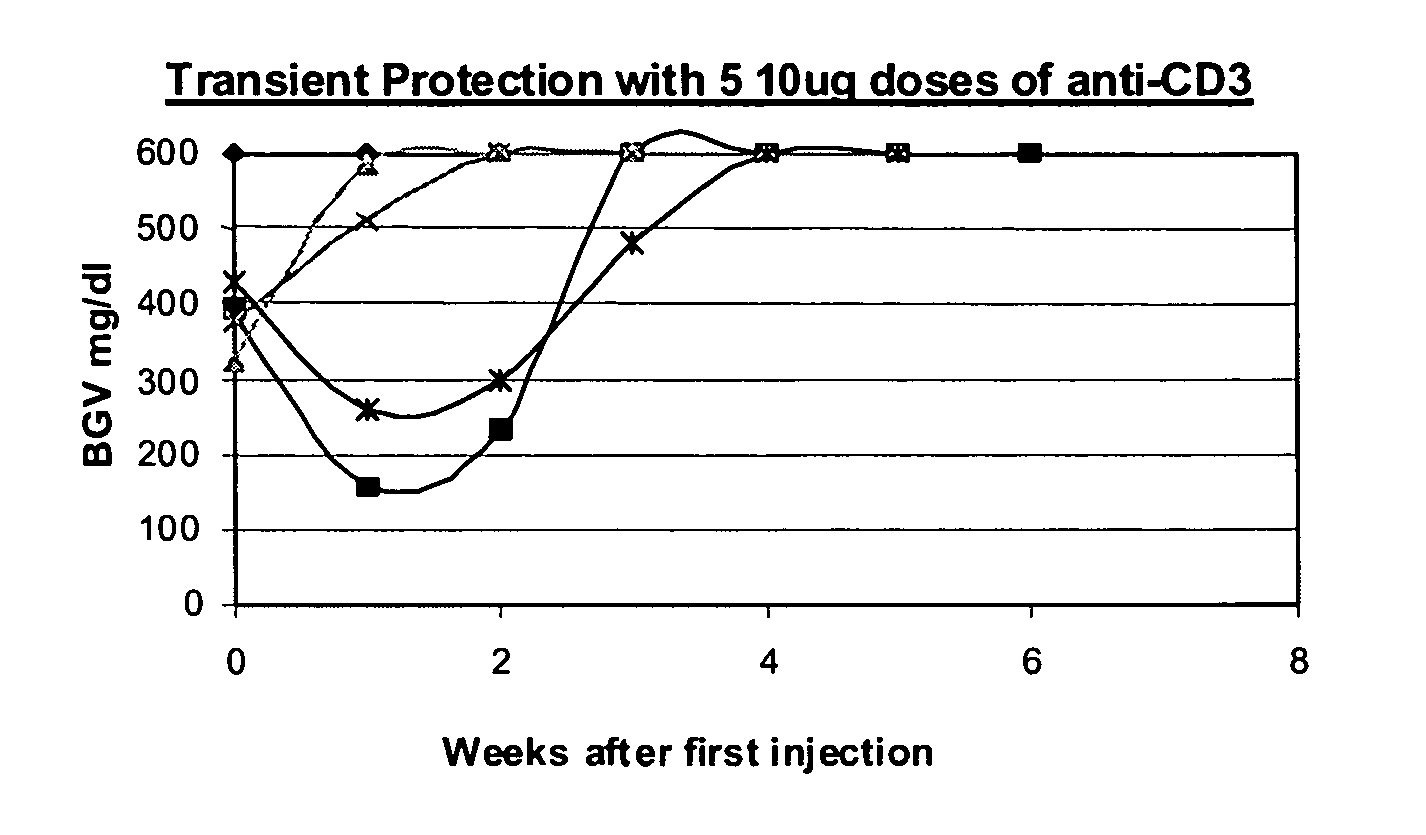
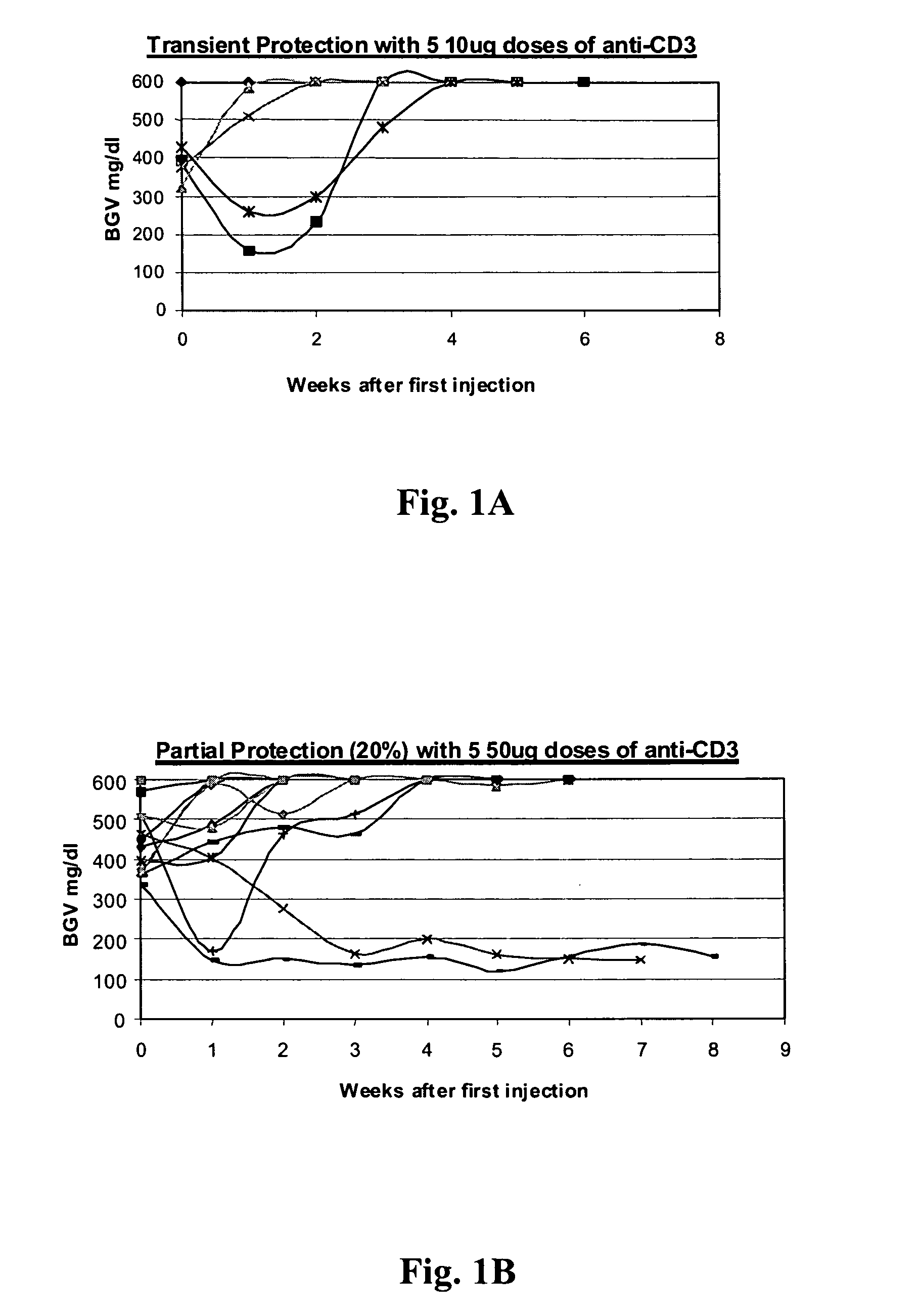
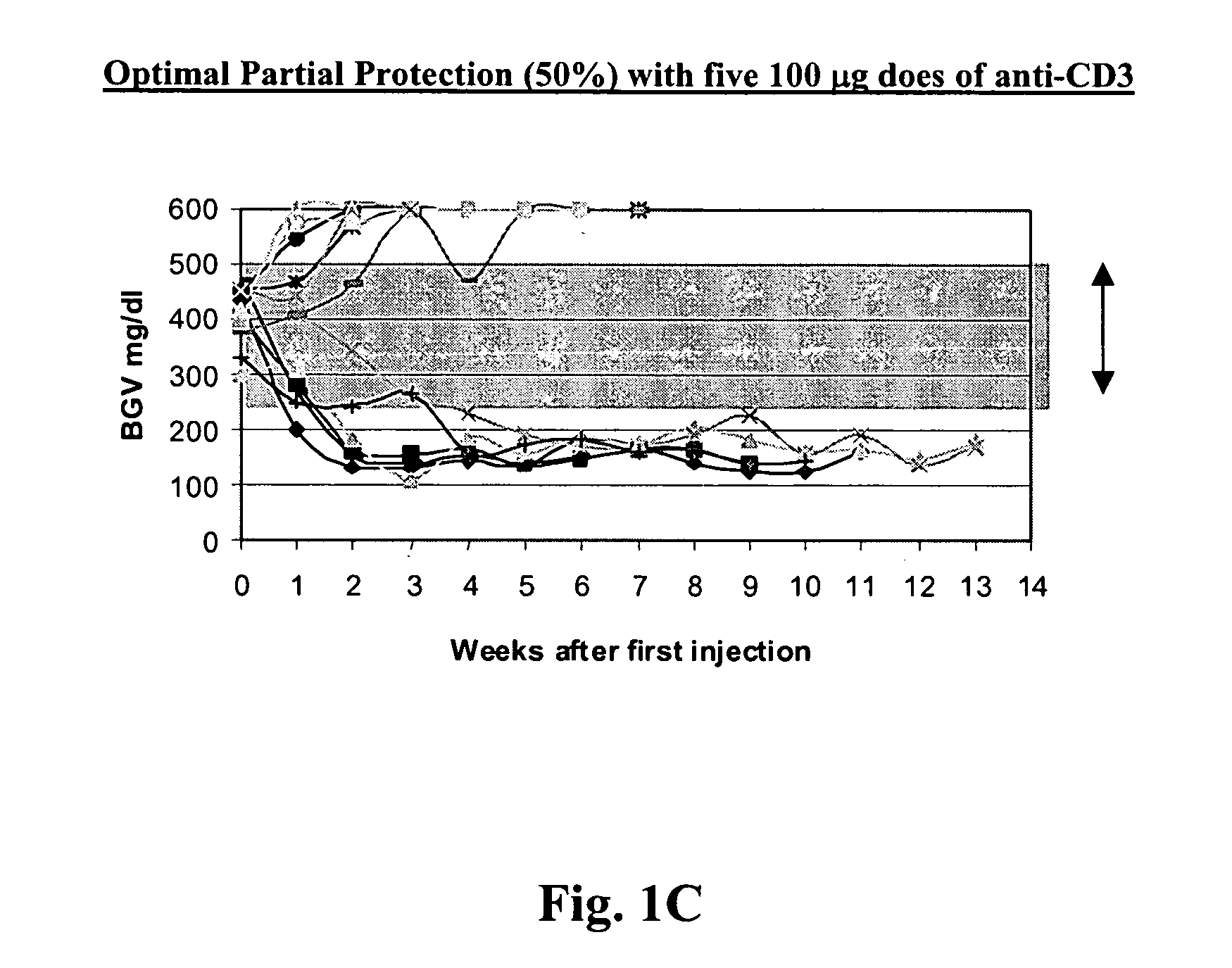
The invention provides methods for treating autoimmunity and for reestablishing tolerance. The methods involve the coadministration of anti-CD3 antibodies and self-antigens. The coadministration has the potential to provide a synergistic effect of protecting or reducing autoaggressive immune processes and / or of reestablishing tolerance towards self-antigens. An underlying rationale behind the methods is that the administration of self-antigens together with anti-CD3 antibodies can alter the response to those self-antigens and prevent progression of autoimmunity. By rechallenging with the autoantigens and stimulating the non-pathogenic response, the blockade of the autoimmune process can be maintained. Preclinical evidence provided herein shows that the combination of anti-CD3 and autoantigen is synergistic in reversing autoimmune diabetes, and therefore, suggests that combination therapy of anti-CD3 and self-antigen may provide synergistic protection in reversing other autoimmune disorders.
Owner:THE UCSF DIABETES CENT +2
Immunoconjugates with improved efficacy for the treatment of diseases
InactiveUS20070196274A1Improve therapeutic efficacyHigh detection sensitivityIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntibody conjugateCD11a
The invention provides therapeutic or diagnostic antibodies with modified N— or C-terminal sequences that are enriched with lysine or tyrosine residues. These lysine or tyfosine residues can be used to couple radioisotopes, cytotoxic agents, or detectable labels. The increased stoichiometric ratios of these agents in the antibody conjugates lead to improved therapeutic efficacy or enhanced detection sensitivity. Non-limiting examples of antibodies suitable for the present invention include anti-CD22, anti-ErbB2, anti-VEGF, anti-EGFR, anti-VEGFR, anti-Her-3, anti-Her-4, anti-CEA, anti-CTLA-4, anti-CD4, anti-CD3, anti-CD20, anti-TNF-a, anti-CD11a, anti-Lewis Y antigen, anti-TrailR, anti-IL2R, anti-CD30, anti-CD146, anti-CD147, anti-alpha V integrin beta, anti-CD19, anti-GD2, anti-3H11, anti-EBV, anti-HIV, anti-HBV, anti-HCV, and other disease-specific antibodies.
Owner:WELSON PHARMA
Methods and materials for modulation of the immunosuppressive activity and toxicity of monoclonal antibodies
InactiveUS20060002933A1Eliminate concernsReduced activityPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectActivation cells
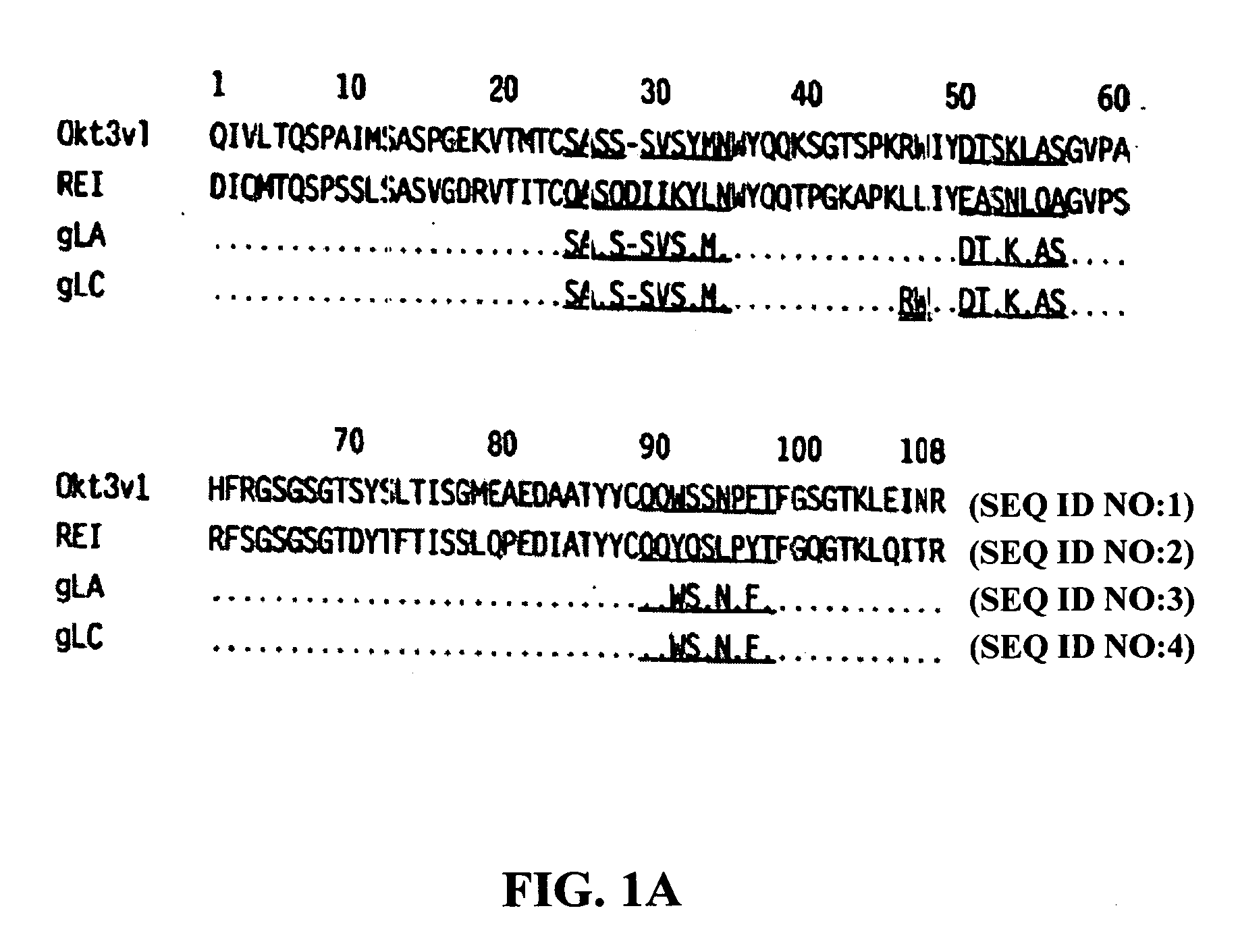
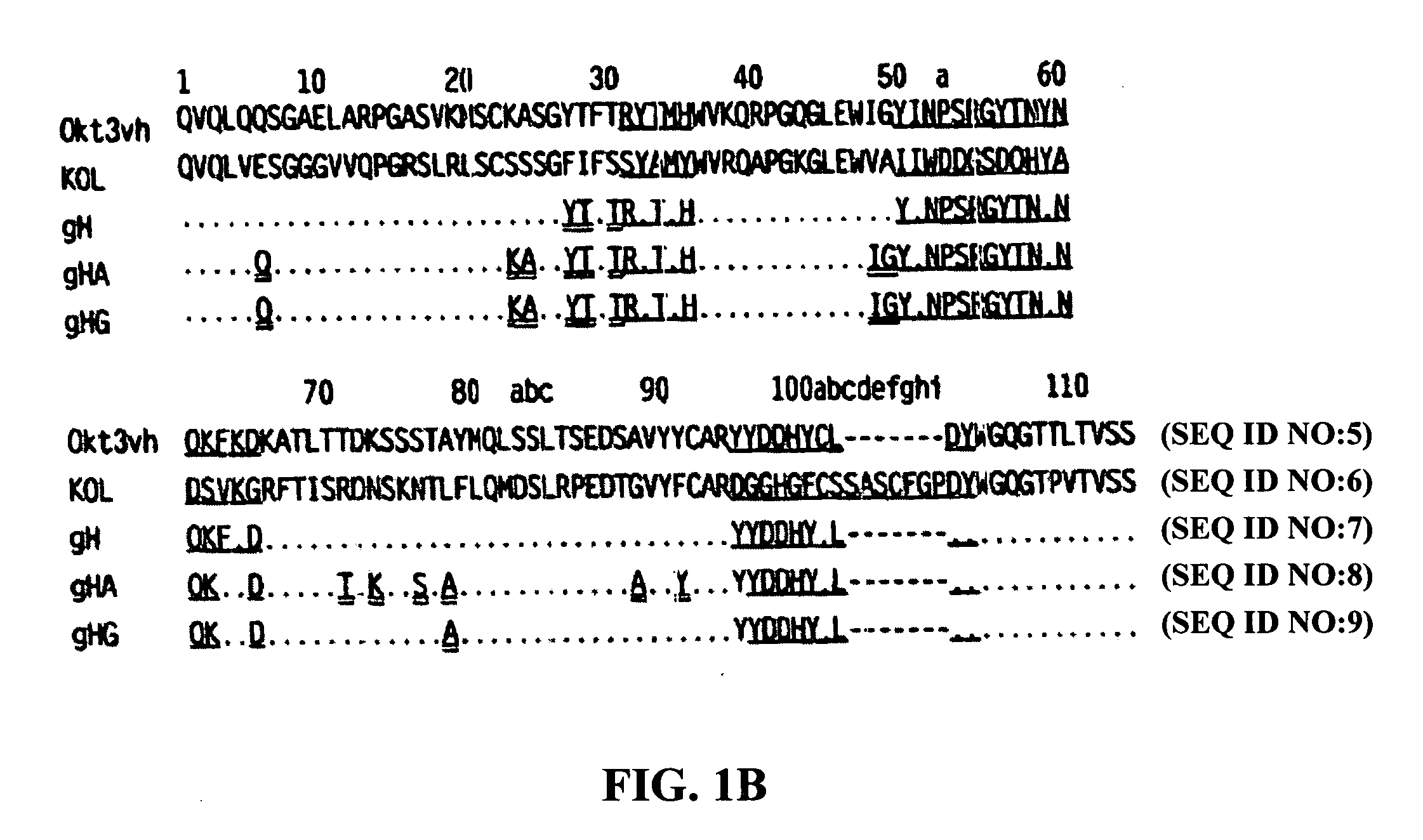
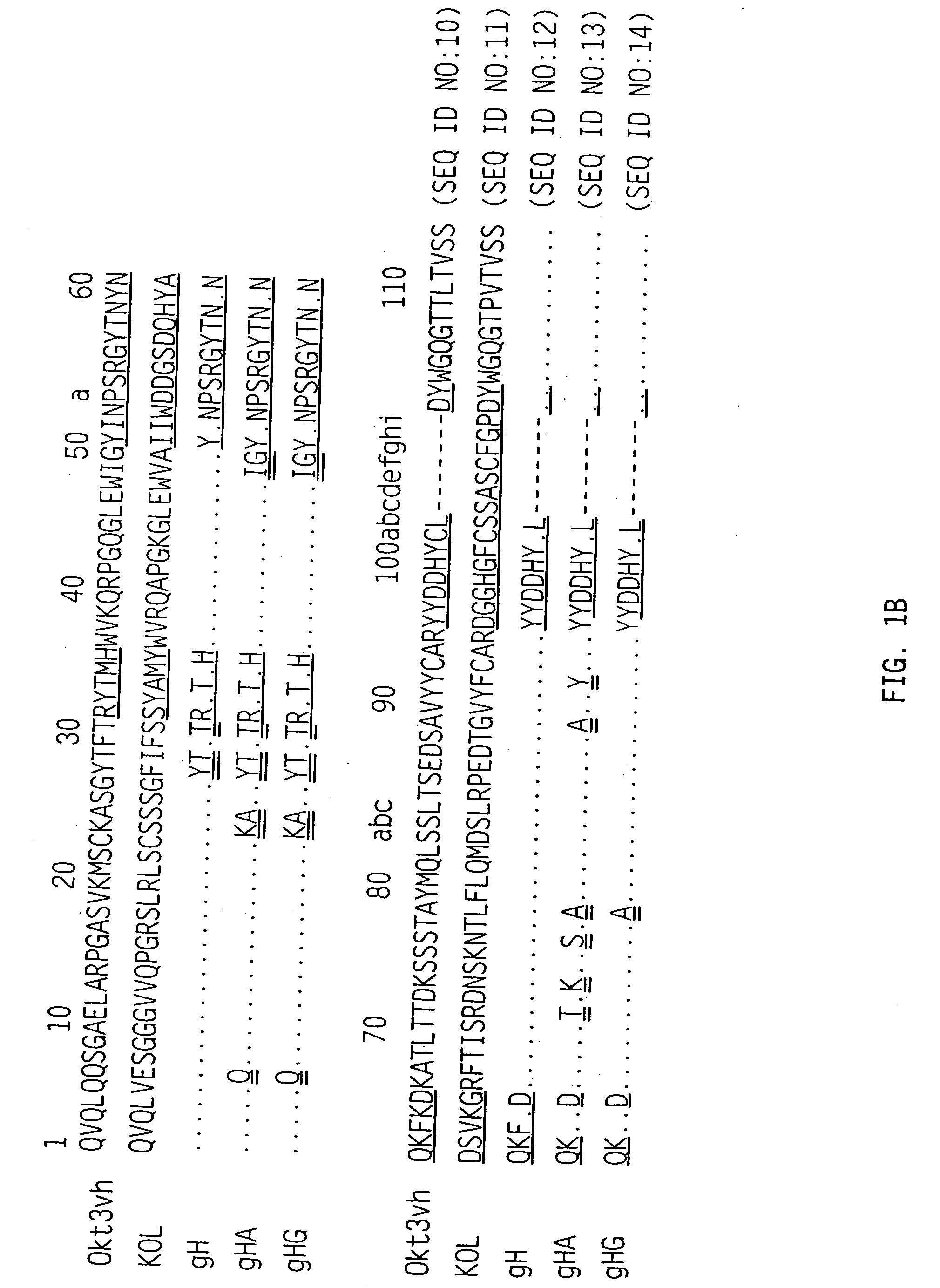
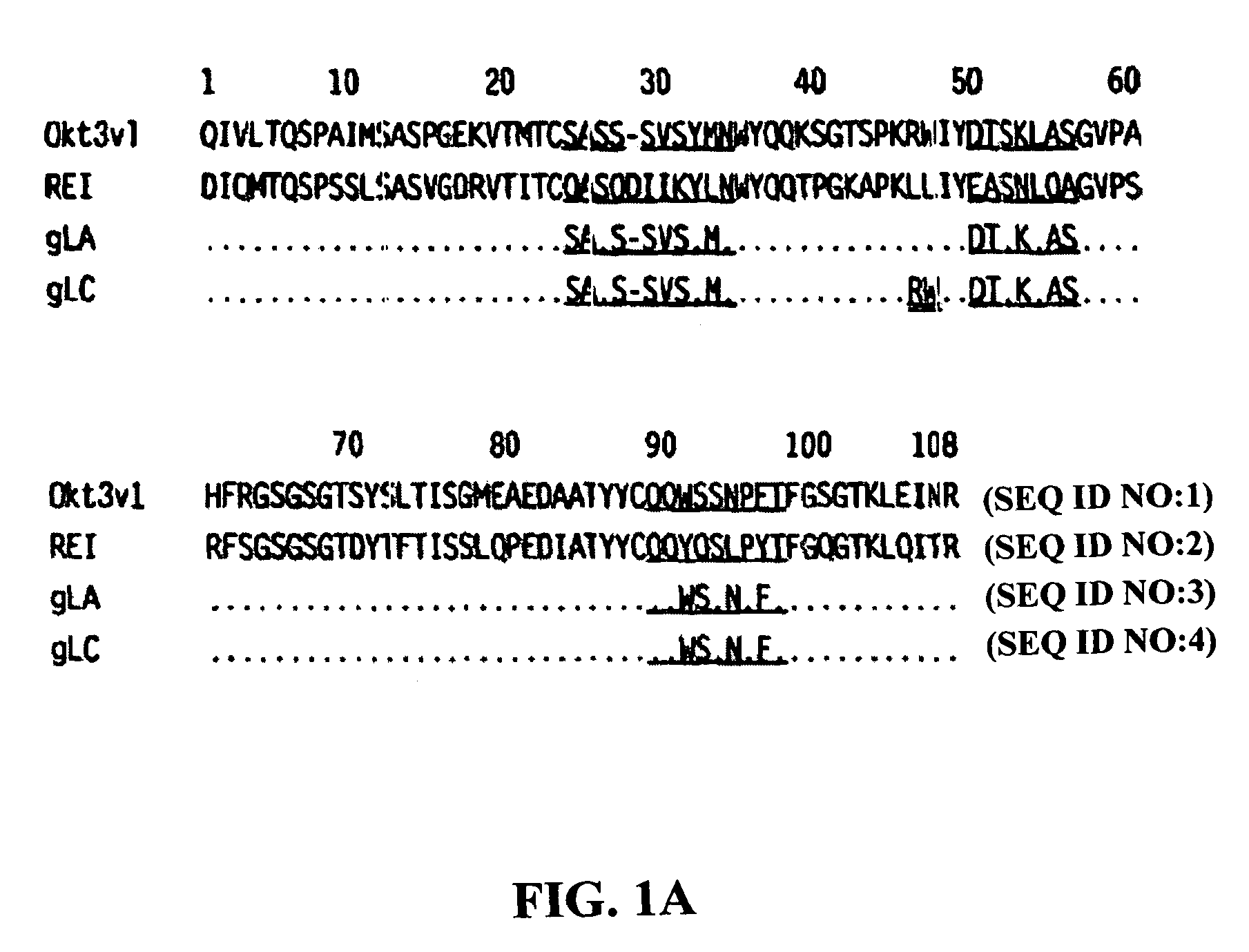
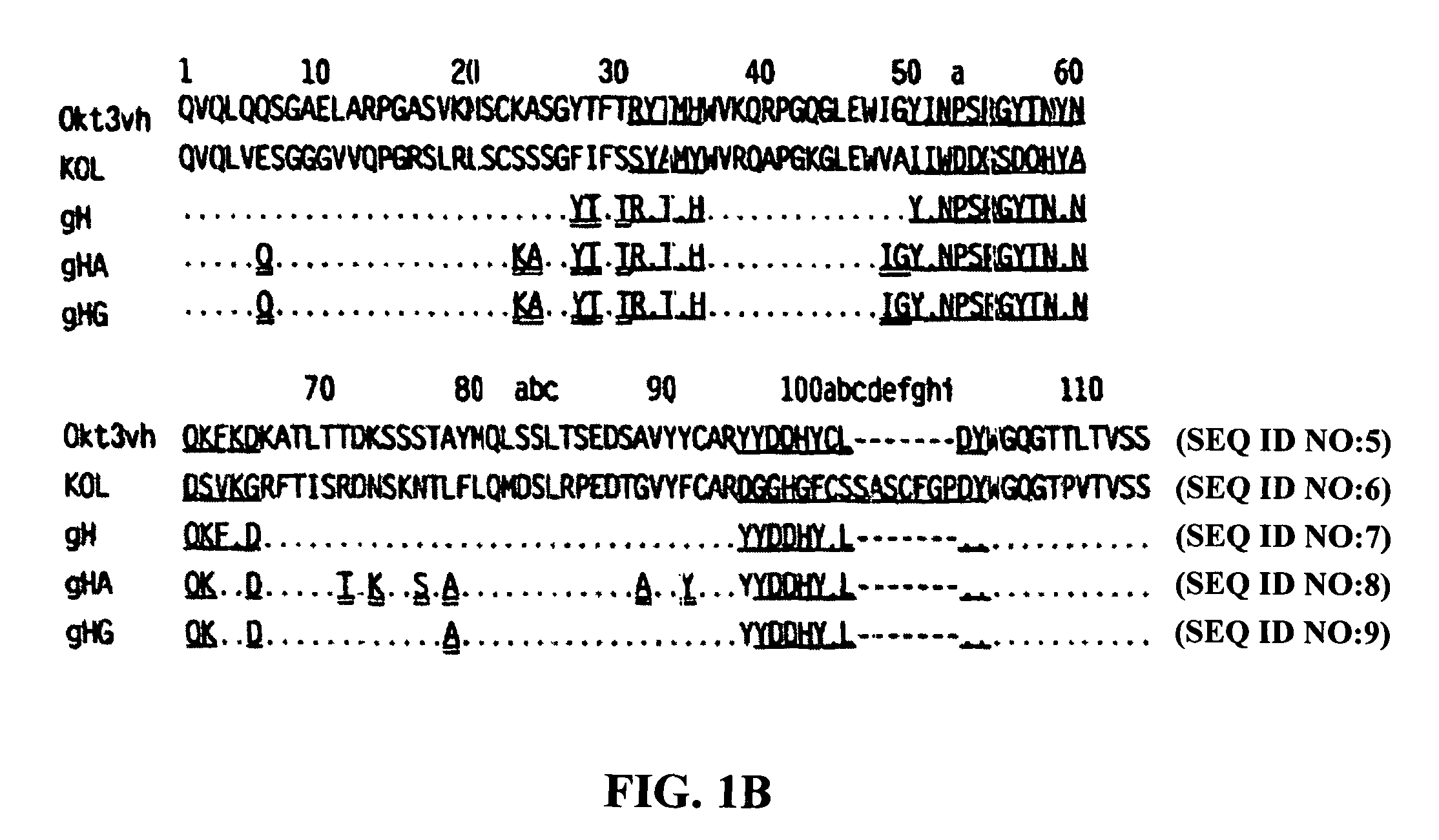
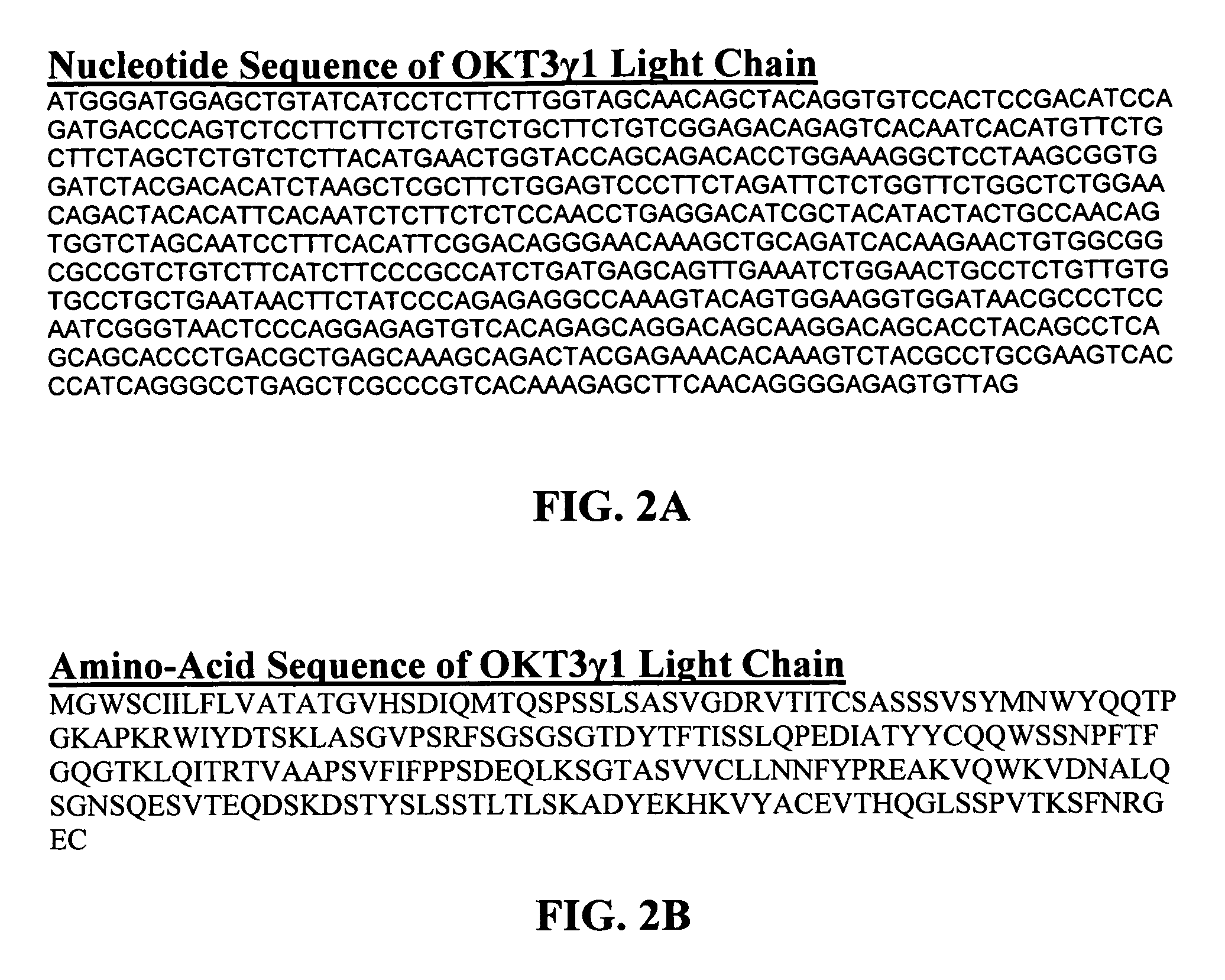
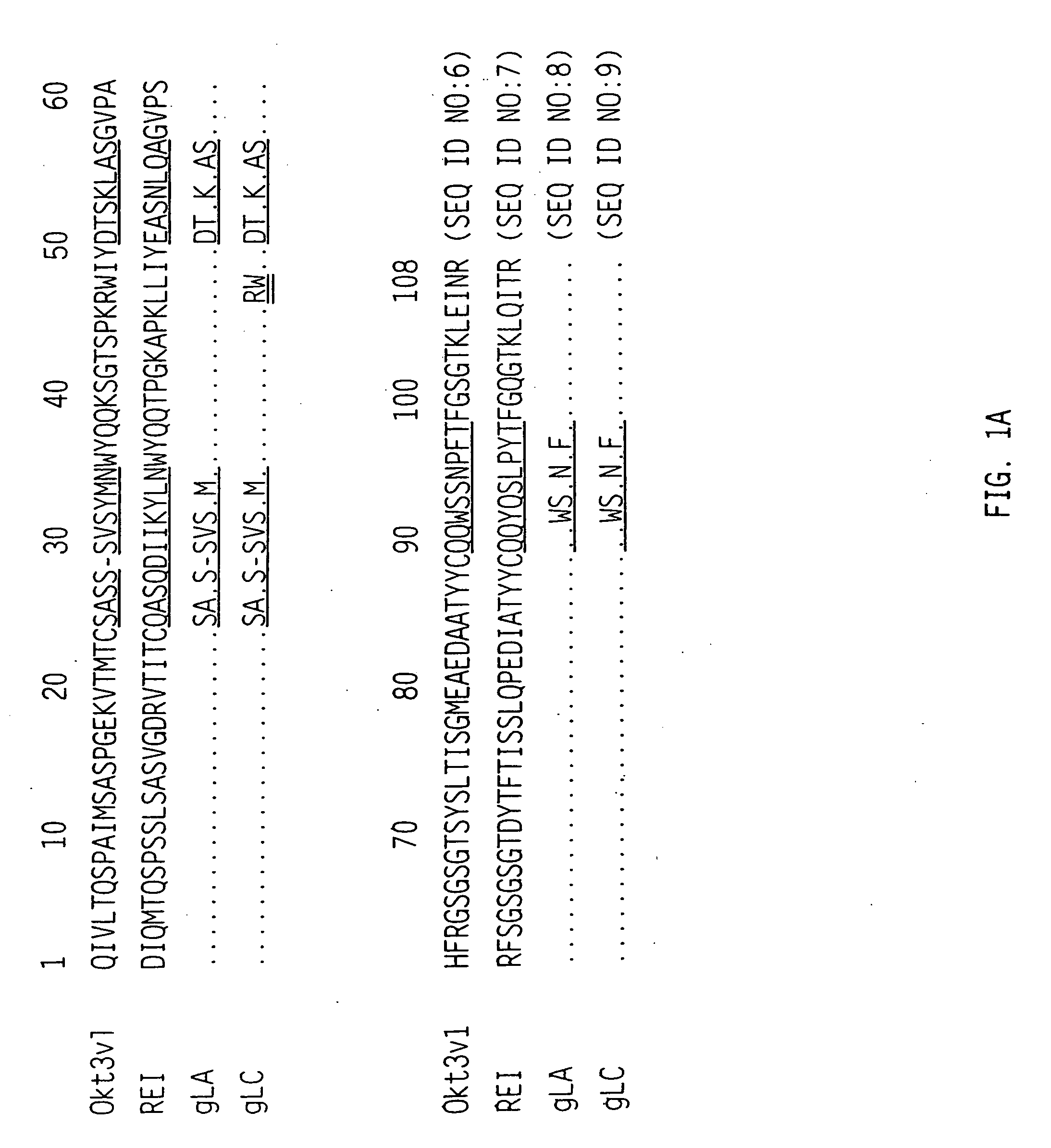
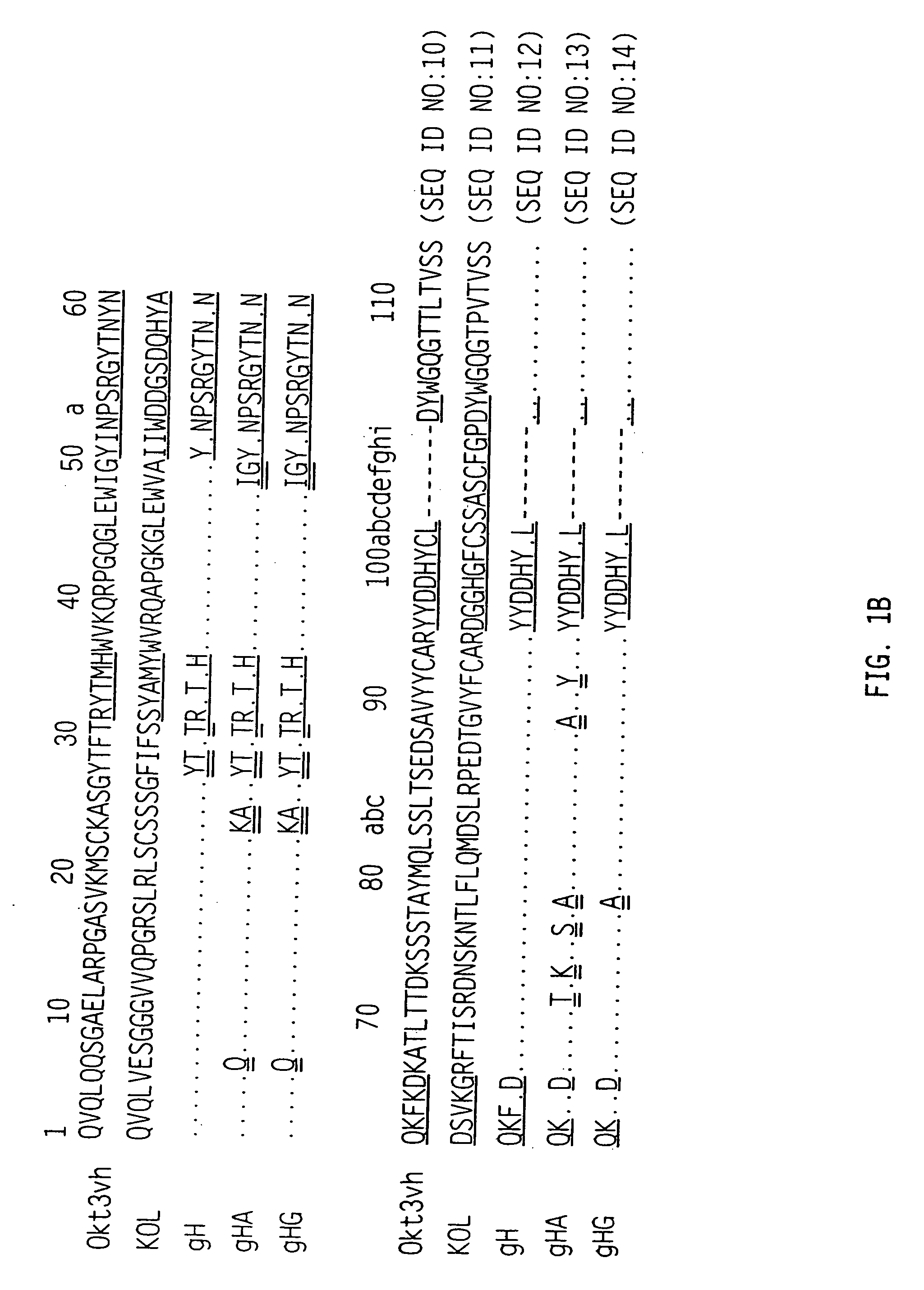
The binding specificity of the murine OKT3 has been transferred into a human antibody framework in order to reduce its immunogenicity. “Humanized” anti-CD3 mAbs, such as gOKT3-5 and gOKT3-7, have been shown to retain, in vitro, all the properties of native OKT3, including T cell activation which has been correlated, in vivo, with the severe side-effects observed in transplant recipients after the first administration of the mAb. Disclosed are modified versions of humanized anti-CD3 mAbs that do not have the property of T cell activation. Further dislosed are methods of using such mAbs.
Owner:BLUESTONE JEFFREY +2
Universal donor chimeric antigen receptor cells
InactiveUS20160237407A1Good curative effectImprove localizationPolypeptide with localisation/targeting motifImmunoglobulin superfamilyProgenitorCord blood stem cell
Disclosed are allogeneic cells useful for the treatment of cancer in a universal donor, off the shelf, manner. In one embodiment of the invention cord blood derived T cell progenitors are matured with anti-CD3 and anti-CD28, interleukin-7 and transfected with a construct encoding a chimeric antigen receptor (CAR) targeting a tumor antigen or a tumor endothelial associated antigen on the antigen binding domain. The intracellular domain containing CD3 zeta chain and at least one shRNA domain encoding a transcript which generates at least one siRNA capable of inhibiting expression of HLA I and / or HLA II. In another embodiment mesenchymal stem cells are transfected with CAR to enhance migration into tumors and induce tumor death, reduction of inflammation, or immune sensitization. In another embodiment universal donor CAR-MSC are disclosed.
Owner:BATU BIOLOGICS
Fully human anti-CD3 monoclonal antibodies
InactiveUS20060275292A1Prevent rejectionImprove survivalImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAutoimmune conditionAutoimmune disease
Non-toxic anti-CD3 antibody is useful for the treatment, prevention, and reversal of human autoimmune disease. Anti-CD3 antibody is generated by immunizing xenogenic mice capable of developing fully human antibodies. Because the inventive antibodies are not derived from other species, they do not the invoke the immune responses typically associated with humanized or grafted antibodies.
Owner:AMGEN FREMONT INC
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS8663634B2Treating and preventing and ameliorating symptomSlow and reduce damagePeptide/protein ingredientsAntipyreticDiseaseAutoimmune responses
The present invention provides methods of treating, preventing or ameliorating the symptoms of T cell-mediated immunological diseases, particularly autoimmune diseases, through the use of anti-CD3 antibodies. In particular, the methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-CD3 antibodies.
Owner:PROVENTION BIO INC
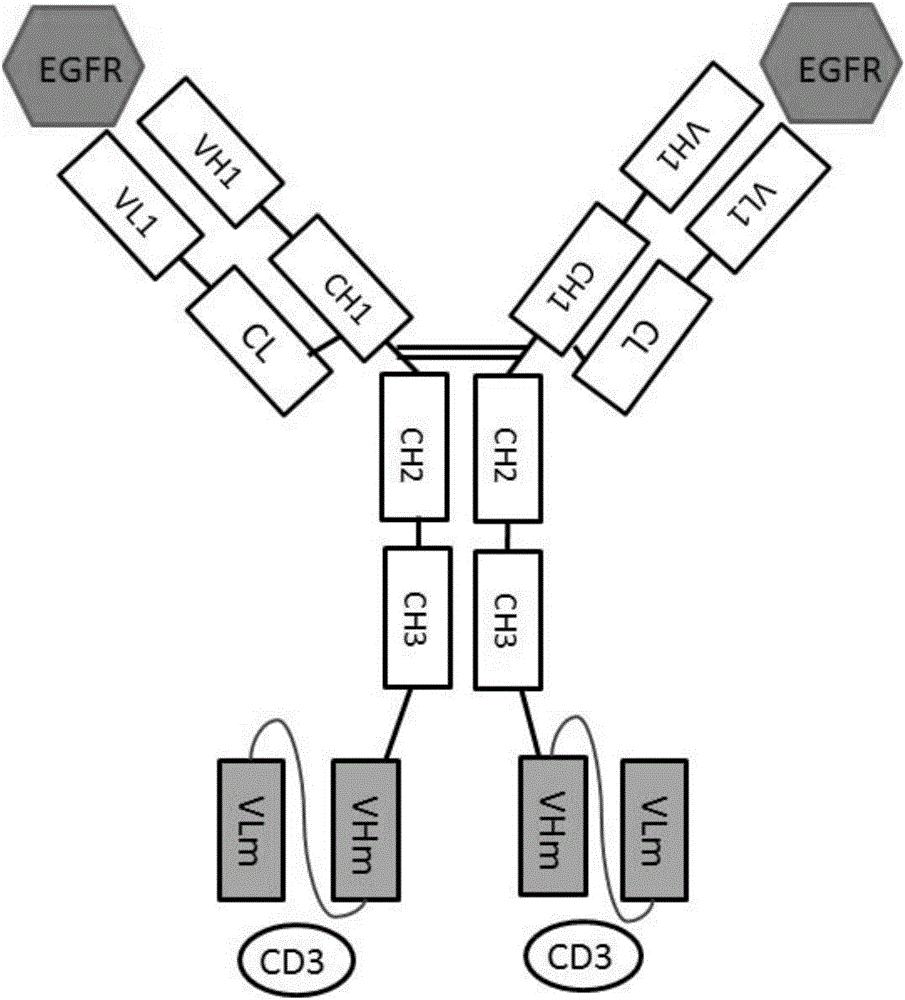
Anti-EGFR (epidermal growth factor receptor) and anti-CD3 (cluster of differentiation 3) bispecific antibody and application thereof
ActiveCN106632681AConducive to biological functionsConducive to biological functionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntigen
Owner:BEIJING DONGFANG BIOTECH +1
Growth method for natural killer cells
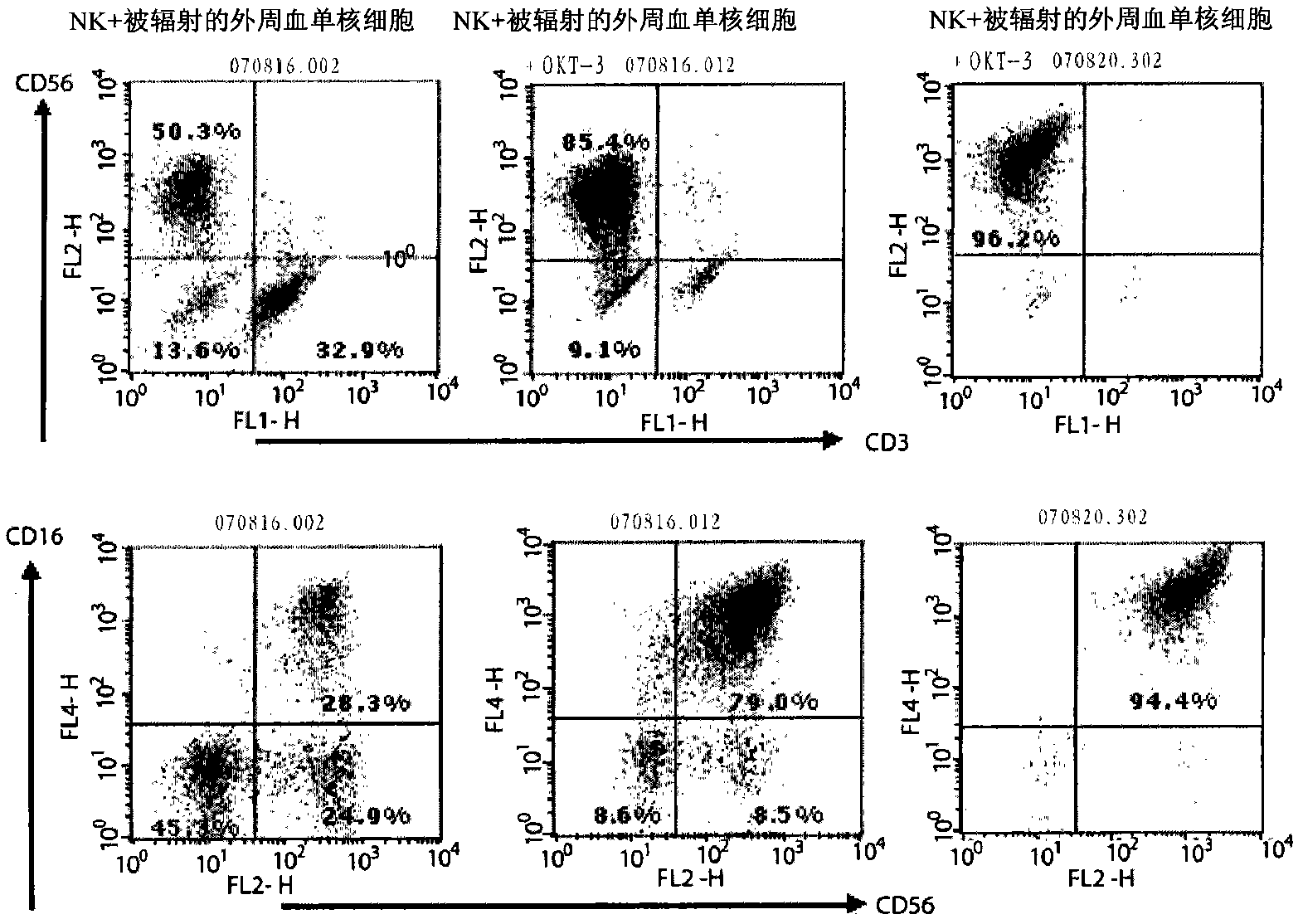
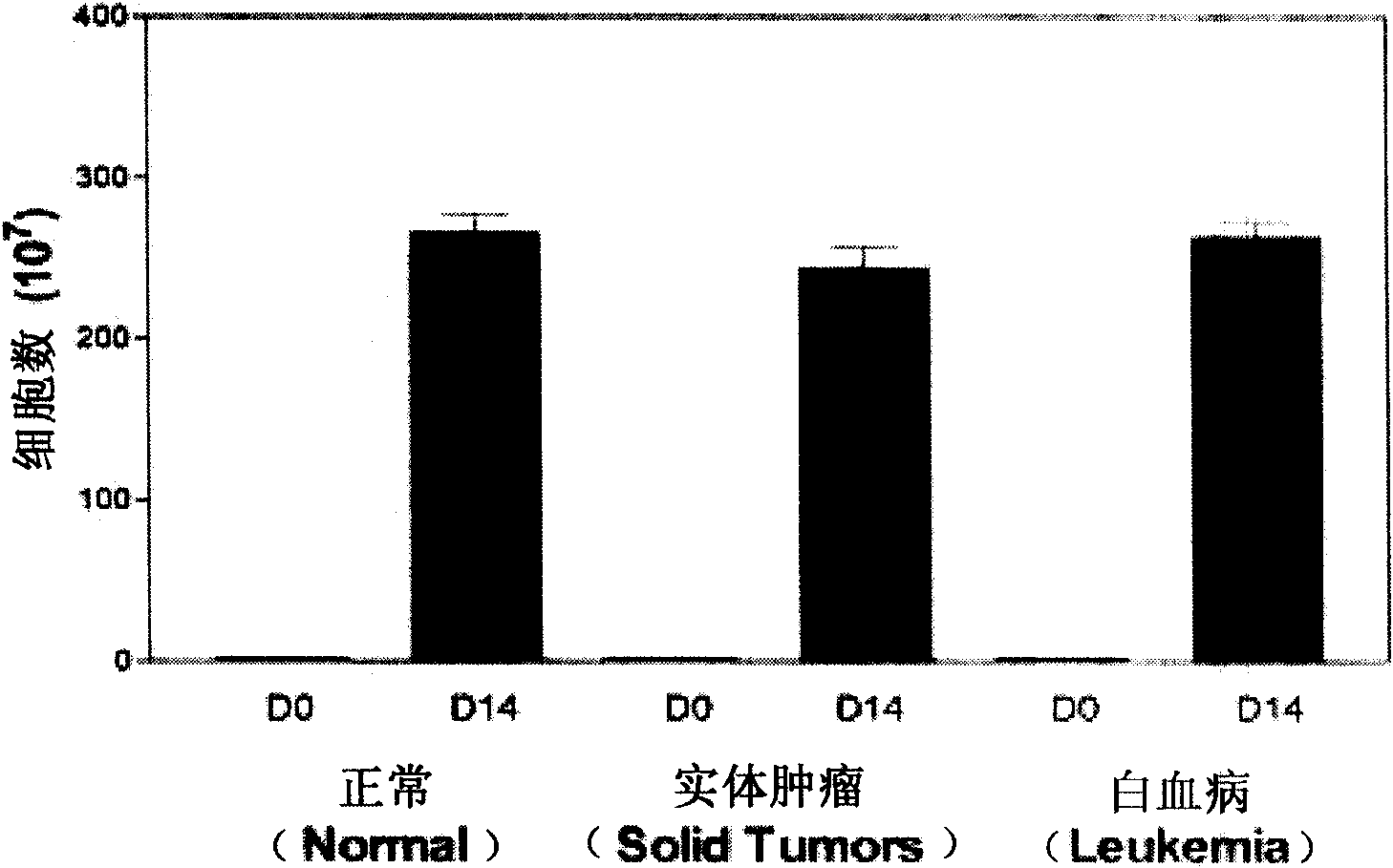
The present invention relates to an improved growth method for natural killer cells (NK cells). More specifically, the present invention relates to a growth method for natural killer cells, which comprises a step of culturing natural killer cells in a medium containing anti-CD3 antibodies and interleukin proteins in the presence of peripheral blood leukocytes. The present invention provides an innovative growth method for natural killer cells, which can obtain a large amount of natural killer cells by remarkably increasing the growth rate compared with conventional growth methods for natural killer cells.
Owner:GREEN CROSS LAB CELL CORP +1
Bispecific asymmetric heterodimers comprising anti-cd3 constructs
Disclosed herein are isolated multi-specific heteromultimer constructs that bind to CD3 expressed on T-cells and to an antigen expressed on B-cells. The multi-specific heteromultimer constructs are capable of bridging T- and B-cells and mediating killing of B-cells. The multi-specific heteromultimer constructs are based on a heterodimeric Fc scaffold or on a segmented albumin scaffold. Also disclosed herein are multi-specific heteromultimer constructs that bind to HER2 and HER3.
Owner:ZYMEWORKS BC INC
Methods and materials for modulation of the immunosuppressive activity and toxicity of monoclonal antibodies
InactiveUS20060292142A1Reduced activityLow affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectActivation cells
The binding specificity of the murine OKT3 has been transferred into a human antibody framework in order to reduce its immunogenicity. “Humanized” anti-CD3 mAbs, such as gOKT3-5 and gOKT3-7, have been shown to retain, in vitro, all the properties of native OKT3, including T cell activation which has been correlated, in vivo, with the severe side-effects observed in transplant recipients after the first administration of the mAb. Disclosed are modified versions of humanized anti-CD3 mAbs that do not have the property of T cell activation. Further dislosed are methods of using such mAbs.
Owner:BLUESTONE JEFFREY +2
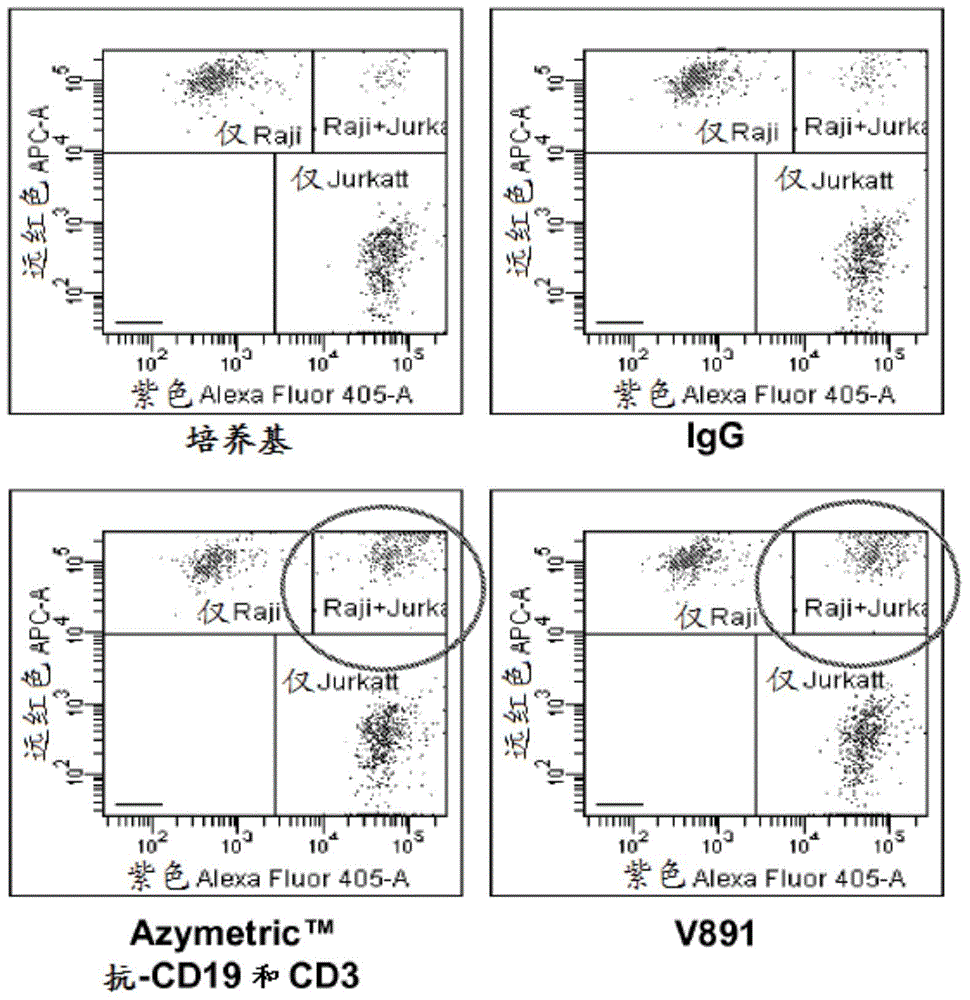
Anti-CD3/anti-CD19 dual-specific antibody and application thereof
InactiveCN105017422AHigh affinityAvoid side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSingle-Chain AntibodiesSide effect
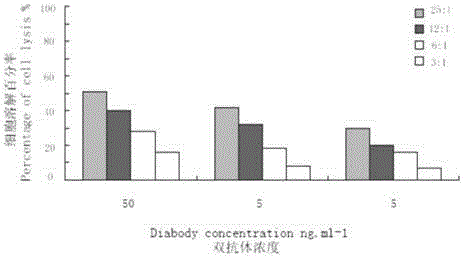
The invention discloses an anti-CD3 / anti-CD19 dual-specific antibody and an application thereof. The anti-CD3 / anti-CD19 dual-specific antibody comprises the combined structural domain of a completely-humanized anti-CD3 single-chain antibody and a completely-humanized anti-CD19 single-chain antibody. The variable region amino acid sequence of heavy chains and light chains of the completely-humanized anti-CD3 single-chain antibody is shown as the SEQ ID NO:7-8. The variable region amino acid sequence of heavy chains and light chains of the completely-humanized anti-CD19 single-chain antibody is shown as the SEQ ID NO:15-16. The anti-CD3 / anti-CD19 dual-specific antibody has high affinity to CD3 molecules and CD19 molecules, and has the effect of conducting mediated activation on T lymphocytes to kill tumor cells. Moreover, the heavy chains and the light chains of the antibody molecules are completely humanized, and thus plenty of side effects are avoided.
Owner:SHANDONG BAYONN PHARMA TECH
Methods of treatment of Ulcerative Colitis and Crohn's disease with anti-CD3 antibodies
InactiveUS20070224191A1Prevent proliferationInhibition of activationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsUlcerative colitisAutoimmune disease
The present invention provides a method of treating autoimmune diseases. In particular, it provides a method for the treatment of ulcerative colitis or Crohn's disease comprising administering to a subject a therapeutically effective amount of a pharmaceutical formulation comprising an antibody, wherein said antibody binds to CD3.
Owner:PDL BIOPHARMA INCORPORATED
Gene therapy for the prevention of autoimmune disease
Autoimmune disease is treated by the delivery of a suppressive agent to the site of disease. Delivery is accomplished by introducing an expression vector encoding the suppressive agent into cells targeted for such sites, and administering the genetically modified cells to the patient. Suppressive agents of particular interest include IL-4; and anti-CD3 antibodies, particularly single chain anti-CD3 antibodies. Cells of interest for delivery include T cells and T cell hybridomas, where the T cell antigen receptor recognizes epitopes associated with the autoimmune disease. Alternatively, dendritic cells are used as delivery vectors.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
A method for cultivating self activated lymphocyte
InactiveCN101603029ALittle side effectsImproved prognosisCancer antigen ingredientsBlood/immune system cellsCancer cellCD16
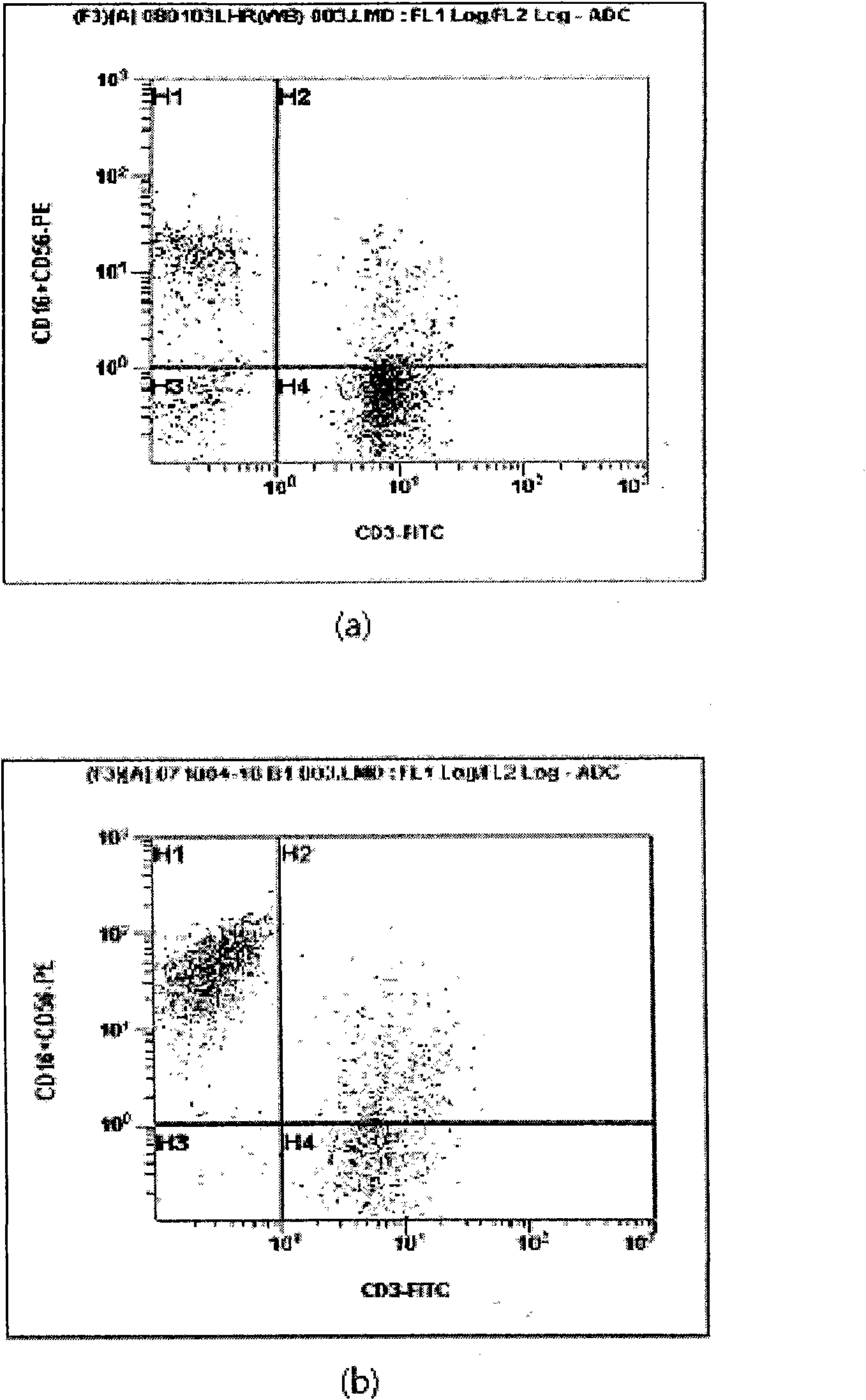
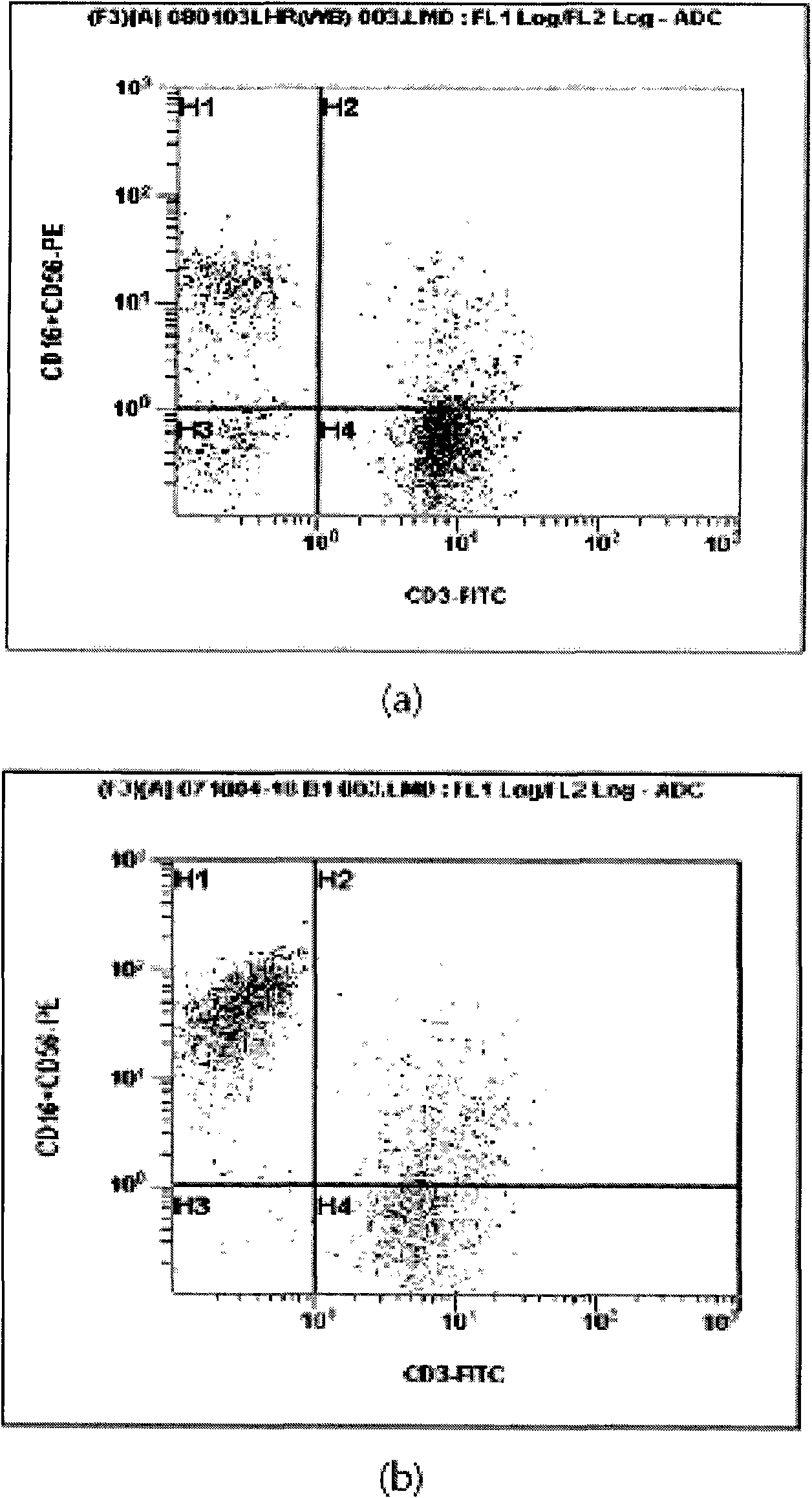
Provided is a method of culturing self-activated lymphocytes applicable to the treatment of malignant tumors. The method raises the percentage's of natural killer (NK) cells of the lymphocytes and evenly activate the NK cells, T cells and natural killer T (NKT) cells, and thus can be used to effectively eliminate various kinds of cancer cells. The method of culturing self- activated lymphocytes involves: extracting lymphocytes from human peripheral blood; performing a first culturing step of culturing the extracted lymphocytes in a culture fluid to which IL-2, L-glutamine and autochthonous plasma are added, in the presence of anti-CD3, anti- CD 16, and anti-CD56 antibodies; and performing a second culturing step of culturing the mixed culture fluid resulting from the first culturing step in the presence of anti-CD3, anti-CD16, and anti-CD56 antibodies after being admixed with a culture fluid to which IL-2, L-glutamine and autochthonous plasma are added.
Owner:NKBIO
Anti human ovarian cancer-anti cd3 bispecific antibody
InactiveUS20050255115A1Low toxicityImprove efficiencyAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionBispecific antibody
The present invention provides an anti-ovarian cancer bispecific antibody. Said antibody includes two polypeptide domains connected by a polypeptide linker, one is anti-ovarian cancer antibody, or its Fab fragment, single complementarity determining region (CDR) antibody or single chain Fv (scFv) and the other is anti-CD3 antibody, or its Fab fragment, single CDR antibody or scFv. The present invention also provides DNA sequences encoding said antibody, an expression vector containing said DNA sequence, and a host cell containing said expression vector.
Owner:DONGGUAN HAOFA BIOTECH DEVAL +2
A medium composition for cultivating self activated lymphocyte
InactiveCN101603028AEnhance killing activityLittle side effectsCancer antigen ingredientsBlood/immune system cellsCD16Cell culture media
Provided is a medium composition for culturing self- activated lymphocytes applicable to the treatment of malignant tumors, to which anti-CD3, anti-CD16 and anti-CD56 antibodies are added along with interleukin2 (IL-2) to evenly activate natural killer (NK) cells, T cells and natural killer T (NKT) cells, and thus the medium composition can be used to culture im- munocytes that can effectively treat various kinds of malignant tumors. The medium composition includes a cell culture medium and additives added to the cell culture medium, wherein the additives include interleukin2 (IL-2), anti-CD3 antibodies, anti-CD16 antibodies, and anti-CD56 antibodies.
Owner:NKBIO
Fusion protein of anti-cluster of differetiation (CD3) antibody Fv segment and interleukin 3, method for preparing fusion protein and application of fusion protein
InactiveCN102796198APreserve associativityEnhance killing activityFungiBacteriaGenetic engineeringBiology
The invention discloses a fusion protein of an anti-cluster of differetiation (CD3) antibody Fv segment and an interleukin 3, a method for preparing the fusion protein and an application of the fusion protein, relates to the fusion proteins of anti-CD3 star-delta IL3 and anti-CD3-delta IL3 and encoded genes thereof, and further relates to a genetic engineering construction method and an application of the strengthened fusion protein.
Owner:中国医学科学院血液病医院
Method for treating tumors, infectious diseases, and autoimmune diseases with an activated HLA matching donor-originating lymphocyte
InactiveUS20080014174A1Effective treatmentHigh antineoplastic effect and antiviral effectBiocidePeptide/protein ingredientsAbnormal tissue growthAutoimmune condition
According to the present invention, HLA matching donor-originating activated lymphocytes highly effective in the treatment of patients with tumors, viral infections and autoimmune diseases are prepared by collecting cells contained in peripheral blood originating from an HLA matching donor or peripheral blood originating from an HLA matching donor achieving micro-chimerism and by stimulating and propagating the cells thus collected with, for instance, interleukin 2 and anti-CD3 antibodies or the like. The HLA matching donor-originating activated lymphocytes thus prepared can be administered to an HLA matching patient with a tumor, a viral infection or an autoimmune disease for therapeutic or preventive purposes.
Owner:LYMPHOTEC
Peptide mimotopes of the cd3 t-cell co-receptor epsilon chain and uses thereof
ActiveUS20190070248A1Optimization of binding propertyGenerate and inhibit immune responseImmunoglobulin superfamilyPeptide/protein ingredientsAntigenBinding domain
The present invention provides molecules that mimic antigenic determinants of the CD3 (cluster of differentiation 3) T-cell co-receptor epsilon chain (CD3ε). These molecules compete with CD3ε for binding to a CD3ε binding domain. e.g. a CD3ε binding domain of an antibody, and are capable of detecting antibodies against CD3ε. The mimotopes of the invention may be used to generate or inhibit immune responses in animals and preferably humans. Additionally, they may serve as tools for anti-CD3ε antibody purification and the detection of anti-CD3ε antibodies in biological samples.
Owner:JPT PEPTIDE TECH +1
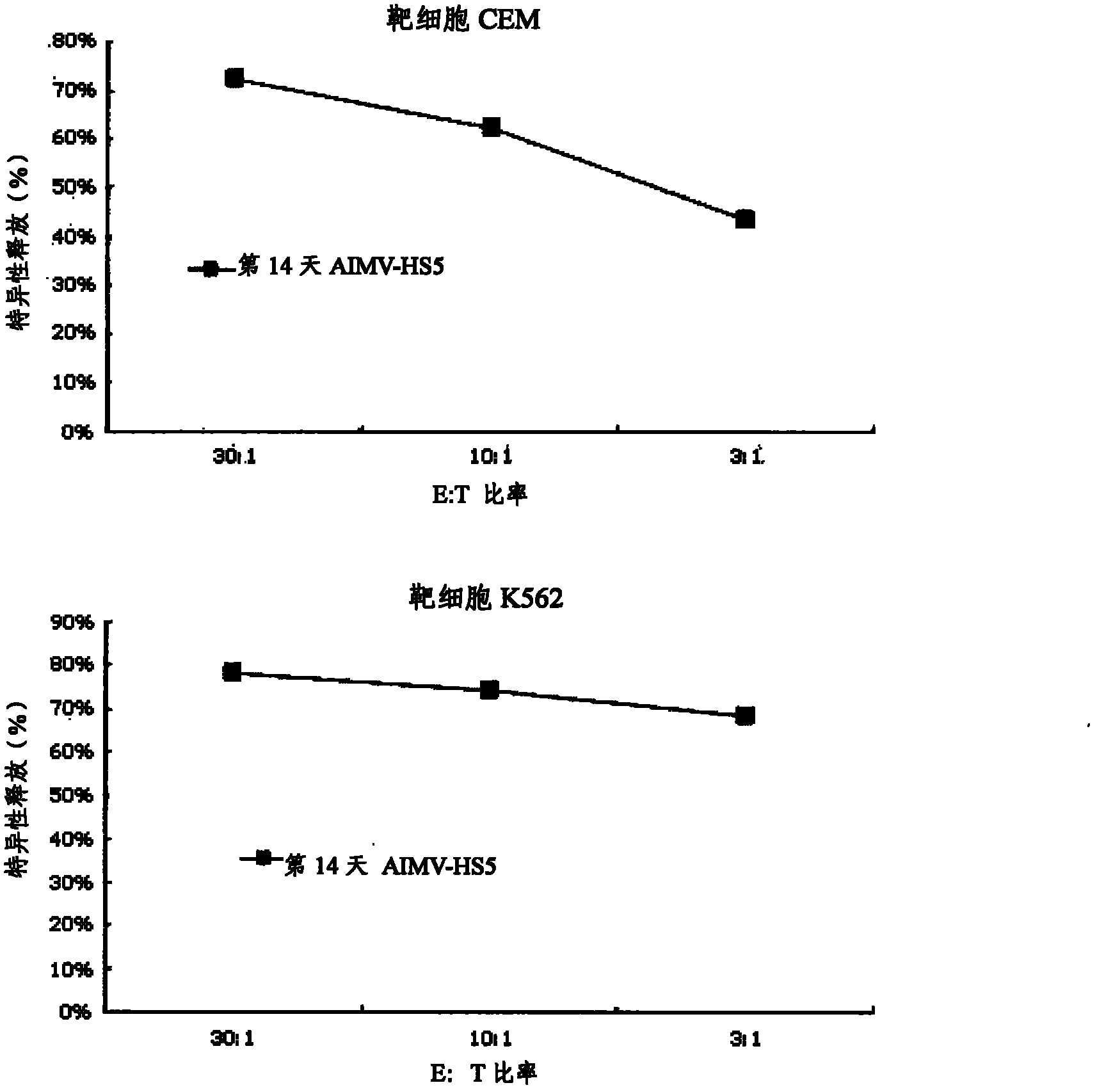
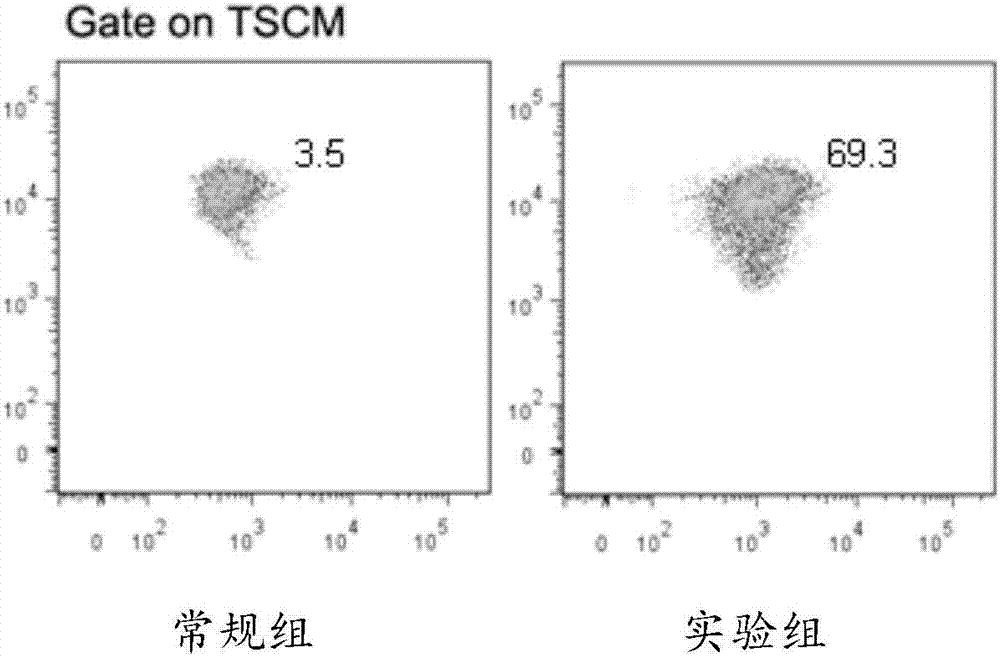
Lymphocyte population with memory stem T cells as main component and in-vitro efficient amplification method of lymphocyte population
ActiveCN107475192AReduce freezingReduce dosageCulture processBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
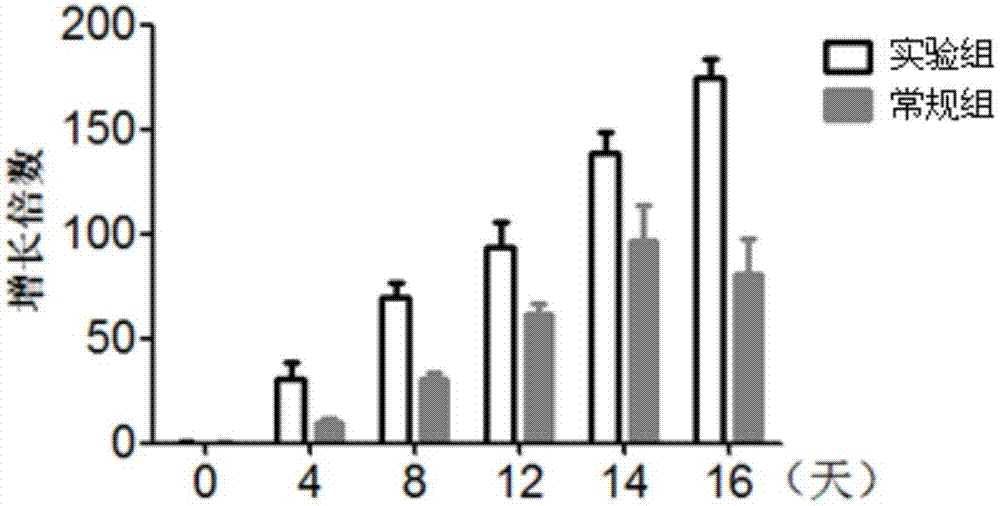
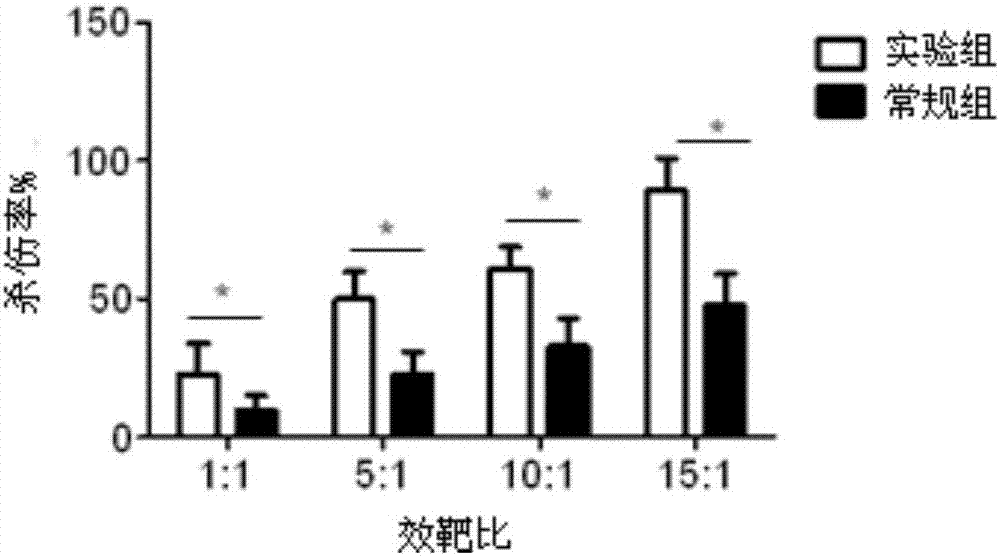
The invention discloses a lymphocyte population with memory stem T cells as a main component and an in-vitro efficient amplification method of the lymphocyte population. The method comprises the steps as follows: peripheral blood mononuclear cells from healthy individuals are transferred into a culture flask precoated with anti-CD3 mAb, anti-CD28McAb and Novonectin, a serum-free medium containing IL-1alpha and IFN-gamma is added, a combination of multiple cytokines including VC, NAC, RAPA, IL-7, IL-15 and IL-21 is added, and then the serum-free medium and the combination of the multiple cytokines are supplemented; and the lymphocyte population with the memory stem T cells as the main component can be obtained after culture for 12 to 16 days. The combined application of the cytokines can have synergistic effect on amplification of lymphocytes, the amplification times are increased, and the in-vitro killing property and survival time are increased obviously; the method has the advantages of high safety, low cost and the like.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com