Growth method for natural killer cells
A technology of natural killer cells and lymphocytes, applied in animal cells, hybrid cell preparation, vertebrate cells, etc., can solve problems such as lack of security and failure to provide proliferation methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of feeder cells and isolation of natural killer cells (NK cells)
[0055] (1) Preparation of feeder cells
[0056] Take 20ml of peripheral blood from a healthy person, and put 5ml of the collected blood into a conical tube with a volume of 15ml. Add 5ml of normal saline to the blood and stir evenly with a pipette. Add 5ml of lymphocyte separation solution (ficoll) (GE healthcare, Uppsala, 17-1440-03) to a new 15ml conical tube with a volume capacity, and carefully add After the above-mentioned stirred (diluted) blood was centrifuged at 2000 rpm at room temperature for 30 minutes (Hanil Group, Korea, Union32-R).
[0057] After taking out the immune cell layer formed between the lymphocyte separation solution and plasma and moving it to a new 15ml conical tube, add HBSS to 10ml, mix the cells evenly, and then centrifuge at 1200rpm for 10 minutes. The supernatant was removed by vacuum suction. Then add 10ml of HBSS and repeat the centrifugation p...
Embodiment 2
[0066] Example 2: Culture of isolated natural killer cells
[0067] Nascent cells were cultured in 12-well plates (Falcon). Put 500ul of the feeder cells prepared in Example 1-(1) into the wells, and add 500ul of the natural killer cells separated in Example 1-(2) into the wells containing the feeder cells.
[0068] After adding IL-2 (Norvatis) cytokine at a concentration of 500 U / ml and OKT-3 antibody (ebioscience, 16-0037) at a concentration of 500 U / ml to each well containing cells, shake the well plate carefully to make the cells Mix well with cytokines.
[0069] The orifice plate was placed in a humid incubator at 37° C. containing 5% carbon dioxide for 5 days of culture, at this time, no culture medium or cytokines were added.
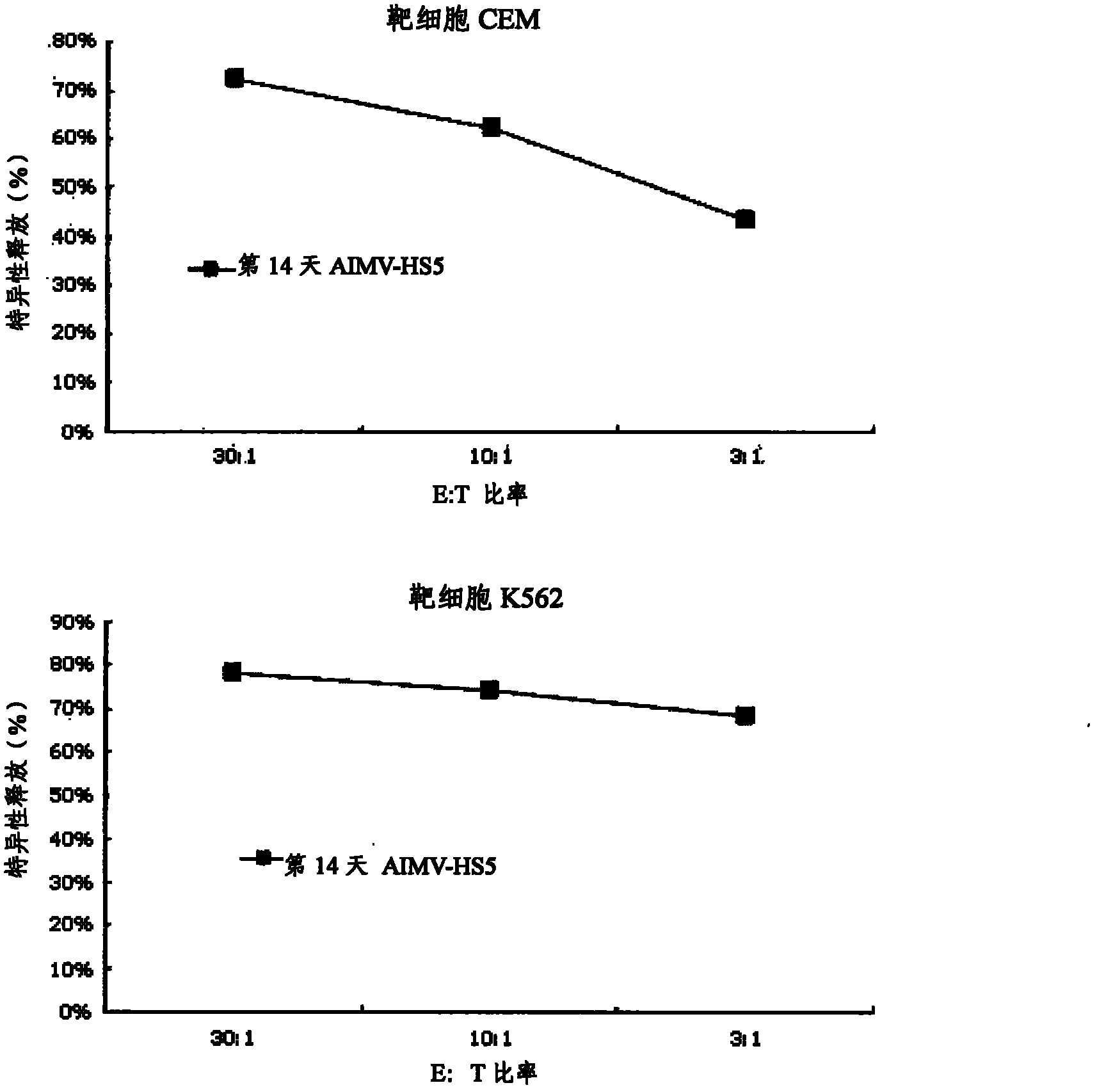
[0070] Counting from the culture day to day 5, pipette all the cells in the wells containing the cells into a 15 ml conical tube. 1 ml of cell culture solution was added to each well from which the cells were removed, and the remaining cells w...
Embodiment 3
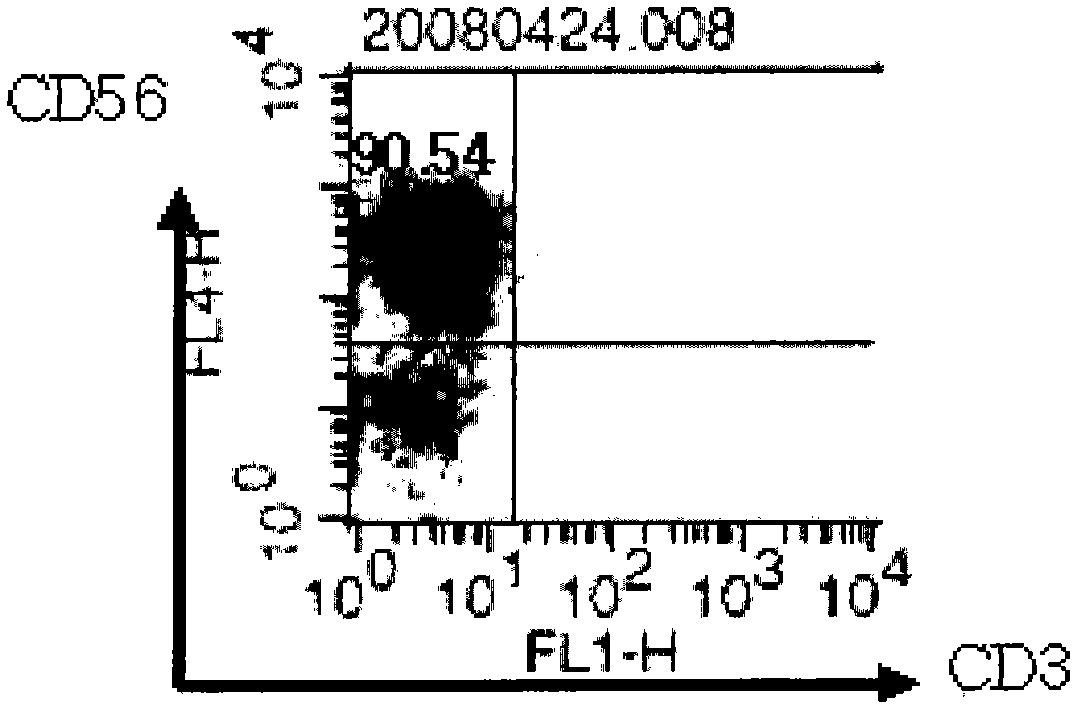
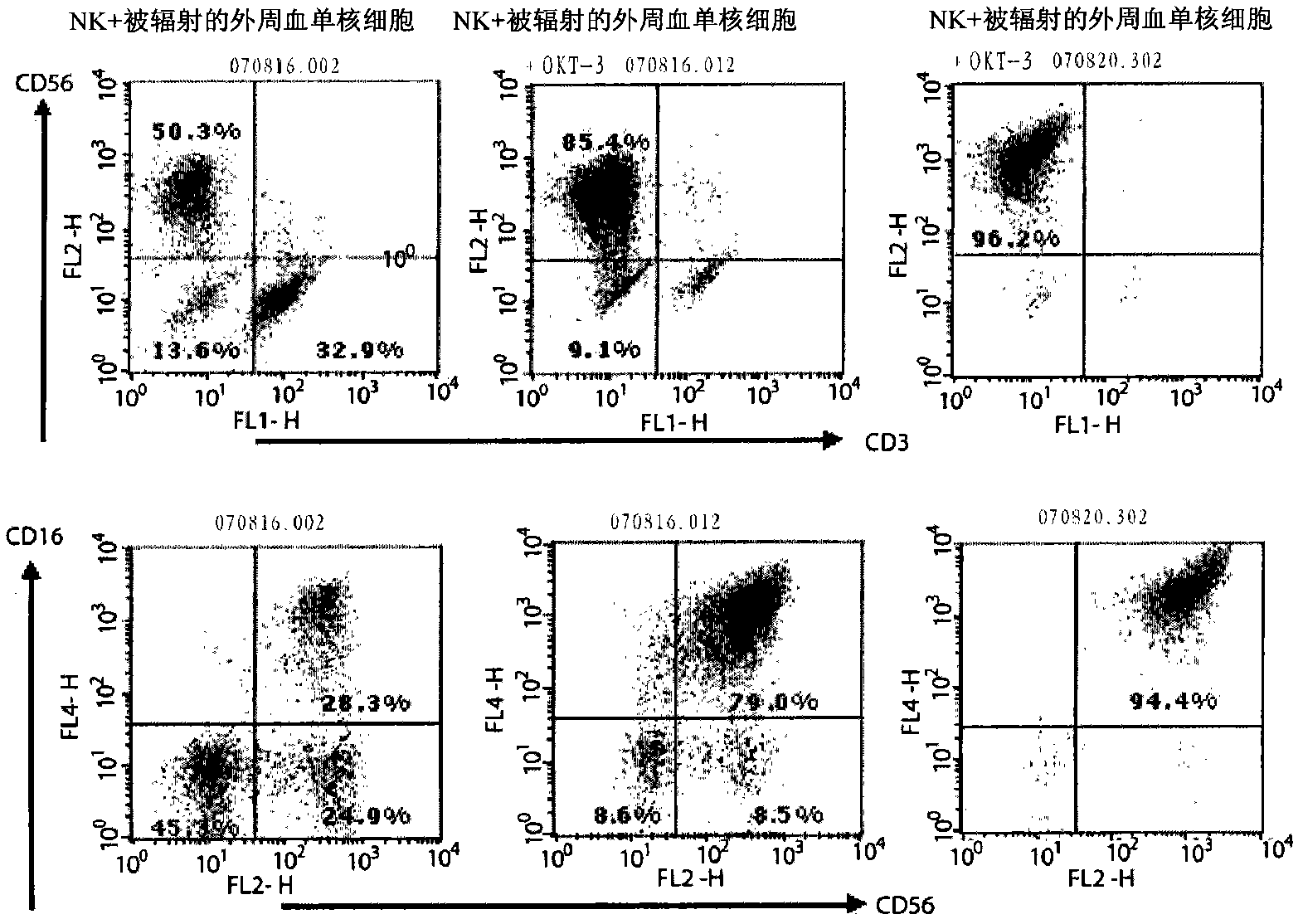
[0076] Example 3: Analysis of the surface morphology of the obtained NK-cells
[0077] After removing OKT-3, only IL-2 was treated alone. On the 7th and 10th day, a part of the cells were harvested for surface morphology analysis.
[0078] Collect the cells before, during, or after the culture, centrifuge at 12000 rpm for 5 minutes, and remove the culture solution by vacuum suction. After diluting with 1 ml of FACS buffer (2.5% FBS+PBS), the number of cells was determined and diluted to 5×10 with FACS buffer. 6 cells / ml. 100 ul of the diluted cell solution was added to a FACS tube (Falcon), and antibodies were added as follows.
[0079] Test tube 1: no staining
[0080] Tube 2: anti-human CD3-FITC (BD Pharmingen, 5555339) + anti-human CD56-APC (BD Pharmingen, 555518) + anti-human CD16-PE (BD Pharmingen, 555407)
[0081] Tube 3: Anti-CD16-FITC (color control) (BD Pharmingen, 555406)
[0082] Tube 4: Anti-CD56-PE (color control) (BD Pharmingen, 555516)
[0083] Tube 5: ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com