Detection and applications of microRNA serum marker of biliary atresia and cholestatic infant hepatitis syndrome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
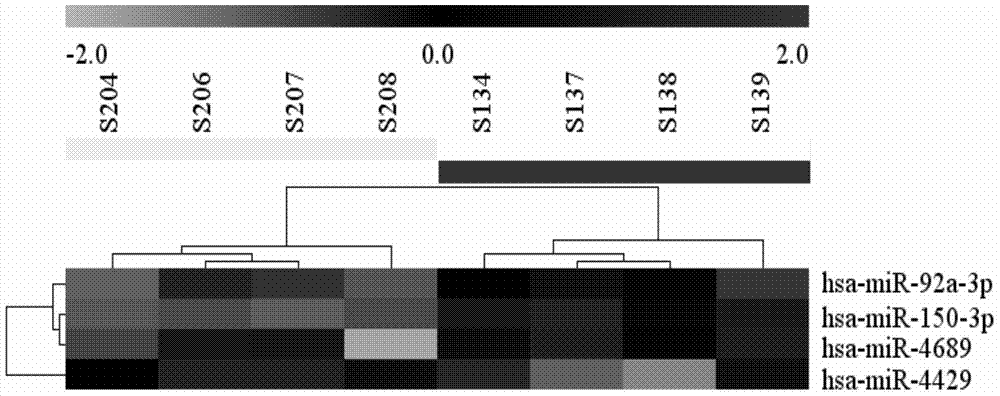
Image
Examples
Embodiment 1
[0076] The preparation of embodiment 1 RNA sample
[0077] Serum samples from 40 biliary atresia and 35 cholestatic infantile hepatitis syndrome were obtained from the Children's Hospital of Fudan University. All the above-mentioned specimens were obtained with the approval of the ethics committee of the WHO partner organization authorized by the Shanghai Municipal Government. Using mirVanaTM PARISTM (miRNA specially used for extracting blood) (Cat#AM1556, Ambion, Austin, TX, US) and according to the standard operating procedure provided by the manufacturer, the total RNA extraction of the sample was carried out, and the extracted total RNA was passed through Agilent Bioanalyzer2100 (Agilent technologies, Santa Clara, CA, US) After passing the electrophoresis quality inspection, it is ready for use. Gene chip: microRNA expression profile chip, using miRNA expression profile chip (single-channel chip) from Agilent Co., Ltd.
Embodiment 2
[0078] Extraction and labeling of embodiment 2microRNA
[0079] The miRNAs were extracted with Ambion's miRNAs extraction kit, and the specific operations were performed according to the corresponding instructions. Samples were labeled with T4 RNA ligase according to Thomson's method.
[0080] In short, here's how:
[0081] (1) Obtain total RNA from Example 1, then use Ambion's miRNAs extraction kit to extract miRNA; take out 5'-phosphate-cytosine-uracil-cy3-3' of 1.4ug miRNA and 500ng ( Dharmacon, Chicago, USA) and 2 units of T4RNA ligase (NEB, Ipswich, USA), incubated at 4°C for 2 hours to label miRNA. An equal amount of corresponding negative control was set up for each miRNA sample.
[0082] (2) The labeled RNA was precipitated with 0.3M sodium acetate and 2.5 volumes of ethanol, and then resuspended with 15u1 hybridization solution containing 3X SSC, 0.2% SDS and 15% formamide. All hybridizations were repeated twice, and the hybridization was performed with LifterSlip ...
Embodiment 3
[0084] Example 3 Quantitative PCR kit detects microRNA expression
[0085] (1) microRNA reverse transcription
[0086] Using ABI Reverse Transcription Kit (Applied Biosystems),
[0087] The reaction system is as follows:
[0088] 100mM dNTPs (with dTTP)0.2μl
[0089] MultiScribe TM Reverse Transcriptase, 50U / μL1μl
[0090] 10×Reverse Transcription Buffer1μl
[0091] RNase Inhibitor, 20U / μL0.13μl
[0092] RT Primer for microRNA (1μl each)
[0093] RNA (10ng / μl) 3μl
[0094] Water 1.67μl
[0095] Total10μl
[0096] (2) MicroRNA fluorescence quantitative PCR detection
[0097] Using TaqMan MicroRNA Kit (Applied Biosystems),
[0098] The reaction system is as follows:
[0099] TaqMan MicroRNA Assay (20×) 0.5μl (probe halved)
[0100] Template: 2ul diluent (stock solution diluted 10 times)
[0101] PCR Mix10ul
[0102] Water 7.5ul
[0103] Total20ul
[0104] The reaction conditions are as follows:
[0105] 95 degrees 5min
[0106] 95 degrees 10s40cycles
[0107]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com