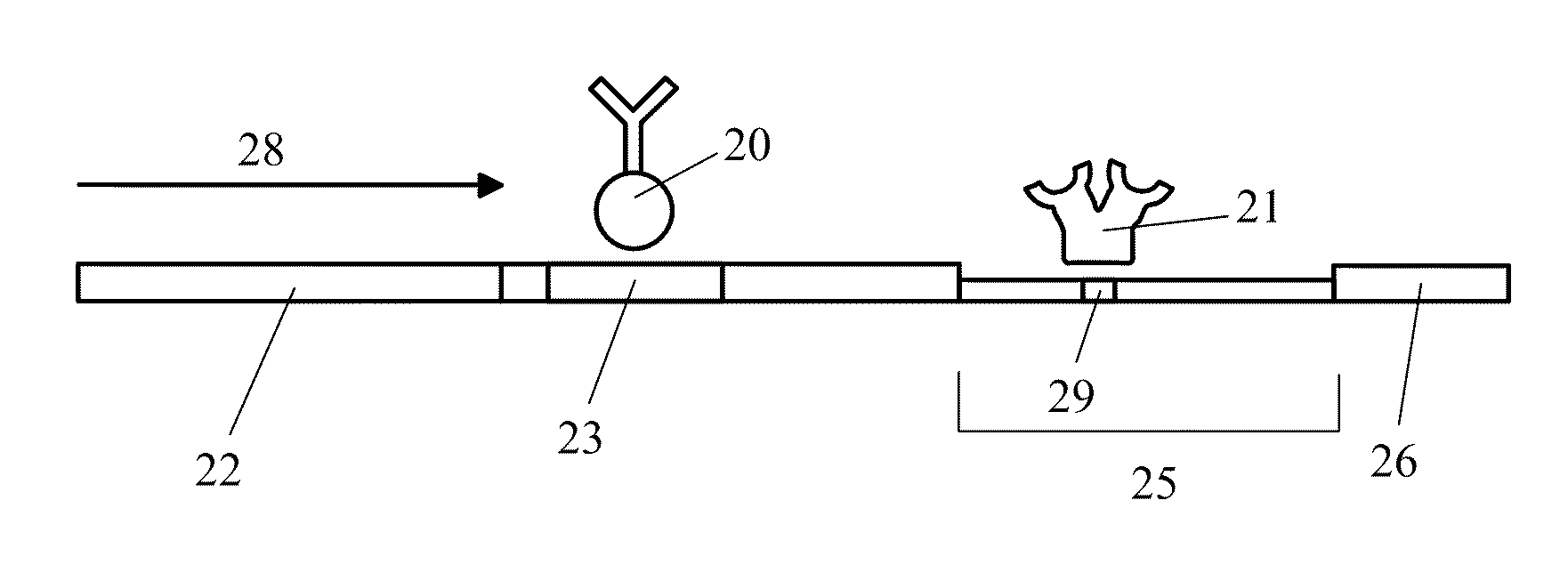
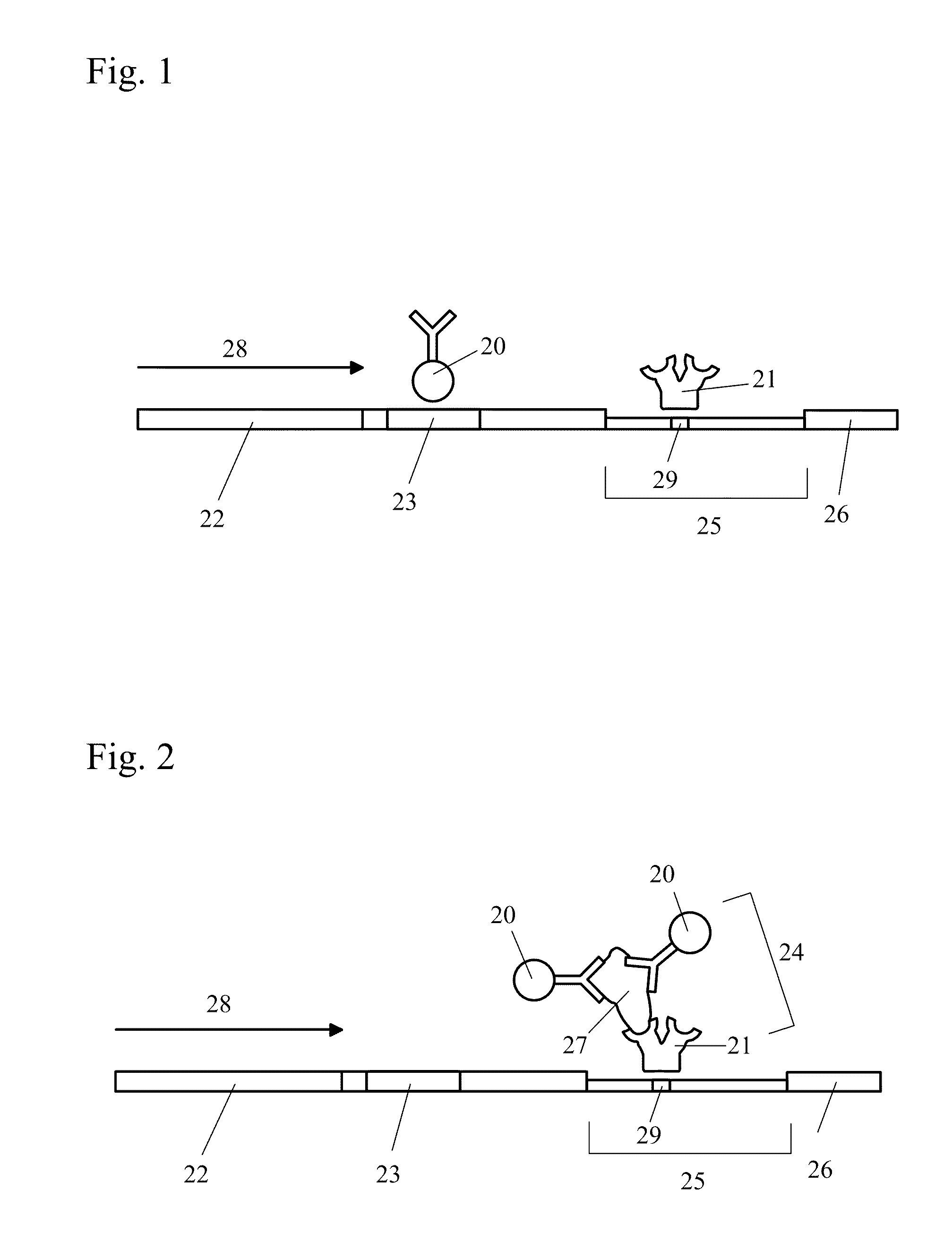
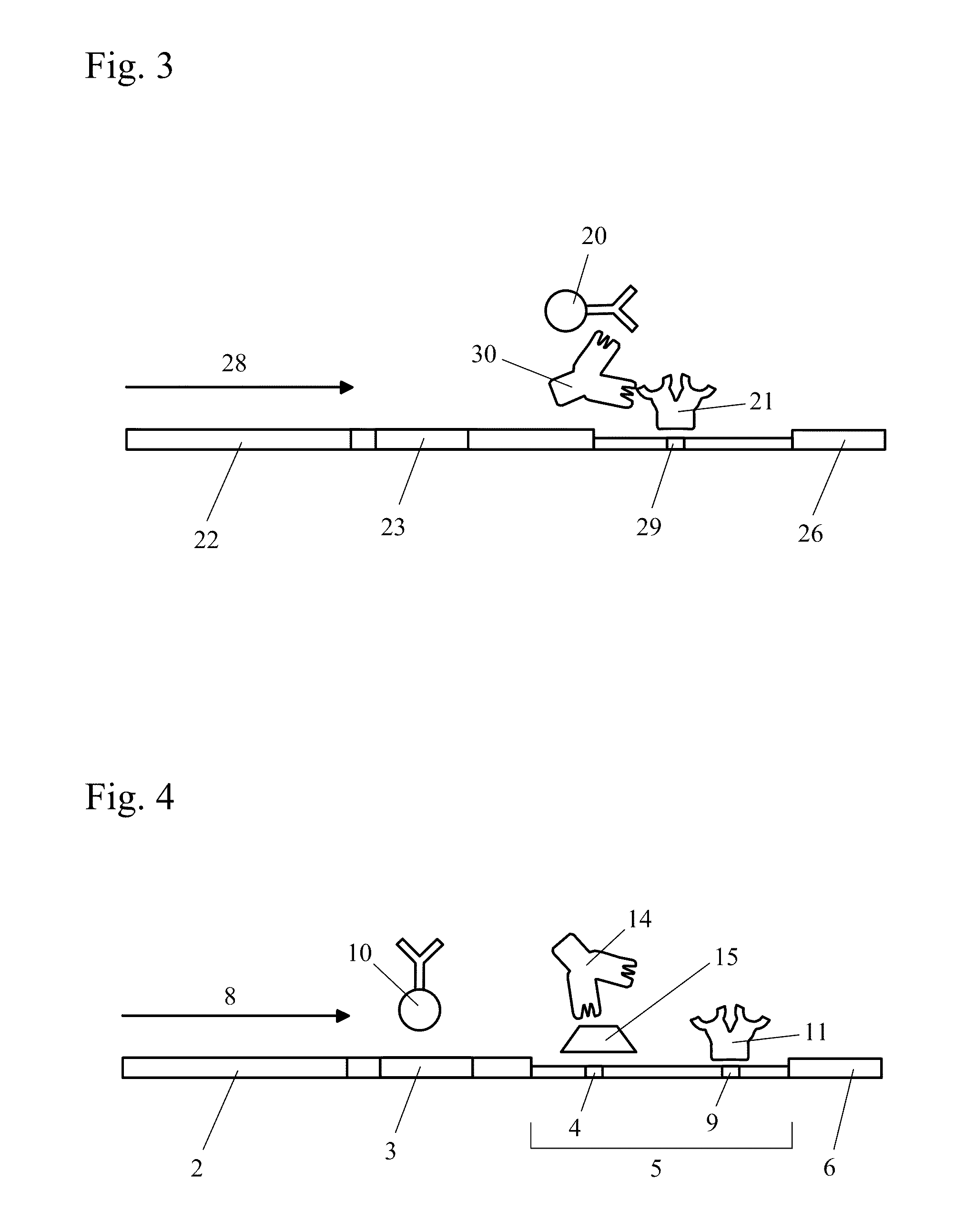
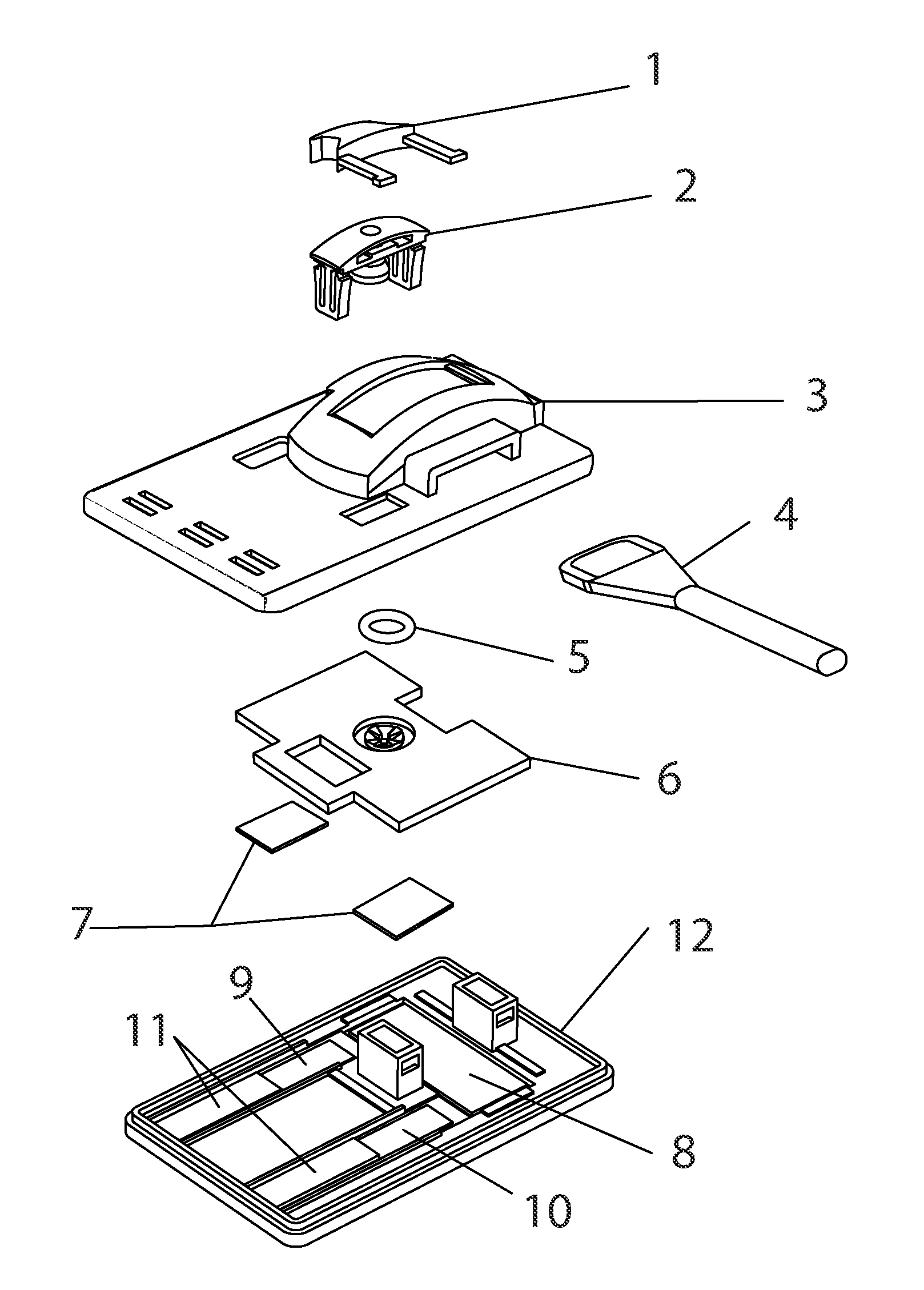
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Lateral flow immunoassay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
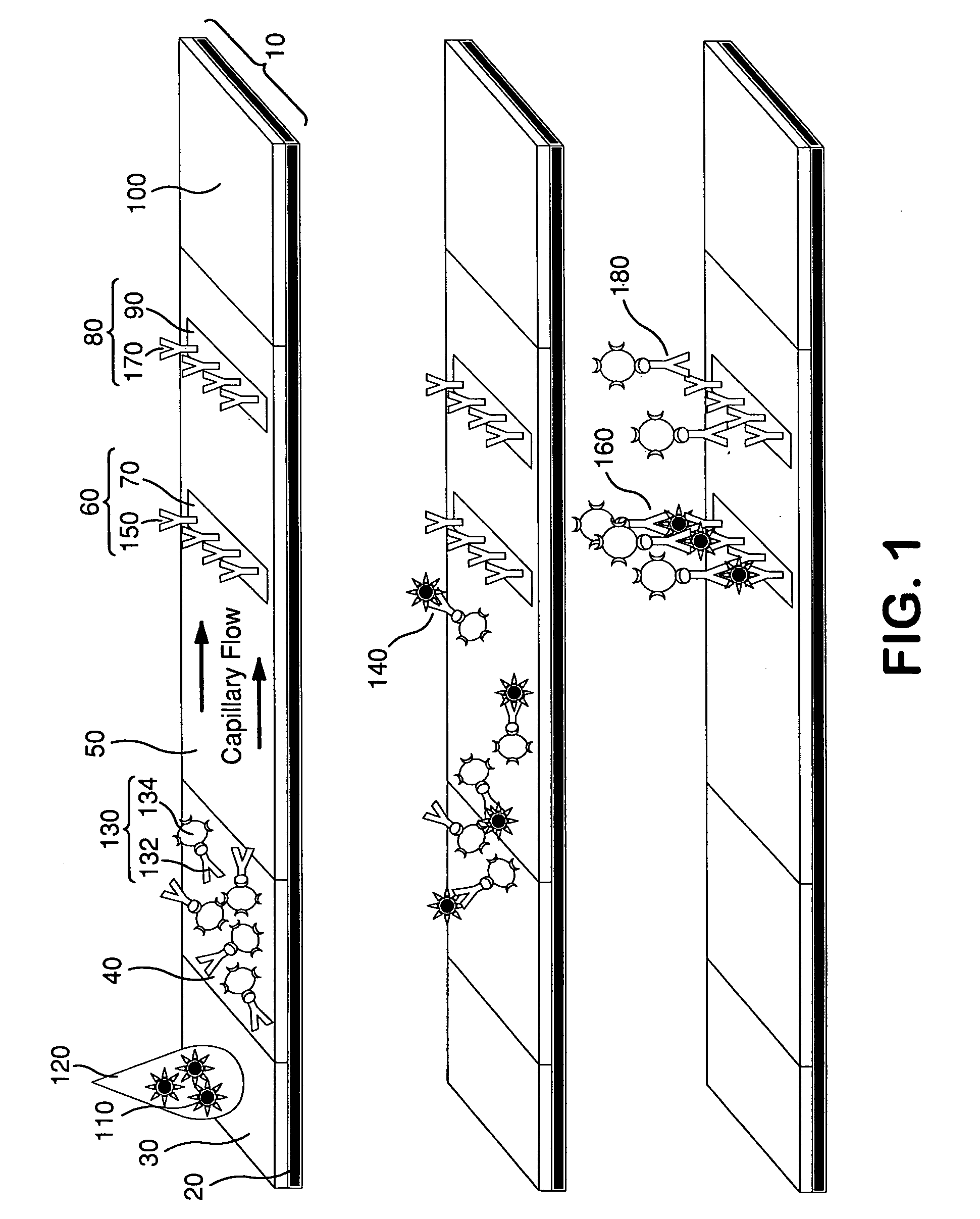
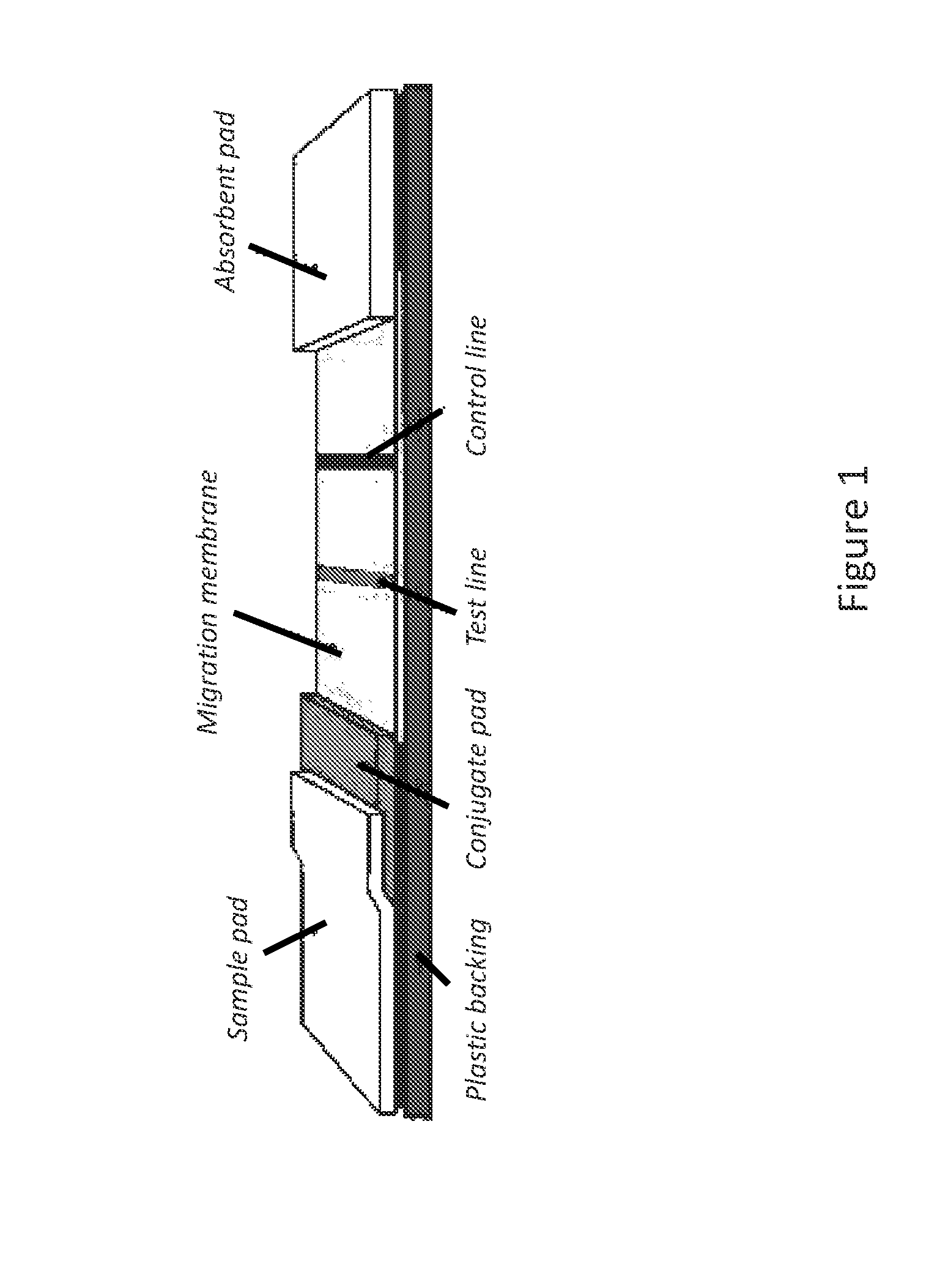
The lateral flow immunoassay (LFI) is an assay platform that is ideally suited for point-of-care (POC) use. Often termed a "dipstick" assay, the LFA format can be used for detection of antibody or antigen in a clinical sample. The LFI is particularly effective for diagnostics for global health and meets...
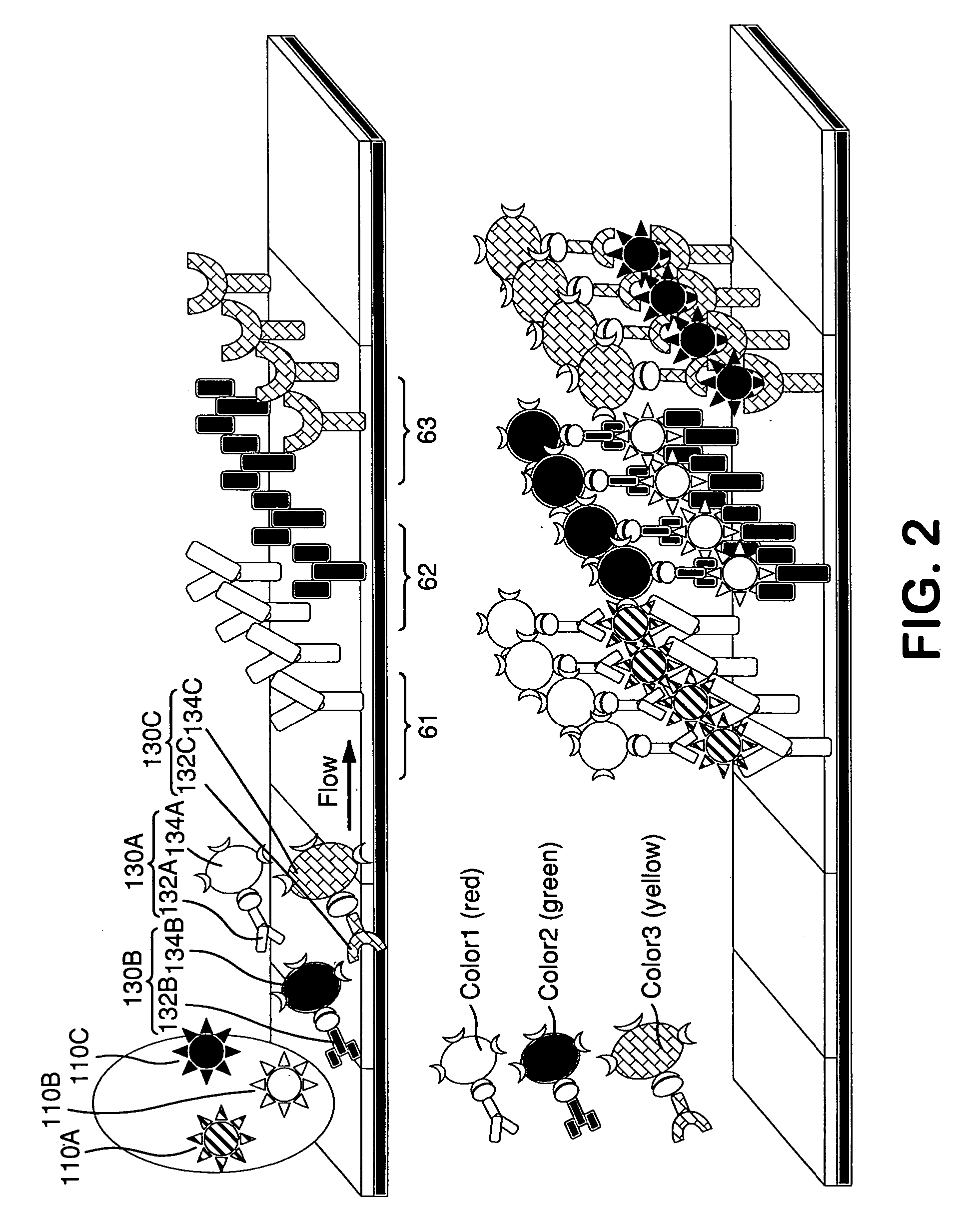
Diagnostic assays including multiplexed lateral flow immunoassays with quantum dots
InactiveUS20050250141A1Reduce non-specific effectsBioreactor/fermenter combinationsBiological substance pretreatmentsQuantum dotLateral flow immunoassay
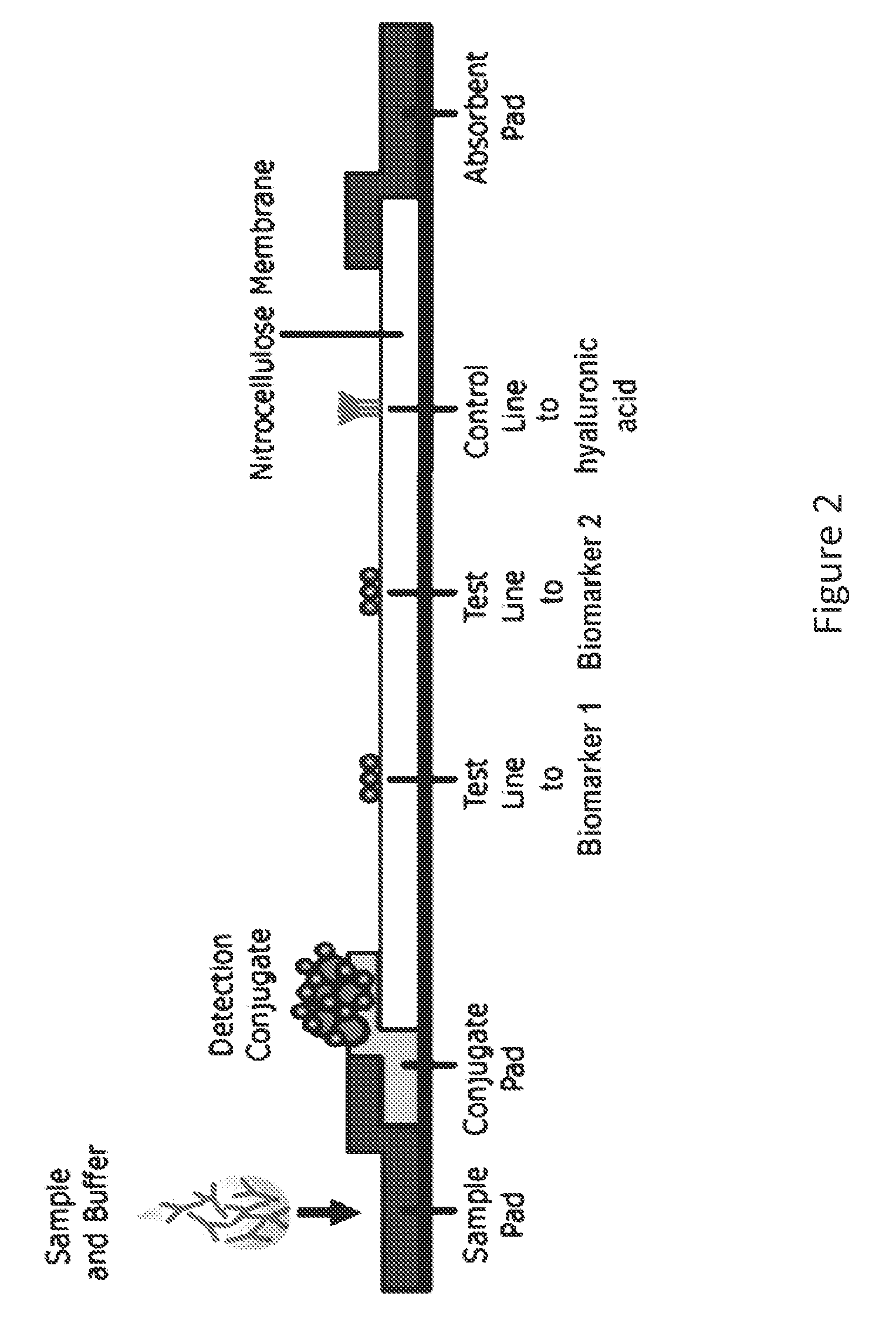
Multiplexed lateral flow assays, related methods, and devices are disclosed which are capable of simultaneously detecting multiple analytes. The assays are preferably immunoassays and can be multiplexed spatially, spectrally, and both spatially and spectrally. Multiplexed assays are disclosed employing quantum dots for applications including the detection of human proteins and the monitoring of microorganisms relevant to water contamination. The invention is widely adaptable to a variety of analytes such as biowarfare agents, human clinical markers, and other substances.
Owner:CALIFORNIA INST OF TECH
Two step lateral flow assay methods and devices
ActiveUS20070020768A1Easy to optimizeEasy to detectEnzymologyDisease diagnosisAntigenLateral flow immunoassay
Lateral flow assay devices and methods for detecting a first member of a specific binding pair in a sample which comprises a plurality of nonspecific binding pair members are adapted for two step determinations. In one embodiment, a two step lateral flow assay method for identifying IgE antibodies in a sample comprises applying a sample to a sample port of a device, wherein the device is adapted to deliver the sample to a lateral flow matrix having a plurality of IgE antigen species immobilized at respective positions at a first location The two step method further comprises allowing the sample to travel along the lateral flow matrix through the immobilized plurality of IgE antigen species to a second location downstream of the first location, applying liquid buffer to the lateral flow matrix to mobilize labeled reagent which is adapted to bind anti-IgE antibody and is dried on the lateral flow matrix at a location upstream of the delivery of the filtered sample to the lateral flow matrix, and allowing labeled reagent mobilized by the liquid buffer to travel along the lateral flow matrix through the immobilized plurality of IgE antigen species to a location downstream of the first location. Further embodiments comprise additional lateral flow immunoassay devices and methods for identifying IgE antibodies in a sample.
Owner:PHADIA AB
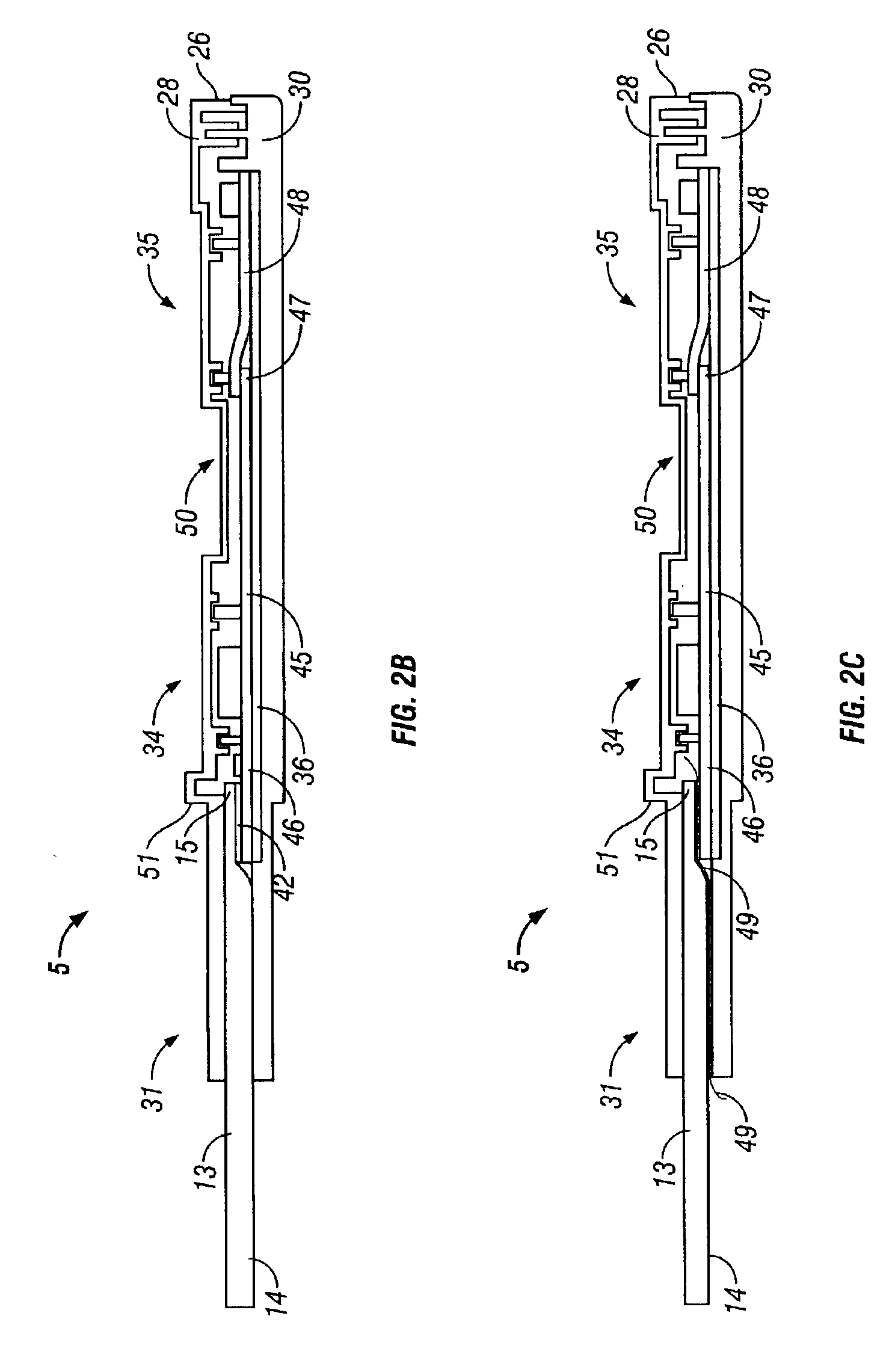
Method to increase specificity and/or accuracy of lateral flow immunoassays
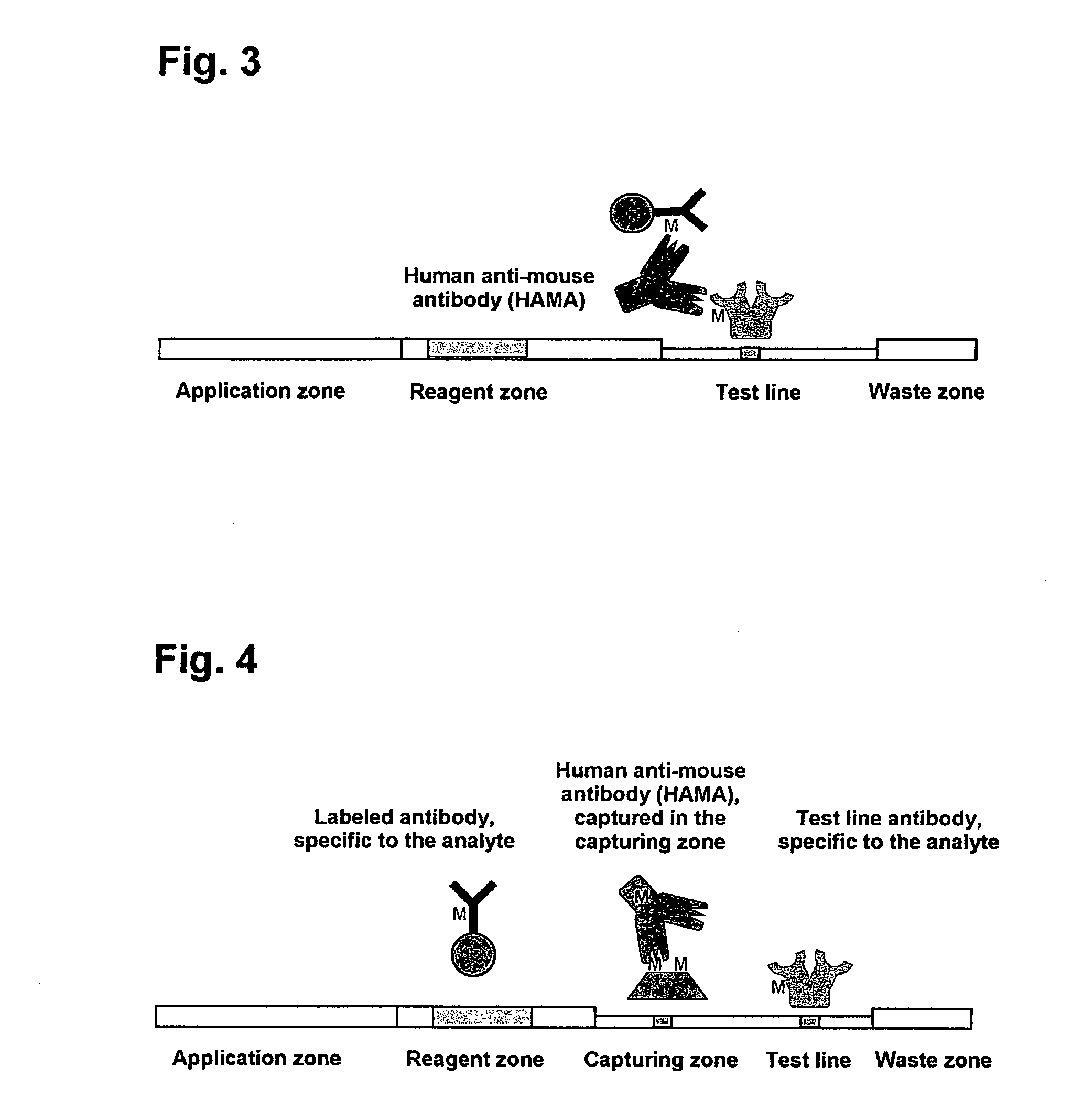
The present invention includes methods and devices for preventing interfering substances from affecting the accuracy of a lateral flow immunoassay. In preferred embodiments, a test strip includes a capturing zone that includes at least one mobile capturing reagent that separates at least one interfering substance from the analyte. The capturing zone is preferably located upstream of the sample application zone. In some embodiments, the reagent / conjugate zone is also located upstream of the sample application zone. The capturing zone may be located upstream, downstream, or overlapping with the reagent / conjugate zone in these embodiments. In other preferred embodiments, one or more mobile capturing reagents are included in the elution medium / running buffer. In yet other embodiments, the capturing reagent is incorporated into a sample collection device of a sample collection system, preferably separate from the chromatographic test strip. A lysis zone is also included in some preferred embodiments.
Owner:RAPID PATHOGEN SCREENING INC
Fluid collection and application device and methods of use of same
The present invention is a device for conveniently collecting and storing liquid samples, as well as applying an aliquot of the sample to a test device, such as a lateral flow immunoassay. The present invention comprises an absorbent swab and a dropper container containing an expression means for expressing the collected sample from the swab. After the collected sample has been expressed into the reservoir of the device, the device is capped. The device can be inverted over a test device, the sides of the reservoir squeezed and an aliquot of the sample applied, in a drop-wise manner, to the sample application well of the test device. The present invention is particularly useful for collecting viscous samples, such as saliva or oral fluid, in a drug of abuse testing setting.
Owner:ABBOTT RAPID DIAGNOSTICS INT UNLTD
In situ lysis of cells in lateral flow immunoassays
ActiveUS20100015634A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteLysis
Devices and methods incorporate lysis agents into a point-of-care testing device. The sample is loaded, and then the sample travels until it encounters a lysis agent. The lysis agent is preferably pre-loaded onto the collection device. In a preferred embodiment, the initially lysis agent is localized between the sample application zone and the conjugate zone. The lysis agent is preferably soluble or miscible in the sample transport liquid, and the lysis agent is solubilized and activated upon contact with the sample transport liquid. The sample transport liquid then contains both lysis agent in solution or suspension and sample components in suspension. Any lysis-susceptible components in a sample, then being exposed in suspension to the lysis agent, are themselves lysed in situ. The running buffer then carries the analyte, including any lysis-freed components, to the detection zone.
Owner:RAPID PATHOGEN SCREENING INC
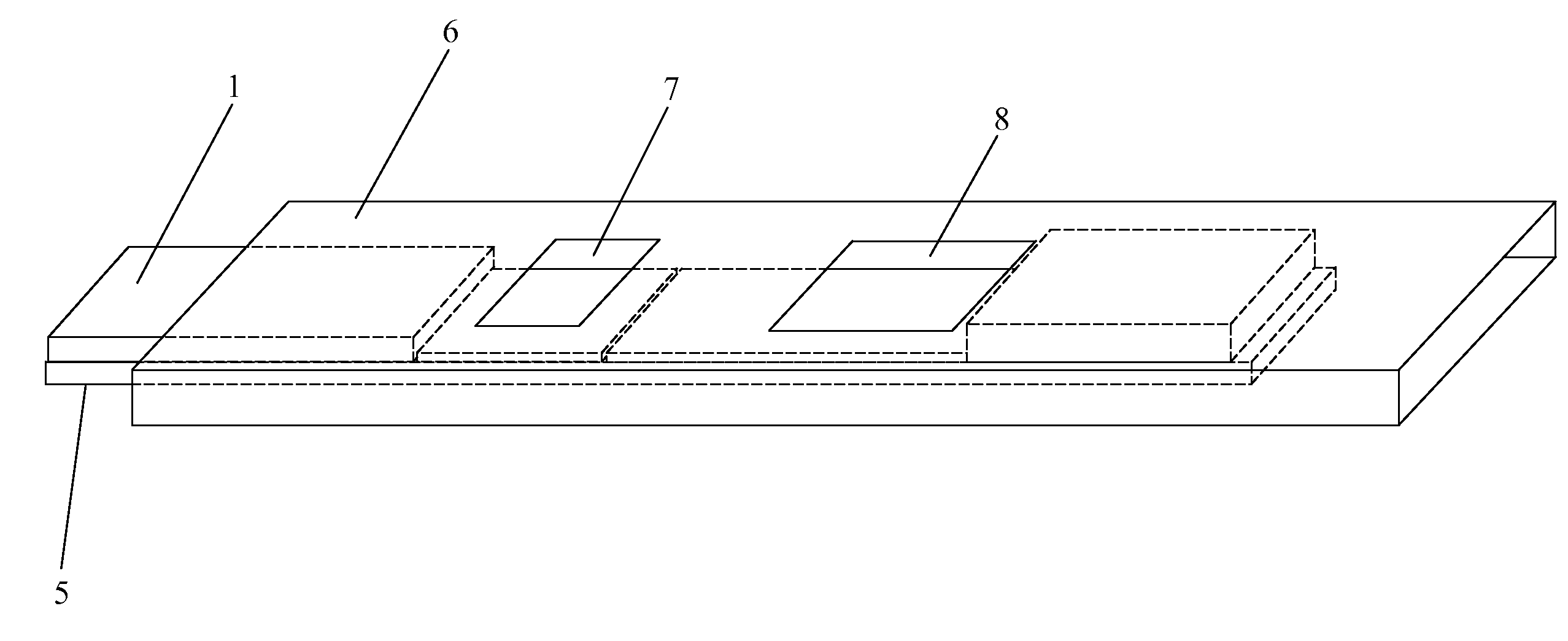
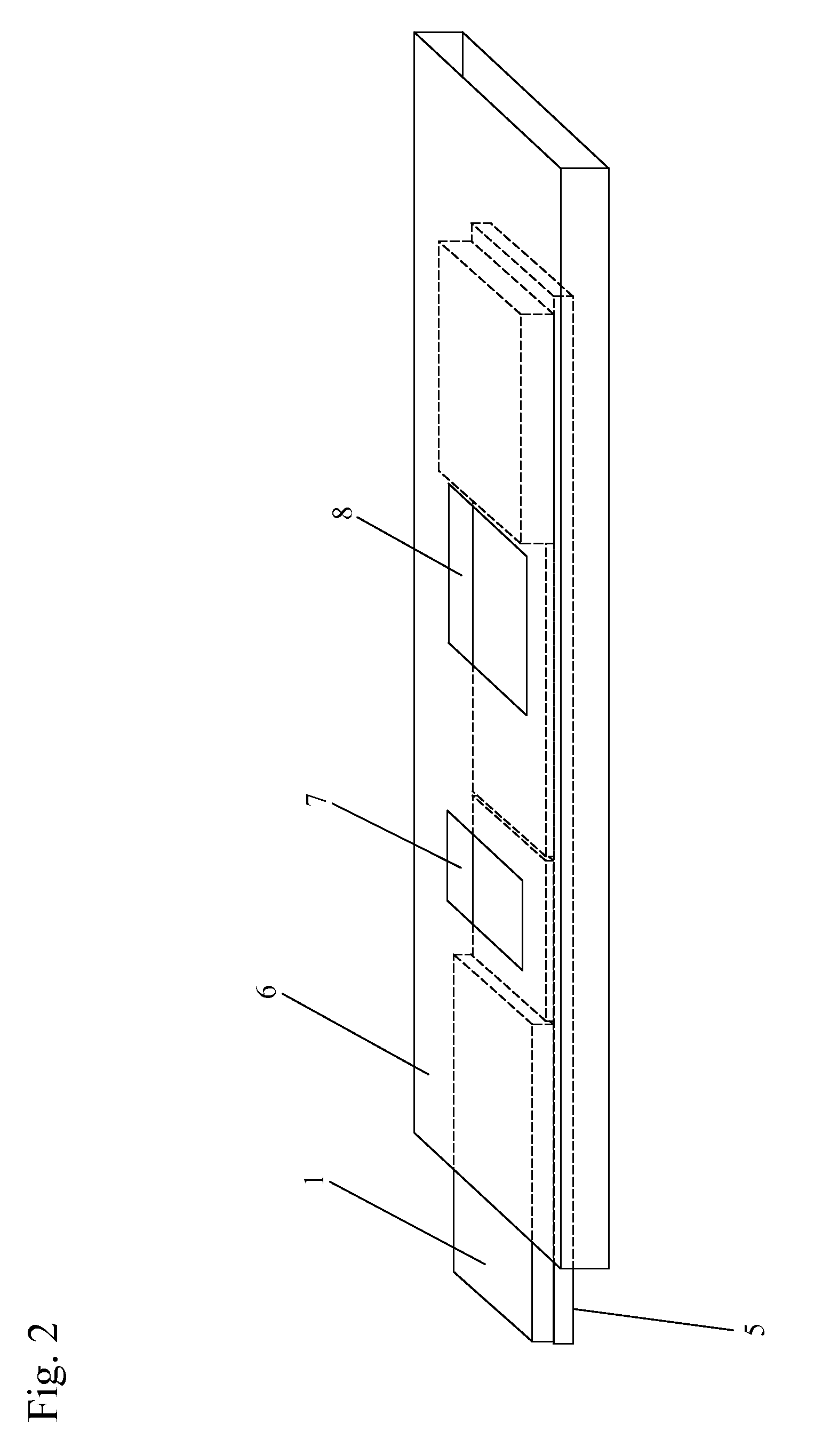
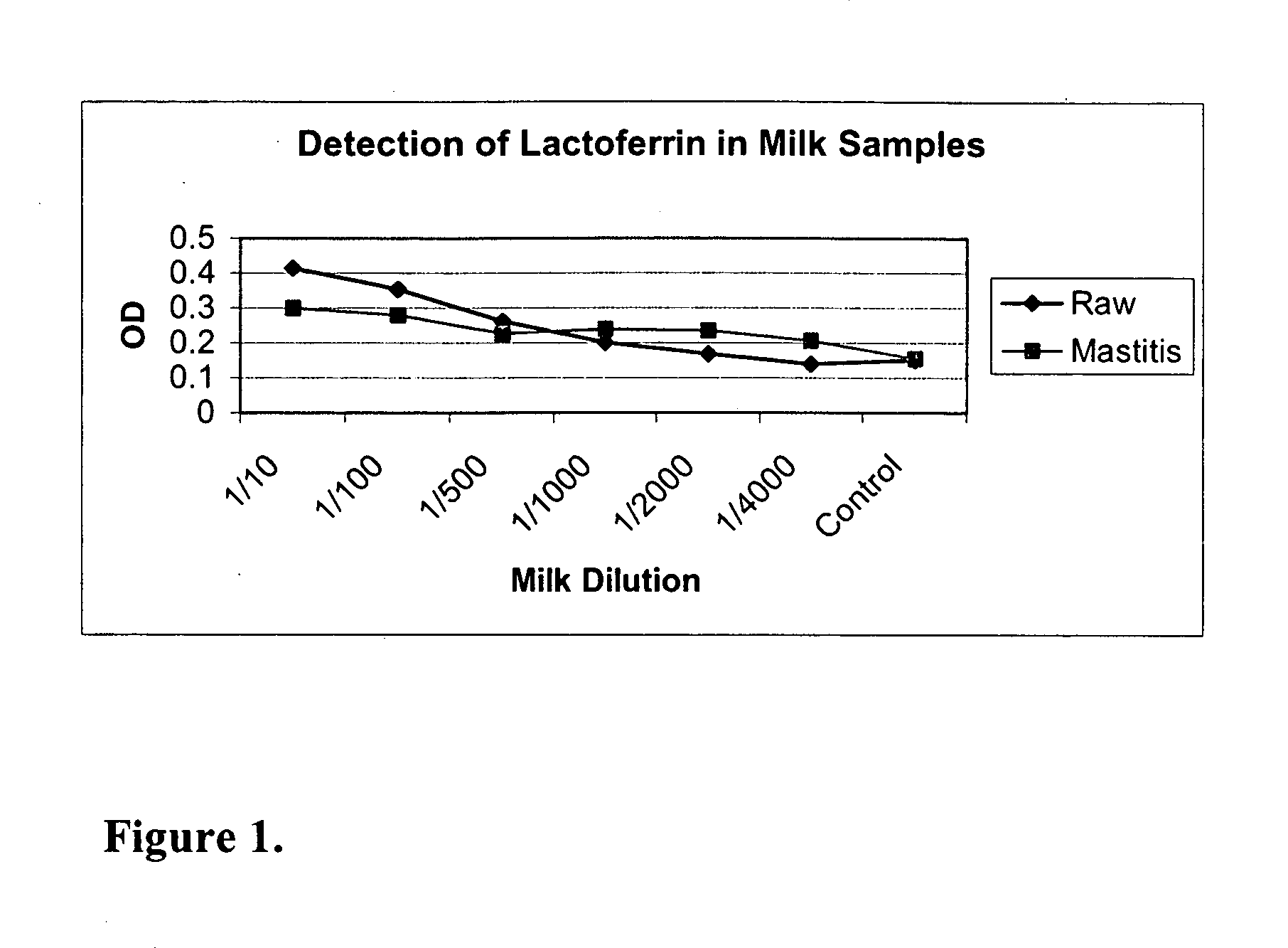
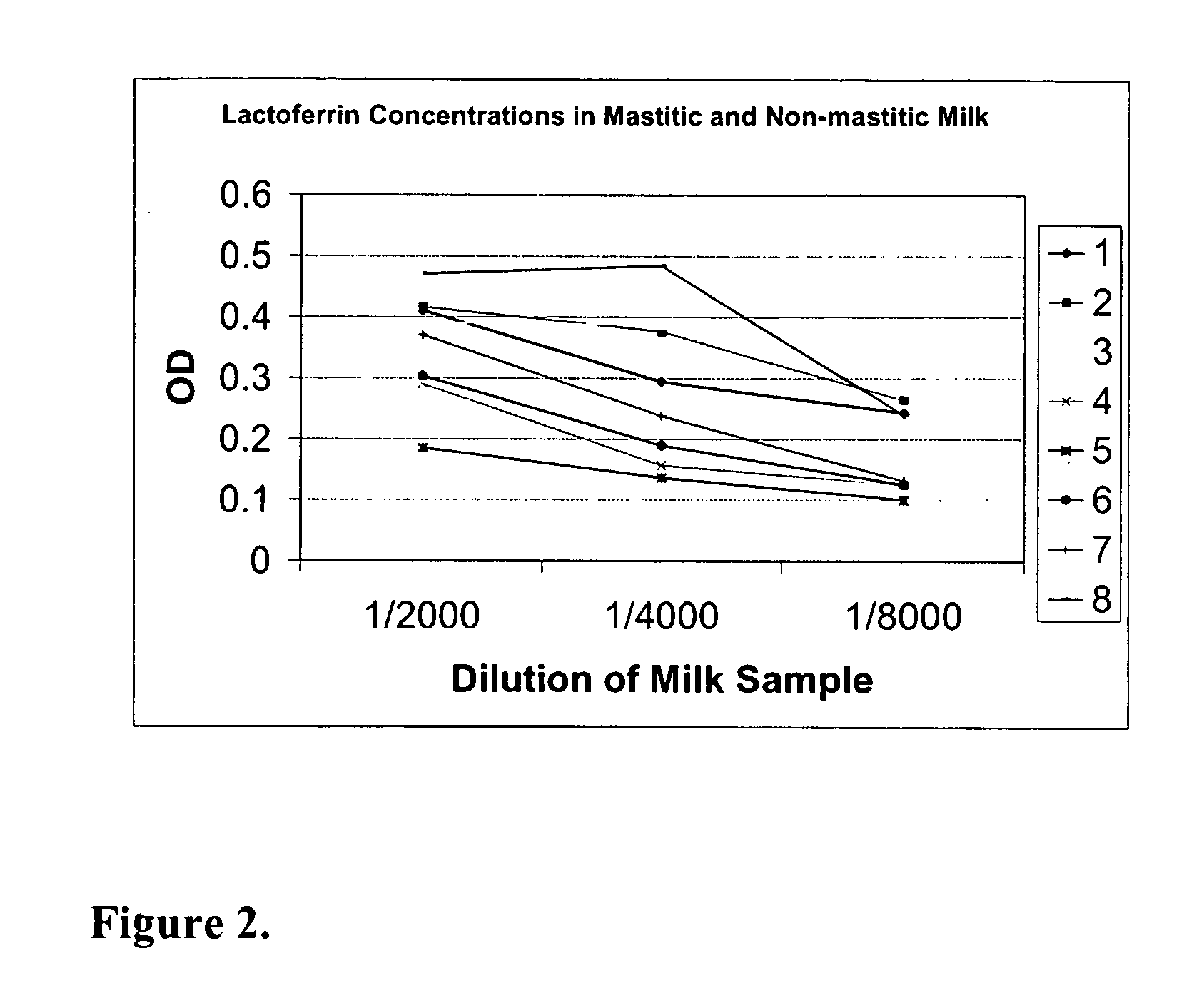
Methods, compositions, devices, and kits for detecting mastitis
InactiveUS20050260695A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMilk sampleMonoclonal antibody
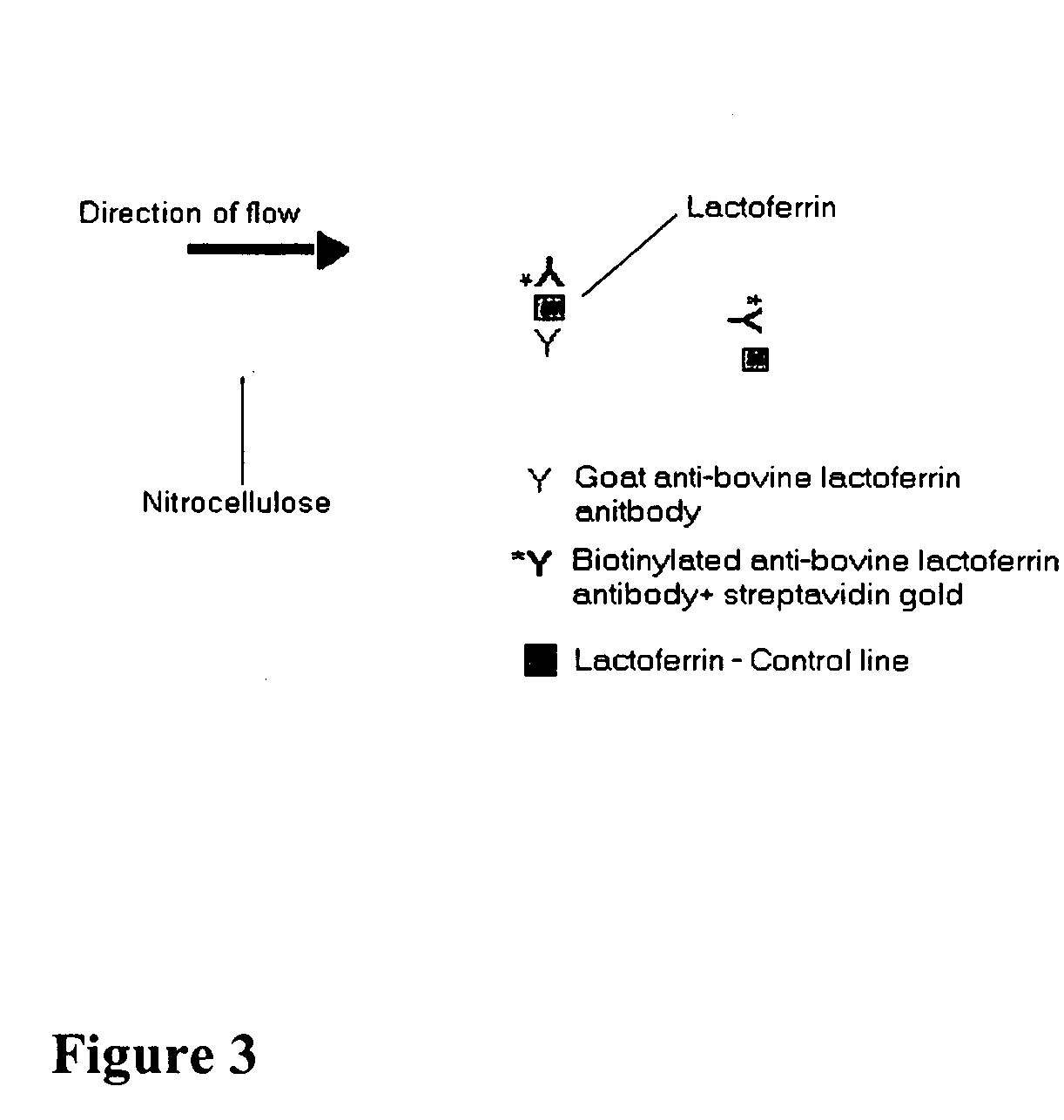
The present invention includes compositions, kits and methods useful for the detection of mastitis in an animal. These agents and methods are primarily directed to a method of detecting the presence of mastitis, including sub-clinical mastitis, in cows, involving incubating a sample of milk from the cow with an agent that binds to lactoferrin such as, e.g., a monoclonal antibody specific for lactoferrin, and then detecting bound lactoferrin. The invention includes lateral-flow immunoassay methods and devices for assessing the presence of lactoferrin in milk samples.
Owner:GENPRIME
Fluorescence lateral flow immunoassay
InactiveUS20060263907A1Inhibit migrationFacilitates resuspensionBioreactor/fermenter combinationsBiological substance pretreatmentsAssayFluorescence
The present invention relates to devices, kits, instruments and methods for conducting lateral flow assays. A naturally hydrophilic membrane a fluorescent or luminescent label are used in the present devices, kits, instruments and methods. Preferably, a single naturally hydrophilic membrane and / or a fluorescent or luminescent particle label is used.
Owner:BECKMAN COULTER INC
Method to increase specificity and/or accuracy of lateral flow immunoassays
InactiveUS20070059682A1Microbiological testing/measurementMaterial analysisChemistryLateral flow immunoassay
The present invention relates to a method for detecting an analyte in a sample, wherein the sample to be analyzed is applied to a chromatographic carrier. After separating from an interfering substance which may be present in the sample, the analyte of interest is detected on the carrier by means of an immunological assay. Further, a test strip for carrying out the method of the invention is provided. The invention further relates to a method for reducing interference in a method for detecting an analyte on a chromatographic carrier.
Owner:RAPID PATHOGEN SCREENING INC
In situ lysis of cells in lateral flow immunoassays
Devices and methods incorporate lysis agents into a point-of-care testing device. The sample is loaded, and then the sample travels until it encounters a lysis agent. The lysis agent is preferably pre-loaded onto the collection device. In a preferred embodiment, the initially lysis agent is localized between the sample application zone and the conjugate zone. The lysis agent is preferably soluble or miscible in the sample transport liquid, and the lysis agent is solubilized and activated upon contact with the sample transport liquid. The sample transport liquid then contains both lysis agent in solution or suspension and sample components in suspension. Any lysis-susceptible components in a sample, then being exposed in suspension to the lysis agent, are themselves lysed in situ. The running buffer then carries the analyte, including any lysis-freed components, to the detection zone.
Owner:RAPID PATHOGEN SCREENING INC
Integrated confirmation sample in a body fluid test device and method of using
InactiveUS6875185B2Small sizeEasy to useAnalysis using chemical indicatorsVaccination/ovulation diagnosticsAntigenSingle sample
A lateral flow immunoassay contact chemical test device and method integrates sample collection, prescreen testing, and confirmation sample collecting and storing with a single device and a minimum of steps. While particularly advantageously used with an oral fluid sample absorbed directly from a person's mouth, (in which constant monitoring of a collection process is made possible), the test device may be used with any of a variety of sample fluids. Prescreen testing and confirmation testing are performed on a single sample. Prescreen testing is performed by a lateral flow assay. The sample collection pad is subsequently separated from the wicking path to prevent continued migration from, and backflow into the sample collection pad, so that the confirmation sample is preserved in the sample collection pad. A multitude of antigens can be detected with a single device.
Owner:BRANAN MEDICAL
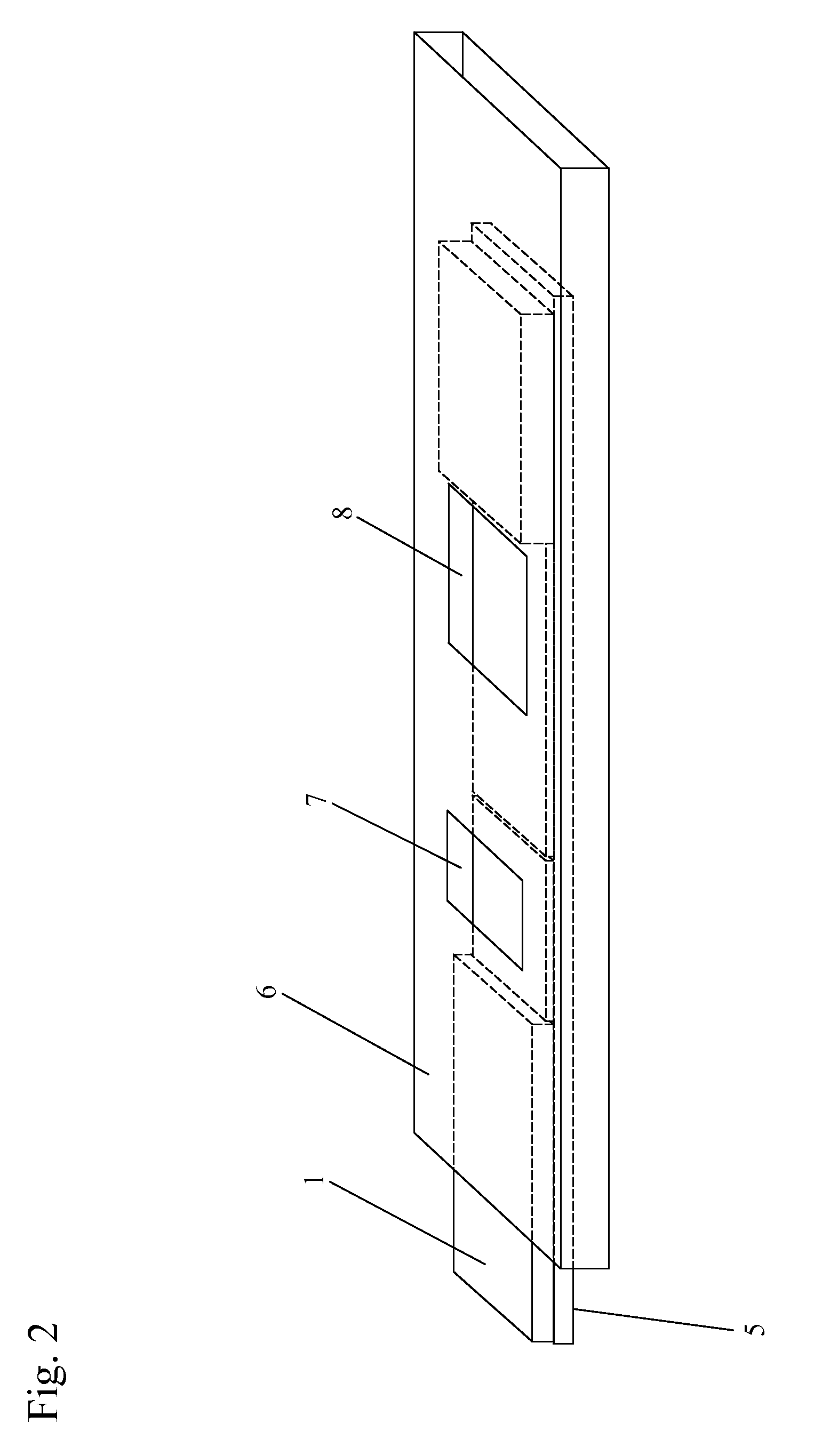
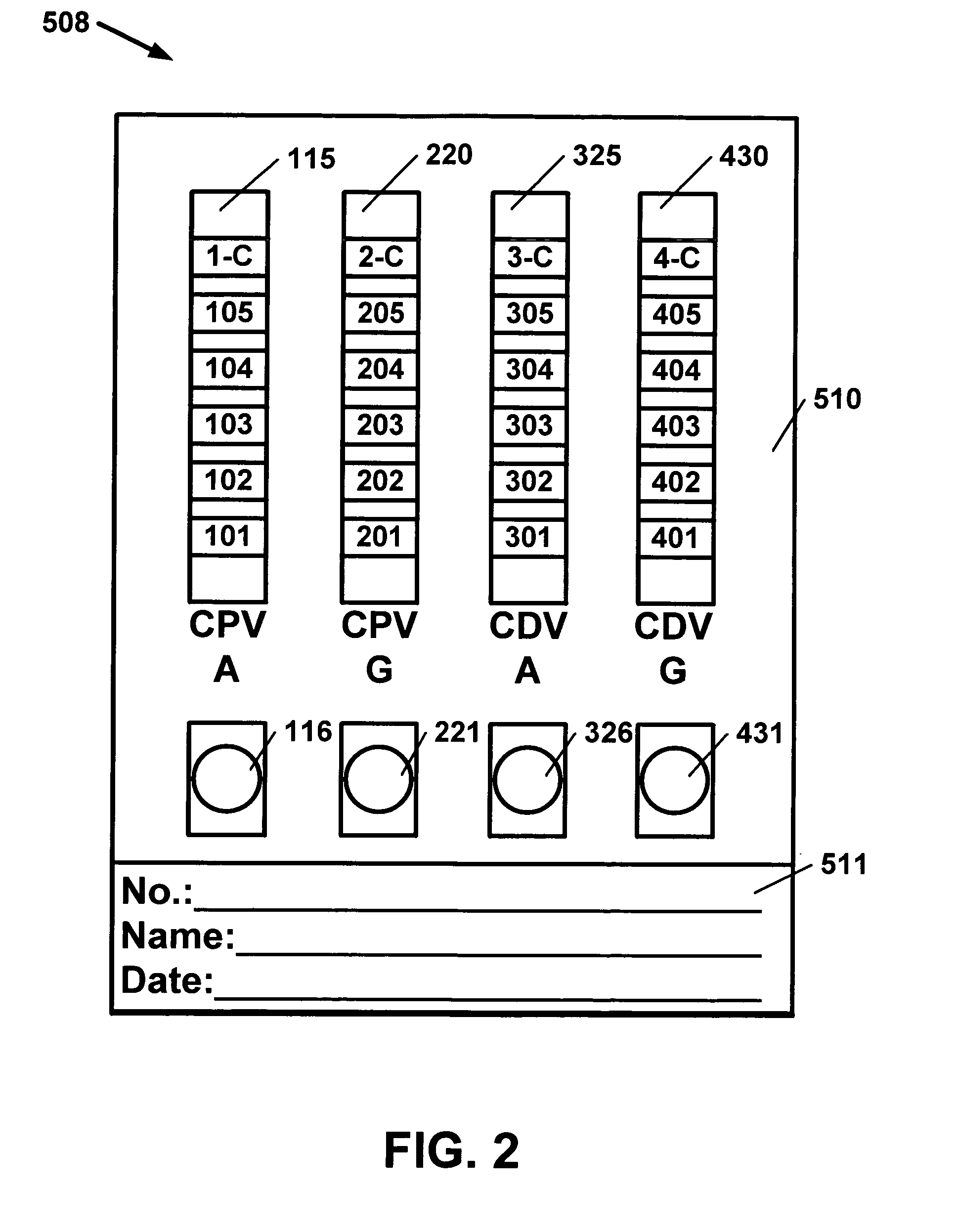
Antibody detection method and device for a saliva sample from a non-human animal
InactiveUS20130309656A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSaliva sampleCanine distemper virus CDV
A rapid test apparatus, system, and method of use utilizing lateral flow immunoassay (LFIA) detection of a selected ligand in a liquid sample from a body fluid such as saliva in a pet in which antibodies and their complimentary antigens are used with detection-nanoparticles to provide a visual or measurable end point indicator in which the method measures the exposure to viruses in the canine from Canine Parvovirus (CPV) and / or Canine Distemper virus (CDV).
Owner:DAVIS DAVID C
Lateral Flow Immunoassay With Encapsulated Detection Modality
InactiveUS20090155811A1High sensitivityThe result is moreAnalysis by material excitationBiological testingWhole blood unitsLateral flow immunoassay
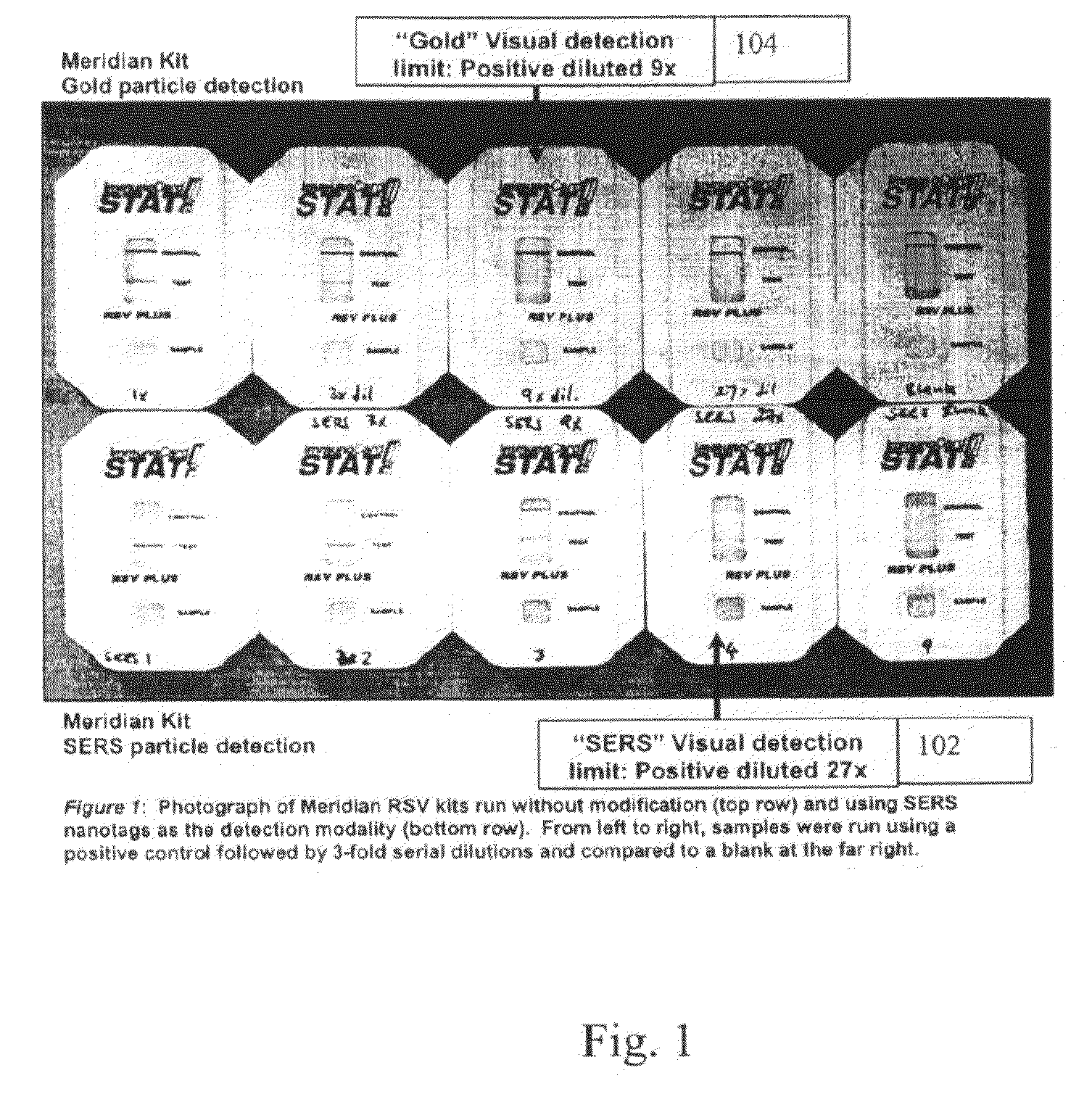
A lateral flow immunoassay featuring encapsulated metal particles. The encapsulated particles may use SERS nanotags as the detection modality. The use of encapsulated particles as a detection modality, in particular encapsulated SERS tags increases the sensitivity of an LFI prepared for visual reading and introduces the ability to obtain substantially more sensitive qualitative results or quantitative results through the analysis of a SERS spectrum read from an LFI prepared in accordance with the present invention. The use of SERS as detection modality also enhances the ability of an LFI device to be used for a multiplexed test. Other aspects of the present invention include LFI devices specifically configured to test whole blood, a reader for the detection and interpretation of a multiplexed assay and the hardware and software components used to implement the reader.
Owner:BECTON DICKINSON & CO
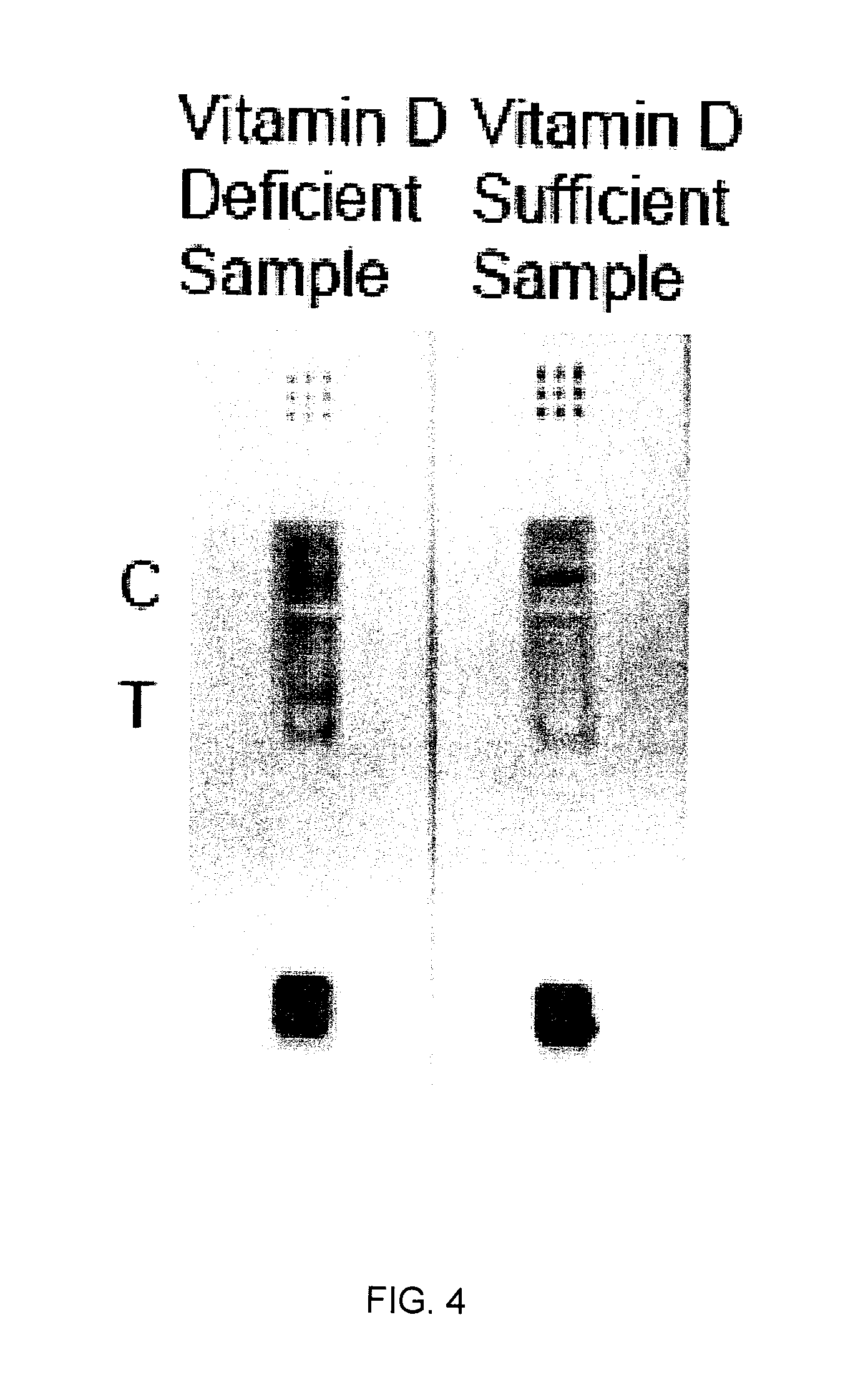
Lateral flow immunoassay for detecting vitamins
The invention is directed to a method for detecting analytes, particularly vitamins, using a lateral flow immunoassay. The method may be used to detect vitamin D, particularly 25-hydroxy vitamin D3 or 1,25-dihydroxy vitamin D3. The method involves obtaining a fluid sample from a subject; applying the sample to a lateral flow test strip comprising a conjugate pad capable of releasing a labelled antibody against vitamin D, and a detection membrane comprising a first capture reagent specific to the antibody and immobilized on a test band; and detecting the presence or absence of vitamin D in the sample. The presence of a detectable signal in the test band is indicative of vitamin D deficiency.
Owner:NANOSPEED DIAGNOSTICS
Method to increase specificity and/or accuracy of lateral flow immunoassays
The present invention includes methods and devices for preventing interfering substances from affecting the accuracy of a lateral flow immunoassay. In preferred embodiments, a test strip includes a capturing zone that includes at least one mobile capturing reagent that separates at least one interfering substance from the analyte. The capturing zone is preferably located upstream of the sample application zone. In some embodiments, the reagent / conjugate zone is also located upstream of the sample application zone. The capturing zone may be located upstream, downstream, or overlapping with the reagent / conjugate zone in these embodiments. In other preferred embodiments, one or more mobile capturing reagents are included in the elution medium / running buffer. In yet other embodiments, the capturing reagent is incorporated into a sample collection device of a sample collection system, preferably separate from the chromatographic test strip. A lysis zone is also included in some preferred embodiments.
Owner:RAPID PATHOGEN SCREENING INC
Methods and devices to enhance sensitivity and evaluate sample adequacy and reagent reactivity in rapid lateral flow immunoassays
ActiveUS20110143365A1Reduce chanceBioreactor/fermenter combinationsBiological substance pretreatmentsIntravenous gammaglobulinLateral flow immunoassay
Methods and devices for rapid lateral flow immunoassays to detect specific antibodies within a liquid sample while also validating the adequacy of the liquid sample for the presence of immunoglobulin and the integrity and immunoreactivity of the test reagents that detect the antibodies of interest, without requiring instrumentation. The methods and devices provide for delivery of a diluted liquid sample to a single location that simultaneously directs the liquid flow along two or more separate flow paths, one that serves as a positive control to confirm that all critical reagents of the test are immunoreactive, and that the sample being tested is adequate, and the other to detect specific antibodies if present.
Owner:BUCHANAN
Combination assay for alcohol and drugs of abuse
A test apparatus that allows testing for the presence of both drugs of abuse and alcohol within a single device. In general, the apparatus of the present invention includes a lateral flow immunoassay screening for various drugs of abuse and a reactive pad including an enzyme as a test for alcohol. The two types of tests are contained within the same housing and these tests are performed using the same sample source from a subject being tested.
Owner:BRANAN MEDICAL
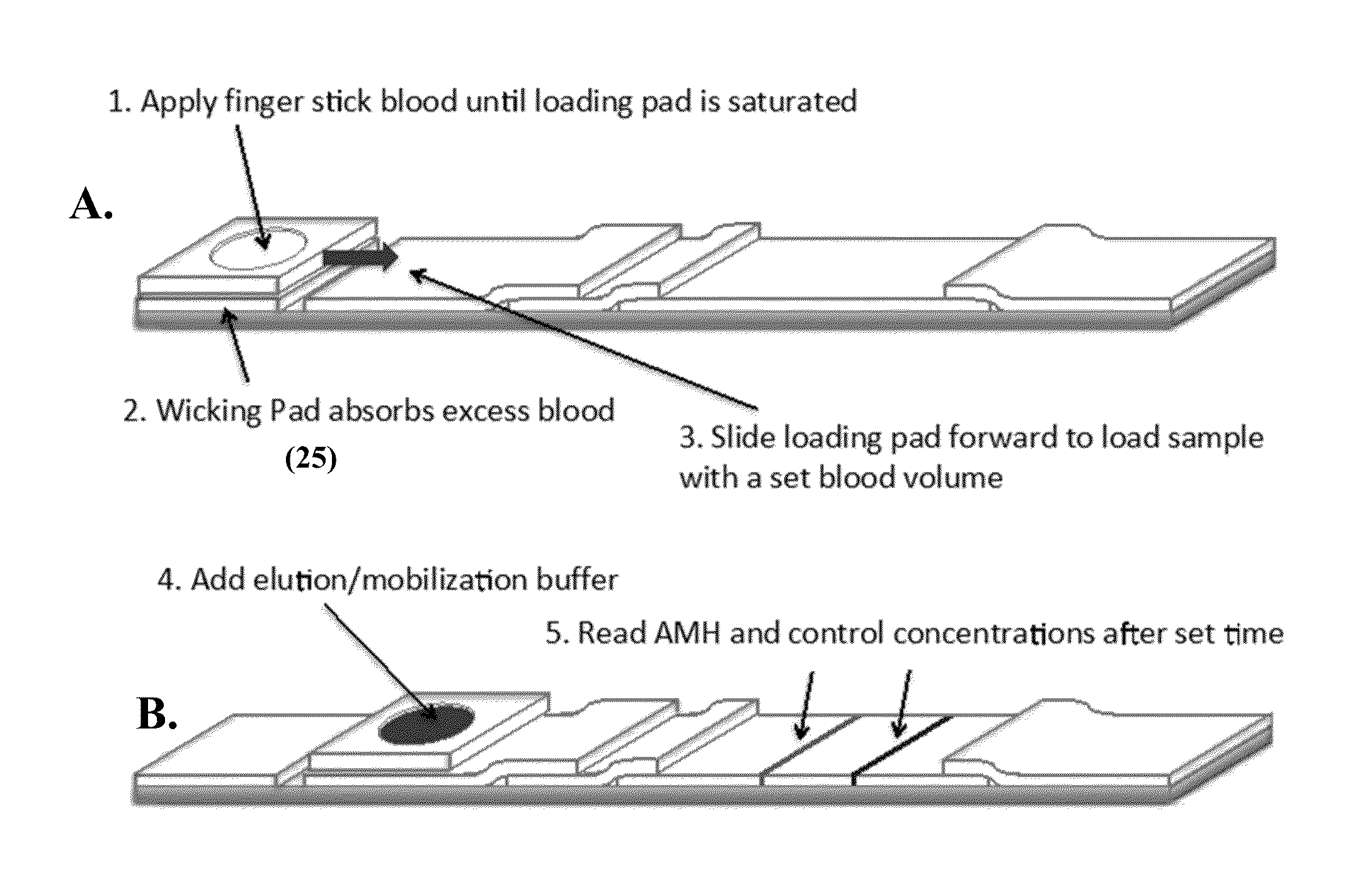
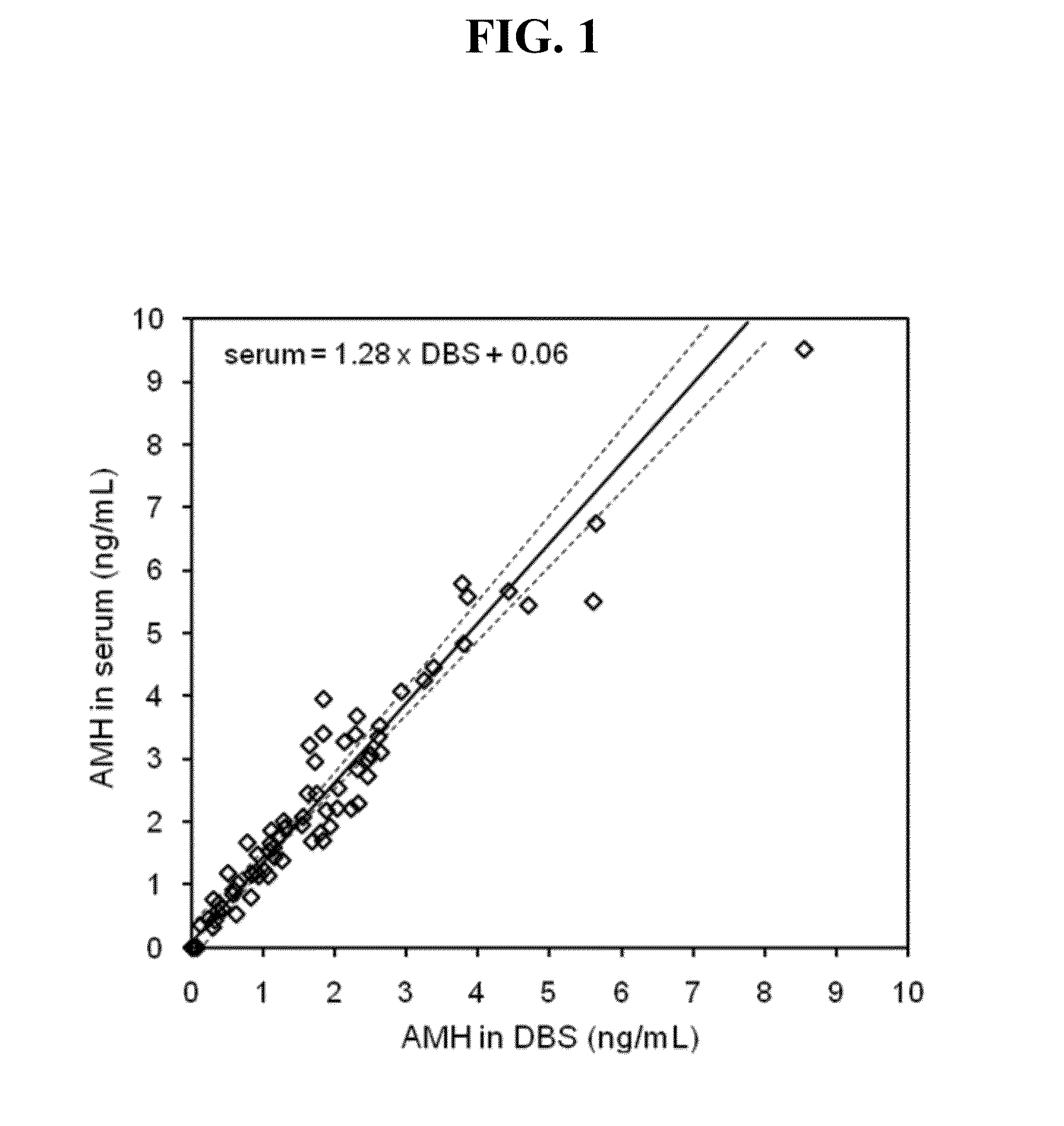
Anti-mullerian hormone detection in whole blood
InactiveUS20130224771A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAntibody conjugatePhysiology
The present invention provides methods, kits, compositions, and devices for detecting Anti-Mullerian hormone (AMH) in whole blood samples. In certain embodiments, the methods, kits, compositions, and devices employ immunoassays that generate a colorimetric or fluorescent signal (e.g., using antibodies conjugated to gold nanoparticles or fluorescent particles) where the signal generated is proportional to the approximate concentration of AMH in a whole blood sample. In particular embodiments, the present invention provides quantitative or semi-quantitative lateral flow immunoassay devices and kits for detecting AMH at home (e.g., in order for women to estimate their ovarian age or diagnose polycystic ovarian syndrome).
Owner:NORTHWESTERN UNIV
Antibody and immunoassays for determining the presence of Δ9-Tetrahydrocannabinol
Antibodies having specific binding for the parent THC (Δ9-THC) and its major metabolites are provided which present a significant increase in sensitivity of immunoassays such as lateral flow immunoassays and ELISA for THC. The present invention also provides a rabbit hybridoma producing the antibody as a monoclonal antibody, a recombinant antibody, further molecularly engineered recombinant antibodies against parent Δ9-THC and its metabolites and cell lines producing the recombinant antibodies. The invention also provides applications of the antibody in immunoassays, particularly lateral flow immunoassays, specifically applications in detecting THC in body fluids, particularly saliva, and kits for determining the presence of THC.
Owner:AGILENT TECH INC
System for detecting infection in synovial fluid
ActiveUS20150011412A1Improve abilitiesBioreactor/fermenter combinationsPeptide librariesProteomics methodsRadioimmunoassay
The invention provides methods and systems for detecting a biomarker in a synovial fluid wherein the system also includes a control to ensure that the test sample is indeed synovial fluid. The biomarkers and the control for synovial fluid can be identified using proteomic methods, including but not limited to antibody based methods, such as an enzyme-linked immunosorbant assay (ELISA), a radioimmunoassay (RIA), or a lateral flow immunoassay.
Owner:CD DIAGNOSTICS
Sensor integration in lateral flow immunoassays and its applications
Lateral flow immunoassay devices for determining the concentration of an analyte in a sample and methods for measuring analyte concentration in sample using such lateral flow immunoassay devices.
Owner:ROBERT BOSCH GMBH
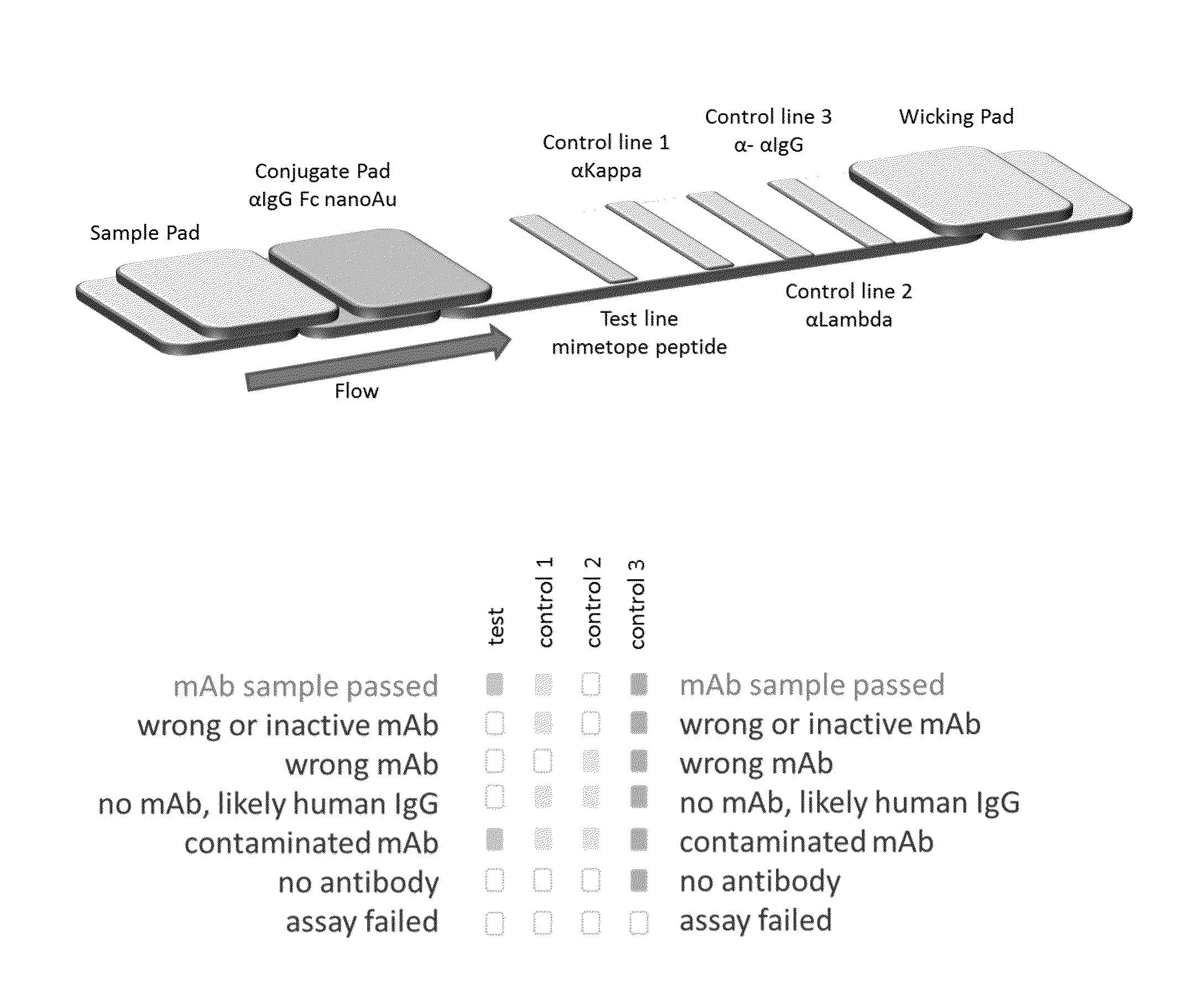
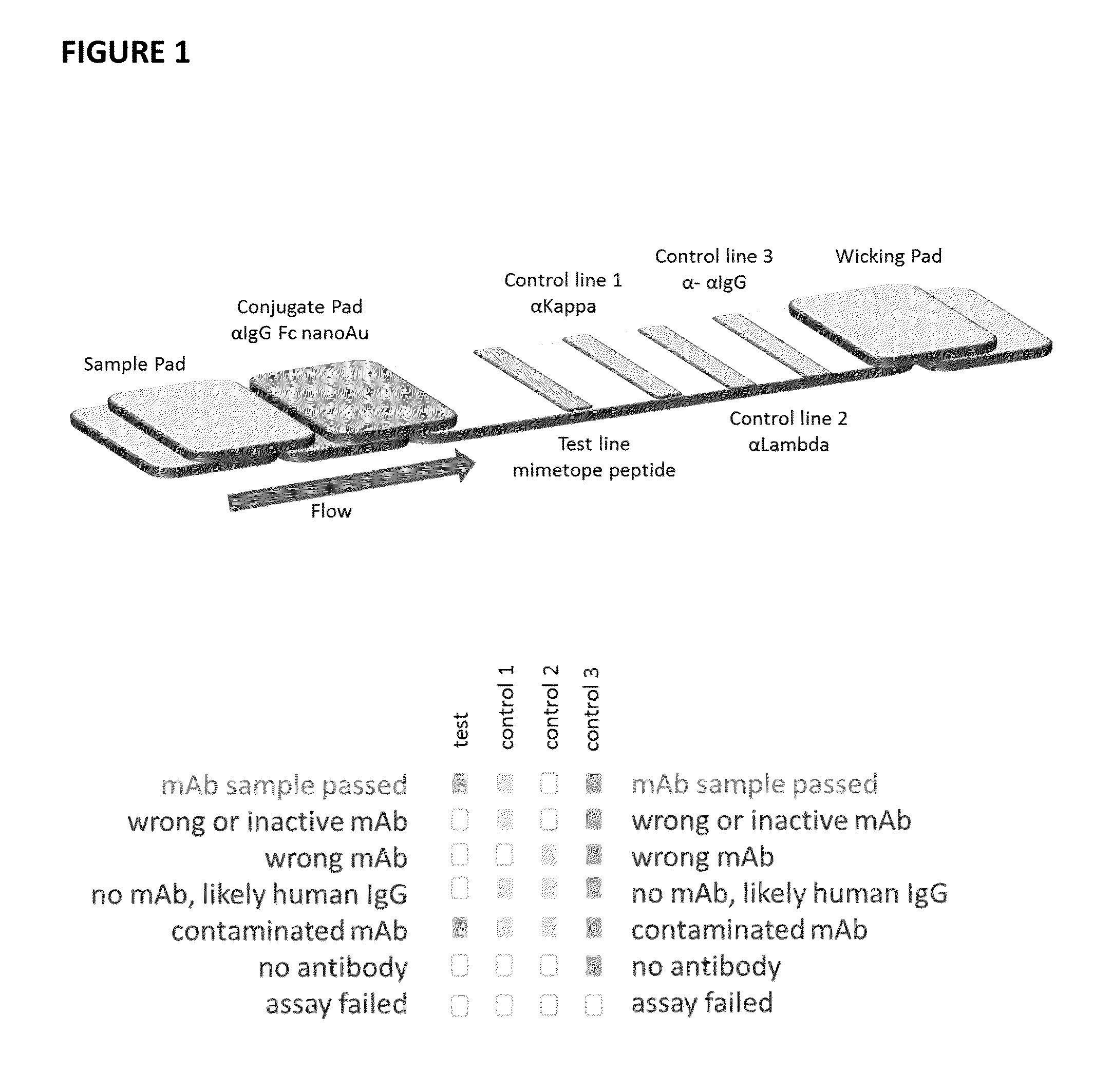
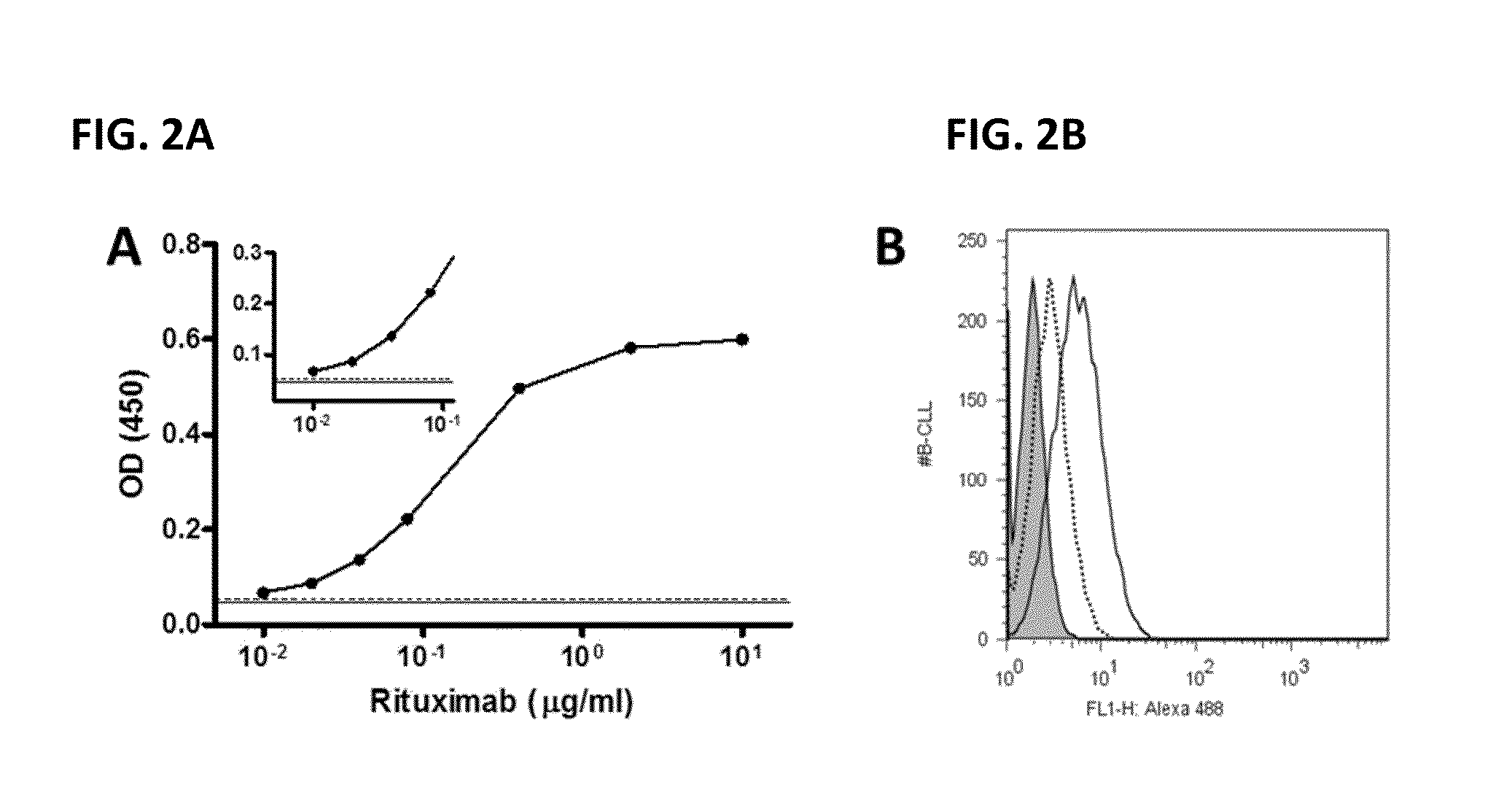
Lateral flow immunoassay
InactiveUS20150293086A1Bioreactor/fermenter combinationsBiological substance pretreatmentsLateral flow immunoassayChemistry
Disclosed is a test device for determining the presence or integrity of a biologic comprising a sample pad for receiving the biologic, a conjugate pad, and a test membrane comprising at least one test line comprising an immobilized mimetope, and methods of using the same.
Owner:ABREOS BIOSCI
Methods, compositions, and kits for the detection of bacteria in a sample
InactiveUS20060014227A1Bioreactor/fermenter combinationsBiological substance pretreatmentsLateral flow immunoassayMicrobiology
The present invention includes compositions, kits, and methods useful for the detection of bacteria. These agents and methods are primarily directed to a method of detecting the presence of bacteria in a sample, involving incubating the sample with an agent that binds to bacteria, such as, e.g., an agent specific for peptidoglycan or a component thereof, and then detecting bound bacteria. The invention includes lateral-flow immunoassay methods and devices for assessing the total bacterial load in a liquid sample.
Owner:GENPRIME
Methods, compositions, and kits for the detection of bacteria in a sample
InactiveUS20080153114A1Bioreactor/fermenter combinationsBiological substance pretreatmentsLateral flow immunoassayMicrobiology
Owner:GENPRIME
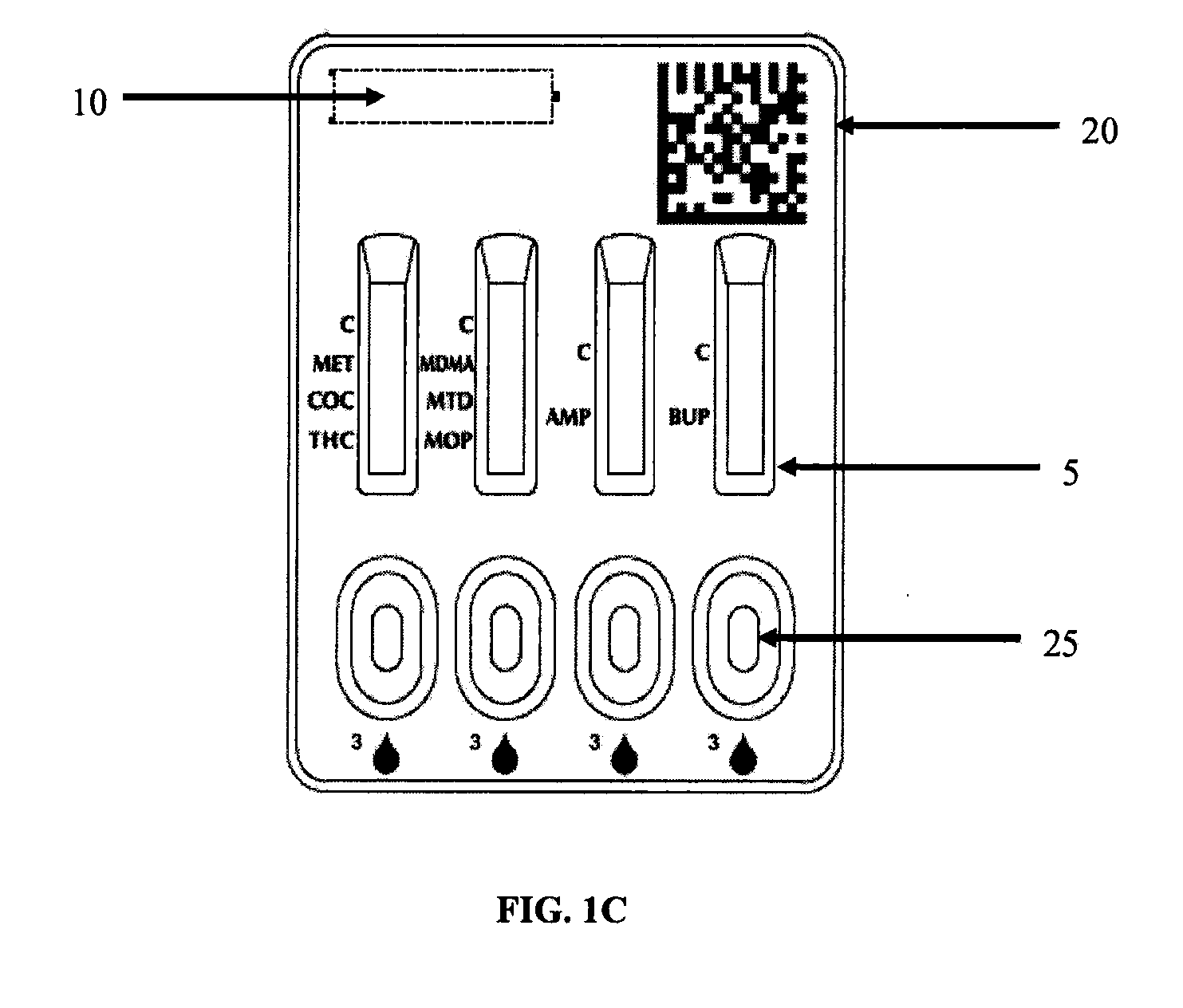
System and Method for Lateral Flow Immunoassay Testing
A lateral flow immunoassay system is provided that includes a housing having a base portion forming a first chamber therein and a body portion formed with three openings in fluid communication with the first chamber, a vial containing a buffer agent, and a sample collector for introducing a sample fluid into the first chamber via the second opening. The vial is mounted to the housing such that a dispensing side extends into the first opening for dispensing the buffer agent therefrom into the first chamber. The housing allows the buffer agent and sample fluid to be mixed within a reaction well formed within the first chamber to form a test sample mixture. The body portion is configured to receive a receiving end of an elongated holder in the third opening and allow a test strip secured in the holder to be brought into communication with the text sample mixture.
Owner:HEALGEN SCI LLC
Sampling and assay device together with methods for use thereof
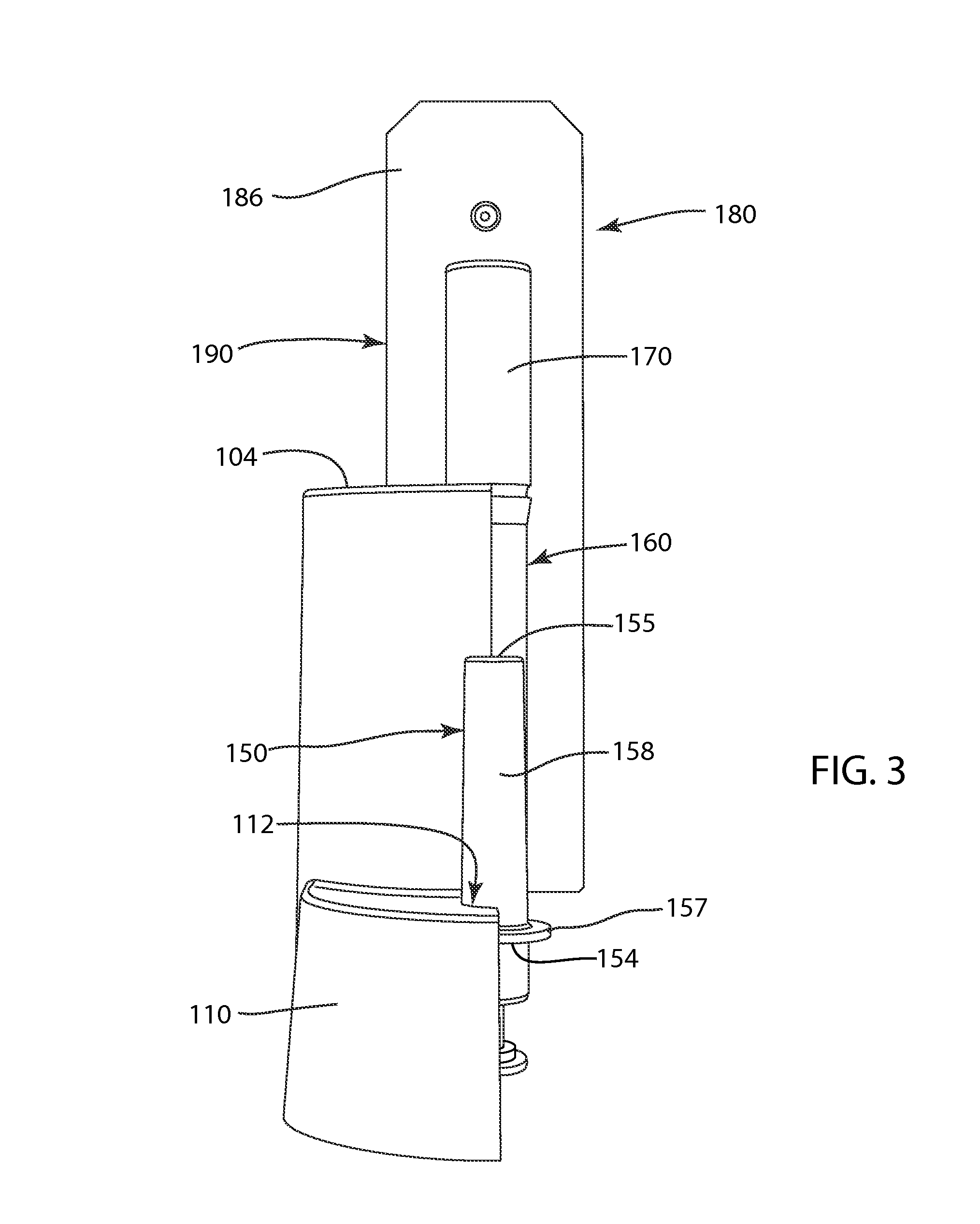
InactiveUS20050181521A1Easy to useSimple to useSamplingLaboratory glasswaresLateral flow immunoassayMedicine
An immunochemical sampling device, kits including the sampling device, processes for production of the sampling device, and methods for use of the sampling device in lateral flow immunoassays. The sampling device comprises a solid support that is partially wrapped in a porous carrier, then covered in a hydrophobic cover. At its distal end, the porous carrier comprises a labeled binding reagent that is retained in the solid support until released into controlled flow with the liquid sample when the sampling device is brought into contact with a test strip.
Owner:ANI BIOTECH
Magnetic lateral flow immunoassay for rapid detection of TTX and preparation of detection test strip
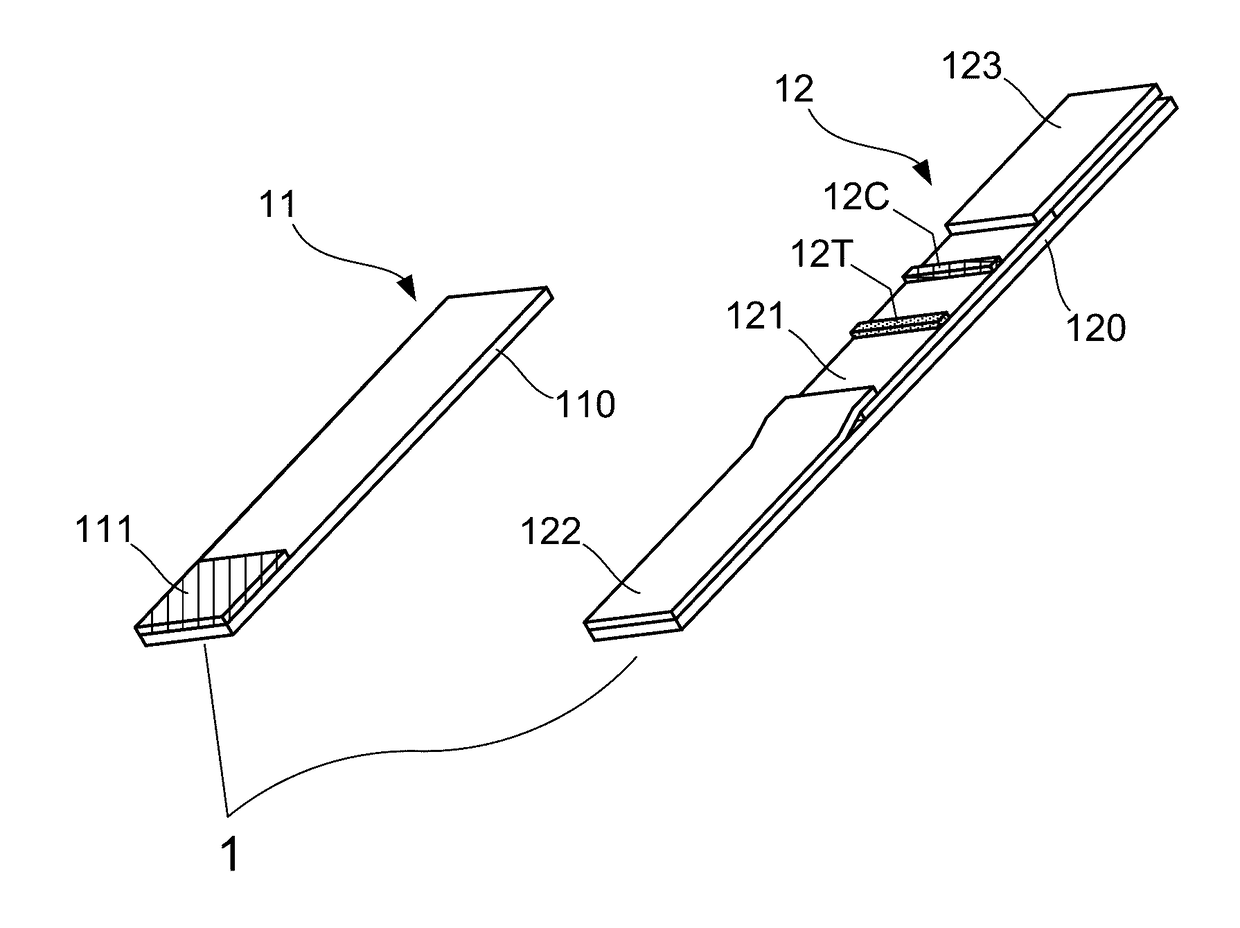
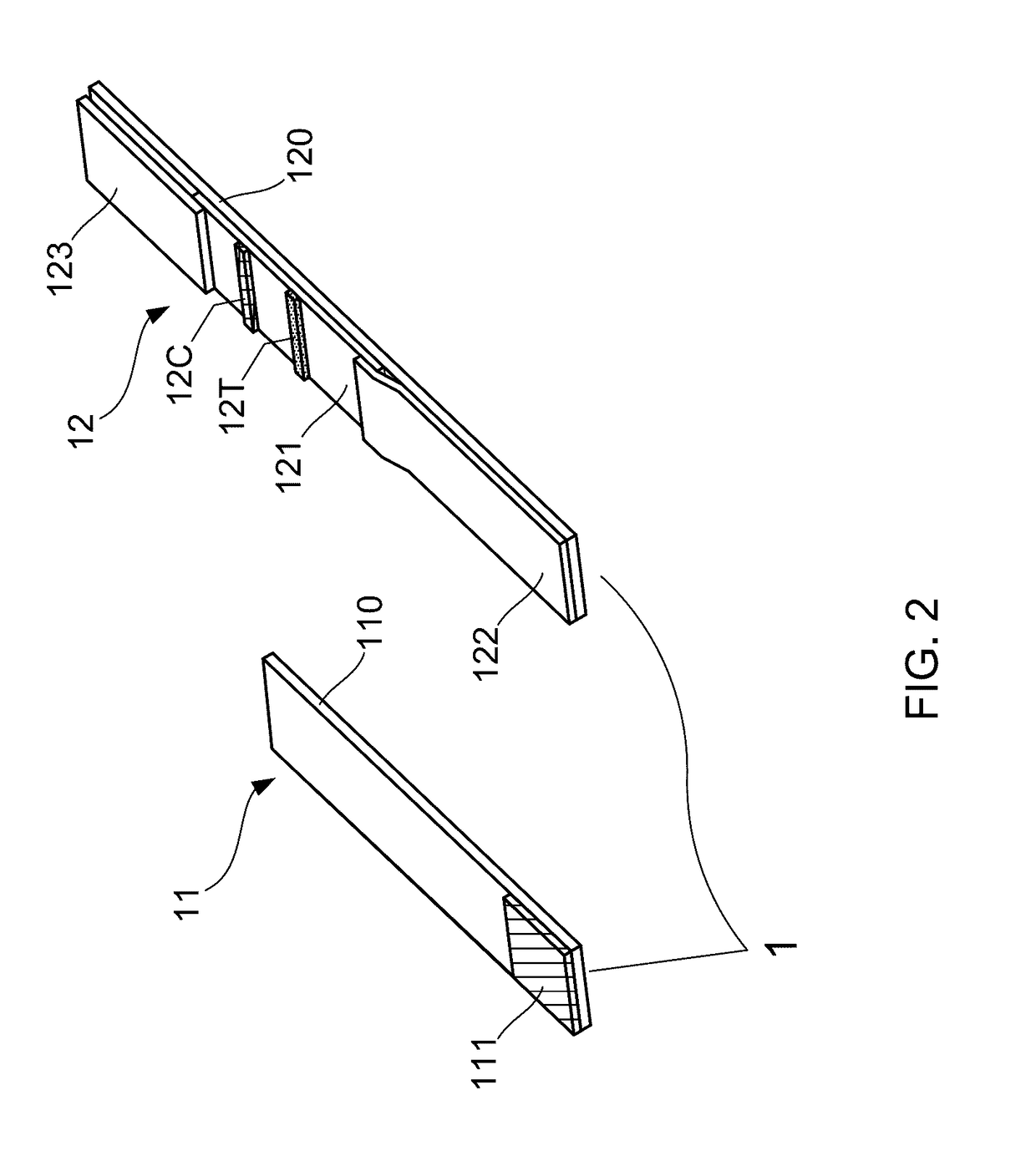
ActiveCN102426222AMeet qualitative testing needsQuick checkTesting foodLateral flow immunoassayControl line
The invention relates to a magnetic lateral flow immunoassay (LFIA) for a rapid detection of Tetrodotoxin (TTX) and a preparation method of a detection test strip, belonging to the technical field of TTX detection. The magnetic LFIA for the rapid detection of TTX is characterized in that: a sample pad, a combination pad labeled by magnetic beads and modified by anti-TTX specific rat monoclonal antibody, a chromatography membrane pre-coated with a detection line T of TTX-BSA and a control line C of IgG, a water absorbent pad are successively pasted on a base plate staggeredly by the distance between adjacent part being 1 mm, then a transparent plastic sealing membrane is coated on the top layer to form a magnetic LFIA test strip for the rapid detection of TTX; immunomagnetic beads are captured by chromatography, the rapid qualitative detection of TTX is realized according to the formed 1-2 macroscopic color developing strips after sample chromatography; the prepared magnetic LFIA test strip through a magnetism analyzer is detected, and the quantitative determination of TTX can be realized according to the detection values of the formed magnetic signal of the detection line and control line after sample chromatography.
Owner:SHANGHAI OCEAN UNIV
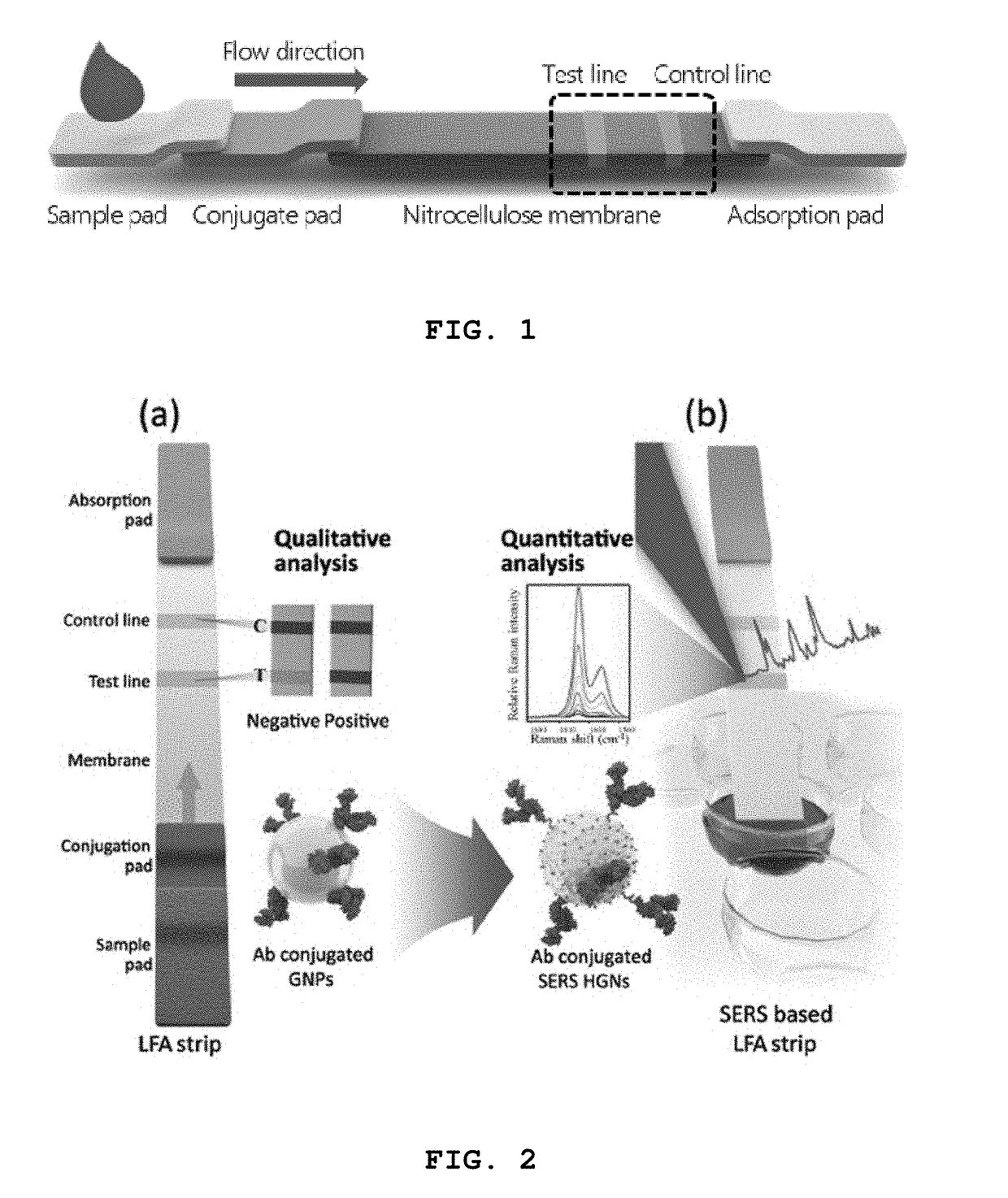
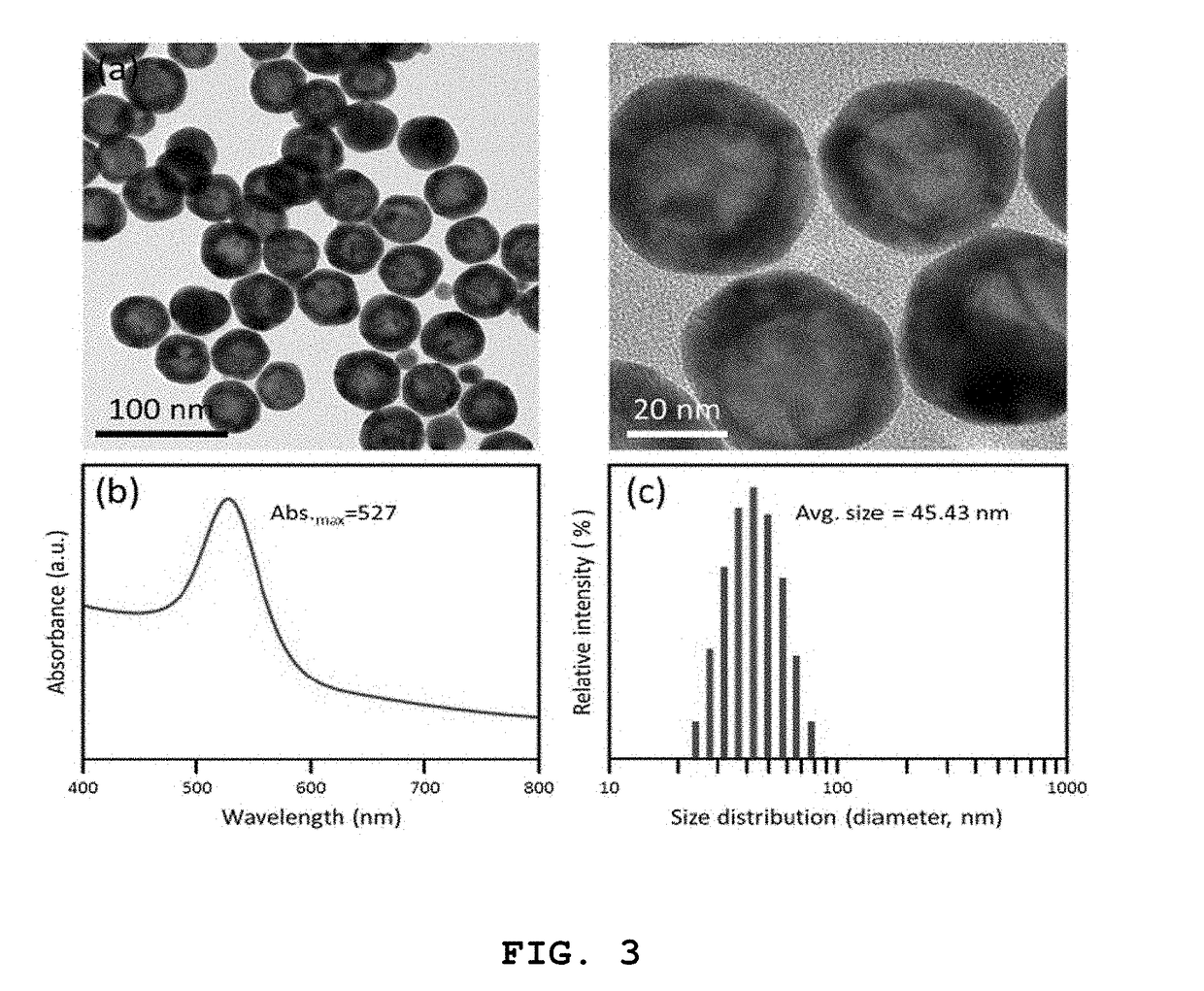
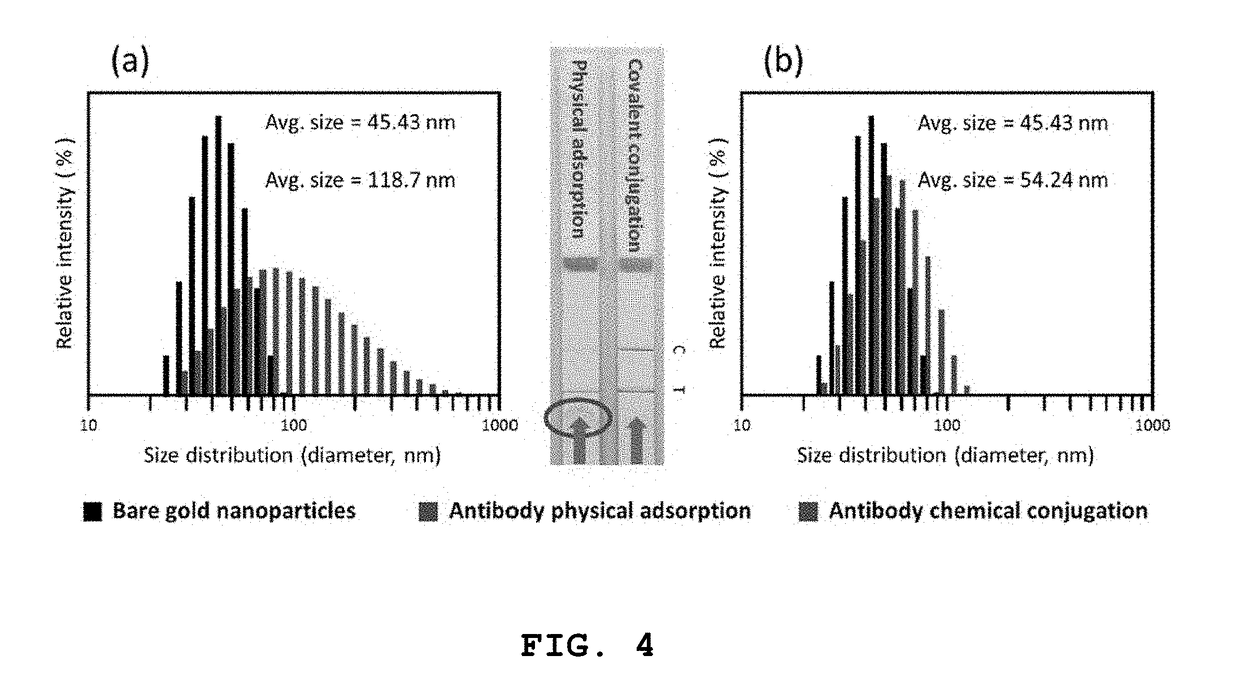
High-sensitivity lateral flow immunoassay strip based on surface-enhanced raman scattering and detection method using the same
InactiveUS20190049384A1Good reproducibilityMaterial analysis by observing effect on chemical indicatorRaman scatteringImmuno assayLateral flow immunoassay
The present disclosure relates to a surface-enhanced Raman scattering (SERS) lateral flow immunoassay strip containing: a sample pad into which a sample containing a target material is introduced; a conjugate pad containing a hollow metal nanoprobe for surface-enhanced Raman scattering, on which an antibody that can be coupled to the target material and a Raman marker are immobilized; and a detection pad including a detection region to which a secondary antibody that can be coupled to the target material coupled to the hollow metal nanoprobe is immobilized. Use of the SERS-based lateral flow immunoassay strip according to the present disclosure enables high-sensitivity quantitative analysis and qualitative analysis of the target material from Raman signal measurement depending on the concentration of the target material.
Owner:IND UNIV COOPERATION FOUND HANYANG UNIV ERICA CAMPUS
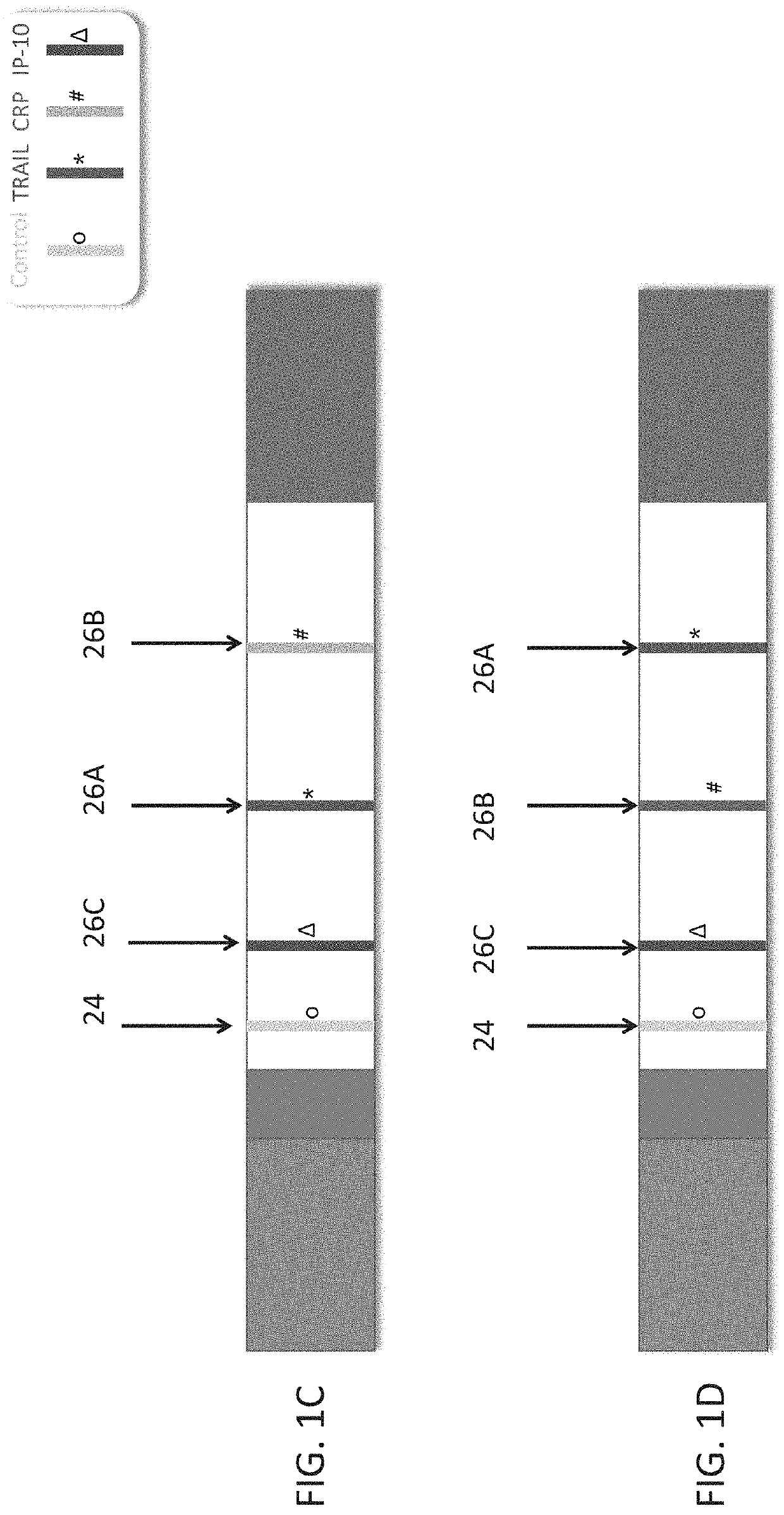
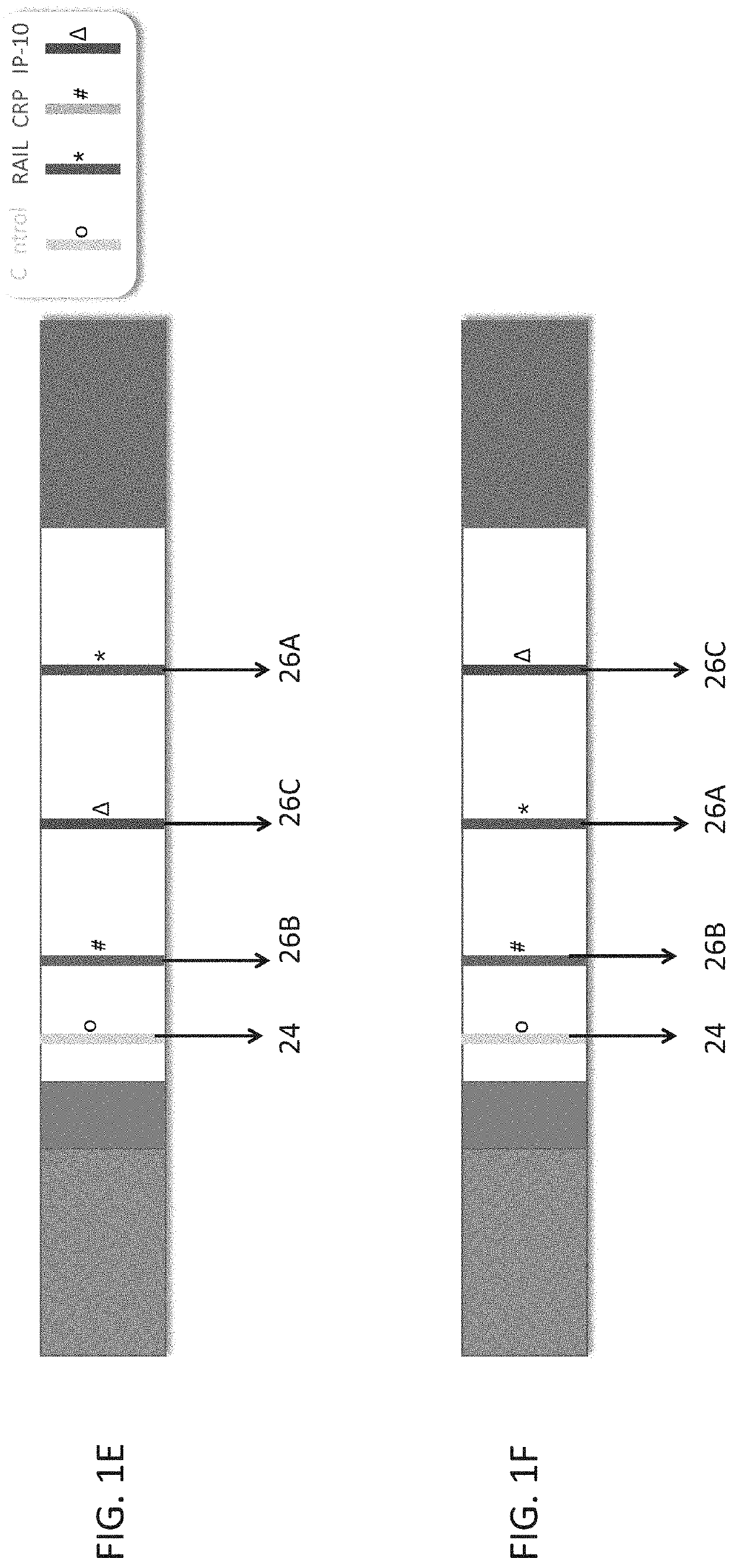
Measuring trail by lateral flow immunoassay
InactiveUS20200124593A1Microbiological testing/measurementDisease diagnosisLateral flow immunoassayChemistry
A method of measuring the amount of TNF-related apoptosis-inducing ligand (TRAIL) polypeptide in a fluid sample of a subject in need thereof is disclosed. The method uses a lateral flow immunoassay (LFI) device.
Owner:MEMED DIAGNOSTICS
Immunoassay kit
InactiveUS20170074873A1Easy to analyzeMaterial analysis using immobilised reagentsMaterial analysis by observing effect on chemical indicatorTest sampleLateral flow immunoassay
Differing from conventional lateral flow immunoassay test strips, the present invention provides an immunoassay kit comprising a release strip and a reaction strip. When using this immunoassay kit to complete a competitive-type immunochromatographic test or a sandwich-type immunochromatographic test for a test sample, it needs to firstly put the release strip into the test sample for releasing a specific antibody (or antigen) conjugating with a marker into the test sample, so as to make the first antibody connect to a target object in the test sample; after that, the reaction strip is eventually put into the test sample after the release strip is removed out of the test sample. Therefore, the qualitative analysis result of the competitive / sandwich-type immunochromatographic test can be easily obtained by determining whether the test line shows the color reaction or not.
Owner:REGA BIOTECH
Method and system utilizing lateral flow immunoassay test device with integrated quality assurance label
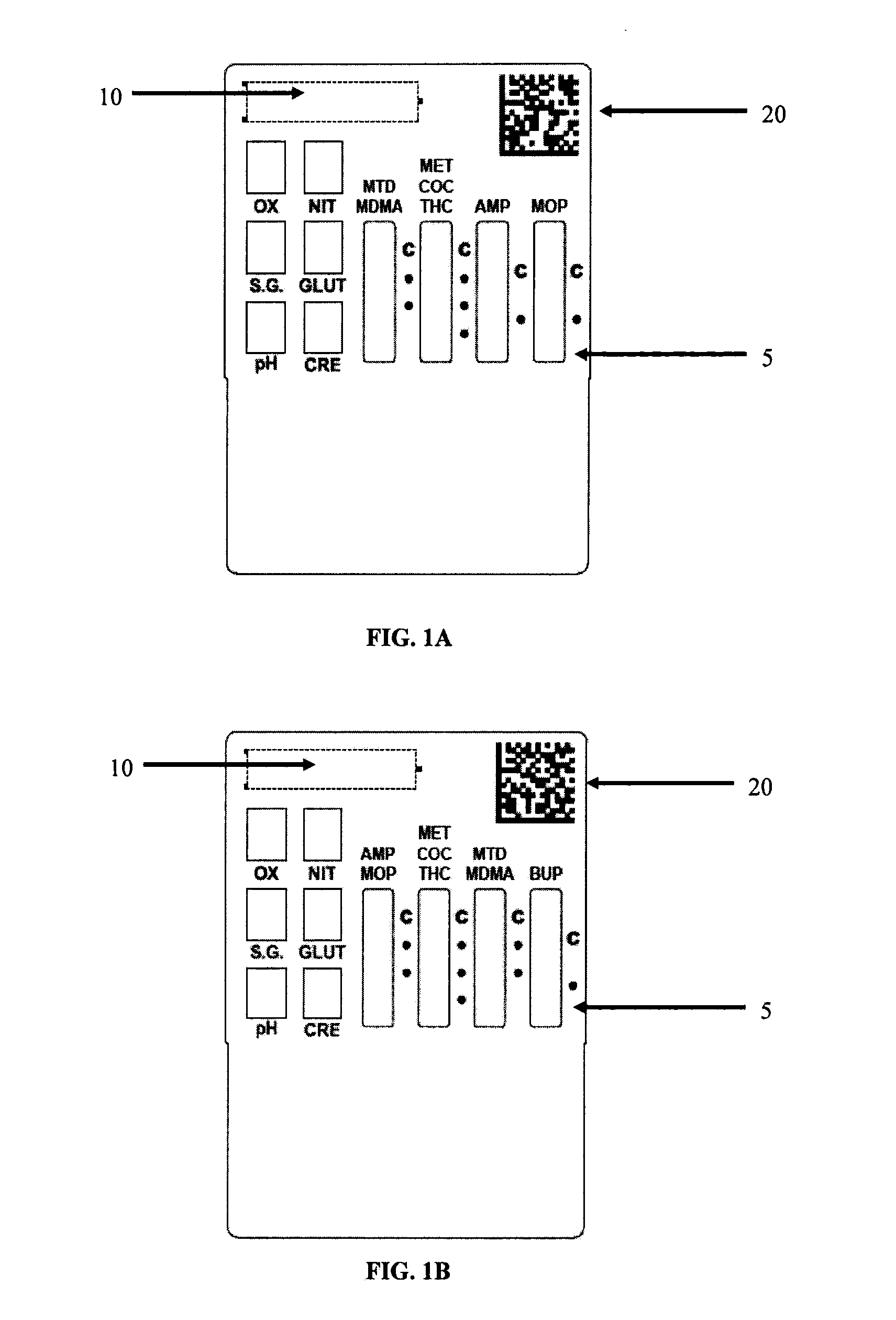
InactiveUS20120122236A1Improve test result reliabilityGood test resultComponent separationAnalysis by subjecting material to chemical reactionAnalyteQuality assurance
The present invention provides a method and system for performing analyte analysis with improved reliability of test results through use of quality assurance labels for accuracy and robust results.
Owner:ALERE SAN DIEGO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com