Cell slide for detecting acetylcholine receptor autoantibody in human body fluid as well as preparation method and application of cell slide
An acetylcholine receptor, autoantibody technology, applied in receptors/cell surface antigens/cell surface determinants, biochemical equipment and methods, cells modified by introducing foreign genetic material, etc., can solve whether it is necessary or not. Conclusion, inability to distinguish Rapsyn and AchR antibody positive, no immunofluorescence detection kit, etc., to achieve high sensitivity and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1, construction of recombinant vector
[0034] By PCR or artificial synthesis method, each subunit gene of AchR α1(P3A+), α1(P3A-), β1, δ1, δ2, δ3, δ4, ε, γ and Rapsyn(longer), (wherein, α1( P3A+), α1 (P3A-), δ1, δ2, δ3, δ4 sequences such as SEQ.ID.NO.1, SEQ.ID.NO.2, SEQ.ID.NO.3, SEQ.ID.NO.4, Shown in SEQ.ID.NO.5 and SEQ.ID.NO.6, the sequence numbers of other sequences on NCBI are: NM_000747.3, NM_000080.4, NM_005199.5, NM_005055.5) connected by molecular cloning On pCDNA3.1, the constructed recombinant vector is sequenced correctly and extracted for later use;
[0035] Step 2. Cell transfection
[0036] (1) 293T cell culture: 10% FBS-DMEM high-glucose medium was prepared by DMEM high-glucose medium and FBS at a ratio of 9:1, and passaged at a ratio of 1:5 to 1:6 when the cells were confluent, placed at 37°C, Cultivate overnight in a cell culture incubator with 5% CO2;
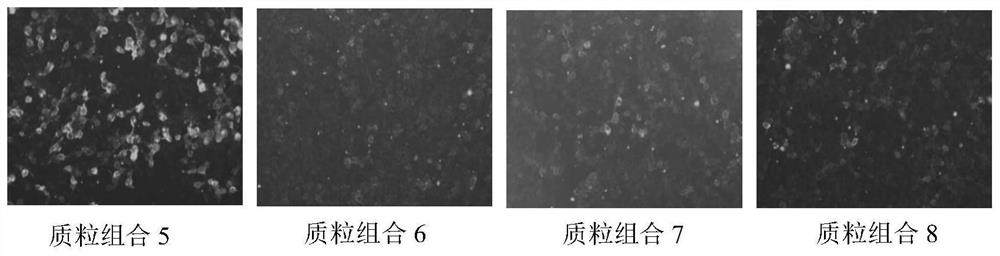
[0037] (2) When the cell density is 30% to 40%, transfect each gene according to the combin...
Embodiment 2
[0058] 1. Construction of recombinant vector
[0059] By PCR or artificial synthesis, connect Rapsyn (shorter, the sequence number on NCBI is: NM_032645.5), and the gene is connected to pCDNA3.1 by molecular cloning method, and the rest of the required vectors are all constructed in Example 1 Vector, the constructed recombinant vector is sequenced correctly and extracted for later use;
[0060] 2. Cell transfection
[0061] (1) 293T cell culture: 10% FBS-DMEM high-glucose medium was prepared by DMEM high-glucose medium and FBS at a ratio of 9:1, and passaged at a ratio of 1:5 to 1:6 when the cells were confluent, placed at 37°C, Cultivate overnight in a cell culture incubator with 5% CO2;
[0062] (2) When the cell density is 30% to 40%, transfect each gene according to the combination of Table 2 and Table 3:
[0063] Transfection System 1 (Table 1):
[0064] Get the recombinant vectors pCDNA3.1-α1 (P3A-), pCDNA3.1-β1, pCDNA3.1-δ1, pCDNA3.1-ε, pCDNA3.1-γ, pCDNA3. 1-rapsyn...
Embodiment 3
[0104] 1. 293T cell culture: 10% FBS-DMEM high-glucose medium was prepared by DMEM high-glucose medium and FBS at a ratio of 9:1, and passaged at a ratio of 1:5 to 1:6 when the cells were confluent, and placed at 37°C for 5 Culture overnight in a cell culture incubator with %CO2;
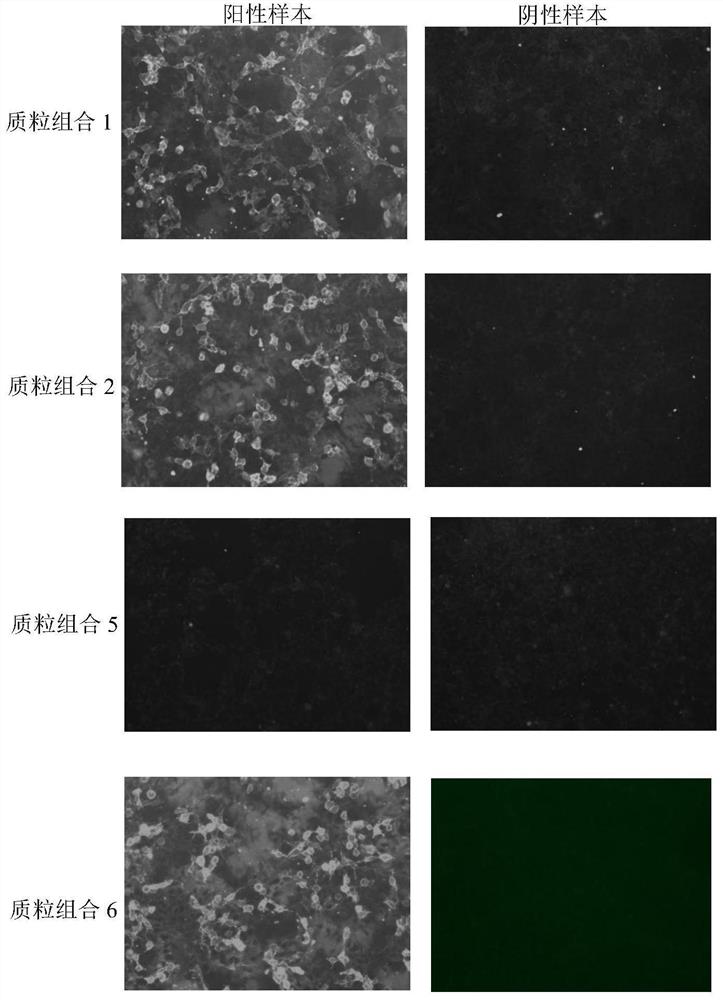
[0105] 2. When the cell density is 30%-40%, take the vector constructed in Example 1 and carry out transfection according to the combination of the following three methods, replace with fresh culture medium after 4 hours, and fix the cells after 48 hours.
[0106] (1) Adult + fetal type: pCDNA3.1-α1(P3A-), pCDNA3.1-β1, pCDNA3.1-γ, pCDNA3.1-δ1, pCDNA3.1-ε, 17-T2A, plasmids according to 2 :1:1:1:1:24 ratio for transfection, the total amount of transfection plasmid: 6ug;
[0107] (2) Adult type: pCDNA3.1-α1(P3A-), pCDNA3.1-β1, pCDNA3.1-γ, pCDNA3.1-δ1, 17-T2A, plasmids according to 2:1:1:1:24 Ratio for transfection, the total amount of transfected plasmids: 6ug;
[0108] (3) Fetal type: pCDNA3.1-α1(P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com