One-step chemiluminiscence quantitative detection kit for hyaluronic acid
A chemiluminescence reagent and hyaluronic acid technology, which is applied in the field of immunoanalysis medicine and chemiluminescence kits, can solve the problems of many interference factors, radioactive pollution, poor repeatability of reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of the hyaluronic acid one-step chemiluminescence quantitative detection kit of the present invention
[0030] 1. Preparation of HA calibrator.
[0031] 1. 50mmol / L pH7.4 Phosphate Buffer (PB):
[0032] Na 2 HPO 4 12H 2 O 14.5 g
[0033] NaH 2 PO 4 2H 2 O 1.48 g
[0034] Dissolve in 1000ml deionized water, and measure its pH value to be 7.4.
[0035] Add 5% calf serum to the standard diluent.
[0036] 2. Accurately dilute the HA concentrated antigen to several concentrations of 25, 50, 100, 200, 400, 800 ng / ml, S0 is the calibrator diluent, a total of 7 bottles.
[0037] 2. Preparation of HA-coated plates
[0038] Buffer 1: Coating Solution
[0039] 50mmol / L pH7.4PB
[0040] Buffer 2: Blocking Solution
[0041] 50mmol / L pH7.4PB
[0042] 10% sucrose
[0043] 0.5%BSA
[0044] 0.1% preservatives.
[0045] Preparation of coated plate: Dilute HA-BSA to 50mmol / L pH7.4 PB coating solution in proportion, add 100μl / well to the microwell p...
Embodiment 2
[0052] Embodiment 2: The operation method of the hyaluronic acid one-step chemiluminescence quantitative detection kit of the present invention.
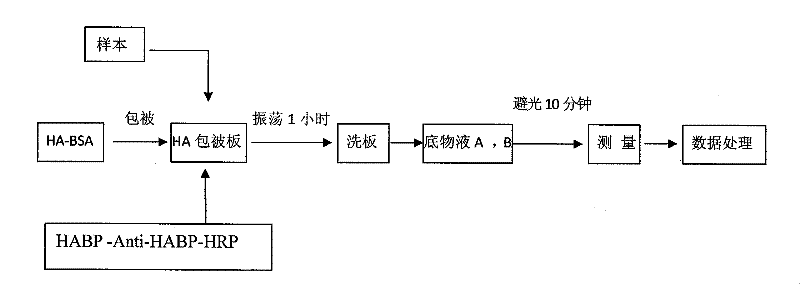
[0053] The specific operation method of the hyaluronic acid one-step chemiluminescence quantitative detection kit prepared in the above embodiment 1 is:
[0054] a. Add 50 μl of HA calibrator or sample to be tested to each well of the microplate.
[0055] b. Add 50 μl of HA enzyme conjugate to each well of the microplate.
[0056] c. Place the microplate on a shaker and shake it at room temperature for 60 minutes.
[0057] d. Discard the liquid in the microwell plate, add washing solution to each well, let it stand for 30 seconds, and then blot or spin dry. So repeated washing 5 times. Buckle the microplate dry on paper when finished.
[0058] e. Add 50 μl (1 drop) of substrate solution A and substrate solution B to each well, shake the microwell plate to mix after adding the samples.
[0059] f. Measure the luminescence value ...
Embodiment 3
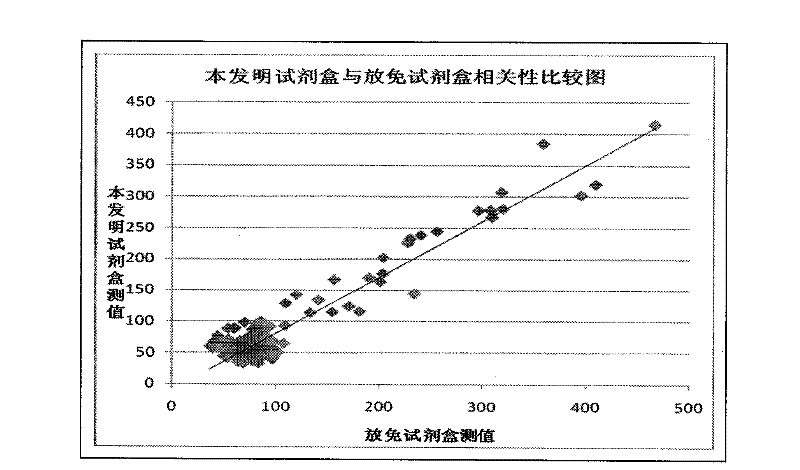
[0060] Example 3 Methodological identification of the one-step chemiluminescence quantitative detection kit for hyaluronic acid of the present invention
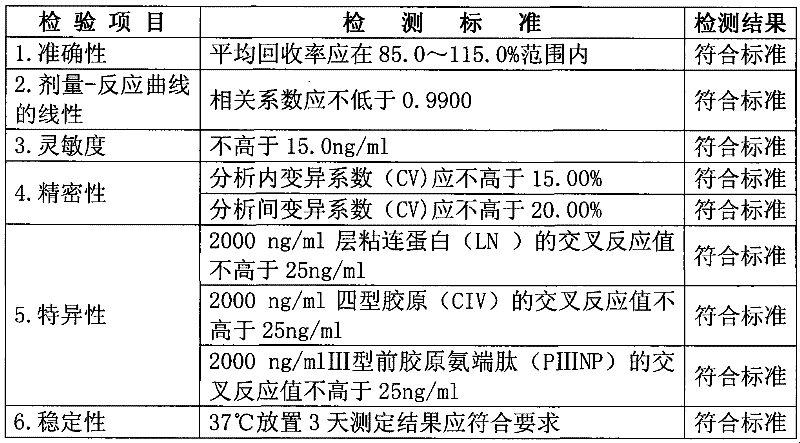
[0061] Table 1.
[0062] Table 1 The methodological identification results of the kit
[0063]
[0064]
[0065] It shows that the accuracy, specificity, precision, sensitivity and stability of the kit of the present invention are fully qualified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com