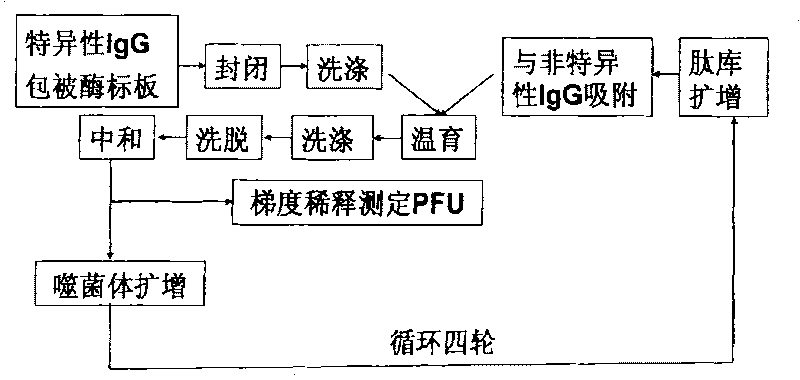
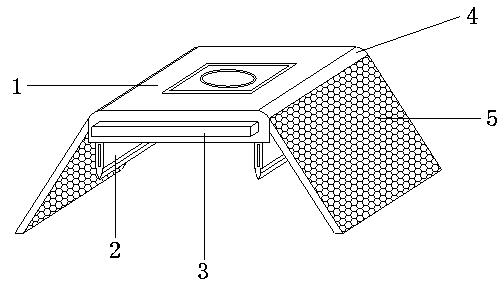
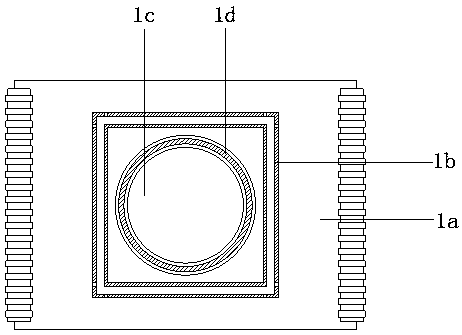
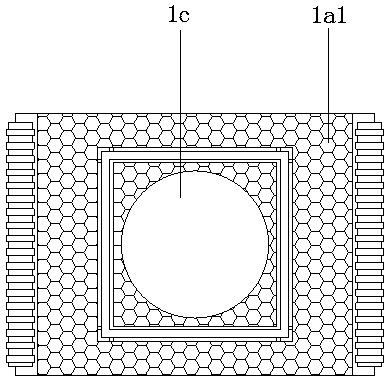
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Enation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enations are scaly leaflike structures, differing from leaves in their lack of vascular tissue. They are created by some leaf diseases. Also found on some early plants such as Rhynia, where they are hypothesized to aid in photosynthesis.
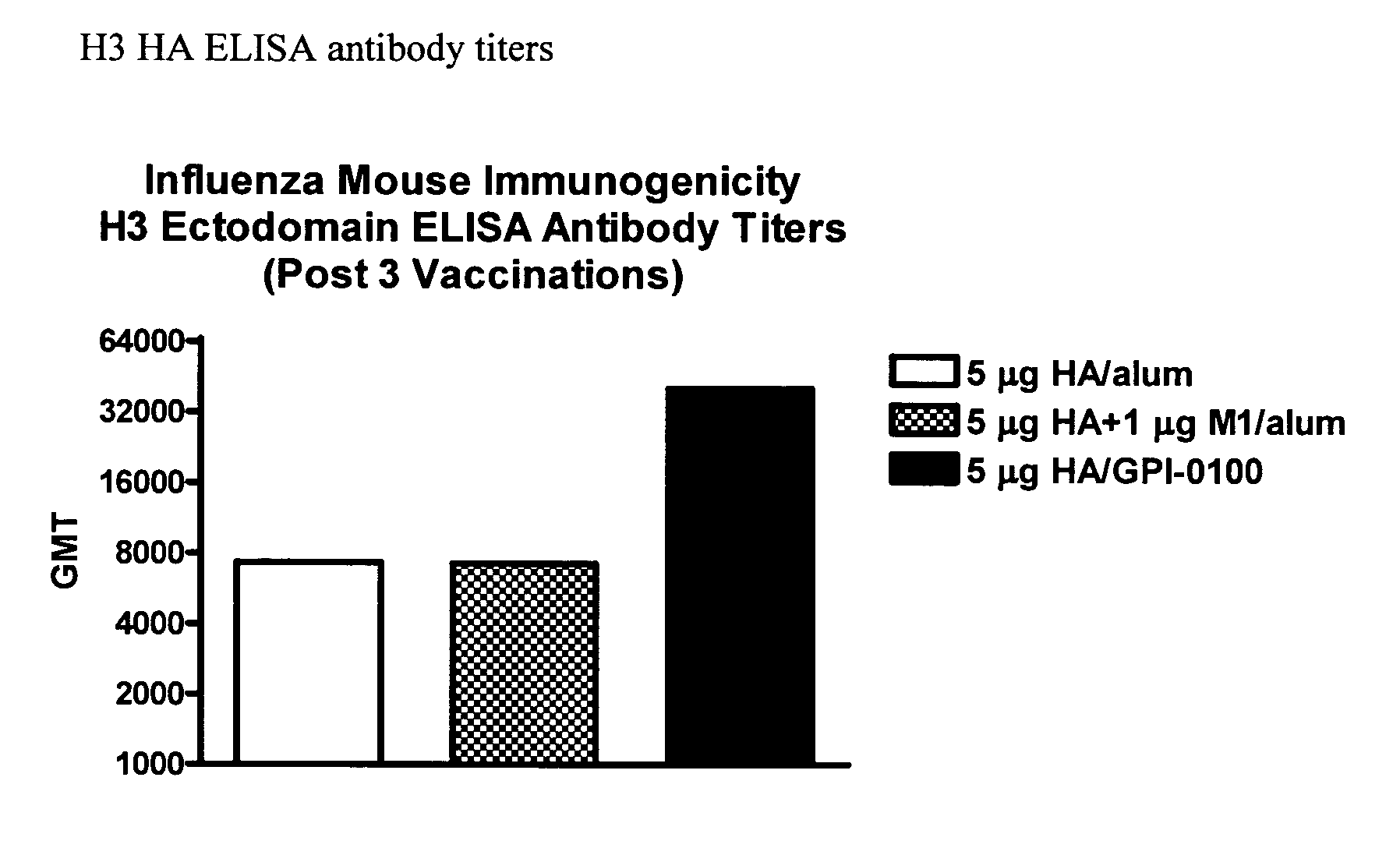
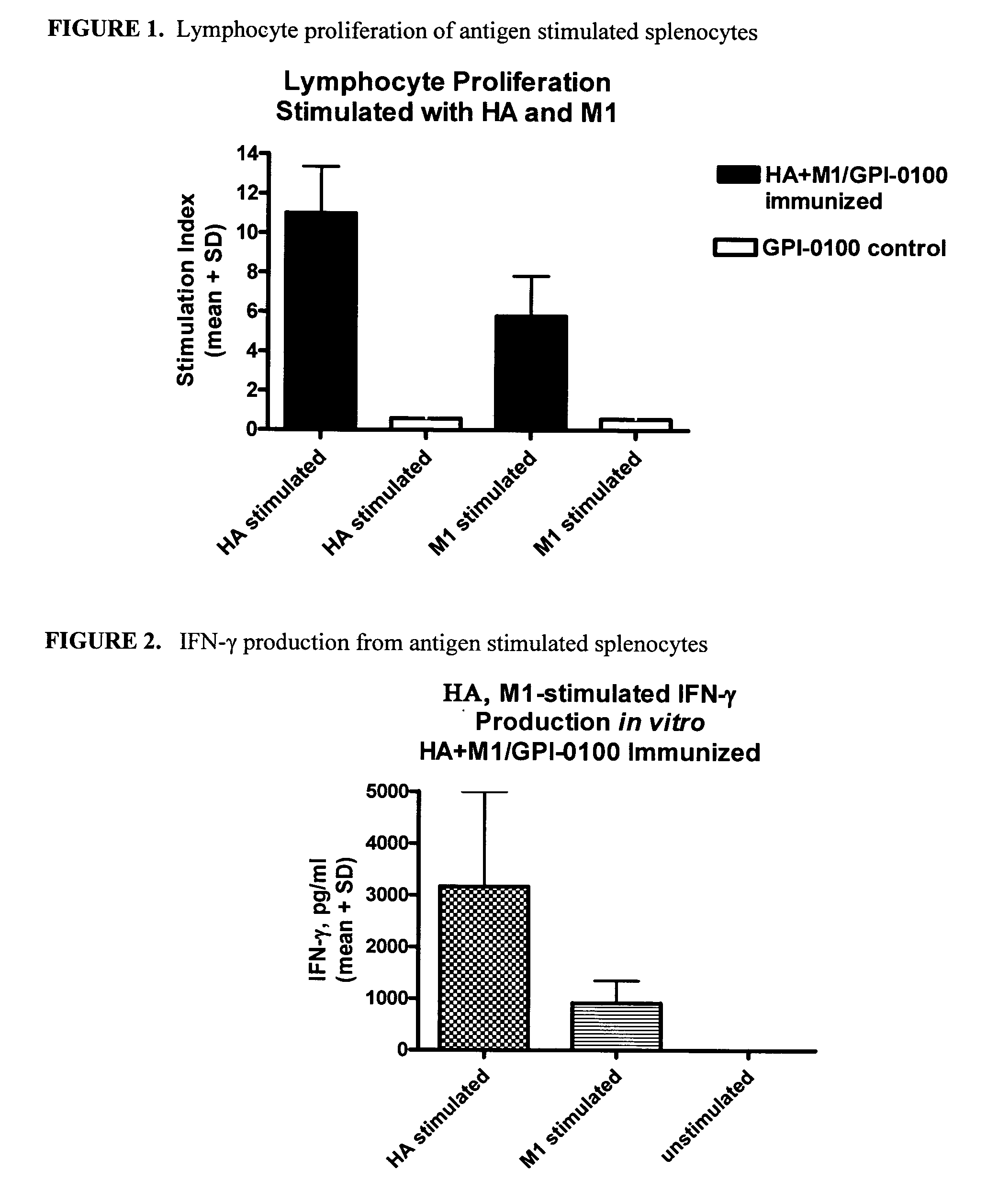
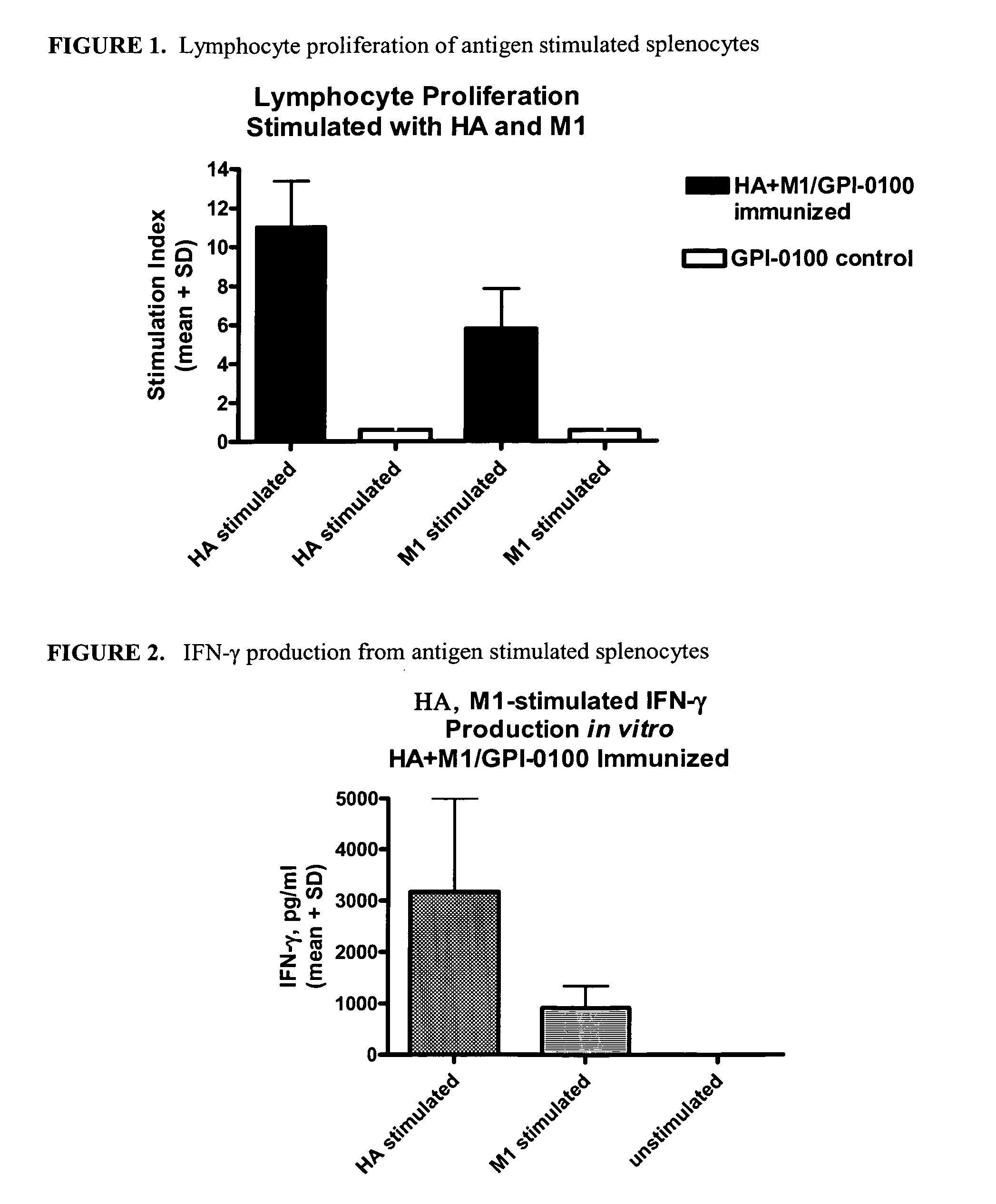
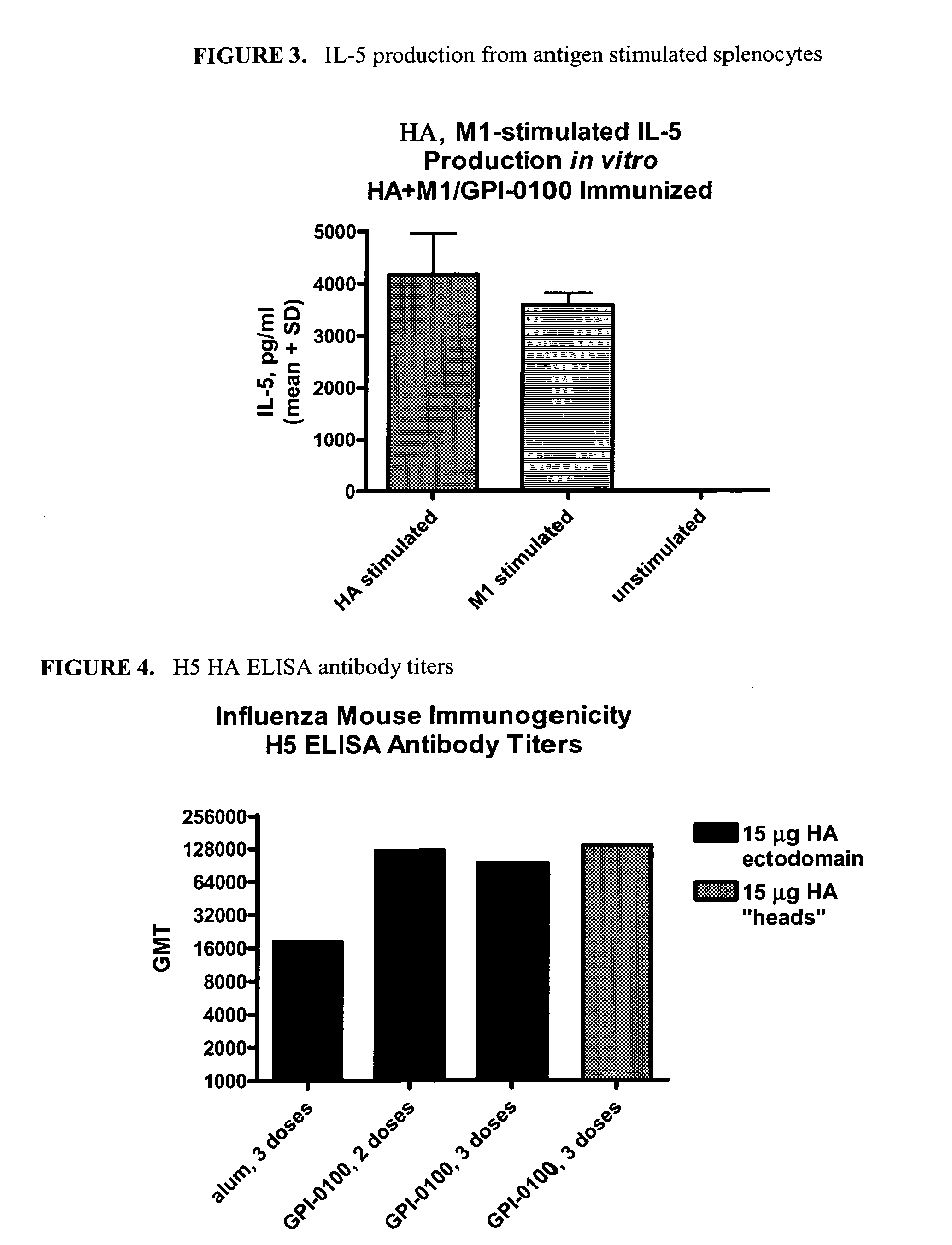
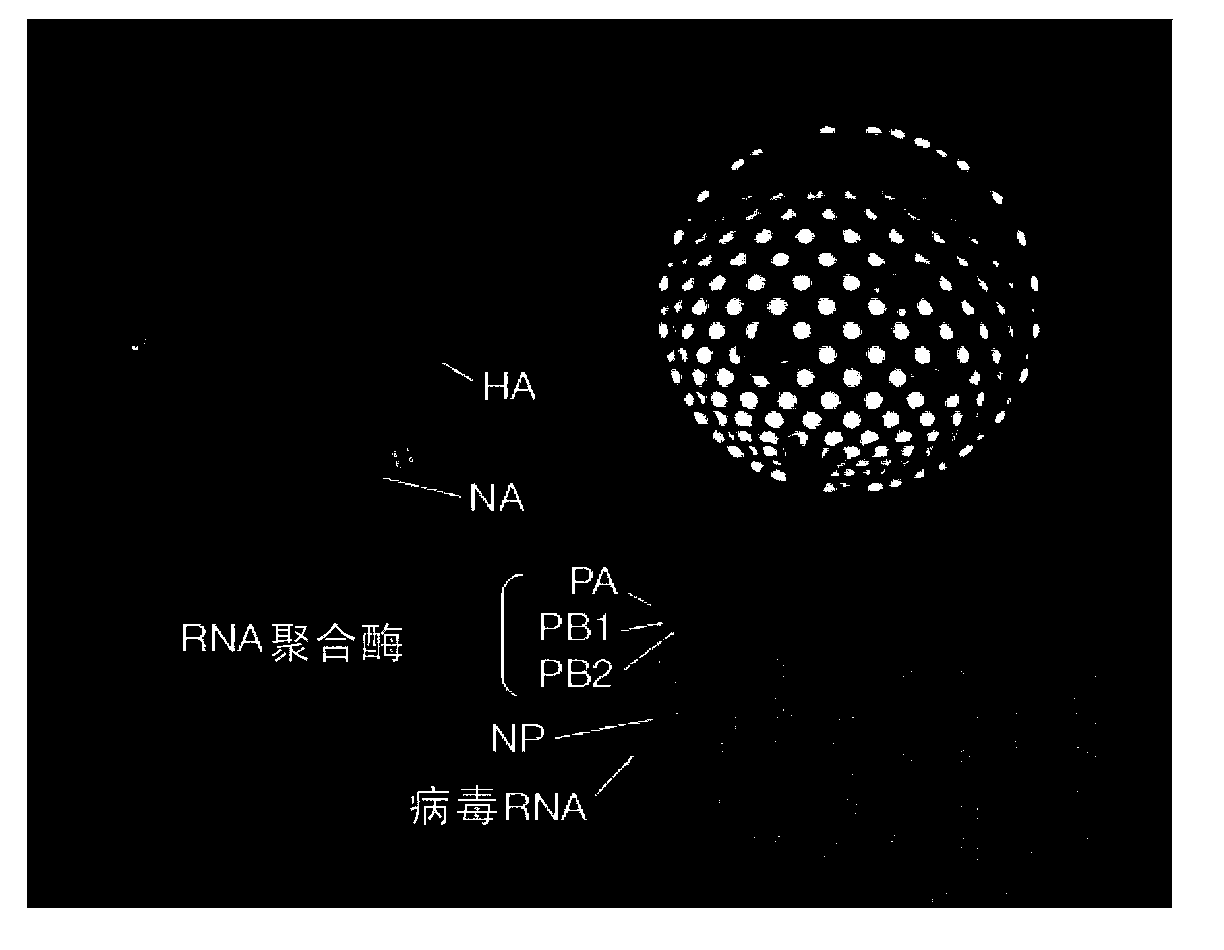
Influenza recombinant subunit vaccine
InactiveUS20070042002A1Improving immunogenicityImprove efficacySsRNA viruses negative-senseVirus peptidesAdjuvantEnation
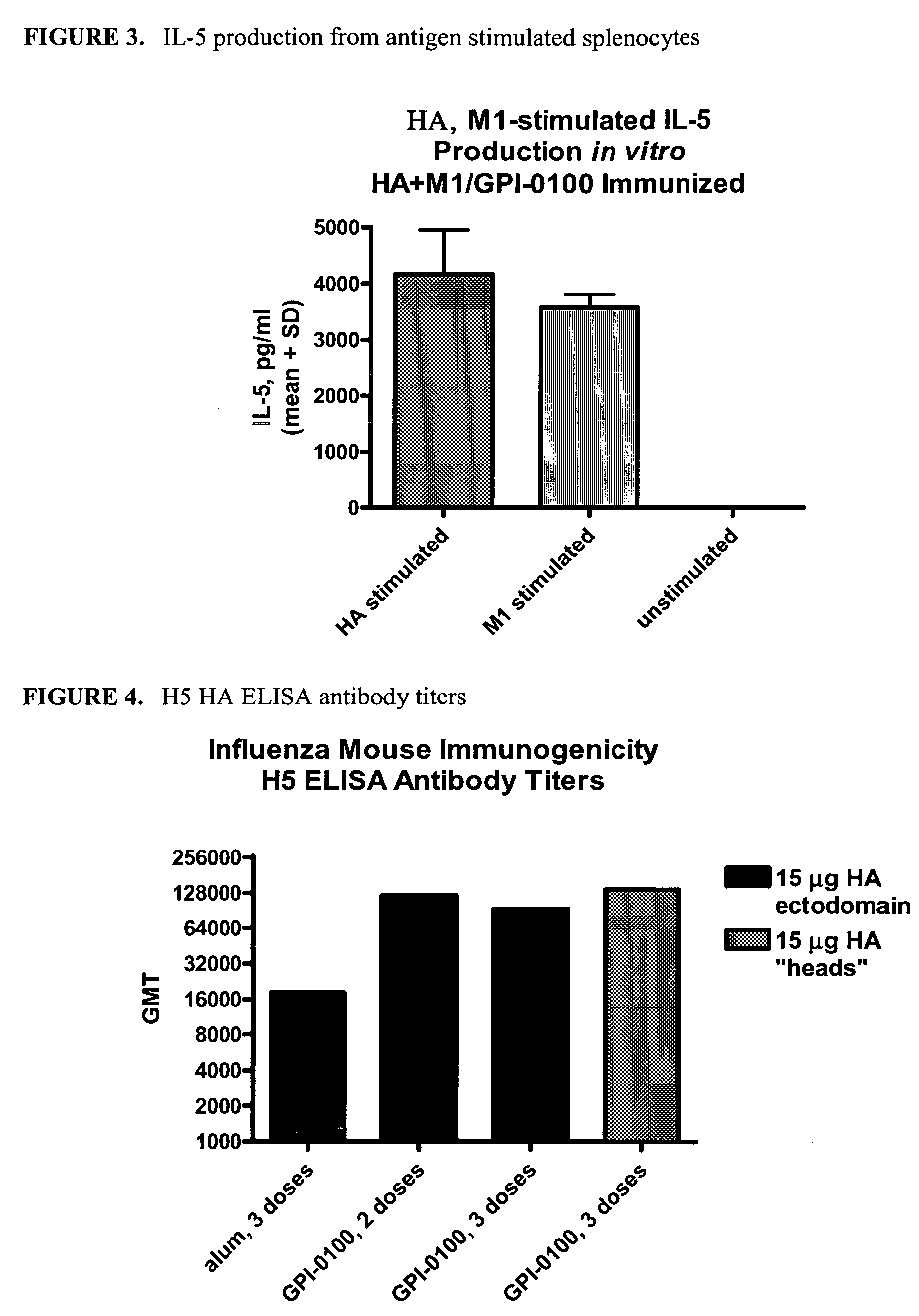
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:HAWAII BIOTECH INC
Influenza recombinant subunit vaccine
InactiveUS20080008725A1Avoids potential degradationAvoiding lysis of the host cellsSsRNA viruses negative-senseViral antigen ingredientsAdjuvantEnation
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:MERCK SHARP & DOHME CORP
Influenza recombinant subunit vaccine
InactiveUS20070042001A1Avoids potential degradationAvoiding lysis of the host cellsSsRNA viruses negative-senseVirus peptidesAdjuvantEnation
The invention provides influenza proteins, including subunit proteins and immunogenic compositions that can be utilized, with or without adjuvants, as vaccines to protect against influenza infection in animal models and humans. The recombinant proteins are expressed from transformed insect cells that contain integrated copies of the appropriate expression cassettes in their genome. The invention uses a Drosophila melanogaster expression system to provide high yields of recombinant subunit proteins with native-like conformation.
Owner:HAWAII BIOTECH INC
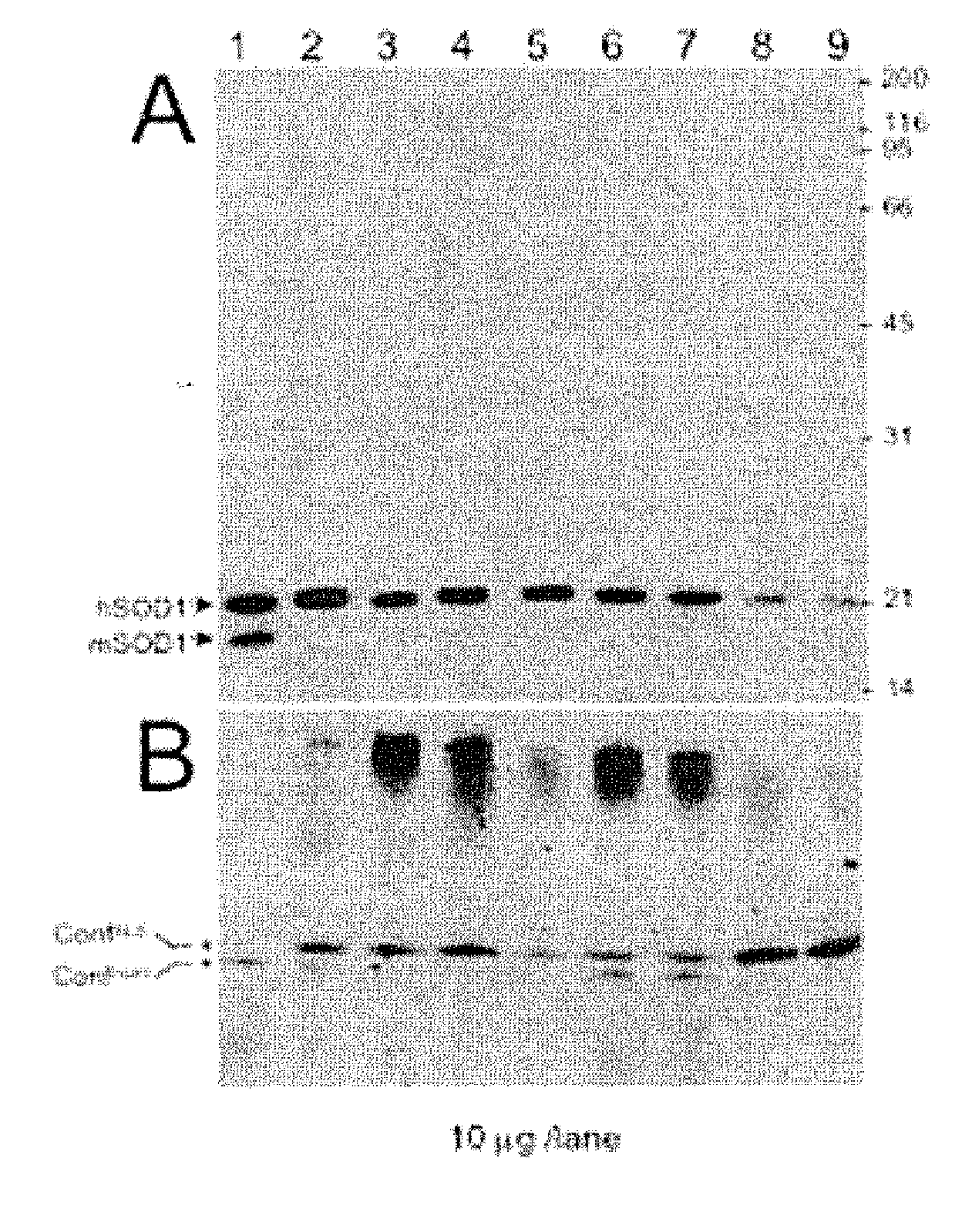
Biomarkers for als
InactiveUS20070202537A1Peptide/protein ingredientsDisease diagnosisAmyotrophic lateral sclerosisEnation
Provided are methods for diagnosing sporadic and familial forms of amyotrophic lateral sclerosis (“ALS”) that detect conformers or conformer patterns of the copper-zinc superoxide dismutase-1 (“SOD-1”) enzyme that are common to sporadic or familial ALS individuals but distinct from SOD-1 conformers of normal individuals. Methods of identifying candidate drugs that modulate SOD-1 conformer formation also are provided.
Owner:PROSETTA CORP
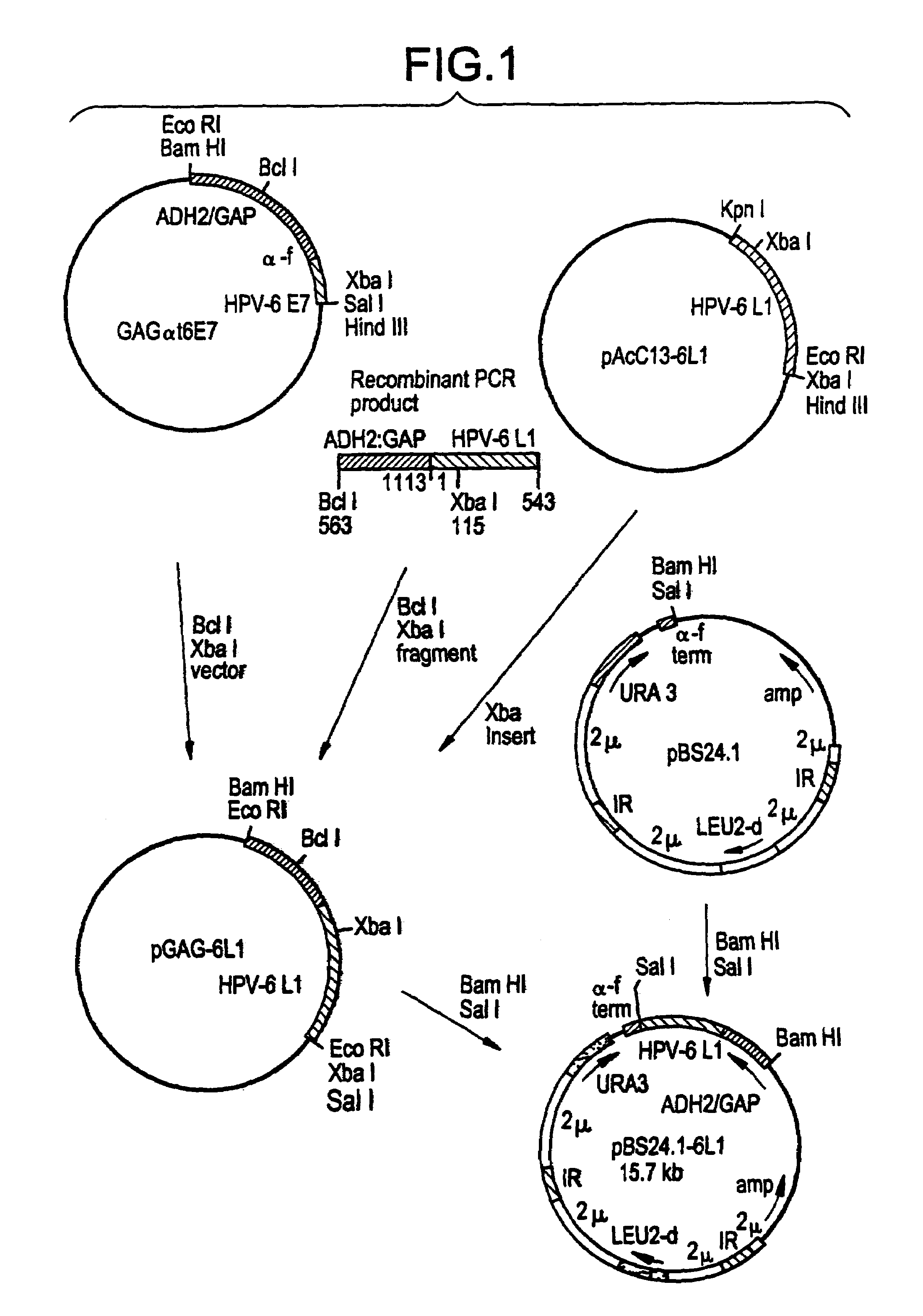
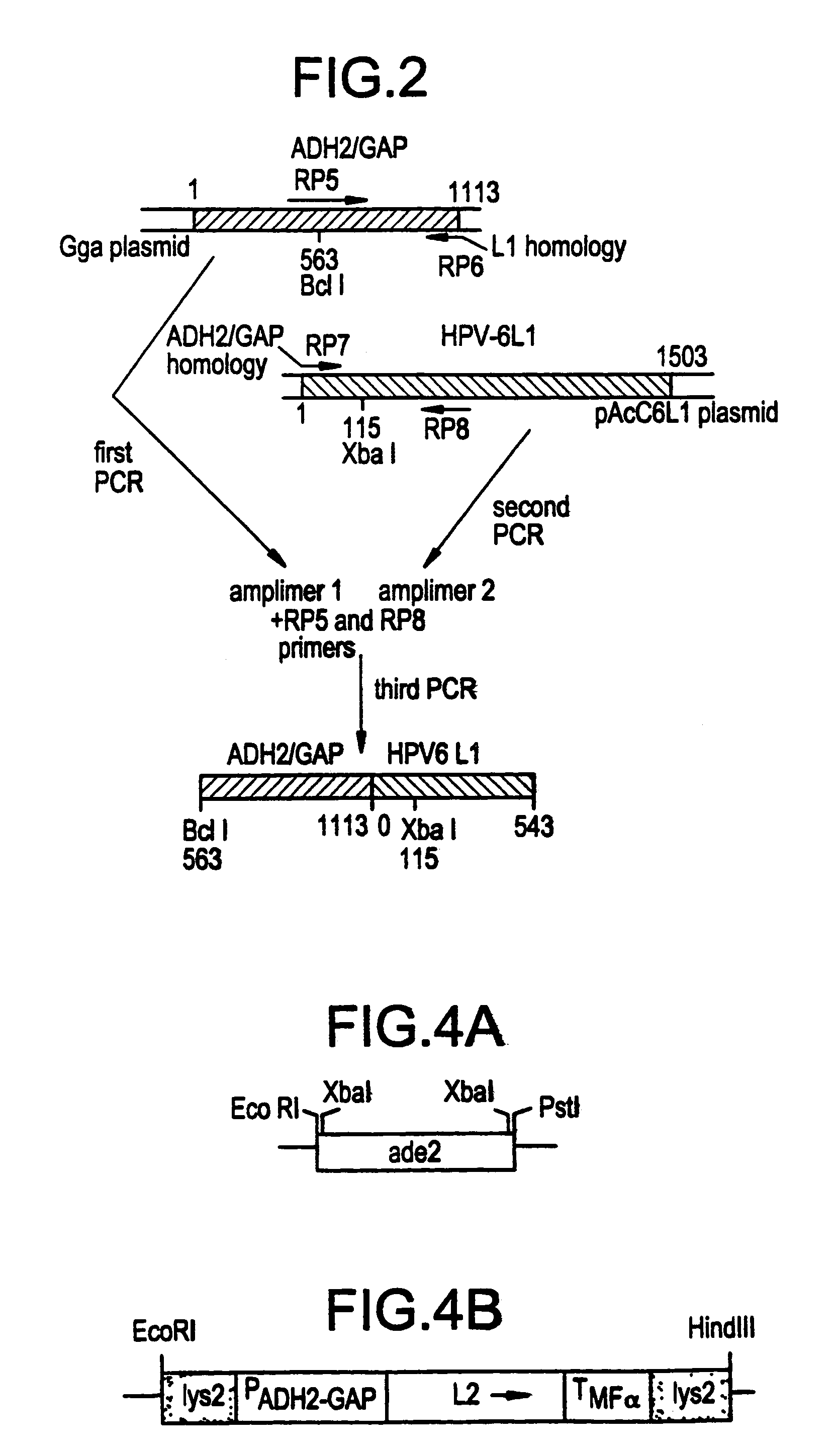
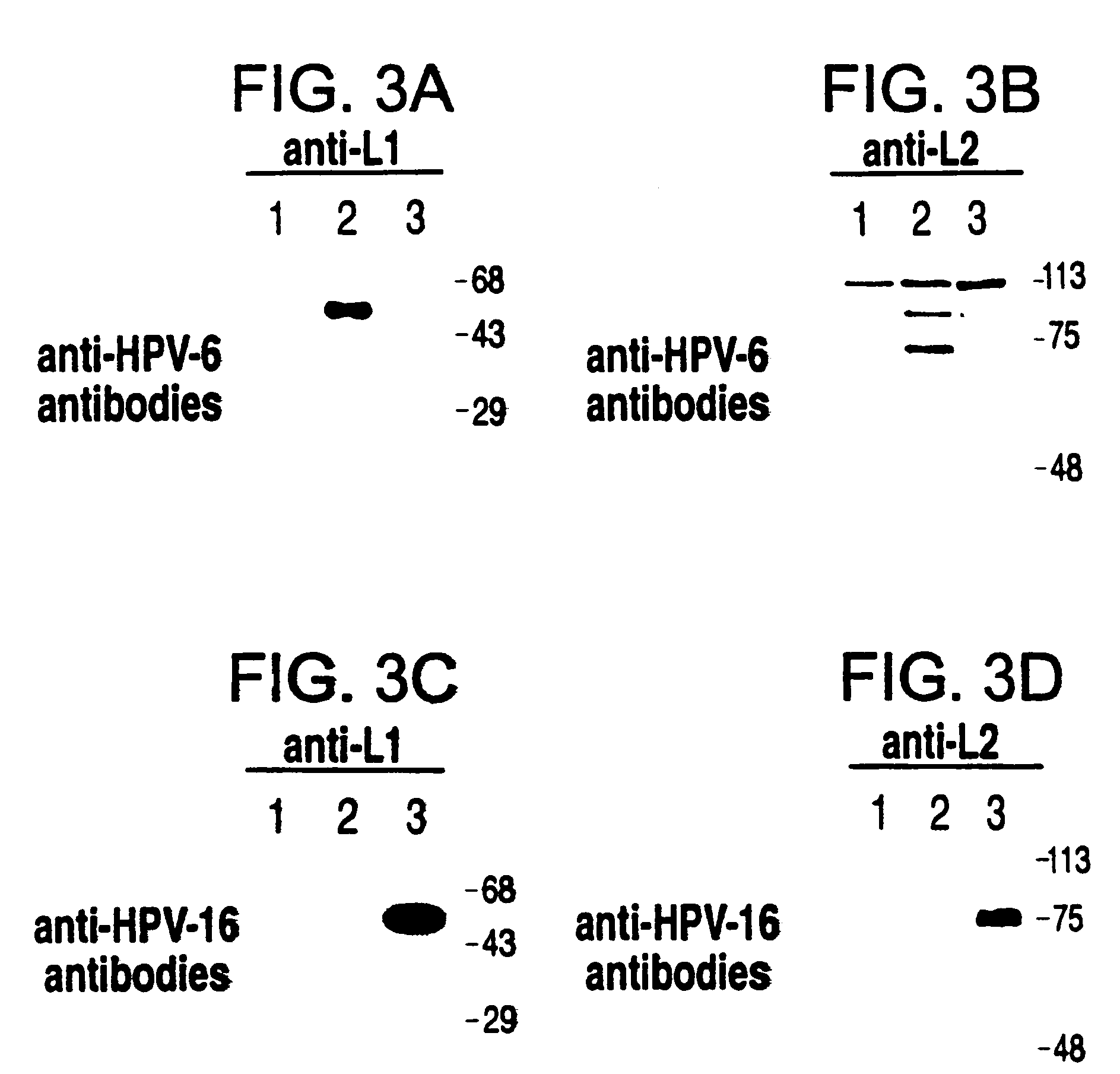
Method for producing yeast expressed HPV types 6 and 16 capsid proteins
Mosaic VLPs of viral capsid proteins from different virus types are described, as are methods of making the same. Specifically, a diploid yeast strain that coexpresses the L1 and L2 capsid proteins of both HPV-6 and HPV-16 as mosaic VLPs is described. The mosaic VLPs induced the production conformational antibodies against both L1 proteins upon administration to mice.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
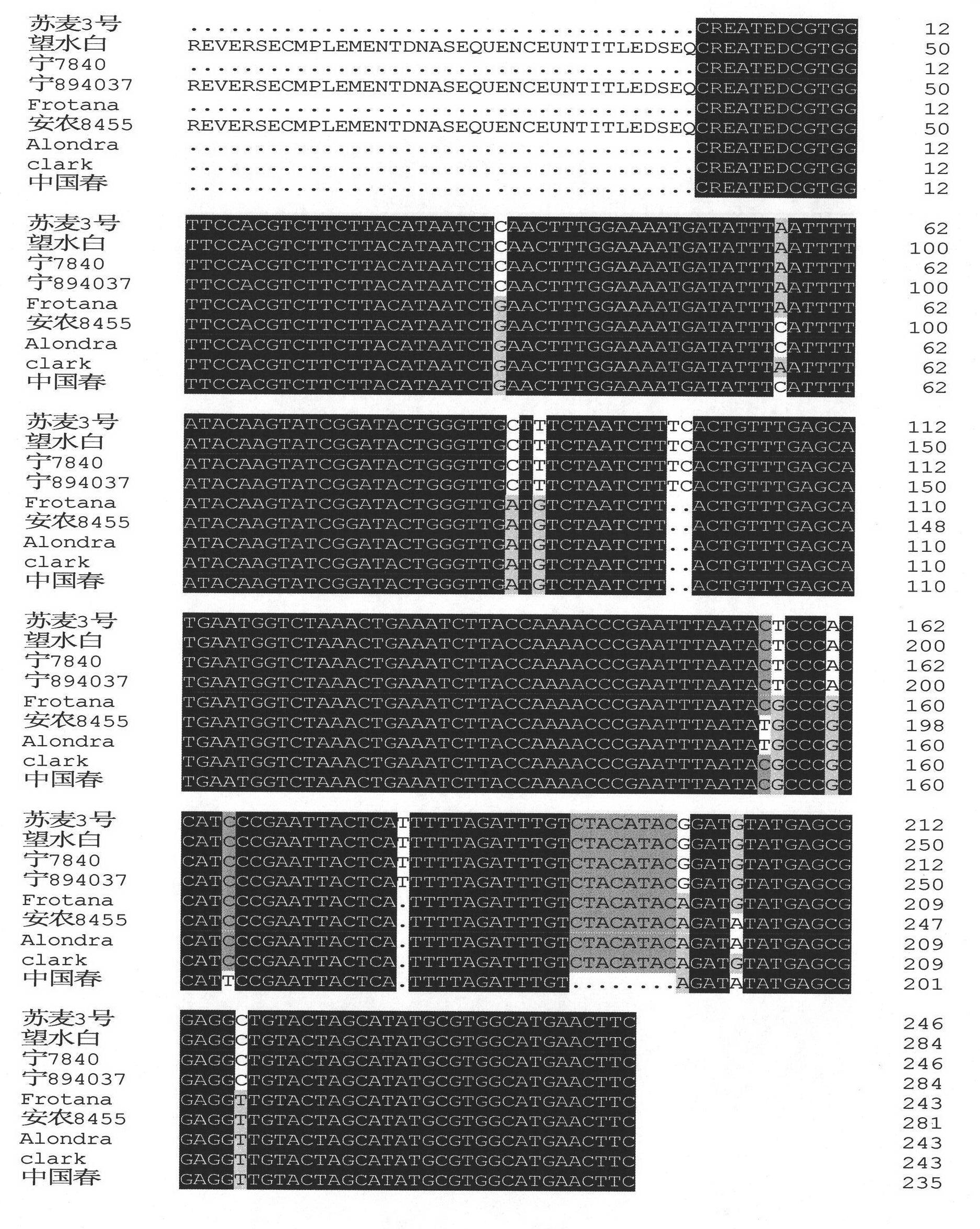
SSCP marker closely linked with major wheat scab resistance QTL and application thereof
InactiveCN101892307AReduce the waste of manpower and material resourcesImprove breeding efficiencyMicrobiological testing/measurementMicroorganism based processesSingle-strand conformation polymorphismSequence analysis
The invention relates to a single strand conformation polymorphism (SSCP) marker closely linked with a major wheat scab resistance quantitative trait locus (QTL). The SSCP marker is characterized in that: scab resistance candidate genes of a scab resistance QTL in a 3BS area of a wheat variety or strain resisting scab is PCR amplified by using a primer; after denaturalization, a PCR-amplified product has different single strand conformation; and the SSCP marker closely linked with the major wheat scab resistance QTL is established for detecting a genotype of the wheat variety or strain during breeding. The SSCP marker has the advantages of: overcoming the disadvantages that the wheat scab resistance screening can only be authenticated in a flowering period and is easily influenced by the environment in conventional breeding, predicting and screening wheat plants with the scab resistance by detecting a molecular marker at a seedling stage, eliminating disease plants, reducing waste of labor and materials and improving the breeding efficiency. Compared with an ABI DNA sequence analysis meter-based marker, the SSCP marker closely linked with the major wheat scab resistance QTL has the advantages of simple and convenient operation, low cost and same sensitivity.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
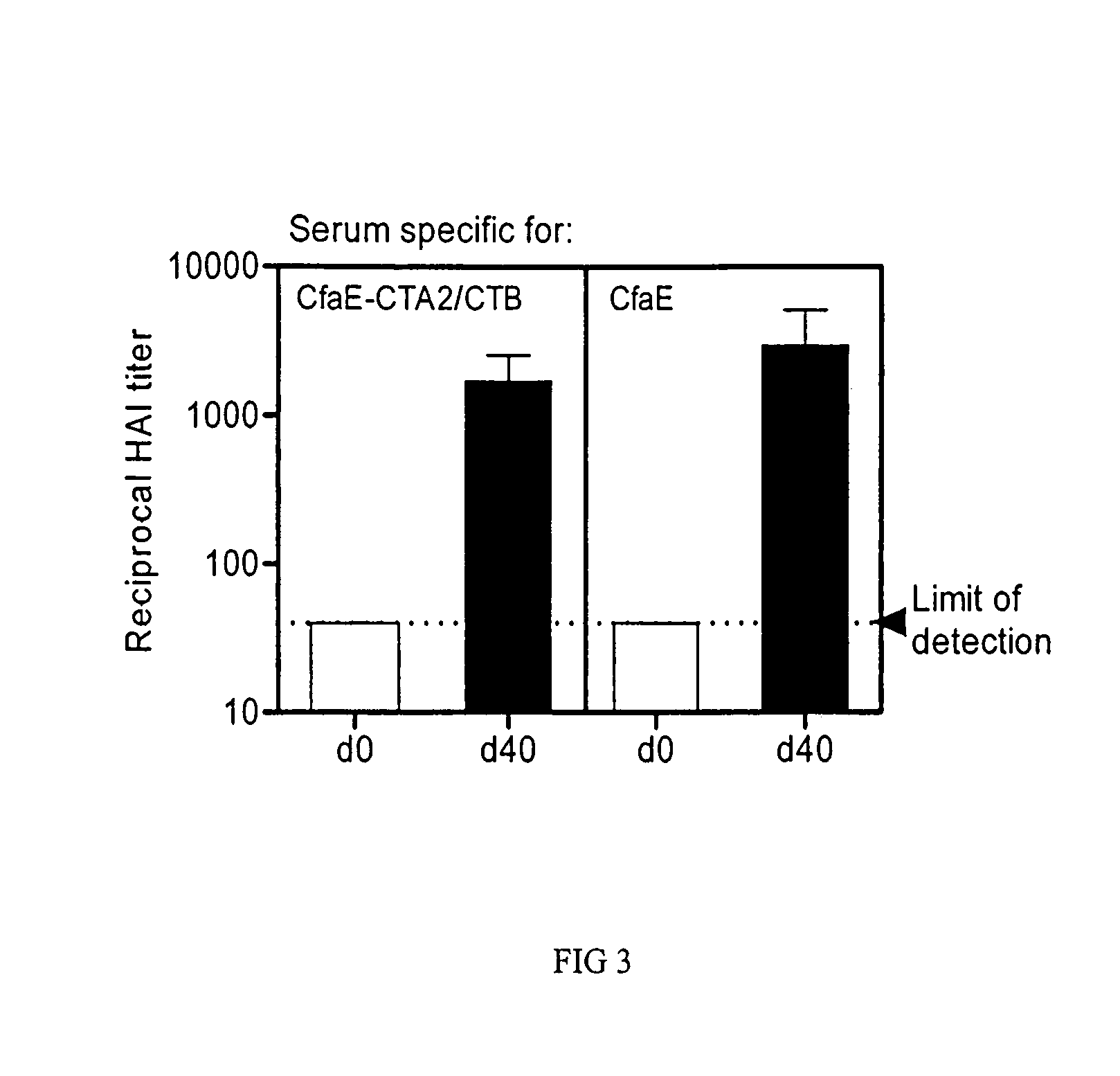
Adhesin-enterotoxin chimera based immunongenic composition against enterotoxigenic Escherichia Coli
The inventive subject matter relates to an immunogenic composition composed of a chimeric molecule including a conformationally stable adhesin from Escherichia coli fused to a bacterial toxin A subunit, such as cholera toxin A subunit or heat-labile Escherichia coli toxin A subunit. The invention also relates to the adhesin-toxin chimera noncovalently associated with a toxin B subunit of the same or different species as the A subunit. The invention also relates to a method of utilizing an adhesin / toxin fusion composition to elicit an immune response.
Owner:UNIV OF COLORADO THE REGENTS OF
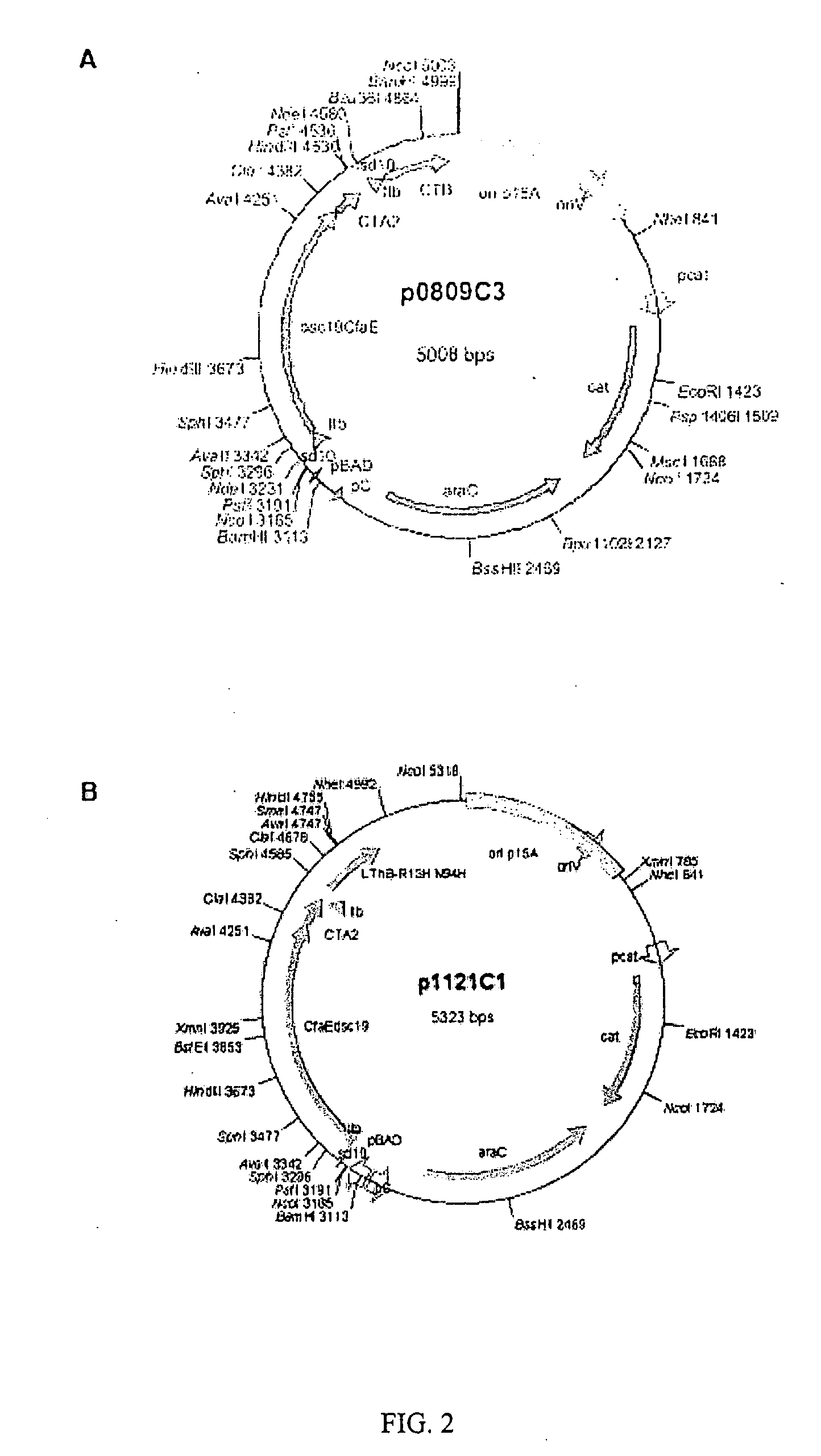
Yeast expressed classical swine fever virus glycoprotein e2 and use thereof
InactiveUS20100028384A1Induce productionProtection against CSFV infectionSsRNA viruses positive-senseViral antigen ingredientsEnationBULK ACTIVE INGREDIENT
A glycoprotein E2 of classical swine fever virus (CSFV) expressed in a recombinant yeast system. The recombinant E2 protein (yE2) is able to form a homodimer, exhibits glycosylation conformation and possesses correct immunogenicity. An anti-CSFV vaccine can be provided with yE2 as a major active ingredient to induce high titers of neutralizing antibody in vaccinated pigs, and to induce a protection against CSFV infection.
Owner:MAO XING BIOLOGICAL TECH
Antibody against hemagglutinin of influenza A H1N1 virus
ActiveCN102241768AMicroorganism based processesImmunoglobulins against virusesHemagglutininInfluenza A (H1N1) virus
The invention relates to monoclonal antibody against hemagglutinin of influenza A H1N1 virus. The invention further provides a hybridoma cell strain secreting the monoclonal antibody. The invention further provides a kit containing the monoclonal antibody. With the present invention, the monoclonal antibody combines with conformational epitope of the hemagglutinin of the influenza A H1N1 virus; the monoclonal antibody has high affinity with a HA1 fragment of the hemagglutinin of the influenza A H1N1 virus; the monoclonal antibody does not generate cross reactions with other proteins; the monoclonal antibody has very high specificity and sensitivity.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
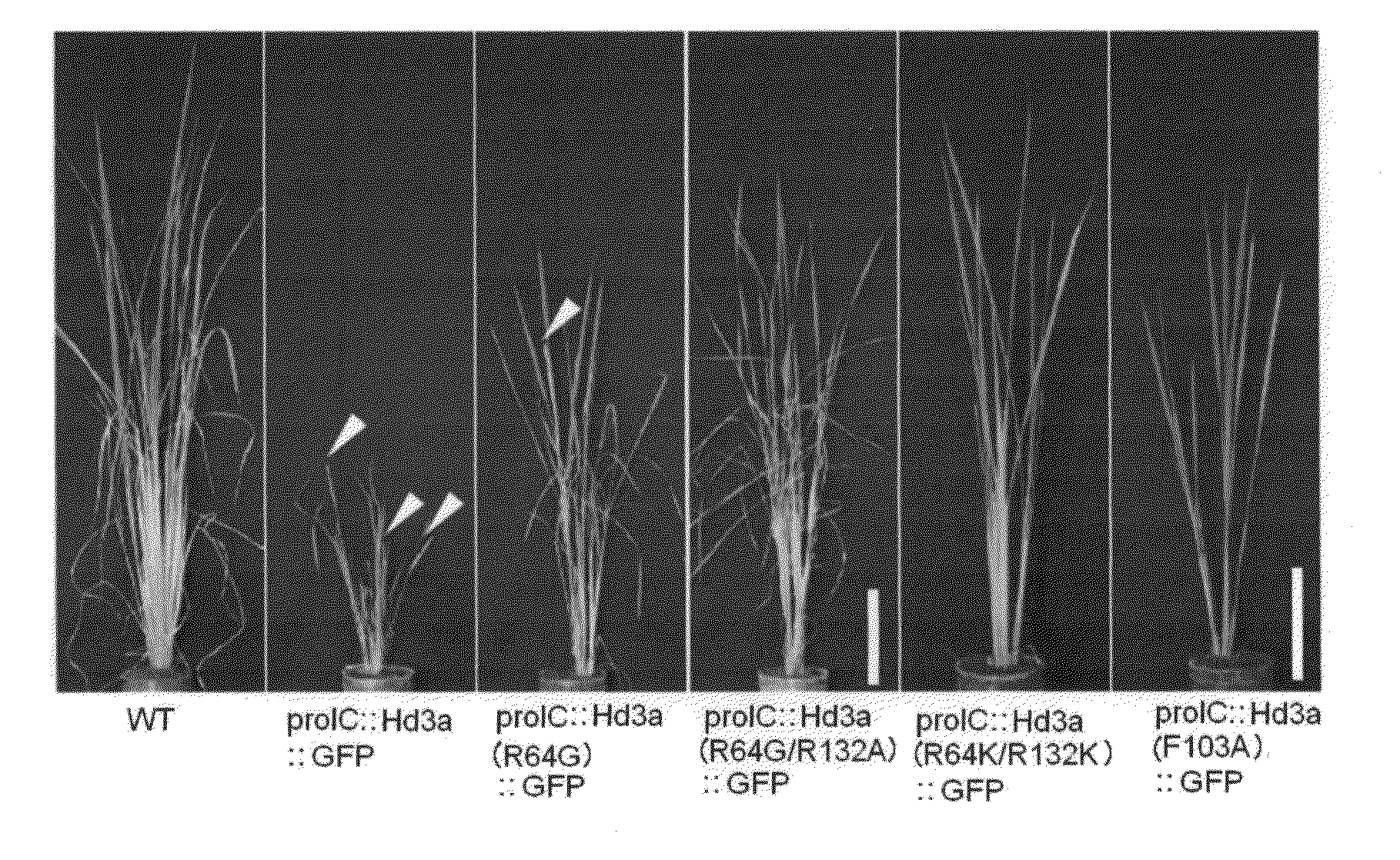
Florigen-activating complex
InactiveUS20130019345A1Increase productionImprove farming efficiencyFrom normal temperature solutionsVector-based foreign material introductionEnationBiochemistry
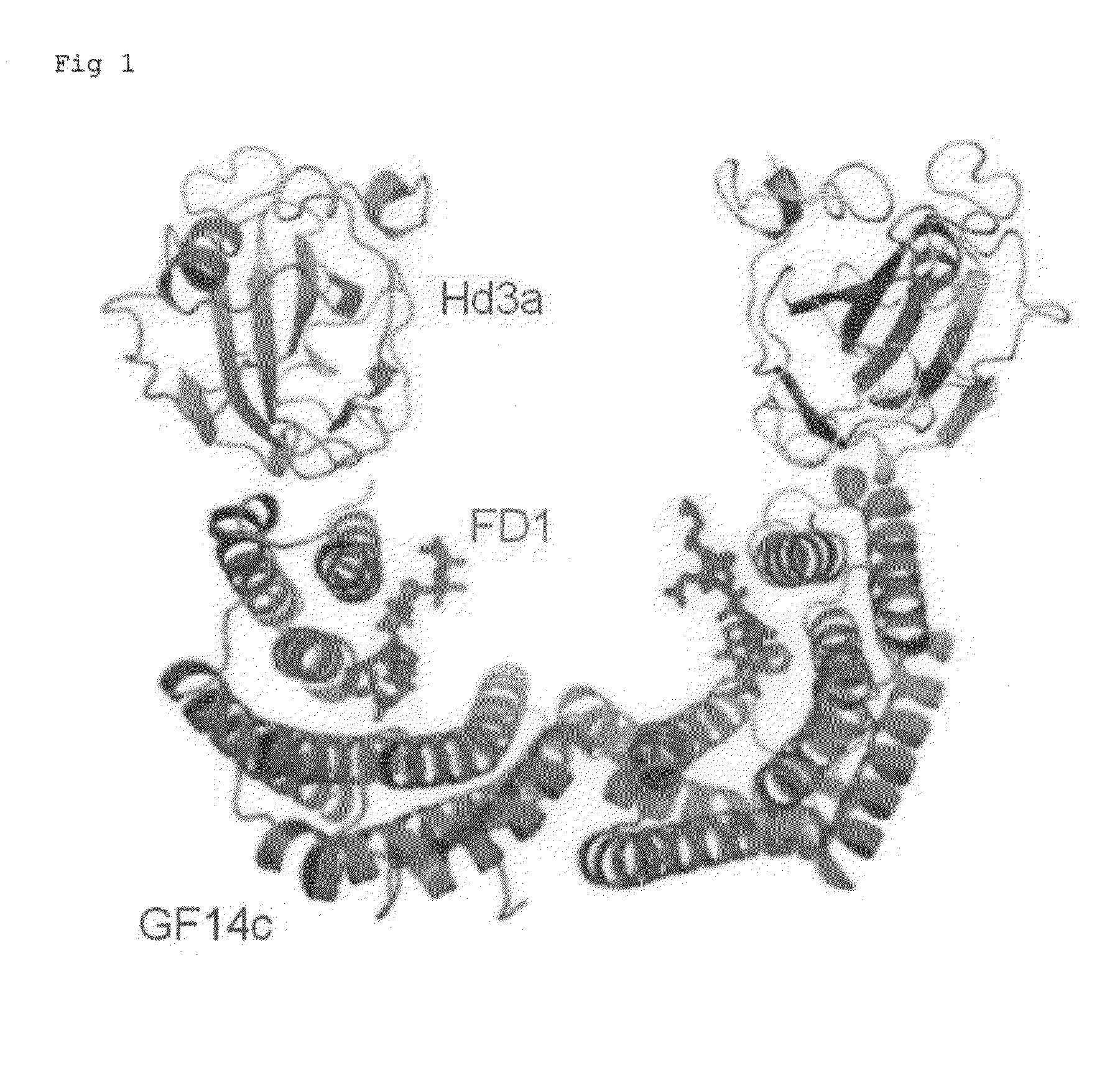
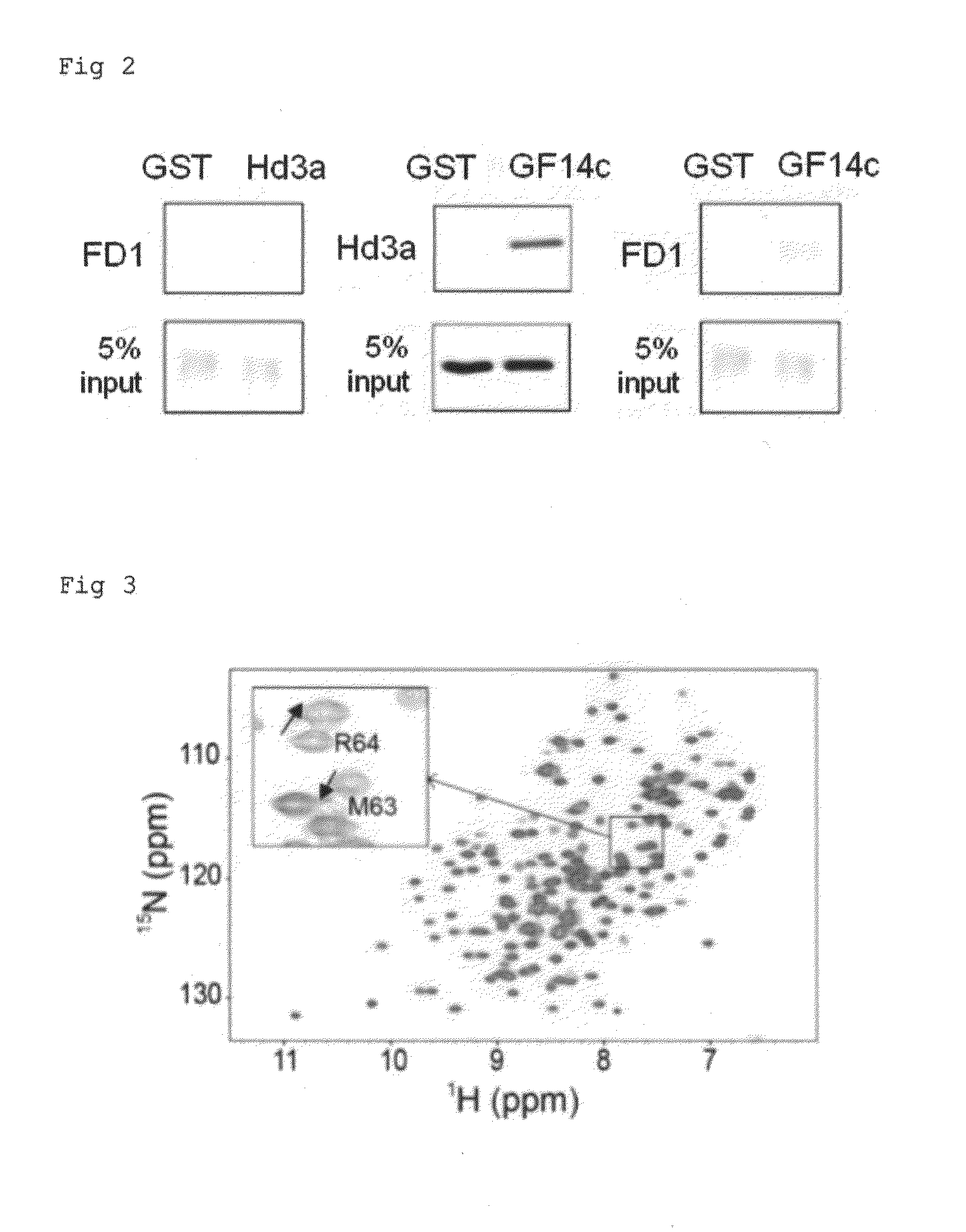
Provided is a crystal of a florigen activation complex, including a florigen, a 14-3-3 protein, and a bZIP transcription factor bound to each other. In addition, flowering of a plant is regulated by controlling mechanisms of interactions among those proteins utilizing the crystal. These are achieved as follows. With attention focused on the fact that Hd3a is bound to FD1 via GF14c to form a florigen activation complex, a crystal of the florigen activation complex is produced, conformational information is obtained through the use of the crystal of the florigen activation complex, and the flowering of a plant is regulated by controlling mechanisms of interactions among the florigen and the like utilizing such conformational information.
Owner:NARA INSTITUTE OF SCIENCE AND TECHNOLOGY
Adhesin-enterotoxin chimera based immunongenic composition against enterotoxigenic Escherichia coli
The inventive subject matter relates to an immunogenic composition composed of a chimeric molecule including a conformationally stable adhesin from Escherichia coli fused to a bacterial toxin A subunit, such as cholera toxin A subunit or heat-labile Escherichia coli toxin A subunit. The invention also relates to the adhesin-toxin chimera noncovalently associated with a toxin B subunit of the same or different species as the A subunit. The invention also relates to a method of utilizing an adhesin / toxin fusion composition to elicit an immune response.
Owner:UNIV OF COLORADO THE REGENTS OF
Single chain antibody against mutant p53
InactiveUS7288637B2Strong specificityImprove effectivenessAnimal cellsSugar derivativesEpitopeSingle-Chain Antibodies
More than 90% of mutations found in the p53 protein produce a conformational change in p53 which results in the exposure of an epitope, which is otherwise hidden in the hydrophobic core of the molecule. A single chain antibody (scFv) which specifically recognizes this common mutant epitope in mutant p53 but not in wild type p53 is disclosed. Also described are a DNA molecule encoding the scFv, pharmaceutical compositions comprising the antibody and methods of treatment using the pharmaceutical compositions.
Owner:RAMOT AT TEL AVIV UNIV LTD
Traditional Chinese medicine monomer having function of promoting growth of motoneuron
The invention belongs to the research and development field of neuro-protective medicament, and discloses a traditional Chinese medicine monomer capable of promoting growth of motoneuron. In the aspect of isolated cell level, the traditional Chinese medicine compound, can obviously promote enation of motoneuron; in the aspect of integral animal level, the compound can obviously promote functional rehabilitation of impaired sciatic nerve and femoral nerve. The invention can be applied to medicinal development for amyotrophic lateral sclerosis and relevant motoneuro symptoms.
Owner:CHINA PHARM UNIV
H3 type flu virus hemagglutinin space conformation simulation antigen epitope and application thereof
InactiveCN101186644ACell receptors/surface-antigens/surface-determinantsMaterial analysisHemagglutininEpitope
The invention relates to polypeptide biological technical field, which discloses a spatial conformation mimic antigen epitope of H3-type influenza virus hemagglutinin, and relative application. The sequence of the spatial conformation mimic antigen epitope is SEQ ID No. 1. The invention utilizes anti-recombination HA1 rabbit serum IgG selection phage 12 peptide library, uses phage ELISA, DNA sequencing, western bolt, and software analysis to analyze selected colony, and selects the small peptide from random phage 12 peptide library, which can be specially combined with anti-recombination HA1 rabbit serum IgG. The inventive spatial conformation mimic antigen epitope can prepare diagnostic reagent box of H3-type influenza virus.
Owner:CHINA PHARM UNIV
Mite antigenic rice
InactiveUS20100285043A1Lower Level RequirementsInduce immunological toleranceBiocideVaccinesGenetically modified riceRice plants
An objective of the present invention is to provide methods for accumulating in rice seeds a partial peptide of a mite antigen protein comprising several T cell epitopes, or a mite antigen peptide that has been modified to not form a conformation to be recognized as an antigen, and plants which have accumulated these peptides.To achieve the above objective, the present inventors have tried to generate seeds (rice) of rice plants that have accumulated mite antigen peptide variants. As a result, the present inventors developed genetically modified rice plants which express and accumulate these mite antigen peptide variants, and demonstrated their effect on asthma, in particular, by orally feeding mice with these variants to induce immunological tolerance, thereby completing the present invention.
Owner:NAT INST OF AGROBIOLOGICAL SCI
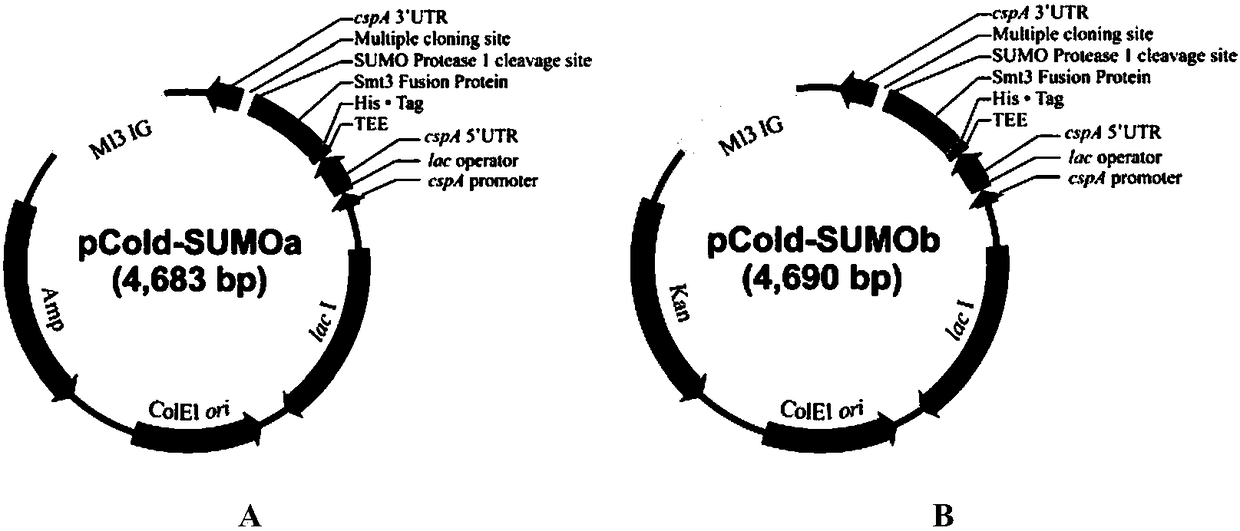
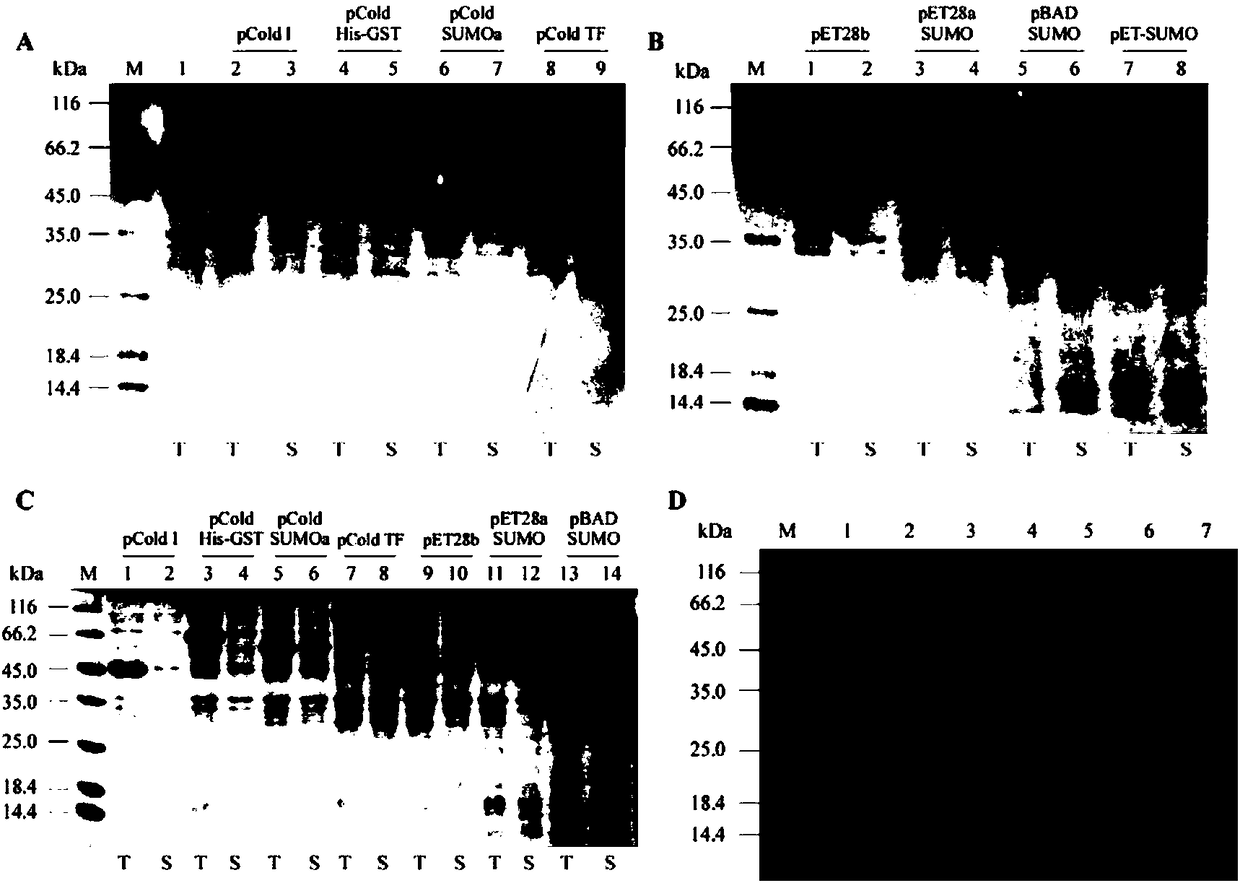
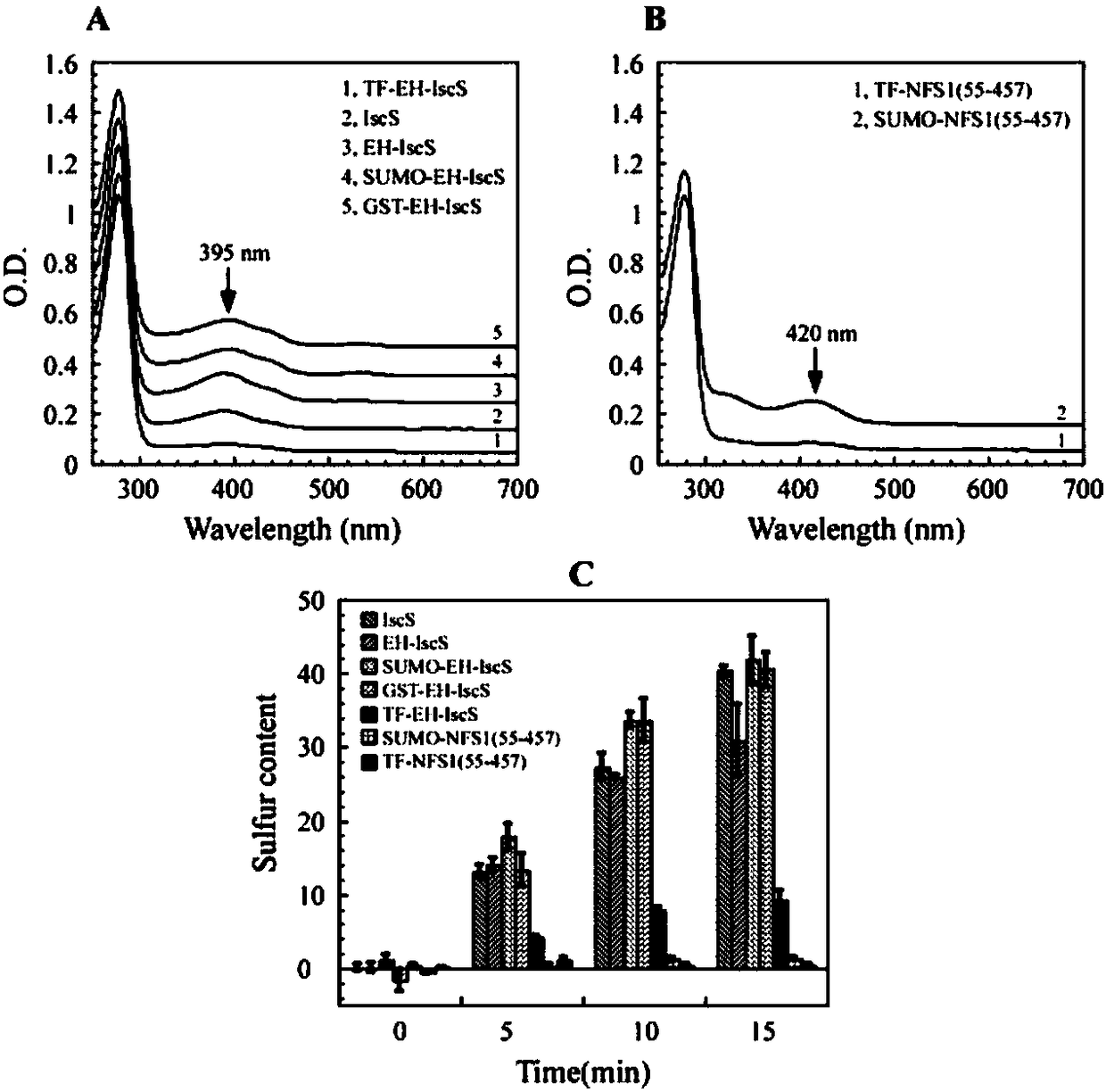
Construction method and application of Escherichia coli cold shock assistant dissolving type expression plasmids
The invention relates to a construction method of Escherichia coli cold shock assistant dissolving type expression plasmids, and a method for preparing a water-soluble heterogenous polypeptide by applying the Escherichia coli cold shock assistant dissolving type expression plasmid. The cold shock assistant dissolving type expression plasmids for realizing small ubiquitin modified protein (SUMO) and exogenous gene fusion expression under the control of a promoter of a cold shock gene is constructed by using a seamless cloning technology. A chimeric cysteine desulfurase is cloned into the plasmids, such as widely-known pCold I and PET28 series plasmids, to obviously improve the water solubility, the stability and the enzyme activity of recombinant proteins. The SUMO has no influences on thetarget protein space conformation of widely-known pCold TF plasmids. The cutting efficiency of an SUMO label is more than 95% when enzyme digestion is performed at 25 DEG C for 1 h according to a ratio of Ulp1 protease to the recombinant protein of 1 U : (0.5-1) mg, so the Escherichia coli cold shock assistant dissolving type expression plasmids are very suitable for preparing proteins having natural N ends in the fields of bioengineering pharmacy and structural biology.
Owner:HUNAN DANWEI BILOGICALTECH
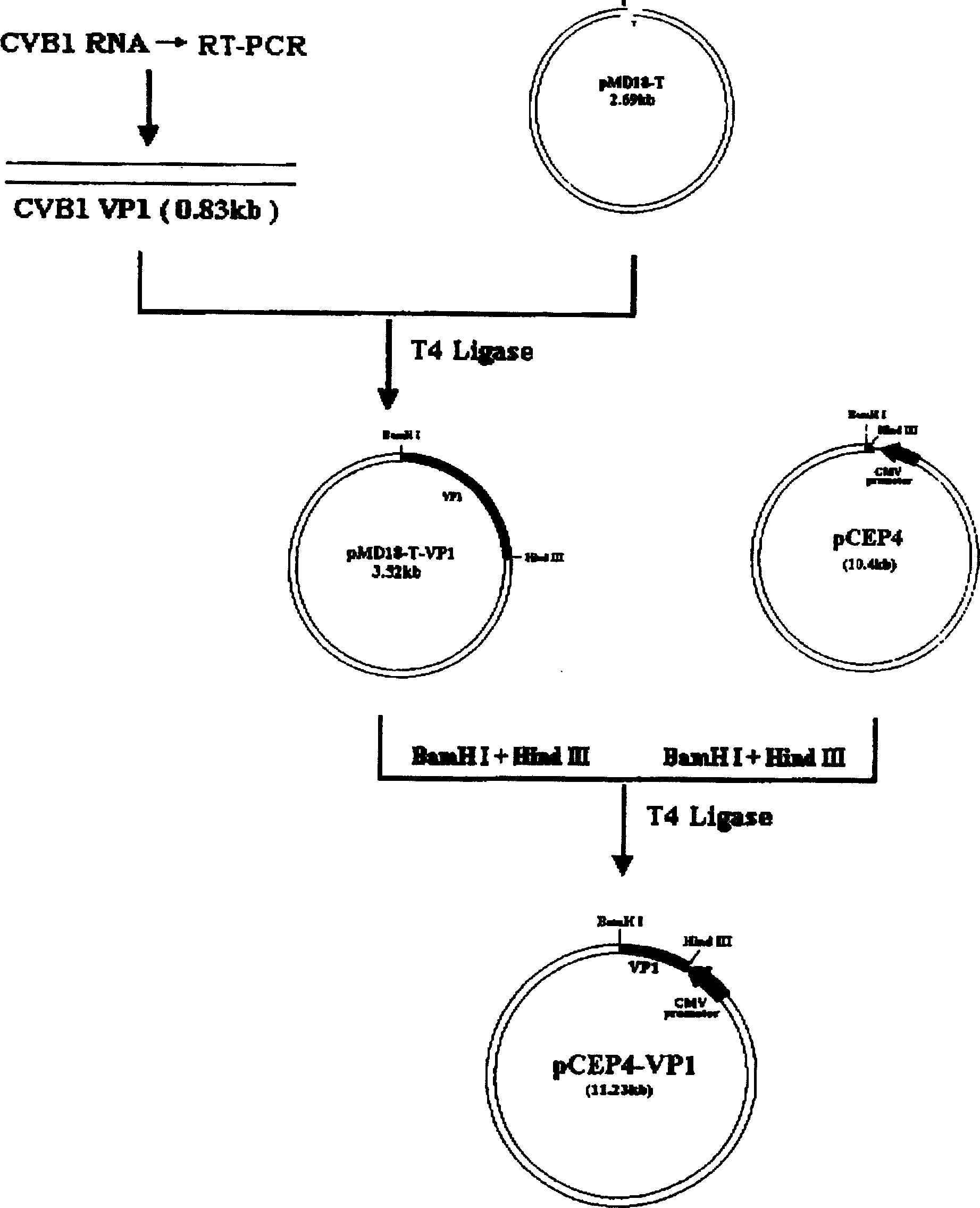
Group-B type-III Coxsackie virus gene vaccine
InactiveCN1772306AAvoid preparative purificationImmune response intactViral antigen ingredientsGenetic material ingredientsAntigen epitopeAntigen
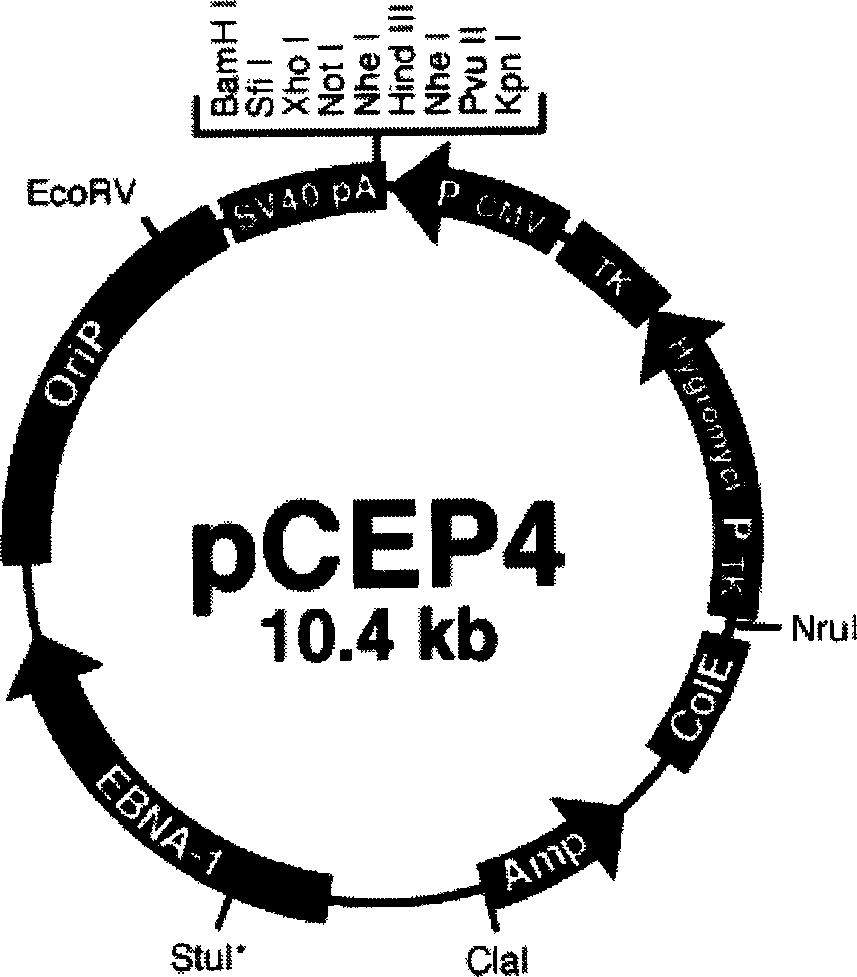
The group-B type-III coxsackie virus gene vaccine is one kind of vaccine for preventing coxsackie virus infection. The present invention aims at providing CVB3 virus gene vaccine as one kind of pcCVP4-CVB1VP1 plasmid comprising VP1 gene with CVB3 coding main neutral antigen and plasmid pCEP4 as eukaryotic expression vector. Compared with traditional vaccines, the gene vaccine of the present invention has the following advantages: direct DNA inoculation without complicated antigen preparing and purifying process, integrated and lasting immune response with antigen polypeptide submission similar to that in natural infection and no antigen epitope altering, common physical and chemical characteristics with capability of embedding several destination genes in the identical plasmid to form combined vaccine, simple preparation process with low cost and high safety and stability for easy storing and transportation.
Owner:HARBIN MEDICAL UNIVERSITY
Cross-reactive staphylococcus aureus antibody
InactiveUS20150086539A1Improved cross-reactiveImproved cross-neutralizing potencyAntibacterial agentsLibrary screeningStaphylococcus cohniiAlpha-toxin
The subject relates to a cross-neutralizing antibody comprising at least one polyspecific binding site that binds to alpha-toxin (Hla) and at least one of the bi-component toxins of Staphylococcus aureus, its medical and diagnostic use, method of producing the antibody, including an isolated nucleotide sequence, plasmids and host cells as used in the production of the antibody; and further an isolated conformational epitope recognized by a specific cross-neutralizing antibody.
Owner:ARSANIS BIOSCI
Anti-influenza-virus broad-spectrum-neutrality neutralizing molecule 3E1
ActiveCN103665156ALow immunogenicityHigh affinityFungiBacteriaHemagglutininComplementarity determining region
The invention relates to an anti-influenza-virus broad-spectrum-neutrality neutralizing molecule 3E1 capable of neutralizing multiple influenza virus subtypes. The functions of the antibody provided by the invention are determined by specific gene sequences of genes in light chain and heavy chain variable regions; and the antibody can be combined with HA2 subunit of influenza virus hemagglutinin (HA) with native conformation, and can prevent multiple influenza virus subtypes from infecting permissive cells. By utilizing the variable region genes or complementary determining region (CDR) genes, different forms of gene engineering antibodies can be modified and produced in any expression system using prokaryotic and eukaryotic cells.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Non-toxic clostridium perfringens beta-toxin genetic engineering vaccine and production method thereof
InactiveCN109701007AHigh expressionIncreased expression of solubleAntibacterial agentsBacterial antigen ingredientsVaccine ProductionClostridium perfringens beta toxin
The present invention relates to a non-toxic clostridium perfringens beta-toxin genetic engineering vaccine. The prepared clostridium perfringens beta-toxin recombinant subunit vaccine is produced bycodon-optimized production and multiple-amino-acid-mutation- containing recombinant clostridium perfringens beta-toxin proteins, thus maximally retains integrity and spatial conformation of natural toxin proteins, keeps immunogenicity, and also avoids biosafety hazards brought by single amino acid mutations. The vaccine also has advantages of simple preparation technology, low immune dose, excellent vaccine efficacy, etc., greatly reduces biosafety risks in vaccine production processes compared with current commercial clostridium perfringens natural toxin inactivated vaccines in China, and isan ideal candidate vaccine for upgrading of current type B and type C clostridium perfringens toxin vaccines in China; and when the vaccine and other antigens are commonly used to prepare a combined vaccine, dose of the combined vaccine cannot be increased and the combined vaccine can be prepared.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Group-B type-II Coxsackie virus gene vaccine
InactiveCN1772304ALow costEasy to storeGenetic material ingredientsAntiviralsCOXSACKIE A VIRUSAntigen epitope
The group-B type-II coxsackie virus gene vaccine is one kind of vaccine for preventing coxsackie virus infection. The present invention aims at providing group-B type-II coxsackie virus gene vaccine as one kind of pcDNA3-CVB2VP1 eukaryotic expression plasmid comprising eukaryotic expression system pcDNA3 and CVB2VP1 gene. The gene vaccine of the present invention has the following advantages: direct DNA inoculation without complicated antigen preparing and purifying process, integrated and lasting immune response with antigen polypeptide submission similar to that in natural infection and no antigen epitope altering, common physical and chemical characteristics with capability of embedding several destination genes in the identical plasmid to form combined vaccine, simple preparation process with low cost and high safety and stability for easy storing and transportation.
Owner:HARBIN MEDICAL UNIVERSITY
A kind of preparation and application method of terminalia zebra seed initiator
ActiveCN110637832BShorten germination timeImprove germination rateBiocidePlant growth regulatorsCopper chlorideEnation
The invention discloses the preparation and application method of a seed initiator of Terminalia zebrae. The invention introduces sodium lauryl sulfate and copper chloride into PEG and sodium chloride solution, and strictly controls the preparation parameters to affect the PEG According to the spatial conformation, an initiator that can effectively shorten the germination time of Terminalia zebrascens seeds, increase the germination rate and realize synchronous germination of seeds can be successfully obtained on the basis of only partially removing the endocarp. By adopting the method of the invention, the average germination time of the terminalia twig seeds is shortened by more than 44%, the germination rate is increased by more than 47%, and the synchronization index is increased by more than 19%.
Owner:琼台师范学院
A kind of recombinant bovine prion protein bprp and its preparation method and application
The invention provides a recombined bovine prion protein (bPrP), and a preparation method and an application thereof. The amino acid sequence of the bPrP protein is shown as SEQ ID NO. 2. The bPrP employs a bovine whole blood as a template, adopts nucleotide sequence shown as SEQ ID No. 3 and 4 as primers via a PCR method to obtain the bovine prion protein DNA sequence having enzyme cleavage sites, connects to plasmids of pPROEX-htb, removes redundant enzyme cleavage sites ahead of target genes in expression vectors via a site-directed mutagenesis to obtain the bPrP eukaryotic expression vectors. The recombined bovine prion protein with identical is obtained conformation with natural prion protein by expressing the vector in escherichia coli, purifying and renaturing through high affinity chromatographic column. The amino acid sequence of the bovine prion protein is remarkably similar to that of the natural bovine prion protein (only one glycine is added in N terminal). The bovine prion protein can be used for exploring a monoclonal antibody of the bovine prion protein, and for a diagnostic kit of the bovine prion diseases.
Owner:CHINA AGRI UNIV
H3 type flu virus hemagglutinin space conformation simulation antigen epitope and application thereof
InactiveCN101186644BCell receptors/surface-antigens/surface-determinantsMaterial analysisHemagglutininEpitope
The invention relates to polypeptide biological technical field, which discloses a spatial conformation mimic antigen epitope of H3-type influenza virus hemagglutinin and a relative application. The sequence of the spatial conformation mimic antigen epitope is SEQ ID No. 1. The invention utilizes anti-recombination HA1 rabbit serum IgG selection phage 12 peptide library, uses phage ELISA, DNA sequencing, western bolt, and software analysis to analyze selected clone, and selects the small peptide from random phage 12 peptide library, which can be specially combined with anti-recombination HA1 rabbit serum IgG. The inventive spatial conformation mimic antigen epitope can prepare diagnostic reagent box of H3-type influenza virus.
Owner:CHINA PHARM UNIV
Device for improving yield per unit of beautiful millettia roots
InactiveCN110622748AAvoid breakingAvoid partneringSoil-working equipmentsPlant protective coveringsEnationEngineering
The invention discloses a device for improving the yield per unit of beautiful millettia roots. The device structurally comprises a yield improving maintenance device, a sheath, a fastening plate, a hinge and a protection plate. The device has the advantages that a stem protection sleeve and an enation frame are matched each other, so that rhizome growth of the beautiful millettia roots at the seedling stage is corrected and fixed, rhizome fracture is prevented, standard enation climbing of the beautiful millettia roots is implemented through a rectangular telescopic frame, sunshine obstruction of the beautiful millettia roots caused by overlapping or deviation development of the beautiful millettia roots is avoided, a net plate and a weed pulling mechanism are matched with each other, nutrients in the unit area of the beautiful millettia roots cannot be lost, weeds scrambling for the nutrients of the beautiful millettia roots are effectively removed, so that the yield per unit of thebeautiful millettia roots is effectively improved, the protection plate is adjustable, trapezoid-shaped trenches in the planting position of the beautiful millettia roots are effectively strengthened,and reduction of the survival rate of the beautiful millettia roots caused by falling and loss of soil in raining or irrigating is avoided.
Owner:YULIN NORMAL UNIVERSITY
Antibody against hemagglutinin of influenza A H1N1 virus
ActiveCN102241768BImmunoglobulins against virusesMicroorganism based processesHemagglutininInfluenza A (H1N1) virus
The invention relates to monoclonal antibody against hemagglutinin of influenza A H1N1 virus. The invention further provides a hybridoma cell strain secreting the monoclonal antibody. The invention further provides a kit containing the monoclonal antibody. With the present invention, the monoclonal antibody combines with conformational epitope of the hemagglutinin of the influenza A H1N1 virus; the monoclonal antibody has high affinity with a HA1 fragment of the hemagglutinin of the influenza A H1N1 virus; the monoclonal antibody does not generate cross reactions with other proteins; the monoclonal antibody has very high specificity and sensitivity.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Group-B type-I Coxsackie virus gene vaccine
InactiveCN1772305ALow costEasy to storeViral antigen ingredientsGenetic material ingredientsAntigenAntigen epitope
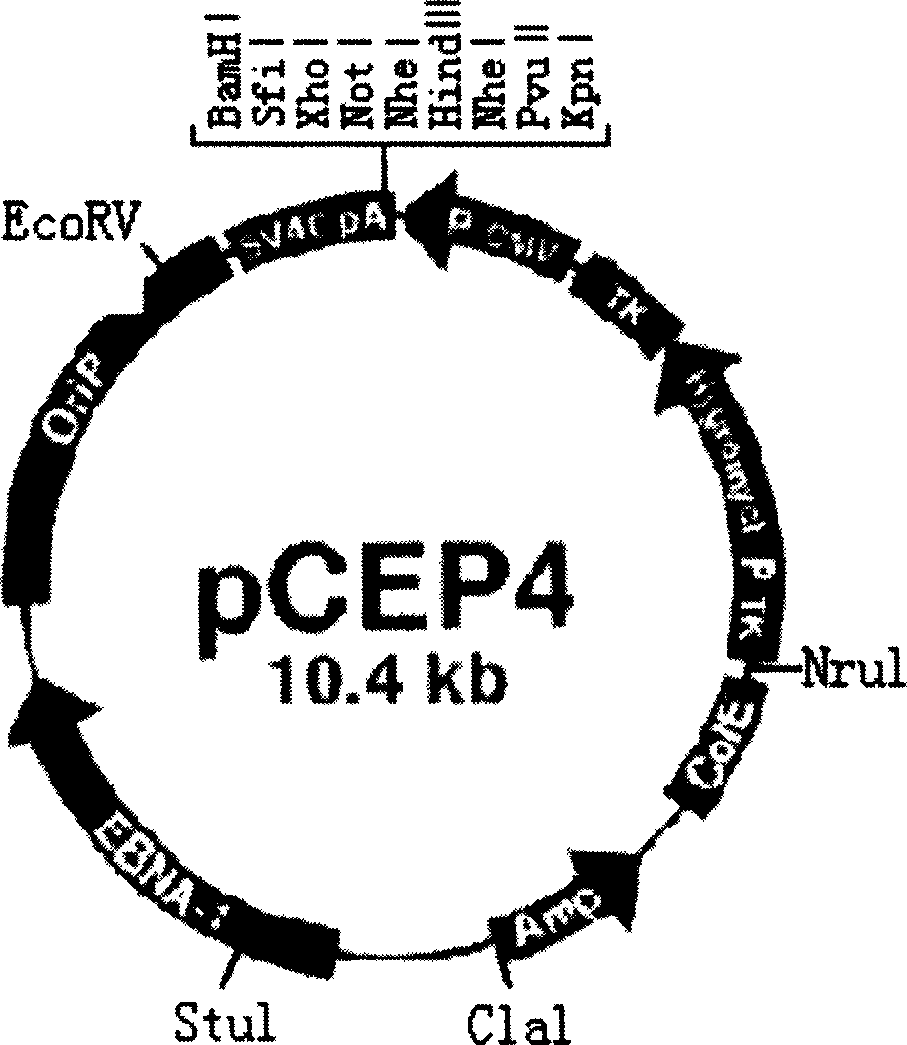
The group-B type-I coxsackie virus gene vaccine is one kind of vaccine for preventing coxsackie virus infection. The present invention aims at providing CVB1 virus gene vaccine as one kind of pcCEP4-CVB1VP1 plasmid comprising VP1 gene with CVB1 coding main neutral antigen and plasmid pCEP4 as eukaryotic expression vector. Compared with traditional vaccines, the gene vaccine of the present invention has the following advantages: direct DNA inoculation without complicated antigen preparing and purifying process, integrated and lasting immune response with antigen polypeptide submission similar to that in natural infection and no antigen epitope altering, common physical and chemical characteristics with capability of embedding several destination genes in the identical plasmid to form combined vaccine, simple preparation process with low cost and high safety and stability for easy storing and transportation.
Owner:HARBIN MEDICAL UNIVERSITY
A broad-spectrum neutralizing molecule 1f2 against influenza virus
ActiveCN103665155BLow immunogenicityHigh affinityFungiBacteriaHemagglutininComplementarity determining region
The invention relates to an anti-influenza-virus broad-spectrum-neutrality neutralizing molecule 1F2 capable of neutralizing multiple influenza virus subtypes. The functions of the antibody provided by the invention are determined by specific gene sequences of genes in light chain and heavy chain variable regions; and the antibody can be combined with HA2 subunit of influenza virus hemagglutinin (HA) with native conformation, and can prevent multiple influenza virus subtypes from infecting permissive cells. By utilizing the variable region genes or complementary determining region (CDR) genes, different forms of gene engineering antibodies can be modified and produced in any expression system using prokaryotic and eukaryotic cells.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
A method for inducing somatic embryos from the embryo
InactiveCN105454042BFor long-term storageTakes a long timePlant tissue cultureHorticulture methodsEnationEvery Two Weeks
Owner:SHANDONG UNIV
A method for inducing somatic embryos of Z.
InactiveCN105393912BShort time to getAvailable for a long timePlant tissue cultureHorticulture methodsPlant hormoneEnation
The present invention relates to a method for inducing somatic embryos of Zinaria chinensis. The inflorescences of spaghetti algae are used as explants, and the surface-sterilized inflorescences are cut into 3-5mm long fragments and inoculated into somatic cells. On the embryo induction medium, conduct induction culture at 15-25°C, dark or weak light conditions, and transfer once every half month. After 15-25 days of culture, the tissue on the inflorescence can be seen to germinate, produce small protrusions, and gradually develop into somatic embryos. No callus phase was experienced during this period. The somatic embryo induction medium is a solid medium with MS and N6 medium as the base medium, supplemented with organic additives, carbon sources, plant hormones (auuxin, or auxin and cytokinin) and sea salt. The method of the present invention can directly induce the somatic embryos of Zinaria spp. It provided important technical support and laid the foundation for the success of seagrass tissue culture.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com