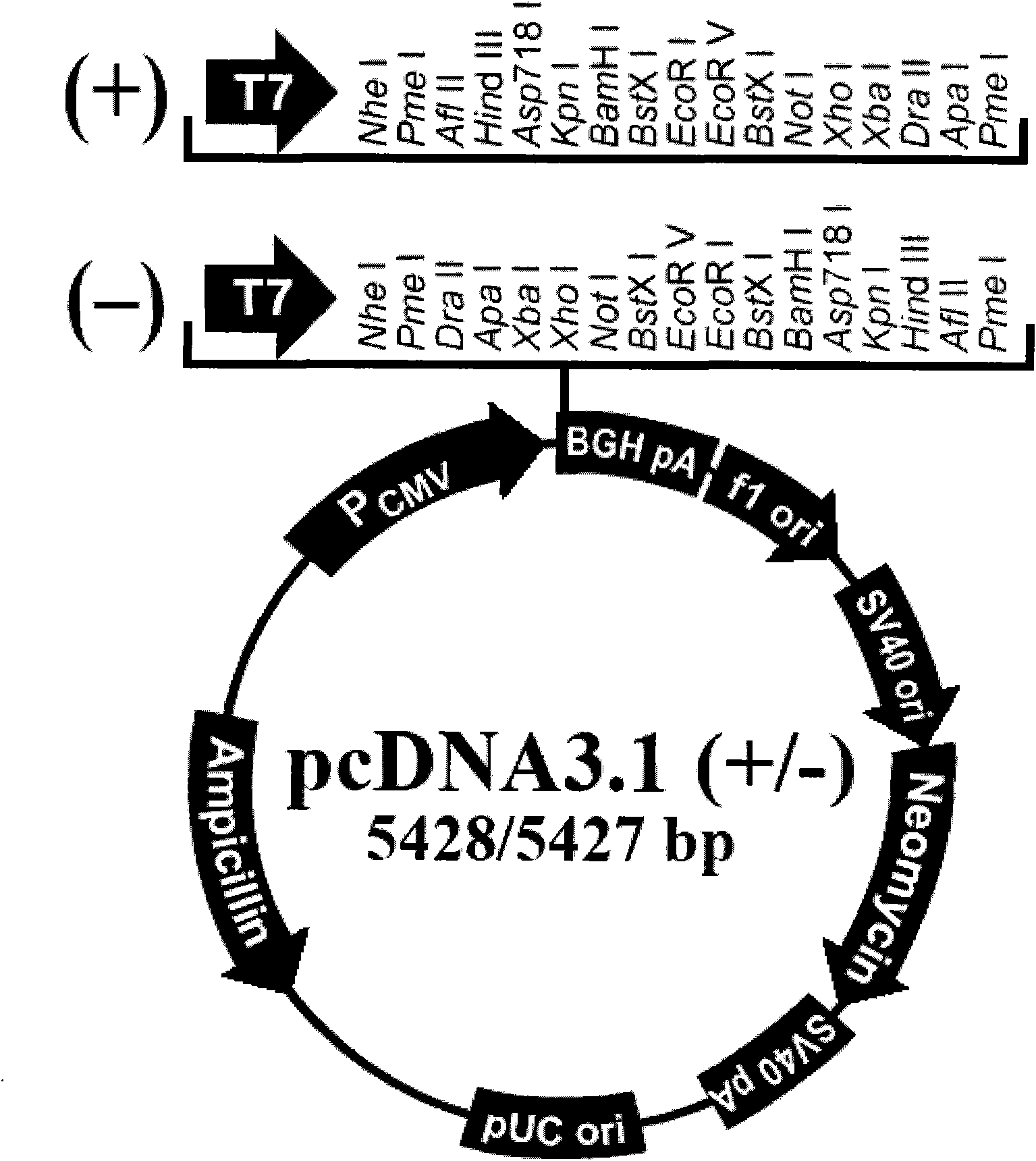
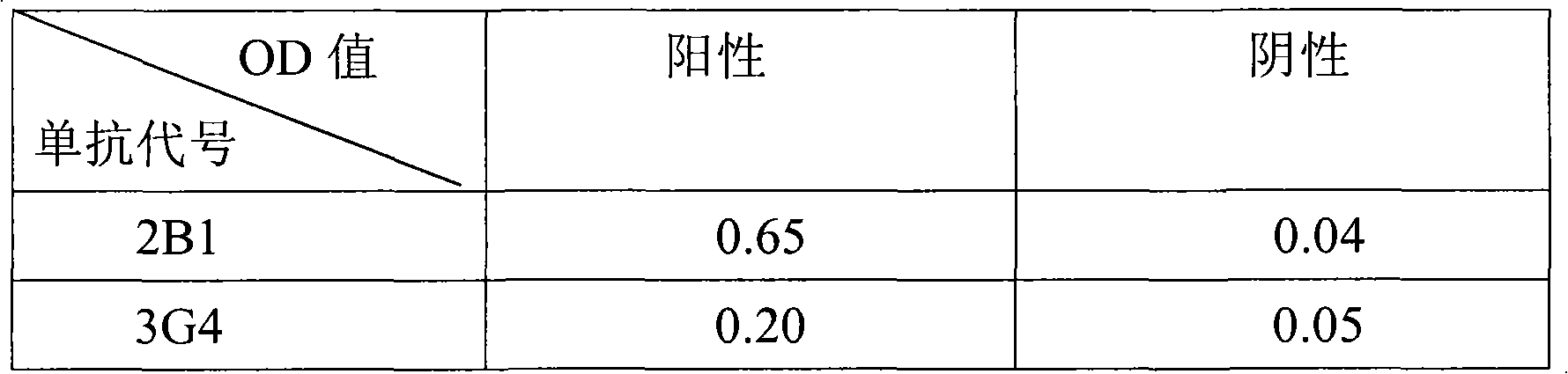
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48 results about "Influenza A (H1N1) virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Influenza A (H1N1) virus is a subtype of influenza A virus and the most common cause of influenza (flu) in humans. Other strains of H1N1 are endemic in pigs (swine flu) and in birds (avian influenza).
Recombinant baculovirus expressing manually modified and synthesized influenza A H1N1 virus HA-NA-M1 gene
InactiveCN101624580AImprove expression levelImprove screening efficiencyGenetic material ingredientsVirus peptidesInfluenza A (H1N1) virusSapovirus
The invention relates to the field of virology, in particular to a recombinant baculovirus which is manually modified and synthesized and contains a main immunogenic gene HA-NA-M1 of an influenza A H1N1 virus. The strain QP-Ac-HNM1 belongs to the baculovirus (Baculovirus) and is preserved in the China Center for Type Culture Collection (CCTCC) with the preserving number of CCTCC-V200912. The recombinant virus is capable of synchronously expressing the HA and NA of the influenza A H1N1 virus and M1 proteins to form virus particles which can be used for developing vaccines so as to prevent human beings and swine from being infected with the influenza A H1N1 virus.
Owner:HUAZHONG AGRI UNIV
Novel Kit for fluorescence quantitative PCR detection of influenza A H1N1 viruses and detecting method therefor
InactiveCN101665842AAccurate detectionHigh detection specificityMicrobiological testing/measurementMicroorganism based processesH1n1 virusInfluenza A (H1N1) virus
The invention discloses a novel kit for fluorescence quantitative PCR detection of influenza A H1N1 viruses, containing a primer, a probe, Taqman 2*universal PRC master mix, 40*multi scribe<TM> and RNase inhibitor mix and nuclease-free water. The primer and the probe have the advantages of good detecting specificity and high sensitivity so that the kit is particularly suitable for the influenza AH1N1 which breaks out at present and has no cross reaction with the Avian flu, swine flu and common seasonal flu and has lower cost.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Primers for detecting influenza virus by RT-PCR and method for detecting influenza virus
InactiveCN101560575AEasy to operateMicrobiological testing/measurementMicroorganism based processesInfluenza A (H1N1) virusQuarantine
The invention discloses primers for detecting influenza virus by RT-PCR and a method for detecting the influenza virus. The primers comprise three pairs of total six oligonucleotide primer sequences such as influenza A virus generality primer, influenza A H1N1 virus H1 generality primer, novel influenza A H1N1 virus H1 specificity primer and the like; and the invention simultaneously discloses treatment of a sample to be detected, an RT-PCR reaction system and reaction condition, and result analysis. The invention can quickly and effectively determine influenza A virus, influenza A H1N1 virus and novel influenza A H1N1 virus, and provide feasible technical support for influenza epidemic early warning mechanisms in the fields such as clinical diagnosis, inspection and quarantine and the like.
Owner:中国疾病预防控制中心病毒病预防控制所
Antibody against hemagglutinin of influenza A H1N1 virus
ActiveCN102241768AMicroorganism based processesImmunoglobulins against virusesHemagglutininInfluenza A (H1N1) virus
The invention relates to monoclonal antibody against hemagglutinin of influenza A H1N1 virus. The invention further provides a hybridoma cell strain secreting the monoclonal antibody. The invention further provides a kit containing the monoclonal antibody. With the present invention, the monoclonal antibody combines with conformational epitope of the hemagglutinin of the influenza A H1N1 virus; the monoclonal antibody has high affinity with a HA1 fragment of the hemagglutinin of the influenza A H1N1 virus; the monoclonal antibody does not generate cross reactions with other proteins; the monoclonal antibody has very high specificity and sensitivity.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Swine influenza A H1N1 virus and use thereof
The invention belongs to the field of microbial virology and provides a swine influenza A H1N1 virus. The virus contains eight fragments, namely HA, NA, NP, PB1, PB2, PA, NS and M, wherein the nucleotide sequences of the eight fragments are shown by the sequences from No.1 to No.8 in a nucleotide sequence table in the description in turn. In the invention, by separate culture of a nasopharyngeal swab sample of a healthy pig, differential item functioning (DIF) and reverse transcription-polymerase chain reaction (RT-PCR) identification and the comparison of full sequences of 8 genes of the virus, a virus is obtained and verified to be a swine influenza A H1N1 virus strain. The swine influenza A H1N1 virus strain is named A / swine / Zhejiang / 26 / 2009(H1N1), with a collection number of CCTCC No.V201016. The strain obtained by the invention supplies valuable genetic information of swine influenza A virus in China, has a great significance for completing a system for monitoring the infection caused by the pathogen and can be used for preparing a reagent for quickly diagnosing infection with the influenza A H1N1 virus.
Owner:FUDAN UNIV
Method for detecting nucleic acids by using dual real-time fluorescent isothermal amplification technology
ActiveCN111394431AStrong specificityAvoid pollutionMicrobiological testing/measurementMicroorganism based processesInfluenza A (H1N1) virusVirus detection
The invention belongs to the technical field of virus detection and discloses a method for detecting nucleic acids by using a dual real-time fluorescent isothermal amplification technology. By adopting the method, existence of RNA of a novel corona virus SARS-VoV-2 and an influenza A H1N1 virus is simultaneously detected through a double-channel double-florescent channel isothermal platform. The method has the advantage of multiplicity, is capable of detecting two viruses in one reaction system, is short in detection cycle, is applicable to rapid clinical detection and diagnosis, and is high in virus detection specificity, high in accuracy, high in detection sensitivity, good in experiment result repeatability and high in precision; and a detection primer has specificity, the detection efficiency can be greatly improved, the amount of reagents in amplification systems can be reduced, and the detection cost can be reduced.
Owner:鲲石生物科技(深圳)有限公司
Combined nucleic acid real-time fluorescent detection method for influenza A H1N1 virus and influenza A virus and kit
InactiveCN101942524ASensitivity is not affectedDoes not affect accuracyMicrobiological testing/measurementMicroorganism based processesFluorescenceInfluenza A (H1N1) virus
The invention provides a combined nucleic acid real-time fluorescent detection method for simultaneously detecting an influenza A H1N1 virus and an influenza A virus and a kit. The combined nucleic acid real-time fluorescent detection method for the influenza A H1N1 virus and the influenza A virus comprises the following steps of: (1) extracting virus RNA; (2) carrying out fluorescent quantitative PCR (Polymerase Chain Reaction) detection; and (3) judging a detecting result. By carrying out multiple sequence comparison and aiming at a conserved gene fragment of the influenza A virus and the influenza A H1N1 virus (infecting in 2009), a primer and a probe with high specificity are designed and used for real-time fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection. The invention can be used for detecting influenza A virus RNA of human influenza, swine influenza, avian influenza, and other influenza viruses, meanwhile specifically detecting the influenza A H1N1 virus (infecting in 2009) RNA and carrying out double analysis so that a detecting result is more reliable.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Application of alkaloid component composition to preparation of medicament for resisting influenza A H1N1 virus
InactiveCN102416014AAbundant raw materialsEasy to produceAntiviralsHeterocyclic compound active ingredientsMedicineInfluenza A (H1N1) virus
The invention relates to application of an alkaloid component composition to preparation of medicaments for resisting influenza A H1N1 virus and can effectively solve a problem of preparation of medicament for resisting influenza A H1N1 virus and controlling H1N1 influenza. A technical scheme is as below: The composition is prepared by mixing 1-carbomethoxy-belta-carboline and 1-deoxymannojirimycin in a mass ratio of 1-5:1-5 and can be effectively used in preparation of medicaments for treating and preventing influenza A H1N1, so as to realize application of the composition to preparation of medicaments for controlling influenza A H1N1. The composition of the invention is simple, can be produced easily from abundant raw materials and effectively applied to preparation of medicaments for treating and preventing influenza A H1N1, so as to solve a problem of drug administration for controlling influenza A H1N1; therefore, the invention is an innovation in medicaments for controlling influenza A H1N1.
Owner:HENAN UNIV OF CHINESE MEDICINE
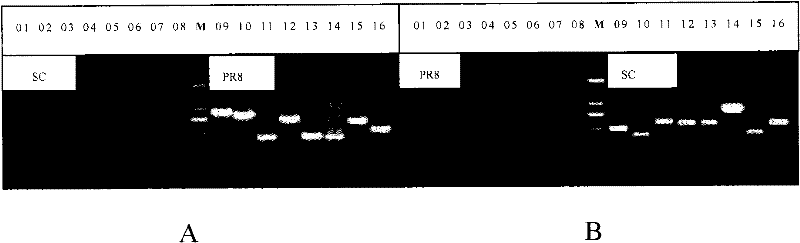
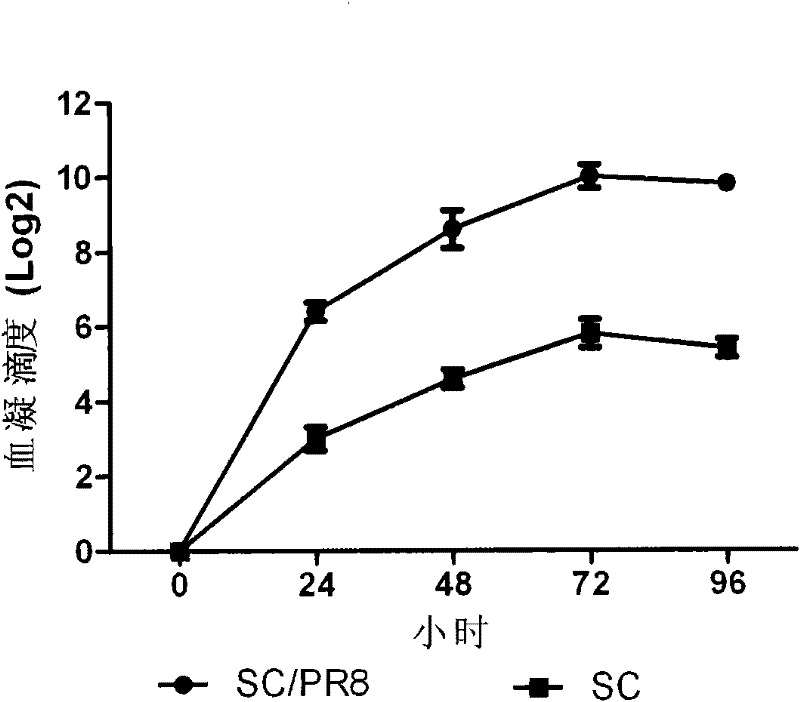
Preparation for reconstruction influenza A H1N1 virus inactivated vaccine strain (SC/PR8), and use thereof
InactiveCN102234637AQuality improvementReduce manufacturing costInactivation/attenuationMicroorganism based processesInfluenza A (H1N1) virusHuman influenza
The invention relates to a reconstruction influenza A H1N1 virus inactivated vaccine strain. The reconstruction influenza A H1N1 virus inactivated vaccine strain is characterized by expressing HA protein and NA protein of the influenza A H1N1 virus. In particular, the reconstruction influenza A H1N1 virus inactivated vaccine strain is SC / PR8. The invention further discloses a method for preparing the reconstruction influenza A H1N1 virus inactivated vaccine strain (SC / PR8), and a use of the reconstruction influenza A H1N1 virus inactivated vaccine strain (SC / PR8) in prevention of animal influenza A H1N1 or human influenza A H1N1.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Primers for determining full-gene sequence of influenza A H1N1 viruses and determination method
ActiveCN102140544AEasy to operateThe result is stableMicrobiological testing/measurementDNA/RNA fragmentationInfluenza A (H1N1) virusEpidemiologic survey
The invention discloses full-gene sequence primers for determining influenza A H1N1 viruses and a determination method. The invention relates to 48 pairs of primers with strong specificity, high sensitivity and good stability, which are used for determining 8 fragments of gene sequences of the influenza A H1N1 viruses, wherein the 8 fragments comprise HA, NA, NP, M1, M2, PA, PB1 and PB2. The method for detection by utilizing the 48 pairs of primers has the advantages of simple operation, short time consumption, strong specificity and high sensitivity and is suitable for identification of the influenza A H1N1 viruses, epidemiological survey and research and the like.
Owner:SUN YAT SEN UNIV
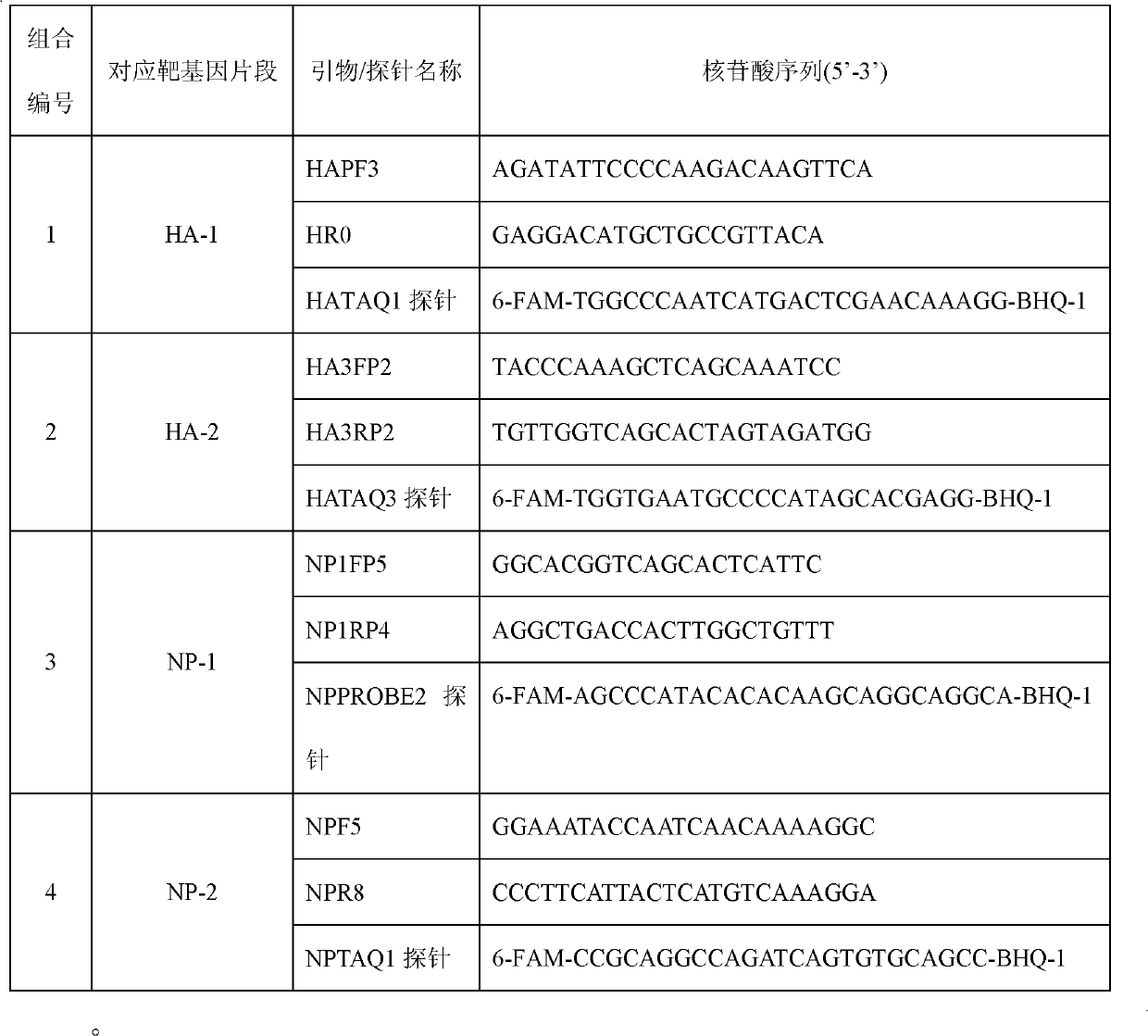
Kit for detecting influenza A H1N1 viruses and detection method
InactiveCN102140539AMicrobiological testing/measurementFluorescence/phosphorescenceInfluenza A (H1N1) virusBioinformatics
The invention provides a kit for detecting influenza A H1N1 viruses and detection method. The kit respectively carries out specificity detection on two different zones (HA-1 and HA-2) on HA gene and two different zones (NP-1 and NP-2) on NP gene of the influenza A H1N1 viruses, which detects four loci of the influenza A H1N1 viruses, and reduces false negative rate caused by virus mutations, thereby achieving the purpose of improving detection rate.
Owner:BEIJING INST OF GENOMICS CHINESE ACAD OF SCI CHINA NAT CENT FOR BIOINFORMATION
Application of Forsythoside A in preparation of anti-influenza A H1N1 virus medicine and preparation thereof
Belonging to the field of traditional Chinese medicines, the invention discloses application of Forsythoside A in preparation of an anti-influenza A H1N1 virus medicine and a preparation thereof. In vivo pharmacodynamic experimental study on the Forsythoside A provided in the invention in treatment of respiratory virus infection and in vitro anti-respiratory virus pharmacodynamic experimental study on the Forsythoside A show that the Forsythoside A has an obvious inhibiting effect on influenza A H1N1 viruses.
Owner:LUNAN PHARMA GROUP CORPORATION
Tibetan medicine composition used for viral pneumonia
InactiveCN106913802AReduce mortalityLower lung indexAntiviralsRespiratory disorderInfluenza A (H5N1) VirusInfluenza A (H1N1) virus
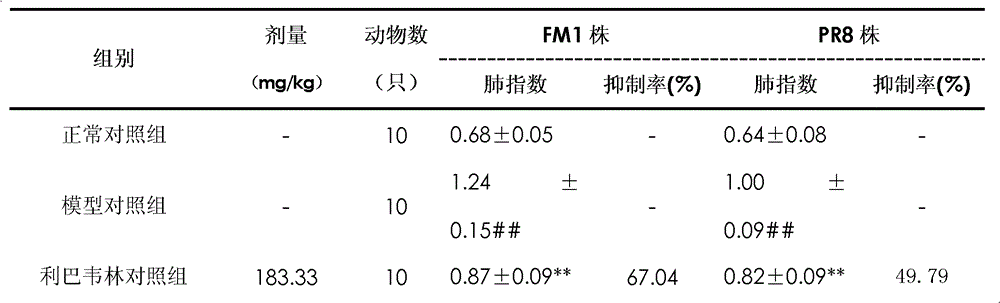
The invention provides a Tibetan medicine composition used for viral pneumonia. Results of animal experiments show that high dose of the Tibetan medicine composition enables the inhibition rate of the lung index of a mouse with pneumonia caused by influenza A H1N1 viruses, influenza A H2N2 viruses, influenza A H5N1 viruses, influenza B viruses and respiratory syncytial viruses (RSVs) to be greater than 40%; the lung index of a model mouse having taken the composition has no significant difference compared with the lung index of a normal mouse from a control group; medium dose and low dose of the composition also have substantial inhibitory effect, with the inhibition rates both greater than 15%; the Tibetan medicine composition can substantially reduce the mortality of mice caused by infection with RSVs and substantially resist reduction in the spleen indexes and thymus indexes of mice infected with RSVs; and the Tibetan medicine composition can substantially improve the immunologic functions of mice with RSV viral pneumonia and reduce the mortality of the mice.
Owner:SHANDONG JINHE DRUG RES DEV
Kit for detecting multiple influenza viruses by polymerase chain reaction (PCR) microarray
ActiveCN102337352AEasy to operateShort timeMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceInfluenza A (H1N1) virus
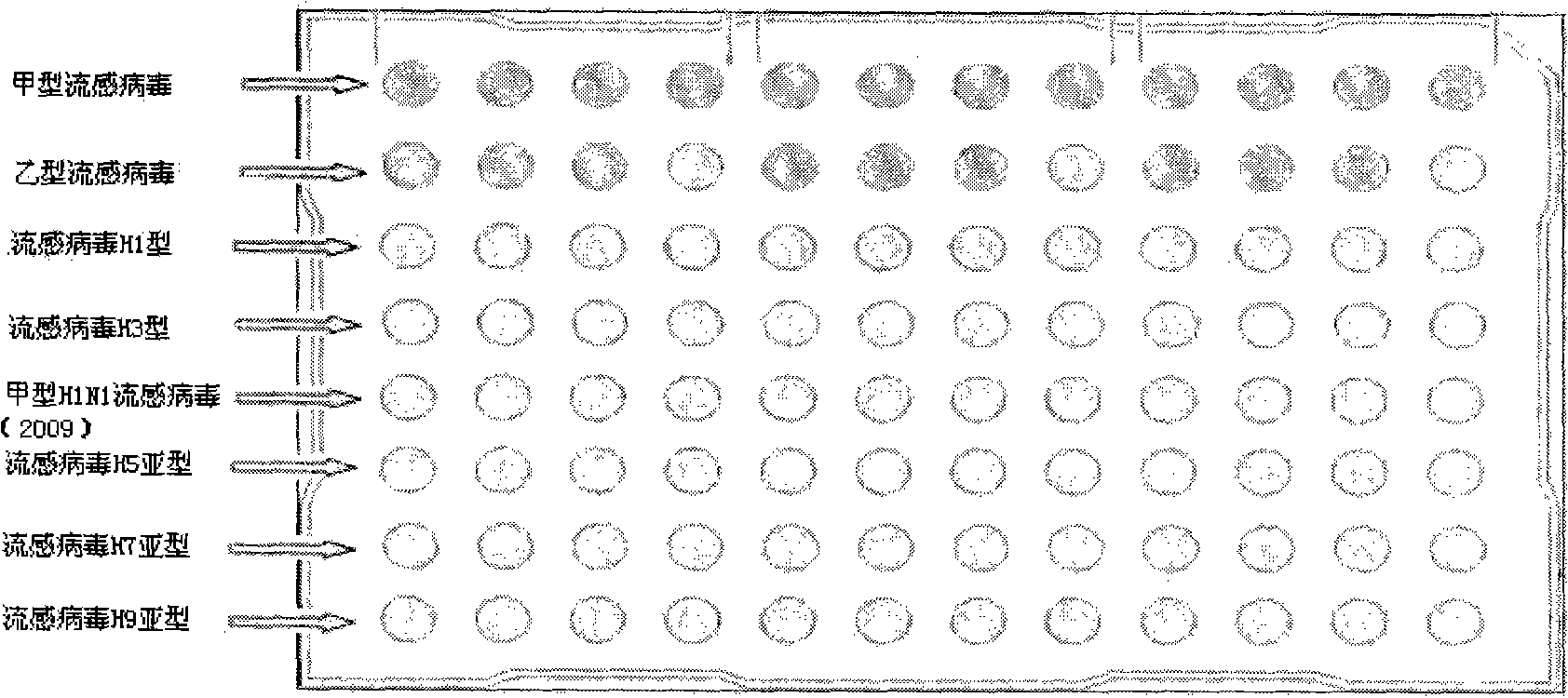
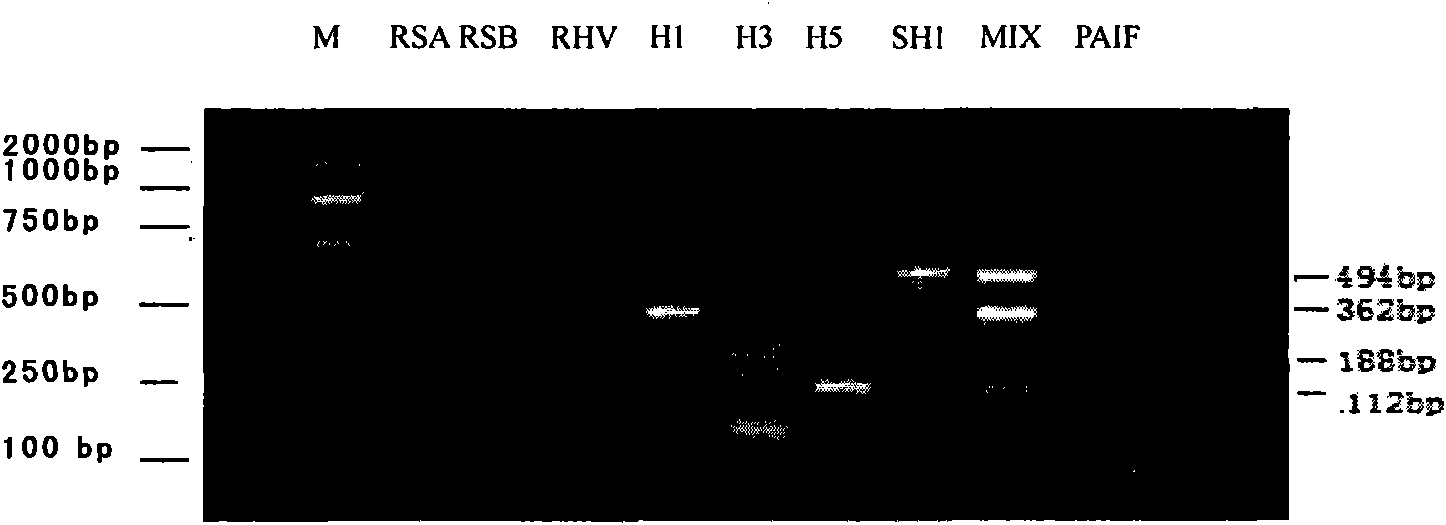
The invention relates to a kit for detecting multiple influenza viruses by a polymerase chain reaction (PCR) microarray. The kit comprises mainly a PCR microarray reaction plate, a PCR reaction solution, and a packaging box for centralized and partitioned packaging of reagent bottles or reagent tubes. Through the combination of a real-time fluorescence PCR technology and a microarray technology, on the single PCR microarray reaction plate, the kit can detect eight influenza viruses comprising influenza A virus, influenza B virus, seasonal influenza H1 subtype virus, seasonal influenza H3 subtype virus, influenza A H1N1 virus, highly pathogenic avian influenza H5 subtype virus, highly pathogenic avian influenza H7 subtype virus, and highly pathogenic avian influenza H9 subtype virus simultaneously. A result of detection adopting the kit can be utilized for differential diagnosis and monitoring of pathogens capable of causing influenza.
Owner:DAAN GENE CO LTD
Preparation method and application of bionic xanthium sibiricum type antibacterial and antiviral plant fibers
ActiveCN112195649ASimple processEasy to operateLiquid/solution decomposition chemical coatingFilament-forming treatmentBiotechnologyStaphylococcus
The invention belongs to the technical field of preparation of antibacterial and antiviral fiber materials, and discloses a preparation method of bionic xanthium sibiricum type antibacterial and antiviral plant fibers. The surfaces of plant fibers are finely fiberized by a mechanical pulping method to prepare the xanthium sibiricum type plant fibers, so that the specific surface area and porosityof the plant fibers are increased, therefore, the in-situ oxidation generation amount of polyaniline on the surfaces of the plant fibers is increased; and then a glucose solution is used for assistingthe reinforced polyaniline in reducing silver ammonia ions on the surfaces of the plant fibers in situ to generate nano-silver, and the xanthium sibiricum type plant fibers with antibacterial and antiviral properties are obtained. The antibacterial rate of the bionic xanthium sibiricum type antibacterial and antiviral plant fiber prepared by the method to escherichia coli, staphylococcus aureus and candida albicans is greater than 99.9%, and the antiviral activity value (Mv) of the bionic xanthium sibiricum type antibacterial and antiviral plant fiber to influenza A H1N1 virus is greater than3.0.
Owner:FUJIAN AGRI & FORESTRY UNIV
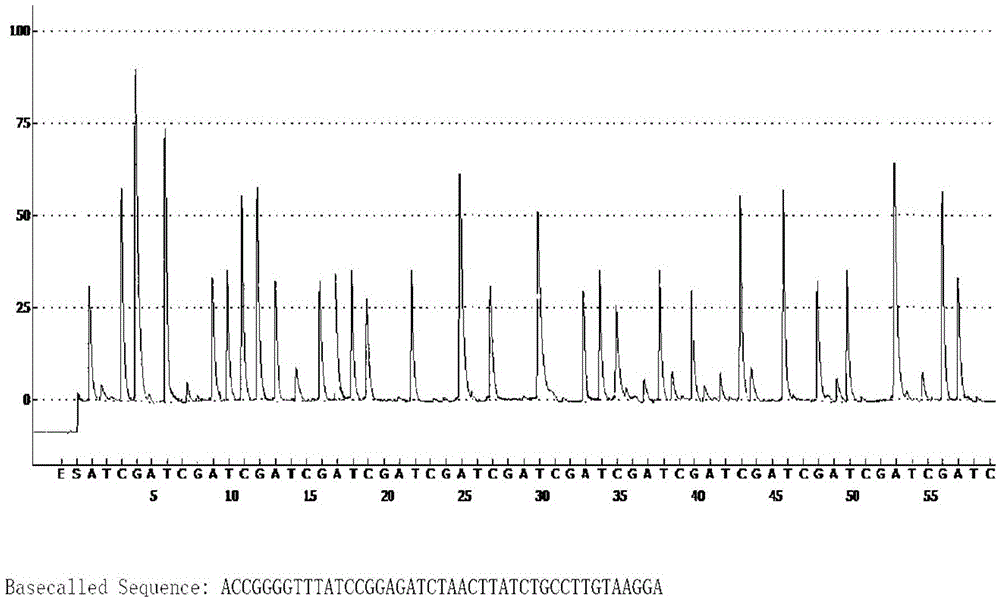
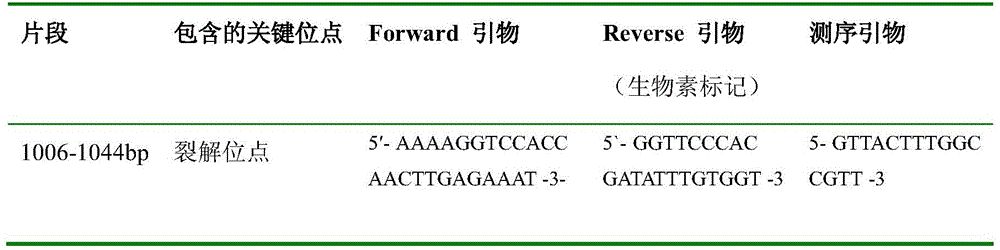
A method for detecting the pathogenicity of influenza A h1n1 virus based on pyrosequencing
The invention relates to a method for detecting the pathogenicity of an influenza A (H1N1) virus based on pyrosequencing. The method comprises the steps of carrying out RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification on a hemagglutinin (HA) gene of an H1N1 virus; carrying out pyrosequencing on a PCR amplification product to judge whether the cleavage site of the HA gene of the influenza A (H1N1) virus has a mutation, wherein the pyrosequencing is carried out in an SQA (Sequence Analysis) mode, and a nucleotide sampling sequence is shown as AGCT. The mutation of the cleavage site of the HA gene of the influenza A (H1N1) virus can be detected at rather high accuracy through comparing a detected result with the sequence of the cleavage site of the HA gene of a standard strain of the influenza A / H1N1 virus. The invention provides a simple and rapid experimental scheme for determining the virulence, pathogenicity and host range of the virus, the complex experiment steps related to complete genome sequencing are omitted, the variation direction of the virus can also be accurately mastered, the virus is conveniently monitored, an infection source is isolated, a transmission route is cut off, and the further development of an epidemic situation is stopped.
Owner:山东国际旅行卫生保健中心
Primers for determining full-gene sequence of influenza A H1N1 viruses and determination method
ActiveCN102140544BEasy to operateThe result is stableMicrobiological testing/measurementDNA/RNA fragmentationInfluenza A (H1N1) virusEpidemiologic survey
The invention discloses full-gene sequence primers for determining influenza A H1N1 viruses and a determination method. The invention relates to 48 pairs of primers with strong specificity, high sensitivity and good stability, which are used for determining 8 fragments of gene sequences of the influenza A H1N1 viruses, wherein the 8 fragments comprise HA, NA, NP, M1, M2, PA, PB1 and PB2. The method for detection by utilizing the 48 pairs of primers has the advantages of simple operation, short time consumption, strong specificity and high sensitivity and is suitable for identification of the influenza A H1N1 viruses, epidemiological survey and research and the like.
Owner:SUN YAT SEN UNIV
Application of glabrous sarcandra glabrous herb extract in reducing susceptibility of influenza virus
ActiveCN102793731BReduce morbidityReduce mortalityAntiviralsRespiratory disorderInflammatory factorsInfluenza A (H1N1) virus
The invention provides novel application of a glabrous sarcandra glabrous herb extract, belongs to the field of medicines, and provides an active extract of a medicinal material glabrous sarcandra glabrous herb through water extraction, alcohol precipitate and drying. 100g of the extract comprise 5 to 20g of tannin, and 0.02 to 1g of isofraxidin. The extract is confirmed and proved due to the effect that the incidence rate and death rate of a low influenza A H1N1 virus FM1 strain infected restraint stress loaded mouse can be reduced based on the glabrous sarcandra glabrous herb extract, and the lung tissue viral load of an influenza virus loaded mouse and inflammatory factor effects can be remarkably reduced by the extract. The glabrous sarcandra glabrous herb extract can be used for preparing medicaments applied to susceptibility of a human body to the influenza virus, relieving pneumonia and other respiratory inflammations due to secondary of body susceptible virus, pulmonary dysfunction and other clinical symptoms, and can be used for particularly effectively preventing, reducing or treating various clinical symptoms caused by virus injection occurring in an influenza virus susceptible body.
Owner:GUANGZHOU BAIYUNSHAN JINGXIUTANG PHARM CO LTD
Establishment of prokaryotic expression and purification as well as test method of reconstruction influenza A H1N1 virus nonstructural protein NS1
InactiveCN101603046AEngineering antibody technology is simpleLow costVirus peptidesMicroorganism based processesEscherichia coliInfluenza A (H1N1) virus
The invention belongs to the research field of zoonosis, relating to a differential diagnosis method. Nonstructural protein NS1 gene in a disclosed influenza A H1N1 California virus strain (A / California / 08 / 2009(H1N1)) cDNA sequence is taken as the research content, a gene synthesis method is used to obtain a gene fragment construction prokaryotic expression carrier Pgex-6P-1-NS1; positive recombinant plasmid is converted to colibacillus to obtain a recombination strain (Escherichia coli BL21 rosseta / Pgex-6p-1-NS1); the nonstructural protein NS1 of the purified H1N1 is obtained by a plurality of chromatography methods, and an enzyme linked immunosorbent assay differential diagnosis method is established in a manner that the expression protein obtains specificity through a immunization host animal specific to polyclonal or monoclonal antibody of the H1N1 nonstructural protein NS1, which is used for distinguishing patients suffering from influenza A H1N1 virus infection and other patients suffering from influenza virus infections.
Owner:CUSABIO TECH LLC
Pterodontic acid derivatives as well as preparation method and application thereof
PendingCN111978198AImprove stabilityGood anti-influenza virus activityOrganic compound preparationCarboxylic acid esters preparationInfluenza A (H1N1) virusViral infectious disease
The invention relates to pterodontic acid derivatives as well as a preparation method and application thereof, and belongs to the technical field of natural pharmaceutical chemistry. A series of pterodontic acid derivatives including C-5, C-12 and C-13 derivatives and the like are prepared by the method. The pterodontic acid derivatives prepared by the method have the advantages of being good in influenza A H1N1 virus resisting effect, good in stability, high in yield and the like. The derivatives play an important role in preparing medicines for treating virus infectious diseases such as influenza and the like. Meanwhile, the preparation method is simple and easy to implement, high in universality and easy to apply and popularize, and the yield of derivatives is larger than or equal to 80%.
Owner:YUNNAN UNIV OF TRADITIONAL CHINESE MEDICINE
Kit for identifying subtypes of influenza A virus
InactiveCN101875933AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationInfluenza A (H5N1) VirusHuman Influenza A Virus
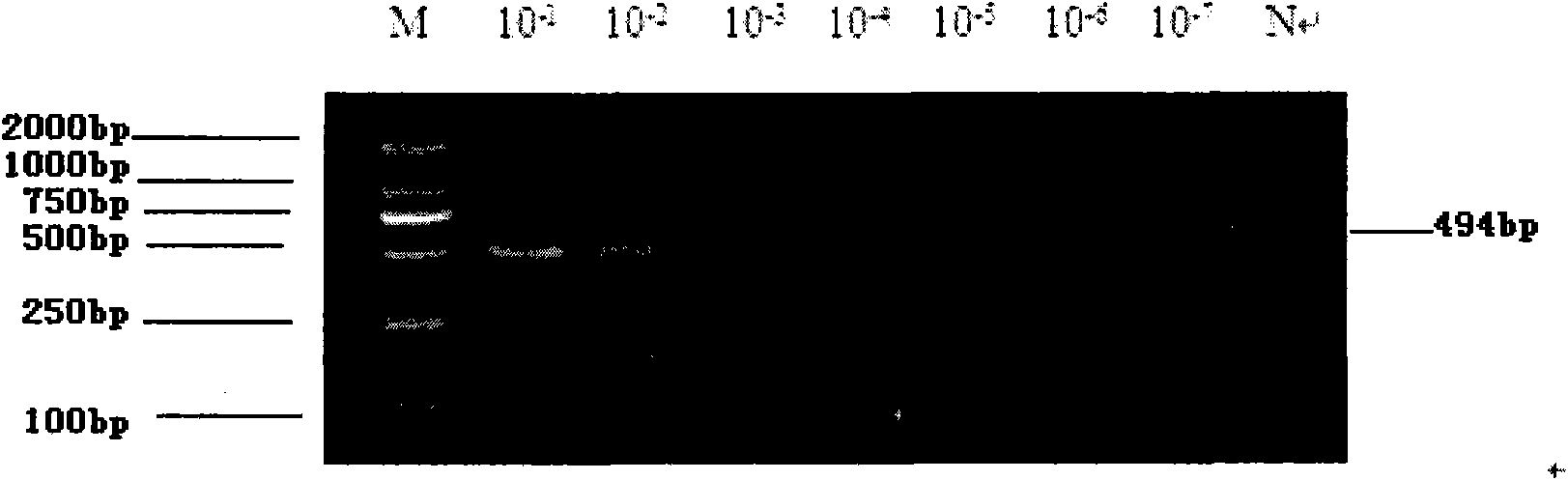
The invention discloses a kit for identifying subtypes of influenza A virus. The kit comprises the following primer pairs: one primer of which the sequence is shown in a sequence 5 in a sequence table and the other primer of which the sequence is shown in a sequence 6 in the sequence table; and the subtypes of influenza A virus are subtypes of new influenza A H1N1 virus, influenza H1N1 virus, influenza A H5N1 virus or influenza A H3N2 virus. Experiments prove that the method has the advantages of reliable detection results, high sensitivity (capable of detecting 103 copy number of the new influenza A H1N1 virus to the lowest), and good specificity; and particularly, the method can also separate the subtypes of the new influenza A H1N1 virus from the subtypes of common seasonal H1N1 influenza virus, which cannot be realized by the conventional technology. Besides, the method has the advantages of no need of precious equipment, simple and quick operation, and capacity of realizing high-flux quick detection. The kit and the PCR reagent have the advantages of high sensitivity, good specificity, wide sources and low cost. The invention provides a simple, feasible and effective method for early diagnosis of the infections of human influenza viruses.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Microbial antibacterial deodorant preparation, and preparation method and application thereof
InactiveCN111500490AImprove stabilityProlong the action timeBiocideGas treatmentBiotechnologyInfluenza A (H1N1) virus
The invention belongs to the field of microorganisms, and particularly relates to a microbial antibacterial deodorant preparation, and a preparation method and application thereof. The microbial antibacterial deodorant preparation disclosed by the invention is prepared from functional bacteria including bacillus aceticus, halomonas, lactobacillus casei and saccharopolyspora chrysosporium; and theratio of colony count of the acetobacter aceticus to the halomonas to the lactobacillus casei to the saccharopolyspora chrysosporium is (3-7):(3-8):(1-6):(0.5-2). The microbial preparation disclosed by the invention does not contain chemical additives; all functional bacteria surviving in the microbial preparation are dominant bacteria; a microorganism interspecific competition principle is utilized; the killing effect of metabolic products of the strains on pathogenic microorganisms is also achieved; the microbial antibacterial deodorant preparation shows excellent antibacterial and virus inactivation effects, the antibacterial effects on pseudomonas aeruginosa, B hemolytic streptococcus, shigella dysenteriae, staphylococcus aureus and escherichia coli all reach 93% or above, and the inactivation rate on influenza A H1N1 viruses reaches 99% or above.
Owner:SHENZHEN HE MIN BIOTECH CO LTD
Primer, probe and method for detecting resistance mutation of influenza A H1N1 viruses
InactiveCN101928787AIncreased sensitivityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceInfluenza A (H1N1) virusFluorescence
The invention discloses a specific amplification primer and a fluorescent probe for detecting the resistance mutation of influenza A H1N1 viruses, application thereof, and a method for detecting the resistance mutation of the influenza A H1N1 viruses. When the method of the invention is in use, an operation process is simple and a result is visual and clear; the primer and the probe have high sensitivity and high specificity; whether the influenza flu is a resistant mutant can be identified in the influenza detection and monitoring quickly; and thus, labor and material consumption is greatly reduced.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Immunoassay reagent for assaying influenza A H1N1 virus antigen
ActiveCN102041265AHigh sensitivityImprove featuresSerum immunoglobulinsGenetic material ingredientsHemagglutininInfluenza A (H1N1) virus
The invention provides an influenza A H1N1 virus hemagglutinin (HA) protein deoxyribonucleic acid (DNA) sequence-containing recombinant expression vector and a DNA vaccine expressed by the recombinant expression vector aiming at an influenza A H1N1 virus antigen. A synthesized influenza A H1N1 virus HA gene is inserted into a plasmid vector to construct the influenza A H1N1 virus HA gene sequence-containing recombinant expression vector, and recombinant plasmid DNA is extracted to prepare an influenza A H1N1 virus DNA vaccine; and the influenza A H1N1 virus DNA vaccine is used for immunizing a mouse to prepare a monoclonal antibody. The invention also provides an immunological method and an immunoassay reagent for assaying the influenza A H1N1 virus antigen. In the invention, a monoclonal antibody cell strain and a polyclonal antibody with high specificity and affinity are prepared; and the prepared immunoassay reagent has high sensitivity and high specificity.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Antibacterial and antiviral plant fiber prepared from zinc chloride/urea eutectic system as well as method and application of antibacterial and antiviral plant fiber
PendingCN114134705ALow costSimple processBiochemical fibre treatmentVegetal fibresBiotechnologyInfluenza A (H1N1) virus
The invention relates to the field of natural high polymer materials, and discloses an antibacterial and antiviral plant fiber prepared from a zinc chloride / urea eutectic system and a method and application thereof. The method comprises the following steps: mixing zinc chloride and urea, heating and melting to obtain a zinc chloride / urea eutectic system, cooling, sequentially adding water and fibrillated plant fibers, heating for reaction, and filtering and washing to obtain the nano-zinc oxide coated antibacterial and antiviral plant fibers. The method is simple in preparation process, easy to operate and low in energy consumption, the plant fibers are good in antibacterial and antiviral performance, the bacteriostasis rate of the plant fibers to escherichia coli, staphylococcus aureus and candida albicans is larger than 99.9%, and the antiviral activity value (Mv) of the plant fibers to influenza A H1N1 viruses is larger than 3.0. The additional value of the plant fiber is improved, and the application of the plant fiber in the fields of spinning, medical treatment, food packaging and the like is promoted.
Owner:FUJIAN AGRI & FORESTRY UNIV
Application of terpenoids in preparation of anti-influenza virus drugs
ActiveCN112891329AExcellent anti-influenza virus activityExcellent anti-influenza virus effectHydroxy compound active ingredientsEther/acetal active ingredientsInfluenza A (H1N1) virusMedicine
The invention relates to the field of medicinal chemistry, and particularly discloses application of terpenoids in preparation of anti-influenza virus drugs. The terpenoid has a structure as shown in a general formula I. The inventor carries out activity screening on the terpenoids with the structure as shown in the general formula I, and finds that the terpenoids with the structure as shown in the general formula I have excellent anti-influenza virus activity for the first time; and in the compounds, the compounds such as the cycloenoic acid and the like have a very remarkable inhibition effect on influenza viruses. The in-vitro and in-vivo anti-influenza virus experiment screening shows that the inhibition effect of the cycloenoic acid on the influenza A H1N1 virus is equivalent to that of a positive control drug oseltamivir phosphate, and even is stronger than that of oseltamivir phosphate.
Owner:JINAN UNIVERSITY
Recombinant baculovirus expressing manually modified and synthesized influenza A H1N1 virus HA-NA-M1 gene
InactiveCN101624580BImprove expression levelImprove screening efficiencyGenetic material ingredientsVirus peptidesInfluenza A (H1N1) virusSapovirus
The invention relates to the field of virology, in particular to a recombinant baculovirus which is manually modified and synthesized and contains a main immunogenic gene HA-NA-M1 of an influenza A H1N1 virus. The strain QP-Ac-HNM1 belongs to the baculovirus (Baculovirus) and is preserved in the China Center for Type Culture Collection (CCTCC) with the preserving number of CCTCC-V200912. The recombinant virus is capable of synchronously expressing the HA and NA of the influenza A H1N1 virus and M1 proteins to form virus particles which can be used for developing vaccines so as to prevent human beings and swine from being infected with the influenza A H1N1 virus.
Owner:HUAZHONG AGRI UNIV
Primer group for detecting influenza a H1N1 virus in respiratory secretions of children
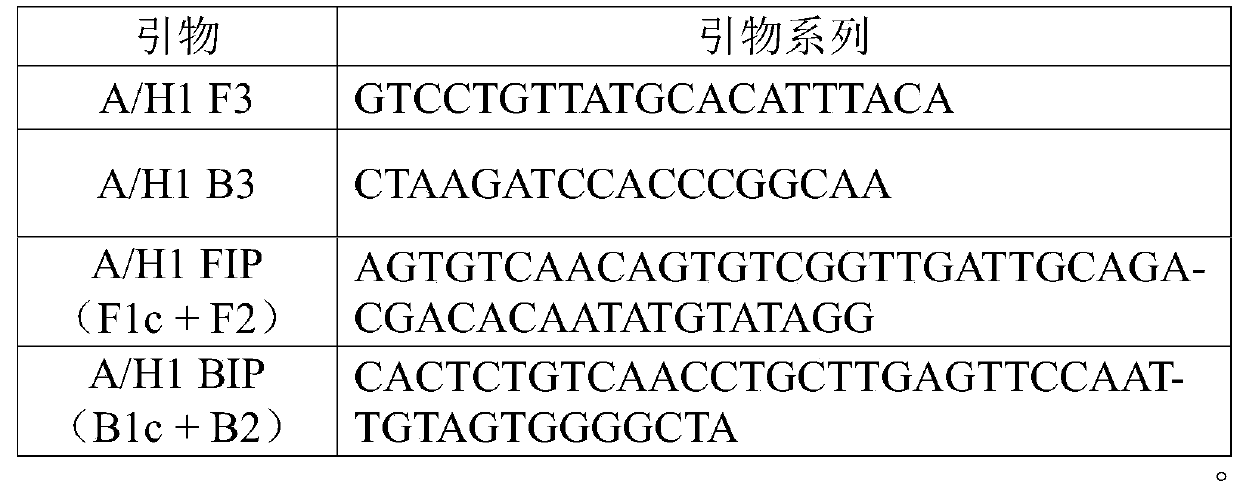
InactiveCN110106289ASimple and fast operationHigh sensitivityMicrobiological testing/measurementMicroorganism based processesRespiratory secretionH1n1 virus
The invention provides a primer group for detecting the influenza a H1N1 virus in respiratory secretions of children, and further relates to a fast quantitative detection method using the primer groupand a fast quantitative detection kit containing the primer group. The primer group is composed of an outer primer pair F3 / B3 and an inner primer pair FIP / BIP. The detection method and the kit are easy to operate, fast, accurate, easy to popularize and high in sensitivity, no expensive instruments are needed, the detection method and the detection kit can be used in detection of on-site pathogensand primary hospitals, and the primer group has an important significance for monitoring and controlling the influenza a virus of the children.
Owner:SHANGHAI CITY JIADING DISTRICT CENT HOSPITAL
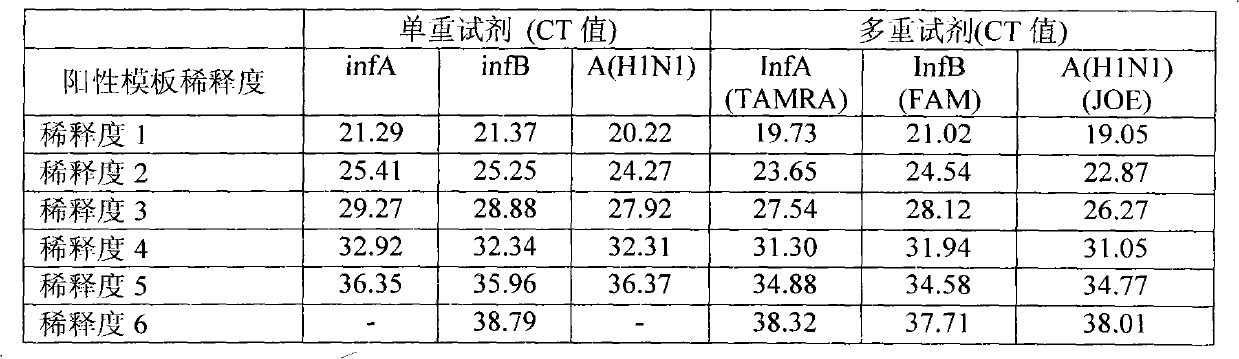
One-tube method with multiplex detection for human Influenza A and B and new Influenza A H1N1 virus and kit
ActiveCN101942525BEnable multiple detectionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationMultiplexInfluenza A antigen
The invention provides a one-tube method with multiplex fluorescent PCR detection for human Influenza A and B and new Influenza A H1N1 virus. The method adopts the primers of which the sequence is shown in SEQ ID NO: 1-6, and also adopts the probes of which the sequence is shown in SEQ ID NO: 7-9. The invention also provides a kit for one-tube method with complex fluorescent PCR detection for human Influenza A and B and new Influenza A H1N1 virus. The kit comprises the primers and the probes. The invention adopts InfA / InfB / A (H1N1) specific primers and Taqman probes, and uses FAM / JOE / TAMRA multiplex fluorescein labels to realize the multiplex detection for the human Influenza A and B and new Influenza A H1N1 virus. The invention has the advantages of high specificity, high sensitivity, high speed, simple and convenient operation, low cost and the like, can be used as a multiple-detection reagent for scientific research and clinical application.
Owner:广东省南山医学发展基金会
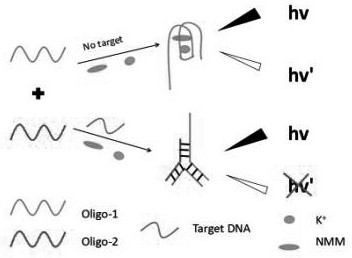
A closed dna fluorescent biosensor and its application in detecting influenza A h1n1 virus
ActiveCN109266784BHigh sensitivityStrong specificityMicrobiological testing/measurementInfluenza A (H1N1) virusSingle strand
Owner:GUANGDONG OCEAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com