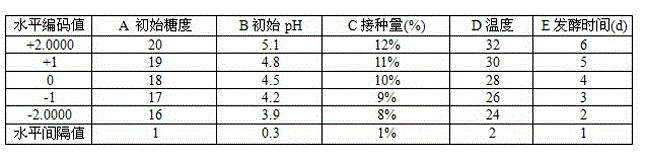
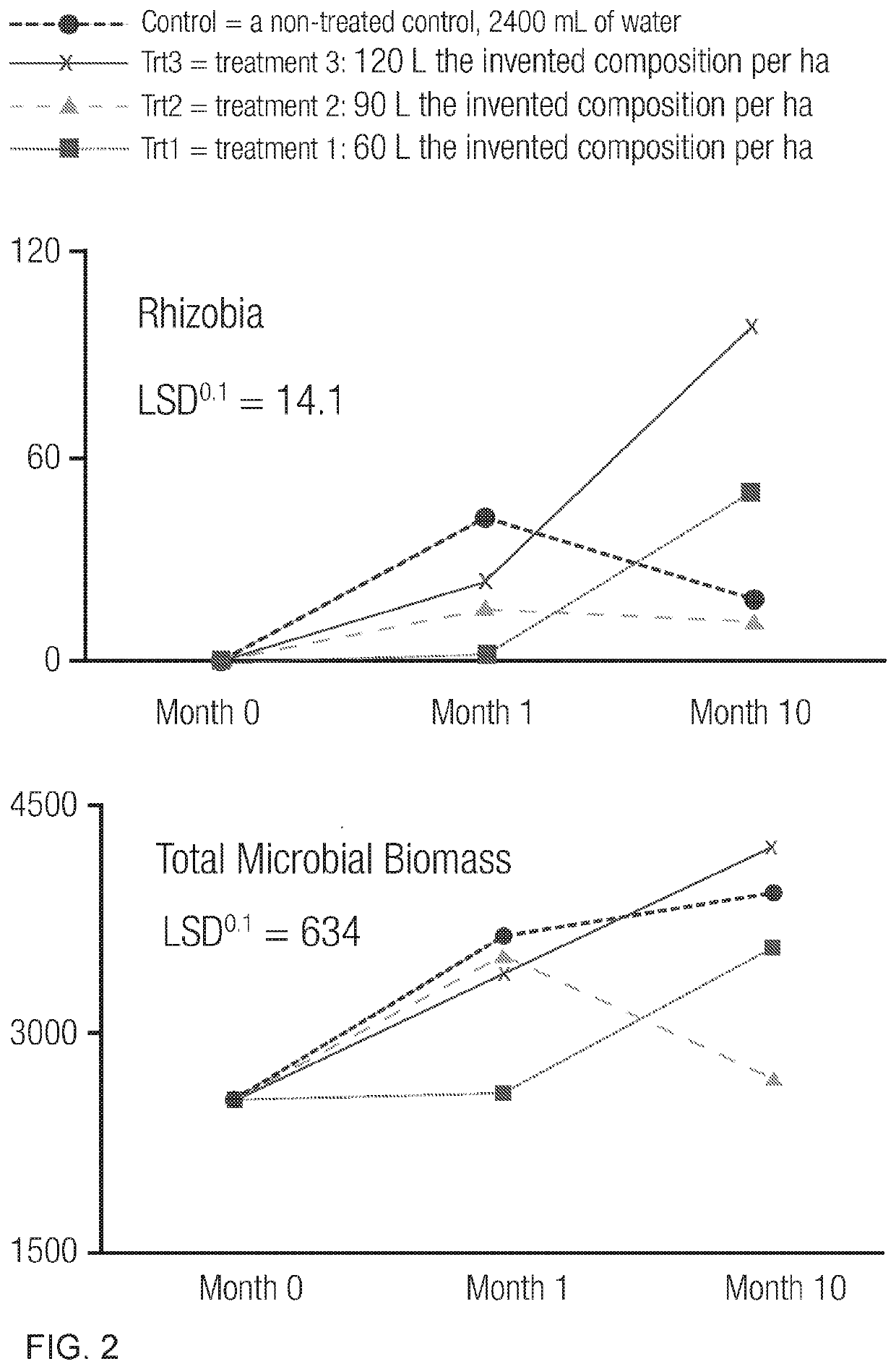
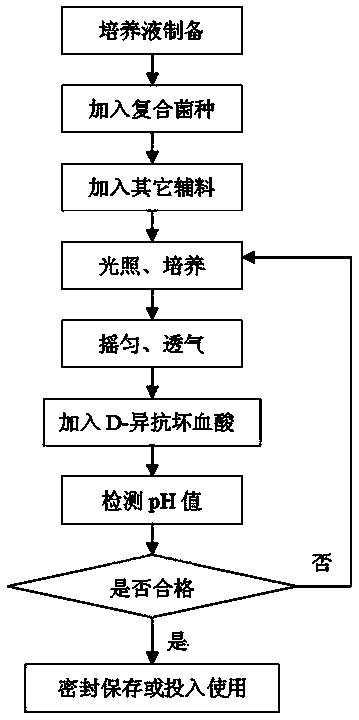
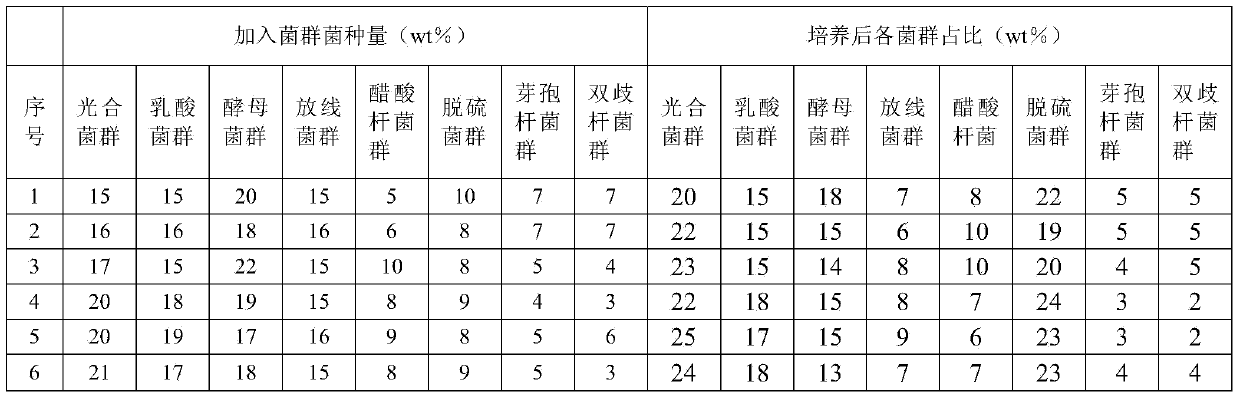
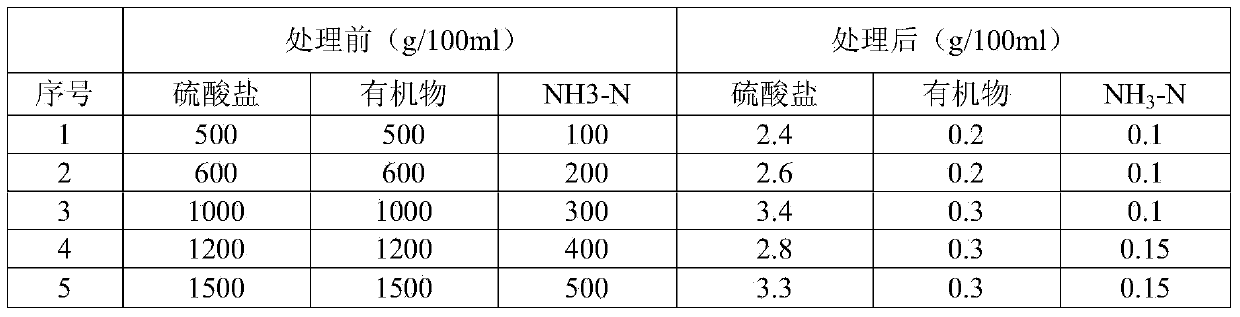
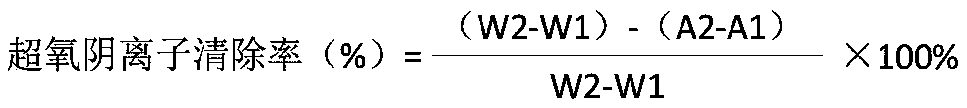Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
116 results about "Acetobacter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acetobacter is a genus of acetic acid bacteria. Acetic acid bacteria are characterized by the ability to convert ethanol to acetic acid in the presence of oxygen. Of these, the genus Acetobacter is distinguished by the ability to oxidize lactate and acetate into carbon dioxide and water. Bacteria of the genus Acetobacter have been isolated from industrial vinegar fermentation processes and are frequently used as fermentation starter cultures.
Organic fertilizer prepared by waste phosphate tailing powder and preparation method thereof
The invention discloses organic fertilizer prepared by waste phosphate tailing powder, which comprises the following raw materials by weight: 20-35 parts of phosphate tailing powder, 20-40 parts of crop straw powder, and 20-40 parts of soy sauce residues. The preparation method comprises the following steps: well mixing the crop straw powder and the soy sauce residues according to the weight ratios, adding water with a material-water ratio of 1:2-1:3, inoculating 4-5% of aspergillusniger, 2-3% of lactobacillus, 3-4% of acetobacter, and 4-6% of bacillus megaterium, performing fermentation at 28-32 DEG C for 3-5 days; adding 15-20% of water, performing fermentation at 50-65 DEG C for 10-15 days; drying, and packaging. The organic fertilizer of the invention has comprehensive nutrition, and lasting fertilizer efficiency, can improve the physical and chemical properties and biological activity of soil, and has low production cost.
Owner:GUANGXI DONGXING DINGKANG PLASTIC IND
Citrus fruit vinegar prepared by composite strain mixed fermentation and preparation method thereof
ActiveCN104694371AFull of nutritionModerately sourMicroorganism based processesVinegar preparationBiotechnologyAcetobacter
The invention relates to a citrus fruit vinegar prepared by composite strain mixed fermentation, belonging to the technical field of fruit vinegar brewage. The preparation method is characterized by comprising the following steps: 1) preparing a composite microzyme strain seed solution; 2) preparing a composite acetobacter strain seed solution; 3) preparing a glutinous rice saccharified solution; 4) preparing a citrus juice; 5) preparing a fermentation liquid; 6) carrying out alcohol fermentation; 7) carrying out acetic fermentation; and 8) aging at room temperature for 2-3 months, filtering with diatomite, and clarifying to obtain the citrus fruit vinegar finished product. By using the citrus and glutinous rice as the raw materials and adopting the fruit / grain co-brewage and composite strain mixed fermentation, the prepared citrus fruit vinegar has the advantages of abundant nutrition, favorable mouthfeel, pleasant fruital aroma and no bitterness.
Owner:LISHUI UNIV
Microbial-based composition and method of use
A microbial-based composition provides a co-cultured microorganism consortium in culture medium that includes one or more Acetobacter sp., Bacillus sp., Bifidobacterium sp., Enter ococus sp., Gluconacetobacter sp., Lactobacillus sp., Rhodopseudomonas sp., Saccharomyces sp., Pichia sp., and Trichoderma sp., as well as a carbon source and chlorine-free water. In some embodiments, the microbial-based composition is useful in the agricultural industry as a plant growth promoting and silage-enhancing agent. For plant growth promotion applications, the microbial-based composition may be applied to the foliar surface of a plant or to the plant growth medium, such as soil or hydroponic solution, surrounding the plant. In silage operations, the microbial-based composition may be applied to a cut plant product during one or more stages in the silage process.
Owner:SUSTAINABLE COMMUNITY DEV LLC
Blueberry kombucha beverage and preparation method thereof
InactiveCN103211253AImprove stabilityExtended shelf lifeFood preparationBiotechnologySodium bicarbonate
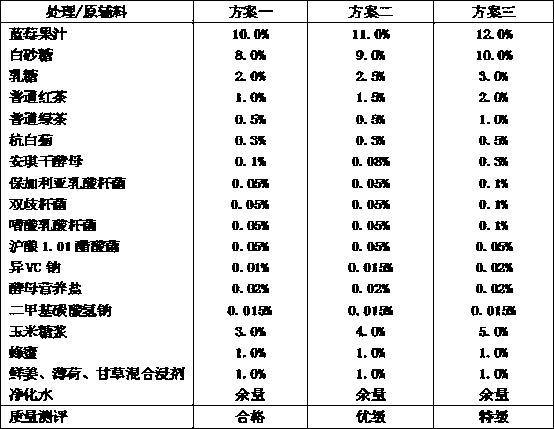
The invention discloses a blueberry kombucha beverage and a preparation method thereof. Raw materials and auxiliary materials comprise: blueberry fruit juice, white sugar, lactose, common black tea, Chrysanthemum morifolium, corn syrup, honey, mixed infusion of fresh ginger, mint and licorice, purified water, angel dried yeast, lactobacillus bulgaricus, bifidobacteria, lactobacillus acidophilus, Shanghai wine 1.01 acetobacter rancens, sodium erythorbate, yeast nutrient salt and dimethyl sodium bicarbonate. The raw materials and auxiliary materials need pretreatment steps of fermenting for 3-4t with a sandwich fermentation cylinder, shearing 5-10min with a high speed shearing machine, and then fermenting for 3-4t. A quantitative fermentation technology is used, and clarifying filtration and aseptic filtration are co-used, to raise biological stability of products. By regulation, product efficacies are strengthened, and goods performances are raised. Low temperature degasification and aseptic cold-filling are preformed, to guarantee product characteristics and quality.
Owner:HEILONGJIANG JINDI WINE IND
Biological deodorant and preparation method thereof
InactiveCN104258440AImprove adsorption capacityImprove working environmentBio-organic fraction processingDeodrantsBiotechnologyMicrobial agent
The invention discloses a biological deodorant. The biological deodorant is characterized by comprising the following raw materials in percentage by mass: 20%-30% of a complex microbial agent, 40%-50% of organic acid and 30%-40% of calcium superphosphate, wherein the complex microbial agent comprises saccharomyces cerevisiae, bacillus subtilis, germany lactic acid bacteria, nitrobacteria, thiobacillus thiooxidans, acetobacter oxydans and an adsorption material; and the organic acid is selected from one or more of oleic acid, linoleic acid, linolenic acid, arachidonic acid, polybasic carboxylic acid, oxalic acid, malic acid, amino acid, sulfoacid and sulfinic acid. According to the biological deodorant, the original foul gas in garbage can be quickly and effectively adsorbed, removed and covered, the work environment of a worker in a composting plant is improved, composting and stabilizing of organic garbage are accelerated, and novel foul gases such as ammonia and hydrogen sulfide generated and released in the composting process are prevented from being reduced.
Owner:WUHAN POLYTECHNIC UNIVERSITY +1
A strain of Acetobacter gluconicum and screen purification method thereof
InactiveCN101503663AGood yieldLess acid productionBacteriaMicrobiological testing/measurementDeterminative bacteriologyBiotechnology
The invention discloses a gluconoacetobacter sp. strain and a screening and purifying method. The screening and purifying method is characterized in that: defective fruits are used as raw materials; cycloheximide is used to suppress the growth of molds and other fungi, and plate enrichment cultivation, gradient dilution plate coating, primary screening, second screening and other methods are adopted for screening and purification to obtain the strain generating bacteria cellulose. According to the description in the ninth version of Bergey's Manual of Determinative Bacteriology, the strain is determined as a novel strain by the analysis of physiological and biochemical properties and 16 Sr DNA sequence. The invention strain obtained by the method has a wide selection range of carbon sources and nitrogen sources, a maximum yield of bacteria cellulose at a pH value of 6.5 and normal temperature, and a higher yield than that of common cellulose-generating bacteria, and has better implementation prospect in the industrial production of bacteria cellulose.
Owner:SICHUAN UNIV
Eucommiaulmoides Oliver fermentation extract and preparation method thereof and application in cosmetics thereof
ActiveCN111317694AGood effectAntioxidant is goodCosmetic preparationsAntipyreticBiotechnologyPectinase
The invention relates to a preparation method of Eucommiaulmoides Oliver fermentation extract, the Eucommiaulmoides Oliver fermentation extract obtained by the method, and a use of the Eucommiaulmoides Oliver fermentation extract for the preparation of cosmetics. The preparation method includes the following steps that a step of preparation of a composite strain is carried out, in the step, the composite strain is obtained by pre-culture of Acetobacter pasteurium, Lactobacillus plantarum and Saccharomyces cerevisiae separately; a step of enzymatic hydrolysis of Eucommiaulmoides Oliver powder to prepare a fermentation matrix is carried out, in the step, leaves of Eucommiaulmoides Oliver are washed, dried, crushed into powder and mixed with water, then Eucommiaulmoides Oliver enzymatic hydrolysate after enzymatic hydrolysis is obtained by adding protease, cellulase and pectinase for enzymatic hydrolysis, and the fermentation matrix is obtained based on the Eucommiaulmoides Oliver enzymatic hydrolysate; a step of fermentation is carried out, in the step, the composite strain is inoculated into the fermentation matrix containing the Eucommiaulmoides Oliver enzymatic hydrolysate after enzymatic hydrolysis and fermented under the condition of low dissolved oxygen to obtain fermentation broth; and a step of purification is carried out, in the step, the obtained fermentation broth is centrifuged and filtered to obtain the Eucommiaulmoides Oliver fermentation extract.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Carbonized bacterial cellulose-cadmium sulfide composite photocatalytic material as well as preparation method and application thereof
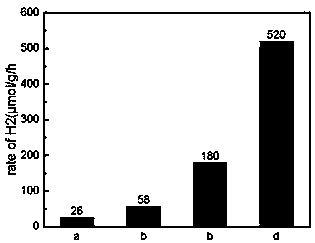
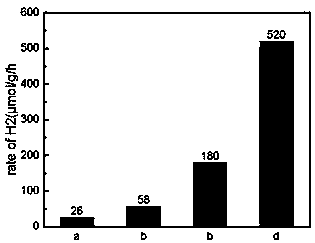
ActiveCN109999835AImprove photocatalytic hydrogen production performanceEasy to operateCatalyst activation/preparationHydrogen productionFreeze-dryingThiourea
The invention relates to a carbonized bacterial cellulose-cadmium sulfide composite photocatalytic material as well as a preparation method and application thereof. The photocatalytic material is prepared by using the following methods: 1) inoculating Gluconacetobacter xylinus into a culture medium A, carrying out dynamic culture for 96 hours, collecting a bacterial cellulose product, washing, drying, and further carrying out vacuum freeze drying so as to obtain bacterial cellulose aerogel; 2) dissolving a fixed amount of thiourea and cadmium sulfide into ultrapure water, adding a proper amount of the bacterial cellulose aerogel, and carrying out a hydrothermal reaction at a high temperature and high pressure; 3) carrying out high-temperature calcining on the bacterial cellulose aerogel-cadmium sulfide compound product in a tubular furnace, thereby obtaining the carbonized bacterial cellulose-cadmium sulfide composite photocatalytic material. The preparation method provided by the invention has the advantages of being simple in operation, low in cost, and the like. The obtained carbonized bacterial cellulose-cadmium sulfide composite photocatalytic material can be used as a catalyst in water hydrogen generation of photocatalysis splitting.
Owner:WUHAN UNIV OF TECH
Municipal house refuse termenting method
InactiveCN1470481AHigh decay heatShort fermentation timeBio-organic fraction processingMicroorganismsLactobacillusLitter
The fermentation method of urban domestic refuse is characterized by that the composite liquid bacterial preparation or composite solid bacterial preparation containing photosynthetic bacteria, acetobacter, microzyme and lactic acid bacteria and refuse are mixed, then undergone the processes of anaerobic fermentation and aerobic souring treatment to shorten fermentation time, prevent the production of malodorous pollutant and reduce the breeding of flies and mosquitoes.
Owner:四川国策环保实业工程股份有限公司
BC/G/MPc ternary composite catalyst and in-situ synthesis method thereof
ActiveCN111298840AEffective dispersionHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsWater contaminantsPtru catalystAcetobacter
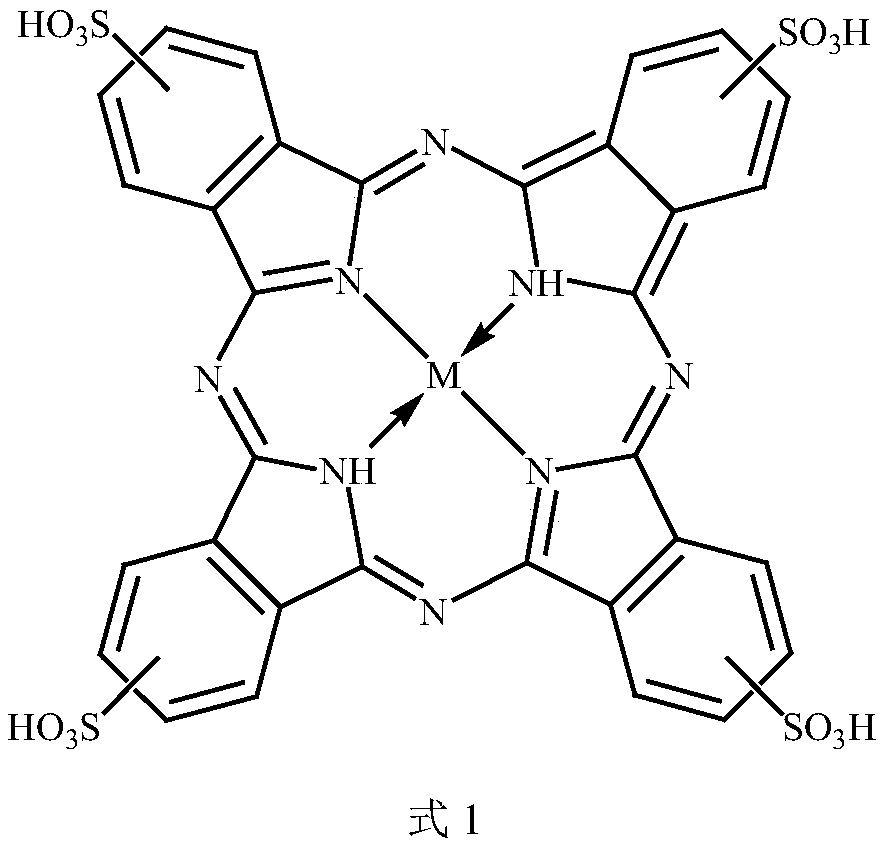
The invention discloses a BC / G / MPc ternary composite catalyst and an in-situ synthesis method thereof. The composite catalyst takes bacterial cellulose / graphene as a carrier, the metal phthalocyanineis tetrasulfonic metal phthalocyanine, and the central metal M of the metal phthalocyanine is one of manganese, iron, cobalt, nickel, copper or zinc ions; immobilization between the metal phthalocyanine and the bacterial cellulose / graphene is realized through pi-pi interaction between the graphene and metal phthalocyanine molecules; the synthesis method comprises the following steps: adding a graphene dispersion liquid subjected to ultrasonic treatment and a tetrasulfonic metal phthalocyanine solution into a bacterial cellulose basal culture medium, and statically culturing acetobacter xylinumat 30 DEG C; and cleaning the obtained product with a hydrochloric acid solution, a sodium hydroxide solution and ultrapure water to obtain the BC / G / MPc ternary composite catalyst. The bacterial BC / G / MPc ternary composite catalyst prepared by adopting the technical scheme has the advantages of simple equipment, simple and convenient process, easiness in post-treatment, easiness in operation and the like.
Owner:杭州沁欣环保科技有限公司
Acetobacter pasteurium and its application method in producing citrus vinegar
The invention discloses a acetobacter pasteurium and its application method in producing citrus vinegars and resulting citrus vinegar products. The invention provides acetobacter pasteurium named LSYY001.2, preserved in CGMCC on 14th June, 2010, preservation number: CGMCC No.4954. Method of the invention for producing citrus vinegar comprises: selecting materials, peeling citruses, removing segment membranes, crushing, filtering, adding yeasts for alcoholic fermentation, adding LSYY001.2 for acetic acid fermentation, then filtering. The acetobacter pasteurium provided by the invention not only ferments alcohol for producing acetic acid, but simultaneously produces iso-limonin hydrolase, thus realizing removing bitter of the citrus vinegar.
Owner:丽水市鱼跃酿造食品有限公司
Gene-nourishing and repairing bioorganic nutrient solution
InactiveCN102488213AGet through fastEasy rescueFood preparationBiotechnologyRadix Astragali seu Hedysari
The invention discloses a gene-nourishing and repairing bioorganic nutrient solution. The bioorganic nutrient solution of the invention takes gene-nutrishing Ganoderma lucidum, rice sprouts, black bean sprouts, sesame sprouts, and five viscera-nourishing, Radix Polygoni Multiflori Preparata, Polygonatum sibiricumt, acanthopanax senticosus, wolfberry fruits, Fructus alpiniae oxyphyllae, and brain-nourishing goose eggs, walnut kernels, oxide resistant haws, winter jujubes, qi replenishing and blood nourishing radix astragali, Chinese Angelica, channel dredging and collateral activating radix aucklandiae, Ligusticum chuanxiong, as well as honey and drinking water as raw materials. Under the effects of beneficial strains such as acetobacter schutzenbachii, beer yeast, Lactobacillus lactis andthe like, multiple beneficial strain groups are subjected to aerobic-anaerobic combined deep processing. The gene-nourishing and repairing bioorganic nutrient solution in the invention is suitable for all groups, and has a plurality of health care functions like virus and bacterium resistance, immunity enhancement, physical strength recuperation, fatigue resistance, sleep improvement, and age defying, etc.
Owner:HENAN BENCAO BIOTECH
Acetobacter orientalis and method for producing astragalus polysaccharide through same
InactiveCN105420143AImprove stabilityTo promote metabolismBacteriaMicroorganism based processesBiotechnologyAstragalus polysaccharide
The invention provides acetobacter orientalis. The preservation name of the acetobacter orientalis is Acetobacter orientalis, the acetobacter orientalis is preserved in China Center for Type Culture Collection on July 16, 2015, and the preservation serial number is CCTCC NO: M 2015455. The acetobacter orientalis has good stability, is high in metabolic capability and safety and has good medicinal value. The invention further provides a method for producing astragalus polysaccharide through the acetobacter orientalis. The content of the astragalus polysaccharide obtained through fermented culture is not lower than 30%.
Owner:YUZHOU TIANYUAN BIOTECH CO LTD
Complex flora for sewage treatment and culture method of complex flora
The invention provides a complex flora for sewage treatment and a culture method of the complex flora in order to solve the problem that the sewage treatment cost is increased due to the fact that the complex flora for the sewage treatment is susceptible to survival conditions, is poorer in stability and is required to be supplemented continuously in the prior art. The complex flora for the sewage treatment comprises the following ingredients by weight percentage: 16-25% of photosynthetic flora, 13-20% of lactic acid flora, 12-20% of yeast flora, 5-10% of ray flora, 3-12% of acetobacter flora, 15-26% of desulfuration flora, 1-6% of bacillus flora and 1-6% of bifidobacterium flora. The complex flora for the sewage treatment and the culture method of the sewage treatment have the beneficial technical effects that the complex flora can be fed at one time, the strain depression problem is better solved, the treatment effect is good, and the complex flora is safe to use and the like. The culture method of the complex flora for the sewage treatment is simple to operate and wide in condition, and a survival rate is high.
Owner:CHONGQING RAINBOW SMART ENERGY CO LTD
Novel gluconacetobacter strain having cellulose producing activity
Disclosed herein is a novel gluconacetobacter strain having cellulose producing activity. Specifically, the present invention relates to a novel gluconacetobacter strain producing nano-structured cellulose in a highly efficient manner. The cellulose produced by the strain, due to its superb thermodynamic properties, can be characterized as nano-structured bacterial cellulose and therefore utilized as a bio-nano-fiber. Particularly, the cellulose can be impregnated with a resin to form a cellulose-based resin which can be effectively adapted for a substrate for a liquid crystal display (LCD).
Owner:SAMSUNG DISPLAY CO LTD
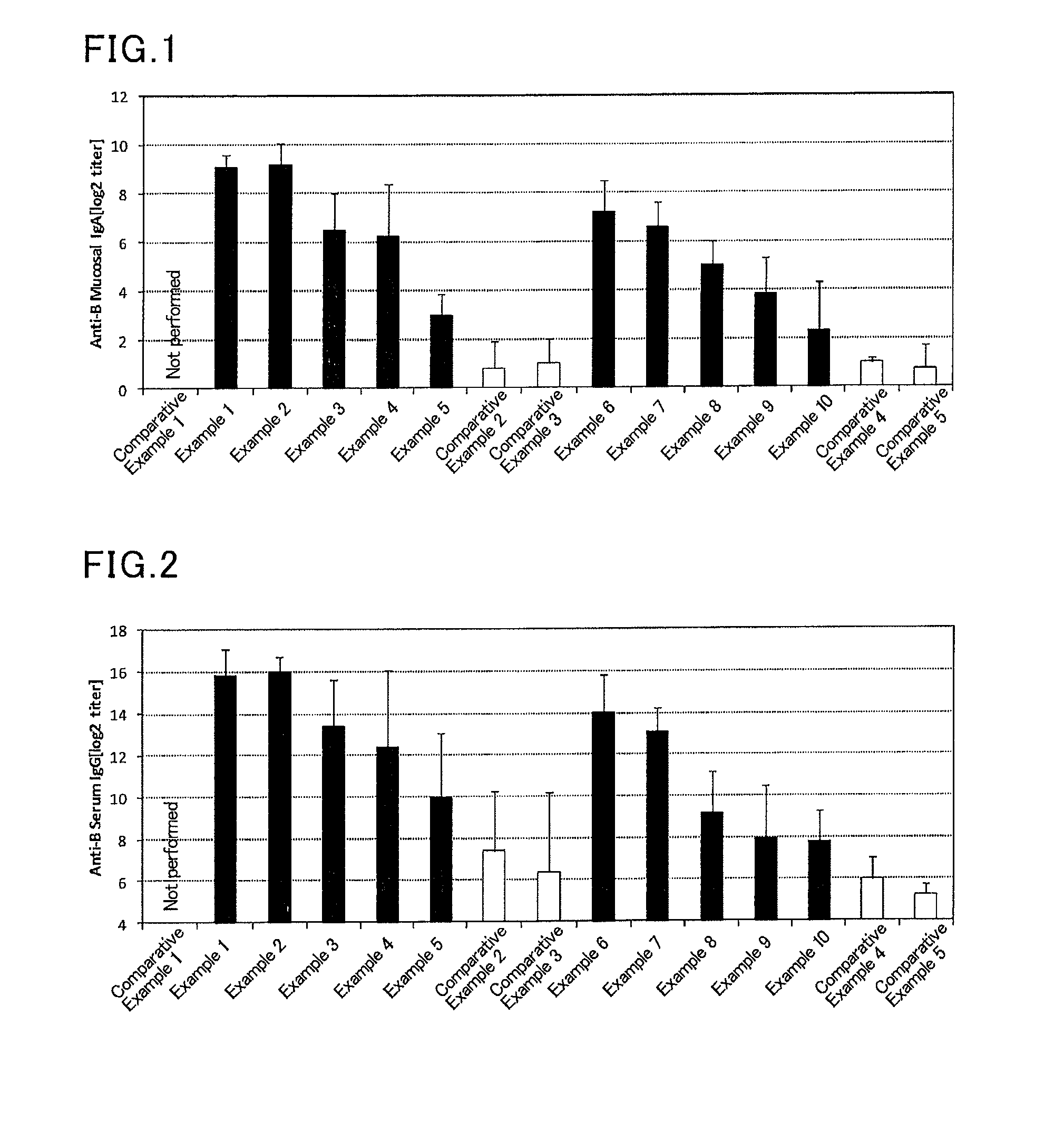
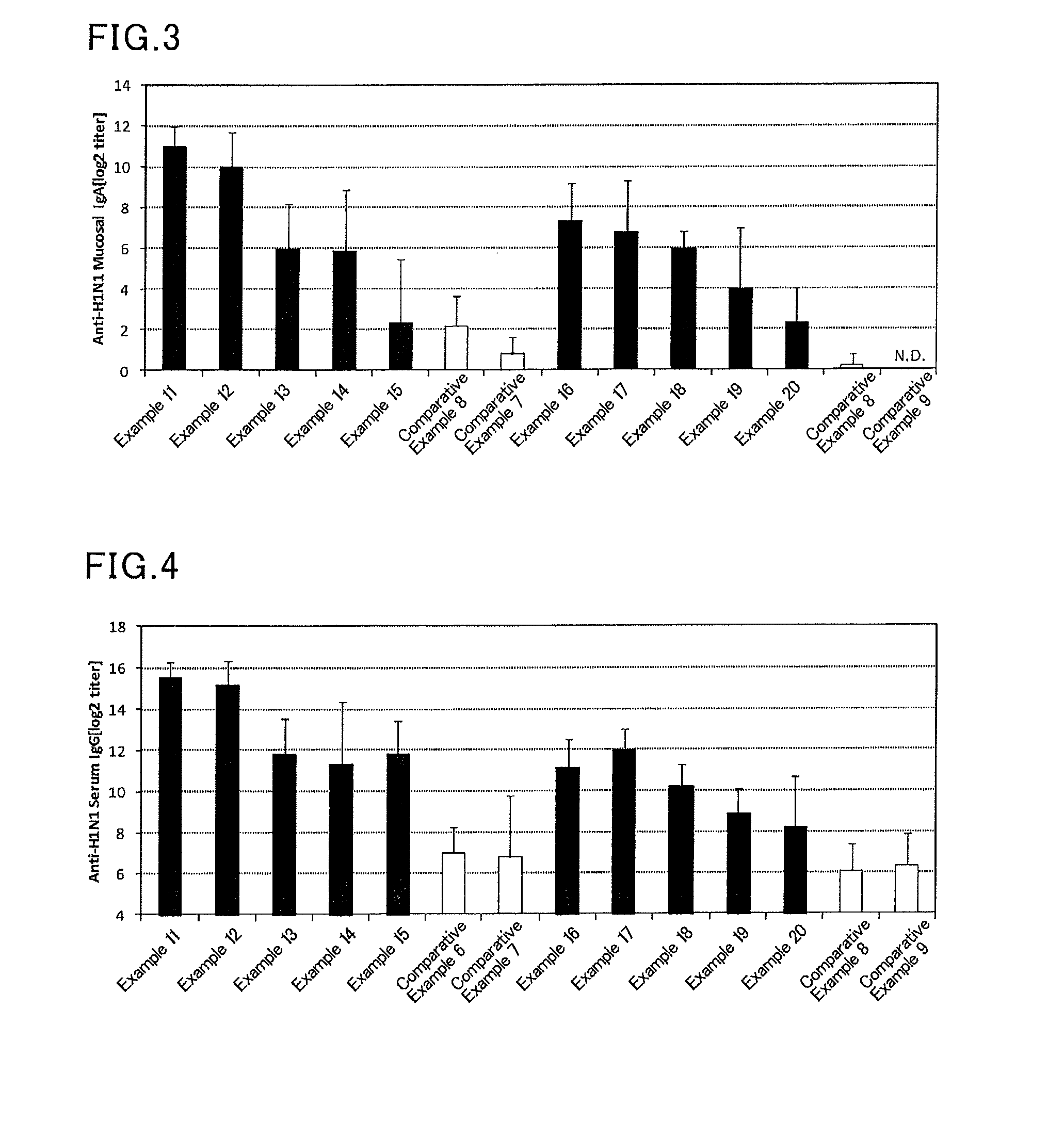
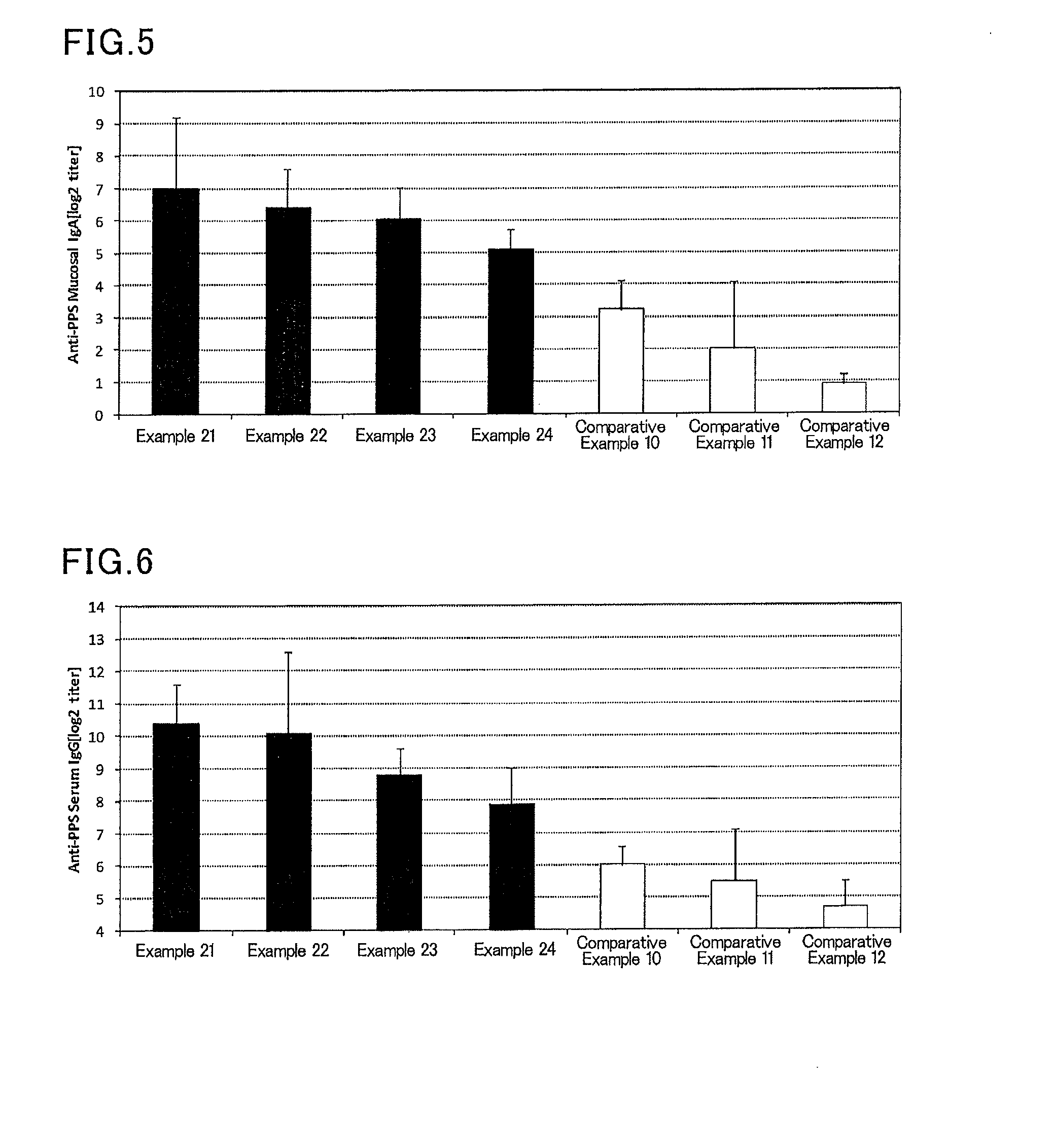
Mucosal vaccine composition
InactiveUS20160213773A1Safe and effectiveSsRNA viruses negative-senseBacterial antigen ingredientsMucosal Immune ResponsesXanthomonas
The present invention aims at providing a vaccine composition capable of being administered to an intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, or rectal mucous membrane, that is safe, useful as a prophylactic or therapeutic agent for infectious diseases or cancers, and capable of effectively inducing the systemic immune response and mucosal immune response. The present invention provides a mucosal vaccine composition to be administered to at least one mucous membrane selected from the group consisting of a human or animal intraoral mucous membrane, ocular mucous membrane, ear mucous membrane, genital mucous membrane, pharyngeal mucous membrane, respiratory tract mucous membrane, bronchial mucous membrane, pulmonary mucous membrane, gastric mucous membrane, enteric mucous membrane, and rectal mucous membrane, the mucosal vaccine composition containing: at least one antigen; and as an adjuvant, a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt thereof, wherein a mass ratio between the adjuvant and the antigen (total mass of the adjuvant / total mass of the antigen) is 0.002 to 500.
Owner:NITTO DENKO CORP
Preparation method of apple enzyme functional beverage
The invention discloses a preparation method of an apple enzyme functional beverage, and belongs to the technical field of food. The preparation method comprises the steps of taking apples, white granulated sugar and purified water as raw materials, inoculating a fermentation preparation for three times of fermentation in sequence, firstly inoculating saccharomyces cerevisiae with the inoculationamount of 0.1-0.2%, fermenting for 5 d, and sterilizing; inoculating Acetobacter pasteurianus with the inoculation amount of 1.2-1.8%, fermenting for 3 d, and sterilizing; finally inoculating lactobacillus plantarum with the inoculation amount of 1.2-1.8%, fermenting for 4 days, and sterilizing. Compared with the gamma-aminobutyric acid prepared by simultaneous fermentation of the three bacteria,the content of the gamma-aminobutyric acid is low, and the content of total phenols and crude polysaccharides is high. The apple enzyme functional beverage disclosed by the invention is wide in raw material source, low in price, unique in sweet and sour taste and mellow in mouthfeel; the product has stable quality, no preservative, rich metabolite and easy absorption by human body. The apple enzyme functional beverage can clear toxins in the body, clear free radicals, delay aging, resist cancer, maintain beauty and keep young, enhance memory and improve immunity.
Owner:HEBEI AGRICULTURAL UNIV.
Method for producing astragalus polysaccharide through acetobacter orientalis
InactiveCN105420144AIncrease contentLow priceBacteriaMicroorganism based processesBiotechnologyAstragalus polysaccharide
The invention provides a method for producing astragalus polysaccharide through acetobacter orientalis. The method includes the following steps that 1, a fermentation culture medium of astragalus polysaccharide is prepared; 2, an MRS bouillon culture medium is prepared; 3, enlarged culture of cetobacter orientalis is performed; 4, a fermentation reaction is performed; 5, separation and determination are performed. The method for producing astragalus polysaccharide is economical and efficient; fermentation raw materials used in the method are low in price and easy to purchase, the fermentation process is simple and easy to control, fermentation equipment and production conditions are not specially required, equipment and production conditions of a conventional fermentation plant can be adopted for production, and industrialized mass production can be easily achieved.
Owner:YUZHOU TIANYUAN BIOTECH CO LTD
Method for enhancing efficacy of enzyme using lactobacillus bulgaricus
ActiveCN107788517AStrengthen the probiotic effectSpecial fruity aromaBacteriaLactobacillusClearance rateAmyl acetate
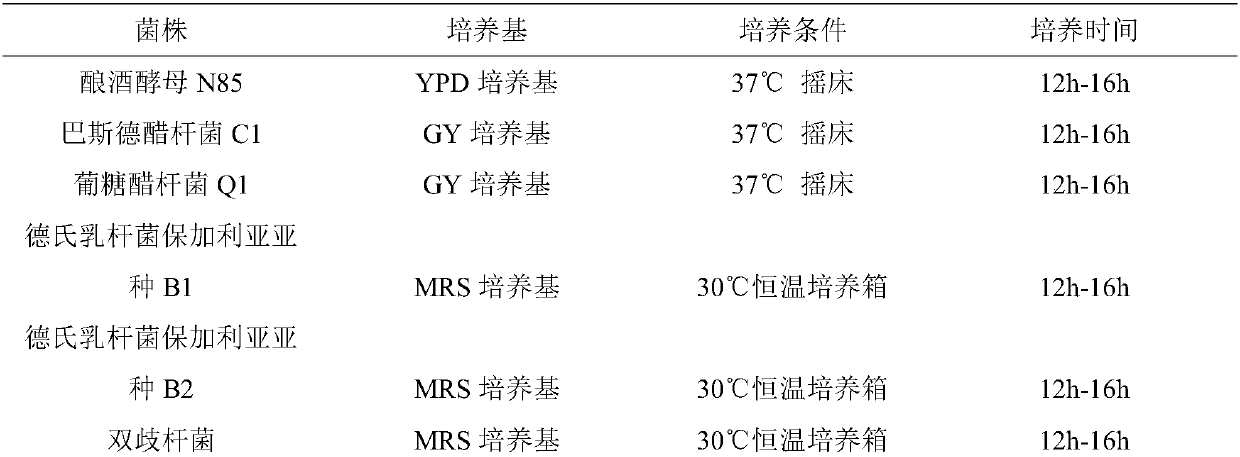
The present invention discloses a method for enhancing an efficacy of an enzyme by using lactobacillus bulgaricus and belongs to the technical field of fermented food. Saccharomyces cerevisiae N85, acetobacter gluconicum Q1 and lactobacillus delbrueckii subsp. bulgaricus B1 are added into fruit and vegetable raw materials, and the health preserving efficacy of the enzyme is enhanced, so that the prepared enzyme has a reducing power increased by 43.3% compared with that in the control group, a DPPH clearance rate also increased from 88.25% to 93.09%, a lactic acid content increased by 36.1%, anacetic acid content increased by 35.2%, and a protease activity increased by 11.9% compared with that in the control group, increases a content of volatile substances, has an ethyl acetate content increased by 85.1% and an amyl acetate content increased by 89.1%, has a special fruit aroma, and is increased in enzyme antibacterial ability.
Owner:北京姿美堂生物技术股份有限公司
Black fungus mycelia and black garlic vinegar
ActiveCN105505735AHigh nutritional valueImproves antioxidant activityVinegar preparationAcetobacterFiltration
The invention relates to black fungus mycelia and black garlic vinegar. The black fungus mycelia and black garlic vinegar is prepared by the following method: putting garlic with peel into an incubator, and performing fermentation at high temperature so as to obtain black garlic; peeling the black garlic, thoroughly cleaning the peeled black garlic, adding water, and grinding the cleaned black garlic with the water into black garlic serosity with a colloid mill; performing pasteurization, inoculating the activated black fungus mycelia fluid into the black garlic serosity, adding magnesium sulphate, and performing incubation in a fermenter; after black fungus mycelia grows out of the black garlic serosity, adding basic liquor of white spirit for blending so as to obtain a solution, regulating the alcoholic strength of the solution to 4-5 degrees, inoculating mixed bacteria of lactic acid bacteria and acetobacter diazotrophicus to the solution with the alcoholic strength of 4-5 degrees for fermentation, performing sealing and aging fermentation on liquor at 5-8 DEG C for 2-3 months, and performing filtration and filling so as to obtain the black fungus mycelia and black garlic vinegar. According to the black fungus mycelia and black garlic vinegar disclosed by the invention, the nutrient substances of the black funguses and the nutrient substances of the black garlic are utilized, so that the black garlic vinegar is rich in flavor substances, acid and smooth in mouth feel, and rich and mellow in vinegar fragrance; the black fungus mycelia is inoculated, so that the black fungus mycelia and black garlic vinegar is extremely high in antioxidant activity capacity, enables skins of people to be rosy and glowing, and can dredge and lubricate intestines and stomachs.
Owner:HUBEI UNIV OF TECH
Microorganism modifier and preparation method and application thereof
The invention discloses a microorganism modifier and a preparation method and application thereof. The microorganism modifier is prepared from: Bacillus subtilis, Saccharomyces cerevisiae, Clostridiumbutyricum, Lactobacillus plantarum, Pediococcus pentosaceus, Enterococcus faecalis, Acetobacter, Bifidobacterium, Lactobacillus, Lactobacillus acidophilus, Lactobacillus casei, Photosynthetic bacteria, Actinomycetes, Clostridium butyricum and Bacillus coagulans. According to the microorganism modifier, various bacterial groups are cooperated and interacted to produce good effects, and the microorganism modifier can be widely used in breeding of various poultry or livestock. Meanwhile, the microorganism modifier can significantly enhance the immunity of poultry or livestock, promote nutrient absorption, increase feed utilization rate, and improve meat quality; and the microorganism modifier has a good therapeutic effect on poultry or livestock that have common diseases or even serious diseases, and the economic benefits of farmers can be significantly improved.
Owner:广东理想生物科学研究有限公司
Phthalocyanine catalyst for dye wastewater treatment and preparation method thereof
ActiveCN113318790AHigh catalytic efficiencyExcellent catalytic oxidation degradation activityOrganic-compounds/hydrides/coordination-complexes catalystsWater contaminantsGluconacetobacter intermediusPtru catalyst
The invention discloses a phthalocyanine catalyst for dye wastewater treatment and a preparation method thereof. According to the preparation method, silver ions are reduced and attached to a phthalocyanine-nanocellulose binary catalyst by using a microorganism. The microorganism is gluconacetobacter intermedius, acetobacter xylinum or acetobacter hansenii; phthalocyanine is sulfamic acid group metal phthalocyanine; and silver ions are provided by silver nitrate, silver acetate or silver lactate. The preparation method comprises the following steps: 1, preparing a glucose culture solution containing phthalocyanine; 2, culturing microorganisms in the culture solution; 3, preparing a mixed solution containing silver ions and glucose; 4, putting a product obtained in the step 2 in the mixed solution obtained in the step 3, and adding the microorganisms; and 5, cleaning the product obtained in the step 4 to obtain the catalyst. According to the above technical scheme, reduction of silver ions on the surface of phthalocyanine is achieved, a chemical reducing agent, a protective agent and an organic solvent do not need to be added in the process, and the method has the advantages of simple operation, mild reaction conditions, green process, low cost and the like.
Owner:浙江庞氏塑业有限公司
Plant growth stimulator
A plant growth stimulator useful for promoting and maintaining plant growth, wherein said stimulator is post-microbial culture inoculation nutrient medium consisting of black tea and a carbohydrate source, wherein the culture is consisting of Acetobacter xylinum, Candida sp. and Zygosaccharomyces rouxii, and a process for the preparation of the plant growth stimulator, and further, a method of promoting and maintaining the growth of plants using the plant stimulator having multiple disease resistance activity.
Owner:COUNCIL OF SCI & IND RES
Castanea mollissima vinegar and preparation technique thereof
InactiveCN106010932ASimple preparation processRaw materials are easy to obtainVinegar preparationYeastAcetobacter
The invention discloses a Castanea mollissima vinegar and a preparation technique thereof. The Castanea mollissima vinegar is prepared from Castanea mollissima, wheat, distillery yeast, sugar, Acetobacter, glutinous rice, longan, Chinese wolfberry and red date. The preparation technique comprises the following steps: raw material treatment, Castanea mollissima fermentation, wheat fermentation, glutinous rice and longan fermentation, mixed distillation, blending, aging, sterilization and the like. The Castanea mollissima vinegar has the subtle fragrance of wheat and the rich fragrance of glutinous rice, and has the effects of nourishing the stomach, strengthening the spleen, tonifying the kidney, strengthening tendons, promoting blood circulation, arresting bleeding, resisting aging and prolonging the life.
Owner:莫丽婷
Preparation method of bacterial cellulose, bacterial cellulose-chitosan composite gel skincare mask and preparation method thereof
InactiveCN112877384AHigh mechanical strengthGood value for moneyCosmetic preparationsToilet preparationsBiotechnologyAcetobacter
The invention discloses a preparation method of bacterial cellulose. The method comprises the following steps of: preparing a soybean residue biological fermentation culture medium by using soybean residues as a basic raw material, namely soybean residues for short, adding 10-18% of a gluconacetobacter xylinum seed solution and amygdalin into the soybean residue biological fermentation culture medium, adding 12-25 mL of a gluconacetobacter xylinum seed solution into every 100 mL of the soybean residue biological fermentation culture medium, wherein the pH is 4.8-6.0, the culture temperature is 28-35 DEG C, the culture time is 12-18 days, the use amount of amygdalin is 150-450 U / mL, and obtaining a bacterial cellulose membrane material. The invention further discloses a bacterial cellulose-chitosan composite gel skin care mask and a preparation method thereof. The mask is good in tensile strength and high in mechanical strength, has a certain inhibition effect on bacteria and fungi, and is good in biocompatibility.
Owner:HARBIN UNIV OF SCI & TECH +1
Snake feed added with Chinese herbal medicine
InactiveCN106343216ALow costImprove survival rateAnimal feeding stuffAccessory food factorsAnimal scienceAcetobacter
The invention discloses a snake feed added with Chinese herbal medicine, which is prepared from the following raw materials by weight: 40-50 parts of chicken, 10-20 parts of fish, 8-12 parts of pork, 450-500 parts of marine fish, 25-35 parts of small frogs, 25-35 parts of eggs, 10-12 parts of enzyme preparations, 6-8 parts of milk powder, 5-7 parts of bone meal, 2-4 parts of earthworm powder, 2-4 parts of lysine, 0.03-0.05 part of yeast, 0.02-0.04 part of acetobacter, 0.05-0.07 part of organic selenium, 3-5 parts of medicament and 2-4 parts of attractant. The material used for the preparation wherein is low in cost, avoiding the living creatures caused by the impact of the bacteria on the snake to improve the survival rate of the snake, in contrast with living snake feed. The prepared feed is in the shape of round bar, conducive to the snake's swallowing; the added Chinese herbal medicine can prevent the snake from cold or stomach trouble, so as to ensure its healthy growth.
Owner:张松波
Preparation method of heavy metal ion adsorbent based on modified bacterial cellulose
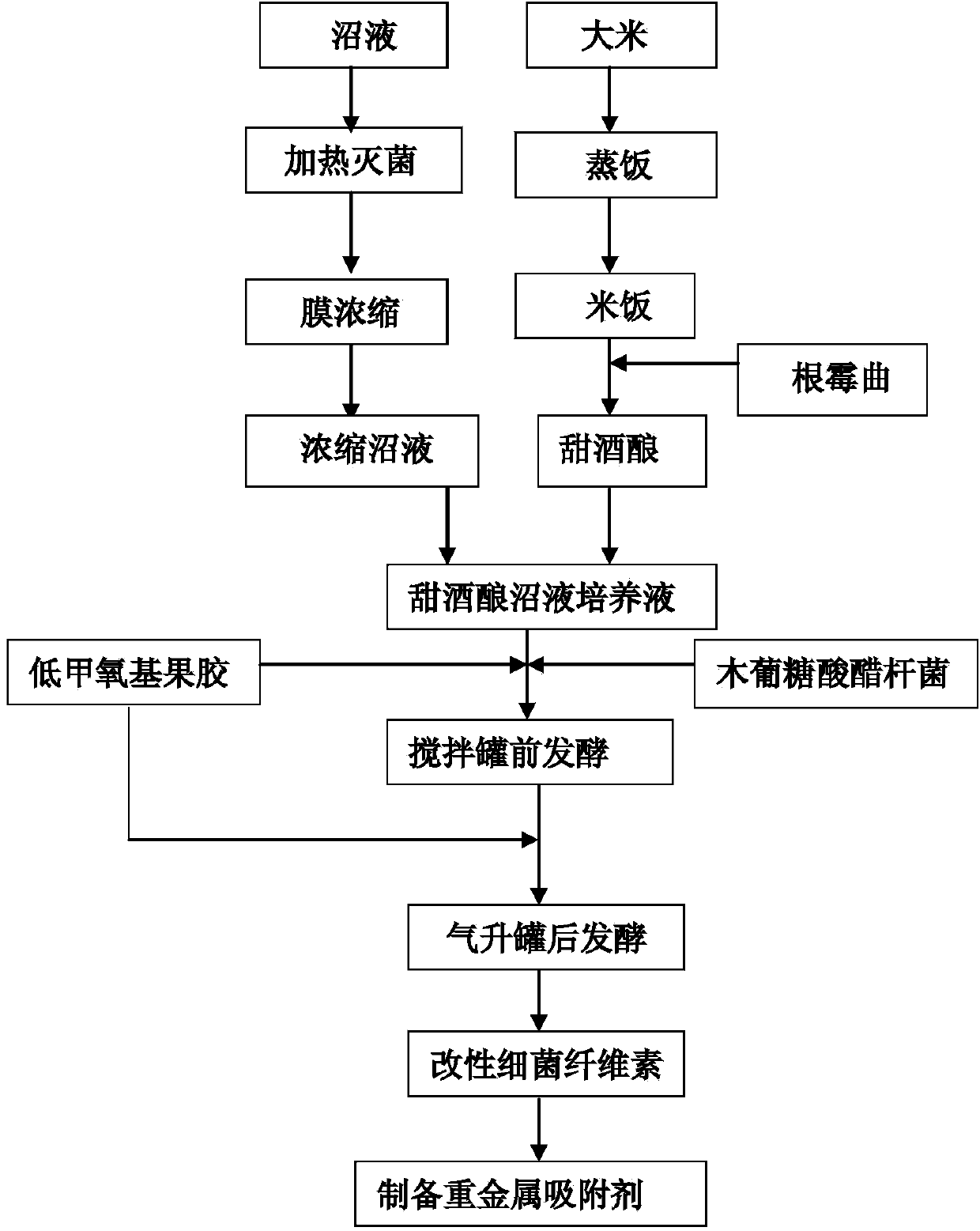
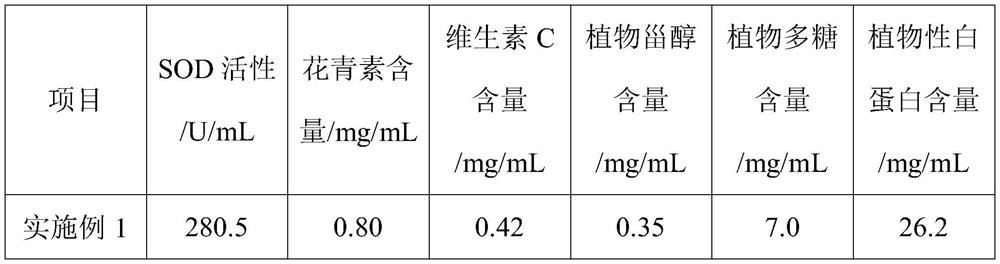
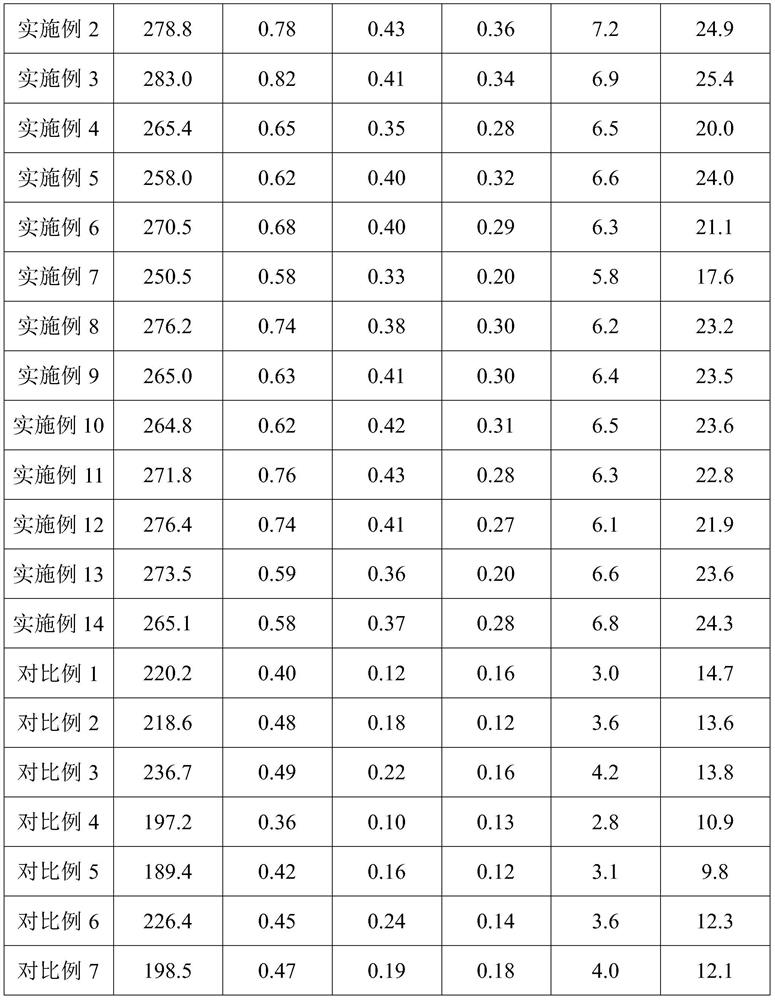
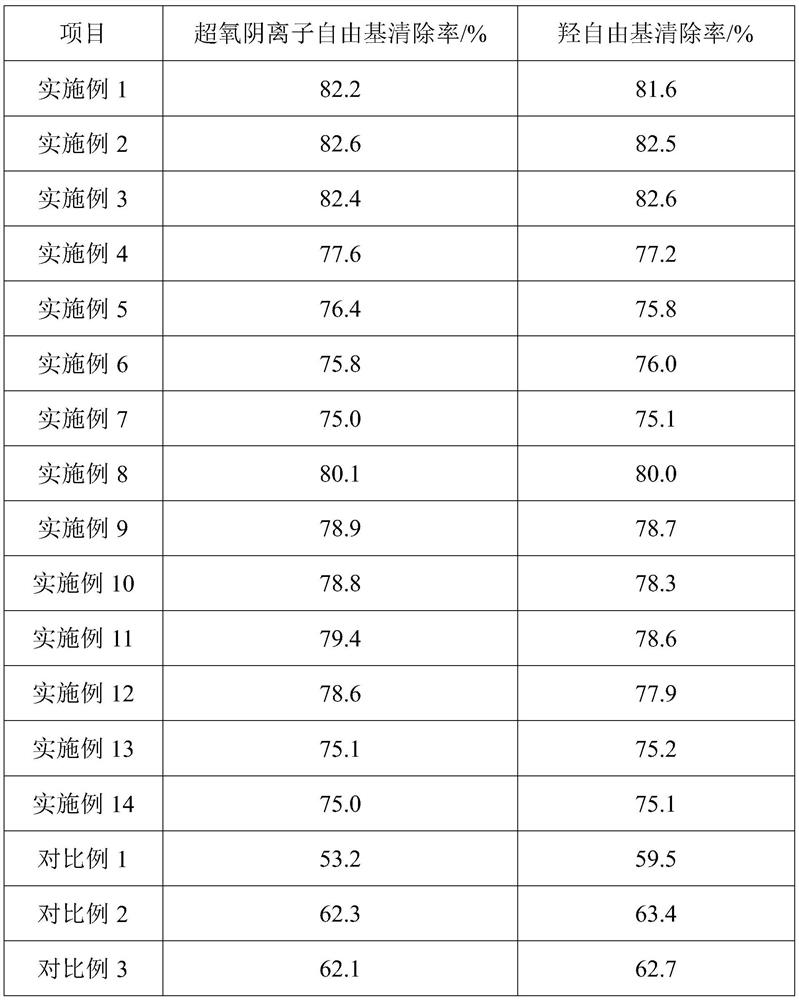
ActiveCN103990441AThe ability to adsorb metal ions is not strongImprove performanceOther chemical processesWater contaminantsFood industryAirlift reactor
The invention discloses a preparation method of a heavy metal ion adsorbent based on modified bacterial cellulose. The preparation method comprises the following steps: adding xylose gluconic acetobacter seed solution into a culture solution fermentation tank filled with sweet wine brewing biogas slurry, adding low-methoxyl pectins solution for fermenting and culturing into a front fermentation mash solution; adding the front fermentation mash solution into an airlift reactor, replenishing the low-methoxyl pectins solution, fermenting and culturing by using the airlift reactor to obtain a mature post-fermentation mash containing micelle modified bacterial cellulose; separating out the micelle modified bacterial cellulose, washing the micelle modified bacterial cellulose for a plurality of times and the immersing the micelle modified bacterial cellulose into NaOH solution, carrying out water bath till the micelle modified bacterial cellulose is semitransparent; finally washing the micelle modified bacterial cellulose with deionized water, drying the micelle modified bacterial cellulose, grinding the micelle modified bacterial cellulose to be greater than 40 meshes and taking the micelle modified bacterial cellulose as the heavy metal ion adsorbent. By adopting the preparation method, the process is simple; the prepared heavy metal ion adsorbent is non-toxic, good in biocompatibility, good in absorption effect on heavy metal ions and applicable to the food industry or the pharmaceutical industry.
Owner:FUJIAN NORMAL UNIV
Medicine for external application used for treating gout and preparation method
ActiveCN102793829APain reliefLower uric acid levelsMedical devicesSkeletal disorderBiotechnologyPolygonum fagopyrum
The invention relates to a medicine for external application used for treating gout and a preparation method, the medicine for external application comprises the following traditional Chinese medicines: smallflower galinsoga flower, buckwheat root, philippine flemingia root, glabrousleaf pittosporum leaf and Paris polyphylla Smith; the above traditional Chinese medicines are respectively immersed in a boiled rice vinegar, and are inoculated to Acetobactersp. GLYSM66 CCTCC NO: M2011238, after fermenting through different fermentation times, and then a fermentation broth is blended to prepare the medicine. The medicine for external application has the function of dredging the channel and activating meridian, can rapidly reduce the uric acid level in blood of the gout patients, and has the advantages of fast pain relieving effect, good curative effect, low cost, no toxic and side effects and simple preparation method.
Owner:莫寿科
Preparation method of dragon fruit extracting solution, dragon fruit extracting solution prepared by preparation method and applications of dragon fruit extracting solution
ActiveCN112022765AActivate biological activityHigh extraction rateCosmetic preparationsHair cosmeticsBiotechnologyAcetobacter
The invention provides a preparation method of a dragon fruit extracting solution, the dragon fruit extracting solution prepared by the preparation method and applications of the dragon fruit extracting solution. The preparation method includes the followings steps: (1) mixing dragon fruit pulp and water, performing squeezing and filtering, and adopting an ion exchange resin column to perform decolorization so as to obtain decolorized dragon fruit squeezing liquid; (2) mixing part of the decolorized dragon fruit squeezing liquid obtained in the step (1) and yeast, and performing fermentation under an anaerobic condition to obtain dragon fruit fermentation broth I; and mixing the rest of the decolorized dragon fruit squeezing liquid obtained in the step (1), acetobacter and lactobacillus plantarum, and performing fermentation under an anaerobic condition to obtain dragon fruit fermentation broth II; and (3) mixing the dragon fruit fermentation broth I and the dragon fruit fermentation broth II obtained in the step (2), and performing fermentation under an anaerobic condition to obtain the dragon fruit extracting solution. The method does not damage the original nutrients of dragon fruits, and the prepared dragon fruit extracting solution is high in biological activity.
Owner:广州善合化工有限公司
Preparation method of edible bacterial cellulose/dietary fiber
InactiveCN109259216ARich nutritional structureFragrant and pleasantMicroorganism based processesFermentationFruit juiceDigestion
The invention discloses a preparation method of edible bacterial cellulose / dietary fiber, and belongs to the technical field of food biology. The preparation method comprises the following steps that1, mixed fruits are washed cleanly and squeezed to be juice, the juice is subjected to centrifugal filtration, and pectase, cellulase and other nutrient matters are added into the filtered juice, andthe mixed serves as a fermentation culture medium; 2, gluconacetobacter xylinum is inoculated, the obtained product is placed in a 30 DEG C constant temperature culture box to be cultured for 10 d, and a bacterial cellulose membrane is formed; and 3, the bacterial cellulose membrane obtained through fermentation is cut to be blocks and stirred and soaked in normal temperature water for 3-5 times,and soaking is conducted for 30-40 min each time; and the sample is placed at the temperature of 90-95 DEG C and stirred for 20-40 min by using a sodium alginate solution with the concentration being0.15-0.3%, and then standing and soaked by using calcium lactate with the concentration being 0.15-0.3% for 20-40 min at the normal temperature. The method utilizes compound fruit juice to serve as afermentation original liquid culture medium to ferment and produce bacterial cellulose. As a novel dietary fiber, bacterial cellulose is rich in nutrient structure, sour and sweet and tasty, has the attractive fragrance and pleasant color and glossiness, supplements a mass of vitamins, mineral substances and part of constant and trace elements which are needed by the human body while promoting human digestion, makes up the defects existing in common dietary fiber, and has the good quality advantage and marketing prospects.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com