Gene chip for detecting enterohemorrhagic escherichia coli, use method and detection kit
A technology of Escherichia coli and gene chip, which is applied in the field of gene chip and its use method and detection kit, can solve the problems of complicated operation, missed detection or false positive result, easy to be affected by human factors, etc. Accurate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Probe design and preparation
[0056] 1. Sequence acquisition
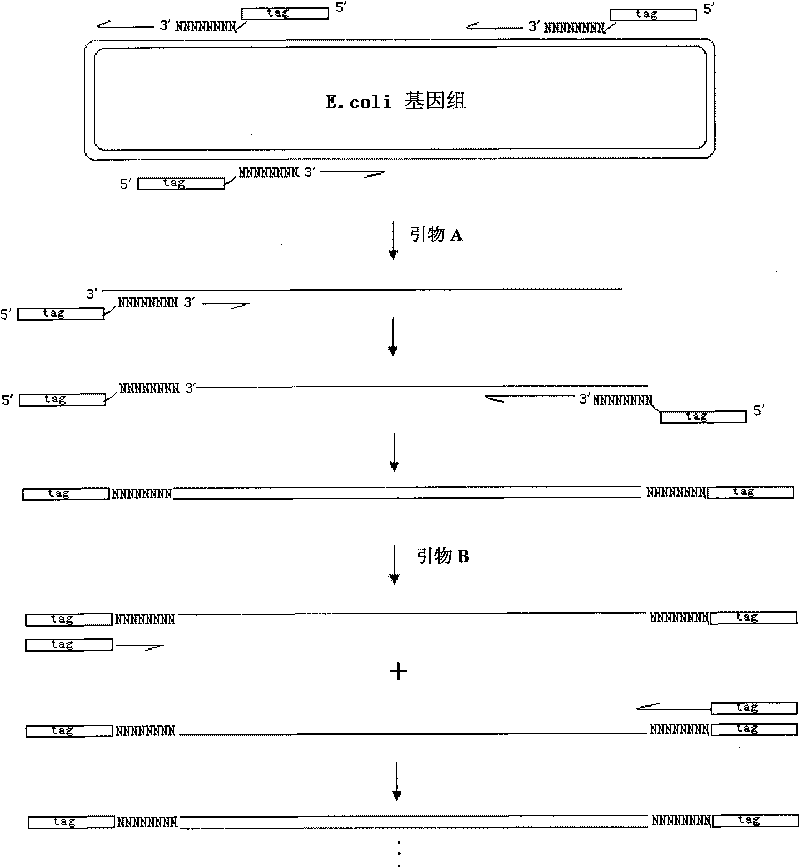
[0057] The genes involved in the present invention include the 16S rRNA gene of bacteria, the oligosaccharide unit processing enzyme genes wzx and wzy of Escherichia coli of 19 different O serogroups (Escherichia coli O8 corresponds to wzt, wzm genes), and ETEC unique The enterotoxin coding genes elt, estA (including human estA (h) and porcine est (p)), estB genes. In addition, due to the high sequence similarity between the wzx and wzy genes of Escherichia coli O148 and the corresponding genes of Shigella dysenteriae 1 (Shigella dysenteriae 1), the rfpB gene region unique to Shigella dysenteriae 1 Two probes were designed to distinguish Escherichia coli O148 and Shigella dysenteriae type 1. The sources of these gene sequences are detailed in Table 1.
[0058] Table 1 Description of sequence sources
[0059]
[0060]
[0061]
[0062] 2. Probe Design
[0063] Enter the O antigen ol...
Embodiment 2
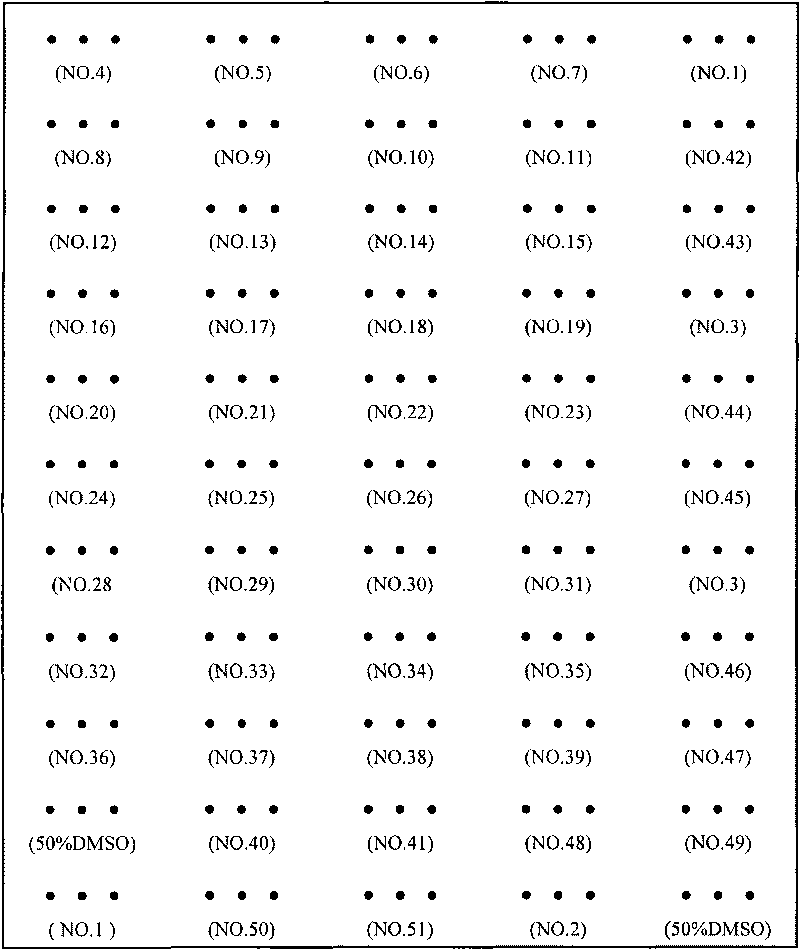
[0075] Example 2 Gene Chip Preparation - Chip Spotting
[0076] 1. Dissolving the probe: Dissolve the probe dry powder tube (synthesized by Aoke) synthesized in Example 1 above. The steps are as follows: centrifuge the above-mentioned probe dry powder tube at 12000 rpm for 10 minutes (be careful not to open the lid of the dry powder tube before centrifugation), then add 1xSpotting Buffer (add 20μl to 1OD), and mix well with a shaker Centrifuge quickly to remove the liquid on the tube wall. After standing at room temperature for 1 hour, take 1.5 μl to measure OD, measure the nucleic acid concentration (ng / μl) of the probe (single strand), and then use the following formula to dilute the probe to a final concentration of 1 μg / μl.
[0077] formula:
[0078] 2. Adding plates: add the 51 dissolved probes to the corresponding positions in the 384-well plate, and add 10 μl of probe solution to each well.
[0079] 3. Spotting: Spot the probes in the above-mentioned 384-well p...
Embodiment 3
[0088] Example 3 Rapid detection of O serogroup and virulence factor types of enterotoxigenic Escherichia coli using the prepared gene chip
[0089] In the method for detecting enterotoxigenic Escherichia coli using the above-mentioned prepared gene chip, the present invention has made a series of experiments on factors such as detection steps and detection conditions, such as the ratio of each component in the whole genome random primer PCR reaction mixture, The template concentration, the hybridization temperature of the chip hybridization reaction, the hybridization time, and the main reagent formulations, such as hybridization solution, washing solution, etc. (refer to the following formula for details), are all excellent ratios obtained after gradient experiments. The experimental procedure of the gene chip for the detection of enterotoxigenic Escherichia coli is also the result of optimization. The following is a specific description of a preferred embodiment of the de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com