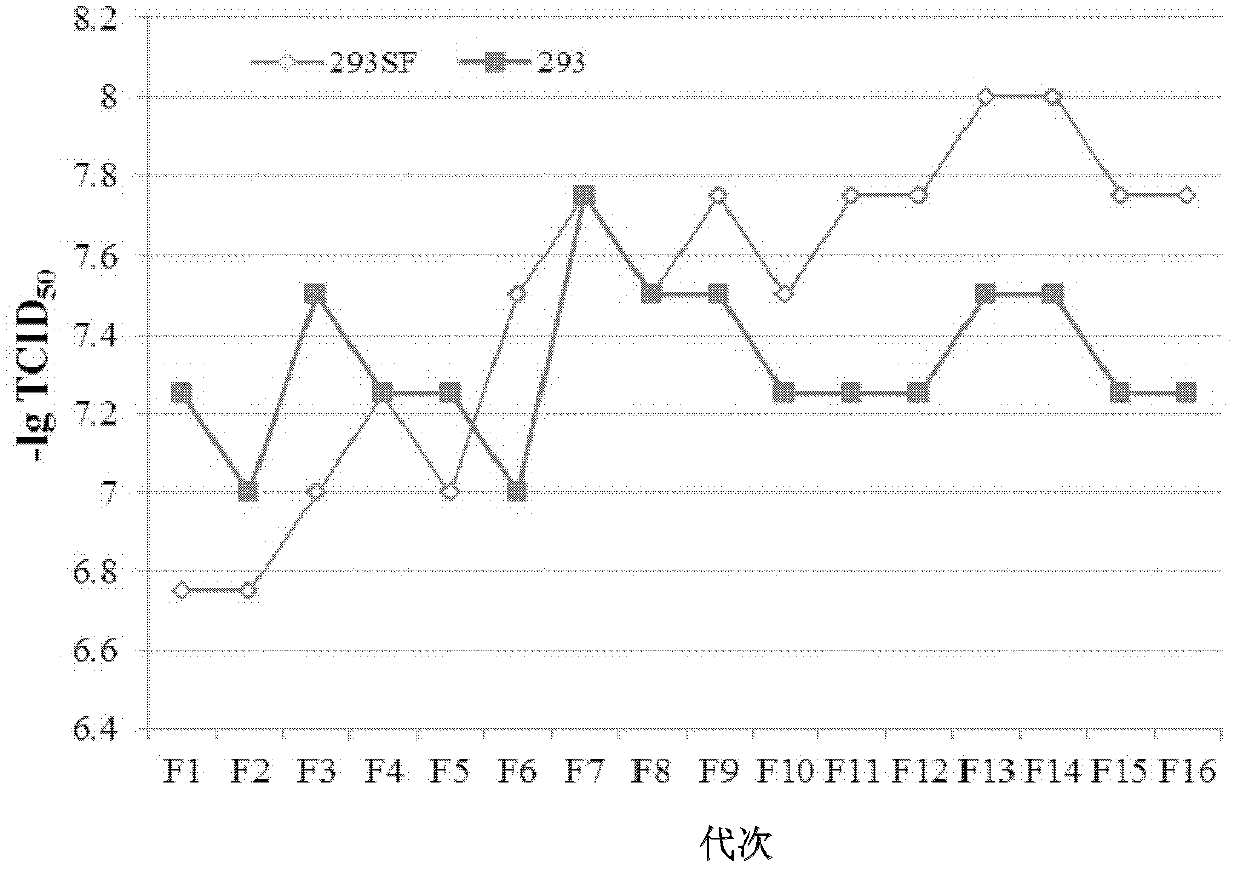
HEK (human embryonic kidney) 293 cell line applicable to serum-free culture and application thereof
A serum-free culture and cell line technology, applied in the biological field, can solve the problems of relying on serum culture, low yield, and difficult purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1 Materials and methods
[0037] 1.1 Cells, antibodies, media, viruses and other materials
[0038] The HEK293 cell line is preserved and provided by our laboratory; the monoclonal antibody HQ06 against classical swine fever virus E2 protein is prepared by our laboratory; serum-free culture medium 293 SFM II, DMEM, calf serum (FBS), GlutaMAX TM-1 were purchased from Life Technology Company; cell culture consumables were purchased from Corning Company; poly-lysine (Poly-L-Lysine, PDL) was purchased from Sigma Company; Recombinant adenovirus expressing the E2 gene of classical swine fever virus, the specific construction process is recorded in the application date of September 30, 2010, the application number is 201010297576.0, and the invention name is "adenovirus / alphavirus replicon chimeric vector swine fever vaccine and its application" in the patent literature.
[0039] 1.2 Culture method
[0040] Before the cells are adapted to culture, they are cultured with 10%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com