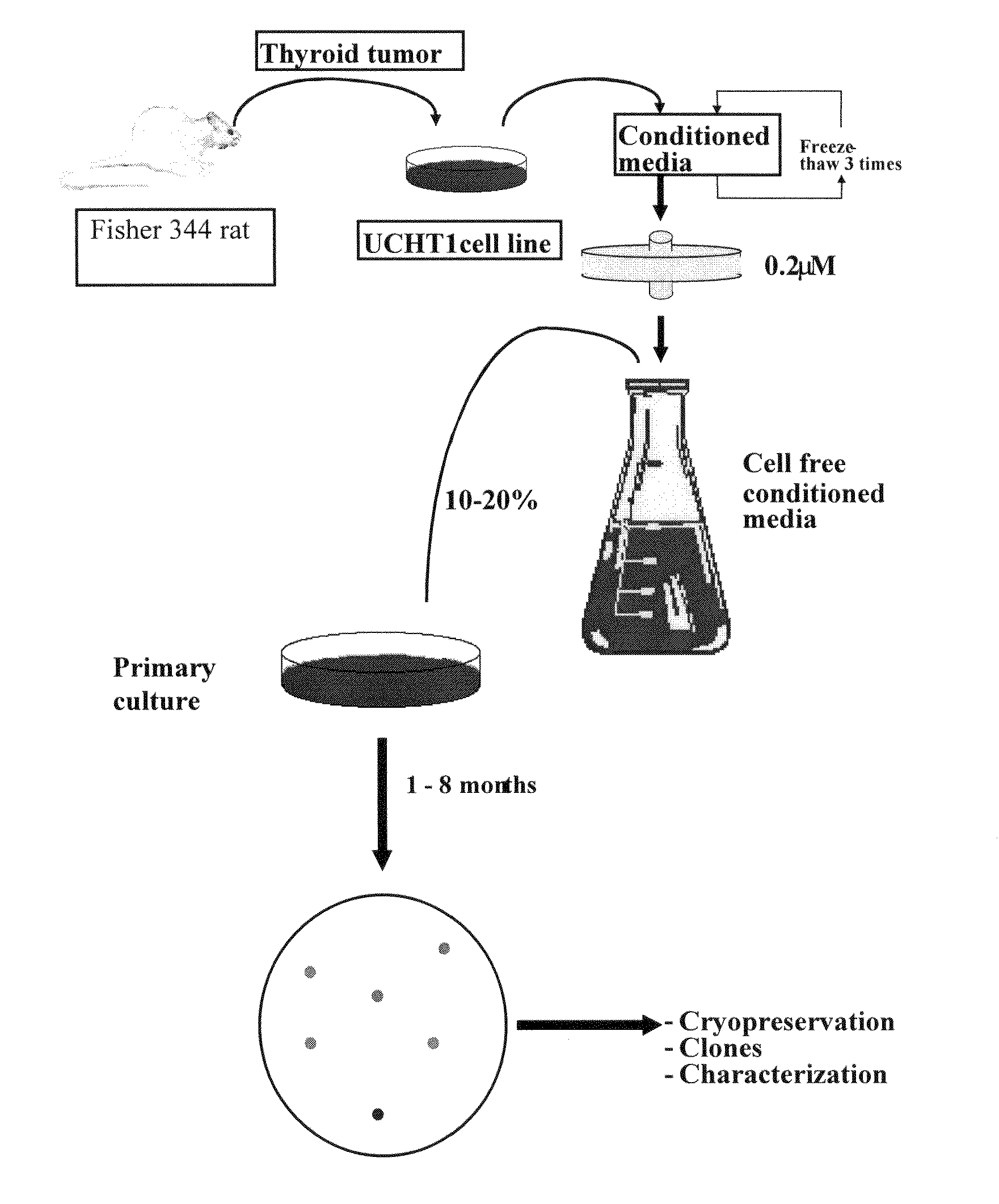
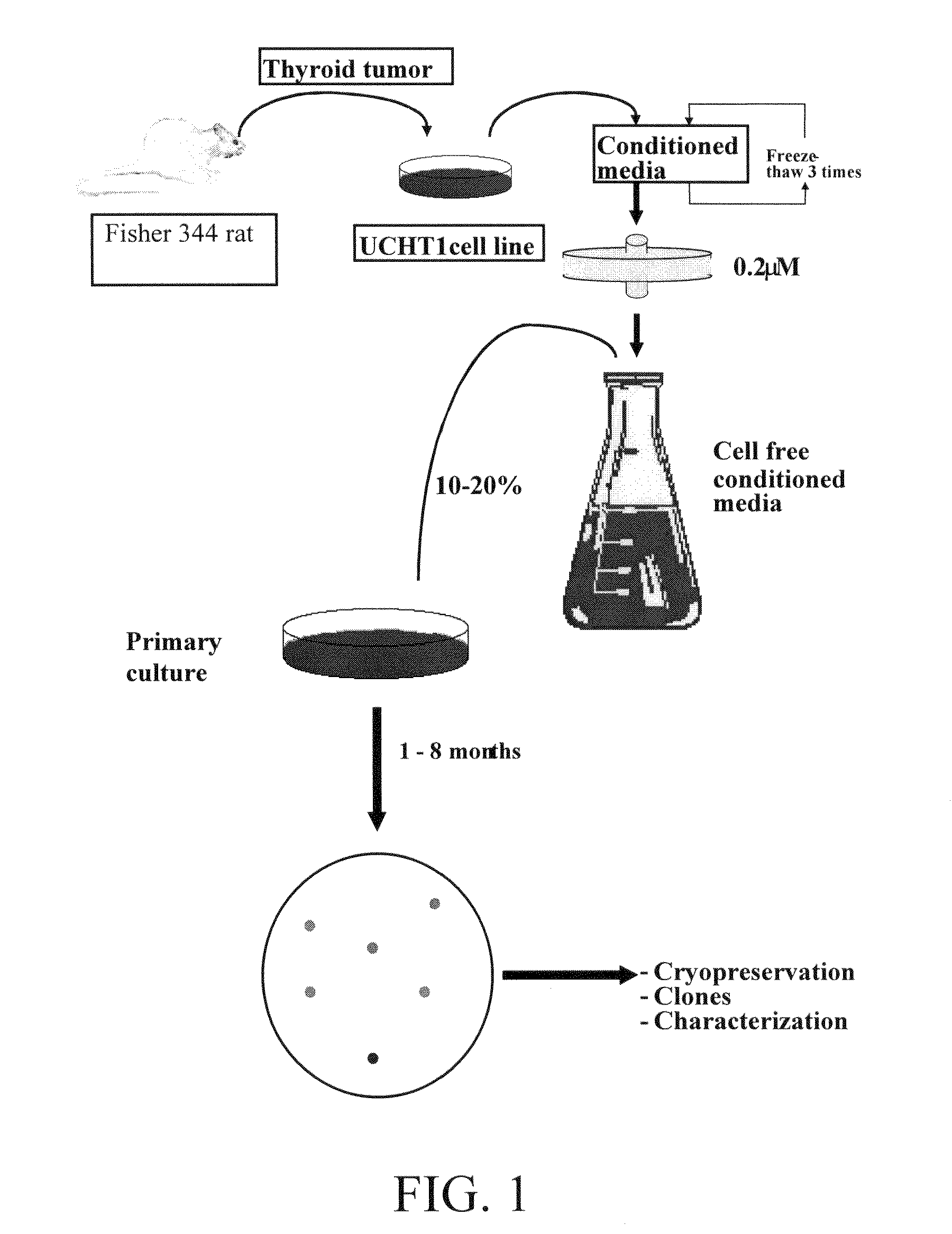
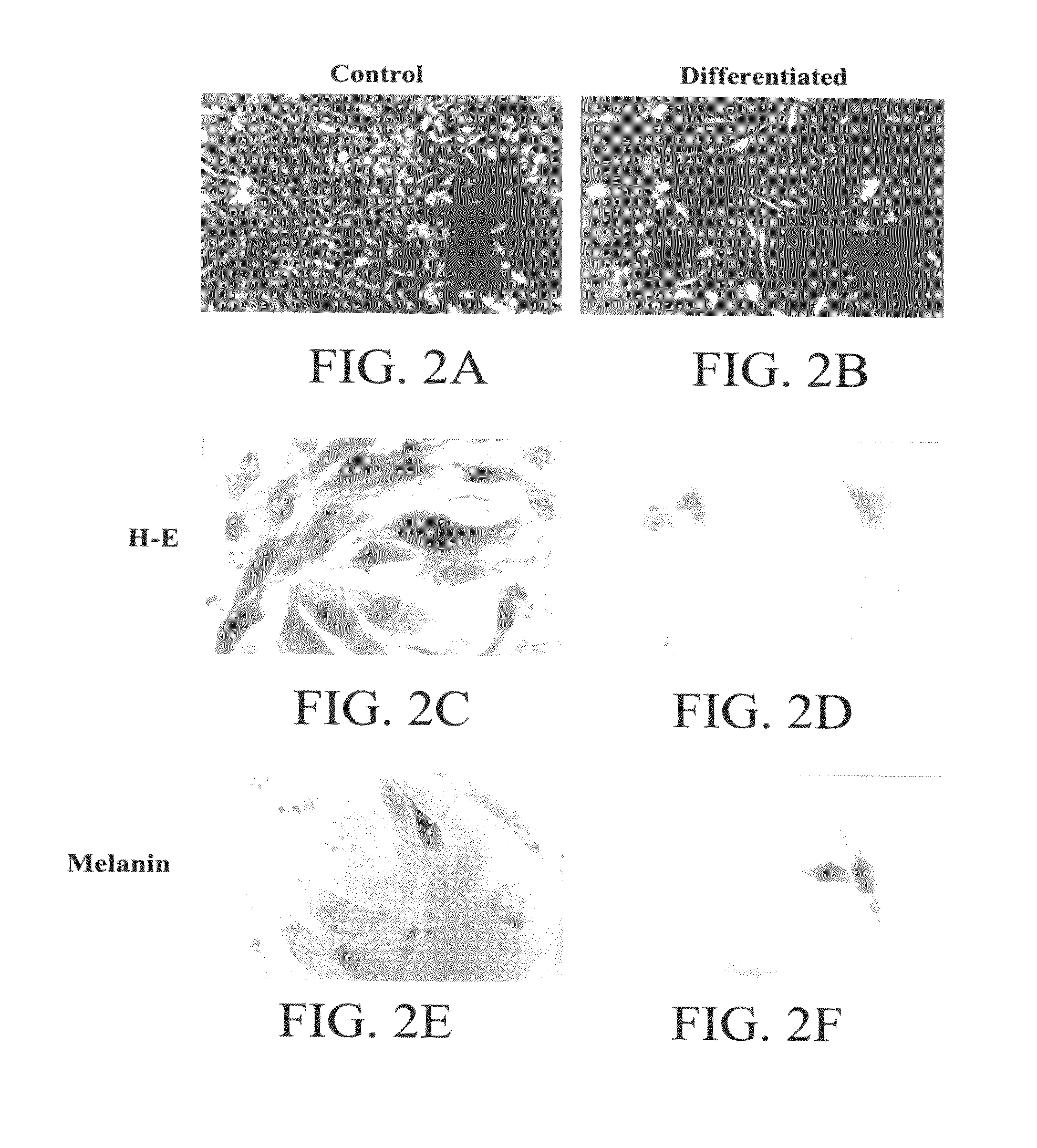
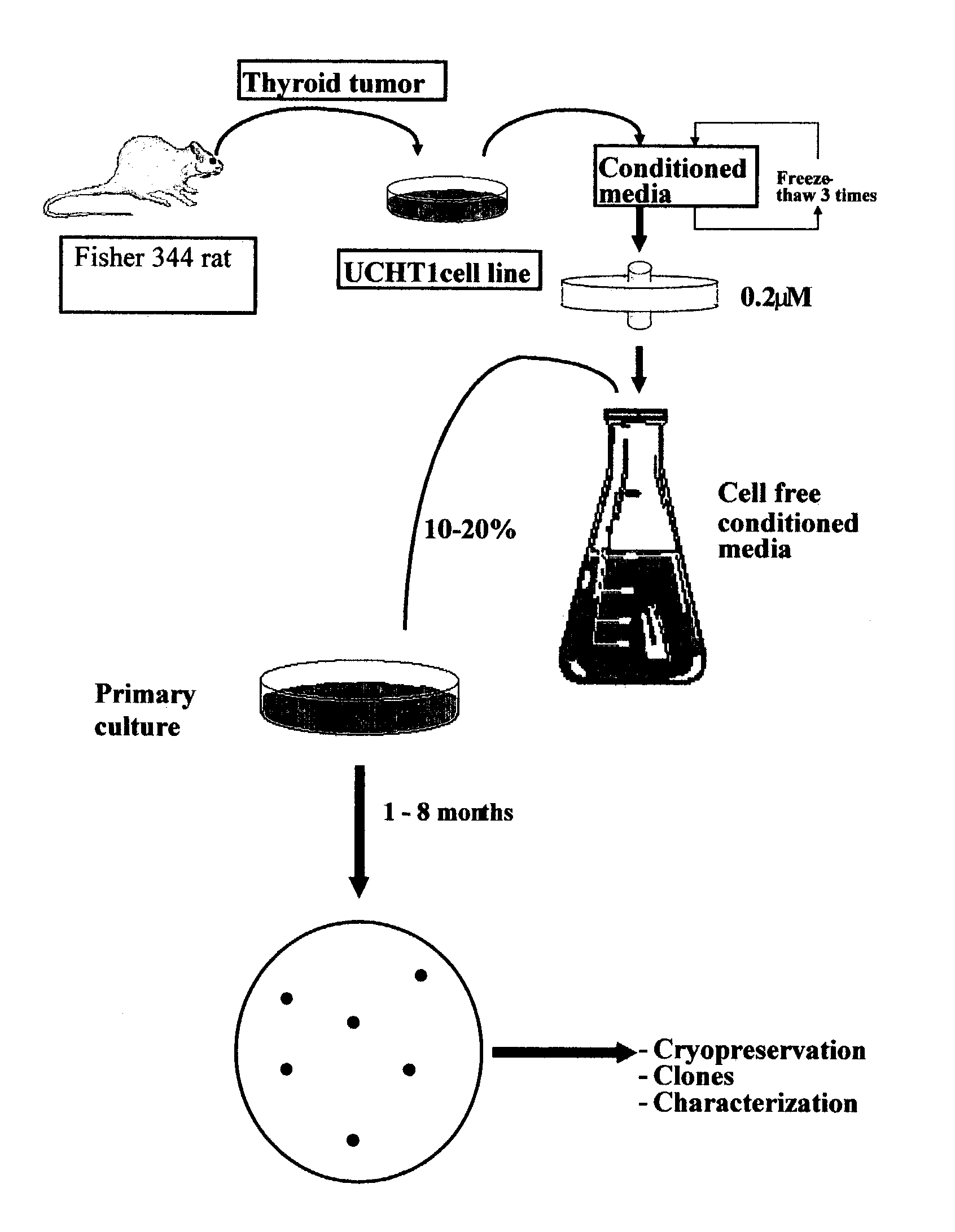
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
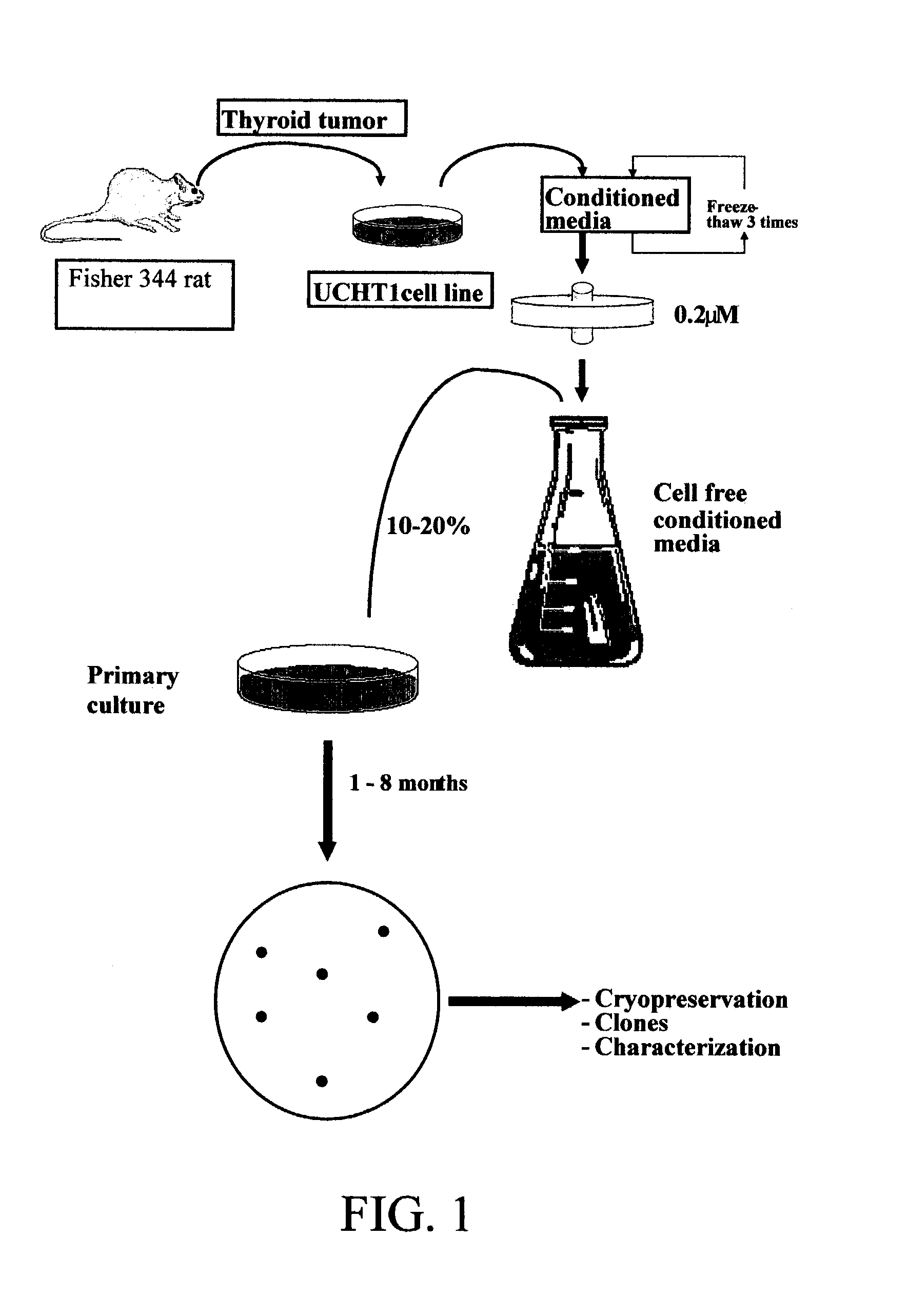
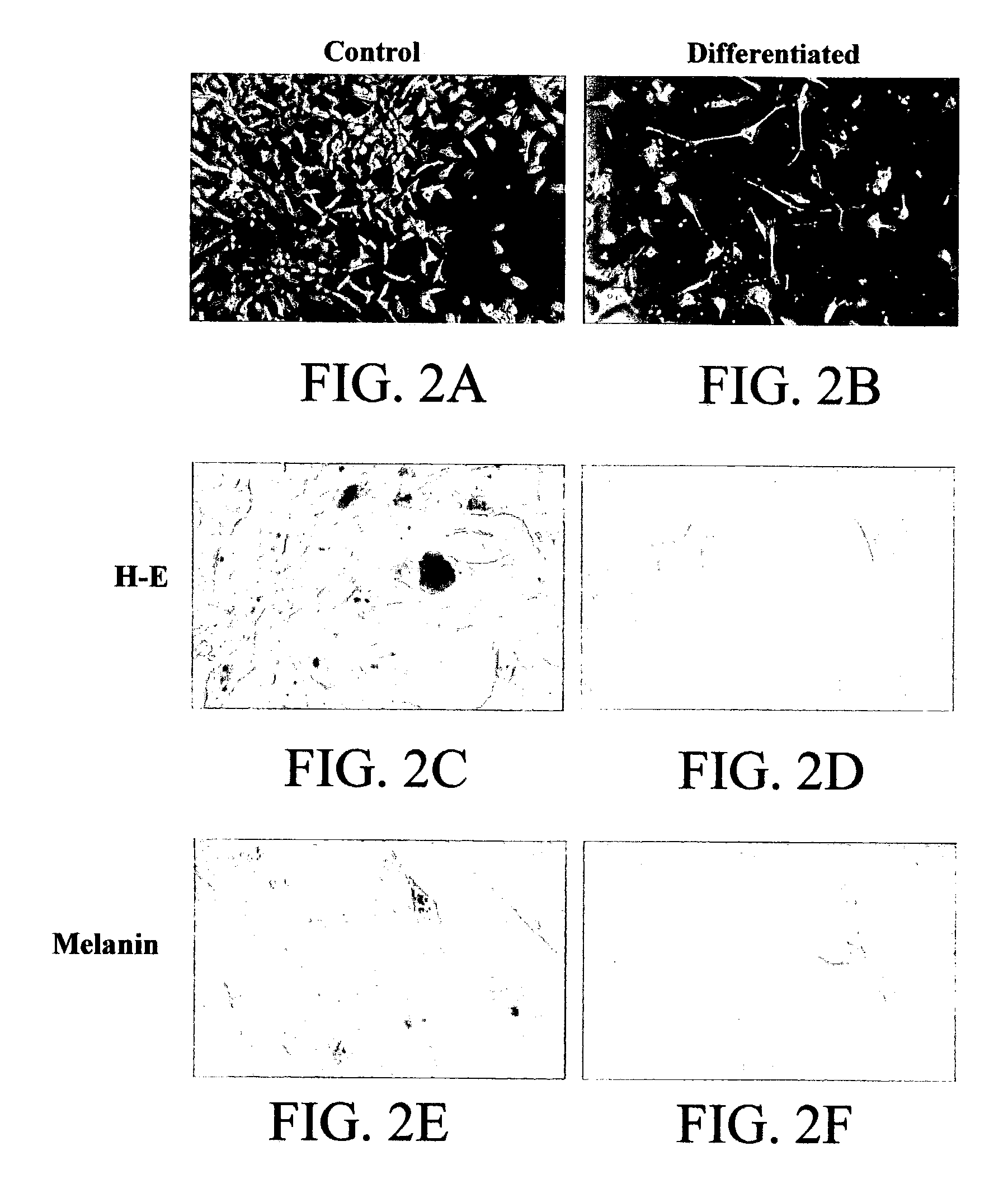
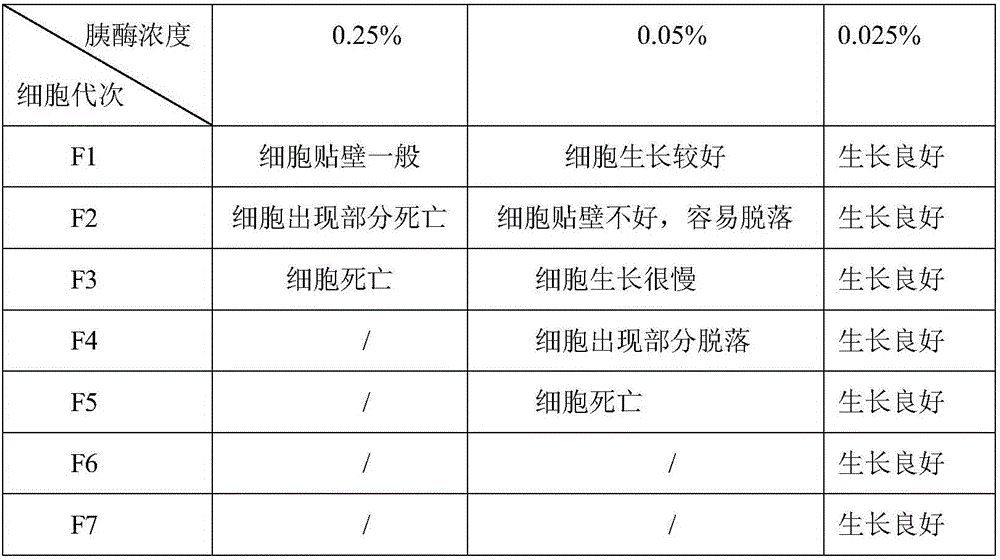
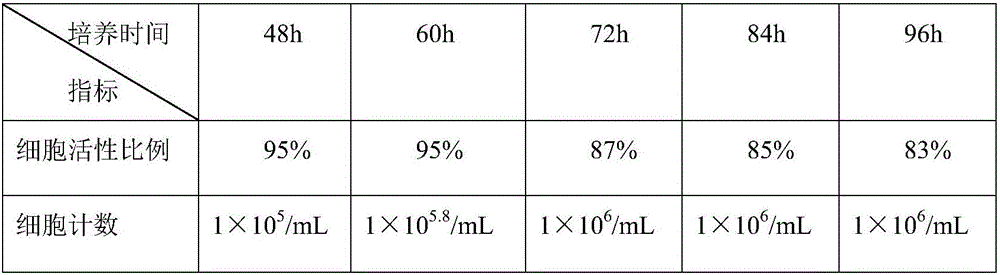
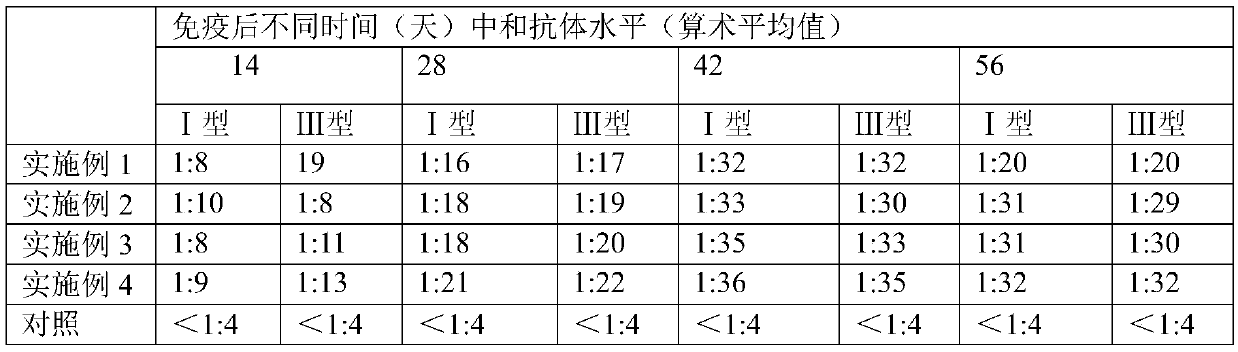
49 results about "Continuous cell line" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
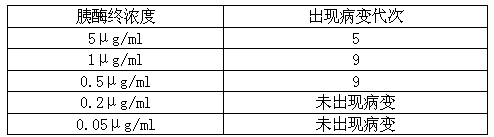
However, some cell lines become immortal through a process called transformation, which can occur spontaneously or can be chemically or virally induced. When a finite cell line undergoes transformation and acquires the ability to divide indefinitely, it becomes a continuous cell line.
Altered strain of the modified vaccinia virus ankara (mva)
InactiveUS20030013190A1Ease of mass productionEfficient and large-scale productionBiocideGenetic material ingredientsAdjuvantVirulent characteristics
The invention provides new strains of the Modified Vaccinia Virus Ankara (MVA) that have a strongly reduced virulence for most mammals, especially humans, but nevertheless grows in cells of a continuous cell line approved for the production of a therapeutic agent such as a vaccine. The invention also provides a method for producing said adapted MVA strains. The adapted MVA can be used e.g. for parenteral immunization, as a vector system, or in the active or inactivated from as an adjuvant or as a regulator of the unspecific components of the immune system.
Owner:BAVARIAN NORDIC AS
Altered strain of the modified vaccinia virus ankara (MVA)
InactiveUS6682743B2Ease of mass productionEfficient and large-scale productionBiocideGenetic material ingredientsVector systemVirulent characteristics
The invention provides new strains of the Modified Vaccinia Virus Ankara (MVA) that have a strongly reduced virulence for most mammals, especially humans, but nevertheless grows in cells of a continuous cell line approved for the production of a therapeutic agent such as a vaccine. The invention also provides a method for producing said adapted MVA strains. The adapted MVA can be used e.g. for parenteral immunization, as a vector system, or in the active or inactivated from as an adjuvant or as a regulator of the unspecific components of the immune system.
Owner:BAVARIAN NORDIC AS
Avian influenza virus inactivated vaccine and preparation method thereof
InactiveCN102600464AAchieve mass productionIncrease volumeAnimal cellsAntiviralsAdjuvantVaccine Production
The invention discloses an avian influenza virus inactivated vaccine and a preparation method thereof. Adaptive avian influenza viruses are adaptive to screening and domestication of a continuous cell line, and after the viruses are adaptive to cells, the number of the viruses is continuously increased until the viruses are inoculated into a cell culture bottle or a bioreactor to culture. After the obtained culture is inactivated, the obtained culture and mineral oil adjuvant are mixed and emulsified so as to prepare the vaccine. A virus reproduction method for producing an avian vaccine by means of utilizing a large quantity of chicken embryo is omitted, resource consumption is reduced, environmental pollution is decreased, biosafety is guaranteed, cost is lowered, and production efficiency is improved. By the aid of the preparation method, high-potency viruses can be produced to prepare corresponding vaccines, and requirements of fast and high-efficient vaccine production are met.
Owner:SINOPHARM YANGZHOU VAC BIOLOGICAL ENG CO LTD +1
Proliferated cell lines and uses thereof
ActiveUS20080175828A1Increase proliferation potentialEnhance proliferation potentialSenses disorderPeptide/protein ingredientsProgenitorCell therapy
The subject invention pertains to tumor cell lines useful for increasing the proliferation potential of any human or animal cell in culture, thereby providing immortalized or continuous cell lines and cultures. The invention also concerns proliferation factors, and compositions containing the factors, which are capable of increasing the proliferation potential of any human or other animal cell in culture. The subject invention further pertains to a method for proliferating cells in culture by containing cells with the proliferation factors. The proliferated cells can range in plasticity and can include, for example, blast cells, fertilized ova, non-fertilized gametes, embryonic stem cells, adult stem cells, precursor or progenitor cells, and highly specialized cells. Optionally, the cells can be induced to cease proliferation. The proliferated cells of the subject invention are useful for cell therapy, cell / gene therapy, biological production of molecules, and as in vitro models for research, toxicity testing, and drug development.
Owner:UNIV OF SOUTH FLORIDA +1
Proliferated cell lines and uses thereof
ActiveUS7416885B2Increase proliferation potentialEnhance proliferation potentialSenses disorderAntipyreticProgenitorDrug development
The subject invention pertains to tumor cell lines useful for increasing the proliferation potential of any human or animal cell in culture, thereby providing immortalized or continuous cell lines and cultures. The invention also concerns proliferation factors, and compositions containing the factors, which are capable of increasing the proliferation potential of any human or other animal cell in culture. The subject invention further pertains to a method for proliferation cells in culture by contacting cells with the proliferation factors. The proliferated cells can range in plasticity and can include, for example, blast cells, fertilized ova, non-fertilized gametes, embryonic stem cells, adult stem cells, precursor or progenitor cells, and highly specialized cells. Optionally, the cells can be induced to cease proliferation. The proliferation cells of the subject invention are useful for cell therapy, cell / gene therapy, biological production of molecules, and as in vitro models for research, toxicity testing, and drug development.
Owner:UNIV OF SOUTH FLORIDA +1
Method for producing avian adenovirus inactivated vaccine through LMH clone line
InactiveCN106798918AGood immune effectReduce manufacturing costViral antigen ingredientsInactivation/attenuationAntigenEmulsion
The invention relates to a method for producing avian adenovirus inactivated vaccine through an LMH clone line. The method comprises the following steps: firstly, preparation continuous cell line LMH cell; secondly, virus-seed reproduction; thirdly, virus collection, concentration and purification; fourthly, virus content determination; fifthly, inactivation and emulsification of virus, and forming emulsion inactivated vaccine. Compared with the traditional technical method for producing avian adenovirus through cell culture, the method has the advantages that high-quality inactivated vaccine with higher antigen titer through the optimization of the concentration of digestive LMH cell pancreatin-EDTA, the culture time of LMH cell and the inoculation concentration and harvest time of virus, injection immunization is performed at different doses, and all the vaccine protection rates reach 100 percent, so that the immune efficiency of the avian adenovirus type IV inactivated vaccine produced through the method is high, and the vaccine has complete immune protection effect on avian adenovirus type IV.
Owner:广州博恒生物科技有限公司
Application of chicken liver cancer cell system as duck plague virus host
ActiveCN105420198APromote growthMicroorganism based processesDsDNA virusesCancer cellCell culture media
The invention belongs to the technical field of duck plague virus culturing, particularly relates to a new host cell of the duck plague virus, and discloses application of a chicken liver cancer cell system as a duck plague virus host. A traditional method for culturing the duck plague virus through the duck embryo fibroblast primary cell is limited by duck embryo supply and the hatching day age, and wastes time and labor during preparation. The established method for culturing the duck plague virus chicken liver cancer cell system is good in virus growth. The method for culturing the virus through a continuous cell line is not influenced by duck embryo supply, and has the advantages of being instantly available and capable of saving time and labor. The duck plague virus can grow and reproduce on a chicken liver cancer cell system continuous cell culture medium, and by means of a newly-found virus copying host cell, a new technical approach, method and material are provided for research of related fields.
Owner:JINLING INST OF TECH
Method for producing avian encephalomyelitis virus inactivated vaccine
InactiveCN104689311AImprove adaptabilityHigh potencyAntiviralsViruses/bacteriophagesImmunogenicityInactivated vaccine
The invention discloses a method for producing an avian encephalomyelitis virus inactivated vaccine. The method comprises the following steps: a. selecting cell lines to serve as cells for preparing the vaccine; b. passing and culturing the cells for preparing the vaccine; c. breeding a virus seed for production; d. producing venom for preparing the vaccine; and e. preparing the vaccine. According to the method disclosed by the invention, continuous cell lines are used as the cells for preparing the vaccine, and sensitive cell lines are screened to reinforce the match of the virus and the cells. The method disclosed by the invention has the advantages of simple and stable production process, easiness in operation, small batch difference and low cost, and the safety and immunogenicity of the produced avian encephalomyelitis virus inactivated vaccine are good.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
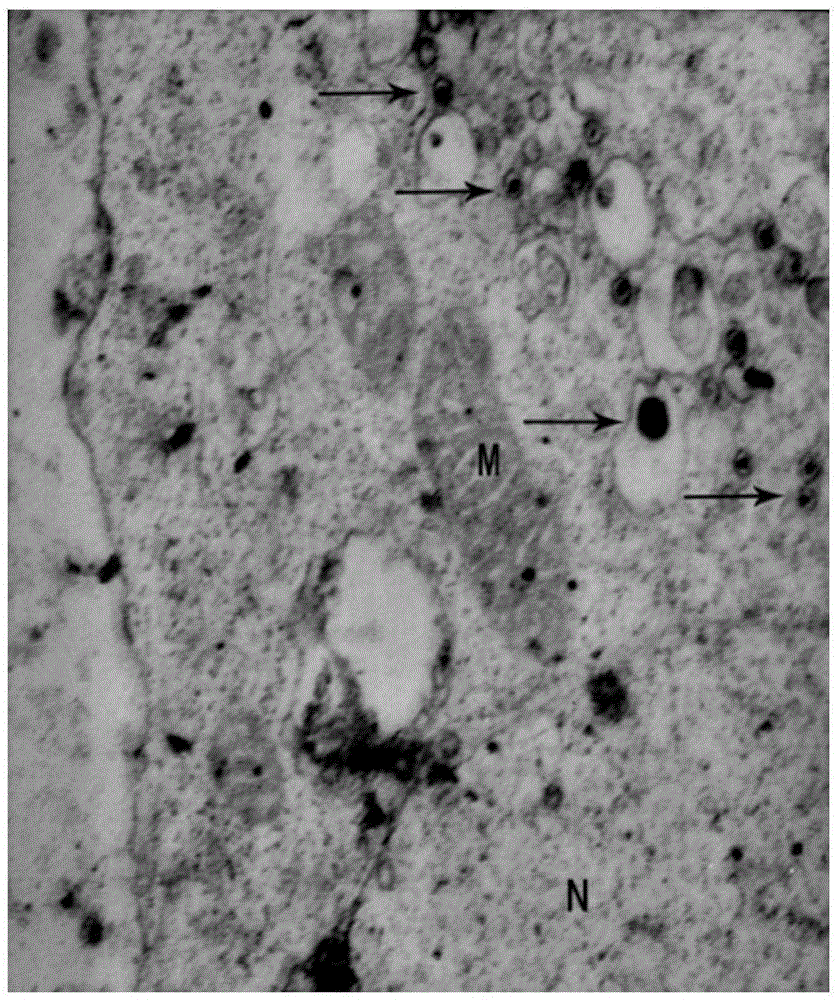
Production of Homogeneous Cell Line Highly Permissive To Porcine Circovirus Type 2 (PCV2) Infection
Continuous cell lines that are highly permissive to infection by porcine circovirus type 2 (“PCV2”) are described. PCV2 is the causal agent of post-weaning multi-systemic wasting syndrome (“PMWS”) in pigs. PMWS has emerged as a major disease that poses a significant threat to the economics of global swine industry. The highly permissive cell lines of this invention provide efficient and reliable sources of PCV2 for use in development of vaccines, therapies and diagnostic agents for PMWS.
Owner:TEMASEK LIFE SCIENCES LABORATORY
Preparation method and product of swine fever live vaccine
ActiveCN102294029ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsImmunofluorescenceVirus multiplication
The invention discloses a preparation method and product of a swine fever live vaccine. The preparation method comprises the following steps: (1) culturing a swine-derived continuous cell line; (2) inoculating the swine-derived continuous cell line into a seed virus for producing the swine fever live vaccine, thereby obtaining a swine fever weak-virus vaccine strain; (3) carrying out virus multiplication on the swine fever weak-virus vaccine strain; (4) determining the virus value of the multiplied virus suspension by an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the qualified virus suspension to carry out vaccine preparation and freeze-drying. Since the swine fever live vaccine is prepared from the cell line, the difference of quality among different batches is small, and the invention has the characteristics of simple and stable technique, high yield, low cost and the like, is easy to operate, and has feasibility and amplification of industrialized mass production. In addition, the immunofluorescence method, which has the advantages of high detection sensitivity, high speed, high specificity, high accuracy, high repetitiveness and reliable result, is used for determining the virus value of the multiplied virus suspension. The swine fever live vaccine disclosed by the invention can 100% protect the attack of swine fever strong virus.
Owner:华威特(江苏)生物制药有限公司
Preparation method of avian influenza virus and fowl adenovirus combined inactivated vaccine
ActiveCN107050448APromote proliferationImprove vaccine effectivenessSsRNA viruses negative-senseViral antigen ingredientsBiotinAvian adenovirus
The invention belongs to the technical field of veterinary biological products and particularly relates to a preparation method of avian influenza virus subtype H9 and fowl adenovirus 4 combined inactivated vaccine. LHM (Leghorn male hepatocarcinoma) continuous cell line is used as carrier cells to perform viral multiplication, culture medium A composed of glutamine, recombinant human insulin, human serum albumin, transferrin, biotin and growth factors is used to perform culturing, and a viral liquid is collected and inactivated to prepare the vaccine. The multiplication of avian influenza virus subtype H9 and fowl adenovirus 4 by the LHM continuous cell line has no need for additional pancreatin, the background of LHM cells is clear, no extraneous pathogens occur, the multiplication is easy, the process can be effectively simplified, and the cost can be reduced; in addition, the avian influenza virus and fowl adenovirus combined inactivated vaccine has high viral content, good stability and high safety and is an ideal avian influenza virus and fowl adenovirus combined inactivated vaccine.
Owner:广东渔跃生物技术有限公司 +2
Production method of pseudorabies virus vaccine
ActiveCN103550772AWill not be subject to supplyReduce manufacturing costInactivation/attenuationMicroorganism based processesInfected cellFreeze and thaw
The invention discloses a production method of a pseudorabies virus vaccine. The production method comprises the following steps: (1) adopting a cell of a continuous cell line, subjecting the cell to digestive passage, and continuously culturing the cell in a cell culture flask by using a cell growth medium; (2) diluting a virus seed into 10 times by using a cell maintenance medium, and inoculating a cell monolayer to obtain an infected cell virus liquid, that is, a virus seed for production; (3) preparing the cell monolayer formed in the step (1) into a cell suspension through digestion, inoculating the cell suspension into a bioreactor, and adding a microcarrier into the bioreactor; (4) performing virus-introduction operation on the cell, wherein the introduced virus seed is the virus seed produced in the step (2); and (5) harvesting liquid in the reactor together with the microcarrier until all of the cells on the microcarrier fall off and a dissolved oxygen value increases obviously, placing the liquid and the microcarrier under conditions of minus 20 DEG C, repeatedly freezing and thawing the liquid and the microcarrier twice, and removing the microcarrier and cell fragments to prepare the pseudorabies virus vaccine. The production method of the pseudorabies virus vaccine is short in production cycle and large in yield.
Owner:成都史纪生物制药有限公司
Bovine viral diarrhoea virus inactivated vaccine and preparation method thereof
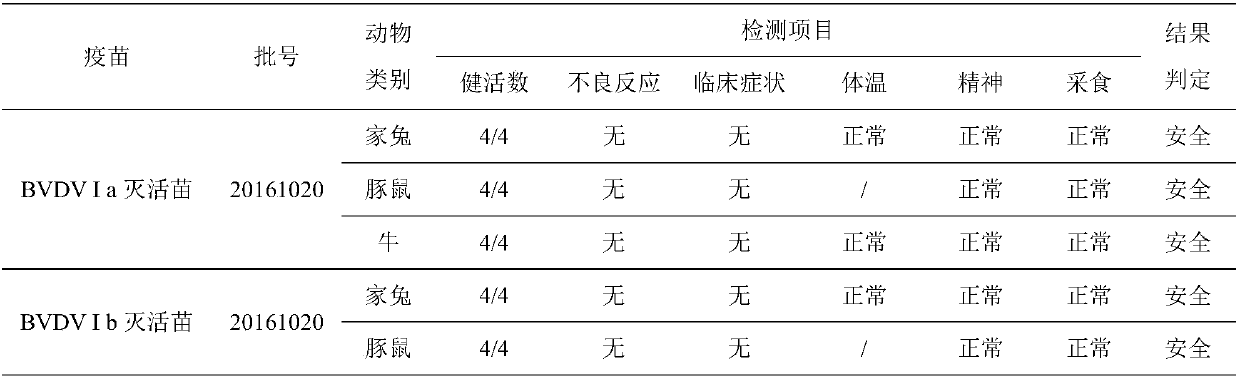
The invention relates to an inactivated vaccine of a bovine viral diarrhoea virus (BVDV) and a preparation method of the inactivated vaccine, and belongs to the field of veterinary biological products. The vaccine is composed of four gene subtypes including BVDV I a, BVDV I b, BVDV II a and BVDV II b. The bovine viral diarrhoea inactivated vaccine is prepared by carrying out virus proliferation byusing a continuous cell line cultured by cell spinner bottles, full suspension and micro carriers, and optimizing a vaccine preparation process and the like. Safety and effectiveness testing resultsof the vaccine show that bovines are free of local and systemic adverse reactions after the bovine is immunized by the bovine viral diarrhoea virus inactivated vaccine; all the bovines receive immuneprotection; the vaccine is safe and reliable, and is capable of preventing diseases caused by different BVDV gene subtypes. The vaccine also comprises various types of monovalent inactivated vaccines,bivalent inactivated vaccines and tetravalent inactivated vaccines prepared from the four gene subtypes above.
Owner:QILU ANIMAL HEALTH PROD
Method for suspension culture of infectious bronchitis virus by continuous cell line
ActiveCN108300704AHigh titerImproving immunogenicitySsRNA viruses positive-senseRecovery/purificationInfectious laryngotracheitis virusInfectious bronchitis virus
The invention provides a method for suspension culture of an infectious bronchitis virus by a continuous cell line. The method comprises the following steps that 1, EB66 cells are taken for recovery and secondary culture; 2, the EB66 cells obtained in the step 1 are subjected to infectious bronchitis virus inoculation culture; 3, the EB66 cells after the virus inoculation are sampled every 6 to 12h; virus EID50 is determined; when the virus EID50 reaches the highest value, the virus is harvested and stored; the cultured infectious bronchitis virus is obtained. The EB66 cells are used as a culture medium of the infectious bronchitis virus; the EB66 cells providing a full suspension continuous cell line is used for performing efficient virus production; the culture titer of the infectious bronchitis virus is effectively improved, so that the large-scale culture of the infectious bronchitis virus vaccine is realized; the virus culture cost is reduced.
Owner:ZHAOQING INST OF BIOTECHNOLOGY CO LTD +3
Pelodiscus sinensis heart cell continuous cell line and establishing method and ultra-low-temperature cryopreservation method thereof
ActiveCN105039241AMaintain biological propertiesMeet resourcesDead animal preservationArtificial cell constructsHeart cellsGermplasm
The invention relates to a pelodiscus sinensis heart cell continuous cell line which is preserved in the common microorganism center of China Committee for Culture Collection of Microorganisms and has a preservation number of CGMCC No.10597, and further relates to an establishing method and ultra-low-temperature cryopreservation method of the pelodiscus sinensis heart cell continuous cell line. The establishing method includes the following steps of firstly, conducting primary culture, wherein a pelodiscus sinensis heart is cut into tissue small pieces after being preprocessed, trypsin is inoculated into a culture bottle after being digested at the room temperature, and subculture is not started until cells are fully laid on 90% or more of the bottle bottom; secondly, conducting subculture, wherein air is blown on the bottle bottom through a trypsin digesting method to make adherent cells fall down so that subculture can be conducted. By means of the established pelodiscus sinensis heart cell continuous cell line, the research on viral diseases of pelodiscus sinensis and other turtle animals and the research on related therapy drugs for instance the analysis of chromosome and the research of iridescent virus can be conducted on the level of molecular cells, and the requirements for pelodiscus sinensis germplasm resource conservation and theoretical research and application can be met.
Owner:ZHEJIANG WANLI UNIV
Duck reovirus vaccine and preparation method thereof
ActiveCN109718370AClear cell backgroundClear backgroundViral antigen ingredientsAntiviralsAntigenPrimary cell
The invention discloses a duck reovirus vaccine. The inactivated vaccine is obtained by inoculating duck reovirus virus seed on continuous cell line BHK-21 cells. A Reed-Muench method is used to determine that the virus titer can reach 107.0-107.5 TCID50 / 0.1mL. The stable production and high titer of antigen are the most important factors in the preparation of vaccines. The duck reovirus proliferation is carried out by using BHK-21 cells, so that the virus titer is 10 times or above as high as that of a conventional duck embryo method and a primary cell method. If the virus proliferation is carried out by using a bioreactor, the virus titer can reach 100-time level. The duck reovirus inactivated vaccine disclosed by the invention has the advantages of high yield, stable quality, good immune effect and huge application prospect, and immune ducks can generate a high-level serum neutralizing antibody.
Owner:广东渔跃生物技术有限公司
Method for culturing gosling plague virus by use of goose embryo continuous cell line and bioreactor
ActiveCN103320393AAvoid influenceEliminate potential safety hazardsMicroorganism based processesViruses/bacteriophagesSmall footprintEmbryo
The invention discloses a method for culturing gosling plague virus by use of a goose embryo continuous cell line and a bioreactor, belonging to the technical field of veterinary biological products. The method comprises the following steps of: 1) culture of master cells; 2) suspension culture of cells for virus reproduction; and 3) culture and harvesting of virus. The method disclosed by the invention can shorten the production period and reduce personnel allocation, and realizes low pollution probability and small floor area; and the obtained virus has high titer and small inter-batch difference.
Owner:浙江美保龙生物技术有限公司
Duck astrovirus vaccine and preparation method thereof
ActiveCN109692329AClear cell backgroundClear backgroundSsRNA viruses positive-senseViral antigen ingredientsAntigenImmune effects
The present invention discloses a duck astrovirus vaccine which is an inactivated vaccine obtained by inoculating duck astrovirus type I and type III virus seeds on a continuous cell line BHK-21 cell.The duck astrovirus type I and type III are propagated by the BHK-21 passage cell, and the virus titer is stable to 106.5-107.0TCID50 / 0.1mL by a Reed-Muench method. The stable production and high titer of antigen are the most important factors in the preparation of the vaccine, the virus titer of duck astrovirus type I and type III proliferation with the BHK-21 cell is more than 10 times that ofa conventional duck embryo method and a primary cell method. If a bioreactor is used for virus proliferation, the virus titer can reach 100 times. The duck astrovirus (type I and III) inactivated vaccine disclosed in the invention has high yield and stable quality, and the immune duck can produce a high-level serum neutralizing antibody, the immune effect is good, and the application prospect is great.
Owner:广东渔跃生物技术有限公司
Continuous cell line for the production of vaccines
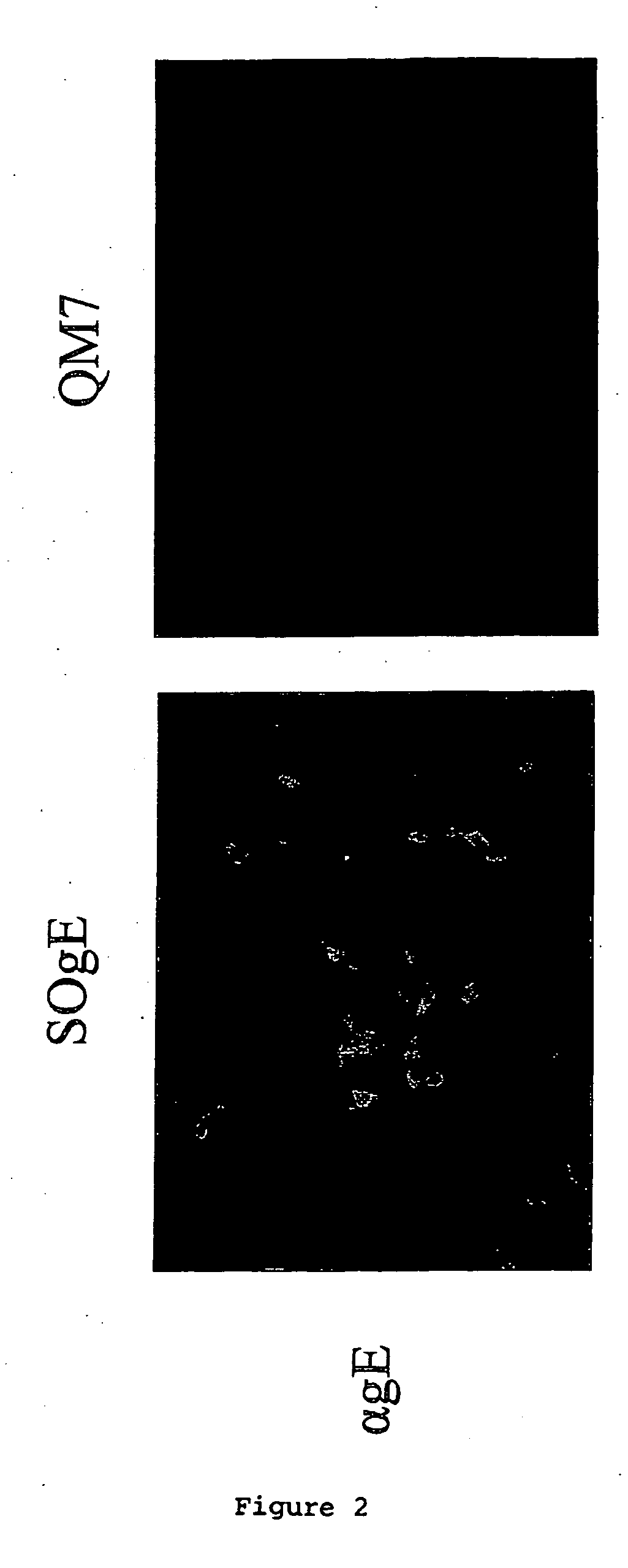
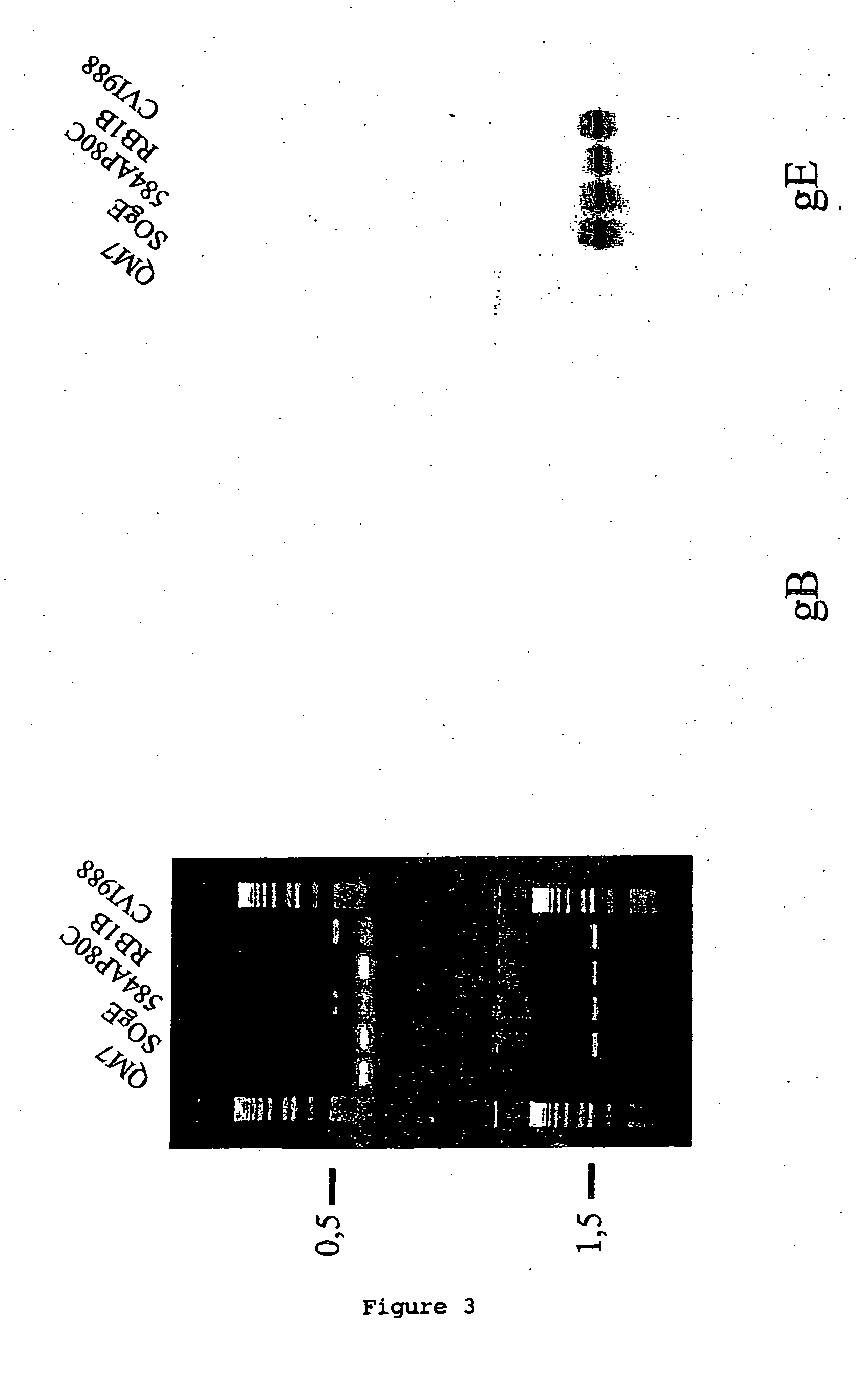
Provided is a method for producing a continuous cell line capable of supporting the growth of a Marek's disease virus (MDV) strain comprising infecting or transfecting a cell with a vector comprising a nucleic acid or fragment thereof of an MDV strain and culturing the cell or a progeny thereof under conditions suitable for the expression of the nucleic acid and propagation of the cell or progeny. The nucleic acid may comprise an MDV glycoprotein gE gene or functional fragment. Also provided is a method of generating, isolating, and / or maintaining a hypervirulent, very virulent, very virulent plus, virulent, and / or avirulent MDV strain comprising infecting a described cell with the MDV strain or strains, and culturing the cell under conditions suitable for the propagation of the cell and the generation, maintenance and isolation of the MDV strain or strains. Also provided is a method to prepare a vaccine capable of inducing protection against disease, preferably an MDV associated disease in an avian, including culturing a continuous cell according to the invention and harvesting cell culture components therefrom.
Owner:OSTERRIEDER NIKOLAUS +1
Medicine composition for curing tumor
The present invention relates to a medicine composition for curing tumor. Its composition includes (wt%) 2-30% of genistein, 0-4% of soybean aglycone, 0-1% of biochain and 65-98% of auxiliary material. The composition can be used as new medicine preparation cor curing tumor, and can be made into soft capsule, hard capsule, dripping pill and injection. The medicine composition has obvious action for inhibiting continuous cell line of mammary cancer, carcinoma of stomach and liver cancer, and can inhibit tumor metastasis, and it is safe and has no toxic side effect.
Owner:SHANDONG SHENGBANG MEDICINE SCI & TECH
Hybridoma cell lines and monoclonal antibodies recognizing Prox1
Monoclonal antibodies to Prox1 of vertebrates (pfam05044.5; NM_002763.3) and two continuous cell lines for their production are disclosed. These antibodies are particularly useful in immunoassays to detect the presence of Prox1 protein in vertebrate tissues and cells.
Owner:UNIVERSITY OF DELAWARE
Method for culturing chlamys farreri trochophore cell line
The invention discloses a method for culturing a chlamys farreri trochophore cell line. A small amount of nutrient factors for keeping cell metabolism are added in a special cell culture medium for chlamys farreri trochophore, so that the good growth state of chlamys farreri trochophore larva in vitro culture cells is realized, and the stable subcultring of the cells can be realized along with cell division. The method is simple to operate and low in cost; the complete dissociation of the cells is realized by use of the combination of soaking in a special calcium-free sea-water iso-osmia buffer solution for the chlamys farreri trochophore and a mechanical method, without any digestion by enzymes; the method is little in damage of the chlamys farreri trochophore larva in vitro culture cells, low in cost, high in efficiency and repeatable; a stable marine invertebrate cell in vitro culture method is established and can realize the in vitro multiplication and the subcultring of the cell, the chlamys farreri trochophore cell line is the first continuous cell line of marine shells, and the method has an important guiding significance for cell line establishment of other shells except the chlamys farreri.
Owner:OCEAN UNIV OF CHINA
Method for preparing vaccine by Newcastle disease virus cultured by using chick embryo continuous cell line and bioreactor
ActiveCN103386127AAvoid influenceAvoid the adverse effects of greater stressViral antigen ingredientsMicroorganism based processesNewcastle disease virus NDVEmbryo
The invention provides a method for preparing a vaccine by Newcastle disease virus cultured by using a chick embryo continuous cell line and a bioreactor, and belongs to the technical field of animal biological products. The method comprises the following steps: 1) performing passage and culture on a master cell; 2) multiplying a cell-adapted virus seed; 3) culturing the cell for multiplying virus in a suspending manner; 4) culturing and harvesting the virus; 5) preparing a chick Newcastle disease live vaccine; and 6) preparing the chick Newcastle disease live vaccine. The method adopted for preparing vaccines is simple and stable in production process, high in virus content, small in differences among batches, is easy to operate and control the quality, and can remarkably improve the yield and quality of vaccines; the produced chick Newcastle disease vaccines with high safety and high immune efficacy have a complete immune protection effect on the attack of virulent Newcastle disease virus.
Owner:浙江美保龙生物技术有限公司
Preparation method of trivalent inactivated vaccine
InactiveCN107308447AAchieve mass productionNo addedSsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusAvian adenovirus
The invention belongs to the technical field of veterinary biological products and particularly relates to a preparation method of a trivalent inactivated vaccine for avian influenza virus, aviadenovirus and avian reovirus. The trivalent inactivated vaccine for avian influenza virus subtype H9, an aviadenovirus type 4 and avian reovirus type 2 is produced by adopting a virus high-adaptability continuous cell line LMH cell. The produced trivalent inactivated vaccine for the avian influenza virus subtype H9, the aviadenovirus type 4 and the avian reovirus type 2 is used for immunizing SPF (Specific Pathogen Free) chickens, so that a high-level HI (Hemagglutination Inhibiting) antibody for resisting the avian influenza virus subtype H9 and a high-level neutralizing antibody for resisting the aviadenovirus type 4 and the avian reovirus type 2 can be generated. The trivalent inactivated vaccine provided by the invention has high titer and complete immune protecting effect on the avian influenza virus subtype H9, the aviadenovirus type 4 and the avian reovirus type 2.
Owner:广州博恒生物科技有限公司
Cell line of dengue virus type-2 replicor with dual-reporter gene and application
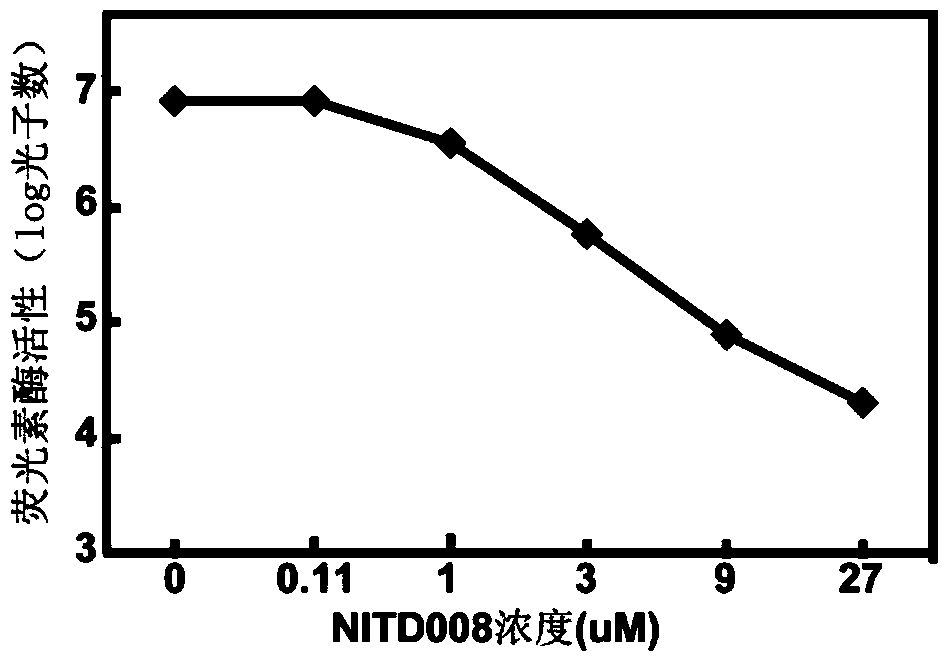
ActiveCN103805567AEfficient expressionCopy does not affectMicrobiological testing/measurementMicroorganism based processesForeign proteinMechanism of action
The invention discloses a cell line of dengue virus type-2 replicor with a dual-reporter gene and an application. By constructing a dengue virus type-2 replicor with the dual-reporter gene and transfecting cells, a cell line of dengue virus type-2 replicor with the dual-reporter gene, CCTCC NO:C2011124 is finally obtained. Experiments of indirect immunofluorescence, RT-PCR, detection of luciferase activity and medicine inhibition prove that, the BHK continuous cell line BHK-21 screened can stably express the virus body protein and foreign protein, realizes stable passage, and has wide application values in studies of the dengue virus drug screening as well as mechanism of action, vaccine and diagnostic reagent of anti-DENV virus drugs.
Owner:嘉兴实践医学科技有限公司
Bovine rotavirus, bovine coronavirus combined inactivated vaccine and preparation method thereof
ActiveCN110124027APrevent diarrheaSeedling process optimizationSsRNA viruses positive-senseViral antigen ingredientsMaternal antibodyBovine rotavirus
The invention relates to a bovine rotavirus, a bovine coronavirus combined inactivated vaccine and a preparation method thereof. The active ingredient of the vaccine of the present invention comprisesinactivated antigen of bovine rotavirus and bovine coronavirus, and the antigenic component of the combined inactivated vaccine can be subjected to virus propagation through a continuous cell line (aiming at bovine rotavirus and bovine coronavirus) or microcarrier suspension culture (aiming at bovine rotavirus), and a seedling process and the like can be optimized. The vaccine safety, effectiveness test and antibody tracking results show that no adverse reaction is generated after immunization of cattle with the combined inactivated vaccine of the present invention, and no abortion is inducedafter immunization of pregnant cattle. Immunization of cattle produces immune protection, and newborn calves can obtain high levels of maternal antibodies through breast milk. The results show that the vaccine of the invention is safe and reliable and can be used for preventing the generation of yak diarrhea.
Owner:QILU ANIMAL HEALTH PROD
Viral latency model
InactiveUS20100216116A1Improve latencyIncrease percentagePeptide/protein ingredientsMicrobiological testing/measurementViral latencyAnti virals
The invention relates generally to the field of virology. More particularly, the present invention relates to in vitro models for viral latency. In particular to latently infected cultures of primary and continuous cell lines, and to the use thereof in methods to identify anti-viral compounds. More in particular to identify compounds which are either able to modulate the induction of viral latency in the aforementioned cell cultures, or which are able to retain the viruses in the aforementioned cells in their latent form. Other aspects of the invention are directed to antiviral compounds identified using the models and methods of the present invention, as well as to the use thereof in treating latent infections such as for example latent Herpes Simplex Virus (HSV) infections.
Owner:UNIV GENT
Hybridoma cell lines and monoclonal antibodies recognizing Prox1
Monoclonal antibodies to Prox1 of vertebrates (pfam05044.5; NM_002763.3) and two continuous cell lines for their production are disclosed. These antibodies are particularly useful in immunoassays to detect the presence of Prox1 protein in vertebrate tissues and cells.
Owner:UNIVERSITY OF DELAWARE
Method for culturing gosling plague virus by use of goose embryo continuous cell line and bioreactor
ActiveCN103320393BEliminate potential safety hazardsUniform purity and qualityMicroorganism based processesViruses/bacteriophagesSmall footprintEmbryo
Owner:浙江美保龙生物技术有限公司
Method for culturing chlamys farreri trochophore cell line
The invention discloses a method for culturing a chlamys farreri trochophore cell line. A small amount of nutrient factors for keeping cell metabolism are added in a special cell culture medium for chlamys farreri trochophore, so that the good growth state of chlamys farreri trochophore larva in vitro culture cells is realized, and the stable subcultring of the cells can be realized along with cell division. The method is simple to operate and low in cost; the complete dissociation of the cells is realized by use of the combination of soaking in a special calcium-free sea-water iso-osmia buffer solution for the chlamys farreri trochophore and a mechanical method, without any digestion by enzymes; the method is little in damage of the chlamys farreri trochophore larva in vitro culture cells, low in cost, high in efficiency and repeatable; a stable marine invertebrate cell in vitro culture method is established and can realize the in vitro multiplication and the subcultring of the cell, the chlamys farreri trochophore cell line is the first continuous cell line of marine shells, and the method has an important guiding significance for cell line establishment of other shells except the chlamys farreri.
Owner:OCEAN UNIV OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com