Preparation method of trivalent inactivated vaccine
A technology of inactivated vaccines and virus seeds, which is applied in the field of veterinary biological products, can solve the problems of low virus titer and high production cost, and achieve the effect of ensuring added and complete immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
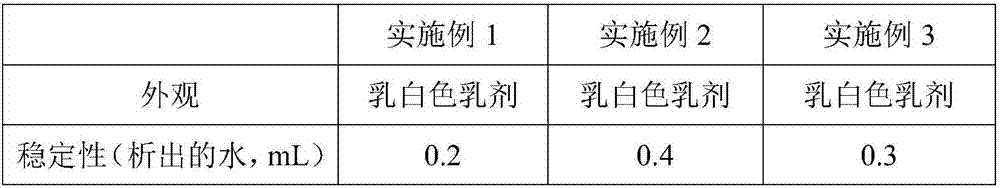
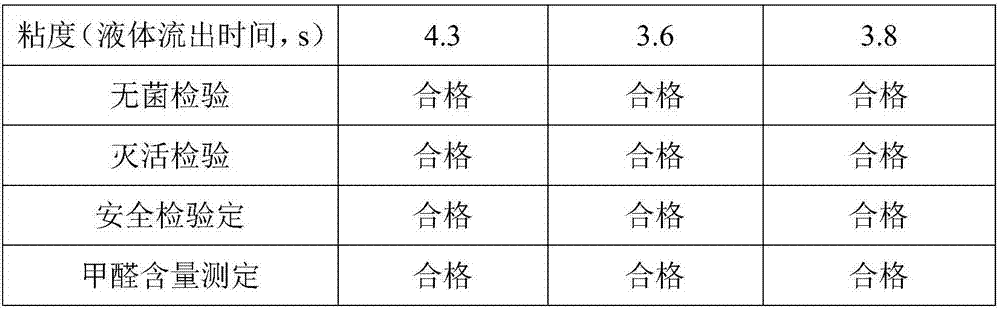
Embodiment 1
[0031] Embodiment 1 A kind of preparation method of avian influenza virus, avian adenovirus and avian reovirus triple inactivated vaccine
[0032] The preparation method of described avian influenza virus, avian adenovirus and avian reovirus triple inactivated vaccine comprises the following steps:
[0033] S1. Preparation of cells: culture LMH cells for seedling production with serum-free cell growth medium until the density of LMH cells reaches 1.2×10 6 cells / mL, subcultured in a microcarrier bioreactor, wherein the amount of microcarriers used was 8.5g / L; the serum-free cell growth solution was composed of the following components and their contents: DMEM basic culture solution 85% v / v, ornithine 0.5mmol / L, behenic acid 0.55g / L, myristic acid 0.4g / L, guanosine 1.0mg / L, dihydrotanshinone I 5.5mg / L, lysine 4g / L, glycine 0.85g / L, phenylisoleucine 2.5g / L, arginine 3g / L, threonine 1.5g / L, valine 2.5g / L, transferrin 7.5g / mL, mugwort leaf polysaccharide 0.12g / L, pyridoxine hyd...
Embodiment 2
[0040] Embodiment 2 A kind of preparation method of avian influenza virus, avian adenovirus and avian reovirus triple inactivated vaccine
[0041] The preparation method of described avian influenza virus, avian adenovirus and avian reovirus triple inactivated vaccine comprises the following steps:
[0042] S1. Preparation of cells: use serum-free cell growth medium to cultivate LMH cells for seedling production until the density of LMH cells reaches 1.5×10 6 cells / mL for subculture; the serum-free cell growth solution is composed of the following components and their contents: 90% v / v of DMEM basic culture solution, 0.6mmol / L of ornithine, and 0.75g / L of behenic acid , myristic acid 0.5g / L, guanosine 1.5mg / L, dihydrotanshinone I 7mg / L, lysine 5.5g / L, glycine 1.0g / L, phenylisoleucine 3.5g / L, arginine Acid 5.0g / L, threonine 3g / L, valine 3.5g / L, transferrin 10g / mL, mugwort leaf polysaccharide 0.15g / L, pyridoxine hydrochloride 6mg / L, biotin 1.5mg / L, Zinc sulfate 0.8mg / L, sodium...
Embodiment 3
[0049] Embodiment 3 A kind of preparation method of avian influenza virus, avian adenovirus and avian reovirus triple inactivated vaccine
[0050] The preparation method of described avian influenza virus, avian adenovirus and avian reovirus triple inactivated vaccine comprises the following steps:
[0051] S1. Preparation of cells: use serum-free cell growth medium to culture LMH cells for seedling production until the density of LMH cells reaches 1.0×10 6cells / mL for subculture; the serum-free cell growth solution is composed of the following components and their contents: 90% v / v of DMEM base culture solution, 0.3mmol / L of ornithine, and 0.15g / L of behenic acid , myristic acid 0.3g / L, guanosine 0.5mg / L, dihydrotanshinone I 3.5mg / L, lysine 1.5g / L, glycine 0.75g / L, phenylisoleucine 1g / L, arginine Acid 2.0g / L, threonine 1g / L, valine 2g / L, transferrin 5g / mL, mugwort polysaccharide 0.05g / L, pyridoxine hydrochloride 3mg / L, biotin 0.1mg / L, sulfuric acid Zinc 0.3mg / L, sodium bica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com