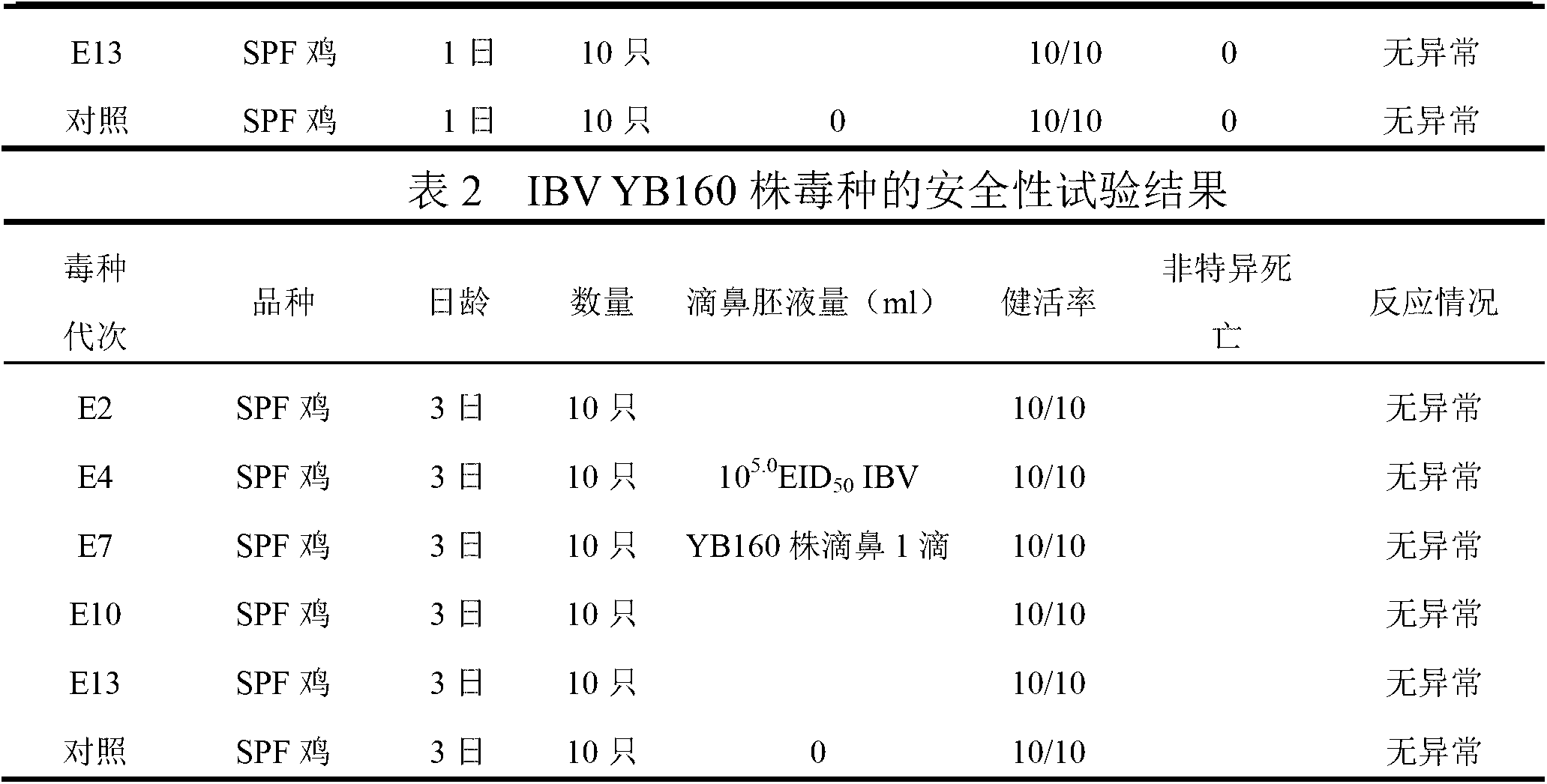
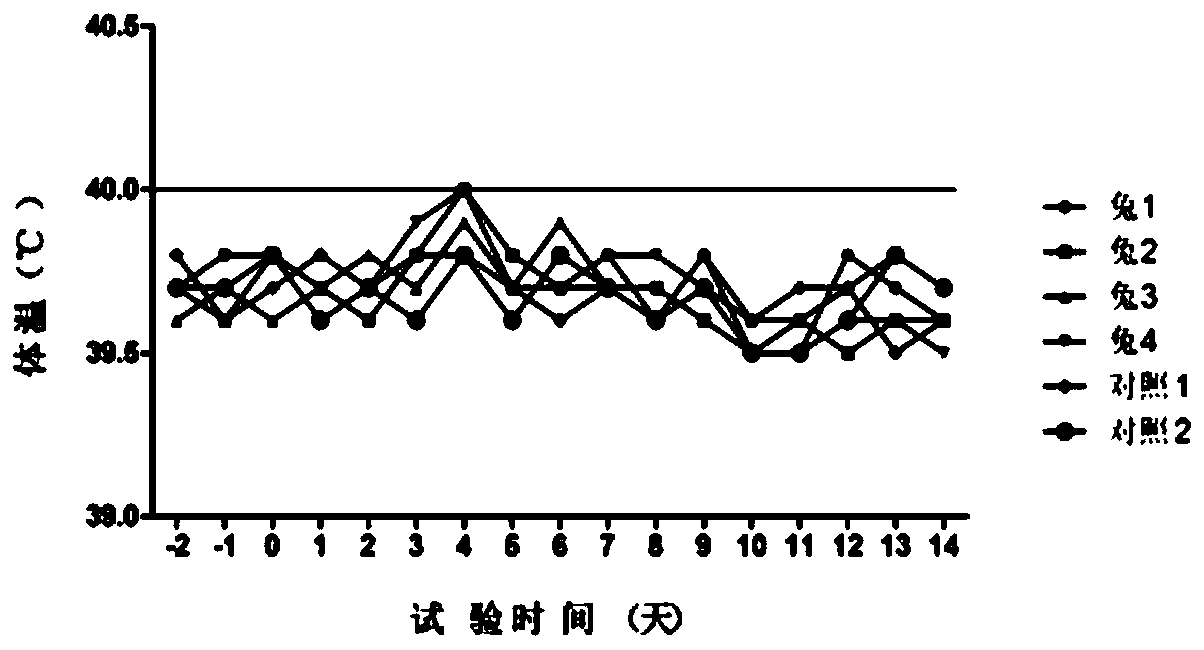
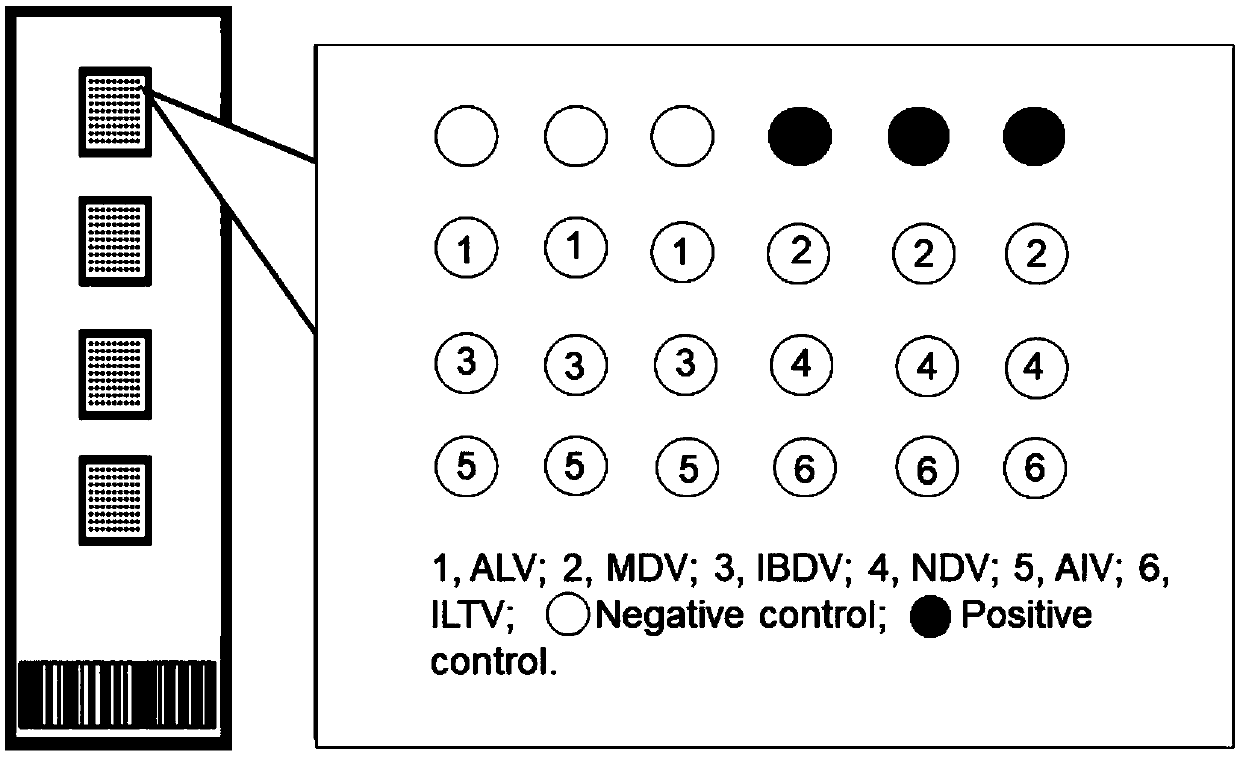
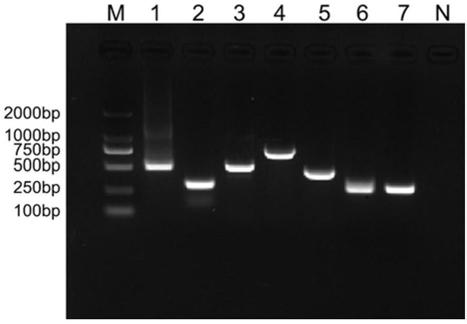
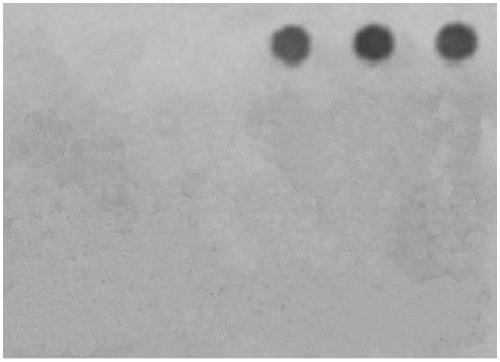
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Infectious laryngotracheitis virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The disease is caused by Gallid herpesvirus I, commonly known as infectious laryngotracheitis virus (ILTV). It has been reported from most areas of the USA in which poultry are intensively reared, as well as from many other countries.
Recombinant avian herpesvirus useful in vaccine production
InactiveUS6913751B2SsRNA viruses negative-senseSsRNA viruses positive-senseInfectious laryngotracheitisInfectious laryngotracheitis virus
The present invention provides a novel avian herpesvirus (NAHV) vector and recombinant vaccines made therefrom that are useful to immunize avian species against Marek's disease, infectious laryngotracheitis and Newcastle disease. Methods of immunizing an avian species against Marek's disease, infectious laryngotracheitis and Newcastle disease are also provided.
Owner:SCHERING PLOUGH ANIMAL HEALTH
GeXP (Gene Expression) rapid detection kit capable of simultaneously identifying six virus of chicken respiratory disease
ActiveCN102899423AStrong specificityImprove throughputMicrobiological testing/measurementMicroorganism based processesBovine respiratory diseaseInfectious bronchitis virus
The invention discloses a GeXP (Gene Expression) rapid detection kit capable of simultaneously identifying six virus of chicken respiratory disease. The GeXP rapid detection kit is used basing on a CeXP system and contains seven PCR (Polymerase Chain Reaction) primer pairs, the specificity for simultaneously detecting avian influence virus, H5, H7 and H9 sub type of the avian influence virus, Newcastle disease virus, infectious bronchitis virus and infectious laryngotracheitis virus is strong, the sensitivity can be up to 100 copy / mu l, and compared with an identifying result of regular test methods such as virus isolation and hemagglutination inhibition, the coincidence rate is up to 100%. According to the GeXP rapid detection kit disclosed by the invention, a simple and high-throughput detection kit and a detection system are provided for the detection of common main chicken viral respiratory disease, the actual needs are accordant, and the application prospect is wide.
Owner:GUANGXI VETERINARY RES INST
Triple egg yolk antibody against newcastle disease virus, avian influenza virus and infectious bronchitis virus
ActiveCN103342749ADecreased levels of maternal antibodiesInvulnerableEgg immunoglobulinsImmunoglobulins against virusesInfectious bronchitisInfectious laryngotracheitis virus
The invention discloses a triple egg yolk antibody against newcastle disease virus, avian influenza virus and infectious bronchitis virus and a preparation method and application thereof. The preparation method of the egg yolk antibody comprises the following steps of: (1) selecting a hen; (2) performing triple inactivation immunization of the hen obtained by the step (1) by use of newcastle disease virus, infectious bronchitis virus and avian influenza virus; (3) taking the egg laid by the hen and separating the egg yolk liquid; and (4) separating and purifying an egg yolk antibody from the egg yolk liquid obtained by the step (3). The triple egg yolk antibody disclosed by the invention is of great value in treating and preventing newcastle disease, avian influenza and infectious bronchitis.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Infectious bronchitis virus vaccine
ActiveUS7291342B2SsRNA viruses positive-senseViral antigen ingredientsFowlInfectious bronchitis virus
Embodiments of the present invention generally relate to a novel attenuated infectious bronchitis virus (IBV) of GA-98 isolate. Further, other embodiments of the present invention generally relate to methods of immunizing avian against an infectious bronchitis virus. As well, further embodiments relate to method of making a vaccine and / or immunogenic composition for protecting avian, such as poultry, from an infectious bronchitis virus of strain GA-98.
Owner:INTERVET INT BV
ILTV gD glycoprotein nucleotide sequence and amino acid sequence, recombined virus bacterin thereof and application of the bacterin
InactiveCN101210248AReduce economic lossEase of mass productionViral antigen ingredientsGenetic material ingredientsInfectious laryngotracheitisNucleotide
The invention provides a novel vaccine with the same immunoefficiency as the prior infectious laryngotracheitis virus (ILTV) attenuated vaccine and capable of overcoming dormant infection induced by the attenuated vaccine. The invention provides the nucleotide sequence and amino acid sequence of an ILTV glycoprotein D (gD) and a preparation method, as well as a recombinant vaccine containing a viral vector (fowlpox virus vector) and ILTV gD. The fowlpox virus is obtained by homologous arm recombination of ILTV gD. The invention also provides the preparation method of the recombinant vaccine. The invention clones gD gene of ILTV Henan isolate, and performs animal experiments of the immunoprotection of the recombinant fowlpox virus. The novel vaccine has the same immunoefficiency as the prior ILTV attenuated vaccine and is capable of overcoming the shortcomings of the attenuated vaccine. The invention is convenient for large-scale production, and provides a foundation for prevention and elimination of infectious laryngotracheitis (ILT).
Owner:HENAN AGRICULTURAL UNIVERSITY
Detection method for infectious bovine rihinotracheitis virus in aerosol
ActiveCN103981283ASimple and fast operationEasy to operateMicrobiological testing/measurementMicroorganism based processesInfectious laryngotracheitis virusInfectious bovine rhinotracheitis virus
The invention discloses a detection method for the infectious bovine rihinotracheitis virus in an aerosol. The method comprises the following steps: (1) acquisition of an aerosol sample; (2) extraction of genome total DNA of the aerosol sample; (3) detection: a step of carrying out PCR amplification; (4) establishment of a standard curve and a melting curve: a step of establishing the standard curve of positive standard plasmid and the melting curve of an amplification system; and (5) judgment: a step of judging whether the aerosol sample contains the infectious bovine rihinotracheitis virus. The invention further discloses specific primers (as shown in SEQ ID No. 3 and 4) and a kit (composed of the specific primers, the infectious bovine rihinotracheitis virus positive standard plasmid pEASY-T3-D, a SYBRGreenI real-time fluorescent quantitative PCR reagent and ddH2O). The method provided by the invention is applicable to detection of an infectious bovine rihinotracheitis virus aerosol sample and to detection of samples like clinical blood, milk and tissue and has wide application prospects.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Infectious chicken bronchitis vaccine
ActiveCN103007272ALittle side effectsAntiviralsAntibody medical ingredientsInfectious bronchitisSide effect
The invention relates to a live vaccine for infectious chicken bronchitis. The live vaccine consists of an antigen and a vaccine adjuvant, wherein the antigen comprises infectious chicken bronchitis viral strain with a collection code CGMCC No. 6752. The prepared vaccine can be used to prevent glandular stomach-type infectious chicken bronchitis. Furthermore, the infectious chicken bronchitis virus constituting the vaccine is a low virulent strain obtained by passage several times, thus reducing the side effect caused by the existing infectious bronchitis virus as the antigen.
Owner:YEBIO BIOENG OF QINGDAO
Infectious Bronchitis Virus Vaccine
ActiveUS20080026449A1SsRNA viruses positive-senseViral antigen ingredientsFowlInfectious bronchitis virus
Owner:INTERVET INT BV
MRT-PCR (multiple reverse transcription-polymerase chain reaction) kit for discriminating and detecting important avian economic animal viruses and application of mRT-PCR kit
ActiveCN104805217AHigh sensitivityReduce lossesMicrobiological testing/measurementMicroorganism based processesAnimal virusRespiratory virus
The invention discloses an mRT-PCR (multiple reverse transcription-polymerase chain reaction) kit for discriminating and detecting important avian economic animal viruses and an application of the mRT-PCR kit. The kit comprises primer pairs for detecting an AIV (avian influenza virus) M gene, AIV H7,H9 and H5 subtypes, an NDV (newcastle disease virus), an ILTV (infectious laryngotracheitis virus), an IBDV (infectious bursal disease virus) and an EDS (egg drop syndrome) virus. The mRT-PCR kit has the characteristics of high sensitivity, high specificity and the like when used for detecting AIVs and other common respiratory viruses, can be used for immediately detecting and discriminating important and popular subtypes of the AIVs and five avian respiratory pathogens most popular in China and has a great significance in zoonosis control, monitoring, prevention and control of avian mixed infection, biosafety of farms, reduction of loss of the breeding industry, food safety, quarantine and the like.
Owner:山西隆克尔生物制药有限公司 +2
Artificially weakened infectious bronchitis virus and application thereof
ActiveCN103146654AImproving immunogenicityGood immune protectionMicroorganism based processesAntiviralsInfectious bronchitisInfectious laryngotracheitis virus
The invention relates to an artificially weakened infectious bronchitis virus and application thereof. The artificially weakened infectious bronchitis virus uses the infectious bronchitis virus (IBV) which has good immunogenicity and is separated by an inventor to be passaged and attenuated, so as to obtain an attenuated strain FNO-E55 as a virus seed for production to prepare a safe and effective infectious bronchitis live vaccine. The strain used in the invention can effectively prevent occurrence of infectious bronchitis resulted from the infectious bronchitis virus (793 / B serotype) by aiming at a prevalence situation of the current domestic infectious bronchitis.
Owner:ZHEJIANG YEBIO BIOTECH
Method for suspension culture of infectious bronchitis virus by continuous cell line
ActiveCN108300704AHigh titerImproving immunogenicitySsRNA viruses positive-senseRecovery/purificationInfectious laryngotracheitis virusInfectious bronchitis virus
The invention provides a method for suspension culture of an infectious bronchitis virus by a continuous cell line. The method comprises the following steps that 1, EB66 cells are taken for recovery and secondary culture; 2, the EB66 cells obtained in the step 1 are subjected to infectious bronchitis virus inoculation culture; 3, the EB66 cells after the virus inoculation are sampled every 6 to 12h; virus EID50 is determined; when the virus EID50 reaches the highest value, the virus is harvested and stored; the cultured infectious bronchitis virus is obtained. The EB66 cells are used as a culture medium of the infectious bronchitis virus; the EB66 cells providing a full suspension continuous cell line is used for performing efficient virus production; the culture titer of the infectious bronchitis virus is effectively improved, so that the large-scale culture of the infectious bronchitis virus vaccine is realized; the virus culture cost is reduced.
Owner:ZHAOQING INST OF BIOTECHNOLOGY CO LTD +3
Recombinant Herpesvirus and Uses Thereof
ActiveUS20070212377A1Shortening the gB geneEfficient processSugar derivativesViral antigen ingredientsInfectious laryngotracheitis virusA-DNA
A recombinant herpesvirus (excluding infectious laryngotracheitis virus) having a DNA that encodes a polypeptide comprising 429 amino acids at the amino terminal end of a protein encoded by the gB gene of infectious laryngotracheitis virus or a polypeptide in which one or a plurality of amino acids have been deleted, added, or substituted in said polypeptide.
Owner:ZEON CORP
Recombinant avian herpesvirus useful in vaccine production
InactiveUS7314715B2SsRNA viruses negative-senseViral antigen ingredientsInfectious laryngotracheitis virusInfectious laryngotracheitis
The present invention provides a novel avian herpesvirus (NAHV) vector and recombinant vaccines made therefrom that are useful to immunize avian species against Marek's disease, infectious laryngotracheitis and Newcastle disease. Methods of immunizing an avian species against Marek's disease, infectious laryngotracheitis and Newcastle disease are also provided.
Owner:SCHERING PLOUGH ANIMAL HEALTH
Test paper for detecting newcastle disease and infectious bronchitis viruses in one step
InactiveCN101852802AStrong specificityHigh sensitivityMaterial analysisInfectious bronchitisGlass fiber
The invention discloses a test paper for detecting newcastle disease and infectious bronchitis viruses in one step and a preparation method and application thereof. The preparation method comprises the following steps of: preparing a coating membrane, namely diluting monoclonal antibody or polyclonal antibody against the newcastle disease and infectious bronchitis viruses and mouse IgG against antibody by using coating buffer solution, spraying the three antibodies in parallel on the middle part of a nitrocellulose membrane respectively, and forming a detection line for chicken infectious bronchitis virus, a detection line for newcastle disease virus and a control line against mouse IgG after the three antibodies permeate into the nitrocellulose membrane; preparing a glass fiber membrane coated with gold-labeled antibody; sticking the coating membrane on the middle part on a bottom lining, and sticking the glass fiber membrane and a piece of water absorption paper on the left and right on the coating membrane; and sticking a sample pad on the glass fiber membrane. The test paper has the advantages of strong specificity, high sensitivity, high detection speed, low cost, simple and convenient operation and wide application.
Owner:QINGDAO VLAND BIOTECH INC +1
Laying hen immune process
InactiveCN104855305AImmune program controlKeep healthyAvicultureInfectious laryngotracheitisBird flu
The invention relates to a laying hen immune process. According to the specific procedures of the laying hen immune process, Marek vaccine is injected for laying hens which are 1 day old; ND-IB vaccine and triad vaccine are injected for laying hens which are 6 to 7 days old; bursa of fabricius vaccine is injected for laying hens which are 12 days old, bird flu vaccine H5 and henpox vaccine POX are injected for laying hens which are 17 days to 18 days old, bursa of fabricius vaccine is injected for laying hens which are 22 day old, ND-IB vaccine is injected for laying hens which are 26 days old, infectious rhinitis vaccine is injected for laying hens which are 32 days old, ND-IB vaccine and infectious laryngotracheitis vaccine are injected for laying hens which are 39 days to 40 days old, bird flu vaccine H5 is injected for laying hens which are 80 days old, ND-IB vaccine is injected for laying hens which are 90 days old, vaccine H9 and brain pox vaccine are injected for laying hens which are 110 days old, bird flue H5 vaccine is injected for laying hens which are 120 days old, and ND-IB vaccine, triad vaccine and insect expelling vaccine are injected for laying hens which are 130 days old. The laying hen immune process has the advantages that the laying hen diseases can be effectively controlled, and the health of the laying hens is ensured.
Owner:HAIAN TINGTING AGRI & SIDELINE PRODSCO
Infectious laryngotracheitis virus (ILTV) vaccine using recombinant newcastle disease virus vector
ActiveUS20170049880A1SsRNA viruses negative-senseViral antigen ingredientsInfectious bronchitis virusInfectious laryngotracheitis virus
In this study, for the first time, protective efficacy of gD against ILTV challenge was evaluated. Immunization with recombinant Newcastle disease virus expressing ILTV gD induced a higher level of neutralizing antibodies and offered complete protection to chickens against lethal ILTV challenge. Uses of recombinant NDV as a vaccine vector are also described.
Owner:MIRADA MEDICAL +1
Infectious bronchitis virus attenuated vaccine strain
ActiveCN102994460ANo reversion of virulence was seenGenetically stableMicroorganism based processesAntiviralsLaboratory cultureInfectious laryngotracheitis virus
The invention relates to an infectious bronchitis virus attenuated vaccine strain. The preservation number of the virus strain is China General Microbiological Culture Collection Center (CGMCC) No.6752. The screened attenuated vaccine strain is used for preparing vaccines for preventing and treating infectious bronchitis. The infectious bronchitis virus attenuated vaccine strain can be passed in chicken bodies continuously whether artificial infection or horizontal transmission infection, the phenomenon of virulence enhancement does not occur, the heredity is stable, and the strain conforms to the standard of no virulence enhancement of infectious bronchitis virus attenuated vaccine strains and can be developed commercially.
Owner:YEBIO BIOENG OF QINGDAO
Adaptation of attenuated infectious bronchitis virus (IBV) to embryonic kidney cells and vaccine thereby produced
Disclosed are methods for preparing a vaccine against infection by infectious bronchitis virus (IBV). The methods typically include passing a heterogeneous attenuated population of IBV in chicken embryonic kidney cells, and optionally may include further passaging the heterogeneous attenuated population of IBV in embryonated chicken eggs (ECE) in order to obtain passaged attenuated population of IBV. Also disclosed are passaged attenuated populations of IBV in which the populations display a desired degree of homogeneity. Also disclosed are vaccines comprising the passaged attenuated populations of IBV and methods of vaccination comprising administering the disclosed vaccines.
Owner:AUBURN UNIV
Ovo immunization against infectious bronchitis
The present invention is directed to processes and compositions for protecting host animals (e.g., chickens) from exposure to virulent infectious bronchitis virus. In ovo administration of live, avirulent strains of IB at appropriate dosage levels on a per egg basis provides an effective and efficient vaccination having acceptable safety and efficacy features.
Owner:WYETH
Primer combination and GeXP detection method for simultaneously identifying 5 bovine viral dermatitis viruses
InactiveCN105969913APromote healthy developmentImprove throughputMicrobiological testing/measurementMicroorganism based processesDisease epidemiologyInfectious laryngotracheitis virus
The invention discloses a primer combination and GeXP detection method for simultaneously identifying 5 bovine viral dermatitis viruses. The primer combination is composed of a primer pair I, a primer pair II, a primer pair III, a primer pair IV and a primer pair V. The invention also discloses a GeXP detection method for simultaneously identifying foot-and-mouth disease virus, bluetongue virus, vesicular stomatitis virus, bovine viral diarrhoea virus and infectious bovine rhinotracheitis virus. The GeXP detection method can simultaneously identify the 5 bovine viral dermatitis viruses. The method has the characteristics of higher flux, higher specificity and higher sensitivity, can be used for monitoring of bovine disease epidemiology and differential diagnosis of unexpected epidemic situations, and ensures the healthy development of cattle raising industry.
Owner:GUANGXI VETERINARY RES INST
Mutant spike protein extending the tissue tropism of infectious bronchitis virus (IBV)
ActiveUS20160060303A1SsRNA viruses positive-senseVirus peptidesInfectious laryngotracheitis virusInfectious bronchitis virus
The present invention provides an infectious bronchitis virus (IBV) spike protein (S protein) which is based on an S protein from an IBV strain with restricted tissue tropism, but which comprises the sequence XBBXBX in the part of the S2 protein corresponding to residues 686 to 691 of the sequence given as SEQ ID No. 2, where B is a basic residue and X is any amino acid; and which comprises at least one of the following amino acid substitutions with reference to the position numbering of SEQ ID NO:2: Leucine (L) to Phenylalanine (F) at position 578 Asparagine (N) to Serine (S) at position 617 Asparagine (N) to Serine (S) at position 826 Leucine (L) to Phenylalanine (F) at position 857 and Isoleucine (I) to Valine (V) at position 1000 such that an IBV virus comprising the S protein has extended tissue tropism. The present invention also provides a virus comprising such an S protein.
Owner:THE PIRBRIGHT INST
Avian infectious laryngotracheitis-fowl pox combined active vaccine
ActiveCN103182080AExtended storage timeReduce side effectsAntiviralsAntibody medical ingredientsFiberInfectious laryngotracheitis
The avian infectious laryngotracheitis virus and fowl pox virus are cultured by using chicken whole embryo fibroblasts. Particularly, the avian infectious laryngotracheitis virus is successfully cultured by using the chicken whole embryo fibroblasts, the avian infectious laryngotracheitis virus and the fowl pox virus cultured by using the chicken whole embryo fibroblasts are obtained, and a combined freeze-drying active vaccine after a proper freeze-drying protectant is added. The combined freeze-drying active vaccine can be inoculated for immunization in a subalar and subcutaneous inoculation manner, so that side effects of the avian infectious laryngotracheitis virus can be alleviated, immune operations can be simplified, stress response can be reduced and production cost can be lowered. At the same time, the freeze-drying protectant prepared by a novel formula can effectively prolong storage time of the active vaccine and provide convenience for storing and transporting the products.
Owner:PU LIKE BIO ENG
Detection kit for chicken infectious bronchitis indirect ELISA antibody
InactiveCN105092839ASimple indirect ELISA methodSpecific indirect ELISA methodMaterial analysisMaternal antibodyInfectious bronchitis virus Antibody
The invention discloses a detection kit for chicken infectious bronchitis indirect ELISA antibody, belonging to the field of biotechnology. According to the invention, genetically engineered bacterium capable of steadily expressing partial nucleoprotein of chicken infectious bronchitis virus is utilized for inducible expression of recombined N160 protein which is subjected to affinity chromatographic purification, and the purified recombined N160 protein is taken as a coating antigen, so as to establish a simple and convenient and specific indirect ELISA method. The invention further provides a specific, sensitive, quick and convenient-to-operate kit for detecting the antibody of the chicken infectious bronchitis virus, which can be used for quickly diagnosing whether the chicken flocks are infected by the chicken infectious bronchitis virus and monitoring the immune chicken serum antibody level and the chicken maternal antibody level in actual production, so as to evaluate the degree of risk that the chicken flocks are infected by the infectious bronchitis virus and provide reference for the formulation of an infectious bronchitis immune procedure.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Combined trivalent live vaccine for Newcastle disease and infectious chicken bronchitis
ActiveCN103007275ATo achieve the goal of one needle and two defensesLittle side effectsViral antigen ingredientsAntiviralsDiseaseInfectious bronchitis
The invention relates to a combined trivalent living body vaccine for Newcastle disease and infectious chicken bronchitis. The live vaccine consists of an antigen and a vaccine adjuvant, wherein the antigen comprises an infectious chicken bronchitis virus YB160 strain with a collection code CGMCC No. 6752, an infectious chicken bronchitis virus H120 strain, and a Newcastle disease virus. The prepared combined trivalent vaccine can simultaneously prevent Newcastle disease and infectious chicken bronchitis, and achieves the purpose of preventing two diseases by one injection. Furthermore, the infectious chicken bronchitis virus constituting the vaccine is a low virulent strain obtained by passage for times, thus reducing the side effect caused by the existing infectious bronchitis virus as the antigen.
Owner:YEBIO BIOENG OF QINGDAO
Vaccine against infectious bronchitis virus
ActiveUS20170360921A1SsRNA viruses positive-senseViral antigen ingredientsInfectious bronchitis virusInfectious laryngotracheitis virus
Embodiments of the present invention relate to an infectious bronchitis virus (IBV) and an immunogenic composition comprising an IBV, respectively, wherein the ORF 3a and / or the ORF 3b and / or the ORF 5a and / or the ORF 5b is inactivated. Furthermore, aspects of the present invention relate to methods for immunizing a subject comprising administering to such subject the immunogenic composition of the present invention. Moreover, embodiments of the present invention relate to methods of treating or preventing clinical signs caused by IBV in a subject of need, the method comprising administering to the subject a therapeutically effective amount of an immunogenic composition according to the embodiments of the present invention.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Primer composition assisting identification of H9 subtype avian influenza virus and chicken infectious bronchitis virus and applications thereof
InactiveCN104232624AEasy to operateEfficient use ofMicrobiological testing/measurementDNA/RNA fragmentationInfectious bronchitis virusAgricultural science
The invention discloses a primer composition assisting identification of an H9 subtype avian influenza virus and a chicken infectious bronchitis virus and applications thereof. A specific primer pair comprises a single-stranded DNA molecule shown as a sequence 3 in a sequence table and a single-stranded DNA molecule shown as a sequence 4 in the sequence table. The invention also discloses the primer composition. The primer composition comprises a primer pair A and the specific primer pair. The primer pair A comprises a single-stranded DNA molecule shown as a sequence 1 in the sequence table and a single-stranded DNA molecule shown as a sequence 2 in the sequence table. The specific primer pair can identify the chicken infectious bronchitis virus. By combining double RT-PCR, the primer composition can simultaneously identify the H9 subtype avian influenza virus and the chicken infectious bronchitis virus and has advantages of high sensitivity, high specificity, high universality, short needed time, low cost, and the like. A plurality of clinical samples can be detected. The primer composition has characteristics of simple operation, liable popularization, and convenient basic level operation and application.
Owner:CHINA AGRI UNIV
Method for secretory expression of honeybee melittin signal peptide-mediated IBV (infectious bronchitis virus) N protein
InactiveCN105602990AHigh expression yieldReduce hydrolysisPolypeptide with localisation/targeting motifSsRNA viruses positive-senseAntigenStructural protein
The invention discloses a method for secretory expression of littin signal peptide-mediated IBV N protein, that is firstly, IBV N gene HBM-N fusing honeybee melittin signal peptide is obtained, then a recombination transfer vector pFast-HBM-N and recombination bacmid rHBM-N are constructed, and finally, the recombination bacmid transfers Sf9 insect cells for secretory expression of IBV N protein. According to the method, the honeybee melittin signal peptide is introduced before IBV N protein, N protein efficient secretory expression is realized, the obtained IBV N protein has excellent biological activity, hydrolysis of endogenous protease to target protein is reduced with the method, purification is facilitated, the foundation is laid for further study of N protein biological functions, development of novel diagnostic antigen, development of novel vaccine and the like, and at the same time, a new thought is provided for expression of other structural protein of the IBV.
Owner:GUANGXI UNIV
Primer group for identifying mycoplasma bovis, bovine viral diarrhea virus and infectious bovine rhinotracheitis virus and application thereof
ActiveCN106754911AHigh sensitivityHigh clinical application valueMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceBovine Viral Diarrhea Viruses
The invention discloses a primer group for identifying a mycoplasma bovis, a bovine viral diarrhea virus and an infectious bovine rhinotracheitis virus and an application thereof. According to the invention, a primer composition formed by a primer pair A, a primer pair B and a primer pair C is protected. The primer pair A is formed by a primer F1 as shown in a sequence 1 and a primer R1 as shown in a sequence 2; the primer pair B is formed by a primer F2 as shown in a sequence 3 and a primer R2 as shown in a sequence 4; and the primer pair C is formed by a primer F3 as shown in a sequence 5 and a primer R3 as shown in a sequence 6. By adopting the primer pair A, the primer pair B and the primer pair C, the mycoplasma bovis, the bovine viral diarrhea virus and the infectious bovine rhinotracheitis virus are detected through triple two-temperature PCR, and the primer group has the advantages of being good in specificity, high in sensitivity, good in universality, convenient and fast, and can be used for clinical differential diagnosis and epidemiological investigation. A novel technique is provided for prevention and control of cattle diseases, and the primer group has very high clinical application value.
Owner:GUANGXI VETERINARY RES INST
Bovine viral diarrhea, bovine infectious rhinotracheitis, bovine parainfluenza triple inactivated vaccine and preparation method thereof
ActiveCN106729692BSsRNA viruses negative-senseSsRNA viruses positive-senseAntigenBovine Viral Diarrhea Viruses
The invention relates to a triple inactivated vaccine for preventing bovine viral diarrhea, infectious bovine rhinotracheitis and bovine parainfluenza and preparation method thereof. The active ingredients of the vaccine include the inactivated antigens of bovine viral diarrhea virus, infectious bovine rhinotracheitis virus and bovine parainfluenza type-3 virus; and in the triple inactivated vaccine, virus multiplication is performed by use of a passage cell line of cell spinner bottle, full suspension and micro-carrier culture, and the vaccine preparation technology and the like are optimized. The safety and efficacy test results of the vaccine indicate that after bovine is immunized with the triple inactivated vaccine provided by the invention, local and whole-body adverse reaction is avoided, and all bovines gain immune protection, thus the vaccine is safe and reliable, an aim of 'one injection and three preventions' is achieved, and economic loss is reduced.
Owner:QILU ANIMAL HEALTH PROD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com