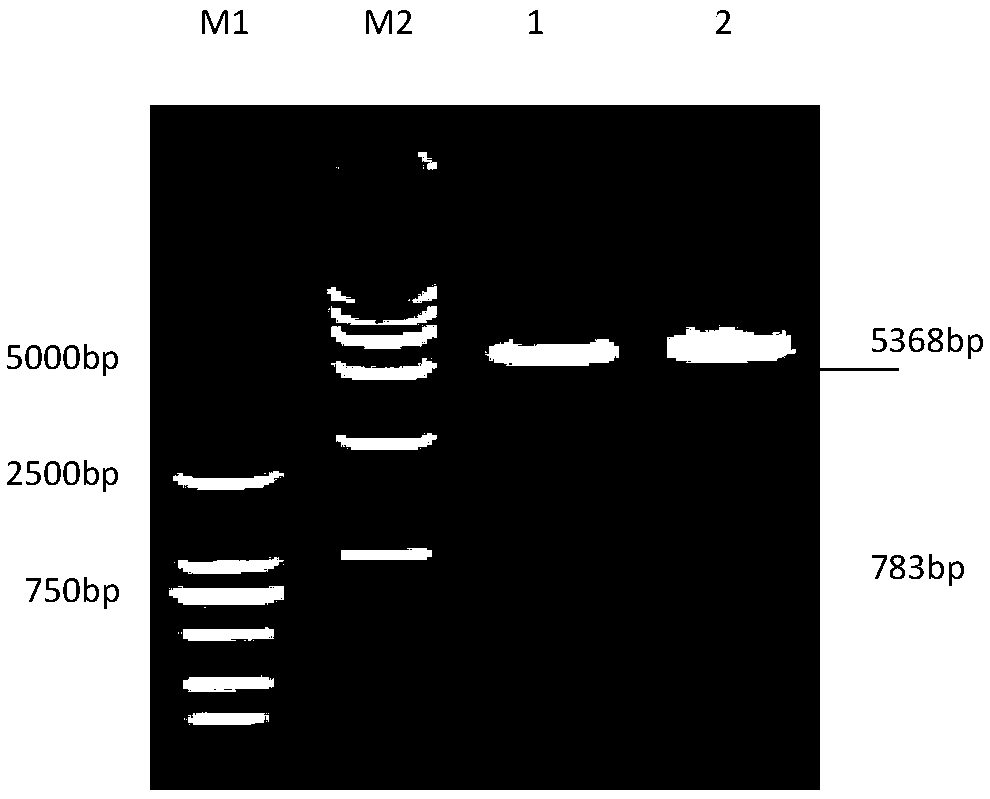
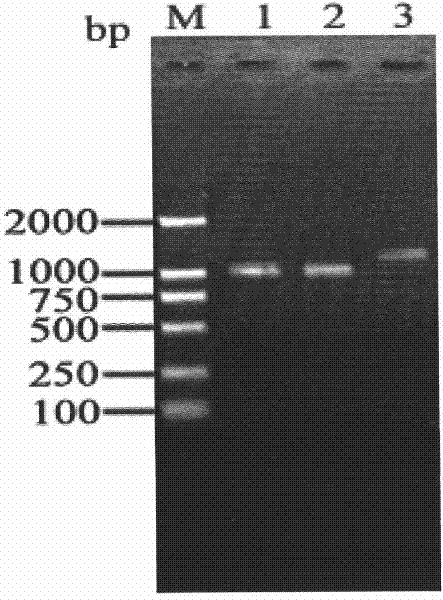
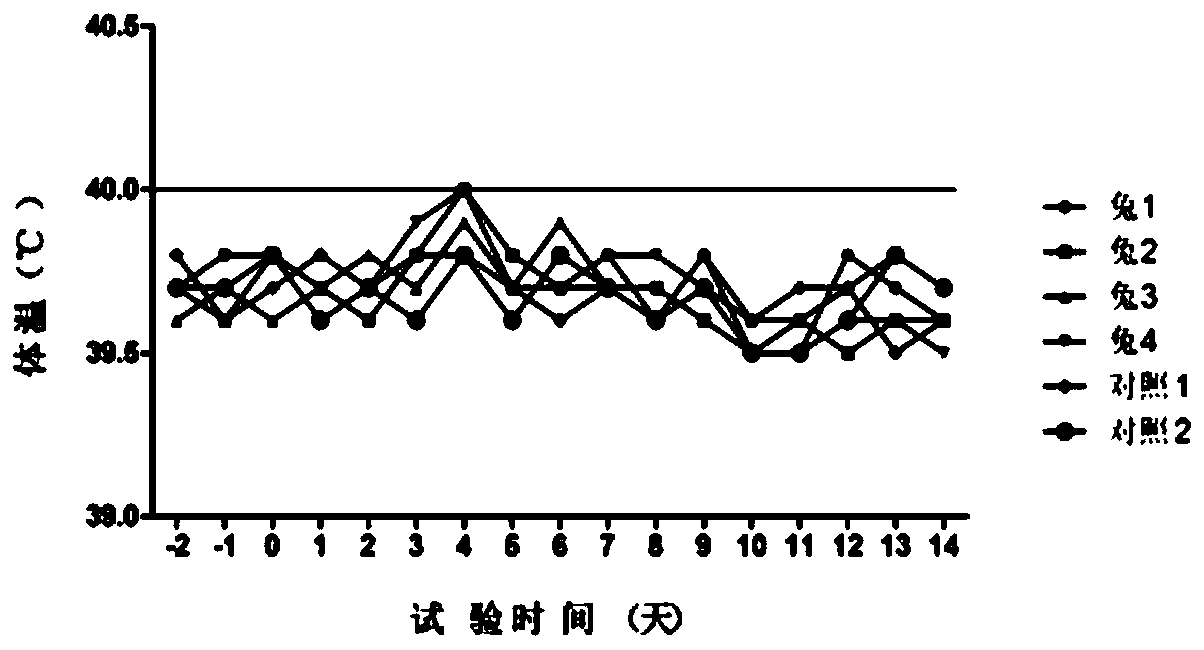
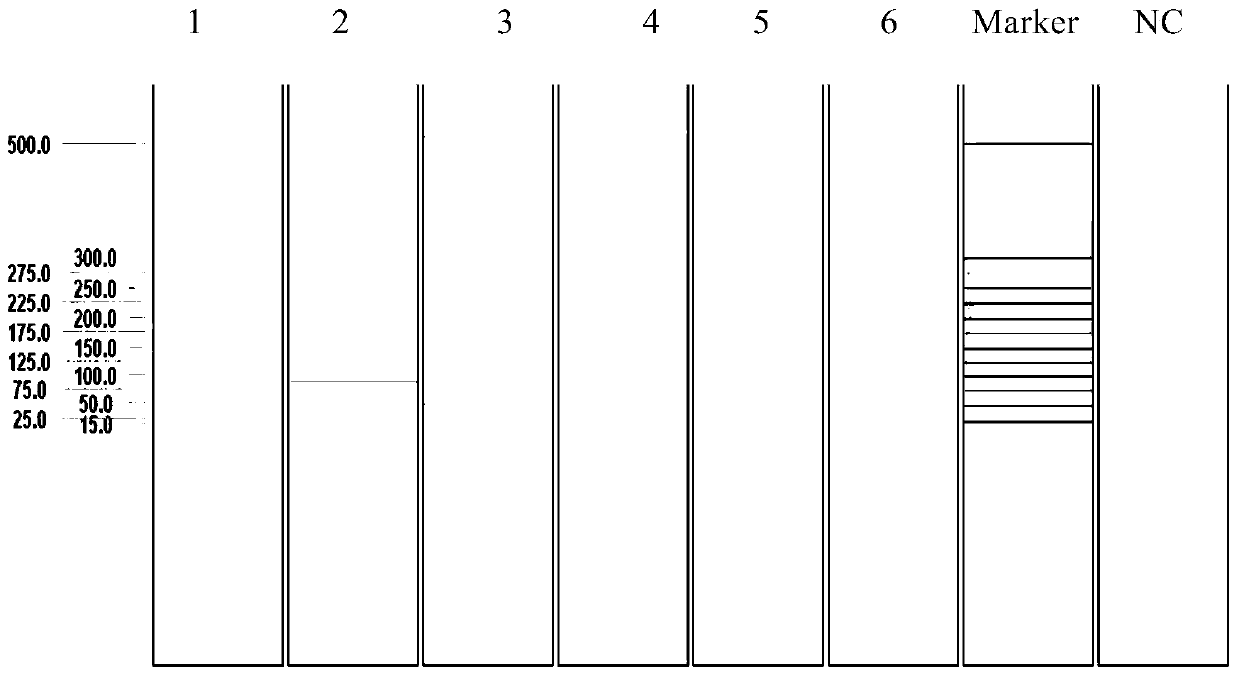
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Infectious bovine rhinotracheitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infectious bovine rhinotracheitis virus gD protein and application thereof
InactiveCN105753947AHigh sensitivityStrong specificityVirus peptidesBiological material analysisCelluloseSerum samples
The invention discloses a test strip for detecting infectious bovine rhinotracheitis, which comprises a PVC backboard, a sample pad, a gold mark pad, a cellulose membrane and a water absorbing pad. The test strip is characterized in that infectious bovine rhinotracheitis virus gD protein according to the claim 1, marked by colloidal gold, is coated on the gold mark pad; and the infectious bovine rhinotracheitis virus gD protein and a rabbit anti-infectious bovine rhinotracheitis virus gD protein antibody are separately coated on a detection line and a quality control line of the cellulose membrane. The test strip for detecting the infectious bovine rhinotracheitis virus antibody in a bovine serum sample is high in sensitivity, is good in specificity, is quick and convenient to detect, is easy to use, and is free of special apparatus equipment, can detect results within 10 minutes, and is very convenient for being popularized and used in the basic-level breeding unit.
Owner:NBGEN
Primer, probe and kit for detecting infectious bovine rhinotracheitis viruses
ActiveCN104862419AHigh sensitivityImprove featuresMicrobiological testing/measurementMicroorganism based processesForward primerInfectious bovine rhinotracheitis virus
The invention relates to a primer, a probe and a kit for detecting infectious bovine rhinotracheitis viruses, and discloses a primer and probe combination used for detecting the infectious bovine rhinotracheitis viruses according to an RPA technology, the forward primer sequence of the primer and probe combination is shown as SEQ ID No.1; the reverse primer sequence is shown as SEQ ID No.2; the probe sequence is shown as SEQ ID No.3. The invention further discloses the kit for detecting the infectious bovine rhinotracheitis viruses. The primer and the probe are adopted for detection, a clinical sample is only subjected to virus DNA crude extraction and RPA isothermal amplification, the result can be displayed on a lateral flow chromatography test strip, a heat circular reaction is not needed, and amplification needs not to be performed in a PCR instrument; the primer, the probe and the kit have the advantages of being high in sensitivity, strong in specificity, simple in reaction procedure, short in detection time, and suitable for clinical field detection in a non-lab environment, and have a wide application prospect.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting five cow disease viruses
ActiveCN105002301AGuaranteed sensitivityEnable multiple detectionMicrobiological testing/measurementMicroorganism based processesLeucosisBovine Viral Diarrhea Viruses
The invention discloses a multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses. The multiple-connection probe is shown in sequence tables from SEQ ID NO:1 to SEQ ID NO:10. The primer is shown in sequence tables from SEQ ID NO:11 to SEQ ID NO:12. The primer, the probe and / or the multiple-connection probe amplification detection kit including the primer and the probe can detect the five vital cow disease pathogenies including the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses at the same time, the detection time and cost are saved, and epidemic diseases can be diagnosed in time.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Dot immunogold filter kit for detecting IBR (infectious bovine rhinotracheitis) virus antibody and detection method thereof
The invention discloses a dot immunogold filter kit for detecting an IBR (infectious bovine rhinotracheitis) virus antibody and a detection method thereof. The kit comprises a) an infectious bovine rhinotracheitis virus antigen, b) a gold marked goat anti-bovine antibody, c) a cleaning solution, and d) a confining liquid. The method comprises the following steps: dotting the infectious bovine rhinotracheitis virus antigen on a nitrocellulose film; closing, and adding a serum sample to be detected; cleaning, and detecting the infectious bovine rhinotracheitis virus antibody by using the gold marked goat anti-bovine antibody as colloidal gold marked protein. Detection of the infectious bovine rhinotracheitis virus antibody by adopting the kit disclosed by the invention has the advantages of specificity, sensitivity, quickness, reliability, intuitive effect, easily determined result and the like, special equipment is not required, and the detection result can be preserved for inspection.
Owner:INSPECTION & QUARANTINE TECH CENT OF FUJIAN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Infectious bovine rhinotracheitis vaccine and preparation method thereof
InactiveCN102049043AAntiviralsAntibody medical ingredientsInfectious bovine rhinotracheitis virusTiter
The invention relates to an infectious bovine rhinotracheitis vaccine and a preparation method thereof, belongs to the technical field of veterinary biological products, and is used for solving the problem to control the endemic infectious bovine rhinotracheitis by a vaccine. The vaccine is prepared by the following steps of: separating virus from a tissue of aborted fetus of cow infected with the infectious bovine rhinotracheitis; breeding; detecting titer; inactivating and emulsifying and the like. The vaccine can be used for controlling the endemic infectious bovine rhinotracheitis.
Owner:TIANJIN RINGPU BIO TECH
Detection kit for detecting infectious bovine rhinotracheitis virus antibody
InactiveCN106405093ALow costEasy to produceBiological material analysisInfectious bovine rhinotracheitis virusMonoclonal antibody
The invention provides a detection kit for detecting an infectious bovine rhinotracheitis virus antibody. The detection kit comprises an elisa plate for enveloping a monoclonal antibody and an IBRV antigen of infectious bovine rhinotracheitis virus gD protein, an IBRV positive standard serum, a negative serum, an enzyme labeling secondary antibody, a substrate developing solution and a stop solution. Infectious bovine rhinotracheitis viruses in infected MDBK cell lysates are captured in the elisa plate by using the monoclonal antibody of the infectious bovine rhinotracheitis virus gD protein, and the optimal enveloping concentrations of the monoclonal antibody and the virus antigen are determined through a square titration method. The detection kit provided by the invention has good specificity and sensitivity, is high in neutralization coincidence rate, and has a good application prospect.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Traditional Chinese medicine for preventing infectious bovine rhinotracheitis
InactiveCN105596627ACompatibility is reasonableImprove the immunityRespiratory disorderPlant ingredientsInfectious bovine rhinotracheitisEuphorbia
The invention provides a traditional Chinese medicine for preventing infectious bovine rhinotracheitis, belongs to the field of traditional Chinese medicine and pharmacy technology, and concretely relates to the traditional Chinese medicine for preventing infectious bovine rhinotracheitis. The invention provides the traditional Chinese medicine with good prevention effects for improving immunity of cattle and preventing infectious bovine rhinotracheitis. The traditional Chinese medicine comprises the following components in parts by mass: 15-30 parts of cassia twigs, 1-3 parts of cucurbits, 1-3 parts of cyperus, 1-2 parts of peach seeds, 2-4 parts of radishes, 3-6 parts of Japanese cayratia herbs, 3-6 parts of garden euphorbia herbs, 3-6 parts of nux vomica, 3-8 parts of moutan barks, and 2-10 parts of dandelion.
Owner:周晓丽
gg and TK gene-deleted recombinant infectious bovine rhinotracheitis virus and application
ActiveCN101818130ALow toxicityImmunity has no effectMicroorganism based processesAntiviralsDiseaseAnimal virus
The invention belongs to the fields of animal virology and animal genetically engineered technology, and in particular relates to a gG and TK gene-deleted recombinant infectious bovine rhinotracheitis virus, a genetic engineering vaccine and application. The recombinant infectious bovine rhinotracheitis virus strain IBRV delta gG / delta TK prepared by the invention is preserved in China Center for Type Culture Collection, with the preservation number of CCTCC No: V200915; and gG and TK genes are deleted in the strain, and the strain does not comprise foreign genes. An intermediate strain, namely recombinant infectious bovine rhinotracheitis virus GAZH-2009-01 is preserved in China Center for Type Culture Collection, with the preservation number of CCTCC No: V200910; and gG and TK genes are deleted in the strain and the strain comprises reporter gene EGFP. The invention also discloses application of the recombinant infectious bovine rhinotracheitis virus IBRV delta gG / delta TK in preparing the genetic engineering vaccine for the infectious bovine rhinotracheitis and preventing and controlling infectious bovine rhinotracheitis virus diseases.
Owner:HUAZHONG AGRI UNIV
Mycoplasma bovis immune related protein, detection kit containing same and application thereof to detection of mycoplasma bovis antibody
The invention discloses a mycoplasma bovis immune related protein, a detection kit containing the same and application thereof to detection of a mycoplasma bovis antibody. The mycoplasma bovis immunerelated protein is named p28 protein, and the amino acid sequence of the protein is shown in SEQ ID NO.2. A sensitivity test proves that the mycoplasma bovis serum antibody ELISA detection kit (MbH kit) provided by the invention can detect positive serum with the dilution multiple of 1:2560 at the minimum; and a specificity test proves that the kit has the specificity of 97.8% and has no specificreaction with the positive serums of contagious bovine pleuropneumonia (CBPP), foot-and-mouth disease (FMD), bovine tuberculosis (MB), bovine viral diarrhea (BVDV) and infectious bovine rhinotracheitis (IBRV), and has good stability and high accuracy. The invention provides a new technical means for detecting a mycoplasma bovis antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Infectious bovine rhinotracheitis delta TK/delta gE gene deletion mark live vaccine and preparation method
InactiveCN102649948AGood virulence factorOther immunogenicity is goodMicroorganism based processesAntiviralsDiseaseInfectious bovine rhinotracheitis
The invention belongs to the technical field of animal-borne disease genetic engineering and specifically relates to an infectious bovine rhinotracheitis delta TK / delta gE gene deletion mark live vaccine and a preparation method. The strain is preserved at China Center for Type Culture Collection and the preservation number is CCTCCNO: V201104. A recombined infectious bovine rhinotracheitis strain is lack of TK and gE genes and an EGFP (Enhanced Green Fluorescent Protein) express box is inserted. The toxicity of the prepared infectious bovine rhinotracheitis delta TK / delta gE gene deletion mark live vaccine is greatly reduced; the potential virus is difficult to activate; the safety is high; besides, except being lack of the TK and gE genes, the deletion strain can still express the other toxic genes; and the immunogenicity is excellent.
Owner:HUAZHONG AGRI UNIV
Infectious bovine rhinotracheitis virus gD protein antigen epitope polypeptide, and inhibitor, monoclonal antibody and application of polypeptide
InactiveCN107586322AVirus peptidesImmunoglobulins against virusesAdjuvantInfectious bovine rhinotracheitis virus
The invention belongs to the field of molecular biology and medicine, and specifically discloses an infectious bovine rhinotracheitis virus gD protein antigen epitope polypeptide and an application ofthe polypeptide in preparation of a reagent or a medicament for detecting or treating infectious bovine rhinotracheitis. The invention provides a monoclonal antibody for resisting the infectious bovine rhinotracheitis virus gD protein, and simultaneously the antigen epitope of the infectious bovine rhinotracheitis virus gD protein is screened out as <323>GEPKPGPSPDADRPE<337> (the shortest epitopesequence is 7 amino acid peptide fragments: <323>GEPKPGP<329>). The recombinant protein based on the antigen epitope can specifically be used to detect infectious bovine rhinotracheitis serum; in addition, a small-molecule inhibition drug designed based on the antigen epitope can block virus infection; and meanwhile, the multi-copy repeated epitope vaccine constructed on the basis of the antigenepitope can induce a high-titer gD protein antibody under the assistance of an appropriate adjuvant, and has a relatively high neutralizing antibody titer. The invention lays a foundation for establishing a detection method and researching and developing vaccines for the infectious bovine rhinotracheitis.
Owner:HAINAN UNIVERSITY
Traditional Chinese medicine composition for preventing and treating infectious bovine rhinotracheitis and application thereof
InactiveCN106177156AGood curative effectQuick resultsAntiviralsFungi medical ingredientsTreatment effectLicorice roots
The invention provides a traditional Chinese medicine composition for preventing and treating infectious bovine rhinotracheitis. The traditional Chinese medicine composition is prepared from, by weight, 15-25 parts of puffball, 25-35 parts of fructus arctii, 25-35 parts of radix scrophulariae, 25-35 parts of radix bupleuri, 15-25 parts of radix isatidis, 15-24 parts of rhizoma cimicifugae, 25-34 parts of radix scutellariae, 15-30 parts of rhizoma coptidis, 15-25 parts of radix platycodi, 30-40 parts of fructus forsythiae, 18-25 parts of herba menthae, 25-32 parts of herba schizonepetae, 15-20 parts of herba ephedra, 18-25 parts of kudzuvine roots and 25-35 parts of licorice roots. The Chinese herbs are compounded to achieve the effects of clearing away heat and toxic materials, relieving sore throat, eliminating swelling, invigorating the spleen and replenishing qi, and then active substances of the Chinese herbs directly arrive at lesions. The traditional Chinese medicine composition has the advantages of being good in treatment effect, capable of quickly achieving the effects and high in cure rate in application for preventing and treating the infectious bovine rhinotracheitis.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Traditional Chinese medicine granules for treating respiratory bovine infectious rhinotracheitis and preparation method thereof
InactiveCN103405504AEnhanced wind-dispelling and pain-relievingEnhanced nasal openingAntiviralsGranular deliverySide effectCurative effect
The invention discloses traditional Chinese medicine granules for treating respiratory bovine infectious rhinotracheitis and a preparation method thereof. The granules comprise the following components in parts by weight: 7-10 parts of radix angelicae, 6-9 parts of honeysuckle, 5-9 parts of cocklebur fruit and 4-7 parts of flos magnoliae. The preparation method comprises the following steps: extracting volatile oil of radix angelicae and flos magnoliae, and collecting distilled aqueous liquor by another container; adding 10 times of water to distilled dregs, honeysuckle and cocklebur fruit to decoct for twice, for 2 hours and 1.5 hours, respectively; combining the decoction liquids, filtering, combining the water-decocted filtrate and distilled water collected by another container, concentrating to relative density of 1.25-1.30, adding moderate amount of saccharose powder, uniformly mixing to prepare granules; and spraying volatile oil of radix angelicae and flos magnoliae to the granules, and uniformly mixing to obtain the granules. The traditional Chinese medicine granules for treating respiratory bovine infectious rhinotracheitis disclosed by the invention is remarkable in curative effect, and free from medicine dregs, toxic and side effects and tolerance.
Owner:TIANJIN SHENGJI GRP CO LTD
Bovine viral diarrhea-bovine infectious rhinotracheitis bivalent subunit vaccine and preparation method and application thereof
PendingCN107174660AImproving immunogenicityImprove securitySsRNA viruses positive-senseViral antigen ingredientsAntigenAdjuvant
The invention discloses a bovine viral diarrhea-bovine infectious rhinotracheitis bivalent subunit vaccine and a preparation method and application thereof, and belongs to the technical field of animal vaccines and animal biological products. The vaccine comprises bovine viral diarrhea virus E2 protein, bovine infectious rhinotracheitis virus gD protein and pharmaceutically acceptable adjuvant. The preparation method for the vaccine comprises the following steps that: 1) preparing the bovine viral diarrhea virus E2 protein and the bovine infectious rhinotracheitis virus gD protein; 2) mixing the bovine viral diarrhea virus E2 protein and the bovine infectious rhinotracheitis virus gD protein prepared in the 1) to prepare antigen liquid; 3) carrying out mixing emulsion on the anti-agent liquid with ISA 201VG at an volume ratio of 46:54. The vaccine has the advantages of high immunogenicity, high safety and no immunity interference; in addition, cows can be effectively prevented and protected from the infection of the bovine viral diarrhea virus and the bovine infectious rhinotracheitis virus, an effect of two prevention functions by one injection can be achieved, time and labor are saved, and cost is saved.
Owner:NOVO BIOTECH CORP
Bovine viral diarrhea-infectious bovine rhinotracheitis bivalent subunit fusion vaccine and identification method thereof
ActiveCN112125961AImprove securityNo immune interferenceSsRNA viruses positive-senseViral antigen ingredientsAntigen epitopeAntigen
The invention relates to the technical field of biology, and particularly provides a bovine viral diarrhea-infectious bovine rhinotracheitis bivalent subunit fusion vaccine and an identification method thereof. The inventor optimizes genes of BVDV-NS3 and IBRV-VP8, and performs fusion expression on antigen epitope proteins of the BVDV-NS3 and the IBRV-VP8 to obtain polypeptide fragment compositions coded by SEQ ID NO.1 and SEQ ID NO.2. The polypeptide fragment compositions can be used for preparing bivalent subunit fusion vaccines, good protective efficacy can be generated after animals are immunized, the purpose of preventing bovine viral diarrhea and infectious bovine rhinotracheitis at the same time is achieved, and the effect of preventing two diseases by one injection is achieved. Meanwhile, a detection kit provided by the invention can be used for rapidly identifying and diagnosing whether cattle are immunized with vaccine strains or infected wild strains, so that the purification of bovine viral diarrhea virus and infectious bovine rhinotracheitis virus is realized.
Owner:天康制药股份有限公司
Anti-IBRV single-chain antibody and preparation method and application thereof
InactiveCN107312087ASmall molecular weightEasy constructionImmunoglobulins against virusesAntiviralsEscherichia coliWAS PROTEIN
The invention provides an anti-IBRV single-chain antibody which is protein consisting of a heavy chain variable region shown as SEQ ID NO.1, a light chain variable region shown as SEQ ID NO.2 and a connecting peptide for connecting the two regions and having the characteristic of resisting an infectious bovine rhinotracheitis peplos gD protein antibody. The invention also provides a preparation method of the single-chain antibody. The single-chain antibody can be specifically combined with IBRV and can be also specifically combined with IBV gD protein in an escherichia coli expression product. An indirect immunofluorescence assay shows that the single-chain antibody can identify the IBRV infected in the bovine kidney cell MDBK, and the single-chain antibody has the good application value when applied to infectious bovine rhinotracheitis detection and development of treatment preparations.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Nucleic acid, kit and droplet type digital PCR method for detecting infectious bovine rhinotracheitis virus
InactiveCN111560471ARealize precise quarantineHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesForward primerInfectious Disorder
The invention provides a nucleic acid for detecting infectious bovine rhinotracheitis virus, a kit and a droplet type digital PCR method. The nucleic acid comprises a forward primer, a reverse primerand a probe, the sequence of the forward primer is shown as SEQ ID NO. 1, the sequence of the reverse primer is shown as SEQ ID NO. 2, and the sequence of the probe is shown as SEQ ID NO. 3. The sensitivity of the droplet type digital PCR detection kit for detecting the infectious bovine rhinotracheitis virus is 1copies / mu L, and only the infectious bovine rhinotracheitis virus can be specificallydetected. When the kit is used for detecting suspected samples of infectious bovine rhinotracheitis viruses, results show that the accuracy of the kit is higher than that of existing qPCR, detectionof trace viruses can be achieved, and absolute quantification can be conducted on nucleic acid without a standard curve. The detection method provided by the invention can realize high-specificity, high-sensitivity and absolute quantitative detection of the infectious bovine rhinotracheitis virus.
Owner:北京市动物疫病预防控制中心
Bovine viral diarrhea, bovine infectious rhinotracheitis, bovine parainfluenza triple inactivated vaccine and preparation method thereof
ActiveCN106729692BSsRNA viruses negative-senseSsRNA viruses positive-senseAntigenBovine Viral Diarrhea Viruses
The invention relates to a triple inactivated vaccine for preventing bovine viral diarrhea, infectious bovine rhinotracheitis and bovine parainfluenza and preparation method thereof. The active ingredients of the vaccine include the inactivated antigens of bovine viral diarrhea virus, infectious bovine rhinotracheitis virus and bovine parainfluenza type-3 virus; and in the triple inactivated vaccine, virus multiplication is performed by use of a passage cell line of cell spinner bottle, full suspension and micro-carrier culture, and the vaccine preparation technology and the like are optimized. The safety and efficacy test results of the vaccine indicate that after bovine is immunized with the triple inactivated vaccine provided by the invention, local and whole-body adverse reaction is avoided, and all bovines gain immune protection, thus the vaccine is safe and reliable, an aim of 'one injection and three preventions' is achieved, and economic loss is reduced.
Owner:QILU ANIMAL HEALTH PROD
Antibody detection kit for infectious bovine rihinotracheitis virus and application thereof
ActiveCN109374886AEasy to operateReduce the difficulty of operationMaterial analysisPositive controlInfectious bovine rhinotracheitis virus
The invention provides an antibody detection kit for an infectious bovine rihinotracheitis virus. The detection kit is composed of a recombinant gD antigen coated elisa plate, negative control, positive control, a horse radish peroxidase marked IBR specific monoclonal antibody, a sample diluting solution, a washing solution, a primer solution and a terminating solution. By means of the kit, whether a cattle is infected with infectious bovine rihinotracheitis or not can be detected within a relatively short time, and a corresponding management policy is made. For a complex situation of currentanimal disease prevention and control, the kit has a very wide market prospect and can play a great role in basic level detection and government regulation.
Owner:北京纳百生物科技有限公司
Multiplex Ligation Probe Amplification Detection Kit, Primers and Probes for Simultaneous Detection of Five Bovine Disease Viruses
ActiveCN105002301BGuaranteed sensitivityEnable multiple detectionMicrobiological testing/measurementMicroorganism based processesMultiplex ligation-dependent probe amplificationBovine Viral Diarrhea Viruses
The invention discloses a multiple-connection probe amplification detection kit, primer and probe for simultaneously detecting the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses. The multiple-connection probe is shown in sequence tables from SEQ ID NO:1 to SEQ ID NO:10. The primer is shown in sequence tables from SEQ ID NO:11 to SEQ ID NO:12. The primer, the probe and / or the multiple-connection probe amplification detection kit including the primer and the probe can detect the five vital cow disease pathogenies including the bluetongue viruses, the infectious bovine rhinotracheitis viruses, the bovine viral diarrhea viruses, the enzootic bovine leucosis viruses and the foot and mouth disease viruses at the same time, the detection time and cost are saved, and epidemic diseases can be diagnosed in time.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Bovine infectious rhinotracheitis virus multi-epitope recombinant chimeric protein and application thereof
ActiveCN110003343AStatistical analysis Significant differenceImprove immunityBacteriaViral antigen ingredientsAntigen epitopeInfectious bovine rhinotracheitis virus
The invention provides a bovine infectious rhinotracheitis virus multi-epitope recombinant chimeric protein and an application thereof. The recombinant chimeric protein is formed by rigid linker series connection of a tetanus toxin general T cell epitope polypeptide P2, a bovine infectious rhinotracheitis virus gB antigen epitopes gB-A and gB-B, bovine infectious rhinotracheitis virus gC antigen epitopes gC-A and gC-B, bovine infectious rhinotracheitis virus gD antigen epitopes gD-A, gD-B and gD-C and bovine IL-6. The anti-bovine infectious rhinotracheitis virus antibody level induced by the bovine infectious rhinotracheitis virus multi-epitope recombinant chimeric protein provided by the invention is significantly higher than that of other control groups, and the recombinant chimeric protein is indicated to have good immunogenicity.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
Feed composition for preventing cattle infectious rhinotracheitis
InactiveCN102370098AReduce morbidityReduce economic lossAnimal feeding stuffBiotechnologyAnimal science
The invention discloses a feed composition for preventing cattle infectious rhinotracheitis. The feed composition is prepared from raw materials in parts by weight as follows: 1.5 to 2.5 parts of pale butterflybush flower, 1.5 to 2.5 parts of gamdir vine, 40 to 45 parts of maize, and 54 to 60 parts of straws. A method for preparing the feed composition disclosed by the invention comprises the steps of: firstly, preparing the pale butterflybush flower, the gamdir vine and the maize in parts by weight into a powdered matter of 10-40 meshes; cutting the straws in parts by weight into sections less than 3 centimeters; and then uniformly agitating all the raw materials. Compared with the feed, the feed composition for preventing the cattle infectious rhinotracheitis, disclosed by the invention, can be used for obviously reducing the morbidity of the cattle infectious rhinotracheitis, and obviously reducing the economic loss of the cattle industry due to the cattle infectious rhinotracheitis.
Owner:广州品悦生物科技有限公司
Primer composition, kit and method for detecting mycoplasma bovis and infectious bovine rhinotracheitis viruses
InactiveCN110878381AHigh amplification efficiencyA large amountMicrobiological testing/measurementMicroorganism based processesInfectious bovine rhinotracheitis virusInfectious bovine rhinotracheitis
The invention discloses a primer composition, a kit and a method for detecting mycoplasma bovis and infectious bovine rhinotracheitis viruses. The primer composition designed and screened in the invention comprises two groups of specific primers and probes. Under the condition of an optimized reaction system and under optimized reaction conditions, mycoplasma bovis and infectious bovine rhinotracheitis viruses can be simultaneously identified and detected in the same reaction tube; and the primer composition, the kit and the method have the characteristics of good specificity, high sensitivity, interference resistance, no pollution, convenience and quickness in operation, low cost and the like.
Owner:GUANGXI VETERINARY RES INST
Chinese medicinal composition for treating cat infectious bovine rhinotracheitis
InactiveCN102895489AImprove personal hygieneReduce in quantityAntiviralsAluminium/calcium/magnesium active ingredientsDiseaseSkunk
The invention provides a Chinese medicinal composition for treating cat infectious bovine rhinotracheitis. The Chinese medicinal composition comprises the following components in parts by weight: 1-450 parts of coptis root, 1-450 parts of baikal skullcap root, 1-400 parts of figwort, 1-300 parts of rhubarb, 1-250 parts of skunk bugbane, 1-250 parts of gypsum and 1-250 parts of common anemarrhena. A Chinese veterinarian dialectical therapy method is adopted; coptis root has the effects of clearing heat, drying dampness, purging intense heat and detoxifying; baikal skullcap root is used for treating symptoms such as a warm heat disease, upper respiratory tract infection, cough with lung heat and the like; figwort has the effects of removing pathogenic heat from blood, purging intense heat, detoxifying and nourishing yin; rhubarb has the effects of eliminating food retention in stomach, clearing away damp-heat, discharging fire, cooling blood, removing blood stasis and detoxifying; and coptis root, baikal skullcap root, figwort, rhubarb, skunk bugbane, gypsum and common anemarrhena co-act with each other, so that cat infectious bovine rhinotracheitis can be treated effectively.
Owner:TIANJIN JIHE TECH
Bivalent live vaccine for bovine viral diarrhea and infectious bovine rhinotracheitis and preparation method of bivalent live vaccine
PendingCN112807424AEfficient immune functionReduce manufacturing costSsRNA viruses positive-senseViral antigen ingredientsBovine Viral Diarrhea VirusesAnimals vaccines
The invention discloses preparation and application of a bivalent live vaccine for bovine viral diarrhea and infectious bovine rhinotracheitis, and belongs to the technical field of animal vaccines and veterinary biological products. The vaccine comprises a live bovine viral diarrhea virus NMM strain and an infectious bovine rhinotracheitis virus JSM strain. The preparation of the vaccine comprises the following steps of 1) obtaining two effective strains and carrying out attenuated culture; 2) carrying out suspension culture on MDBK cells to respectively prepare virus antigens; and (3) proportioning according to the concentration of a titer of 10<4.7>TCID<50>, sub-packaging, adding 20% of a freeze-drying protective agent, and freeze-drying. According to the bivalent live vaccine for bovine viral diarrhea and infectious bovine rhinotracheitis, the same immune effect of inactivated vaccines with respective virus titers of 10<7.3>TCID<50> and 10<7.8>TCID<50> can be obtained under the concentration of 10<3.5>TCID<50>, and the results of safety evaluation and potency test evaluation of over-dose inoculation show that the bivalent live vaccine for bovine viral diarrhea and infectious bovine rhinotracheitis has the characteristics of low cost, high efficacy and safe inoculation.
Owner:华威特(江苏)生物制药有限公司
Application of Oritavancin Phosphate in Preparation of Drugs for Prevention and Treatment of Bovine Infectious Rhinotracheitis
ActiveCN109550041BAntiviralsSaccharide peptide ingredientsOritavancinInfectious bovine rhinotracheitis virus
The invention provides an application of oritavancin phosphate in the preparation of a drug for preventing and treating bovine infectious rhinotracheitis. Oritavancin phosphate can inhibit and kill bovine infectious rhinotracheitis in an in vitro MDBK cell model The virus can effectively inhibit the invasion and replication of bovine infectious rhinotracheitis virus, and has low cytotoxicity. It also lays the foundation for the development of highly effective and specific anti-bovine infectious rhinotracheitis virus drugs and provides a new perspective.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI +1
Application of ertapenem sodium in preparation of medicine for preventing and treating bovine infectious rhinotracheitis
InactiveCN109646435BEnhanced inhibitory effectDirect killOrganic active ingredientsAntiviralsDiseaseErtapenem sodium
The invention provides the application of ertapenem sodium in the preparation of medicines for preventing and treating bovine infectious rhinotracheitis. Ertapenem sodium can inhibit and kill bovine infectious rhinotracheitis virus on the MDBK cell model in vitro, can effectively inhibit the invasion and replication of bovine infectious rhinotracheitis virus, and has low cytotoxicity, and can be used as a class of The new drug against bovine infectious rhinotracheitis virus has laid a foundation for the prevention and control of bovine infectious rhinotracheitis disease and drug research and development, and has also laid an experimental foundation for the development of highly effective and specific anti-bovine infectious rhinotracheitis virus drugs. Offer new perspectives.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI +1
Application of oritavancin diphosphate to preparation of medicines for preventing and treating infectious bovine rhinotracheitis
ActiveCN109550041AAntiviralsSaccharide peptide ingredientsDiseaseInfectious bovine rhinotracheitis virus
The invention provides an application of oritavancin diphosphate to preparation of medicines for preventing and treating infectious bovine rhinotracheitis. On a MDBK cell model of in-vitro experiment,the oritavancin diphosphate can restrain and kill infectious bovine rhinotracheitis viruses, can effectively restrain invasion and duplication of the infectious bovine rhinotracheitis viruses, is small in cytotoxicity, can be used as a new medicine for resisting the infectious bovine rhinotracheitis viruses, establishes foundation for prevention and control of infectious bovine rhinotracheitis diseases and research and development of medicines, also establishes experiment foundation and provides new view for development of high-efficiency specific infectious bovine rhinotracheitis virus resisting medicines.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI +1
Detection method of infectious bovine rhinotracheitis virus
InactiveCN112981010AShort detection timeEasy to operateMicrobiological testing/measurementMicroorganism based processesInfectious bovine rhinotracheitis virusInfectious bovine rhinotracheitis
The invention belongs to the technical field of biology, and particularly relates to a detection method of an infectious bovine rhinotracheitis virus. The detection method comprises a specific target sequence as shown in SEQ ID NO. 1, a kit comprises a fluorescent quantitative PCR detection primer pair and a probe, the primer pair is as shown in SEQ ID NO. 2 and SEQ ID NO. 3, and the sequence of the probe is as shown in SEQ ID NO. 4. The TaqMan probe fluorescent quantitative PCR kit for detecting the infectious bovine rhinotracheitis virus has high specificity and sensitivity, can be used for screening various clinical samples to detect infectious bovine rhinotracheitis positive samples, and has the characteristics of short detection time, high detection sensitivity and high stability.
Owner:XINJIANG AGRI UNIV
A gene Gbabc
ActiveCN106047902BGood antigenicityImprove immune activityAntibody mimetics/scaffoldsVirus peptidesImmunocompetenceAntigenicity
The invention discloses a gene gBabc which is obtained from main antigen glycoprotein gB of infectious bovine rhinotracheitis virus. The gene can express fusion protein which is high in immunogen activity, and finally, the gene can make a colloidal gold immunochromatography test strip which is used for detecting an infectious bovine rhinotracheitis antibody. The target gene disclosed by the invention, through successful and efficient expression as well as separation and purification, can prepare fusion antigen protein gBF for detecting the infectious bovine rhinotracheitis antibody, and experiments prove that the fusion protein is high in antigenicity and immunocompetence; therefore, a colloidal gold immunochromatography for the infectious bovine rhinotracheitis antibody is established.
Owner:HEBEI AGRICULTURAL UNIV.
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com