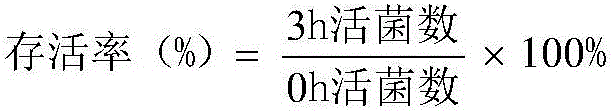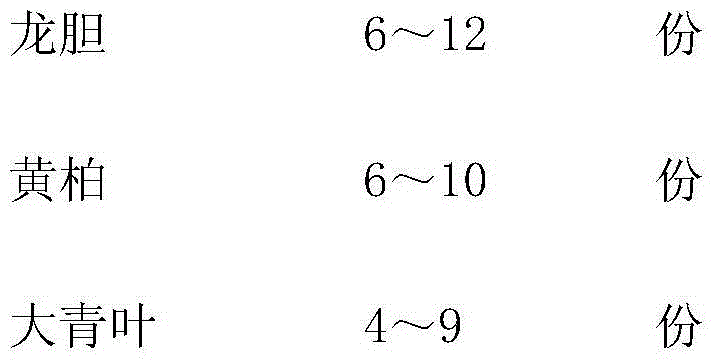Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
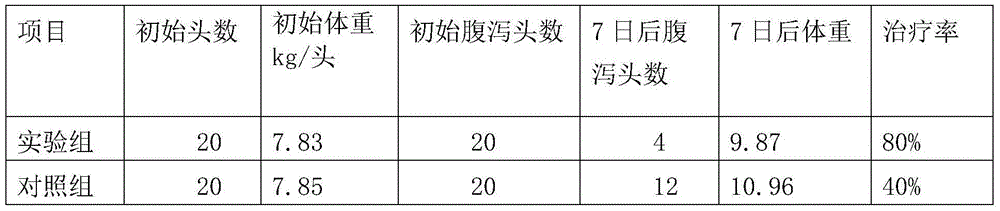
113 results about "Viral diarrhea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral diarrhea is actually caused by several different viruses, and the technical name for it is viral gastroenteritis. These viruses get into a person’s stomach through a variety of methods, usually involving contact with an infected person. They inflammation, which eventually leads to vomiting and diarrhea.
Traditional Chinese medicine mixture for treating livestock and poultry virosis and preparation method thereof
ActiveCN101554414AEasy to takeAbsorb quickly and playDigestive systemAntiviralsHigh grade feverRadix isatidis
The invention relates to a traditional Chinese medicine mixture for treating livestock and poultry virosis and a preparation method thereof. The traditional Chinese medicine mixture contains the contents according to the parts by weight: 10-40 of radix isatidis, 10-40 of folium isatidis, 7-20 of honeysuckle, 3-9 of radix bupleuri, 1-6 of radix lithospermi, 5-20 of forsythia, 5-20 of scutellaria baicalensis, 3-9 of lightyellow sophora root, 2-7 of coptis chinensis, 4-11 of raidx astragali and 1-6 of liquorice. The traditional Chinese medicine mixture has reasonable compositions, convenient mixture taking, obvious effects and no mixture residues, conforms to the safe requirement of animal-derived foods and can be effectively applied to preventing and curing the infective livestock and poultry virosis of chicken flu, newcastle diseases, duck hepatitis viruses, swine fever, virus diarrhea, and the like. The traditional Chinese medicine mixture has the advantages of low cost, simple preparation process and no environmental pollution and is suitable for industrial production.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
Test paper for one-step detection for pig virus diarrhoea disease pathogen
The invention discloses a test strip which is used for testing porcine viral diarrhea pathogen in one step, and the test strip comprises a support layer, an adsorption layer and a protection layer. The adsorption layer has a sample fiber layer, a gold antibody fiber layer, a cellulose film layer and a hygroscopic material layer at the handle end sequentially from a test end. The cellulose film layer has a test blot and a contrast blot, and the gold antibody fiber layer is adhered with at least one of monoclonal antibodies or polyclonal antibodies of anti-TGEV, anti-PDEV and anti-RV with colloid gold mark; the test blot is printed by at least one of polyclonal antibody liquid or monoclonal antibody liquid of anti-TGEV, anti-PDEV and anti-RV, and the contrast blot is printed by IgG solution of sheep or rabbit anti-mouse or IgG solution of sheet or rabbit anti-porcine. When used for testing, the test strip has the advantages of strong specificity, high sensitivity, convenient and rapid operation and direct and accurate testing result. The test strip is especially suitable for fast identifying and diagnosing on site.
Owner:HENAN ACAD OF AGRI SCI
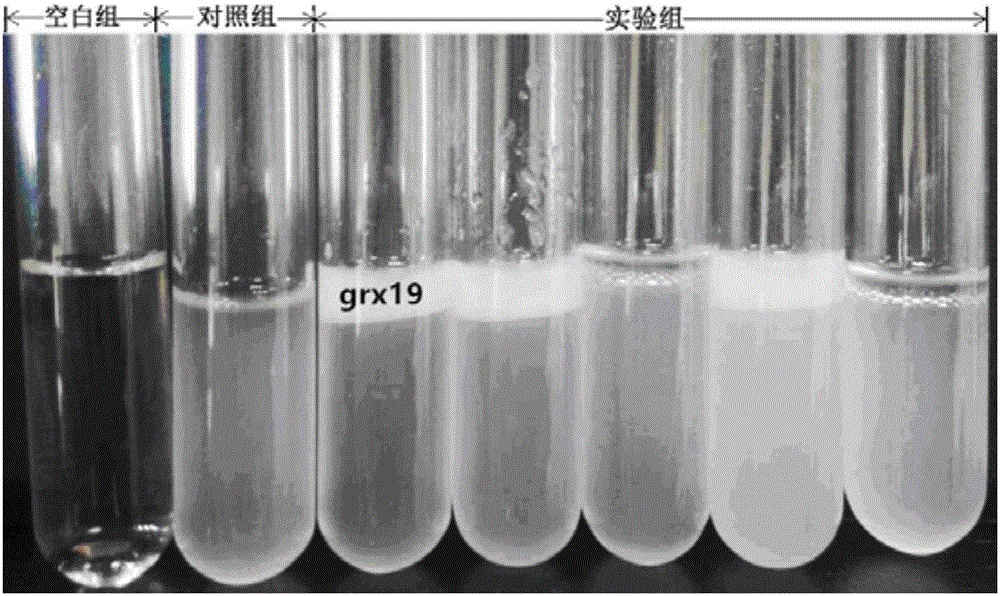
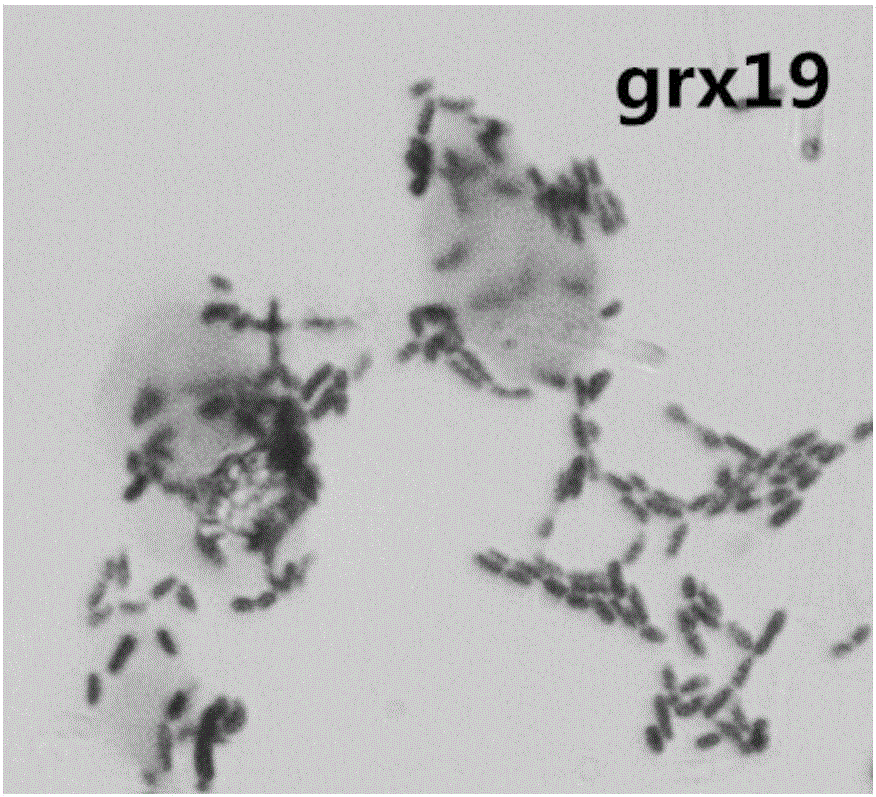
Application of lactobacillus rhamnosus grx19 in regulation of intestinal floras
InactiveCN105920049AExcellent intestinal colonization abilityStructural regulationMilk preparationDigestive systemCrypt cellLactobacillus rhamnosus
The invention relates to new application of lactobacillus rhamnosus grx19, and discloses application of the lactobacillus rhamnosus in preparation of functional foods and drugs for regulating intestinal floras and preventing viral diarrheas. The lactic acid bacteria grx19 disclosed by the invention has good acid and bile salt tolerance and pathogenic bacteria inhibition, higher surface hydrophobicity, higher self-aggregation ability, and obvious adhesion for small intestinal epithelial crypt cells (IEC-6 cells) of rats shown by cell research. The lactobacillus grx19 disclosed by the invention has the effects beneficial to human health like general lactobacilli, and further can regulate the structures of the intestinal floras and prevent the viral diarrheas, thereby being used for production of the functional foods or drugs.
Owner:YANGZHOU UNIV
Preparation and application method for treating swine viral diarrhea biological agent
InactiveCN103694348ALow costHigh recovery rateEgg immunoglobulinsDigestive systemSwine Transmissible GastroenteritisHigh activity
The invention relates to a preparation and application method for treating a swine viral diarrhea biological agent, and belongs to the technical field of biological agents. The method specifically comprises the following steps of: (1) preparing for immunogen preparation, namely, getting swine transmissible gastroenteritis and porcine epizootic diarrhea duplex inactivated vaccine which consists of two viral protein; (2) performing immune procedure; (3) detecting valence of antibody; (4) purifying an egg yolk antibody; and (5) identifying the egg yolk antibody. The preparation and application method has the advantages that the water diluting method is performed and the method is safe, reliable, out of pollution, and low in cost; the treated high-immunity egg can gain the egg yolk antibody with high activity, purity and output and stable performance; the operation method is simple, feasible, and reliable; the industrial production can be carried out conveniently.
Owner:刘聚祥
A pueraria root scutellaria and coptis extract
InactiveCN101156907AReduce diarrheaSlow downAntibacterial agentsDigestive systemMedicineBacillary dysentery
The invention provides Gegenqinlian extractive which is obtained by mixing four medicines of kudzuvine roots, baikal skullcap roots, goldthread roots, and licorice roots in the weight ratio of 8: 3: 3: 2, extracting effective parts and refining. The extractive has the main effective parts in the following weight percentage: total isoflavone of the kudzuvine roots is 13.5 to 21.0 percent, total flavone of the baikal skullcap roots is 20.5 to 32.5 percent, total alkaloid of the goldthread roots is 10.5 to 18.5 percent, and glycerrhizic acid is 2.0 to 5.0 percent. Through adding medicinal auxiliary materials into the extractive, and adopting a conventional method, various peroral preparation for treating bacillary dysentery, acute enteritis, and infantile virus diarrhea can be prepared.
Owner:SOUTHERN MEDICAL UNIVERSITY
Pure natural traditional Chinese medicine feed additive as well as preparation method and application thereof
InactiveCN104171629AOvercome shortcomings that affect utilizationOvercome the disadvantages of exploitingAnimal feeding stuffNatural productImmunity
The invention relates to a pure natural traditional Chinese medicine feed additive and a preparation method thereof and belongs to the filed of animal feed additives capable of preventing diarrhea of piglets. The pure natural traditional Chinese medicine feed additive is a compound feed additive prepared by mixing medicine extracts capable of improving immunity of the piglets, preventing bacterial and viral diarrhea of the piglets and improving appetite with a natural mineral carrier in a certain ratio. The pure natural traditional Chinese medicine feed additive has the main effects of improving the immunity of the piglets, preventing and treating diarrhea, increasing survival rate, promoting digestive absorption, improving daily gain and feed utilization efficiency, reducing production cost and improving meat quality. All the raw materials required by the pure natural traditional Chinese medicine feed additive are natural, safe, non-toxic, rich and low in cost, so that the pure natural traditional Chinese medicine feed additive is convenient for popularization and application.
Owner:BEIJING JIAHE AGRI BIOTECH
DPO (Dual Priming Oligonucleotide) primer group for porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus detection and application of DPO primer group
ActiveCN108060269ALarge annealing temperature rangeStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationReal-Time PCRsPorcine rotavirus
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
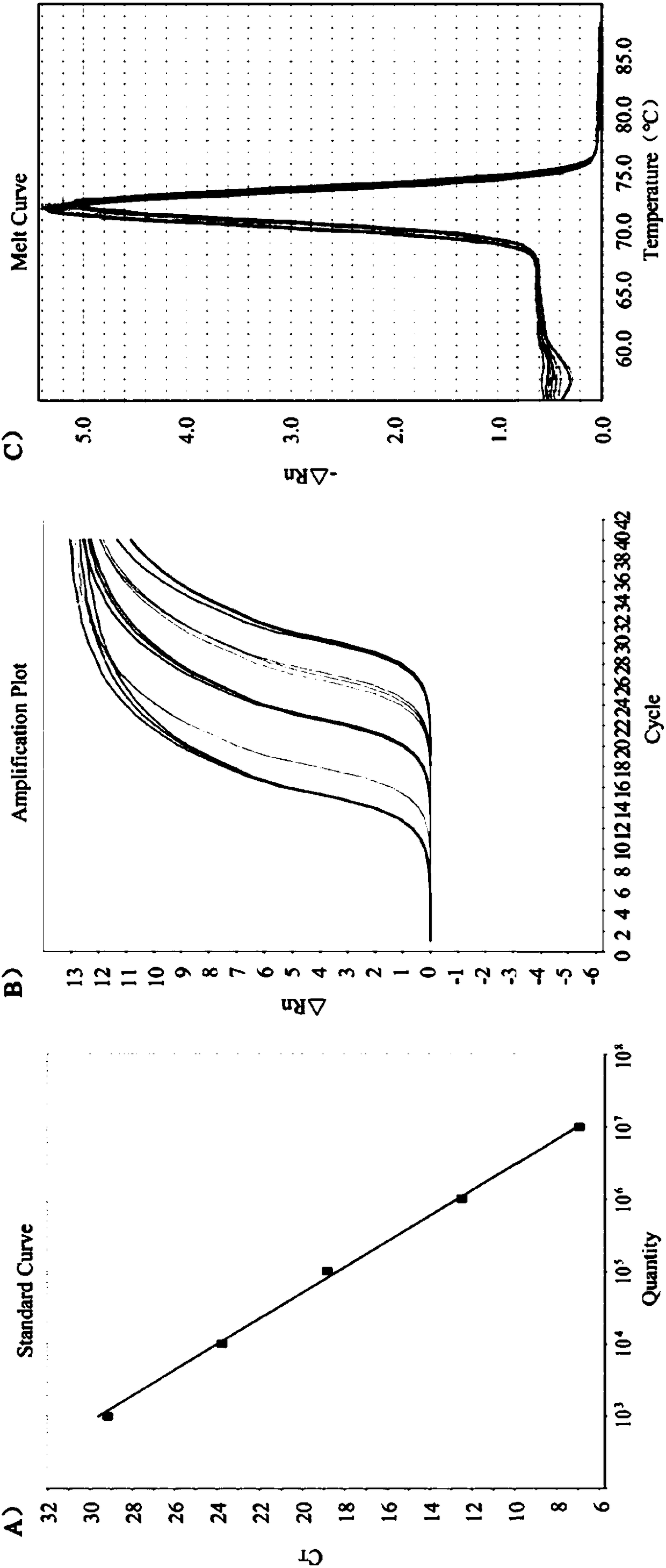
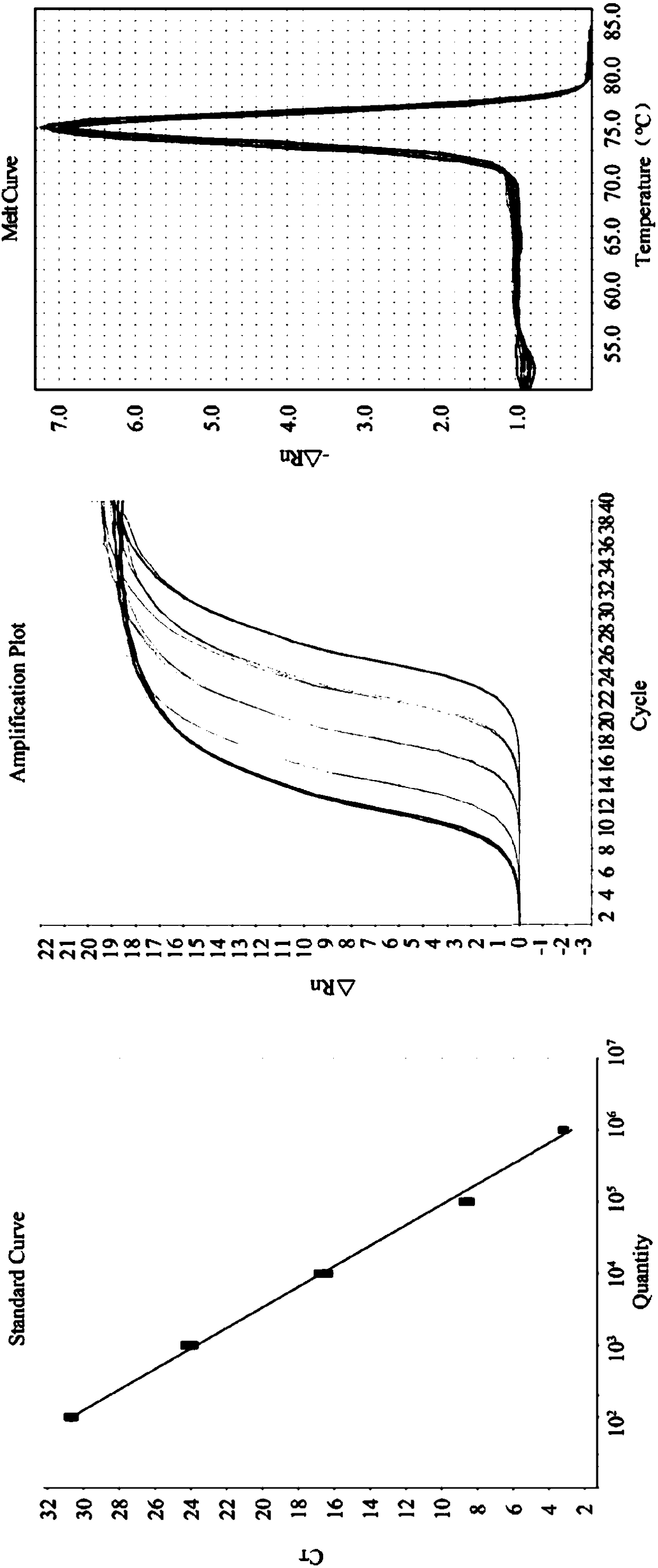
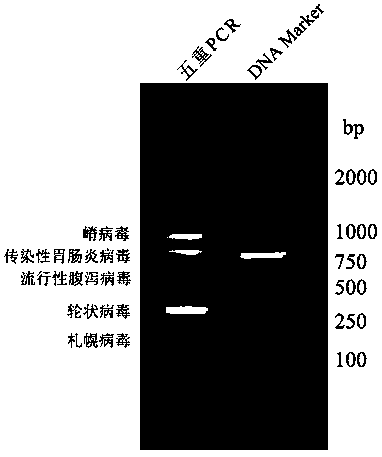
Quintuple RT-PCR detection kit for porcine viral diarrhea viruses
ActiveCN105506182AAccurate judgmentRapid diagnosisMicrobiological testing/measurementMicroorganism based processesTotal rnaPorcine rotavirus
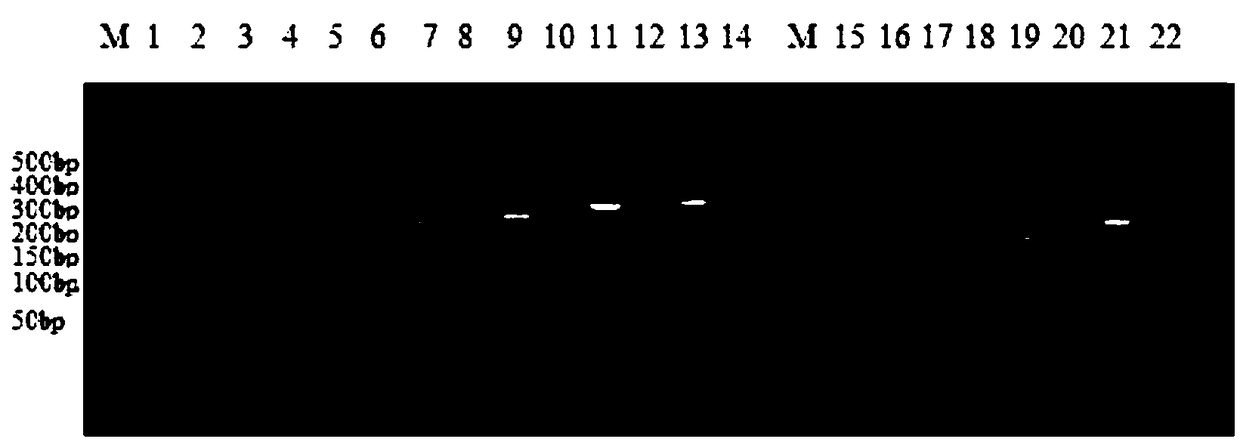
The invention discloses a quintuple RT-PCR detection method and kit for porcine epidemic diarrhea viruses, porcine transmissible gastroenteritis viruses, porcine rotaviruses, porcine sapoviruses and porcine kobuviruses. The kit comprises ten specificity amplification primers. In the using process of the kit, total RNA of a sample to be detected is subjected to reverse transcription to become cDNA through a 6-basic-group random primer, a detection reaction system in the kit is used for RCR amplification with the cDNA as a template, and whether the sample to be detected is infected with one kind of pathogens or is under mixed infection is determined according to different sizes of amplified PCR segments of different pathogens. On the condition of ensuring specificity and sensitivity, the kit has the advantages of being easy and quick to operate and lowering detection cost and labor intensity, and is suitable for field sample detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Formula for traditional Chinese medicines for treating different types of porcine diarrhea
InactiveCN106039248ANo pollutionGood treatment effectAntibacterial agentsDigestive systemSide effectOfficinalis
The invention discloses a formula for traditional Chinese medicines for treating different types of porcine diarrhea. The traditional Chinese medicines in the formula comprise 30-40 parts of rhizoma atractylodis, 30-40 parts of rhizoma atractylodis macrocephalae, 20-30 parts of cortex magnoliae officinalis, 20-30 parts of rhizoma alismatis, 15-20 parts of pericarpium citri reticulatae, 20-30 parts of poria, 20-30 parts of polyporus, 20-40 parts of herba agastaches rugosae, 10-20 parts of herba periliae, 10-20 parts of radix angelicae dahuricae, 15-30 parts of rhizoma pinelliae, 15-30 parts of radix platycodonis, 10-20 parts of pericarpium arecae, 7-10 parts of ramulus cinnamomi, 7-10 parts of rhizoma zingiberis recens and 15-20 parts of radix glycyrrhizae. The formula has the advantages that traditional Chinese medicine compositions are pure traditional Chinese medicine compositions without other antibiotics, and obvious effects of treating bacterial diarrhea, viral diarrhea and particularly, epidemic porcine diarrhea and cold-dampness type diarrhea due to other factors can be realized; the traditional Chinese medicines are free of medicine residues or toxic and side effects or medicine resistance and high in safety on the premise that optional chemical components and the antibiotics are omitted, and recurrence can be prevented; the formula is low in cost and hopefully can be widely popularized in actual animal husbandry and Chinese herbal medicines, and the like.
Owner:李业静
Antidysenteric premixed agent for roaster
ActiveCN1899437AHeat-clearing and detoxifying toolsAntidiarrheal and dysentery deviceDigestive systemPlant ingredientsEpidemic diarrheaMedicine
The antidysenteric pre-mixed preparation for sucking pig is prepared with the materials including wolf's milk 30-60 weight portions, Chinese goldthread 6-10 weight portions, rhubarb 2-5 weight portions, eclipta 10-15 weight portions and phellodendron bark 7-10 weight portions. The antidysenteric pre-mixed preparation may be used in treating viral diarrhea, mixed viral and bacterial diarrhea of various kinds of animal, and has obvious curative effect on pig's epidemic diarrhea.
Owner:江西和泽生物科技有限公司
Chinese medicinal oral solution for treating calf viral diarrhea and preparation method thereof
InactiveCN103272088AQuick resultsDefinite curative effectDigestive systemPharmaceutical delivery mechanismBaical Skullcap RootLicorice roots
The invention discloses a Chinese medicinal oral solution for treating calf viral diarrhea and a preparation method thereof. The Chinese medicinal oral solution is prepared from the following components in parts by weight: 5-8 parts of Chinese pulsatilla roots, 4-6 parts of ash barks, 4-6 parts of baical skullcap roots, 3-5 parts of golden threads, 3-5 parts of honeysuckle flowers, 2-4 parts of weeping forsythiae capsules, 2-4 parts of amur corktree barks, 2-4 parts of plantain seeds, 1-3 parts of areca seeds and 1-3 parts of licorice roots. The Chinese medicinal oral solution is obtained through a decoction method. According to the Chinese medicinal oral solution for treating calf viral diarrhea, the traditional Chinese medicines are reasonably formulated, so that a function of treating viral diarrhea is realized, and the Chinese medicinal oral solution for treating calf viral diarrhea is provided for the cultivation industry and has the characteristics of quick response and exact curative effect, and medicine residues and toxic and side effects are avoided; and moreover, the preparation method is easy and is suitable for the requirement of scale industrial production.
Owner:TIANJIN SHENGJI GRP CO LTD
Traditional Chinese medicinal composition for pigs and application of traditional Chinese medicinal composition
ActiveCN103432267AImprove survival rateLow priceAntibacterial agentsHeavy metal active ingredientsBiotechnologyPig farms
The invention relates to the technical field of veterinary medicines, and in particular relates to a traditional Chinese medicinal composition for pigs. The traditional Chinese medicinal composition consists of 6-14 parts of smoked plum, 6-14 parts of myrobalan, 4-8 parts of rhizoma picrorhizae, 2-6 parts of sanguisorba carbon, 2-6 parts of astragalus membranaceus, 8-12 parts of halloysitum rubrum, 6-10 parts of anemone chinensis, 2-6 parts of rhizoma atractylodis and 2-6 parts of liquorice. The traditional Chinese medicinal composition can be powder, granules or oral administration liquid. The traditional Chinese medicinal composition for the pigs, which is disclosed by the invention, is a pure traditional Chinese medicinal composition, does not contain any chemical medicaments, has no residue and no toxic or side effect, is high in safety and difficultly generates drug resistance; an application test in a pig farm shows that the traditional Chinese medicinal composition has good treating effects of bacterial diarrhea, viral diarrhea, nutritional diarrhea and diarrhea caused by other various reasons of piglets; the pharmaceutical effect is quick to take, a quick respond is realized; the using amount of medicines is small; the cost is reduced; the survival rate of piglets is obviously improved; the treatment rate can be over 95 percent; the price is low; the medication safety is realized; the drug resistance can be avoided; the traditional Chinese medicinal composition can be popularized and applied.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
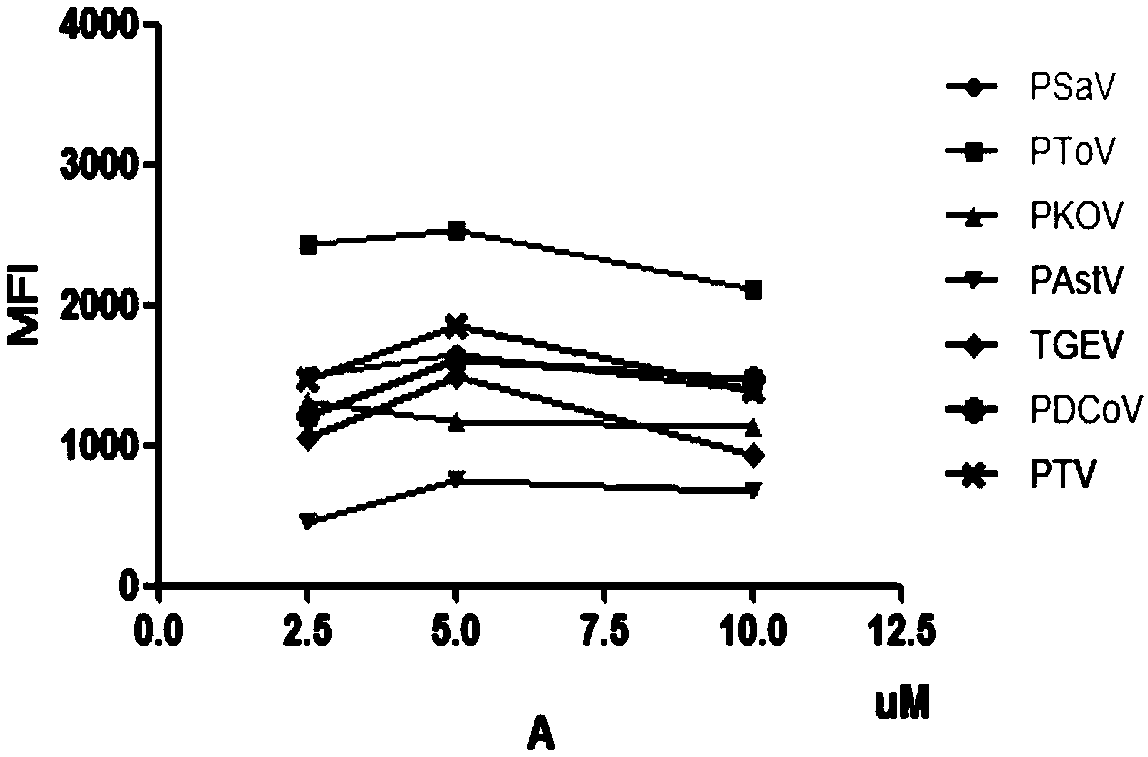
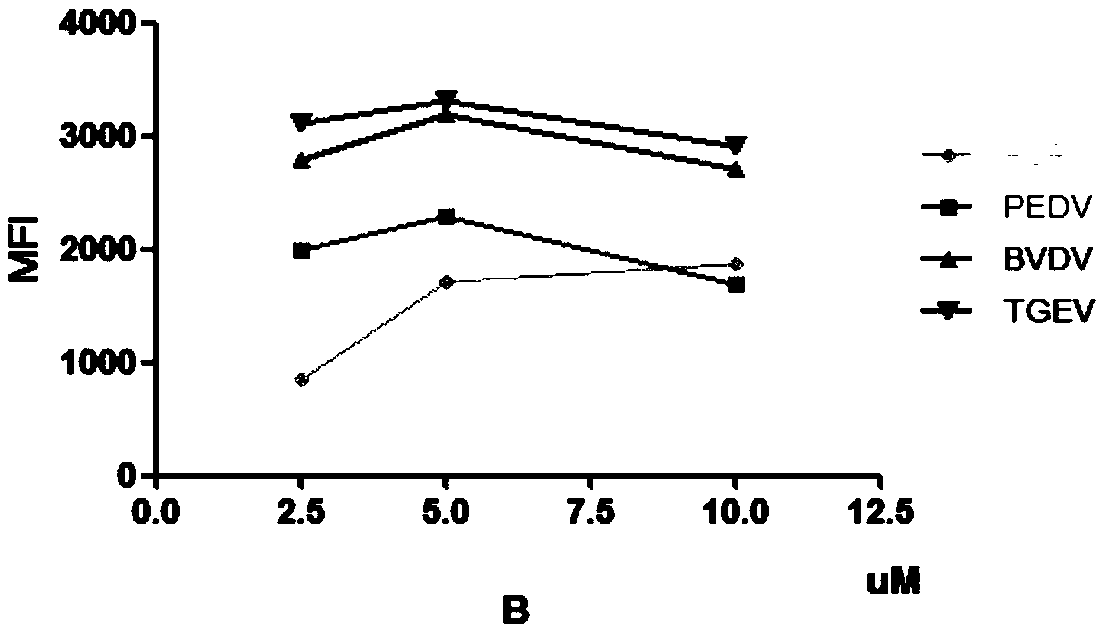
Multiple liquid-phase chip detection method for swine viral diarrhea pathogens and construction of multiple liquid-phase chip detection method
ActiveCN108913812AAchieve qualitativeEasy to detectMicrobiological testing/measurementMicrosphereBiotin
The invention provides a multiple liquid-phase chip detection method for swine viral diarrhea pathogens and construction of the multiple liquid-phase chip detection method. The method comprises the following steps: according to the gene sequences of the swine viral diarrhea pathogens, screening out suitable TAG sequences, and coupling the suitable TAG sequences to a microballoon; marking TAG complementary sequences to 5'terminals of upstream primers of PCR primers of the pathogens, and marking biotin at the 5'terminals of downstream primers of the PCR primers; and carrying out multiple PCR amplification by utilizing a modification primer, carrying out hybridization on the PCR amplification products and the TAG sequences coupled to the microballoon, detecting the MFI value, and judging thefeminine gender or masculine of the detection result according to the MFI value. With the method, the detection specificity and sensitivity are improved, the qualitative and quantitative detection formultiple pathogens of the swine viral diarrhea is realized, and in addition, the method has the advantages of high throughput, time and labor conservation, strong specificity, high sensitivity and the like, and can satisfy the demand of simultaneously detecting various pathogens.
Owner:SHANGHAI ACAD OF AGRI SCI
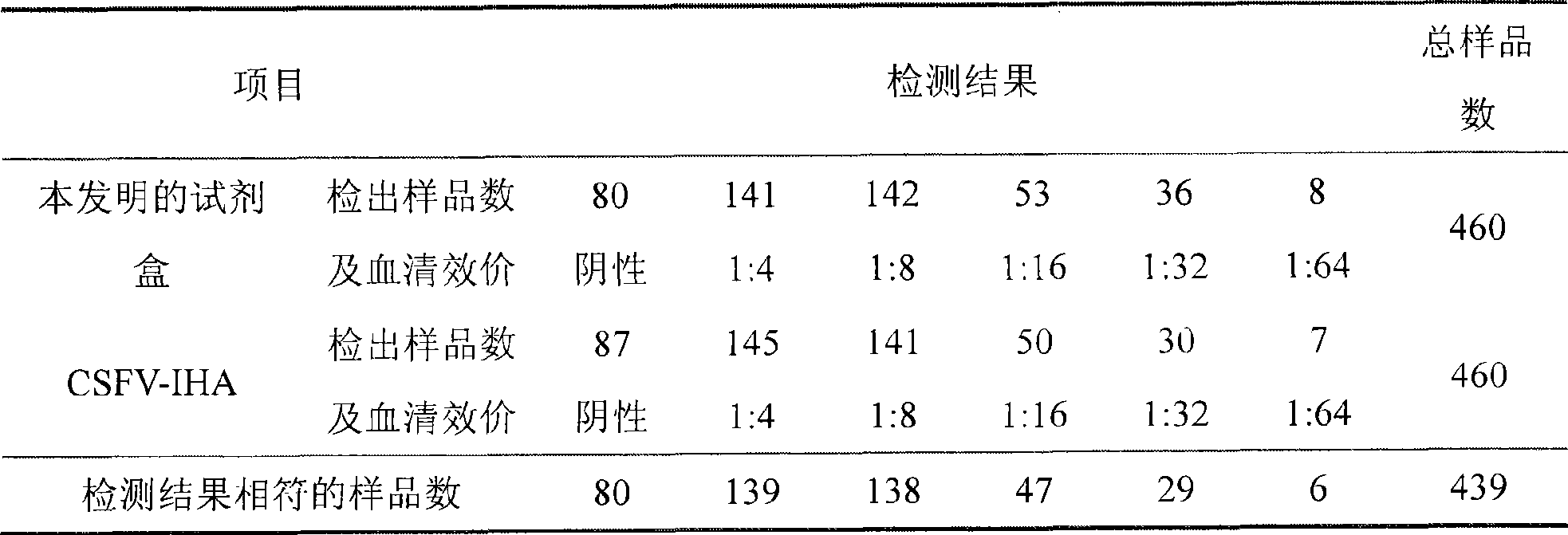
Hog cholera indirect hemagglutination detection kit and preparation
InactiveCN101487017AAccurate responseStrong specificityVirus peptidesMicroorganism based processesEscherichia coliNucleotide
The invention relates to a preparation method of a recombinant protein, an indirect classical swine fever hemagglutination test kit that is prepared by the protein, and a preparation method of the test kit. The preparation method of the protein comprises following steps: a segment with the gene homeology degree comparing with a bovine viral diarrhea-mucosal disease virus (BVDV) that is lower than 35 percent is selected from a conservative region of a classical swine fever virus (CSFV) gene, thus obtaining a nucleotide sequence S1 that is corresponding to an amino acid A1; a forth amino acid in the nucleotide sequence S1 is modified to an hydrophilic amino acid, thus obtaining an amino acid A2; a corresponding nucleotide sequence S2 of A2 is modified, thus respectively obtaining three new nucleotide sequences; the three new nucleotide sequences are orderly and serially connected by connecting nucleotides, thus obtaining a sequence S9 that is corresponding to an amino acid sequence A3; and a nucleotide sequence S10 is obtained by processing S9 correspondingly, and after artificially synthesizing and digesting S10, connecting S10 with a carrier pGEX-4T-1 and inducing the recombinant carrier into colon bacillus for induction expression, the recombinant protein is obtained by separating and purifying an expression product.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Anti-diarrhea piglet feed
InactiveCN109043181AControl bacteriaControl viral diarrheaFood processingAnimal feeding stuffDiseasePhytase
The invention discloses an anti-diarrhea piglet feed. The feed is prepared from the following components in parts by weight: 670 to 680 parts of soybean meal, 65 to 75 parts of corn germ meal, 20 to 28 parts of stone powder, 18 to 24 parts of soybean oil, 75 to 85 parts of expanded soybean, 28 to 32 parts of fishmeal, 27 to 33 parts of 70% lysine, 0.55 to 0.65 of methionine, 3.2 to 3.6 parts of threonine, 18 to 20 parts of calcium hydrogen phosphate, 15 to 17 parts of salt, 3.15 to 3.25 parts of choline chloride, 18 to 22 parts of premix compound, 1.15 to 1.25 parts of feed additive, 0.55 to 0.65 parts of phytase, 4.5 to 5.5 parts of enzyme preparation, 0.35 to 0 .45 parts of antioxidant, and 1.1 to 1.3 parts of sweetener. The anti-diarrhea piglet feed has the advantages that by using theanti-diarrhea piglet feed disclosed by the invention to feed piglets, the bacterial and viral diarrhea of the piglets can be effectively controlled, and at the same time, the occurrence of yellow-white dysentery and red dysentery of the piglets is reduced, and the disease resistance of the piglets is improved; food intake and appetite of the piglets are improved, piglet growth is promoted, energyand nutrient conversion to muscle tissues is promoted, protein synthesis and deposition are promoted, the ketone body lean rate is increased, the piglet body shape is improved, feed conversion is improved, free radicals are scavenged, meat color is improved, various stress responses of the piglets are relieved, and non-specific immunity of the piglets is increased.
Owner:淮北华大农牧科技有限公司
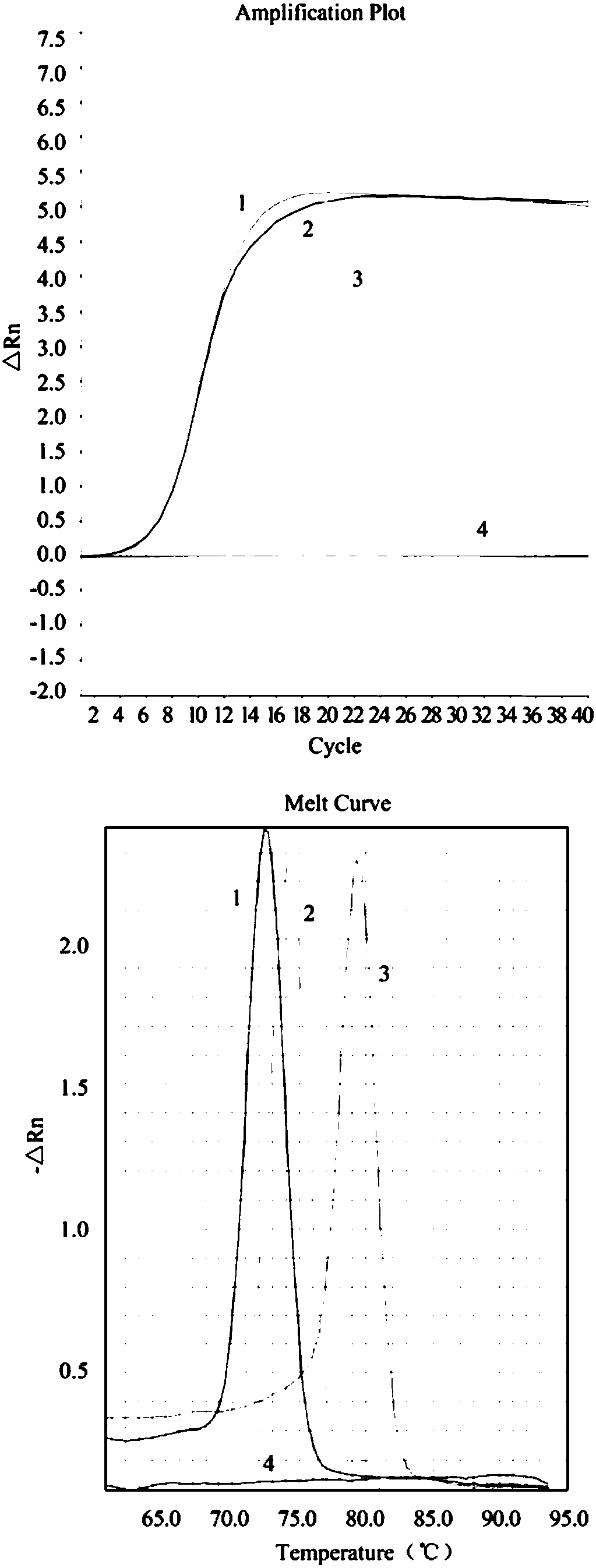
Real-time fluorogenic quantitative PCR detection kit for bovine viral diarrhea-diarrhoea virus and special primer and probe thereof
InactiveCN106167837AAchieving identification of bovine viral diarrhea-mucosal disease virus (BVDV)Achieving the purpose of swine fever virus (CSFV)Microbiological testing/measurementDNA/RNA fragmentationVaccine ProductionCow milk
The invention discloses a real-time fluorogenic quantitative PCR detection kit for type-I and type-II bovine viral diarrhea-diarrhoea virus and a special primer thereof and a TaqMan probe. The detection kit has advantages of simple operation, strong specificity, high sensitivity and good repeatability for BVDV detection. The detection kit can be used not only for identification of type-I and type-II bovine viral diarrhea-diarrhoea virus and hog cholera virus but also for accurate quantification of type-I and type-II bovine viral diarrhea-diarrhoea virus. The detection kit can be used in fields of detection of raw materials and semi-finished products in vaccine production and detection of dairy products, etc.
Owner:JINYUBAOLING BIO PHARMA CO LTD
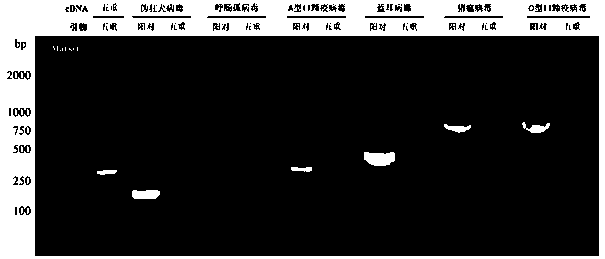
Bovine rotavirus, coronavirus, and viral diarrhea virus multi-connected RT-PCR detection method
ActiveCN110079637AShorten the timeReagent savingMicrobiological testing/measurementAgainst vector-borne diseasesBovine rotavirusElectrophoresis
The invention discloses a bovine rotavirus, coronavirus, and viral diarrhea virus multi-connected RT-PCR detection method, which is characterized in that according to the detection method, RNA of a to-be-detected sample is taken as an RNA template, and a specificity upstream primer and a specificity downstream primer designed according to hereditary characteristics of the bovine rotavirus, coronavirus, and viral diarrhea virus are taken as a primer to perform RT-PCR proliferation to obtain a proliferation product. Then, electrophoresis detection is performed on the proliferation product with 1.5% agarose gel, and a result is recorded; according to the results of the electrophoresis detection, whether the to-be-detected sample contains the bovine rotavirus, coronavirus, and viral diarrhea virus is determined. The bovine rotavirus, coronavirus, and viral diarrhea virus detection method achieves has the beneficial effect of accurately, rapidly and efficiently distinguishing and diagnosingthe three viral disease with RT-PCR.
Owner:INNER MONGOLIA UNIV FOR THE NATITIES
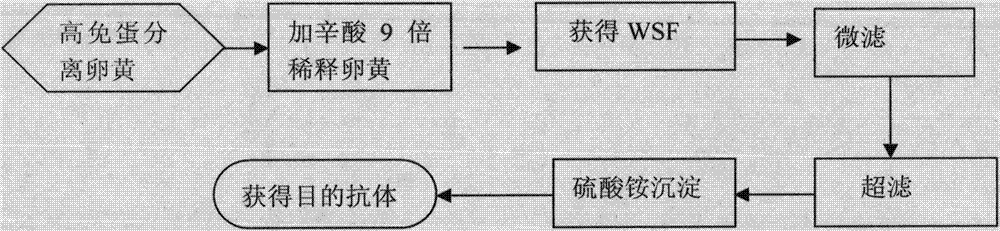
Preparation method of compound egg yolk antibody medicinal preparation for preventing and treating piglet virus epidemic disease
A compound yolk antibody preparation for preventing the viral diseases of piglet, including viral diarrhea of piglet is prepared through providing the vaccines of infectious gastroenteritis, epidemic diarrhea and rotavirus, immunizing with them sequentially by three turns, collecting immunized eggs, taking yolk, diluting with aseptic buffer liquid of phosphate, treating by hydroxypropylmethyl cellulose phthalate, filtering, and spraying drying.
Owner:SHANGHAI JIAO TONG UNIV
Traditional Chinese medicine composition for preventing piglet dysentery and preparation method thereof
InactiveCN102743657ACompatibility is reasonableGood treatment effectDigestive systemPlant ingredientsBiotechnologyEscherichia coli
The invention discloses a traditional Chinese medicine composition for preventing piglet dysentery and a preparation method thereof. The traditional Chinese medicine composition, by weight, comprises 5-20 parts of bulk pharmaceutical chemicals hawthorn, 5-20 parts of malt, 5-25 parts of pericarpium citri reticulatae, 5-25 parts of medicated leaven, 10-30 parts of radix bupleuri, 10-30 parts of root of Chinese pulsatilla and 10-30 parts of root of common peony. Firstly needed bulk pharmaceutical chemicals are processed to be clean, then drying and smashing are carried out, various bulk pharmaceutical chemical powder are weighted to be sufficiently and evenly mixed, finally split charging is carried out. The traditional Chinese medicine composition can effectively prevent and treat various irritability diarrheas, trophism diarrheas and yellow and white dysentery caused by escherichia coli, has obvious effects on viral diarrheas, can protect and repair intestinal mucosa and recovers digestion capability of piglets.
Owner:河南中亚神鹏医药科技有限公司
Primer combination for identifying bovine viral diarrhea disease virus and bovine rotavirus and application thereof
ActiveCN106987657AEfficient amplificationStrong specificityMicrobiological testing/measurementMicroorganism based processesBovine rotavirusDisease
The invention discloses a primer combination for identifying bovine viral diarrhea disease virus (BVDV) and bovine rotavirus (BRV) and application thereof. The primer combination provided by the invention consists of single chain DNA molecules shown in sequences 1-8 in a sequence table; 5' end of a single chain DNA molecule shown in the sequence 3 is connected with fluorophore A, and 5' end of a single chain DNA molecule shown in the sequence 7 is connected with fluorophore B. According to the primer combination disclosed by the invention, the fluorophores are introduced into an LAMP method firstly, a dual-fluorescence RT-LAMP method for identifying BVDV and BRV can be established; by color observation of amplified products, two viruses can be simultaneously identified and diagnosed. The dual- RT-LAMP method built by the invention has good specificity, and can effectively amplify target genes without amplification to other pathogenic nucleic acid, so that the sensitivity is good, and 100 mixed template copy / reaction can be detected at minimum; therefore, the primer combination is a convenient, quick and low-cost diagnosis method, and is suitable for large-scale epidemiologic investigation.
Owner:GUANGXI VETERINARY RES INST
Pestivirus mutants and vaccines containing the same
The present invention is directed to attenuated pestivirus mutants, which have a reduced ability to replicate as exhibited by a small plaque size. The mutations are in the 5′ nontranslated region of the viral genome. These mutant viruses are useful as live vaccines in the control of bovine viral diarrhea, border disease and classical swine fever.
Owner:INTERVET INT BV
Mycoplasma bovis immune related protein, detection kit containing same and application thereof to detection of mycoplasma bovis antibody
The invention discloses a mycoplasma bovis immune related protein, a detection kit containing the same and application thereof to detection of a mycoplasma bovis antibody. The mycoplasma bovis immunerelated protein is named p28 protein, and the amino acid sequence of the protein is shown in SEQ ID NO.2. A sensitivity test proves that the mycoplasma bovis serum antibody ELISA detection kit (MbH kit) provided by the invention can detect positive serum with the dilution multiple of 1:2560 at the minimum; and a specificity test proves that the kit has the specificity of 97.8% and has no specificreaction with the positive serums of contagious bovine pleuropneumonia (CBPP), foot-and-mouth disease (FMD), bovine tuberculosis (MB), bovine viral diarrhea (BVDV) and infectious bovine rhinotracheitis (IBRV), and has good stability and high accuracy. The invention provides a new technical means for detecting a mycoplasma bovis antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Swine umbilical cord blood swine fever virus wild strain diagnosing primer group, kit containing same and application thereof
ActiveCN106498091AExtensiveStrong characteristicMicrobiological testing/measurementDNA/RNA fragmentationBorder disease virusPositive control
The invention discloses a swine umbilical cord blood swine fever virus wild strain diagnosing primer group, a kit containing the same and application of the swine umbilical cord blood swine fever virus wild strain diagnosing primer group. Swine umbilical cord blood swine fever virus wild strain diagnosing primer are shown in SEQ ID NO: 1-SEQ ID NO: 4. The swine umbilical cord blood swine fever virus wild strain diagnosing primer group can be applied to producing a swine umbilical cord blood swine fever virus wild strain diagnosing kit. The swine umbilical cord blood swine fever virus wild strain diagnosing kit is composed of an outer set of primers, an inner set of primers, negative control, positive control and 2*PCR (polymerase chain reaction) Mixture. The swine umbilical cord blood swine fever virus wild strain diagnosing primer and the kit containing the swine umbilical cord blood swine fever virus wild strain diagnosing primer group can specifically diagnose swine umbilical cord blood swine fever virus wild strains and distinguish swine fever virus vaccine strains from swine fever virus vaccine strains as well as swine fever virus from bovine viral diarrhea-mocosal virus and border disease virus, and meanwhile, are high in sensibility, good in repeatability and reliable in detection results. The detection method through the swine umbilical cord blood swine fever virus wild strain diagnosing primer group is simple in process, short in detecting time and simple in result evaluation.
Owner:湖南国测生物科技有限公司
Pharmaceutical composition as well as preparation method and application thereof
InactiveCN104546908ALower doseLittle side effectsPowder deliveryDigestive systemEarly weaningMontmorillonite
The invention discloses a pharmaceutical composition, which comprises montmorillonite andzinc oxide at the weight ratio of (0.25-4) to 1. The invention further provides a preparation method of the pharmaceutical composition and an application of the pharmaceutical composition in preparation of medicines for preventing and / or treating piglet diarrhea. A pharmacological experiment proves that the pharmaceutical composition disclosed by the invention is capable of effectively preventing piglet diarrhea and improving the growth performance and the digestion function of piglets. In addition, the pharmaceutical composition disclosed by the invention does not need to use antibiotic in viral diarrhea of primary piglets and diarrhea of early weaning piglets; the piglets are high in survival rate and free of sequela; and the pork is relatively safe and healthy.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Compound propolis composition used for treating pig viral diarrhea and preparation method thereof
InactiveCN102600457ANo pollution in the processNo drug residueAnthropod material medical ingredientsPeptide/protein ingredientsSide effectPropolis
The invention provides a compound propolis composition used for treating pig viral diarrhea and a preparation method thereof and relates to the field of animal medicine. The preparation method provided by the invention comprises the following steps of: (1) respectively carrying out superfine grinding on various components (except for interferon) in a prescription, and respectively sieving by virtue of a 700-mesh sieve; (2) weighing 15-35 parts by weight of propolis, 10-20 parts by weight of porcine interferon, 10-35 parts by weight of granatum, 15-40 parts by weight of fructus chebulae, 10-40 parts by weight of scutellaria baicalensis, and uniformly mixing for 5-10 minutes; (3) weighing 10-35 parts by weight of dark plum, 10-35 parts by weight of Chinese knotweed herb, 15-35 parts by weight of round cardamom, 15-35 parts by weight of dried ginger, 15-35 parts by weight of potentilla chinensis, and uniformly mixing for 5-10 minutes; and (4) mixing compositions obtained by the steps (2) and (3), and continuously mixing uniformly for 10-15 minutes, thus the compound propolis composition is obtained. The compound propolis composition provided by the invention has the beneficial effects that the prescription is reasonable, the preparation method is simple, the compound propolis composition has no toxic or side effect, no pollution, no drug residue and no drug resistance, the superfine grinding efficiency and the biological availability are high, the curative effect is obvious, and clinical application shows that the compound propolis composition achieves a special effect when being used for treating pig viral diarrhea.
Owner:QINGDAO LVMAN BIOLOGICAL ENG
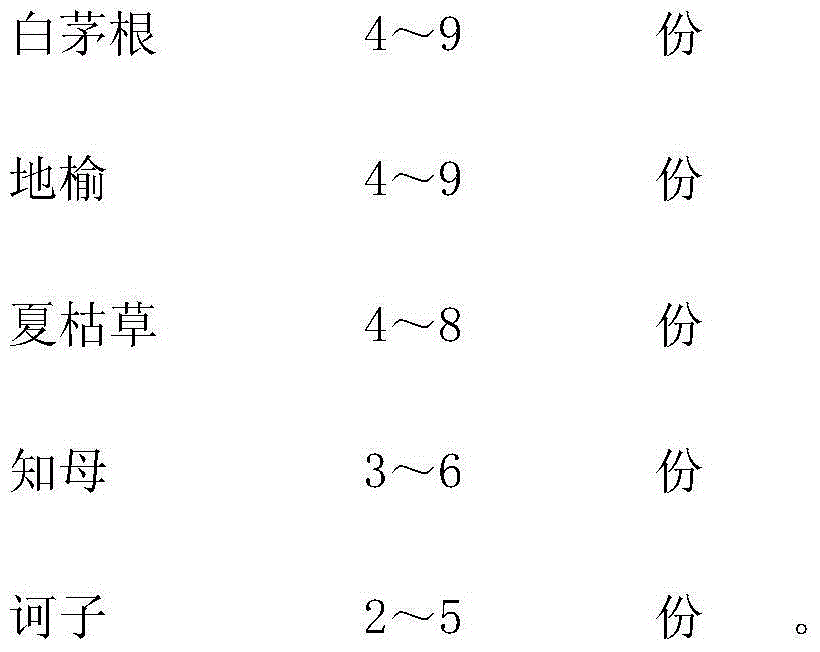
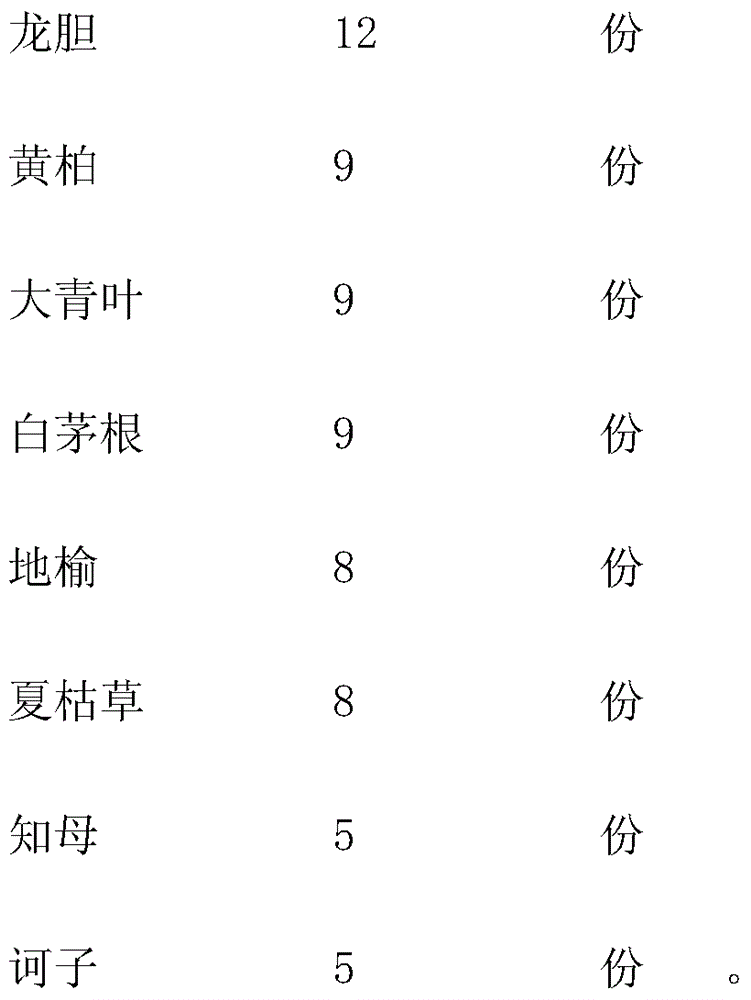
Traditional Chinese medicinal oral liquid for treating bovine viral diarrhea and preparation method for traditional Chinese medicinal oral liquid
InactiveCN104398896ALBody temperature returns to normalDigestive systemPharmaceutical delivery mechanismSide effectGentiana scabra
The invention relates to traditional Chinese medicinal oral liquid for treating bovine viral diarrhea. The traditional Chinese medicinal oral liquid comprises gentiana scabra, amur cork tree barks, folium isatidis, lalang grass rhizome, sanguisorba officinalis, prunella vulgaris, anemarrhena asphodeloides and medicine terminalia fruits. A finished product is prepared by weighing each component according to a proportion, adding water for decoction twice, mixing filtrate, performing concentration, adding ethanol for standing, performing concentration again, adding water for cold storage, finally adding sodium benzoate, and performing filling and sterilization. When the traditional Chinese medicinal oral liquid is used, diseased cattle is fed according to the body weight of the diseased cattle, and after the traditional Chinese medicinal oral liquid is continuously used for a period of time, the condition of the diseased cattle is controlled, body temperature is recovered to be normal, mucus in manure is avoided, and the digestive tract and lymph nodes are recovered to be normal after the diseased cattle is dissected; tests show that harm to viruses to the cattle can be effectively suppressed and medicinal residues and toxic or side effects are avoided.
Owner:DINGZHENG ANIMAL PHARMA TIANJIN
Compound cattle feed and preparation method thereof
InactiveCN105661057AImprove immune functionImprove anti-stressFood processingAnimal feeding stuffBiotechnologyAnimal science
The invention provides a compound cattle feed and a preparation method thereof.The compound cattle feed comprises main materials according to a mixture ratio (according to parts by weight): 50-60 parts of barley, 40-60 parts of corn, 30-50 parts of soybean, 20-30 parts of peanut meal, 20-30 parts of rapeseed meal, 10-15 parts of rice bran, 10-15 parts of bone meal and 5-13 parts of a traditional Chinese medicine additive.A production process of the compound cattle feed includes: preparation of the traditional Chinese medicine additive, preparation of a premix, granulating and dewatering, and packaging.The compound cattle feed prepared herein can improve cattle immunity and prevent and improve bovine viral diarrhea, has the advantages of safety, high efficiency, low toxicity and low residue and has a promising market prospect.
Owner:FUJIAN NIUZHUANG FOREST & ANIMAL HUSBANDRY CO LTD
Use of 17-ketosteroid compounds, as well as derivatives, metabolites and precursors for treatment of hapatitis C type virus and other togavirus infections
The present invention provides 17-keto steroid compounds and their derivatives, metabolites and precursors and pharmaceutically acceptable salts thereof for the treatment or prevention of hepatitis C virus and / or hepatitis G virus in patients requiring such treatment, these compounds Collectively referred to as "compounds of the present invention". In addition, the present invention provides methods of treating or preventing togavirus infections including one or more of alphaviruses, flaviviruses (such as yellow fever virus), hepatitis C virus, and hepatitis G virus, Infection with rubella virus or pestiviruses (such as bovine viral diarrhea virus). In addition, the present invention provides combination therapy comprising the administration of one or more compounds described herein and the administration of one or more compounds selected from the group consisting of plasma concentration-enhancing compounds, macrophage stimulating factors, oxidative agents, ribavirin, and alpha interferon compounds and / or oxygen supply. The compounds of the invention may also be used to alleviate or alleviate one or more symptoms associated with togavirus infection.
Owner:HOLLIS EDEN PHARMA
PAPN gene site-directed modified pig
The invention discloses a pAPN gene site-directed modified pig. The pAPN gene site-directed modified pig is obtained by a plurality of technologies, such as constructing a gene vector of porcine aminopeptidase N (pAPN) by adopting a method in genetic engineering and performing cell transfection. The pAPN gene site-directed modified pig is mainly applied in two aspects: 1, the infection of viral diarrhea and K88 of the pig is thoroughly eliminated from a genetic angle, the disease control cost of pig farming is reduced, the pollution to environment caused by pig farming is reduced, and a method for performing healthy cultivation and reducing the abuse of antibiotics is provided; 2, the gene site-directed modified pig is the trial test before researching the screening and clinic of the pathogenesis and related intervention and treatment of related diseases, such as human cancers.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Deer-derived bovine viral diarrhea inactivated vaccine and preparation method thereof
InactiveCN108300703AMeet the virus requirements for seedling productionSsRNA viruses positive-senseViral antigen ingredientsBacteroidesMicroorganism
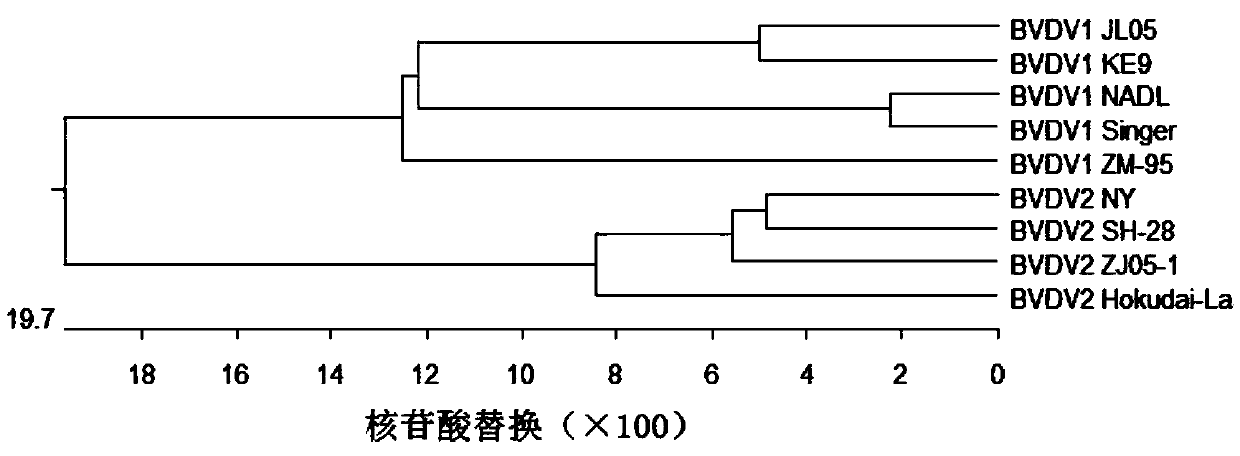
The invention discloses a deer-derived bovine viral diarrhea inactivated vaccine and a preparation method thereof, relates to the technical field of vaccines, and fills the blank of a vaccine specialfor viral diarrhea / mucosal diseases of sika deer. The deer-derived bovine viral diarrhea inactivated vaccine JL05 is preserved in the CGMCC on January 11, 2018, with a collection number of CGMCC No.15283. The deer-derived BVDVJL05 virus is subjected to passage by using MDBK cells and then is subjected to plaque cloning to purify the virus; detection shows that the content of the deer-derived BVDVJL05 virus is 107.2 TCID50 / ml or more, and no germ, mold, mycoplasma or exogenous virus pollution is caused; the deer-derived BVDV JL05 virus meets a virus requirement for vaccine preparation. The virus is inactivated by BEI and then is mixed and emulsified with a 206 adjuvant to prepare the vaccine; safety and potency inspection shows that the vaccine is safe to the sika deer, and the challenge protection rate can be up to 100 percent.
Owner:JILIN AGRICULTURAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com