Swine umbilical cord blood swine fever virus wild strain diagnosing primer group, kit containing same and application thereof
A technology of swine fever virus and umbilical cord blood, applied in the biological field, can solve problems such as false negatives, insufficient sensitivity, and limited template content in amplification results, and achieve the level of protection evaluation, strong broadness and specificity, and reliable technical support Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
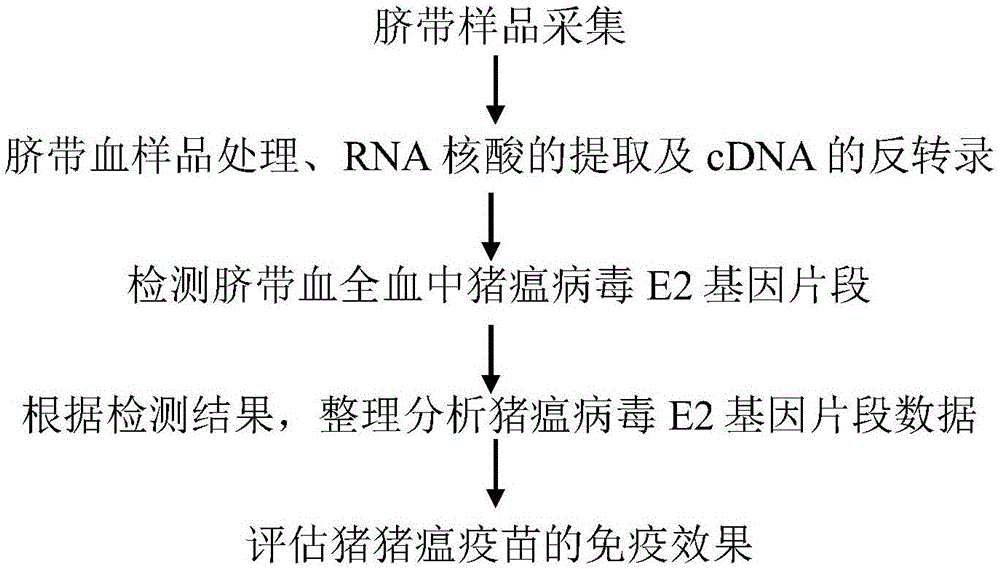
[0067] The flow chart of kit of the present invention being used for diagnosing porcine umbilical cord blood swine fever virus wild strain infection is as follows figure 1 shown. Concretely comprise the following steps: (1) umbilical cord blood sample collection; (2) umbilical cord blood sample processing, extraction of nucleic acid RNA and reverse transcription of cDNA; (3) detection of swine fever virus E2 gene fragment in umbilical cord blood whole blood; (4) ) According to the test results, sort out and analyze the data of the classical swine fever virus E2 gene fragment; (5) evaluate the immune effect of the swine fever vaccine.
[0068] 1. Extraction of total RNA of CSFV in umbilical cord blood
[0069] (1) Collection of umbilical cord blood
[0070] Cord blood collection involves the following steps:
[0071] a. Take a clean penicillin bottle and cork, clean it, boil and sterilize it for 30 minutes, dry it and collect it for later use;
[0072] b. When the piglets a...
Embodiment 2
[0121] In the sow farm of manufacturer A, all the swine fever vaccines were immunized 21 days before production, and the sows were randomly divided into groups. Piglet umbilical cord blood of pigs, according to the method described in implementation 1, carries out the diagnosis of pig umbilical cord blood CSFV wild strain, evaluates the immune effect of CSF vaccine, and the situation of piglets infected with CSFV. The result is as figure 2 shown.
[0122] From figure 2 It can be seen that the positive control amplified a 272bp band, the negative control had no 272bp band amplification, and there was no specific 272bp band amplification in the umbilical cord blood of samples 1-6, indicating that the umbilical cord blood of samples 1-6 There is no swine fever virus, sows do not have swine fever virus detoxification and piglet infection, and the sows have a high level of health, which further indicates that the sows' swine fever vaccine immunity is effective and the immunizat...
Embodiment 3
[0124] In the sow farm of manufacturer B, all the swine fever vaccines were vaccinated 21 days before production, and the sows were randomly divided into groups. Piglet umbilical cord blood of pigs, according to the method described in implementation 1, carries out the diagnosis of pig umbilical cord blood CSFV wild strain, evaluates the immune effect of CSF vaccine, and the situation of piglets infected with CSFV. The result is as image 3 shown.
[0125] From image 3It can be seen from the figure that the positive control amplified a 272bp band, the negative control had no 272bp band amplification, and there was no specific 272bp band amplification in the umbilical cord blood of samples 1-20, indicating that the umbilical cord blood of samples 1-20 There is no swine fever virus in the pig, there is no detoxification of swine fever virus and piglet infection in sows, and the health level of sows is high, which further shows that the immunization effect of swine fever vacci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com