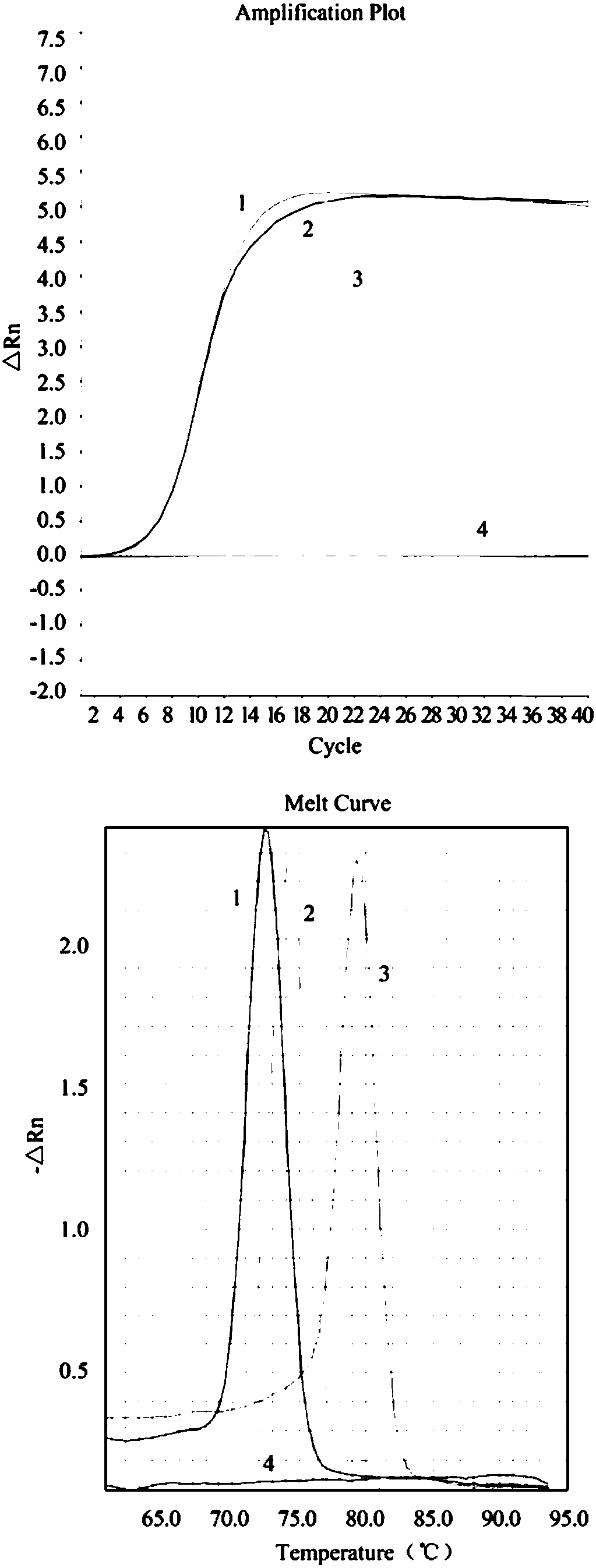
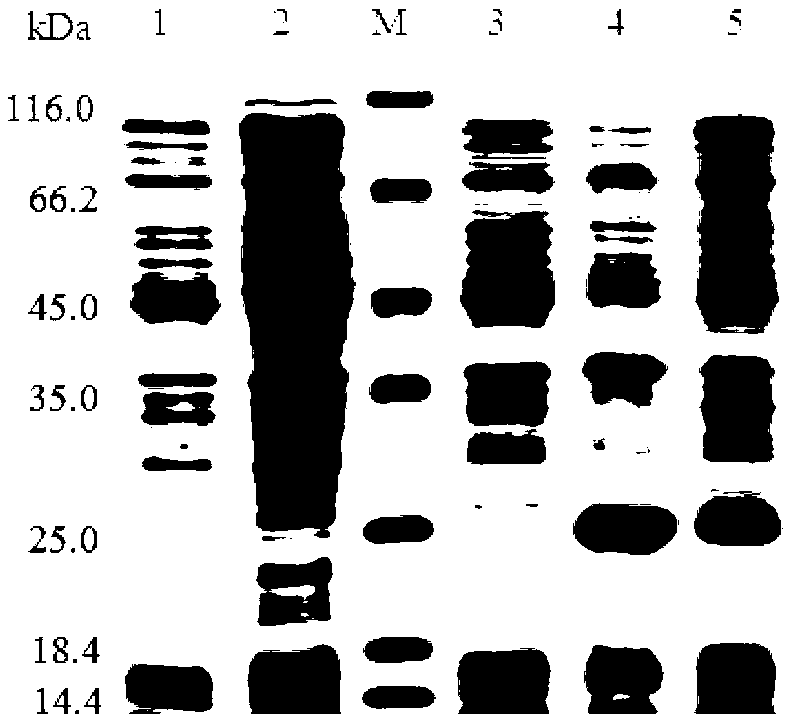Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
93 results about "Porcine rotavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus
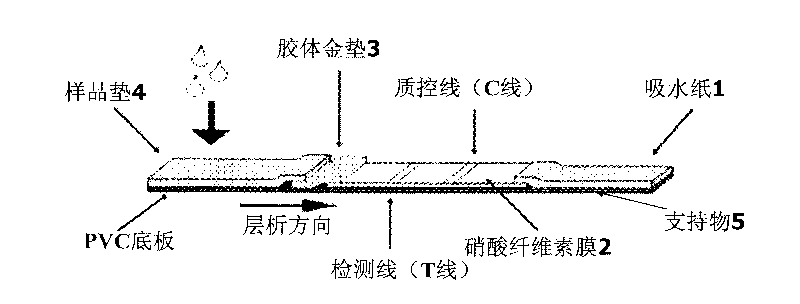
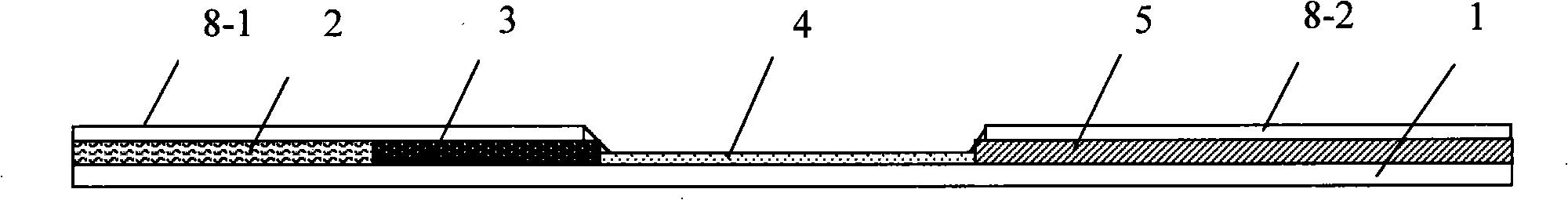
The invention discloses a colloidal gold immunochromatographic test strip for detecting wild-type classical swine fever virus, which consists of water absorbent paper (1), a cellulose nitrate membrane (2), a colloidal gold pad (3), a sample pad (4) and a support (5), wherein the cellulose nitrate membrane contains a detection line which is formed by coating monoclonal antibody HQ06 of anti-classical swine fever virus E2 protein and a quality control line which is formed by coating rabbit anti-mouse IgG antibody; and the colloidal gold pad is combined with colloidal gold-labeled monoclonal antibody 6E10 of the anti-classical swine fever virus E2 protein. The test strip does not react with C-strain of classical swine fever virus, bovine viral diarrhea virus, porcine reproductive and respiratory syndrome virus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus, pseudorabies virus, porcine parvovirus and porcine circovirus type 2, and can accurately and sensitively identify the wild-type classical swine fever virus, thereby having good specificity, sensitivity and repeatability.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
Triple vaccine of pig transmissible gastroenteritis, pig epidemic diarrhea and pig rotavirus
InactiveCN101491673AAvoid pollutionDoes not destroy nutrientsViral antigen ingredientsDigestive systemDiseaseCytopathic effect
The invention provides a method for preparing triple vaccine for preventing porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus. The method comprises the following steps: inoculating a host-cell line with a 90 percent grown monostratum against a porcine transmissible gastroenteritis virus, a porcine epidemic diarrhea virus and a porcine rotavirus respectively, and adding a cell maintenance media into the host-cell lines respectively to be cultured at 37 DEG C; after cytopathic effect reaches over 75 percent, collecting viruses to be stored at 20 DEG C below zero for standby; mixing the viruses according to 10 TCID50 in 1:1:1, and simultaneously adding Freund's complete adjuvant and immunopotentiator into the mixture to inactivate the mixture by formaldehyde at 37 DEG C for 24 hours; and adding an oil adjuvant into the mixture to prepare a vaccine of water-oil-water preparation. The method can be used for preparing the triple vaccine for preventing the porcine transmissible gastroenteritis, the porcine epidemic diarrhea and the porcine rotavirus so as to solve the problem that the diseases do not have an effective medicine to treat currently.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Honeysuckle composition for resisting virus diseases of pigs
The invention discloses a honeysuckle composition for resisting virus diseases of pigs, and relates to a feed additive or Chinese veterinary medicine special for preventing and controlling the virus diseases of the pigs and capable of promoting the growth of the pigs. The composition consists of the following components: 30 to 60 portions of honeysuckle extract, 5 to 20 portions of Chinese thorowax root extract, 5 to 20 portions of baical skullcap root P.E, 5 to 20 portions of dandelion P.E, and 10 to 25 portions of licorice extract. The composition is Chinese medicinal powder of which the granularity is between 200 and 300 meshes and which is yellow or brown-yellow, and has the following content of the active components: 5 to 20 percent of chlorogenic acid, 2 to 10 percent of baicalin and5 to 15 percent of glycyrrhizic acid. When used, the composition is mixed with a feed in a ratio of 0.02-0.2 percent and then is fed to animals. The composition has the effect of preventing and controlling highly pathogenic blue ear pig disease, porcine rotavirus disease, influenza, infectious bronchitis and the like, and virus diseases caused by porcine circovirus PCV, foot-and-mouth disease virus and the like, and can effectively promote the growth of the live pigs.
Owner:新乡博凯生物技术有限公司
Detection kit and application thereof
ActiveCN105527437AHigh sensitivityBiological material analysisPorcine rotavirusPorcine Transmissible Gastroenteritis
The invention provides a detection kit containing two anti porcine epidemic diarrhea virus monoclonal antibodies PEDV-McAB1 and PEDV-McAB2, two anti porcine transmissible gastroenteritis virus monoclonal antibodies and two anti porcine rotavirus monoclonal antibodies; the kit can be applied for simultaneous detection of porcine epidemic diarrhea viruses, porcine transmissible gastroenteritis viruses and / or porcine rotaviruses with non-diagnostic purpose, moreover, the detection sensitivity of the kit for simultaneous detection of two or three kinds of viruses is higher than that of single detection of one kind of virus, and false positive results are avoided.
Owner:LUOYANG PULIKE WANTAI BIOTECH +2
Porcine rotavirus delta VP8* subunit recombinant protein and applications thereof
InactiveCN103304642AStrong immune responseFast titerViral antigen ingredientsVirus peptidesVp4 geneInclusion bodies
The invention relates to a porcine rotavirus delta VP8* subunit recombinant protein and an encoding gene of the protein. The invention further provides a recombinant protein formed after increasing tetanus toxin T cell epitope P2 into the recombinant protein, and an encoding gene. The delta VP8* protein is 64th-site to 223th-site amino acid in the VP8* and can effectively stimulate an organism to produce specific serum antibody, humoral immune response is good, the problem that the VP4 gene can not conduct prokaryotic expression due to overlarge fragment can be overcome, the protein can be expressed as a soluble protein in vitro, so that the problem that the VP8* is expressed as an inclusion body in vitro can also be overcome; the T cell epitope P2 (830th-site to 844th-site amino acid of TT) in the tetanus toxin is induced into the delta VP8* subunit recombinant protein, so that the immunity efficacy of the protein can be greatly improved, the faster and stronger neutralizing antibody titer can be induced, and high-titer rotavirus cross neutralizing antibody can also be induced.
Owner:HEILONGJIANG BAYI AGRICULTURAL UNIVERSITY
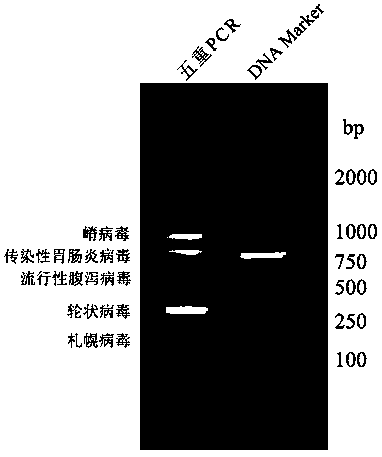
Multiplex PCR primer group for detecting porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus simultaneously
ActiveCN103409558ANo cross reactionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationPcr methodPorcine rotavirus
The invention discloses a multiplex PCR primer group for detecting porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus simultaneously, and belongs to the field of virus detection. The primer group comprises three pairs of primers, wherein the primer sequences of the first pair of primers used for detecting the porcine epidemic diarrhea virus are respectively SEQ ID NO:1 and SEQ ID NO:2, the primer sequences of the second pair of primers used for detecting the porcine transmissible gastroenteritis virus are respectively SEQ ID NO:3 and SEQ ID NO:4, and the primer sequences of the third pair of primers used for detecting the porcine rotavirus are respectively SEQ ID NO:5 and SEQ ID NO:6. Through the application of the sequences of the primers, different strains of the porcine epidemic diarrhea virus, the porcine transmissible gastroenteritis virus and the porcine rotavirus can be detected simultaneously through the multiplex PCR method, and the detection results of the porcine epidemic diarrhea virus, the porcine transmissible gastroenteritis virus and the porcine rotavirus are masculine, while the defection results of other common pig-derived viruses are feminine, in conclusion, the primer group is strong in specificity, and good in repeatability; the PCR detection is carried out after the virus cDNA is subjected to gradient dilution, which shows that the sensitivity of the primers is high.
Owner:哈尔滨威科赛斯生物科技有限公司
Kit for detecting porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus
ActiveCN104232801AEfficient detectionQuick checkMicrobiological testing/measurementMicroorganism based processesPorcine rotavirusVirology
The invention discloses a kit for detecting a porcine epidemic diarrhea virus, a porcine transmissible gastroenteritis virus and a porcine rotavirus. The gene detection kit disclosed by the invention can accurately and effectively detect the porcine epidemic diarrhea virus, the porcine transmissible gastroenteritis virus and the porcine rotavirus, and is strong in specificity, high in sensitivity, short in time consumption, fast in detection and good in application prospects.
Owner:SICHUAN AGRI UNIV
DPO (Dual Priming Oligonucleotide) primer group for porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus detection and application of DPO primer group
ActiveCN108060269ALarge annealing temperature rangeStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationReal-Time PCRsPorcine rotavirus
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
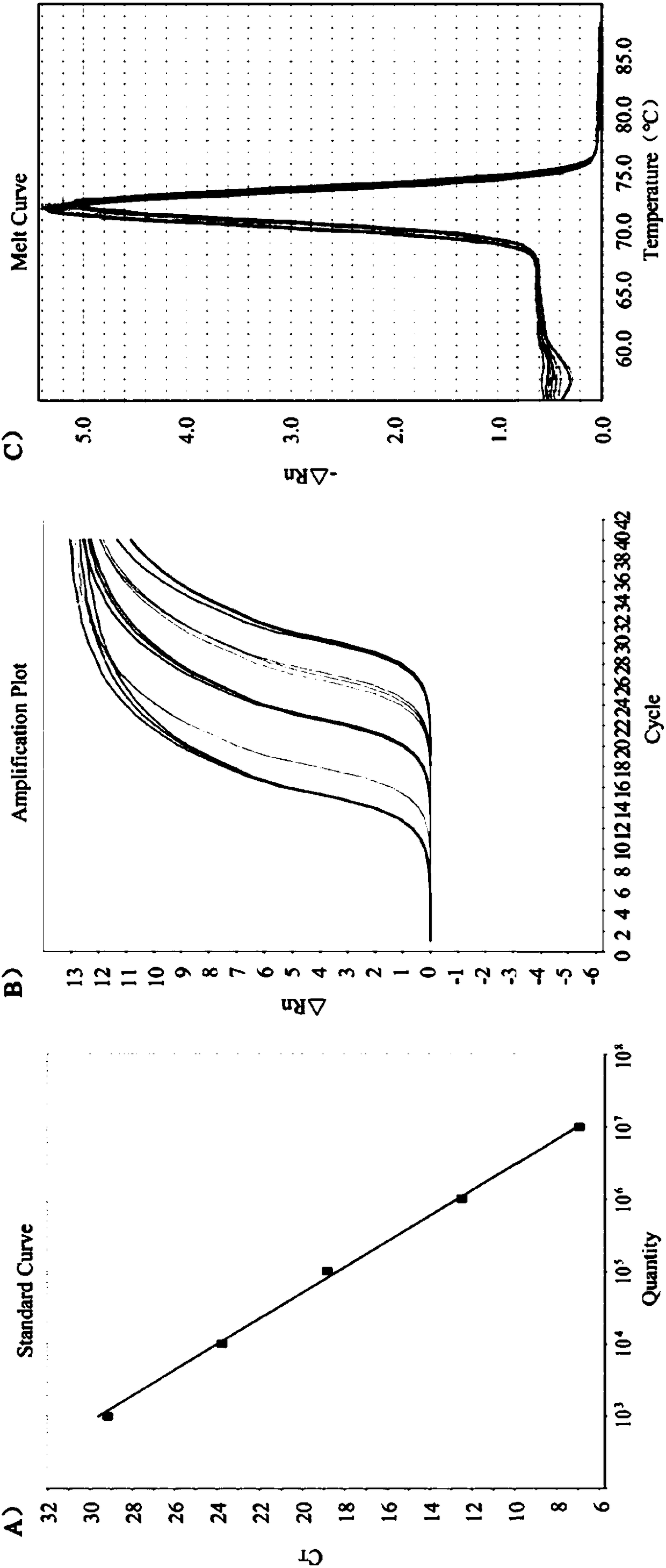
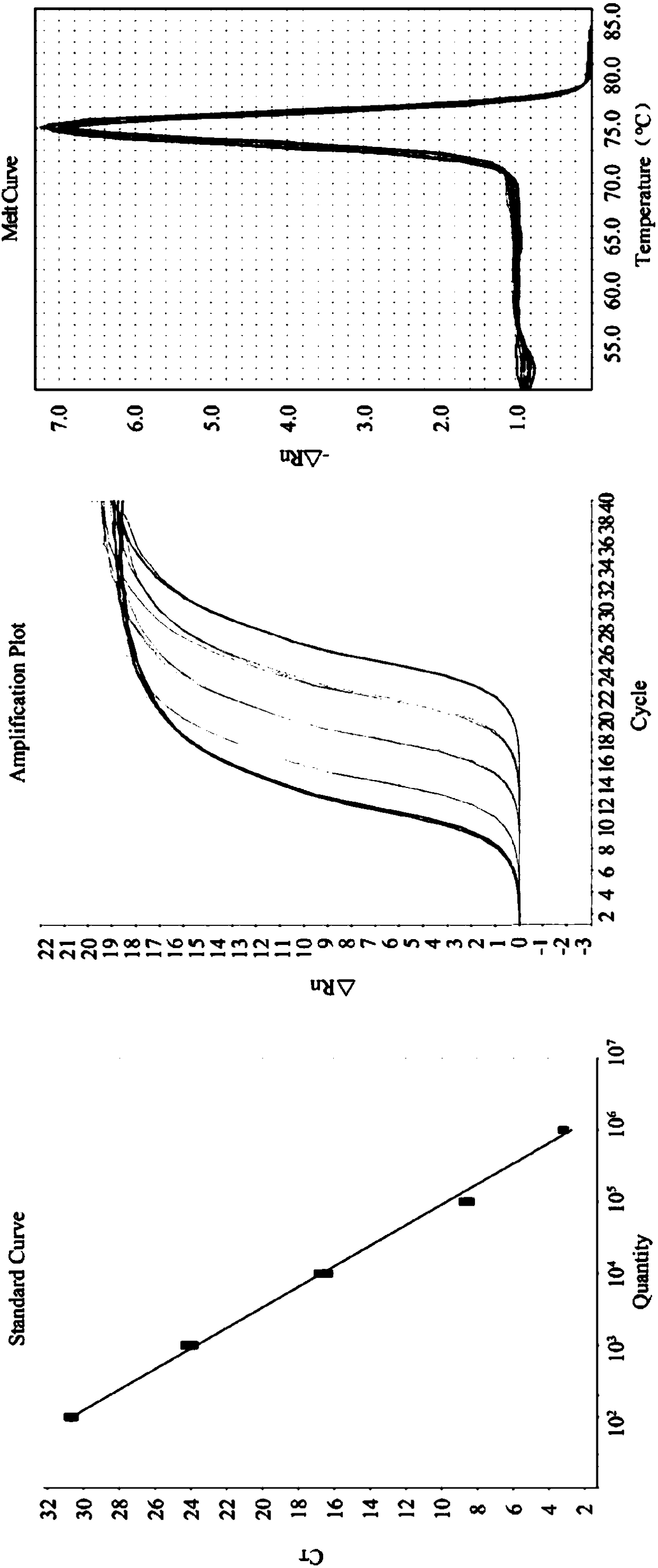
Quintuple RT-PCR detection kit for porcine viral diarrhea viruses
ActiveCN105506182AAccurate judgmentRapid diagnosisMicrobiological testing/measurementMicroorganism based processesTotal rnaPorcine rotavirus
The invention discloses a quintuple RT-PCR detection method and kit for porcine epidemic diarrhea viruses, porcine transmissible gastroenteritis viruses, porcine rotaviruses, porcine sapoviruses and porcine kobuviruses. The kit comprises ten specificity amplification primers. In the using process of the kit, total RNA of a sample to be detected is subjected to reverse transcription to become cDNA through a 6-basic-group random primer, a detection reaction system in the kit is used for RCR amplification with the cDNA as a template, and whether the sample to be detected is infected with one kind of pathogens or is under mixed infection is determined according to different sizes of amplified PCR segments of different pathogens. On the condition of ensuring specificity and sensitivity, the kit has the advantages of being easy and quick to operate and lowering detection cost and labor intensity, and is suitable for field sample detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Test paper strip for detecting one or more porcine virus diarrhea disease antibody
The present invention relates to a test strip which is used for testing a kind of or a plurality of kinds of disease antibodies of porcine virus diarrhea; the test strip comprises a supporting layer and a reaction reagent carrier absorbing layer which is pasted on the supporting layer; the reaction reagent carrier absorbing layer comprises a testing end fiber layer, a fiber layer of absorbing gold-labeled SPA protein or gold-labeled antigen which corresponds to the antigen to be tested, and a cellulose layer, which are arranged at the sample end in sequence, and an absorbent material layer which is positioned at the handle end; the cellulose layer contains one, two or three testing print(s) which are printed by anyone, any two or three of the purified transmissible gastroenteritis virus TGEV solution, the porcine epidemic diarrhea virus PDEV solution and the porcine rotavirus RV, and the cellulose layer also contains the contrast prints which are printed by anyone, any two or three of the anti-SPA protein IgG solution of the sheep or the rabbit or the anti-TGEV, anti-PDEV, anti-RV IgG solution of the sheep or the rabbit; and the invention provides a test strip for testing a kind of or a plurality of kinds of disease antibodies of the porcine virus diarrhea, which has the advantages of accurate and rapid detection, convenient operation and low costs.
Owner:HENAN ACAD OF AGRI SCI
Fourfold RT-PCR detection primer and reagent kit of four porcine epidemic diarrheaviruses
ActiveCN110093461AStrong specificityRepeatableMicrobiological testing/measurementMicroorganism based processesEpidemic diarrheaAstrovirus gastroenteritis
The invention relates to a detection primer and reagent kit of four RT-PCR of porcine epidemic diarrheaviruses, transmissible gastroenteritis of swine, porcine rotaviruses and porcine deltacoronaviruses, and belongs to the technical field of molecular detection. The fourfold RT-PCR detection primer comprises detection primers PEDV F and PEDV R of porcine epidemic diarrhea viruses, detection primers TGEV F and TGEV R of the transmissible gastroenteritis of swine, detection primers PoRV F and PoRV R of the porcine rotaviruses and detection primers PDCoV-F and PDCoV-R of the porcine delta coronaviruses. The primer is high in specificity, has repeatability, is high in sensitivity and is high in clinical reliability.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Gene chip and kit for detecting porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus
ActiveCN104293977AEfficient detectionQuick checkNucleotide librariesMicrobiological testing/measurementPorcine rotavirusTransmissible gastroenteritis virus
The invention discloses a gene chip and kit for detecting porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus. The gene chip disclosed by the invention comprises a solid phase carrier and a probe fixed on the solid phase carrier, wherein the probe comprises any one or two oligonucleotide fragments shown in SEQ ID NO.1 or 2, any one or two oligonucleotide fragments shown in SEQ ID NO.3 or 4, and any one or two of oligonucleotide fragments shown in SEQ ID NO.5 or 6. The kit disclosed by the invention comprises the gene chip and reagents for amplifying genes of porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus. The gene chip and detection kit disclosed by the invention can accurately and effectively detect the porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus and are good in specificity, high in sensitivity, low in time consumption, high in detection speed and good in application prospects.
Owner:SICHUAN AGRI UNIV
Breeding method for breeding pigs
InactiveCN104542463APrevention of swine feverPrevention of Foot and Mouth DiseaseAntibacterial agentsSsRNA viruses positive-senseAnimal sciencePig breeding
The invention discloses a breeding method for breeding pigs, and relates to the technical field of pig breeding. In the breeding pig breeding process, the breeding pigs in different phases such as piglet phase, reserve swine phase and sow phase are subjected to immunization by various vaccines in different time stages. According to the breeding method, a normative immunization program is provided, so that swine fever, foot-and-mouth diseases, pseudorabies, porcine reproductive and respiratory syndrome, parvovirus, epidemic diarrhea, contagious gastroenteritis porcine rotavirus, epidemic encephalitis B, haemophilus parasuis, porcine circovirus and the like which are common in the piglets, the reserve swines and the sows can be effectively prevented.
Owner:刘长华
Novel porcine rotavirus vaccine VP6 antigen
The present invention provides a porcine rotavirus VP6 antigen gene, the amino acid sequence of which is SEQ ID NO: 2; a nucleotide fragment encoding the above antigen, a sequence of which is SEQ ID NO: 1. The invention obtains a novel porcine rotavirus VP6 gene through screening, and constructs an Escherichia coli BL21 (DE3) host bacterium capable of expressing porcine rotavirus VP6 gene 1 by using the pET28a(+) expression vector. After SDS‑PAGE analysis, the recombinant target protein was expressed. The recombinant protein is purified to prepare a genetically engineered subunit vaccine, which has a significant immune effect on porcine rotavirus.
Owner:青岛宏昊生物科技有限公司
Construction of carriers for prokaryotic expression and eukaryotic expression of candidate gene of porcine rota virus vaccines
InactiveCN102041266ABiologically activeNormal biological activityViral antigen ingredientsMicroorganism based processesCandidate Gene Association StudyPorcine rotavirus vaccine
The invention discloses a construction method for carriers for prokaryotic expression and eukaryotic expression of candidate gene of porcine rotavirus vaccines VP4 and VP7, comprising: cloning and prokaryotic expression of genes of porcine rotavirus VP4, construction and transfection of the recombinant vector of genes of rotavirus VP4 and VP7 and the expression carriers of insect cell sf9, and the construction of a recombinant vector of genes of rotavirus VP4 and VP7 and the expression vector of yeast cells GS115.
Owner:SHANGHAI ACAD OF AGRI SCI
Vaccine diluent and preparing method and application thereof
InactiveCN106466295ASimple methodEasy to operateSsRNA viruses negative-senseOrganic active ingredientsDiseaseSwine Transmissible Gastroenteritis
The invention relates to the field of animal biological products, in particular to a vaccine diluent and a preparing method and application thereof. The vaccine diluent is prepared from 0.1-100g / L Carbomer and 0.1-200g / L levamisole; the adopted solvent is any one kind of water, normal saline or phosphate buffer. The vaccine diluent can solve the problems that a neutralizing antibody is low in potency after a vaccine is immunized, long in producing time and short in antibody maintenance time, and can be matched with a livestock and poultry attenuated live vaccine to use, such as a single vaccine or a mixed vaccine of viral vaccines of a swine fever live vaccine, a swine pseudorabies live vaccine, a porcine reproductive and respiratory syndrome live vaccine, a porcine epizootic diarrhea live vaccine, a swine transmissible gastroenteritis live vaccine, a porcine rotavirus disease live vaccine, a fowl pox live vaccine, a new castle disease live vaccine and the like.
Owner:SICHUAN HUASHEN ANIMAL BIOLOGICAL PRODS
Porcine Deltacoronavirus and swine transmissible gastroenteritis virus multiplex RT-PCR detection primer and detection method
ActiveCN105400910ARapid differential diagnosisMicrobiological testing/measurementAgainst vector-borne diseasesAgricultural scienceSwine Transmissible Gastroenteritis
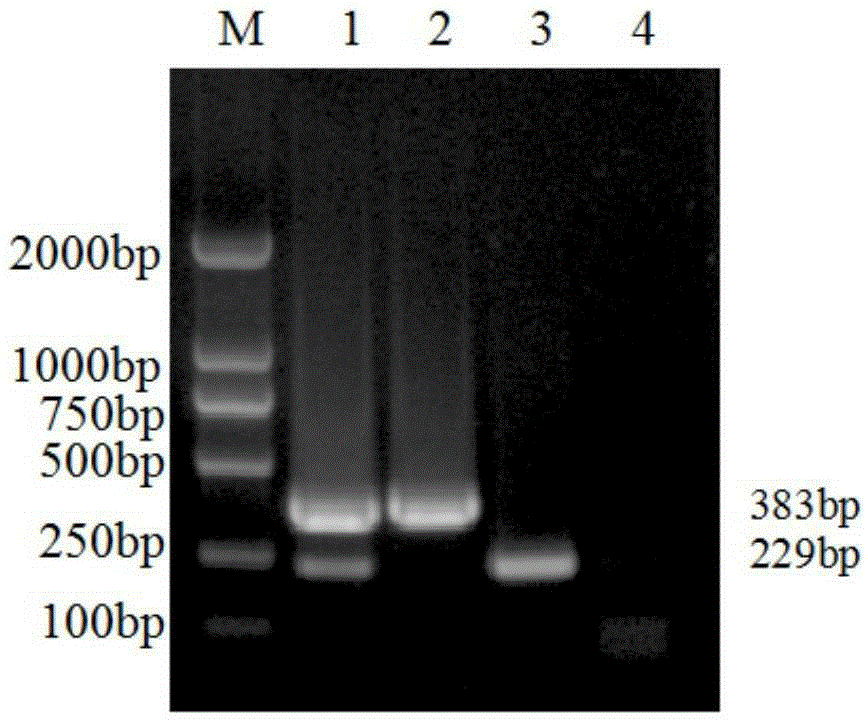
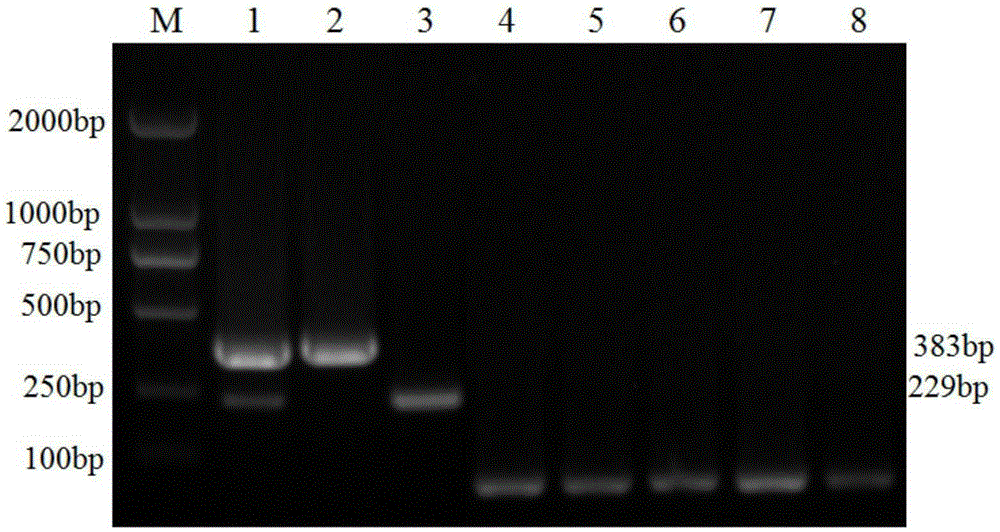
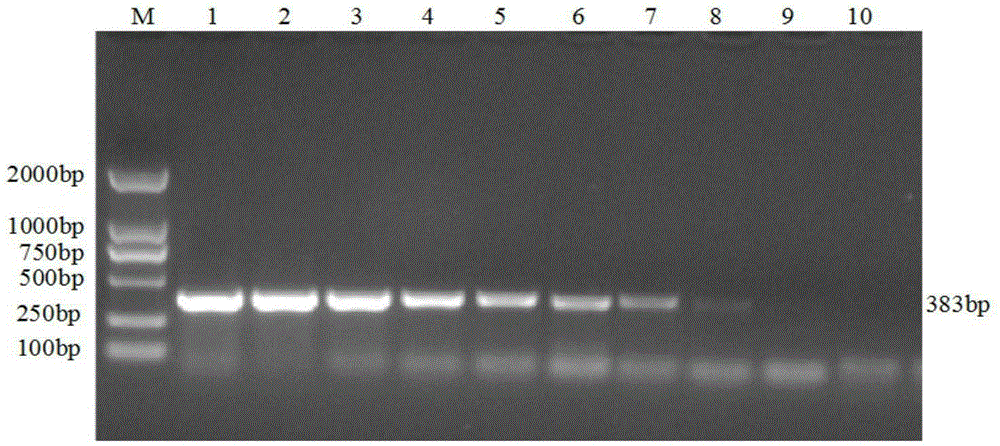
The invention discloses a porcine Deltacoronavirus (PDCoV) and swine transmissible gastroenteritis virus (TGEV) multiplex RT-PCR detection primer. The primer sequence of PDCoV is expressed as follows: upstream primer P1: 5'-ATGGCTACTGGCTGCGTTAC-3', downstream primer P2: 5'-GCGTTTCCTGGGCTGATT-3', and partial PDCoV gene segments are amplified by 383 bp. The primer sequence of TGEV is expressed as follows: upstream primer P3: 5'-CCCTCCAGCAAGGTTCAA-3', downstream primer P4: 5'-GCAACCCAGACAACTCCA-3', and partial TGEV gene segments are amplified by 229 bp. The detection limits of multiplex RT-PCR on PDCoV and TGEV are 4.05*101 copy per microliter and 5.47*102 copy per microliter respectively, and amplified results on porcine epidemic diarrhea virus, bocavirus, porcine reproductive and respiratory syndrome virus and porcine rotavirus are negative. Multiplex RT-PCR detection results of 57 clinical samples show that the positive rate of being infected with the two viruses at the same time is 1.75%, the PDCoV infection positive rate is 19.30%, and the TGEV infection positive rate is 1.75%.
Owner:HENAN AGRICULTURAL UNIVERSITY
Multiple PCR detection primer group and kit for rapidly differentiating PCV1 type from PCV3 type
InactiveCN107653346AIncreased sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationPorcine rotavirusTransmissible gastroenteritis virus
The invention discloses a multiple PCR detection primer group and kit for rapidly differentiating the PCV1 type from the PCV3 type. The primer group includes primers L1, L2 and L3, wherein L1 represents 5'-ACCAGCGCACTTCGGCAGCGGCAGCA-3', L2 represents 5'-GCATTGTTCACCAGTACCCA-3', and L3 represents 5'-AAGTCCCCTTCCTGCGTCCGCTAATCT-3'. The special kit has very high sensitivity, and minimum detection quantities for the PCV1 type and the PCV3 type are 1*10<-7> ng / mL and 2*10<-7> ng / mL respectively; the specificity is high, and the amplification results of porcine reproductive and respiratory syndromevirus, classical swine fever virus, porcine pseudorabies virus, porcine parvovirus, porcine epidemic diarrhea virus, transmissible gastroenteritis virus and porcine rotavirus are negative; the repeatability is good, the same sample is detected for 3 times at an interval of one month, and the same result is obtained; moreover, the coincidence rate can reach 95% or above compared with methods of virus isolation, IFA and the like, and the primer group and the kit can be widely used in clinical typing detection of the PCV1 type and the PCV3 type.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Lactobacillus gene engineering subunit vaccine strain capable of stably expressing porcine rotavirus VP4 protein and preparation method of lactobacillus gene engineering subunit vaccine strain
InactiveCN107988130AImproving immunogenicityBacteriaViral antigen ingredientsMucosal Immune ResponsesVp4 gene
The invention discloses a lactobacillus gene engineering subunit vaccine strain capable of stably expressing porcine rotavirus VP4 protein and a preparation method of the lactobacillus gene engineering subunit vaccine strain. The vaccine strain is obtained through the following steps: on the basis of lactobacillus casei with uracil phosphoribosyl transferase, UPP gene deleted, the porcine rotavirus VP4 gene is inserted between a termination codon and a terminator of the neuronspecificenoluse gene of lactobacillus casei through homologous recombination, and the vaccine strain is not provided with an antibiotic selection marker. The experiment proves that after the constructed lactobacillus gene engineering subunit vaccine strain capable of stably expressing the porcine rotavirus VP4 proteinis used for immunizing animals through oral administration, the local mucosal immune response can be induced, the mucous membrane antibody IgA is generated, the body is induced to generate humoral immune response, the serum antibody IgG is then generated, good immunogenicity is displayed, and the constructed lactobacillus gene engineering vaccine strain not labelled by antibiotic resistance accords with the development concept of 'no pollution and environmental protection' of veterinary vaccines.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Multiplex RT-PCR (Reverse Transcription-Polymerase Chain Reaction) primer probe group for real-time fluorescent quantitative detection of four porcine diarrhea viruses, kit and detection method
PendingCN113462820AThe test result is accurateReal-time quantitative detectionMicrobiological testing/measurementAgainst vector-borne diseasesMultiplexPig farms
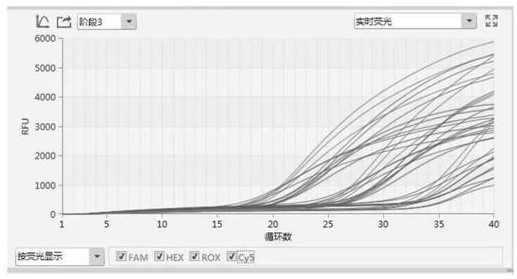
The invention discloses a multiplex RT-PCR (reverse transcription-polymerase chain reaction) primer probe group for real-time fluorescent quantitative detection of four porcine epidemic diarrhea viruses. The multiplex RT-PCR primer probe group comprises an upstream primer, a downstream primer and a specific fluorescent probe of a porcine epidemic diarrhea virus M gene, an upstream primer, a downstream primer and a specific fluorescent probe of a swine transmissible gastroenteritis virus S gene; an upstream primer, a downstream primer and a specific fluorescent probe of a porcine rotavirus VP6 gene; and an upstream primer, a downstream primer and a specific fluorescent probe of a porcine D-type coronavirus M gene. The kit assembled on the basis of the primer probe group has the advantages of high sensitivity, high specificity, low pollution and real-time detection, and provides a reliable technology and product for early warning, early diagnosis and prevention and control monitoring of clinical diarrhea virus in a first-line pig farm.
Owner:HEBEI UNIV OF ENG
LAMP (Loop-Mediated Isothermal Amplification) detection method of PRV (Porcine Rotavirus) inverse transcription and application
InactiveCN104694670AImprove efficiencyIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseUltraviolet
The invention discloses an LAMP (Loop-Mediated Isothermal Amplification) detection method of PRV (Porcine Rotavirus) inverse transcription and an application. The detection method comprises the following steps: (1) building a RT-LAMP reaction system, and setting a negative control at the same time, wherein the RT-LAMP reaction system is 25 [mu] L and comprises 2 [mu] L of template, 0.5 [mu]L of 0.8 [mu]M FIP primer, 0.5 [mu]L of 0.8 [mu]M BIP primer, 0.25 [mu]L of 0.2 [mu]M of F3 primer, 0.25 [mu]L of 0.2 [mu]M of B3primer,2[mu]L of dNTP (Diethyl-Nitrophenyl Thiophosphate), 5 [mu]M of MgSO4 (Magnesium Sulfate), 8UBst of DNA polymerase and 1*ThermoPol Buffer, and 1[mu]L of MLV (Murine Leukemia Virus) reverse transcriptase, and supplementing the volume to 25 [mu]L by sterile water; and the negative control template is sterile water; (2) after reacting for 20-60min at 61-65 DEG C in a constant temperature water bath boiler, terminating for 20 min at 80 DEG C, carrying out AGE (Agarose Gel Electrophoresis) identification on an amplification product or adding 2 [mu]L of SYBR Green dye, and observing a result in an UV lamp (Ultraviolet Lamp) or by naked eyes. According to the LAMP detection method provided by the invention, a convenient, fast and accurate molecular biological diagnosis method can be provided for the clinical diagnosis and epidemiological investigation of the PRV.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Porcine rotavirus G4-G5-G9 type trivalent inactivated vaccine as well as preparation method and application thereof
ActiveCN114854697AAdvantages of Guaranteed Seed PoisonImprove stabilityViral antigen ingredientsMicroorganism based processesAdjuvantPreventive vaccination
The invention provides a porcine rotavirus G4-G5-G9 type trivalent inactivated vaccine as well as a preparation method and application thereof, three genotype strains of current PoRV epidemic are adopted as seed viruses, are inactivated by BEI, and are emulsified with an adjuvant ISA201VG to prepare the PoRV trivalent inactivated vaccine. The trivalent inactivated vaccine prepared by the research has the characteristics of high immune protection rate, good stability, high yield, safety and reliability, and can be used for emergency vaccination when a PoRV epidemic situation outbreaks.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Porcine rotavirus (PRV) VP fusion protein reconstruction body as well as preparation method and application thereof
ActiveCN108359015AGut-piercing activityAvoid enteringPolypeptide with localisation/targeting motifBacteriaMucosal Immune ResponsesIntraperitoneal route
The invention discloses a porcine rotavirus (PRV) VP fusion protein reconstruction body as well as a preparation method and application thereof. The fusion protein VP8-VP7-TAT provided by the invention comprises truncation fragments VP8 and VP7 of a PRV coat shell structure protein VP4 and eleven core amino acids bonded to a TAT protein transduction peptide basic amino acid enrichment region at anend C. A mouse is immunized with the fusion protein VP8-VP7-TAT by means of intraperitoneal injection or oral intragastric administration, so that the organism can be effectively inducted to generatea humoral immune response and a mucosal immune response, and high immunogenicity is achieved. Through fusion expression of PRV proteins VP8, VP7 and TAT, a novel method is provided for the preventionof PRV infection, and a basis is laid for the development of a novel PRV oral vaccine.
Owner:WUHAN UNIV
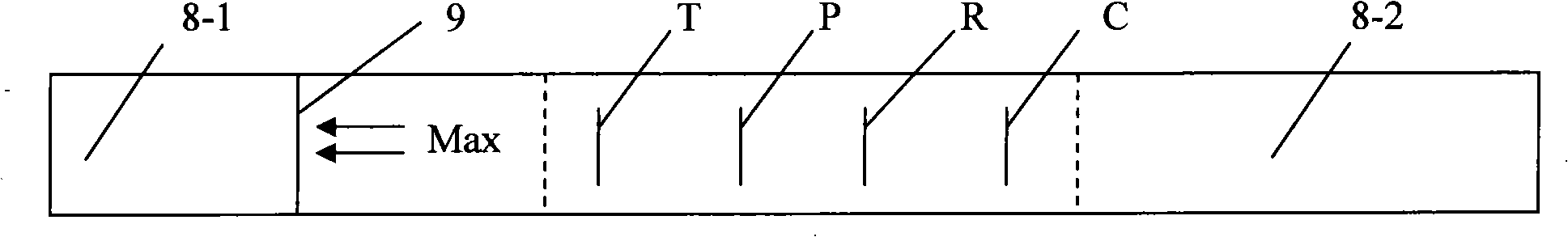
Immune test paper for detecting group A rotaviruses, and its making method
An immune test paper for detecting group A rotaviruses comprises a sample pad, a bonding pad, a cellulose nitrate film, a water absorbing pad and a back lining, the sample pad, the bonding pad, the cellulose nitrate film and the water absorbing pad are boned on the back lining, two ends of the bonding pad have a lapping joint with the sample pad and the cellulose nitrate film respectively, one end of the cellulose nitrate film far from the binding pad has a lapping joint with the water absorbing pad, the cellulose nitrate film is provided with a detection line and a quality control line which are spaced, the bonding pad is provided with a group A porcine rotavirus monoclonal antibody A-fluorescent marker, the detection line is formed by a group A monoclonal porcine rotavirus antibody B, and the quality control line is formed by goat anti-mouse IgG. The invention also provides a making method of the immune test paper for detecting group A rotaviruses.
Owner:SHENZHEN INST OF ADVANCED TECH
Porcine rotavirus strain and inactivated vaccine prepared from strain as well as application
ActiveCN107988170AImproving immunogenicityImprove securityViral antigen ingredientsMicroorganism based processesDiseasePharmacy
The invention discloses a porcine rotavirus strain and an inactivated vaccine prepared from the strain as well as application. The invention firstly discloses a separated porcine rotavirus zyh25 strain, of which the microbial preservation number is CGMCC No. 13792. The invention further discloses application of the porcine rotavirus zyh25 strain in the preparation of a vaccine for preventing porcine rotavirus diseases. Moreover, the invention discloses a vaccine composition for preventing the porcine rotavirus diseases, and the vaccine composition is prepared from an effective quantity of inactivated porcine rotavirus zyh25 strain used for prevention and treatment and an adjuvant which can be accepted in pharmacy. The porcine rotavirus zyh25 strain separated in the invention has good immunogenicity when serving as a vaccine strain, can generate a relatively high antibody level after used for immunizing an animal and can tolerate the attack of a virulent strain. The porcine rotavirus zyh25 strain can be used for serum-free full-suspended continuous production, and the prepared inactivated vaccine has the advantages of low production cost, small difference between product batches andthe like.
Owner:JILIN ZHENGYE BIOLOGICAL PROD
Porcine rotavirus strain, inactivated vaccine prepared from porcine rotavirus strain and application of porcine rotavirus strain
ActiveCN113186170AImprove securityImproving immunogenicityViral antigen ingredientsBiological material analysisAdjuvantMicrobacterium
The invention discloses a porcine rotavirus strain as well as an inactivated vaccine prepared from the porcine rotavirus strain and application of the porcine rotavirus strain, and firstly discloses a separated porcine A-type rotavirus RVA / Pig-tc / CHN / SCMY-A3 / 2017 / G9P [23] type strain (A3 strain for short), the microbial preservation number of the strain being V202132. The invention also discloses a preparation method of the porcine rotavirus inactivated vaccine, which comprises the following steps: mixing the virus liquid containing the A3 strain with the inactivating agent, inactivating the strain, and mixing the strain with the adjuvant to obtain the porcine rotavirus inactivated vaccine. The inactivated vaccine prepared from the separated porcine rotavirus can generate a higher antibody level and can resist the attack of virus strains.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Porcine rotavirus VP6 and VP7 combined vaccine
InactiveCN107875379AImprove immunityViral antigen ingredientsVirus peptidesImmune effectsRotavirus RNA
The invention provides a porcine rotavirus VP6 and VP7 combined vaccine, which comprises vaccine antigen proteins and a vaccine adjuvant. The antigen proteins are a VP6 protein having an amino sequence represented by SEQ ID No.2 and a VP7 protein having an amino sequence represented by SEQ ID No.5. On the basis that a novel VP6 antigen gene is obtained through screening, the VP6 antigen protein isrecombinant expressed, and the expressed VP6 antigen protein and existing VP7 protein are used to produce a gene engineering subunit vaccine. The gene engineering subunit vaccine has a good immune effect on conventional porcine rotavirus and newly screened strains of rotavirus.
Owner:青岛宏昊生物科技有限公司
A 5-plex rt-PCR detection kit for porcine viral diarrhea virus
ActiveCN105506182BAccurate judgmentRapid diagnosisMicrobiological testing/measurementMicroorganism based processesTotal rnaPorcine rotavirus
The invention discloses a quintuple RT-PCR detection method and kit for porcine epidemic diarrhea viruses, porcine transmissible gastroenteritis viruses, porcine rotaviruses, porcine sapoviruses and porcine kobuviruses. The kit comprises ten specificity amplification primers. In the using process of the kit, total RNA of a sample to be detected is subjected to reverse transcription to become cDNA through a 6-basic-group random primer, a detection reaction system in the kit is used for RCR amplification with the cDNA as a template, and whether the sample to be detected is infected with one kind of pathogens or is under mixed infection is determined according to different sizes of amplified PCR segments of different pathogens. On the condition of ensuring specificity and sensitivity, the kit has the advantages of being easy and quick to operate and lowering detection cost and labor intensity, and is suitable for field sample detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Composition containing probiotics metabolite
InactiveCN108888762AEmission reductionInhibition of reproductionAntibacterial agentsPowder deliveryEscherichia coliMetabolite
The invention relates to a composition containing probiotics metabolite. The composition is prepared from, by weight, 1-10 parts of probiotics metabolite, 2-3 parts of oligosaccharide and 3-8 parts ofpowder or liquid containing egg yolk antibody. Compared with the prior art, the composition containing the probiotics metabolite has the advantages that the waste discharge can be reduced, the wasteis turned into treasure, the bacteria reproduction can be selectively inhibited, and the composition is used for preventing or curing gastrointestinal infection caused by escherichia coli of piglets,salmonella, clostridium perfringens, porcine transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine rotavirus and the like.
Owner:FUJIAN AONONG BIOLOGICAL TECH GRP CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com