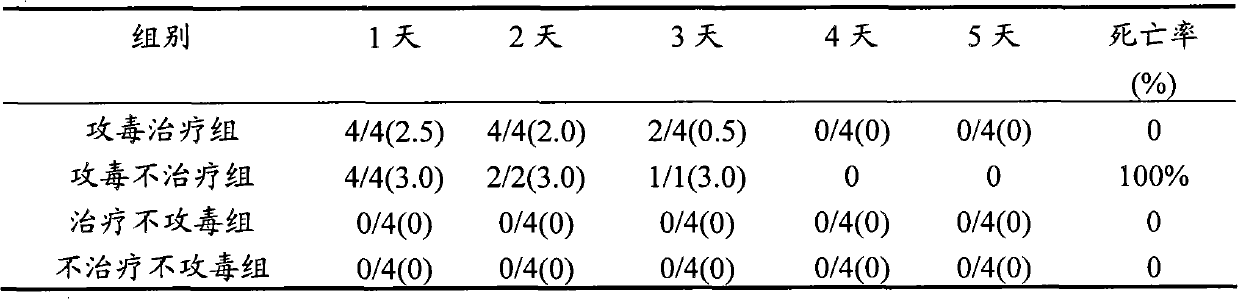
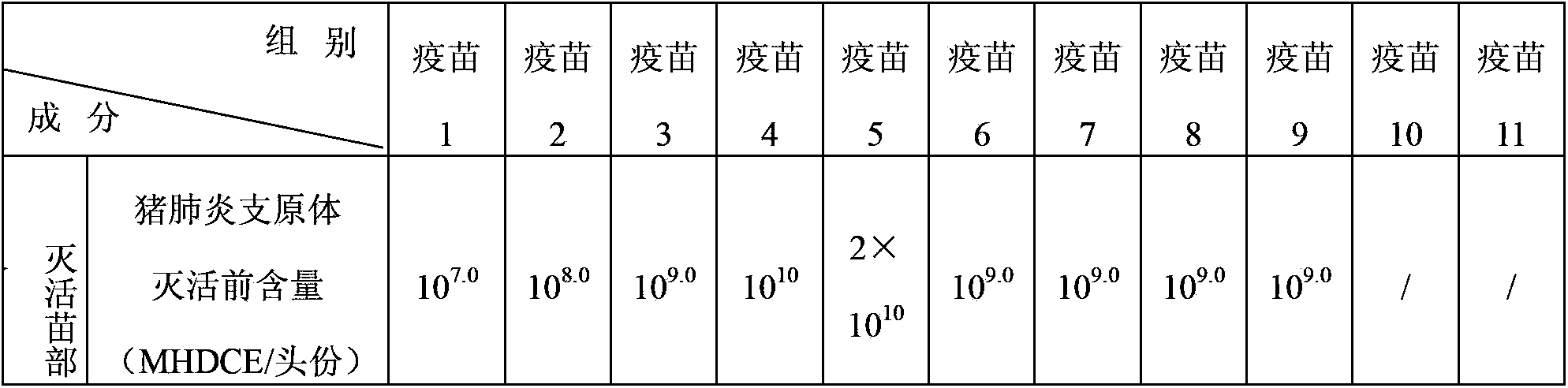
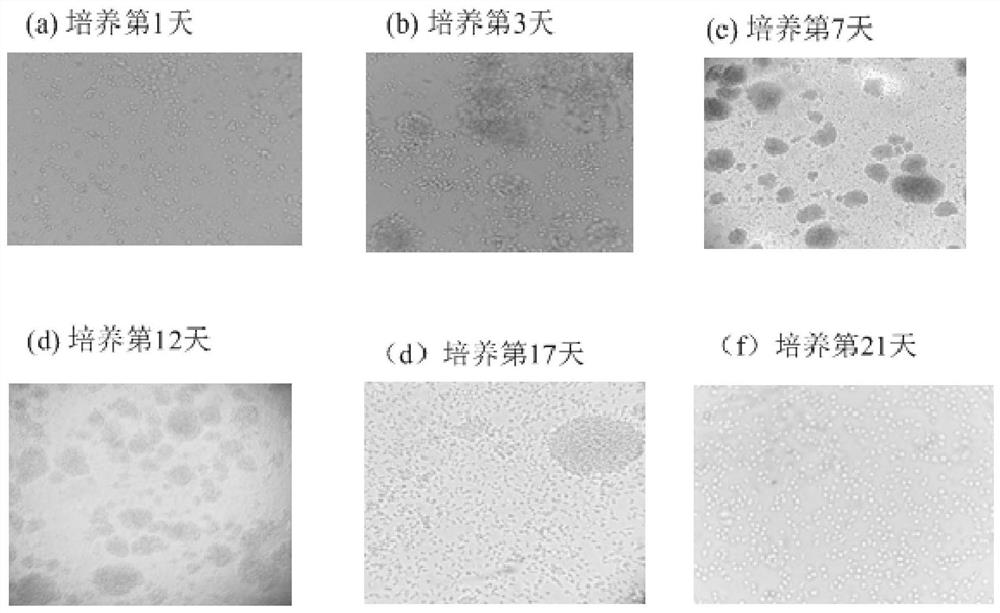
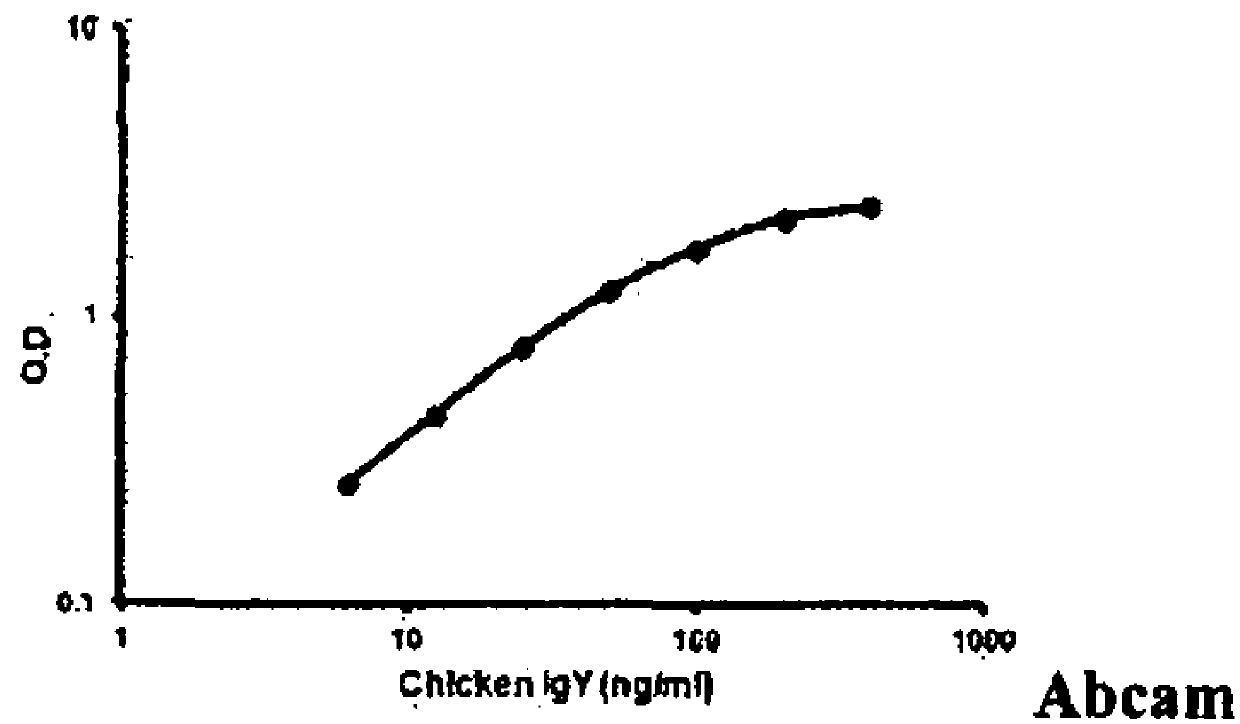
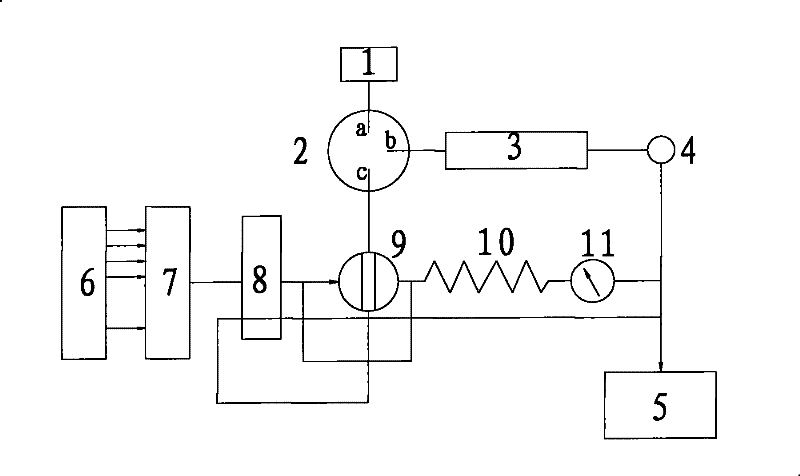
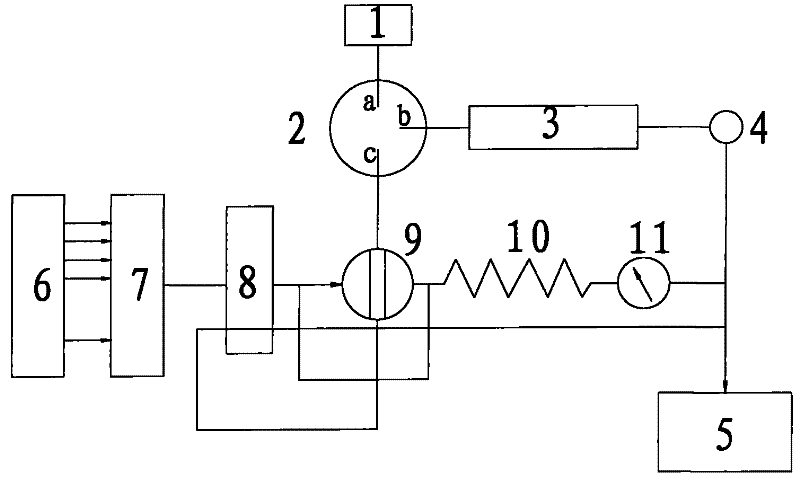
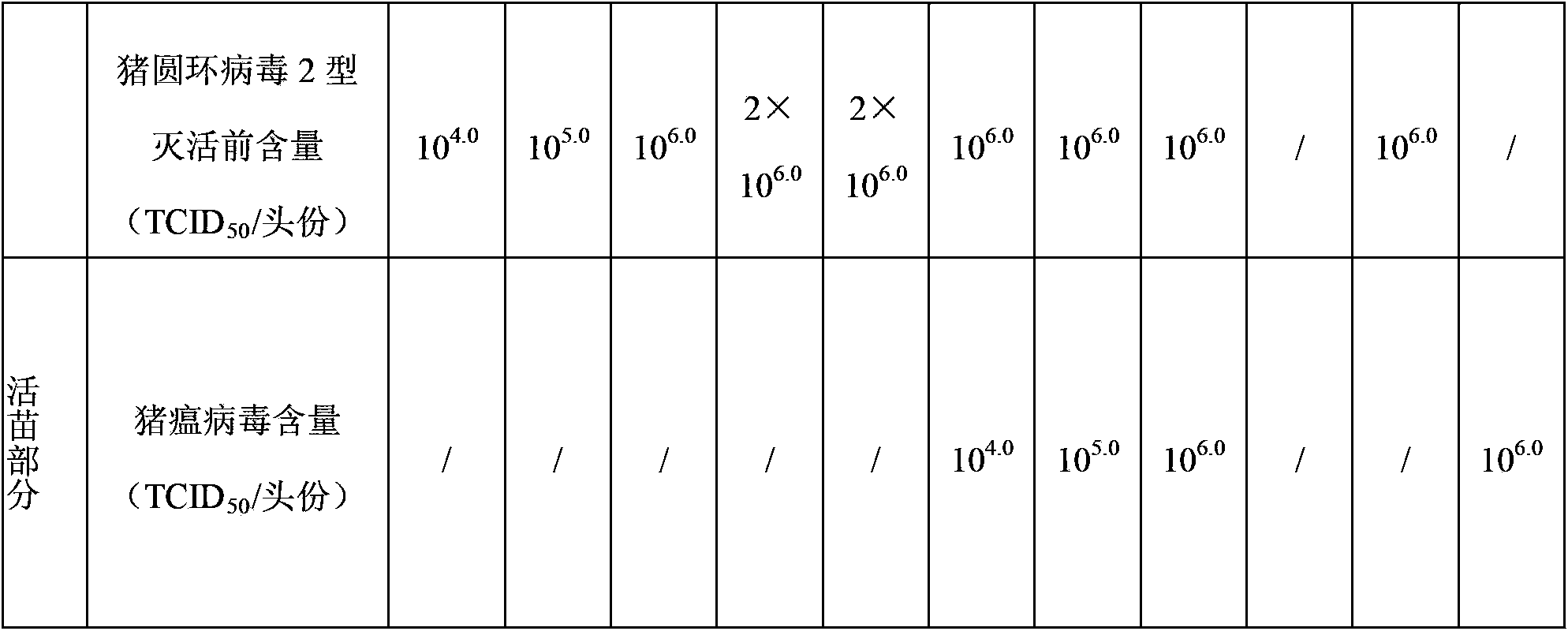Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Immunization program" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
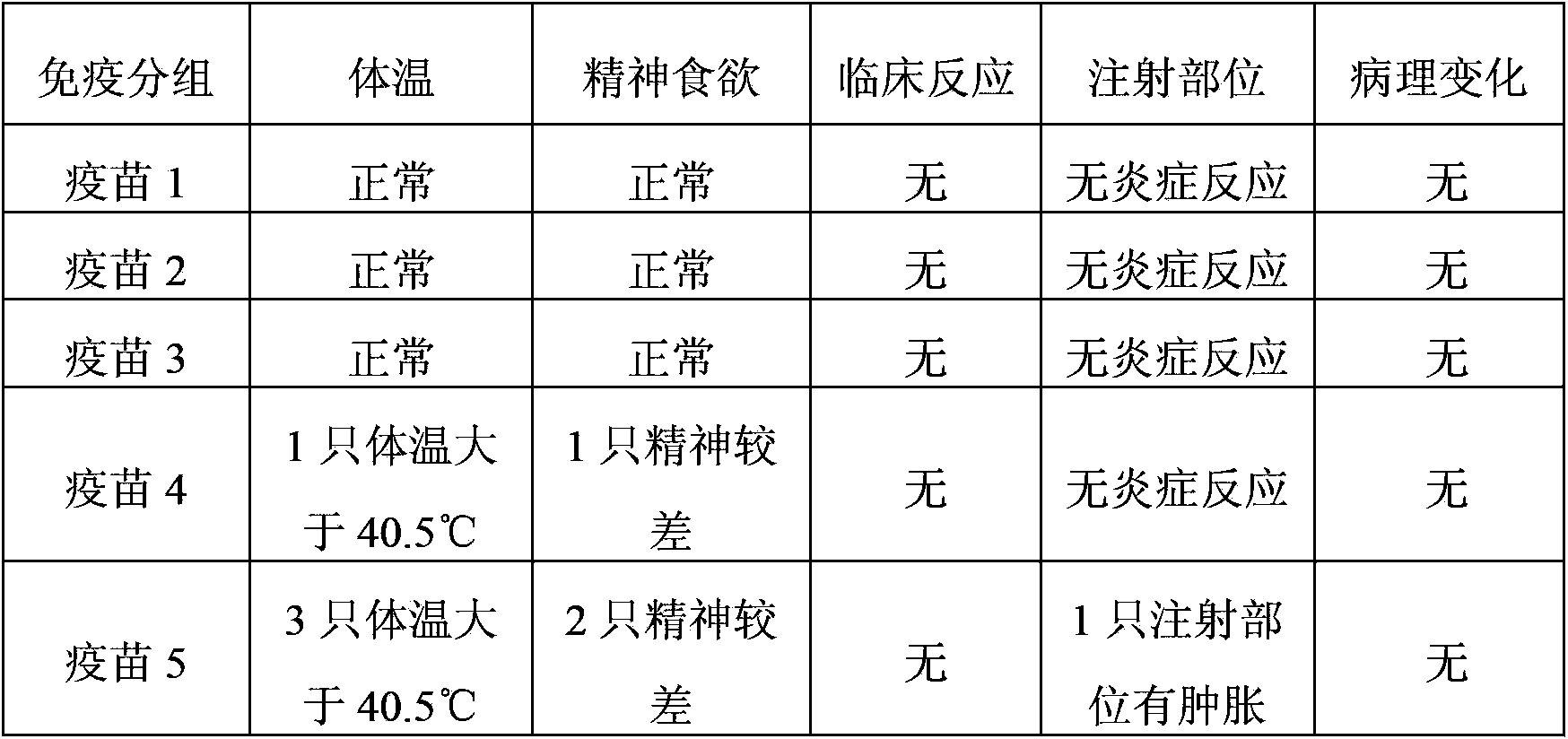
The goal of the Ohio Department of Health (ODH) Immunization Program is to reduce and eliminate vaccine-preventable diseases among Ohio's children, adolescents and adults. The Immunization Program provides the following services:
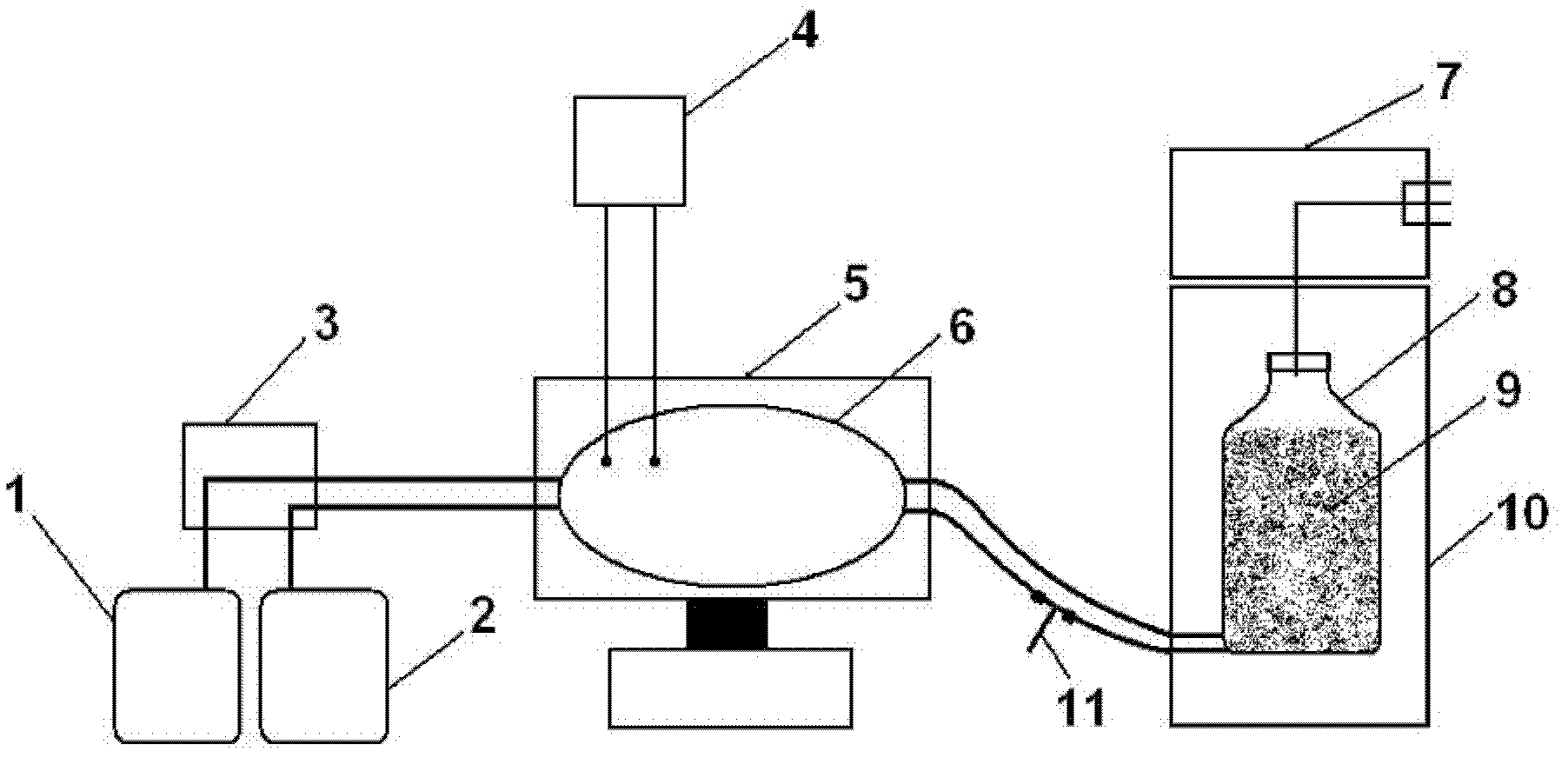
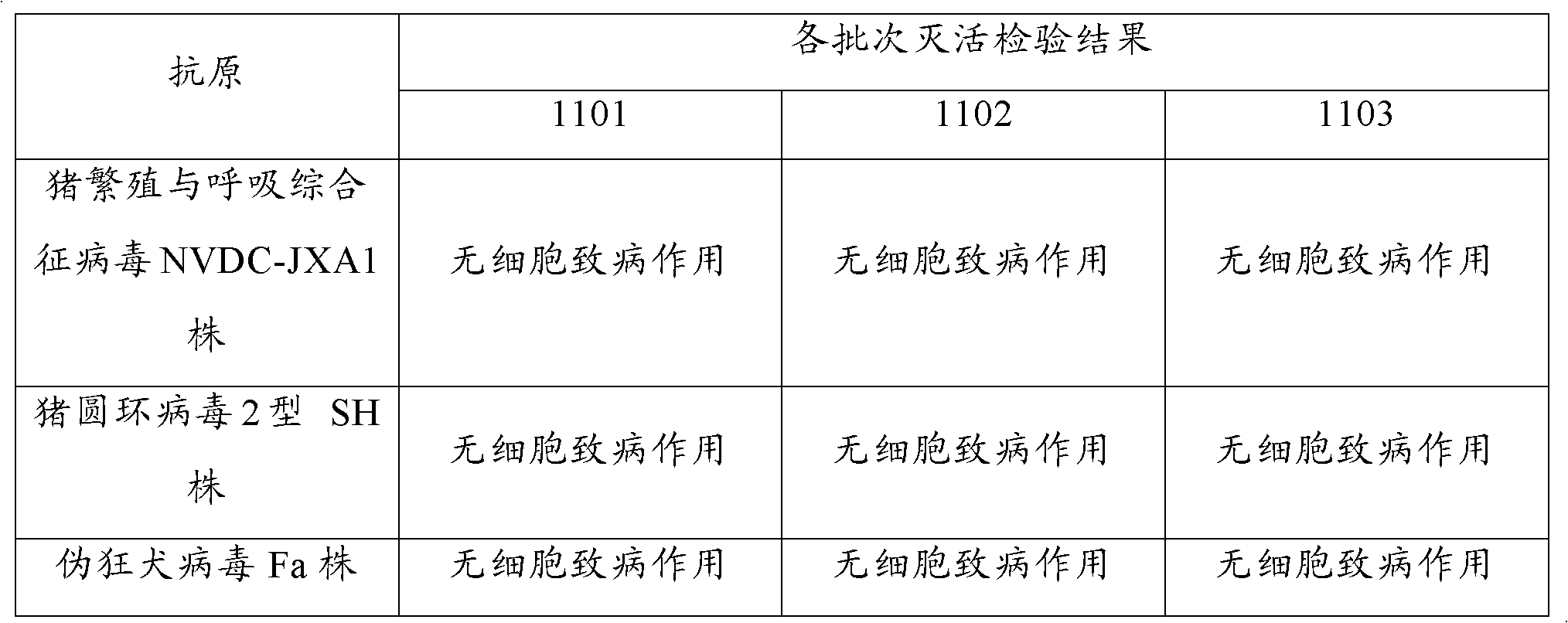
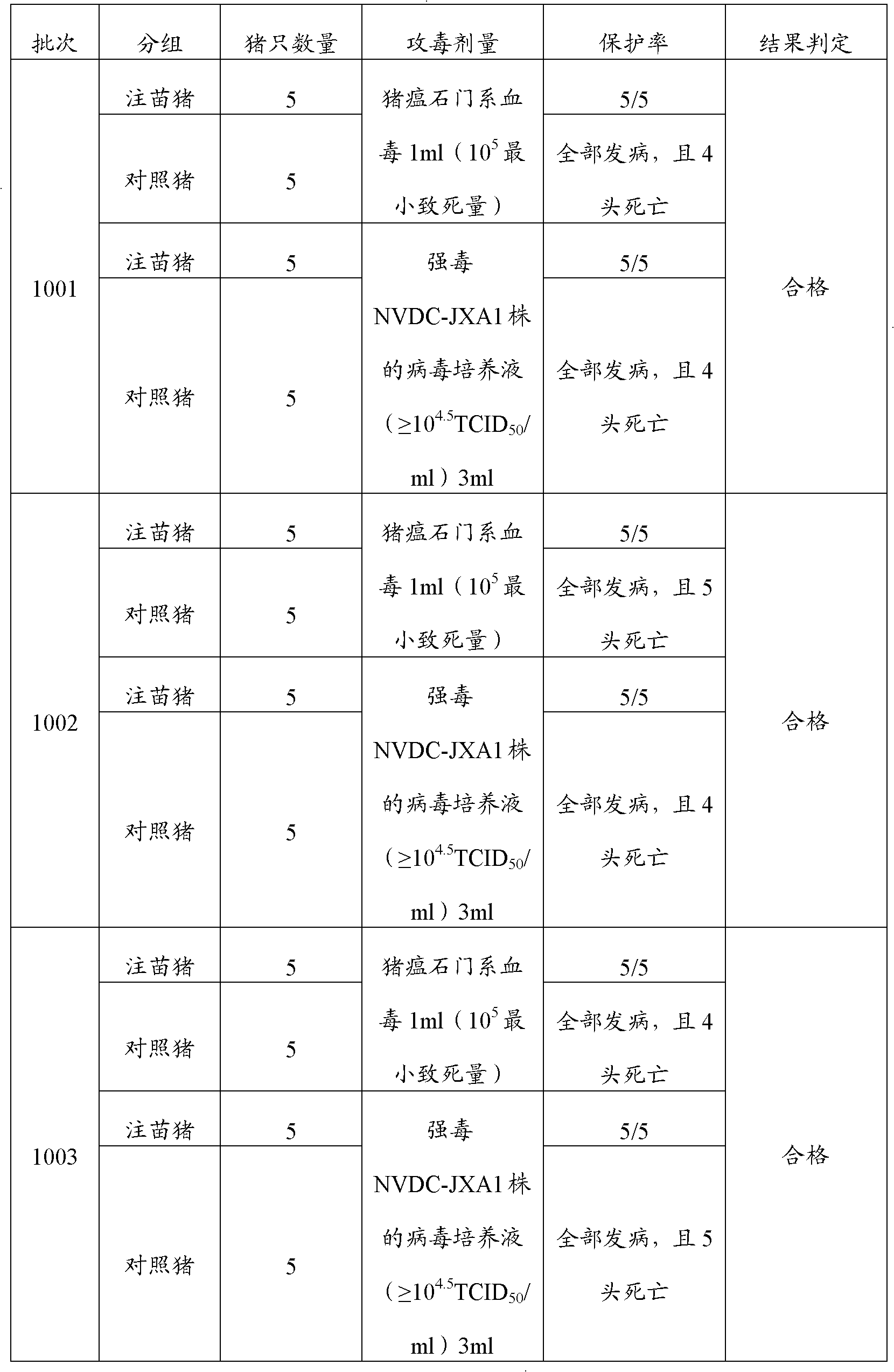
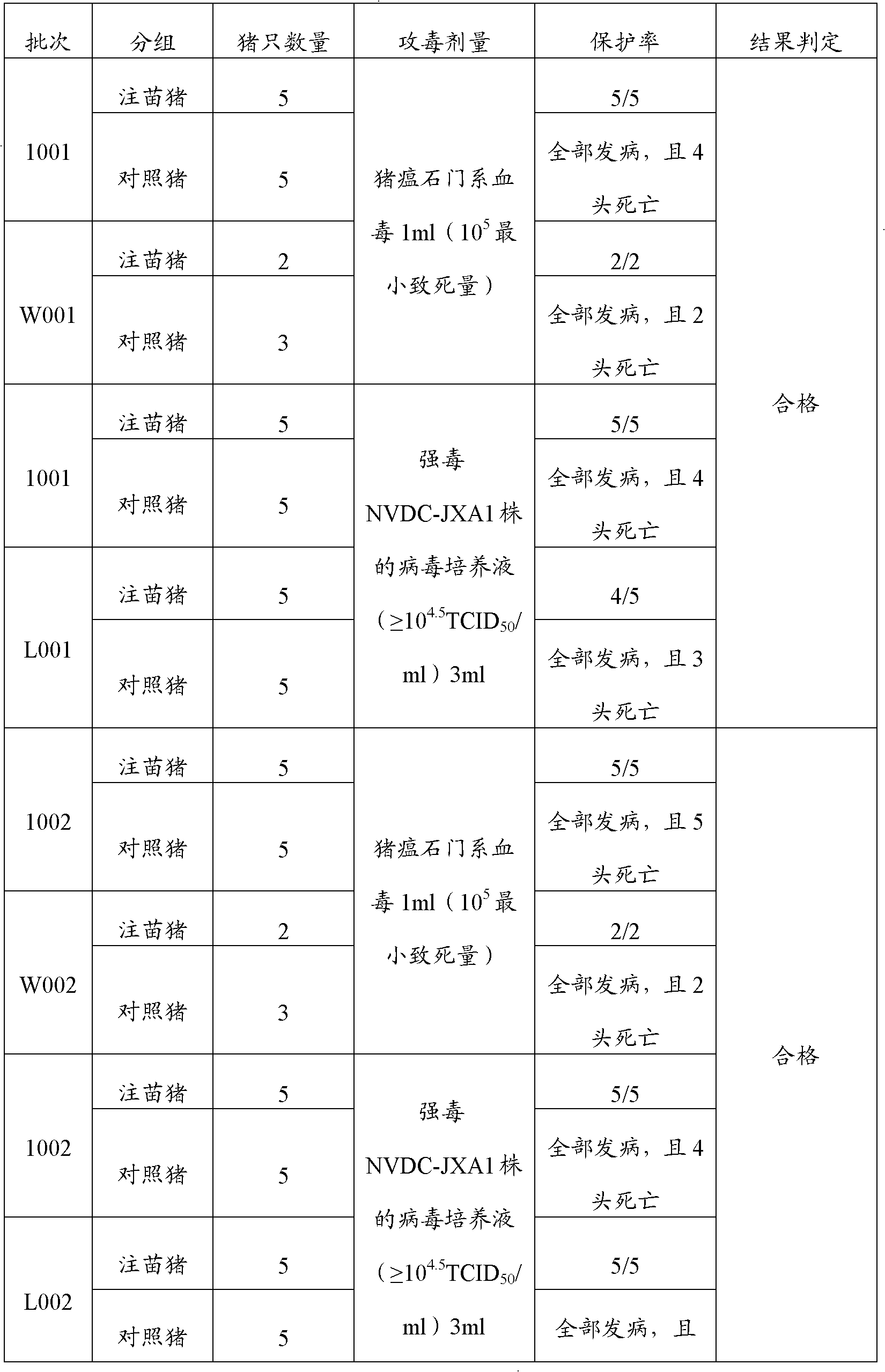
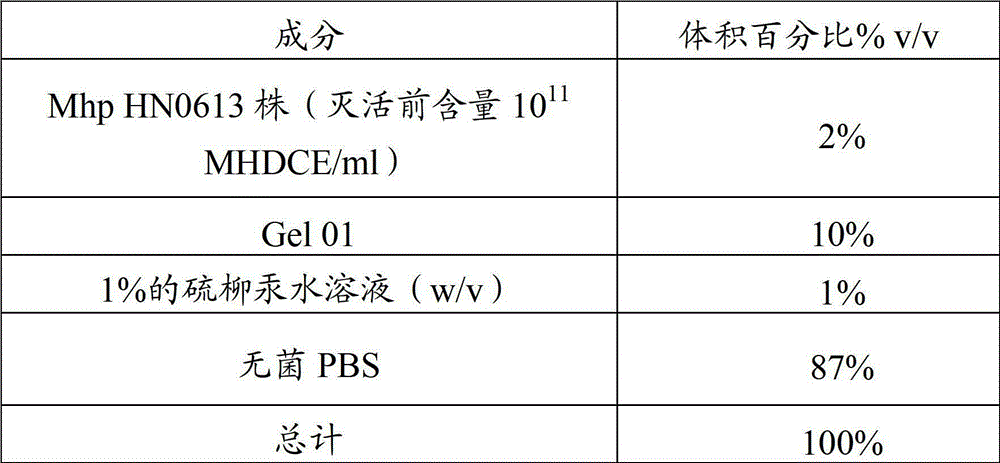
Porcine transmissible gastroenteritis virus (PTGEV) and porcine epidemic diarrhea virus (PEDV) dual yolk antibody and preparation method thereof
ActiveCN101948536AEasy to produceReduce manufacturing costEgg immunoglobulinsDigestive systemYolkFreeze-drying
Owner:PU LIKE BIO ENG
Dry-breeding method and breeding facilities for cherry valley ducks
The invention relates to full control technology and breeding facilities under a dry-breeding method for cherry valley ducks. The technical scheme is that: the dry-breeding method for the cherry valley ducks comprises breeding management, epidemic disease control management and sewage treatment, wherein the breeding management comprises feed amount control, number control on male and female ducks and breeding density control; the epidemic disease control management comprises a step of regularly replacing bath water in a bath pool and a step of making an immunization program for the cherry valley ducks; and the sewage treatment comprises a step of regularly draining the bath water in the bath pool and a step of performing three-level settlement, aeration and filtering on the sewage.
Owner:HUZHOU ZHONGWANG FOWL IND
Vaccine composition, preparation method and application thereof
ActiveCN103908665AReduce the number of vaccinationsIncrease costAntibacterial agentsBacterial antigen ingredientsDiluentWater soluble
The invention relates to a vaccine composition, which contains classical swine fever virus antigen, porcine circovirus type 2 antigen, mycoplasma hyopneumoniae antigen and a vaccine adjuvant. The invention also provides a method for preparing the vaccine composition, a water-soluble vaccine adjuvant is used for preparing a mixture of the porcine circovirus type 2 antigen and mycoplasma hyopneumoniae antigen, and the mixture is taken as a diluent for diluting classical swine fever virus antigen. The vaccine composition has the advantages of simple preparation method, high titer content of the vaccine, and convenient and fast immunization, one time immunization can simultaneously preventing or treating swine fever, porcine circovirus type 2 antigen and mycoplasma hyopneumoniae, immunization cost is reduced, the immunization program is saved, and the application is more economic and reliable.
Owner:PU LIKE BIO ENG
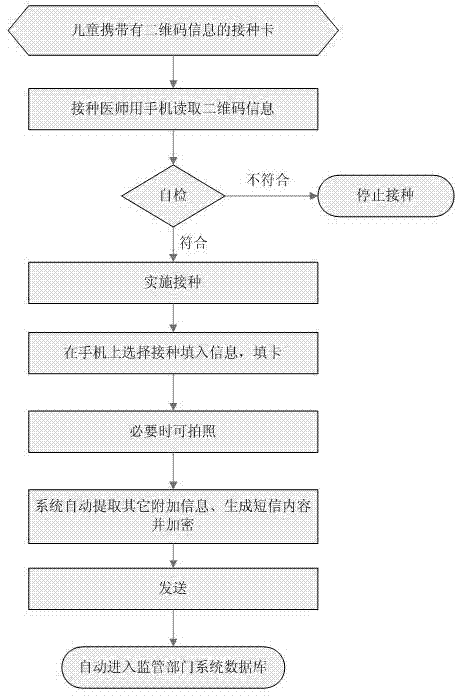
Children immunization program information management system
InactiveCN104331761AAccurate informationSimplify the entry processResourcesInformation processingRelevant information
The invention discloses a children immunization program information management system which comprises a children's preventive vaccination certificate, a doctor mobile communication platform and a database. The preventive vaccination certificate is printed with two-dimension codes comprising vaccination children related information; the mobile communication platform is equipped with a preventive vaccination information processing program, wherein the processing program comprises a medicine usage registration module, a vaccination information input module and a vaccination information sending module. The medicine usage registration module is used for recording information of medicine used by a doctor every time; the vaccination information input module is for reading the two-dimension code information on the children's preventive vaccination certificate and for carrying out selection and confirmation on vaccination site, vaccination mode and vaccination medicine information of each vaccination child every time; the vaccination information sending module is for carrying out encryption on the input vaccination information and sending the information to the database, vaccination data being automatically generated when the information is sent; and the database can automatically decode the received vaccination information and can dynamically carry out statistical analysis.
Owner:刘勇军 +1
A kind of egg yolk antibody with high biological activity and preparation method thereof
InactiveCN102276720AGood control effectEnsure safetyAntibacterial agentsEgg immunoglobulinsYolkBacterial disease
The invention discloses a highly biologically active yolk antibody and a preparation method thereof. The antibody in the present invention uses aerolysin and extracellular protease, the main pathogenic factors of pathogenic Aeromonas hydrophila, as immunogens respectively, and simultaneously immunizes laying hens with an appropriate immunization program to obtain highly immune eggs, and the titer can be as high as 1:212, the antibody was purified by octanoic acid-ammonium sulfate method and then spray-dried. The antibody titer obtained according to the invention is high, and can be used to control fish bacterial diseases, especially the immune protection effect on fish with hemorrhagic diseases can be as high as 90%. Moreover, the mass production of antibodies by this method is low in cost, labor-saving and workshop-saving, high in social benefits, and broad in prospects.
Owner:JIANGSU AGRI ANIMAL HUSBANDRY VOCATIONAL COLLEGE
A method of cultivating quail for eggs using internal and external ecological technology
InactiveCN102293180AReduce pollutionReduce manufacturing costAnimal feeding stuffAnimal housingDiseaseAnimal science
A method for raising quails for eggs using internal and external ecological technology discloses a method for ecologically raising quails for eggs, which uses natural organic disinfectants instead of daily disinfectants to improve the internal and external environment of quail houses and make the air quality comply with NY / T391 ( Environmental technical conditions for green food production areas) requirements; adding lactic acid bacteria to the quail feed for eggs instead of medicine and health care can regulate the normal flora of the gastrointestinal tract of the egg quail body, maintain the micro-ecological balance, and inhibit the growth and reproduction of spoilage bacteria and the production of spoilage products in the intestinal tract. Effectively reduce harmful gases such as ammonia in quail houses and the odor of feces, improve the breeding environment, and improve the health of laying quails; establish a reasonable immunization program through pathogen investigation and antibody detection to control the occurrence of diseases. After adopting the method, the internal and external environment of the poultry house is greatly improved, the resistance of laying quail to diseases and the prevention of infectious diseases are enhanced, the occurrence of epidemic diseases is controlled, the yield of quail eggs is increased, and the quality of quail eggs is improved.
Owner:河南鹑都实业有限公司
Breeding method for breeding pigs
InactiveCN104542463APrevention of swine feverPrevention of Foot and Mouth DiseaseAntibacterial agentsSsRNA viruses positive-senseAnimal sciencePig breeding
The invention discloses a breeding method for breeding pigs, and relates to the technical field of pig breeding. In the breeding pig breeding process, the breeding pigs in different phases such as piglet phase, reserve swine phase and sow phase are subjected to immunization by various vaccines in different time stages. According to the breeding method, a normative immunization program is provided, so that swine fever, foot-and-mouth diseases, pseudorabies, porcine reproductive and respiratory syndrome, parvovirus, epidemic diarrhea, contagious gastroenteritis porcine rotavirus, epidemic encephalitis B, haemophilus parasuis, porcine circovirus and the like which are common in the piglets, the reserve swines and the sows can be effectively prevented.
Owner:刘长华
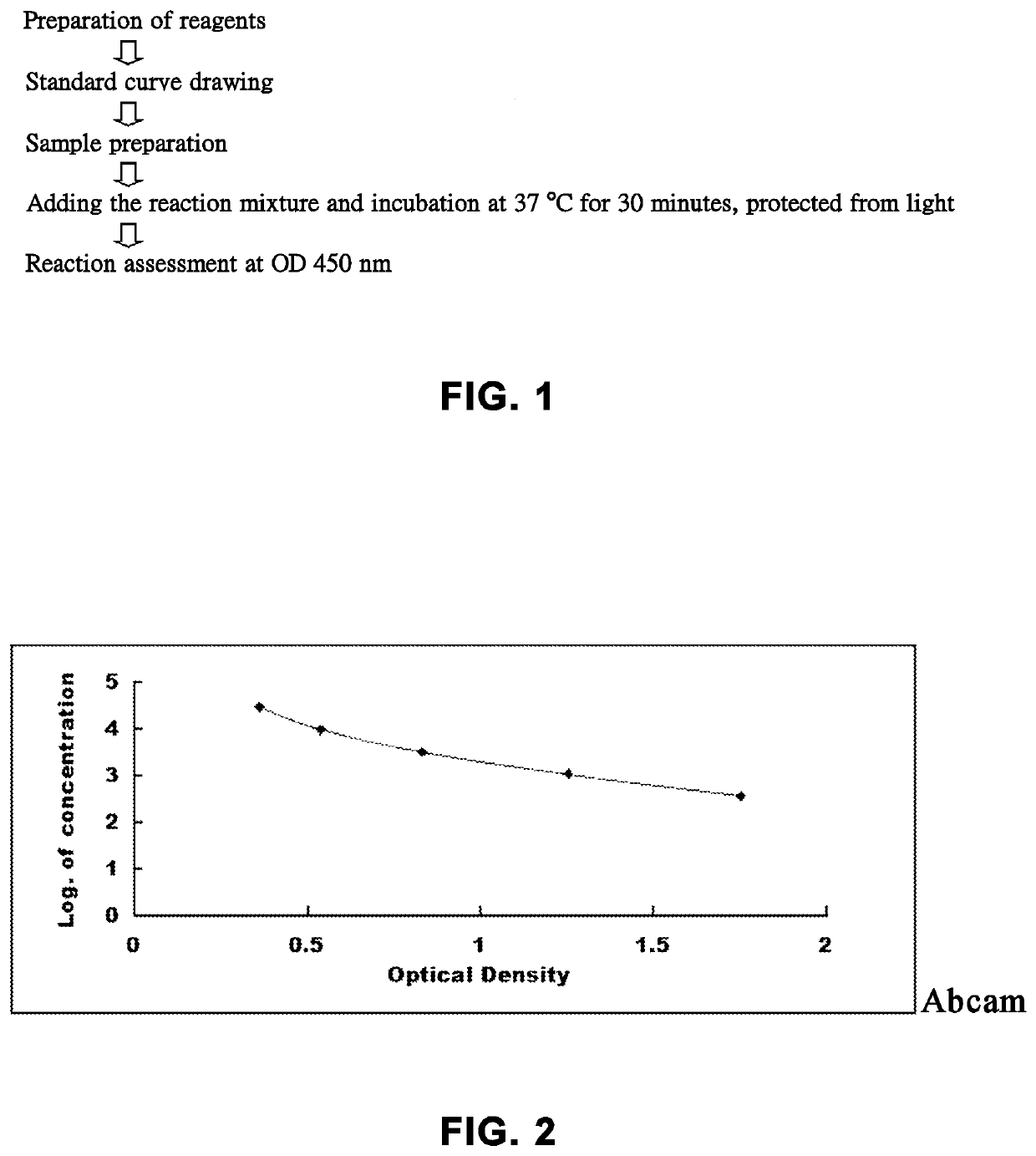
Automatic pipeline for blood cell analysis and POCT immunoassay
InactiveCN110187134AShort detection cycleVersatilityMaterial analysisTest channelFluorescence immunoassay
The invention discloses an automatic pipeline for blood cell analysis and POCT immunoassay, and the pipeline comprises a rack, a first conveying platform, a first mixing device, a first sampling mechanism, a first detector with an analyzing device, a first unloading platform, a first conveying device, a second conveying platform, a relay mechanism, a second mixing device, a second sampling mechanism and a fluorescent immunoassay analyzer with a plurality of test channels, wherein the automatic pipeline for blood cell analysis and POCT immunoassay with the above structure organically combines blood cell analysis and fluorescence immunoassay to achieve detection of blood cells and POCT immunization programs, and the plurality of test channels using the fluorescent immunoassay analyzer can freely combine different test items, thereby providing versatility and expandability, shortening sample detection cycle, improving efficiency, reducing manual intervention and reducing error rates.
Owner:ZHONGSHAN CHUANGYI BIOCHEM ENG
Combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin inactivated poliovirus and preparation thereof
InactiveCN102178949ASimplified and expanded immunization programsReduce the number of vaccinationsAntibacterial agentsAntiviralsDiseaseTetanus toxoids
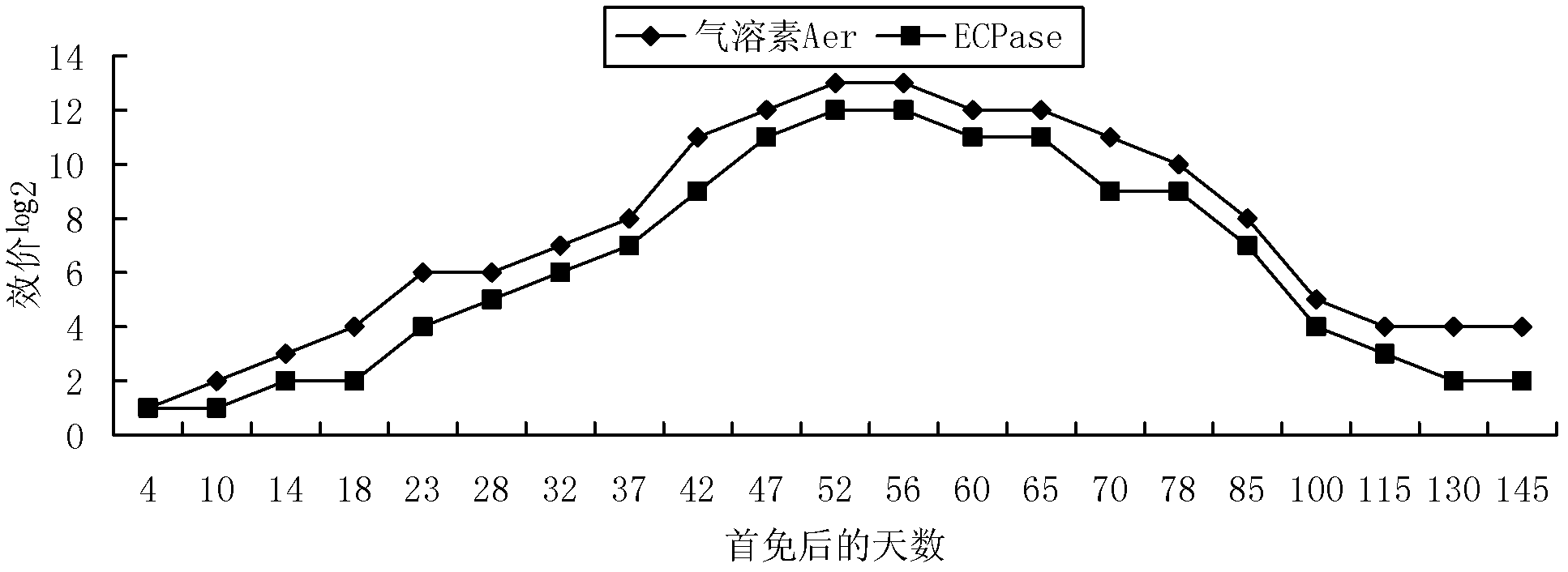
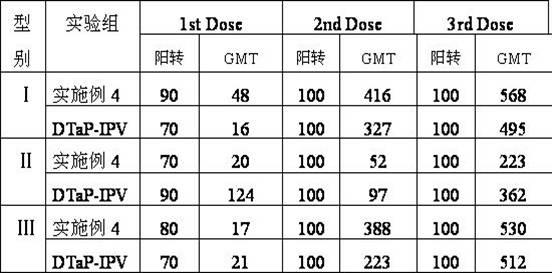
The invention provides combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin (DTaP-sIPV) inactivated poliovirus and preparation thereof. The DTaP-sIPV is characterized in that each 100ml of the combined vaccine comprises the following components: 400-1800ug (PN) of acellular pertussis (AP) stock solution, 300-700Lf of tetanus toxoid (TT), 1000-2500Lf of diphtheria toxoid (DT), 126-154mg of Al(OH)<3>, 3000-6000DU of sIPV I, 5700-7100DU of sIPV II, 4500-9000DU of sIPV III, 765-935mg of NaCl, 0-600mg of 2-phenoxyethanol and the balance of H<2>O. Compared with the existing products, the DTaP-sIPV has the advantages of higher biological safety, better side reaction and the like; and the DTaP-sIPV has the beneficial effects of preventing a plurality of target diseases, reducing inoculating needles, simplifying immunization programs, improving inoculation rate, reducing opportunity of cross infection, being popular with a majority of parents and children, saving various expenses and facilitating smooth promotion of immunization plan.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Trivalent inactivated vaccine of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and porcine pseudorabies virus and preparation method thereof
InactiveCN102973932AIncrease productionImprove securityViral antigen ingredientsAntiviralsImmune effectsMultivalent Vaccine
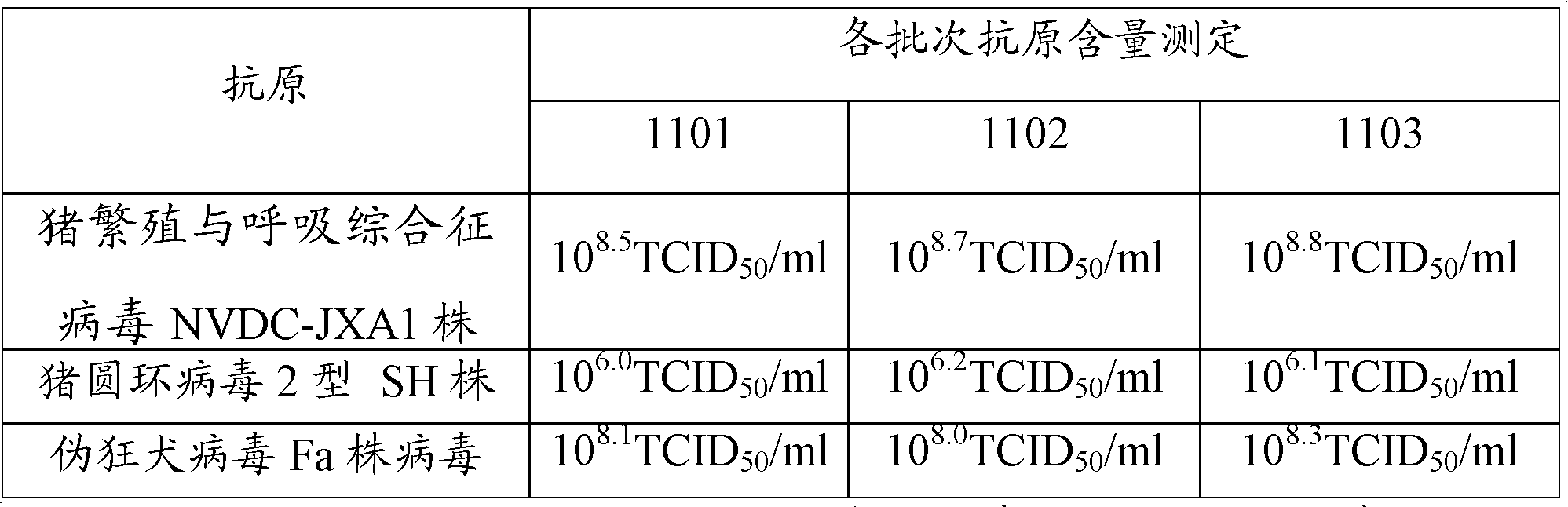
The present invention provides a trivalent inactivated vaccine of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and porcine pseudorabies virus. Before inactivation, the contents of the three viruses are respectively greater than 10<8.5>TCID[50] / ml 10<6.0>TCID[50] / ml and 10<8.0>TCID[50] / ml; And after the inactivation, the volume ratio of the three antigens is 1:1:1. According to the present invention, via a large number of detailed tests, the contents and ratio of the three viral antigens are selected; and immune effects are measured in a large number of experimental animals and swine themselves, to ensure that the phenomenon of immune interference does not occur among the various immune components in the multivalent vaccine. Compared with the existing three individual vaccines of the same three virus, wherein three injections are need to prevent the three diseases caused by the three virus by using the three individual vaccines, the trivalent inactivated vaccine of the present invention is economical and practical, and simplifies the immunization procedure, and reduces the cost of epidemic prevention. The present invention realizes preparation and application of multivalent inactivated vaccine of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and porcine pseudorabies virus, which has not been achieved for all the time in the field.
Owner:PU LIKE BIO ENG
Tilapia mossambica streptococcus agalactiae oral attenuated freeze-dried vaccine
InactiveCN104906568AEasy to useReduce stressAntibacterial agentsPowder deliveryStreptococcus agalactiaeStreptococcus mastitidis
The invention discloses a tilapia mossambica streptococcus agalactiae oral attenuated freeze-dried vaccine and its preparation method. According to the invention, tilapia mossambica streptococcus agalactiae attenuated bacterial strain YM001 is performed with steps of enlarge cultivation, thalline collection and freeze-drying preparation to obtain the oral attenuated vaccine. The immunizing dose, immunization program and a vaccine usage method of the vaccine can be determined through tests. The immune efficacy, security and maneuverability of the vaccine can be detected and confirmed through field tests, the result shows that the vaccine has the advantages of high protection effectiveness, good security, convenient usage and low cost, the vaccine can be used for preventing tilapia mossambica streptococcus agalactiae, and has high business value.
Owner:GUANGXI ACADEMY OF FISHERY SCI
Integration of meningococcal conjugate vaccination
ActiveUS9402915B2Antibacterial agentsCarrier-bound antigen/hapten ingredientsCarrier proteinDiphtheria vaccination
Conjugated meningococcal capsular saccharides will be introduced into immunization schedules in the near future, but the phenomenon of “carrier suppression” must first be addressed, particularly where multiple conjugates are to be used. It has been found that diphtheria toxoid and its derivatives (such as CRM197) can safely be used as the carrier protein, even where multiple meningococcal conjugates are administered at the same time and where a patient has previously been exposed to the carrier protein, either in the form of a previous immunogen (e.g. in a DTP vaccine) or as a previous carrier protein (e.g. in a Hib or pneumococcal conjugate vaccine). The invention provides a method for immunizing a patient, comprising administering multiple conjugates of meningococcal capsular saccharides, wherein each conjugate comprises a diphtheria toxoid (or derivative thereof) carrier protein, and the capsular saccharide, and wherein the patient has been pre-immunized with a diphtheria toxoid (or derivative thereof).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
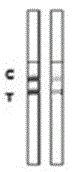
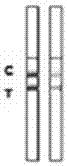
Rabbit pestivirus antibody rapid detection card and preparation method thereof
The invention relates to a rabbit pestivirus antibody rapid detection card and a preparation method thereof. The preparation method comprises the following concrete steps: (1) preparing an anti-rabbit-pestivirus VP60 monoclonal antibody, concretely performing immunization program, cell fusion, hybridomas screening and cloning, and antibody preparation and purification; and (2) preparing the rabbit pestivirus antibody rapid detection card, concretely decocting a colloidal gold solution, marking the anti-rabbit-pestivirus VP60 monoclonal antibody, processing a colloidal-gold pad, processing a sample pad, and determining C and T lines. The rabbit pestivirus antibody rapid detection card is capable of rapidly, sensitively and accurately detecting rabbit pestivirus antibody, and overcomes the problems that domestic rabbit pestivirus antibody detection mainly employs erythrocyte hemagglutination and ELISA detection.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Meningococcal conjugate vaccination
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
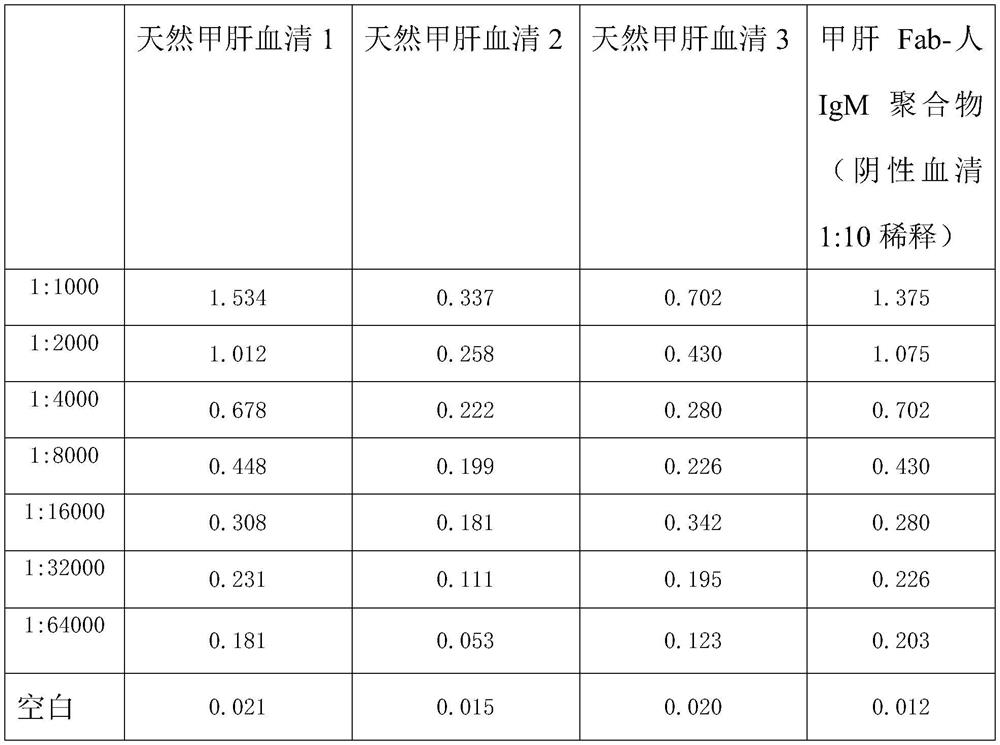
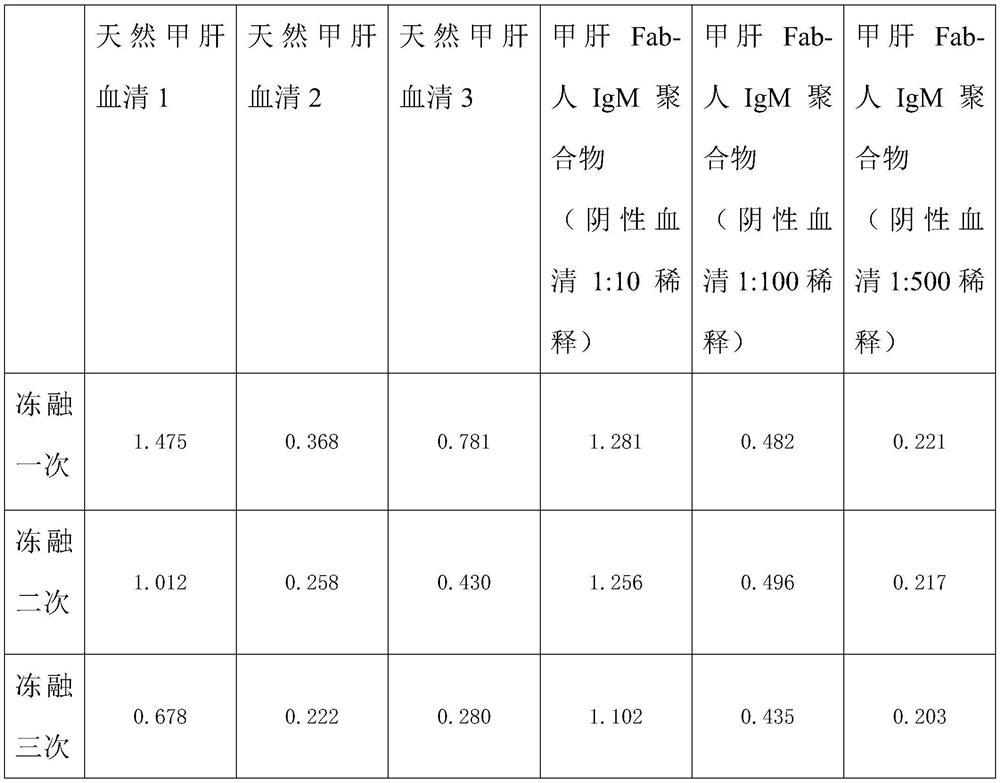
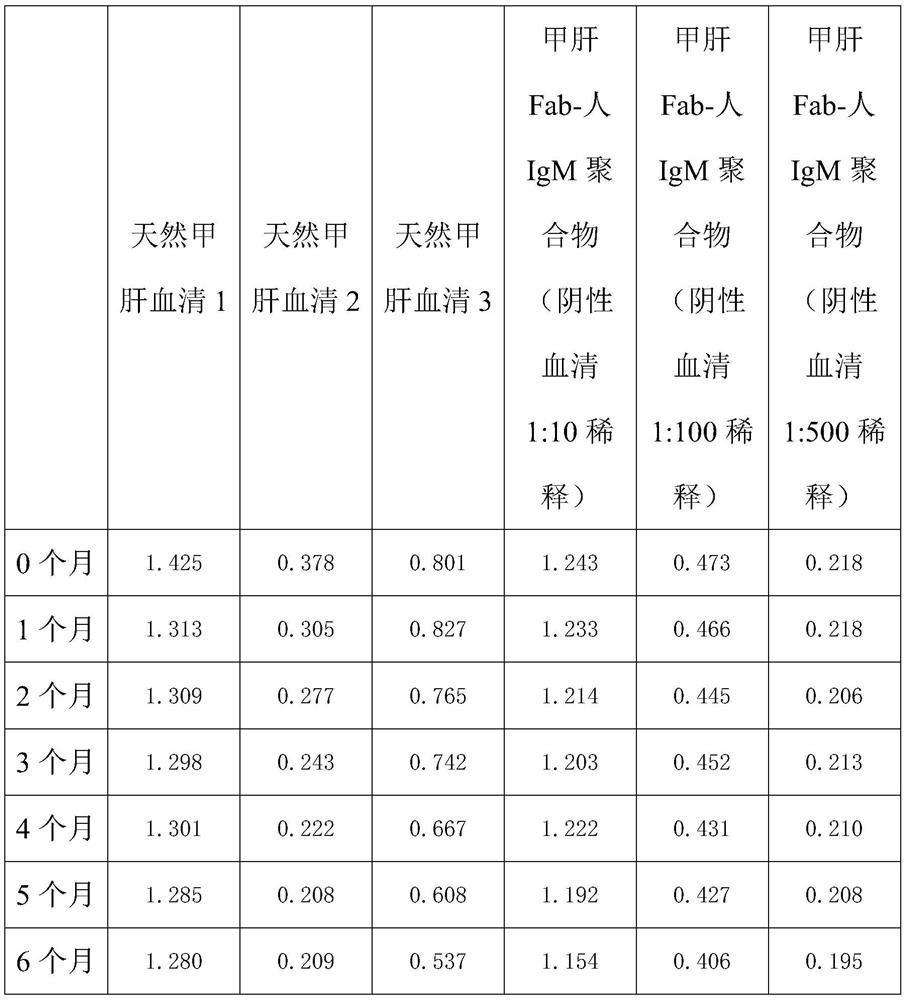
Method for preparing IgM (Immunoglobulin M) calibrator
PendingCN114544295AHigh affinityImprove stabilityPreparing sample for investigationIgm antibodyAntiserum
The invention discloses a method for preparing an IgM calibrator, and relates to the field of IgM calibrators, the method comprises the following steps: 1, obtaining a sheep polyclonal antibody: immunizing a sheep according to a fixed immunization program, and extracting serum when the titer is qualified; 2, purifying the sheep polyclonal antibody: purifying the extracted sheep polyclonal antibody serum by using affinity chromatography; 3, cutting the sheep polyclonal antibody: cutting the sheep polyclonal antibody IgG by using an enzyme, purifying a Fab fragment in the sheep polyclonal antibody, and using the purified Fab fragment for subsequent coupling; 4, coupling of the human IgM and the Fab fragment: coupling the human IgM and the Fab fragment of the sheep polyclonal antibody by using a chemical method; 5, detection: carrying out affinity test and aging test on a product obtained by coupling the human IgM and the Fab fragment of the sheep polyclonal antibody; the prepared specific IgM is added into negative serum according to a certain concentration to obtain the IgM antibody serum, and the IgM antibody serum can be used for research and development of in-vitro diagnosis IgM kits and calibration products of the specific IgM serum.
Owner:武汉原谷生物科技有限责任公司
Vaccine composition containing swine mycoplasmal pneumonia antigen and swine streptococcosis antigen, and preparation method and application thereof
ActiveCN103861095APreserve immune efficiencyLow costAntibacterial agentsBacterial antigen ingredientsImmune effectsAdjuvant
The invention provides a vaccine composition containing a swine mycoplasmal pneumonia antigen and a swine streptococcosis antigen, and a preparation method and an application thereof. The vaccine composition includes an immunizing dose of the swine mycoplasmal pneumonia antigen, an immunizing dose of the swine streptococcosis antigen, and an adjuvant. The vaccine composition has a simple immunization program, can effectively control the swine mycoplasmal pneumonia antigen and the swine streptococcosis antigen, has an immune effect equivalent to the immune effect realized through respective injection of single vaccines, and also has the characteristics of small side reaction, long immune period, less time consumption, and less labor consumption; and the vaccine composition also has the advantages of simple production technology, low immune cost and strong practicality.
Owner:PU LIKE BIO ENG
Nursery pig breeding method for large-scale pig farms
InactiveCN107211952AImprove survival rateGrow fastAccessory food factorsVeterinary instrumentsPig farmsDisease
The invention relates to a breeding method for nursery pigs in a large-scale pig farm, which includes the following technical points: the selection of feed must have a corresponding change in nutritional ratio, and in the early growth stage of nursery pigs, the feed for nursery pigs needs to increase the protein ratio in an appropriate amount; Vaccine injection to ensure the quality of the vaccine. According to the specific situation, combined with the epidemic period of different diseases and the growth law of antibodies in the nursery pigs, scientifically and reasonably formulate the immunization plan; when the density of pigs is high, pay special attention to the pigs The guarantee of a good living environment can effectively inhibit the growth of viruses and the like. The method of the invention is simple and low in cost, can realize large-scale breeding of nursery pigs, and greatly improves the growth cycle and survival rate of nursery pigs.
Owner:重庆问天农业科技有限公司
Preparation technology of anti-rabies virus chicken yolk antibody
InactiveCN104402994AGood antiviral effectNo side effectsEgg immunoglobulinsImmunoglobulins against virusesYolkBiting
A preparation technology of an anti-rabies virus chicken yolk antibody comprises the steps of immune inoculated vaccine chicken selection, immune inoculated vaccine dosage determination, immunization program, yolk separation method and skill, yolk antibody water-soluble ingredient liquid WSF preparation, yolk antibody separating extraction, yolk antibody extract product aseptic treatment, PEG solution preparation and dialysis bag treatment. The yolk antibody (IgY) is an easily available and cheap polyclonal antibody, has a good antivirus effect and has no toxic side effects, and the purified IgY is a modern ideal immunopotentiator and treatment agent, is convenient to prepare, is safe and reliable, is prepared through immunizing birds with a rabies vaccine, through generating an anti-rabies virus polyclonal antibody by bird B lymphocytes under the stimulation of rabies viruses and through transferring the anti-rabies virus polyclonal antibody to yolk, can be used for preventing and treating people rabies, and can be especially used for prevention of dog exposure high risk people and emergency prevention after dog biting.
Owner:ZUNYI MEDICAL UNIVERSITY
Treatment method of human peripheral blood T lymphocytes, immunogen preparation and application
ActiveCN113025571ASolve bottlenecksSimplify the production purification processSerum immunoglobulinsImmunoglobulins against animals/humansWhite blood cellT lymphocyte
The invention relates to a treatment method of human peripheral blood T lymphocyte, animmunogen preparation, a preparation method of antiserum, and application. T lymphocytes are separated and purified by using a waste leukocyte filter disc, multiplication culture is carried out, and antigen surface marker identification comparison and activity comparison are carried out on the separated T lymphocytes and the cultured T lymphocytes; the high-activity T lymphocytes subjected to multiplication culture are used as an immunogen, antiserum is prepared by using a healthy pig, the titer after immunization is evaluated by using results of an E rose ring inhibition experiment and a cytotoxicity experiment, and formulating an immunization program, so as to prepare a large amount of antiserum; and anti-plasma is collected for plasma separation, and the plasma separation is used for preparation of ALG products. According to the invention, the limitation problem that fresh whole blood needs to be collected every time in the titer verification of the anti-human T lymphocyte immune globulin preparation raw material and the titer verification of the finished product can be solved, and meanwhile, the bottleneck problem of the anti-human T lymphocyte immune globulin raw material can also be solved.
Owner:武汉中生毓晋生物医药有限责任公司
Composition, Preparation Method And Evaluation Of A Complex Immunogen Named I-SPGA For Production Of Immunological Active Proteins (IAP)
ActiveUS20210121552A1Increased antibody coupling powerEasy to combineBacterial antigen ingredientsEgg immunoglobulinsPotentiatorResistant infection
The present invention relates to the composition and method of preparing an immunogen designated as I-spga consisting of a complex antigen prepared from 18 to 26 species of pathogenic microorganisms isolated from patients, inactivated with binary ethyleneamine (BEI) and formalin, diluted in a SPGA immunopotentiator mixed with QS-21 adjuvant. By inoculating the hens with the I-spga immunogen, hyperimmune eggs (Immunospga) are obtained which contain immunologically active proteins specific to the 18-26 antigens used for immunization. The immune response of the hens is specific to the used antigens by amplification of the antigenic signal by the SPGA immunopotentiator and due to a special immunization program that allows the immune system to act complex and intense: The I-spga complex antigen contains 18-26 microorganisms isolated from patients, bacterial bodies, components from bodies obtained by ultrasonography, cilia, exotoxins, endotoxins, spores, viruses, fungi or yeasts. This pathogenic material is inactivated with BEI and formalin. The I-spga antigen is of three types. The standard I-spga antigen is composed of 18 to 24 antibiotic-resistant bacterial species isolated from patients in Romania. The specific I-spga complex antigen is composed of the I-spga complex antigen containing a mixture of 7-9 strains from a single species of bacteria, fungi or yeasts isolated from patients in Romania mixed with SPGA and QS-21, used for inoculation of hens previously immunized with standard I-spga antigen. The personalized I-spga antigen is composed of patient-derived pathological material containing cellular debris and pathogenic germs inactivated with BEI and formalin and mixed with SPGA and QS-21 and is used to immunize hens previously immunized with the standard I-spga antigen. This now patented technology encompasses a new generation of biological products in which the immune response of the hens to different groups of parenterally inoculated antigens at different time intervals is overlapping. Chicken response is uniform and additional administration of immunogens and SPGA as an immunopotentiator amplifies the antigenic signal and immune response. The I-spga immunogen as well as the immune response contain two markers, G and A, which identify the I-spga antigen used for immunization against the antigens used to produce the Imunoinstant group bio-preparations or similar products. The I-spga immunogen is used to immunize the hens for obtaining immunologically active proteins that can be used to treat immune deficiencies, psoriasis, epidermolysis bullosa, other dermatitises, nosocomial infections, antibiotic-resistant infections in the urinary system of children and grownups.
Owner:FANTANA RAUL SORIN +1
A blood analysis system for highly infectious diseases
InactiveCN101504419BWon't happenHigh biosecurityBiological testingMaterial electrochemical variablesPeristaltic pumpBlood gas analysis
The present invention provides a blood analysis system for highly infectious diseases in order to solve the technical problems of low biological safety and poor real-time performance of the existing blood analysis system for highly infectious diseases, including a sampling mechanism, a measurement and analysis module, A waste liquid treatment device and a liquid circuit system, the measurement and analysis module is composed of an electrolyte measurement module and a biochemical measurement module, the electrolyte measurement module is connected to the sampling mechanism and connected to the waste liquid treatment device through a peristaltic pump, and the biochemical measurement module is composed of A reagent container, a reagent valve, a peristaltic pump, a sampling valve, a reaction pipeline and a detector are sequentially connected to form, the sampling valve is connected to a sampling mechanism, and the detector is connected to a waste liquid treatment device. Its beneficial effect is that the biological safety is high, all the measurement items are carried out in a closed pipeline, there is no injection during the analysis process, and it has a waste liquid disinfection device; the real-time performance is strong, and the analysis of electrolyte items, biochemical items, and immune items are three Combined into one, the instrument is small in size and can be used in the ward.
Owner:SHENZHEN UNIV +1
Bivalent vaccine for porcine reproductive and respiratory syndrome and classical swine fever prevention or treatment, and preparation method thereof
ActiveCN102973933BEasy to prepareHigh titer contentViral antigen ingredientsAntiviralsAntigenDisease
The present invention provides a bivalent vaccine for porcine reproductive and respiratory syndrome (PRRS) and classical swine fever (CSF) prevention or treatment. The bivalent vaccine comprises the following components: PRRS antigen with a content of at least 10<5>TCID50 / unit, and CSF antigen with a content of at least 7500 rabbit infection dose / unit. The bivalent vaccine preparation method comprises: adopting passage cells to culture PRRS viruses and CSF viruses to obtain PRRS antigens and CSF antigens, mixing the two antigen components according to a certain ratio, and auxiliarily adopting a freeze drying protection agent to prepare the freeze drying vaccine. According to the present invention, the two antigens achieve the appropriate ratio, such that the bivalent vaccine achieves or exceeds efficacy of the original single vaccine; the bivalent vaccine has characteristics of simple preparation method, high titer content and convenient and rapid immunization; compared with fractionated immunization, vaccines for PRRS and CSF prevention and treatment through immunization at least 2 times or 3 times, and immunization methods thereof in the prior art, the bivalent vaccine and the immunization method thereof have characteristics of reduced immunization cost, saved immunization program, economy and reliability.
Owner:PU LIKE BIO ENG
Processing method of human peripheral blood T lymphocytes and immunogen preparation and application
ActiveCN113025571BSolve bottlenecksSimplify the production purification processSerum immunoglobulinsImmunoglobulins against animals/humansWhite blood cellT lymphocyte
The present invention relates to a treatment method for human peripheral blood T lymphocytes, preparation and application of immunogen preparations and antiserum, in which discarded white blood cell filter discs are used to separate and purify T lymphocytes, and the T lymphocytes are proliferated and cultured, and the isolated T lymphocytes Compared with the cultured T lymphocytes for antigen surface marker identification and activity comparison; then the expanded and cultured highly active T lymphocytes were used as immunogens, and healthy pigs were used to prepare antiserum, and E rosette inhibition experiments and cell Toxicity test results to evaluate the potency after immunization, formulate immunization procedures, and then a large amount of antiserum can be prepared; then anti-plasma is collected for plasma separation and used for the preparation of ALG products. The present invention can solve the restriction problem that fresh whole blood needs to be collected each time in the potency test of raw materials for preparation of anti-human T lymphocyte immunoglobulin and the potency test of finished products, and can also solve the problem of the bottleneck of the raw material of anti-human T lymphocyte immunoglobulin .
Owner:武汉中生毓晋生物医药有限责任公司
Artificial scale domestication method for sika deer for observation and exhibition
ActiveCN112119975AImprove survival rateAdjust the production cycleAnimal husbandryBlood collectionFed cattle
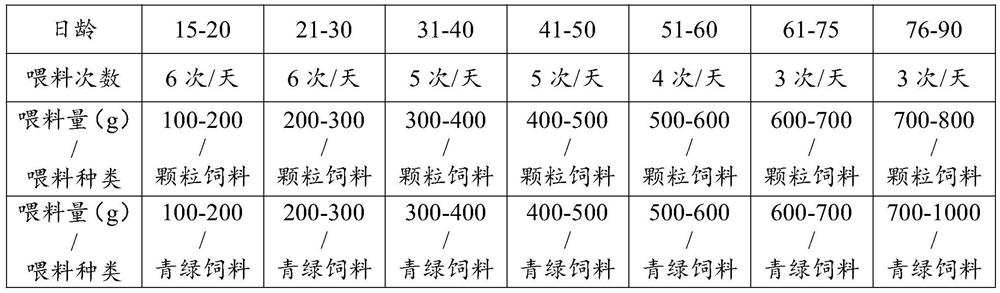
The invention relates to the field of animal domestication, in particular to an artificial scale domestication method for a sika deer for observation and exhibition. The artificial scale domesticationmethod comprises the steps that incubation room temperature and humidity control is carried out; after the one-day-old young deer which is one of two or more fetuses of a female deer and is fed withfirst colostrum is isolated from the female deer, the young deer is fed with bovine colostrum at the age of 1-3 days and is fed with bovine everyday milk at the age of 4-75 days, granular feed and greenish feed are supplemented at the age of 15 days, the young deer is fed with the granular feed and the greenish feed at the age of 76-90 days, and the anus of the young deer is pressed and wiped during feeding; immunizing is carried out according to an immunization program; regular disinfecting is carried out; when the young deer is 3 days old, music is played for the young deer for 1-2 hours ineach of the morning and afternoon every day; the young deer starts to contact crowds at the age of 7 days; the young deer starts to freely move from the age of 15-20 days, wherein the young deer respectively moves for 1-2 h in each of the morning and afternoon every day; after 20 days old, the contact strength is increased until the domestication standard is reached. According to the artificial scale domestication method, the young deer can be domesticated to be in zero-distance contact with a person, such as touch, stroke, feeding and living body non-anesthesia blood collection, and actions such as withering, face fitting, photographing and the like can be performed; and the survival rate of the young deer is 90% or above, and the breeding rate is 80%-90%.
Owner:东丰县梅花鹿产业发展服务中心
A kind of vaccine composition and its preparation method and application
ActiveCN103908665BReduce the number of vaccinationsIncrease costAntibacterial agentsBacterial antigen ingredientsWater solublePorcine circovirus
The invention relates to a vaccine composition, which contains classical swine fever virus antigen, porcine circovirus type 2 antigen, mycoplasma hyopneumoniae antigen and a vaccine adjuvant. The invention also provides a method for preparing the vaccine composition, a water-soluble vaccine adjuvant is used for preparing a mixture of the porcine circovirus type 2 antigen and mycoplasma hyopneumoniae antigen, and the mixture is taken as a diluent for diluting classical swine fever virus antigen. The vaccine composition has the advantages of simple preparation method, high titer content of the vaccine, and convenient and fast immunization, one time immunization can simultaneously preventing or treating swine fever, porcine circovirus type 2 antigen and mycoplasma hyopneumoniae, immunization cost is reduced, the immunization program is saved, and the application is more economic and reliable.
Owner:PU LIKE BIO ENG
A kind of identification method of chicken infectious bronchitis virus
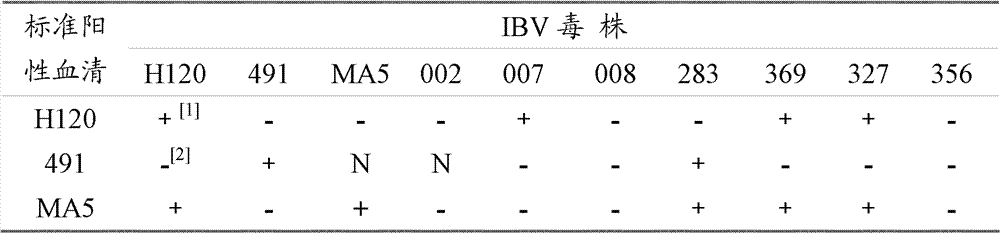
InactiveCN102286638BSmall dispersionAccurate judgmentMicrobiological testing/measurementSerotypeHemagglutination
The invention relates to a method for identifying chicken infectious bronchitis virus (IBV). The method is to inoculate SPF chicken embryos into the allantoic cavity of the sample to be tested, and set the SPF chicken embryos inoculated with PBS buffer solution as a control at the same time. Chicken embryos were incubated at 37°C for 24-30 hours, and then inoculated with attenuated Newcastle disease virus strain at 200-2000 times the half infectious dose (EID50) of chicken embryos, and then incubated at 37°C for 48-72 hours to detect the hemagglutination value of chicken embryo allantoic fluid , according to the hemagglutination value judgment result. This method utilizes the characteristic that IBV virus can interfere with the proliferation of chicken Newcastle disease virus (NDV) in chicken embryos, and can be used for laboratory diagnosis, antibody detection and IBV serotype identification of chicken infectious bronchitis, so as to carry out chicken infectious bronchitis vaccine immunization The evaluation of the effect and the monitoring of the epidemic situation provide a theoretical basis for formulating a scientific immunization program and effectively preventing and controlling the occurrence of the disease.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
A rapid detection card for rabbit plague virus antibody and its preparation method
The invention relates to a rabbit pestivirus antibody rapid detection card and a preparation method thereof. The preparation method comprises the following concrete steps: (1) preparing an anti-rabbit-pestivirus VP60 monoclonal antibody, concretely performing immunization program, cell fusion, hybridomas screening and cloning, and antibody preparation and purification; and (2) preparing the rabbit pestivirus antibody rapid detection card, concretely decocting a colloidal gold solution, marking the anti-rabbit-pestivirus VP60 monoclonal antibody, processing a colloidal-gold pad, processing a sample pad, and determining C and T lines. The rabbit pestivirus antibody rapid detection card is capable of rapidly, sensitively and accurately detecting rabbit pestivirus antibody, and overcomes the problems that domestic rabbit pestivirus antibody detection mainly employs erythrocyte hemagglutination and ELISA detection.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Vaccine composition containing swine mycoplasma pneumonia antigen and porcine streptococcal disease antigen and its preparation method and application
ActiveCN103861095BLow costReduce the number of vaccinationsAntibacterial agentsBacterial antigen ingredientsAntigenImmune effects
The invention provides a vaccine composition containing a swine mycoplasmal pneumonia antigen and a swine streptococcosis antigen, and a preparation method and an application thereof. The vaccine composition includes an immunizing dose of the swine mycoplasmal pneumonia antigen, an immunizing dose of the swine streptococcosis antigen, and an adjuvant. The vaccine composition has a simple immunization program, can effectively control the swine mycoplasmal pneumonia antigen and the swine streptococcosis antigen, has an immune effect equivalent to the immune effect realized through respective injection of single vaccines, and also has the characteristics of small side reaction, long immune period, less time consumption, and less labor consumption; and the vaccine composition also has the advantages of simple production technology, low immune cost and strong practicality.
Owner:PU LIKE BIO ENG
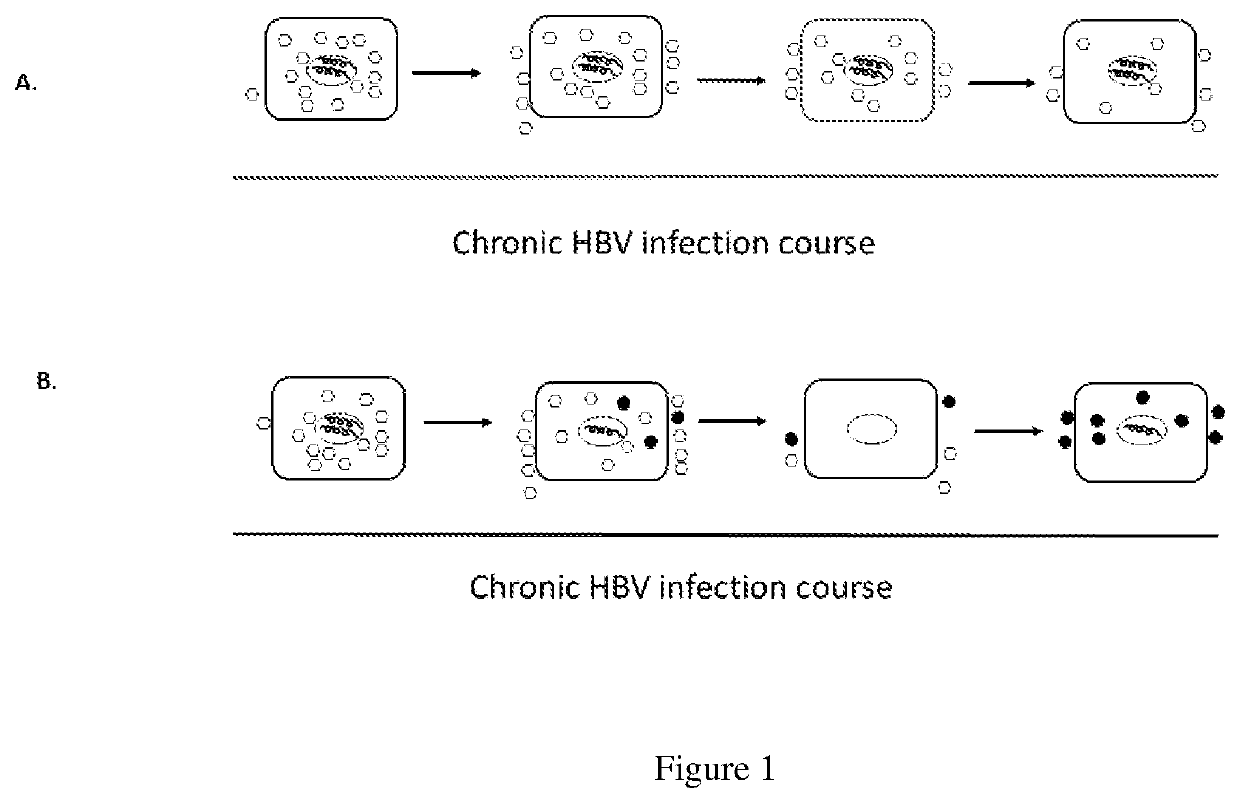
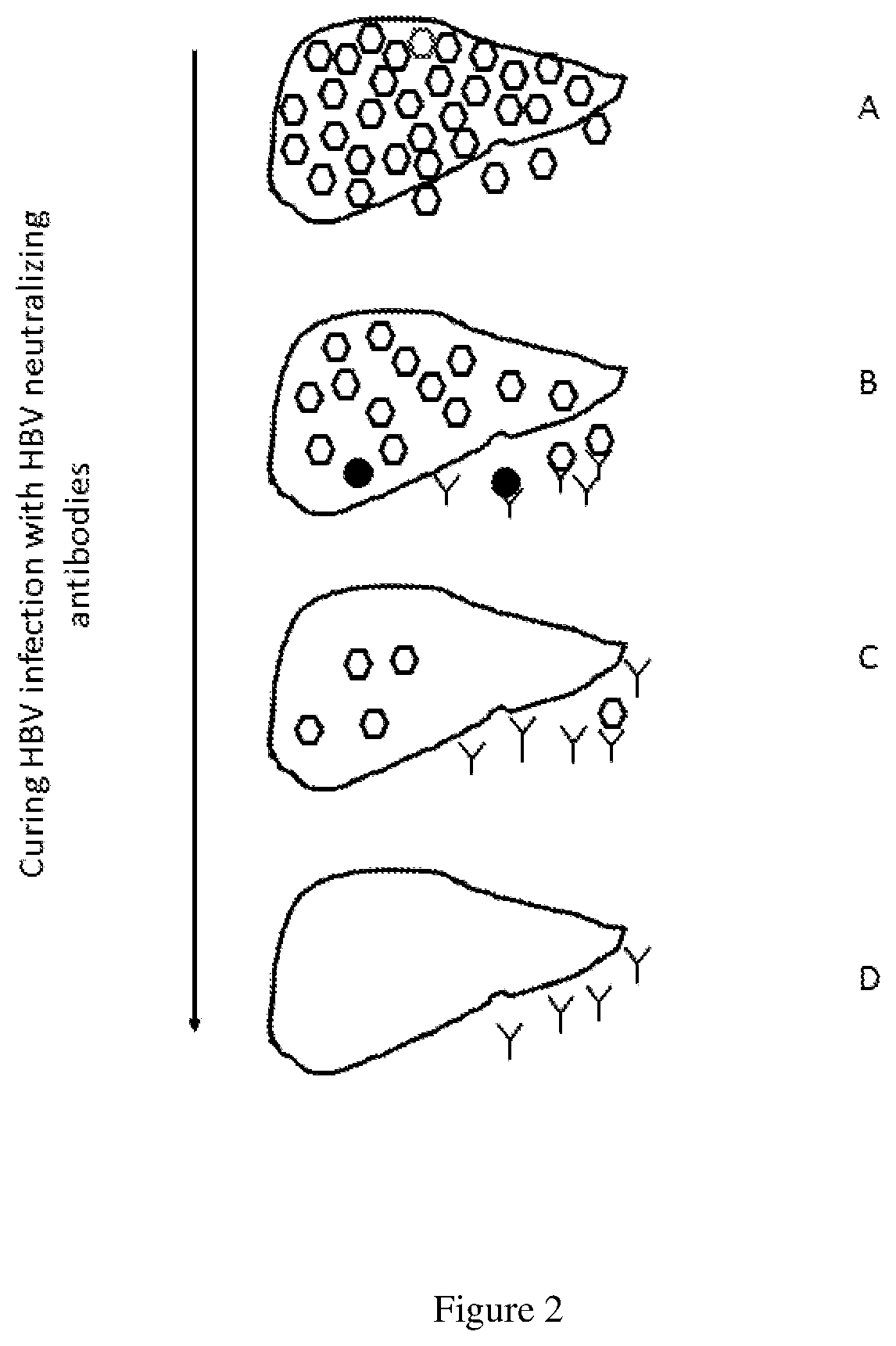
Methods of curing HBV infection and providing complete protection against HBV infection
ActiveUS11136378B2Sufficient levelHigh riskImmunoglobulins against virusesAntibody ingredientsHepatitis B immunizationReplication competent virus
Disclosed are the methods of curing HBV infection and providing complete protection against HBV infection in a simplified HBV immunization schedule. The mechanistic basis for curing HBV infection is founded on the understanding that hepatitis B virus infection is established and prolonged by new rounds of infection with continuously produced viruses, which are not fully neutralized because of insufficient endogenous neutralizing antibodies. The methods of curing HBV infection including chronic HBV infection are aimed to block new rounds of HBV infection. The guidelines for establishing treatment regimens for curing HBV infection, include production or administration of sufficient level of HBV neutralizing antibodies in treated patients. Among the many different possibilities contemplated, the sufficient level of HBV neutralizing antibodies is expressed and maintained by the single injection of the HBV therapeutics that comprises non-replicating viruses or vectors encoding HBV neutralizing antibodies or by multi-injection of exogenous HBV neutralizing antibodies.
Owner:HBVTECH LLC
Composition, preparation method and evaluation of a complex immunogen named I-SPGA for production of immunological active proteins (IAP)
ActiveUS11458196B2Increase coupling powerHigh affinityBacterial antigen ingredientsEgg immunoglobulinsPotentiatorResistant infection
The present invention relates to the composition and method of preparing an immunogen designated as I-spga consisting of a complex antigen prepared from 18 to 26 species of pathogenic microorganisms isolated from patients, inactivated with binary ethyleneamine (BEI) and formalin, diluted in a SPGA immunopotentiator mixed with QS-21 adjuvant. By inoculating the hens with the I-spga immunogen, hyperimmune eggs (Immunospga) are obtained which contain immunologically active proteins specific to the 18-26 antigens used for immunization. The immune response of the hens is specific to the used antigens by amplification of the antigenic signal by the SPGA immunopotentiator and due to a special immunization program that allows the immune system to act complex and intense: The I-spga complex antigen contains 18-26 microorganisms isolated from patients, bacterial bodies, components from bodies obtained by ultrasonography, cilia, exotoxins, endotoxins, spores, viruses, fungi or yeasts. This pathogenic material is inactivated with BEI and formalin. The I-spga antigen is of three types. The standard I-spga antigen is composed of 18 to 24 antibiotic-resistant bacterial species isolated from patients in Romania. The specific I-spga complex antigen is composed of the I-spga complex antigen containing a mixture of 7-9 strains from a single species of bacteria, fungi or yeasts isolated from patients in Romania mixed with SPGA and QS-21, used for inoculation of hens previously immunized with standard I-spga antigen. The personalized I-spga antigen is composed of patient-derived pathological material containing cellular debris and pathogenic germs inactivated with BEI and formalin and mixed with SPGA and QS-21 and is used to immunize hens previously immunized with the standard I-spga antigen. This now patented technology encompasses a new generation of biological products in which the immune response of the hens to different groups of parenterally inoculated antigens at different time intervals is overlapping. Chicken response is uniform and additional administration of immunogens and SPGA as an immunopotentiator amplifies the antigenic signal and immune response. The I-spga immunogen as well as the immune response contain two markers, G and A, which identify the I-spga antigen used for immunization against the antigens used to produce the Imunoinstant group bio-preparations or similar products. The I-spga immunogen is used to immunize the hens for obtaining immunologically active proteins that can be used to treat immune deficiencies, psoriasis, epidermolysis bullosa, other dermatitises, nosocomial infections, antibiotic-resistant infections in the urinary system of children and grownups.
Owner:FANTANA RAUL SORIN +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com