Method for preparing IgM (Immunoglobulin M) calibrator
A calibrator and polyclonal antibody technology, which is used in the preparation, sampling, and instrumentation of test samples. It can solve problems such as non-specific adsorption and false serum results, and achieve the effect of good stability and high affinity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Acquisition of hepatitis A polyclonal antibody
[0036] Obtain 5 mg of hepatitis A antigen for immunizing goats. The immunization procedure was 2 mg for the first time, followed by 1 mg each time, and the immunization interval was 2 weeks. Seven days after the completion of the fourth dose of immunization, the goats were bled, and the polyclonal serum of the goats was collected by centrifugation.
[0037] The polyclonal antibody was purified from polyclonal serum by proteinG and eluted with 100mM Gly pH3.0. The eluate was collected and dialyzed into PBS pH7.6.
[0038] Centrifuge at 12000rpm for 10min, take the supernatant, which is the anti-hepatitis A goat polyclonal antibody, freeze-dry and store.
[0039] 2. Acquisition of Hepatitis A Polyclonal Antibody Fab
[0040] Take goat anti-hepatitis A polyclonal antibody 100mg, papain 5mg, then add 200ul 0.5M cysteine, 200ul 0.1M EDTA pH7.0 and 0.1M phosphate buffer system (pH7.6), then place at 37 ℃, 150rpm constan...
Embodiment 2
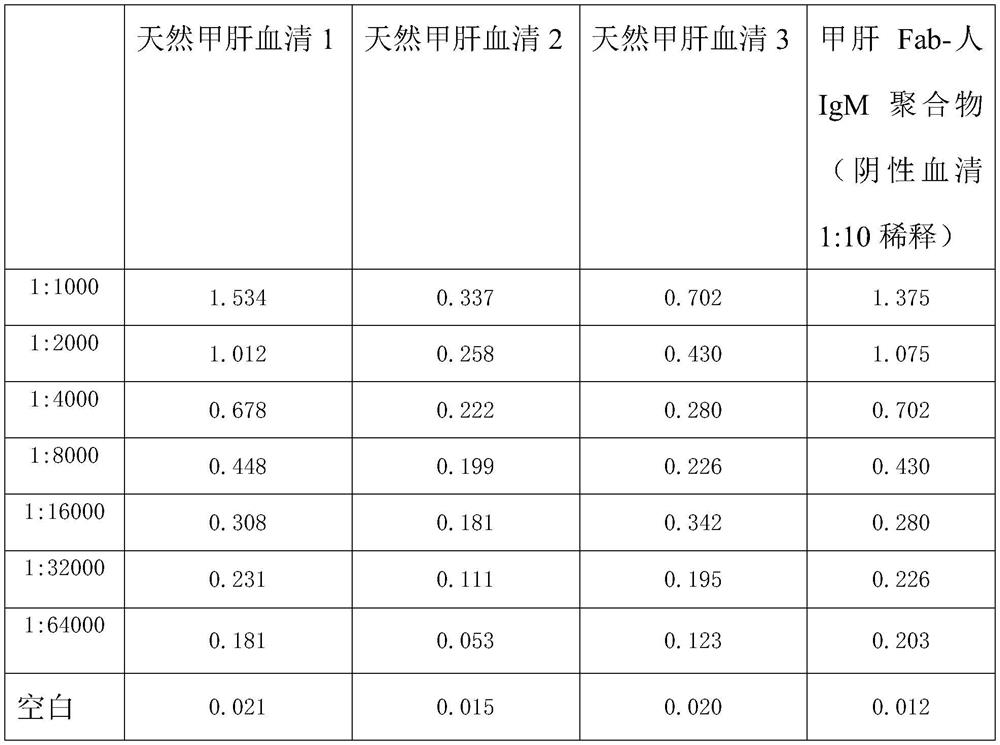
[0050] Compared with the natural hepatitis A IgM antiserum, the prepared hepatitis A Fab-human IgM polymer can react with the hepatitis A natural antigen. Use the enzyme-linked immunoassay kit for comparison as follows:
[0051] Natural Hepatitis A Serum 1 Natural Hepatitis A Serum 2 Natural Hepatitis A Serum 3 Hepatitis A Fab-Human IgM Polymer (Negative Serum Diluted 1:10)
[0052]
[0053] Through the above findings, the prepared hepatitis A Fab-human IgM can react well with the natural hepatitis A antigen and has a high titer, the negative value is similar to the serum negative value, and has no background, and can be used as a calibrator of hepatitis A IgM class.
Embodiment 3
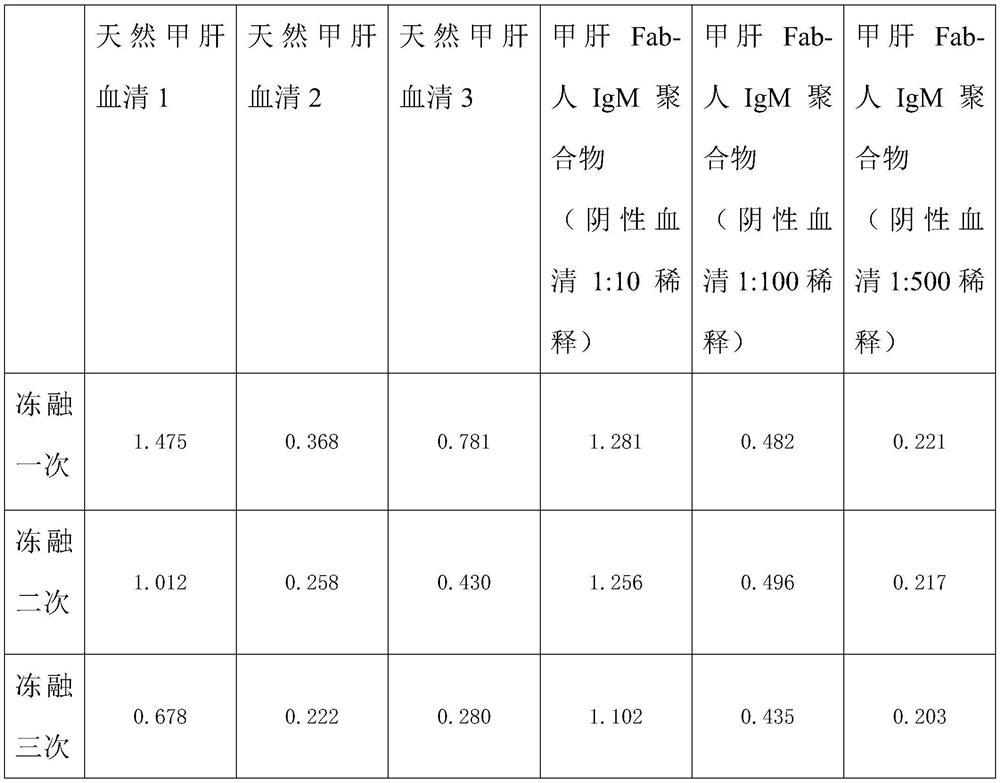
[0055] In order to further compare the stabilizing effects of the hepatitis A Fab-human IgM prepared by the present invention, the negative blank serum was used to dilute this product, and multiple freeze-thaw experiments and long-term stability experiments were performed respectively, and the enzyme-linked immunoassay kit was used for comparison as follows The long-term stable effect of this product.
[0056]
[0057] Repeated freeze-thaw results
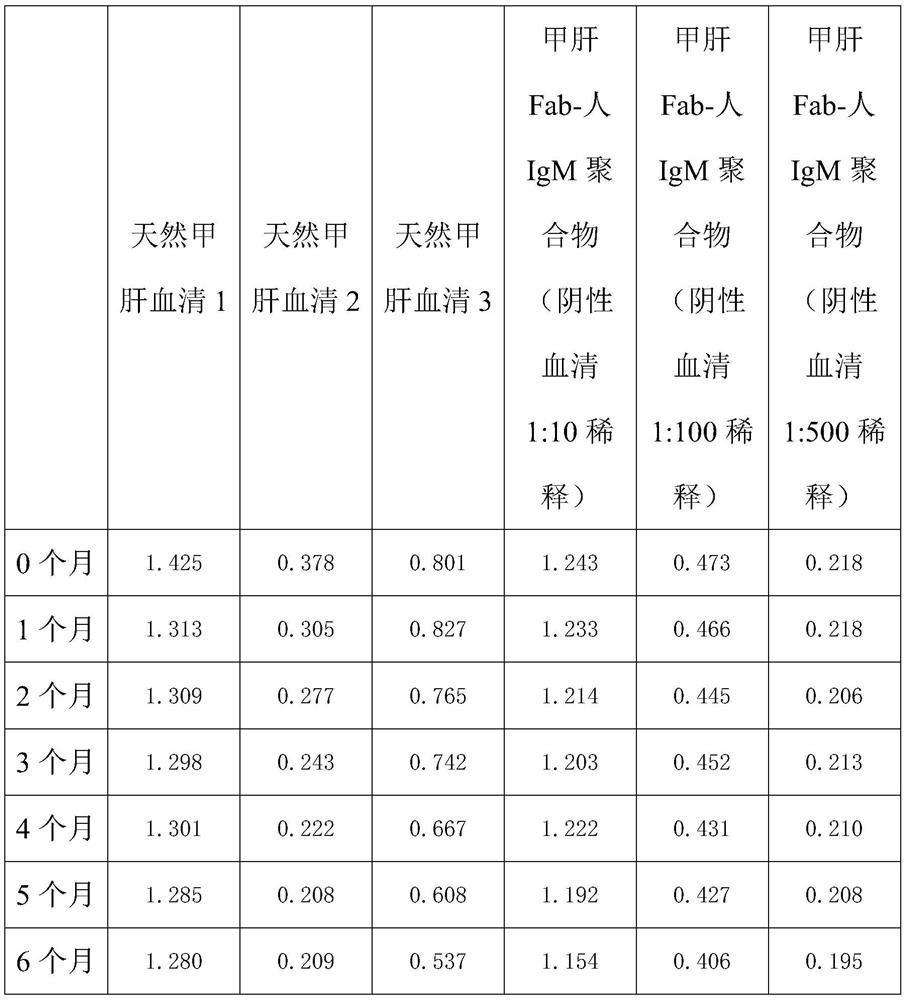
[0058] Long-term stability results (≤-20°C)
[0059]
[0060] Through the above two tables, it is found that the hepatitis A Fab-human IgM prepared by the present invention has more than 80% activity after repeated freezing and thawing three times compared with natural hepatitis A IgM serum, and the activity loss of natural hepatitis A IgM is all at 50% after repeated freezing and thawing three times. % or more; when stored at ≤-20°C, the activity of hepatitis A Fab-human IgM polymer was still ≥85% after 6 months of storage,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com