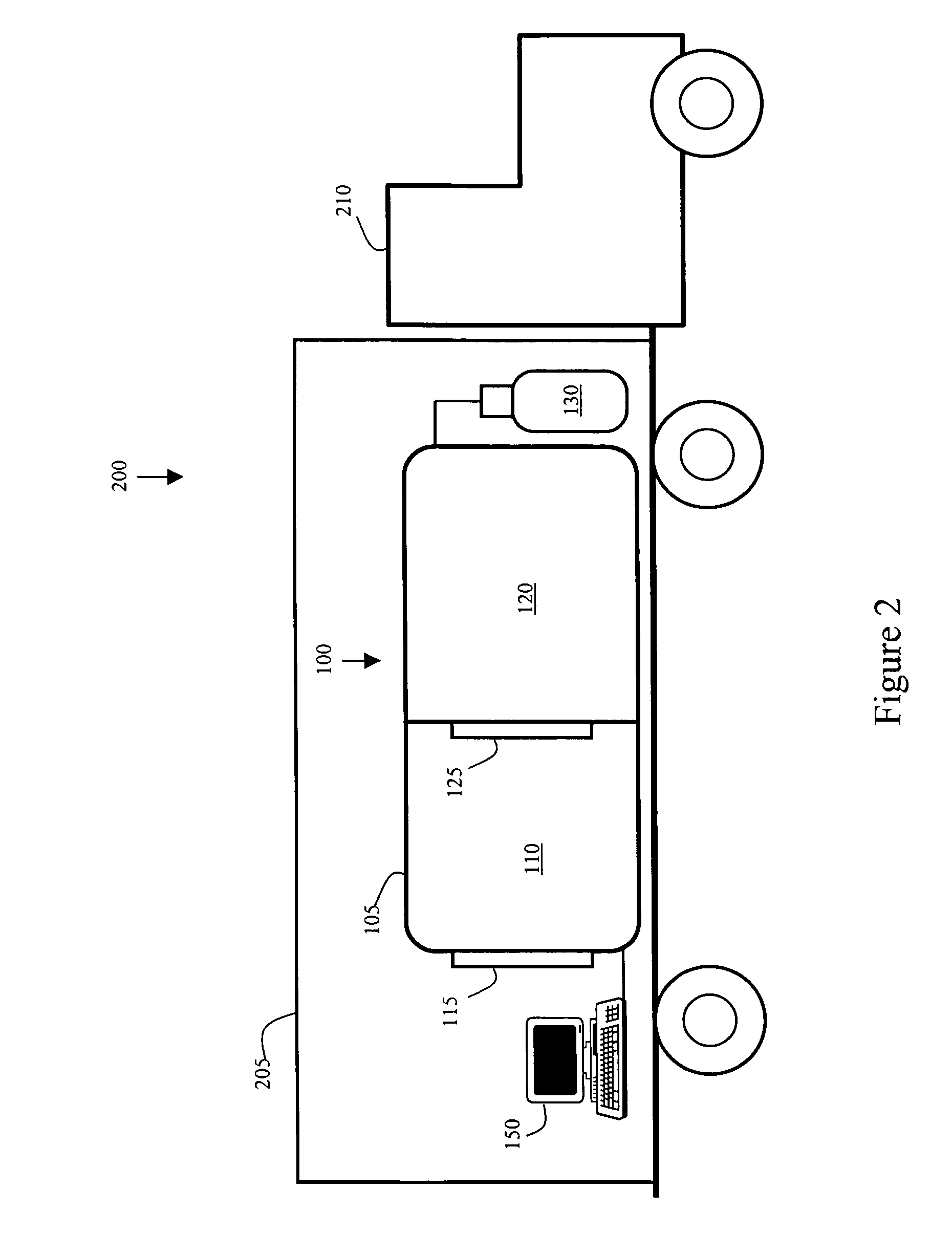
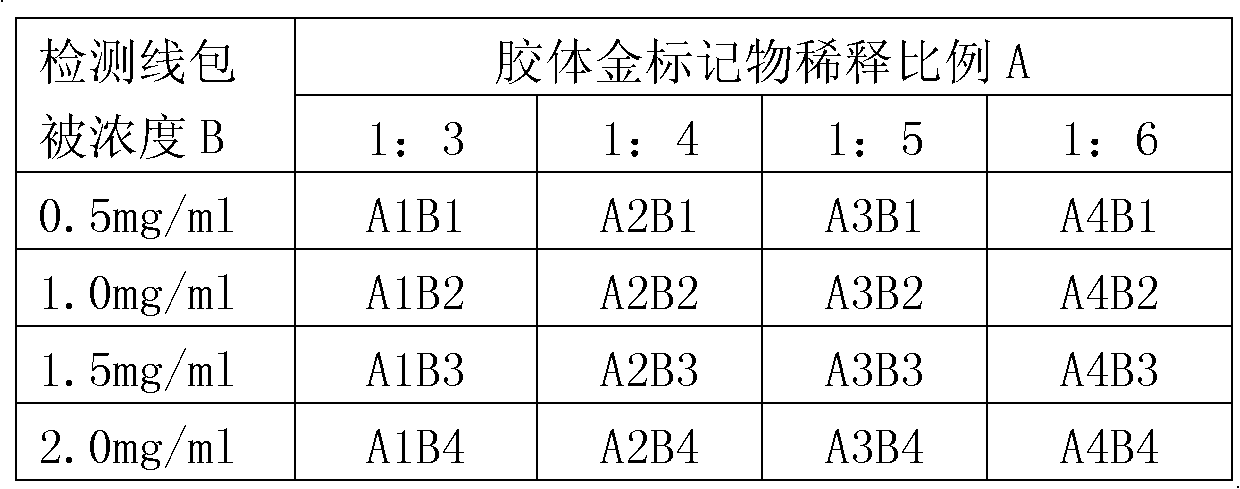
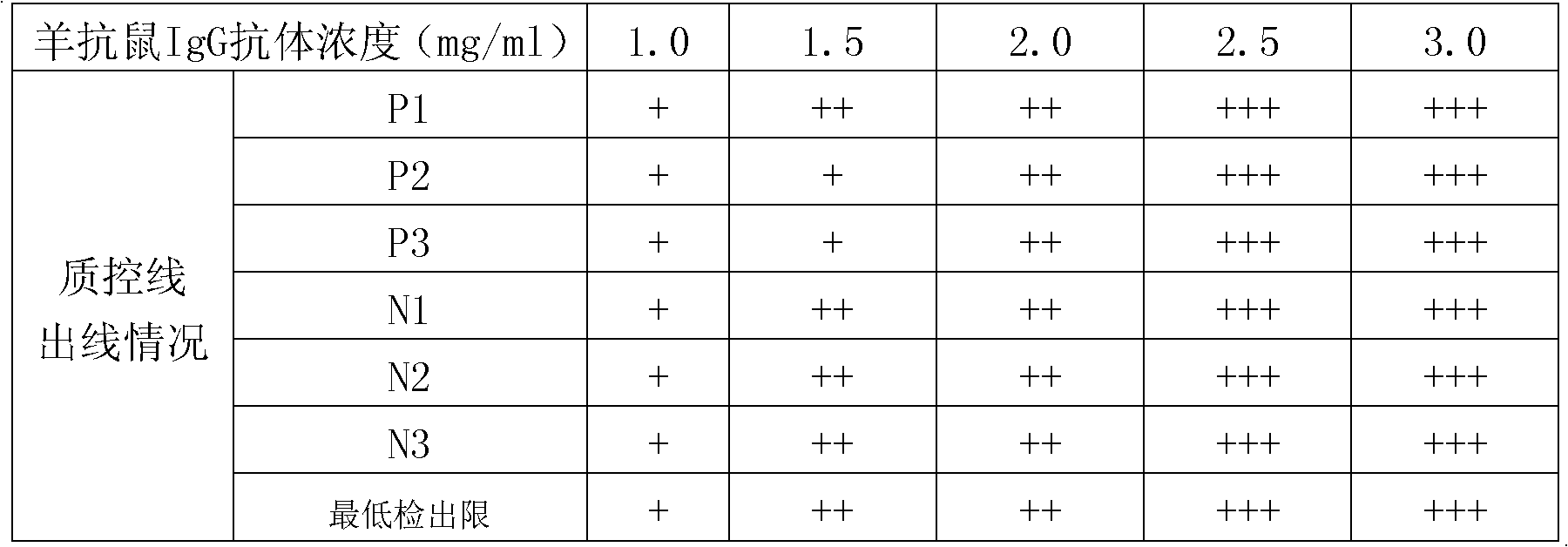
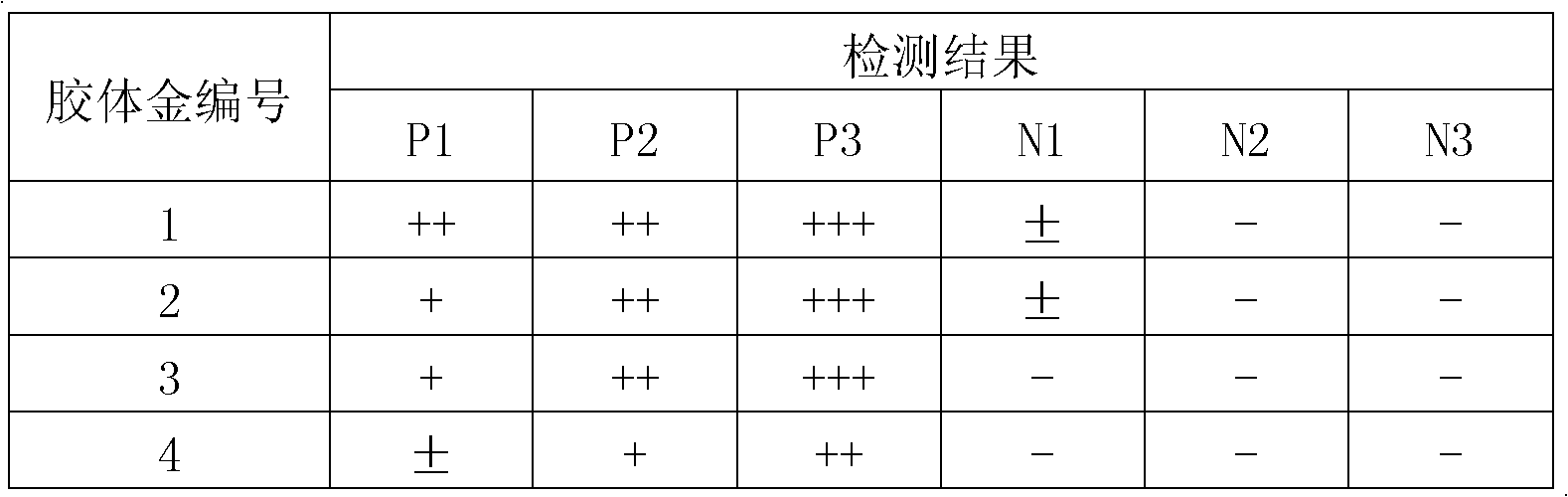
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
186 results about "Hepatitis A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A highly contagious liver infection caused by hepatitis A virus.
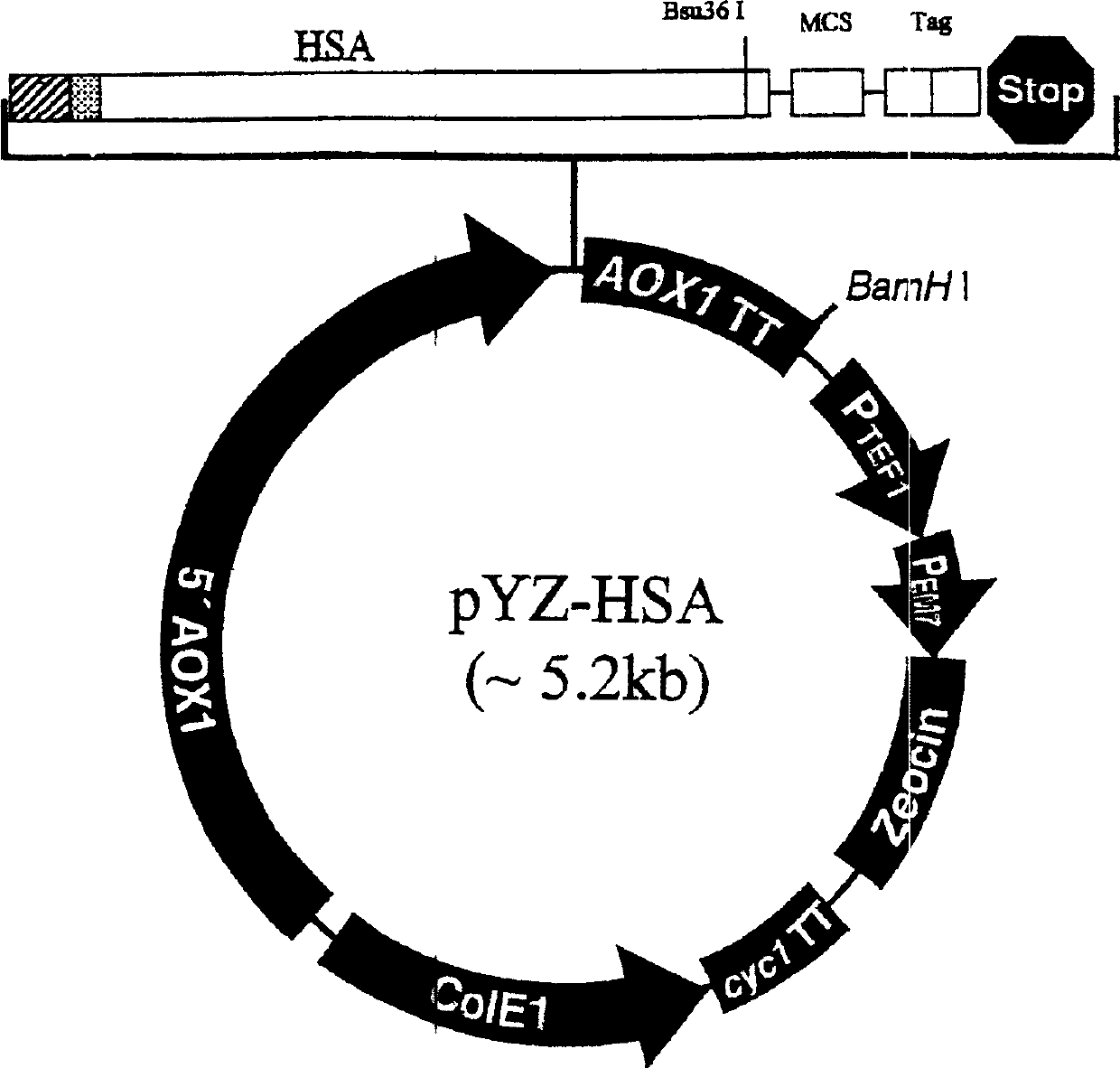
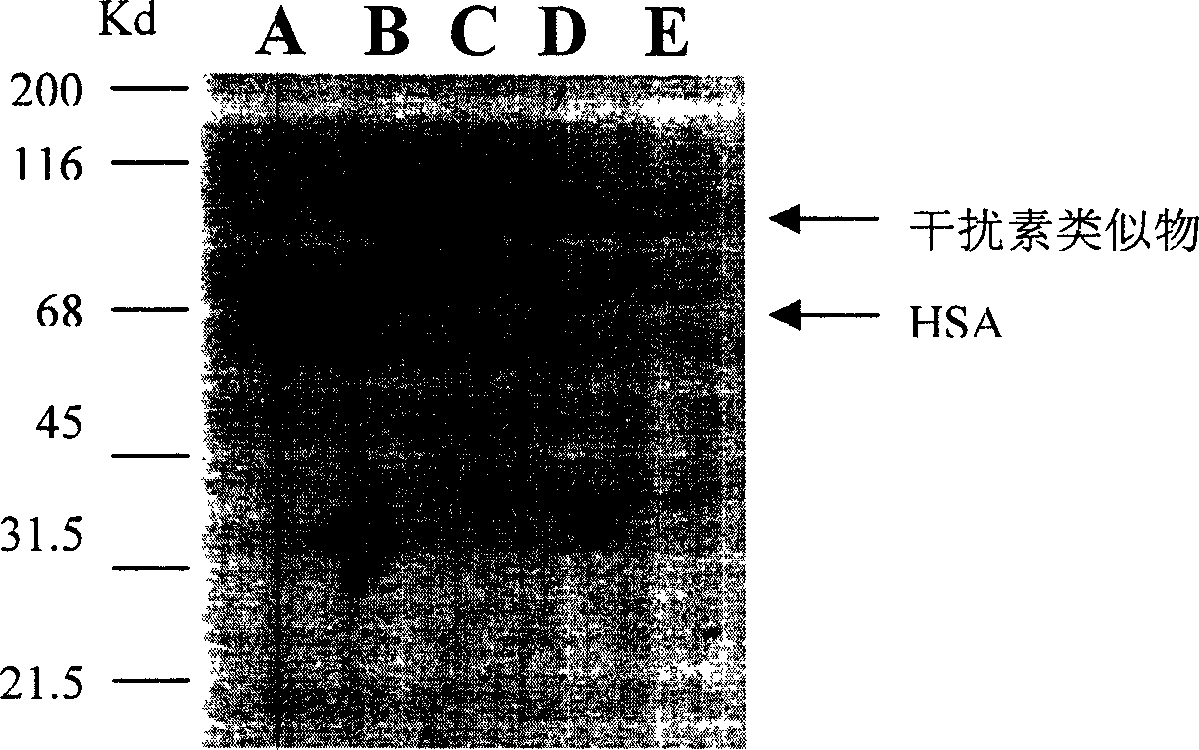
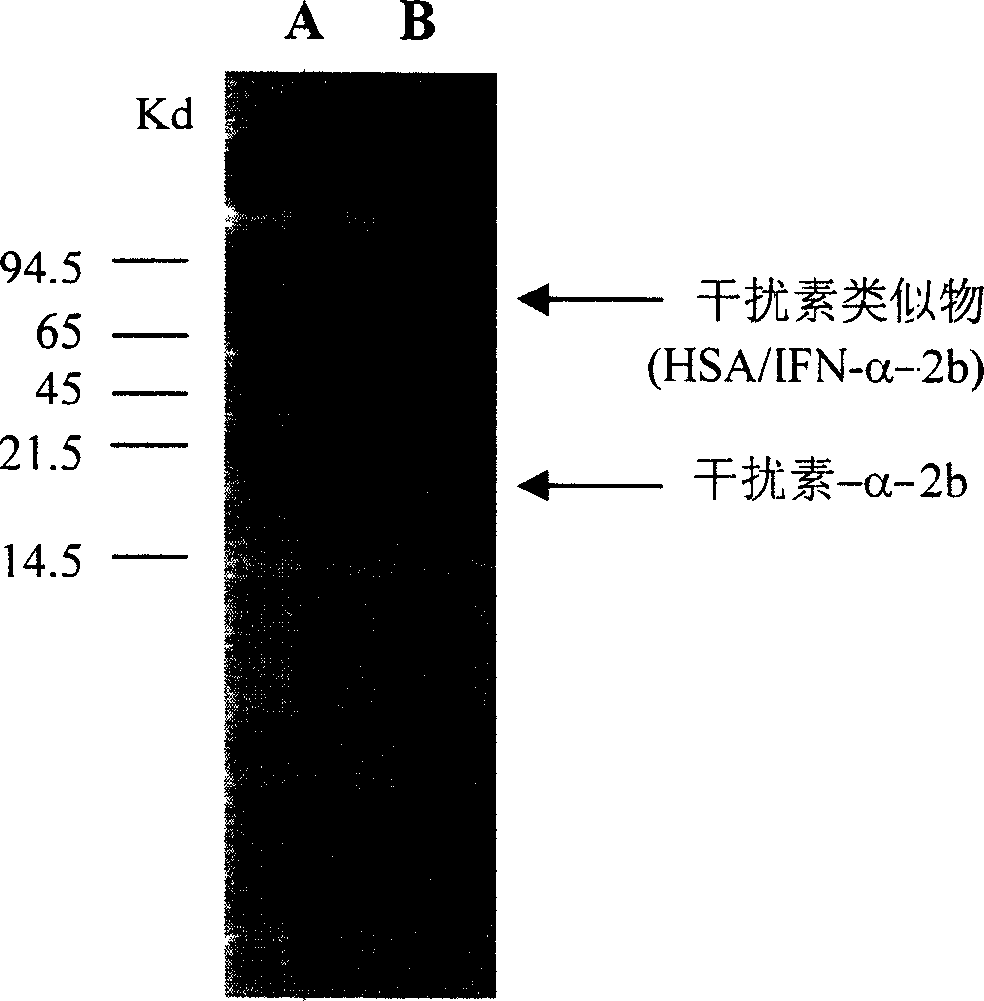
Human interferon analogue with long-lasting biological effects
The invention relates to human interferon analogue with long-lasting biological effects and preparation method and medicinal effect thereof. The human interferon analogue is a syzygial albumin prepared by recombination of interferon and human serum albumin (HSA) in gene engineering method. Proved by animal experiment, the novel interferon has not only anti-virus effect of general interferon but also has prolonged half time as 3 to 10 times as other interferon, therefore, internal action time is prolonged. The long-lasting interferon can be used for treating virulence infection diseases, such as SARS, AIDS, hepatitis b, hepatitis c and hepatitis a, and can also be used for treating leukemia and chromoma. The storage period of the human interferon analogue is 3 to 5 times as the same of the traditional interferon.
Owner:FORTUNEROCK (CHINA) CO LTD ALL RIGHTS RESERVED +1
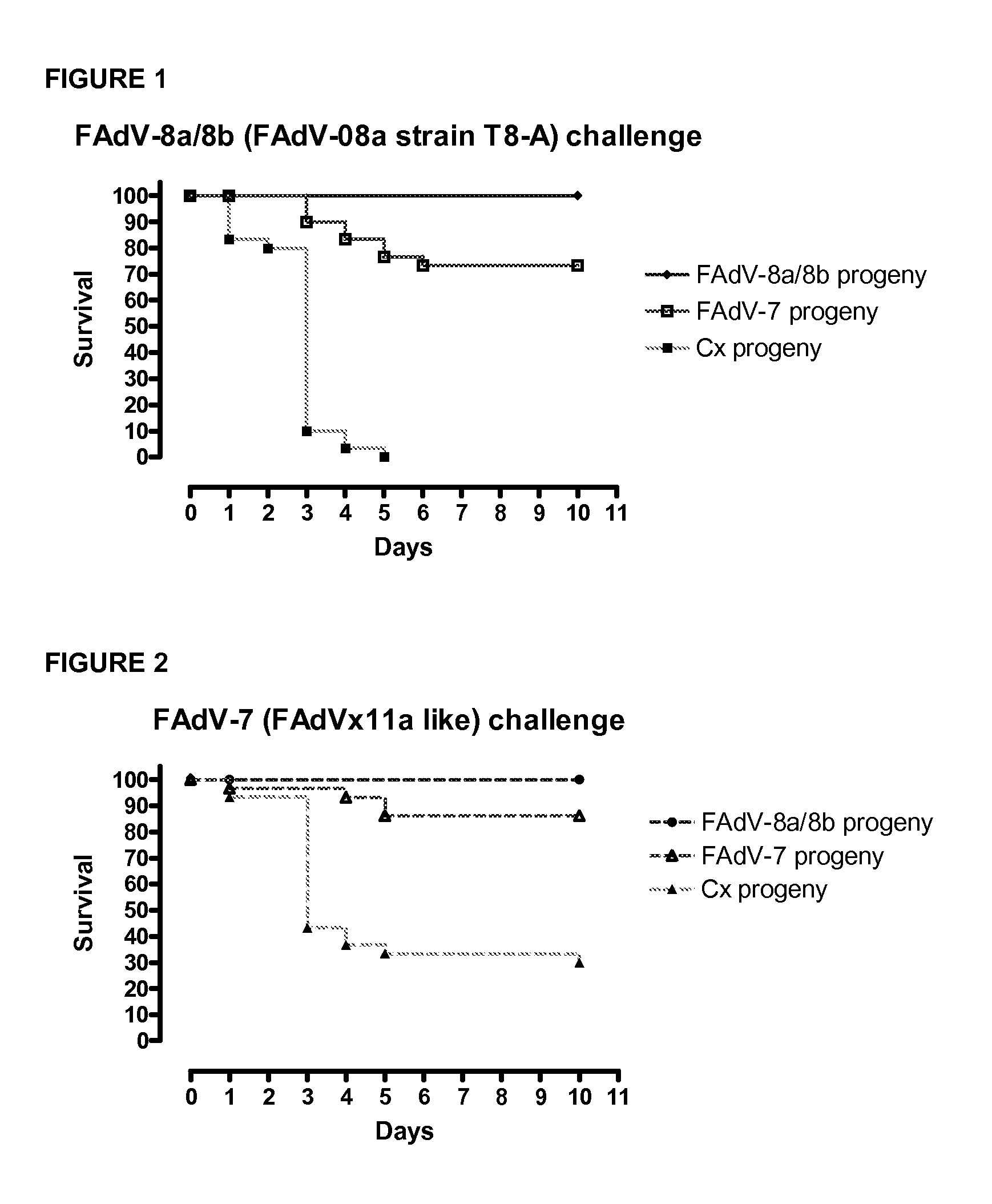
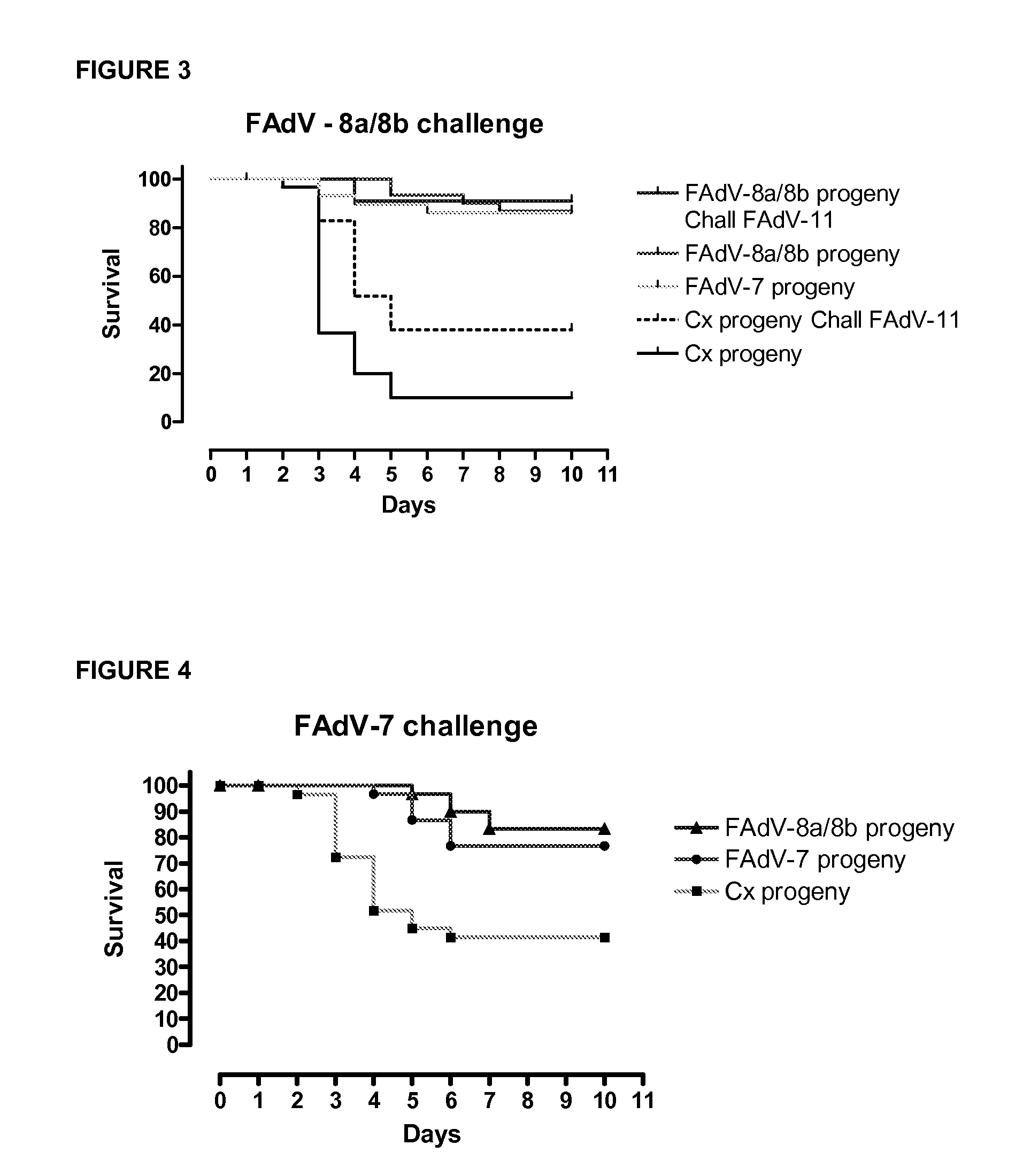
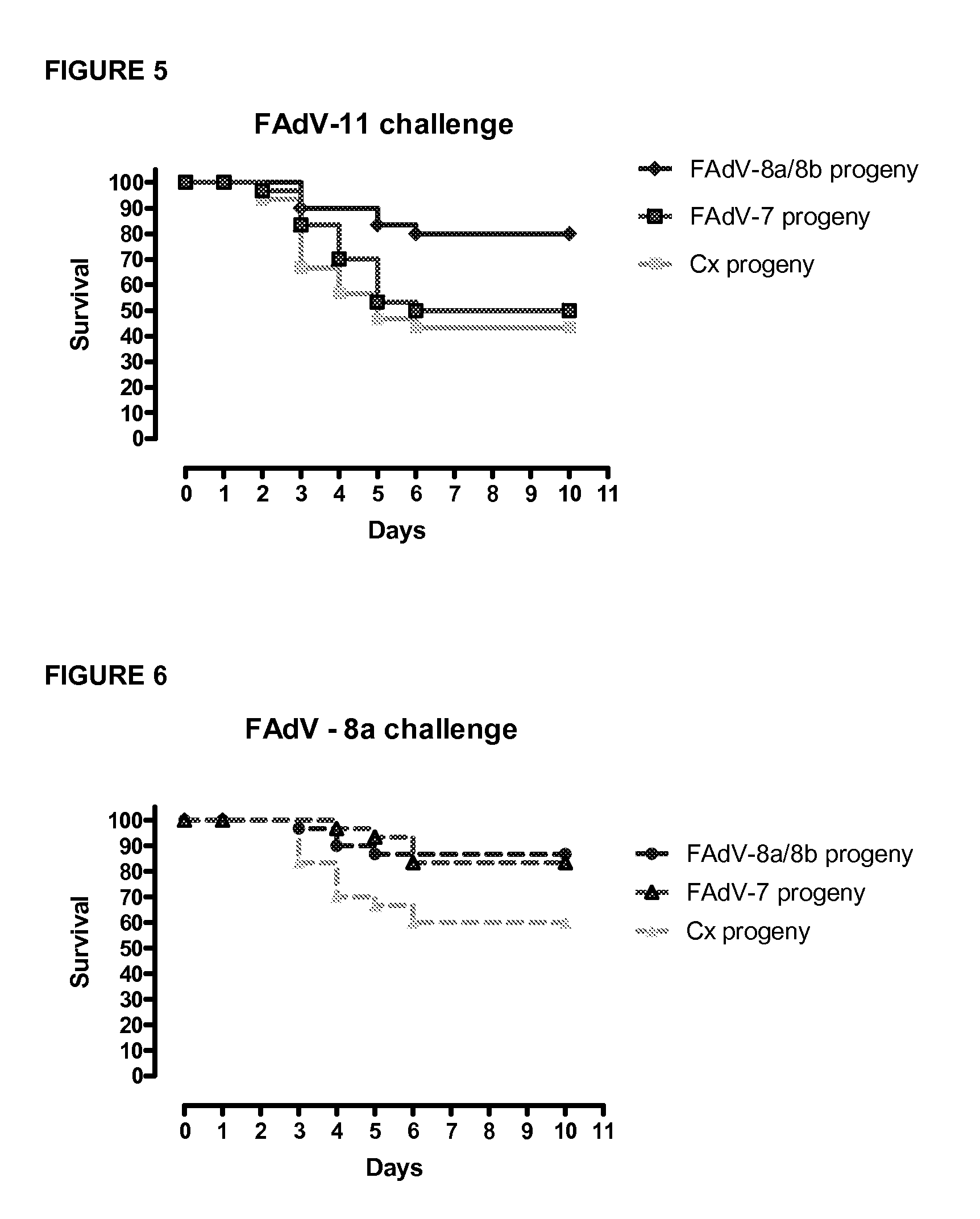
Vaccines for inclusion body hepatitis
A composition comprising an isolated fowl adenovirus (FAdV), wherein the FAdV is a strain selected from FAdV-2, FAdV-7, FAdv-8a, FAdV-8b, FAdV-8a / 8b or FAdV-11 serotype strains; and a suitable carrier and methods for inducing protective immunity in a subject and / or its progeny.
Owner:UNIVERSITY OF GUELPH +1
Washing free gel for disinfection
A non-washing disinfecting gel for disinfecting human skin and surface of object is prepared from triclosan, alcohol, moistening agent, thickening agent, pH regulator and deionized water through dissolving triclosan in alcohol, and sequentially adding others while stirring. It can be used to prevent SARS, hepatitis A, hepatitis B, and AIDS.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Freeze-drying hepatitis A varicella attenuation combined vaccine and producing method thereof
ActiveCN101259269AImprove securityImproving immunogenicityPowder deliveryDigestive systemDiseaseUltrasound attenuation
The present invention provides a freeze-dried hepatitis-A-chicken-pox attenuation combined vaccine and a production thereof. The combined vaccine has the security and the immunogenicity as good as the univalent vaccine and can effectively prevent the hepatitis A and the chicken pox. Two kinds of diseases can be effectively prevented just by one immunization, which can reduce vaccine cost, improve vaccine inoculation efficiency and reduce the bad reaction caused by a plurality of times of injections, and can reduce the vaccine inoculation times but realize the purpose of preventing various diseases.
Owner:长春生物制品研究所有限责任公司
Monoclonal antibody of blood coagulation factor IX
ActiveCN103396494AImmunoglobulins against blood coagulation factorsPeptide preparation methodsDiseaseHeavy chain
The invention discloses a monoclonal antibody of a blood coagulation factor IX. The antibody comprises a light chain and a heavy chain, wherein the variable region amino acid sequence of the light chain is represented by SEQ ID NO.3, and the variable region amino acid sequence of the heavy chain is represented by SEQ ID NO.4. The invention also discloses a preparation method of the antibody. The antibody can specifically identify the blood coagulation factor IX. The invention further discloses a method for purifying the blood coagulation factor IX from blood plasma through utilizing the antibody. The blood coagulation factor IX can be conveniently and efficiently purified from the blood plasma through utilizing the antibody, so the infection risks of the diseases comprising hepatitis A, the hepatitis B, AIDS and the like caused by the patient blood transfusion supplementation of the blood coagulation factor IX are reduced.
Owner:浙江耶大生物医药有限公司
Indirect ELISA method and kit for detecting serum 3-type duck hepatitis virus a antibody
The invention discloses an indirect ELISA method and kit for detecting serum 3-type duck hepatitis virus a antibody and belongs to the technical field of serum antibody detection. The indirect ELISA method includes that serum 3-type duck hepatitis virus VP1 protein is utilized as an envelope antigen with the peridium quantity as 0.1-1mug per hole, HRP-mice anti-duck IgY is utilized as the HRP with the use concentration as 0.2-2mug / mL, and the coloration time is 10 minutes. The kit comprises a VP1 protein antigen envelope board, the HRP-mice anti-duck IgY, sample diluent, a scrubbing solution, TMB solutions A and B and a stop solution. The method and the kit can be used for detecting the serum 3-type duck hepatitis virus a antibody in duck serum and duck egg yolk, and the serum does not have cross reaction with positive serum of other viruses. Compared with a traditional neutral reaction detection method, the method has the advantage of being convenient and accurate.
Owner:WUHAN CHOPPER BIOLOGY
Fluorescent quantitative RT-PCR detection kit and detection method for enterovirus
InactiveCN101407846ANo cross reactionQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceCerebrospinal fluidHand-foot-and-mouth disease
The invention provides a fluorescence quantitative RT-PCR detecting kit for an enterovirus and a detecting method thereof; and the sequences of an upstream primer and a downstream primer and a specific probe of the fluorescence quantitative RT-PCR detecting kit are as follows: the upstream primer EV(YG)F is 5'-GGCTGCGYTGGCGGCC-3', the downstream primer EV(YG)R is 5'-CCAAAGTAGT CGGTTCCGC-3' and the specific probe EV(YG)PB is 5'-CTCCGGCCCCTGAATGCGG-3'. The method has high specificity on detecting the enterovirus and does not have cross reactions with other enteroviruses such as hepatitis A, hives, rubella, parotitis, encephalitis, dengue fever, adenovirus, and the like. The detection sensitivity of the method achieves 0.1TCID50; the method can directly detect the nucleic acid of the enterovirus from the samples of ncurolymph, herpes liquid, dejecta and the like of suspected patients; only about 3h is needed from extracting the nucleic acid of the enterovirus to finishing the detection; and the detecting kit and the detecting method are suitable for the lab early diagnosis of sudden epidemic caused by the infection of the enteroviruses such as the hand-foot-and-mouth disease and the like.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Primer for doubly detecting hepatitis e virus and hepatitis a virus through RT-RPA-lateral flow tomography, probe and kit
ActiveCN108841926AShorten detection timeThe reaction procedure is simpleMicrobiological testing/measurementAgainst vector-borne diseasesControl lineHepacivirus
The invention discloses a RT-RPA-lateral flow tomography kit for doubly detecting hepatitis e virus and hepatitis a virus. The kit comprises an upstream primer, an intermediate probe and a downstreamprimer which are applicable to hepatitis e virus ORF2 gene sequence in the RT-RAP amplification technology, and / or an upstream primer, an intermediate probe and a downstream primer which are applicable to hepatitis a virus VP1 gene sequence, general agents for recombinase polymerase amplification technology, and a lateral flow tomography test strip, wherein the lateral flow tomography test stripcomprises a sample adding pad, a control line, a #1 detecting line and / or a #2 detecting line; the control line, the detecting lines and the primers are combined with a probe label. With the adoptionof the kit, the hepatitis e virus ORF2 gene and / or hepatitis a virus VP1 gene in a sample can be rapidly, sensitively and specifically detected on site in a non-lab environment.
Owner:JINZHOU MEDICAL UNIV
Method of preparing hepatitis A inactivated vaccine
ActiveCN102058882AIncrease production capacityHigh purityAntiviralsViruses/bacteriophagesAdjuvantCell factory
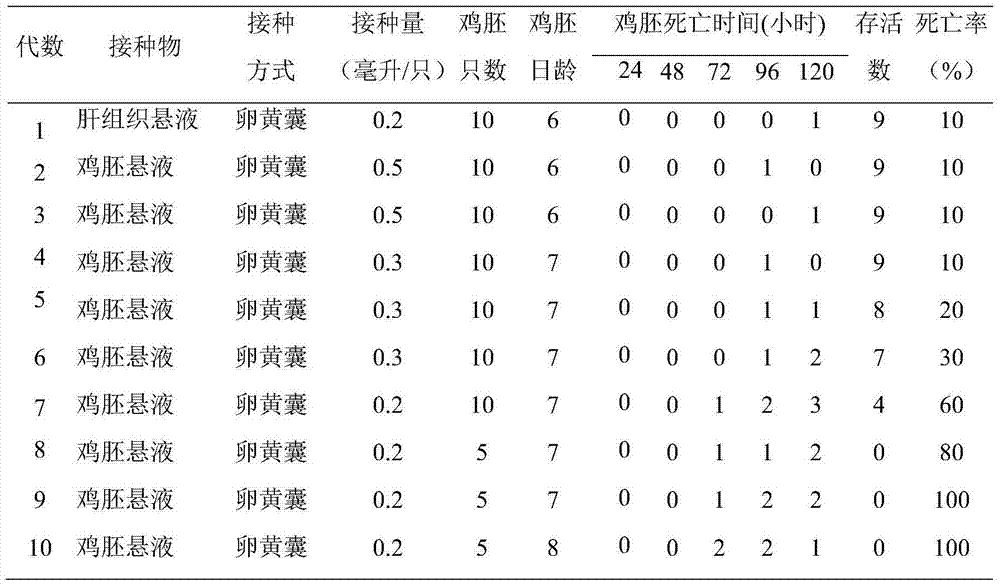
The invention provides a method of preparing a hepatitis A inactivated vaccine, which comprises the following steps of inoculating mixed and absorbed hepatitis A virus strain SH and a human embryo diploid cell MRC-5 to hepatitis A virus propagated in a cell factory, digesting the cell in the virus propagation peak to obtain cell virus liquid, removing impurity proteins of the cell by ultrasonication, chloroform extraction and ultrafiltration through ultrafiltration membranes, degerming and filtering by gel filtration chromatography and purification, and absorbing by an aluminium hydroxide adjuvant so that the hepatitis A inactivated vaccine is prepared. The result of in vivo effectiveness experiments performed on a mouse shows that the hepatitis A inactivated vaccine prepared by the method of the invention has higher ED50 and better immunogenicity than the contract strain. The method of the invention can improve the safety of the vaccine, simplify the technique, shorten the productionperiod, reduce the production cost, and realize the scale production of the hepatitis A inactivated vaccine.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Serum type 3 duck hepatitis A virus live vaccine and preparation method thereof
InactiveCN104726414AInactivation/attenuationMicroorganism based processesDuck hepatitis A virusLiver tissue
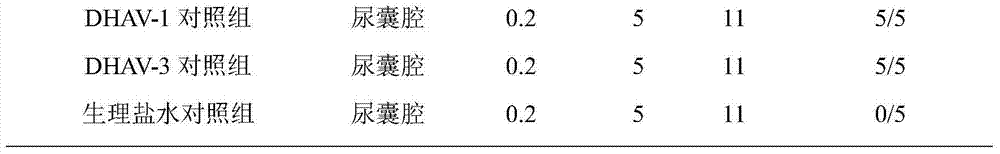
The invention relates to a serum type 3 duck hepatitis A virus (DHAV-3) live vaccine and a preparation method thereof. The serum type 3 duck hepatitis A virus (DHAV-3) can only be separated and cultured by virtue of duck embryos generally at present, which creates a very big obstacle for the research of the virus and the research and manufacturing of a serum type 3 duck hepatitis live vaccine. According to the preparation method provided by the invention, a chick embryo yolk sac inoculation way is adopted, the serum type 3 duck hepatitis A virus is obtained by separating liver tissues of a duck with duck hepatitis, and an attenuated HuB60 strain (CGMCC No.10307) is obtained by virtue of chick embryo continuous passage culture, wherein the attenuated strain can be used for preparing the serum type 3 duck hepatitis A virus (DHAV-3) live vaccine, and can also be mixed with a serum type 1 duck hepatitis attenuated strain (such as A66 strain) according to an appropriate proportion to prepare a duck hepatitis A bivalent live vaccine.
Owner:兆丰华生物科技(南京)有限公司
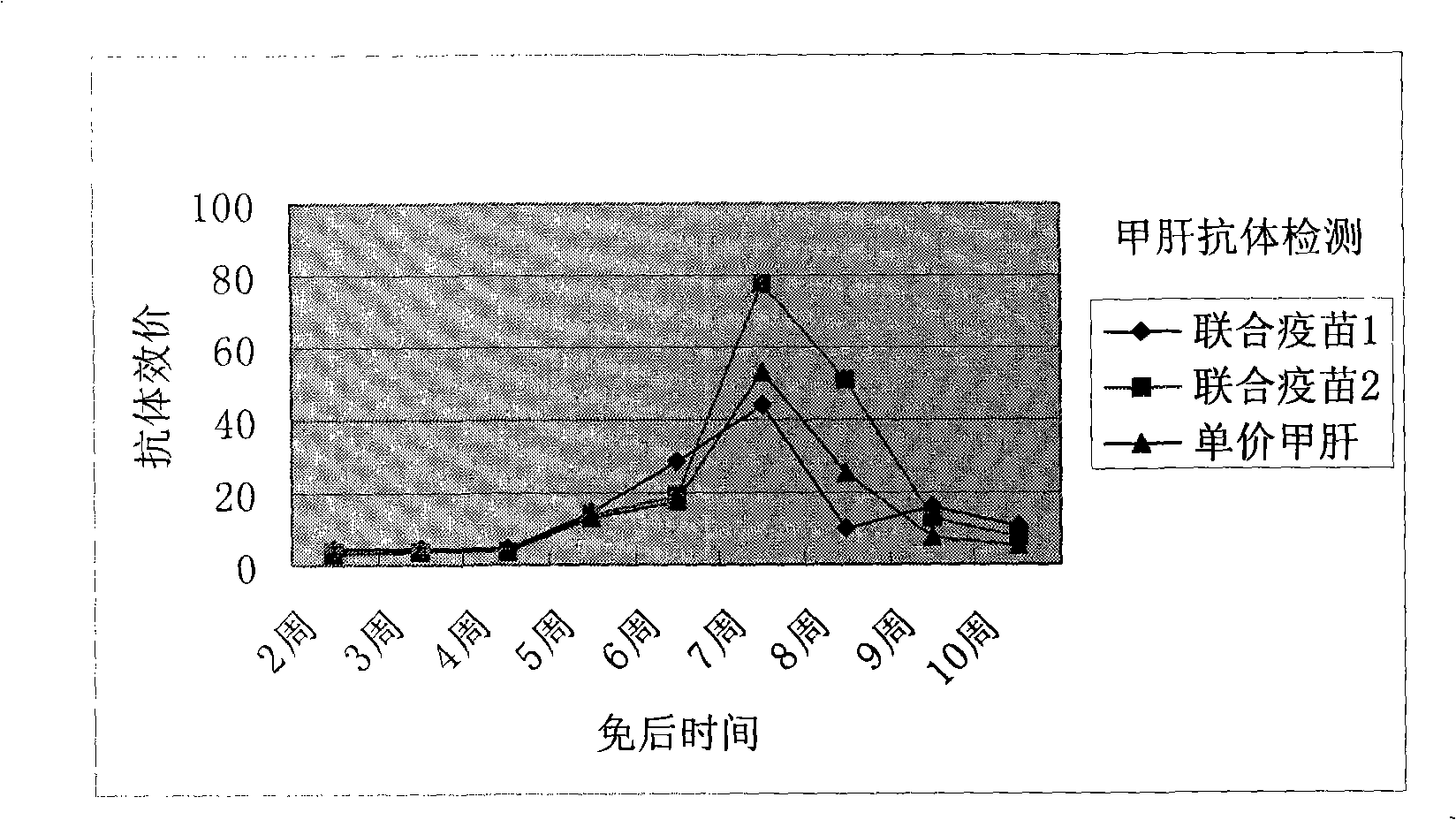
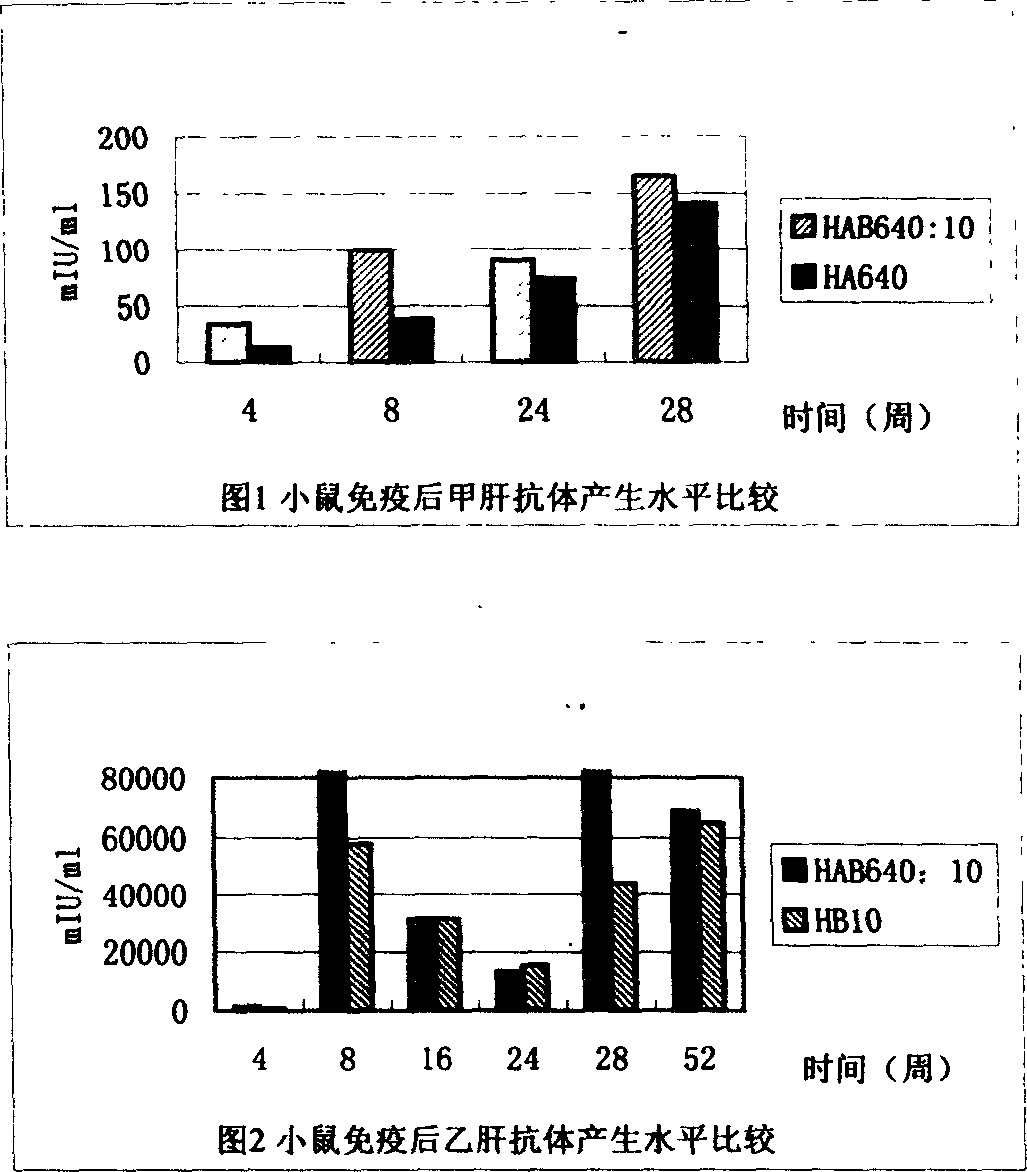
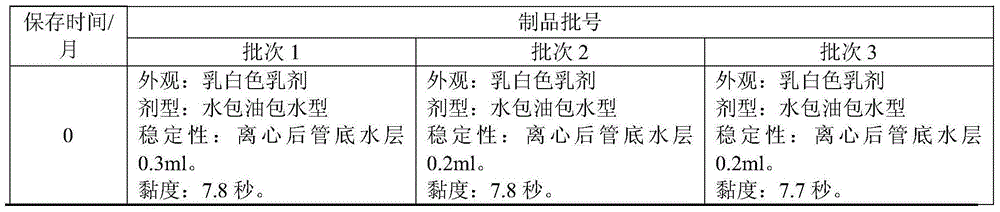
Combined vaccine for hepatitis A, B virus and its preparation method
InactiveCN1596978AAdsorption completeStable physical and chemical propertiesDigestive systemAntiviralsDiseaseVaccine Immunogenicity
A vaccine for preventing both hepatitis A and hepatitis B and its preparing process are disclosed. It has high safety, immunogenicity and stability.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Traditional Chinese medicine for treating acute icteric hepatitis
InactiveCN101152539ACompatibility is simpleLow costDigestive systemAntiviralsDiseaseTherapeutic effect
A traditional Chinese medicine for curing acute hepatitis with jaundice belongs to a traditional Chinese medicine for curing hepatitis. The components contain: 25g of virgate wormwood herb, 15g of tuckahoe, 15g of polyporus, 15g of oriental waterplantain rhizome, 15g of largehead atractylodes rhizome, 15g of chinese thorowax root, 15g of turmeric root tuber, 30g of danshen root; the invention has the auxiliary prescriptions that: for severe heat, 20g of cape jasmine fruit, 20g of rhubarb and 20g of atrina glass are added; for severe dampness, 20g of agastache rugosa, 20g of atractylodes rhizome and 20g of roud cardamon seed are added; for static blood, 25g of red peony root, 25g of tree peony bark and 25g of peach seed are added. The substances in the prescription are made from natural traditional Chinese medicine plus traditional preparing methods. The materials are easy to collect, the prescription and the preparation methods are simple, and the cost of medicine preparation is low. The compatibility of the traditional Chinese medicine is simple; the medicine used in the prescription is fully natural herbal medicine, which is easy to collect and use; the preparation method is simple; the effects for taking and curing are good; the cost of the medicine is low, and the medicine is in particular suitable for the people living in remote villages far from towns; the cost is low for curing the people suffering from acute hepatitis with jaundice, which solves the problem that the local area lacks medical conditions to cure the disease because of low household income and poor living condition.
Owner:LIANG PING
Identification of oligonucleotides for the capture, detection and quantitation of hepatitis A viral nucleic acid
InactiveCN1675378ASugar derivativesMaterial analysis by observing effect on chemical indicatorViral nucleic acidGenome
Hepatitis A virus-specific primers and probes derived from conserved regions of the hepatitis A virus genome are disclosed. Also disclosed are nucleic acid-based assays using the capture oligonucleotides, primers and probes.
Owner:CHIRON CORP
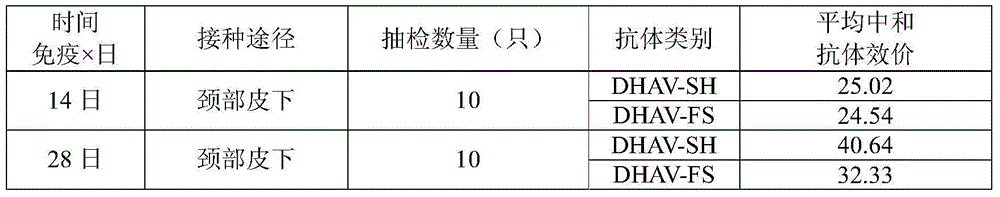
Inactivated vaccine for duck viral hepatitis as well as preparation method and application thereof
ActiveCN104531625AGood securityImmunity is stableMicroorganism based processesAntiviralsViral hepatitis bAluminum Stearate
The invention discloses an inactivated vaccine for duck viral hepatitis as well as a preparation method and an application thereof. The bivalent inactivated vaccine for duck viral hepatitis comprises an antigen and an oil adjuvant, wherein the antigen is prepared by mixing a duck hepatitis viral composition and Tween-80; the duck hepatitis viral composition comprises a duck hepatitis A virus (DHAV) type 1 and a duck hepatitis A virus (DHAV) type 3; the duck hepatitis A virus type 1 is an inactivated duck hepatitis virus type 1 DHAV-SH strain virus liquid; the duck hepatitis A virus type 3 is an inactivated duck hepatitis virus type 3 DHAV-FS strain virus liquid; and the oil adjuvant comprises Montanide ISA206, aluminum stearate and span-80. The bivalent inactivated vaccine for duck viral hepatitis, which is disclosed by the invention, has the following advantages that (1) the inactivated vaccine simultaneously has preventive effects on the duck hepatitis A virus type 1 and the duck hepatitis A virus type 3; (2) the inactivated vaccine is prepared from inactivated virus and has good safety; (3) the inactivated vaccine is convenient to store, transport and use; and (4) the stable immune effect is achieved.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +2
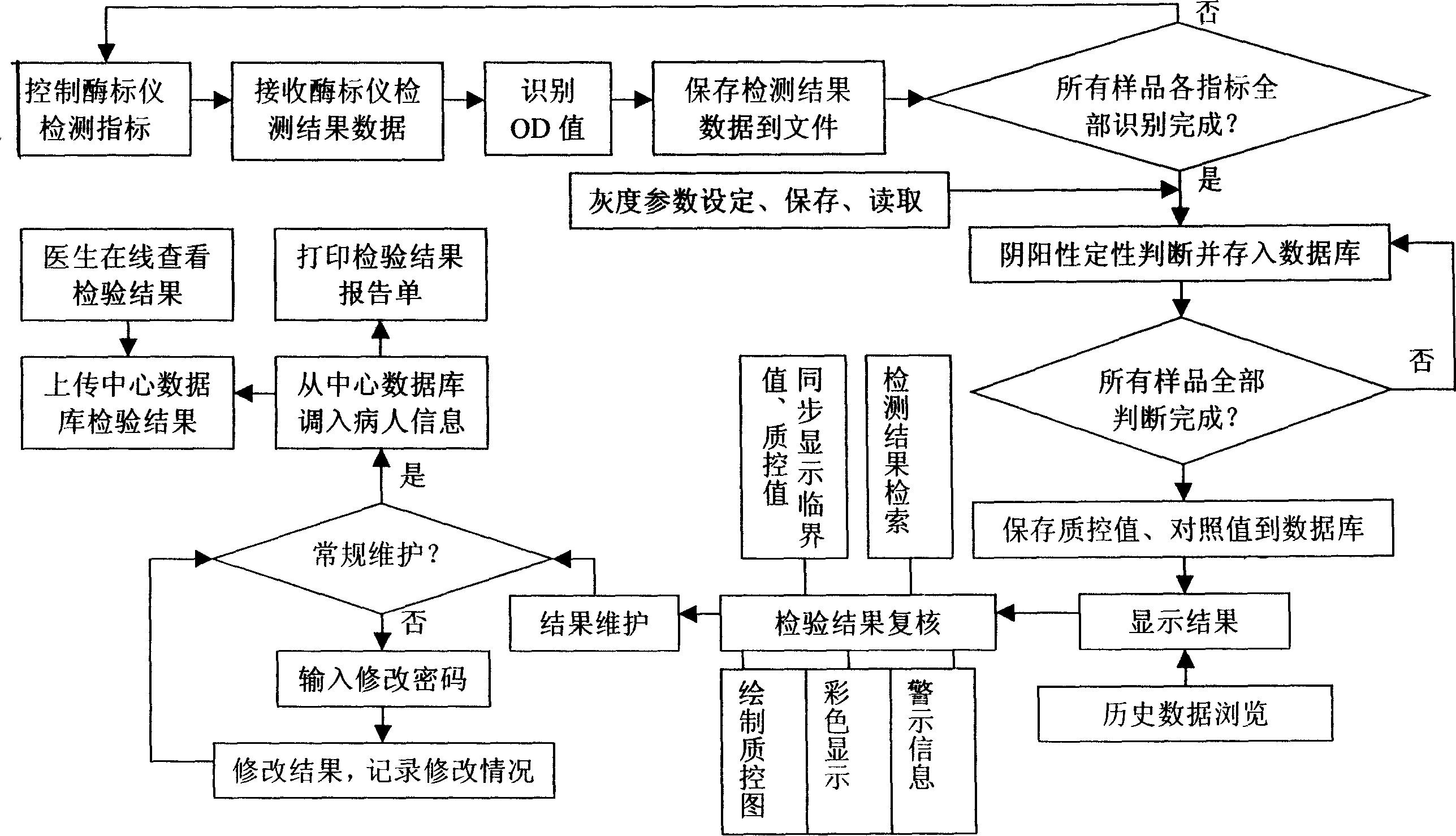
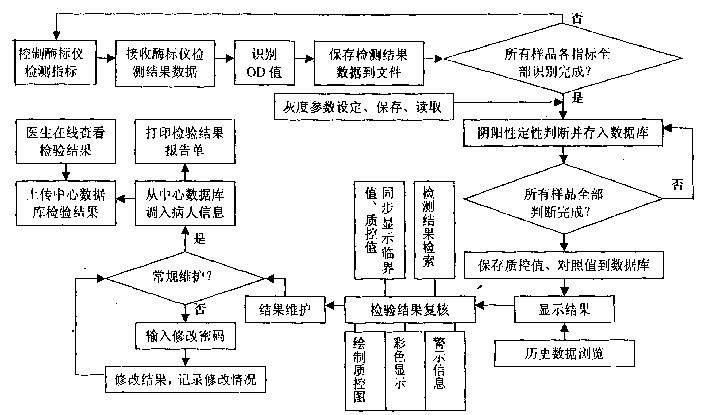
Method for fast detecting marker of hepatitis b virus with accuracy
InactiveCN1519564AAvoid false detectionImprove accuracyMaterial analysisAgainst vector-borne diseasesQuality controlHepatitis B virus
The invention includes following steps: computer controlled testing of enzyme marking device; receiving result data tested by the device; recognizing OD value; saving data of tested result data to file; under region of gray scale set, determining negative or positive and saving them to database; storing quality control value and comparison value to database. The invention provides following functions: displaying all results of samples at a time; checking tested results; maintaining results and printing out tested report form including negative or positive and OD value of each sample at one time. Comparing with current method, the invention possesses features of simple operation, saving half time and labor, reflecting degree of state of an illness and infection. The invention provides online searching and real time quality control to ensure tested report in accuracy, quick and fast transmission. The method is also applicable to hepatitis A, C, D, and E.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hepatitis A virus strain, method for preparing hepatitis A inactivated vaccine and obtained vaccine
InactiveCN1443844AHigh yieldHigh productivityInactivation/attenuationAntibody medical ingredientsVero cellVirus strain
Owner:YUXI WALVAX BIOTECH CO LTD
Rabdosia lophanthide extractive, natural medicine for treating hepatitis
InactiveCN1405177ASimple manufacturing processClear antiviralOrganic active ingredientsOrganic compounds purification/separation/stabilisationBiliary excretionProthoracic gland
A kind of inartificial medicine Rabdosia.serra (Maxim) Hara extraction materials which may cure hepatitis and its preparation method is disclosed in the invention, which acts Rabdosia.serra (Maxim) Hara as materials, adopts one of the hot water, supercriticality CO2 or organic impregnant extracting methods, and executes Rabdosia.serra (Maxim) Hara extracting materials by decompression condensation, vacuum dryness, spray dryness etc. methods. It contains antivirus active component 2-hydroxyl ursocic acid, 2,3-di hydroxyl ursocic acid and antioxygenation active component rosmarinic acid and so on speciality valid components, and so it has restraining HBV copy, protecting immunity liver damnification, advancing cell immunity and increasing spleen, thymus weight, bile drainage of low immunity experiment animals etc. functions.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Combination of medicine for treating hepatitis
A Chinese medicine for treating hepatitides and hepatic disfunction caused by hepatitis and protecting liver is prepared from 8 Chinese-medicinal materials including giant knotweed rhizome, lysimachia, gymstemma pentaphyllum, astragalus root, etc.
Owner:梁珍惠
Hepatitis A inactivated vaccine storage device
InactiveCN112478405AFlexible accessGuaranteed storage stabilityDischarging meansContainers to prevent mechanical damageTGE VACCINEMechanical engineering
The invention discloses a hepatitis A inactivated vaccine storage device, and relates to the technical field of vaccine storage. The hepatitis A inactivated vaccine storage device comprises a storagebox, wherein a supporting plate is fixed to the bottom of the storage box, a steering mechanism is arranged between a hand wheel and a rotating partition plate, a vertical through hole vertically corresponding to a single storage pipe is formed in the top of the storage box, and a pushing mechanism used for vertically pushing a clamping mechanism is arranged in the storage box. According to the hepatitis A inactivated vaccine storage device, the plurality of storage pipes are used for storing a plurality of hepatitis A inactivated vaccines, the clamping mechanism can effectively clamp the storage pipes, so that the storage stability of the hepatitis A inactivated vaccines in the storage pipes is guaranteed; and the steering mechanism can drive the rotating partition plate to rotate, the storage pipes at different positions on the rotating partition plate can rotate to vertically correspond to the vertical through holes, the pushing mechanism is arranged to vertically push the storage pipes, so that the storage pipes extend to the outside of the vertical through holes, and the storage pipes containing the hepatitis A inactivated vaccines can be conveniently and flexibly taken.
Owner:JIANGSU CONVAC BIO TECH CO LTD
Chinese traditional medicine for treating hepatosis and preparation method thereof
InactiveCN101357184AThe formula is simpleExact therapeutic effectDigestive systemAntiviralsSide effectTherapeutic effect
The invention pertains to the field of Chinese traditional medicine, in particular to a traditional Chinese medicine used for treating hepatopathy and a preparation method thereof. The traditional Chinese medicine is made of the following bulk drugs: capillary wormwood herb, giant knotweed, spreading hedyotis herb, red sage root, red peony root, nutagrass flatsedge rhizome, wolfberry fruit and ginseng. The traditional Chinese medicine has the advantages of simple formula, definite treatment effect, few toxic and side effects as will as high cure rate and quick effect. The cure rate of the traditional medicine is over 98% for common hepatitis A and over 80% for hepatitis B and other hepatic diseases.
Owner:朱秀进
Hepatitis
InactiveUS6852505B1Microbiological testing/measurementPreparing sample for investigationScreening methodHepatitis A
A screening method of identifying a compound for treating hepatitis. Also disclosed is a method for evaluating responsiveness of a subject having hepatitis to a drug.
Owner:CHANG GUNG UNIVERSITY
Human source anti-hepatitis A virus gene engineering antibody from CHO cell
InactiveCN1605628AFunction to block infectionGenetic material ingredientsImmunoglobulins against virusesNatural antibodyNeutralizing antibody
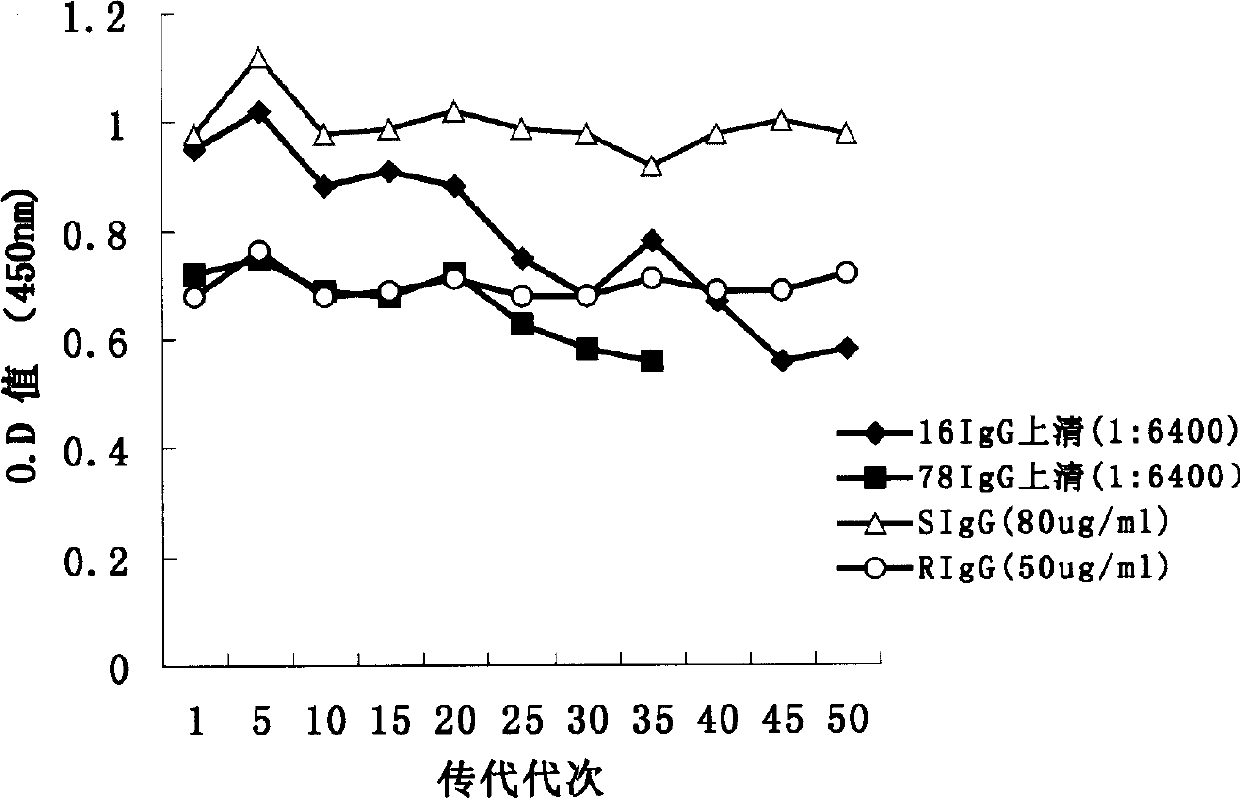
The human-derived anti-Hepatitis A virus genetically engineered antibody produced by CHO cells includes two strains of human-derived anti-Hepatitis A virus neutralizing genetically engineered antibody CHO (Chinese hamster ovary cell) production cell line and the production of human-derived anti-Hepatitis A virus neutralizing for prevention and treatment Genetically engineered antibody preparations. Its product features are fully human antibodies IgG1λ and IgG1κ derived from phage human antibody library screening and human IgG Fc gene recombination in Chinese hamster ovary (CHO) cells stably expressing cells, named ChHAIgG16 and ChHAIgG78 respectively. The molecular weight is about 150kd. The cocktail-type antibody preparation composed of ChHAIgG16 and ChHAIgG78 mixed at a ratio of 3:1 is named HAMaxTMM. Utilizing its functional characteristics of highly neutralizing hepatitis A virus infection and the molecular structure characteristics of whole antibodies similar to natural antibody molecules, it is expected to replace blood product gamma globulin in clinical practice for the prevention and treatment of hepatitis A caused by hepatitis A virus infection .
Owner:中国疾病预防控制中心病毒病预防控制所
Chinese medicine composition for treating hepatitis
InactiveCN1679887APromote repairImprove microcirculationPowder deliveryDigestive systemLiver functionCurative effect
Owner:张庆存
Auxiliary therapeutic agent for C type hepatitis
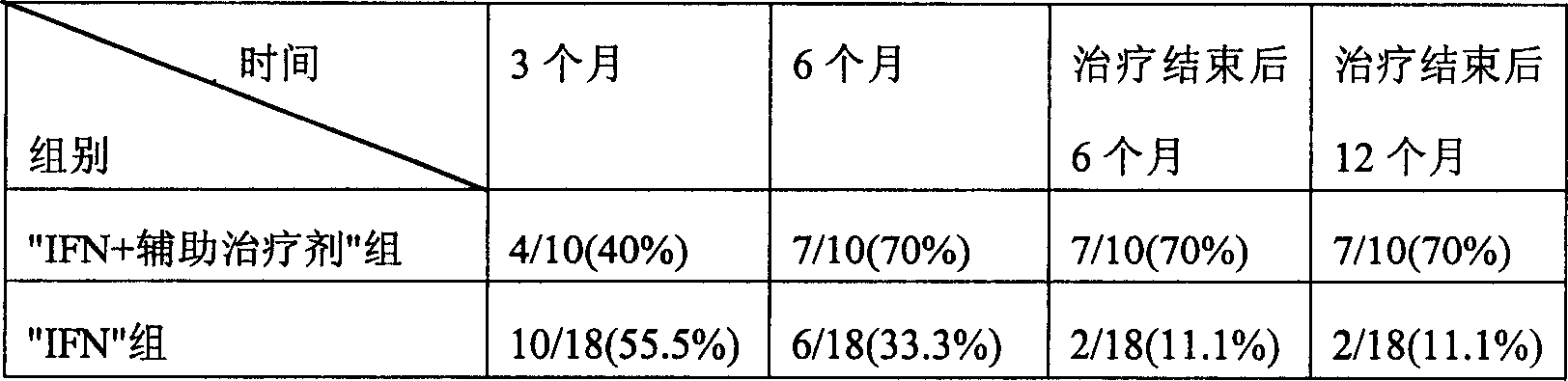
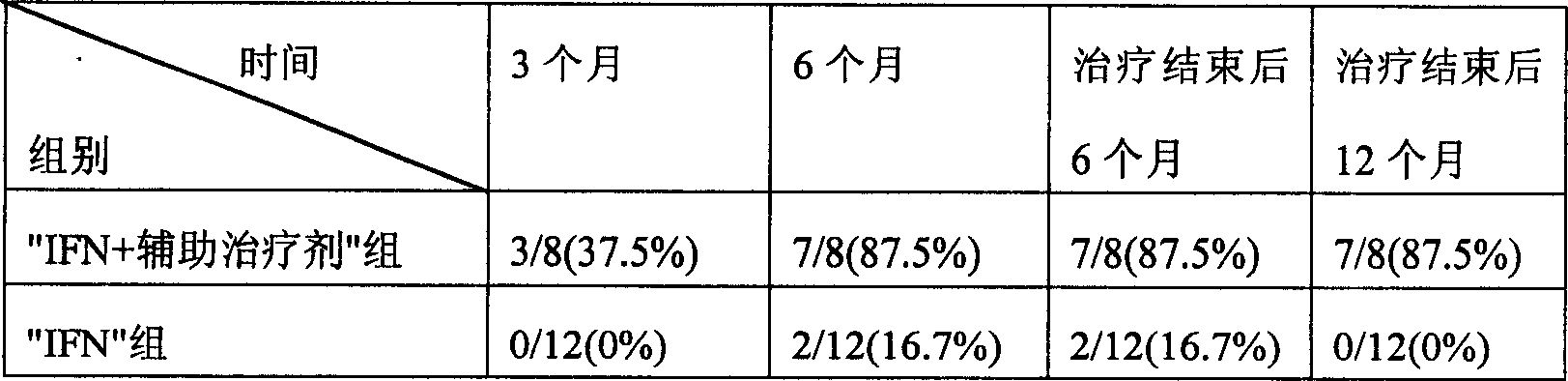
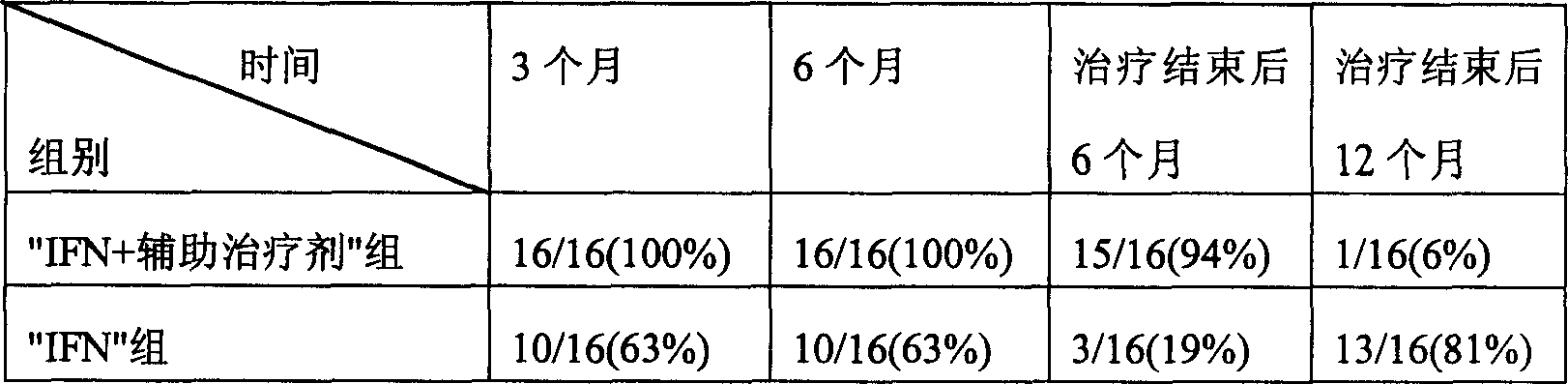
InactiveCN1607003ARaise the ratioIncreased complement concentrationPeptide/protein ingredientsDigestive systemCurative effectInterferon alpha
A auxiliary therapeutic agent for hepatitis C combined treating with a complex therapeutic method (interferon and Ribavirin)for hepatitis C, which is composed of (by weight) 50-90 part of Chinese caterpillar fungus and 10-50 part of astragalus. Said invention can adjust immune function of patient and improve curative effect for hepatitis C.
Owner:TCM BIOTECH INT CORP
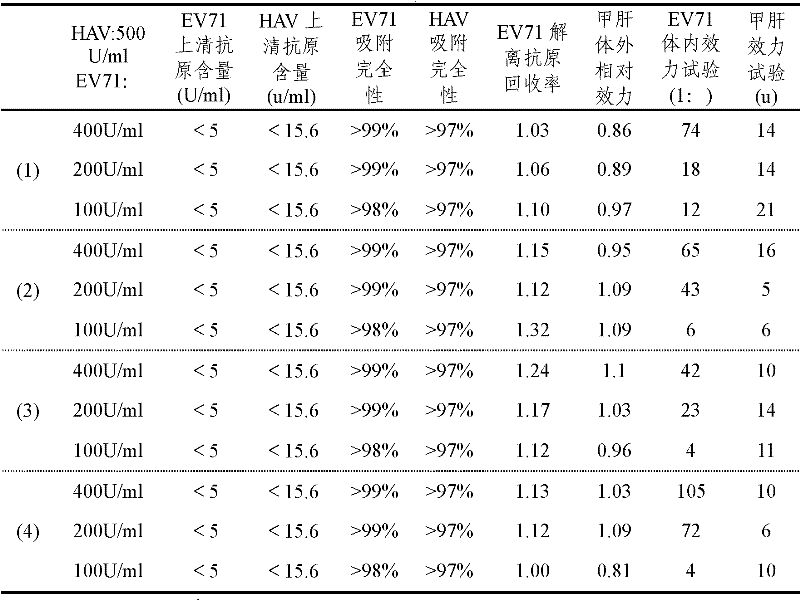
Combined EV71 (enterovirus 71)-HA (hepatitis A) vaccine
ActiveCN102210858AImproving immunogenicityImprove securityAntiviralsAntibody medical ingredientsDiseaseAdjuvant
The present invention discloses a combined EV71 (enterovirus 71)-HA (hepatitis A) vaccine which comprises inactivated enterovirus 71 viruses, inactivated hepatitis A viruses, and a aluminium adjuvant. The invention also provides a method for preparing the combined EV71-HA vaccine; and in the method, the protective agents such as gelatins and the like are not required to be added, the pH adjustment is not required to be performed, and the absorption effect and stability of the prepared vaccine are good. After the vaccine is vaccinated, the hepatitis A virus has an adjuvant effect on EV71 (enterovirus 71), therefore, the immunogenicity of the EV71 (enterovirus 71) is enhanced, and the immunity effect of the combined EV71-HA vaccine is equal to or greater than the immunity effect of a univalent vaccine. The vaccine disclosed by the invention has EV71-HA double immunities and protection performances; when the vaccine disclosed by the invention is vaccinated, the inoculating needle time can be reduced, and the immunization process can be simplified; and meanwhile, the diseases of people and animals arising from enterovirus 71 viruses and hepatitis A viruses can be effectively prevented.
Owner:SINOVAC BIOTECH
Treatment for hepatitis
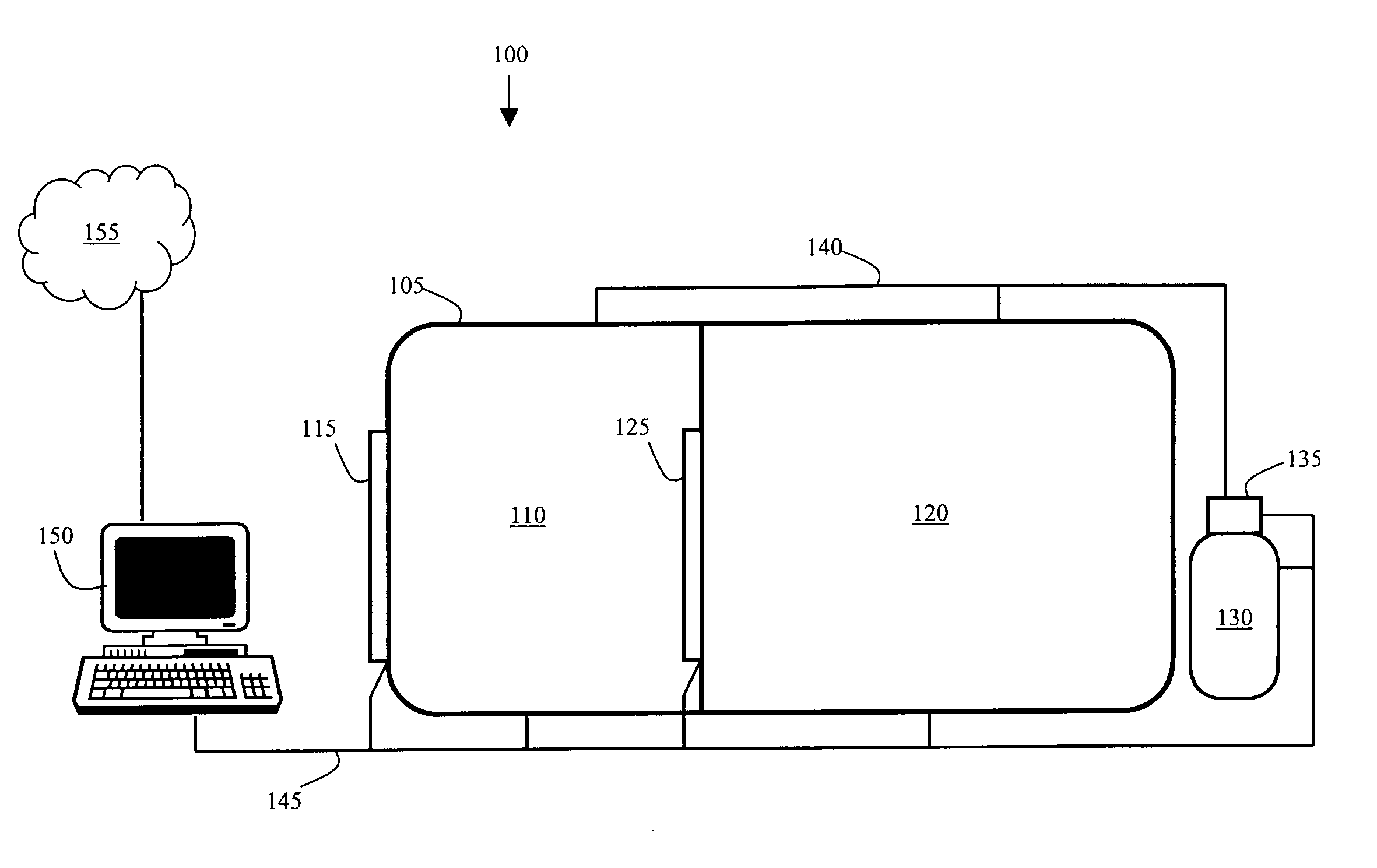
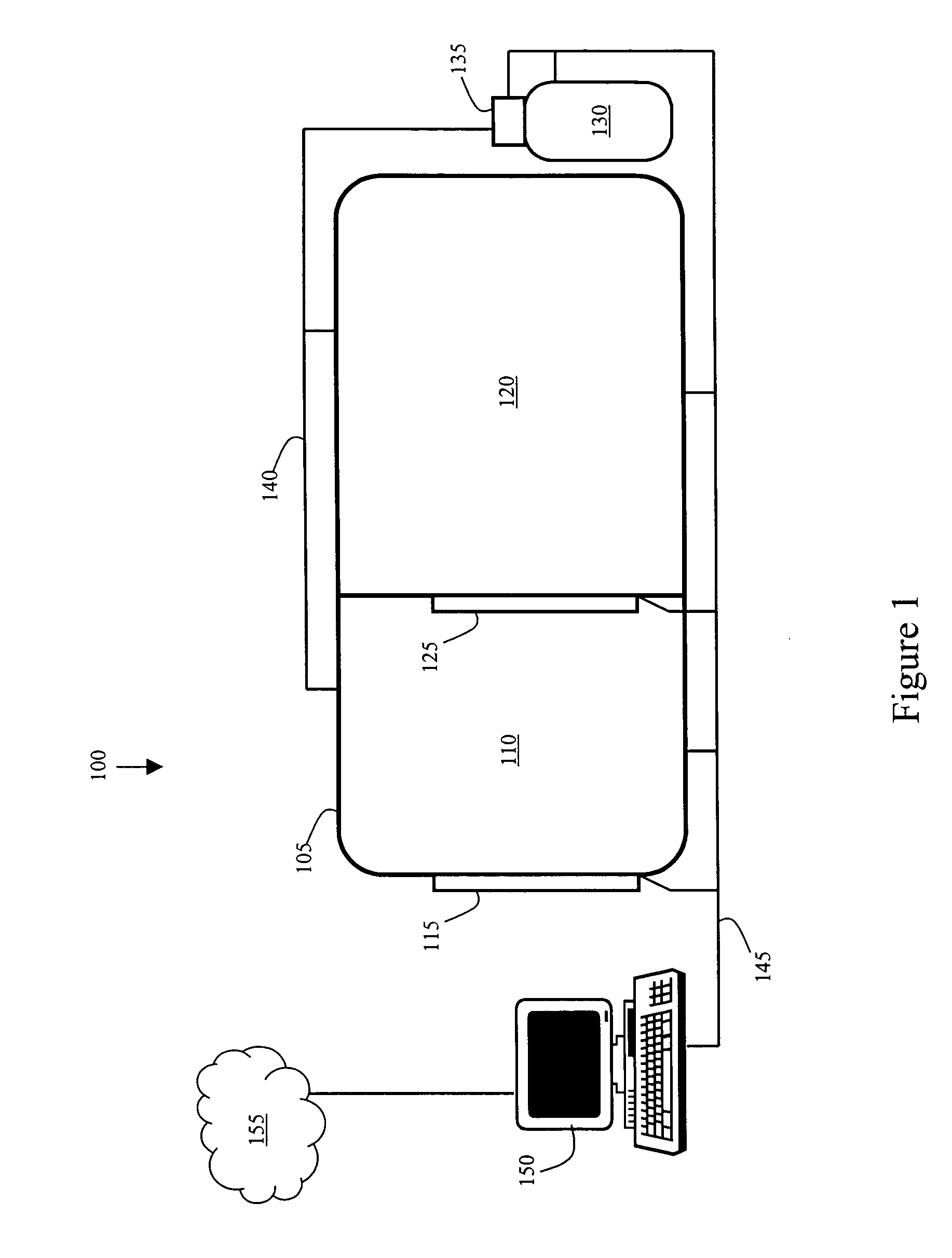
InactiveUS20060266354A1Strengthens a person's immune systemBetter able to fightBreathing protectionTreatment roomsAtmospheric pressureIntensive care medicine
A system for administering a treatment to individuals infected with a virus such as hepatitis. The system includes an enclosure and at least one gas supply that is used to create an altered atmospheric environment within the enclosure. The patients walk into the enclosure, the super-atmospheric environment is created with the gas, and the patients remain in the super-atmospheric environment for a predefined length of time. The individuals are subsequently returned at a safe rate to normal atmospheric pressure. The treatment can be repeated daily, monthly or annually depending on the needs of the patient. The system includes a control unit that stores and runs at least one treatment program that helps determine treatment variables such as amount of pressure, length of time and the type of gas or gases. It is believed that the present viral treatment may be able to be used in combination with medications and other viral treatments.
Owner:GUTHRIE STEPHEN D
Methods and compositions for diagnosis and prognosis of sepsis
InactiveUS20150177260A1Eliminate needBiocideOrganic active ingredientsKexinHepatitis A Virus Cellular Receptor 1
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in sepsis patients and in patients at risk for sepsis. In particular, the invention relates to using assays that detect one or more of WAP four-disulfide core domain protein 2, Hepatitis A virus cellular receptor 1, Interleukin-1 receptor-like 1, and Proprotein convertase subtilisin / kexin type 9 as diagnostic and prognostic in such patients.
Owner:ASTUTE MEDICAL
A colloidal gold method detection kit for hepatitis A virus igm antibody and preparation method thereof
InactiveCN102288758AReduce the effectHigh sensitivityMaterial analysisAgainst vector-borne diseasesHepatitis APolyacrylamide gel electrophoresis
The invention discloses a colloidal gold detection kit for hepatitis A virus IgM antibody, comprising a recombinant antigen HAV-Ag coated on a nitrocellulose membrane detection line, a goat anti-mouse IgG antibody and a gold label coated on a quality control line The mouse anti-human IgM monoclonal antibody labeled with colloidal gold coated on the pad, the recombinant hepatitis A virus antigen concentration was greater than 2mg / ml, and was determined by SDS-PAGE; the goat anti-mouse IgG antibody concentration was greater than 4mg / ml; The concentration of the mouse anti-human IgM monoclonal antibody was greater than 2 mg / ml, which was determined by SDS-PAGE, and the loading amount was two bands under the condition of 10 μl. The beneficial effects of the invention are as follows: the hepatitis A virus IgM antibody detection kit has the characteristics of rapidity, simplicity, accuracy and high sensitivity, and the whole operation time only takes 20 minutes to interpret the results. The colloidal gold rapid detection test strip is based on the multi-epitope recombinant antigen as the raw material. It has the characteristics of easier operation, low cost, good specificity and high sensitivity. It can be detected in a single batch and is easy to popularize. The detection and control effect is obvious.
Owner:北京中检安泰诊断科技有限公司
Protein marker for simultaneously detecting specificity of multiple diseases in blood serum and kit thereof
InactiveCN102183577AExact molecular weightEasy to operateMaterial analysis by electric/magnetic meansDiseaseSyphilis
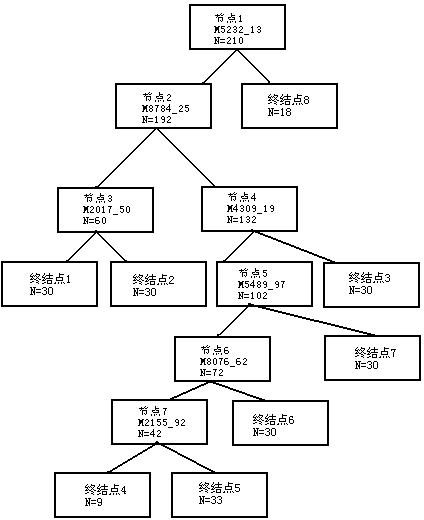
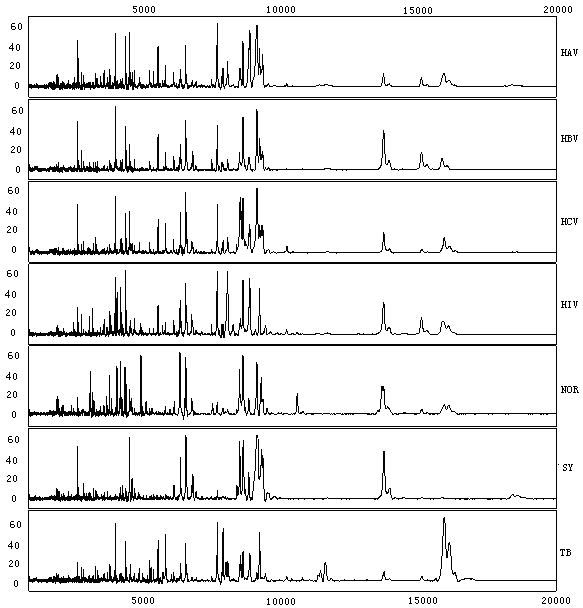
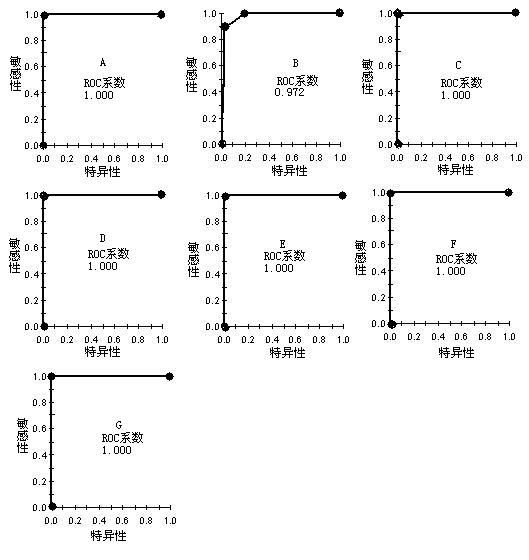
The invention discloses a group of protein marker for simultaneously detecting the specificity of hepatitis A, hepatitis B, hepatitis C, Aids, syphilis and tuberculosis in blood serum, a kit for detecting the group of specific proteins and application thereof. The protein marker for detection the specificity in the invention comprises seven proteins with different molecular weights in a serum proteome map: A: 5232.13, B: 8784.25, C: 2017.5, D: 4309.19, E 5489.97, F: 8076.62 and G: 2155.92. A detection method for the specific proteins comprises the steps of: processing a serum sample to be detected by using a lysis solution; incubating and capturing serum proteins by using a nano magnetic bead with carboxyl as a genin; and eluting the nano magnetic bead, and detecting seven specific proteins in eluted ingredients by using a protein reader. The kit and the detection method in the invention have the advantages of rapidness, sensitivity, specificity, flexibility, simplicity for operation and the like.
Owner:薛芳 +1
Traditional Chinese medicine for treatment of liver diseases
InactiveCN104666627AEffective treatmentPromotes Clearance KillDigestive systemAntiviralsSalvia miltiorrhizaChronic hepatitis
The invention discloses a traditional Chinese medicine for the treatment of liver diseases, which comprises the following raw materials in parts by weight: 11 parts of radix paeoniae alba, 11 parts of herba hyperici japonica, 6 parts of christina loosestrife herbs, 10 parts of panax notoginseng, 10 parts of angelica sinensis, 7 parts of five flavors, 10 parts of salvia miltiorrhiza, 18 parts of herba artemisiae, 15 parts of hawthorn, 11 parts of manyflower glorybower roots, 10 parts of radix polygoni multiflori, 10 parts of radix bupleuri, 8 parts of solanum lyratum, and 15 parts of radix glycyrrhizae. The traditional Chinese medicine disclosed by the invention effectively treats acute or chronic hepatitis B, hepatitis A, hepatitis C, liver ascites, liver cirrhosis, alcoholic liver, and the like.
Owner:郝文旭
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com