Combined EV71 (enterovirus 71)-HA (hepatitis A) vaccine
A combined vaccine and enterovirus technology, applied in the field of medical biology, to achieve the effect of reducing transportation and storage costs, reducing the number of vaccinations, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Preparation of inactivated EV71 stock solution
[0024] Cultivate the Vero cells to be inoculated until they grow into a dense monolayer, then digest the cells, subculture at 1:8 and inoculate the virus until they grow into a monolayer, and inoculate the virus with the EV71 inactivated vaccine virus seeds at a ratio of 1:10 2 CCID 50 / ml inoculated in the well-grown Vero cells, cultured at 33±1°C for 15 days, and harvested the cultured cell virus supernatant according to the cell pathological changes to obtain EV71 virus harvest liquid. The harvest liquid is inactivated by formaldehyde with a final concentration of not more than 250 μg / ml for not less than 6 days, and then purified by ultrafiltration and column chromatography, and the combined chromatographic purified liquid is concentrated by ultrafiltration and then sterilized and filtered to obtain inactivated EV71 stock solution.
Embodiment 2
[0025] Embodiment 2: Preparation of inactivated HAV stock solution
[0026] 2BS cells were cultured in Eagle's MEM medium containing newborn bovine serum. After the cells had grown well, they were digested and subcultured by dilution, and then inoculated with HAV of TZ84 strain, YN5 strain, HM175 strain and Lv8 strain at a MOI of 0.05-1.0. During the period, the maintenance medium was changed according to the growth of the cells, and the maintenance medium was Eagle's MEM medium containing newborn bovine serum. After culturing to the peak of virus proliferation, trypsin or other appropriate methods are used to digest cells containing HAV, and the virus harvest liquid is collected by centrifugation or filtration. The virus is concentrated by ultrafiltration, and then purified by chromatography; after sterilization and filtration, it is inactivated with formaldehyde with a final concentration of no more than 250 μg / ml at 37±1°C for 12 days, and the inactivated virus liquid is in...
Embodiment 3
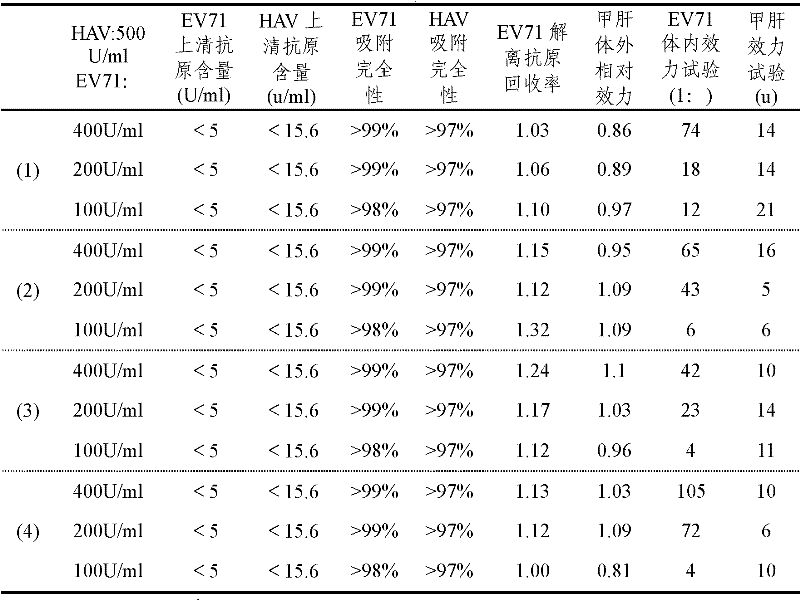
[0027] Embodiment 3: Preparation of EV71 and HAV combined vaccine
[0028] Adsorption method 1: After the diluted inactivated HAV and the diluted inactivated EV71 are adsorbed with aluminum adjuvant respectively, the adsorption products of the two inactivated viruses are mixed to prepare a combined vaccine of EV71 and HAV.
[0029] Adsorption method 2: The diluted inactivated HAV is first adsorbed with an aluminum adjuvant, and then diluted inactivated EV71 is added for adsorption to prepare a combined vaccine of EV71 and HAV.
[0030] Adsorption method 3: The diluted and inactivated EV71 is first adsorbed with aluminum adjuvant, and then diluted and inactivated HAV is added for adsorption to make a combined vaccine of EV71 and HAV.
[0031] Adsorption method 4: Mix the diluted inactivated HAV and the diluted inactivated EV71 first, and then add the mixed solution to the aluminum adjuvant for adsorption to make a combined vaccine of EV71 and HAV. The content of EV71 antigen i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com