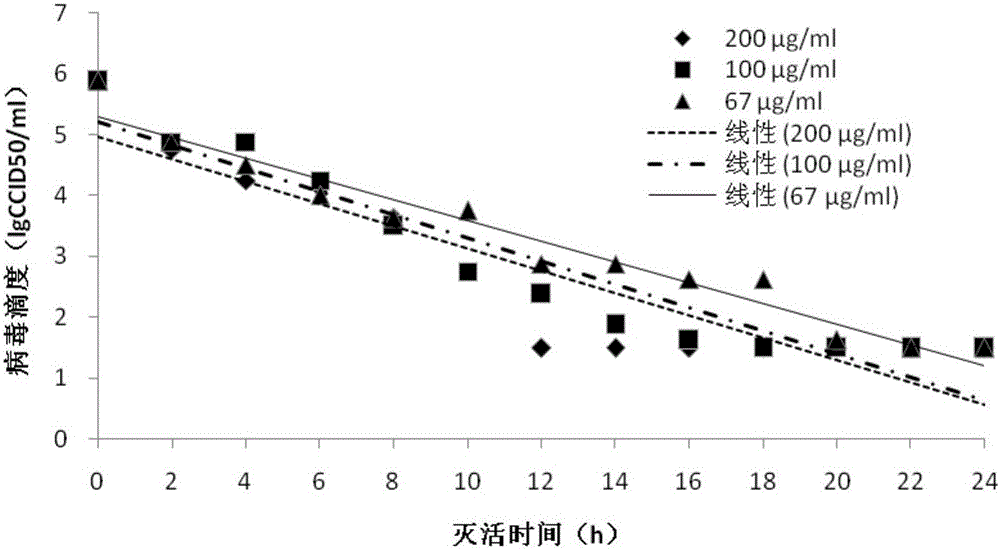
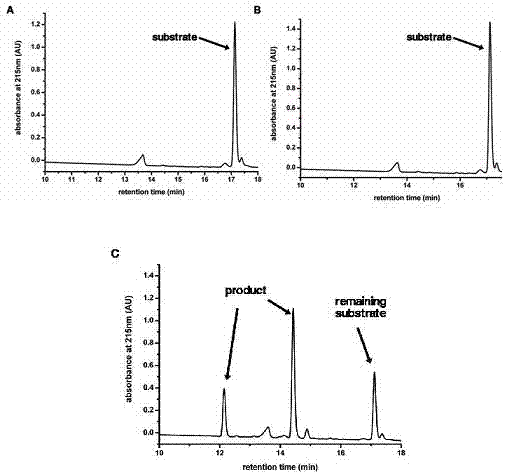
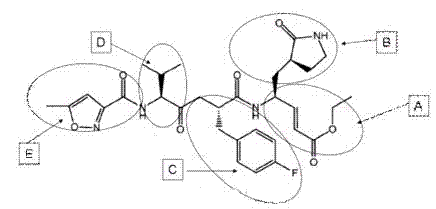
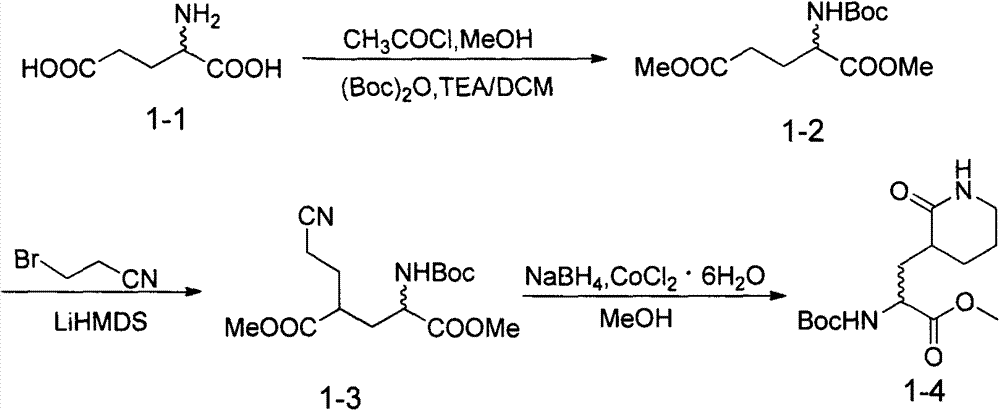
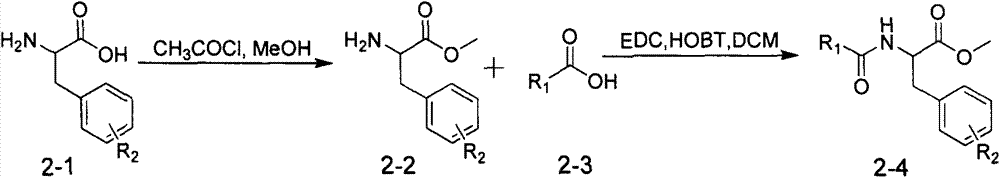
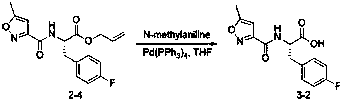Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
236 results about "Enterovirus 71" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enterovirus 71 (EV71) is a virus of the genus Enterovirus in the Picornaviridae family notable for its role in causing epidimics of severe neurological disease and hand, foot, and mouth disease in children. It was first isolated and characterized from cases of neurological disease in California in 1969. Enterovirus 71 infrequently causes polio-like syndrome permanent paralysis.
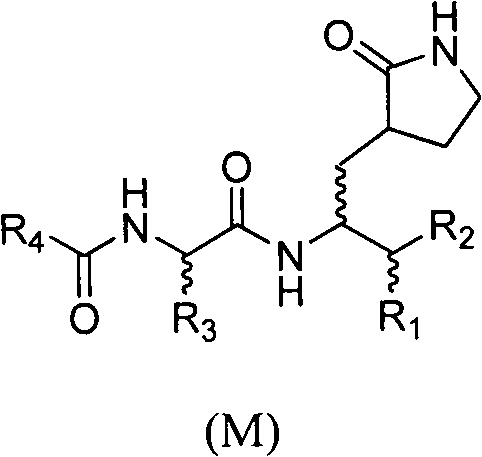
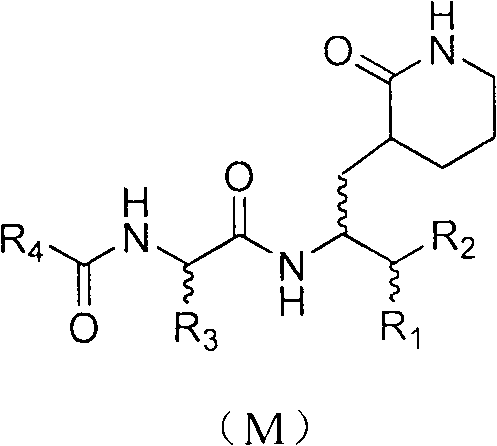
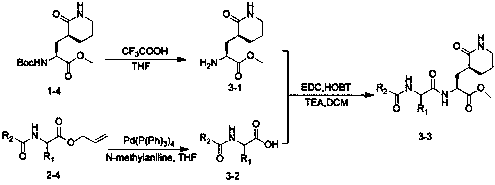
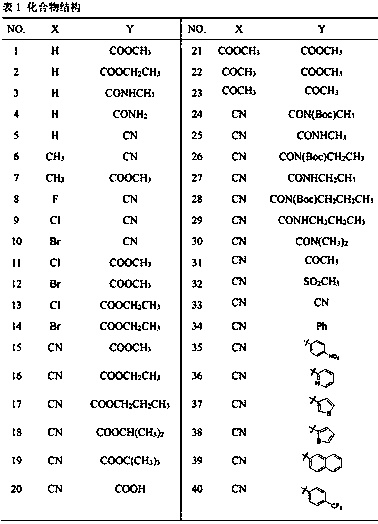
Anti-enterovirus 71 (EV71) valerolactam compounds, preparation method and uses thereof
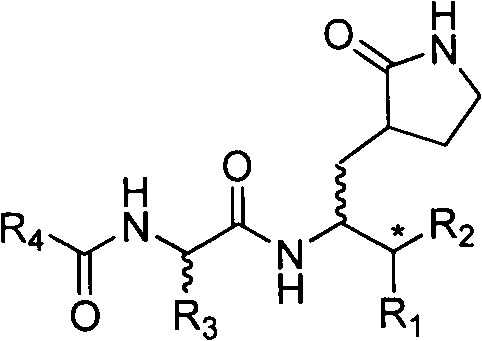
The invention relates to valerolactam enterovirus 71 (EV71) 3C protease inhibitors, wherein a structure general formula of the inhibitors is represented by a compound M, various variables in the structure is defined in an instruction, and EV71 replication is effectively inhibited or blocked with the compounds. The present invention relates to discoveries and applications of the compounds containing the structure represented by the formula (M), optical isomers, metabolites with pharmaceutical activities, pharmaceutically acceptable salts, solvates, and prodrugs thereof in preparations of anti-virus drugs for treatment of hand-foot-mouth disease infection. The present invention further relates to an intermediate of the structure compound represented by the formula (M) and a synthesis method thereof.
Owner:NANKAI UNIV +1
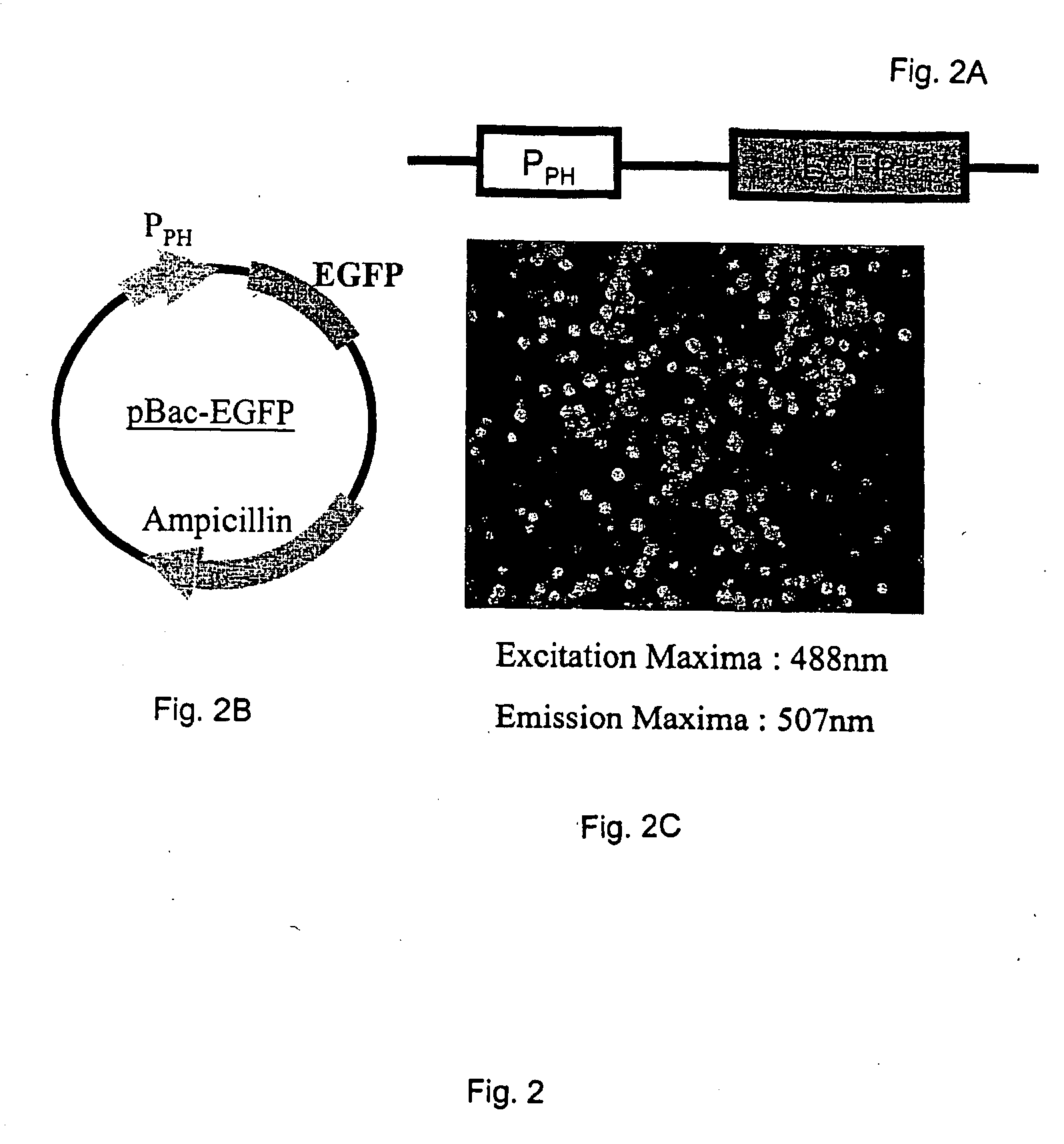
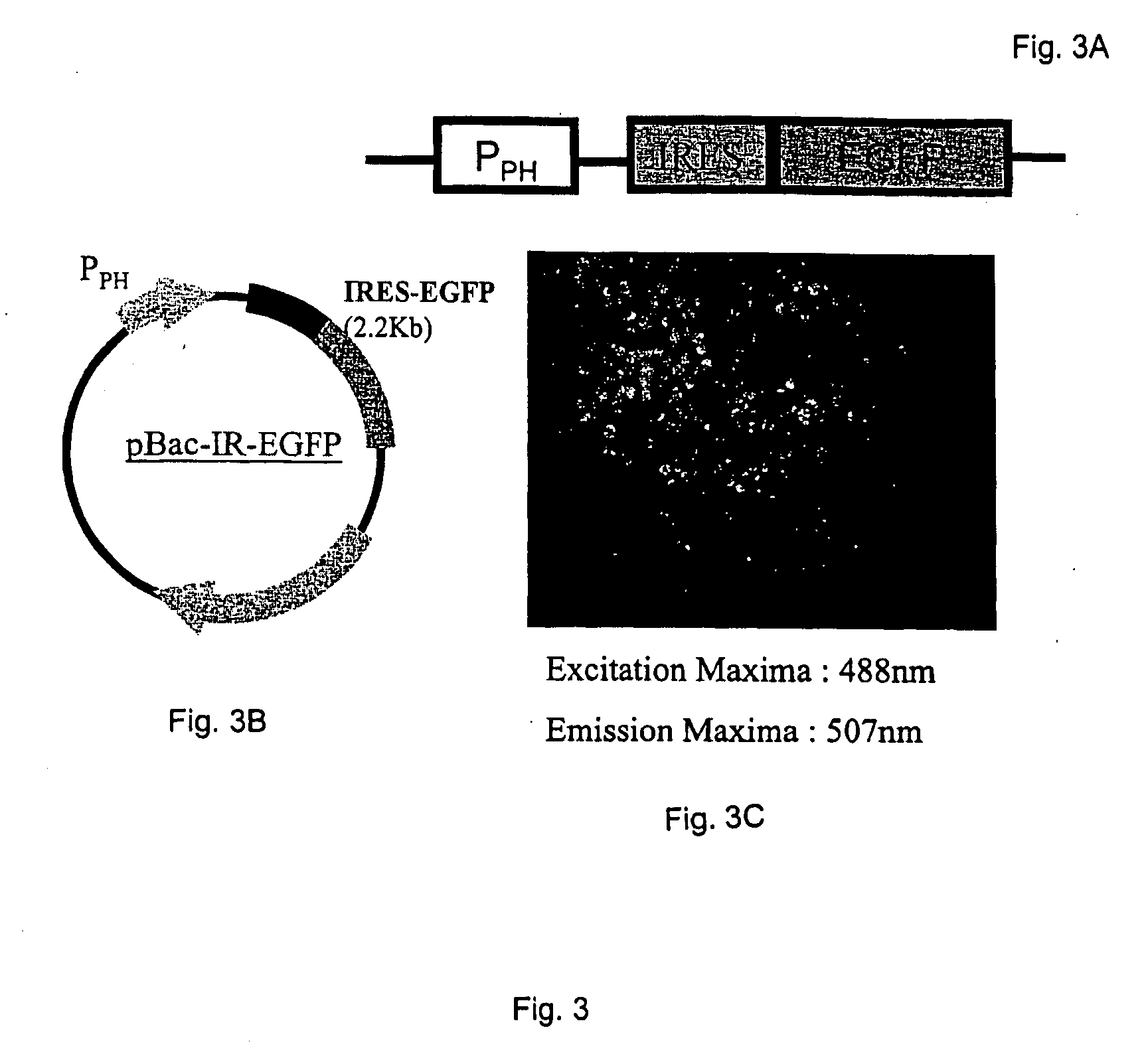
Internal ribosome entry sites for recombinant protein expression
InactiveUS20050112095A1Efficiently translatedConstant ratioBiocideSsRNA viruses positive-senseInternal ribosome entry siteEnterovirus 71
The invention describes compositions and methods for recombinant protein expression in a wide range of cell types, including mammalian, insect, and bacterial cells. The compositions comprise a viral IRES sequence selected from enterovirus 71 (EV71), hepatitis C virus (HCV), or encephalomyocarditis virus (EMCV), or a variant or fragment thereof, or alternatively, a homolog of a viral IRES selected from EV71, HCV, or EMCV, or a variant or fragment thereof. Methods of using the compositions are also described.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Anti-enterovirus 71 (EV71) caprolactam compounds, and preparation method and application thereof
The invention relates to a caprolactam anti-enterovirus 71 (EV71) 3C protease inhibitor with a structural formula shown as compounds (M). Each variable in the structure is defined as the specification. The compounds can effectively inhibit or block replication of enterovirus 71. The invention relates to discovery and application of compounds comprising a structure of formula (M), various optical isomers thereof, metabolites with pharmaceutically activity, pharmaceutically acceptable salts, solvates and prodrugs in preparing antiviral drugs for treating virus infections of hand-foot-and-mouth diseases. The invention also relates to an intermediate and a synthetic method for preparing the compounds having the structure of the formula (M).
Owner:NANKAI UNIV +2
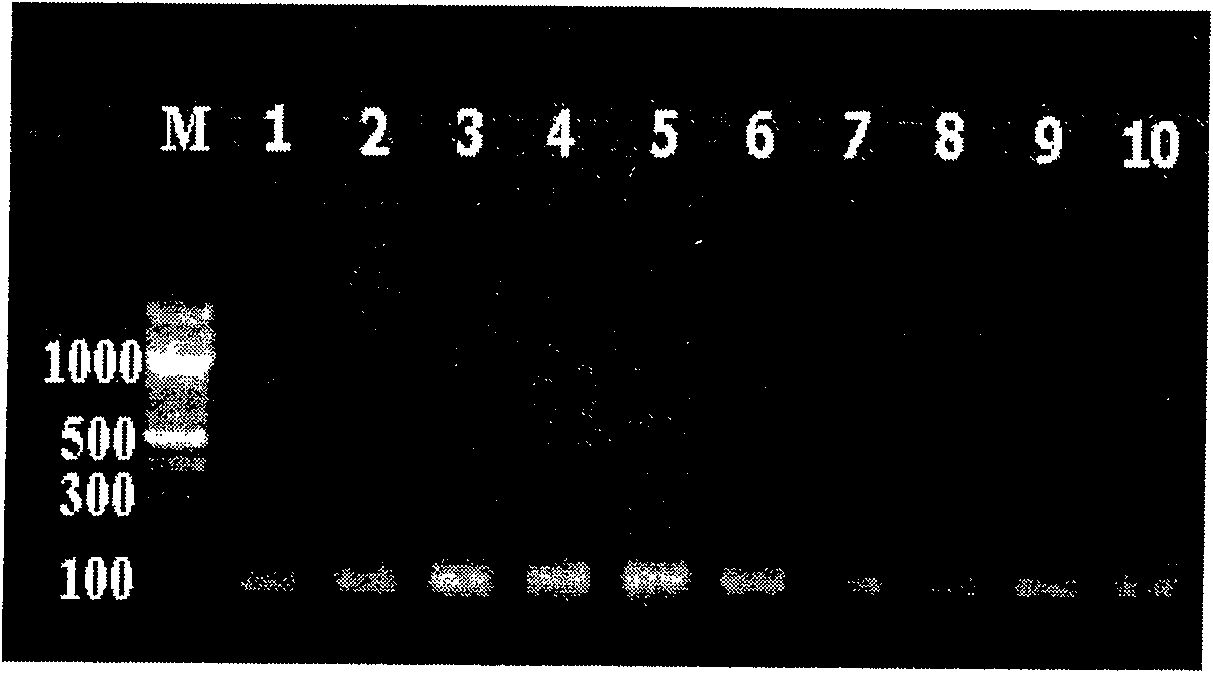
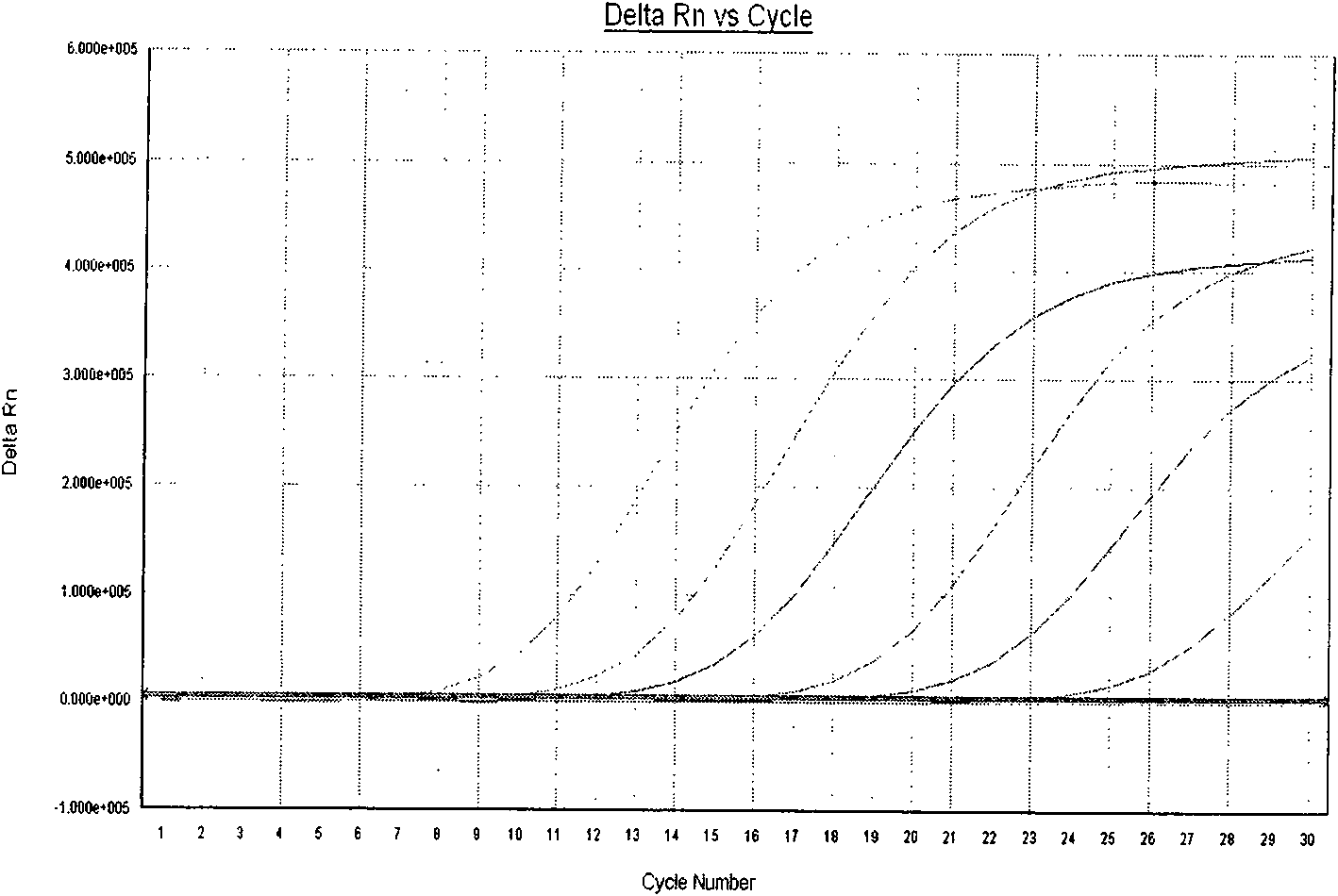
Three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as kit thereof
InactiveCN101886138AEasy to detectHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Reverse transcription polymerase chain reaction
The invention provides a three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as a kit thereof. The method can rapidly and accurately detect the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of enterovirus in a sample. The method comprises the following steps of: (1) acquiring and conveying a sample of an infected patient or a suspected patient; (2) preprocessing the sample and extracting RNA; (3) detecting the sample by adopting a one-step PCR-three-color fluorescent probe in-vitro amplification method; and (4) analyzing the corresponding sample according to the fluorescence intensity of each amplification reaction after the amplification reaction is finished, thereby judging the existence of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus in the acquired sample and being capable of carrying out accurate quantitation (a figure 3) on the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus. The invention realizes the aim of carrying out rapid and accurate combined detection of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus.
Owner:BEIJING SUOAO BIOTECH
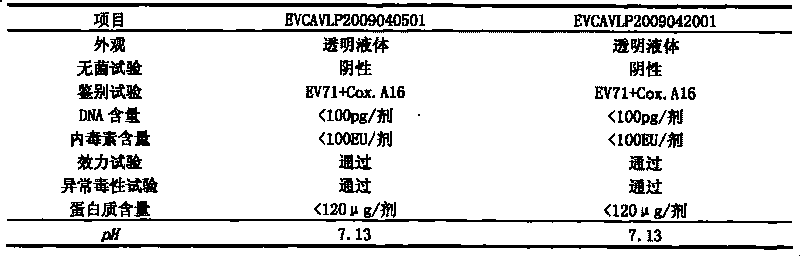
Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695569AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
The invention discloses a univalent and bivalent gene engineered subunit vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: respectively obtaining recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD by gene engineering means, respectively efficiently coexpressing similar SeQ ID No.1 EV71 P1 and Se Q ID No.2 Cox.A16 P1 and 3CD proteins in insect cells, and respectively self-assembling into EV71 VLP and Cox.A16 VLP; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; lysing the cells, ultra-filtering and purifying virus suspension; and further preparing the univalent and bivalent vaccine. The vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Recombinant enterovirus 71 neutralizing antibodies and applications thereof
Provided is an antibody against enterovirus 71 comprising the amino acid sequence shown in SEQ ID NOS: 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, or 26 or functionally active homologues thereof. Also provided are methods for obtaining the antibody comprising (a) selecting a yeast expressing such an antibody from a yeast library, (b) culturing the yeast under conditions that the antibody is expressed, and (c) recovering the antibody from the culture. Also provided is a process for producing the antibody comprising (a) culturing a host cell under conditions that the antibody is expressed, (b) recovering the antibody from the culture, wherein the host cells are transformed or transfected for expressing the antibody against enterovirus 71. Also provided are pharmaceutical compositions comprising an antibody against enterovirus 71 and a pharmaceutically acceptable carrier or diluent, wherein the antibody has an anti-virus agent or detectable label attached thereto.
Owner:DEV CENT FOR BIOTECHNOLOGY
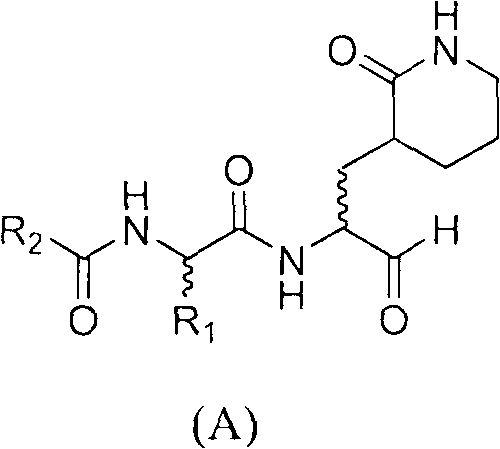
Anti-enterovirus 71 (EV 71) caprolactam aldehyde compound and preparation method and purpose thereof
A structure general formula of a caprolactam aldehyde enterovirus 71 (EV 71) 3C protease inhibitor is as shown in chemical combination A, all variables in the structure are defined in an instruction book, and the compound effectively restrains or blocks the copy of the enterovirus 71. The invention relates to a compound with the structure as shown in formula (A), various types of optical isomers of the compound with the structure as shown in formula (A), drug active metabolites, officinal salt, solvate and discovery and application when the compound with the structure as shown in formula (A), the various kinds of optical isomers of the compound with the structure as shown in formula (A), the drug active metabolites and the officinal salt are used for preparing antiviral drugs for curing hand-foot-mouth virus infection diseases. The invention further relates to an intermediate and a synthetic method of preparing the compound with the structure as shown in formula (A).
Owner:NANKAI UNIV +2
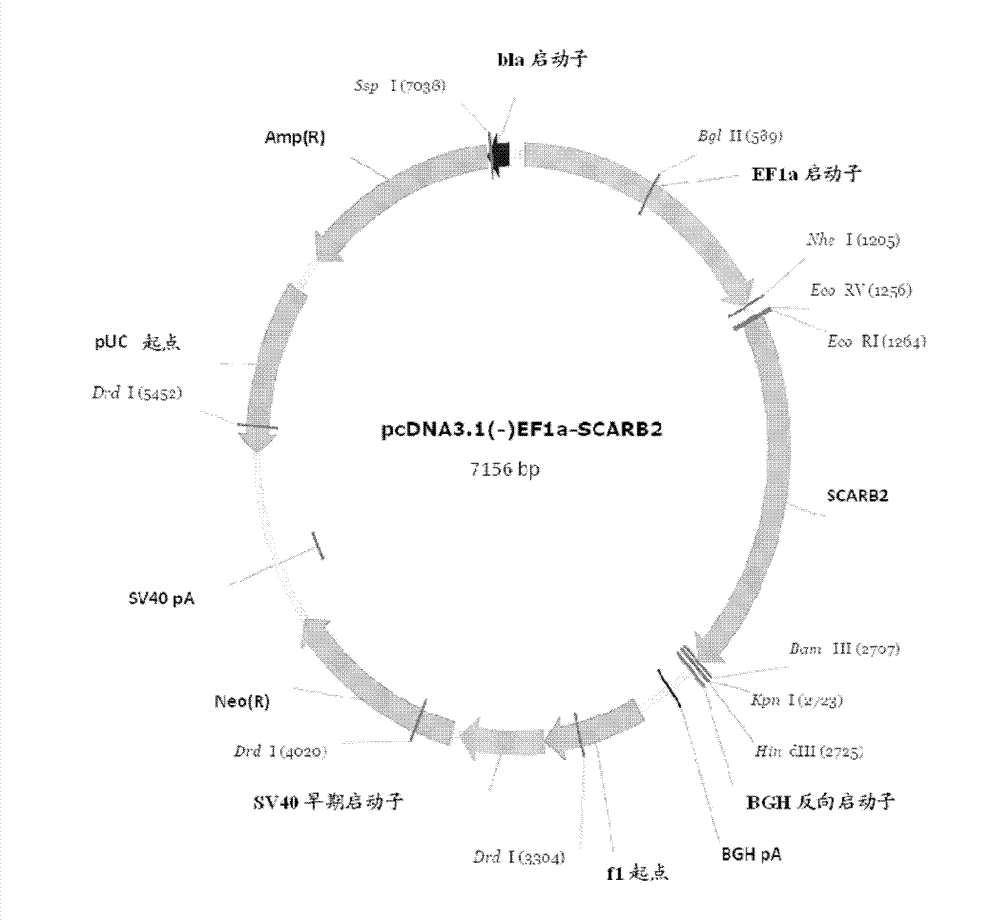
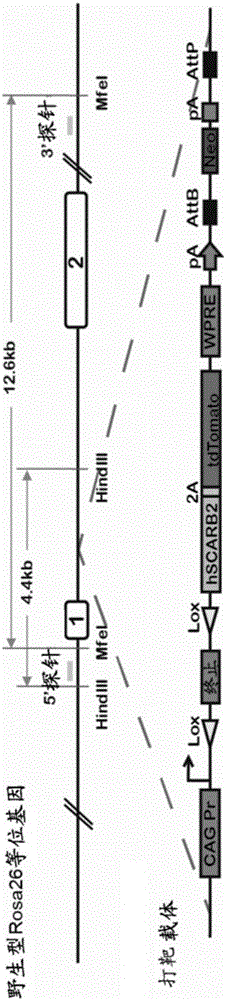
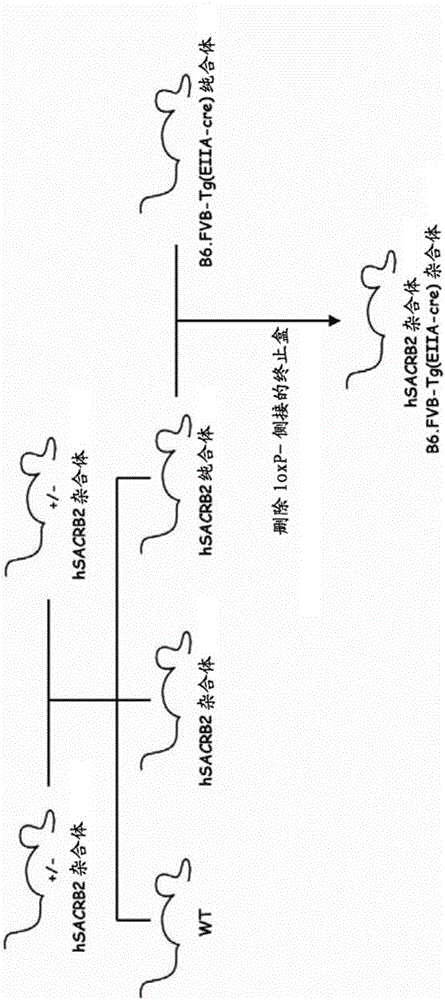
Manufacture of hSCARB 2 transgenic mouse and application of the transgenic mouse as enterovirus infection animal model
InactiveCN103081868AAnimal reproductionMicrobiological testing/measurementHand-foot-and-mouth diseaseEnteroviral infections
The present invention concerns the manufacture of EV71 acceptor SCARB 2 transgenic mouse. The manufactured SCARB 2 transgenic mouse can be used as an animal model infected with the hand-foot-and-mouth disease, such as infected with enterovirus 71 or Coxsackies virus A16, so as to allow for estimation of enterovirus viral vaccine immune protection efficacies and application of enterovirus infection in pathological study.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
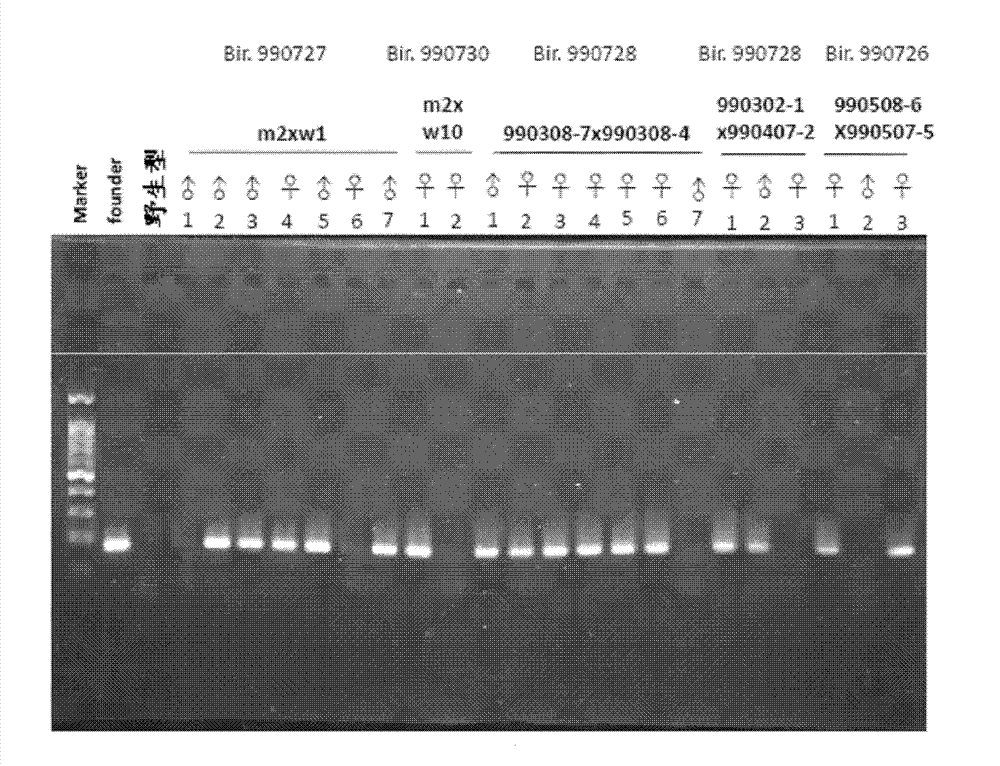
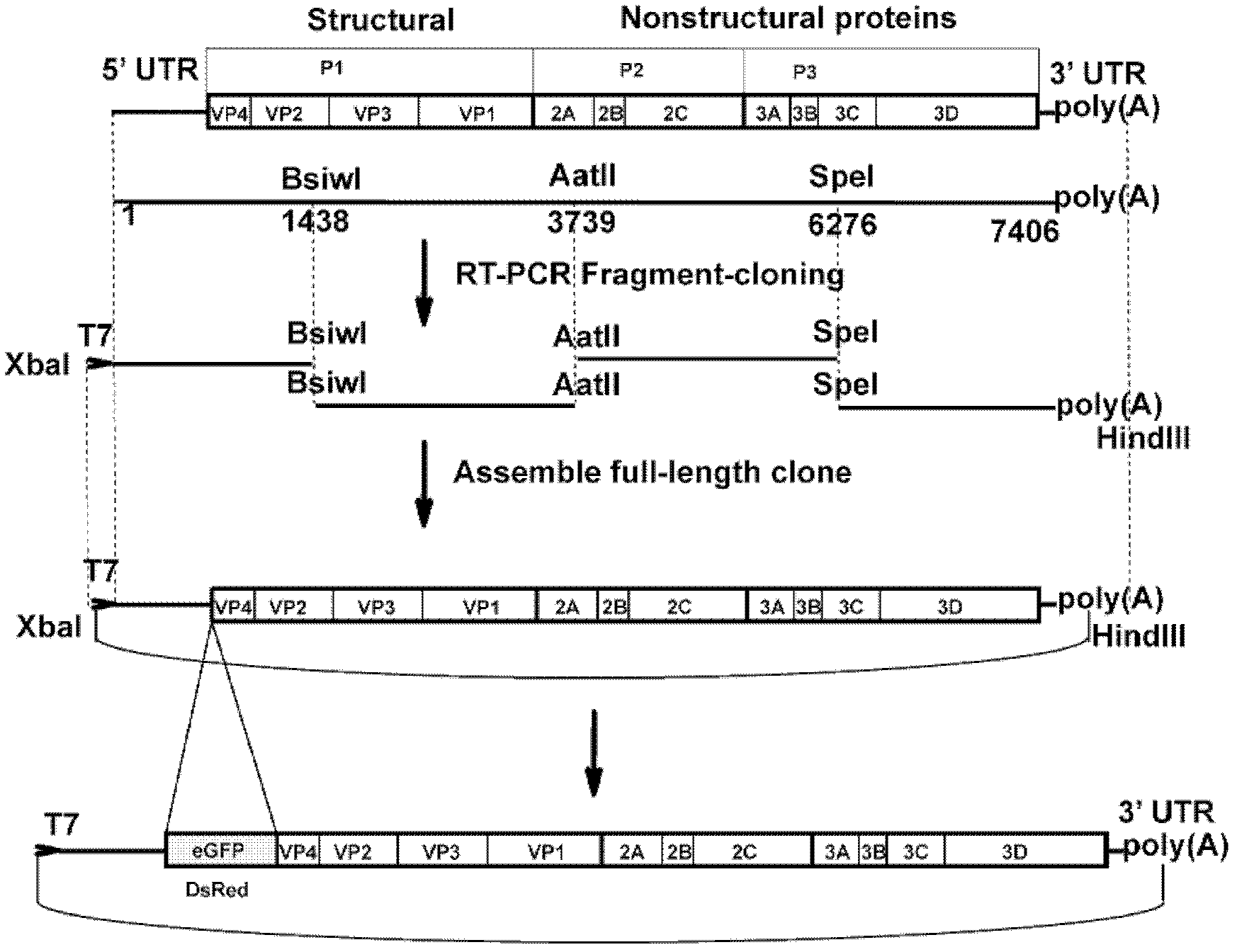
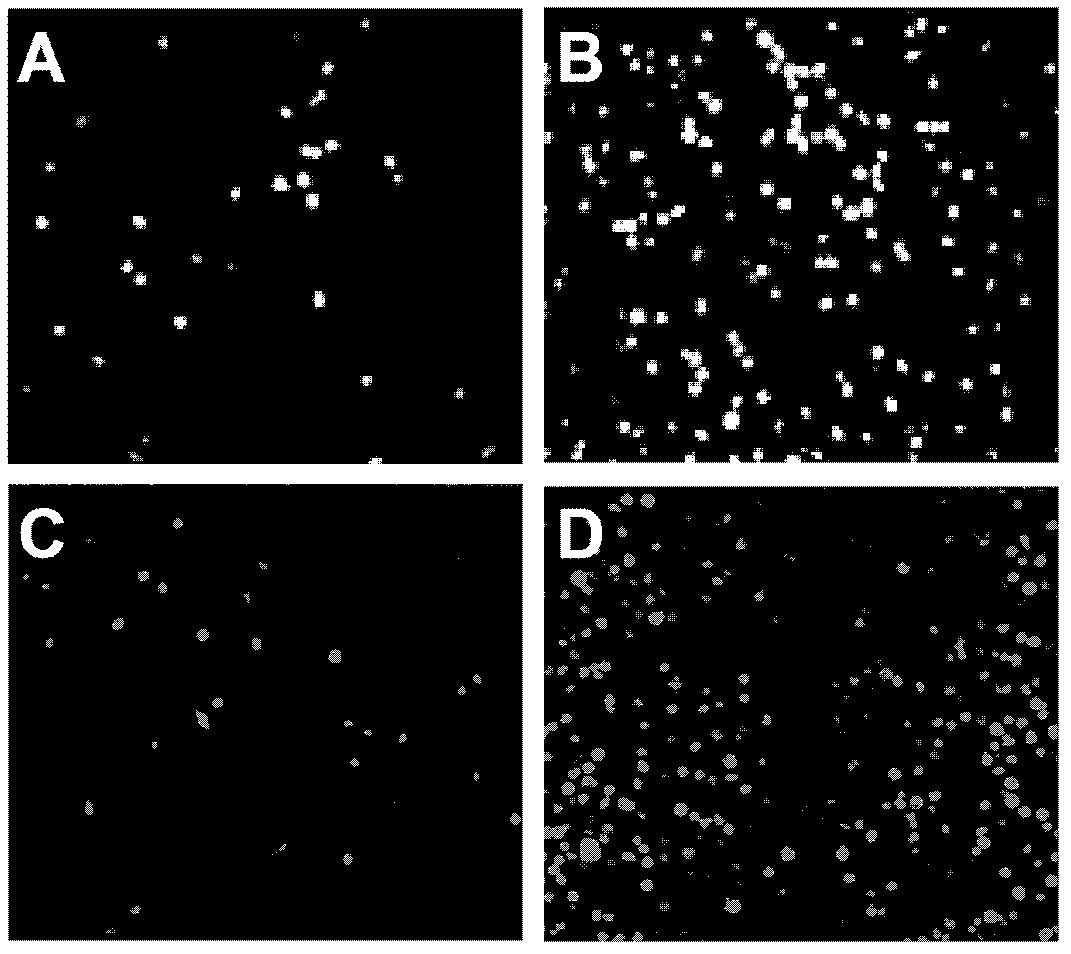
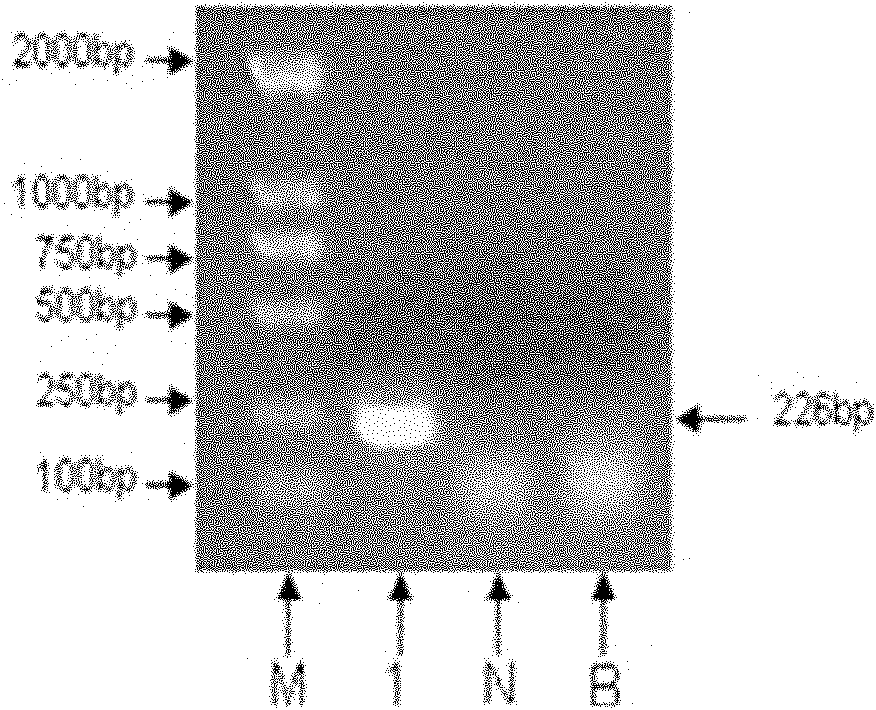
Preparation method and application of enterovirus 71 type full-length infectious clone with tags
InactiveCN102517317AEffective generationEfficiently obtainedMicrobiological testing/measurementViruses/bacteriophagesCytopathic effectTotal rna
The invention discloses a preparation method and application of enterovirus (EV) 71 type full-length infectious clone with tags. The preparation method comprises the following steps of: (1) extracting total RNA; (2) performing reverse transcription-polymerase chain reaction (RT-PCR) amplification on the EV71; (3) constructing EV71 subclone; (4) constructing recombinant clone containing EV71 genes; and (5) constructing the enterovirus 71 type full-length infectious clone with eGFP and DsRed. Tests, such as cytopathic effect, fluorescence detection, gene detection, virus plaque, medicine inhibition and the like, prove that the EV71 full-length infectious clone with the tags is obtained successfully. The invention has high application value in the aspects of research and development of animal models, virus replication and pathogenesis, medicine screening and medicine action mechanism, vaccine and diagnostic reagents and the like.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Enterovirus 71 type specific recombinant protein antigen and application thereof
InactiveCN102558313AIncreased sensitivityImprove featuresBacteriaMicroorganism based processesSerodiagnosesType specific
Owner:SOUTHEAST UNIV
Cell lines for virus production and methods of use
InactiveUS20150374812A1SsRNA viruses positive-senseViral antigen ingredientsExpression geneEnterovirus 71
Provided herein are engineered cell lines. In some embodiments, cells of an engineered cell line have altered expression of a gene and / or altered expression of an miRNA, wherein the altered expression results in increased or decreased production of a virus. The virus is a picomavirus, such as a poliovirus or Enterovirus 71. Also provided herein are methods for using the engineered cells to produce virus, and methods for treating a subject having or at risk of having a viral infection.
Owner:UNITED STATES OF AMERICA +2
Preparation and uses of novel Michael receptor-based enterovirus 71 type inhibitor
The present invention relates to a class of novel Michael receptor-based virus 71 (EV71) 3C protease inhibitors, wherein various variables of the structure general formula (M) are defined in the specification, and the compounds effectively inhibit or block the replication of enterovirus 71. The present invention relates to discovery and applications of the compound containing the structure generalformula (M), various optical isomers, pharmaceutically active metabolites, pharmaceutically acceptable salts, solvates and prodrugs thereof in preparation of antiviral drugs for the treatment of hand-foot-mouth virus infection diseases. The invention relates to an intermediate and a synthesis method for preparing the compound having the structure general formula (M). The formula (M) is defined inthe specification.
Owner:NANKAI UNIV
Application of ganoderma acid y in preparation of medicine for treating or preventing enterovirus 71 infection
InactiveCN102274234AAnti-infectionAnti-inflammatoryOrganic active ingredientsAntipyreticPositive controlTreatment effect
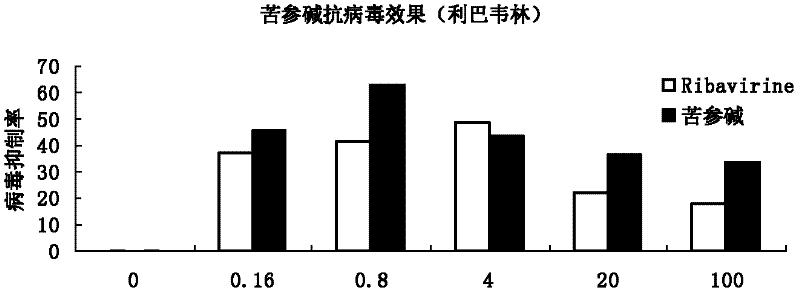
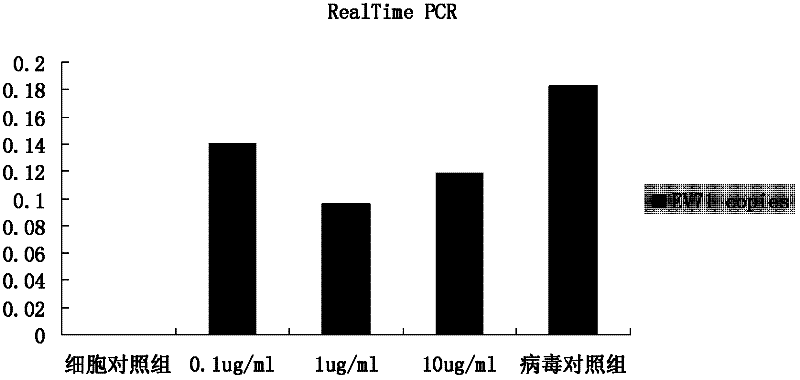
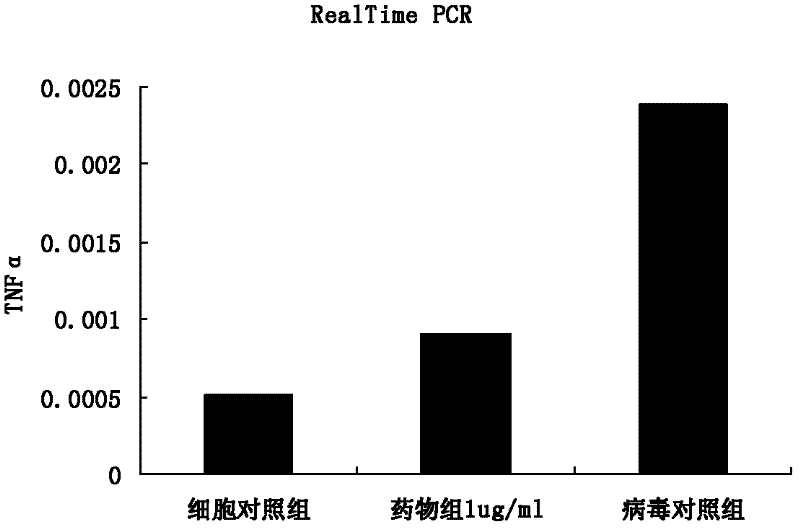
The invention discloses application of ganoderic acid Y to the preparation of a medicament for treating or preventing enterovirus 71 infection. The invention proves that the ganoderic acid Y has a good in-vitro antivirus effect in an enterovirus 71 infection cell test. Meanwhile, the ganoderic acid Y has a certain killing effect on enterovirus 71, can be used for preventing the infection of the enterovirus 71, and has a good treatment effect on cells infected by the enterovirus 71 and a good inhibiting effect on virus replication; and the acting effect of the ganoderic acid Y is more remarkable than ribavirin serving as a positive control medicament. Moreover, the ganoderic acid Y has a good inhibiting effect on inflammatory reactions caused by the enterovirus 71, and the prospect of the development of the medicament into an anti-enterovirus 71 medicament is disclosed.
Owner:WUHAN UNIV
Method for building 7-day-old mouse model infected with enterovirus 71
The invention discloses a method for building a 7-day-old mouse model infected with enterovirus (EV) 71, comprising the following steps: taking 7-day-old suckling mice and injecting liquid virus into the brains of the mice, thus obtaining the mouse model infected with EV 71. The injection volume of the liquid virus is 20mu L and the liquid virus is prepared by the following steps: inoculating the EV71 seed into MA104 cells in terms of inoculation amount of 200mu L / 100mL cell culture bottle, culturing the MA104 cells in 5% of CO2 at 37 DEG C and observing the cytopathic effect every day; on the second day after inoculating EV71, observing that about 70% of the MA104 cells are agglutinated and crimpled, freeze-thawing the cells in the whole bottle and then centrifuging the cells and taking the supernatant, thus obtaining the liquid EV71. The method successfully builds the stable 7-day-old mouse model infected with EV71, provides foundations for the scientific research personnel to research the EV71 etiology and pathogenic mechanism and for screening efficient antivirus drugs and developing vaccines and has broad application prospect.
Owner:INST OF BASIC MEDICINE OF SAMS
Construction method and use of enterovirus 71 type infection model based on hSCARB2 gene knock-in
ActiveCN106554972AIn-vivo testing preparationsVector-based foreign material introductionAntiviral drugPharmaceutical drug
The invention relates to a construction method of an enterovirus 71 type infection model based on hSCARB2 gene knock-in and use of the infection model, and especially provides an effective means for in vivo evaluation of EV71 vaccines and antiviral drugs.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Loop-mediated isothermal amplification assay kit and detection method of hand, foot and mouth disease
InactiveCN102242223ASuitable for field applicationStrong specificityMicrobiological testing/measurementHand-foot-and-mouth diseaseCoxsackievirus a16
The invention belongs to the field of biotechnology, and relates to a loop-mediated isothermal amplification (LAMP) assay kit of Coxsackie A16 (Cox A16) and Enterovirus 71 (EV 71) which are main pathogens of hand, foot and mouth disease, and an establishment method and an application of the assay kit. The assay kit comprises four LAMP primers and LAMP reaction liquid for detecting Coxsackie A16, and four LAMP primers and LAMP reaction liquid for detecting Enterovirus 71. Tests prove that the assay kit has the advantages of good specificity and sensitivity, rapid amplification rapid, high efficiency and simple identification. A detection system provided by the invention can rapidly, conveniently, efficiently, high specifically and high sensitively detect Coxsackie A16 and Enterovirus 71 without complex apparatuses thus can satisfy well clinical detection requirements of hand, foot and mouth disease, and is suitable for a large-scale promotion and an application.
Owner:上海吉美生物工程有限公司
Application of two nitrogen-containing heterocyclic ester compounds to preparation of drugs with resistance to enterovirus 71
ActiveCN106822120ALow priceEasy to buyOrganic active ingredientsAntiviralsAntiviral drugCytopathic effect
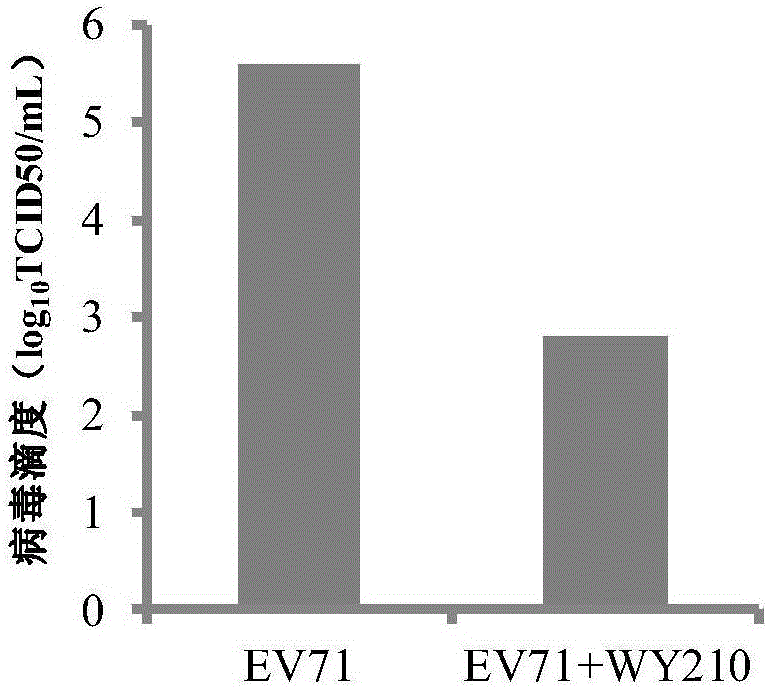
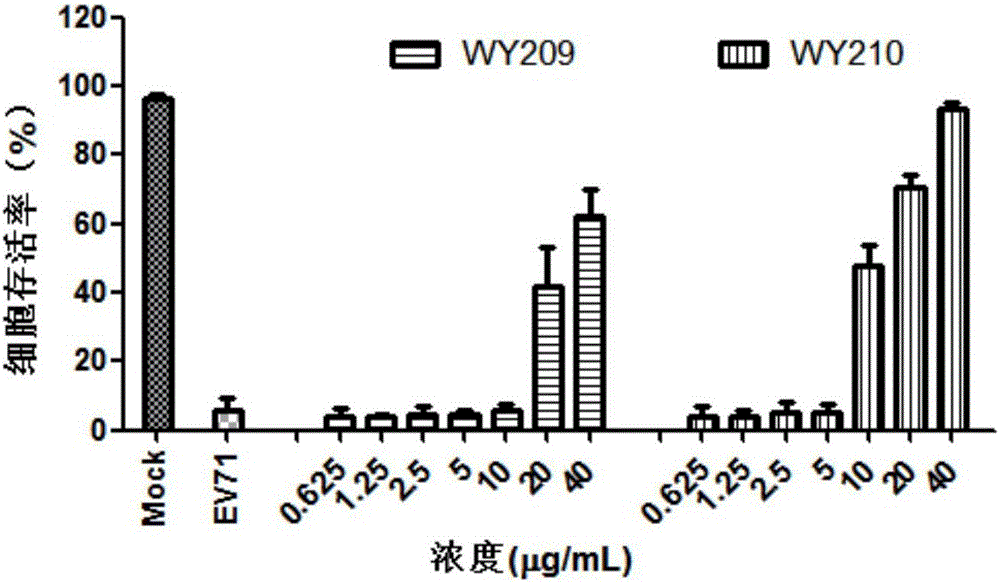
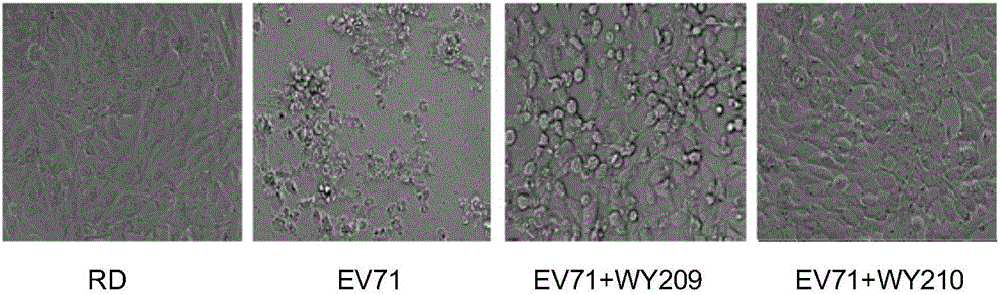
The invention belongs to the field of antiviral drugs and provides application of two nitrogen-containing heterocyclic ester compounds to preparation of drugs with resistance to enterovirus 71 (EV71). The nitrogen-containing heterocyclic ester compounds are compounds expressed as formulas WY209 and WY210. Through research experiment of inhibition of novel nitrogen-containing heterocyclic ester compounds on activity of EV71, WY209 and WY210 can inhibit cytopathic effect (CPE) generated by EV71 in host cells RD; the cell survival rate is increased; the progeny virus yield is reduced; the apoptosis of the host cells caused by infection of EV71 can be inhibited; the application shows that the nitrogen-containing heterocyclic ester compounds have potentials for preparing the drugs with resistance to the EV71 virus; the synthesis process of the compounds is simple, economical and rapid; the large-scale production of the compounds is easy to implement; the compounds have clinical application prospects.
Owner:HUBEI UNIV OF TECH
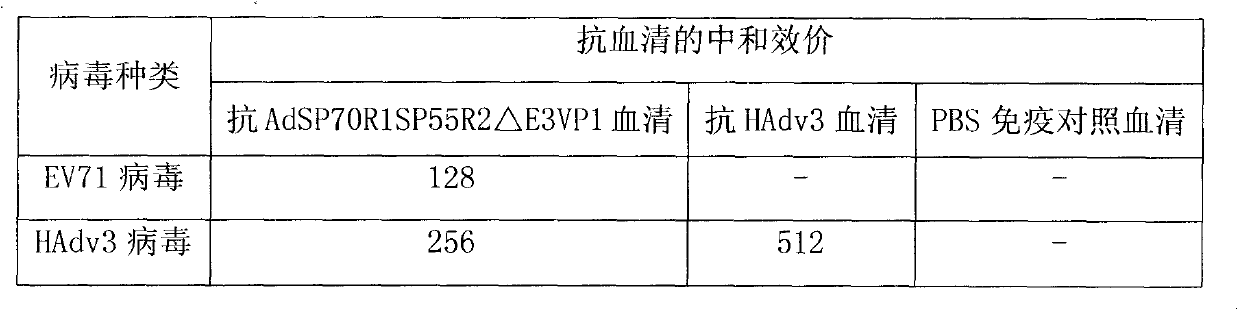
Recombinant human adenovirus 3, and preparation method and application thereof
InactiveCN103966263ARetain major antigenic activityAvoid infectionMicroorganism based processesFermentationEnterovirus 71Genome
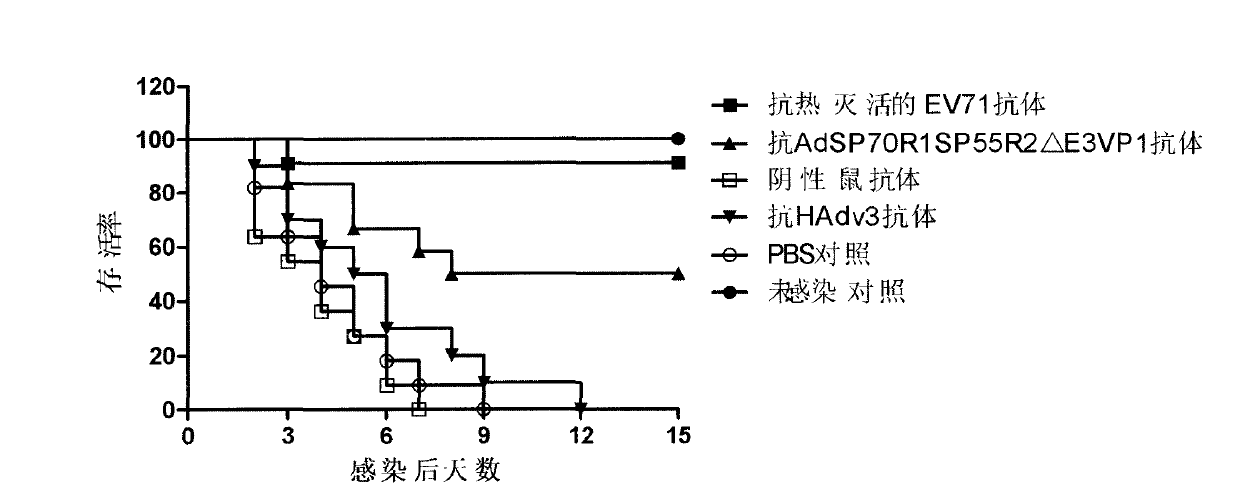
The invention discloses a novel enterovirus 71-recombinant human adenovirus 3 vaccine candidate strain with human adenovirus 3 (HAdv3) as a carrier, and a preparation method thereof. Two EV71 neutralizing epitopes are embedded to the hexon of the human adenovirus 3, and the VP1 protein cassette of EV71 is inserted to the genome E3 region of the human adenovirus 3. The vaccine candidate strain can induce a strong anti-EV71 infection and anti-HAdv3 infection immunization reaction, and can be used for making bivalent vaccines for preventing the EV71 infection and the HAdv3 infection.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +1
Sequence of enterovirns type71 genome and uses thereof
InactiveCN101509003AMicroorganism based processesImmunoglobulins against virusesData informationGenetics
The invention provides an enterovirus 71 complete genome sequence, comprising a nucleotide sequence shown by SEQ ID NO:1. The sequence can be used for preparing a vaccine for preventing the enterovirus 71 and a neutralizing antibody for resisting the enterovirus 71, provides impersonal basis for further acquiring EV71 genetic derivation in South China, provides reliable data information for developing diagnostic reagents and vaccines of enzyme-linked immunosorbent assay (ELISA) for outbreak people in different areas of China, and provides valuable resource for deeply studying genetic variation rules of EV71 and mechanism causing hand-foot-and-mouth disease.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Gene recombinant vaccine for preventing enterovirus 71 infection and preparation method thereof
The invention discloses a gene recombinant vaccine for preventing enterovirus 71 (EV71) infection and a preparation method thereof. The inflection comprises multiple diseases related with the nervous system such as hand-foot-and-mouth disease, paralytic diseases of sterile meningitis, cephalitis and poliomyelitis and the like. Escherichia coli labile enterotoxin B subunit (LTB) is used as an immunological enhancement adjuvant, two fragments of linear neutralizing epitope SP55 and SP70 in EV71 virus coat protein VP1 are used as antigens, prokaryotic expression plasmids of LTB-SP55-SP70 fusion genes are constructed by using gene engineering technology, the plasmids are expressed in escherichia coli, and a recombinant expression product is purified for preparing the EV71 virus gene engineering vaccine.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
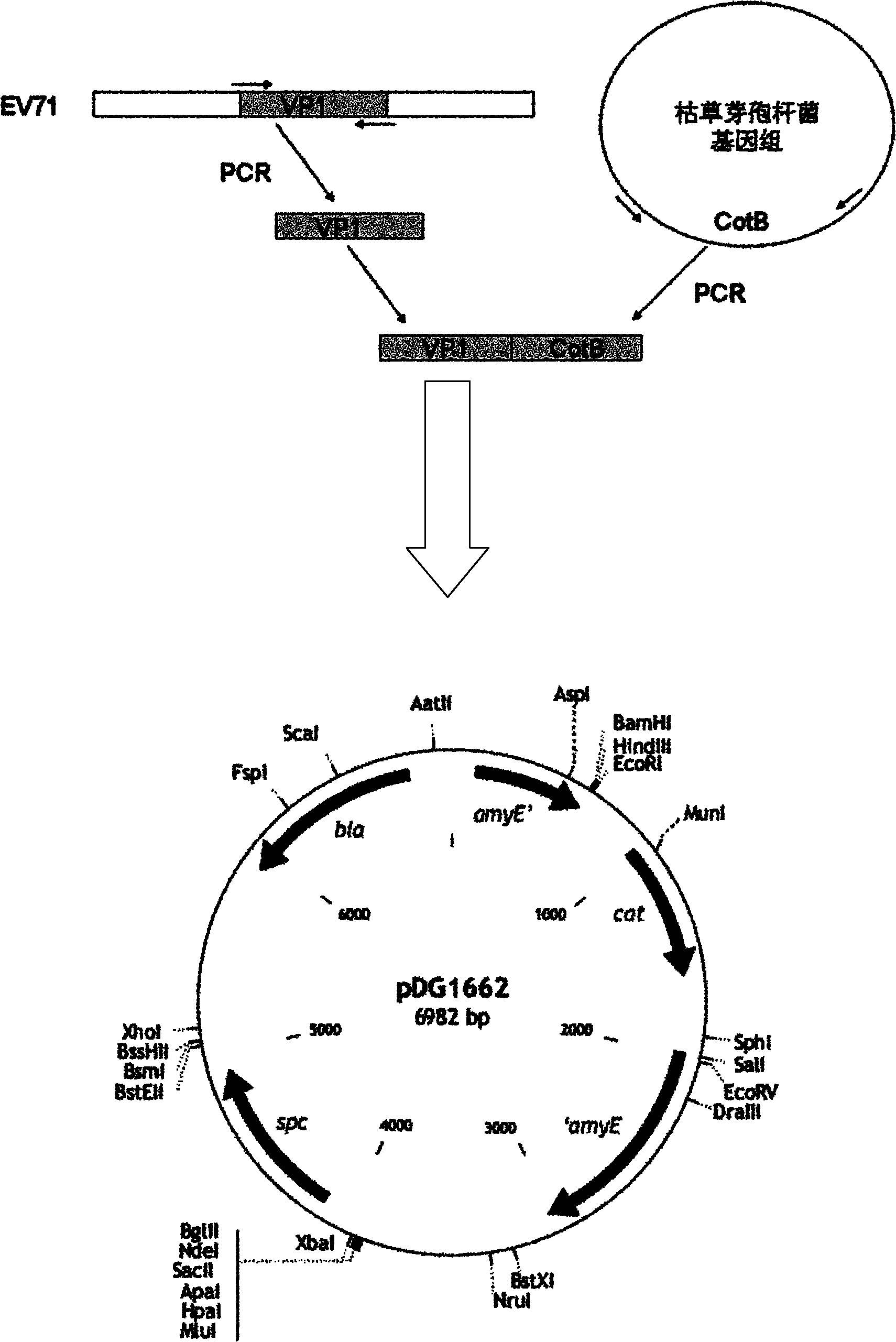
Recombinant bacillus subtilis EV71-VP1 expression vector and preparation method and application thereof
InactiveCN102010876AImprove securityNot infectiousBacteriaMicroorganism based processesEscherichia coliAntigen
The invention discloses a recombinant bacillus subtilis EV71-VP1 expression vector, the vector is an escherichia coli-bacillus subtilis shuttle expression vector, and a coding gene of capsid protein VP1 of EV71 virus is loaded on the vector. The construction method is as follows: (1) amplifying CotB gene regulatory sequence and coding sequence of bacillus subtilis, connecting to a pMD18T vector, and obtaining plasmid pDG1662-CotB; and (2) amplifying enterovirus 71 type capsid protein VP1 antigen sequence, connecting to the plasmid pDG1662-CotB, and obtaining plasmid pDG1662-CotB-VP1 which is the recombinant bacillus subtilis EV71-VP1 expression vector. After introducing the recombinant vector into host cells for culturing, oral vaccines for preventing or treating diseases caused by EV71 can be further prepared.
Owner:INST OF BASIC MEDICINE OF SAMS
Method for establishing enterovirus 71-type intraperitoneal inoculation infection BALB/c suckling mouse model
The invention discloses a method for establishing an enterovirus 71-type (EV71) intraperitoneal inoculation infection BALB / c suckling mouse model. The EV71 infection mouse model is prepared by intraperitoneally injecting virus liquid into a BALB / c suckling mouse. The virus liquid is prepared by the following steps of: inoculating EV71 strains into MA104 cells, performing culture at the temperature of 37 DEG C by using 5 percent carbon dioxide (CO2), and observing the pathological change conditions of the cells every day, wherein on the next day of the EV71 inoculation, agglutination and shrinkage occur on 70 percent of the MA104 cells; and after freeze-thawing the whole bottle of cells, centrifugally taking supernatant to obtain the EV71 virus liquid. By the method, a stable enterovirus EV71 intraperitoneal inoculation infection BALB / c suckling mouse model is established successfully; and foundation is laid for scientific researchers to research EV71 etiology, an EV71 pathogenic mechanism, the screening of high-efficiency anti-virus medicines and the preparation of vaccines. The method has a wide application prospect.
Owner:INST OF BASIC MEDICINE OF SAMS +1
Alkaloid dimer, preparation method thereof and application of alkaloid dimer as antiviral agent
ActiveCN104876945AStrong inhibitory activityOrganic active ingredientsOrganic chemistryEthyl acetateAlkaloid
The invention discloses an alkaloid dimer, a preparation method thereof and application of the alkaloid dimer as an antiviral agent. The method comprises the following steps: firstly, carrying out strain culture on fungus Pestalotiopsis sp.(ZJ-2009-7-6) during preparation, and carrying out fermental cultivation on the fungus; leaching the obtained mycelium with a chloroform-methanol mixed liquid (1 to 1) for three times, carrying out vacuum concentration, and extracting with ethyl acetate for three times, so as to obtain crude extractum; and sequentially carrying out positive slica column chromatography, Sephadex LH-20 gel column chromatography and HPLC (high-performance liquid chromatography) on ethyl acetate-phase crude extractum to obtain a compound shown in a formula I. The antiviral agent provided by the method has the characteristics that the compound shown in the formula I or a pharmaceutically acceptable salt is taken as an effective component for preventing and / or treating diseases caused by herpes simplex virus I (HSV-1) and / or enterovirus 71 (EV71).
Owner:OCEAN UNIV OF CHINA
Enterovirus 71 antigen detection test strip (colloidal gold method)
InactiveCN102243237AImprove featuresHigh sensitivityMaterial analysisCase fatality ratePulmonary edema
The invention relates to the field of biomedicine, and specifically relates to an enterovirus 71 antigen detection test strip (colloidal gold method) and a preparation method and application thereof. Enterovirus 71 can cause hand-foot-and-mouth disease, which has largegeneration proportion of severe infections (viral encephalitis, meningomyelitis virus and pulmonary edema), and a high death rate reaching 10%-25%. The test strip of the invention is used for rapid diagnosis of EV(enterovirus)71 infection. A virus separation and an RT-PCR (reverse transcription-polymerase chain reaction) are methods first used for EV71 antigen detection, but are not suitable for primary clinic usage due to defects of difficult operation and high costs, etc. The invention overcomes the above insufficiencies and provides a reagent, which is highly demanded in clinic detection, simply operated, suitable for various medical disease control sections, and capable of detecting EV71 antigens in human oropharyngeal swabs, bubble liquid, serum or excrement, and also provides the preparation method and application thereof. A technical scheme is as follows: a specimen is dropped on a sample pad, and the EV71 antigen wherein combines with a gold-labeled EV71 polyclonal antibody in a gold-labeled pad and migrates along a chromatography membrane. A detected line captures colloidal gold particles to form a red line visible to naked eyes, so as to realize detection of the EV71 antigen.
Owner:BEIJING BEIER BIOENG
Application of matrine to medicament for treating or preventing enterovirus 71 type infection
InactiveCN102240286AAnti-infectionAnti-inflammatoryOrganic active ingredientsAntipyreticAnti virusTreatment effect
The invention discloses application of matrine to a medicament for treating or preventing enterovirus 71 type infection. The invention proves that the matrine has good anti-virus effect in an in-vitro enterovirus 71 type (EV71) infection cell test. The matrine has certain killing effect on the EV71, can prevent EV71 infection, has good treatment effect on cells infected with EV71, and has good inhibition effect on virus replication, wherein the action effect is more remarkable than that of a positive control medicament ribavirin. In addition, the matrine has good inhibition effect on inflammatory reaction caused by EV71, so the medicament has a prospect of being developed into an anti-EV71 medicament.
Owner:WUHAN UNIV
Combined vaccine for preventing hand-foot-mouth disease
ActiveCN105963692AEnsure safetyNo intellectual property barriersSsRNA viruses positive-senseViral antigen ingredientsHand-foot-and-mouth diseaseFiltration
The invention provides a combined vaccine for preventing a hand-foot-mouth disease. The combined vaccine contains an inactivated EV71 (Enterovirus 71) antigen, an inactivated CA16 (Coxasckievirus A16) antigen and an inactivated CA10 (Coxasckievirus A10) antigen. The hand-foot-mouth disease preventing combined vaccine with good immunogenicity, high safety and high stability is prepared by culturing main epidemic virus strains, namely an EV71 type, a CA16 type and a CA10 type, which cause the hand-foot-mouth disease, performing ultrafiltration concentration, performing sucrose density gradient centrifugation, performing ultrafiltration desugarization and performing sterilization filtration to obtain a purified virus solution, and further preparing the virus solution into the combined vaccine. The combined vaccine provided by the invention can prevent infection of a variety of enteroviruses to a human body at the same time, and a phenomenon of mutual interference among the antigens does not exist and the corresponding immunogenicity is not lower than that excited by a single antigen; after use of the combined vaccine provided by the invention, a vaccinating procedure can be significantly simplified, the vaccinating efficiency is improved and the cost is reduced.
Owner:SINOVAC BIOTECH
Structure and application of Enterovirus 71 3C protease
The invention discloses a structure and application of an Enterovirus 71 (EV71) 3C protease and belongs to the field of RNA virus protein. The invention also discloses an application of the 3C protease and a substrate binding groove thereof in drug design. The protein provided by the invention has a special substrate binding groove and new EV71 virus 3C protease drugs are designed according to the spatial structure of the substrate binding groove, thus the inhibitors aiming at the EV71 virus 3C protease, which are more specific and have better effect, can be obtained, potential drug candidates can be provided for the clinical treatment of hand, foot and mouth disease and the EV71 virus 3C protease has very high application value.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Anti-enteroviral 71(EV71) 4-iminooxazolidine-2-ketone compound as well as preparation method and application of anti-enteroviral 71(EV71) 4-iminooxazolidine-2-ketone compound
The invention discloses a 4-iminooxazolidine-2-ketone enterovirus 71(EV71) 3C protease inhibitor of which the structural general formula is shown as a compound (M), each variable in the structure is defined as the specification, and the compounds are used for effectively inhibiting or stopping the replication of enterovirus 71. The invention relates to discovery and application of a compound with a structure shown as the formula (M) as well as various optical isomers of the compound, a pharmaceutically active metabolite, a medicinal salt, a solvate and a prodrug of the compound in preparation of antiviral drugs for treating hand-foot-mouth virus infection diseases and also relates to an intermediate for preparing the compound with the structure shown as the formula (M) and a synthesis method.
Owner:NANKAI UNIV
Fusion protein for screening and evaluating anti-enterovirus 71 medicine and application of fusion protein
InactiveCN102766607AControl expression efficiencyEasy to observe directlyMicrobiological testing/measurementMicroorganism based processesFluorescenceC-terminus
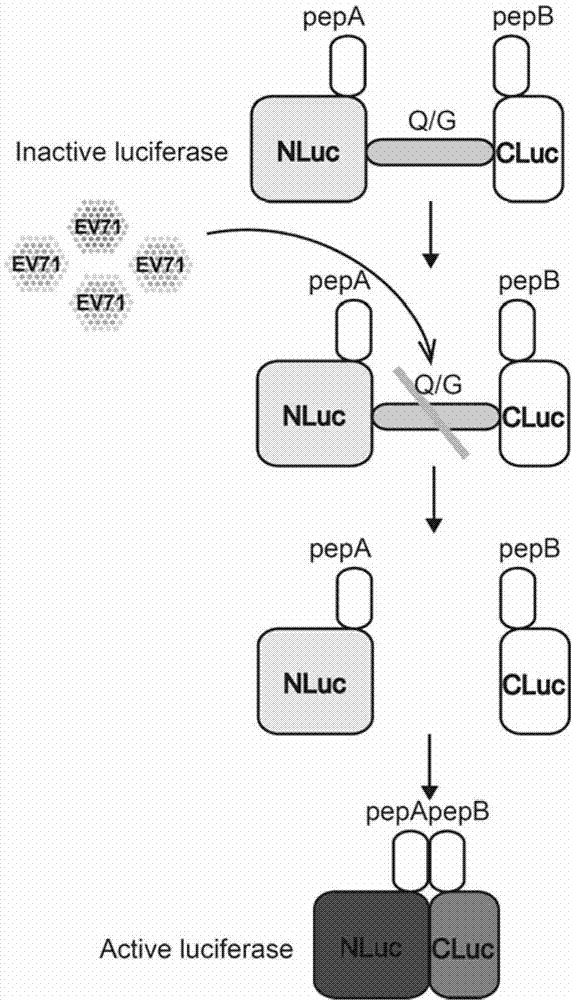
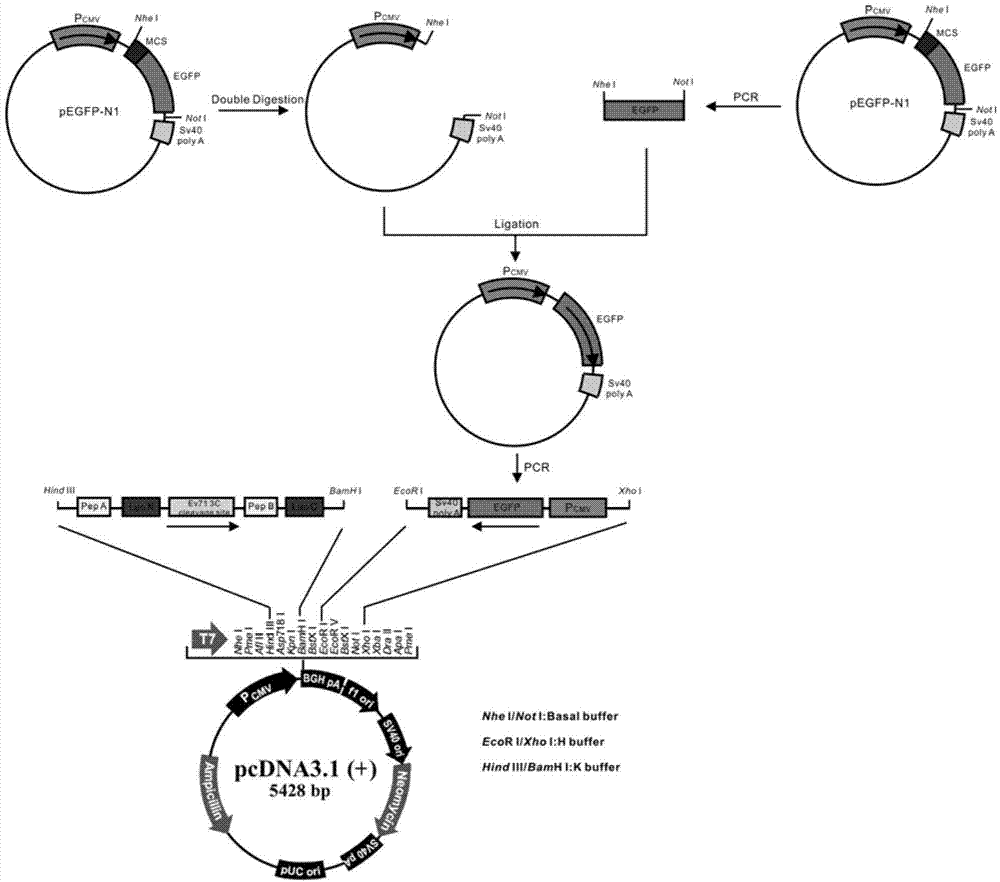
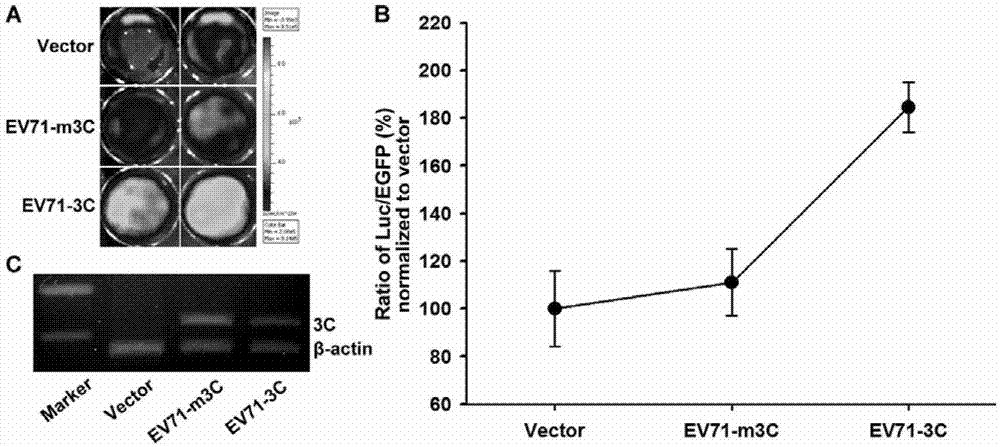
The invention discloses fusion protein for screening and evaluating an anti-enterovirus 71 (EV 71) medicine and application of the fusion protein. According to the fusion protein, firefly luciferase (Fluc) is used as a reporter gene, two micromolecule polypeptides which can be bonded tightly pepA and pepB are bonded with the N end and the C end of the firefly luciferase respectively, the middles of the firefly luciferase are connected by an EV713C protease effect substrate, and a fusion gene is inserted into an eukaryotic expression vector and is expressed to generate the fusion protein. Simultaneously, enhanced green fluorescent protein (EGFP) which is inserted reversely can indicate the transfection efficiency of the EGFP in cells which are transfected in vitro by observing the condition of green fluorescence. By utilizing an indication vector provided by the fusion protein, the reproduction condition of EV 71 can be indicated simply, quickly, flexibly and quantitatively, and the indication vector also can be applied to the screening and evaluation of the anti-EV 71 medicine, has high actual application value and has a broad application prospect in the field of medical science.
Owner:HARBIN MEDICAL UNIVERSITY
Enterovirus 71 type latex agglutination detection kit, preparation and application
The invention relates to an enterovirus 71 type latex agglutination detection kit, preparation and application. The kit comprises the following substances: (1) a carboxylated latex reagent sensitizing a prokaryotic expression enterovirus 71 type VP1 specific antigen; (2) a carboxylated latex reagent sensitizing three strains of monoclonal mixed antibodies aiming at enterovirus 71 type VP1; (3) enterovirus 71 type positive-negative standard serum, an inactivated virus solution and PBS; and (4) a toothpick or a plastic cement rod which contains a glass slide platform for using the sentization latex to perform aggregation reaction and is used to uniformly mix latex and to-be reacted serum. The kit can detect EV71 antigen in samples of different sources, and overcomes the disadvantages that the sensitized antigen is less in amount and unstable, sensitized protein is easy to fall off, and the like when protein sensitizes common latex. The detection method is applicable to enterovirus 71 type serum epidemiological investigation, clinic assistant diagnosis, regulation and control on EV71 virus replication, anti-virus therapy medicine screening and other scientific research fields.
Owner:绍兴市疾病预防控制中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com