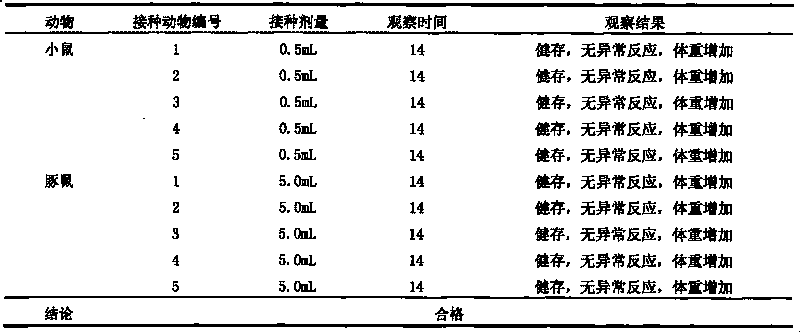
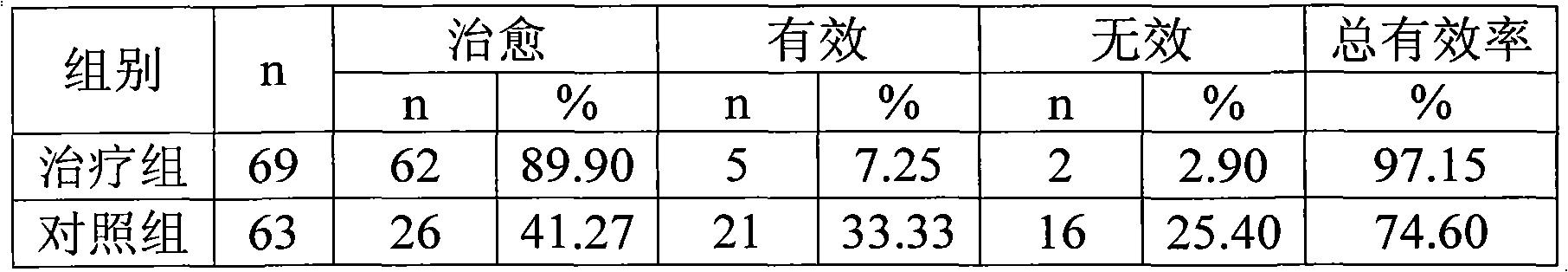
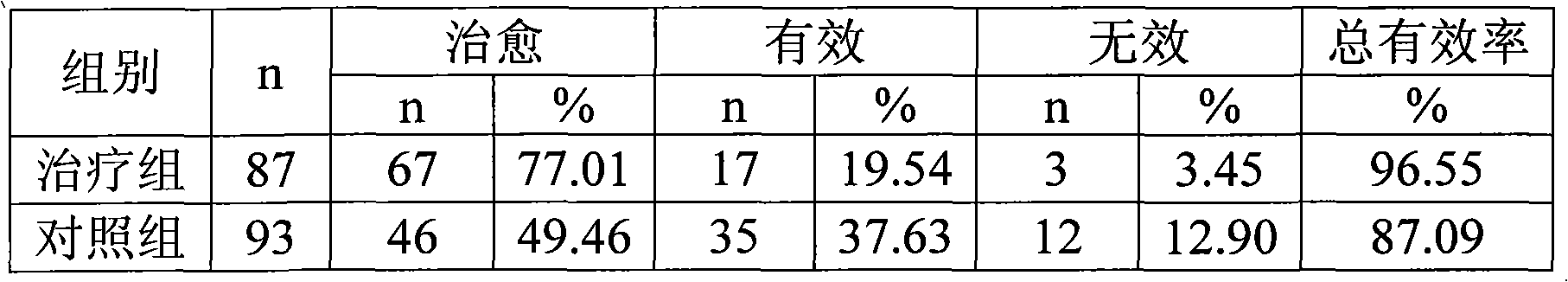
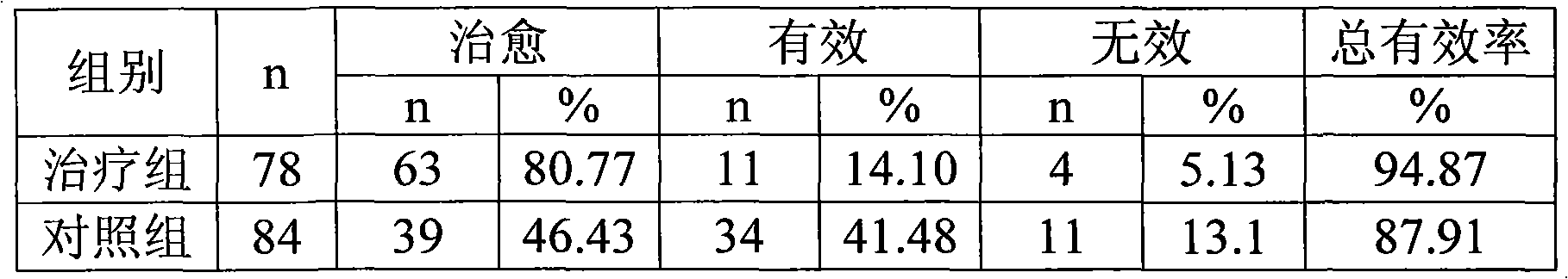
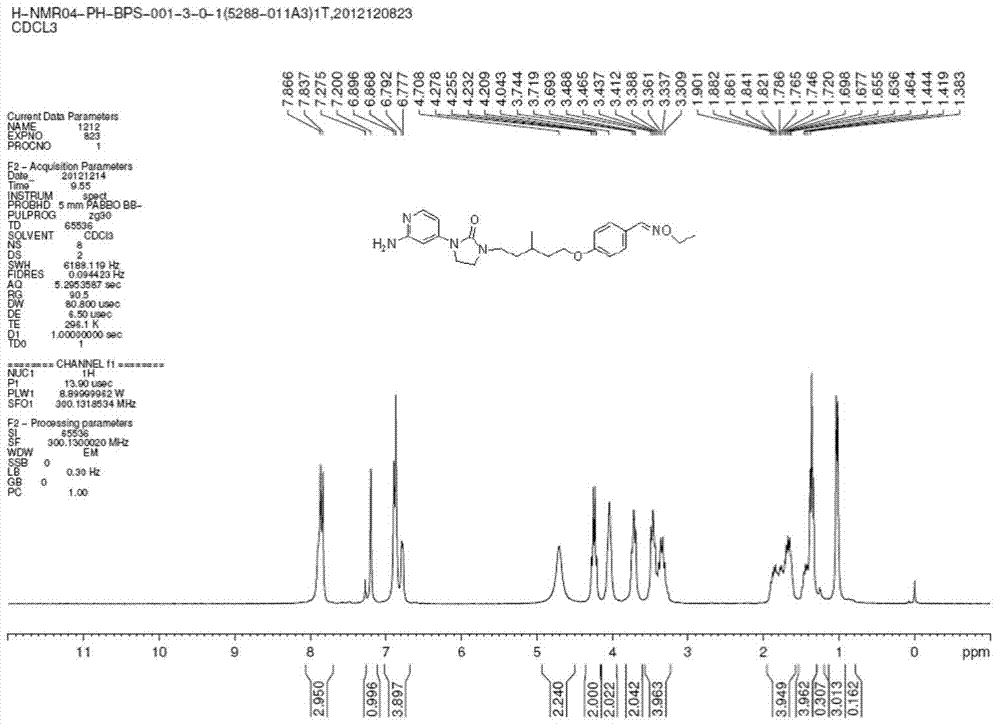
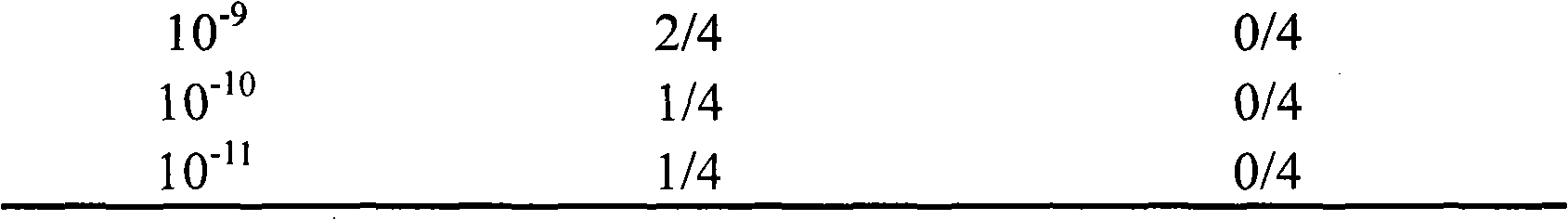Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
402 results about "Hand-foot-and-mouth disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A mild, contagious viral infection caused by coxsackie virus.
Application of Chinese medicinal composition in preparation of medicament for treating hand-foot-and-mouth disease
ActiveCN101637529AEffective treatmentHydroxy compound active ingredientsAntiviralsAnti virusHand-foot-and-mouth disease
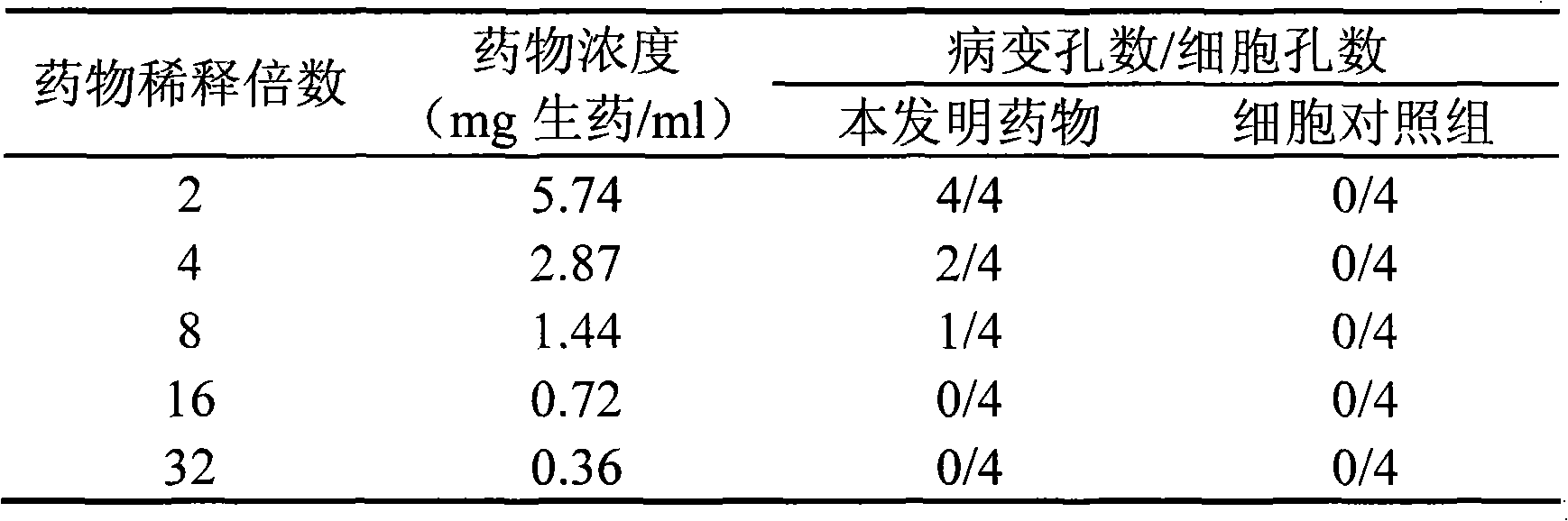
The invention discloses application of Chinese medicinal composition in preparation of medicament for treating hand-foot-and-mouth disease. The Chinese medicinal composition comprises weeping forsythiae capsule, honeysuckle flower, ephedra herb, bitter apricot seed and the like. The Chinese medicinal composition has the function of broad-spectrum anti-virus, and can effectively treat hand-food-and-mouth disease by effectively killing virus, eclipsing fever and diminishing inflammation.
Owner:BEIJING YILING PHARMA
Univalent and bivalent inactivated vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695570AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
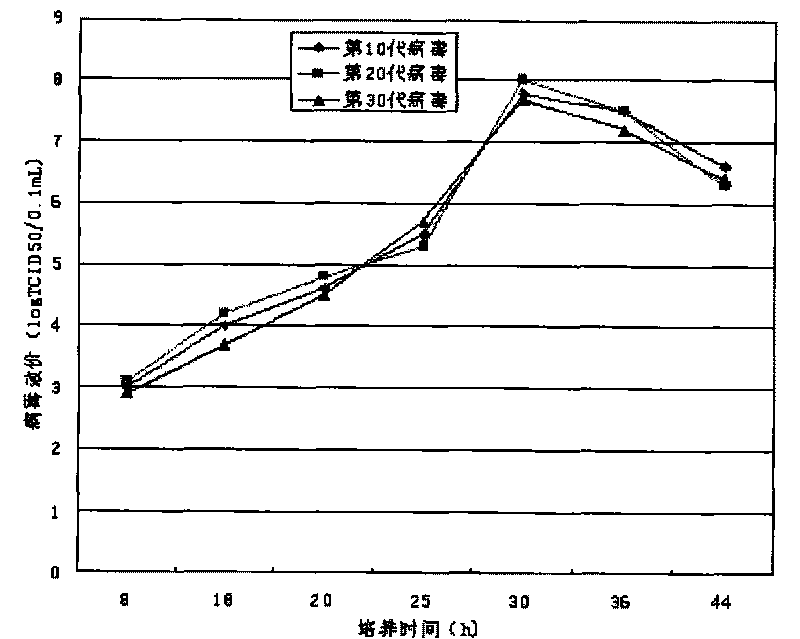
The invention discloses a univalent and bivalent inactivated vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: screening vaccine strains for the hand-foot-and-mouth disease; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; collecting virus suspension; inactivating the virus; ultra-filtering, concentrating and purifying the virus suspension to obtain vaccine stock solution; and finally preparing the univalent and bivalent inactivated vaccine. The univalent and bivalent inactivated vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Diagnostic reagent kit (enzyme-linked immunosorbent assay (ELISA)) for enterovirus (EV) 71-type antibody (immune globulin M (IgM))
InactiveCN102243232AEasy to operateAvoid distractionsColor/spectral properties measurementsEnterovirusAbzyme
The invention relates to the field of biomedicine, in particular to an enzyme-linked immunization diagnostic reagent kit for detecting an enterovirus (EV) 71-type antibody (immune globulin M (IgM)), and a preparation method and application of the diagnostic reagent kit. The probability of hand-foot-and-mouth disease and severe infection (viral encephalitis, viral cerebrospinal meningitis and pulmonary edema) caused by EV71 type is relatively higher, and case fatality rate is relatively higher and can be 10 to 25 percent. The enzyme-linked immunization diagnostic reagent kit of the EV71-IgM antibody can be used for diagnosing the infection of the EV71 type. According to related documents about the detection of the EV71-IgM, EV71 virus cultures serving as indirect enzyme-linked immuno sorbent assay (ELISA) of envelope antigens has defects in such aspects as specificity, sensitivity and stability, and due to high cultivation cost and low efficiency, a large amount of virus cannot be supplied to the market. In order to overcome the defects, the invention provides the reagent kit which is used for detecting the EV71-IgM in human blood serum, required by clinical examination, simple and convenient to operate and applicable to all medical disease control departments, and the preparation method and the application of the reagent kit. The invention has the technical scheme that: firstly, the human blood serum is added into a micro-pore plate, wherein the IgM antibody is obtained by an anti-mu chain which is pre-enveloped on the micro-pore plate, and other uncombined components are washed and removed; secondly, an enzyme labeling object is added, the EV71-IgM in the obtained IgM can be combined with the specificity of an EV71 recombinant antigen which is labeled by horse radish peroxidase (HRP), and after washing, the HRP can react with substrates which are added subsequently; and finally, the aim of detecting the EV71-IgM antibody is fulfilled.
Owner:BEIJING BEIER BIOENG
Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695569AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
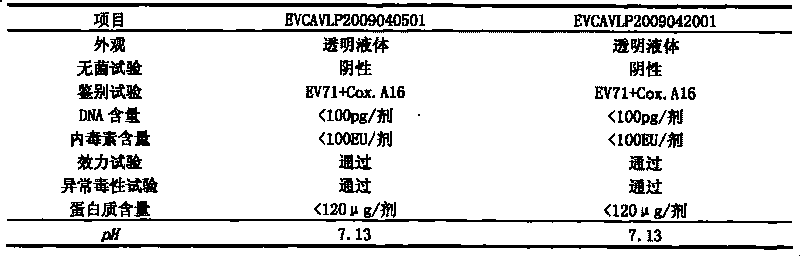
The invention discloses a univalent and bivalent gene engineered subunit vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: respectively obtaining recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD by gene engineering means, respectively efficiently coexpressing similar SeQ ID No.1 EV71 P1 and Se Q ID No.2 Cox.A16 P1 and 3CD proteins in insect cells, and respectively self-assembling into EV71 VLP and Cox.A16 VLP; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; lysing the cells, ultra-filtering and purifying virus suspension; and further preparing the univalent and bivalent vaccine. The vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
American cockroach medicinal composition for curing ulcers or ulcerative inflammation and preparation method thereof
InactiveCN101837018AEasy to prepareThe effective cure rate is remarkableAnthropod material medical ingredientsDigestive systemUlcer careAmerican cockroach
The invention discloses an American cockroach medicinal composition for curing ulcers or ulcerative inflammation. The medicinal composition is mainly prepared from an active ingredient mixture prepared by mixing an American cockroach extract and montmorillonite powder. The weight part ratio of the American cockroach extract to the montmorillonite powder is equal to 0.8-1.2:0.5-6. A preparation method of the American cockroach medicinal composition for curing the ulcers or the ulcerative inflammation comprises the following steps of: preparing the American cockroach extract, mixing active ingredients, and preparing medicaments. The medicinal composition has simple preparation method, fully exerts effects of the American cockroach extract, and has obvious effects of effectively curing diseases such as oral ulcers, recurrent aphthous ulcers, oral lichen planus, oral mucosal injury (behcet disease, herpes simplex, leukoplakia, discoid lupus erythematosus, hand-foot-and-mouth disease, scalds, chemotherapy, radiotherapy and the like) caused by various reasons, reflux esophagitis, eophageal ucers, radiation esophagitis, ulcerative colitis, radiation proctitis, and diarrhea, and hematochezia with mucosal injury, and the like.
Owner:耿福能
ALD and application thereof as EV71 virus and CAV16 virus inhibitor
The invention discloses an ALD derivative or pharmaceutically acceptable salts thereof, and further provides application of the ALD derivative or the pharmaceutically acceptable salts thereof as EV71 virus and CAV16 virus inhibitors and application of the ALD derivative or the pharmaceutically acceptable salts thereof in preparing medicines for treating hand-foot-and-mouth diseases. The structural formula of the ALD derivative is as shown in the specification. The invention further provides a method for preparing the ALD derivative or the pharmaceutically acceptable salts thereof.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Method for determining cross antigen region initiating human antigens in human enterovirus 71-type total protein
The invention belongs to the field of biomedicine, and provides a method for determining a cross antigen region initiating human antigens in human enterovirus 71-type (EV71) total protein. In the method, the human enterovirus 71-type total protein is identified through segmental expression; and cross reaction between immunogenic and inductive antibodies and the human antigens in each segment is performed, and different segments of the human enterovirus 71-type total protein are divided into three classes: (1) strongly-crossed immunogens initiating the human antigens; (2) weakly-crossed immunogens initiating the human antigens; and (3) no crossed immunogens existing between the antibodies and the human antigens. Thus, the method plays a guidance role in developing and preparing human enterovirus 71-type vaccines, and hand-foot-and-mouth disease vaccines having no cross reaction or weak cross reaction with human bodies can be designed according to the method.
Owner:INST OF LAB ANIMAL SCI CHINESE ACAD OF MEDICAL SCI
Preparation method for recombinant coxsackie virus A16 like particle and applications thereof
InactiveCN102533797AIncrease productionFungiInactivation/attenuationYeastHand-foot-and-mouth disease
The invention discloses a preparation method for a recombinant coxsackie virus A16 like particle, which comprises the following steps: (1) cloning a P1 gene and a 3CD gene of a coxsackie virus A16 to a target plasmid to obtain a recombinant expression vector; (2) transforming a target yeast cell by using the recombinant expression vector obtained in the step (1) to obtain a recombinant yeast cellfor expressing the P1 gene and the 3CD gene; and (3) cracking the recombinant yeast cell obtained in the step (2), and separating to obtain the recombinant coxsackie virus A16 like particle. The recombinant coxsackie virus A16 like particle can be prepared in a yeast expression system by the method provided by the invention. Compared with a wild-type P1 gene and a wild-type 3CD gene, the yield ofthe coxsackie virus A16 like particle in the yeast expression system is greatly increased through the optimization of codons of the P1 gene and the 3CD gene, and the recombinant coxsackie virus A16 like particle can be further used for producing candidate preventive vaccines and pharmaceutical compositions for infant hand-foot-and-mouth diseases.
Owner:BEIJING UNIV OF TECH
Fluorescent quantitative kit for detecting coxsackie viruses A6 and A10
The invention provides a fluorescent quantitative kit for detecting coxsackie viruses A6 and A10. The fluorescent quantitative kit is composed of a quantitative reverse transcription-polymerase chain reaction (RT-PCR) reaction liquid, enzyme mixed liquor, primer probe mixed liquor, CVA6 and CVA10 standard substances, CVA6 and CVA10 strong positive reference substances, CVA6 and CVA10 weak positive reference substances, and a negative reference substance. Existence of CVA6 and CVA10 is simultaneously detected from a stool specimen by adopting a one-step real-time fluorescent quantitative RT-PCR technology, and CVA6 and CVA10 primers and fluorescently-labeled probes with high specificity, compared with a single fluorescent quantitative PCR method, the fluorescent quantitative kit is more convenient and faster, real-time in detection, and accurate to quantitate; the cost is saved; early diagnosis is provided clinically; a reference frame is provided for formulation of a clinical treatment scheme; the fluorescent quantitative kit can be applied to laboratory emergency diagnosis, rapid screening and clinical diagnosis of epidemic outbreak caused by coxsackie viruses A6 and A10, and research of epidemiology of a hand-foot-and-mouth disease.
Owner:ZHEJIANG UNIV
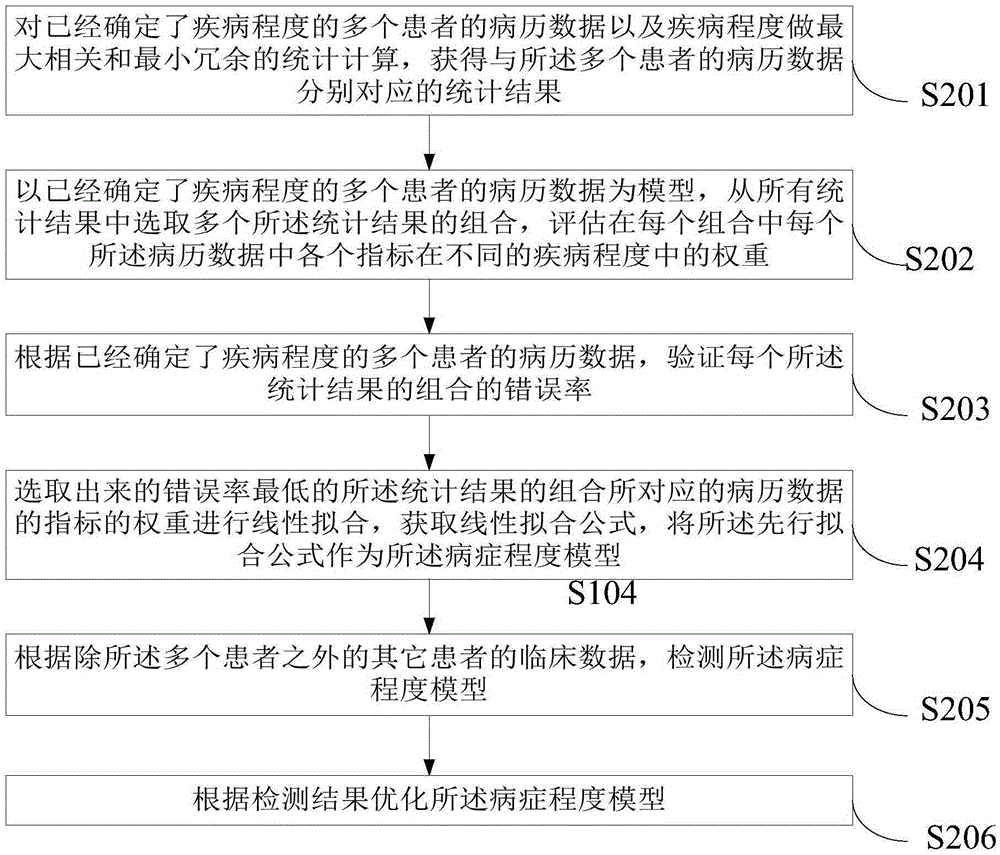
Medical record data processing method, apparatus and system
ActiveCN105389465AReduce delaysMedical data miningSpecial data processing applicationsHand-foot-and-mouth diseaseMedical record
Owner:徐翼 +1
Enterovirus type 71 and use thereof
ActiveCN101717754AViral/bacteriophage medical ingredientsHybrid cell preparationEnterovirusHand-foot-and-mouth disease
The invention relates to an enterovirus type 71 (abbreviated as EV71) and use thereof, in particular to a monoclonal virus strain which is separated by a plaque assay method. The progeny viruses of the monoclonal virus strain show genetic stability. The monoclonal virus strain can be used in the production of a vaccine (particularly the production of a vaccine for infant). The virus strain or the vaccine produced by using the same can be used in the prevention of diseases (such as hand-foot-and-mouth diseases, particularly hand-foot-and-mouth diseases in children) caused by the EV71 and has the characteristics of stable titer, high immunogenicity and small immunizing dose.
Owner:SINOVAC BIOTECH
Anti-enterovirus 71 (EV 71) caprolactam aldehyde compound and preparation method and purpose thereof
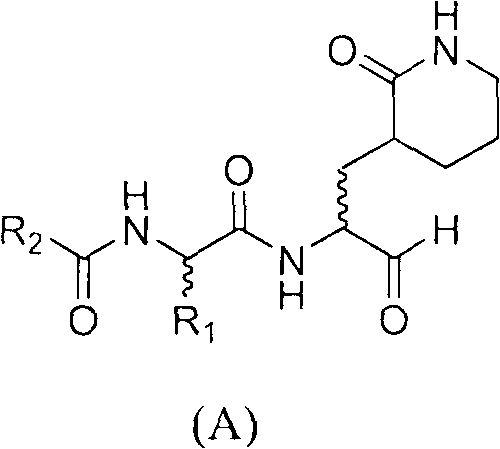
A structure general formula of a caprolactam aldehyde enterovirus 71 (EV 71) 3C protease inhibitor is as shown in chemical combination A, all variables in the structure are defined in an instruction book, and the compound effectively restrains or blocks the copy of the enterovirus 71. The invention relates to a compound with the structure as shown in formula (A), various types of optical isomers of the compound with the structure as shown in formula (A), drug active metabolites, officinal salt, solvate and discovery and application when the compound with the structure as shown in formula (A), the various kinds of optical isomers of the compound with the structure as shown in formula (A), the drug active metabolites and the officinal salt are used for preparing antiviral drugs for curing hand-foot-mouth virus infection diseases. The invention further relates to an intermediate and a synthetic method of preparing the compound with the structure as shown in formula (A).
Owner:NANKAI UNIV +2
Agarose compatible medium for purifying immune globulin of hand-foot-and-mouth disease and preparation method thereof
ActiveCN101602805AEfficient purificationTaller than aliveImmunoglobulins against virusesPeptide preparation methodsHand-foot-and-mouth diseasePurification methods
The invention provides an agarose compatible medium for purifying immune globulin of the hand-foot-and-mouth disease and a preparation method thereof. In the agarose compatible medium, agarose gel is used as a matrix, is activated by an epoxy activating agent, and is coupled with vaccine of the hand-foot-and-mouth disease which is used as petunidin, wherein the agarose gel is made of one of agarose 6FF, agarose 4FF, agarose CL-6B, agarose CL-4B, agarose 6B and agarose 4B. The agarose compatible medium can be used for purifying the immune globulin of the hand-foot-and-mouth disease, has the advantages of good medium selectivity, strong specificity, strong mechanical strength and industrial amplification benefit. Furthermore, the preparation process is simple and is easy to amplify. The medium prepared by the method is adopted for purifying the immune globulin of the hand-foot-and-mouth disease, and has simple and feasible purification method and good separation effect.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
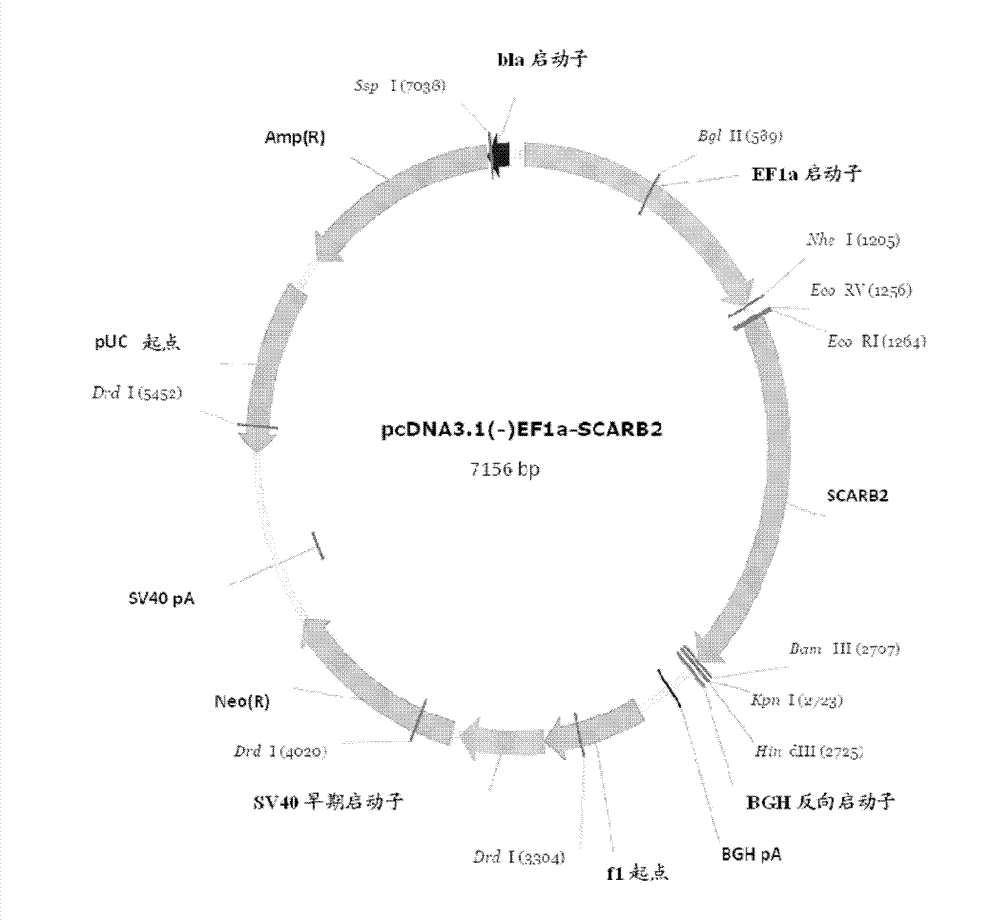
Manufacture of hSCARB 2 transgenic mouse and application of the transgenic mouse as enterovirus infection animal model
InactiveCN103081868AAnimal reproductionMicrobiological testing/measurementHand-foot-and-mouth diseaseEnteroviral infections
The present invention concerns the manufacture of EV71 acceptor SCARB 2 transgenic mouse. The manufactured SCARB 2 transgenic mouse can be used as an animal model infected with the hand-foot-and-mouth disease, such as infected with enterovirus 71 or Coxsackies virus A16, so as to allow for estimation of enterovirus viral vaccine immune protection efficacies and application of enterovirus infection in pathological study.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Chinese medicinal composition for preventing and treating hand, foot and mouth disease in children
The invention relates to a Chinese medicinal composition for preventing and treating hand, foot and mouth disease in children and belongs to the technical field of the traditional Chinese medical science and Chinese medicines. The Chinese medicinal composition is prepared from the following Chinese herbal medicines by weight: 10g of towel gourd vegetable sponge, 30g of rhubarb, 10g of Chinese gall, 35g of common cnidium fruit, 30g of calamine, 25g of salted jellyfish, 5g of wrinkled gianthyssop herb, 4g of gypsum rubrum, 15g of cassia twig, 20g of incised notopterygium rhizome, 18g of heracleum, 15g of clematis root, 10g of bark of oriental variegated coralbean, 8g of fructus amomi, 8g of katsumade galangal seed, 20g of coix seed, 15g of hypericum japonicum, 10g of abrus herb, 12g of borax and 10g of Spanish fly. The Chinese medicinal composition has quick response when used for treating the hand, foot and mouth disease, can take effect in two days, has a good therapeutic effect, and can effectively prevent herpes from ulcerating and relive the pain of the children, and the cure rate reaches over 90 percent; and the Chinese medicinal composition can prevent the hand, foot and mouth disease.
Owner:张成河
Human embryo lung fibroblast strain and method for using human embryo lung fibroblast strain for producing hand-foot-mouth viral vaccine
InactiveCN102911910AAvoid Residual EffectsReduced purification stepsAntiviralsEmbryonic cellsEmbryoViral Vaccine
The invention provides a novel human embryo lung diploid fibroblast strain Walvax-2, CCTCCC201055. The cell strain is sensitive to main epidemic disease viral strains-coxsackievirus 16-type COX.A16 in a group A and enterovirus 71-type EV71 second-strain virus of a hand-foot-mouth disease, and virus output is high. The invention further provides a method for preparing a divalent hand-foot-mouth virus inactivated vaccine and application of the human embryo lung diploid fibroblast strain in preparation of the hand-foot-mouth virus inactivated vaccine. The divalent inactivated vaccine produced by the human embryo lung diploid fibroblast strain can effectively prevent hand-foot-mouth disease.
Owner:云南沃森生物技术股份有限公司
Preparation and uses of novel Michael receptor-based enterovirus 71 type inhibitor
The present invention relates to a class of novel Michael receptor-based virus 71 (EV71) 3C protease inhibitors, wherein various variables of the structure general formula (M) are defined in the specification, and the compounds effectively inhibit or block the replication of enterovirus 71. The present invention relates to discovery and applications of the compound containing the structure generalformula (M), various optical isomers, pharmaceutically active metabolites, pharmaceutically acceptable salts, solvates and prodrugs thereof in preparation of antiviral drugs for the treatment of hand-foot-mouth virus infection diseases. The invention relates to an intermediate and a synthesis method for preparing the compound having the structure general formula (M). The formula (M) is defined inthe specification.
Owner:NANKAI UNIV
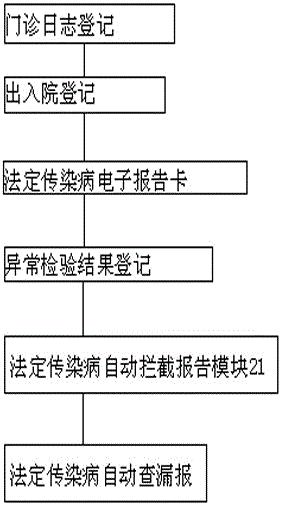
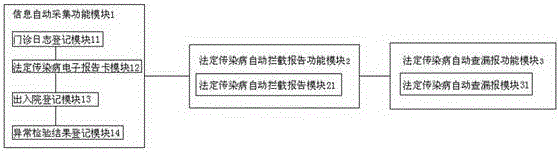
Underreporting preventing management system and method for notifiable infectious diseases such as hand-foot-and-mouth diseases, influenza and the like
InactiveCN106250668AEasy to operateHealthcare resources and facilitiesSpecial data processing applicationsMedical recordHand-foot-and-mouth disease
The invention discloses an underreporting preventing management system and method for notifiable infectious diseases such as hand-foot-and-mouth diseases, influenza and the like. The system comprises three major functional modules of an information automatic acquisition module, a notifiable infectious disease automatic interception and reporting module and a notifiable infectious disease underreporting automatic check module. The underreporting preventing management method for the notifiable infectious diseases such as the hand-foot-and-mouth diseases, the influenza and the like comprises the following steps of automatically acquiring information: registering outpatient logs, forming notifiable infectious disease electronic report cards, forming notifiable infectious disease report cards, registering admission and discharge, and registering abnormal check results; and calling a hospital HIS to form electronic outpatient logs by utilizing an outpatient log registration module, wherein data acquisition sources are registration office information in the HIS, outpatient doctor workstation information, clinic electronic medical record information, electronic medical record information of an inpatient department and the like.
Owner:无锡市疾病预防控制中心
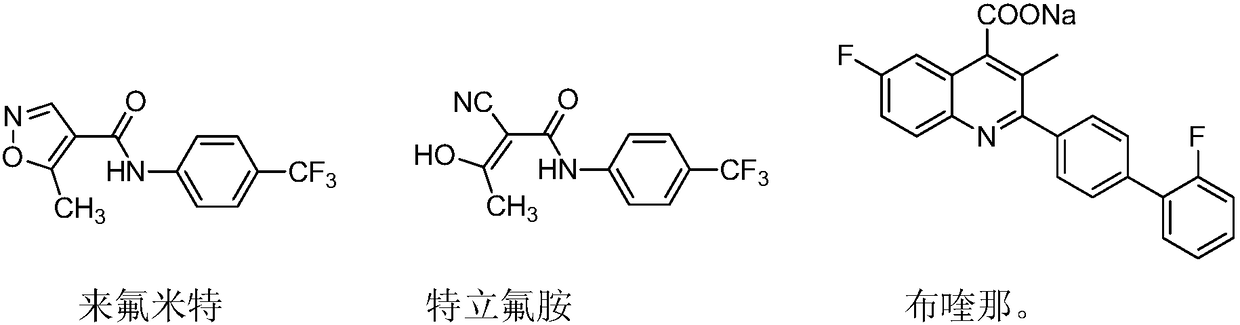
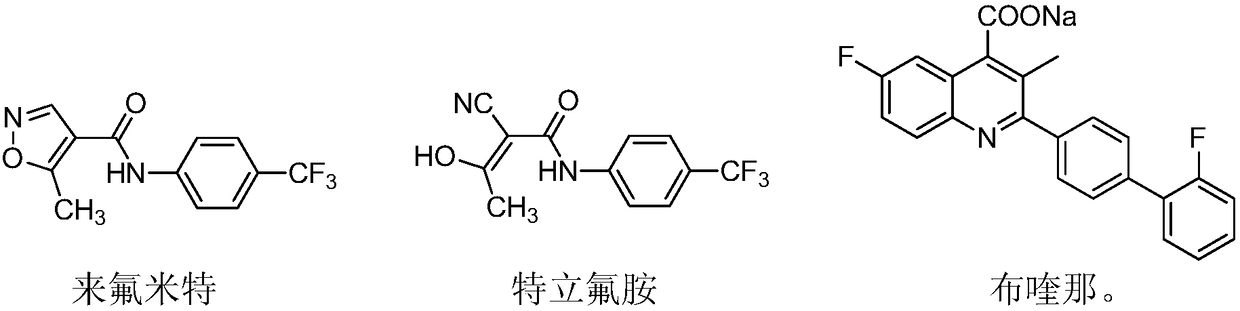
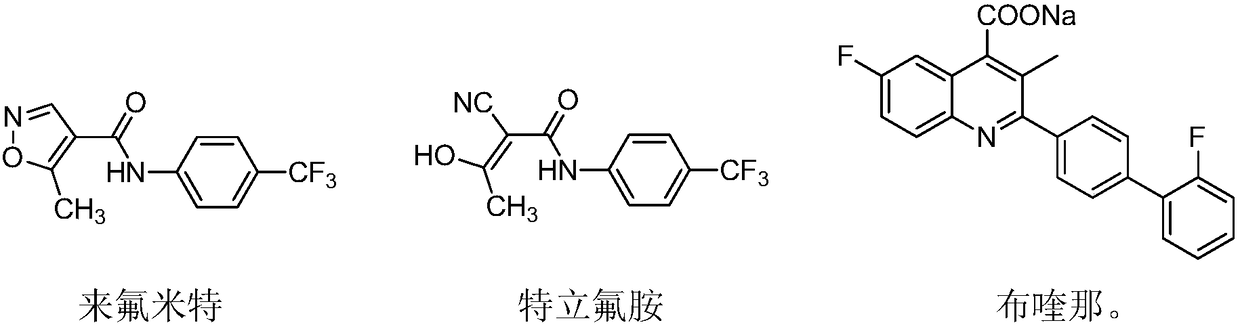
Novel antiviral medicines and application thereof
PendingCN108721281ABroad-spectrum and excellent antiviral activityLow toxicityAntiviralsNitrile/isonitrile active ingredientsLeflunomideRNA virus
The invention discloses application of leflunomide, teriflunomide, brequinar and derivatives thereof to treatment of virus infection, especially RNA virus infection. RNA viruses include but are not limited to influenza viruses, respiratory syncytial viruses, hand, foot and mouth viruses (EV71), dengue viruses (type-2 dengue viruses), Zika viruses and Japanese encephalitis viruses. The medicines have broad-spectrum and excellent antiviral activity and have relatively low toxicity to normal cells.
Owner:EAST CHINA UNIV OF SCI & TECH
Chinese medicament for treating hand-foot-and-mouth diseases and preparation method thereof
InactiveCN103127371AInhibit side effectsNo side effectsAntiviralsPlant ingredientsTree rootSide effect
The invention relates to a Chinese medicament for treating hand-foot-and-mouth diseases, and belongs to the technical field of medicaments. The Chinese medicament is prepared into a decoction from the following raw materials: 3 to 10 g of licorice, 3 to 10 g of folium isatidis, 5 to 15 g of isatis root, 1 g of coptis, 3 to 10 g of tee tree root, 3 to 10 g of bupleurum, 3 to 10 g of dandelion, 3 to 10 g of mint, 3 to 10 g of Chinese violet, 5 to 10 g of dried rehmannia root, 5 to 10 g of honeysuckle, 5 to 10 g of weeping forsythia, 5 to 10 g of pseudostellaria heterophylla, 5 to 10 g of root of red-rooted salvia, 5 to 10 g of honeysuckle stem, 3 to 5 g of phellodendron, 3 to 6 g of gardenia, 3 to 10 g of figwort root, and 3 to 5 g of big fritillaria. Based on the cognition and the treatment principle of the pathogenesis of the hand-foot-and-mouth diseases, the Chinese medicament is prepared by using the traditional Chinese medicinal materials as raw materials through clinical test and repeated verification; and the Chinese medicament emphasizes heat clearing, detoxification and dehumidification, simultaneously proposes tonification and purgation in combination, qi tonification, yin nourishment, heat clearing and collateral dredging, accords with the theory of the traditional Chinese medicine, avoids the side response of unidirectional action of the western medical therapy and does not have toxic or side effect, the effective rate is 98 percent, and the cure rate is over 95 percent.
Owner:李志荃
Hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof
InactiveCN102190725ASignificant effectImprove survival rateImmunoglobulins against virusesAntiviralsTotal proteinPasteurization
The invention relates to a hand-foot-and-mouth disease resistant human immunoglobulin, and preparation and using methods and application thereof in pharmacy. The preparation method comprises the following steps of: separating component I+II+III, I+III and II precipitates in turn by using a low-temperature methanol protein separation method; performing pasteurization on a component II precipitate; refining and purifying; performing dealcholization; preparing; and sterilizing, packaging and performing low-pH incubated inactivation. The product has antibody titer of not less than 1:640 for enteroviruses (including one or more of coxsackie virus, Echo and EV71), the immunoglobulin content is not less than 95.0 percent of the total protein content, and the sum of IgG monomer and dimer is not less than 95 percent; and the using method is that a specific antibody with titer of 160,000-320,000 is intravenously infused. The invention is suitable for industrial production; and the product has high-titer enterovirus resistant specificity, is safe and reliable, and can become an effective medicine for treating the hand-foot-and-mouth disease.
Owner:HUALAN BIOLOGICAL ENG INC
Qualitative detection method for simultaneously detecting three types of main hand-foot-and -mouth disease viruses in water environment
InactiveCN102676700AHigh sensitivityShorten the timeMicrobiological testing/measurementMicroorganism based processesHand-foot-and-mouth diseaseCoding region
The invention discloses a qualitative detection method for simultaneously detecting three types of main hand-foot-and-mouth disease viruses (EV71,CVA10,CVA16) in water environment, according to conservative property of nucleic acid in a section from a 5' noncoding region of RNA (Ribose Nucleic Acid) of the three types of main hand-foot-and-mouth disease viruses (EV71,CVA10,CVA16) to a viral protein capsid coding region VP2, and semi-nested PCR (Polymerase Chain Reaction) primers of the three types of main viruses of the hand-foot-and-mouth disease can be produced. A qualitative PCR detection method for the hand-foot-and-mouth disease viruses in the water environment by the semi-nested PCR primers can be built. The method has the advantages of good specificity and high sensitivity, and can be used for establishing a favorable base for follow-up genotyping.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
Preparation method of room air virus pathogenic-bacteria jinx
ActiveCN101491243AResolve inhibitionSolving the Killing ProblemBiocideGaseous substancesBacterial virusStaphylococcus aureus
The invention discloses a method for preparing an indoor air virus pathogen invincible opponent. The indoor air virus pathogen invincible opponent prepared by menthol, thymol, weeping forsythia oil and radix bupleuri oil has the killing rate of between 76 and 99.9 percent to idemic encephalitis, influenza viruses, hand-foot-and-mouth disease, avian influenza, legionnella, pneumoniae, hepatitis C viruses, hepatitis A viruses, Staphylococcus albus, Staphylococcus aureus, Klebsiella pneumoniae, Candida albicans, typhoid viruses, Escherichia coli, cholera viruses, infectious germs and bacteria, and harmful germs and bacteria. The indoor air virus pathogen invincible opponent is mainly applied to rooms, schools, hospitals, hotels, air conditioners, automobiles, public places, and the like, and has obvious effect on the control of diseases such as influenza, upper respiratory tract infection. The indoor air virus pathogen invincible opponent has outstanding substantive characteristics and broad application prospect.
Owner:上海钮爱环保科技有限公司
Pentacyclic triterpene enterovirus EV71 inhibitors, and medicinal compositions and medicinal use thereof
InactiveCN104840467AOrganic active ingredientsNervous disorderAcute hyperglycaemiaHand-foot-and-mouth disease
The invention relates to the pharmacy field, concretely relates to a use of a series of pentacyclic triterpene compounds as enterovirus EV71 inhibitors, and especially relates to an application in the preparation of medicines for preventing and treating EV71 infection induced hand-foot-and-mouth diseases and complications thereof, such as synanche, myocarditis, pulmonary edema, encephalitis, herpes, septicemia, hypertension, hyperglycemia, cognitive function disorder, poliomyelitis-like paralysis and many nerve system associated diseases. The invention also discloses medicinal compositions of the series of the pentacyclic triterpene compounds as enterovirus EV71 inhibitors.
Owner:CHINA PHARM UNIV
Loop-mediated isothermal amplification assay kit and detection method of hand, foot and mouth disease
InactiveCN102242223ASuitable for field applicationStrong specificityMicrobiological testing/measurementHand-foot-and-mouth diseaseCoxsackievirus a16
The invention belongs to the field of biotechnology, and relates to a loop-mediated isothermal amplification (LAMP) assay kit of Coxsackie A16 (Cox A16) and Enterovirus 71 (EV 71) which are main pathogens of hand, foot and mouth disease, and an establishment method and an application of the assay kit. The assay kit comprises four LAMP primers and LAMP reaction liquid for detecting Coxsackie A16, and four LAMP primers and LAMP reaction liquid for detecting Enterovirus 71. Tests prove that the assay kit has the advantages of good specificity and sensitivity, rapid amplification rapid, high efficiency and simple identification. A detection system provided by the invention can rapidly, conveniently, efficiently, high specifically and high sensitively detect Coxsackie A16 and Enterovirus 71 without complex apparatuses thus can satisfy well clinical detection requirements of hand, foot and mouth disease, and is suitable for a large-scale promotion and an application.
Owner:上海吉美生物工程有限公司
Method for testing quality of antiviral oral liquid for treating hand-foot-and-mouth disease
ActiveCN101843884ASimple methodComponent separationPharmaceutical delivery mechanismHand-foot-and-mouth diseaseMedicine
The invention discloses a method for testing the quality of antiviral oral liquid for treating the hand-foot-and-mouth disease and relates to the technical field of medicaments. In the method for testing the quality, the quality is tested by using a high performance liquid chromatogram single-test multi-evaluation method. 10 ml of antiviral oral liquid contains no less than 60 mu g of (R,S)-goitrin, no less than 1 mg of forsythoside A, no less than 0.25 mg of forsythin and no less than 0.12 mg of forsythingenin. The method has the advantages that: the method is simple and convenient; the content of four active ingredients of the antiviral oral liquid can be tested at the same time; the quality of the antiviral oral liquid and the curative effect of the antiviral oral liquid on the hand-foot-and-mouth disease can be comprehensively evaluated by establishing a quantitative analysis result of the active ingredients of the antiviral oral liquid, so the antiviral oral liquid has a truly-controllable standard; and simultaneously, the medicinal material basis of a Chinese medicinal preparation, namely, the antiviral oral liquid in the aspect of curing the hand-foot-and-mouth disease is put forward in China for the first time.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
Fluorescent quantitative RT-PCR detection kit and detection method for enterovirus
InactiveCN101407846ANo cross reactionQuick checkMicrobiological testing/measurementFluorescence/phosphorescenceCerebrospinal fluidHand-foot-and-mouth disease
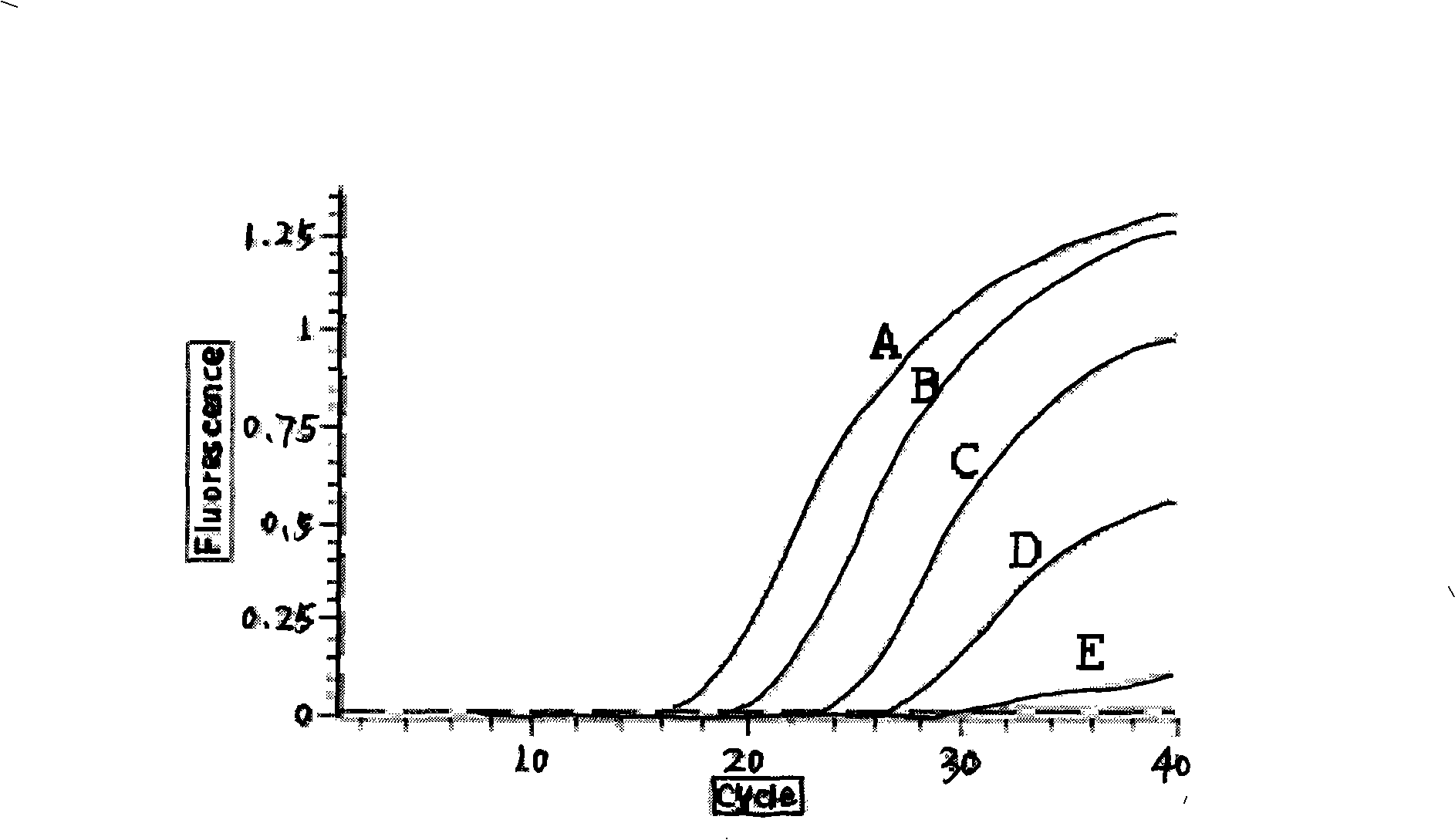
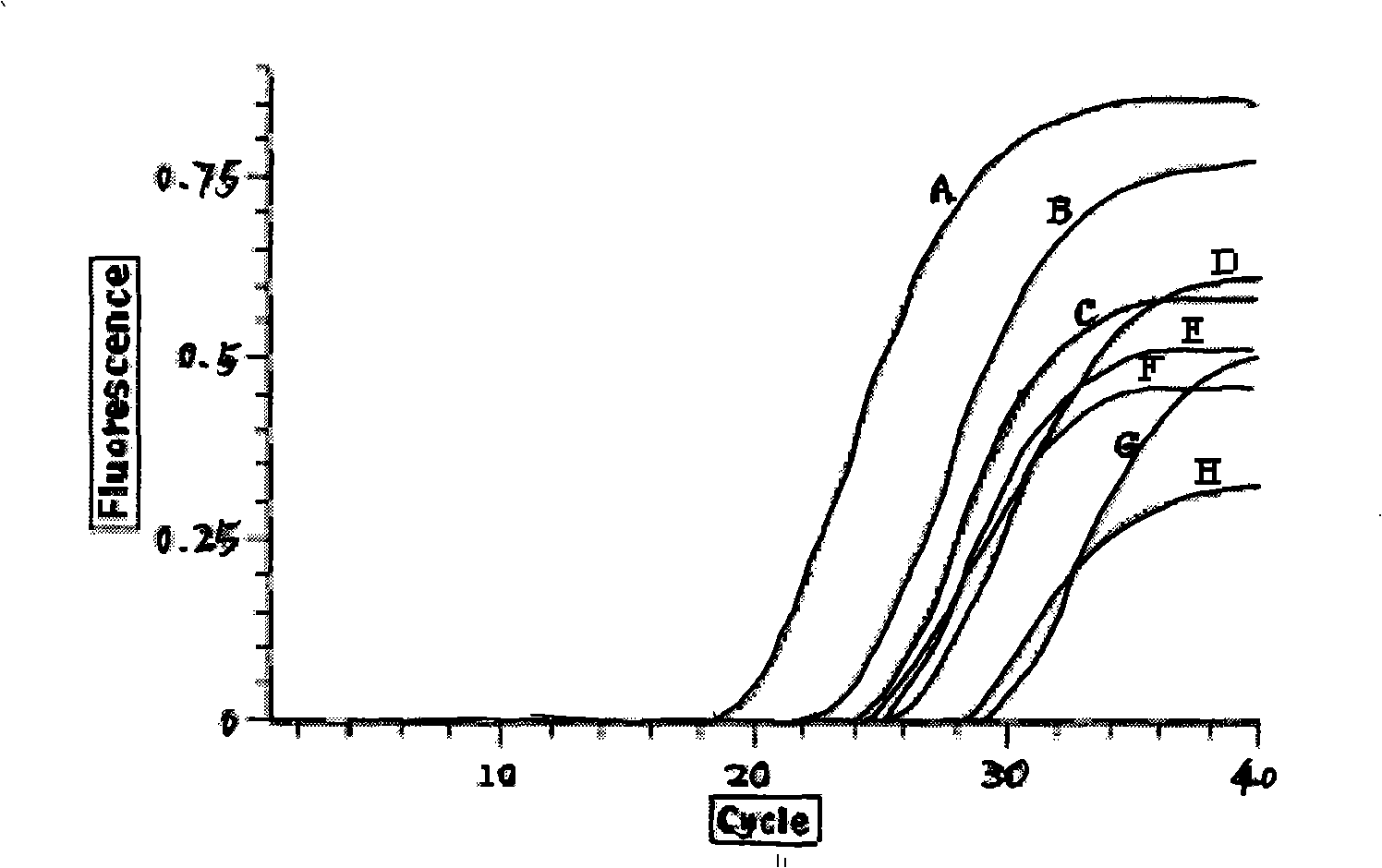
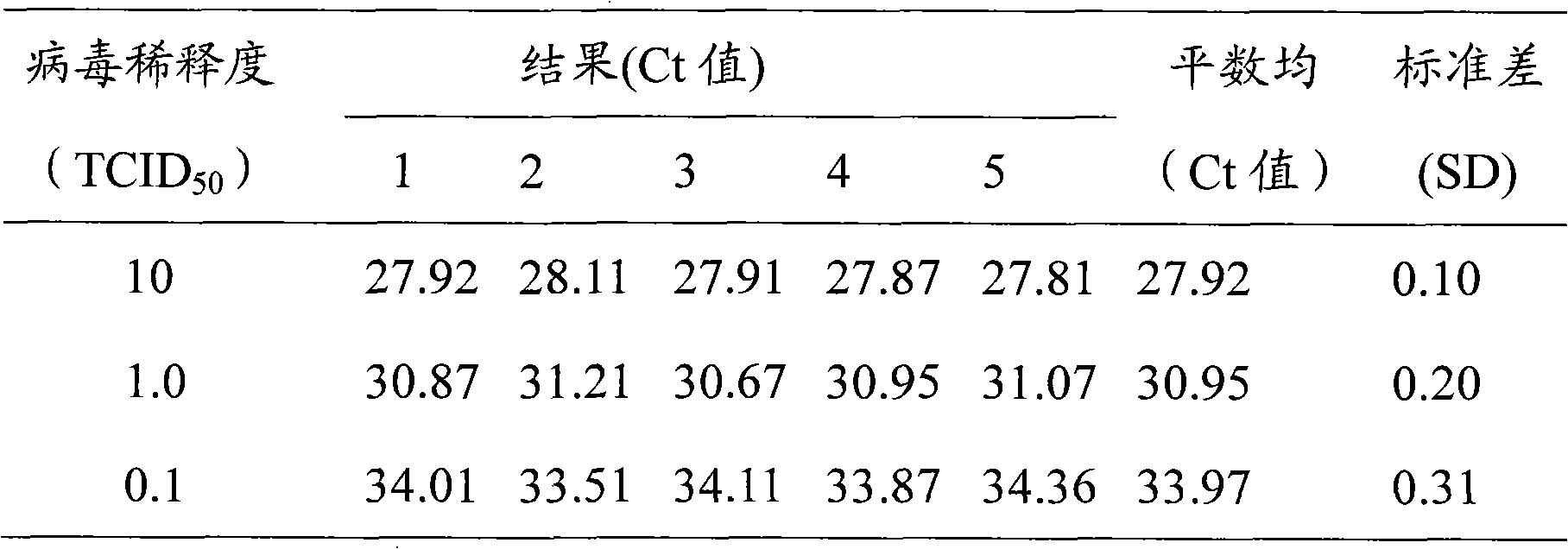
The invention provides a fluorescence quantitative RT-PCR detecting kit for an enterovirus and a detecting method thereof; and the sequences of an upstream primer and a downstream primer and a specific probe of the fluorescence quantitative RT-PCR detecting kit are as follows: the upstream primer EV(YG)F is 5'-GGCTGCGYTGGCGGCC-3', the downstream primer EV(YG)R is 5'-CCAAAGTAGT CGGTTCCGC-3' and the specific probe EV(YG)PB is 5'-CTCCGGCCCCTGAATGCGG-3'. The method has high specificity on detecting the enterovirus and does not have cross reactions with other enteroviruses such as hepatitis A, hives, rubella, parotitis, encephalitis, dengue fever, adenovirus, and the like. The detection sensitivity of the method achieves 0.1TCID50; the method can directly detect the nucleic acid of the enterovirus from the samples of ncurolymph, herpes liquid, dejecta and the like of suspected patients; only about 3h is needed from extracting the nucleic acid of the enterovirus to finishing the detection; and the detecting kit and the detecting method are suitable for the lab early diagnosis of sudden epidemic caused by the infection of the enteroviruses such as the hand-foot-and-mouth disease and the like.
Owner:ZHEJIANG CENT FOR DISEASE CONTROL & PREVENTION
Anti-enteroviral 71(EV71) 4-iminooxazolidine-2-ketone compound as well as preparation method and application of anti-enteroviral 71(EV71) 4-iminooxazolidine-2-ketone compound
The invention discloses a 4-iminooxazolidine-2-ketone enterovirus 71(EV71) 3C protease inhibitor of which the structural general formula is shown as a compound (M), each variable in the structure is defined as the specification, and the compounds are used for effectively inhibiting or stopping the replication of enterovirus 71. The invention relates to discovery and application of a compound with a structure shown as the formula (M) as well as various optical isomers of the compound, a pharmaceutically active metabolite, a medicinal salt, a solvate and a prodrug of the compound in preparation of antiviral drugs for treating hand-foot-mouth virus infection diseases and also relates to an intermediate for preparing the compound with the structure shown as the formula (M) and a synthesis method.
Owner:NANKAI UNIV
Sequence of enterovirns type71 genome and uses thereof
InactiveCN101509003AMicroorganism based processesImmunoglobulins against virusesData informationGenetics
The invention provides an enterovirus 71 complete genome sequence, comprising a nucleotide sequence shown by SEQ ID NO:1. The sequence can be used for preparing a vaccine for preventing the enterovirus 71 and a neutralizing antibody for resisting the enterovirus 71, provides impersonal basis for further acquiring EV71 genetic derivation in South China, provides reliable data information for developing diagnostic reagents and vaccines of enzyme-linked immunosorbent assay (ELISA) for outbreak people in different areas of China, and provides valuable resource for deeply studying genetic variation rules of EV71 and mechanism causing hand-foot-and-mouth disease.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Gene recombinant vaccine for preventing enterovirus 71 infection and preparation method thereof
The invention discloses a gene recombinant vaccine for preventing enterovirus 71 (EV71) infection and a preparation method thereof. The inflection comprises multiple diseases related with the nervous system such as hand-foot-and-mouth disease, paralytic diseases of sterile meningitis, cephalitis and poliomyelitis and the like. Escherichia coli labile enterotoxin B subunit (LTB) is used as an immunological enhancement adjuvant, two fragments of linear neutralizing epitope SP55 and SP70 in EV71 virus coat protein VP1 are used as antigens, prokaryotic expression plasmids of LTB-SP55-SP70 fusion genes are constructed by using gene engineering technology, the plasmids are expressed in escherichia coli, and a recombinant expression product is purified for preparing the EV71 virus gene engineering vaccine.
Owner:中国疾病预防控制中心病毒病预防控制所 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com