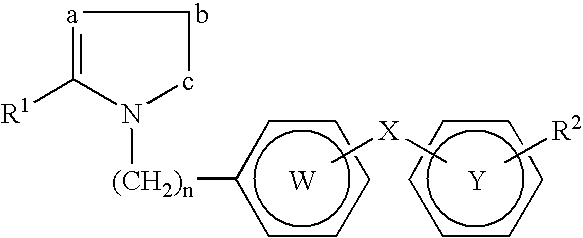Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
161 results about "Pulmonary edema" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A condition where fluid accumulates in lung tissues.
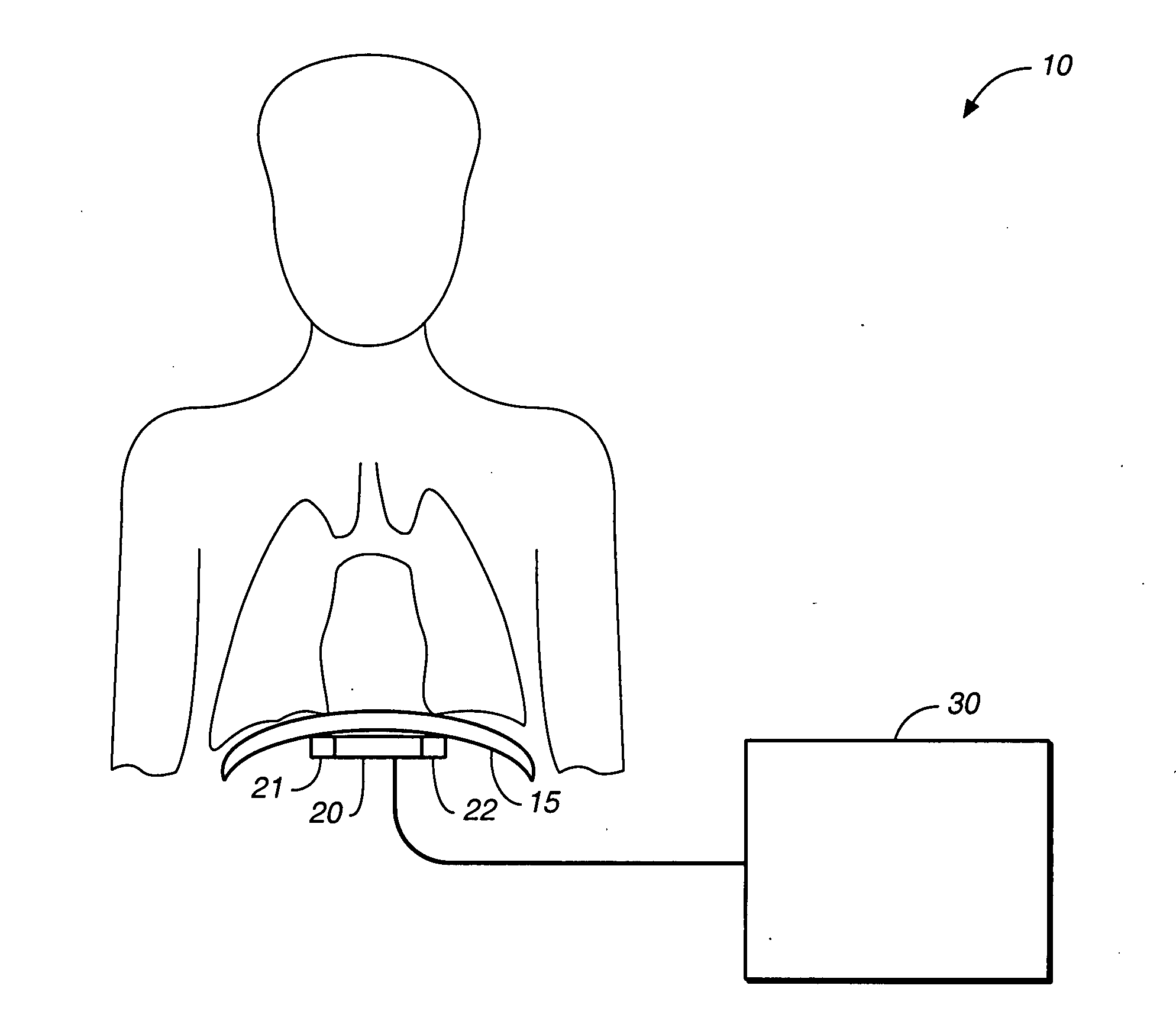
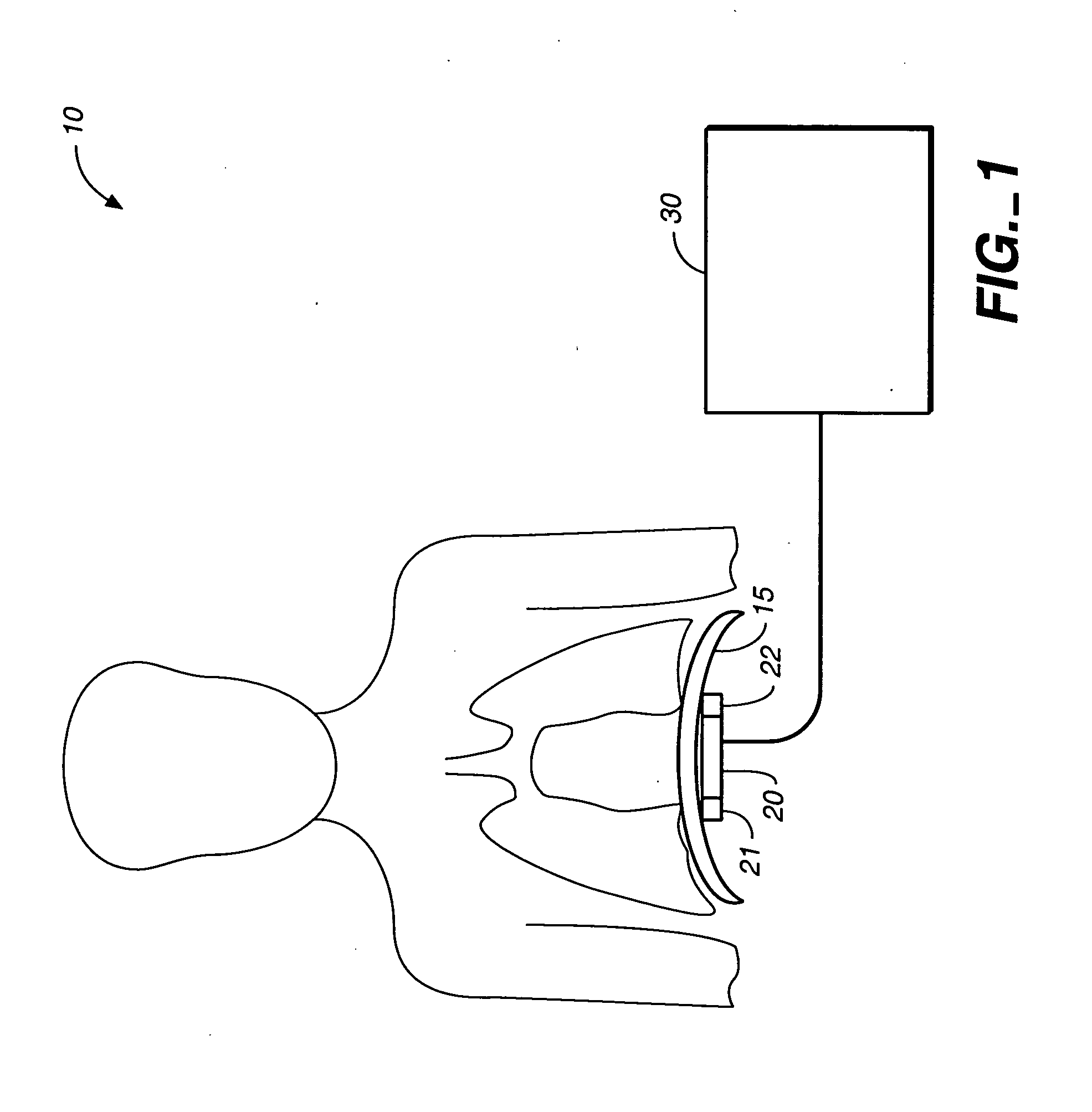
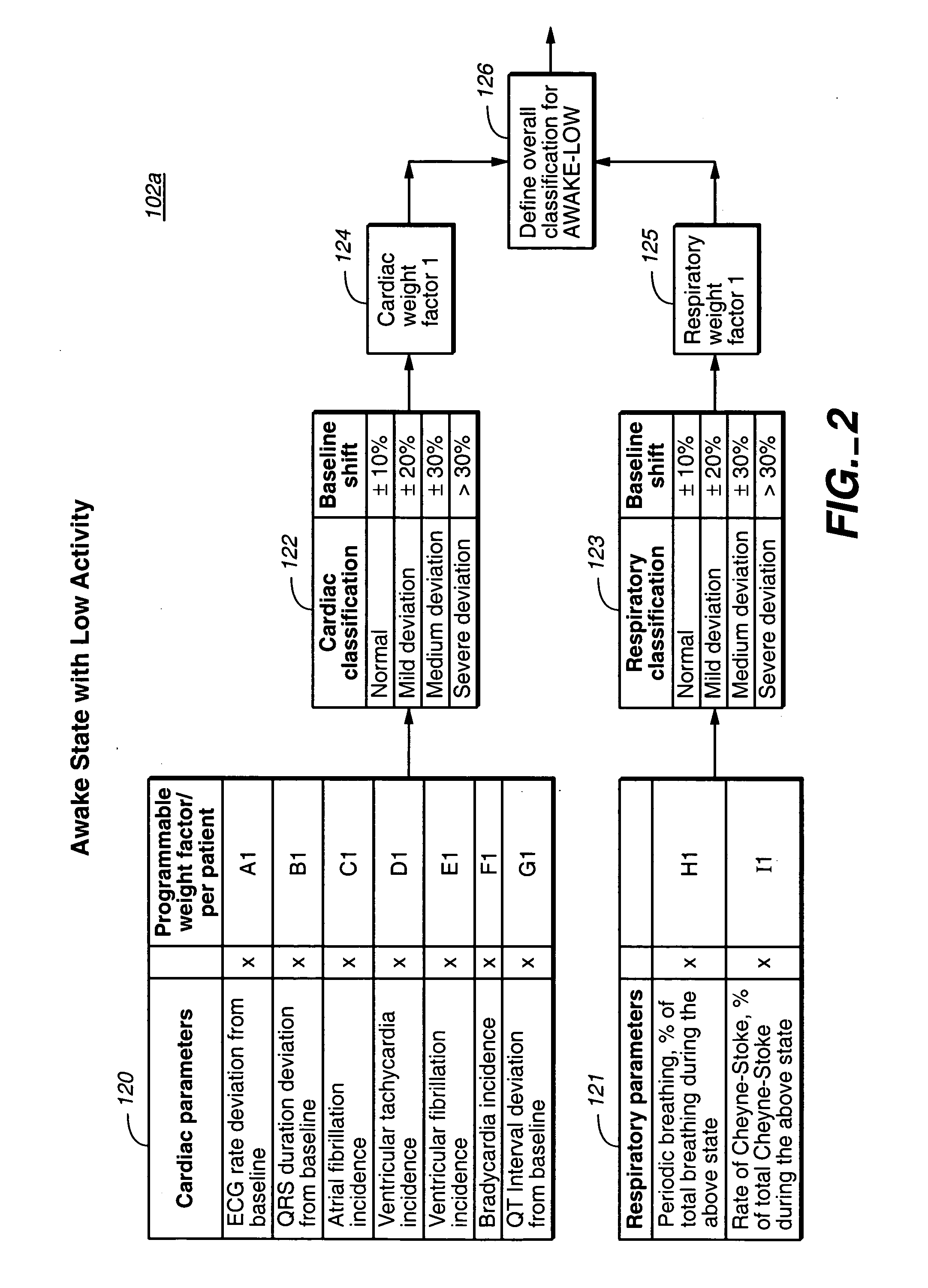
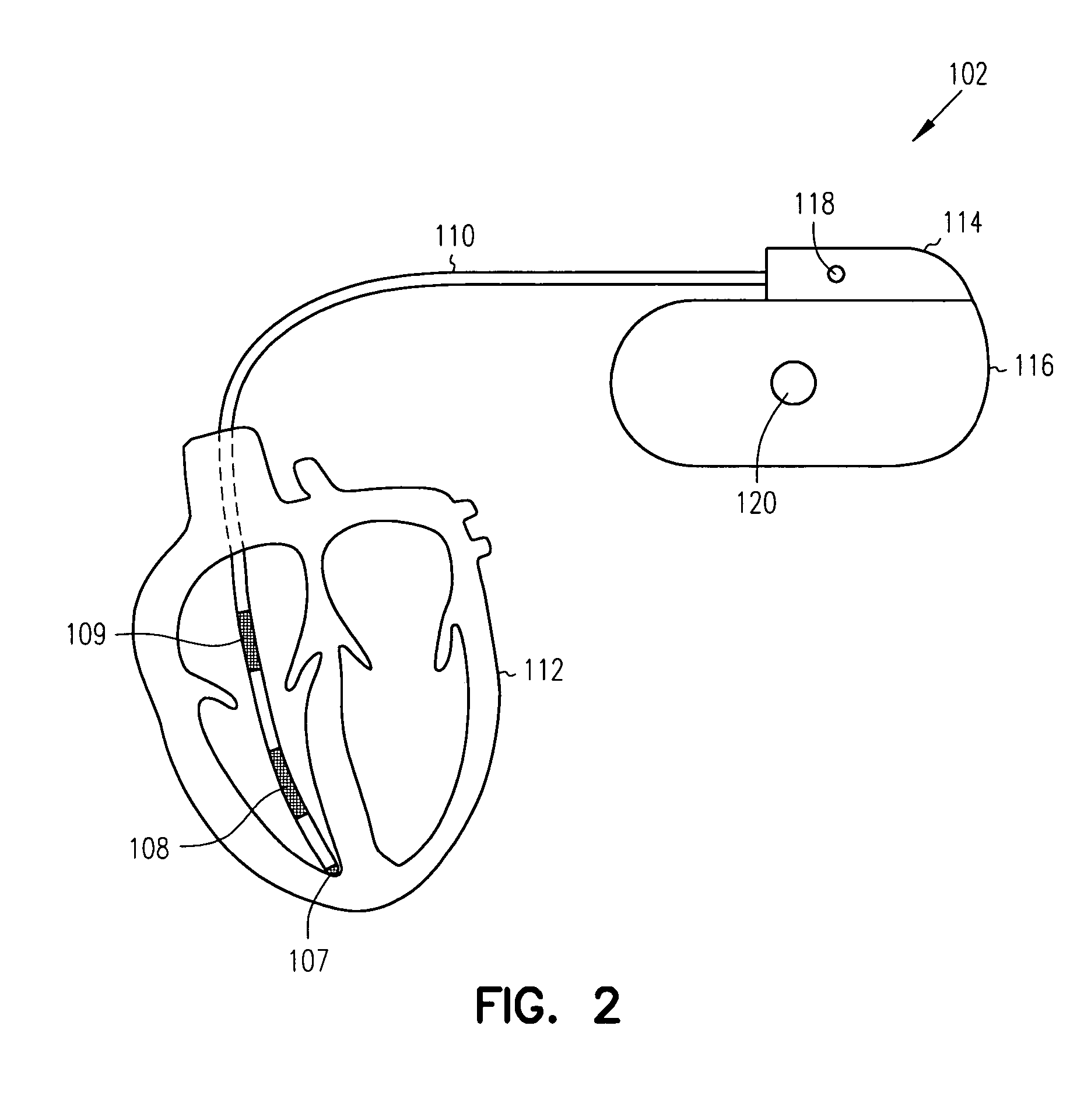
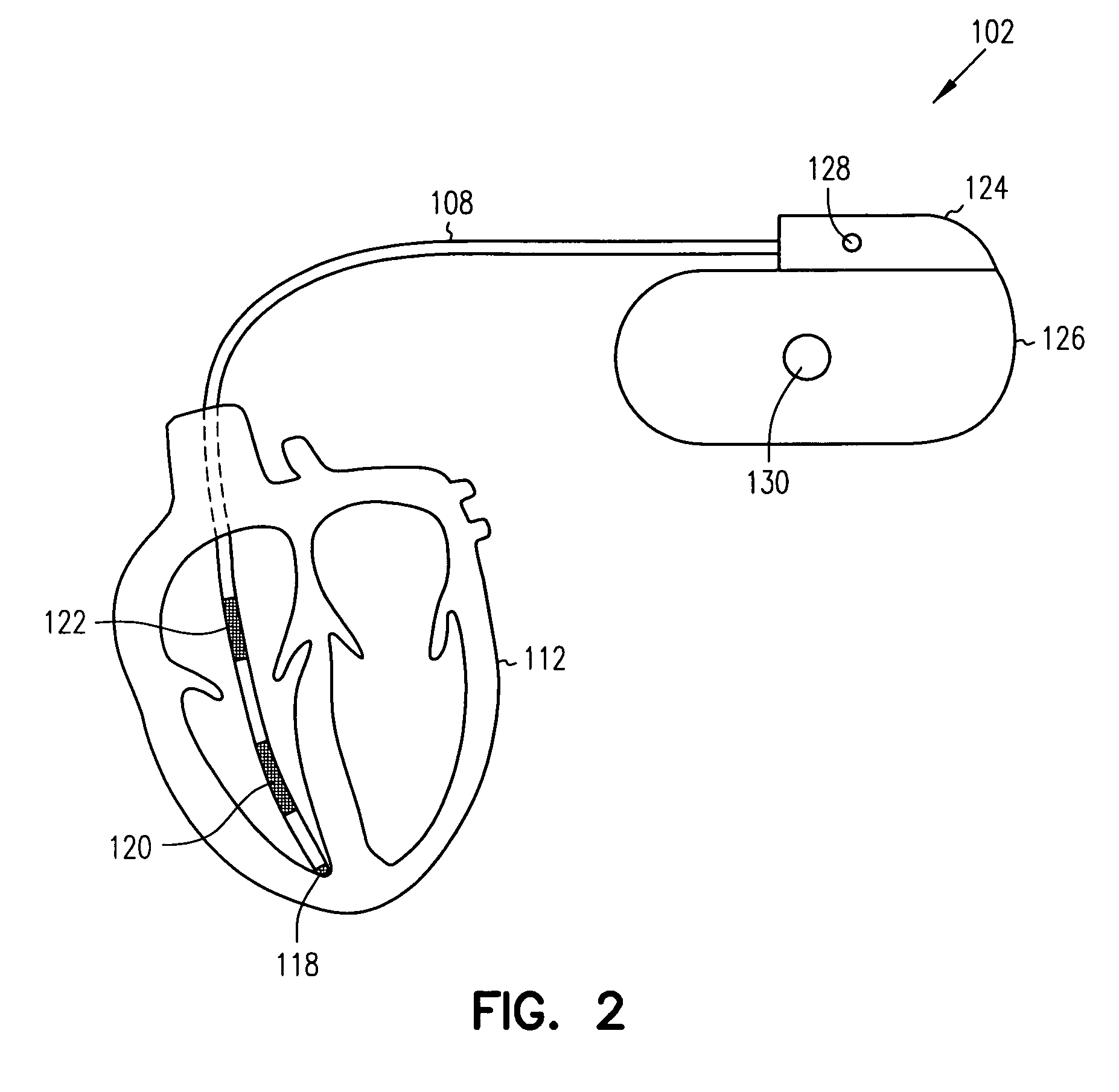
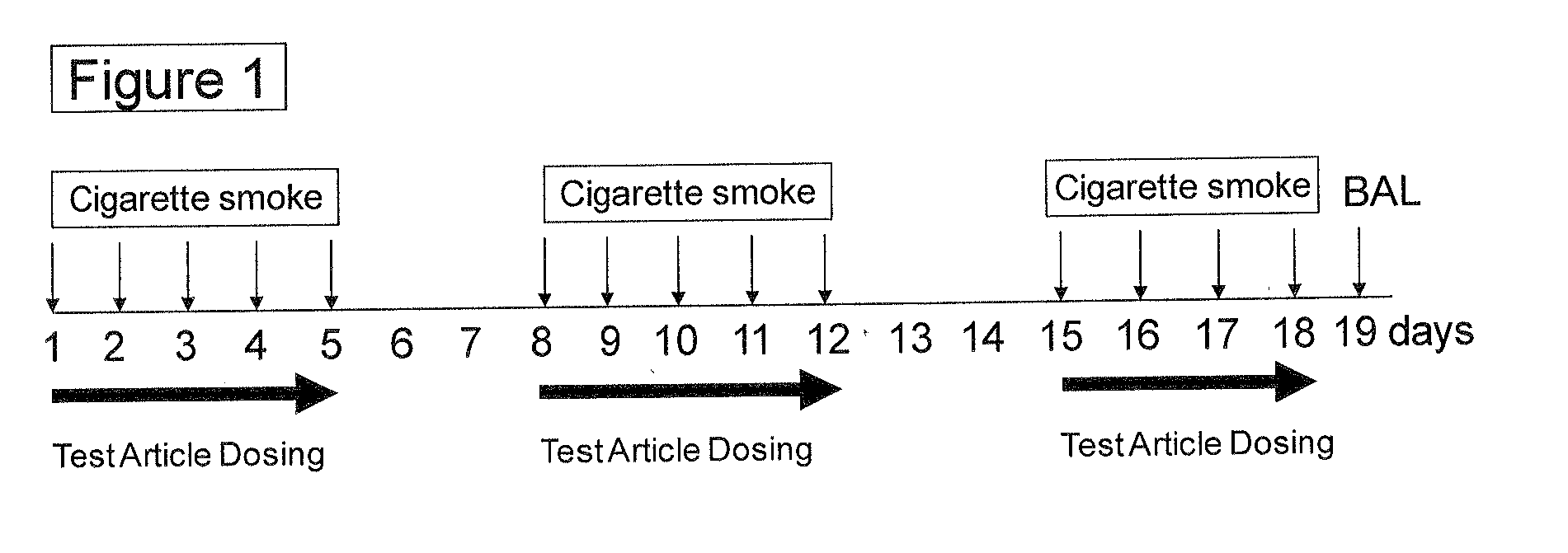
Heart failure patient treatment and management device
InactiveUS20050085734A1Improve severityWorsen conditionElectrotherapyElectromyographyDiseasePatients symptoms
A device and method to detect and manage heart failure patient symptoms is provided. Respiration and / or cardiac parameters may be sensed or observed to determine the status of a patient's condition. These symptoms may be classified for appropriate patient disease management. A patient's activity level may be monitored in conjunction with respiration and / or cardiac parameters to provide additional patient status information. These symptoms may be classified for appropriate patient disease management. Pulmonary edema is one condition that may be determined to exist when a respiration parameter is out of range for a given sensed activity level. If edema is determined to be present, the device may be configured to respond to treat the edema.
Owner:RMX
Pulmonary vein valve implant
InactiveUS20050273160A1Reduced likelihoodReduce severityStentsBronchiPulmonary vasculatureAtrial cavity
The present invention involves placing a valve between the left atrium and the lung to prevent regurgitant flow from increasing the pulmonary pressures, which may lead to pulmonary edema and congestion. Mitral stenosis or poor synchronization of the mitral valve may add additional pressures to the left atrium thus raising the pulmonary pressures and leading to congestion in the lung vasculature. By blocking the additional pressures from the mitral regurgitant flow from reaching the pulmonary circulation, the left atrium may act as a sealed vessel to allow additional aortic output. The valve placement can be intralumenal or attached to the ostium of the atrium. The device can be placed via the vascular conduits or through a surgical procedure into the pulmonary circulation. One or more devices may be placed in each of the four pulmonary veins. Additionally only one, two, three or all four veins may be implanted with the valve as desired by the physician.
Owner:DIRECT FLOW MEDICAL INC
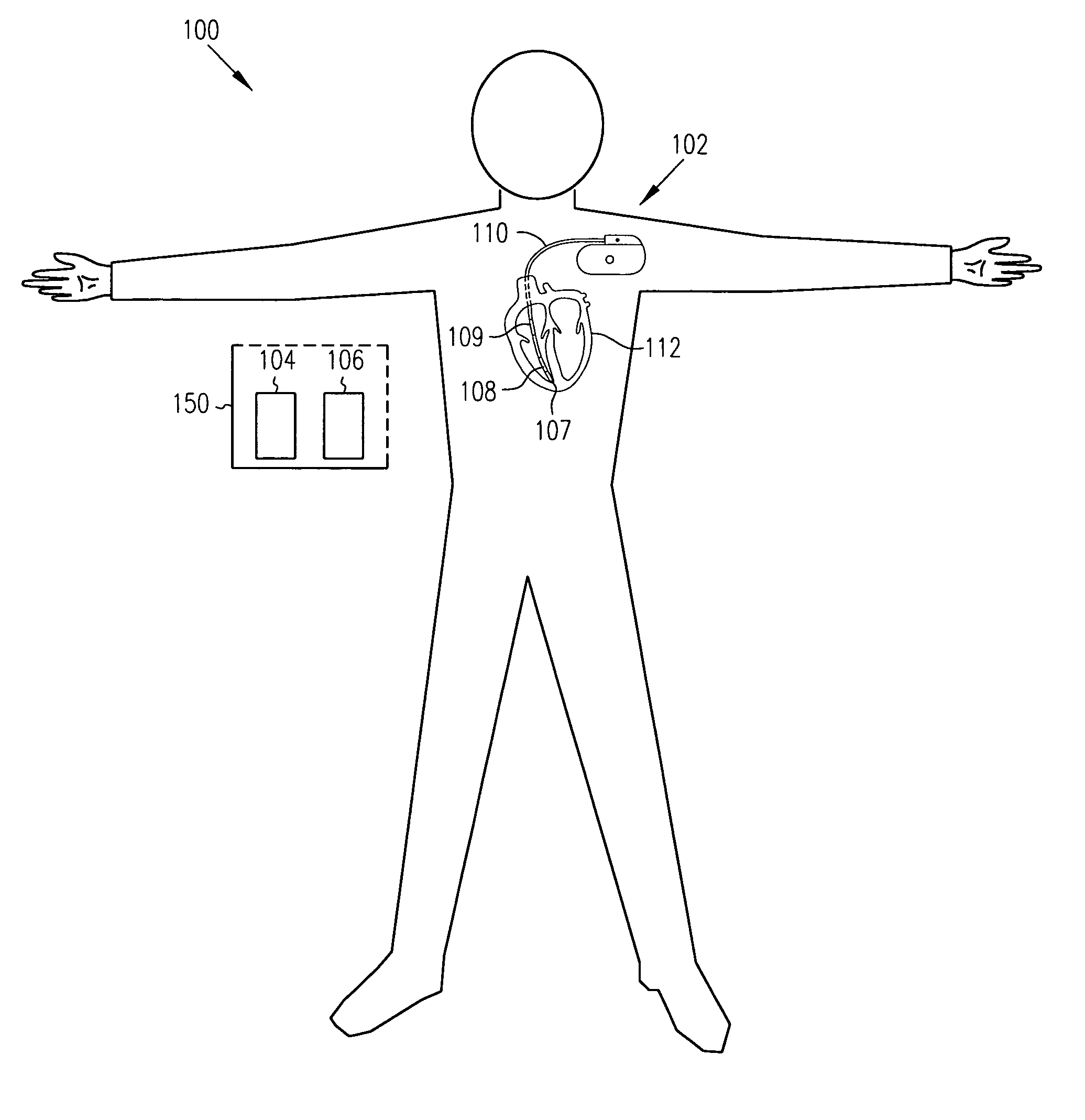
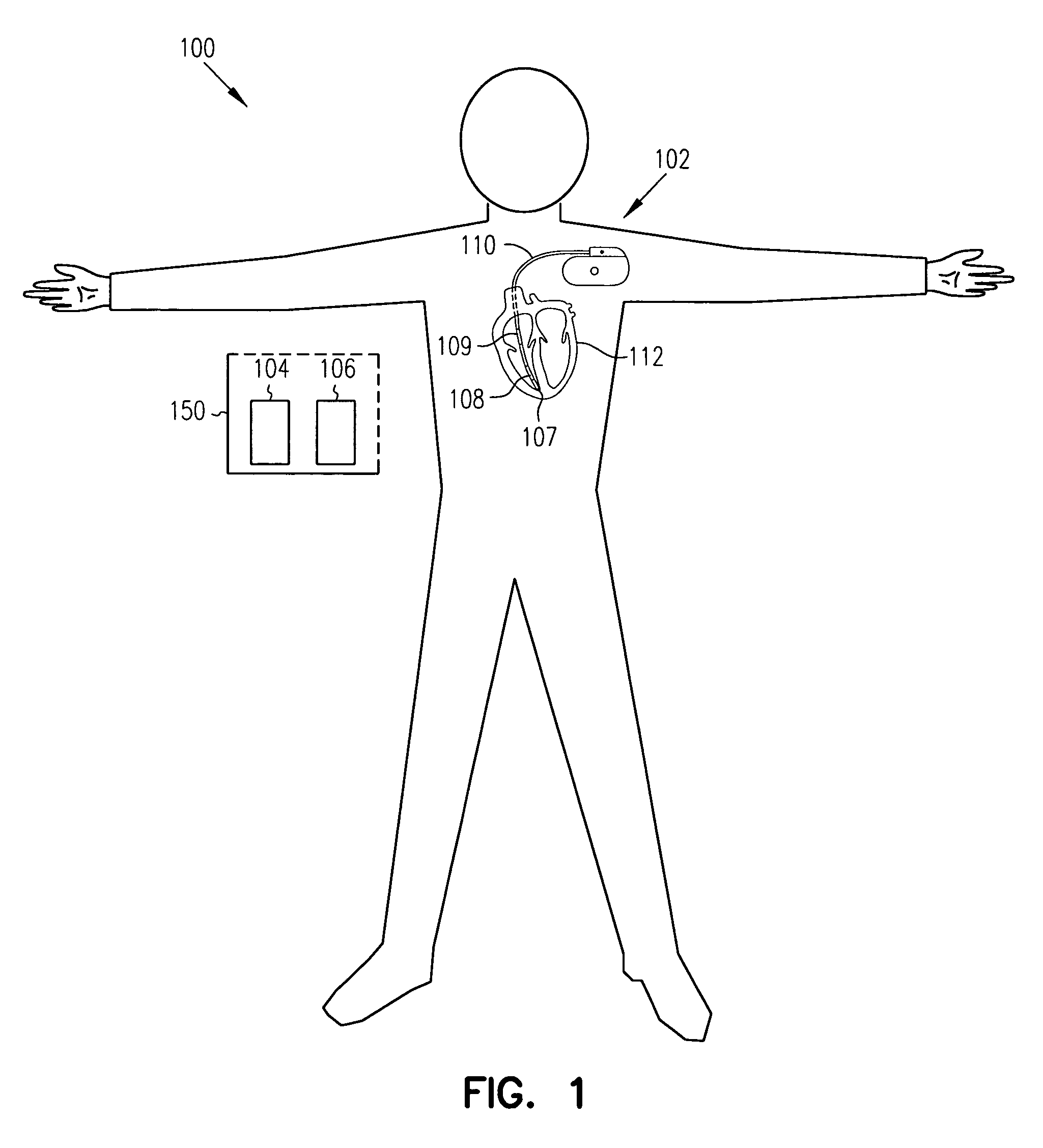
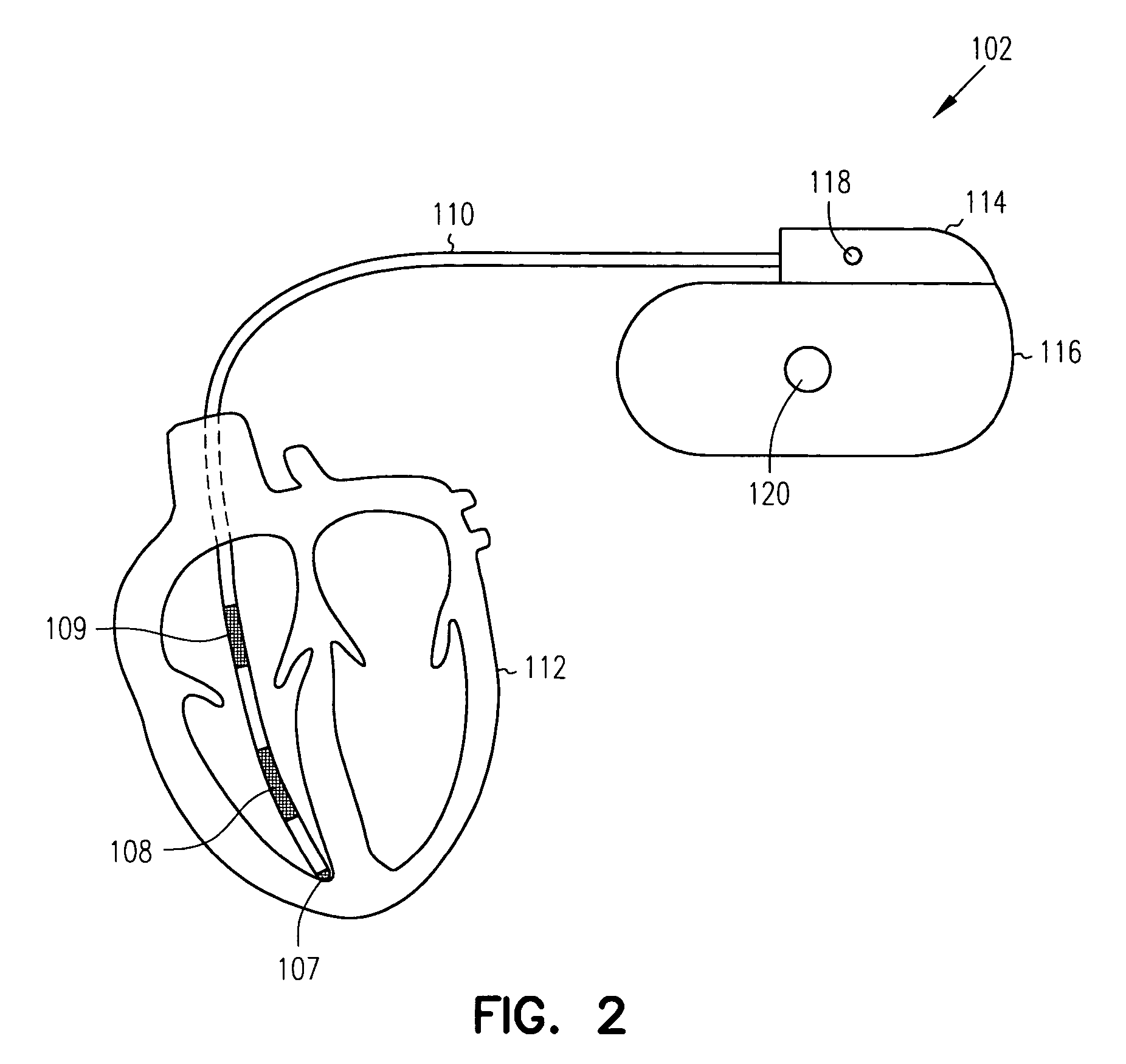
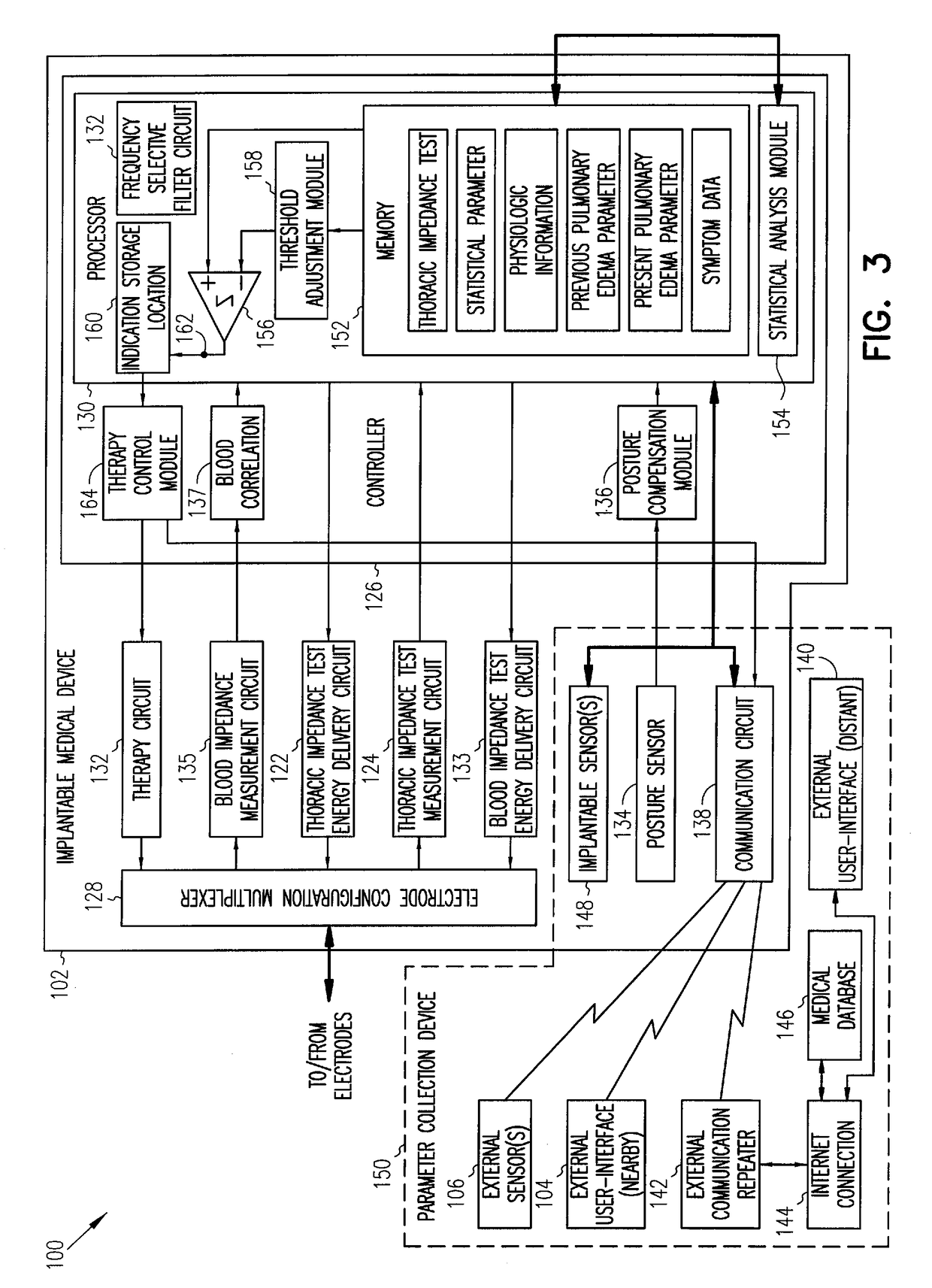
Sensitivity and specificity of pulmonary edema detection when using transthoracic impedance
This patent document discusses, among other things, systems, devices, and methods for enhancing detection of pulmonary edema using, in addition to thoracic impedance, one or a combination of: physiologic information about a subject, at least one statistical parameter, a user-programmable detection level, at least one parameter associated with a previous pulmonary edema event, and patient symptom information about the subject. In one example, a (base) thoracic impedance threshold is modified to an adjusted thoracic impedance threshold. The adjusted thoracic impedance threshold provides an increased sensitivity of pulmonary edema detection as compared to the base thoracic impedance threshold. In another example, an alert is provided to a subject, a caregiver, or other user based on a pulmonary edema indication determined by the present systems, devices, and methods. In a further example, a therapy (provided to the subject) is adjusted or initiated in response to the pulmonary edema indication.
Owner:CARDIAC PACEMAKERS INC
Detection of pleural effusion using transthoracic impedance
Owner:CARDIAC PACEMAKERS INC
Enhancements to the detection of pulmonary edema when using transthoracic impedance
This patent document discusses, among other things, systems, devices, and methods for enhancing detection of pulmonary edema using, in addition to thoracic impedance, one or a combination of: physiologic information about a subject, at least one statistical parameter, a user-programmable detection level, at least one parameter associated with a previous pulmonary edema event, and patient symptom information about the subject. In one example, a (base) thoracic impedance threshold is modified to an adjusted thoracic impedance threshold. The adjusted thoracic impedance threshold provides an increased sensitivity of pulmonary edema detection as compared to the base thoracic impedance threshold. In another example, an alert is provided to a subject, a caregiver, or other user based on a pulmonary edema indication determined by the present systems, devices, and methods. In a further example, a therapy (provided to the subject) is adjusted or initiated in response to the pulmonary edema indication.
Owner:CARDIAC PACEMAKERS INC
Detection of pleural effusion using transthoracic impedance
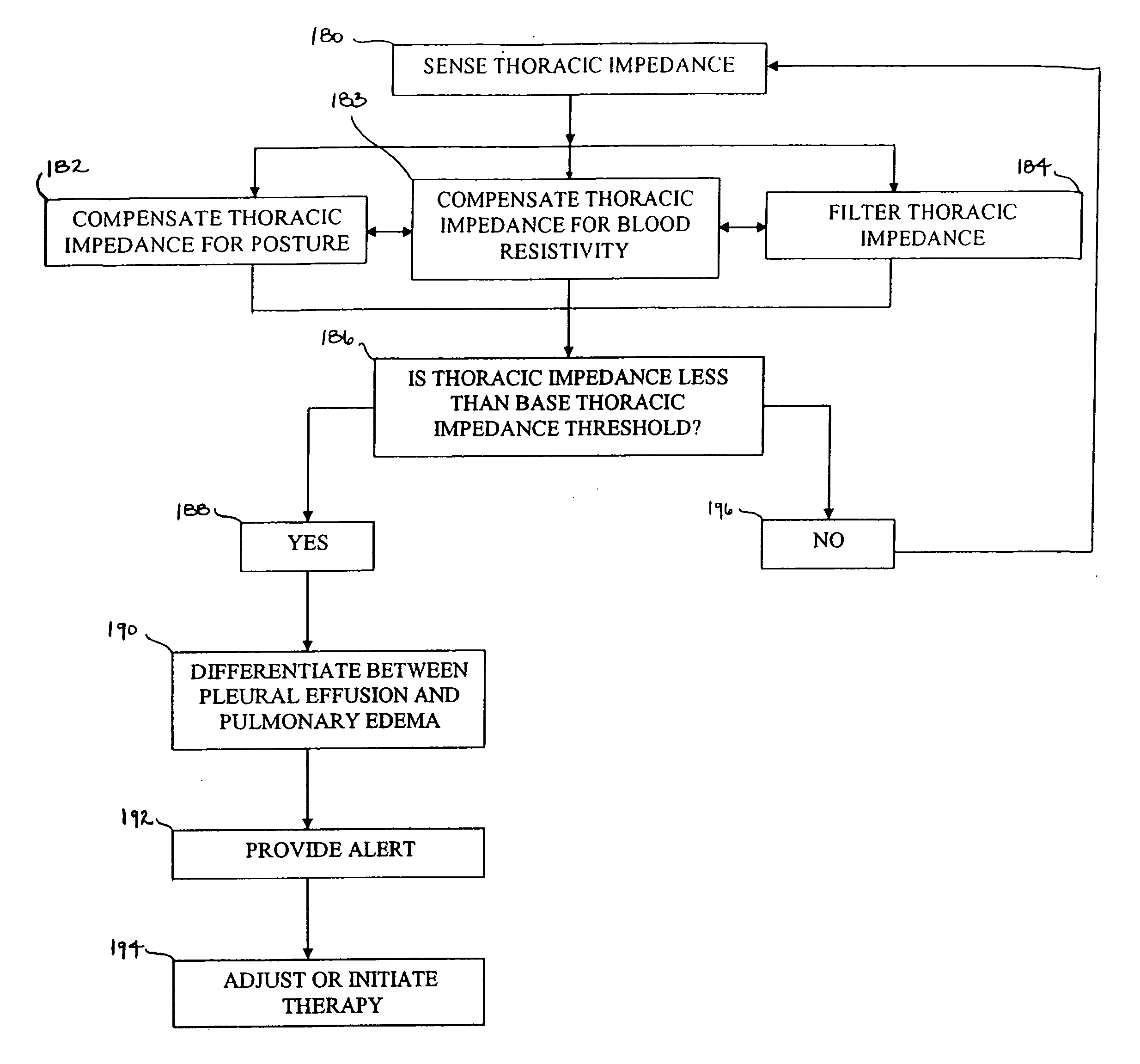
This patent document discusses systems, devices, and methods for increasing a sensitivity or specificity of thoracic fluid detection in a subject and differentiating between pleural effusion and pulmonary edema. In one example, a thoracic impedance measurement circuit senses a thoracic impedance signal. In another example, a processor receives the thoracic impedance signal and determines whether such thoracic impedance signal is “significant.” A significant thoracic impedance signal indicates the presence of thoracic fluid and may be recognized by comparing the thoracic impedance signal (or variation thereof) to a thoracic impedance threshold. When a significant thoracic impedance signal is recognized, the processor is adapted to detect one or both of: a pleural effusion indication and a pulmonary edema indication using one or a combination of: physiologic information, patient symptom information, and posture information. In another example, the thoracic impedance threshold is adjusted using such physiologic, patient symptom, or posture information.
Owner:CARDIAC PACEMAKERS INC
System and method for predicting a heart condition based on impedance values using an implantable medical device
ActiveUS7272443B2Reliably predict onset of heart conditionHeart stimulatorsDiagnostic recording/measuringPulmonary edemaThoracic cavity
Techniques are provided for predicting the onset of a heart condition within a patient based on impedance measurements. Briefly, overloads in fluid levels in the thorax and in ventricular myocardial mass within the patient are detected based on impedance signals sensed using implanted electrodes. The onset of certain heart conditions is then predicted based on the overloads. For example, pulmonary edema arising due to diastolic heart failure is predicted based on the detection of on-going overloads in both fluid levels and ventricular mass. Ventricular hypertrophy is detected based on an on-going ventricular mass overload without a sustained fluid overload. Various other heart conditions may also be predicted based on specific combinations of recent or on-going overloads. Evoked response is exploited to corroborate the predictions. Appropriate warning signals are generated and preemptive therapy is initiated.
Owner:PACESETTER INC
System and method for predicting a heart condition based on impedance values using an implantable medical device
ActiveUS20050216067A1Reliably predict onset of heart conditionHeart stimulatorsDiagnostic recording/measuringPulmonary edemaThoracic cavity
Techniques are provided for predicting the onset of a heart condition within a patient based on impedance measurements. Briefly, overloads in fluid levels in the thorax and in ventricular myocardial mass within the patient are detected based on impedance signals sensed using implanted electrodes. The onset of certain heart conditions is then predicted based on the overloads. For example, pulmonary edema arising due to diastolic heart failure is predicted based on the detection of on-going overloads in both fluid levels and ventricular mass. Ventricular hypertrophy is detected based on an on-going ventricular mass overload without a sustained fluid overload. Various other heart conditions may also be predicted based on specific combinations of recent or on-going overloads. Evoked response is exploited to corroborate the predictions. Appropriate warning signals are generated and preemptive therapy is initiated.
Owner:PACESETTER INC
Implantable fluid management system for the removal of excess fluid
InactiveUS20050273034A1Increased risk of infectionAvoid difficultyWound drainsMedical devicesHydrocephalusPulmonary edema
An implantable fluid management device, designed to drain excess fluid from a variety of locations in a living host into a second location within the host, such as the bladder of that host. The device may be used to treat ascites, chronic pericardial effusions, normopressure hydrocephalus, hydrocephalus, pulmonary edema, or any fluid collection within the body of a human, or a non-human mammal.
Owner:SEQUANA MEDICAL NV
Implantable fluid management system for the removal of excess fluid
ActiveUS20050096582A1Increased risk of infectionAvoid difficultyWound drainsMedical devicesHydrocephalusPulmonary edema
An implantable fluid management device, designed to drain excess fluid from a variety of locations in a living host into a second location within the host, such as the bladder of that host. The device may be used to treat ascites, chronic pericardial effusions, normopressure hydrocephalus, hydrocephalus, pulmonary edema, or any fluid collection within the body of a human, or a non-human mammal.
Owner:SEQUANA MEDICAL NV
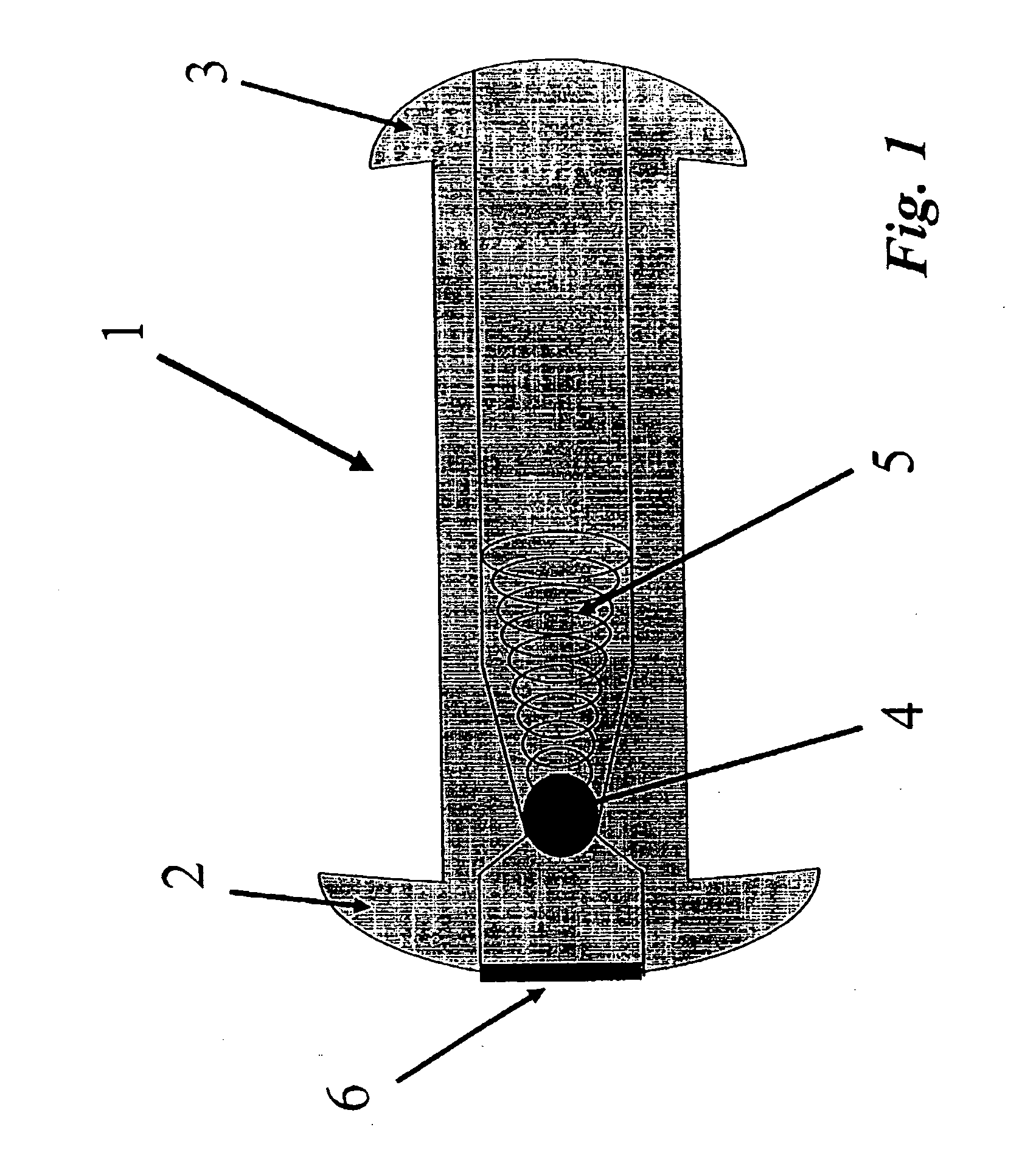
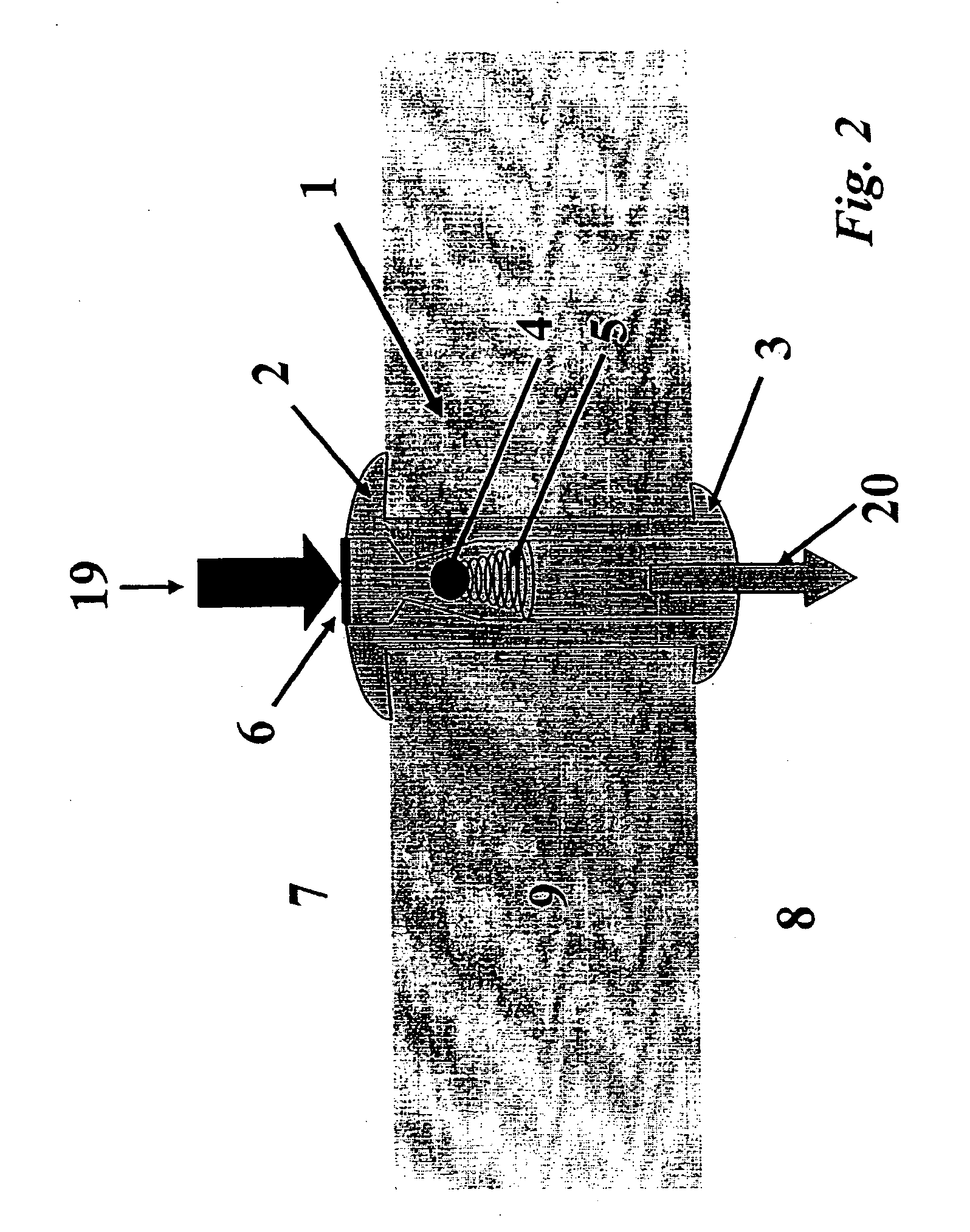
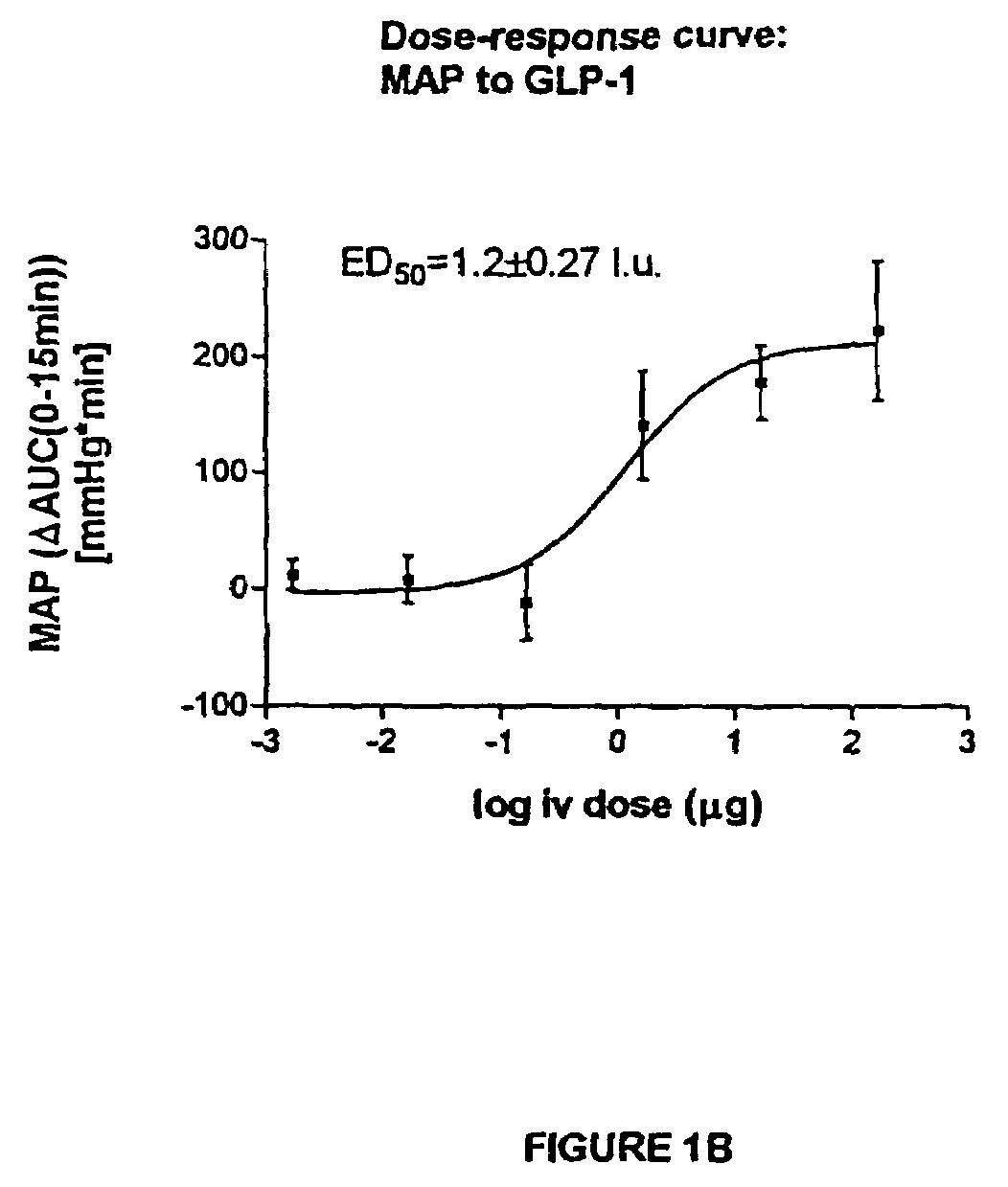
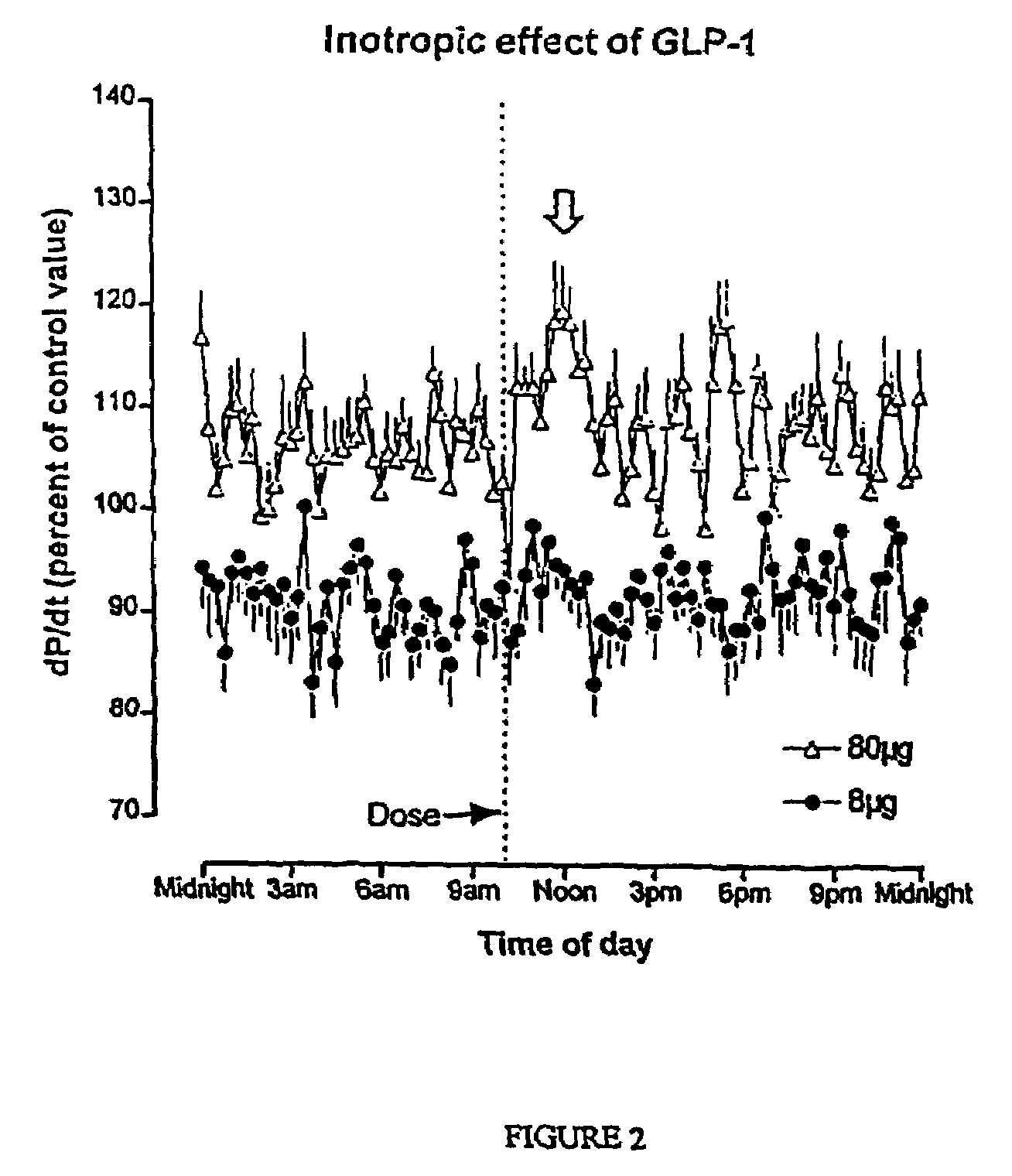
Inotropic and diuretic effects of GLP-1 and GLP-1 agonists
InactiveUS7442680B2Rapid inotropicRapid diuretic effectOrganic active ingredientsPeptide/protein ingredientsMuscle contractionPulmonary edema
Methods for increasing urine flow are disclosed, comprising administration of an effective amount of GLP-1, an exendin, or an exendin or GLP-1 agonist. Methods for increasing urinary sodium excretion and decreasing urinary potassium concentration are also disclosed. The methods are useful for treating conditions or disorders associated with toxic hypervolemia, such as renal failure, congestive heart failure, nephrotic syndrome, cirrhosis, pulmonary edema, and hypertension. The present invention also relates to methods for inducing an inotropic response comprising administration of an effective amount of GLP-1, an exendin, or an exendin or GLP-1 agonist. These methods are useful for treating conditions or disorders that can be alleviated by an increase in cardiac contractility such as congestive heart failure. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:AMYLIN PHARMA INC
Methods of reducing the risk of occurrence of pulmonary edema in children in need of treatment with inhaled nitric oxide
ActiveUS8282966B2Reduces occurrenceReduces riskNitrogen compoundsInorganic active ingredientsInhalationNitric oxide
The invention relates methods of reducing the risk or preventing the occurrence of an adverse event (AE) or a serious adverse event (SAE) associated with a medical treatment comprising inhalation of nitric oxide.
Owner:MALLINCKRODT HOSPITAL PRODUCTS IP LTD
Methods and compositions for disease treatment using inhalation
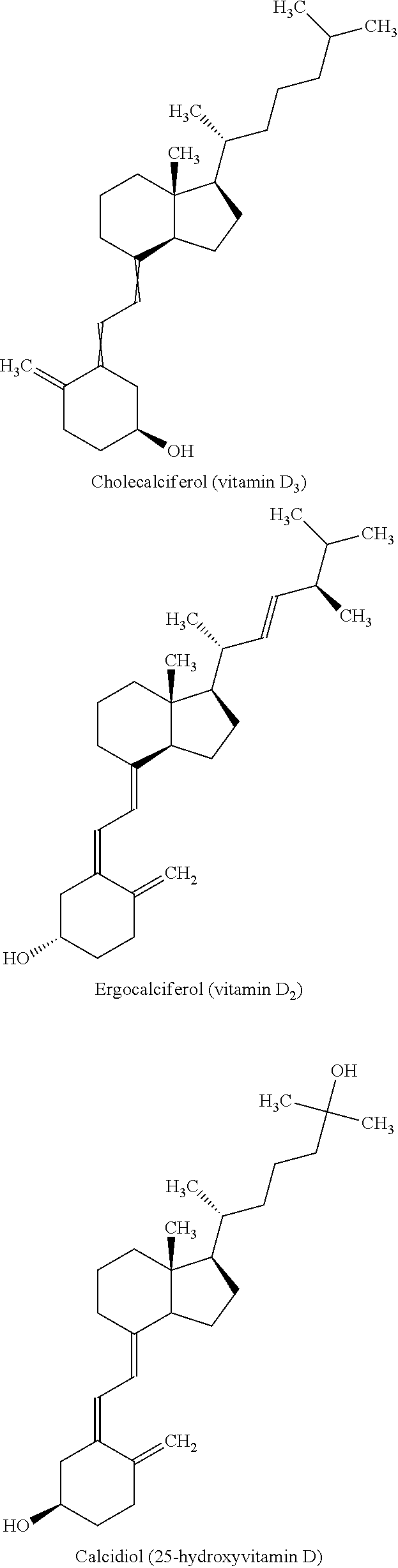
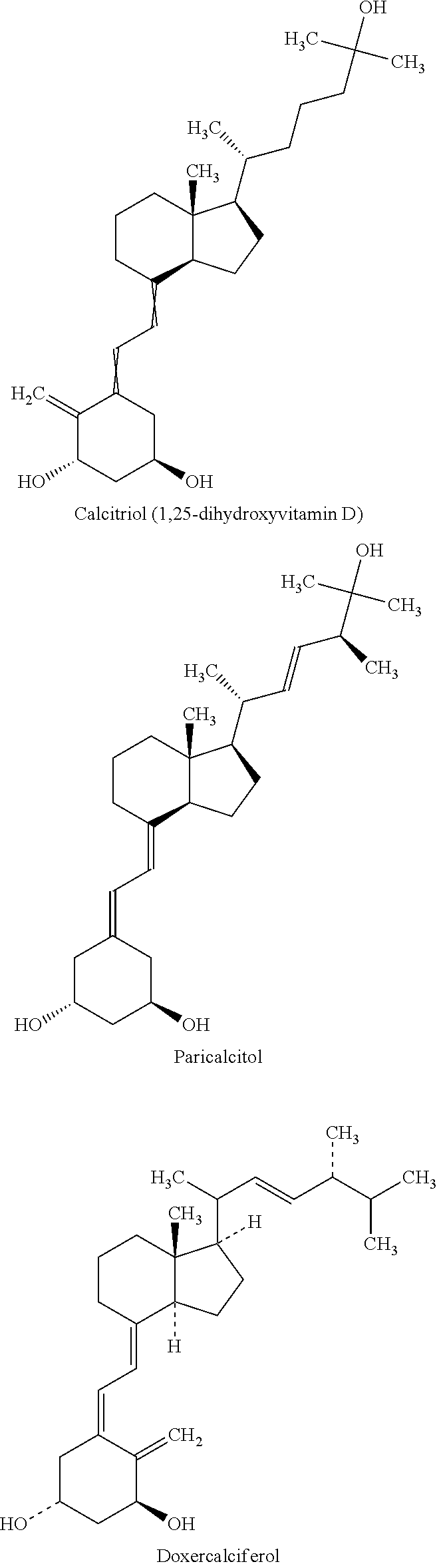
InactiveUS20120077786A1Increasing subject 's convenienceGood treatment complianceBiocideOrganic active ingredientsPulmonary edemaLung cancer
Methods and compositions for the treatment of pulmonary disease using inhalation are provided. In particular, the present disclosure provides novel methods and compositions for treating pulmonary diseases such as asthma, bronchitis, COPD, emphysema, lung cancer, pneumonia and pulmonary edema. In addition, the present disclosure provides novel methods and compositions for treating complications associated with pulmonary disease such as corticosteroid resistance and pulmonary tissue destruction. The compositions of the present disclosure comprise corticosteroid resistance agents including but not limited to vitamin D, calcitriol and equivalents thereof. The compositions of the present disclosure also comprise alveolar development and maintenance agents including but not limited to vitamin A, ATRA and equivalents thereof. The present invention provides effective administration of therapeutic agents to specific airways of the lungs by utilizing controlled site delivery.
Owner:MICRODOSE THERAPEUTX INC
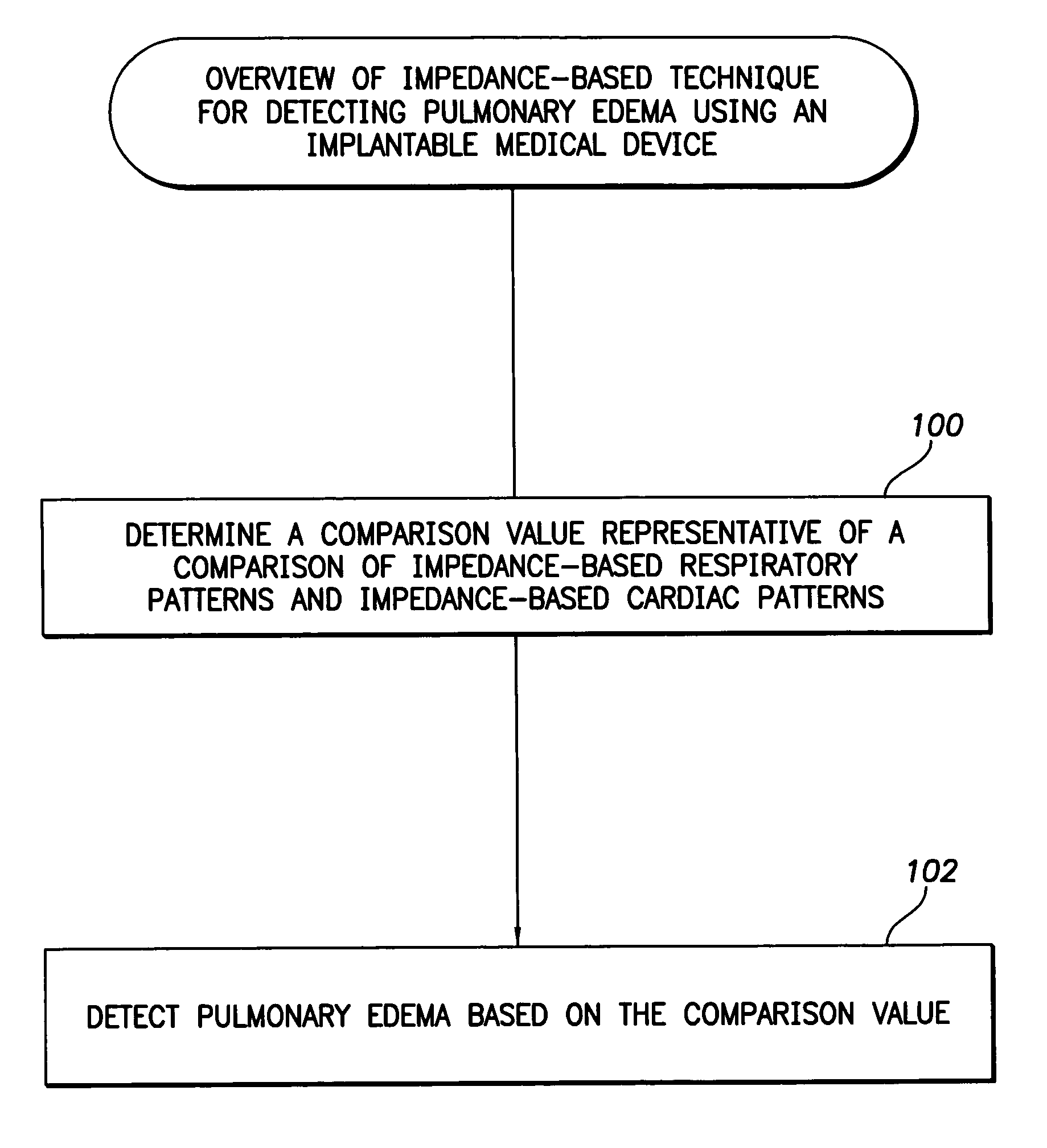
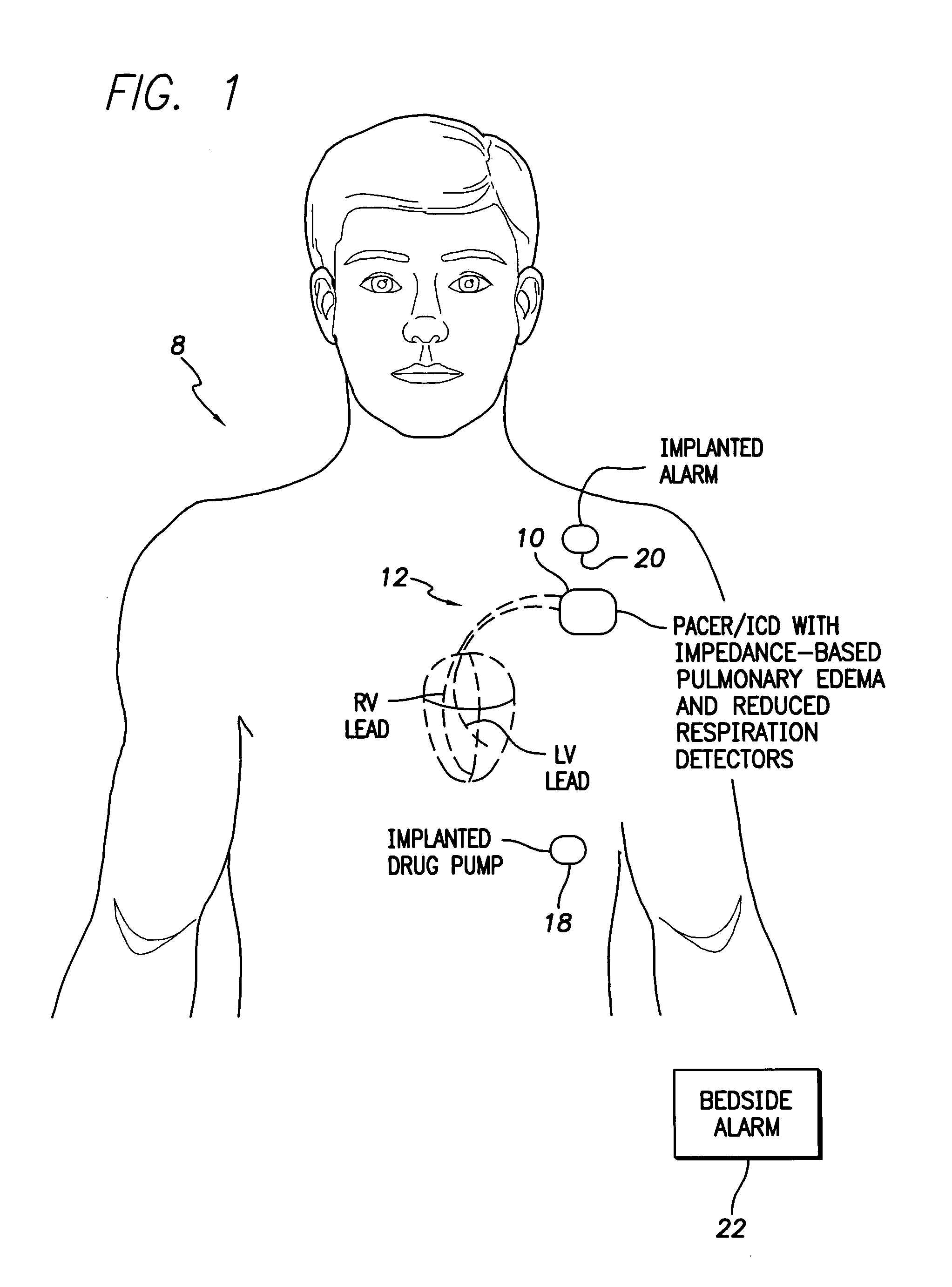
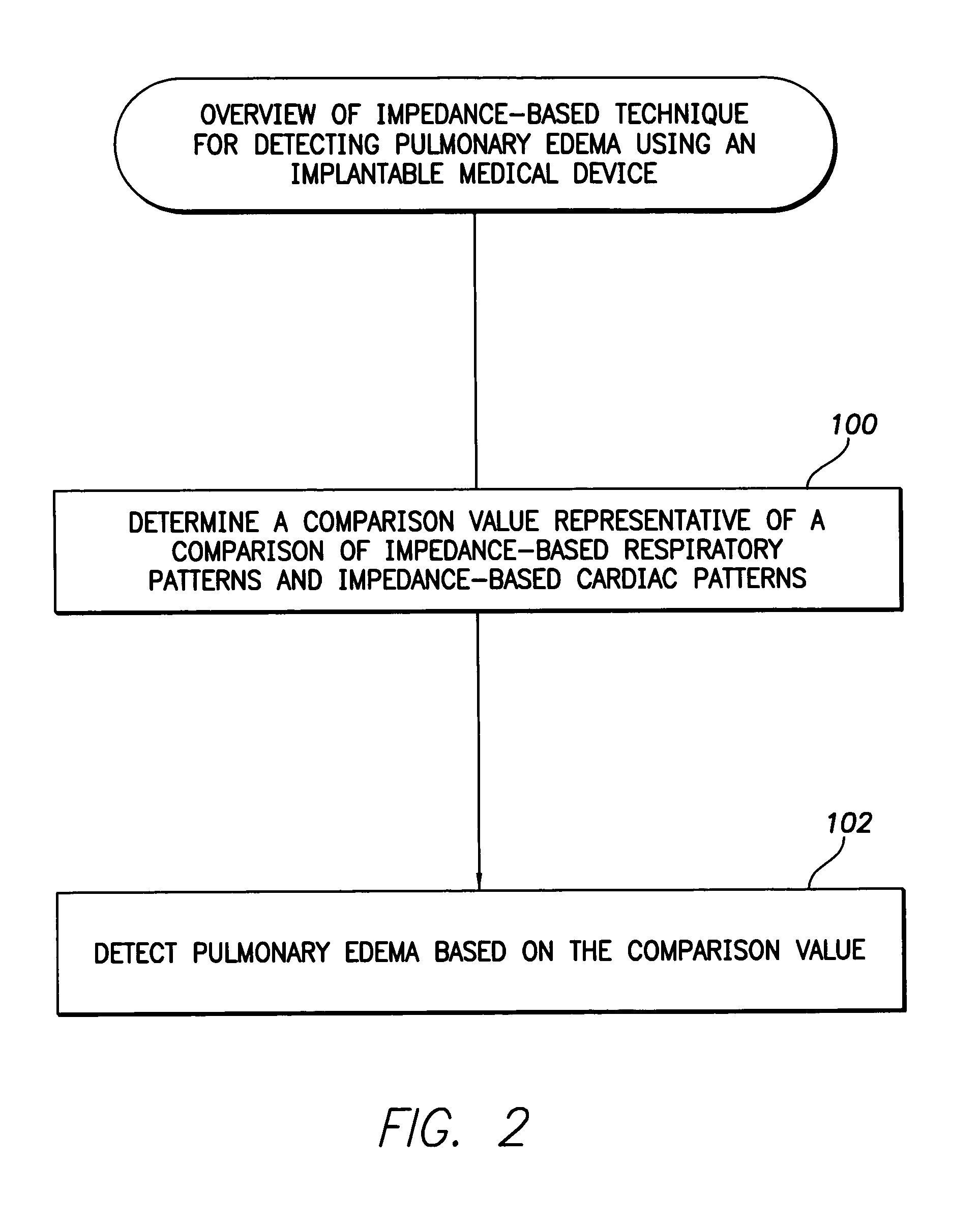
System and method for impedance-based detection of pulmonary edema and reduced respiration using an implantable medical system
Techniques are provided for detecting pulmonary edema based on a comparison of impedance-based respiratory patterns and impedance-based cardiac patterns, i.e. patterns derived from thoracic impedance signals. In one example, a numerical ratio is calculated between average peak-to-peak amplitudes of the respiratory patterns and average peak-to-peak amplitudes of the cardiac patterns. Pulmonary edema is detected if the amplitude ratio falls below a pulmonary edema detection threshold. Techniques are also provided for controlling operation of an impedance-based reduced respiration detector, i.e. a detector which seeks to detect apnea, hypopnea, or the like, based on analysis of respiratory patterns derived from a thoracic impedance signal. If the numerical ratio falls below a minimum reliability threshold, then the reduced respiration detector is deactivated because episodes of reduced respiration cannot then reliably be derived from an analysis of respiration patterns obtained from the thoracic impedance signals.
Owner:PACESETTER INC
Novel PPAR ligands that do not cause fluid retention, edema or congestive heart failure
Methods are provided for treating or prophylactically preventing metabolic disorders in humans without causing, promoting, or aggravating fluid retention, peripheral edema, pulmonary edema, or congestive heart failure, by administration of a therapeutically effective amount of a compound sufficient to partially or fully activate peroxisome proliferator activated receptors (PPARs) and partially or fully inhibit, antagonize or block the activity of angiotensin II type 1 receptors. Metabolic disorders that can be treated or prevented include but are not limited to type 2 diabetes, the metabolic syndrome, prediabetes, and other insulin resistance syndromes. Compounds are provided that antagonize or block the angiotensin II type 1 (AT1) receptor, function as partial or full activators of peroxisome proliferator activated receptors (PPARs), can be used to treat or prevent diseases known to be treatable or preventable by PPAR activators and were not previously recognized to be therapeutic targets for angiotensin II receptor antagonists.
Owner:BETHESDA PHARMA
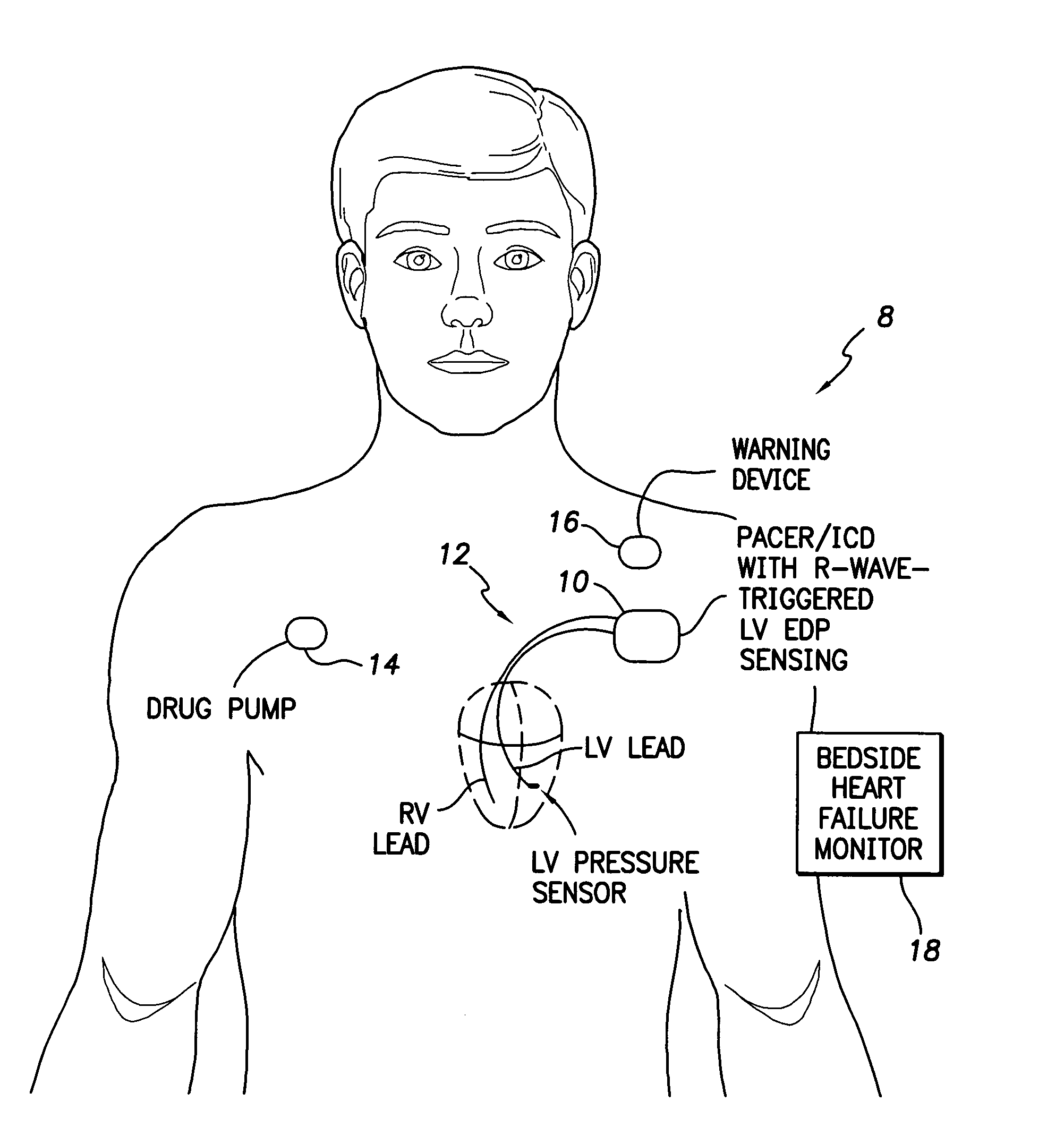
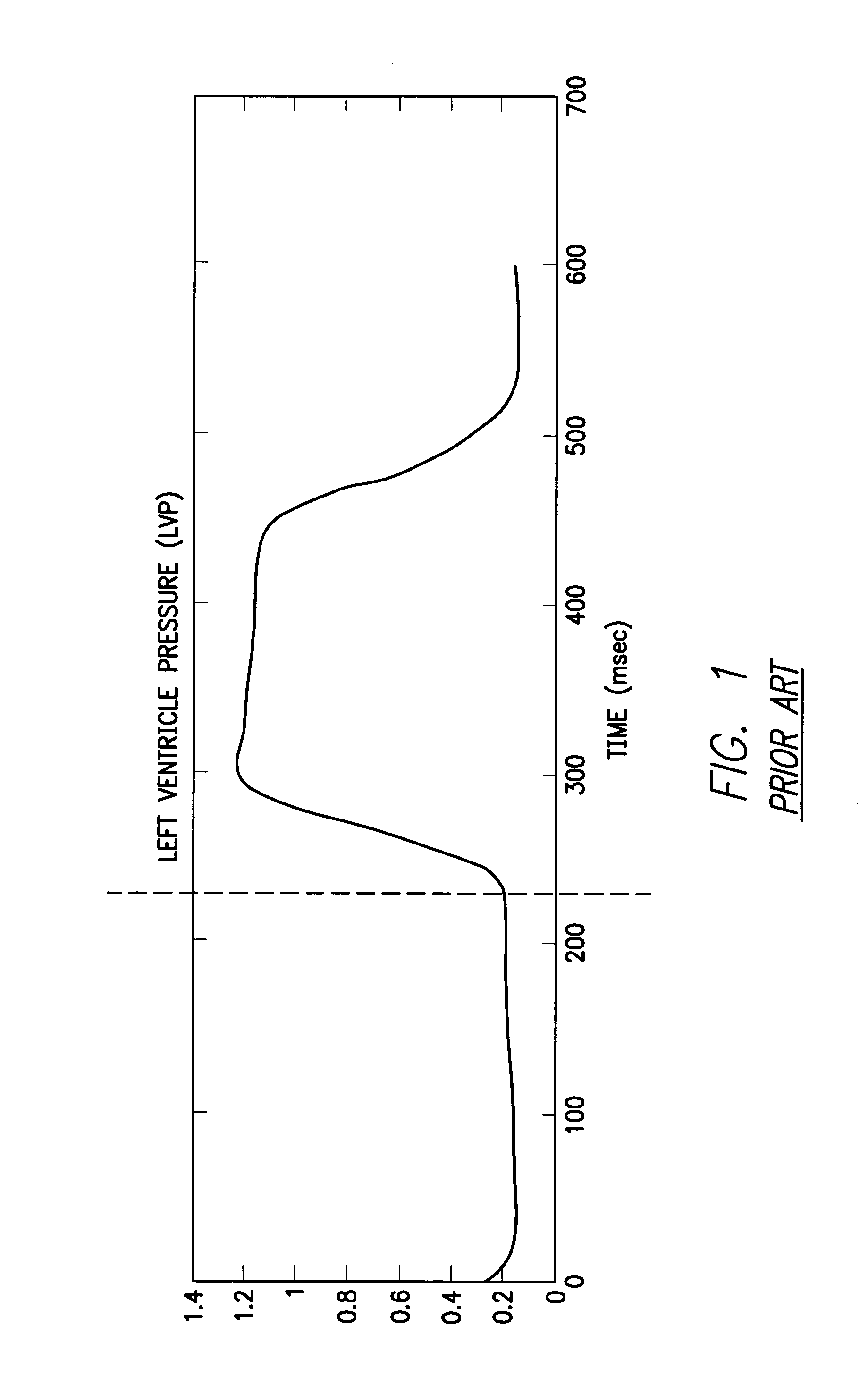
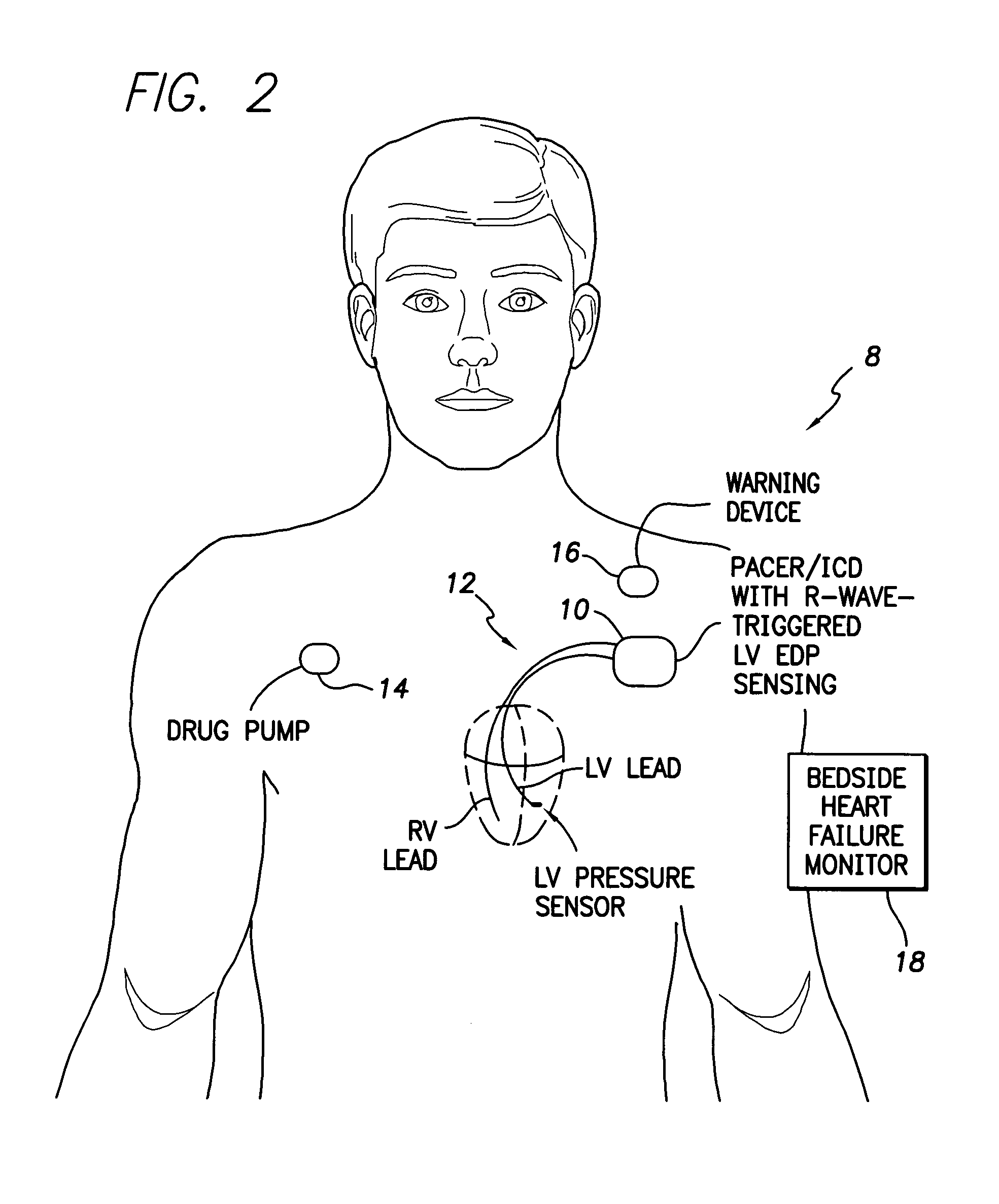
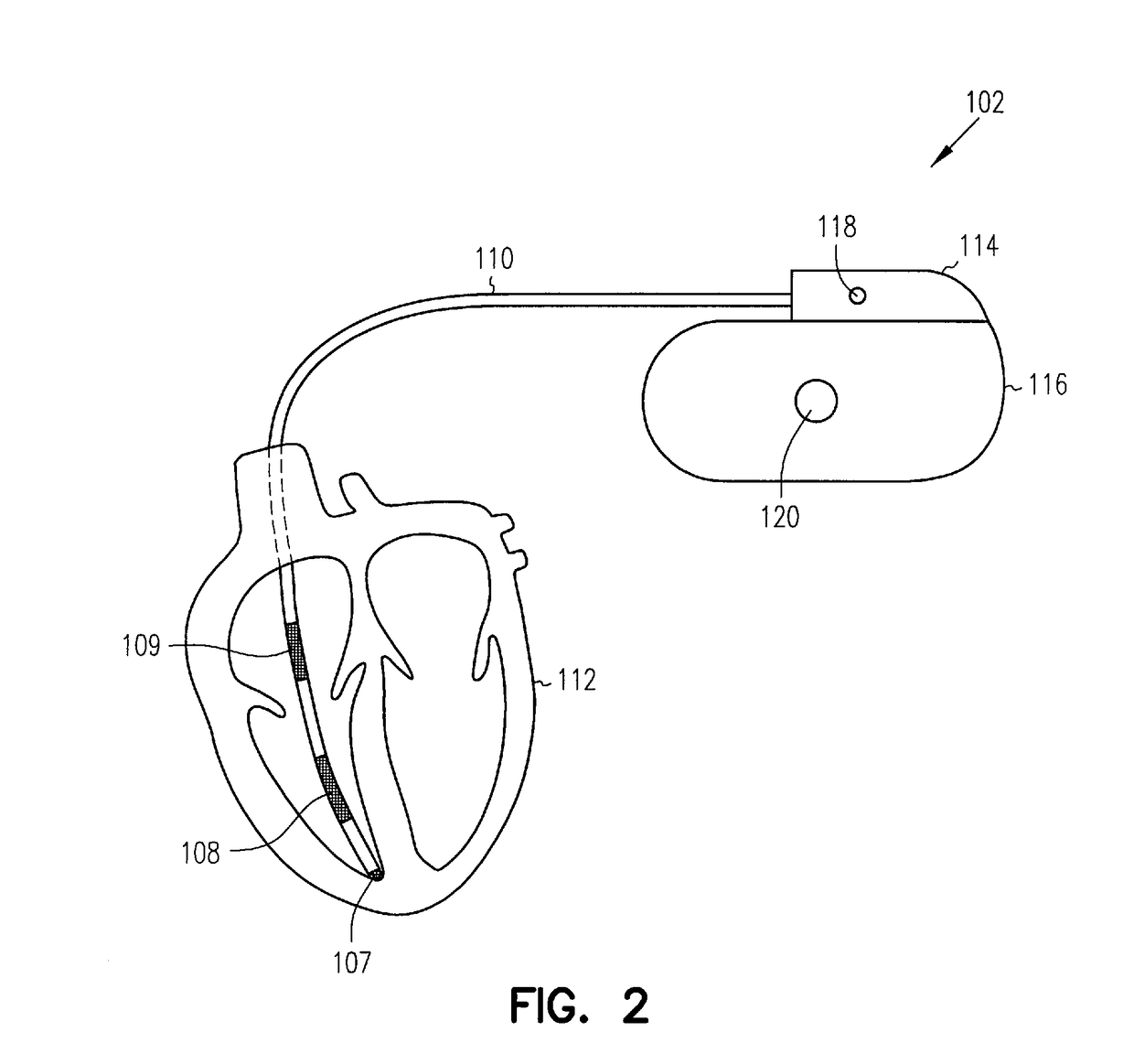
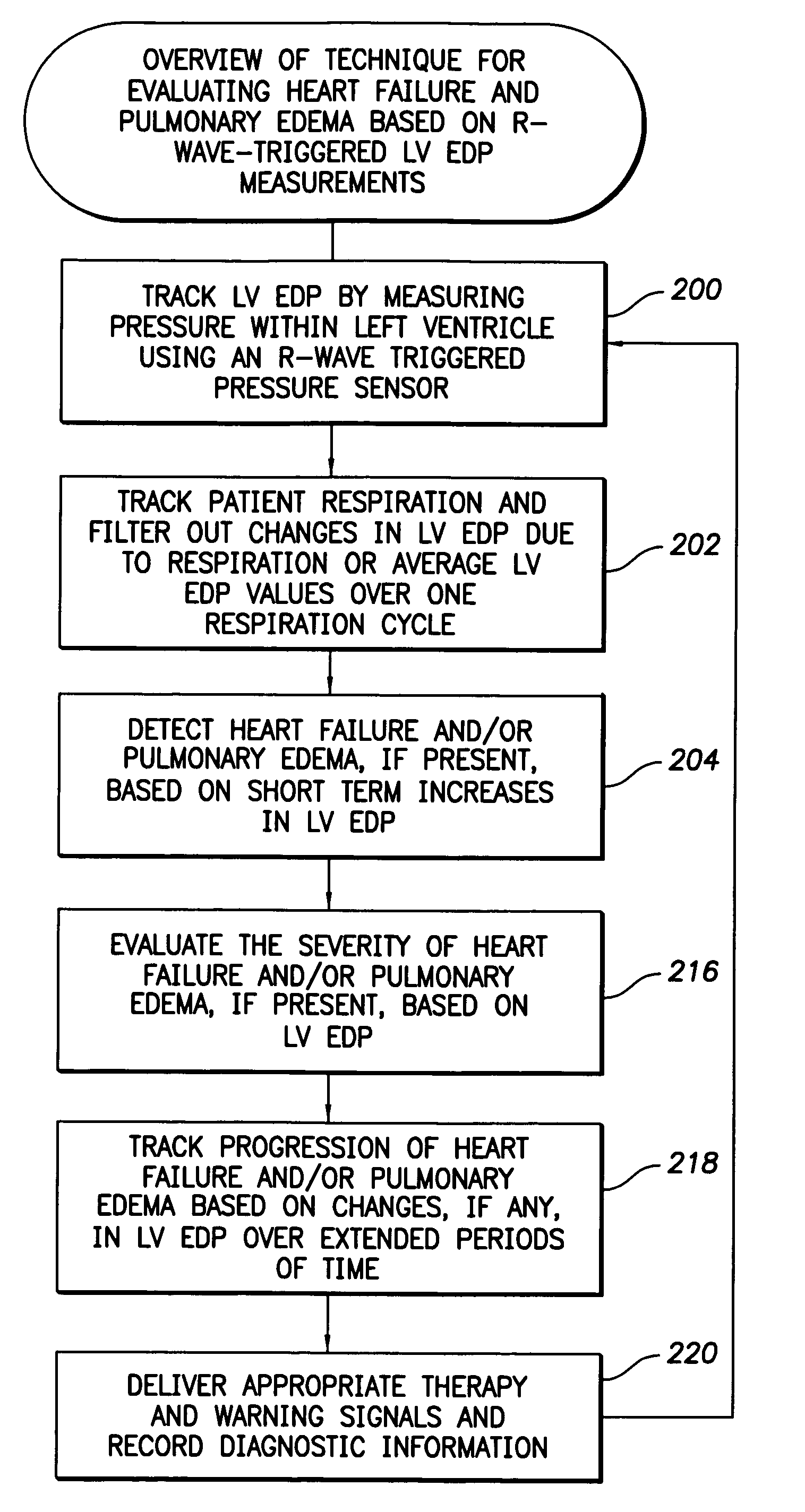
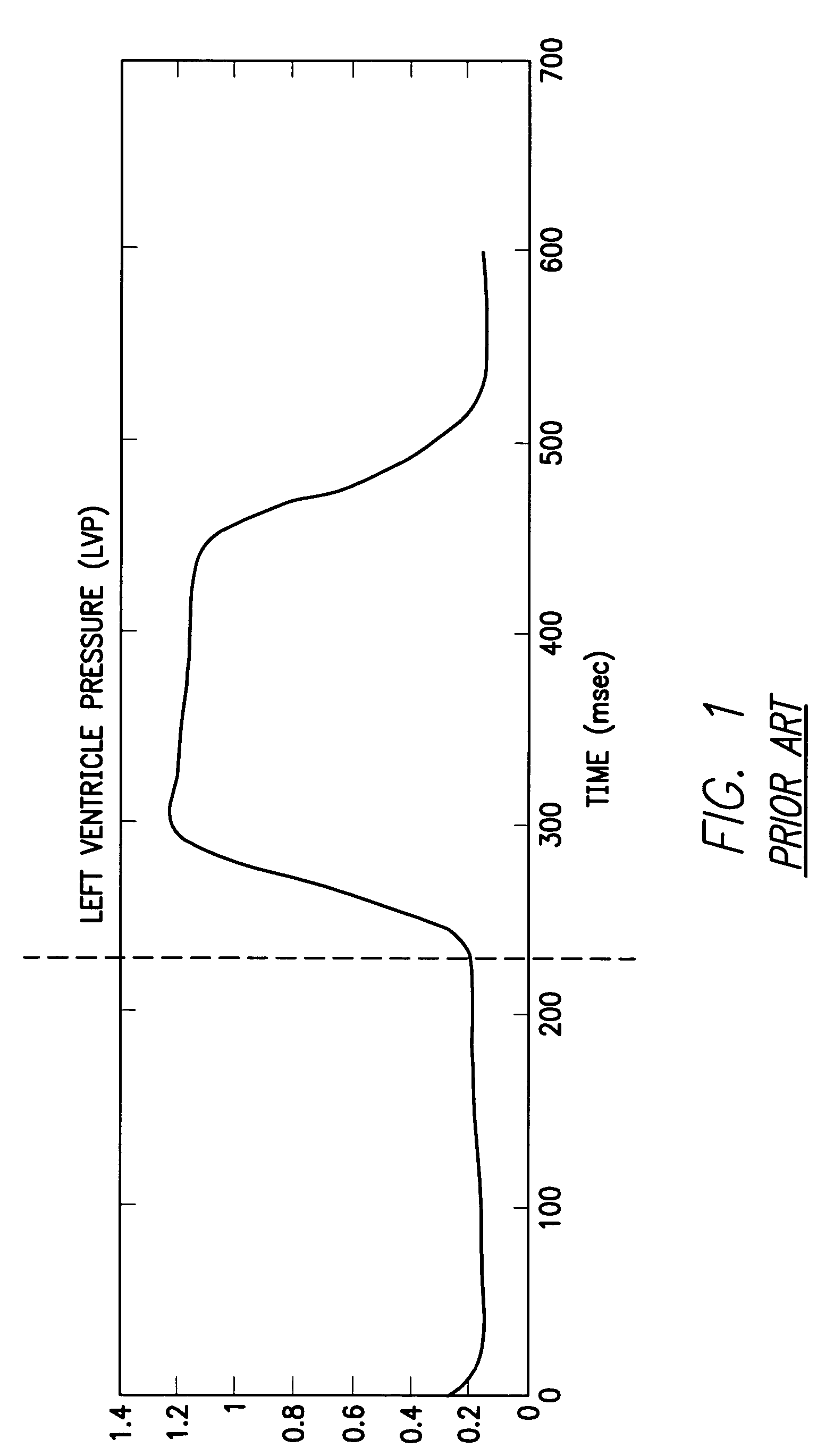
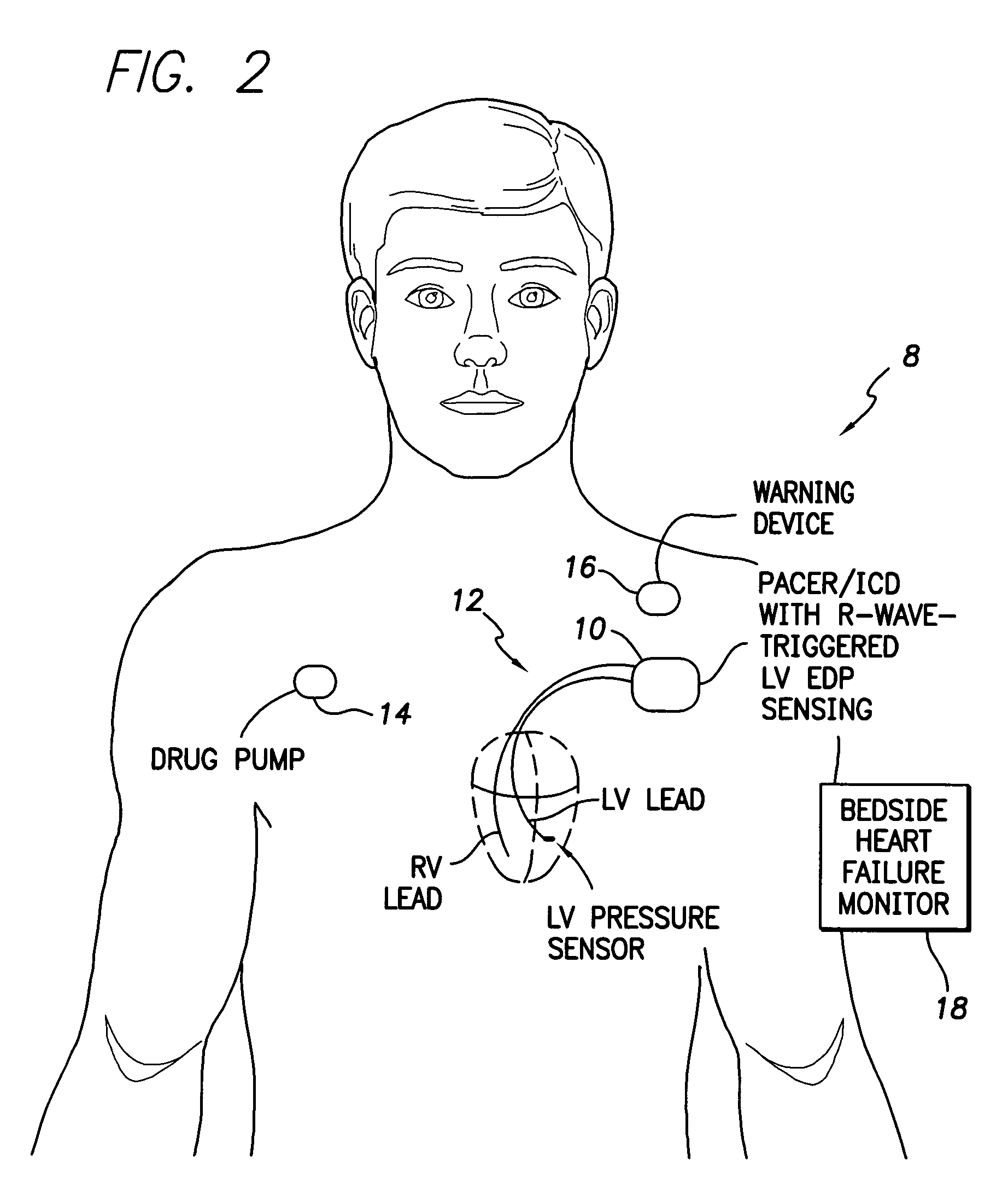
System and method for detecting heart failure and pulmonary edema based on ventricular end-diastolic pressure using an implantable medical device
InactiveUS20060224190A1Easy to detectEliminate needElectrotherapyPressure infusionLeft ventricular sizePulmonary edema
Techniques are provided for detecting left ventricular end diastolic pressure (LV EDP) using a pressure sensor implanted within the heart of a patient and for detecting and evaluating heart failure and pulmonary edema based on LV EDP. Briefly, the peak of the R-wave of an intracardiac electrogram (IEGM) is used to trigger the measurement of a pressure value within the left ventricle. This pressure value is deemed to be representative of LV EDP. In this manner, LV EDP is easily detected merely by measuring pressure at one point within the heartbeat—thereby eliminating any need to track ventricular pressure throughout the heartbeat. Techniques for detecting and evaluating heart failure and pulmonary edema based on the R-wave triggered LV EDP measurements are also set forth herein.
Owner:PACESETTER INC
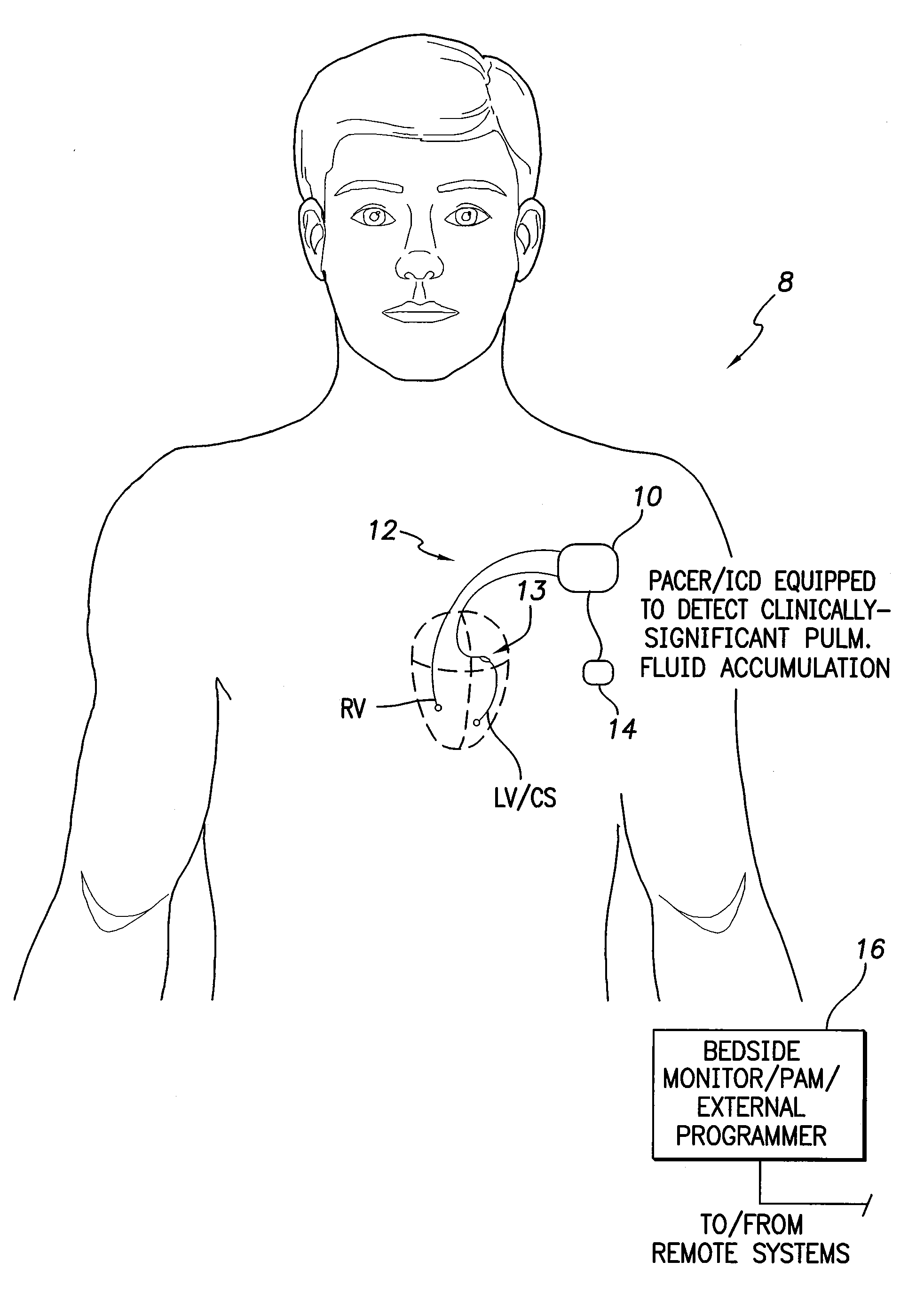
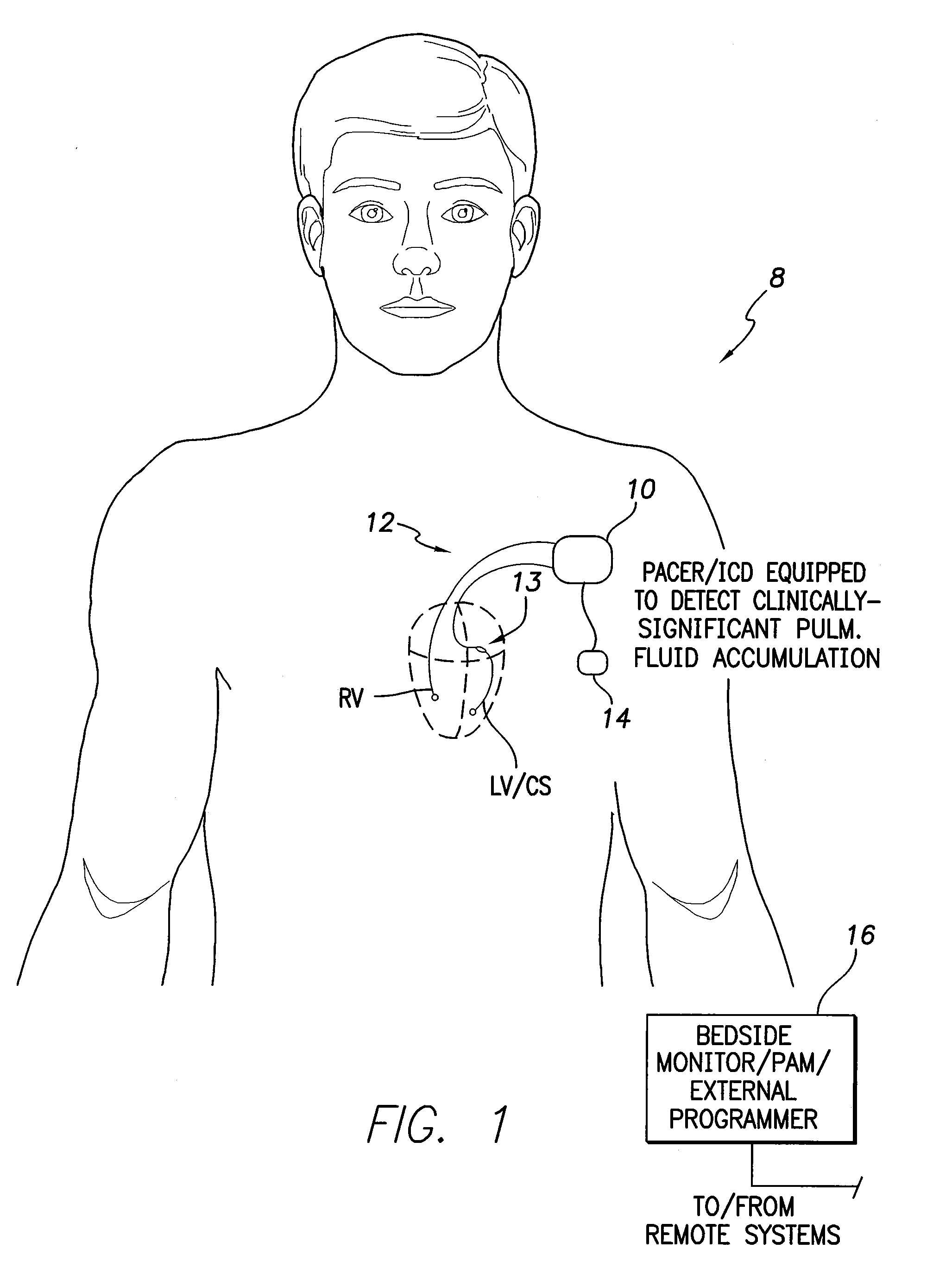
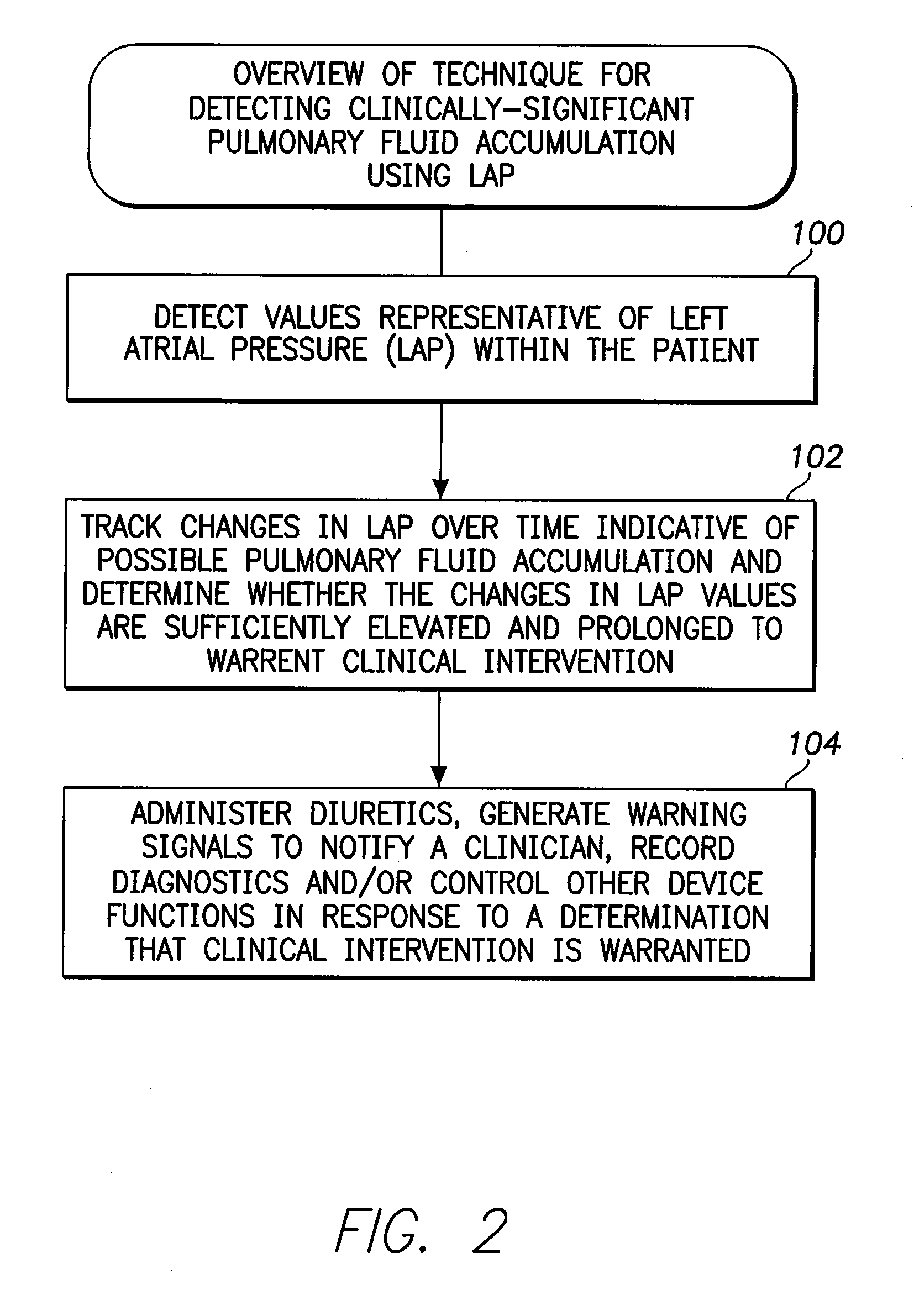
System and Method for Detecting a Clinically-Significant Pulmonary Fluid Accumulation Using an Implantable Medical Device
InactiveUS20120190991A1Evaluation of blood vesselsCatheterCardiac pacemaker electrodeLeft atrial pressure
Techniques are provided for detecting a clinically-significant pulmonary fluid accumulation within a patient using a pacemaker or other implantable medical device. Briefly, the device detects left atrial pressure (LAP) within the patient and tracks changes in the LAP values over time that are indicative of possible pulmonary fluid accumulation within the patient. The device determines whether the changes in LAP values are sufficiently elevated and prolonged to warrant clinical intervention using, e.g., a predictor model-based technique. If the fluid accumulation is clinically significant, the device then generates warning signals, records diagnostics, controls therapy and / or titrates diuretics. False positive detections of pulmonary edema due to transients in LAP are avoided with this technique. Pulmonary artery pressure (PAP)-based techniques are also described.
Owner:PACESETTER INC
Diagnostic reagent kit (enzyme-linked immunosorbent assay (ELISA)) for enterovirus (EV) 71-type antibody (immune globulin M (IgM))
InactiveCN102243232AEasy to operateAvoid distractionsColor/spectral properties measurementsEnterovirusAbzyme
The invention relates to the field of biomedicine, in particular to an enzyme-linked immunization diagnostic reagent kit for detecting an enterovirus (EV) 71-type antibody (immune globulin M (IgM)), and a preparation method and application of the diagnostic reagent kit. The probability of hand-foot-and-mouth disease and severe infection (viral encephalitis, viral cerebrospinal meningitis and pulmonary edema) caused by EV71 type is relatively higher, and case fatality rate is relatively higher and can be 10 to 25 percent. The enzyme-linked immunization diagnostic reagent kit of the EV71-IgM antibody can be used for diagnosing the infection of the EV71 type. According to related documents about the detection of the EV71-IgM, EV71 virus cultures serving as indirect enzyme-linked immuno sorbent assay (ELISA) of envelope antigens has defects in such aspects as specificity, sensitivity and stability, and due to high cultivation cost and low efficiency, a large amount of virus cannot be supplied to the market. In order to overcome the defects, the invention provides the reagent kit which is used for detecting the EV71-IgM in human blood serum, required by clinical examination, simple and convenient to operate and applicable to all medical disease control departments, and the preparation method and the application of the reagent kit. The invention has the technical scheme that: firstly, the human blood serum is added into a micro-pore plate, wherein the IgM antibody is obtained by an anti-mu chain which is pre-enveloped on the micro-pore plate, and other uncombined components are washed and removed; secondly, an enzyme labeling object is added, the EV71-IgM in the obtained IgM can be combined with the specificity of an EV71 recombinant antigen which is labeled by horse radish peroxidase (HRP), and after washing, the HRP can react with substrates which are added subsequently; and finally, the aim of detecting the EV71-IgM antibody is fulfilled.
Owner:BEIJING BEIER BIOENG
System and method for analyzing an impedance course
ActiveUS20090216145A1Easy to implementHigh sensitivityRespiratory organ evaluationHeart stimulatorsEngineeringPulmonary edema
A system which generates a warning signal in the event of looming pulmonary edema and / or looming decompensation. The system has an impedance detection unit for determining impedance values, which represent a transthoracic impedance course, and an impedance analysis unit (78), which is connected to the impedance detection unit. The impedance analysis unit (78) is implemented to determine the degree of modulation for a particular impedance course detected by the impedance detection unit.
Owner:BIOTRONIK SE & CO KG
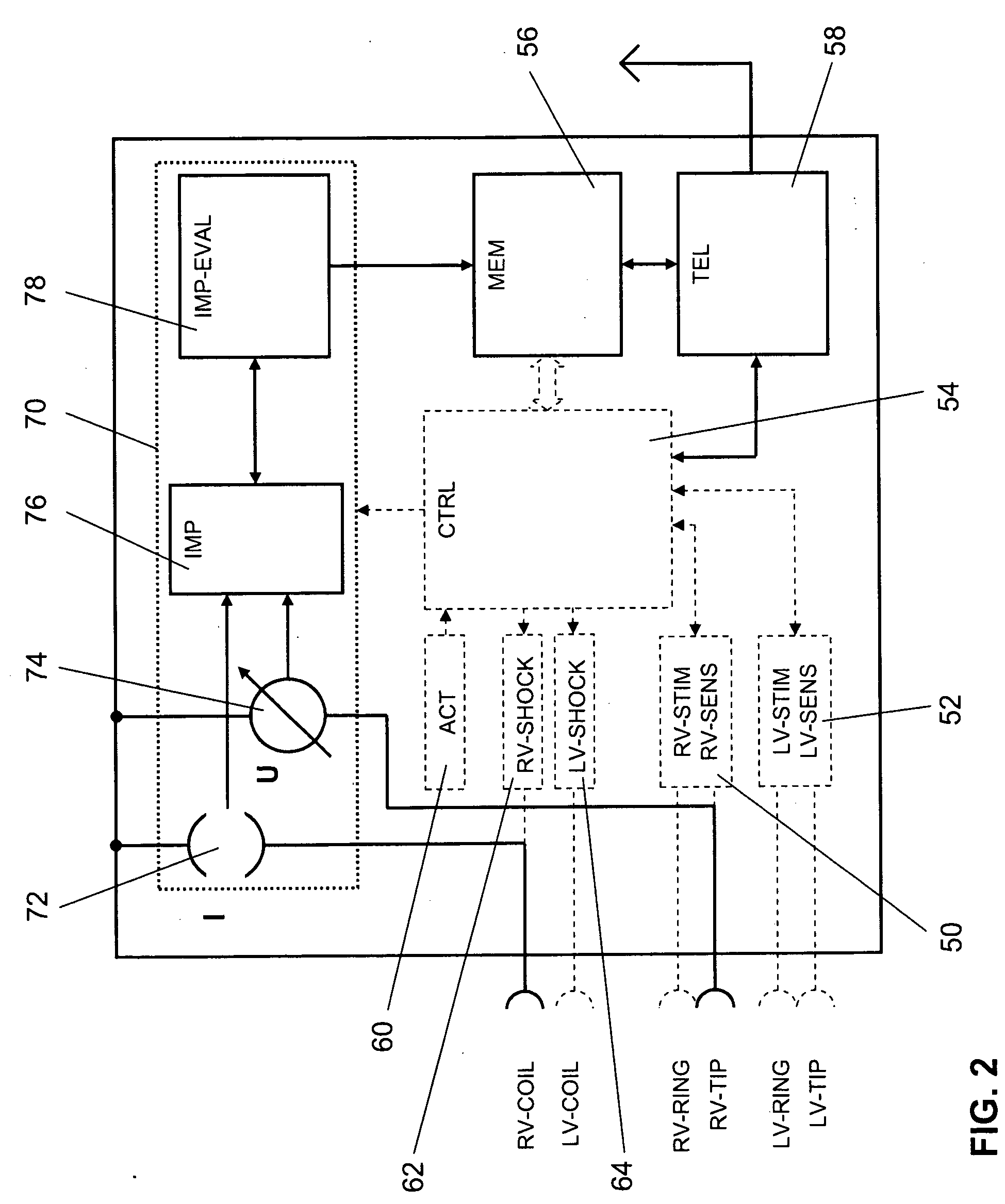
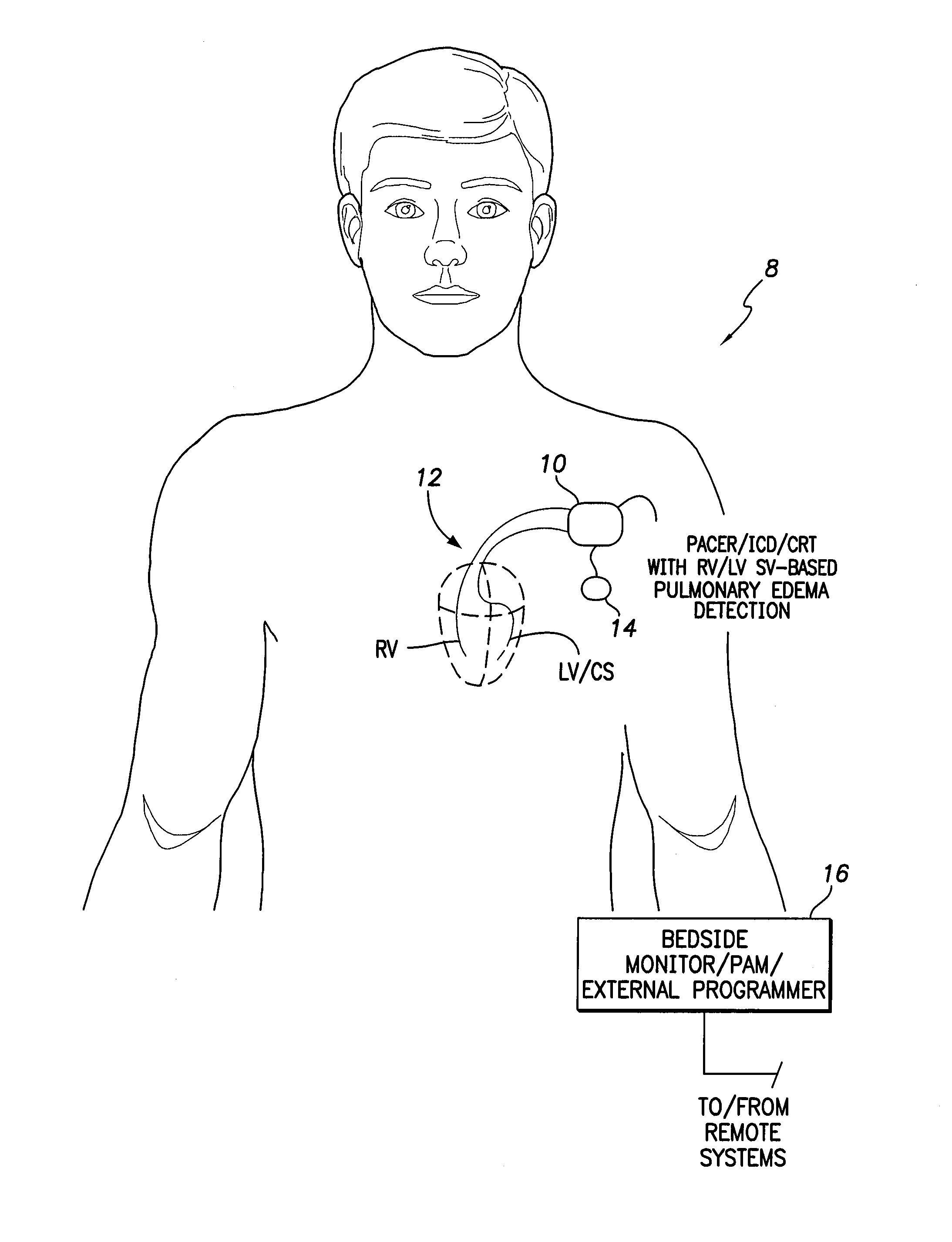
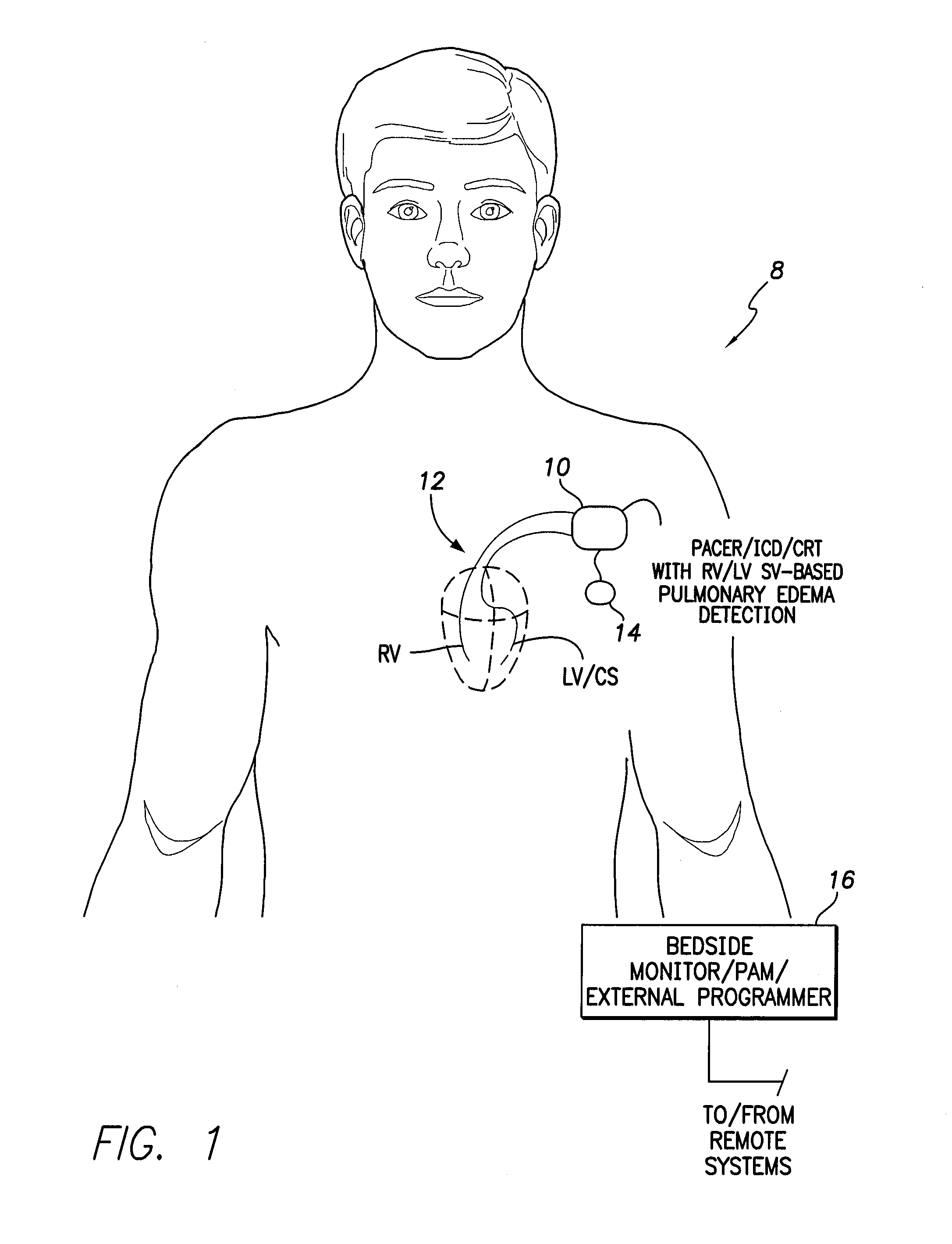
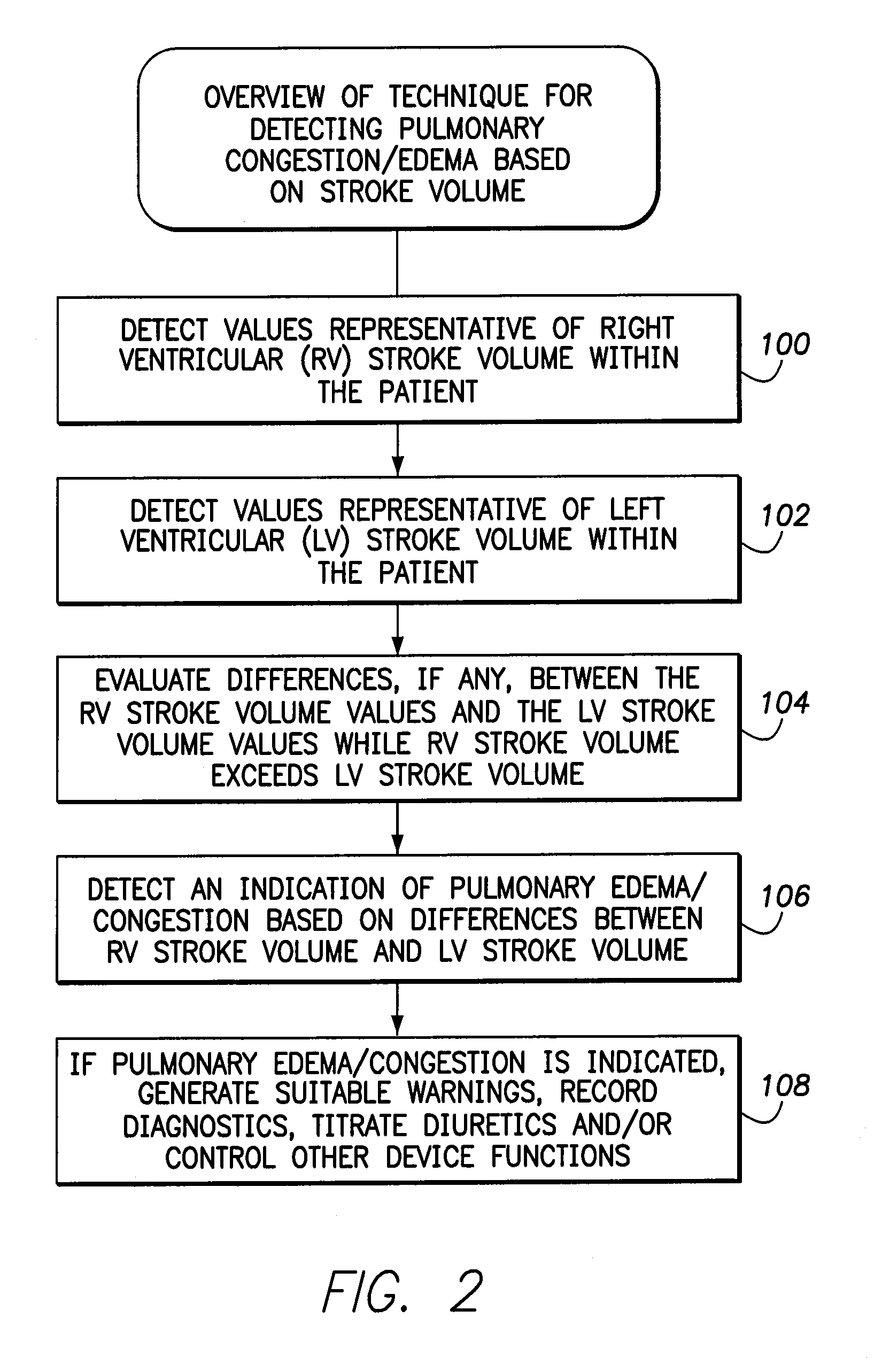
System and method for detecting pulmonary congestion based on stroke volume using an implantable medical device
ActiveUS20130184545A1Large indexGreat imbalanceElectrotherapyElectrocardiographyLeft ventricular sizeLeft atrial pressure
Techniques are provided for detecting pulmonary congestion based on an increase in right ventricular (RV) stroke volume over left ventricular (LV) stroke volume. In one example, the device generates an index based on accumulated differences between RV stroke volume and LV stroke volume while RV stroke volume exceeds LV stroke volume, such that the index is indicative of an ongoing imbalance between RV and LV stroke volume. The index is compared to a suitable threshold to detect a severe imbalance indicative of pulmonary edema. Additionally, techniques are described for estimating RV and LV stroke volumes based on pulmonary artery pressure, left atrial pressure, aortic pressure, LV strain or on various intracardiac or extracardiac impedance measurements.
Owner:PACESETTER INC
PPAR Ligands that do not cause fluid retention, edema or congestive heart failure
Methods are provided for treating or prophylactically preventing metabolic disorders in humans without causing, promoting, or aggravating fluid retention, peripheral edema, pulmonary edema, or congestive heart failure, by administration of a therapeutically effective amount of a compound sufficient to partially or fully activate peroxisome proliferator activated receptors (PPARs) and partially or fully inhibit, antagonize or block the activity of angiotensin II type 1 receptors. Metabolic disorders that can be treated or prevented include but are not limited to type 2 diabetes, the metabolic syndrome, prediabetes, and other insulin resistance syndromes. Compounds are provided that antagonize or block the angiotensin II type 1 (AT1) receptor, function as partial or full activators of peroxisome proliferator activated receptors (PPARs), can be used to treat or prevent diseases known to be treatable or preventable by PPAR activators and were not previously recognized to be therapeutic targets for angiotensin II receptor antagonists.
Owner:BETHESDA PHARMA
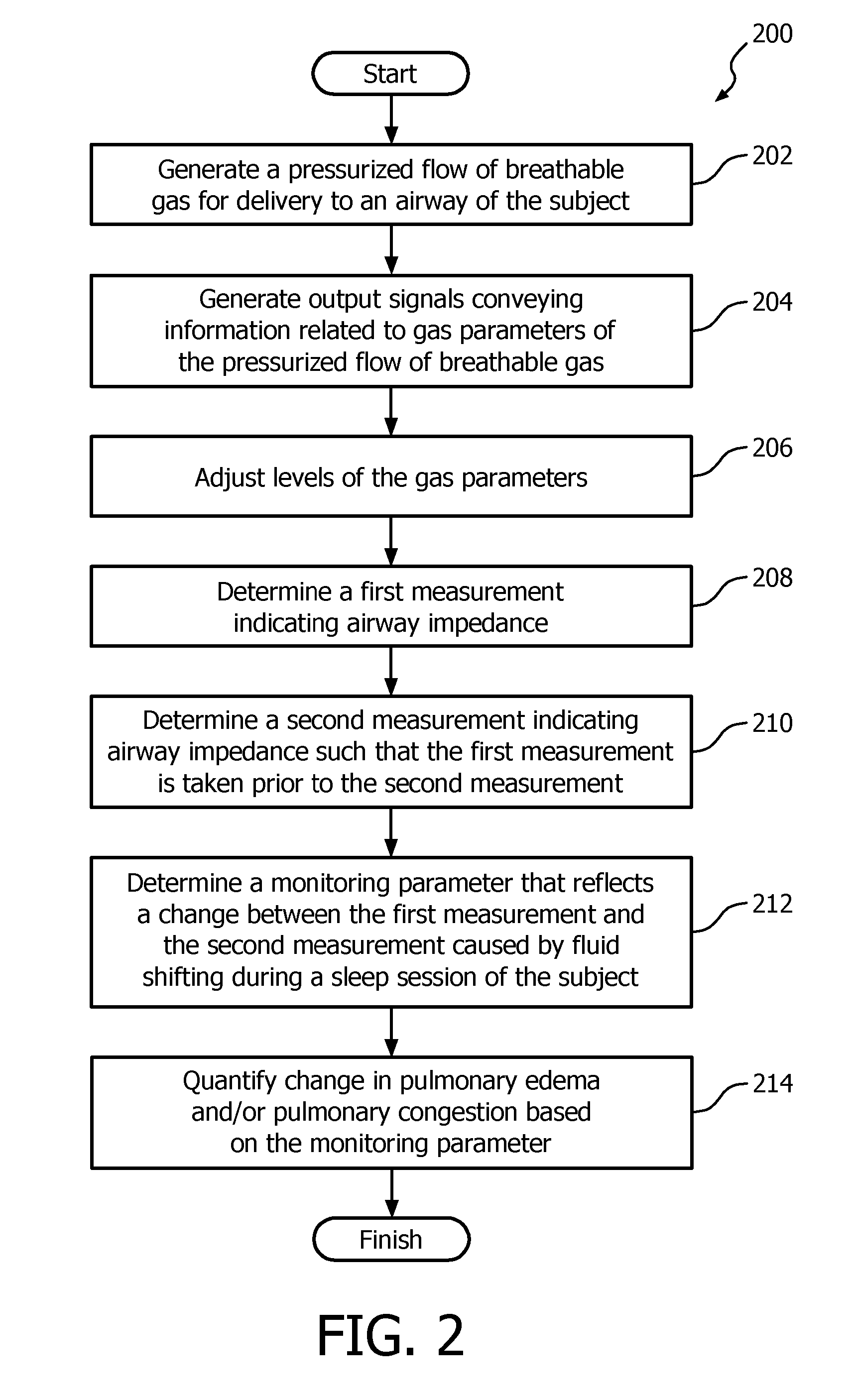
Detection of respiratory disorders
Systems and methods for monitoring pulmonary edema and / or pulmonary congestion are based on changes in impedance parameters that are indicative of lung impedance of a patient. Early detection of changes in the status of a patient pertaining to pulmonary edema and / or pulmonary congestion may be especially beneficial for heart failure patients. Quantification is based on differentials, e.g. within a span of 24 hours, of one or more lung impedance parameters.
Owner:KONINKLJIJKE PHILIPS NV
Device and method for predicting and preventing pulmonary edema and management of treatment thereof
InactiveUS20100106046A1Diagnostic recording/measuringSensorsProphylactic treatmentTherapy management
A device and a method for preventive treatment of evolving pulmonary edema in patients which are at risk of complication associated with pulmonary edema which is based on the monitoring of internal thoracic impedance of the patient. The device extracts the internal thoracic impedance from measured trans-thoracic impedance and is relatively immune to variations in skin / electrode interface impedance. The method includes identification of a stage of interstitial edema development before the appearance of a clinical indication and the beginning of an appropriate medicinal treatment in accordance to variations of the monitored internal thoracic impedance. The method also indicates the appropriate moment for terminating the medicinal treatment and can be applied when the patient and his treating physician are positioned at remote locations.
Owner:SHOCHAT MICHAEL +1
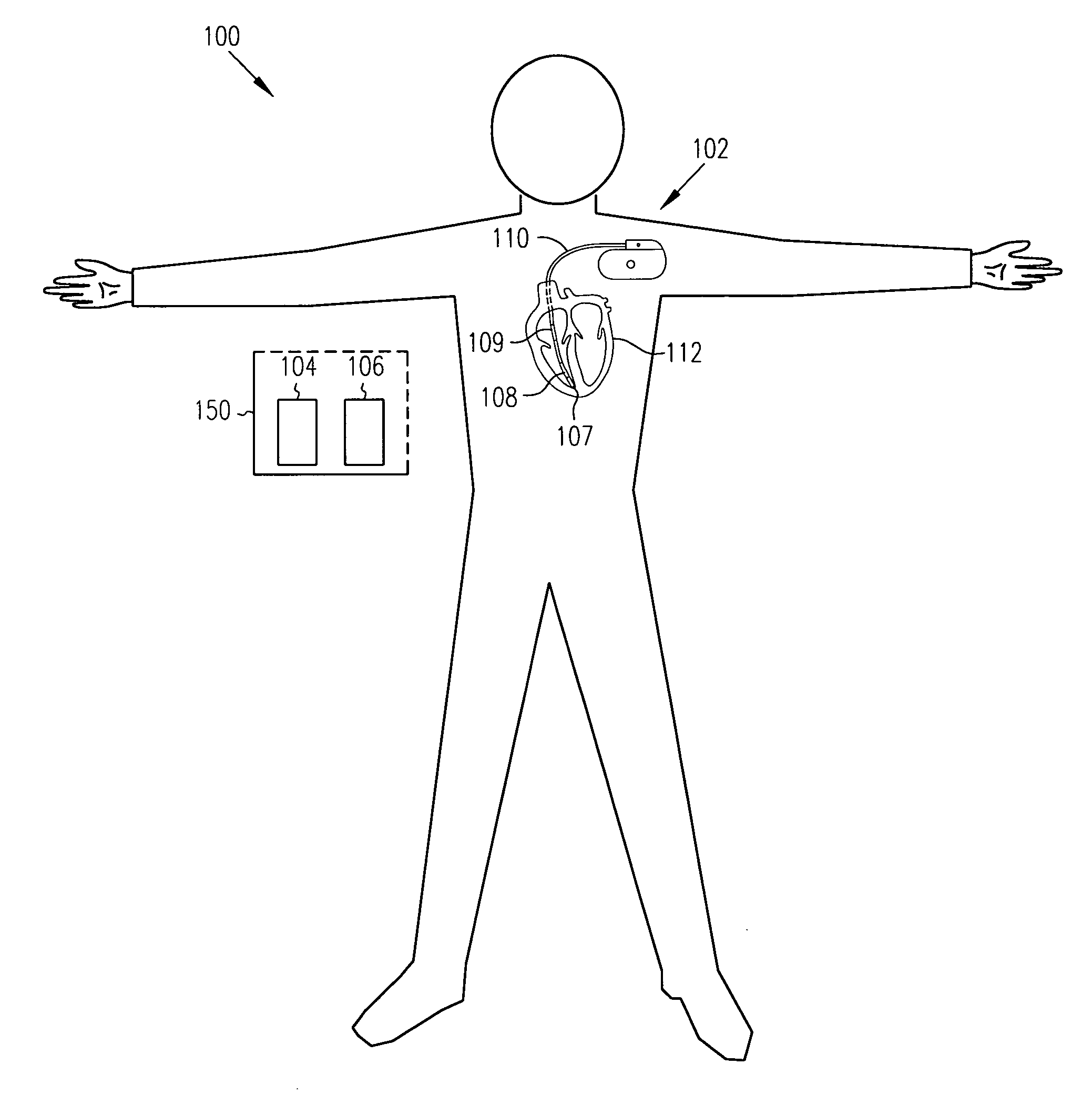
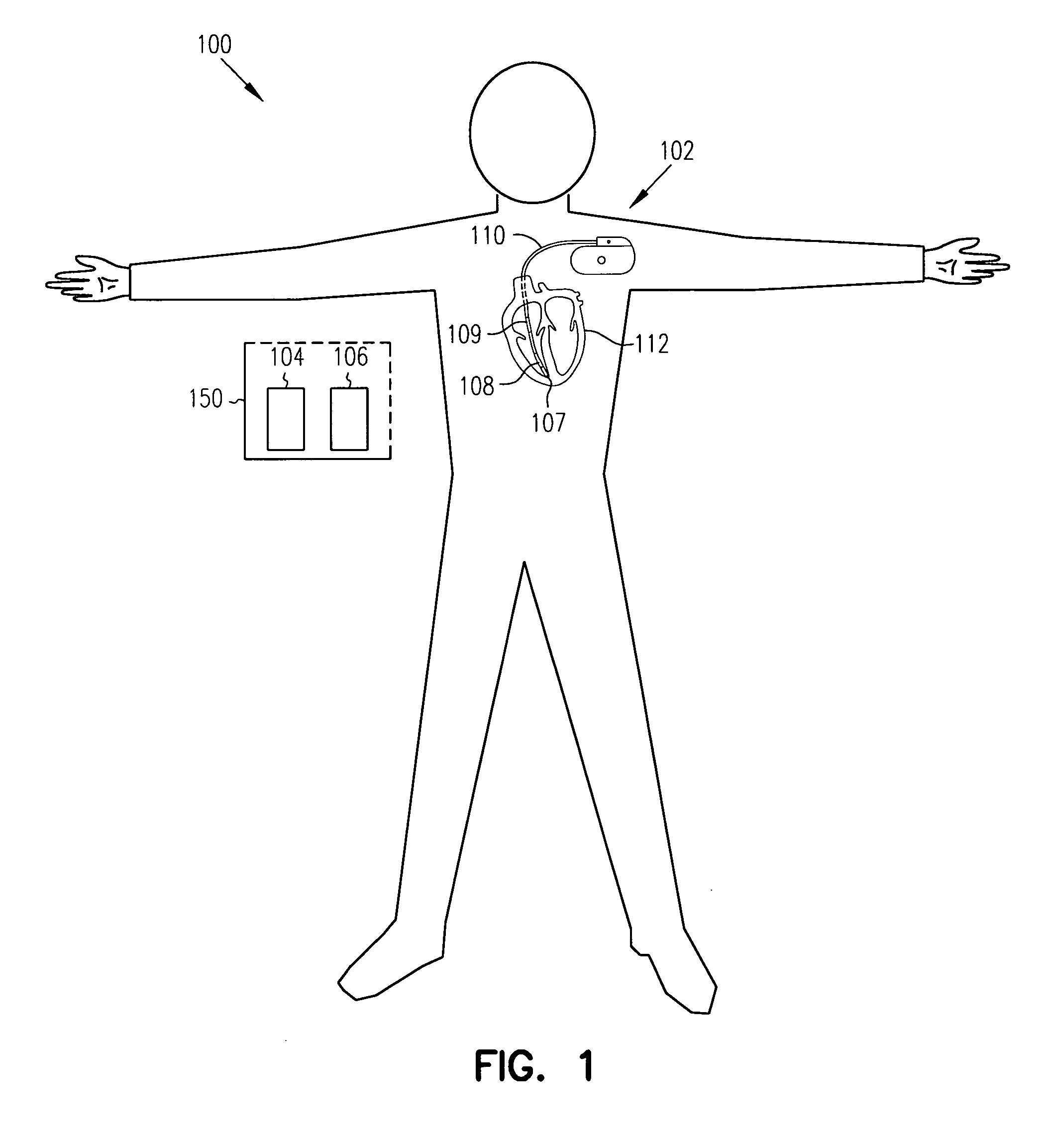
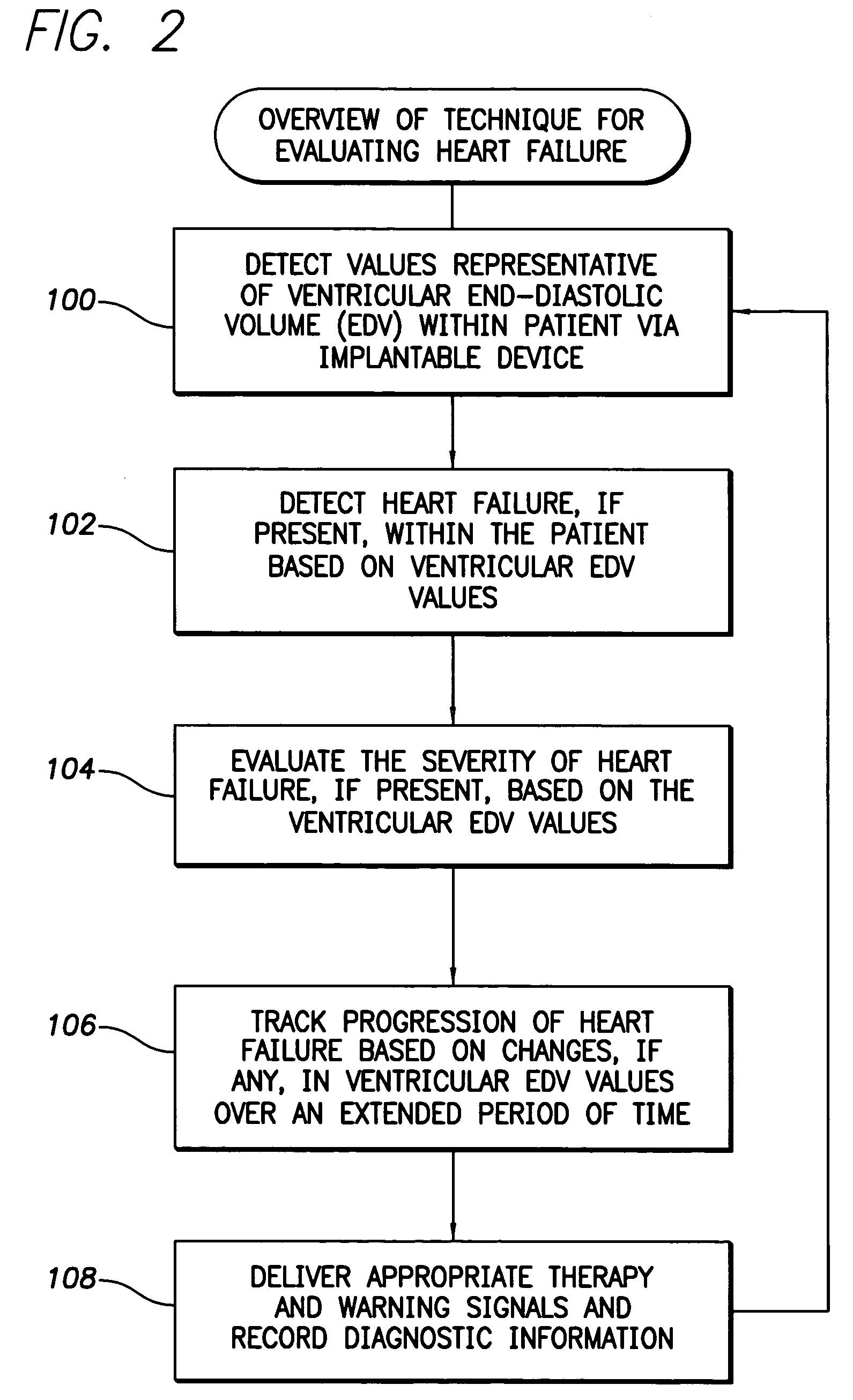
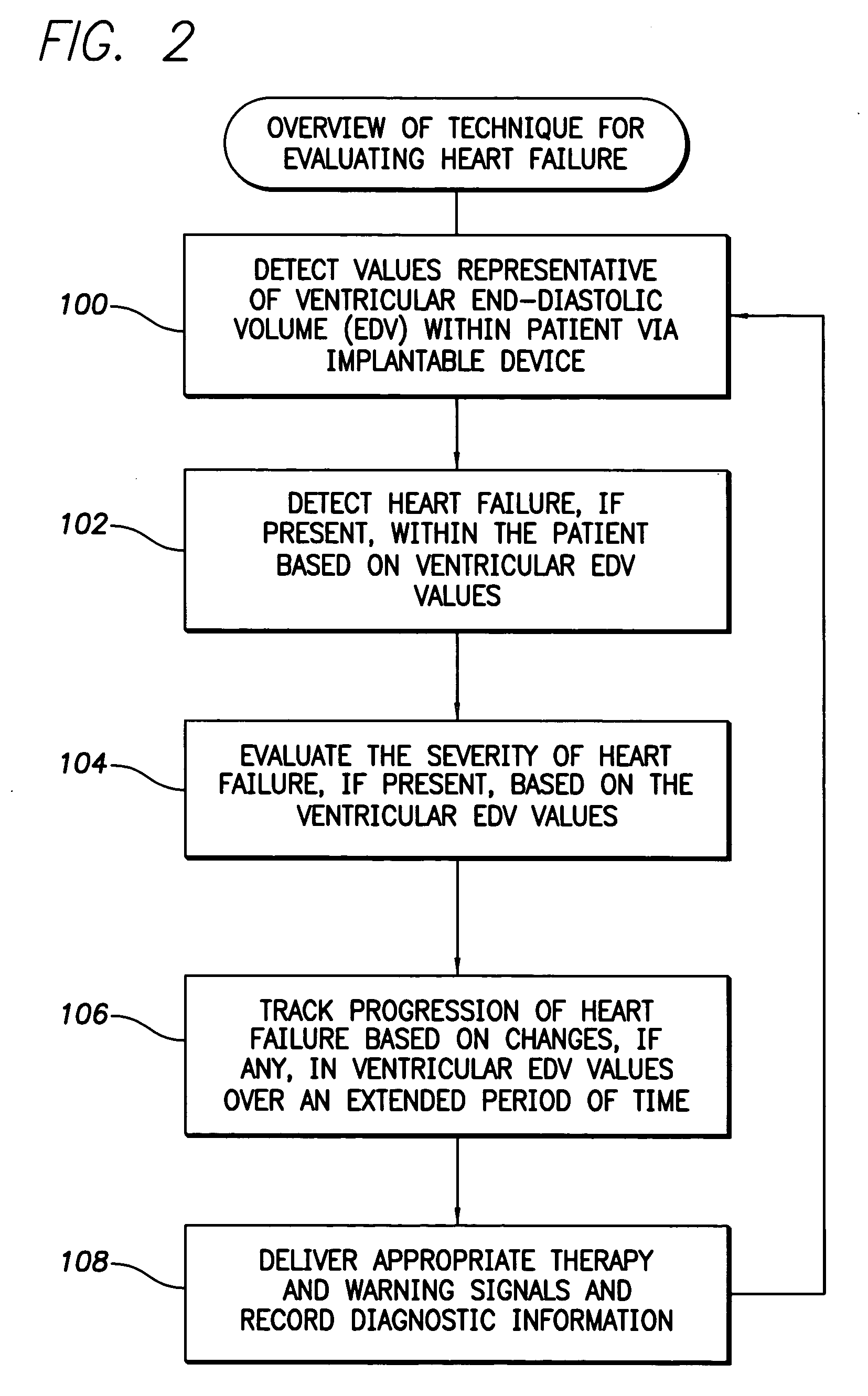
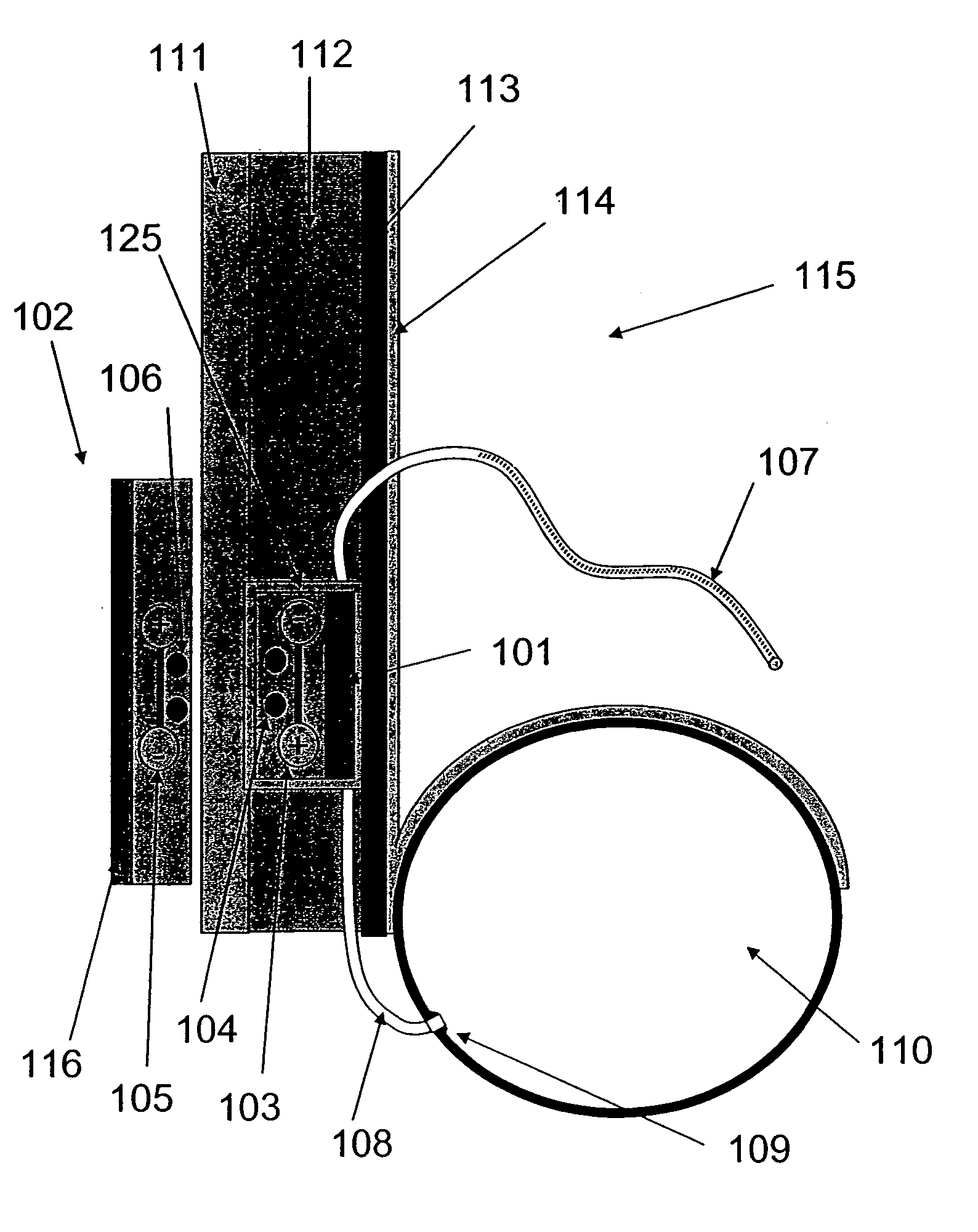
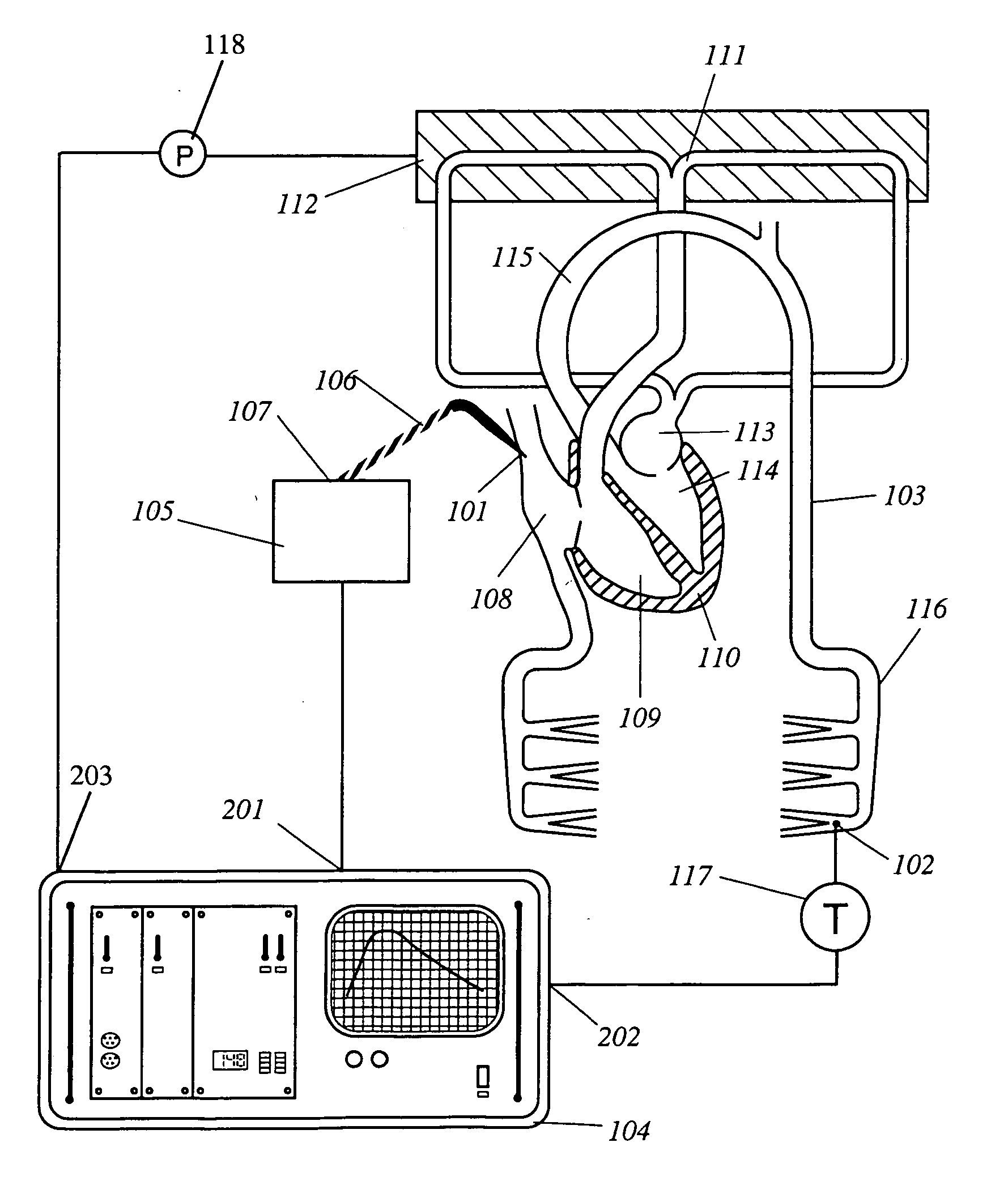
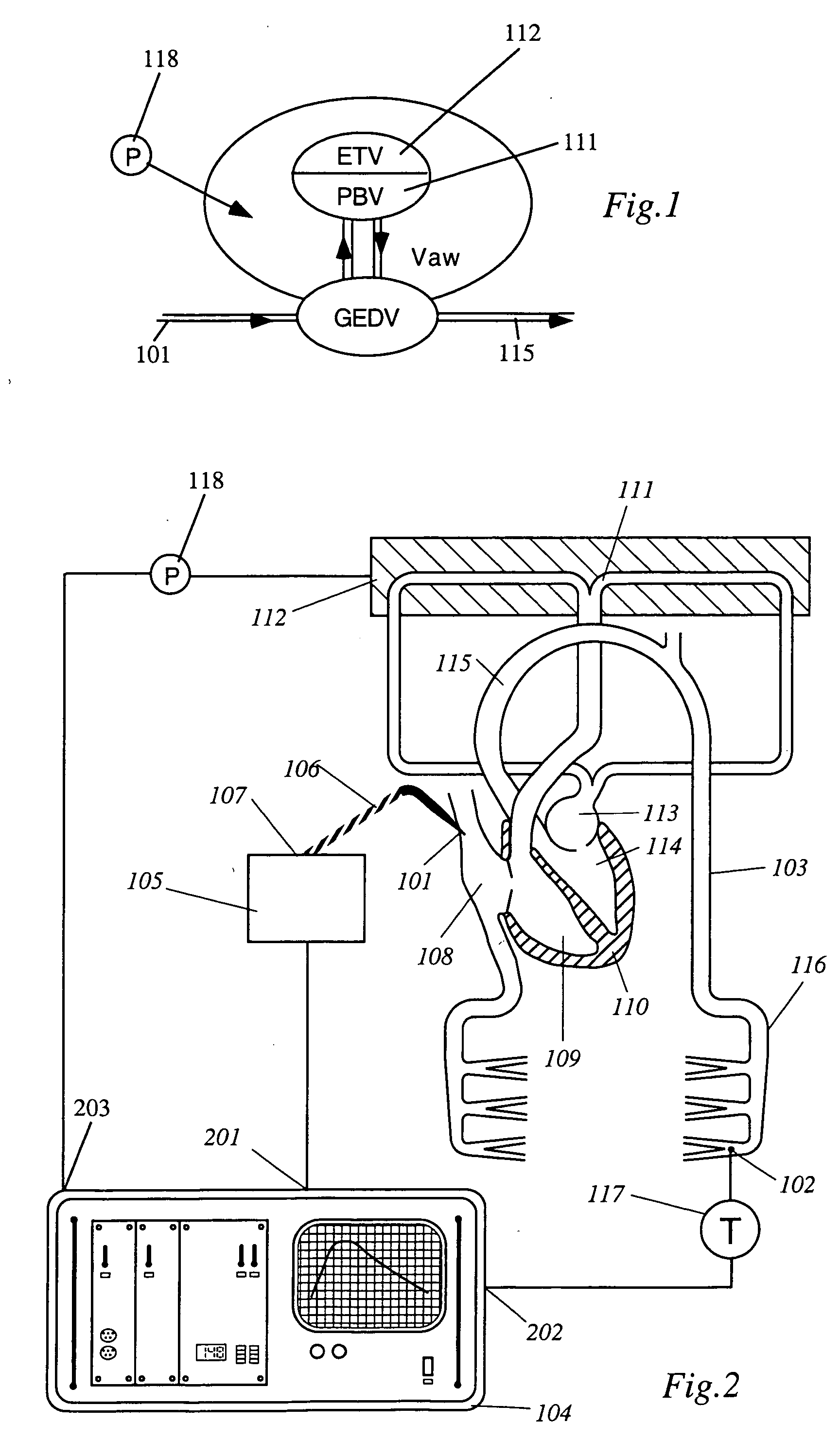
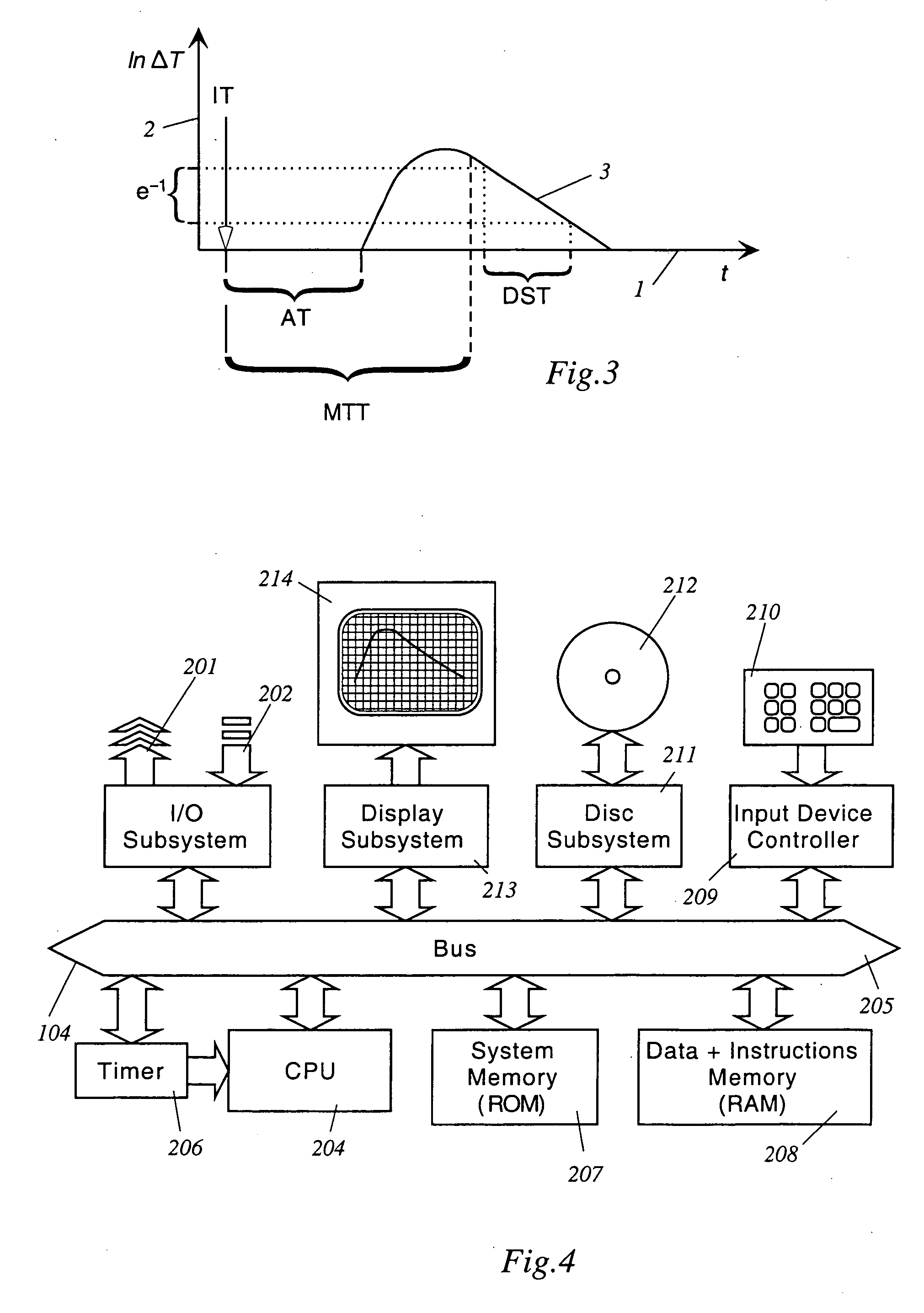
Apparatus, computer system and computer program for determining intrathoracic blood volume and other cardio-vascular parameters
Apparatus, computer system, computer program and storage medium for determining intrathoracic blood volume (ITBV) and other cardio-vascular parameters of a patient by thermodilution measurements. The apparatus comprises a) temperature influencing means (107) for provoking an initial local temperature change in the proximity of a first place (101) of a patient's vascular system (103), thus introducing a travelling temperature deviation to patient's blood stream, b) a temperature sensor device (117) for measuring the local temperature of patient's blood at a second place (102) of patient's vascular system (103) downstream of said first place (101) c) a computer system (104) coupled to said temperature sensor device (117) and adapted to record said patient's local blood temperature measured at said second place (102) as a function of time to determine a thermodilution curve, d) said computer system (104) being further adapted to determine patient's global enddiastolic blood volume (GEDV) and patient's intrathoracic thermovolume (ITTV) from said thermodilution curve, e) said computer system (104) being further adapted to determine patient's intrathoracic blood volume (ITBV) according to the following formula: ITBV=f(GEDV, ITTV, P), ITBV being the intrathoracic blood volume, GEDV being the global enddiastolic blood volume, ITTV being the intrathoracic thermovolume, P being an airway pressure inside patient's lungs. By determining ITBV not only from GEDV but also as a function of ITTV and P enhanced accuracy can be obtained, especially if the patient suffers from pulmonary edema and / or is mechanically ventilated.
Owner:PULSION MEDICAL SYSTEMS SE
Enhancements to the detection of pulmonary edema when using transthoracic impedance
This patent document discusses, among other things, systems, devices, and methods for enhancing detection of pulmonary edema using, in addition to thoracic impedance, one or a combination of: physiologic information about a subject, at least one statistical parameter, a user-programmable detection level, at least one parameter associated with a previous pulmonary edema event, and patient symptom information about the subject. In one example, a (base) thoracic impedance threshold is modified to an adjusted thoracic impedance threshold. The adjusted thoracic impedance threshold provides an increased sensitivity of pulmonary edema detection as compared to the base thoracic impedance threshold. In another example, an alert is provided to a subject, a caregiver, or other user based on a pulmonary edema indication determined by the present systems, devices, and methods. In a further example, a therapy (provided to the subject) is adjusted or initiated in response to the pulmonary edema indication.
Owner:CARDIAC PACEMAKERS INC
System and method for detecting heart failure and pulmonary edema based on ventricular end-diastolic pressure using an implantable medical device
Techniques are provided for detecting left ventricular end diastolic pressure (LV EDP) using a pressure sensor implanted within the heart of a patient and for detecting and evaluating heart failure and pulmonary edema based on LV EDP. Briefly, the peak of the R-wave of an intracardiac electrogram (IEGM) is used to trigger the measurement of a pressure value within the left ventricle. This pressure value is deemed to be representative of LV EDP. In this manner, LV EDP is easily detected merely by measuring pressure at one point within the heartbeat—thereby eliminating any need to track ventricular pressure throughout the heartbeat. Techniques for detecting and evaluating heart failure and pulmonary edema based on the R-wave triggered LV EDP measurements are also set forth herein.
Owner:PACESETTER INC
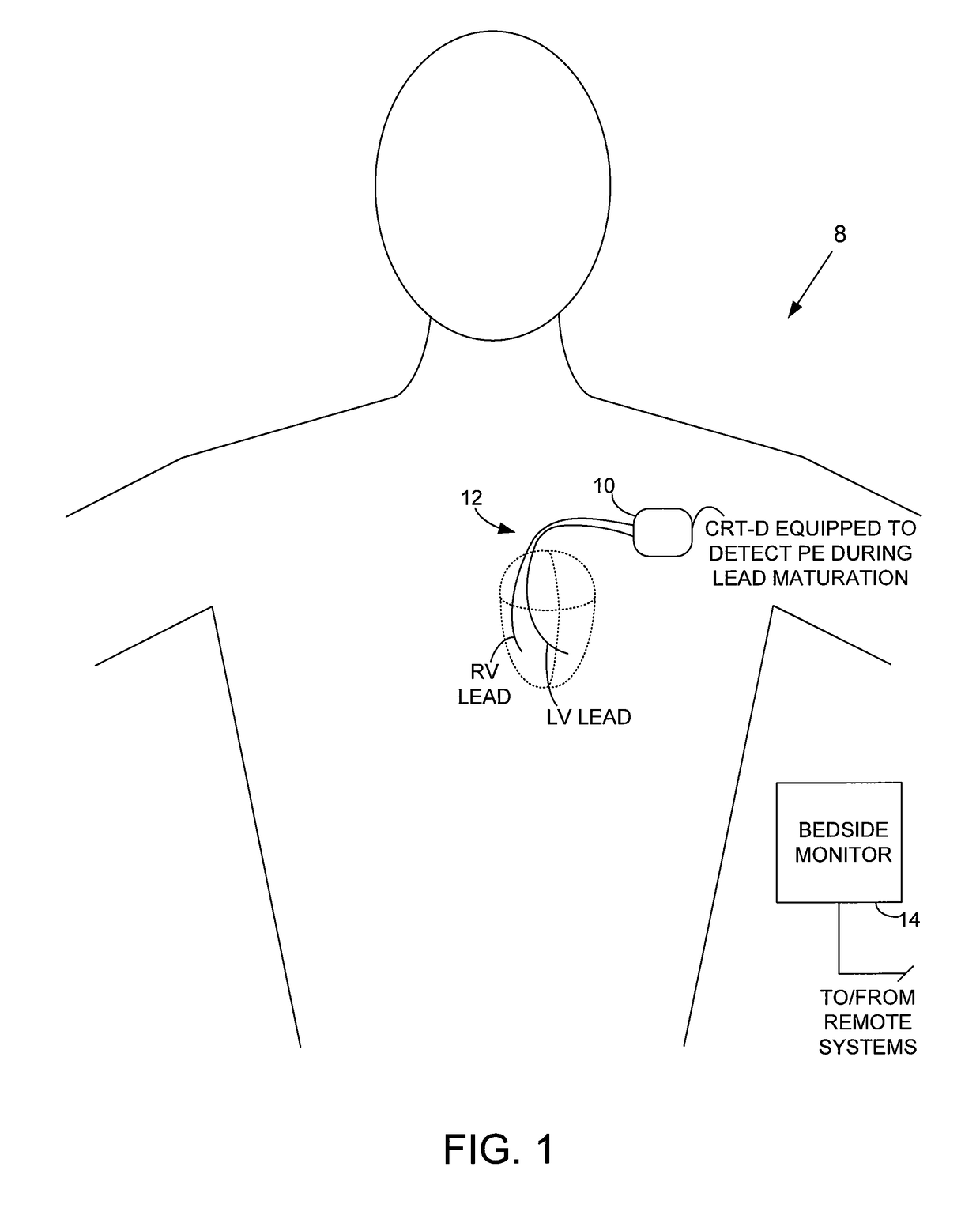
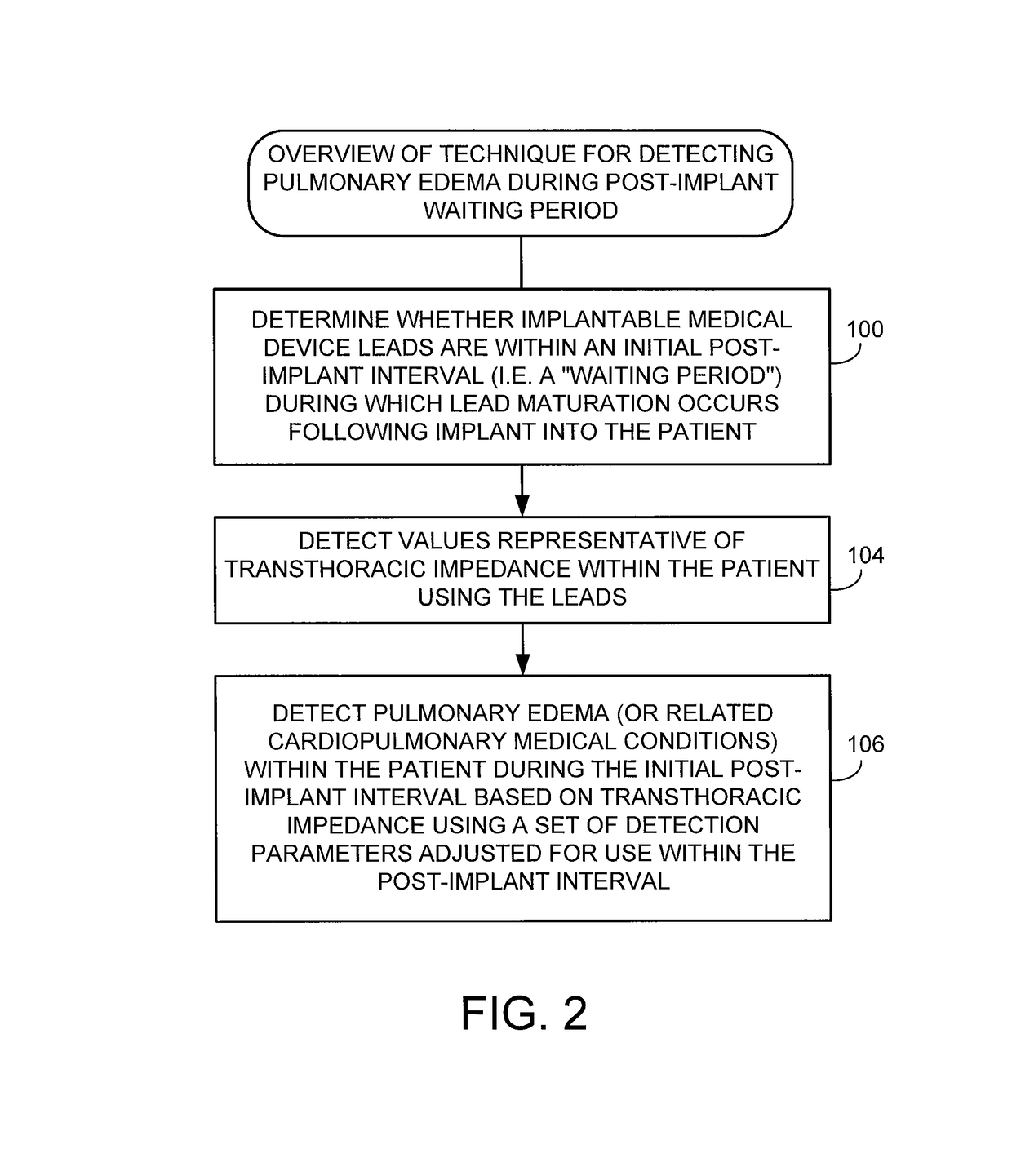
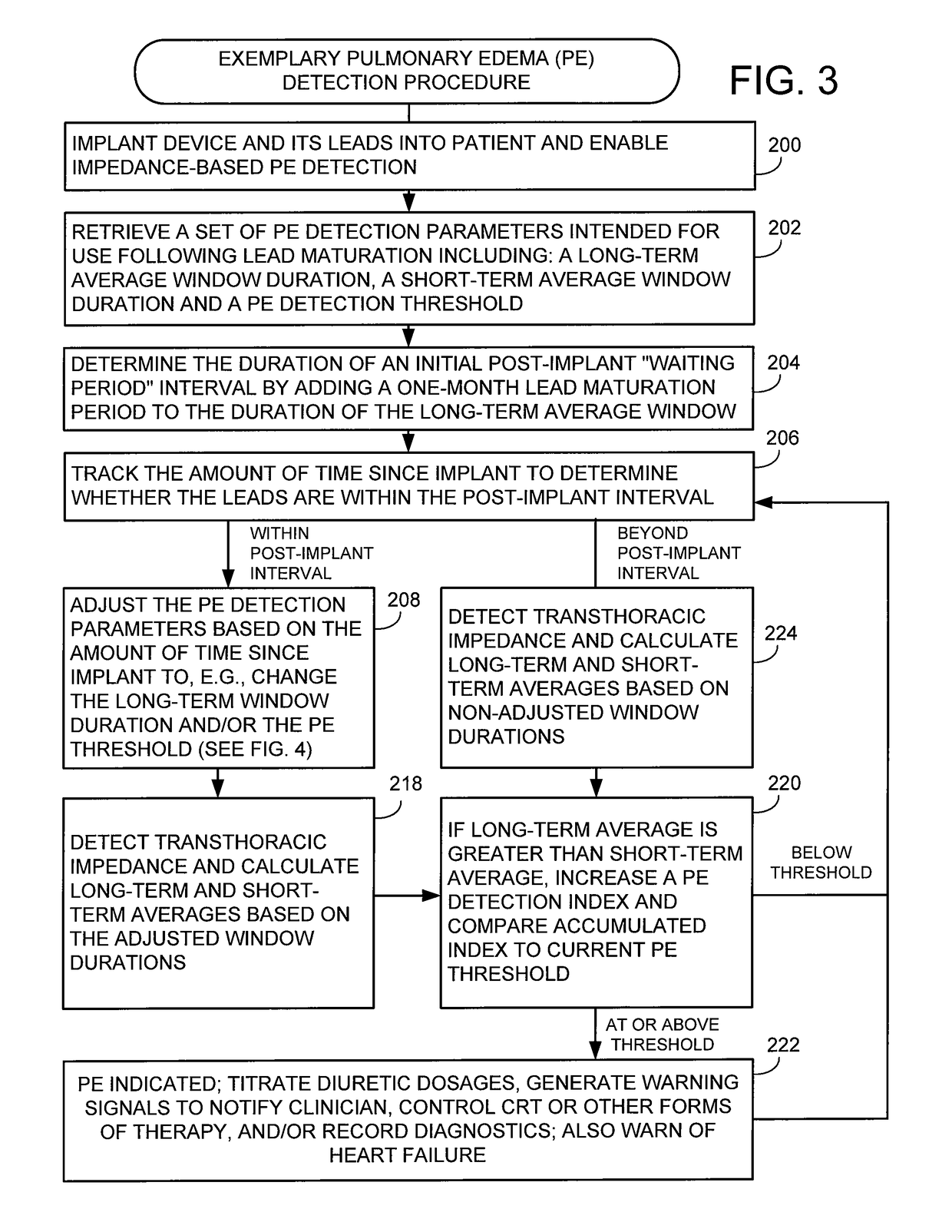
System and method for detecting pulmonary edema based on impedance measured using an implantable medical device during a lead maturation interval
Techniques are provided for use by implantable medical devices such as cardiac resynchronization therapy (CRT) devices for detecting pulmonary edema based on transthoracic impedance sensed using cardiac pacing / sensing leads, wherein detection can be performed while lead maturation occurs. Briefly, the implantable device determines whether the leads are within an initial post-implant interval following implant during which lead maturation generally occurs. The device then detects pulmonary edema or related medical conditions within the patient based on transthoracic impedance using a set of detection parameters adjusted for use during the post-implant interval. Thus, rather than “blanking” impedance data during lead maturation, the device instead detects and processes impedance during this period to identify possible episodes of pulmonary edema so that appropriate measures can be undertaken, such as delivery of warnings or titration of appropriate medications.
Owner:PACESETTER INC
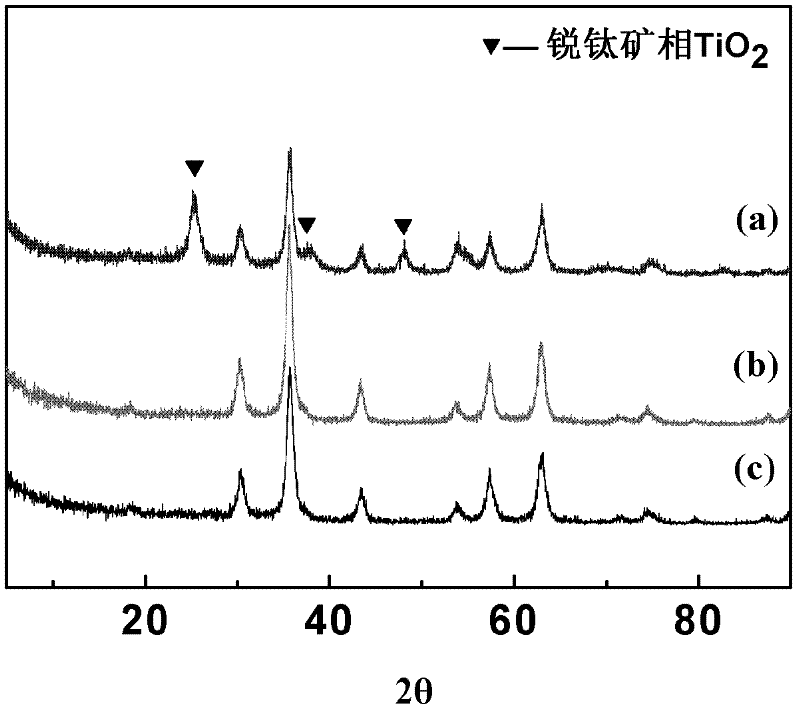
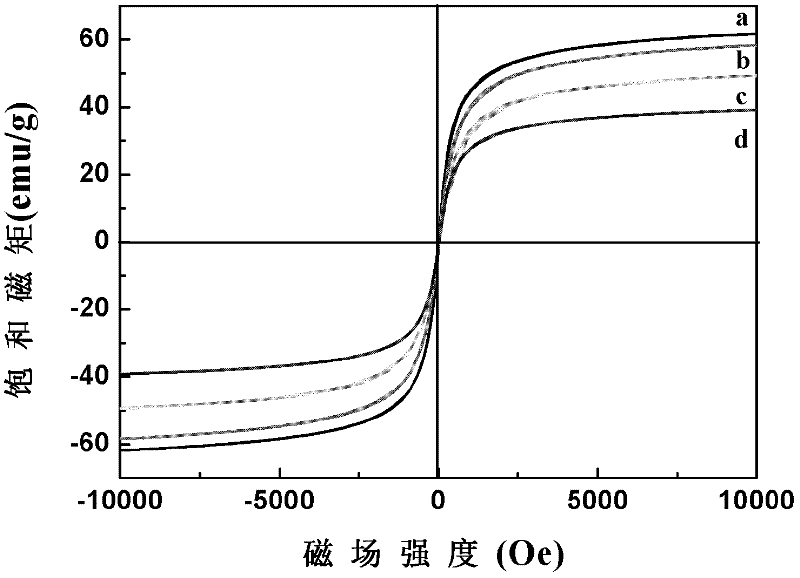
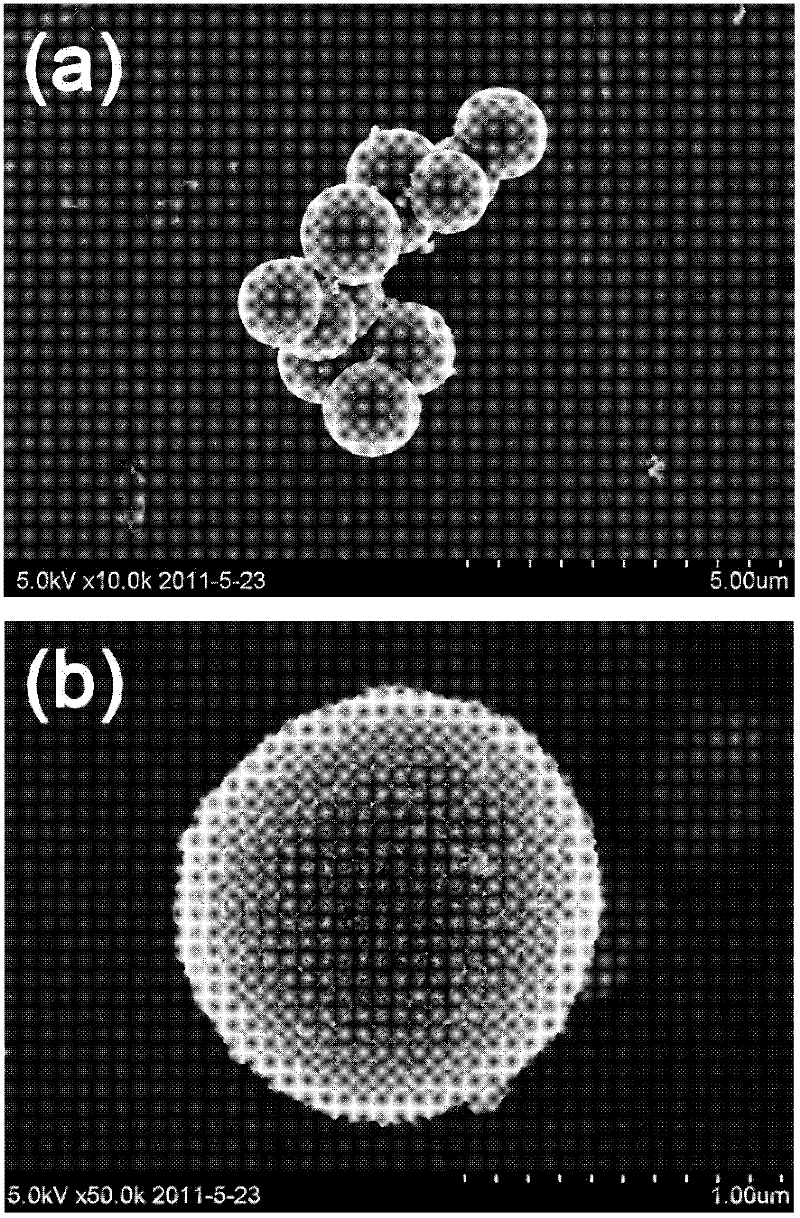
Preparation method for magnetic nanometer microballoon photocatalysis composite materials
InactiveCN102489300AEasy reunionInhibit photocorrosionWater/sewage treatment by irradiationCatalyst activation/preparationAlcoholMicrosphere
The invention relates to a preparation method for magnetic nanometer microballoon photocatalysis composite materials. The preparation method includes steps of (1) preparing Fe3O4 magnetic nanometer particles; (2) preparing Fe3O4 / SiO2 coating magnetic nanometer microballoons from the Fe3O4 magnetic nanometer particles by the aid of a steam phase method; (3) adding the Fe3O4 / SiO2 coating magnetic nanometer microballoons into absolute ethyl alcohol, adding tetra-n-butyl titanate under ultrasonic treatment, transferring solution into a steam phase device to be used as solid phase after continuous ultrasonic treating, and staying the solution at the temperature ranging from 100 DEG C to 200 DEG C for 8 hours to 14 hours; washing and separating solid phase products; and finally drying the solid phase products in a vacuum manner, so that the magnetic nanometer microballoon photocatalysis composite materials are obtained. The preparation method is simple in operation and low in cost, and can realize scale production. The obtained magnetic nanometer microballoon photocatalysis composite materials are high in magnetic particle content, large in saturation magnetic moment and fine in photocatalytic performance, and dye molecules for simulating pulmonary edema, such as acid red, methylthionine chloride and the like, can be effectively degraded.
Owner:DONGHUA UNIV
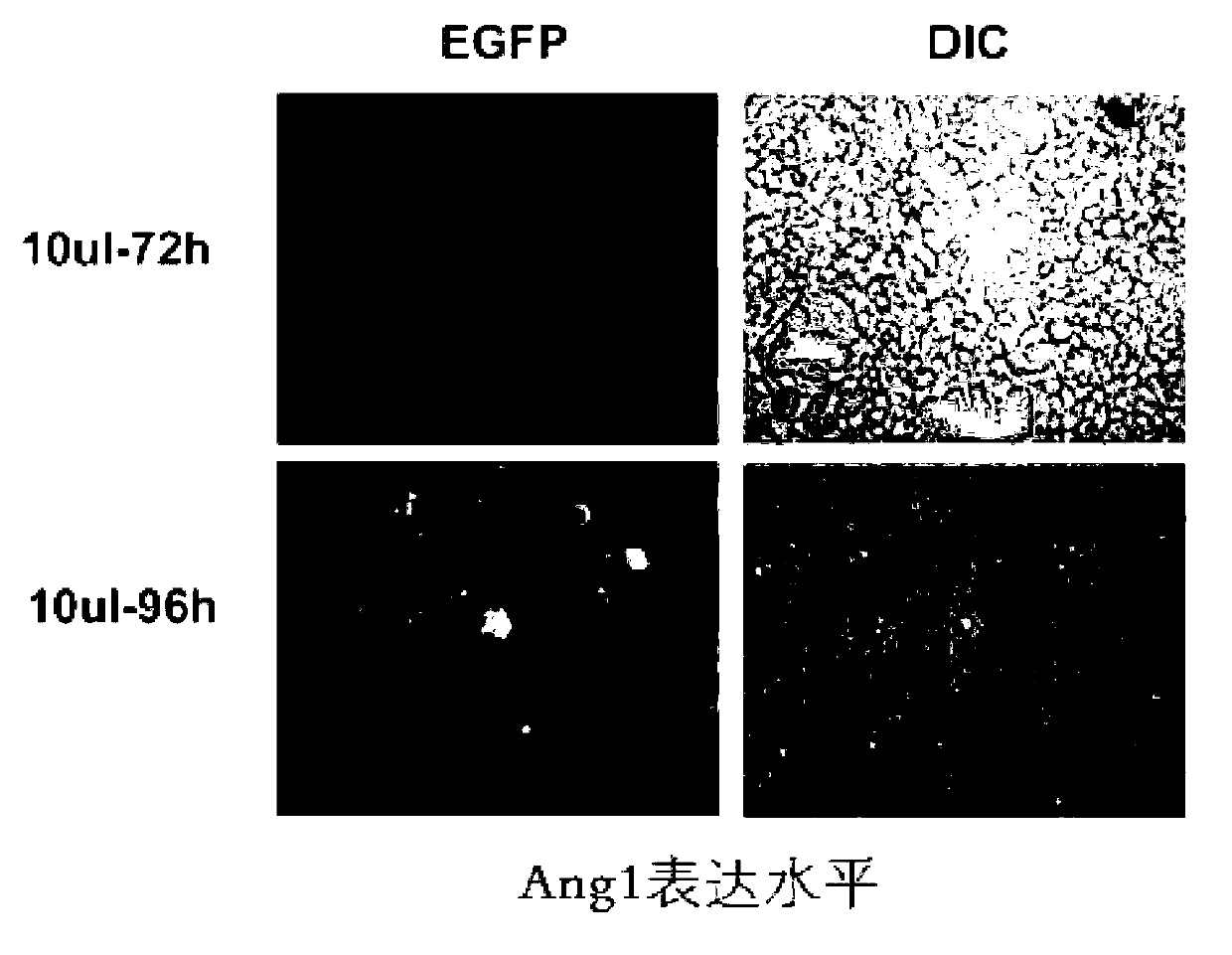
Mesenchymal stem cells genetically modified with angiopoietin 1 gene and construction method and application thereof
The invention discloses mesenchymal stem cells genetically modified with an angiopoietin 1 gene and a construction method and application thereof. The mesenchymal stem cells carry the angiopoietin 1 gene. Tests prove that the mesenchymal stem cells genetically modified with an angiopoietin 1 gene can overexpress angiopoietin 1; after venous re-transfusion, the angiopoietin 1 is overexpressed in the pulmonary tissue, so that pulmonary pathological injuries, pulmonary inflammatory response and pulmonary edema are obviously relieved, and an obvious and long-lasting effect is achieved; and the mesenchymal stem cells genetically modified with angiopoietin 1 gene and the construction method thereof are expected to provide a suitable microenvironment for repair of the pulmonary injuries, and have a wide application prospect.
Owner:SHANGHAI PULMONARY HOSPITAL
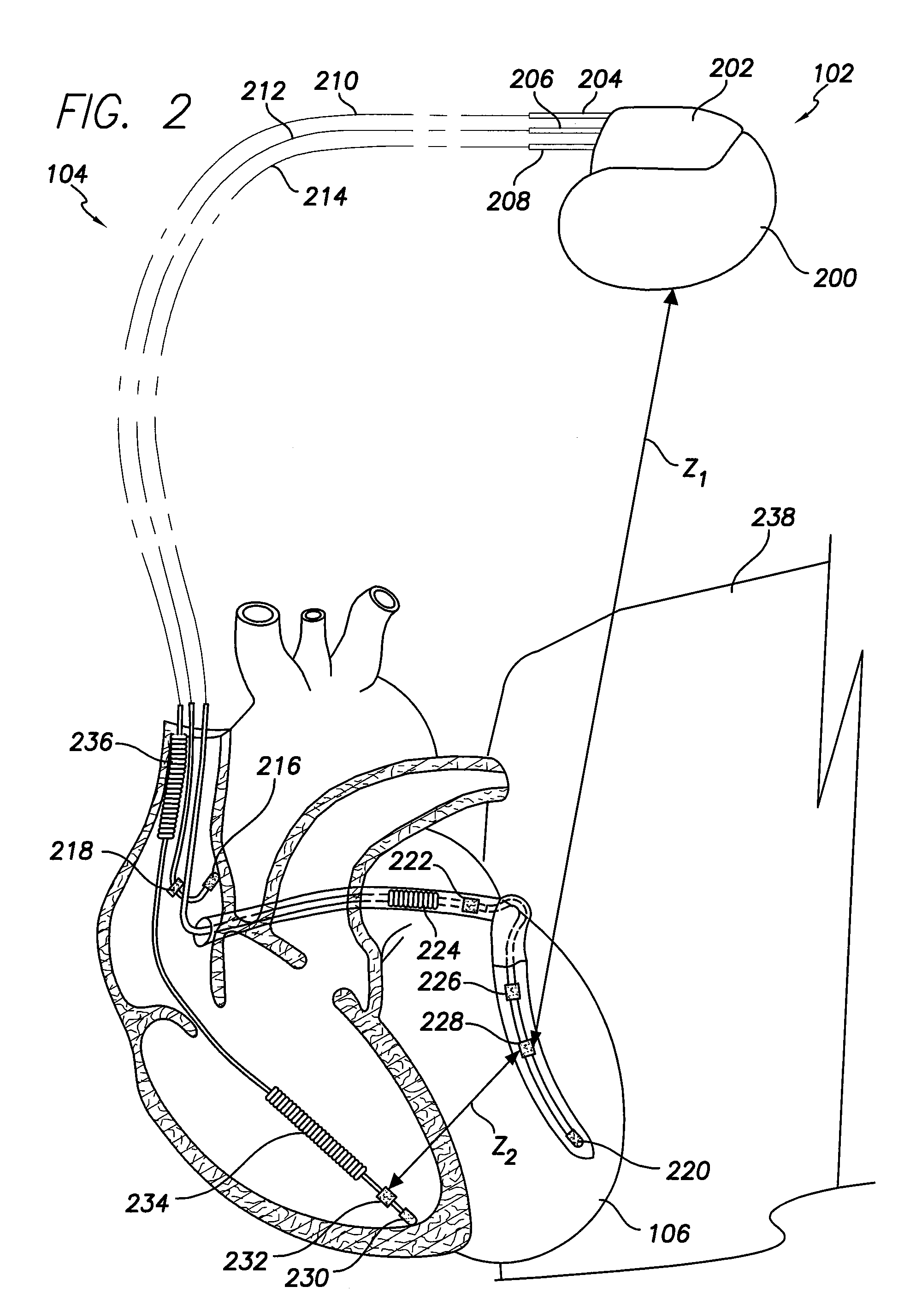
System and method for identifying a potential cause of pulmonary edema
A method of identifying a potential cause of pulmonary edema is provided. The method includes obtaining one or more impedance vectors between predetermined combinations of the electrodes positioned proximate the heart. At least one of the impedance vectors is representative of a thoracic fluid level. The method also includes applying a stimulation pulse to the heart and sensing cardiac signals of the heart that are representative of an electrophysiological response to the stimulation pulse. The method further includes monitoring the cardiac signals and at least one of the impedance vectors with respect to time to identify the potential cause of pulmonary edema.
Owner:PACESETTER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com