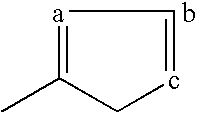
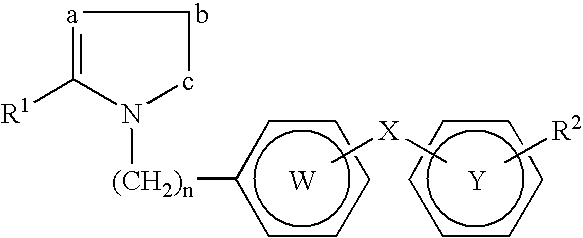Novel PPAR ligands that do not cause fluid retention, edema or congestive heart failure
a technology of ppar and ppar ligand, which is applied in the field of prevention and treatment of cardiovascular diseases and insulin resistance syndrome, can solve the problems of increasing the risk of fluid retention, edema or congestive heart failure, and not being able to predict, so as to improve the ability to treat or prevent inflammatory diseases, prevent and treat inflammatory diseases, and promote or aggravate fluid retention. , the effect of promoting or aggravating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Angiotensin II Type 1 Receptor Blockers That Activate PPARgamma
[0157] PPARγ activity was determined by transactivation assays in CV-1 cells (CCL-70 line from ATCC, Bethesda, Md.) transfected using the GenePorter transfection reagent (Gene Therapy Systems, San Diego, Calif.) to deliver 200 ng of a PPARγ expression plasmid and 1 μg of a luciferase reporter plasmid and 400 ng pCMVSport β-gal (Gibco, Grand Island, N.J.) as an internal control. 24 hr post-transfection, cells were treated with varying concentrations of the test compounds (telmisartan, irbesartan, valsartan, losartan, the active metabolite of losartan, the active forms of candesartan and olmesartan, rosiglitazone, or pioglitazone) and incubated for an additional 24 hr. Cell extracts were assayed for luciferase and β-galactosidase activity using Promega (Madison, Wis.) assay systems. All treatments were performed in triplicate, and normalized for β-galactosidase activity. Agonist concentrations yielding h...
example 2
Measurement of In Vitro Adipocyte Differentiation Activity
[0159] The following examples 2 and 3 provide a generic means to measure adipocyte differentation to determine if one has an insulin-sensitizing agent. A mouse preadipocyte cell line (3T3-L1) obtained from the American Type Culture Collection, and the cells are grown in a Dulbecco's modified Eagle medium (DMEM) containing 4.5 g / L glucose, 50 mg / L streptomycin sulfate, 100,000 units / L penicillin-G, 0.584 g / L L-glutamine, 4 mg / L pantothenate, 8 mg / L D-biotin, and 10 mM HEPES (pH 7.2)] supplemented with 10% fetal bovine serum (FBS). The cells are then plated at 1.5×104 / cm2 in a 96-well tissue culture plate (view plate, 96 white, Packard) coated with type 1 collagen. After the cells had reached confluence, the cells were further cultured with differentiation medium DMEM supplemented with 5% FBS, 100 ng / mL insulin, 0.1 mM isobutylmethylxanthine (IBMX), and 1 mM dexamethasone, and containing various concentrations of compounds for...
example 3
Measurement of In Vivo Insulin-Sensitivity Activity
[0160] The hypoglycemic activity of the test compounds in insulin resistant obese fatty (fa / fa) Zucker rats (Jackson Laboratory, Bar Harbor, Me.). These rats are profoundly insulin resistant with extremely high blood concentrations of insulin. Lean littermates (− / −) are used as controls. Each test compounds is administered to three Zucker rats at 10 mg / kg daily for five days after which blood samples are taken in the non-fasting state. Blood samples are collected, placed in a hematocrit centrifuge tube, and centrifuged to obtain plasma. Insulin in the collected plasma is measured by means of a radioimmuno-assay kit (Linco Research, Inc, St Charles, Mo.). The insulin-sensitizing activity of the test compounds are calculated as follows:
Insulin-sensitivity activity (%)=[(PI in C−PI in T) / PI in C]×100 [0161] where “PI in C” is plasma insulin in control rats and “PI in T” is plasma insulin in rats treated with test compounds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com