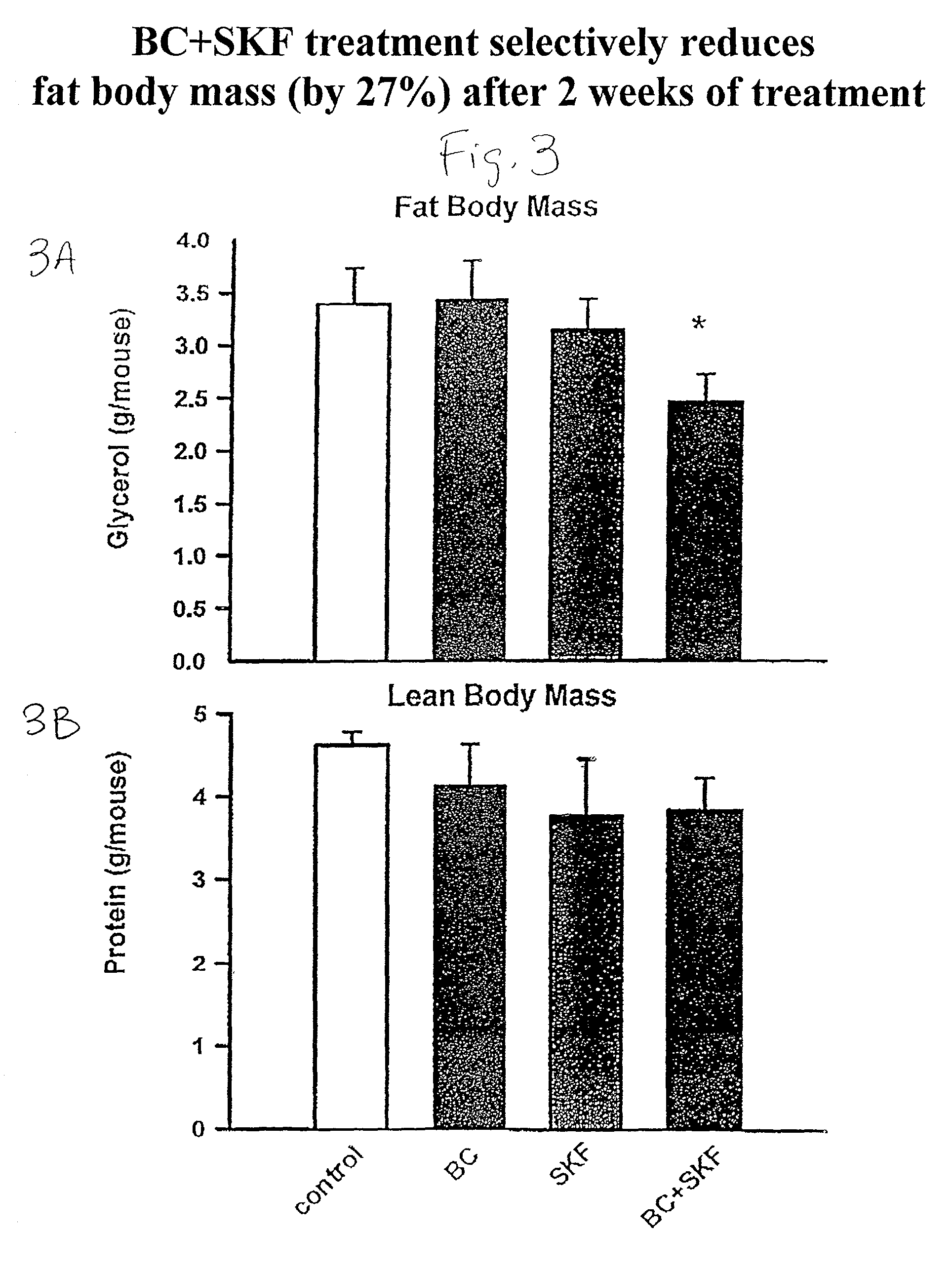
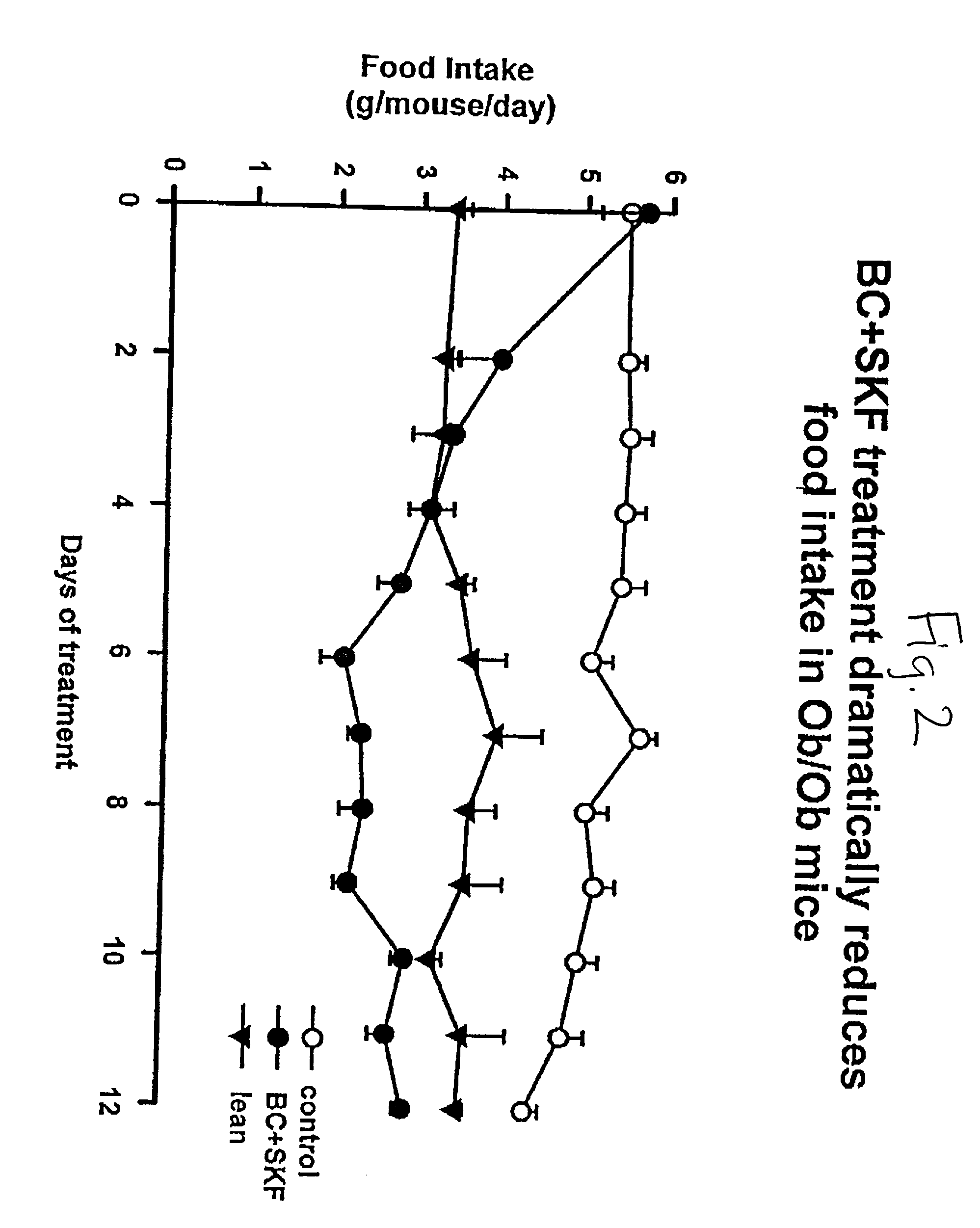
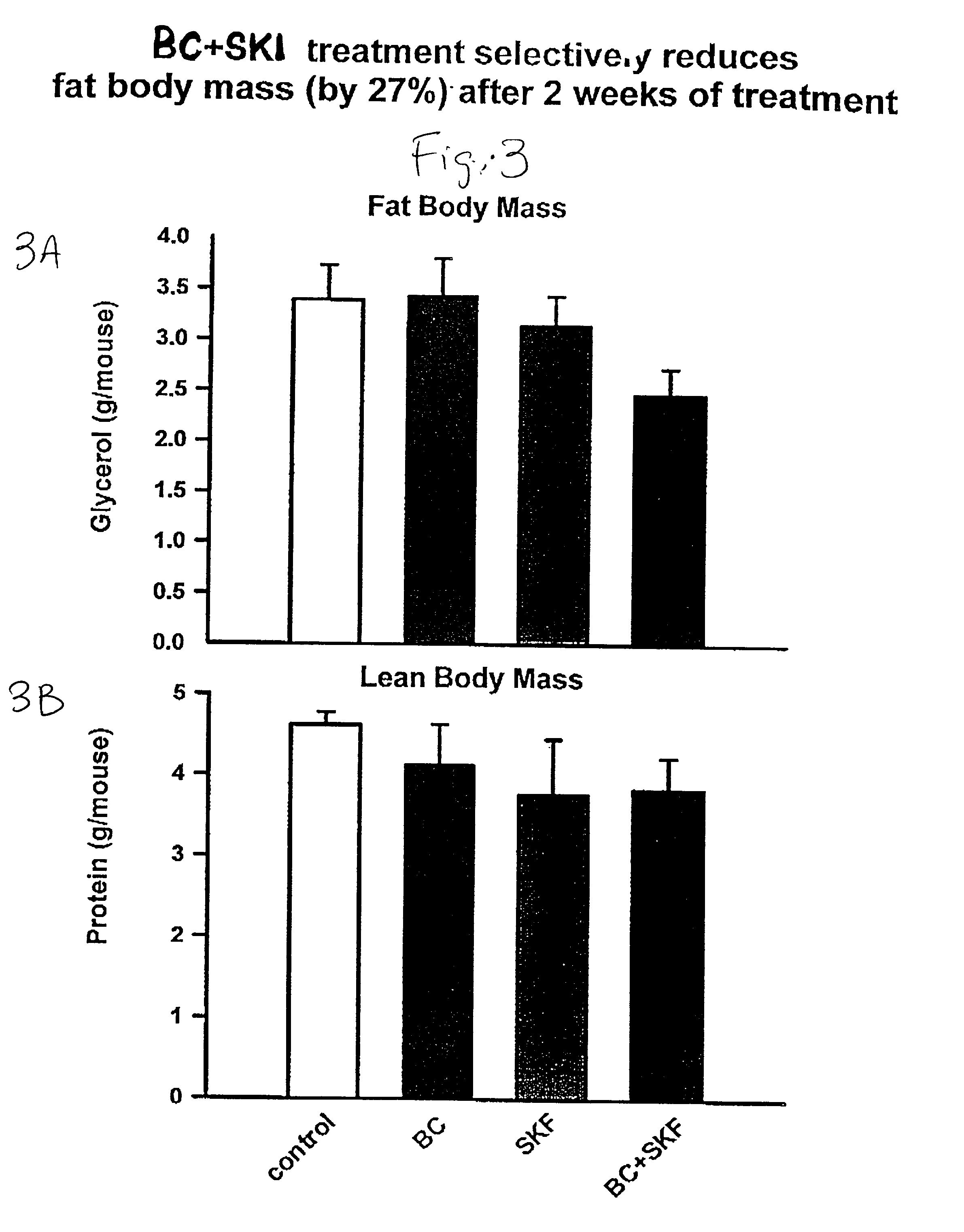
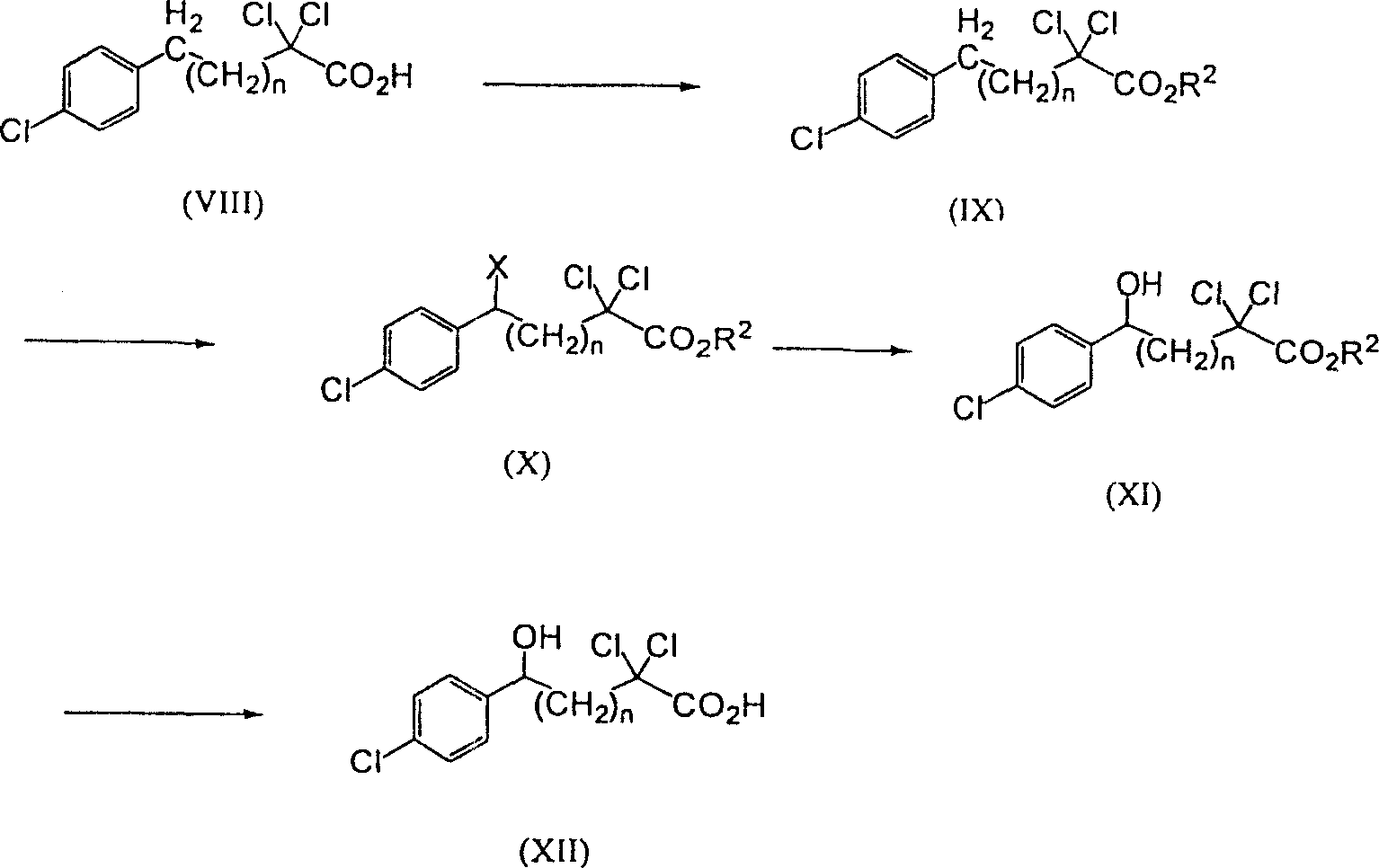
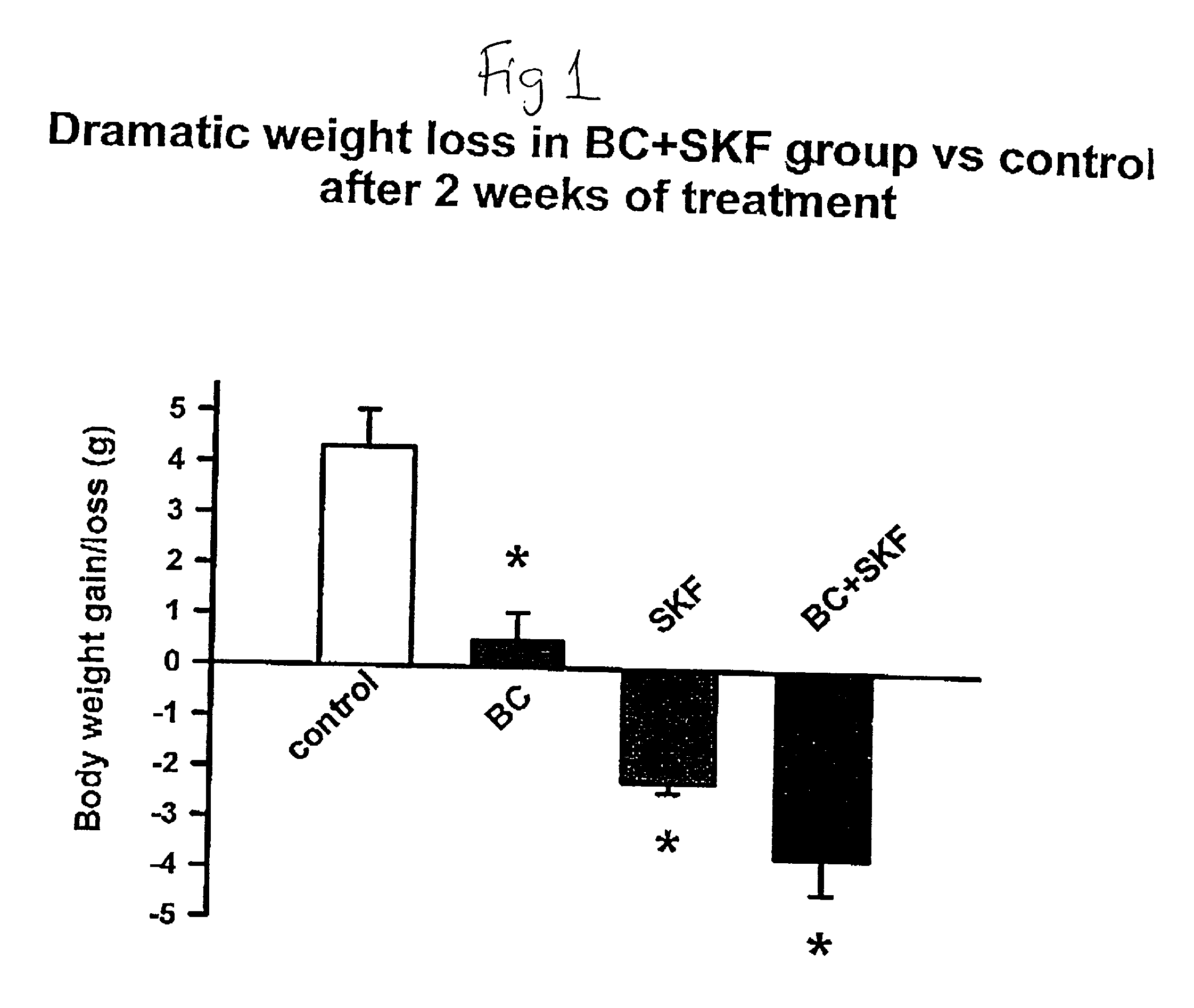Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Plasma insulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The most accurate way to measure insulin resistance is the euglycemic insulin clamp technique, in which insulin is infused to maintain a constant plasma insulin level. Glucose is then infused and, as the plasma level falls because of the action of insulin, more glucose is added to maintain a steady level.
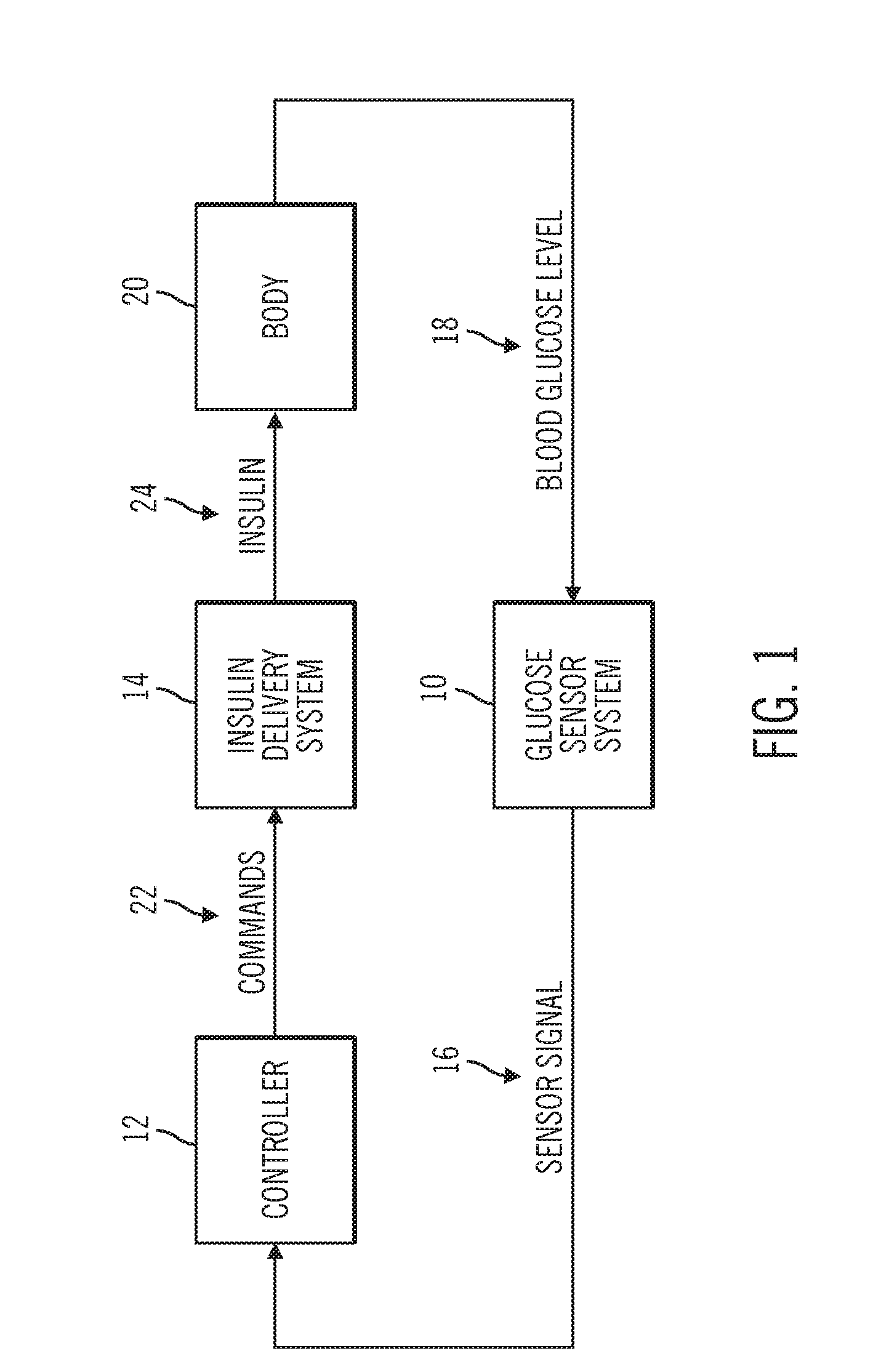
Multivariable artificial pancreas method and system
ActiveUS20160354543A1Easy to controlIncrease heart rateLocal control/monitoringMedical devicesAcute hyperglycaemiaHypoglycemia
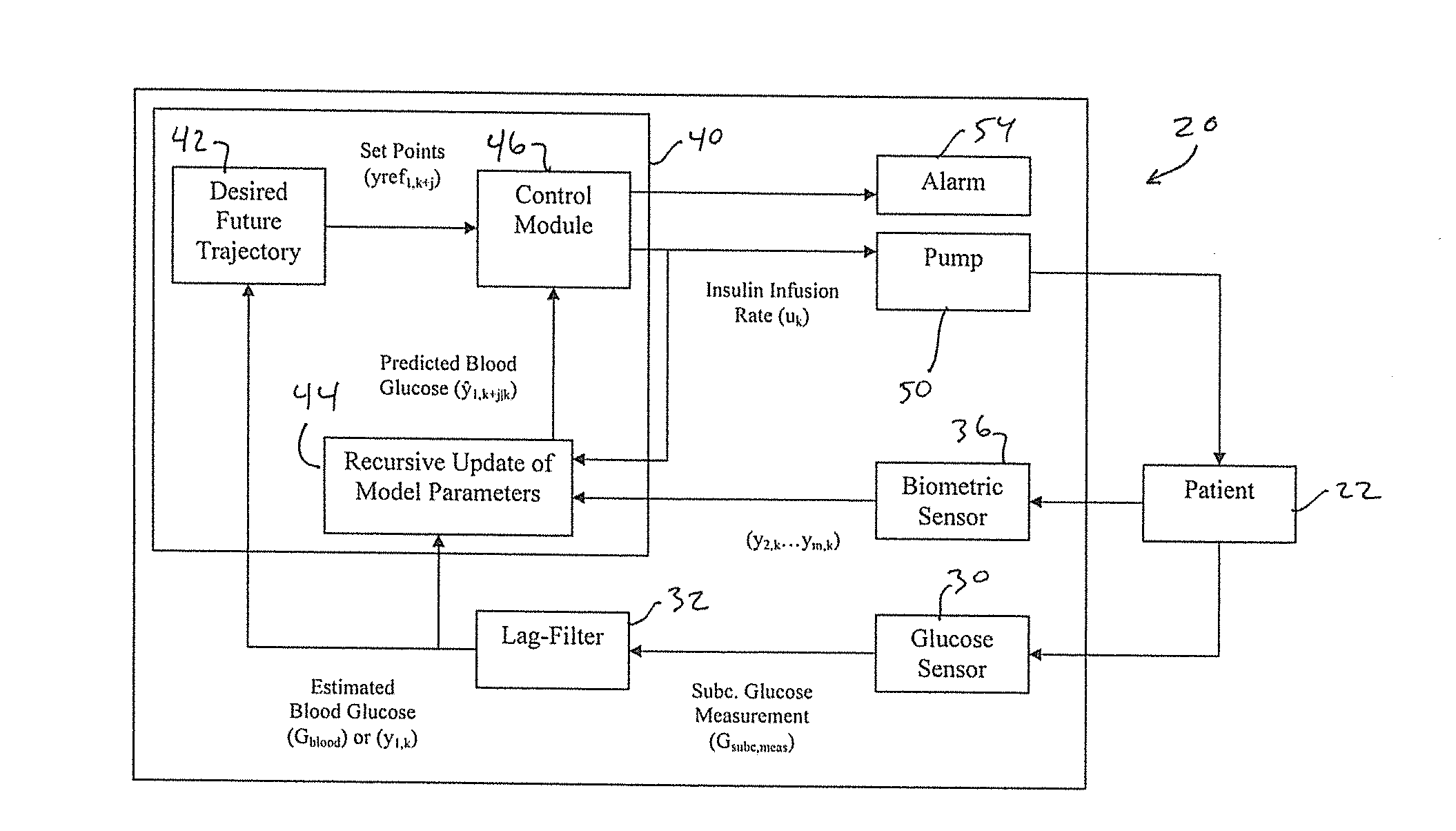
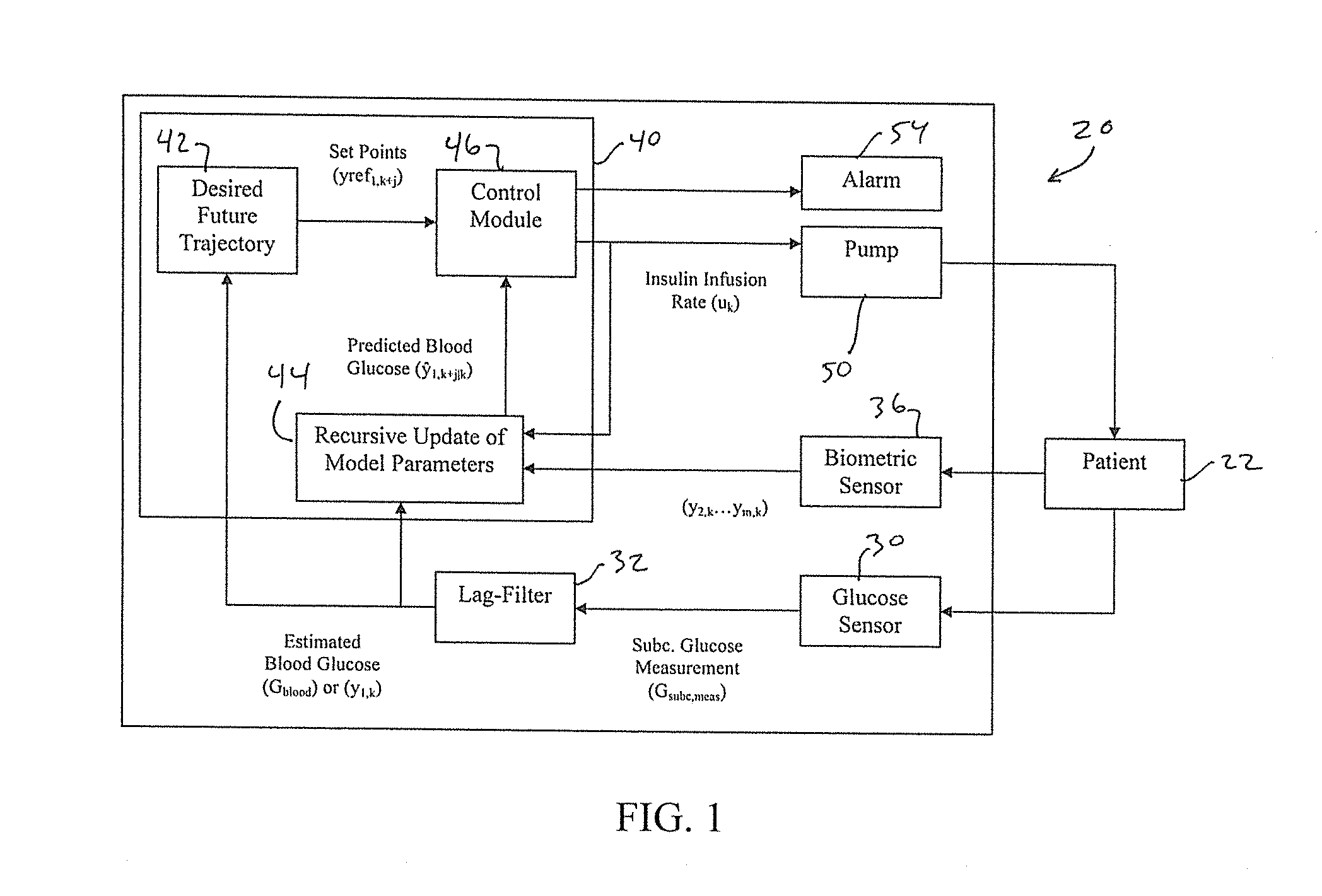
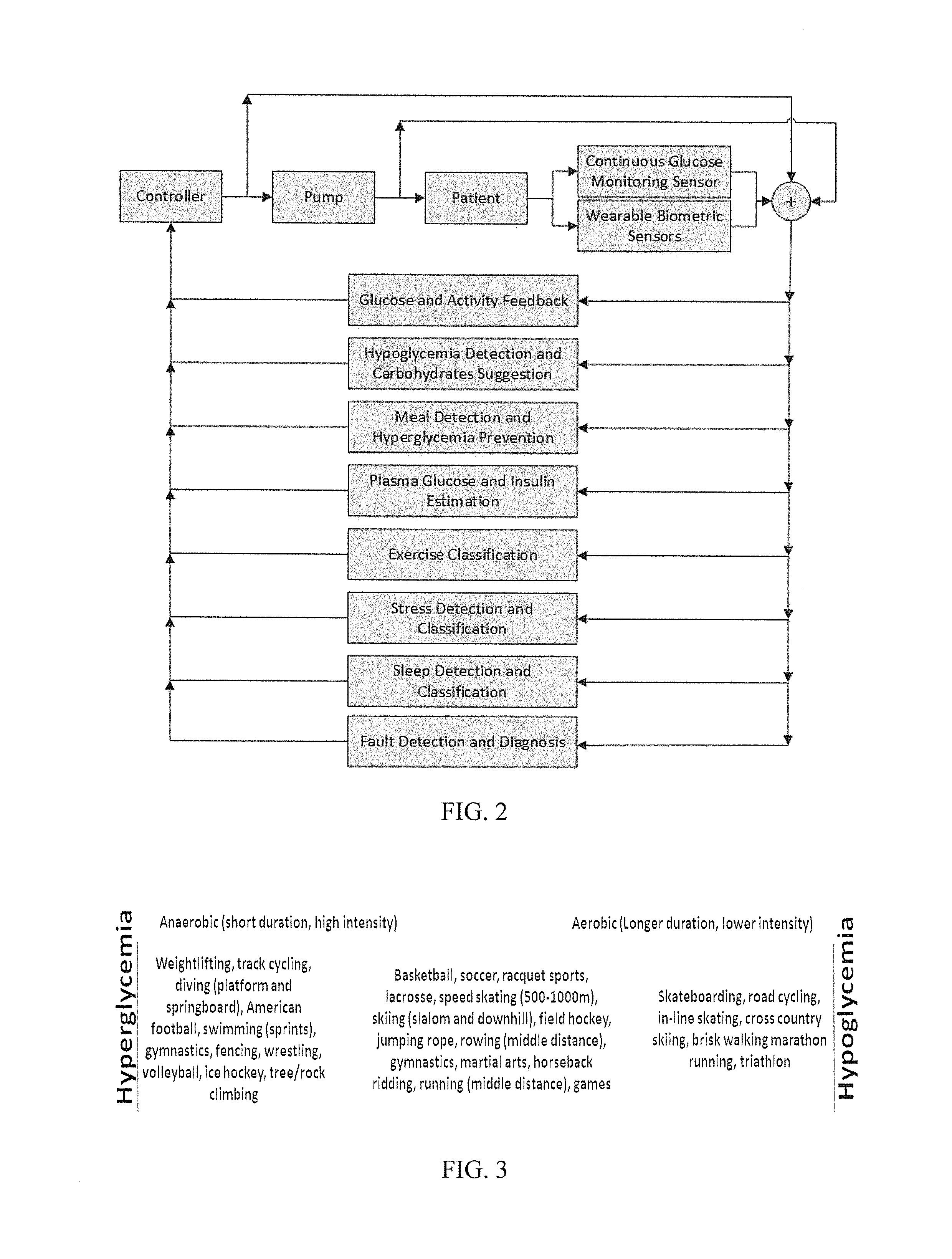
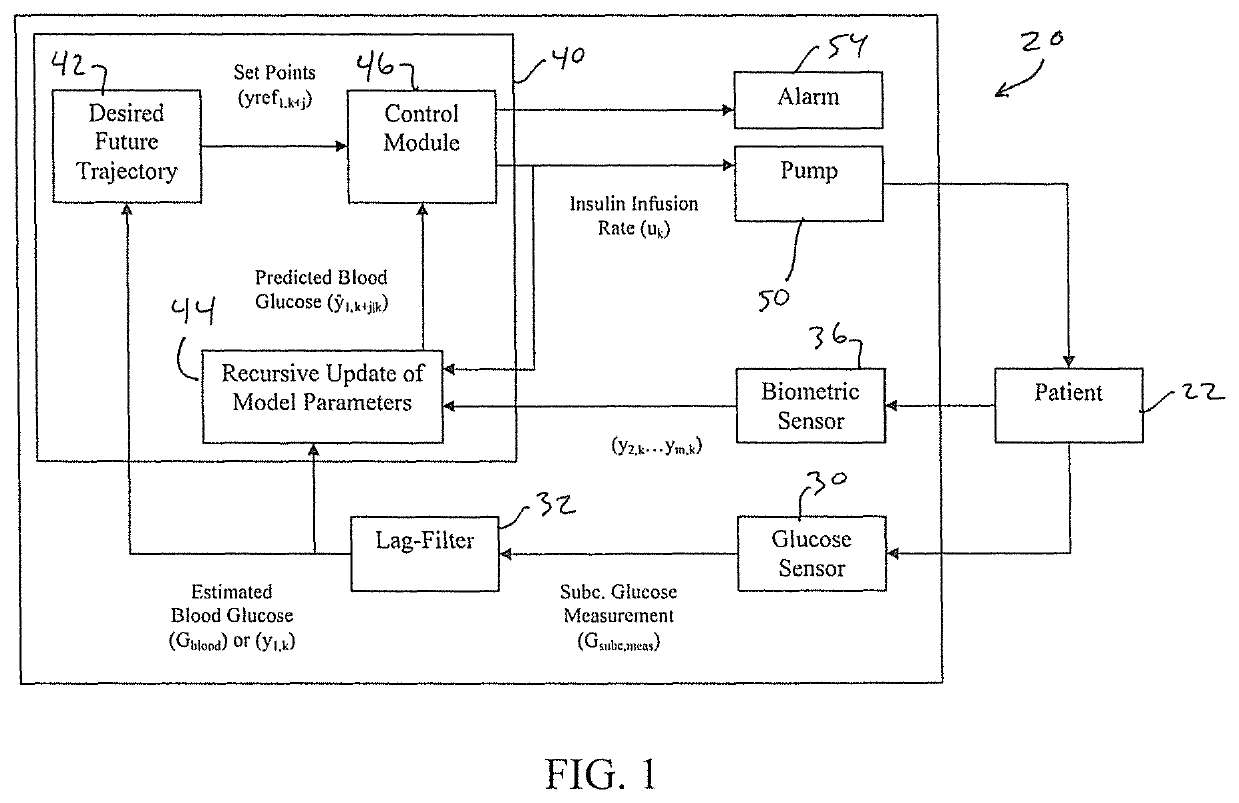
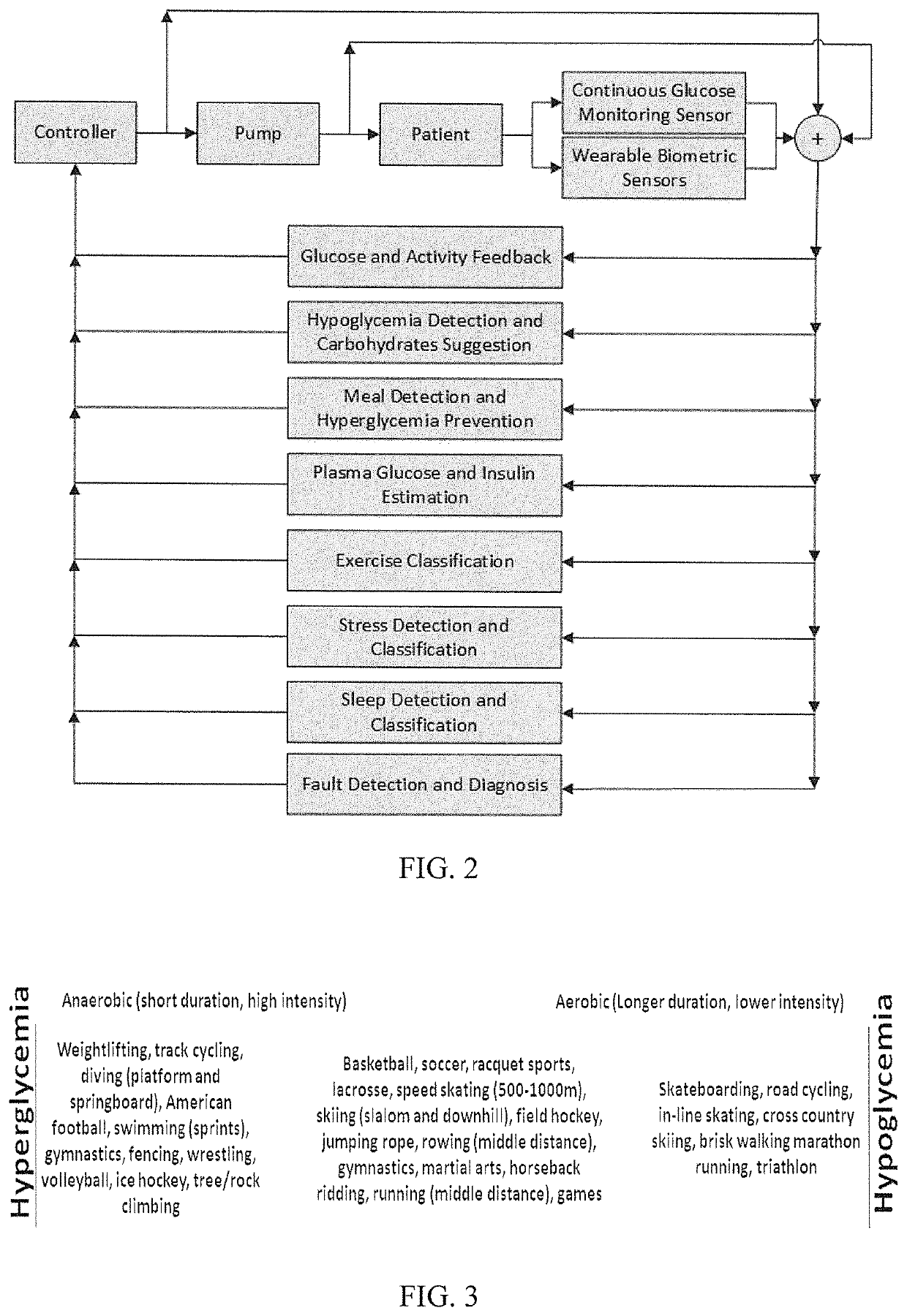
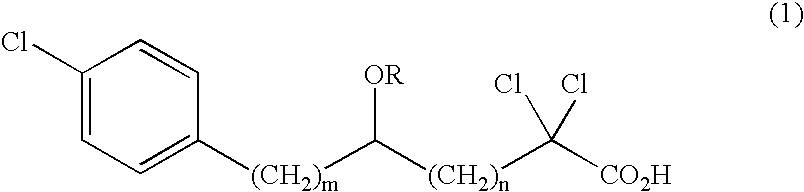
Methods and modules for using physiological (biometric) variables to advance the state of the artificial pancreas. The method and system includes one or more modules for recursive model identification, hypoglycemia early alert and alarm, adaptive control, hyperglycemia early alert and alarm, plasma insulin concentration estimation, assessment of physical activity (e.g., presence, type, duration, expected effects on insulin sensitivity and GC), detection of acute stress and assessment of its impact on insulin sensitivity, detection of sleep and its stages and assessment of sleep stages on GC, sensor fault detection and diagnosis, software and controller performance evaluation and adjustment and / or pump fault detection and diagnosis.
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
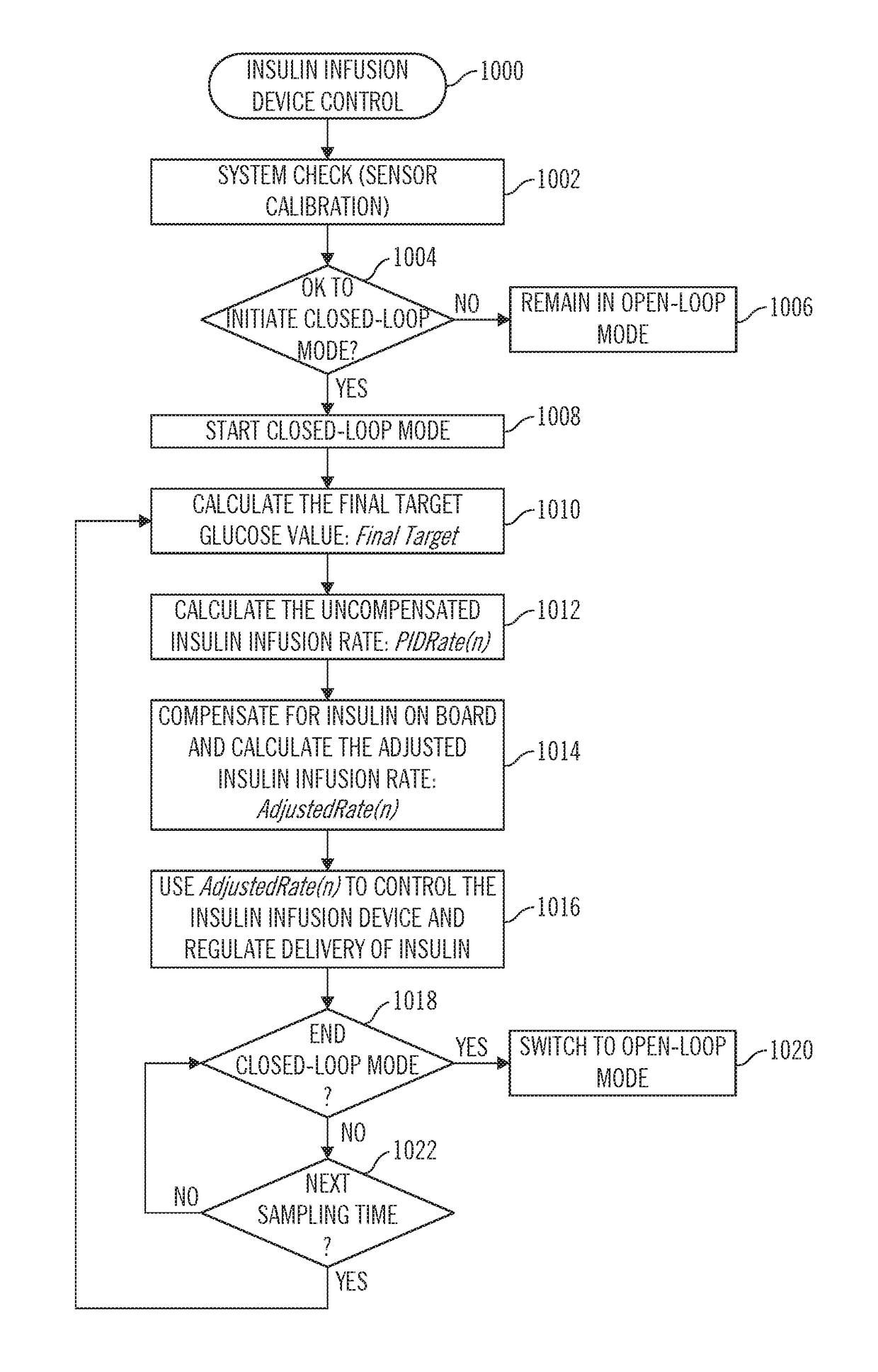
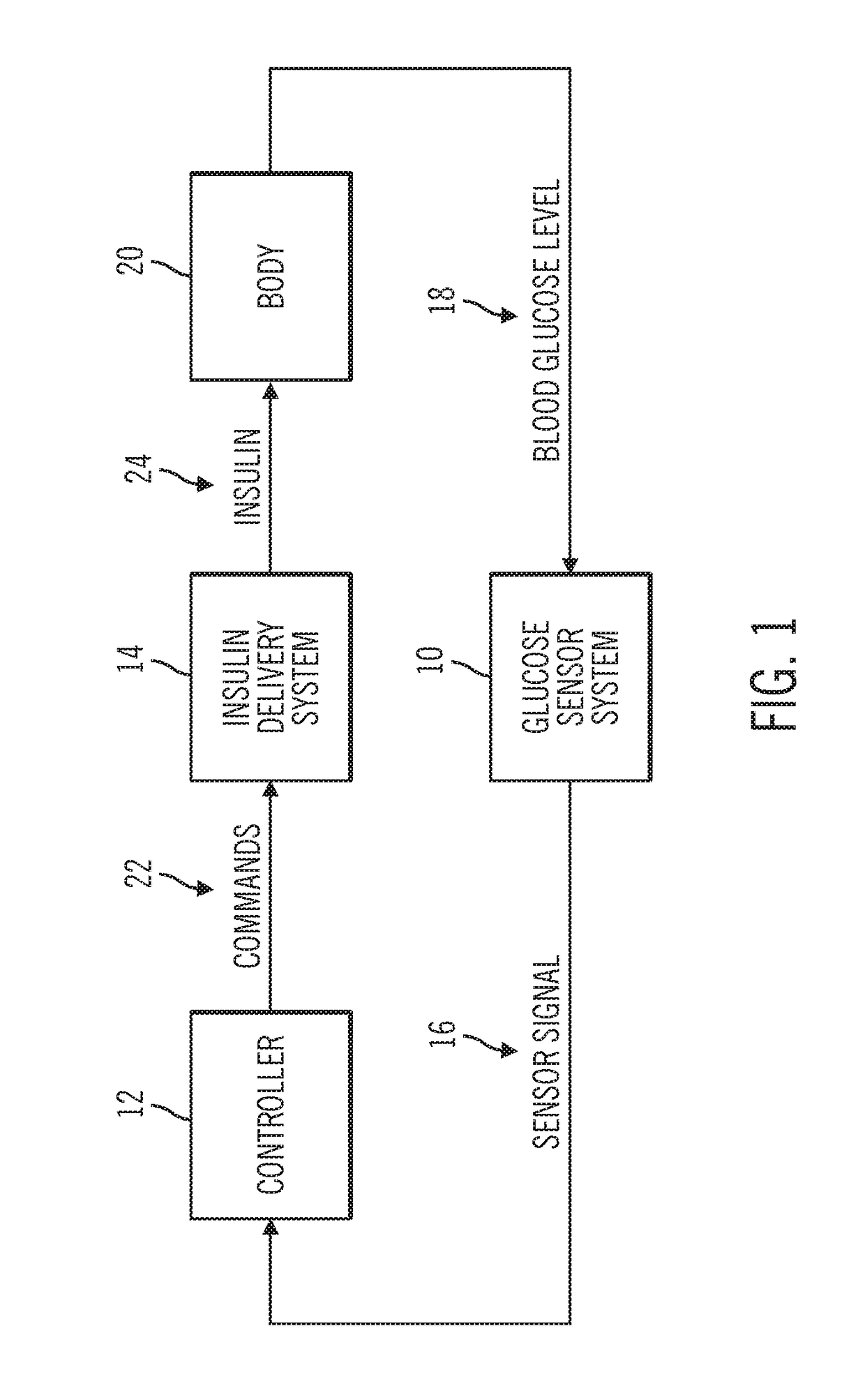
Sensor model supervisor for a closed-loop insulin infusion system
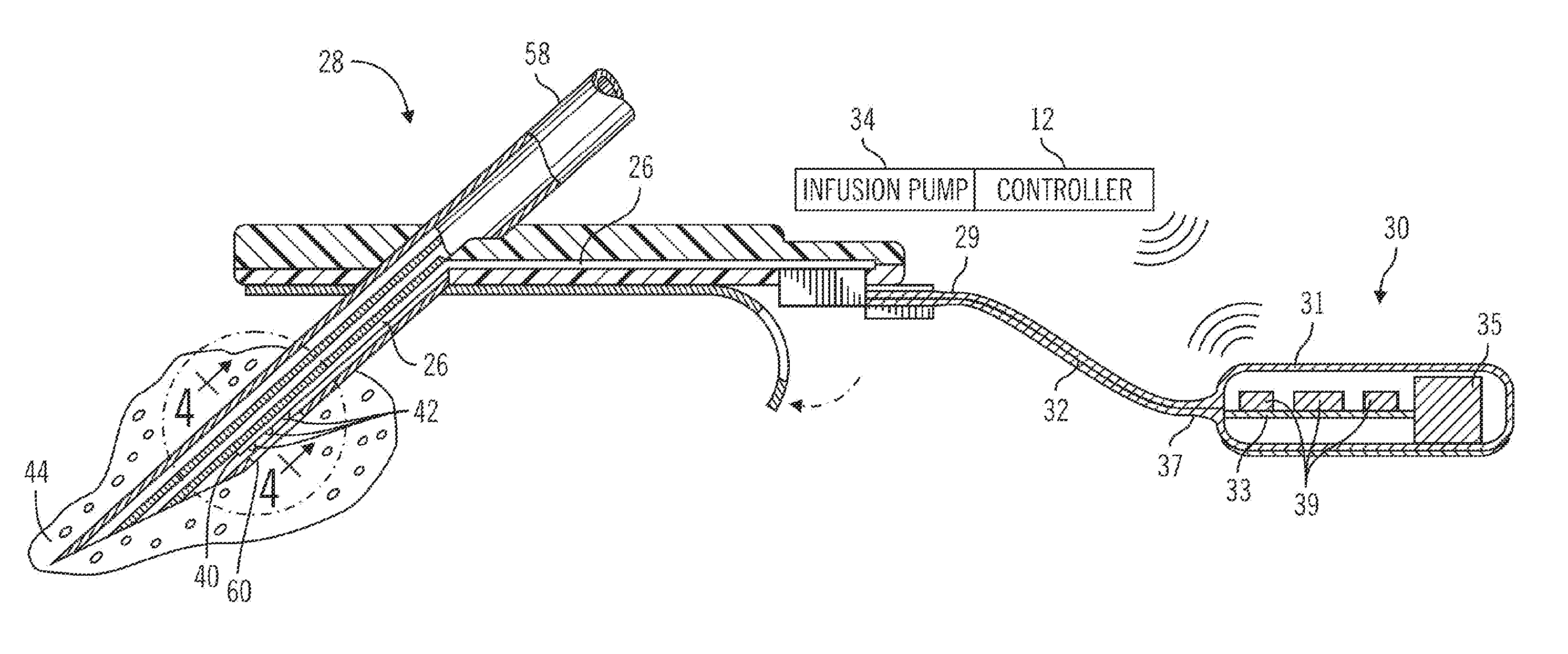
An insulin infusion device includes a processor architecture, and a memory element that stores executable instructions to perform a method of controlling delivery of insulin to a user. The method operates the device in a closed-loop mode to deliver insulin, obtains patient-specific parameters for a current time sample, and estimates a plasma insulin value and a blood glucose value for the user based on at least some of the patient-specific parameters. The estimating is also based on a previously estimated plasma insulin value obtained for a previous time sample, and a previously estimated blood glucose value obtained for the previous time sample. A predicted sensor glucose value is generated for the current time sample, and the closed-loop mode or a safe basal mode is selected for controlling operation of the insulin infusion device in accordance with the selected mode.
Owner:MEDTRONIC MIMIMED INC
Method and composition for the treatment of lipid and glucose metabolism disorders
InactiveUS20020187985A1Improved modification and regulationImprovement of one or more metabolic indicesBiocideAnimal repellantsBlood plasmaPlasma insulin
Disclosed are methods for modifying or regulating at least one of glucose or lipid metabolism disorders which comprises administering to a human or vertebrate subject a D1 dopamine agonist in conjunction with a dopamine D2 agonist where the conjoined administration is effective to improve at least one of the following lipid and glucose metabolic indices: body weight, body fat, plasma insulin, plasma glucose and plasma lipid, and plasma lipoprotein. In preferred embodiments, the administration of the D1 dopamine agonist and the D2 dopamine agonist is conducted at a predetermined time.
Owner:VEROSCI
Method and composition for the treatment of lipid and glucose metabolism disorders
InactiveUS20010016582A1Improved modification and regulationImprovement of one or more metabolic indicesBiocideCarbohydrate active ingredientsBlood plasmaPlasma insulin
Disclosed are methods for modifying or regulating at least one of glucose or lipid metabolism disorders which comprises administering to a human or vertebrate subject a D1 dopamine agonist in conjunction with a dopamine D2 agonist where the conjoined administration is effective to improve at least one of the following lipid and glucose metabolic indices: body weight, body fat, plasma insulin, plasma glucose and plasma lipid, and plasma lipoprotein. In preferred embodiments, the administration of the D1 dopamine agonist and the D2 dopamine agonist is conducted at a predetermined time.
Owner:CINCOTTA ANTHONY H
Method System and Device for Assessing Insulin Sensitivity
ActiveUS20080319381A1Low costHighly profitable product for the manufacturerMedical simulationDrug and medicationsPlasma glucose concentrationBlood plasma
A method and a system for determining insulin sensitivity (IS) is described. In one aspect the method and the system can be implemented by receiving a first parameter corresponding to an insulin dose in a subcutaneous tissue; applying a first kinetic model to obtain a plasma insulin concentration based on the first parameter; receiving a second parameter corresponding to a plasma glucose concentration; determining the insulin sensitivity (IS) based on the plasma insulin concentration and the second parameter.
Owner:ROCHE DIABETES CARE INC
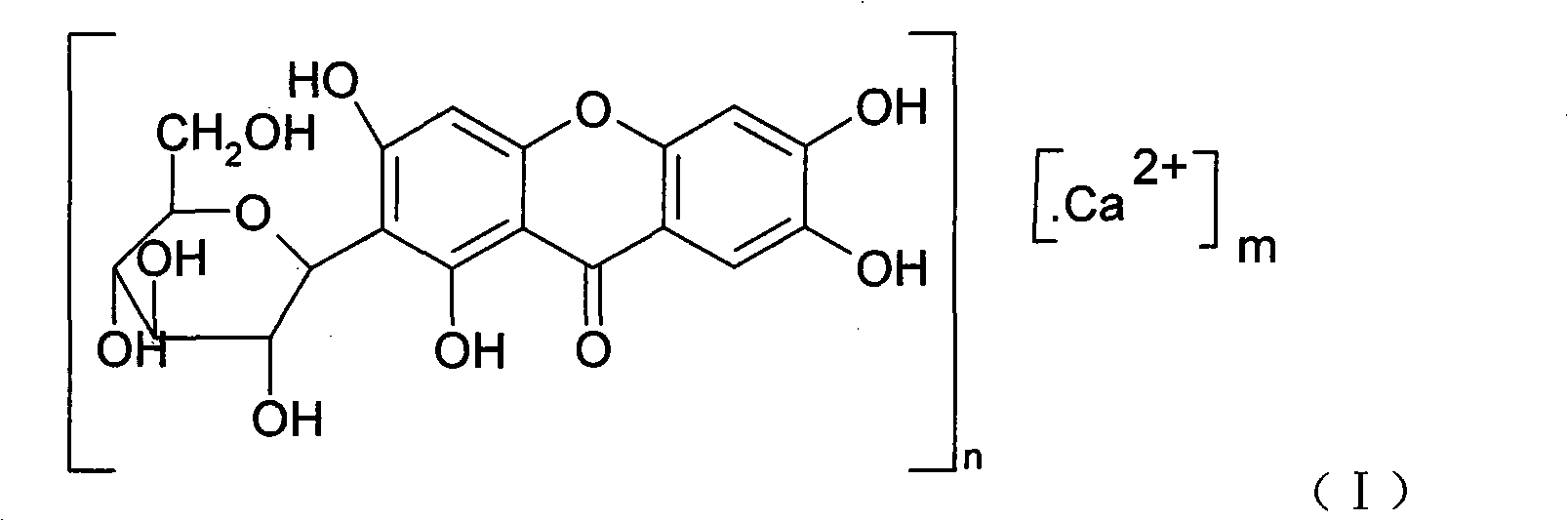
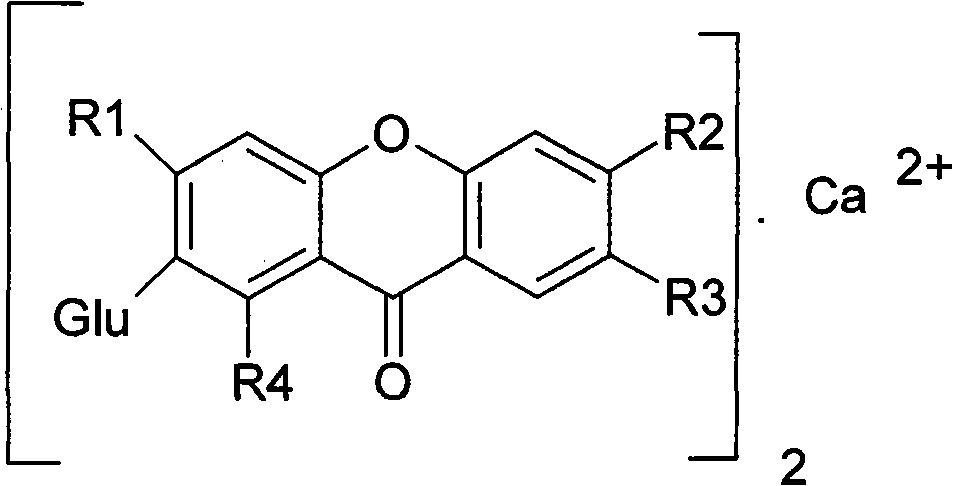
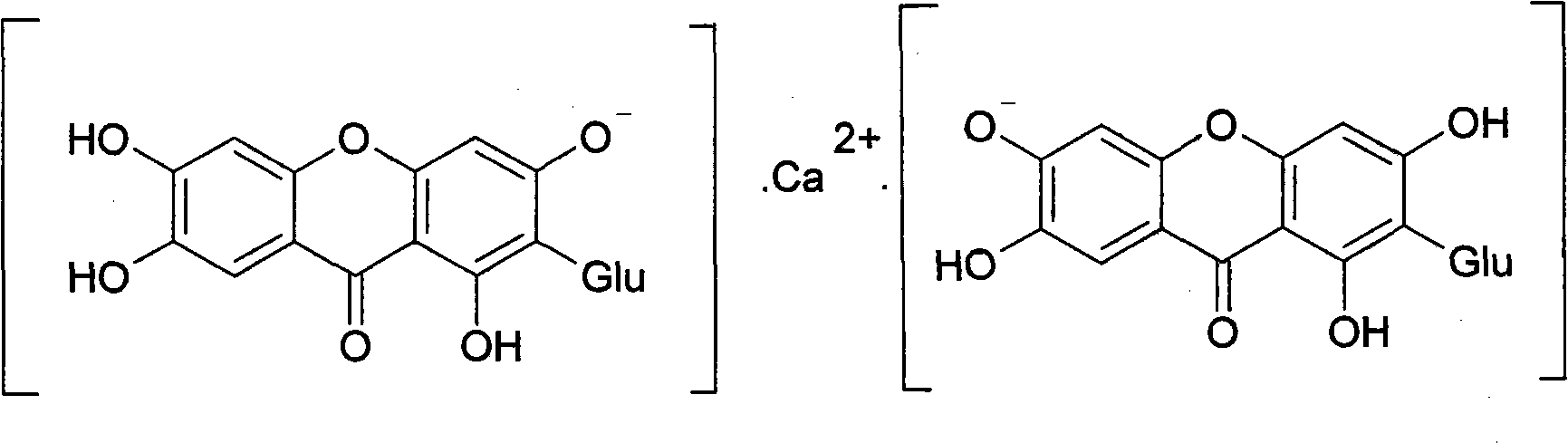
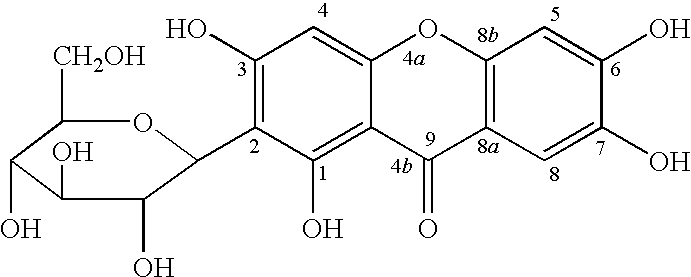
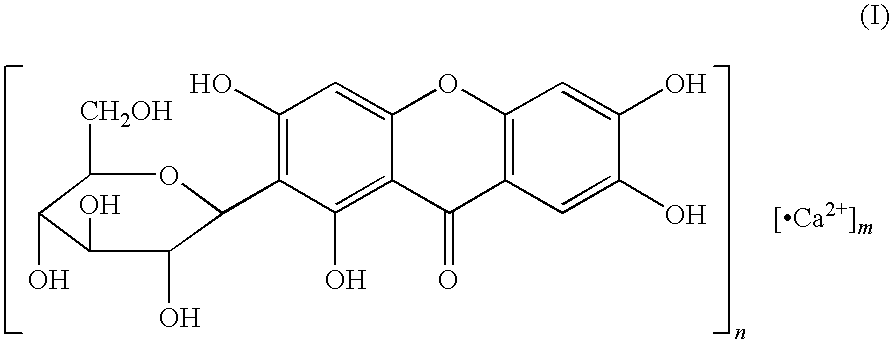
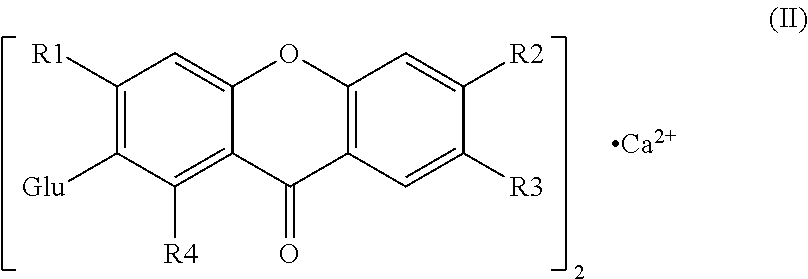
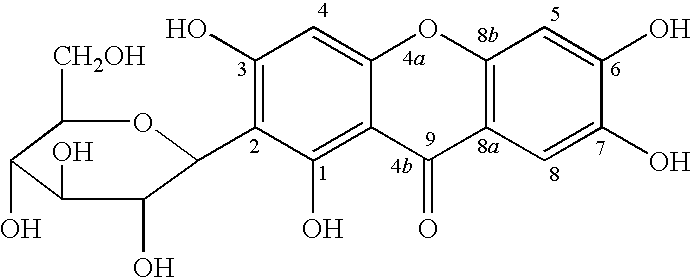
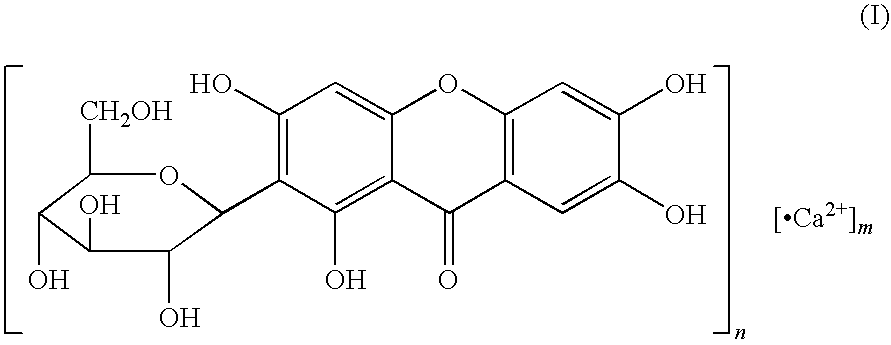
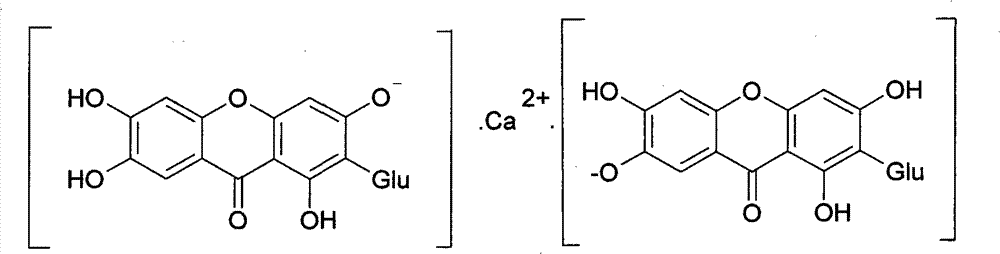
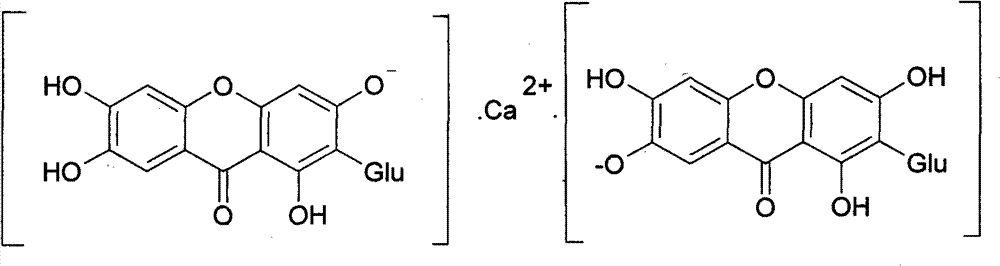
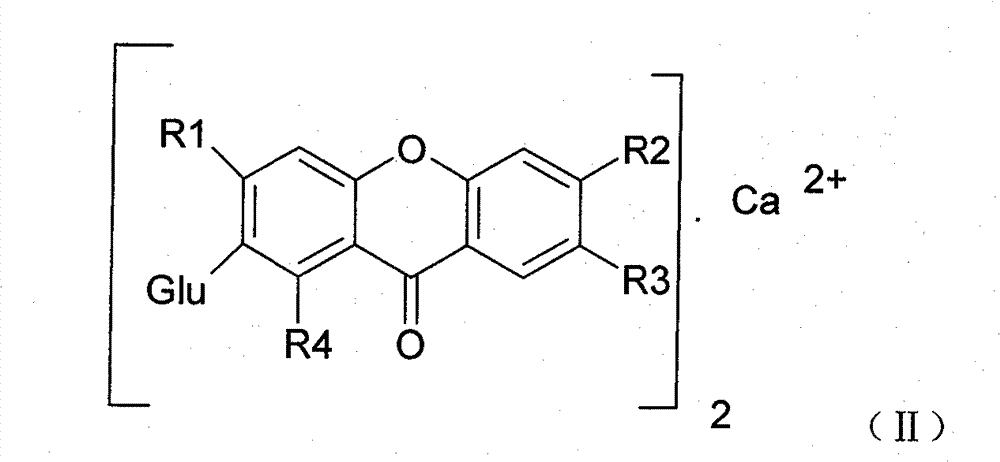
Novel mangiferin calcium salts, the method for its preparation and its use
The present invention provides a mangiferin calcium and its preparation and use. The mangiferin calcium can lower plasma insulin, glucose, lipid, and also can improve the solubility and oral bioavailability of mangiferin and increase insulin sensitivity.
Owner:CHANGZHOU DEZE MEDICAL SCI CO LTD
Application of leonurine to preparation of medicament for treating 2-type diabetes
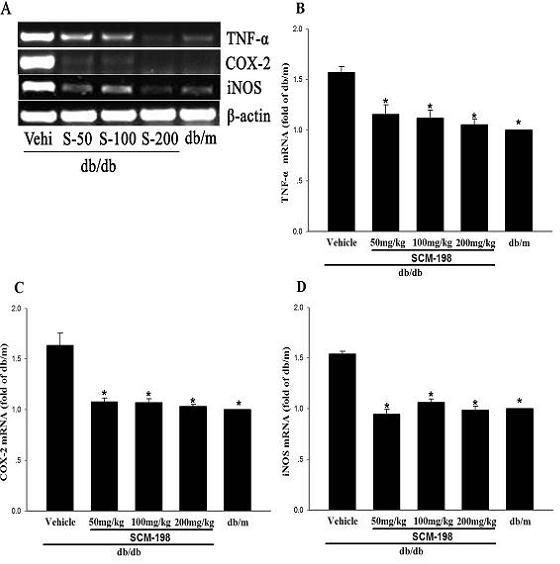
The invention belongs to the field of traditional Chinese medicine manufacturing, and relates to application of leonurine to preparation of a medicament for treating 2-type diabetes. Animal experiments prove that the fasting blood glucose of a 2-type diabetes mouse, i.e., a db / db mouse, can be lowered by the leonurine, and the tolerance of oral glucose is improved; and meanwhile, fasting plasma insulin is increased, the plasma triglyceride is reduced and the content of the plasma high-density lipoprotein is increased. Experiment results also show that the expression of liver glucose metabolic enzymes such as glucokinase, glucose-6-phosphatase and phosphoenolpyruvate carboxyl enzyme is adjusted by the leonurine in an Akt dependent mode; and the biological response of an inflammatory mediator, such as generation of TNF (Tumor Necrosis Factor)-alpha, degradation of IkB-alpha and subsequent phosphorylation of NF-kBp65, is suppressed. Through the leonurine, the inflammatory state of the 2-type diabetes can be corrected, and the symptoms of the 2-type diabetes can be improved; and the leonurine can be used as a treatment medicament to be applied to the treatment of the 2-type diabetes.
Owner:FUDAN UNIV
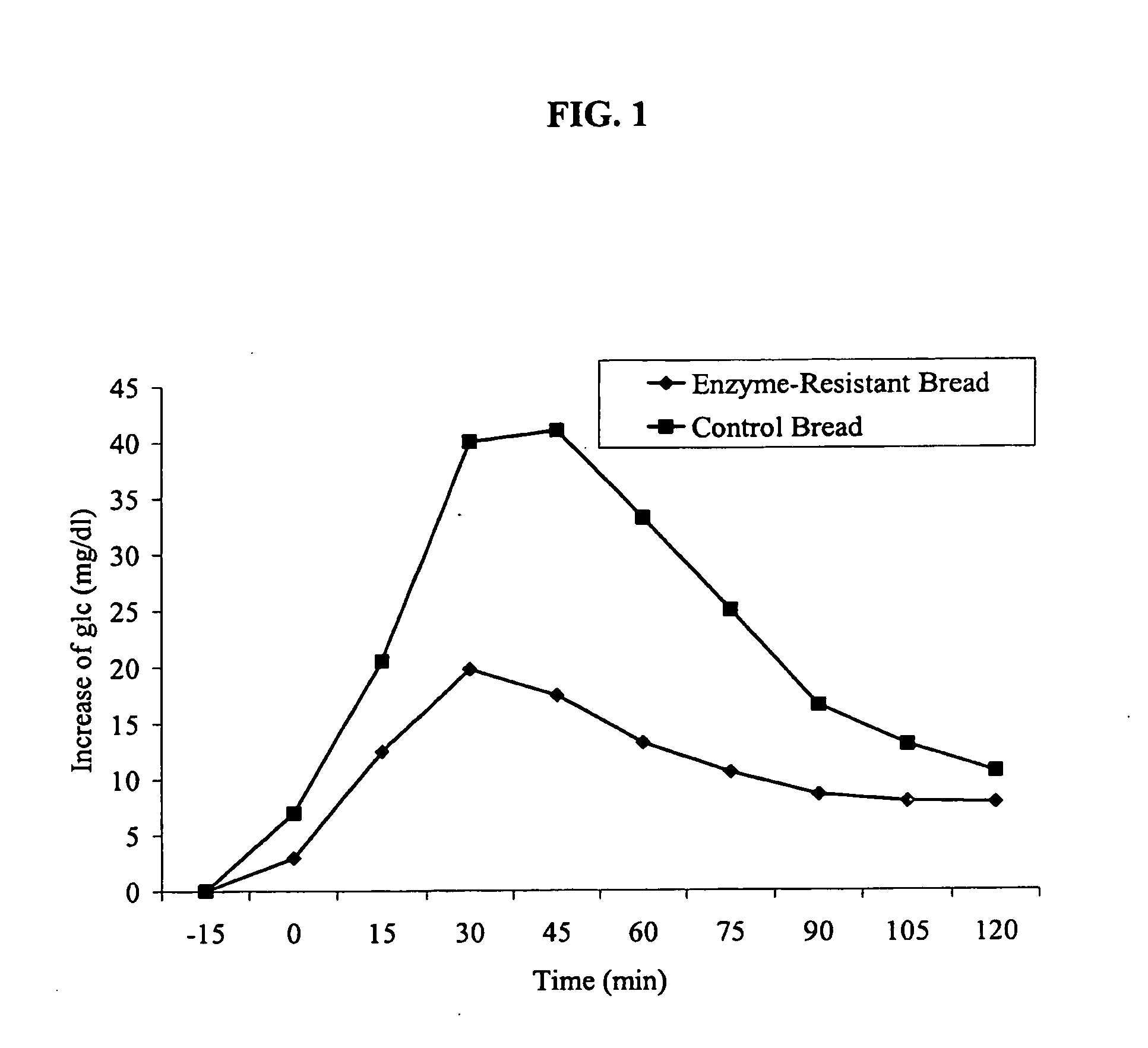
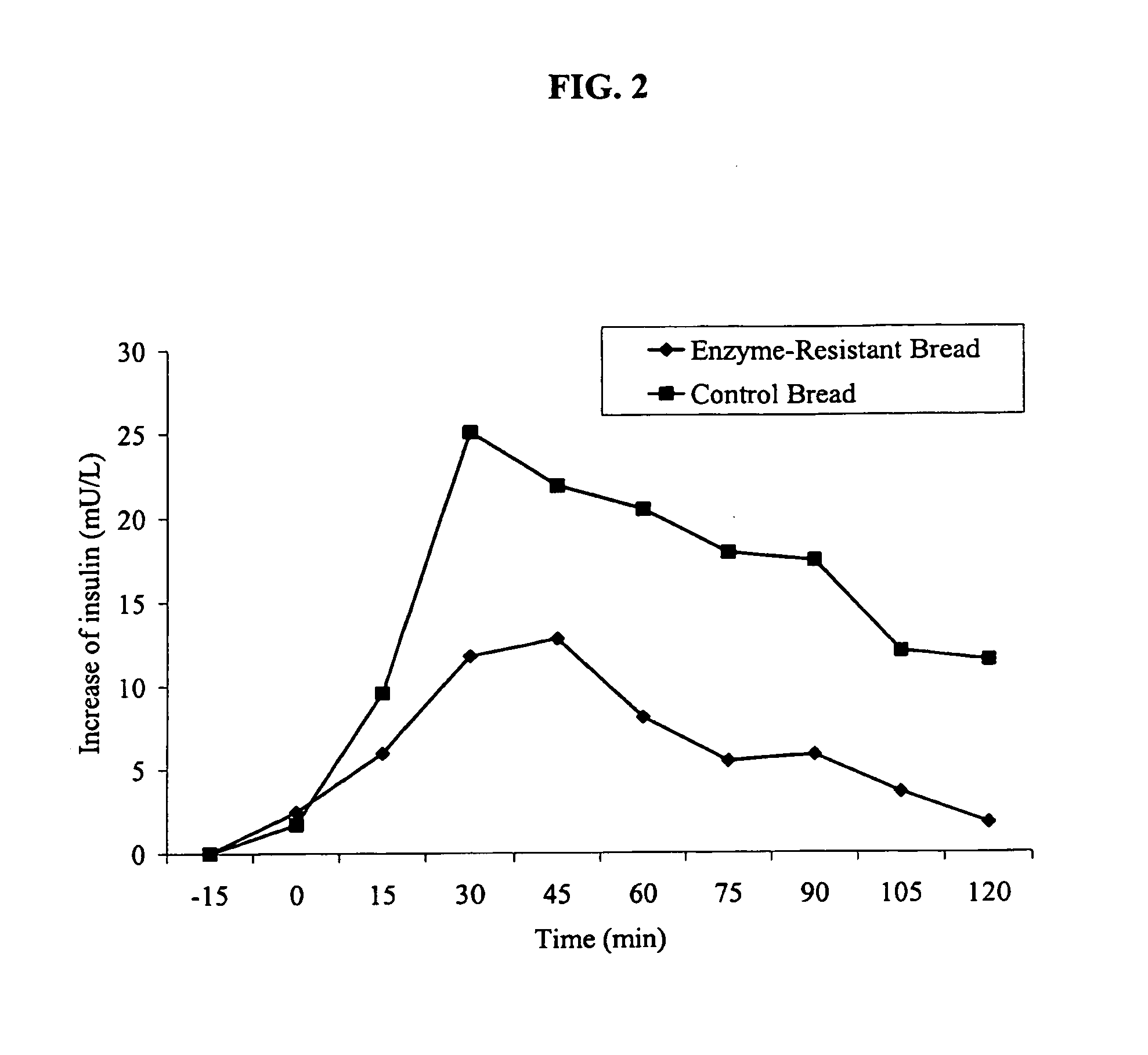
Resistant food starches and methods related thereto
InactiveUS20110256261A1Less timeLess energy-consumingMilk preparationDough treatmentBiotechnologyAdditive ingredient
The present invention provides novel resistant starches, methods to make the resistant starches, and methods to use the resistant starches. These resistant starches may be used as an ingredient in a variety of foods to impart health benefits, such as: decreasing plasma insulin response; decreasing plasma glucose response; increasing colonic fermentation; decreasing the risk of colon cancer; increasing digestive health; decreasing colonic pH. The methods for making the novel modified resistant starches dramatically decrease the cost of producing them.
Owner:IOWA STATE UNIV RES FOUND
Application of pinitol to pharmacy and health care product
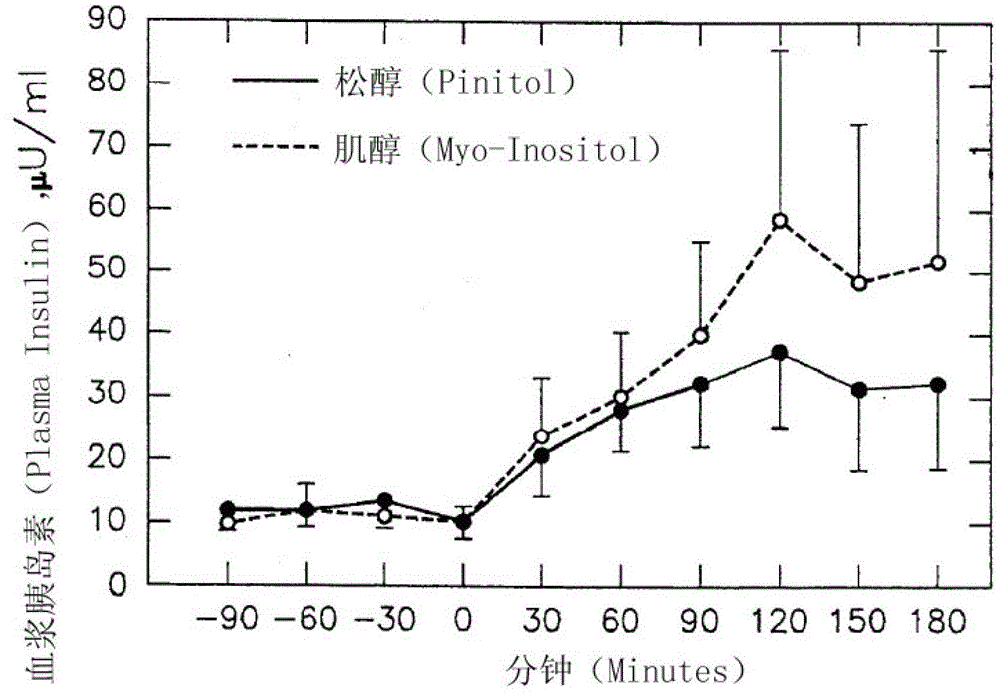
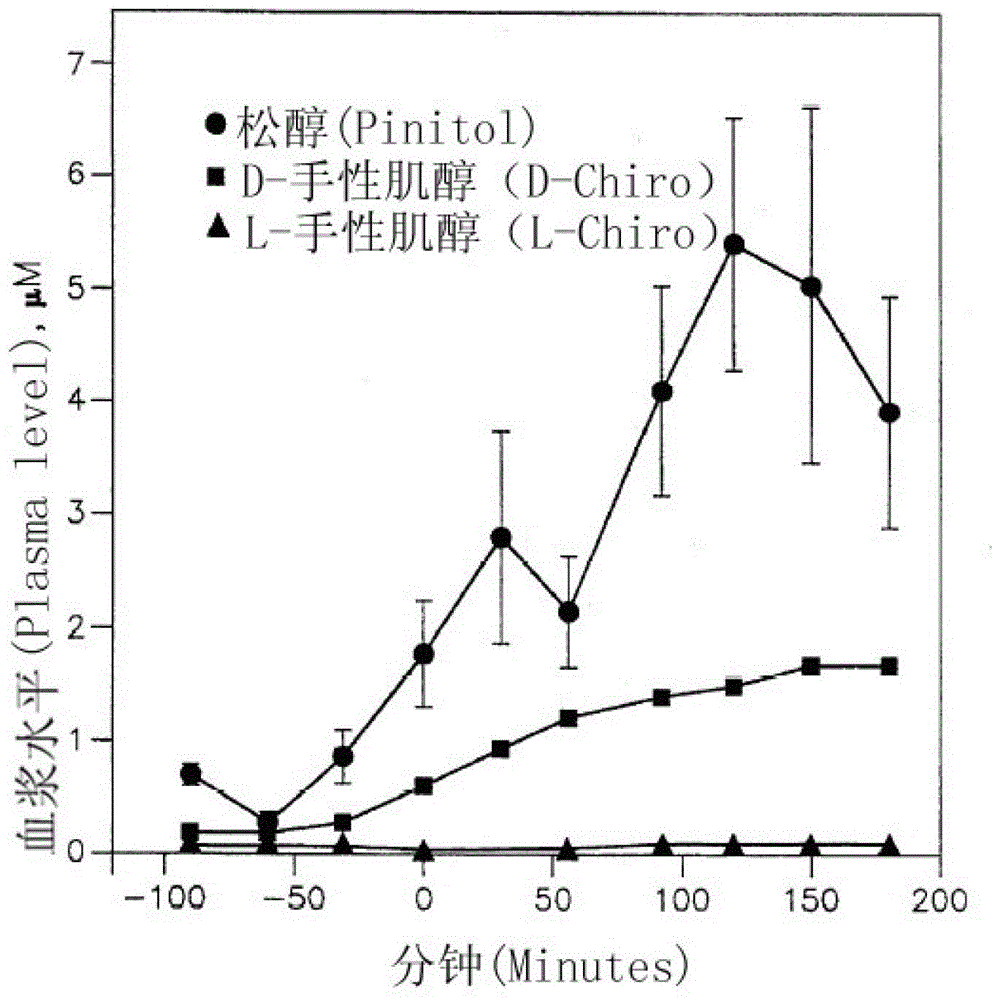
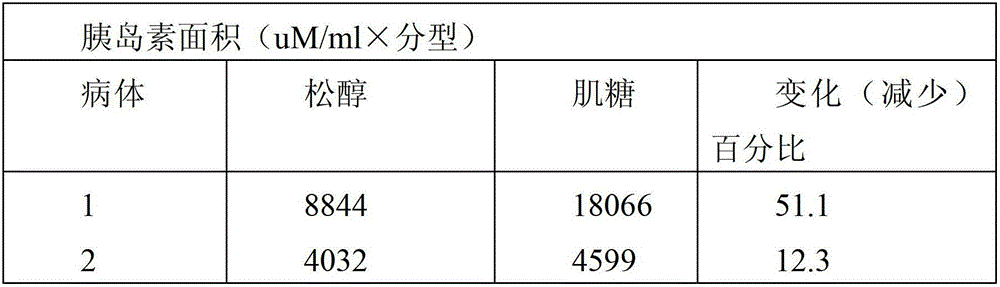
InactiveCN103202824ARich sourcesSimple preparation processAntibacterial agentsHydroxy compound active ingredientsD-chiro-InositolPinitol
The invention provides application of pinitol to pharmacy and health care product. Compared with the prior art, the invention has the beneficial effects that: novel medical application and health care application of a known compound is explored, and a new application field is developed; the product has rich source and simple preparation process, can be prepared into an oral preparation, an injection and tablet, and is convenient for use; the configured drug product or health care product has effects of increasing plasma levels of pinitol and D-chiro-inositol, and decreasing plasma insulin and glucose levels; and the configured drug product or health care product has effects of reducing the blood sugar value, insulin level, plasma lipid and plasma triglyceride.
Owner:SHAANXI HUAXIN ECONOMIC & TRADE
Mangiferin calcium salts, the method for its preparation and its use
ActiveUS8334267B2Improve bioavailabilityImprove solubilityBiocideSenses disorderLipid formationSolubility
The present invention relates to novel mangiferin calcium and its preparation and use. The mangiferin calcium can lower plasma insulin, glucose, lipid, also can improve the solubility and oral bioavailability of mangiferin.
Owner:CHANGZHOU DEZE MEDICAL SCI CO LTD
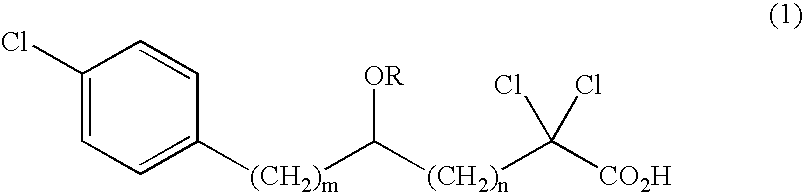
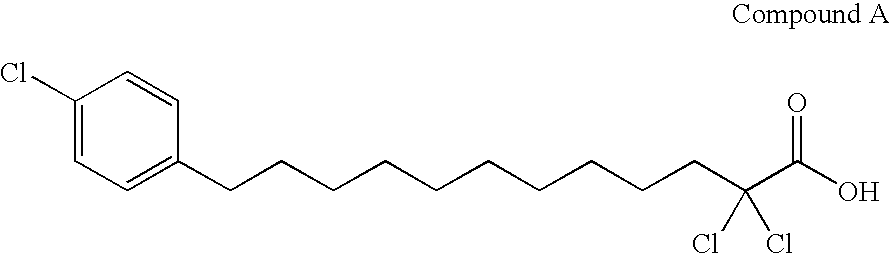
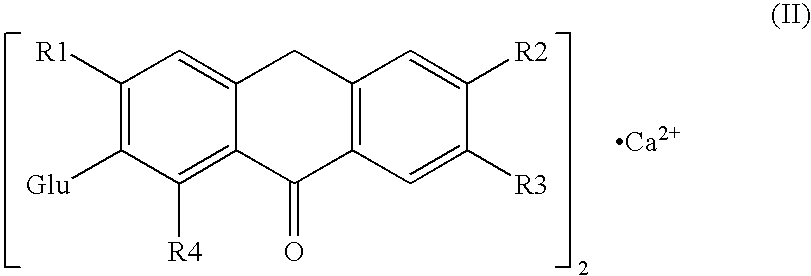
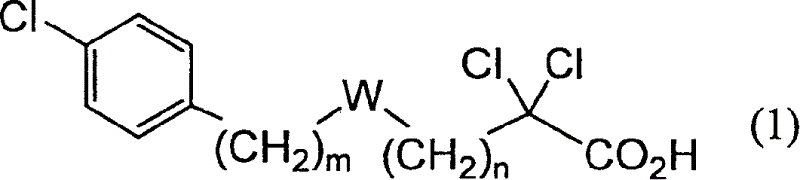
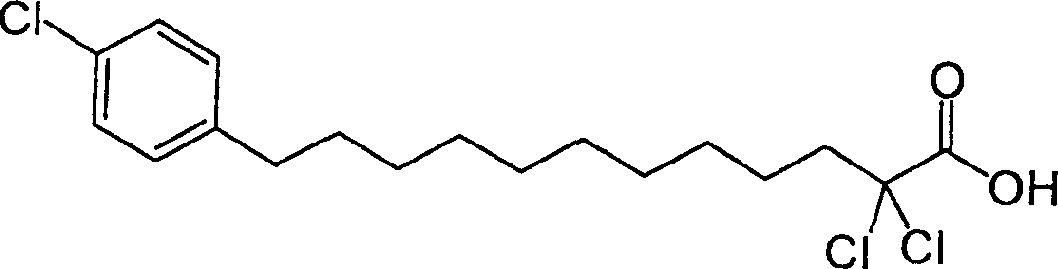
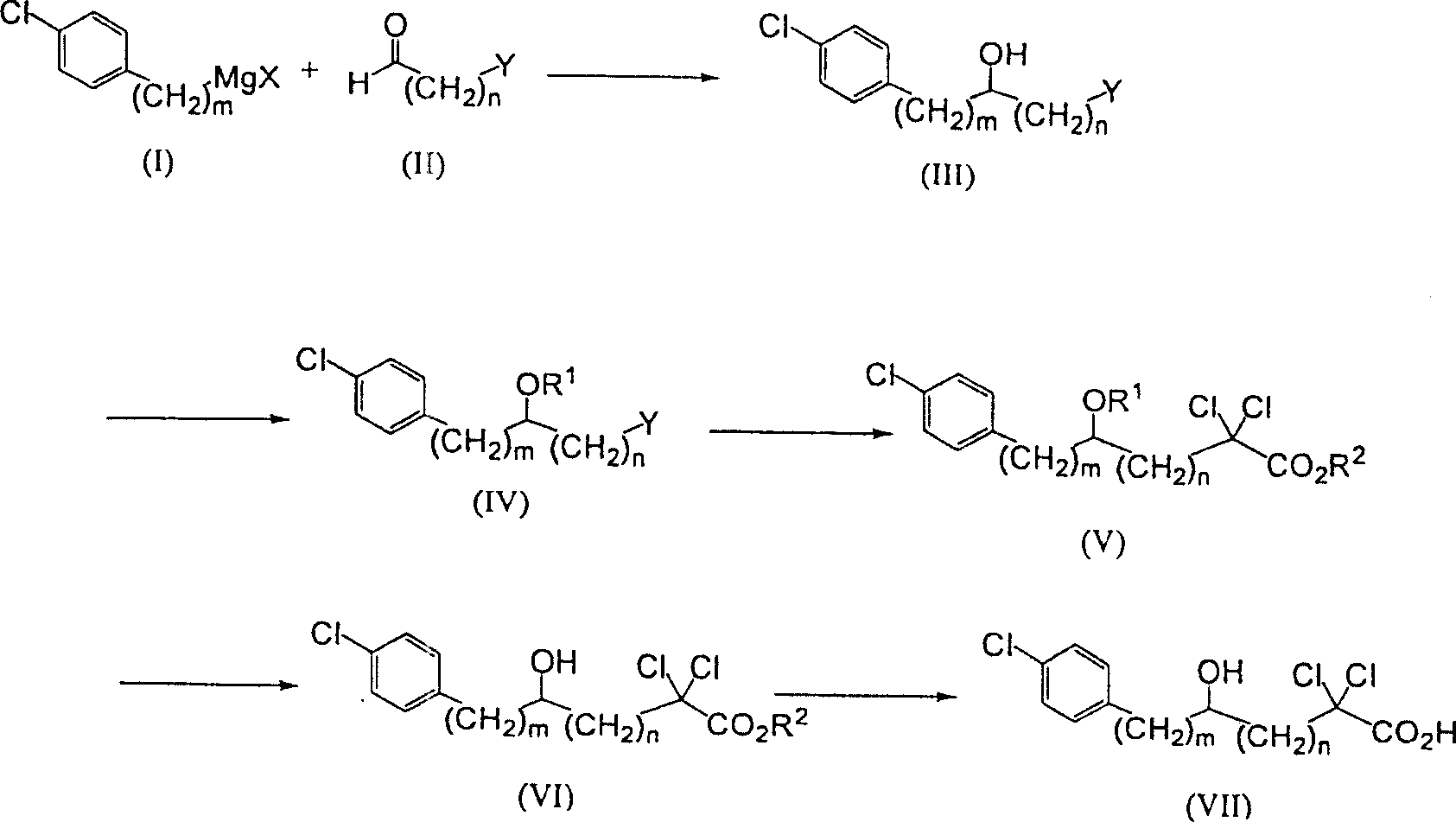
Carboxylic compound and medicine comprising the same
InactiveUS7109242B2Effective treatmentHigh activityBiocideOrganic chemistryHydrogen atomTriglyceride
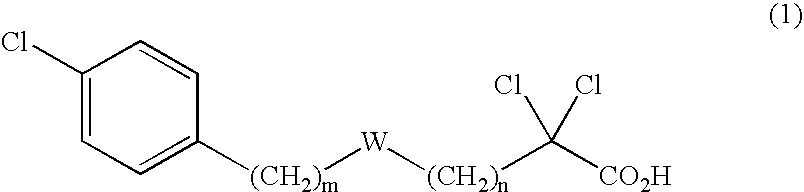
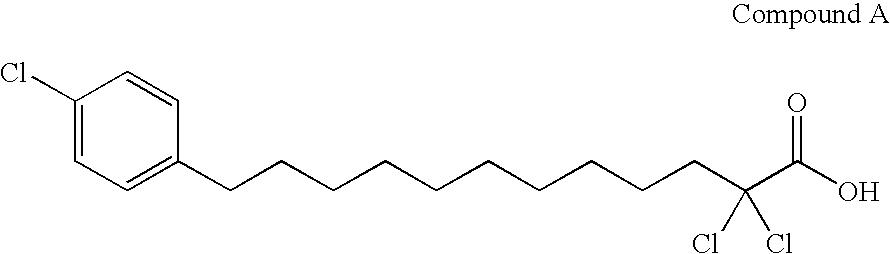
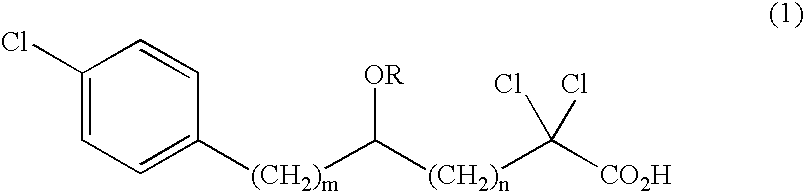
A compound represented by the following general formula (1), a salt thereof, or an ester thereof:wherein m represents an integer of 0 to 4, n represents an integer of 5 to 9, and R represents hydrogen atom or a protective group of hydroxyl group, which has reducing actions of blood glucose, plasma insulin, and triglyceride, and is useful for preventive and / or therapeutic treatment of diabetes, complications of diabetes, hyperlipemia and others.
Owner:KOWA CO LTD
Comprehensive utilization method for potato residues, potato juice and modified starch based on juicing of potatoes
PendingCN107259444APromote staple foodPromote the level of industrializationFood ingredientsSolubilityDigestion
The invention discloses a comprehensive utilization method for potato residues, potato juice and modified starch based on juicing of potatoes. The method comprises the following steps: with cleaned, sorted and sieved potatoes as a raw material, carrying out juicing on the potatoes in the process of extraction of starch from the potatoes; separating starch so as to obtain potato residues and potato juice; and separately conveying the potato residues and the potato juice to respective treating zones for treatment via pipelines or conveyer belts. According to the invention, juicing is carried out on the basis of extraction of starch from the potatoes and then comprehensive treatment is carried out on the products, i.e., the potato residues, the potato juice and the starch, so the industrialization level of the potato industry is improved and application of potatoes as staple food grain is promoted. In the process of processing, flour and potato modified starch adsorb and bond with water molecules, and cross-linking and coagulation occur, so a novel crystal structure is formed; the novel crystal structure is low in solubility in water, enzymatic hydrolysis is reduced, and digestion and absorption are slowed down; and thus, the composition of starch in wheaten food can be changed, and rise in blood sugar and the reaction of plasma insulin after meal are delayed.
Owner:张保华
Nasal formulations of insulin
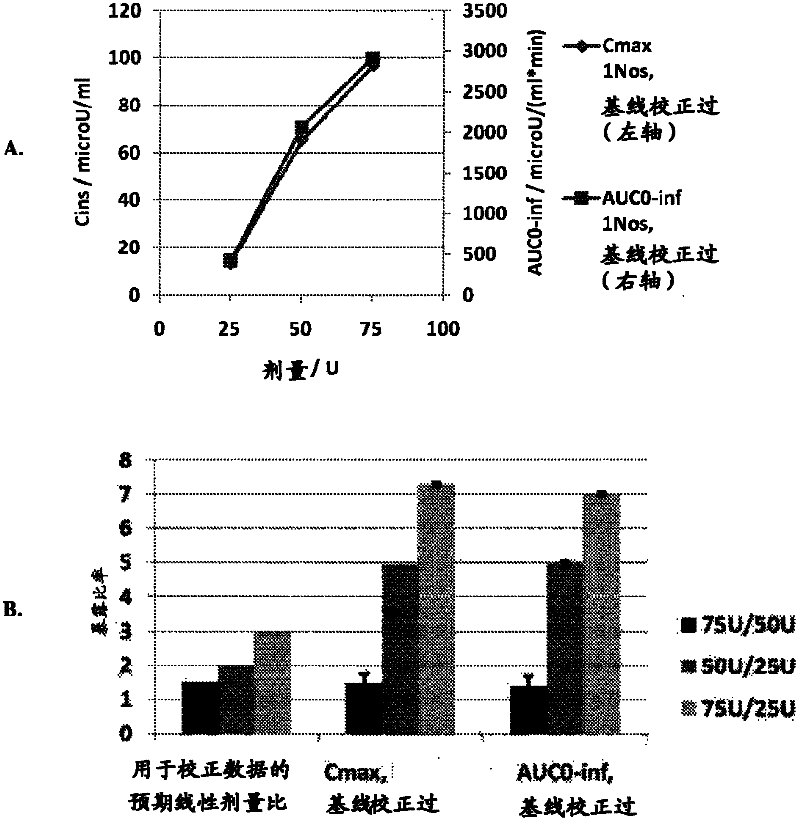
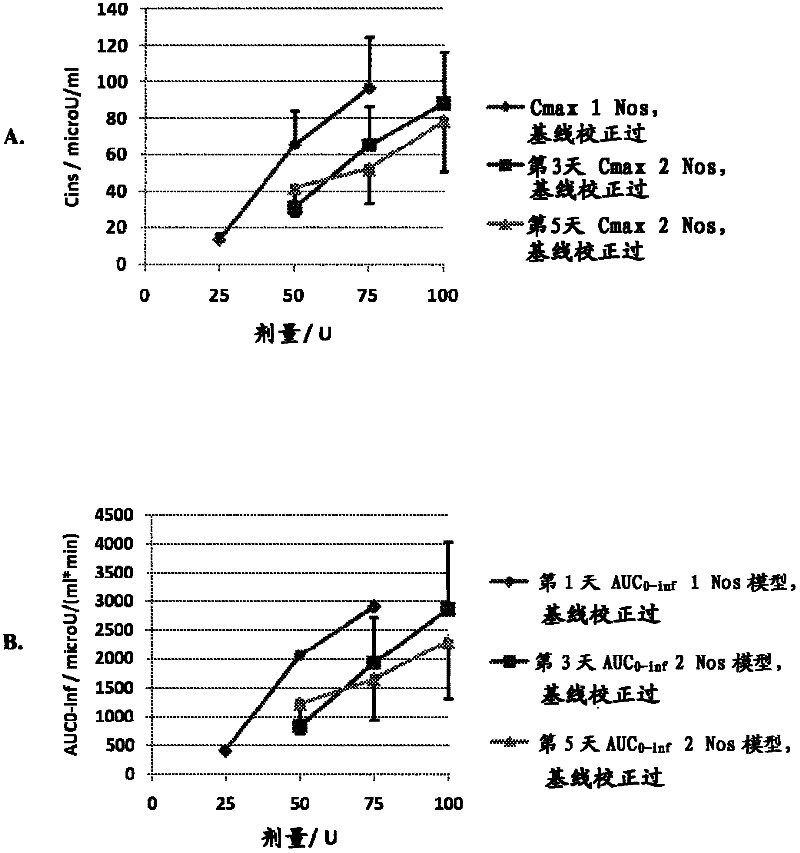
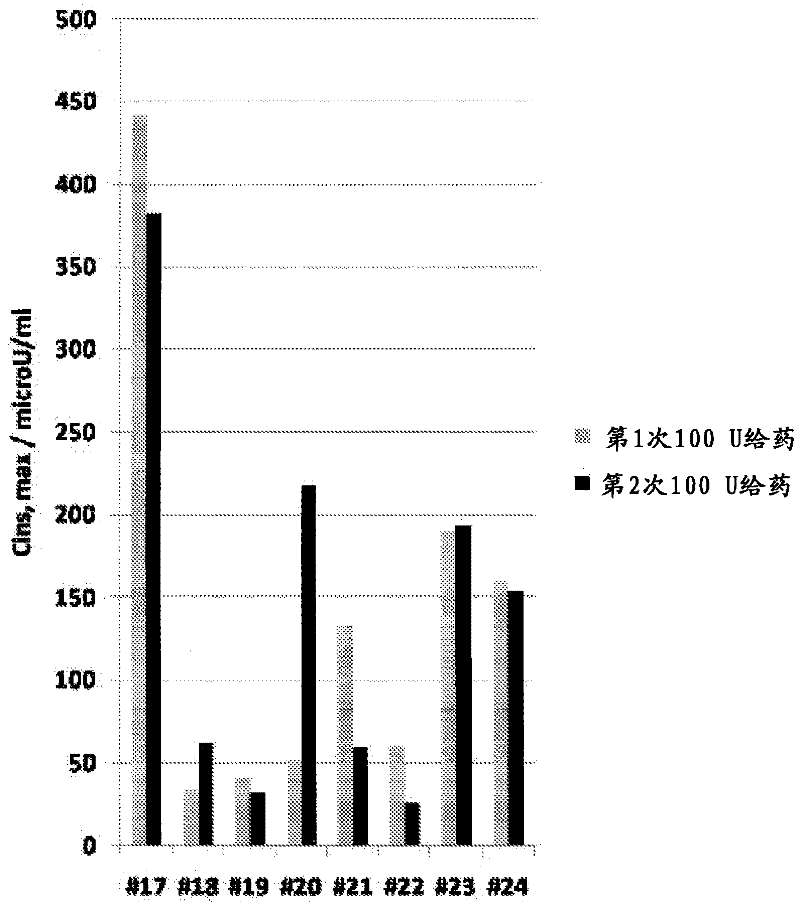
The present invention provides a method for achieving a therapeutically effective plasma levels of insulin by admmisteppg at least two doses of pharmaceutical formulation of insulin sequentially into the same nostril The administration of the second dose in the same nostril gives substantially higher plasma levels of insulin when compared with sequential administration in two different nostpls Without being limited to any specific physiological mechanism, it is believed that the first dose of insulin acts as a loading dose This loading dose is required to achieve the subsequent plasma levels of insulin that are observed with subsequent doses The Cmax of plasma insulin achieved by the methods and formulations of the present invention is at least about 7Q microU / ml when plasma insulin is measured from about 0 to about 45 minutes after administration of a second dose The AUC achieved is at least about 1800 microU / (ml* mm).
Owner:CPEX PHARMACEUTICALS INC
Sensor model supervisor for a closed-loop insulin infusion system
An insulin infusion device includes a processor architecture, and a memory element that stores executable instructions to perform a method of controlling delivery of insulin to a user. The method operates the device in a closed-loop mode to deliver insulin, obtains patient-specific parameters for a current time sample, and estimates a plasma insulin value and a blood glucose value for the user based on at least some of the patient-specific parameters. The estimating is also based on a previously estimated plasma insulin value obtained for a previous time sample, and a previously estimated blood glucose value obtained for the previous time sample. A predicted sensor glucose value is generated for the current time sample, and the closed-loop mode or a safe basal mode is selected for controlling operation of the insulin infusion device in accordance with the selected mode.
Owner:MEDTRONIC MIMIMED INC
Novel mangiferin calcium salts, the method for its preparation and its use
ActiveUS20100249046A1Easy to getImprove bioavailabilityBiocideSaccharide with heterocyclic radicalsSolubilityLipid formation
The present invention relates to novel mangiferin calcium and its preparation and use. The mangiferin calcium can lower plasma insulin, glucose, lipid, also can improve the solubility and oral bioavailability of mangiferin.
Owner:CHANGZHOU DEZE MEDICAL SCI CO LTD
Multivariable artificial pancreas method and system
ActiveUS10646650B2Easy to controlIncrease ratingsMedical devicesCatheterLow glucosePancreatic hormone
Methods and modules for using physiological (biometric) variables to advance the state of the artificial pancreas. The method and system includes one or more modules for recursive model identification, hypoglycemia early alert and alarm, adaptive control, hyperglycemia early alert and alarm, plasma insulin concentration estimation, assessment of physical activity (e.g., presence, type, duration, expected effects on insulin sensitivity and GC), detection of acute stress and assessment of its impact on insulin sensitivity, detection of sleep and its stages and assessment of sleep stages on GC, sensor fault detection and diagnosis, software and controller performance evaluation and adjustment and / or pump fault detection and diagnosis.
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
Carboxylic compound and medicine comprising the same
InactiveUS20050009909A1Reduce and eliminate side effectEffective treatmentBiocideOrganic chemistryCarboxylic acidTherapeutic treatment
A compound represented by the following general formula (1), a salt thereof, or an ester thereof: wherein m represents an integer of 0 to 4, n represents an integer of 5 to 9, and R represents hydrogen atom or a protective group of hydroxyl group, which has reducing actions of blood glucose, plasma insulin, and triglyceride, and is useful for preventive and / or therapeutic treatment of diabetes, complications of diabetes, hyperlipemia and others.
Owner:KOWA CO LTD
Method system and device for assessing insulin sensitivity
ActiveUS9244077B2Low costHighly profitable product for the manufacturerMedical simulationDrug and medicationsPlasma glucose concentrationBlood plasma
A method and a system for determining insulin sensitivity (IS) is described. In one aspect the method and the system can be implemented by receiving a first parameter corresponding to an insulin dose in a subcutaneous tissue; applying a first kinetic model to obtain a plasma insulin concentration based on the first parameter; receiving a second parameter corresponding to a plasma glucose concentration; determining the insulin sensitivity (IS) based on the plasma insulin concentration and the second parameter.
Owner:ROCHE DIABETES CARE INC
Application of pinitol to pharmacy and health care product
InactiveCN103202824BRich sourcesSimple preparation processAntibacterial agentsHydroxy compound active ingredientsPinitolLow insulin
The invention provides application of pinitol to pharmacy and health care product. Compared with the prior art, the invention has the beneficial effects that: novel medical application and health care application of a known compound is explored, and a new application field is developed; the product has rich source and simple preparation process, can be prepared into an oral preparation, an injection and tablet, and is convenient for use; the configured drug product or health care product has effects of increasing plasma levels of pinitol and D-chiro-inositol, and decreasing plasma insulin and glucose levels; and the configured drug product or health care product has effects of reducing the blood sugar value, insulin level, plasma lipid and plasma triglyceride.
Owner:SHAANXI HUAXIN ECONOMIC & TRADE
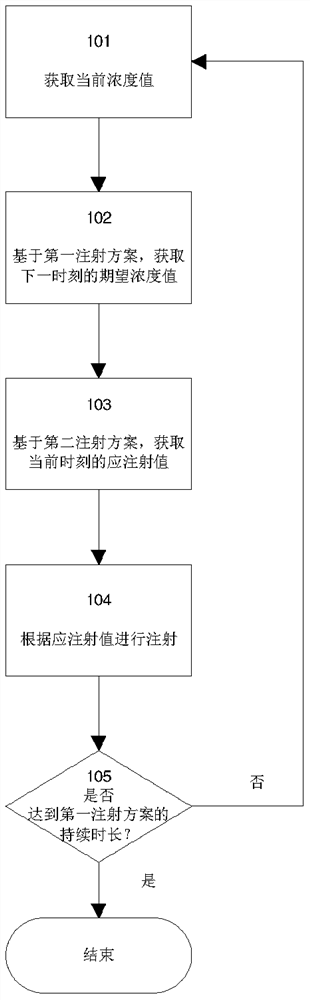
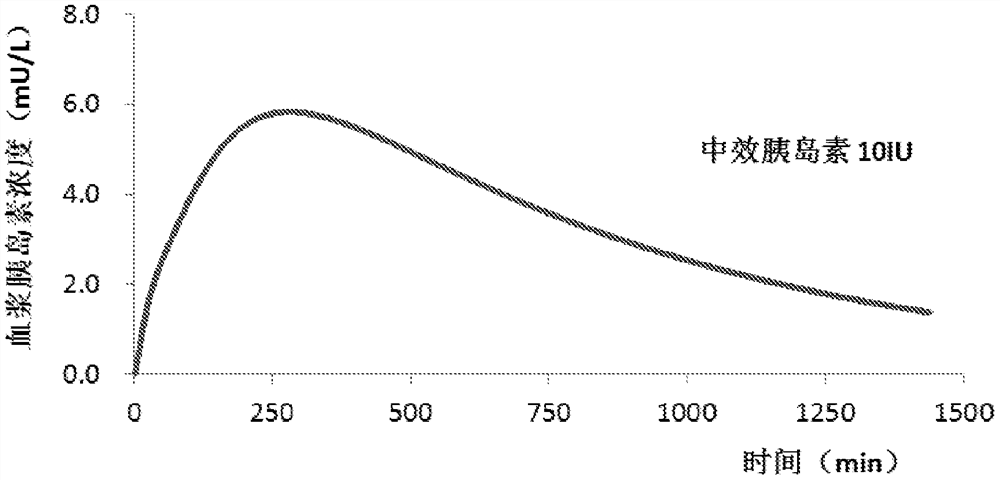
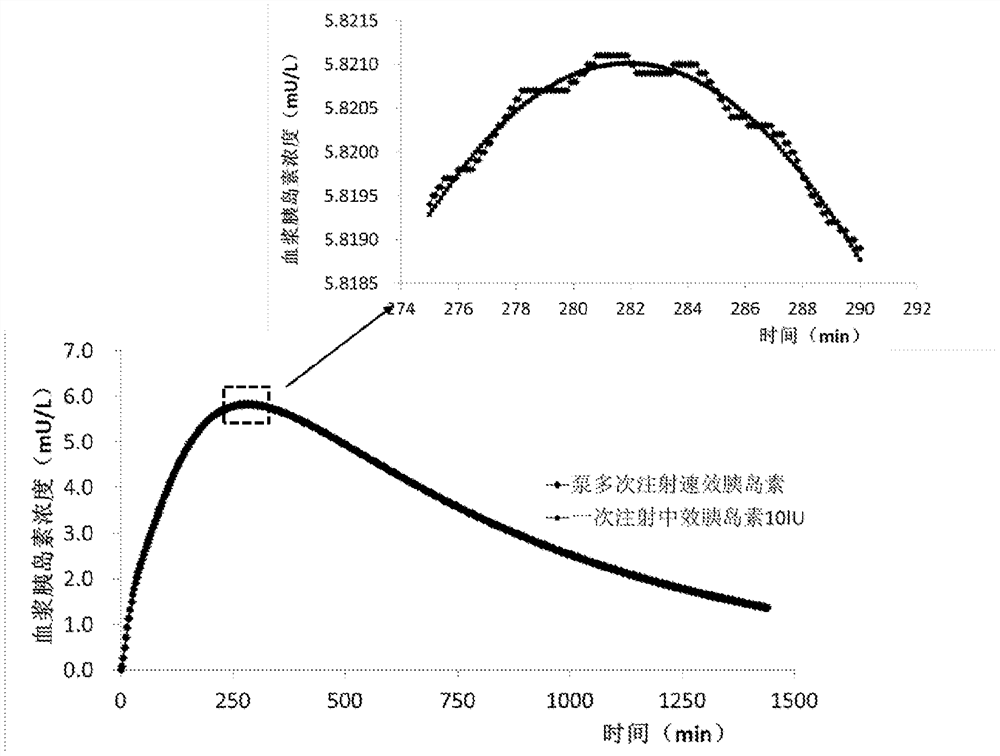
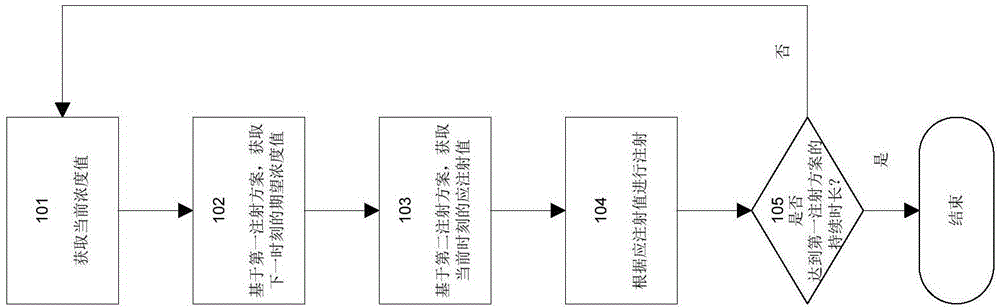
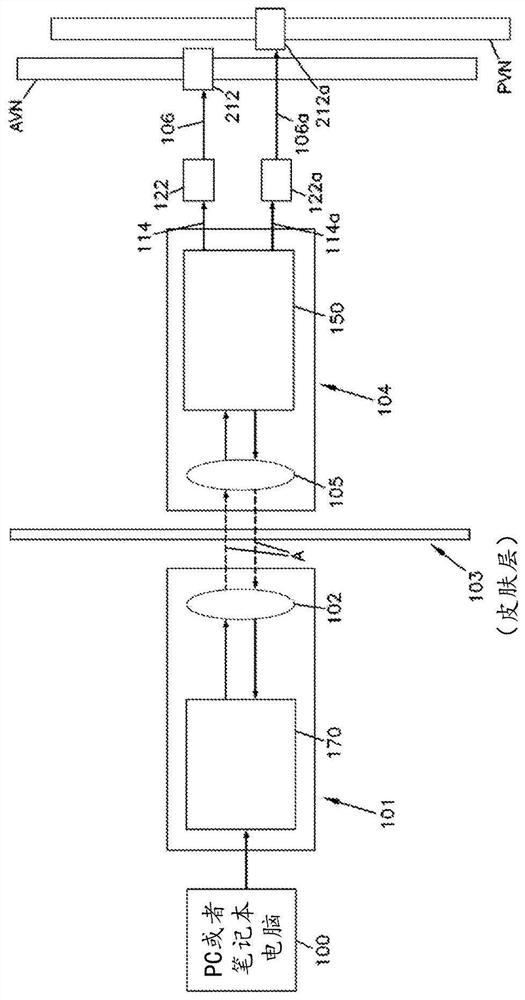
Syringe pump control method
A method for controlling an insulin injection pump, a related control unit (200), and an injection pump (300). The control unit (200) comprises: an expected plasma insulin concentration value acquiring module (202), a current subcutaneous and interstitial insulin concentration value acquiring module (201), a supposed injection value acquiring module (203), an injection instruction sending module (204), and a counting module (205). The injection pump comprises a pump body and the control unit (200), and the control unit (200) is disposed in the pump body.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
An injection pump control method and control unit and an injection pump
InactiveCN105688308AEasy to useAutomatic syringesDrug and medicationsInsulin injectionPlasma insulin
The invention relates to a method for controlling an insulin injection pump and also relates to a relevant control unit and the injection pump. The control unit comprises an anticipated plasma insulin concentration value obtaining module, a current subcutaneous and tissue space insulin concentration value obtaining module, a necessary injection value obtaining module, an injection command transmitting module and a counting module. The injection pump comprises a pump body and the control unit arranged in the pump body. According to the invention, the injection pump can be extremely convenient to use, so that the injection pump can be promoted to benefit vast patients.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
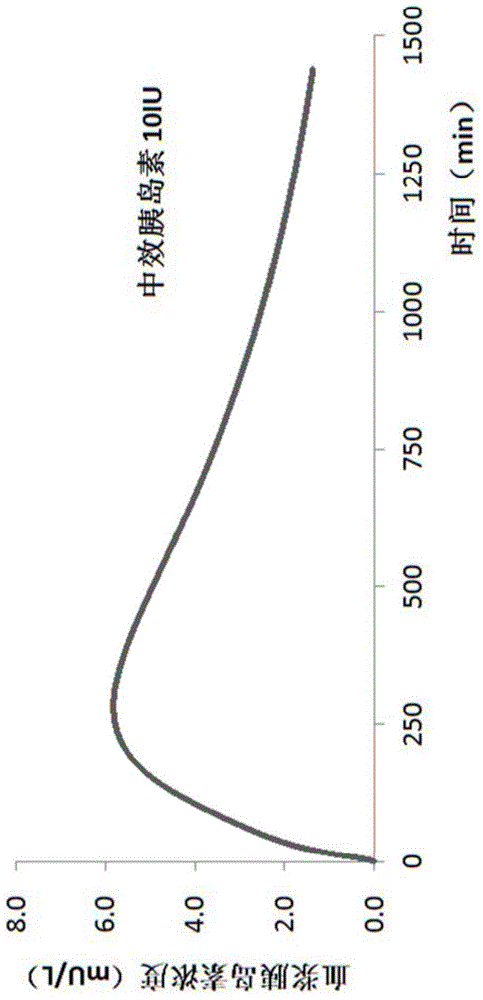
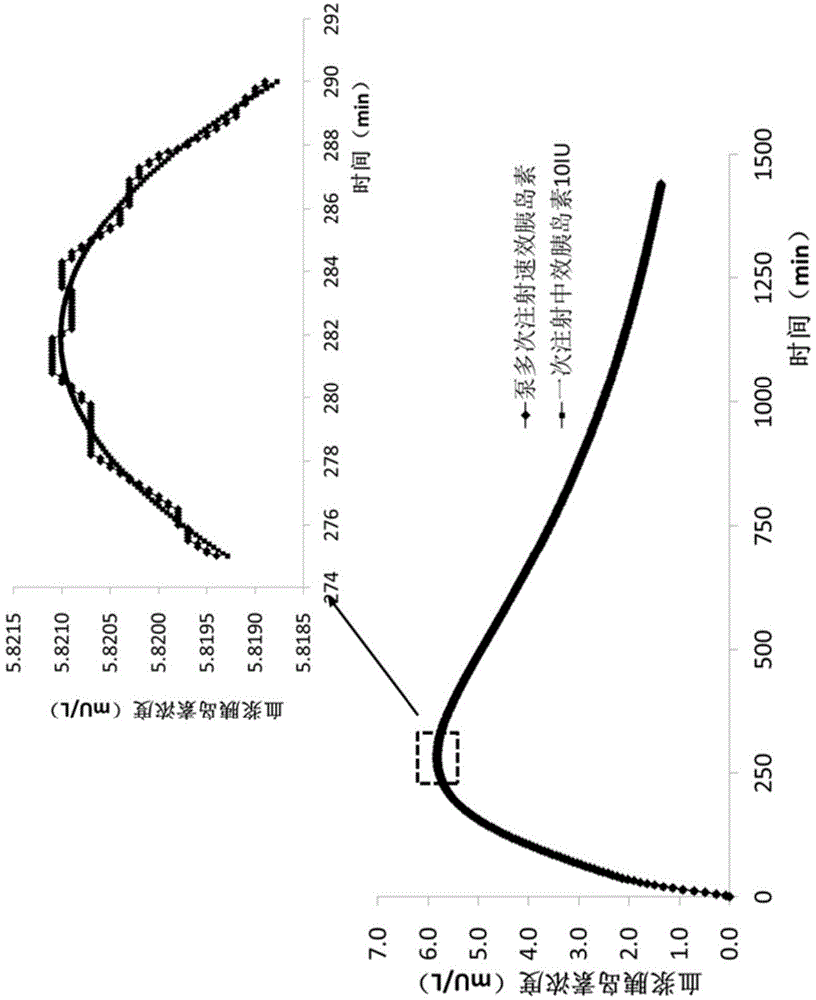
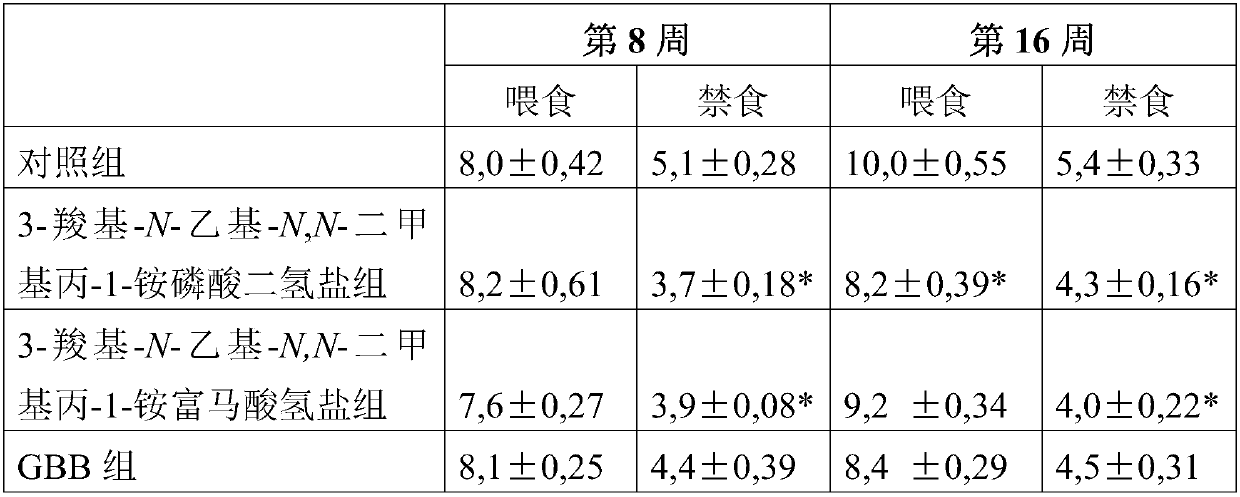
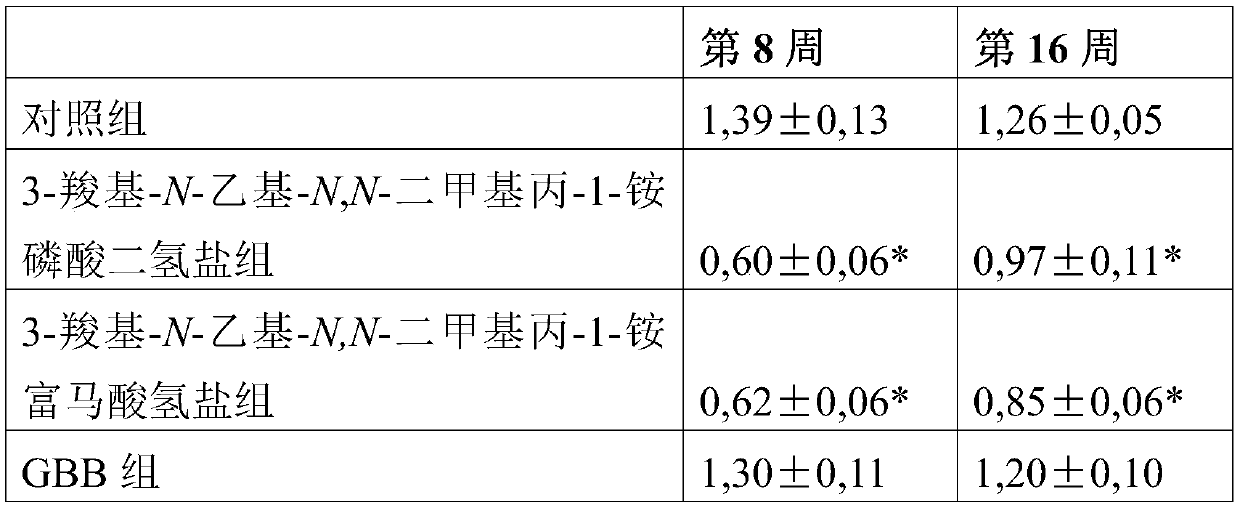
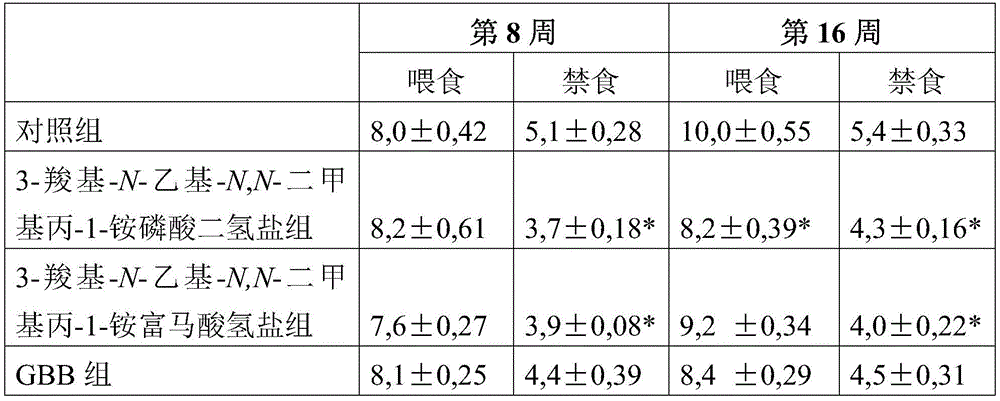
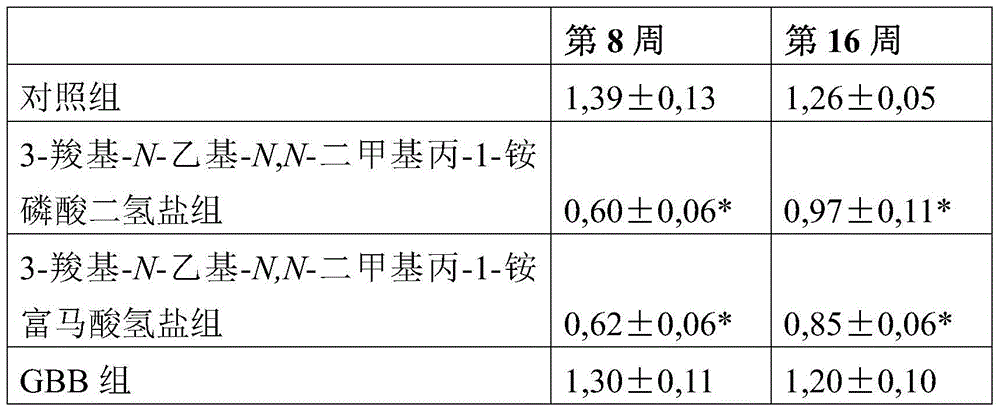
Use of 3-carboxy-n-ethyl-n,n-dimethylpropan-1-ammonium or a pharmaceutically acceptable salt thereof in the prevention and treatment of diabetes
ActiveCN105530929BLower levelOrganic active ingredientsOrganic compound preparationBlood plasmaPlasma insulin
Owner:GRINDEKS
Novel mangiferin calcium salts, the method for preparation and use thereof
The present invention relates to novel mangiferin calcium and its preparation and use. The mangiferin calcium can lower plasma insulin, glucose, lipid, also can improve the solubility and oral bioavailability of mangiferin.
Owner:CHANGZHOU DEZE MEDICAL SCI CO LTD
Use of 3-carboxy-n-ethyl-n,n-dimethylpropan-1-aminium or a pharmaceutically acceptable salt thereof in the prevention and treatment of diabetes
ActiveCN105530929ALower levelOrganic active ingredientsOrganic compound preparationD-GlucoseGlucose polymers
Use of 3-carboxy-N-ethyl-N,N-dimethylpropan-1-aminium and its pharmaceutically acceptable salts to decrease blood plasma levels of insulin and glucose.
Owner:GRINDEKS
Inhibitor of peroxisome proliferator-activated receptor alpha coactivator 1
InactiveCN101166751AControl effectivenessOrganic active ingredientsSugar derivativesDiseaseTolerability
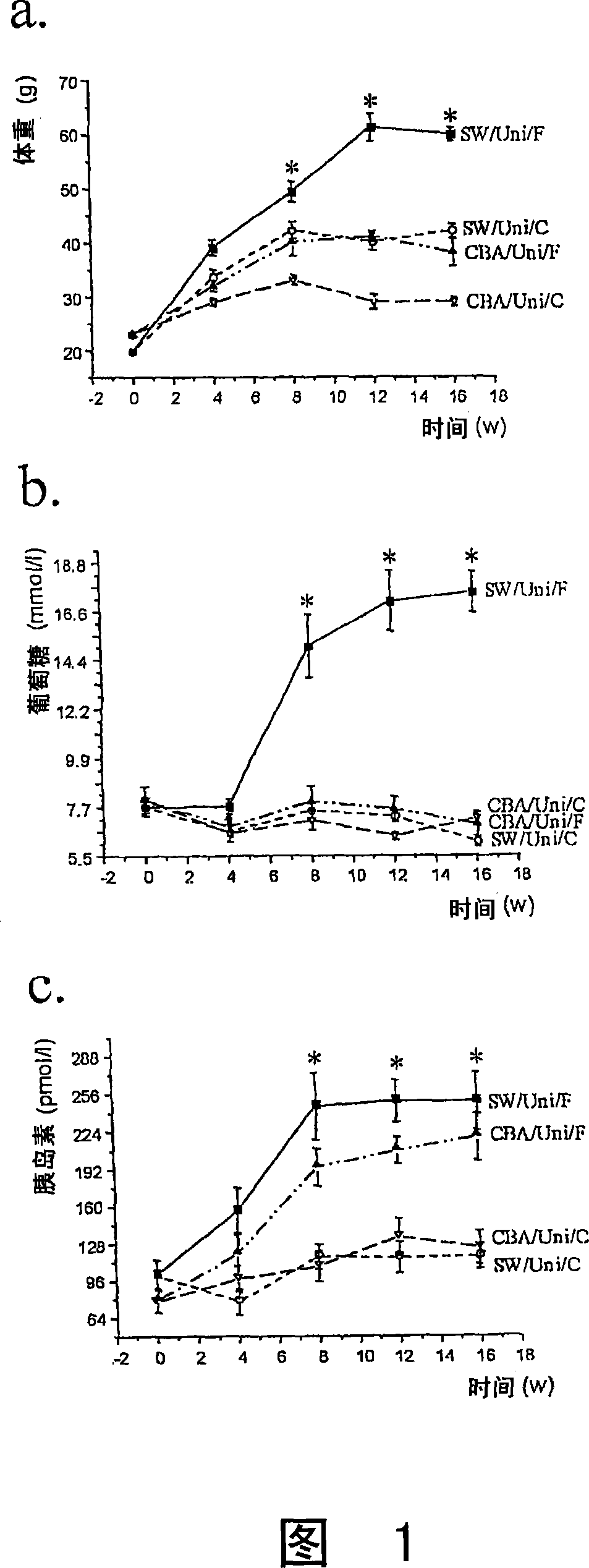
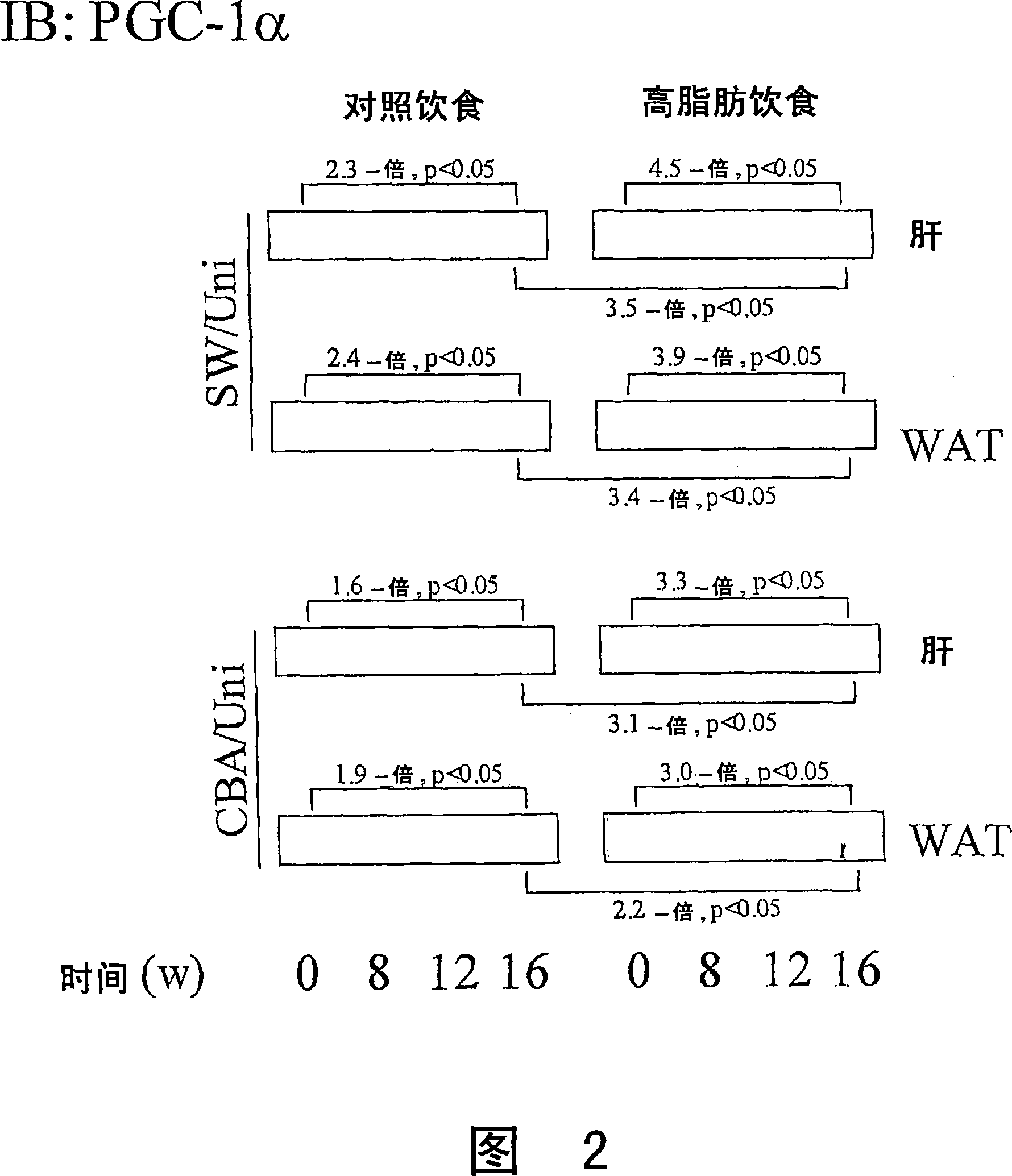
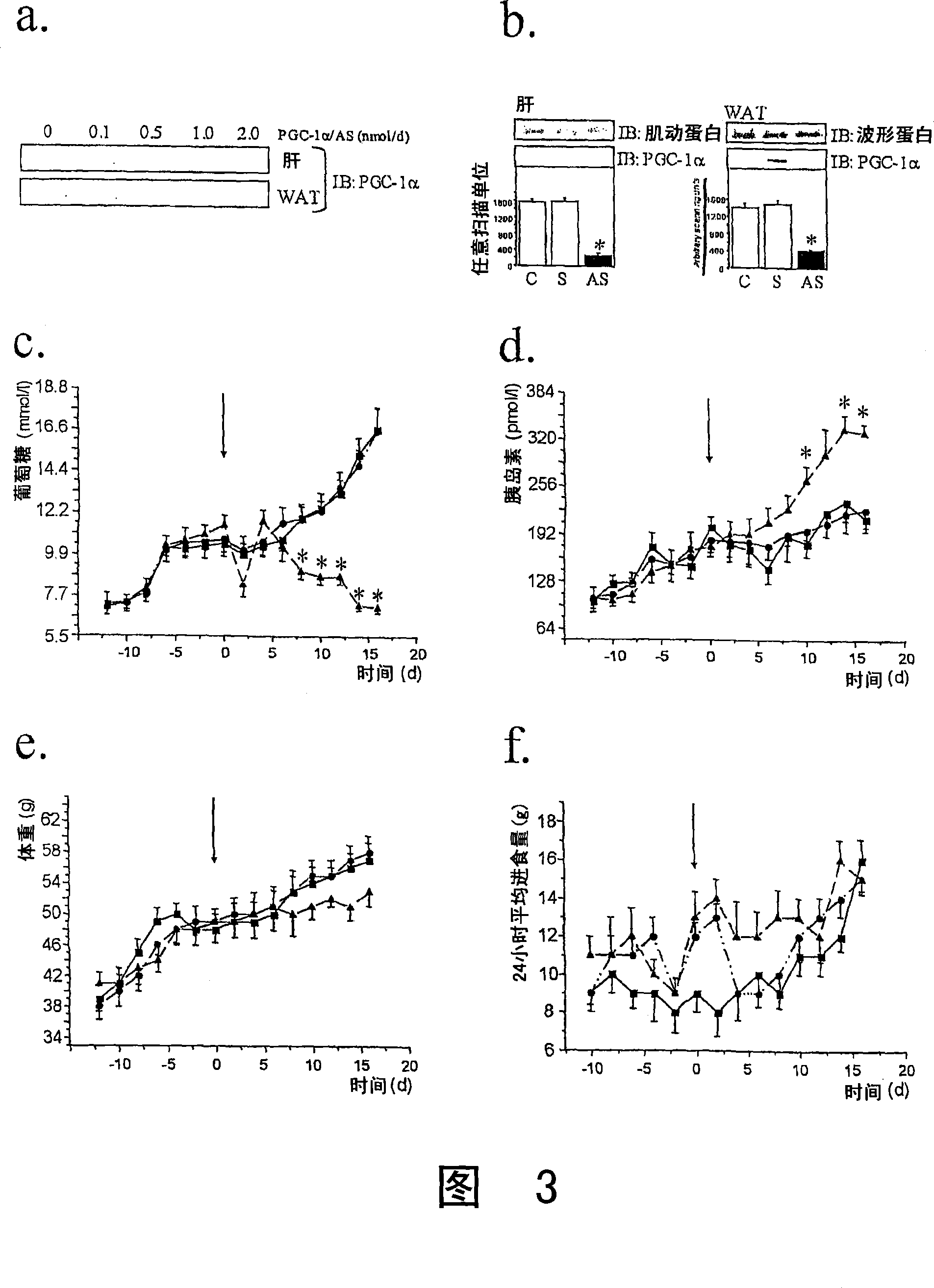
The present invention refers to the use of an antisense DNA oligonucleotide for the messenger RNA of the PGC-la protein, useful as drug for the treatment of diabetes mellitus, insulin resistance and metabolic syndrome. More specifically, the present invention deals with a compound used as drug, through enteral or parenteral route, preferably, with the property of inhibiting the protein expression peroxisome proliferator-activated receptor alpha Coactivator 1 (PGC-la) leading to the reduction of the blood glucose levels. It deals, therefore, with a pharmacological compound that promotes, in diabetic and insulin-resistant individuals, improvement of the glucose serum levels, increase of the plasmatic insulin concentration and reduction of insulin resistance. The present invention presents a more effective control of the glucose levels and acts beneficially on other complications associated to the Diabetes and obesity conditions, according to tests performed in animal models. In this manner, the principal advantage of the present invention over others alike already existing in the market is the effectiveness that controls blood glucose levels and the fact of acting beneficially on other complications that accompany the disease.
Owner:坎皮纳斯州立大学 UNICAMP
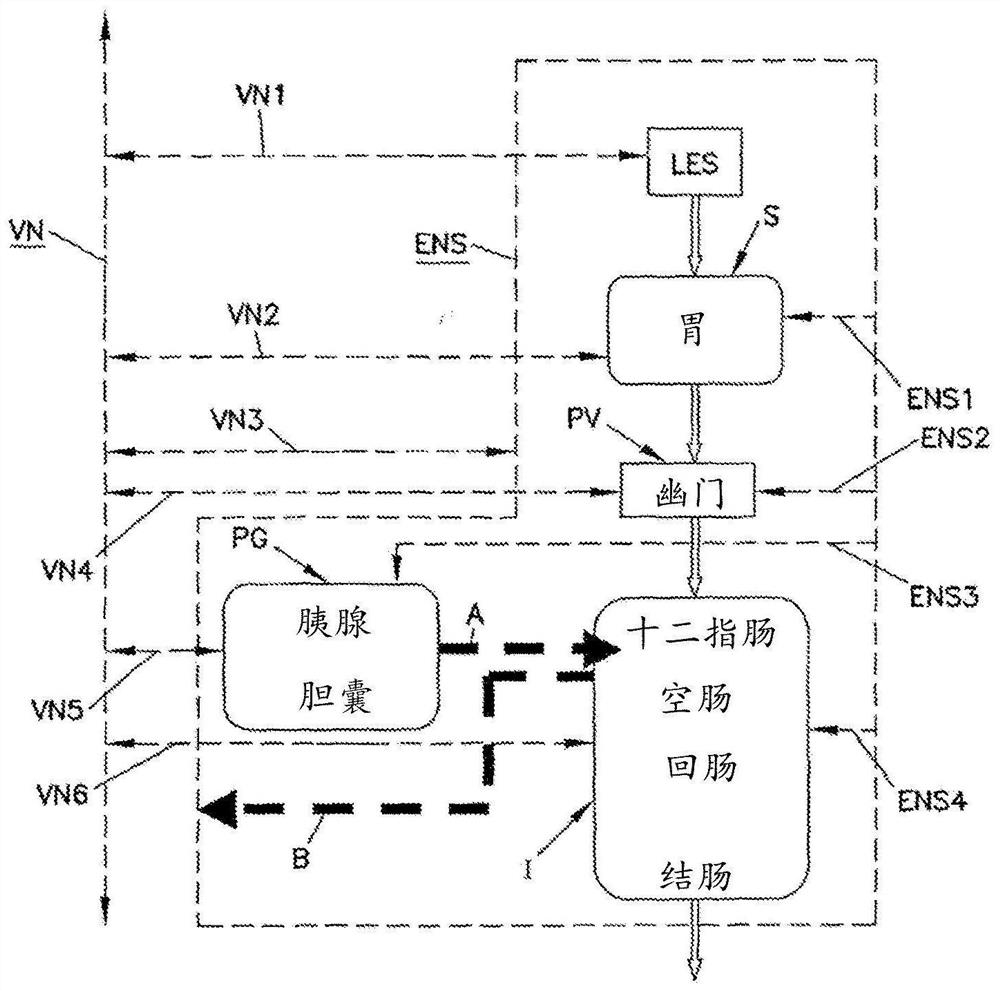
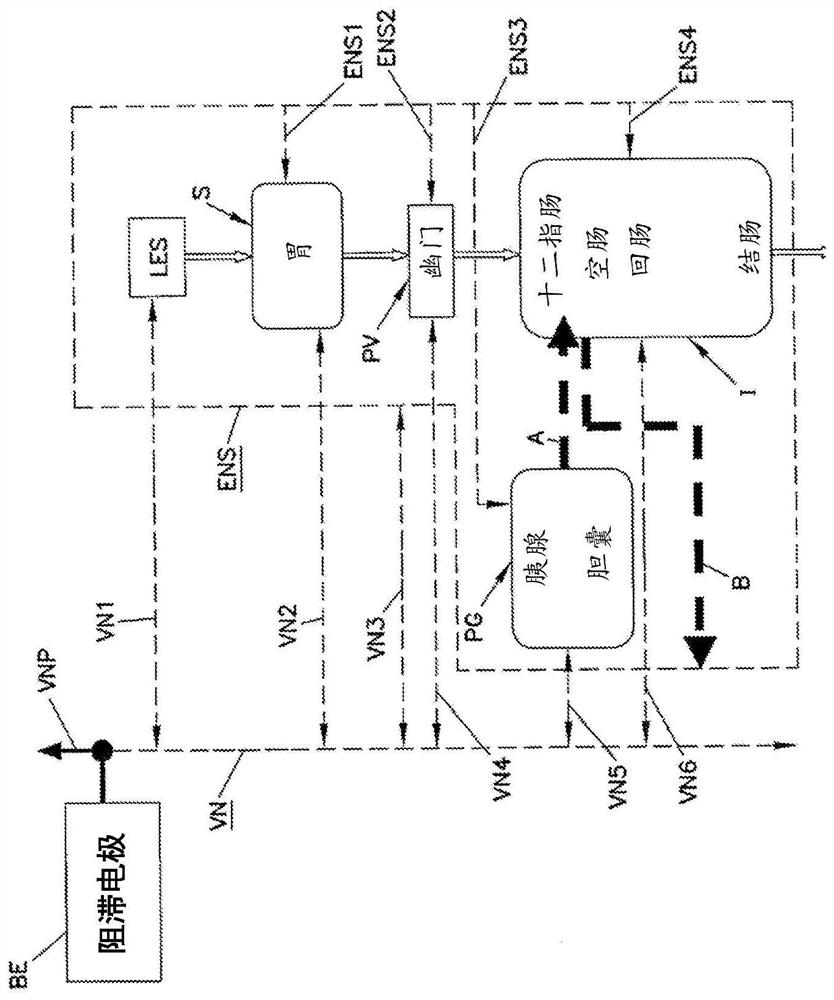
Simultaneous multi-site vagus nerve neuromodulation for improved glycemic control system and methods
Various methods and apparatus for treating a condition associated with impaired glucose regulation in a subject comprising in one embodiment, applying a neural conduction block to a target nerve at ablocking site with the neural conduction block selected to at least partially block nerve pulses. In another embodiment, combinations of down-regulating and or up-regulating with are used to treat impaired glucose regulation. In other embodiments, up-regulation or downregulation of various nerves, such as the vagus and its branches, are used to modify the secretion of insulin and glucagon from thepancreas, thereby controlling glucose levels. In yet further embodiments, combinations of down-regulating and or up-regulating are used to control sensitivity of the liver to plasma insulin and glucagon to treat impaired glucose regulation.
Owner:RESHAPE LIFESCIENCES INC
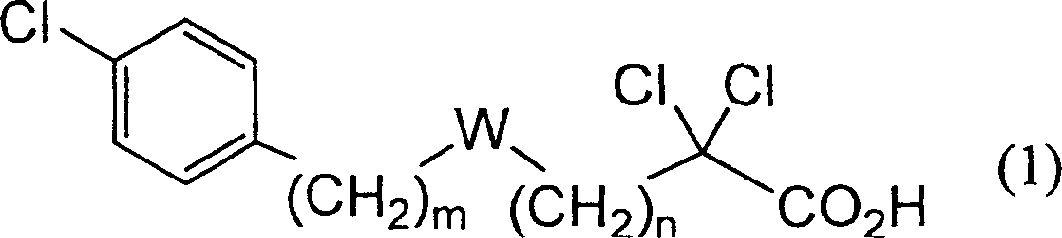
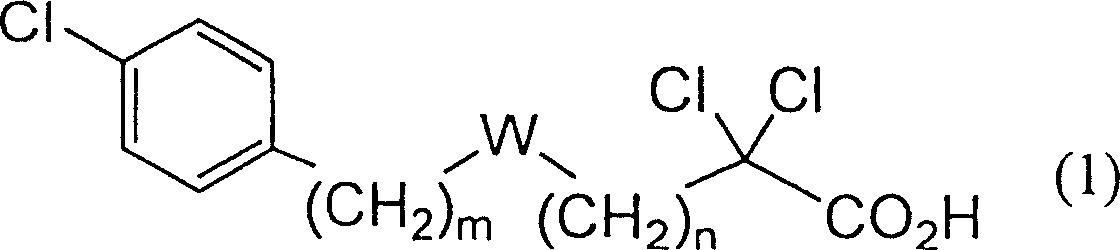
Carboxylic compound and medicine comprising the same
One uses the following general formula (1): [In the formula, m represents an integer of 0 to 4, n represents an integer of 5 to 9, W represents -CH(OR)- (R represents a hydrogen atom or a hydroxyl protecting group) or -C(=O)-], a salt or an ester thereof, which has the effects of lowering blood sugar, lowering plasma insulin and lowering glyceride, and is very useful for the prevention and / or treatment of diabetes, diabetic complications or hyperlipidemia, etc. .
Owner:KOWA CO LTD
Carboxylic compound and medicine comprising the same
Provided are a compound represented by the following general formula (1): [wherein m is an integer of 0 to 4; n is an integer of 5 to 9; and W represents -CH(OR)- (R represents hydrogen or a hydroxy-protecting group) or -C(=O)-], a salt of the compound, or an ester of the compound. The compound, salt, or ester is effective in blood sugar depression, plasma insulin reduction, and triglyceride reduction and is useful for the prevention of and / or treatments for diabetes, complications of diabetes, hyperlipemia, etc.
Owner:KOWA CO LTD
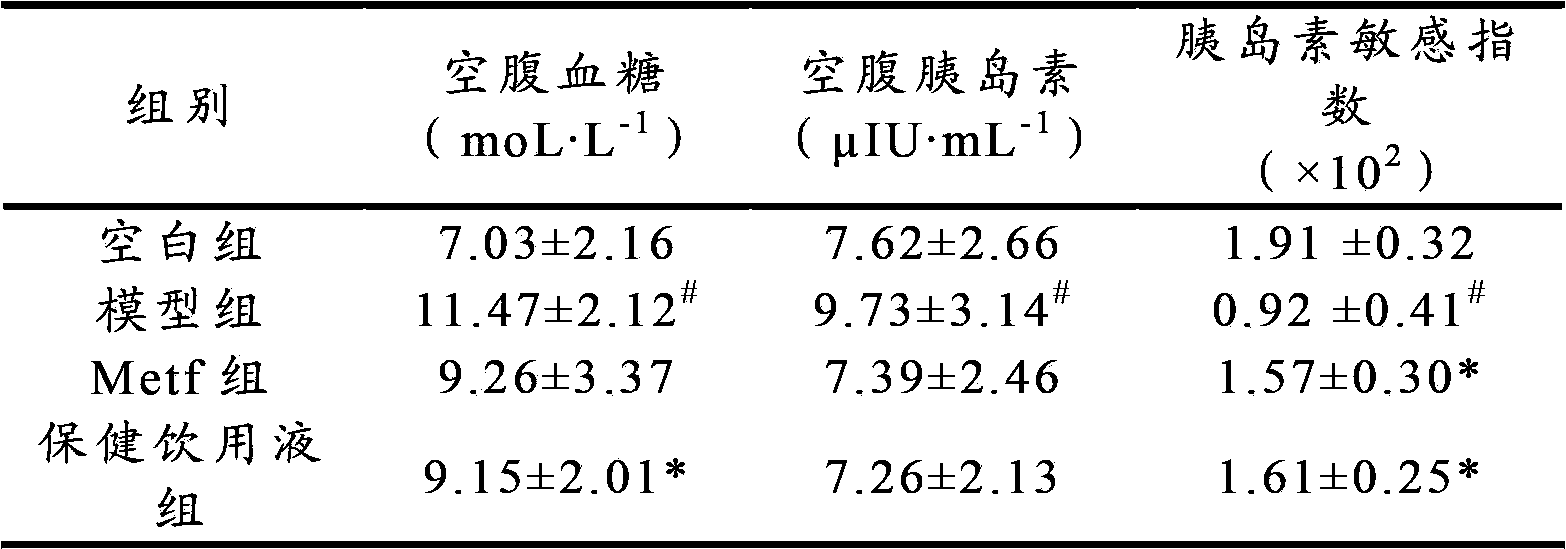
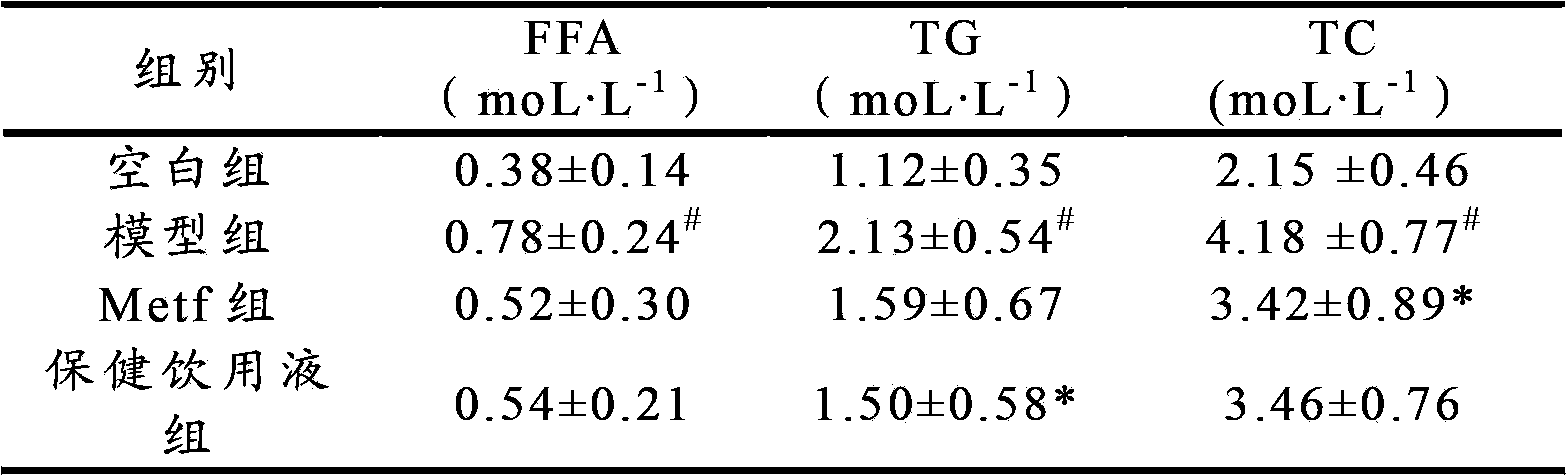
Application of health-care drinking liquid in preparation of medicines or health-care products for preventing or treating II-type diabetes mellitus, diabetes mellitus and diabetic nephropathy
ActiveCN102697807BIncrease contentImprove disease symptomsMetabolism disorderGeneral water supply conservationReverse osmosisSpontaneous diabetes
The invention discloses an application of a health-care drinking liquid in preparation of medicines or health-care products for preventing or treating II-type diabetes mellitus, diabetes mellitus and diabetic nephropathy. The health-care drinking liquid comprises a liquid A and a liquid B; the liquid B is a high-concentration concentrated liquid which is obtained by decompression concentration of concentrated water part separated from deep ocean water through a reverse osmosis apparatus; and the hardness range of the health-care drinking liquid is 200-1000ppm. The health-care drinking liquid is fed to experimental animals with II-type diabetes mellitus in a free drinking manner and can be used for remarkably reducing levels of fasting blood-glucose, fasting plasma insulin, blood lipid and weights in diabetic mice induced by combination of high-glucose and high-fat diets and STZ (Streptozotocin) and improving insulin sensitivities and remarkably improving db / db disease syndromes of spontaneous diabetes model animals and effectively inhibiting diabetic nephropathy lesions, so that the health-care drinking liquid can be applied to preventing, improving or treating II-type diabetes mellitus and diabetic nephropathy diseases. The health-care drinking liquid has the advantages of rich resources and easy industrialization and the like.
Owner:OCEAN UNIV OF CHINA
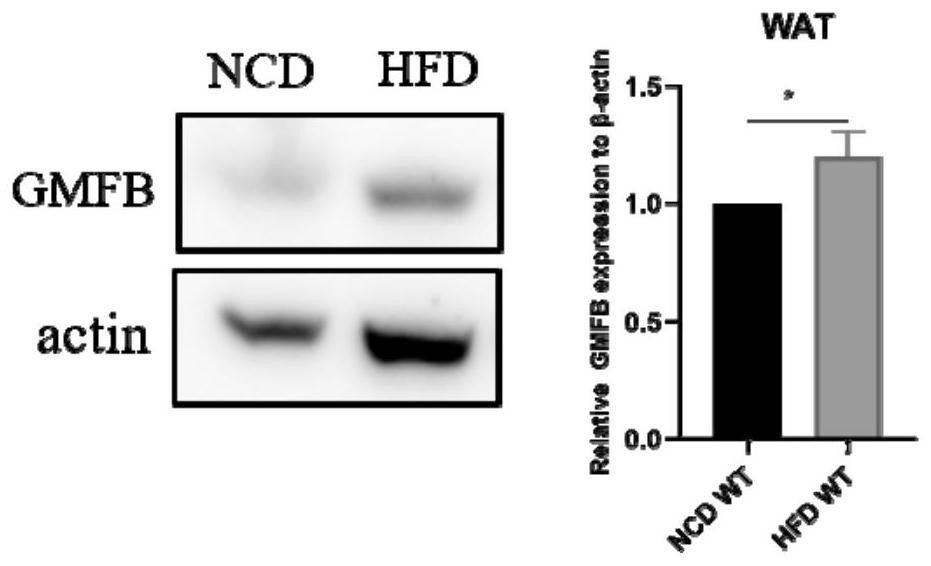
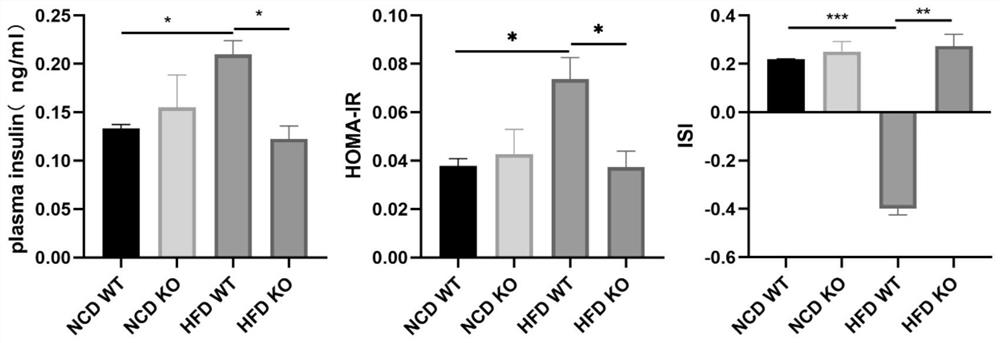
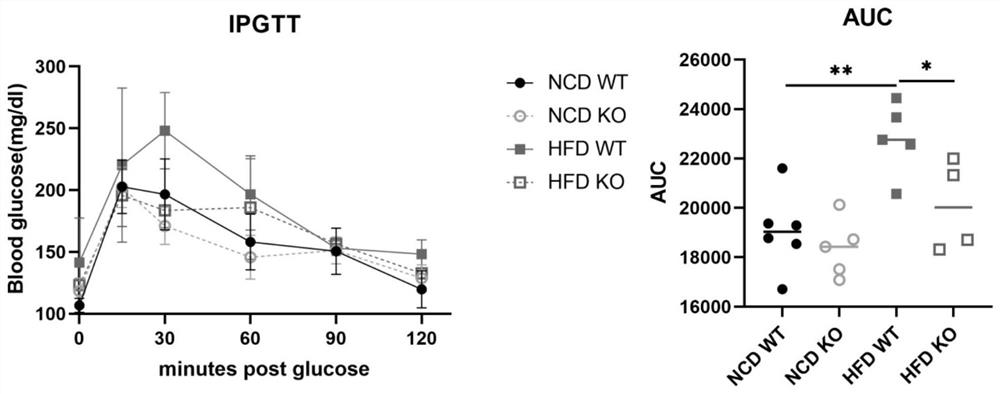
Application of GMFB as insulin resistance biomarker
PendingCN113759127AInhibitory functionImprove the immunityPeptide/protein ingredientsMetabolism disorderInflammatory factorsPhysiology
The invention relates to the technical field of treatment of insulin resistance, and in particular relates to application of a cytokine GMFB, and application of the GMFB as a biomarker of insulin resistance. Experiments discover that GMFB as an inflammatory factor is high in expression in an insulin resistance state caused by high fat diet and is not expressed or extremely low in expression in a normal condition, which indicates that GMFB plays a role in adipose tissues in the insulin resistance state and can inhibit the functions of the adipose tissues. The plasma insulin concentration of a high-fat diet rat after GMFB gene knockout is obviously reduced compared with that of a wild type high-fat diet rat, which indicates that the GMFB gene knockout can improve insulin resistance caused by high-fat diet; GMFB gene knockout obviously improves the glucose tolerance of SD rats; the insulin tolerance is improved; the functions of fat cells are improved.
Owner:TONGJI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com