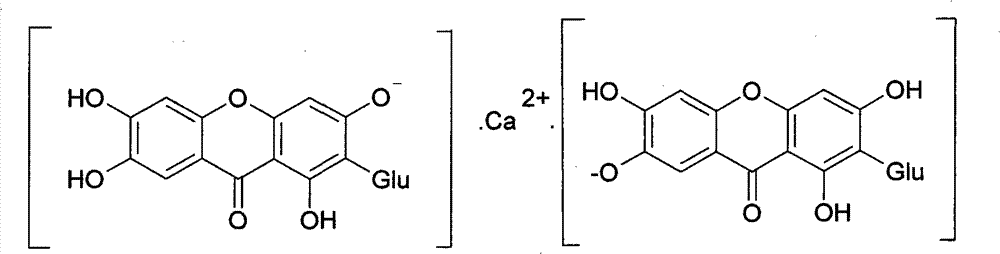
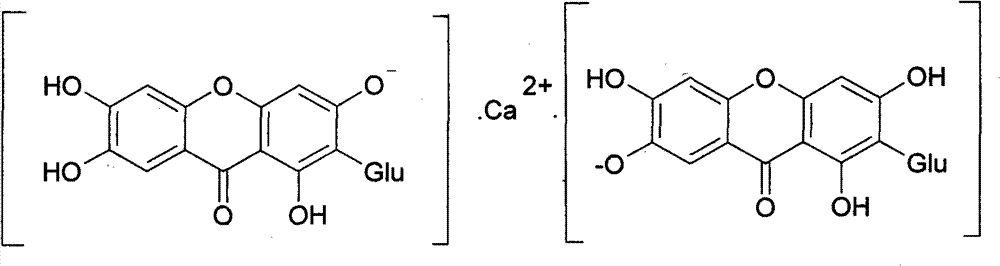
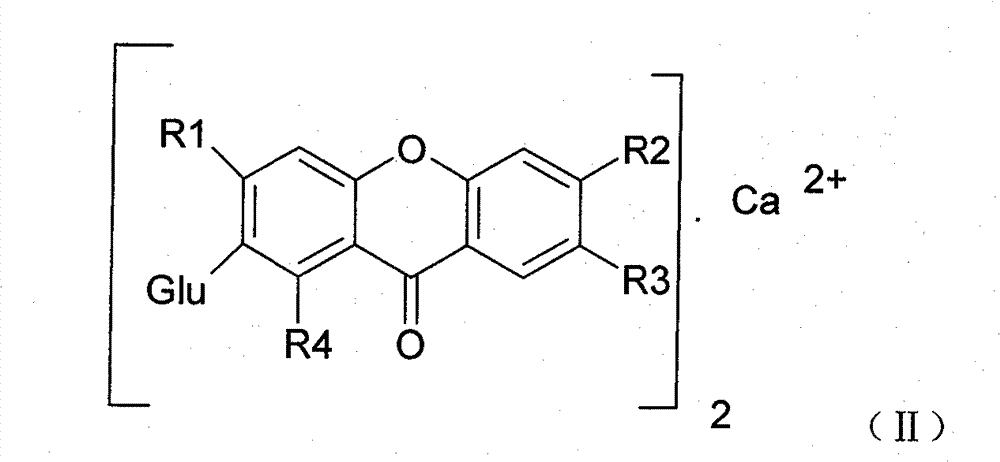
Novel mangiferin calcium salts, the method for preparation and use thereof
A technology of mangiferin calcium salt and mangiferin, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, metabolic diseases, etc., can solve the problems of mangiferin solubility, bioavailability, absorption defects, etc., and achieve stable properties and high yields. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0078] Preparation Example 1: Preparation of Mangiferin
[0079] 100 kg of Zhimu decoction pieces were extracted twice with 80% ethanol at 80°C, concentrated, adsorbed on a macroporous resin, washed with water, and eluted with 40% ethanol. The eluate was concentrated under reduced pressure to obtain crude mangiferin. The crude mangiferin was purified by solvent-dioxane-water recrystallization to obtain pure mangiferin. The mangiferin sample was identified with the mangiferin reference substance, and the obtained sample was determined to be mangiferin. The purity was determined by HPLC to be 98.5%. .
[0080] Compound identification:
[0081] 1 HNMR(DMSO-d 6 ) (δppm), 4.60 (1H, d, J = 9.8 Hz) is a glucose terminal proton, and it exists in the form of B-glycosidic bond; and 6.37 (1H, s), 6.86 (1H, s) and 7.39 (1H, s) 3 aromatic proton signals.
[0082] 13 CNMR(DMSO-d 6 )(δppm): 162.7(C-1), 108.4(C-2), 164.7(C-3), 94.2(C-4), 157.1(C-4a), 102.2(C-4b), 103.5(C -5), 154.9(C-6), 144.6(C-7...
Embodiment 1
[0087] Example 1: Preparation of mangiferin monosodium salt
[0088] Add 42.2g (0.1mol) of mangiferin, 1800ml of water, and 600ml of ethanol into the reactor to fully suspend, add 8.4g (0.1mol) of sodium bicarbonate to water to make a 0.5% (w / v) solution, and slowly add it to the stirring state In the suspension, react until it is clear, filter, add an appropriate amount of absolute ethanol-ethyl acetate (1:1.5v / v) to the solution, stir well, and a large amount of precipitate will precipitate, filter with suction, and dry the solid below 60°C , 31.2 g of light yellow solid mangiferin monosodium salt was obtained, and the yield was 74.0%. The purity of the sample was determined to be 98.6% by HPLC.
Embodiment 2
[0089] Example 2: Preparation of mangiferin monosodium salt
[0090] Add 42.2g (0.1mol) of mangiferin, 1800ml of water, and 900ml of ethanol into the reactor to fully suspend, add 5.30g (0.05mol) of sodium carbonate to water to make a 0.5% (w / v) solution, and slowly add to the stirred mixture In the suspension, react until it is clear, filter, add appropriate amount of acetone to the solution, stir well, a large amount of precipitation will precipitate, filter with suction, and dry the solid below 60°C to obtain 31.4g of light yellow solid mangiferin monosodium salt, yield It is 74.5%. The purity of the sample was determined to be 98.5% by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com