Nasal formulations of insulin
A technology for insulin and pharmaceutical preparations, applied in the field of insulin nasal administration, can solve the problems of complex, low bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
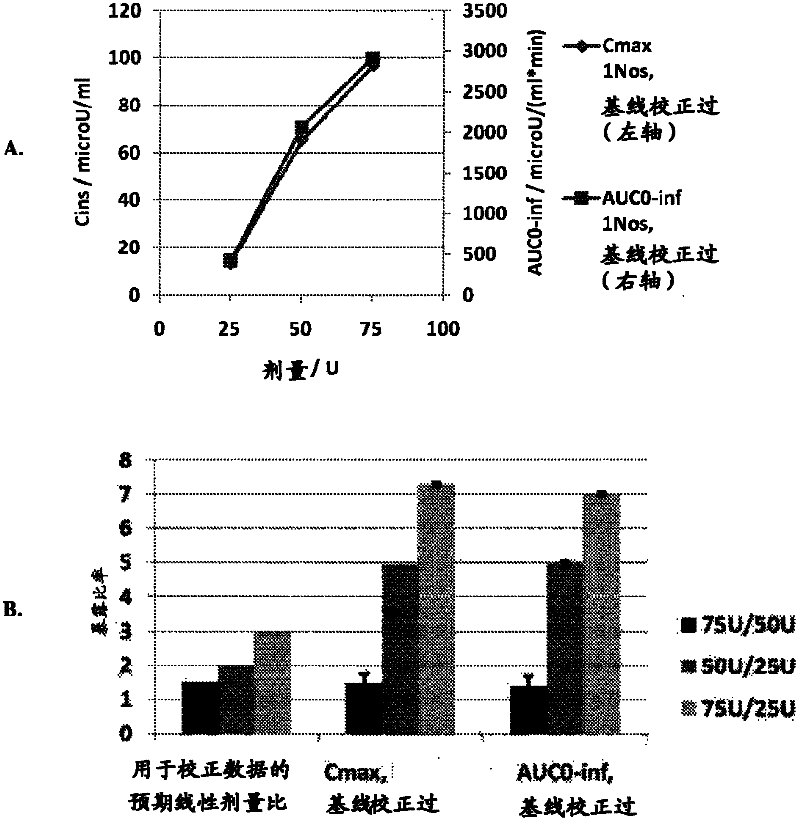
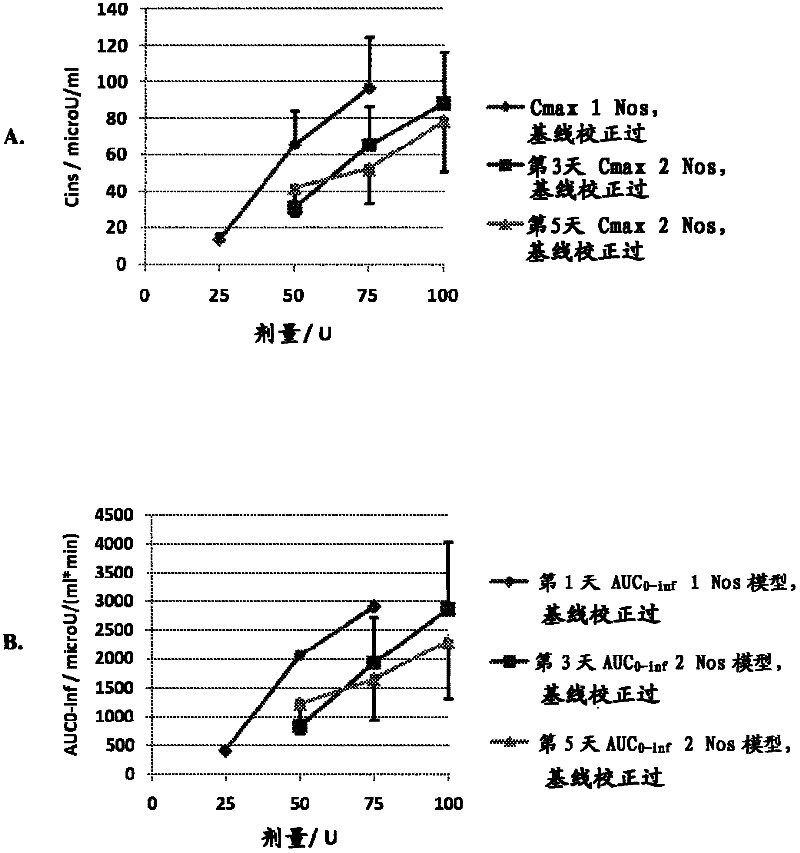
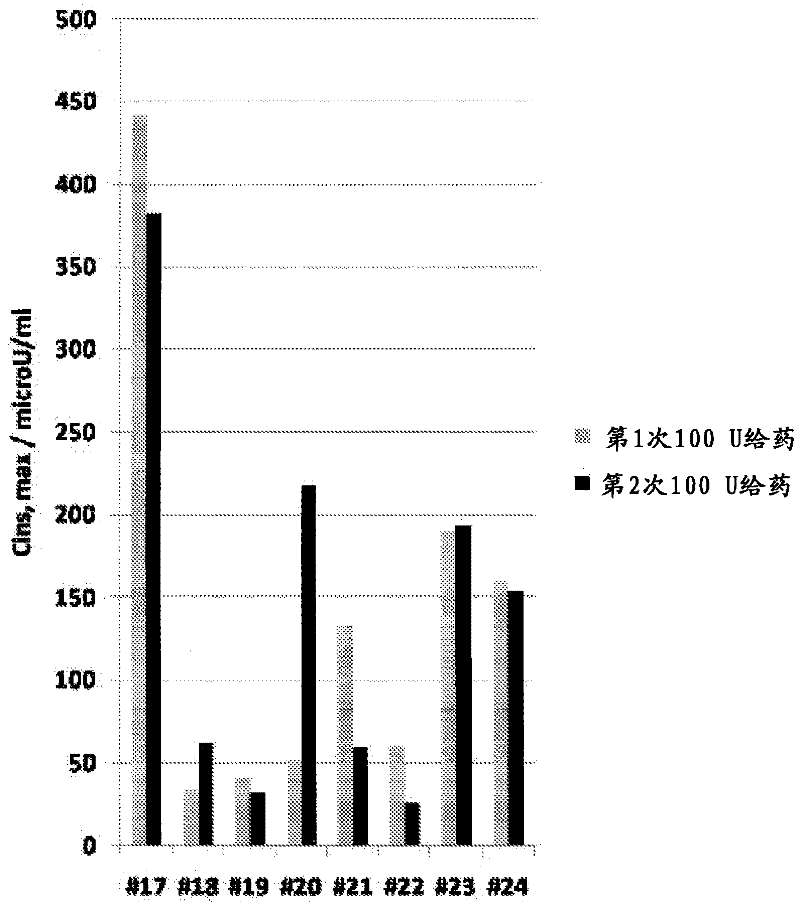
[0050] The goals of this study were to determine the optimal method for intranasal administration of insulin and to characterize the dose-response pharmacokinetics and pharmacodynamics. This study was conducted in accordance with the protocol approved by the Institutional Review Board (IRB).
[0051] Preparations and Equipment
[0052] The formulation tested was an intranasal insulin spray comprising conventional short-acting human recombinant insulin dissolved in water with several common excipients including polysorbate 20, sorbitan monolaurate , cottonseed oil and cyclopentadecanolide (CPE-215). The excipient cyclopentadecanolide is a compound that occurs naturally in plants such as Angelia archangelica root and is a common component in many food, cosmetic and personal hygiene products. It is important that the insulin preparation is left at room temperature for 2 to 10 hours before use. Slowly invert it two or three times. Prime the pump when using the spray for the ...
Embodiment 2
[0072]The glucose clamp technique is a well-established measurement method for measuring the direct effect of insulin on glucose uptake. This method of measurement is achieved by clamping or maintaining a predetermined blood glucose level (eg -100 mg / dl) and by using a regulated rate of glucose infusion to offset the effect of the insulin being tested. Thus, the glucose infusion rate (GIR) becomes a direct measure of the amount of glucose "disappearing" from the plasma per unit time.
[0073] Glucose clamp studies are performed according to the following steps:
[0074] a. Subjects fasted from 11:00 PM the night before (except water).
[0075] b. The subjects kept the sitting position and rested for 5 minutes before collecting vital signs.
[0076] c. Both arms are placed on a heating pad to dilate the vein. An IV catheter was placed in the antecubital vein in one arm for infusion of dextrose 20% and insulin through two separate regulator valves. Another IV catheter was in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com