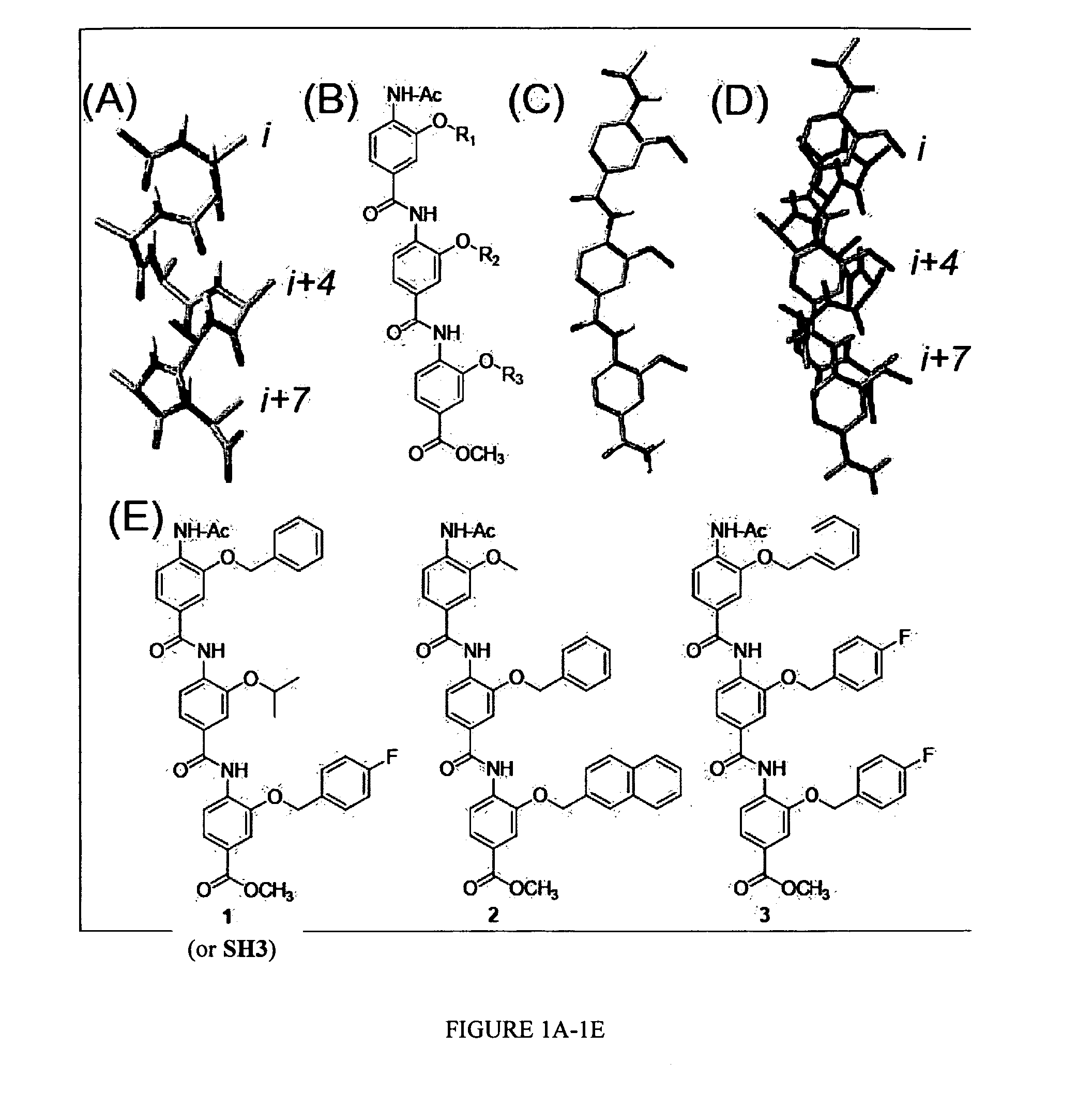
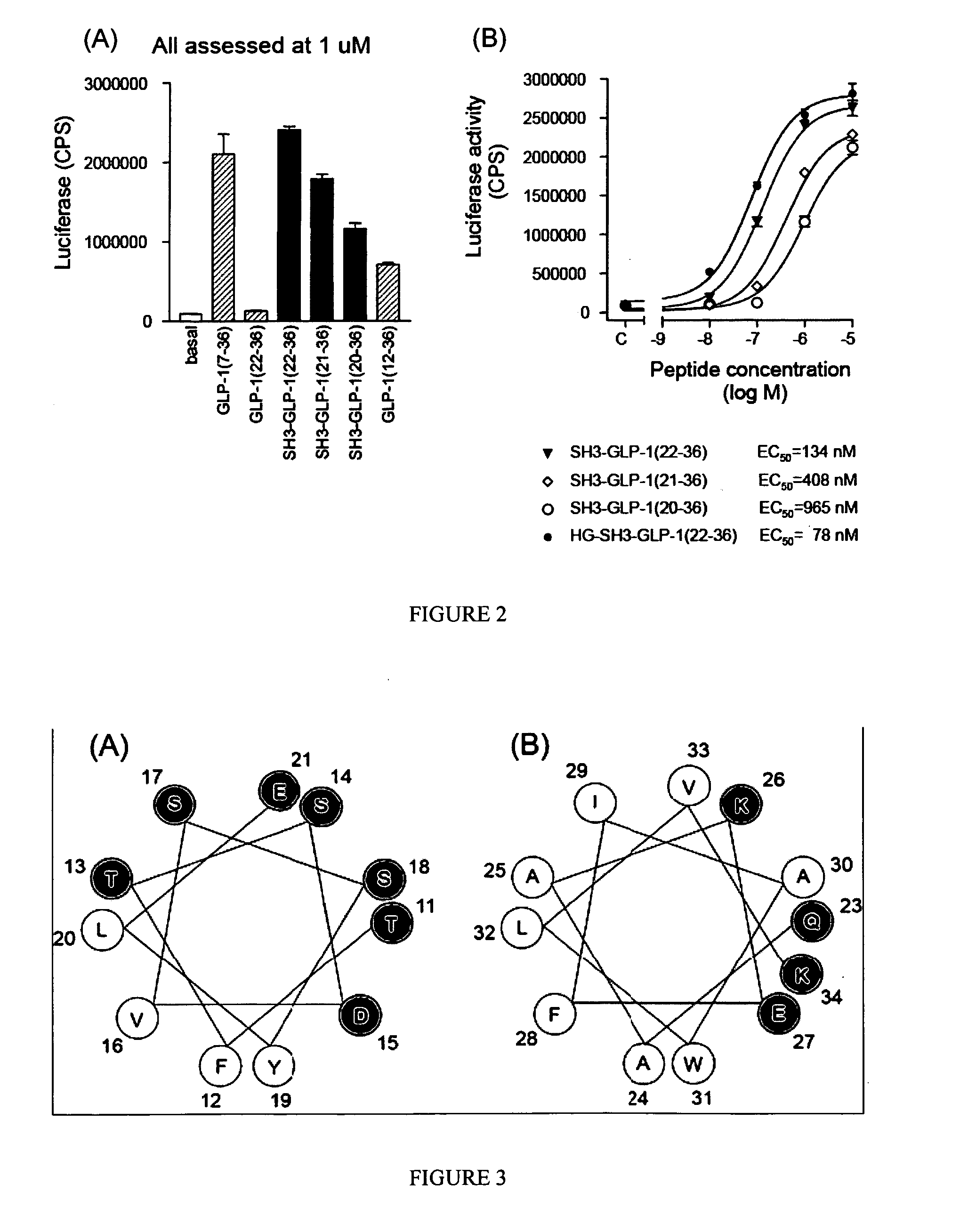
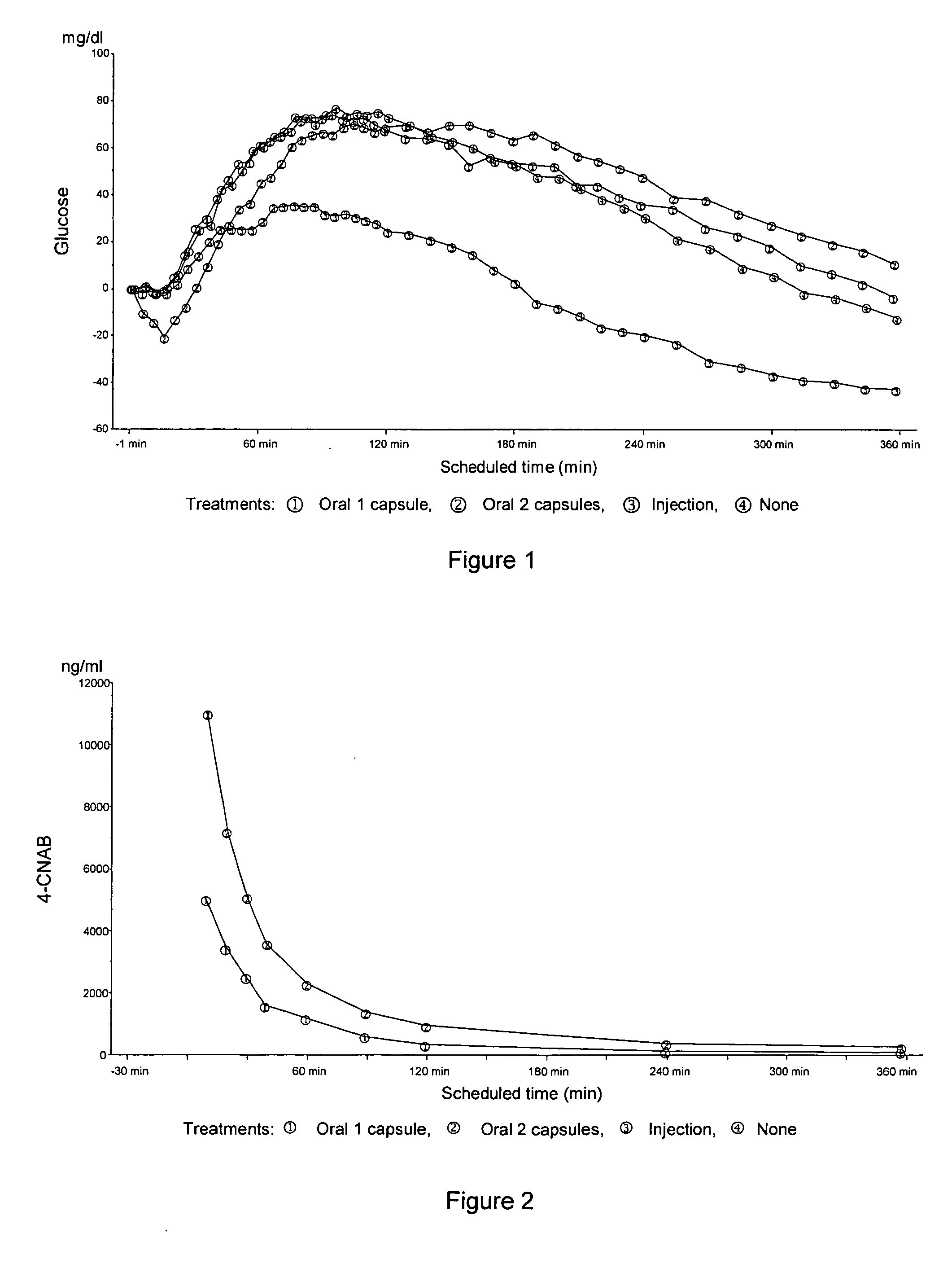
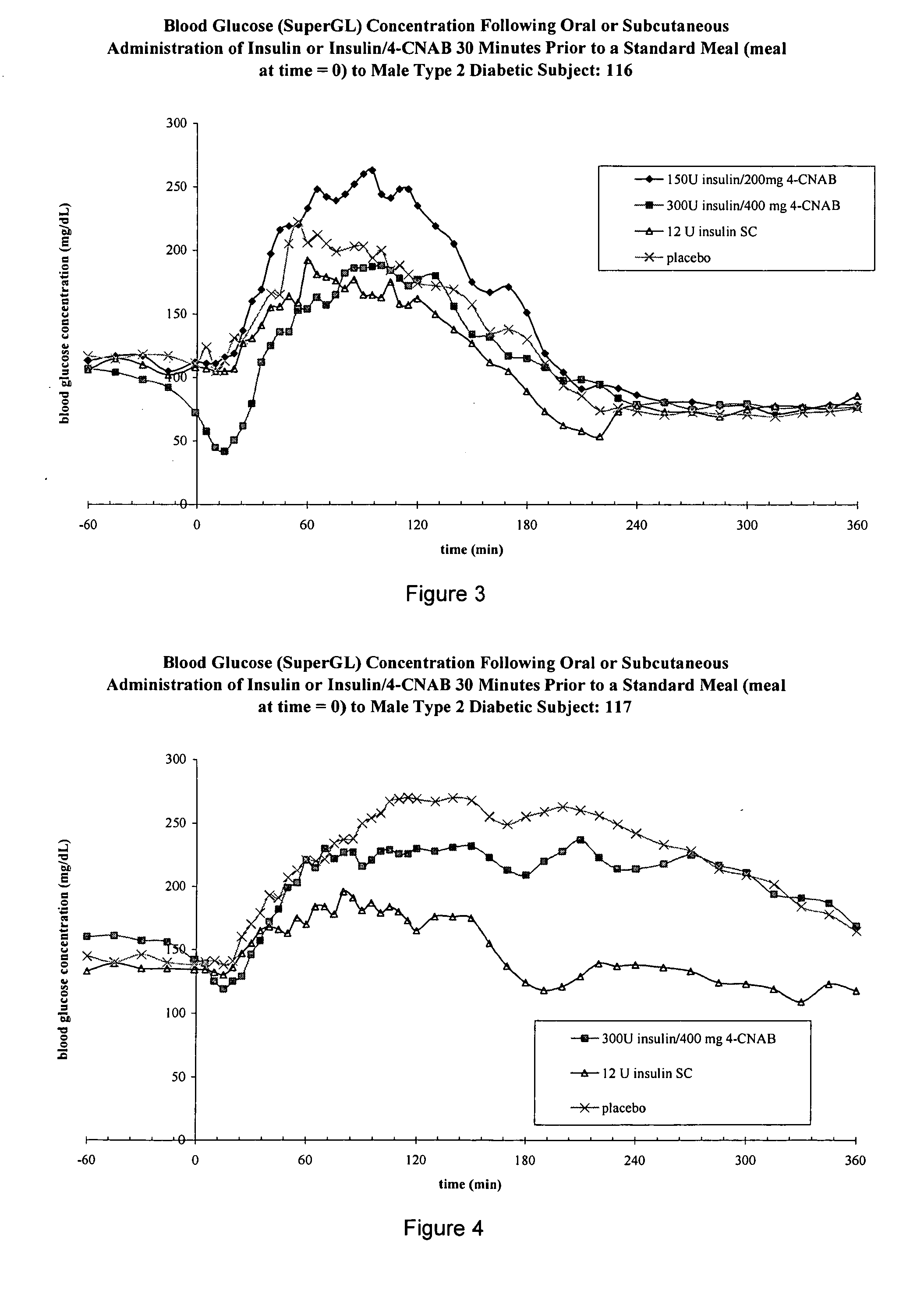
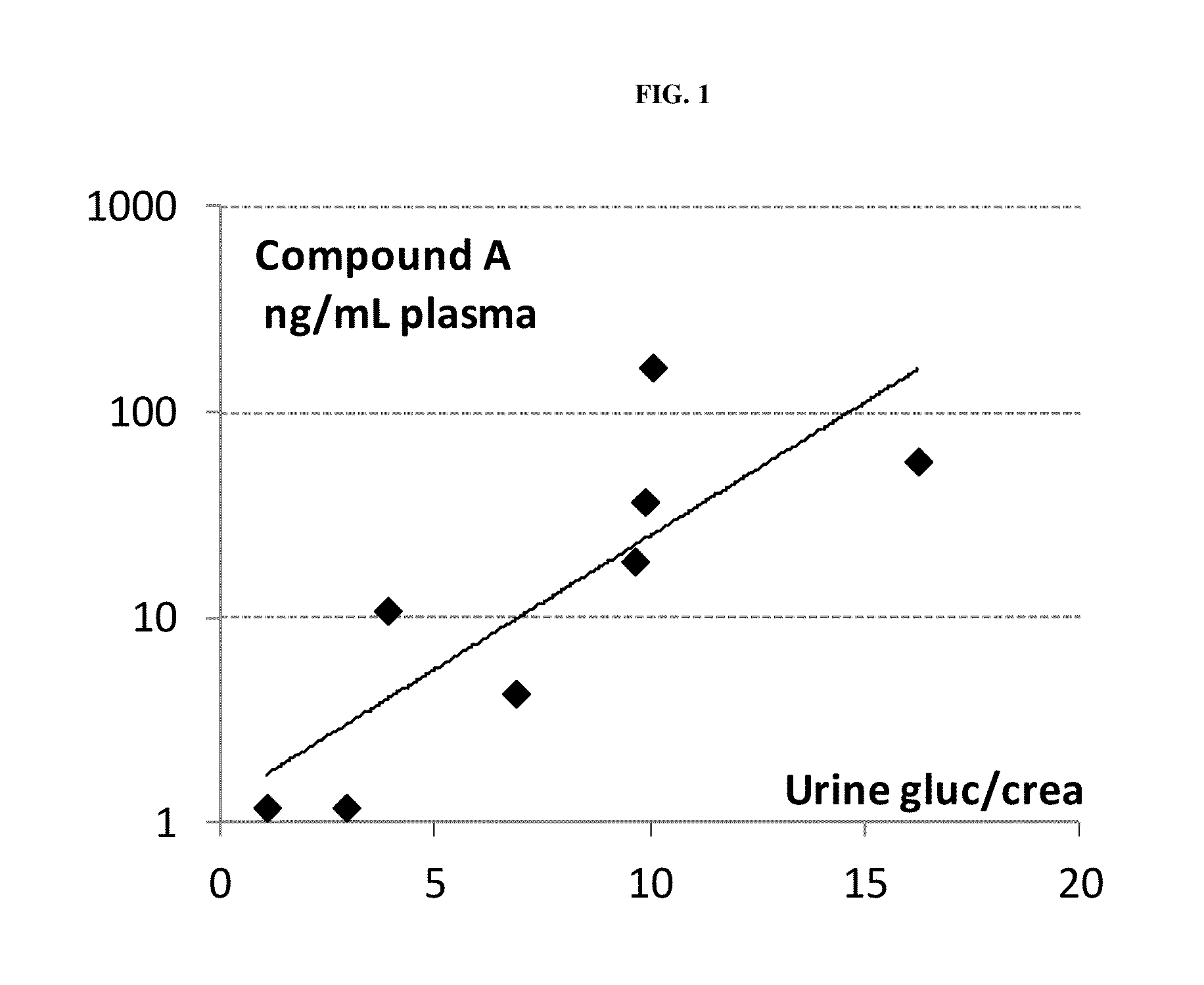
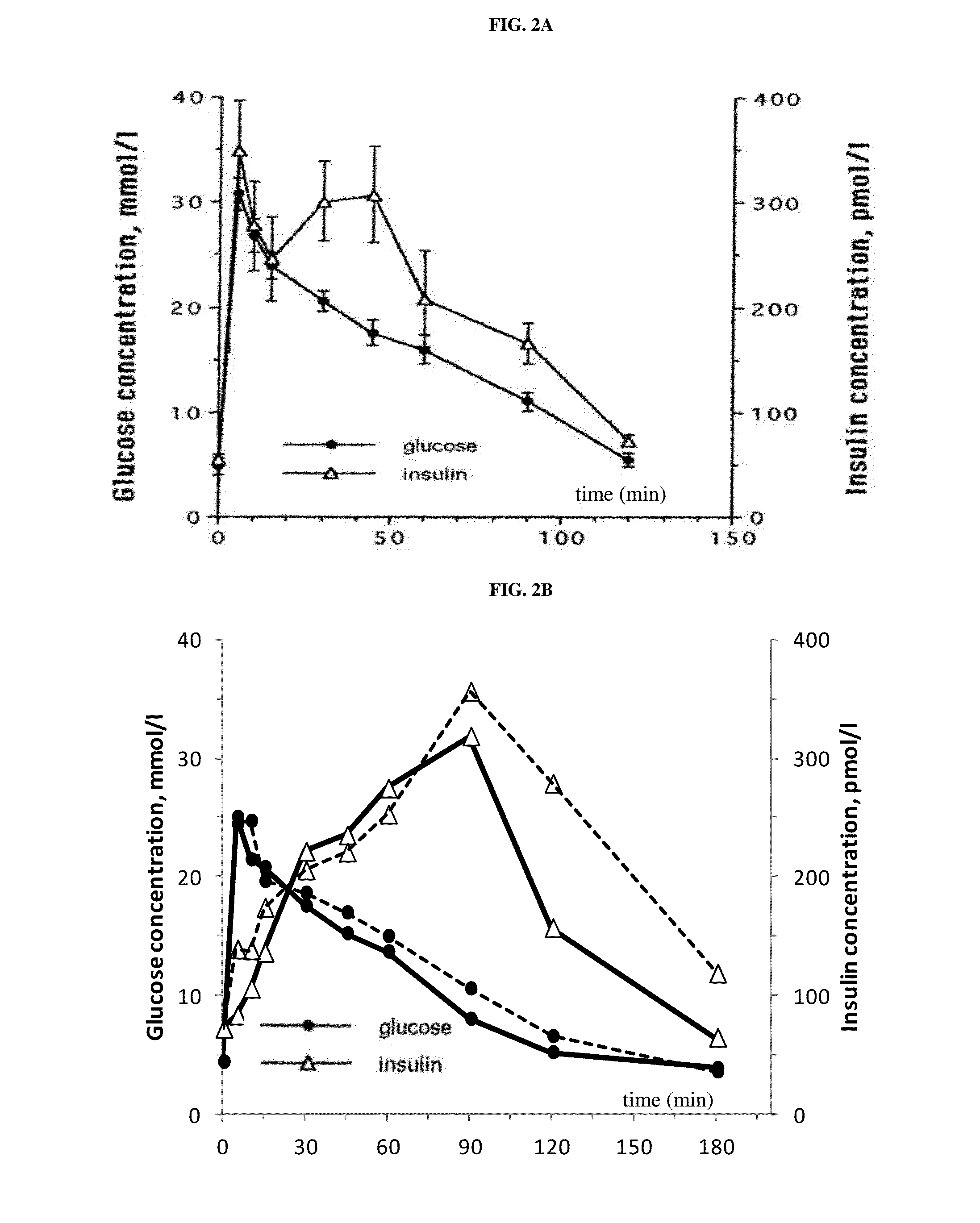
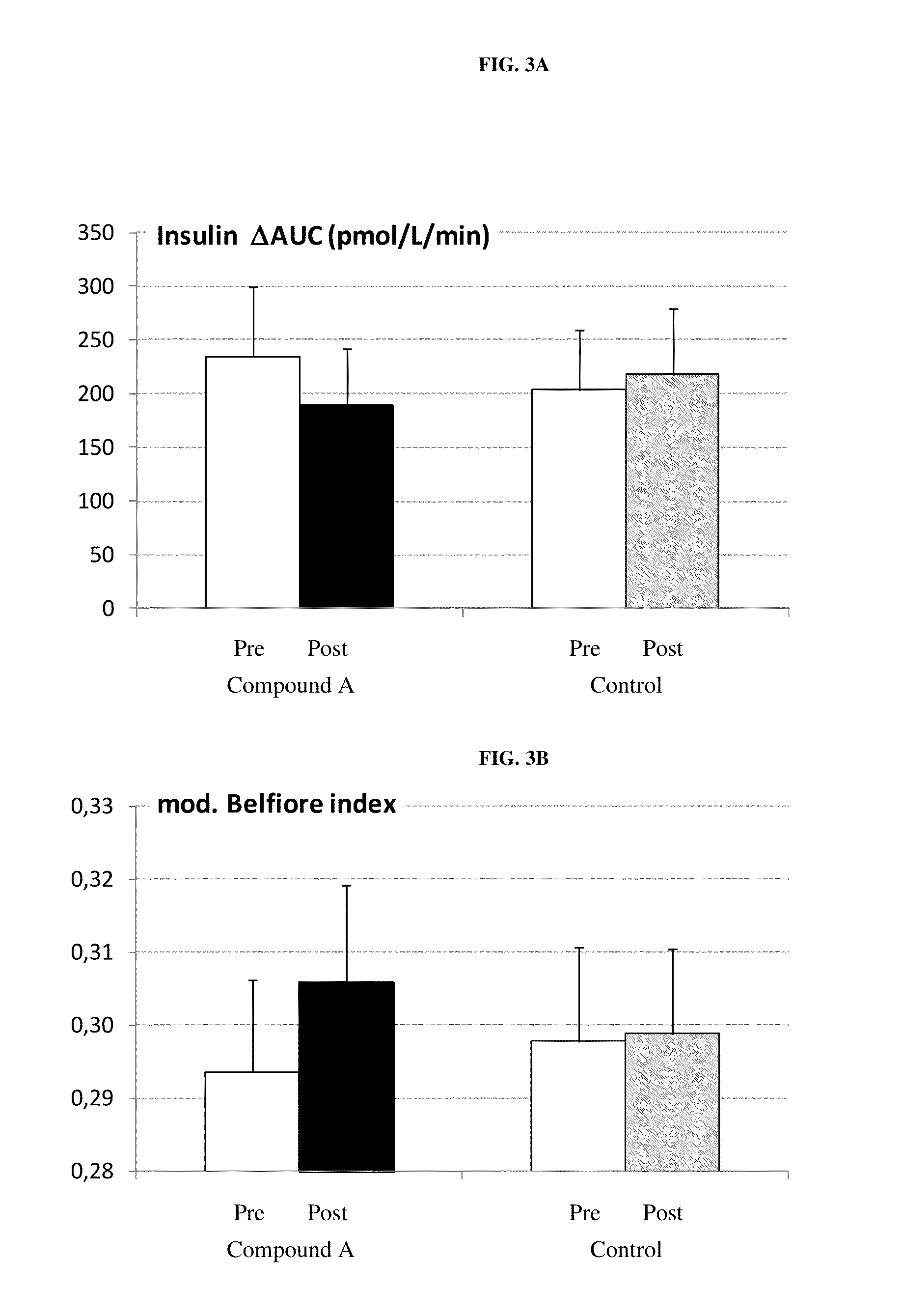
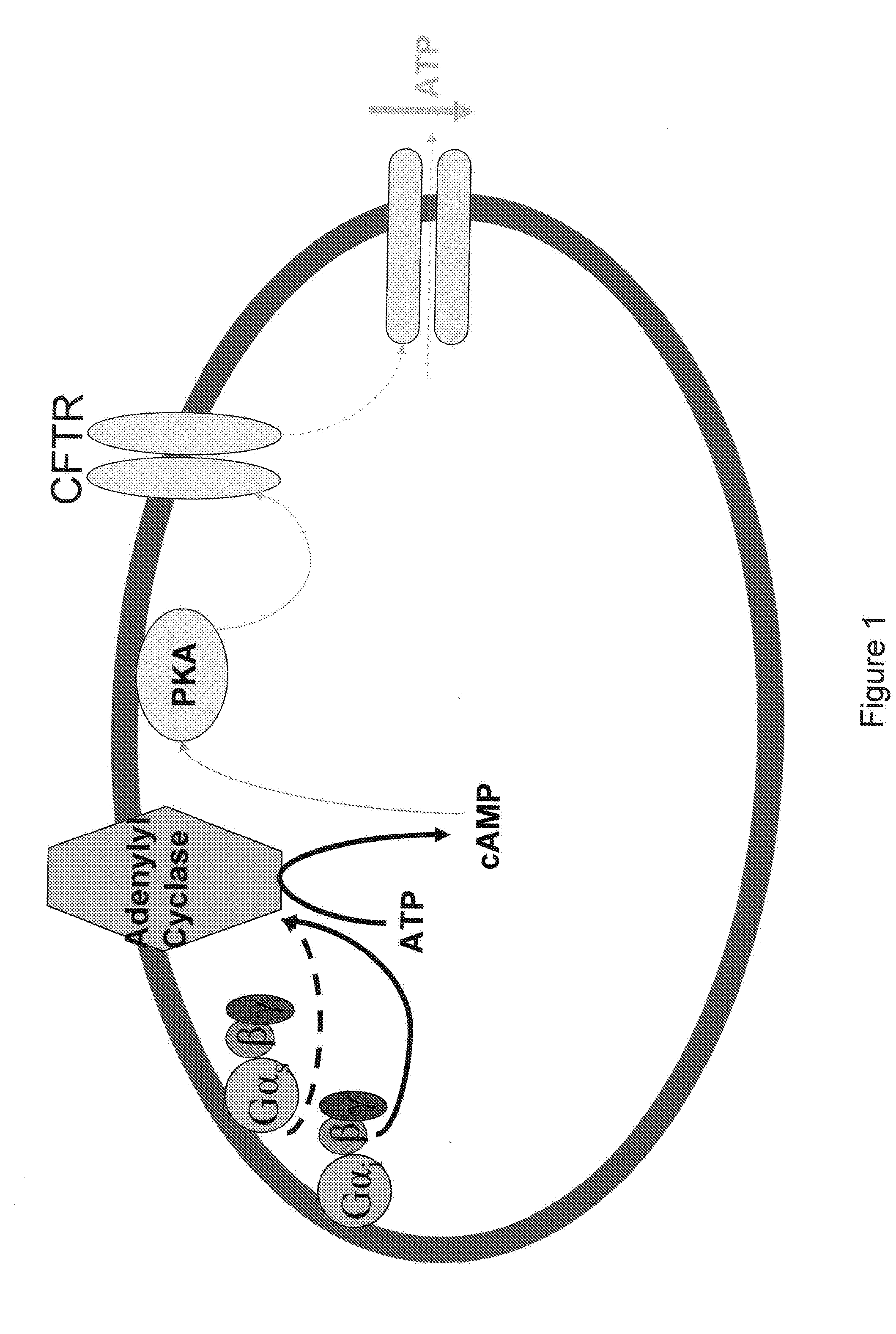
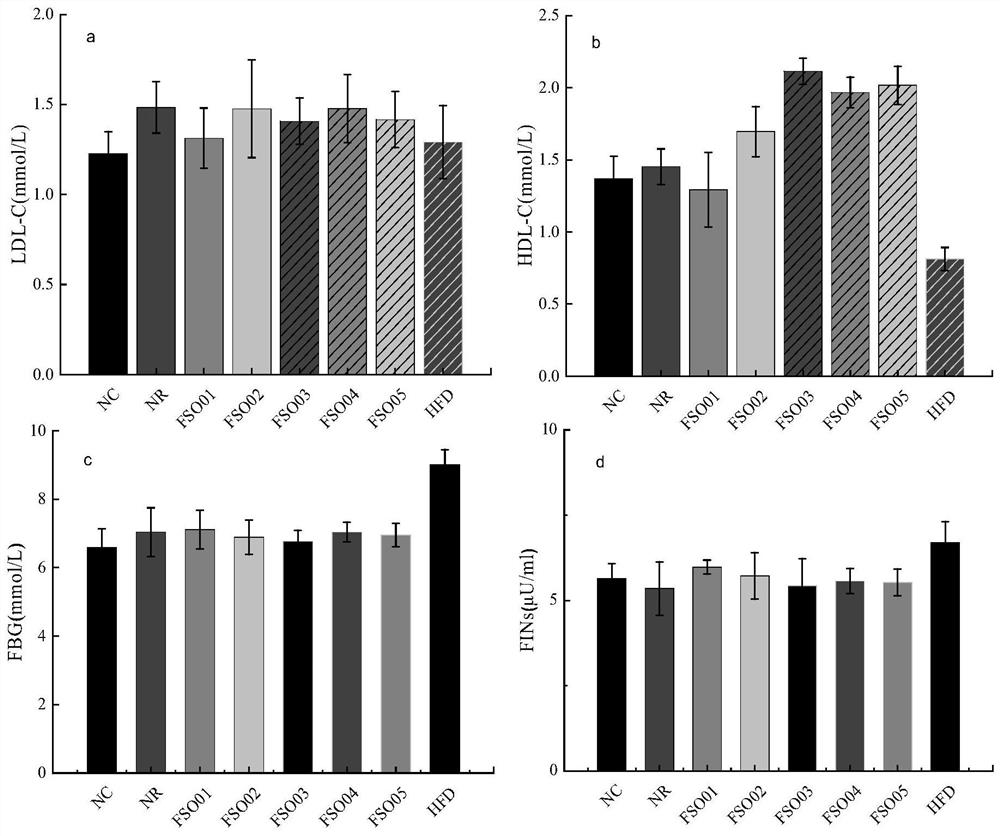
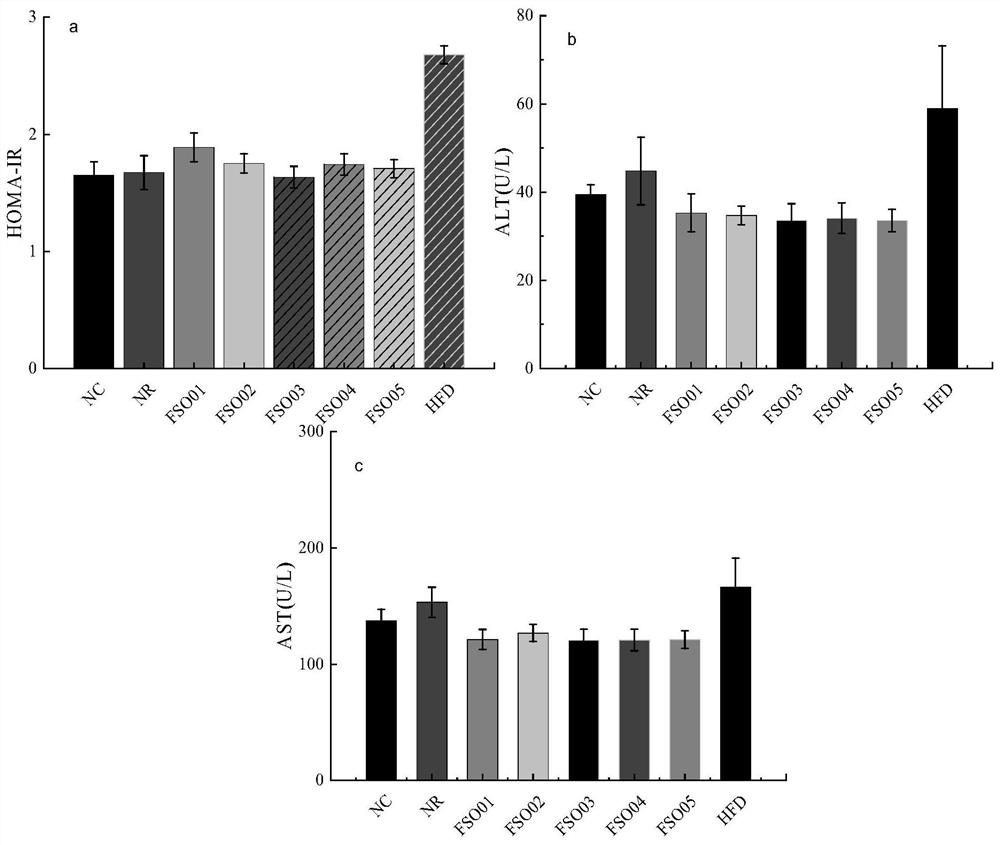
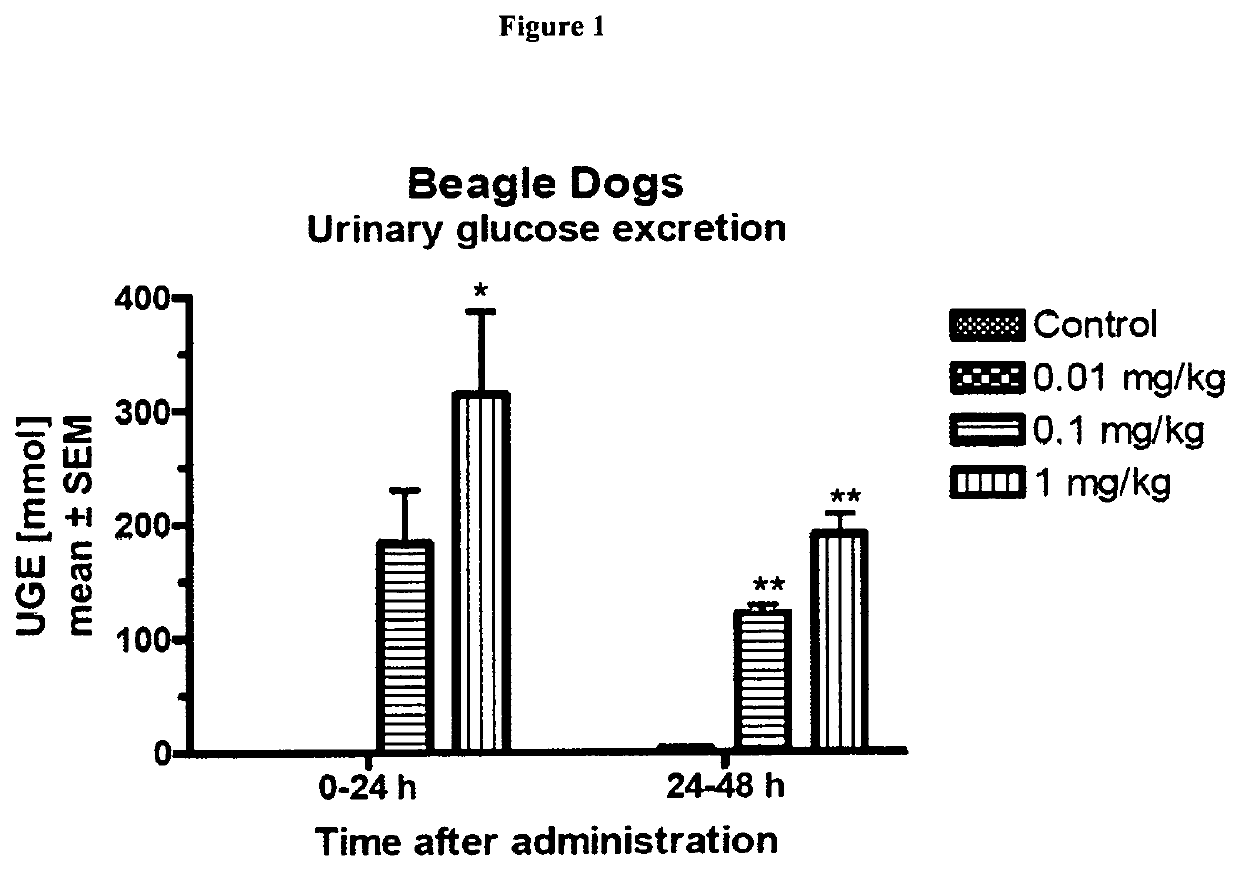
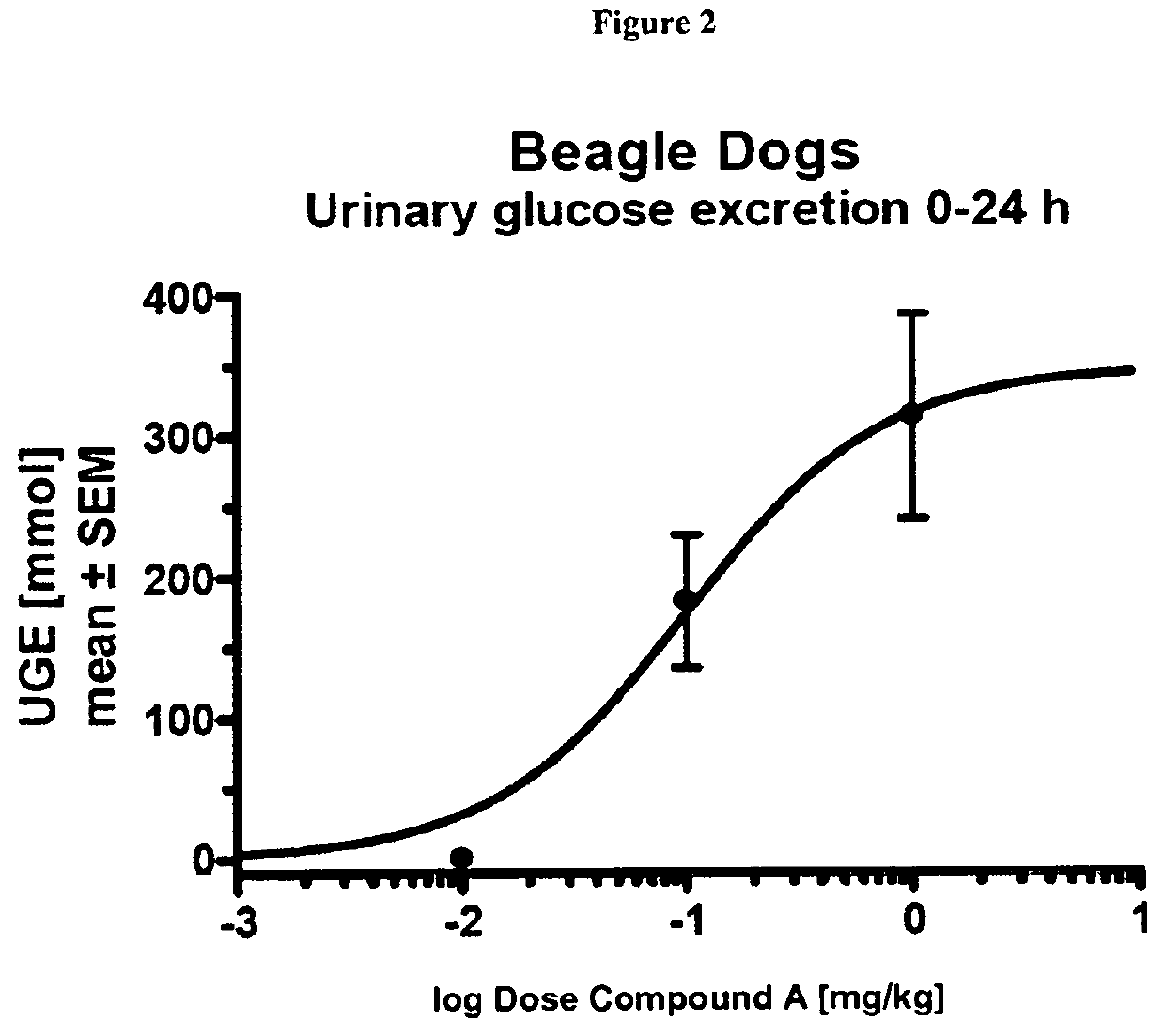
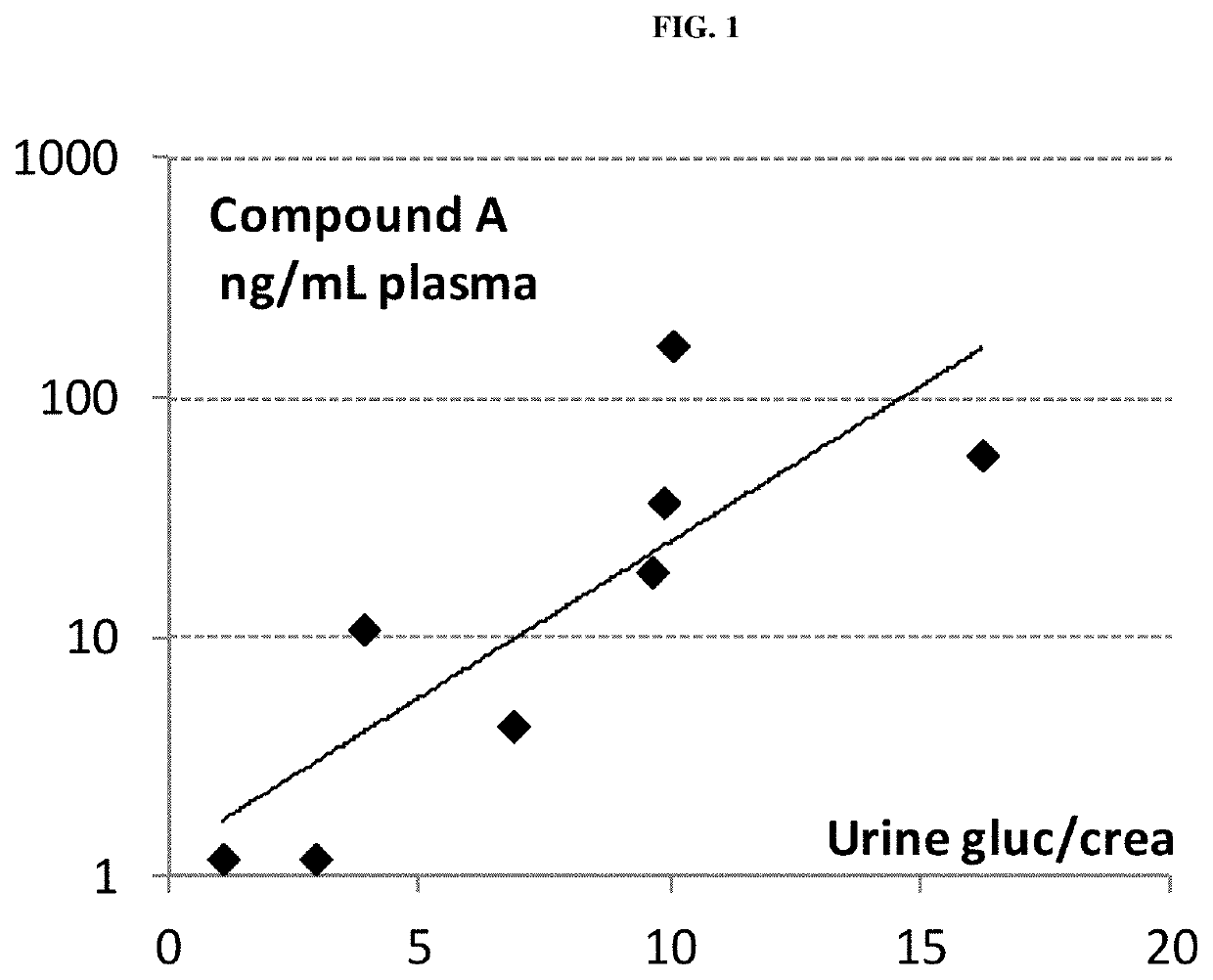
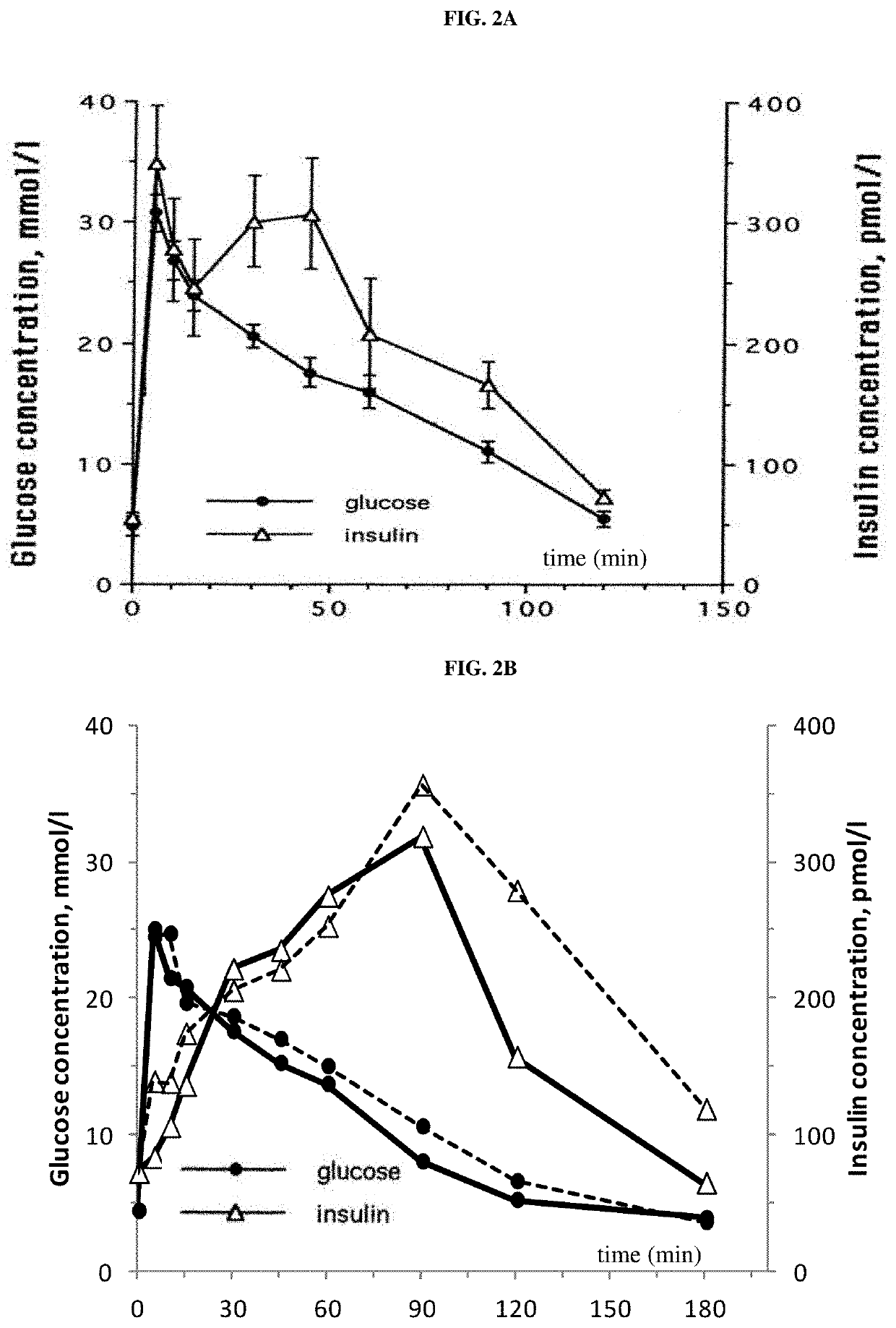
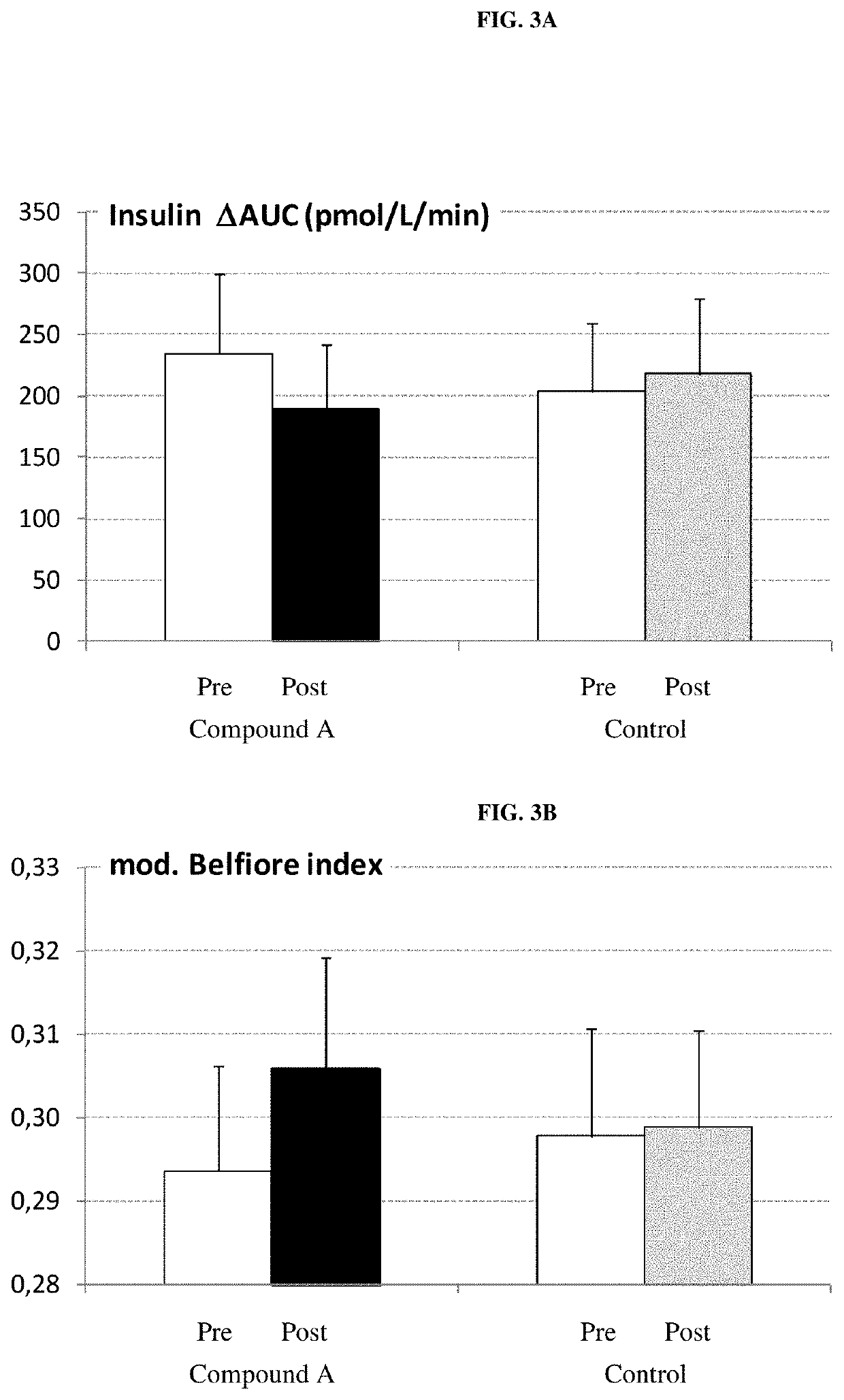
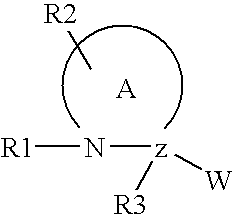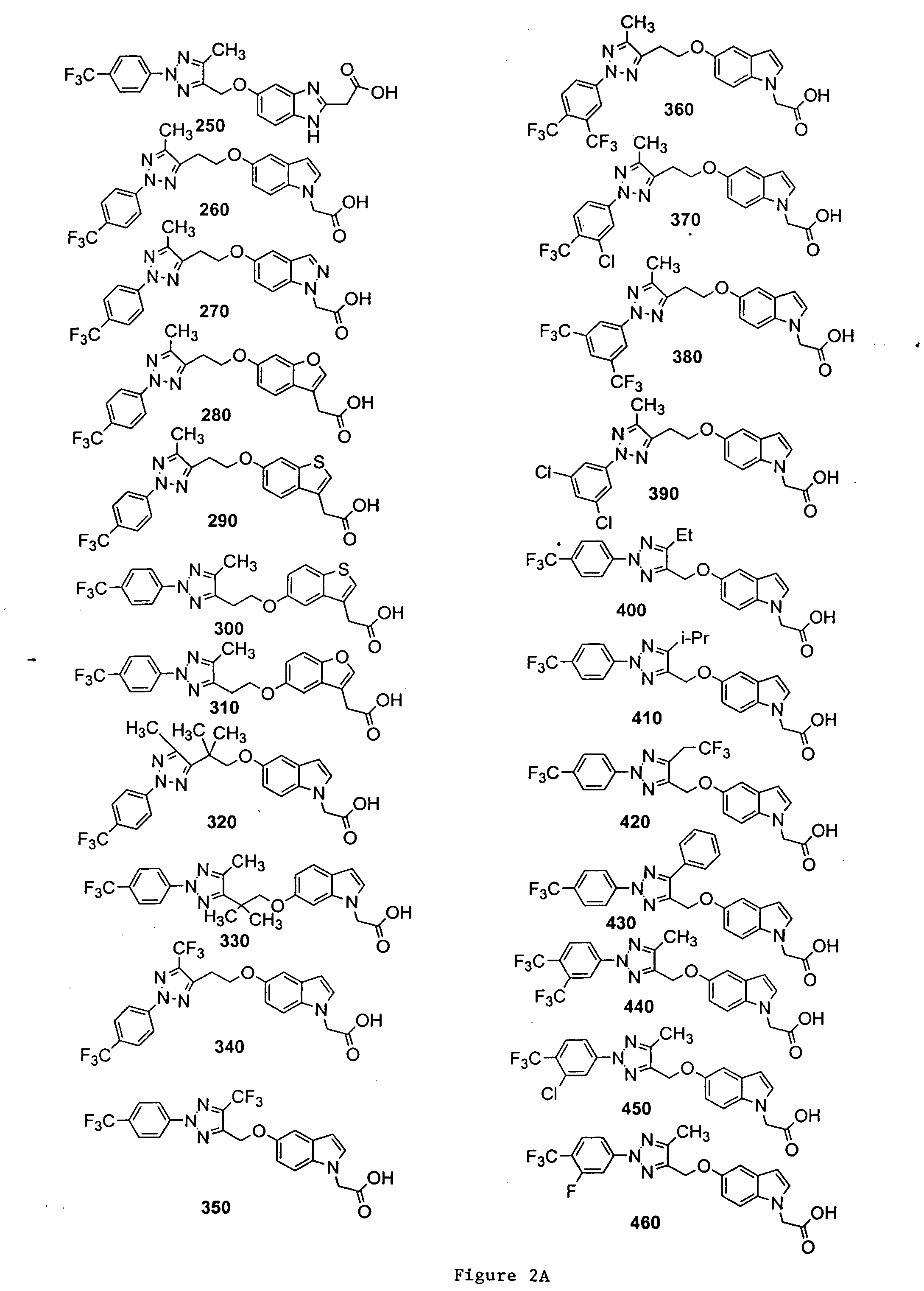Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Insulin blood" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antidiabetic agents
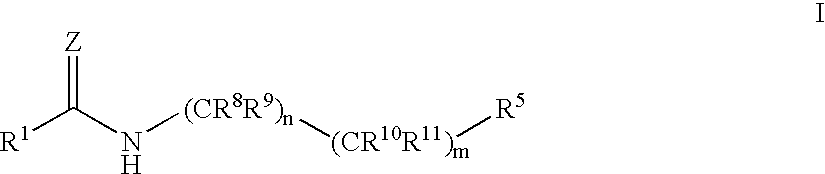
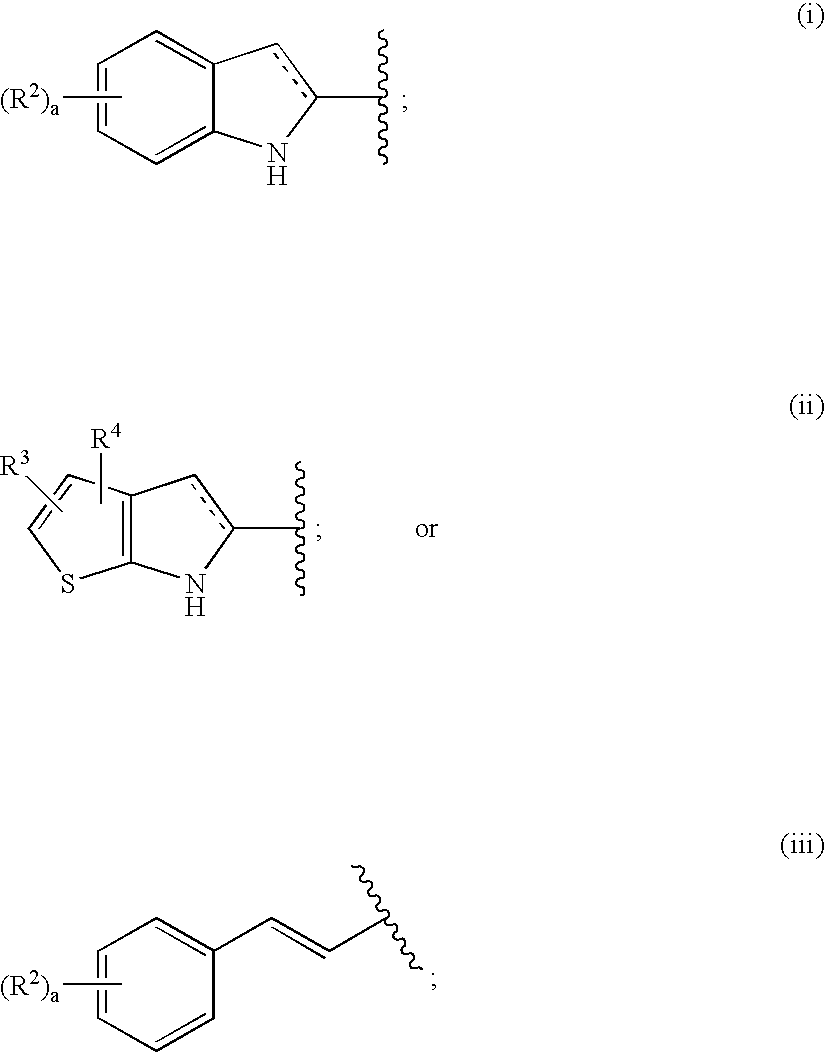
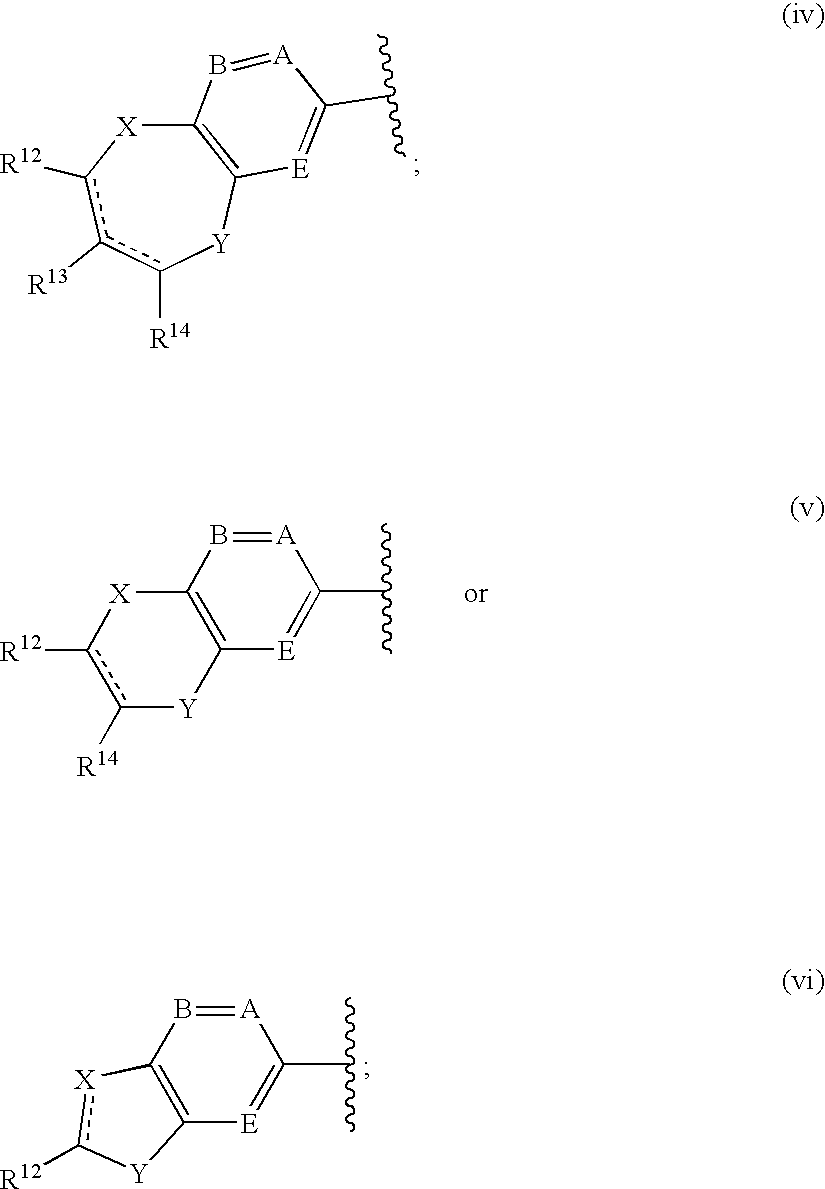
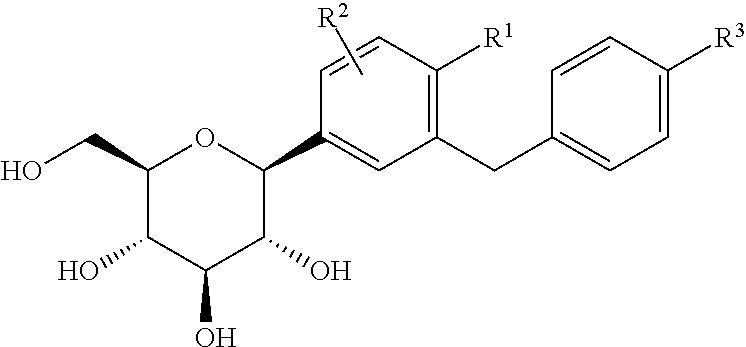
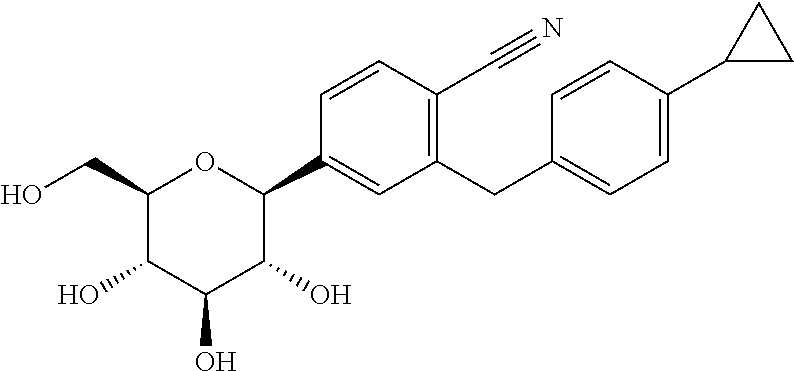
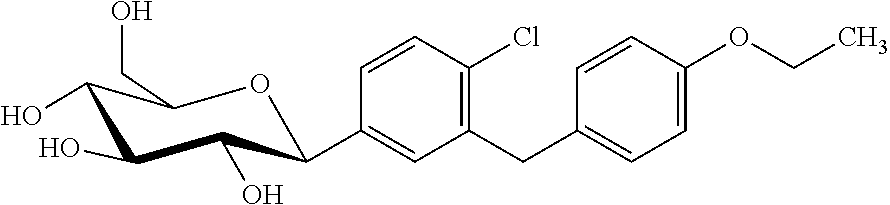
A compound of the formula wherein R1 is: R5 is: and n, m, Z, R8, R9, R10 and R11 are as defined herein, useful in the treatment of diabetes, insulin resistance, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, cataracts, hyperglycemia, hypercholesterolemia, hypertension, hyperinsulinemia, hyperlipidemia, atherosclerosis, and tissue ischemia, particularly, myocardial ischemia.
Owner:GAMMILL RONALD B
Dosage forms useful for modifying conditions and functions associated with hearing loss and/or tinnitus
InactiveUS20020061870A1Increased susceptibilityImprove usabilitySalicyclic acid active ingredientsBiocideWhole bodyActive agent
The invention defines interdependent biofactors and biomolecules, and clinically useful formulations that are comprised of them. The active agents are demonstrated to be complementary in their physiologic functions especially as these relate to the quenching of free radicals and to the support of endothelial physiology, the reduction of hyperinsulinemia and improvements in vascular health. The active components of the invention are selected for inclusion in precise combinations specifically because they improve these various conditions and physiological functions, and by so doing reduce a variety of risks associated with hearing loss and tinnitus. The resulting enhancement of general systemic vascular health, improvement in local VIIIth nerve vascular health, modulation of conditions surrounding blood fluid dynamics, the consequences of hyperinsulinemia, and improvements in free radical defenses, all reduce the potential for cochlear hair cell death and VIIIth nerve atrophy, and the hearing loss and possible deafness that accompany them.
Owner:CHRONORX
Composition and Method for the Treatment of Diseases Affected by a Peptide Receptor
InactiveUS20080300193A1Modifies glucose metabolismIncreased riskDipeptide ingredientsMetabolism disorderDiseasePancreatic hormone
The present invention includes peptidomimetic compound compositions and methods of making and using peptidomimetic compounds to modulate the activity of a peptide receptor for the treatment of one or more of hyperglycemia, insulin resistance, hyperinsulinemia, obesity, hyperlipidemia, hyperlipoproteinemia or other symptoms that relate to the function of the targeted receptor. The peptidomimetic includes an oligo-benzamide compound having at least three optionally substituted benzamides.
Owner:NEW ENGLAND MEDICAL CENT HOSPITALS +1
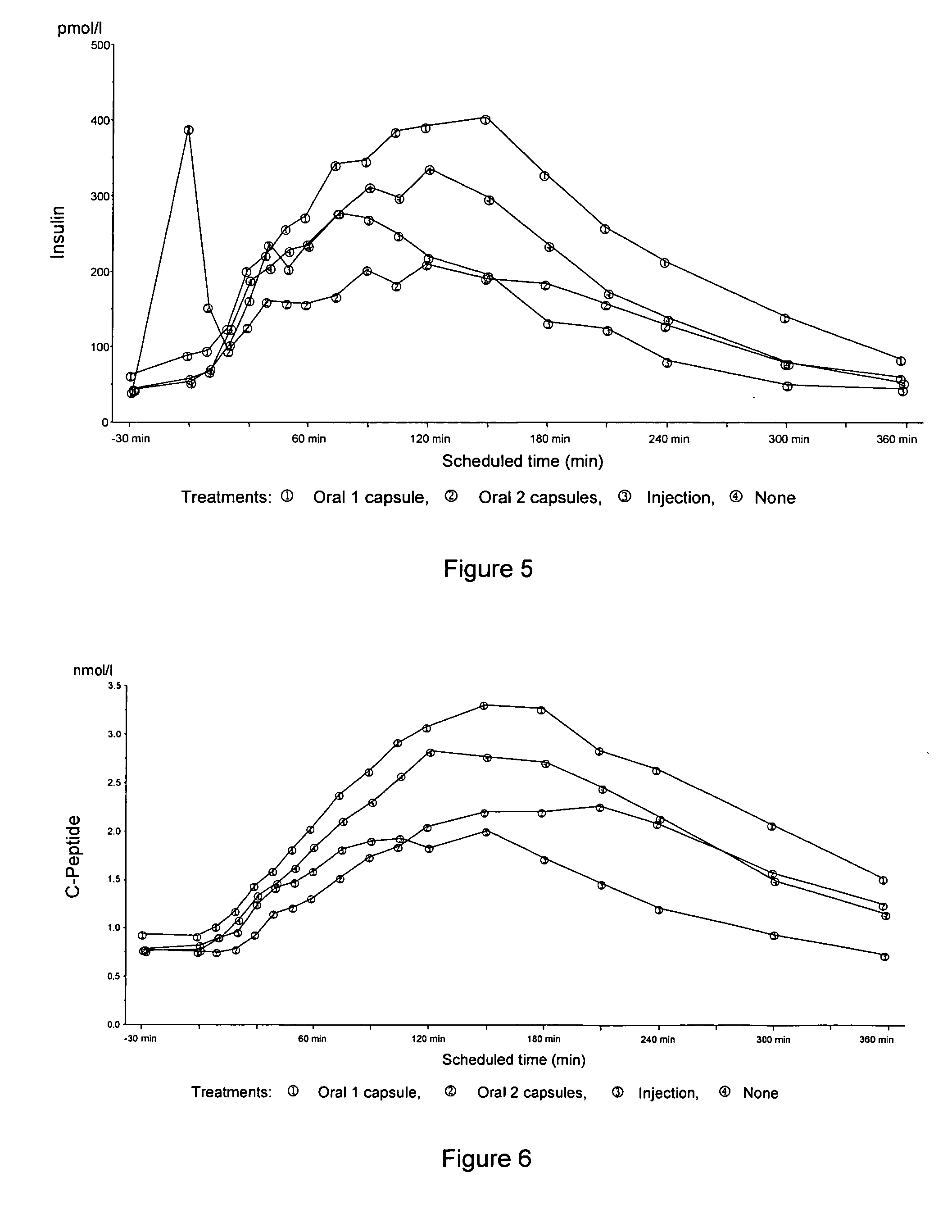
Oral insulin therapies and protocol
InactiveUS20050203001A1Good treatment effectPositive therapeutic effectOrganic active ingredientsPeptide/protein ingredientsLate onset diabetesHypoglycemia
Methods for treating impaired glucose tolerance and early and late stage diabetes in mammals, for prophylactically sparing β-cell function, aiding in preventing β-cell death, preventing the onset of overt diabetes in a mammal with type 2 diabetes, treating the current level of glycemic control dysfunction of a mammal with impaired glucose tolerance or diabetes, comprising orally administering insulin and a delivery agent that facilitates insulin absorption from the gastrointestinal tract at the time of or shortly before mealtime, e.g., within about 10 minutes prior to ingestion of a meal, on a chronic basis. The methods also comprise, in addition to administering a rapid-acting insulin to provide a first insulin peak, administering a slow acting insulin to provide a second insulin peak occurring at a later time but of a longer duration. These methods achieve improved glycemic control without the risks of hypoglycemia, hyperinsulinemia and weight gain and the need for frequent blood glucose monitoring that are normally associated with insulin therapy.
Owner:EMISPHERE TECH INC
Method of regulating glucose metabolism, and reagents related thereto
InactiveUS20040176307A1Long-term abatementLong-term reductionBiocideDipeptide ingredientsLipid storageChylomicron
The present invention provides methods and compositions for modification and regulation of glucose and lipid metabolism, generally to reduce insulin resistance, hyperglycemia, hyperinsulinemia, obesity, hyperlipidemia, hyperlipoprotein-emia (such as chylomicrons, VLDL and LDL), and to regulate body fat and more generally lipid stores, and, more generally, for the improvement of metabolism disorders, especially those associated with diabetes, obesity and / or atherosclerosis.
Owner:1149336 ONTARIO +2
Treatment of metabolic disorders in feline animals
ActiveUS20150164856A1Reduce doseReduce frequencyBiocideNervous disorderAcute hyperglycaemiaDyslipidemia
The present invention relates to one or more SGLT2 inhibitors or pharmaceutically acceptable forms thereof for use in the treatment and / or prevention of a metabolic disorder in a feline animal, preferably wherein the metabolic disorder is one or more selected from the group consisting of: ketoacidosis, pre-diabetes, diabetes mellitus type 1 or type 2, insulin resistance, obesity, hyperglycemia, impaired glucose tolerance, hyperinsulinemia, dyslipidemia, dysadipokinemia, subclinical inflammation, systemic inflammation, low grade systemic inflammation, hepatic lipidosis, atherosclerosis, inflammation of the pancreas, neuropathy and / or Syndrome X (metabolic syndrome) and / or loss of pancreatic beta cell function and / or wherein the remission of the metabolic disorder, preferably diabetic remission, is achieved and / or maintained.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
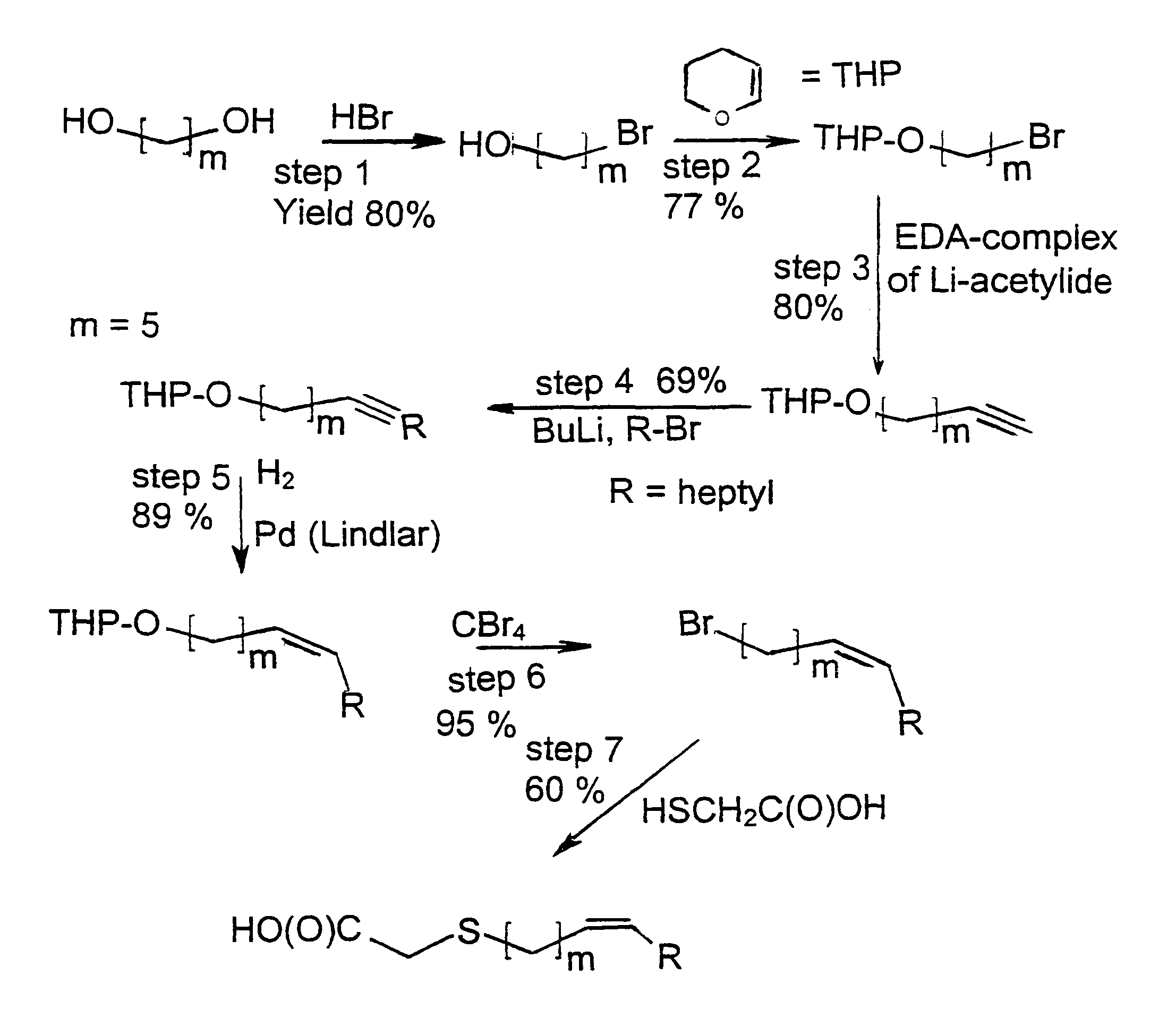
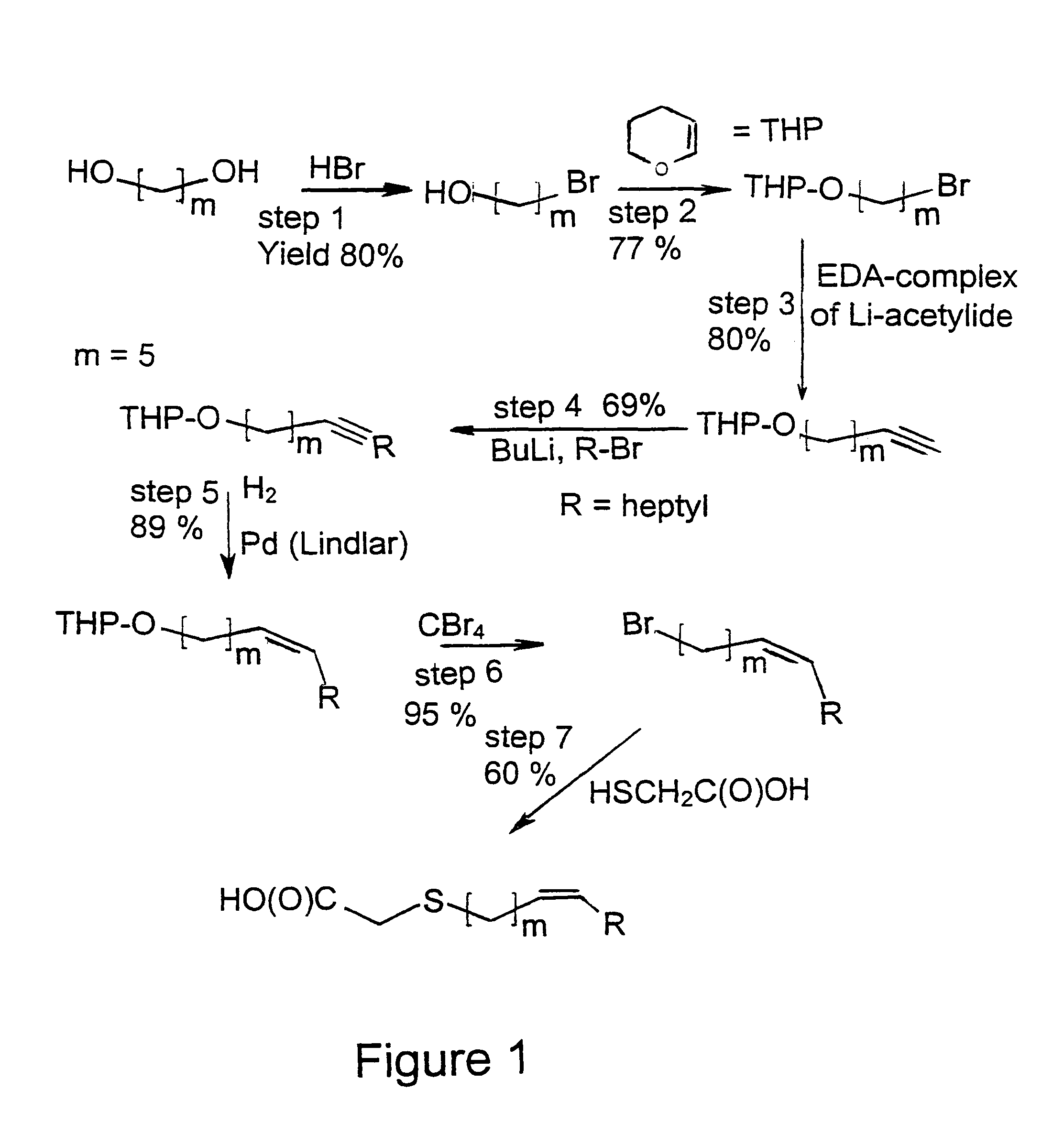
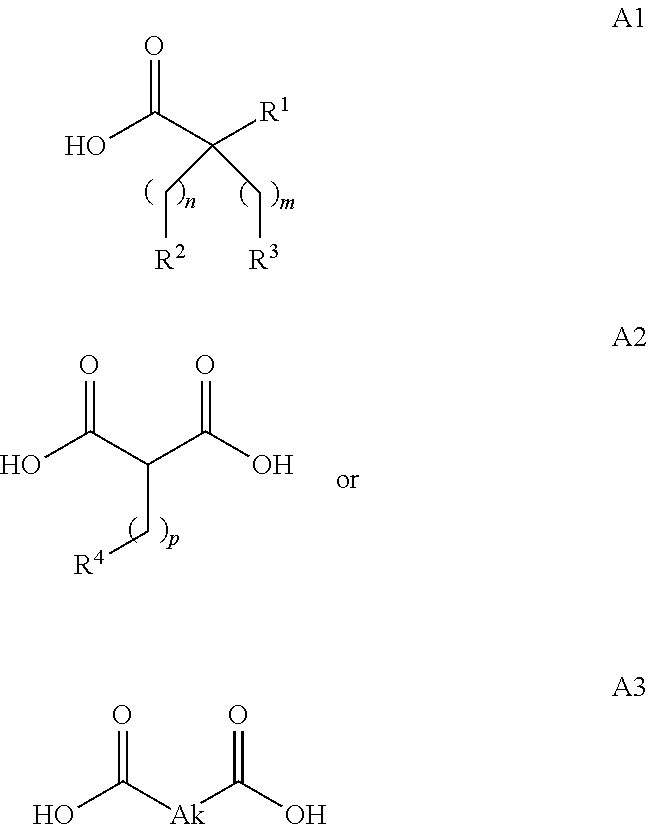
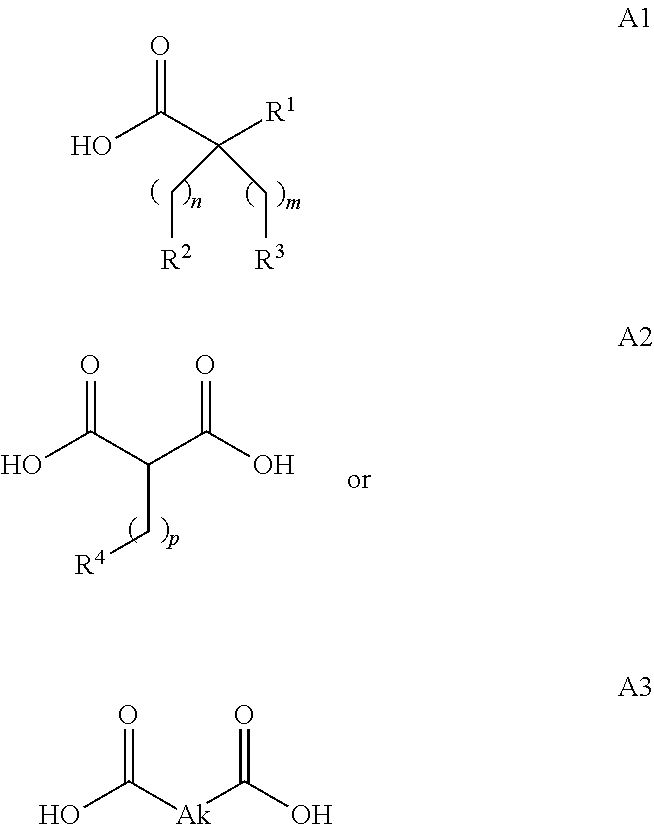
Novel fatty acids and their use in conjugation to biomolecules
The invention provides a conjugate comprising a biomolecule linked to a fatty acid via a linker wherein the fatty acid has the following Formulae A1, A2 or A3:wherein R1, R2, R3, R4, Ak, n, m and p are defined herein. The invention also relates to a method for manufacturing the conjugate of the invention such as GDF15 conjugate, and its therapeutic uses such as treatment or prevention of metabolic disorders or diseases, type 2 diabetes mellitus, obesity, pancreatitis, dyslipidemia, alcoholic and nonalcoholic fatty liver disease / steatohepatitis and other progressive liver diseases, insulin resistance, hyperinsulinemia, glucose intolerance, hyperglycemia, metabolic syndrome, hypertension, cardiovascular disease, atherosclerosis, peripheral arterial disease, stroke, heart failure, coronary heart disease, diabetic complications (including but not limited to chronic kidney disease), neuropathy, gastroparesis and other metabolic disorders. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Insulin pump with dynamic closed-loop control
InactiveCN104667379AAchieve the effect of automatic control injectionStabilize blood sugarMedical devicesPressure infusionMedical equipmentInsulin blood
The invention discloses an insulin pump with a dynamic closed-loop control in the field of medical equipment. The insulin pump comprises an administration mechanism, a driving mechanism, a detection mechanism and a dynamic control module, wherein the administration mechanism is connected with the insulin pump and transmits insulin injection amount information; the driving mechanism is connected with an injection unit of the insulin pump, and transmits and controls the injection amount information; the detection mechanism is connected with a glucometer and transmits human body blood glucose information; and the dynamic control module is connected with the driving mechanism of the insulin pump and transmits insulin-blood glucose level control information. The insulin pump with the dynamic closed-loop control can effectively reduce the unstabilizing factors due to a complex blood glucose system and has the relatively nice robustness after simulation testing. The insulin pump can control the blood glucose level of the diabetics and the injection amount of the insulin in time through model prediction and iterative control, so that the blood glucose level of the diabetics tends to be stable; and therefore, the effect of the insulin pump to control the injection automatically is achieved.
Owner:SHANGHAI JIAO TONG UNIV
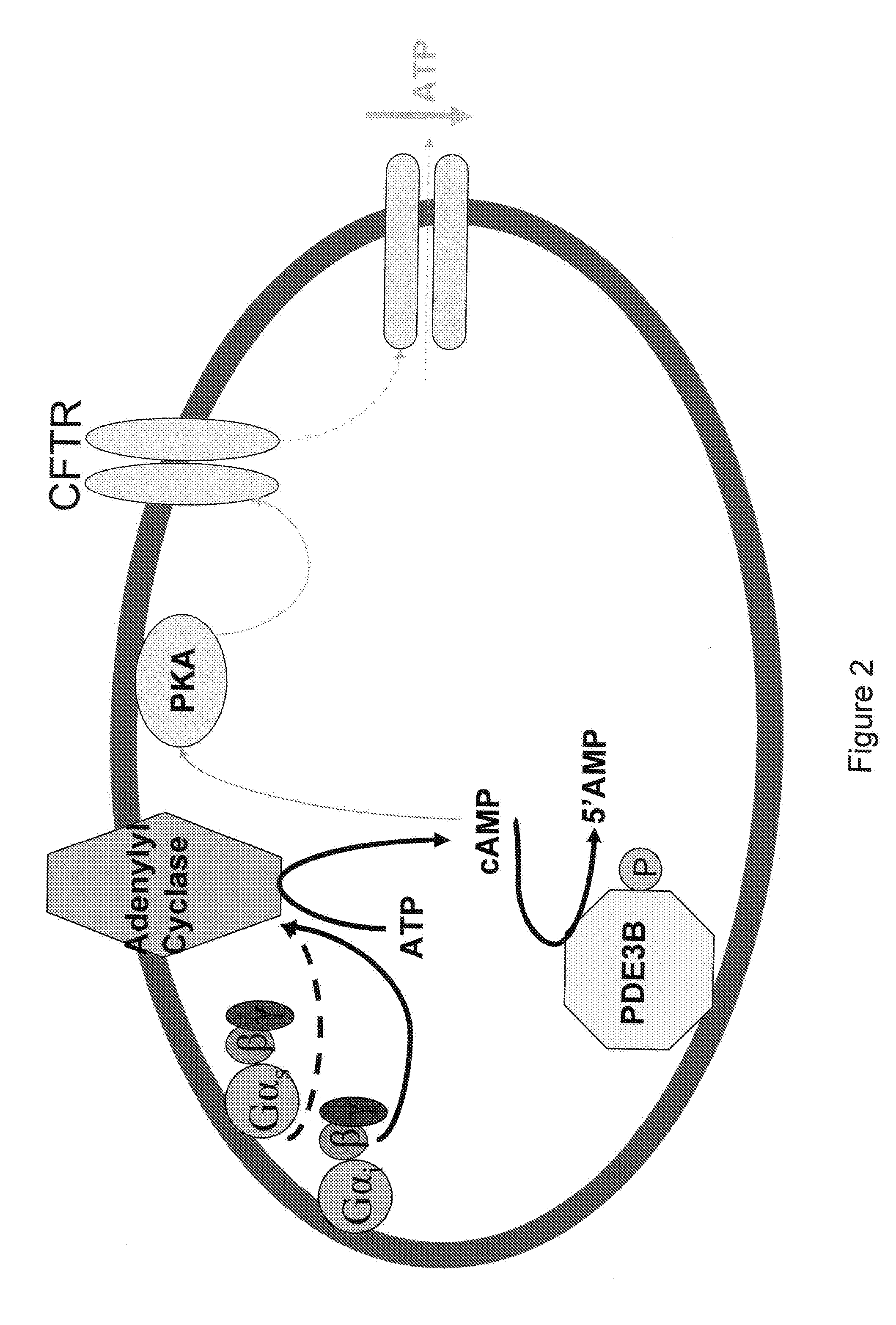
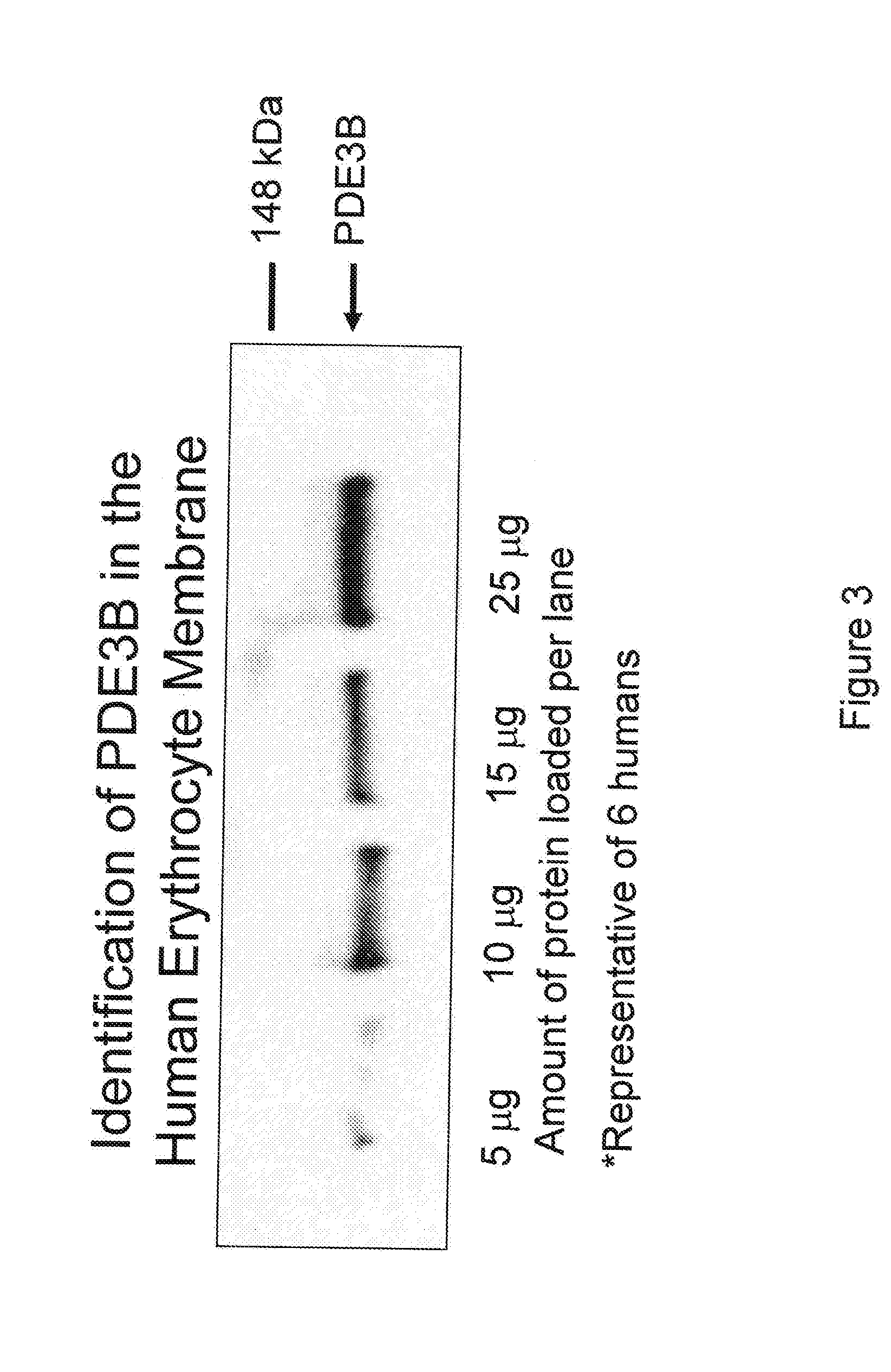
Control of ATP release by red blood cells and therapeutic applications thereof
InactiveUS20070249668A1Increase productionFacilitated releaseBiocideElcosanoid active ingredientsHyperinsulinemiaRed Cell
The invention is based upon the discovery that red blood cells contain phosphodiesterase 3B (PDE3B), and that inhibition of that phosphodiesterase allows for an enhanced accumulation of cAMP and subsequent release of ATP. It was further discovered that RBCs treated with insulin accumulate significantly less cAMP and release significantly less ATP than normal RBCs. Likewise, RBCs of patients suffering from type 2 diabetes (hyperinsulinemia) accumulate significantly less cAMP and release significantly less ATP than normal RBCs. It was further discovered that prostaglandin analogues synergistically work with phosphodiesterase 3B inhibitors to improve or increase cAMP accumulation and ATP release by RBCs. Thus the invention is directed to compositions and methods for improving ATP release by RBCs, via administering PDE3B inhibitor or a combination of PDE3B inhibitor and prostaglandin analogue.
Owner:SAINT LOUIS UNIVERSITY
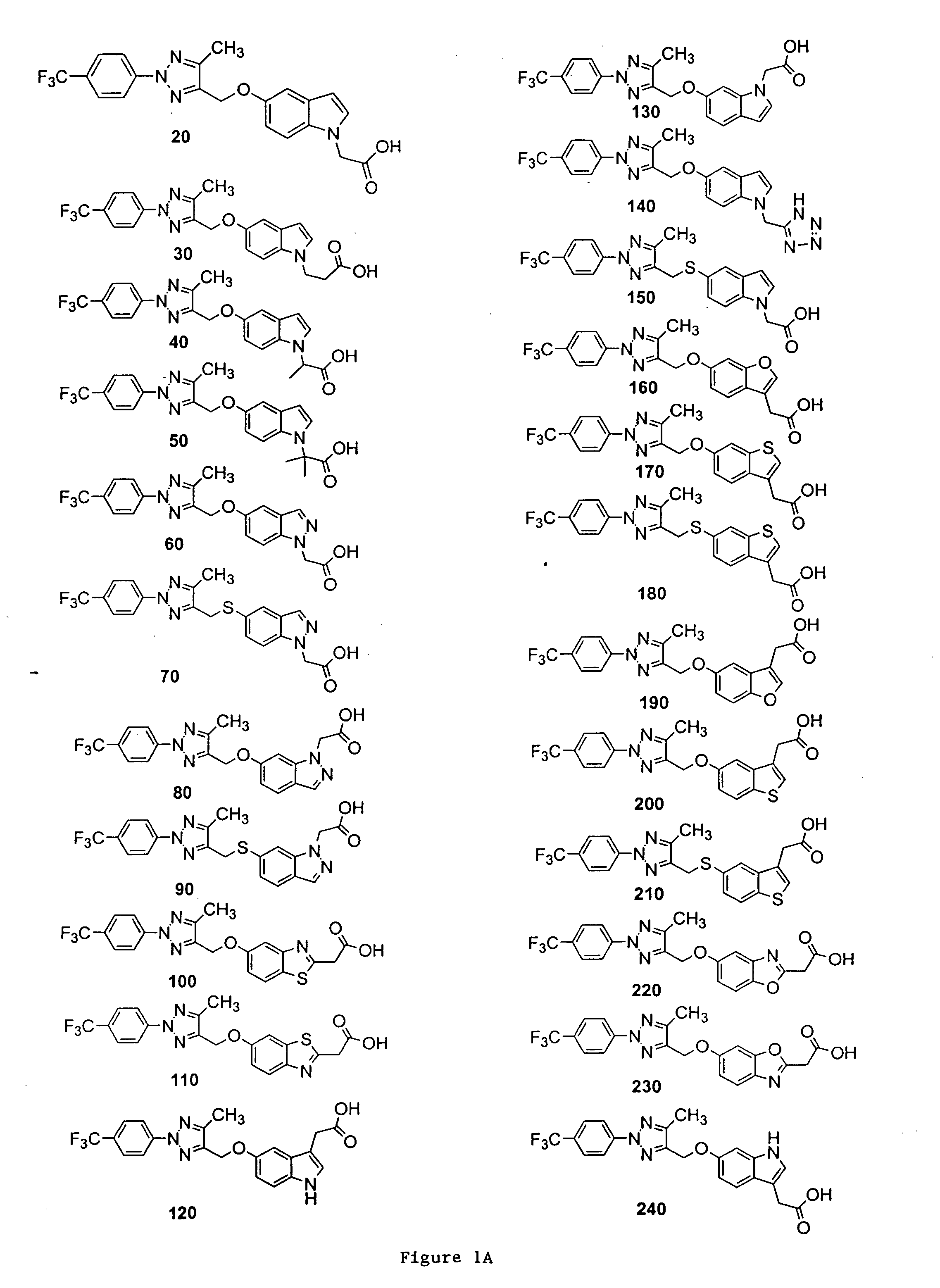
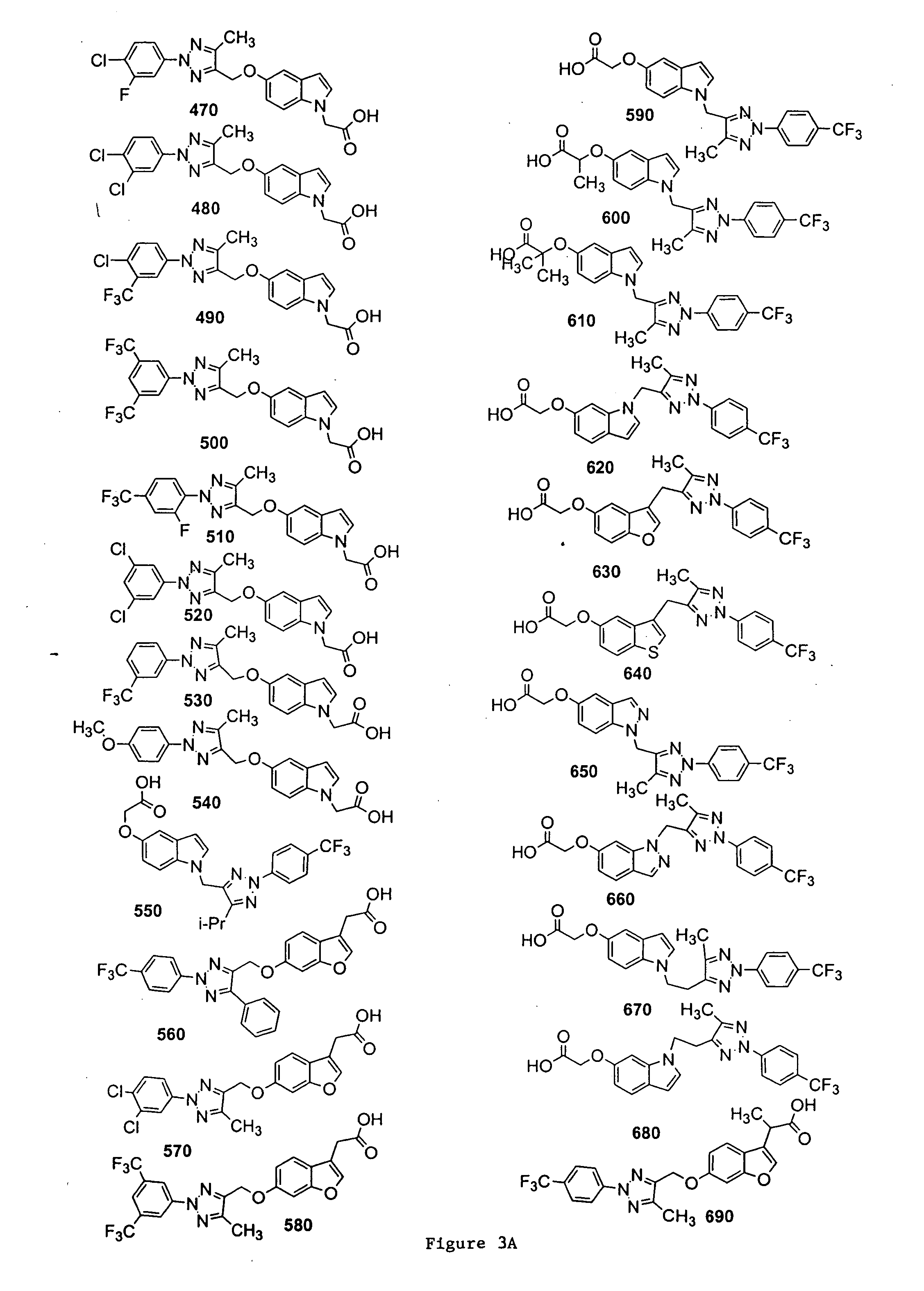
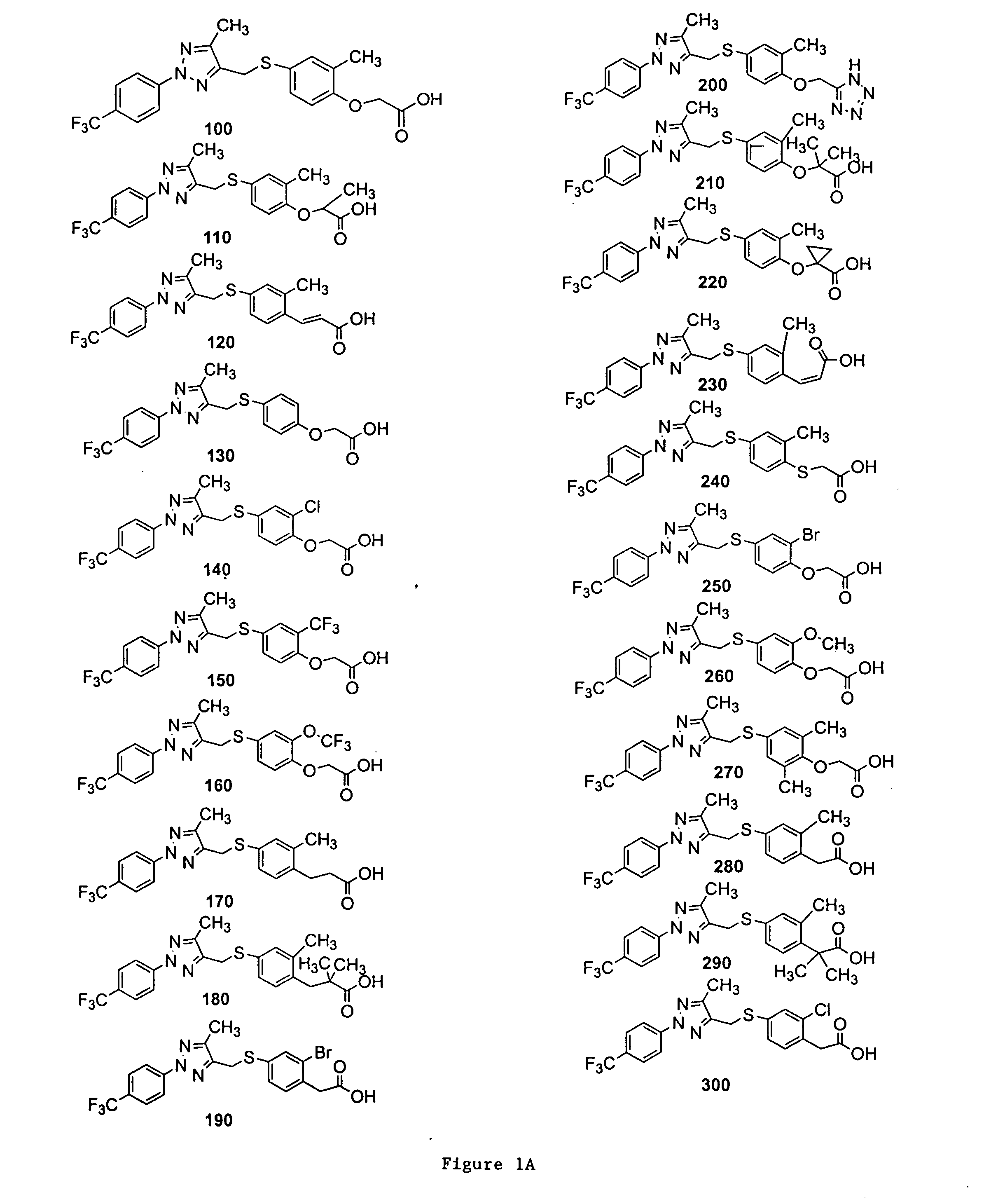
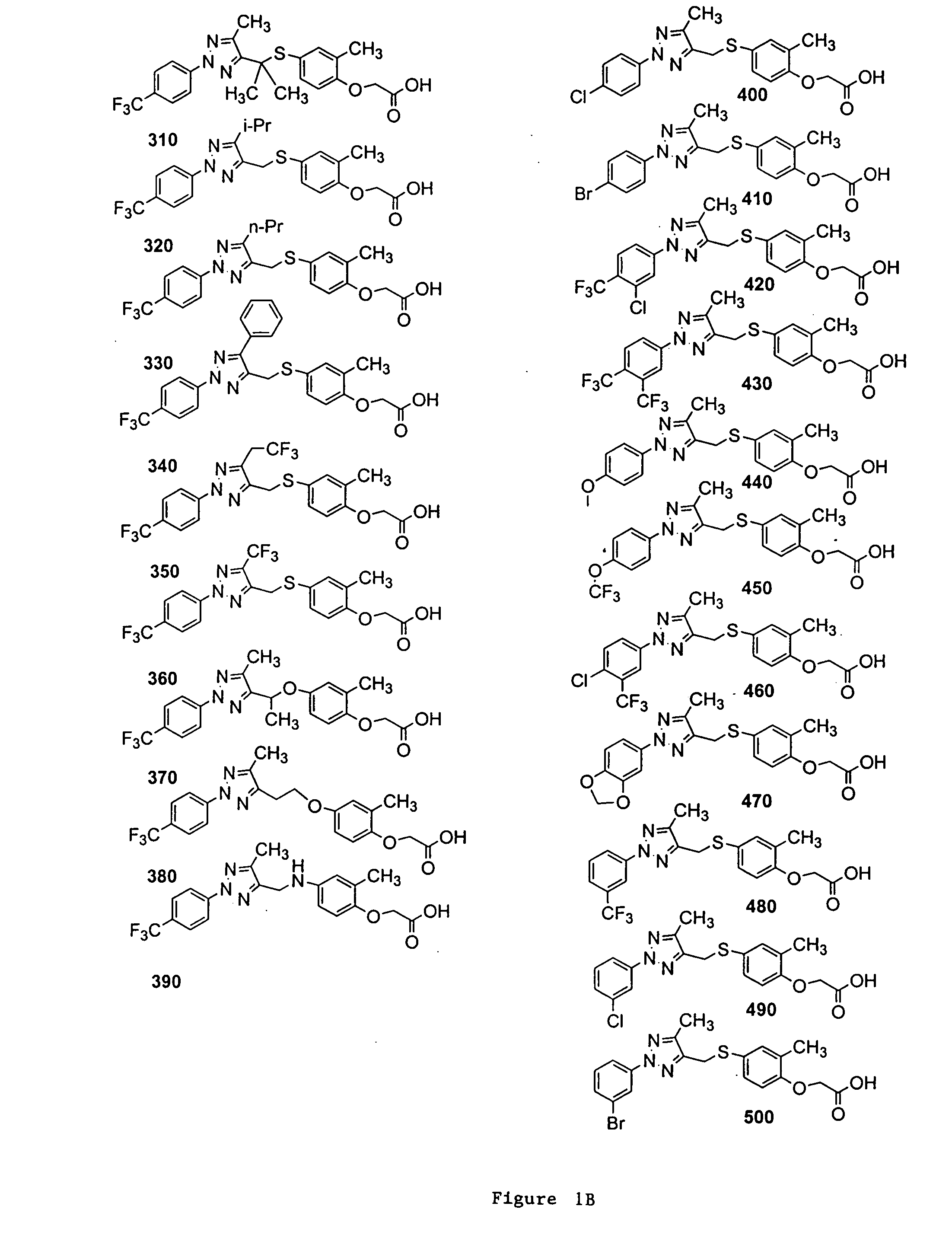
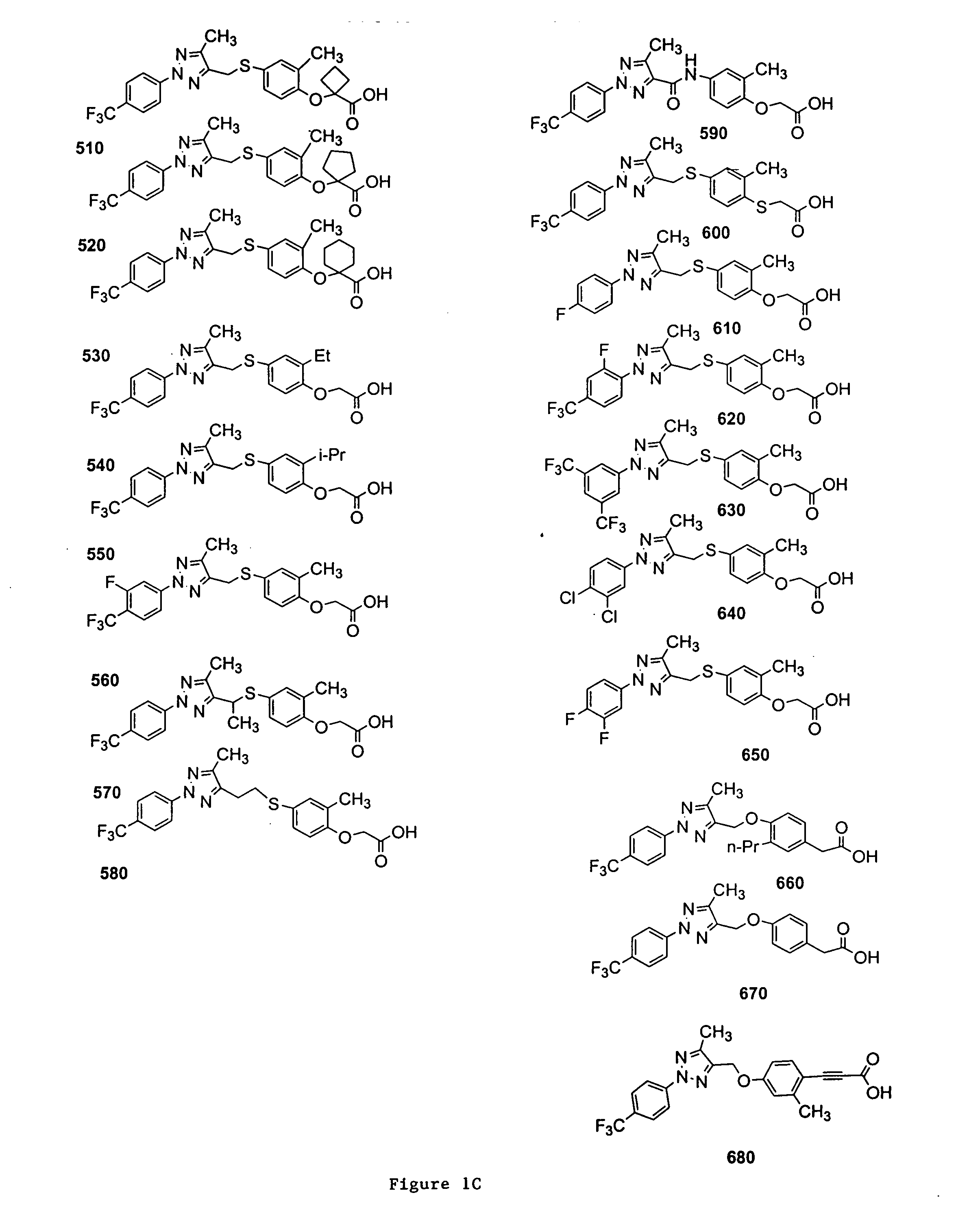
Bicyclic, substituted triazoles as modulators of PPAR and methods of their preparation
The present invention is directed to certain novel triazole compounds represented by Formula I and pharmaceutically acceptable salts, solvates, hydrates, and prodrugs thereof. The present invention is also directed to methods of making and using such compounds and pharmaceutical compositions containing such compounds to treat or control a number of diseases mediated by PPAR such as glucose metabolism, lipid metabolism and insulin secretion, specifically Type 2 diabetes, hyperinsulemia, hyperlipidemia, hyperuricemia, hypercholesteremia, atherosclerosis, one or more risk factors for cardiovascular disease, Syndrome X, hypertriglyceridemia, hyperglycemia, obesity, and eating disorders.
Owner:METABOLEX INC
Substituted triazoles as modulators of PPAR and methods of their preparation
The present invention is directed to certain novel triazole compounds represented by Formula I and pharmaceutically acceptable salts, solvates, hydrates, and prodrugs thereof. The present invention is also directed to methods of making and using such compounds and pharmaceutical compositions containing such compounds to treat or control a number of diseases mediated by PPAR such as glucose metabolism, lipid metabolism and insulin secretion, specifically Type 2 diabetes, hyperinsulemia, hyperlipidemia, hyperuricemia, hypercholesteremia, atherosclerosis, one or more risk factors for cardiovascular disease, Syndrome X, hypertriglyceridemia, hyperglycemia, obesity, and eating disorders.
Owner:CYMABAY THERAPEUTICS
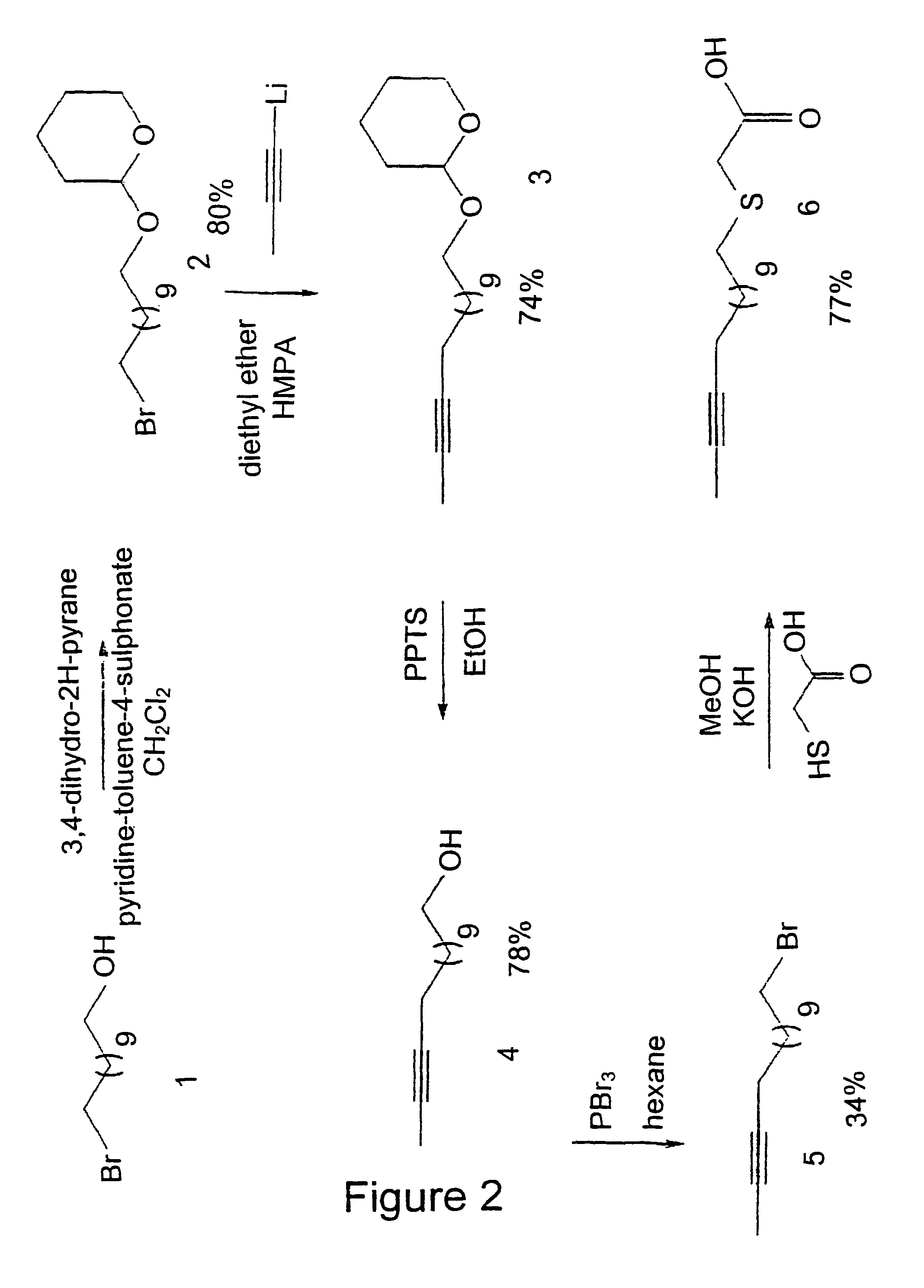
Fatty acids analogous
The present invention relates to novel fatty acid analogous of the general formula (I): R1—[xi—CH2]n—COOR2 wherein R1 is: a C6-C24 alkene with one or more double bonds and / or with one or more triple bonds, and / or a C6-C24 alkyne, and / or a C6-C24 alkyl substituted in one or several positions with one or more compounds selected from the group comprising fluoride, chloride, hydroxy, C1-C4 alkoxy, C1-C4 alkylthio, C2-C5 acyloxy or C1-C4 alkyl, and wherein R2 represents hydrogen or C1-C4 alkyl, and wherein n is an integer from 1 to 12, and wherein i is an odd number and indicates the position relative to COOR2, and wherein Xi independent of each other are selected from the group comprising O, S, SO, SO2, Se and CH2, and with the proviso that at least one of the Xi is not CH2, and with the proviso that if R1 is an alkye, then the carbon-carbon triple bond is positioned between the (ω-1) carbon and the (ω-2) carbon, or between the (ω-2) carbon and the (ω-3) carbon, or between the (ω-3) carbon and the (ω-4) carbon, a salt, prodrug or complex thereof, which can be used for the treatment and / or prevention of syndrome X, obesity, hypertension, fatty liver, diabetes, hyperglycaemia, hyperinsulinemia and stenosis. Further, the invention relates to a nutritional composition comprising said fatty acid analogues, and a method for reducing the total weight, or the amount of adipose tissue in an animal.
Owner:THIA MEDICA AS
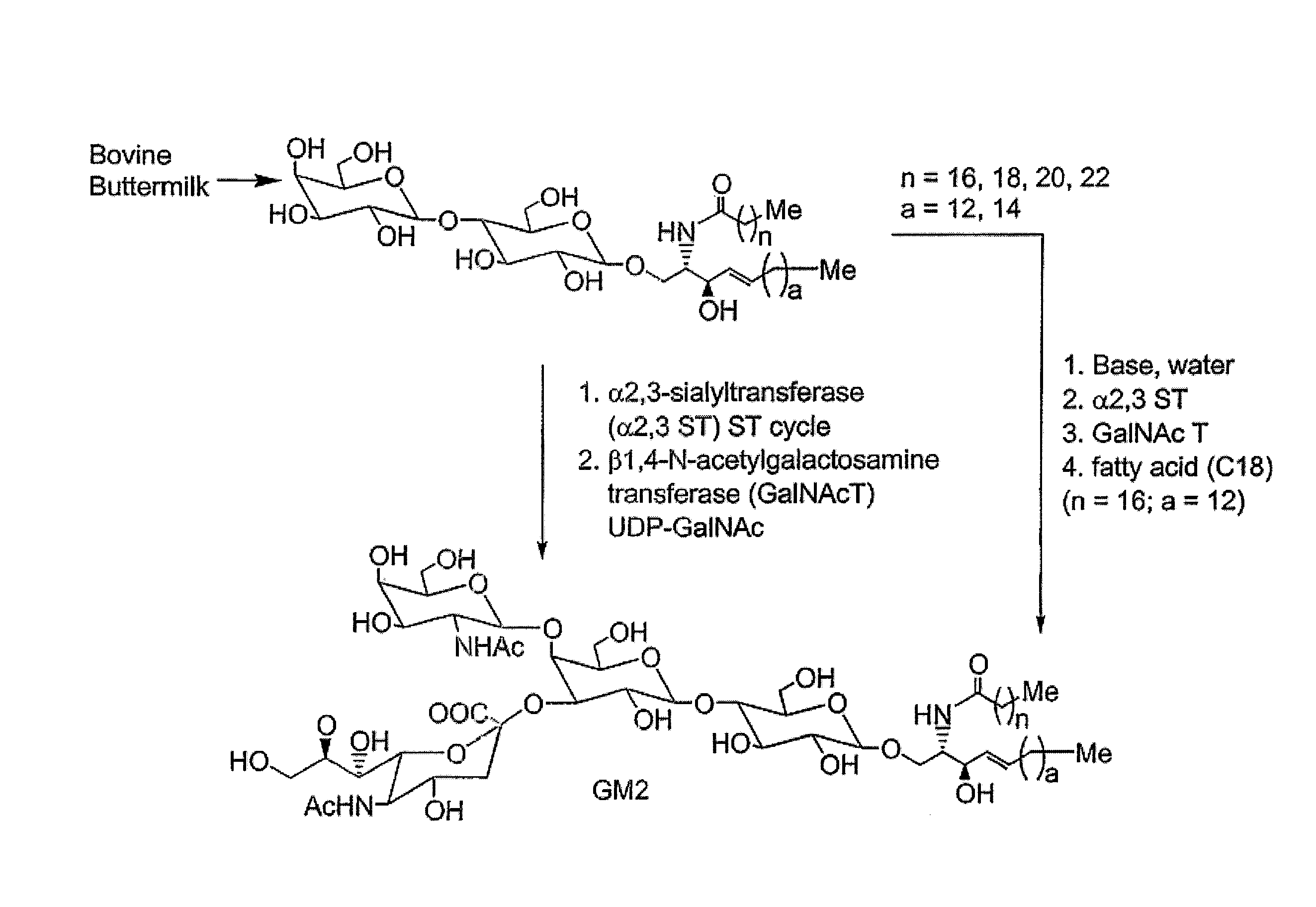
Glycolipids as treatment for disease
InactiveUS20120035120A1High affinityExtended half-lifeBiocideNervous disorderDiseaseHuntingtons chorea
This invention provides compounds, compositions, and methods for treating a disorder selected from cancer, hyperinsulinemia, hypoglycemia, hyperinsulinemia with hypoglycemia, atypical Parkinson's disease, Huntington's disease, multiple systems atrophy, GM3 synthase deficiency, GM2 synthase deficiency or tauopathy.
Owner:SENEB BIOSCI
Functional edible oil as well as preparation method and application thereof
PendingCN112772731AImprove glucose and lipid metabolism disordersGood for healthEdible oils/fatsFatty acid esterificationNutritionCarbon chain
The invention relates to functional edible oil as well as a preparation method and application thereof. The functional edible oil is formed by performing ternary ester exchange on medium- carbon-chain glyceride, linoleic acid grease and linolenic acid grease. On the basis of the mass of fatty acid, the mass ratio of medium-carbon-chain fatty acid in the medium-carbon-chain glyceride to long-carbon-chain fatty acid in the linoleic acid grease and the linolenic acid grease is 2.3-4.0, and the mass ratio of linoleic acid to linolenic acid in the long-carbon-chain fatty acid is 0.5-1.0. The addition amount of the functional edible oil in foods is greater than or equal to 18.00%. The functional edible oil can significantly improve in-vivo glucose and lipid metabolism disorder, balance and supplement in-vivo necessary and functional fatty acids, and rapidly supplement energy, has a freezing point lower than 7.5 DEG C, can meet consumers, particularly, dietary and nutritional requirements of patients with metabolic syndromes such as overweight, obesity, fatty liver, hyperlipidemia, hyperglycemia, hypertension, hyperblood viscosity, hyperuricemia and hyperinsulinemia and athletes can be met, and the functional edible oil can be widely applied to foods and special medical application industries.
Owner:NANCHANG UNIV
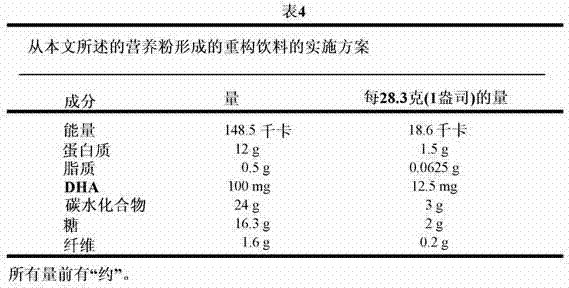
Nutritional composition for pregnant women with a beneficial glucose and insulin profile
The present disclosure is directed to a nutritional powder, a translucent reconstitutable beverage formed therefrom, and methods relating thereto. The nutritional powder and beverage are adapted to include the proper balance of proteins, lipids, carbohydrates, vitamins and minerals appropriate for a pregnant woman. The nutritional compositions further stem the glycemic response and improve glycemia and insulinemia during gestational and lactating periods for preventing or reduce the incidence of glucose intolerance later in life.
Owner:ABBOTT LAB INC
Application of triacetyl-3-hydroxyphenyl adenosine in preparing medicines for improving insulin resistance and related diseases
InactiveCN105663152AIncreased sensitivityImprove the immunityOrganic active ingredientsMetabolism disorderTriacetyl-3-hydroxyphenyladenosineDisease
The invention discloses application of triacetyl-3-hydroxyphenyl adenosine, as shown in Formula (I), in preparing medicines for preventing, relieving or treating insulin resistance and related diseases, wherein the insulin resistance related diseases include type II diabetes, abnormal glucose tolerance, hyperinsulinemia, impaired glucose tolerance, hypertension, coronary disease, obesity and non-alcoholic fatty liver disease. Specifically, the triacetyl-3-hydroxyphenyl adenosine disclosed by the invention can improve the insulin sensitivity of peripheral tissues and the glucose tolerance of a body and the triacetyl-3-hydroxyphenyl adenosine can reduce insulin content, so that insulin resistance is improved (as shown in the Description).
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI +1
Combination treatment of SGLT2 inhibitors and dopamine agonists for preventing metabolic disorders in equine animals
ActiveUS10555958B2Increase insulin sensitivityNormalize the insulin dysregulationMetabolism disorderDigestive systemDiseasePancreatic hormone
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Functional structural oil as well as preparation method and application thereof
PendingCN113287659AImprove glucose and lipid metabolism disordersGood for healthVitamin food ingredientsSkeletal disorderNutritionFood sector
The invention relates to functional structure oil, which is formed by carrying out quaternary ester exchange on camphor tree seed kernel oil, caprylin, linoleic acid grease and linolenic acid grease. The preparation method comprises the following steps: directly carrying out quaternary ester exchange on camphor tree seed kernel oil, caprylin, linoleic acid grease and linolenic acid grease at proper temperature and stirring intensity by taking lipase as a catalyst, and carrying out molecular distillation purification to obtain the functional structure oil. The functional structure oil can significantly improve in-vivo glucose and lipid metabolism disorder, balance and supplement in-vivo necessary and functional fatty acids, and quickly supplement energy, and can meet the requirements of consumers. Particularly, the dietary and nutritional requirements of patients with metabolic syndromes such as overweight, obesity, fatty liver, hyperlipidemia, hyperglycemia, hypertension, hyperblood viscosity, hyperuricemia and hyperinsulinemia and athletes can be met, and the food can be widely applied to the industries of grease powder, milk tea, meal replacement powder, health food, sports nutritious food and food for special medical purposes.
Owner:NANCHANG UNIV
Treatment of metabolic disorders in canine animals
ActiveUS10603300B2Reduce doseReduce frequencyOrganic active ingredientsPowder deliveryPhysiologyPancreatic hormone
The present invention relates to one or more SGLT2 inhibitors or pharmaceutically acceptable forms thereof for use in the treatment and / or prevention of a metabolic disorder in a canine animal, preferably wherein the metabolic disorder is one or more selected from the group consisting of: ketoacidosis, pre-diabetes, insulin dependent diabetes mellitus, insulin resistance diabetes, insulin resistance, obesity, hyperglycemia, hyperglycemia induced cataract formation, impaired glucose tolerance, hyperinsulinemia, dyslipidemia, dysadipokinemia, subclinical inflammation, systemic inflammation, low grade systemic inflammation, hepatic lipidosis, inflammation of the pancreas, metabolic disorder consequences, such as hypertension, renal dysfunction and / or muscoskeletal disorders, and / or Syndrome X (metabolic syndrome), wherein preferably the development of hyperglycemia induced cataract formation is prevented or remission is achieved and / or wherein preferably the development of metabolic disorder consequences, such as hypertension, renal dysfunction and / or muscoskeletal disorders, is prevented or progression is slowed or remission is achieved.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Nasal formulations of insulin
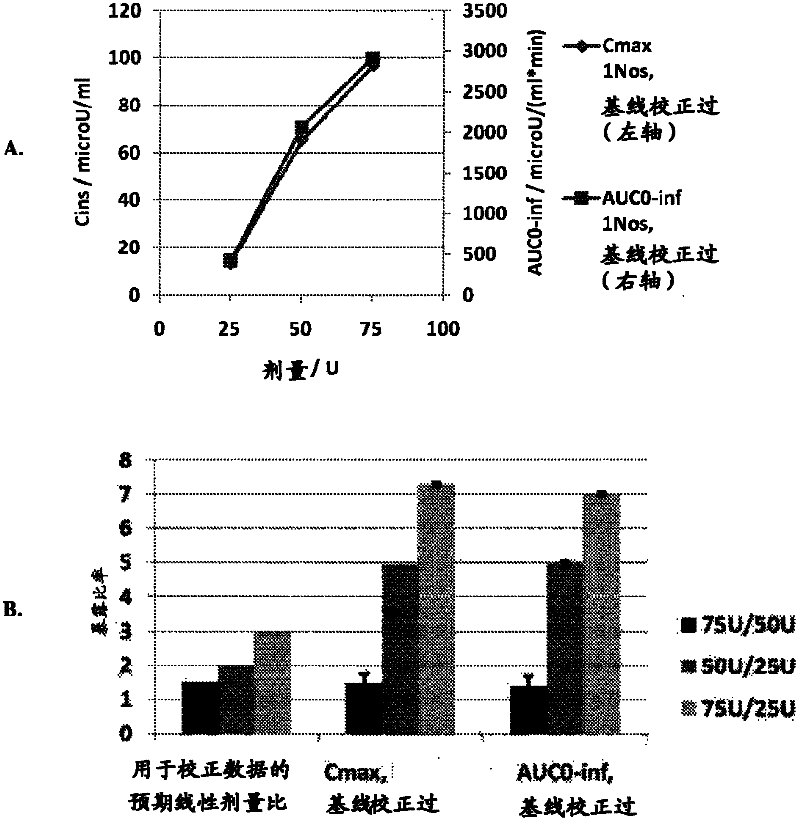
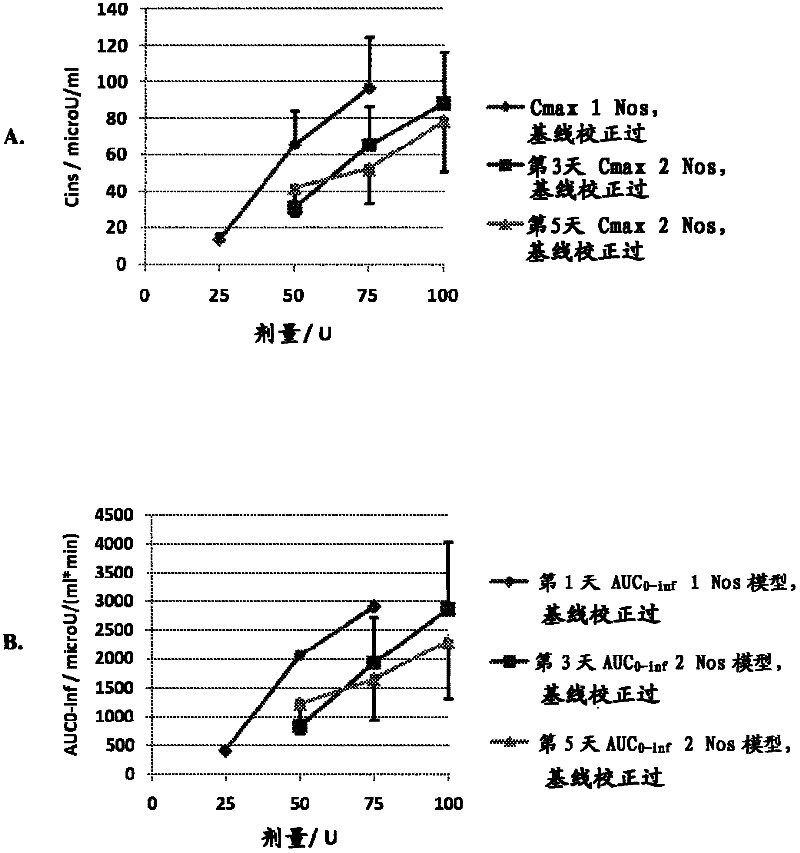
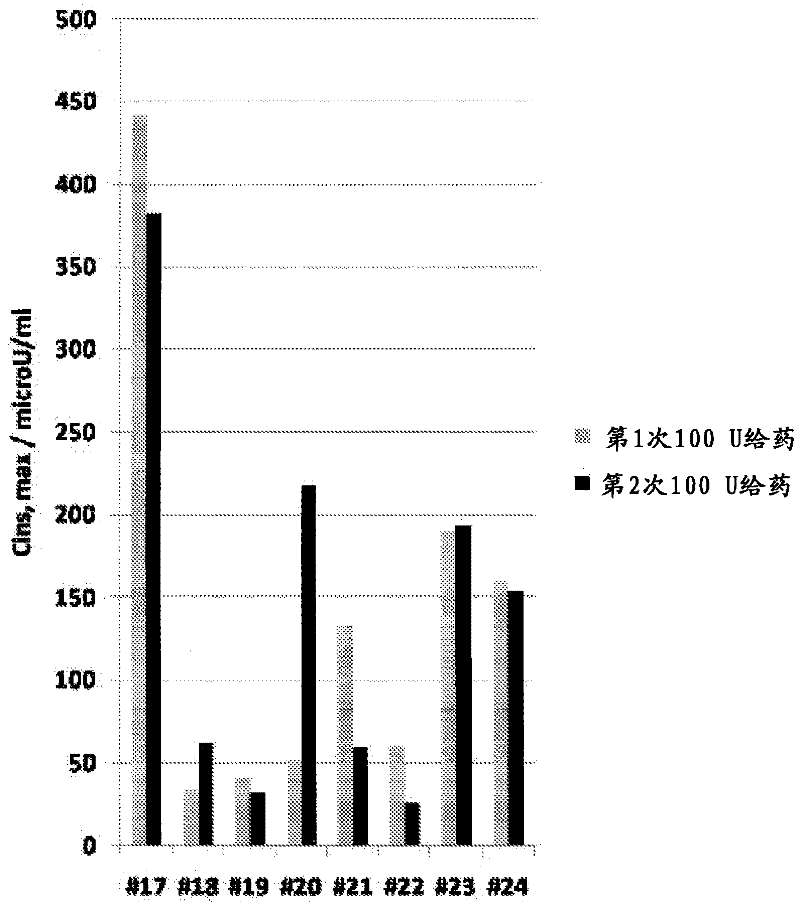
The present invention provides a method for achieving a therapeutically effective plasma levels of insulin by admmisteppg at least two doses of pharmaceutical formulation of insulin sequentially into the same nostril The administration of the second dose in the same nostril gives substantially higher plasma levels of insulin when compared with sequential administration in two different nostpls Without being limited to any specific physiological mechanism, it is believed that the first dose of insulin acts as a loading dose This loading dose is required to achieve the subsequent plasma levels of insulin that are observed with subsequent doses The Cmax of plasma insulin achieved by the methods and formulations of the present invention is at least about 7Q microU / ml when plasma insulin is measured from about 0 to about 45 minutes after administration of a second dose The AUC achieved is at least about 1800 microU / (ml* mm).
Owner:CPEX PHARMACEUTICALS INC
Diagnosis of hyperinsulinemia and type ii diabetes and protection against same based on proteins differentially expressed in serum
InactiveUS20100028326A1Sufficient productionMaintain biological activityOrganic active ingredientsPeptide/protein ingredientsAntagonistIncreased Insulin Secretion
Mouse proteins differentially expressed in serum, in comparisons of normal vs. hyperinsulinemic, hyperinsulinemic vs. type 2 diabetic, and normal vs. type 2 diabetic white adipose tissue have been identified, as have corresponding human proteins. The human molecules, or antagonists thereof, may be used for protection against hyperinsulinemia or type 2 diabetes, or their sequalae.
Owner:OHIO UNIV
Special grease base oil for functional food as well as preparation method and application of special grease base oil
PendingCN112772924AWide melting rangeImprove glucose and lipid metabolism disordersEdible oils/fats with fatty acid estersFood ingredient functionsBiotechnologyNutrition
The invention discloses special grease base oil for a functional food as well as a preparation method and application of the special grease base oil. The special grease base oil is formed by performing ternary ester exchange on medium-carbon-chain glyceride, high-melting-point fat and linolenic acid grease, and performing ternary ester exchange on the medium-carbon-chain glyceride, the high-melting-point fat and the linolenic acid grease under the conditions of temperature and stirring intensity by taking lipase as a catalyst, so as to obtain the special grease base oil for the functional food in one step. The special grease base oil for for the functional food is wide in melting range, can remarkably improve in-vivo glucose and lipid metabolism disorder, balance and supplement in-vivo necessary and functional fatty acids and quickly supplement energy, can meet the requirements of consumers, particularly, dietary and nutritional requirements of patients with metabolic syndromes such as overweight, obesity, fatty liver, hyperlipidemia, hyperglycemia, hypertension, hyperblood viscosity, hyperuricemia and hyperinsulinemia and athletes can be met, and the special grease base oil can be widely applied to grease powder, margarine and sports nutritional foods.
Owner:NANCHANG UNIV
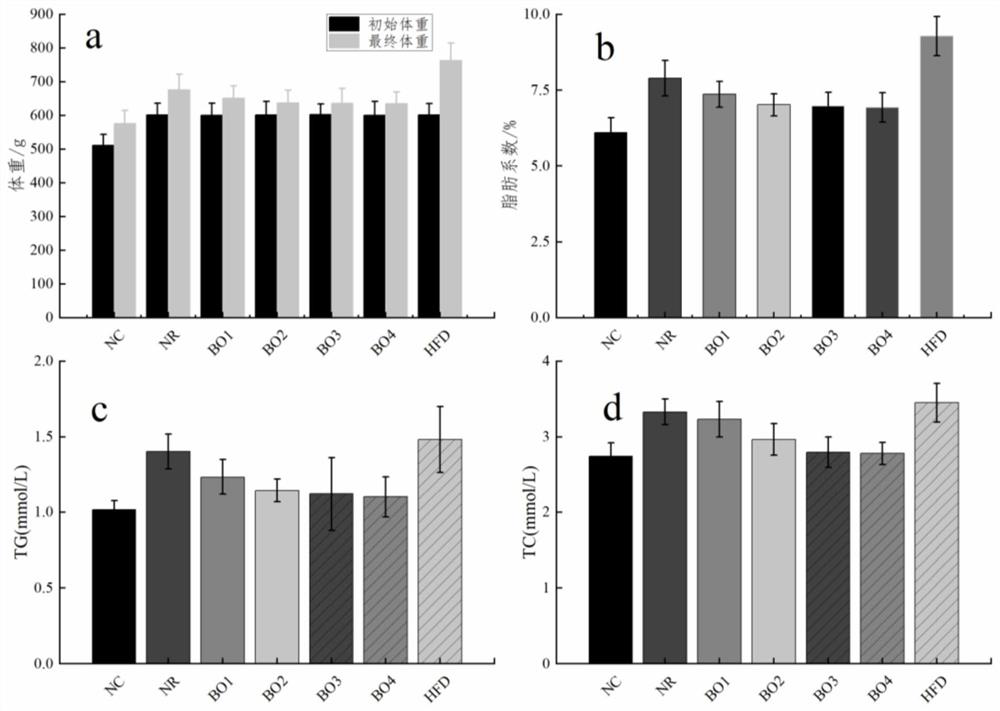
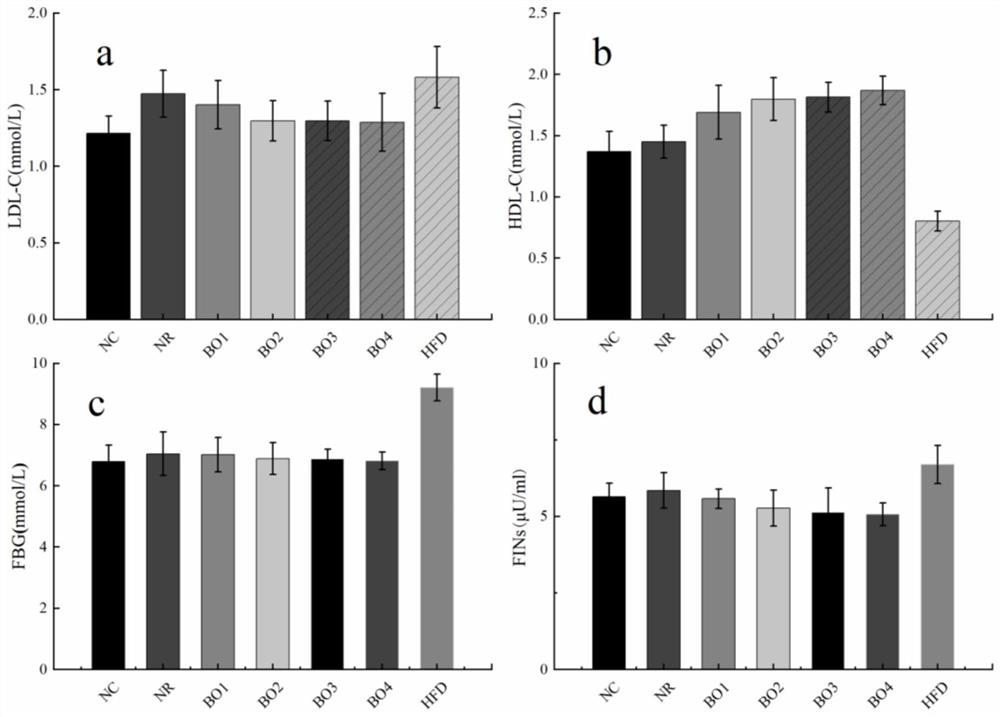
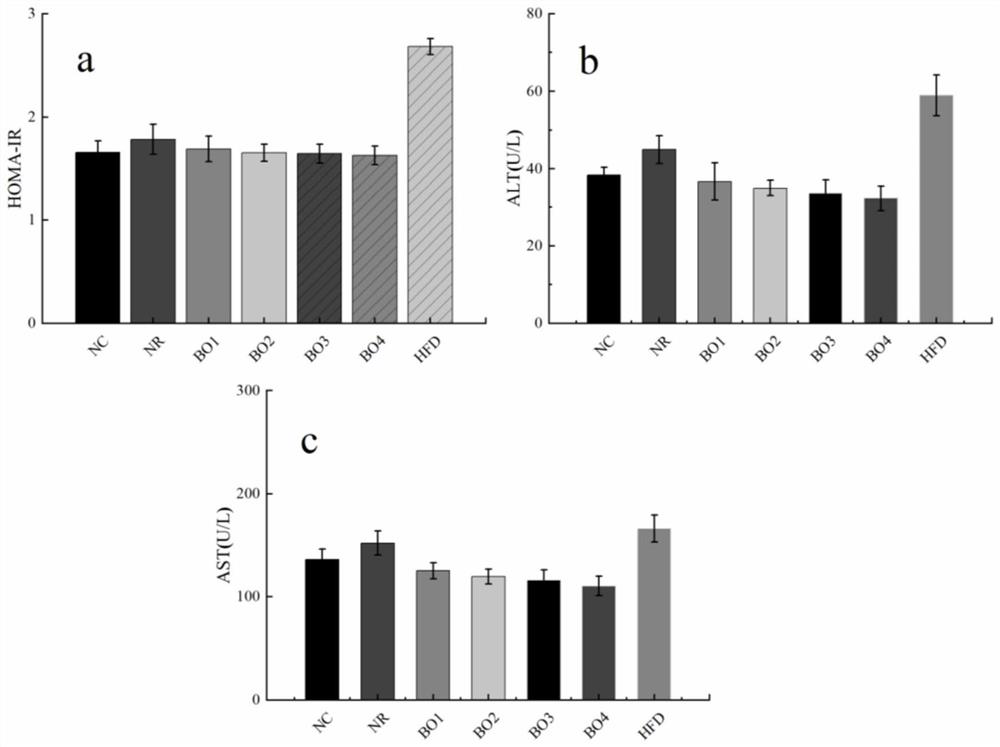
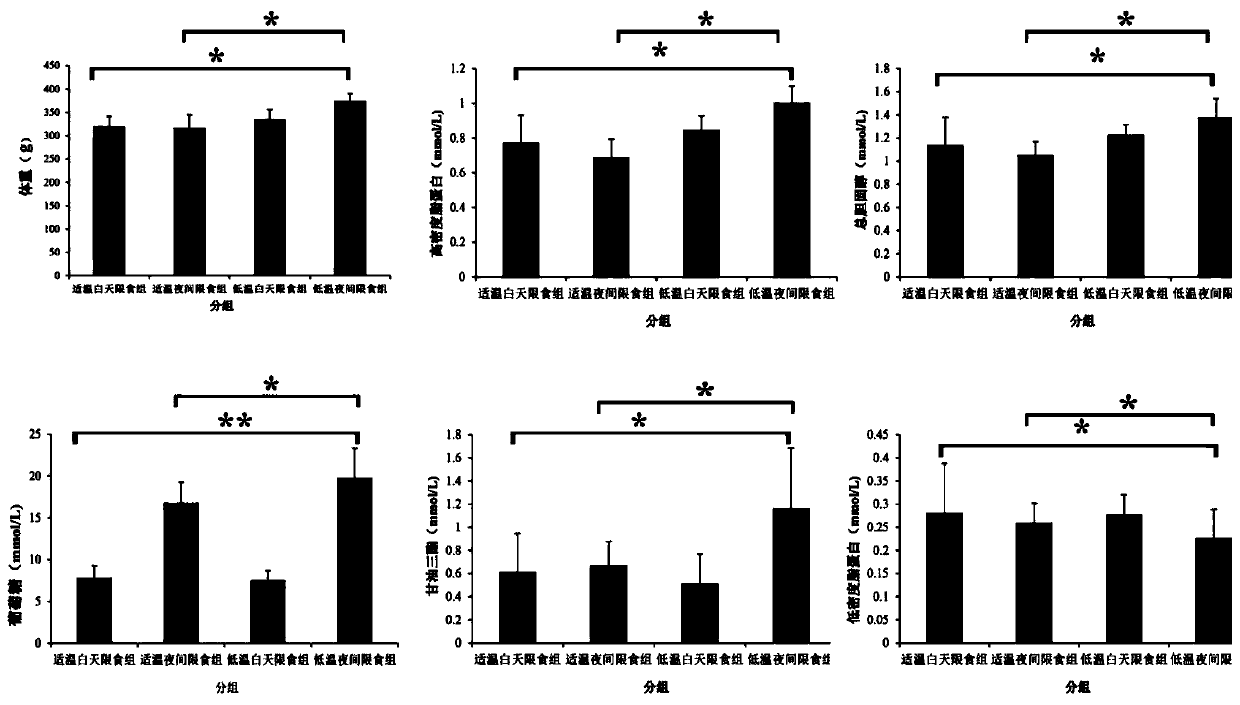
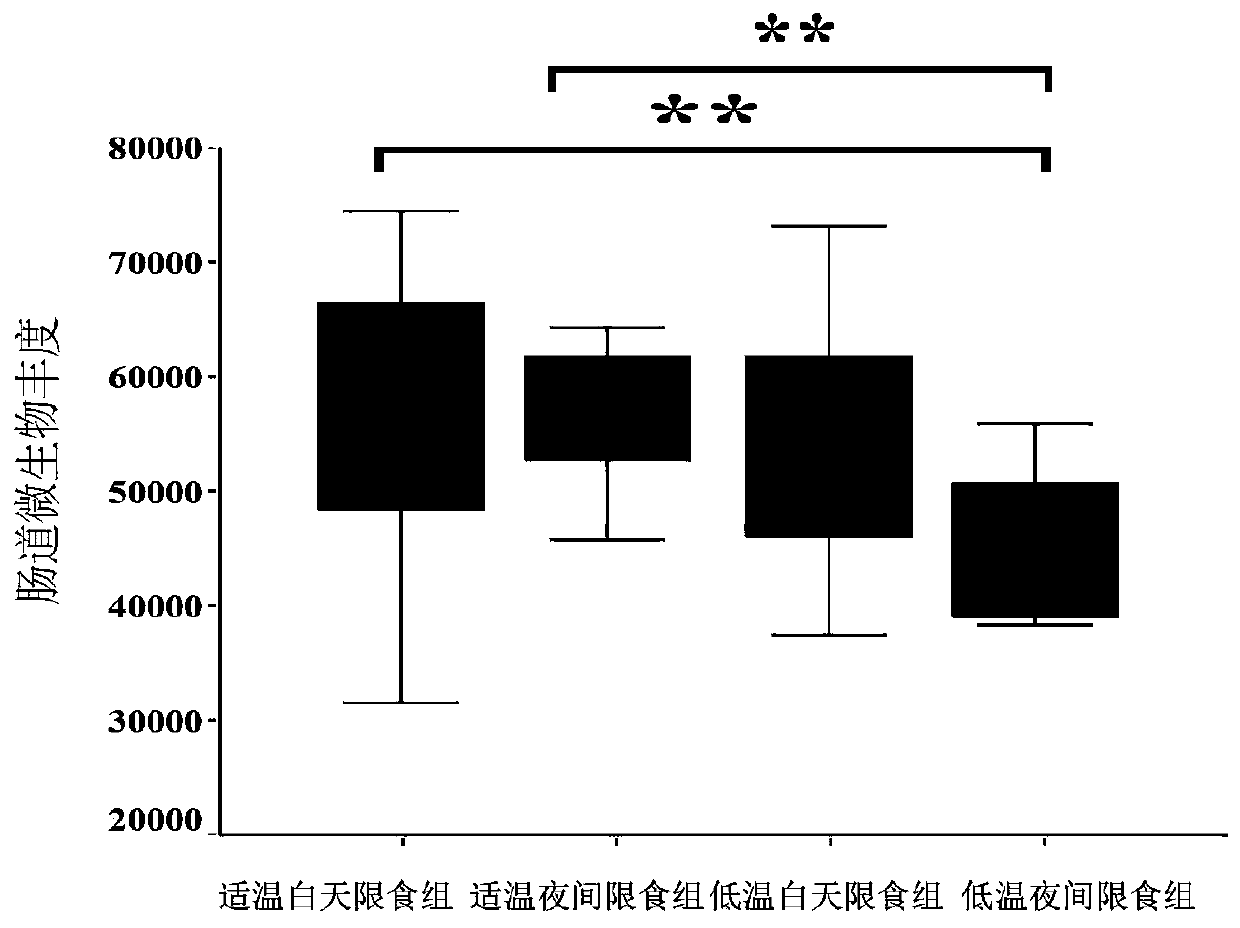
Method for inducing type 2 diabetes animal model through low-temperature and dietary rhythm regulation and control
ActiveCN111557273AReduce abundanceConsistent with early symptomsCompounds screening/testingAnimal husbandryPhysiologyIntestinal microorganisms
The invention discloses a method for inducing type 2 diabetes animal model through low-temperature and dietary rhythm regulation and control. By combining the regulation and control of a low-temperature environment with the change of the dietary rhythm of a feeding feed or independently through the change of a dietary rhythm of the feeding feed, or through only the change of the dietary rhythm ofthe feeding feed, the animal is induced to form type 2 diabetes animal model with weight gain, hyperlipidemia, hyperglycemia, hyperinsulinemia and insulin resistance as main markers. Experimental research results show that SD rats can be induced to form main markers of type 2 diabetes such as weight gain, hyperlipidemia, hyperglycemia, hyperinsulinemia and insulin resistance by low-temperature environment and night time-limited diet, the diversity of intestinal microorganisms can be remarkably reduced, the morbidity mode is close to that of human type 2 diabetes, and the constructed diabetes animal model can be used for studying a morbidity mechanism of type 2 diabetes, searching related targets of early diagnosis, and researching and developing treatment drugs and prevention and treatmentmethods.
Owner:贵州中医药大学
Novel fatty acids and their use in conjugation to biomolecules
Owner:NOVARTIS AG
Treatment of metabolic disorders in feline animals
ActiveUS10617666B2Reduce doseReduce frequencyNervous disorderSaccharide with carbocyclic radicalsDyslipidemiaPhysiology
The present invention relates to one or more SGLT2 inhibitors or pharmaceutically acceptable forms thereof for use in the treatment and / or prevention of a metabolic disorder in a feline animal, preferably wherein the metabolic disorder is one or more selected from the group consisting of: ketoacidosis, pre-diabetes, diabetes mellitus type 1 or type 2, insulin resistance, obesity, hyperglycemia, impaired glucose tolerance, hyperinsulinemia, dyslipidemia, dysadipokinemia, subclinical inflammation, systemic inflammation, low grade systemic inflammation, hepatic lipidosis, atherosclerosis, inflammation of the pancreas, neuropathy and / or Syndrome X (metabolic syndrome) and / or loss of pancreatic beta cell function and / or wherein the remission of the metabolic disorder, preferably diabetic remission, is achieved and / or maintained.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
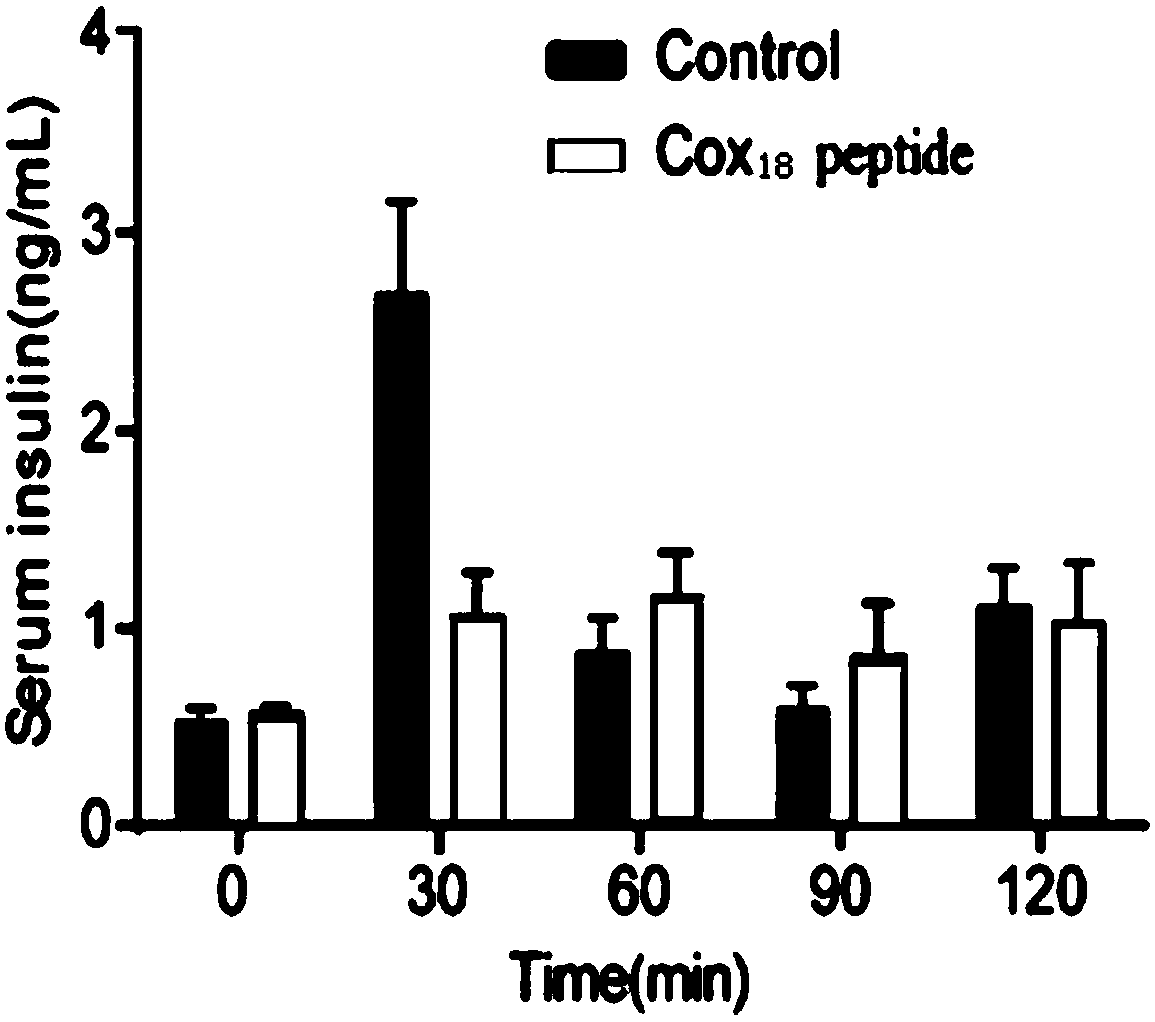
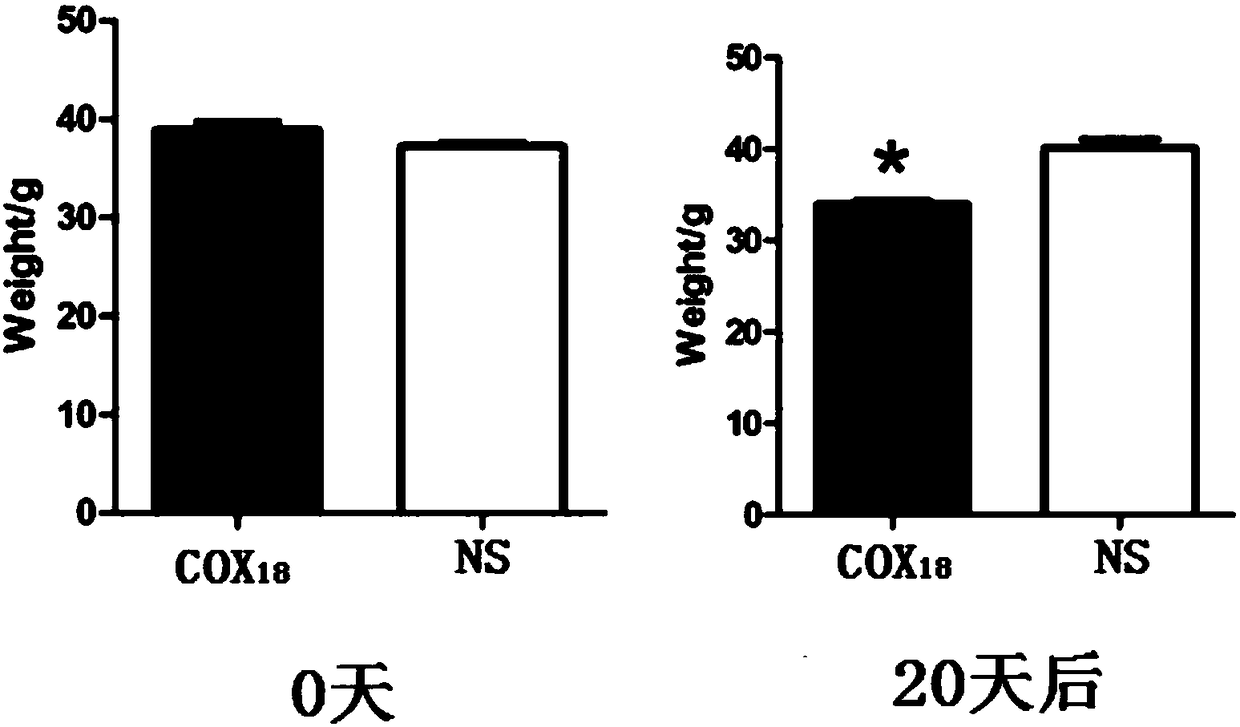
COX18 polypeptide with biological activity as well as synthesis method thereof and application thereof
InactiveCN108315308ASimplify biochemical tedious proceduresLow costPeptide/protein ingredientsMetabolism disorderChemical synthesisSide effect
The invention provides a COX18 polypeptide with biological activity. An amino acid sequence of the polypeptide is as shown in SEQ ID NO. 1: LLPAGWILSH LETYRRPE. The invention further provides a methodfor synthesizing the COX18 polypeptide through a chemical synthesis method, namely an Fmoc method as well as application, and the polypeptide is applicationd for preparing drugs which inhibit sugar induced insulin secretion, resist insulin tolerance, resist hyperinsulinemia and reduce body weight. The invention finds a novel COX18 polypeptide which is 52-69th part of human cytochrome c oxidase 8A, which is different from the precious porcine sequence. Compared with the previous sequence, the sequence has 38.88% of different amino acid. Moreover, the sequence is humanized, and has better homology, fewer immunoreactions and side effects while being applied to a human body.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Treatment of metabolic disorders in canine animals
ActiveUS20200179328A1Reduce doseReduce frequencyOrganic active ingredientsPowder deliveryPhysiologyPancreatic hormone
The present invention relates to one or more SGLT2 inhibitors or pharmaceutically acceptable forms thereof for use in the treatment and / or prevention of a metabolic disorder in a canine animal, preferably wherein the metabolic disorder is one or more selected from the group consisting of: ketoacidosis, pre-diabetes, insulin dependent diabetes mellitus, insulin resistance diabetes, insulin resistance, obesity, hyperglycemia, hyperglycemia induced cataract formation, impaired glucose tolerance, hyperinsulinemia, dyslipidemia, dysadipokinemia, subclinical inflammation, systemic inflammation, low grade systemic inflammation, hepatic lipidosis, inflammation of the pancreas, metabolic disorder consequences, such as hypertension, renal dysfunction and / or muscoskeletal disorders, and / or Syndrome X (metabolic syndrome), wherein preferably the development of hyperglycemia induced cataract formation is prevented or remission is achieved and / or wherein preferably the development of metabolic disorder consequences, such as hypertension, renal dysfunction and / or muscoskeletal disorders, is prevented or progression is slowed or remission is achieved.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Cell culture medium, and preparation method and application thereof
ActiveCN104928231AExcellent self-renewal abilityAvoid immune responseVertebrate cellsArtificial cell constructsNutrientCulture cell
The invention discloses a cell culture medium, and a preparation method and application thereof. The cell culture medium contains a DMEM, an F12 culture medium, sodium selenate, sodium bicarbonate, vitamin C, insulin, a vascular endothelial growth factor and a transforming growth factor beta signaling pathway inhibitor. The cell culture medium does not contain animal-derived ingredients, and can provide adequate nutrients and stable living environment for culture cells; in addition, the cell culture medium can effectively induce and differentiate mesendoderm progenitor cells into progenitor cells of vascular endothelial cells.
Owner:宁波医诺生物技术有限公司
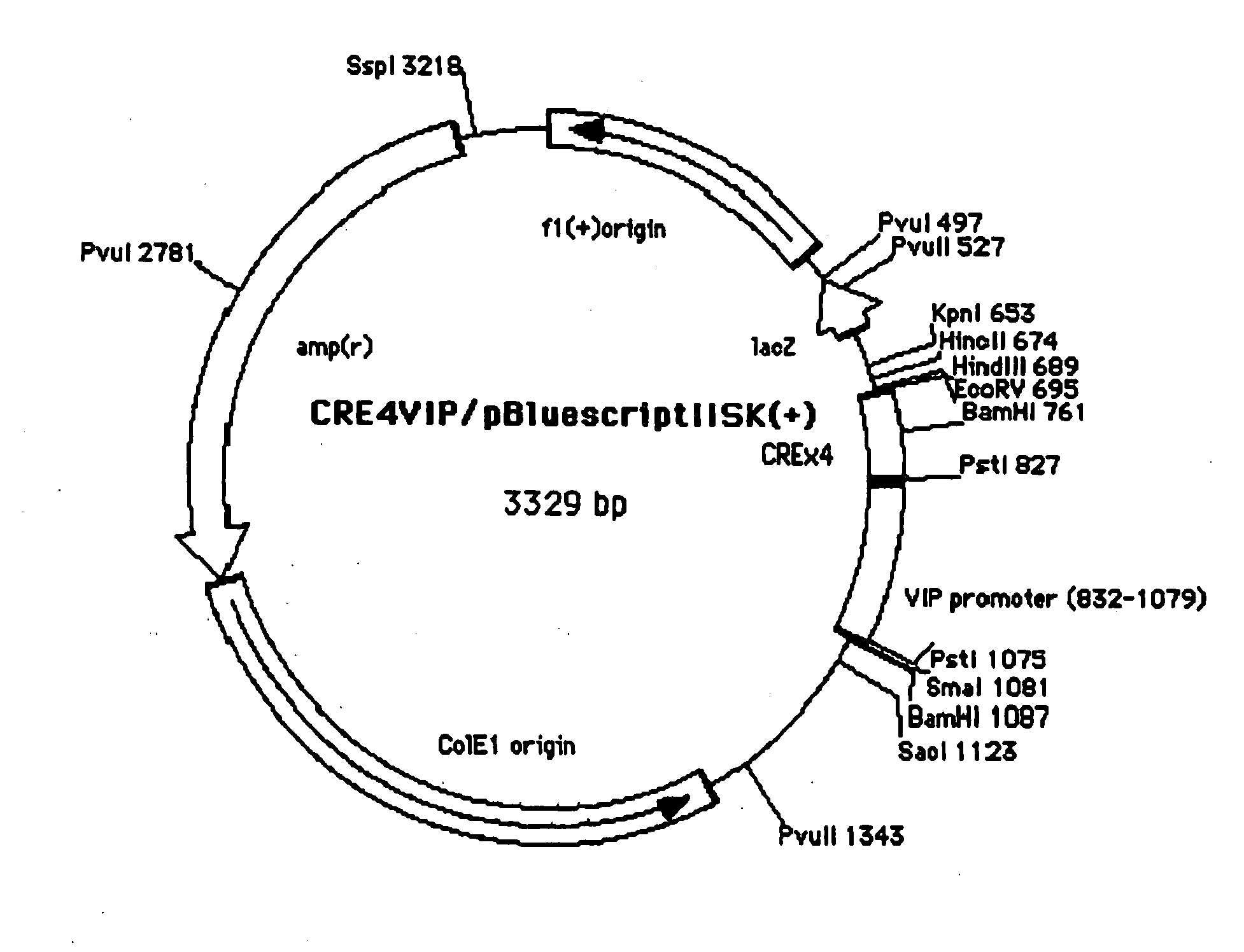
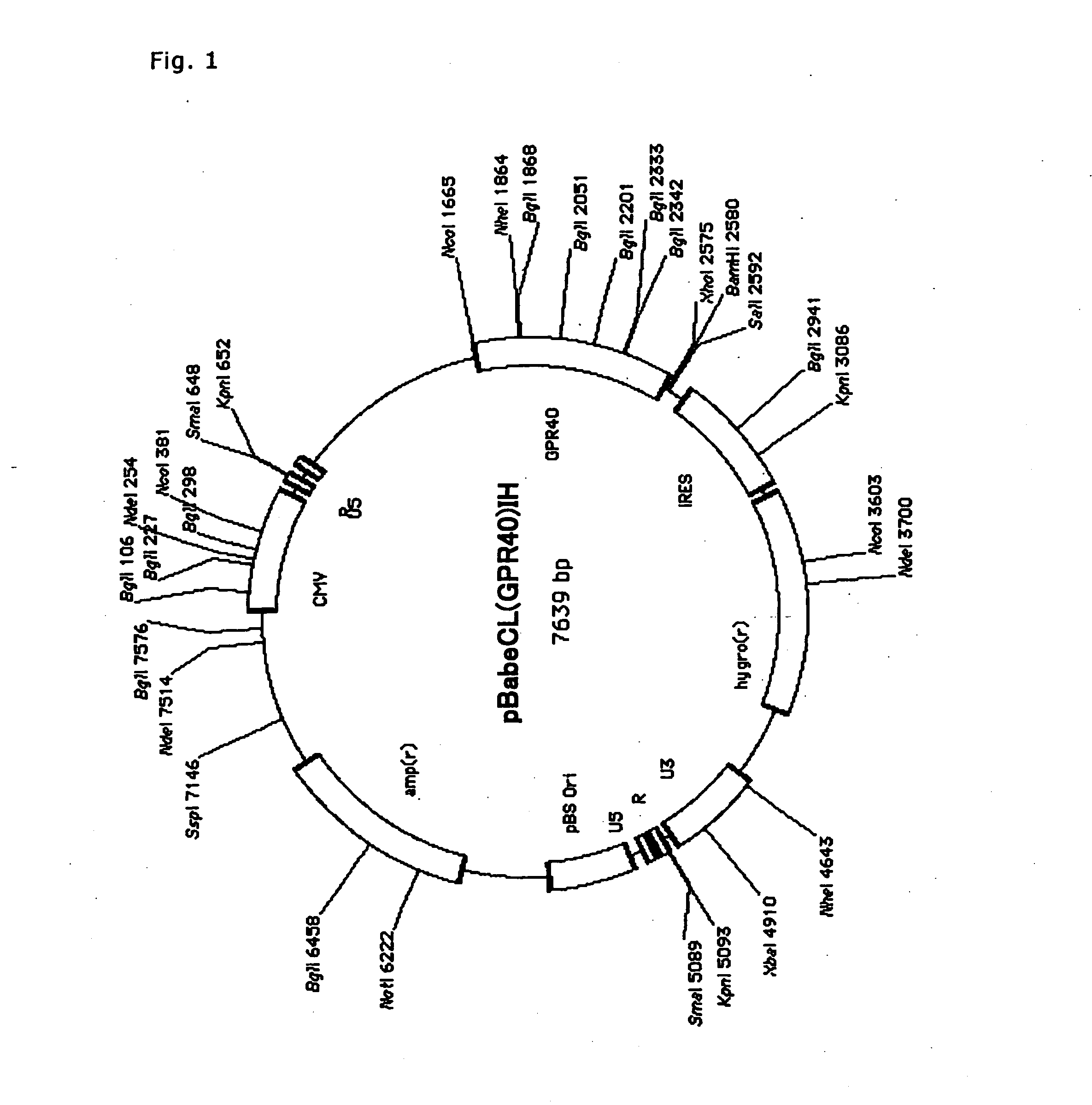
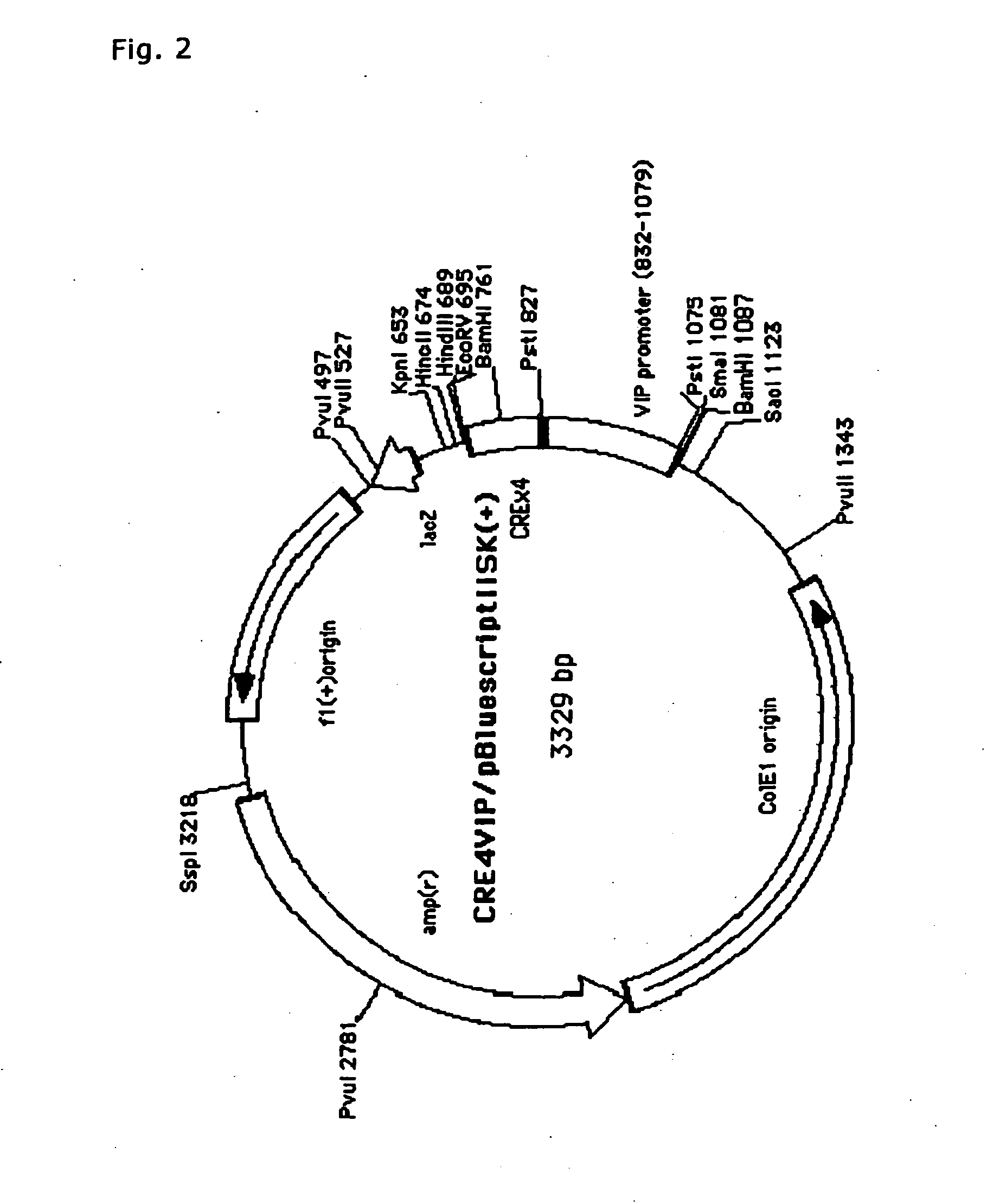
Method of Screening Substance Useful in Treating Disease With the Use of GPR40 and Phospholipase
The present invention relates to a screening method for determining whether a substance of interest is a substance which alters GPR40-mediated cell stimulating activities, comprising using a substance of interest, a biomembrane containing GPR40, or cells containing said biomembrane, and phospholipase or salts thereof. According to the present invention, substances involved in insulin secretion can be screened. In addition, according to the present invention, substance useful for the prevention or treatment of diabetes, diabetic complications and degenerative diseases, hyperglycemia, polyuria, ketonemia, acidosis, insulin resistance, impaired glucose tolerance, neurodegenerative diseases, insulinoma, cancers, hyperinsulinemia, hyperglyceridemia, fatty liver, hypoglycemia due to insulin hypersecretion, arteriosclerosis, hyperlipidemia, cerebral stroke, obesity, various diseases induced by diabetes or obesity, and the like.
Owner:EISIA R&D MANAGEMENT CO LTD
Human umbilical cord mesenchymal stem cell culture medium, human umbilical cord mesenchymal stem cell injection, preparation method and application of human umbilical cord mesenchymal stem cell injection in preparation of medicine for treating cerebral apoplexy
PendingCN114164171APromote proliferationImprove proliferative abilityCulture processPharmaceutical delivery mechanismVitamin CPancreatic hormone
The invention provides a human umbilical cord mesenchymal stem cell culture medium, a human umbilical cord mesenchymal stem cell injection, a preparation method and application of the human umbilical cord mesenchymal stem cell culture medium and the human umbilical cord mesenchymal stem cell injection in preparation of drugs for treating cerebral apoplexy. The human umbilical cord mesenchymal stem cell culture medium comprises a basic culture medium, vitamin C, insulin, platelet-derived growth factors, epidermal growth factors, basic fibroblast growth factors, beta-mercaptoethanol, SAG, folic acid, hypoxia inducible factor-1, protoporphyrin cobalt and simvastatin. The SAG, the hypoxia inducible factor-1, the protoporphyrin cobalt and the simvastatin are creatively combined to be used for preparing the human umbilical cord mesenchymal stem cell culture medium, and the four components are matched with one another, so that the human umbilical cord mesenchymal stem cell culture medium has a remarkable synergistic interaction effect in the aspect of promoting proliferation of umbilical cord mesenchymal stem cells. The human umbilical cord mesenchymal stem cells obtained through culture are high in multiplication capacity, the prepared human umbilical cord mesenchymal stem cell injection can be directly used for treating cerebral apoplexy after resuscitation, and the human umbilical cord mesenchymal stem cell injection has important application value.
Owner:SHANGHAI AISAER BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com