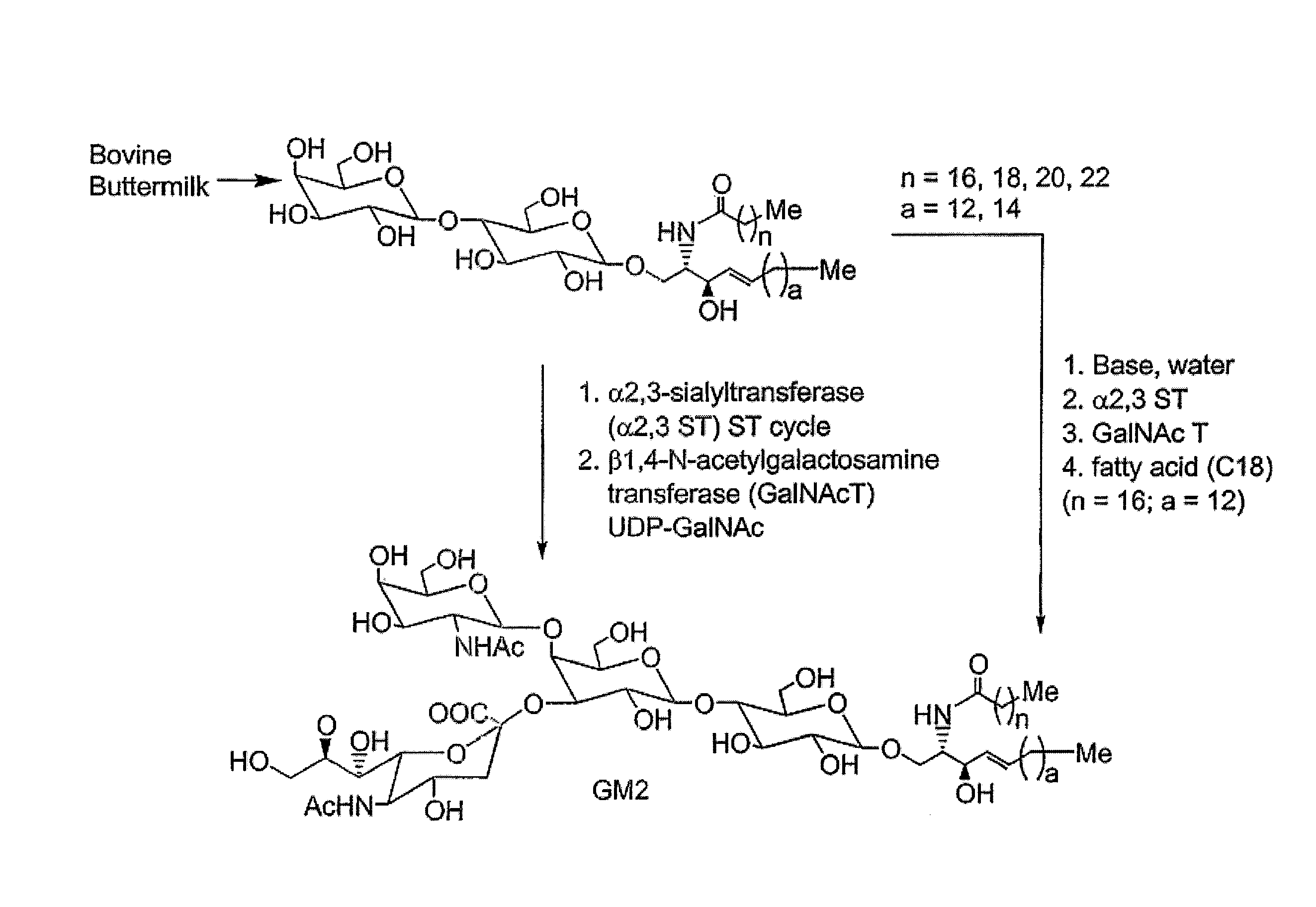
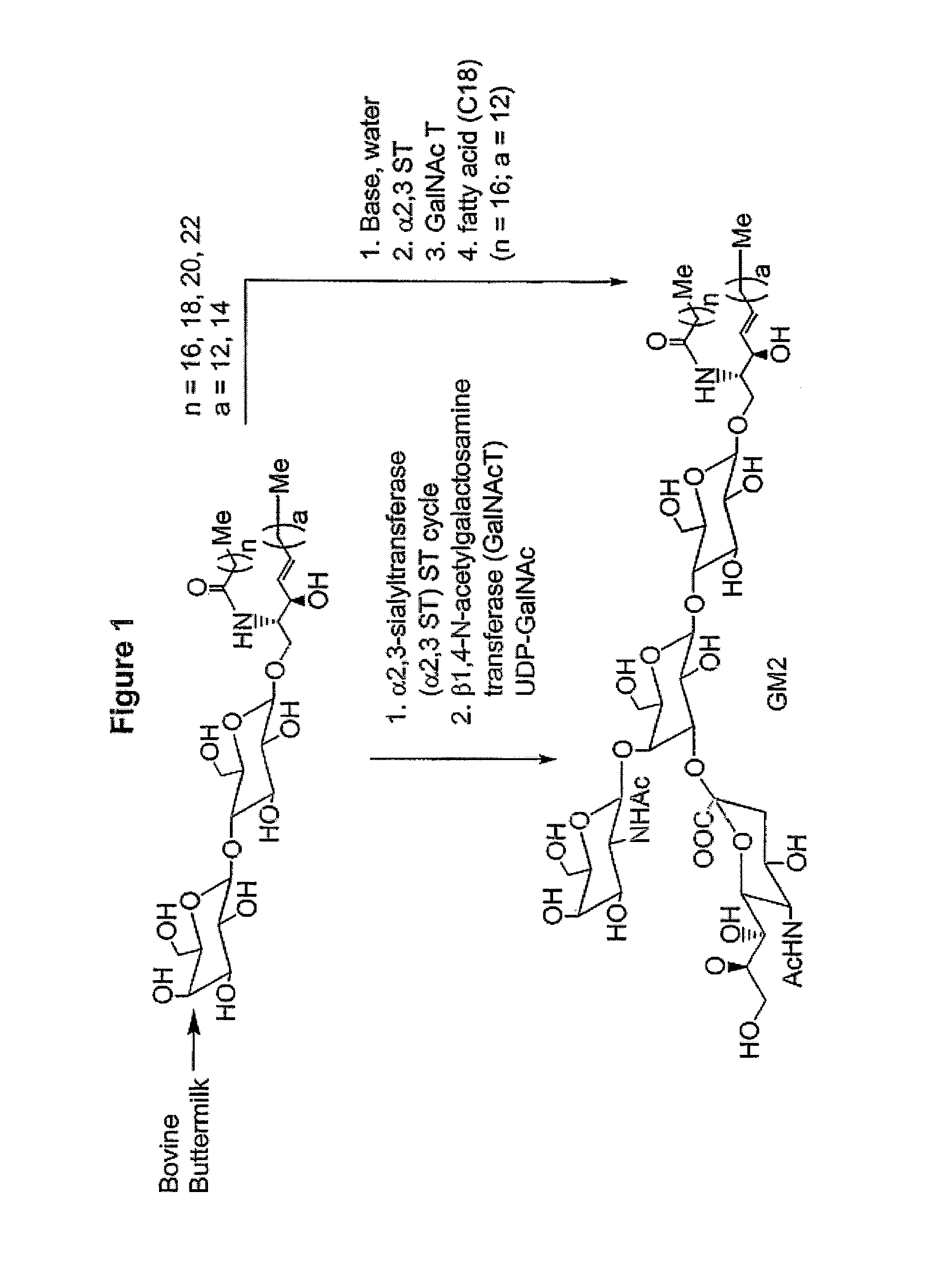
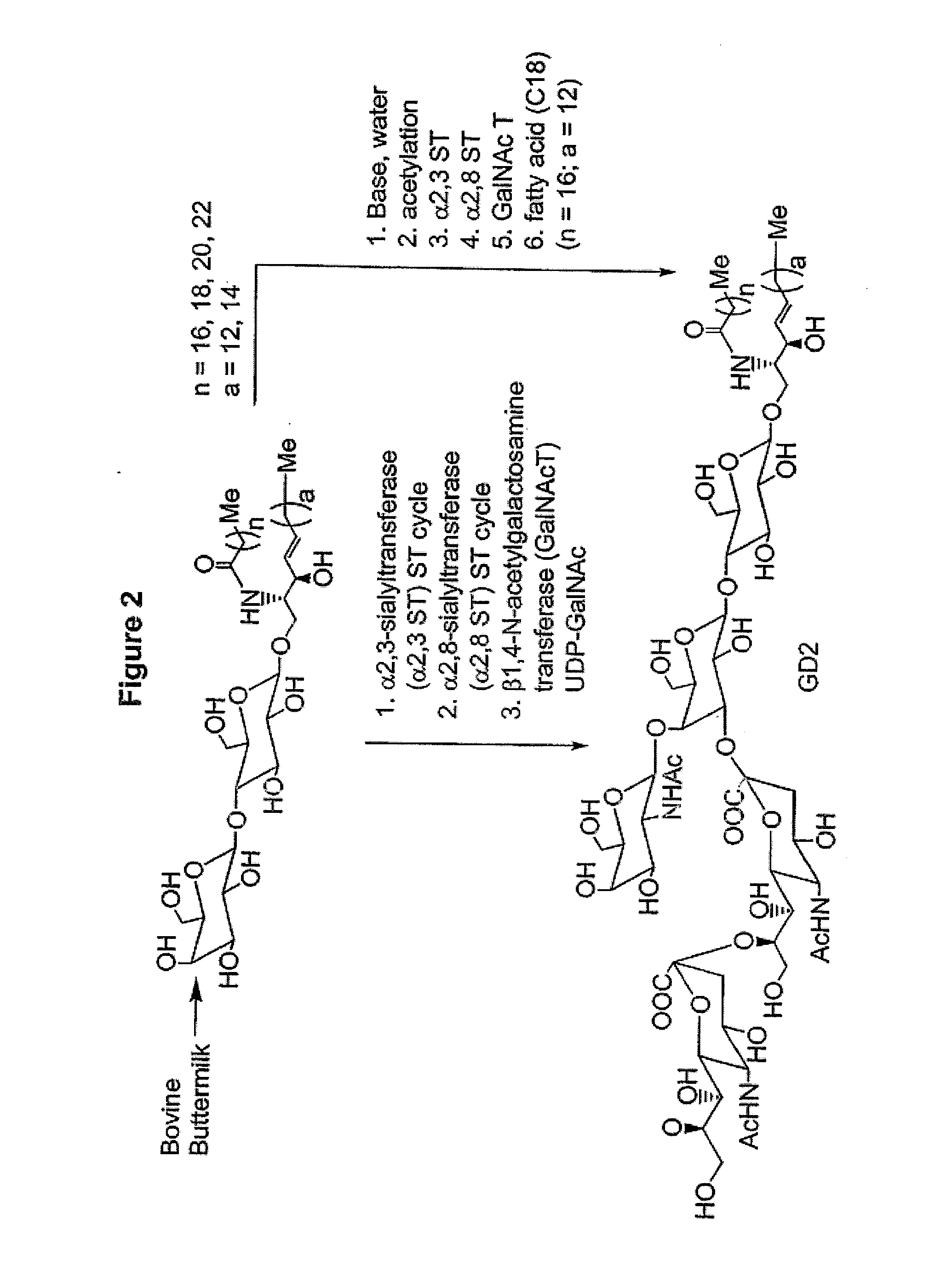
Glycolipids as treatment for disease
a technology of glycolipids and lipids, applied in the field of glycolipids as treatment for disease, can solve the problems of limiting radiation treatment side effects, brain damage or death, and the remission obtained through chemotherapy is often not durable, and achieves the effect of improving the potency of antagonism of the insulin receptor and affinity for the insulin receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of [5-N-acetyl-α-neuraminyl)-(2→3)]-[β-D-galactopyranosyl-(1→4)-O-D-glucopyranose] by Fermentation
[0125]The synthesis of this compound uses the process described by Samain (Samain and Priem, Method for Producing Oligopolysaccharides. WO 01 / 04341 (2001); Priem, Glycobiology, 12:235 (2002); Cottaz, EP Application No. 06291569.9 (2006)) with E. coli JM107-nanA-(Nst-01, pBBnsy) strain using lactose and sialic acid (Kok, J Chem Soc Perkin Trans, 1:2811-2815 (1996)) acid as exogeneous acceptors.
[0126]E. coli JM107-nanA-(Nst-01, pBBnsy) strain was grown at low cell density culture in shake flask fermentation. When the OD540 is about 1, isopropyl 1-thio-β-galactopyranoside (IPTG) and the substrates Neu5Ac and lactose (2 equivalents) were added via a sterile filter and the culture is shaken at 28° C. for 16 hr. After 16 hr, the cells were recovered by centrifugation at 8000 g and 4° C. for 10 min. The supernatant was separated for further TLC analysis while the pellet is resuspende...
example 2
Synthesis of 1-Fluoro-[(5-N-acetyl-α-neuraminyl)-(2→3)]-[β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside]
[0127]The [5-N-acetyl-α-neuraminyl)-(2→3)]-[β-D-galactopyranosyl-(1→4)-β-D-glucopyranose], and DMAP are dissolved in pyridine. Acetic anhydride was added dropwise and then the reaction mixtureis stirred for 2 days. The reaction mixture was concentrated and the residue treated with methanol. After 30 min, the reaction mixture was concetrated again, the residue dissolved in ethyl acetate and the organic solution washed with water, 5% citric acid in water, water and brine. The organic solution was dried with sodium sulfate, filtered and concetrated to dryness yielding a solid used directly for the next step.
[0128]The solid is cooled to −30° C. and then hydrogen fluoride-pyridine was added. The reaction mixture was stirred for 1 hr allowing the reaction to slowly warm to room temperature and then for 4 hrs at room temperature. The reaction mixture was cooled to −10° C. and is then slo...
example 3
Synthesis of [(5-N-acetyl-α-neuraminyl)-(2→3)]-[β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-β-(1→1′)-D-erythro-sphingosine]
[0130]The compound 1-fluoro-[(5-N-acetyl-α-neuraminyl)-(2→3)]-[β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside] and D-erythro-sphingosine are coupled using the procedure as described in Vaughan, J Am Chem Soc, 128:6300-6301 (2006). The reaction was performed in 25 mM NaOAc (pH 5.0) containing 0.2% Triton X-100. A typical reaction mixture contained approximately 10 mM 1-fluoro-[(5-N-acetyl-α-neuraminyl)-(2→3)]-[β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside], 20 mM of the acceptor D-erythro-sphingosine, and 0.5 mg / mL of the appropriate EGC mutant in a total reaction volume. When completed, the product was purified using reversed phase (C-18) chromatography, the eluted product was concentrated to dryness, dissolved in water and freeze-dried to yield a white solid. The product is analyzed by NMR and MS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com