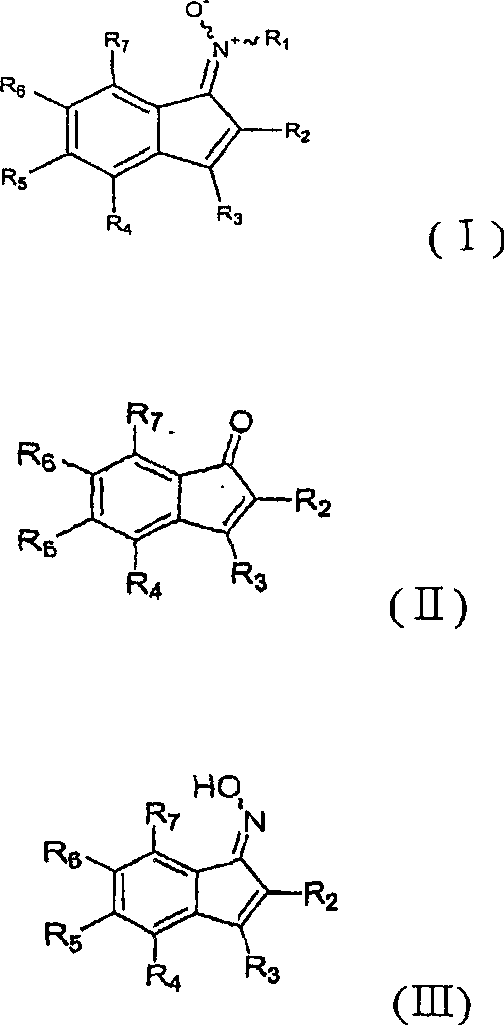
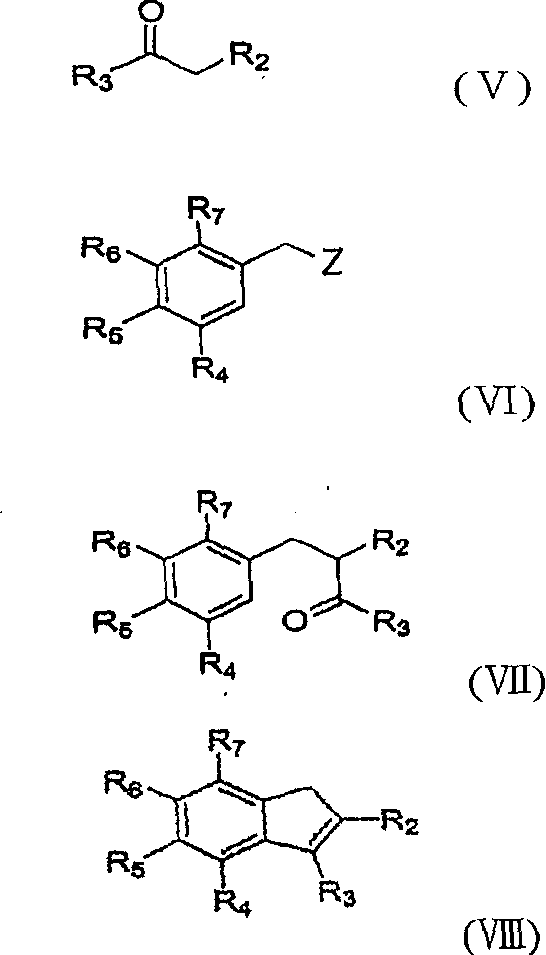
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
65 results about "Increased Insulin Secretion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
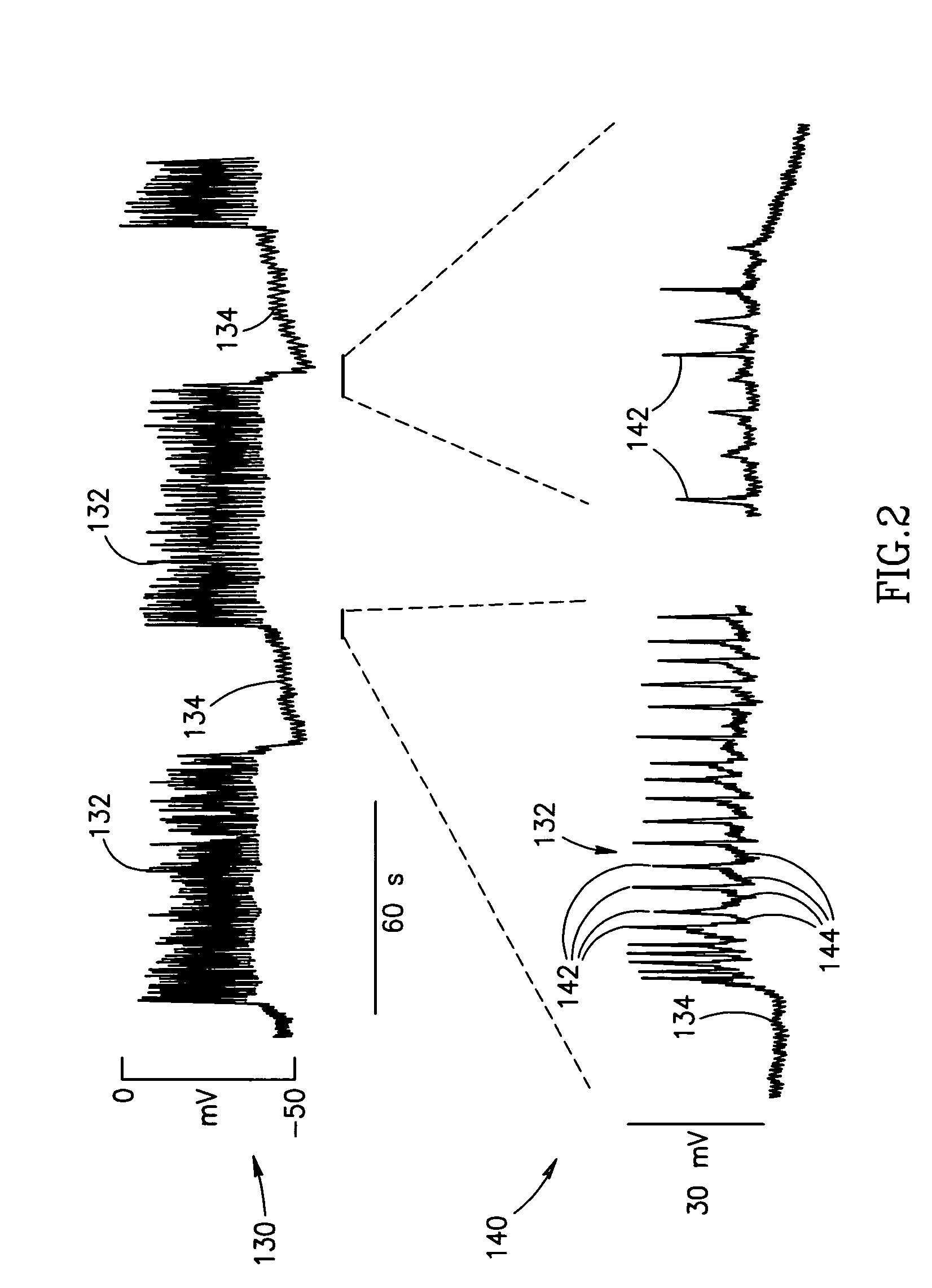
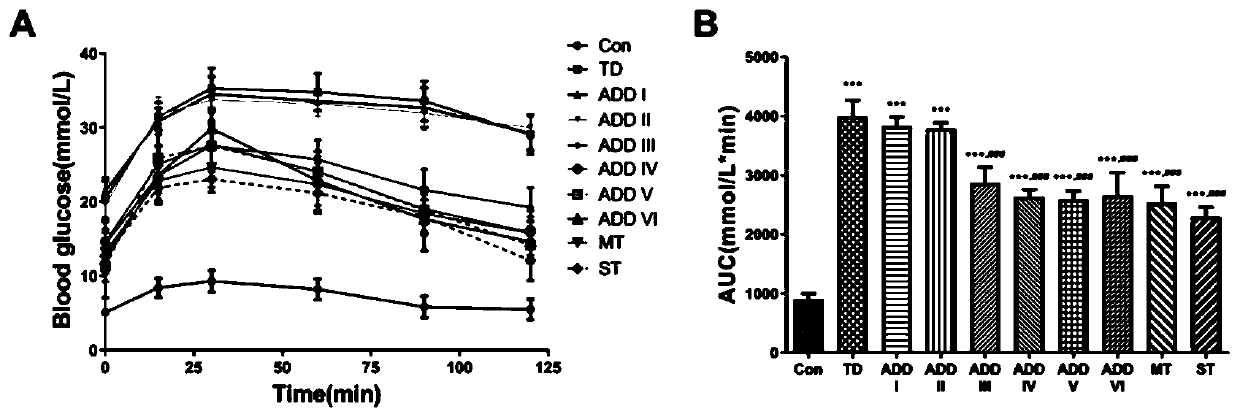
Hyperinsulinism due to diminished sensitivity, associated with diabetes risk. Although many factors influence insulin secretion, the most important control is the amount of glucose moving from the blood into the beta cells of the pancreas. In healthy people, even small rises in blood glucose result in increased insulin secretion.
Blood glucose level control
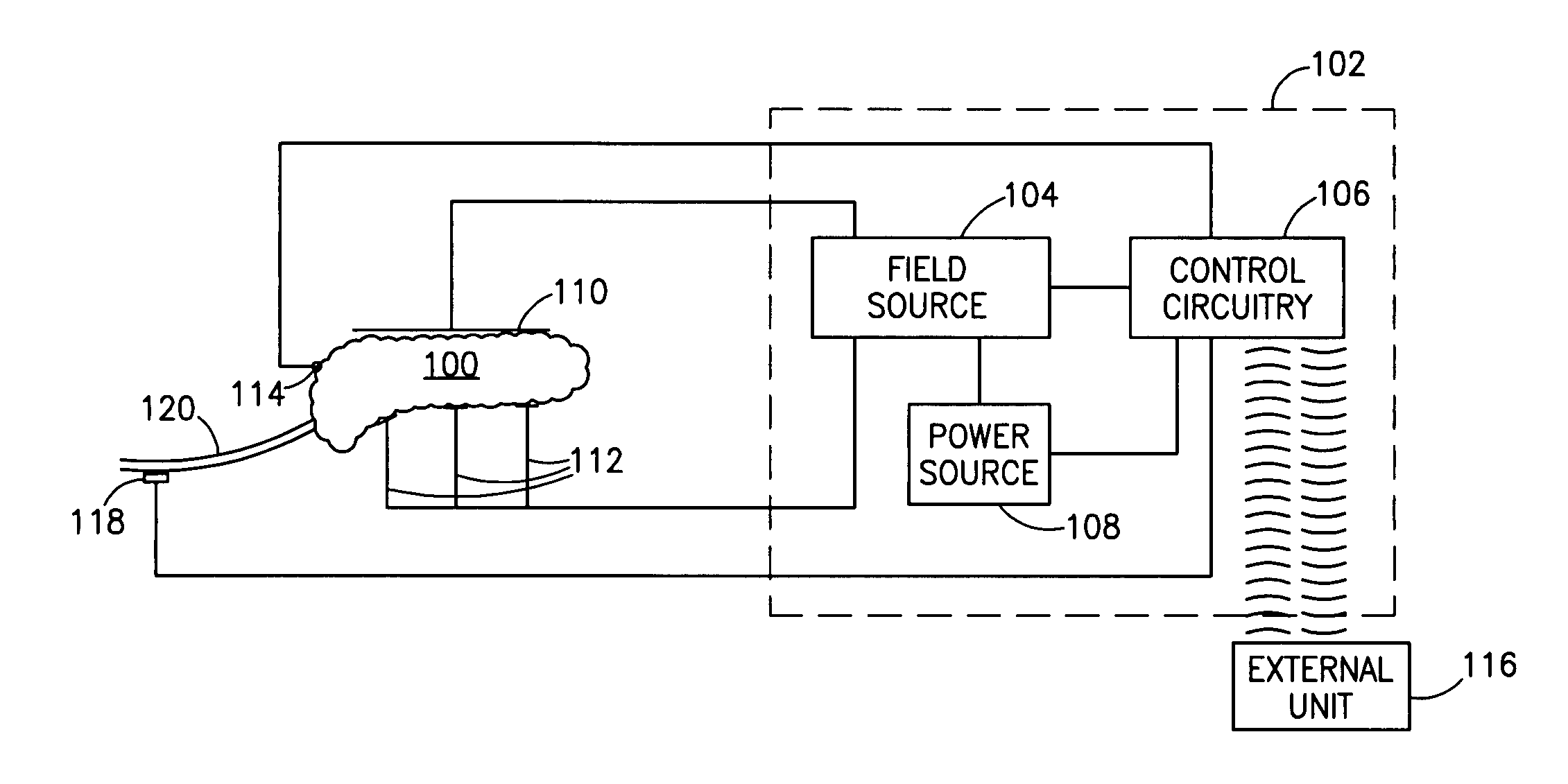
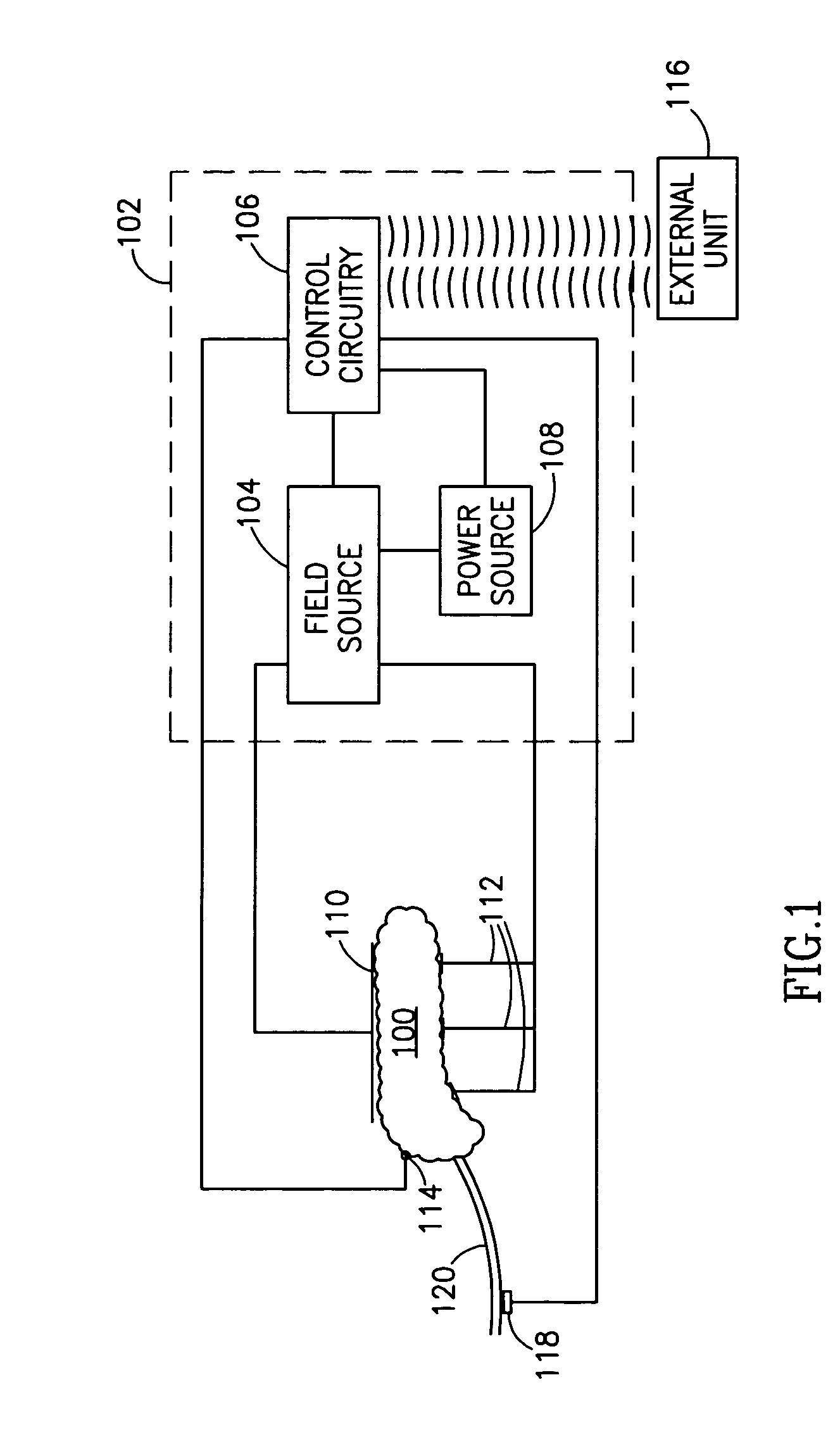
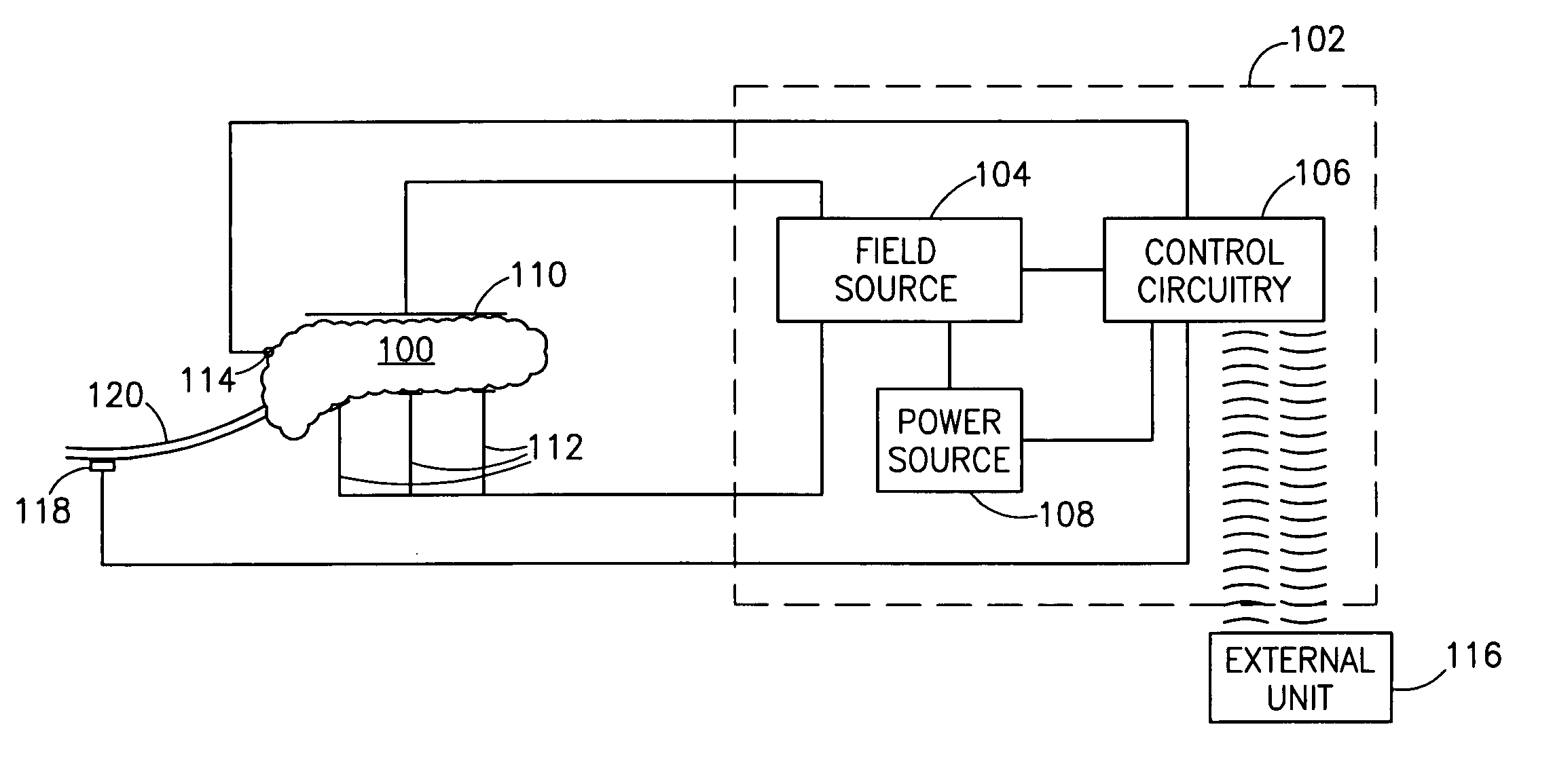
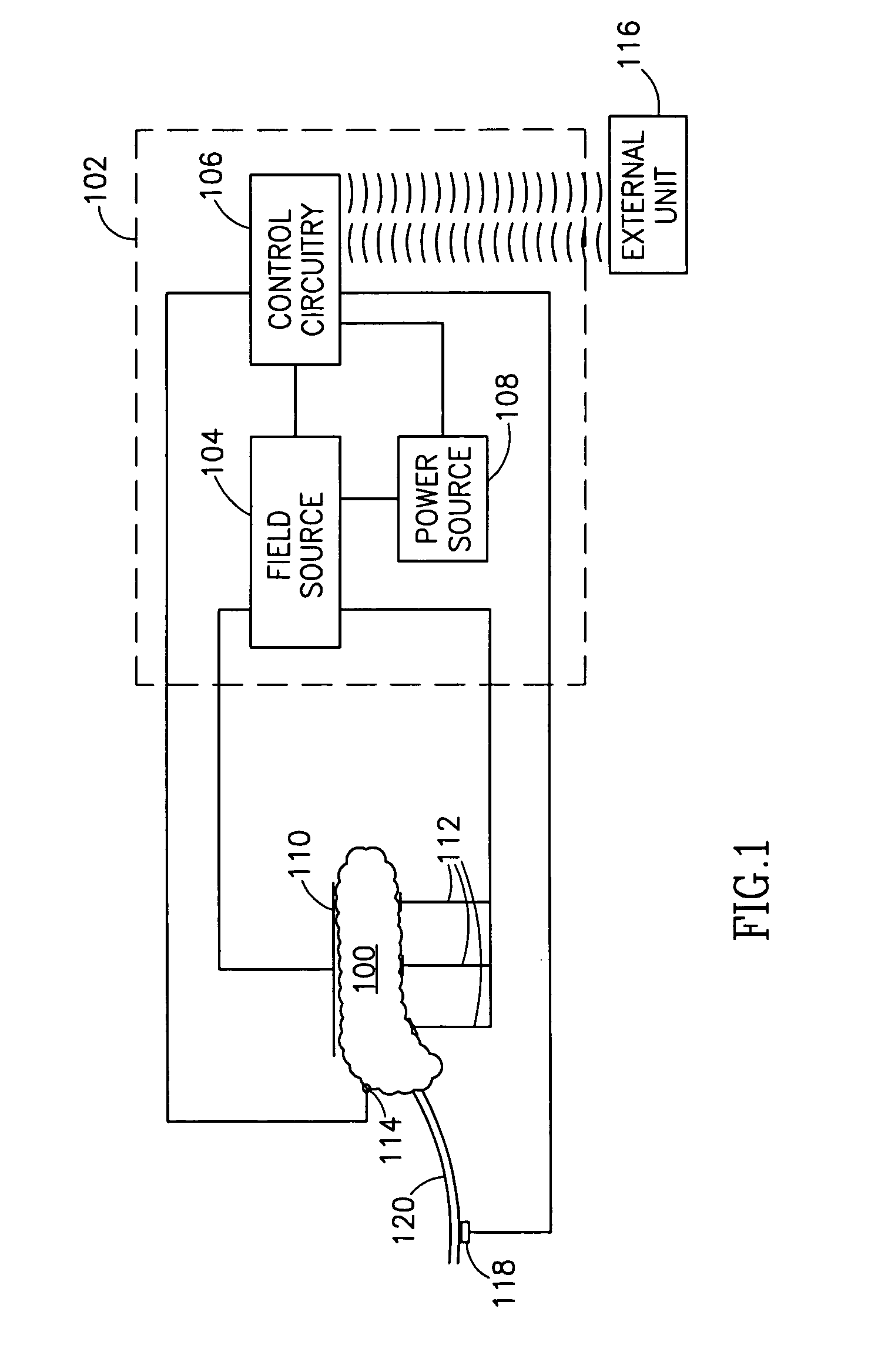
InactiveUS7006871B1Increased insulin secretionAvoiding unacceptable calcium level profileElectrotherapyDiagnostic recording/measuringGlucose sensorsLevel insulin
A pancreatic controller (102), comprising: a glucose sensor (118), for sensing a level of glucose or insulin in a body serum; at least one electrode (110, 112), for electrifying an insulin producing cell or group of cells; a power source (104) for electrifying said electrode with a pulse that does not initiate an action potential in said cell and has an effect of increasing insulin secretion; and a controller (106) which receives the sensed level and controls said power source to electrify said electrode to have a desired effect on said level.
Owner:METACURE
Blood glucose level control
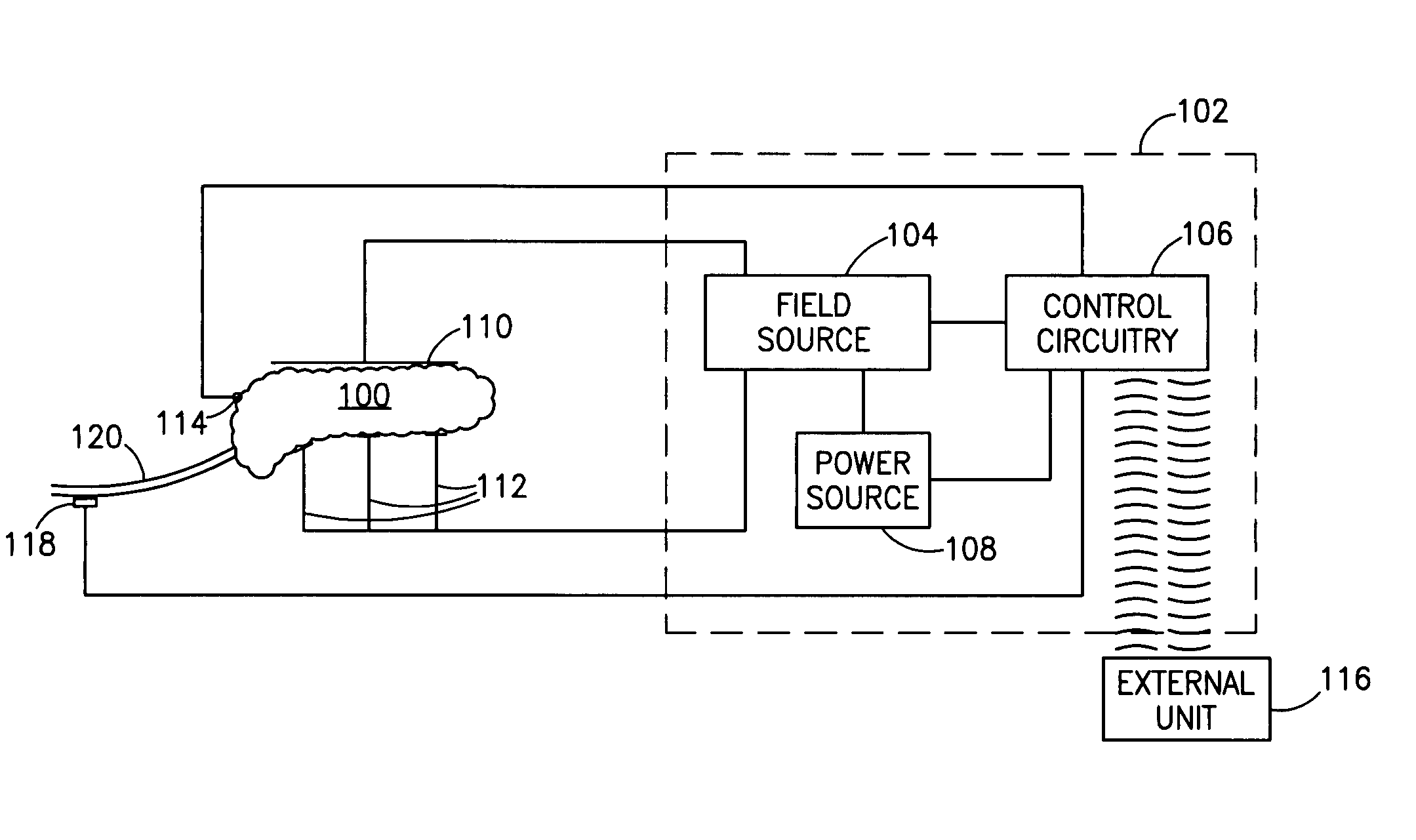
InactiveUS20060184207A1Avoiding unacceptable calcium level profileIncrease secretionElectrotherapyGlucose sensorsAction potential
A pancreatic controller, comprising: a glucose sensor, for sensing a level of glucose or insulin in a body serum; at least one electrode, for electrifying an insulin producing cell or group of cells; a power source for electrifying said electrode with a pulse that does not initiate an action potential in said cell and has an effect of increasing insulin secretion; and a controller which receives the sensed level and controls said power source to electrify said electrode to have a desired effect on said level.
Owner:TYLERTON INT INC
Compounds and methods for treating diabetes
ActiveUS20130190294A1Lower blood sugar levelsStabilize blood sugar levelsBiocideOrganic chemistryAntagonistDisease cause
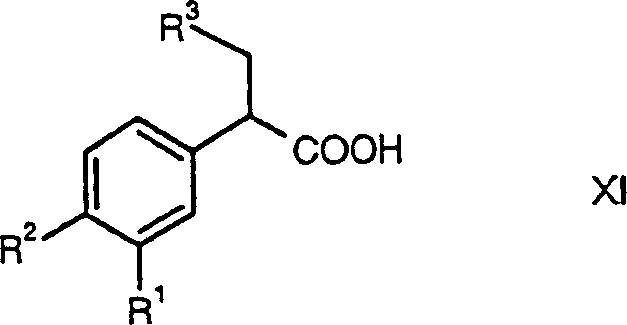
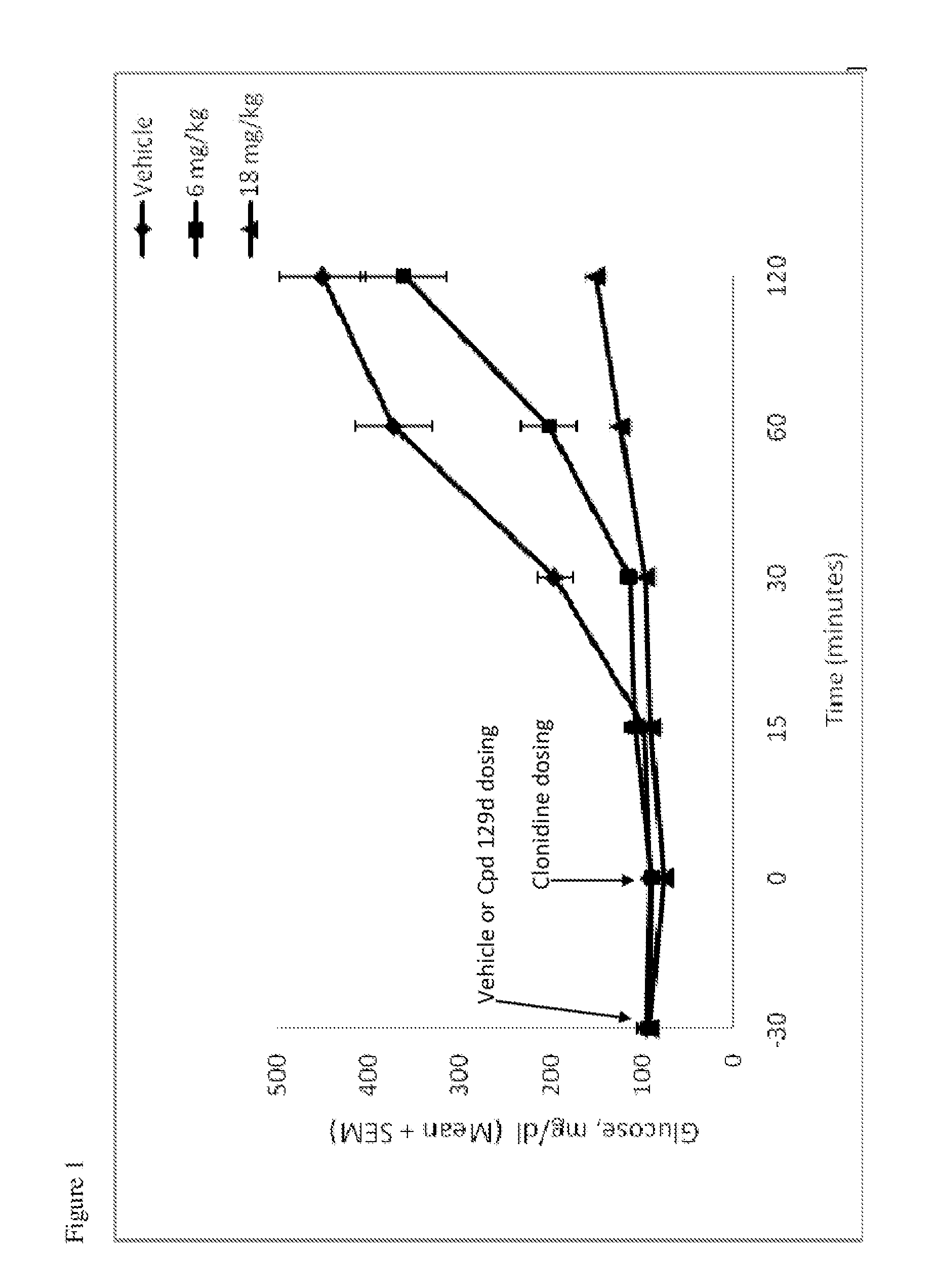
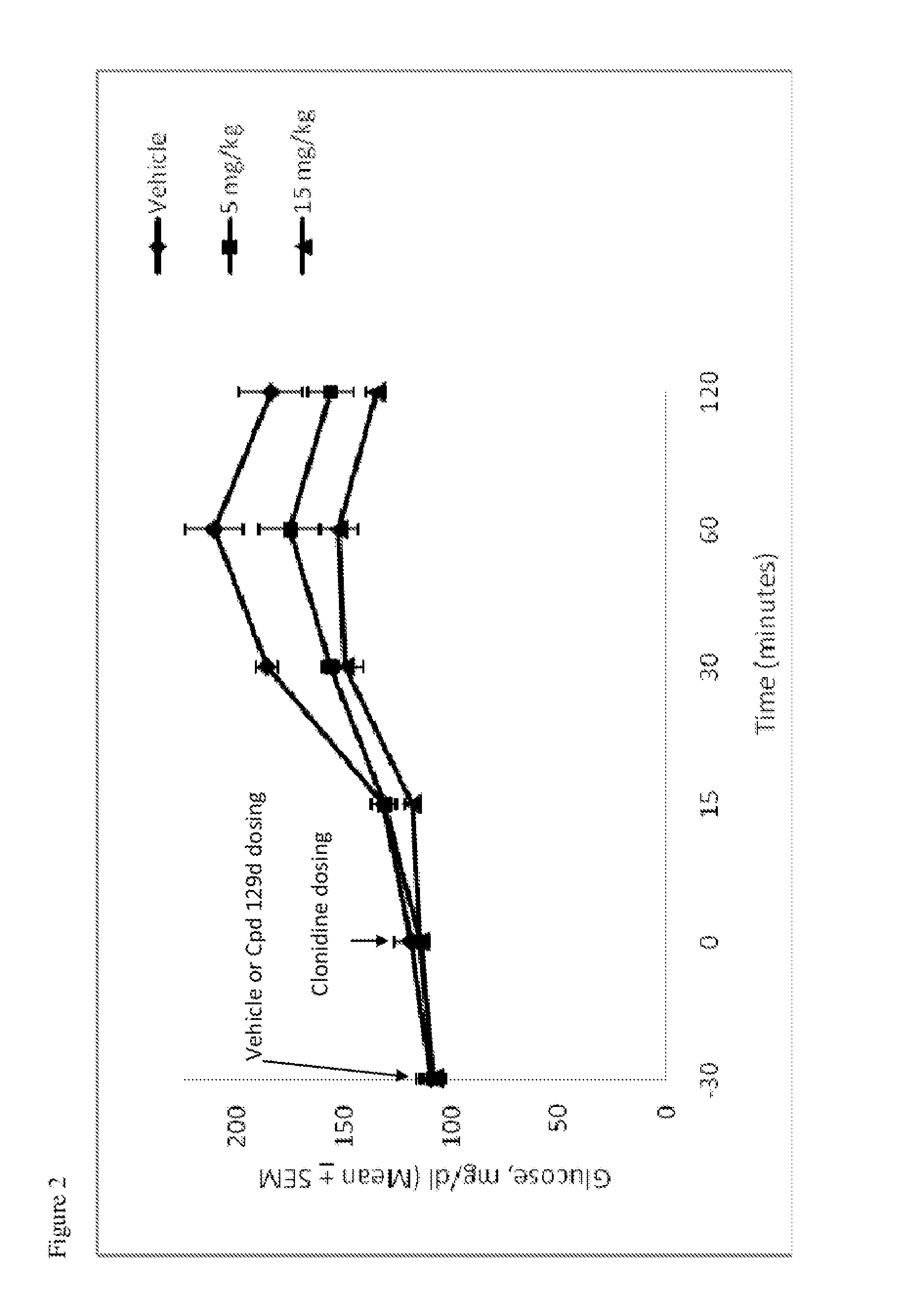
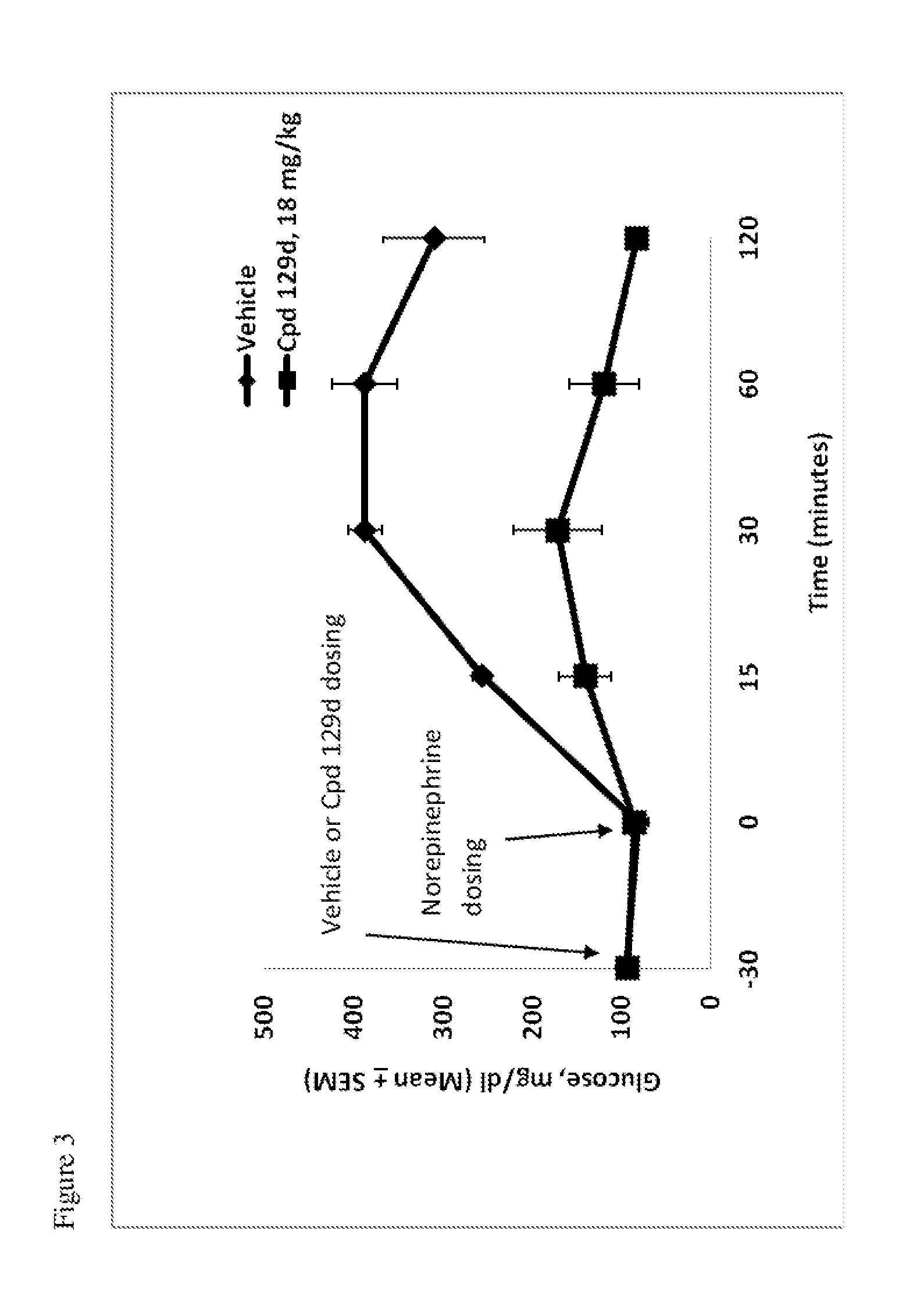
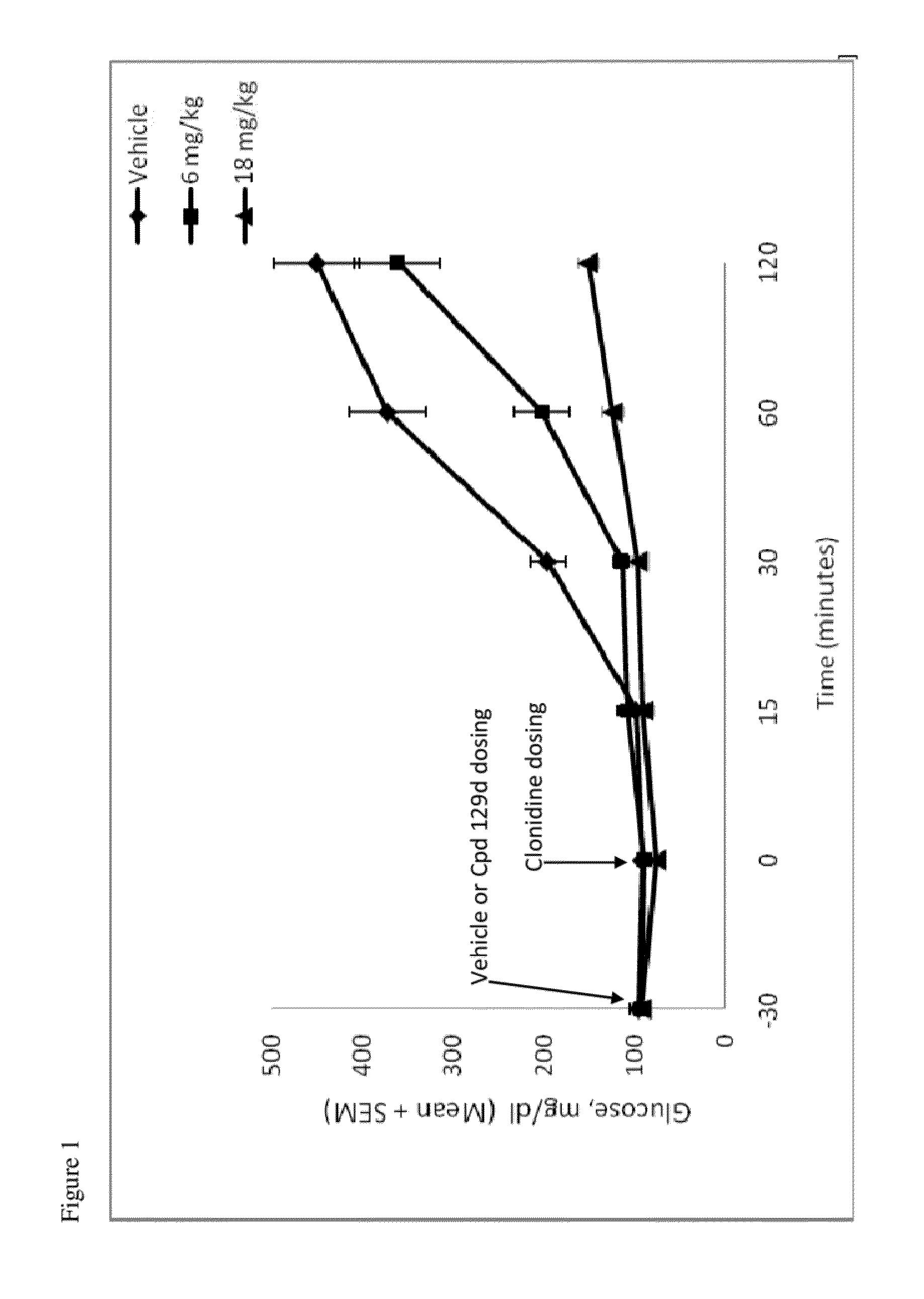
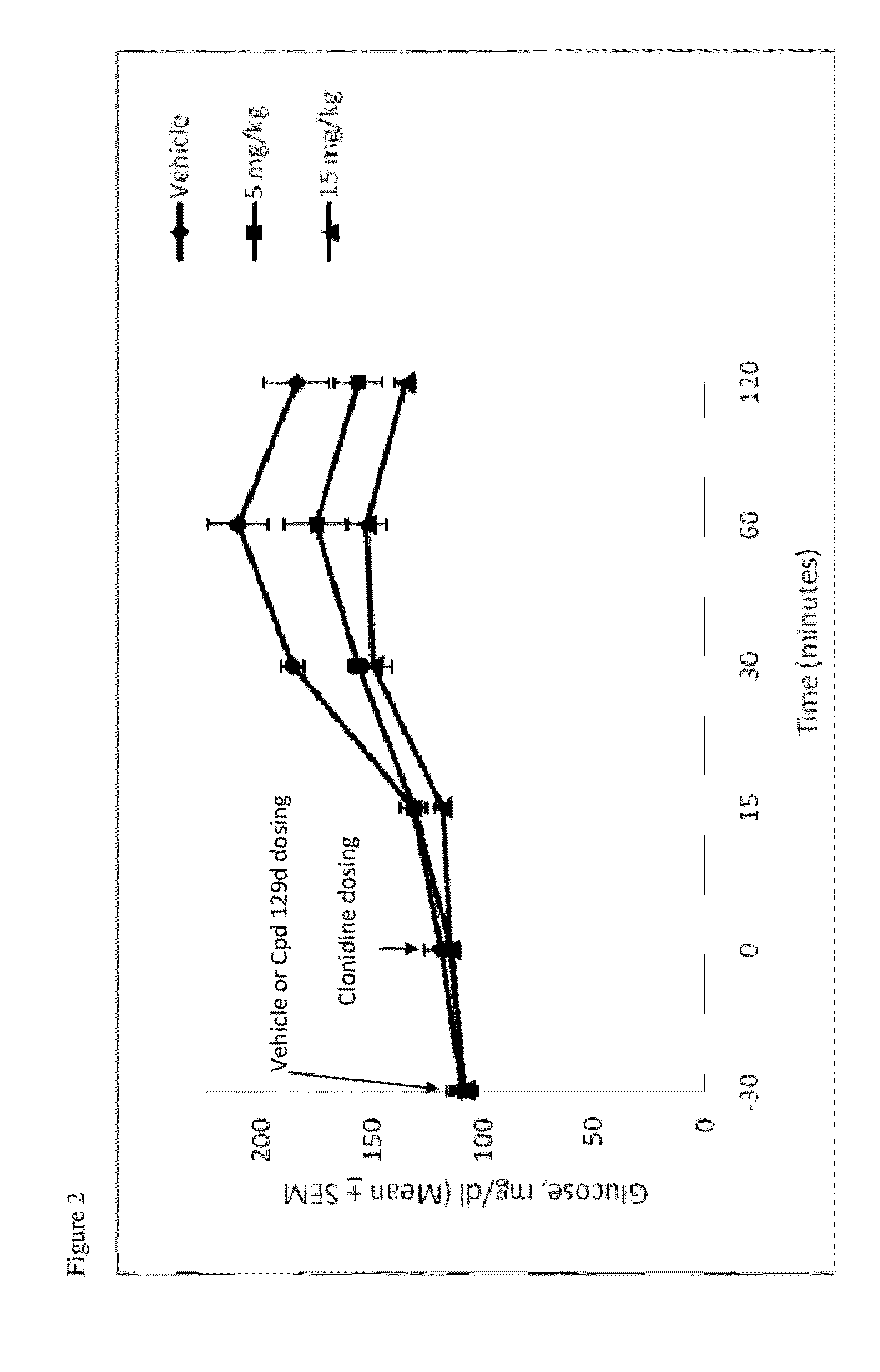
Hydrogenated pyrido[4,3-b]indoles, pyrido[3,4-b]indoles and azepino[4,5-b]indoles are described. The compounds may bind to and are antagonists of the adrenergic receptor α2A. The compounds may also bind to and are an antagonist of the adrenergic receptor α2B; or the compounds are not antagonists of the adrenergic receptor α2B and the compounds are administered in conjunction with a second agent that reduces, or is expected to reduce, blood pressure in an individual. The compounds may find use in therapy, e.g., to regulate blood glucose level, increase insulin secretion and treat diseases or conditions that are, or are expected to be, responsive to an increase in insulin production. Use of the compounds to treat type 2 diabetes is particularly described.
Owner:MEDIVATION TECH INC
Epidermal growth factor increasing insulin secretion and lowering blood glucose
InactiveUS20090312250A1Lower Level RequirementsEGF treatmentPeptide/protein ingredientsMetabolism disorderD-GlucoseGlycemic
The present inventors show that a brief exposure to EGF stimulates insulin secretion glucose-independently via a Ca2+ influx- and PLD2-dependent mechanism. Furthermore, the present invention shows that EGF is a novel secretagogue that lowers plasma glucose levels in normal and diabetic mice, suggesting the potential for EGF treatment in diabetes.
Owner:POSTECH ACAD IND FOUND
Compounds and methods of treating diabetes
InactiveUS20140155384A1Lower blood sugar levelsIncreased insulin secretionBiocideOrganic chemistryDiseaseAdrenergic
Hydrogenated pyrido[4,3-b]indoles, pyrido[3,4-b]indoles and azepino[4,5-b]indoles are described. The compounds may bind to and are antagonists of the adrenergic receptor a2A. The compounds may also bind to and are an antagonist of the adrenergic receptor α2B; or the compounds are not antagonists of the adrenergic receptor α2β and the compounds are administered in conjunction with a second agent that reduces, or is expected to reduce, blood pressure in an individual. The compounds may find use in therapy, e.g., to regulate blood glucose level, increase insulin secretion and treat diseases or conditions that are, or are expected to be, responsive to an increase in insulin production. Use of the compounds to treat type 2 diabetes is particularly described.
Owner:MEDIVATION TECH INC
Compounds and methods of treating diabetes
InactiveUS20140228353A1Lower blood sugar levelsStabilize blood sugar levelsBiocideOrganic chemistryDiseasePancreatic hormone
Hydrogenated pyrido[4,3-b]indoles, pyrido[3,4-b]indoles and azepino[4,5-b]indoles are described. The compounds may bind to and are antagonists of the adrenergic receptor α2A. The compounds may also bind to and are an antagonist of the adrenergic receptor α2B; or the compounds are not antagonists of the adrenergic receptor α2B and the compounds are administered in conjunction with a second agent that reduces, or is expected to reduce, blood pressure in an individual. The compounds may find use in therapy, e.g., to regulate blood glucose level, increase insulin secretion and treat diseases or conditions that are, or are expected to be, responsive to an increase in insulin production. Use of the compounds to treat type 2 diabetes is particularly described.
Owner:MEDIVATION TECH INC
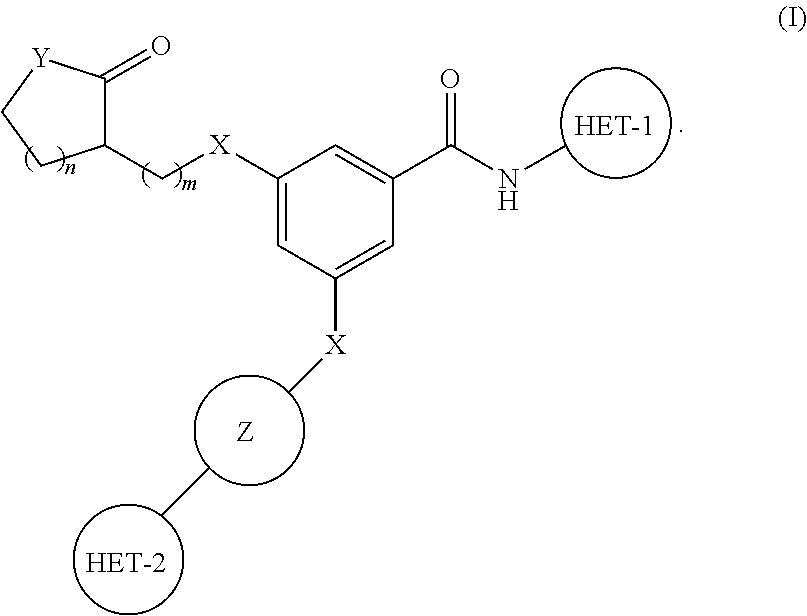
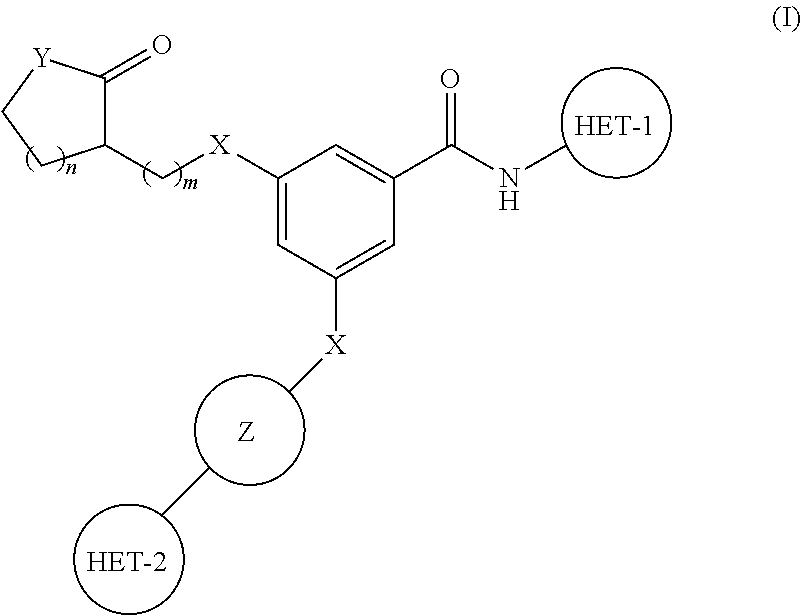
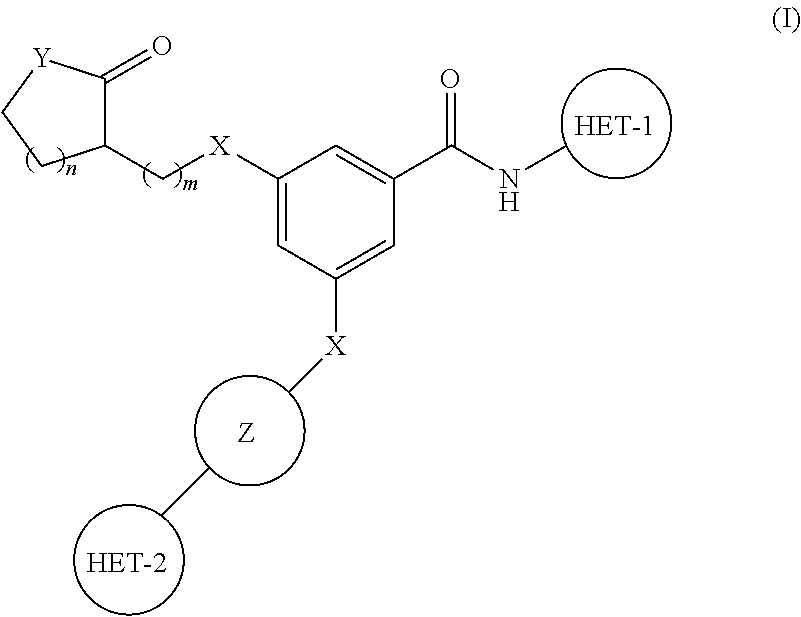
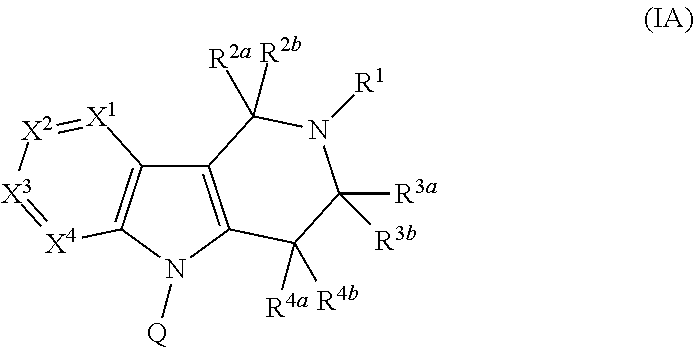
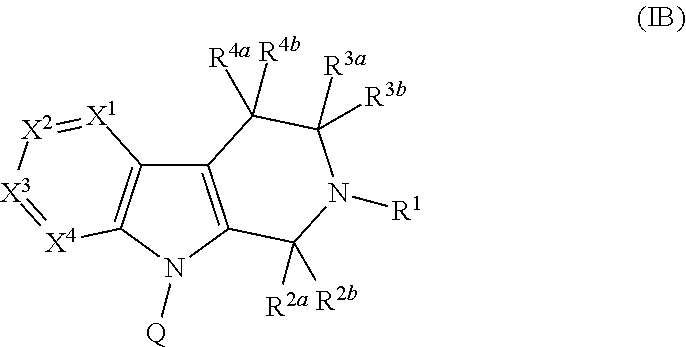
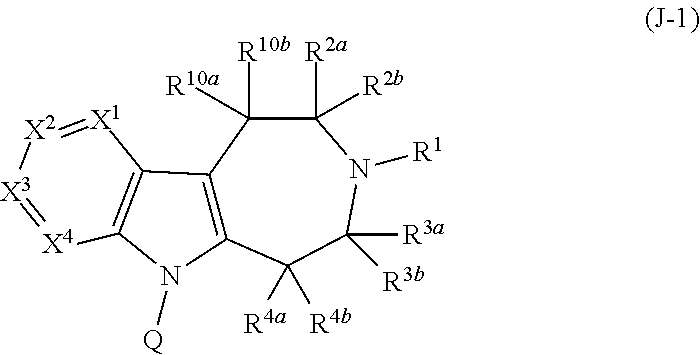
PYRIDO[4,3-b]INDOLE AND PYRIDO[3,4-b]INDOLE DERIVATIVES AND METHODS OF USE
InactiveUS20140206711A1Improve cognitive functionReduce psychotic effectBiocideOrganic chemistryBlood sugarKidney disease
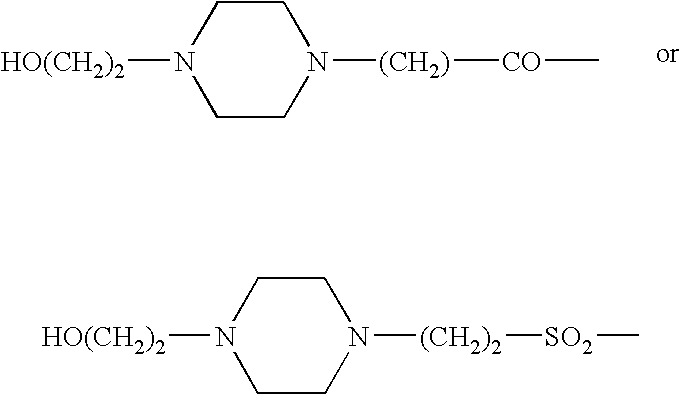
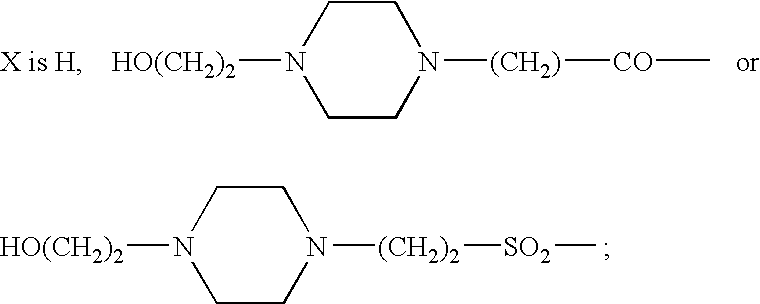
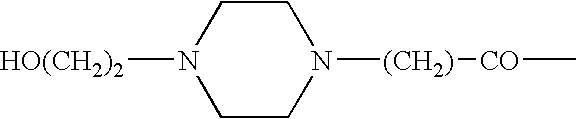
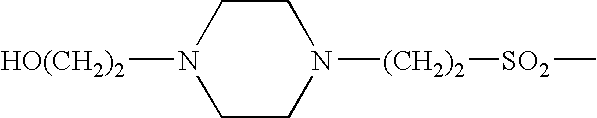
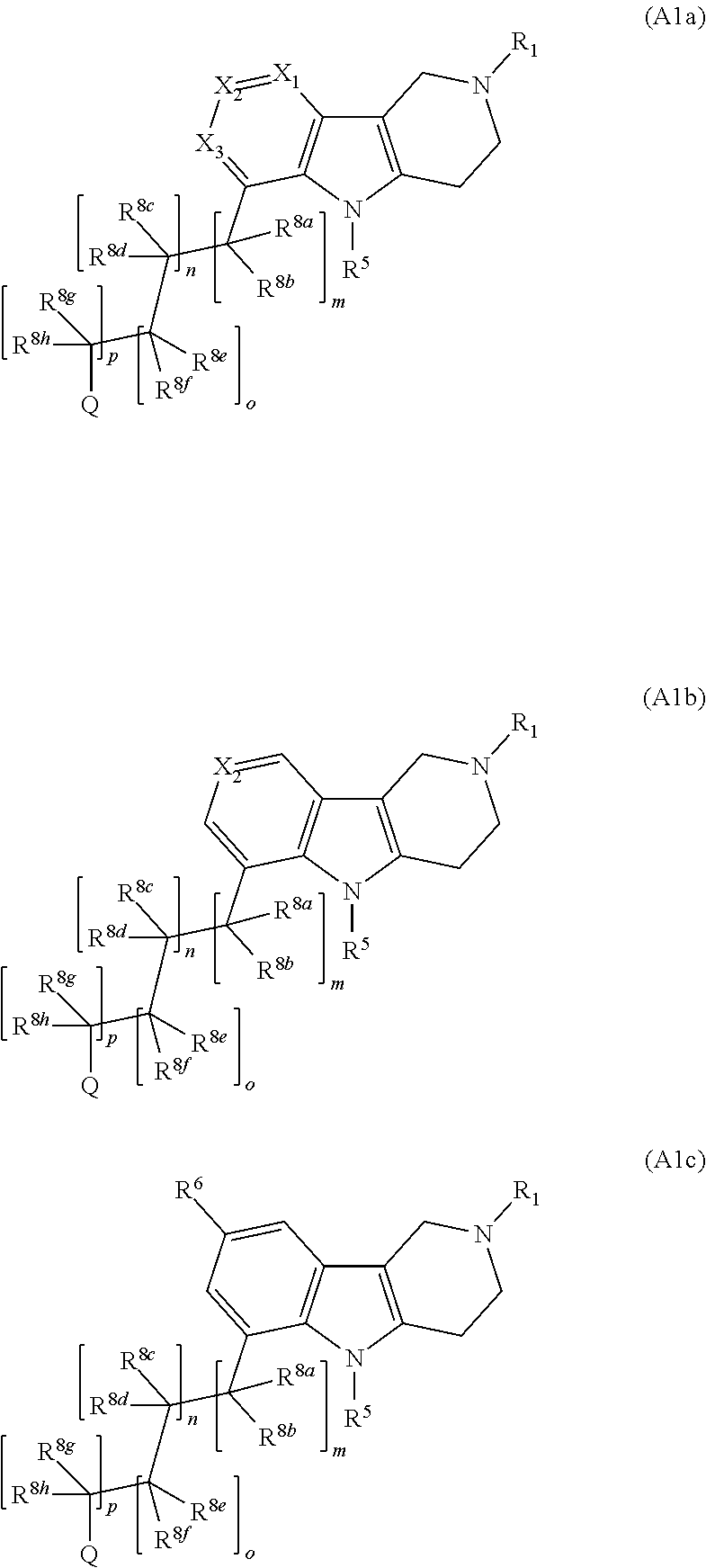
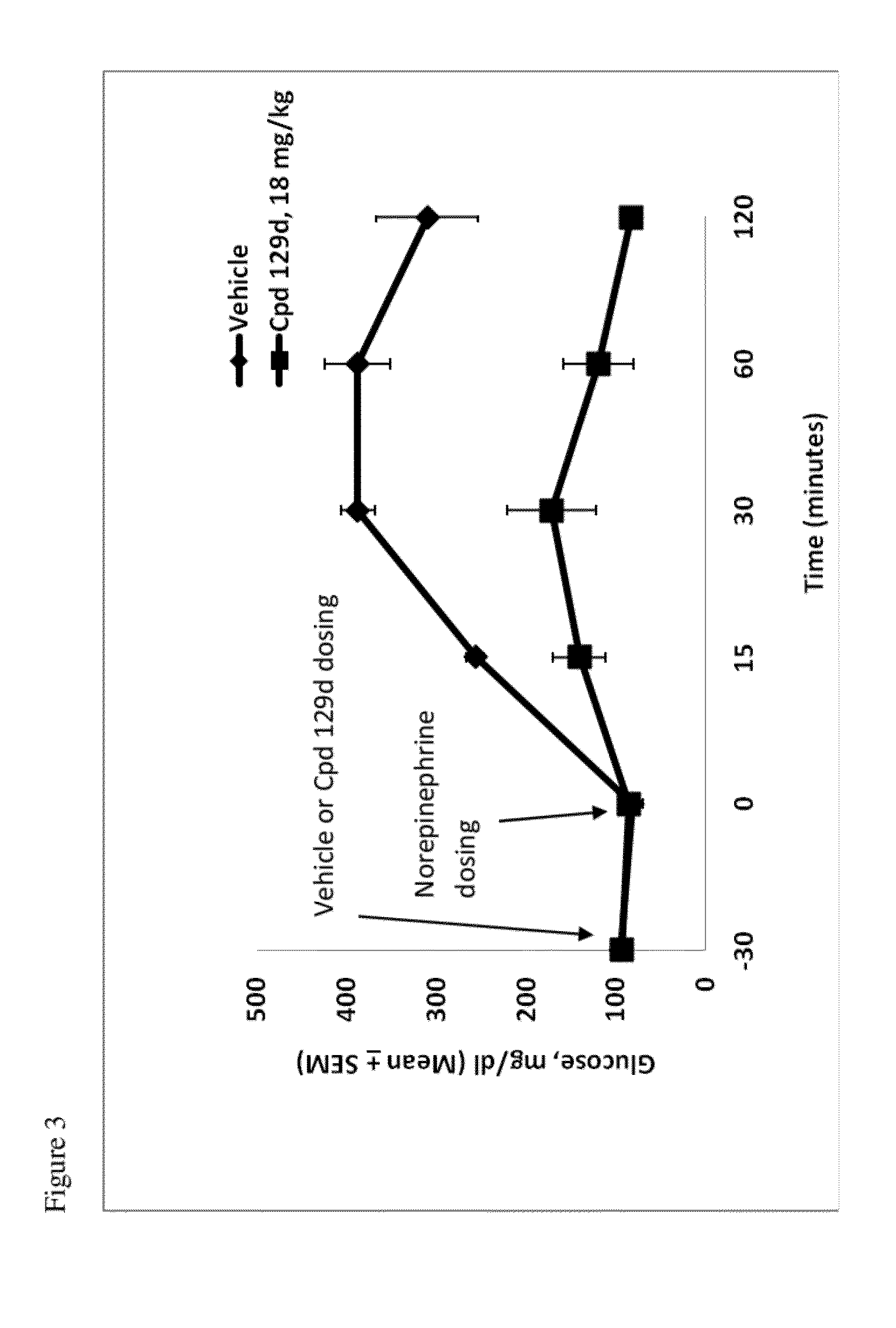
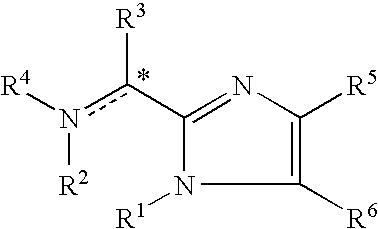
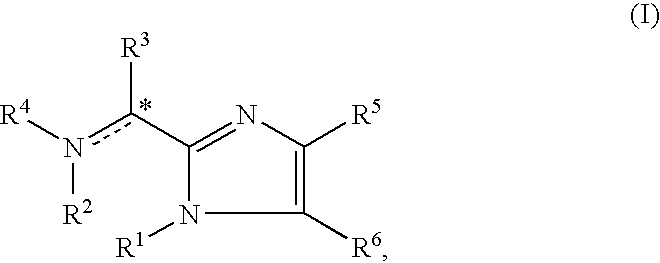
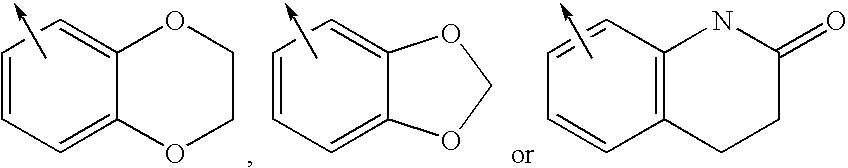
This disclosure is directed to pyrido[4,3-b]indole and pyrido[3,4-b]indole derivatives. Pharmaceutical compositions comprising the compounds are also provided, as are methods of using the compounds in a variety of therapeutic applications, including the treatment of a cognitive disorder, psychotic disorder, neurotransmitter-mediated disorder and / or a neuronal disorder. The compounds may bind to and antagonize receptor α2B, α1B or α2A. The compounds may find use in therapy, e.g., to (i) reduce blood pressure and / or (ii) promote renal blood flow and / or (iii) decrease or inhibit sodium reabsorption, or to regulate blood glucose level, increase insulin secretion and treat diseases or conditions that are, or are expected to be, responsive to an increase in insulin production. The compounds may also be used to treat diseases or conditions that are expected to be responsive to a decrease in blood pressure. Use of the compounds to treat cardiovascular, renal disorders or type 2 diabetes is particularly described.
Owner:MEDIVATION TECH INC
Compounds and methods of treating diabetes
InactiveUS20130053367A1Increased insulin secretionLower blood sugar levelsBiocideOrganic chemistryDiseaseAdrenergic
Hydrogenated pyrido[4,3-b]indoles, pyrido[3,4-b]indoles and azepino[4,5-b]indoles are described. The compounds may bind to and are antagonists of the adrenergic receptor α2A. The compounds may also bind to and are an antagonist of the adrenergic receptor α2B; or the compounds are not antagonists of the adrenergic receptor α2B and the compounds are administered in conjunction with a second agent that reduces, or is expected to reduce, blood pressure in an individual. The compounds may find use in therapy, e.g., to regulate blood glucose level, increase insulin secretion and treat diseases or conditions that are, or are expected to be, responsive to an increase in insulin production. Use of the compounds to treat type 2 diabetes is particularly described.
Owner:MEDIVATION TECH INC
Celosia argentea suponin compound and its pharmaceutical use
InactiveCN1850836AOrganic active ingredientsNervous disorderCoronary artery diseaseCoronary heart disease
This invention relates to natual medicine chemical and medical technique field. It discloses two kind celosin new compounds of celosin A and celosin B structure and its distillation and eperating method from amaranthcea plant celosin. Their chemical general formula is shown as formula (1). This invention also discloses the compound and its derivative medicine aspect application of curing and preventing fatty liver, liver injury, hepatic fibrosis, liver cirrhosis, coronary heart disease, cardiac infarction, myocardial ischemia, cerebral ischemia, cerebral infarction, cerebral apoplexy, diabetes mellitus, hyperinsulinism, insulin resistance disease, obesity, glucose intolerance capability, and / or hypertension adiposity, hyperlipemia, dementia, depression or anxiety, and insomnia diseases.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Imidazolyl derivatives
The present invention is directed to imidazolyl derivatives of the formula: where the substituents are defined in the specification, or a pharmaceutically acceptable salt thereof. The derivatives bind selectively to the somatostatin subtype receptors and elicit either an agonist or antagonist effect from the somatostatin subtype receptors. The derivatives are useful for treating a variety of diseases including acromegaly, restenosis, Crohn's disease, systemic sclerosis, external and internal pancreatic pseudocysts and ascites, VIPoma, nesidoblastosis, hyperinsulinism, gastrinoma, Zollinger-Ellison Syndrome, diarrhea, AIDS related diarrhea, chemotherapy related diarrhea, scleroderma, Irritable Bowel Syndrome, pancreatitis, small bowel obstruction, gastroesophageal reflux, duodenogastric reflux, Cushing's Syndrome, gonadotropinoma, hyperparathyroidism, Graves' Disease, diabetic neuropathy, Paget's disease, polycystic ovary disease, cancer, cancer cachexia, hypotension, postprandial hypotension, panic attacks, GH secreting adenomas or TSH secreting adenomas.
Owner:IPSEN PHARMA SAS
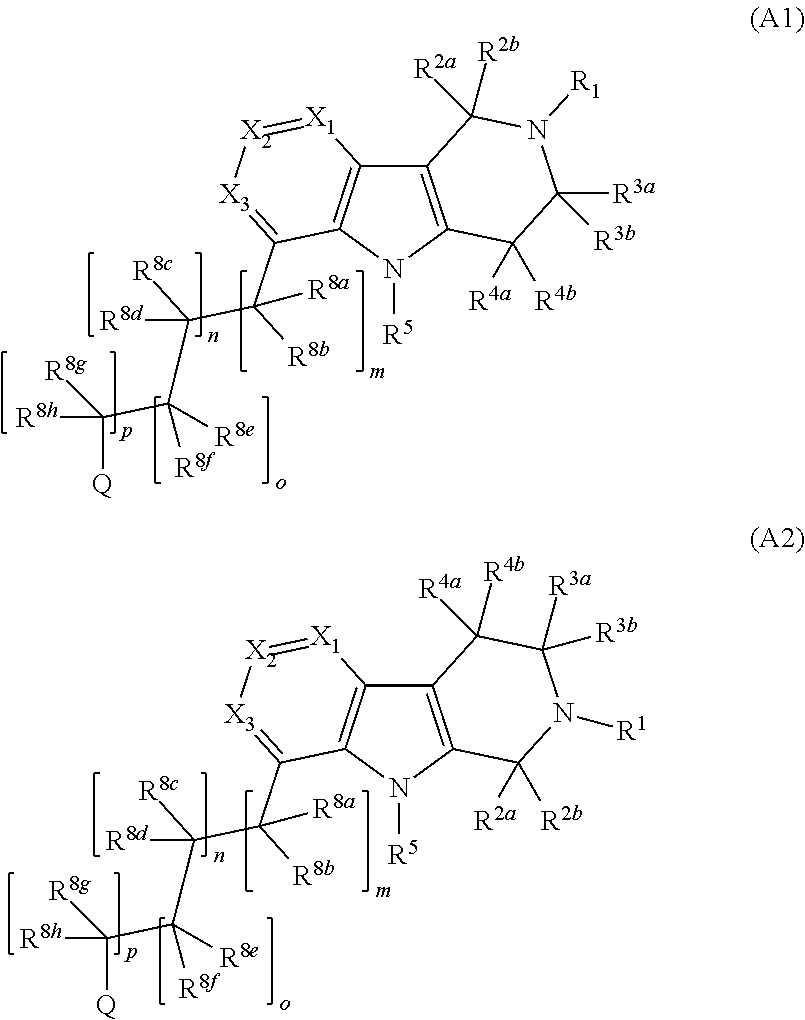
Pyrido[4,3-b]indole and pyrido[3,4-b]indole derivatives and methods of use
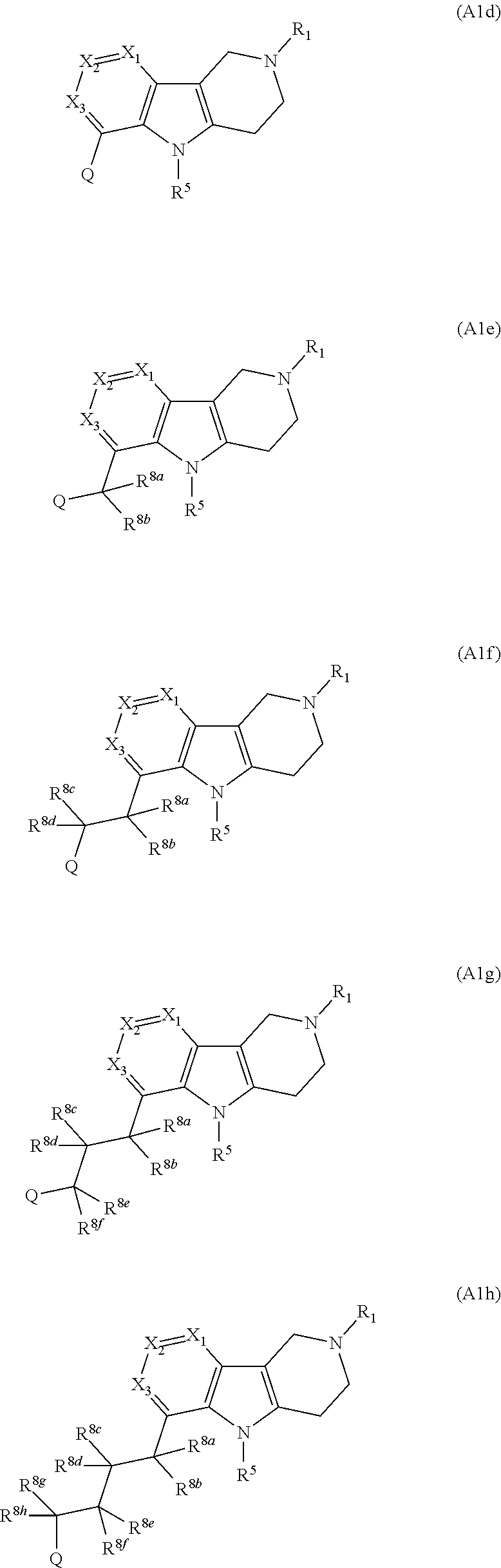
InactiveUS9035056B2Lower systolic blood pressureIncrease heart rateBiocideOrganic chemistryDiseaseInsulin humulin
This disclosure is directed to pyrido[4,3-b]indole and pyrido[3,4-b]indole derivatives. Pharmaceutical compositions comprising the compounds are also provided, as are methods of using the compounds in a variety of therapeutic applications, including the treatment of a cognitive disorder, psychotic disorder, neurotransmitter-mediated disorder and / or a neuronal disorder. The compounds may bind to and antagonize receptor α2B, α1B orα2A. The compounds may find use in therapy, e.g., to (i) reduce blood pressure and / or (ii) promote renal blood flow and / or (iii) decrease or inhibit sodium reabsorption, or to regulate blood glucose level, increase insulin secretion and treat diseases or conditions that are, or are expected to be, responsive to an increase in insulin production. The compounds may also be used to treat diseases or conditions that are expected to be responsive to a decrease in blood pressure. Use of the compounds to treat cardiovascular, renal disorders or type 2 diabetes is particularly described.
Owner:MEDIVATION TECH INC
Blood glucose level control
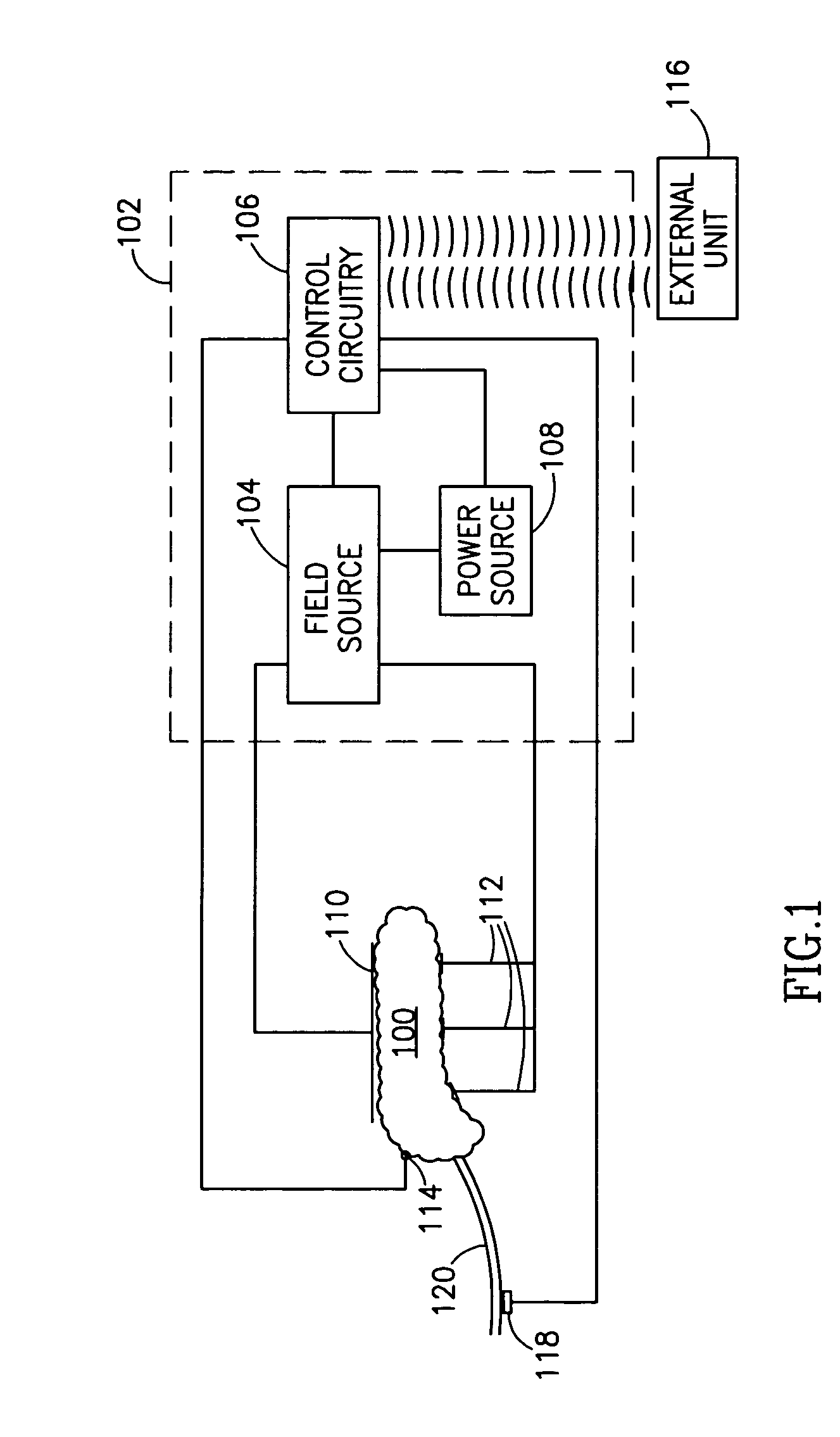
InactiveUS8346363B2Avoiding unacceptable calcium level profileIncrease secretionElectrotherapyGlucose sensorsLevel insulin
A pancreatic controller, comprising:a glucose sensor, for sensing a level of glucose or insulin in a body serum;at least one electrode, for electrifying an insulin producing cell or group of cells;a power source for electrifying said electrode with a pulse that does not initiate an action potential in said cell and has an effect of increasing insulin secretion; anda controller which receives the sensed level and controls said power source to electrify said electrode to have a desired effect on said level.
Owner:TYLERTON INT INC
A composition for treating diabetes or diabesity comprising oxyntomodulin analog
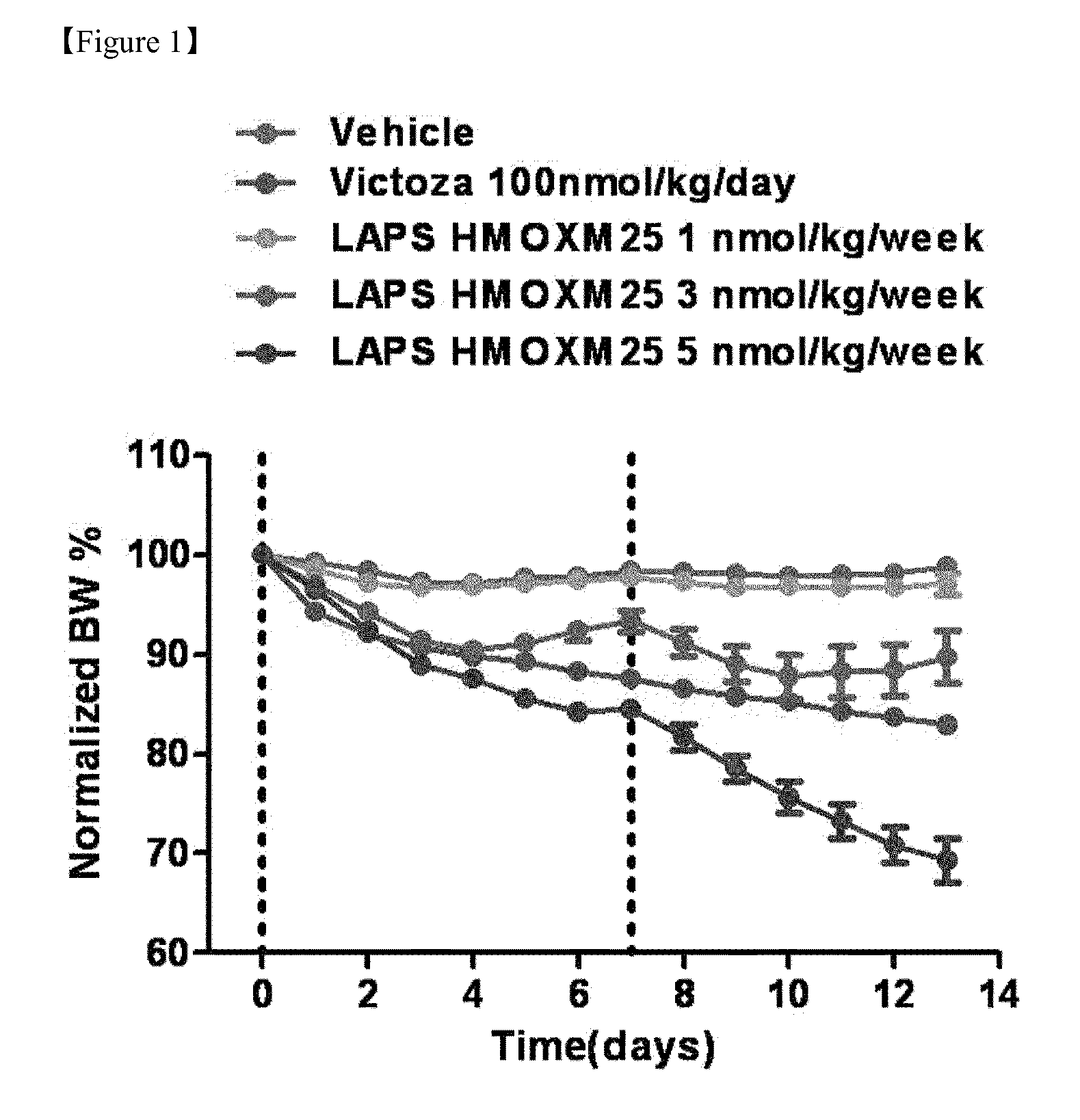
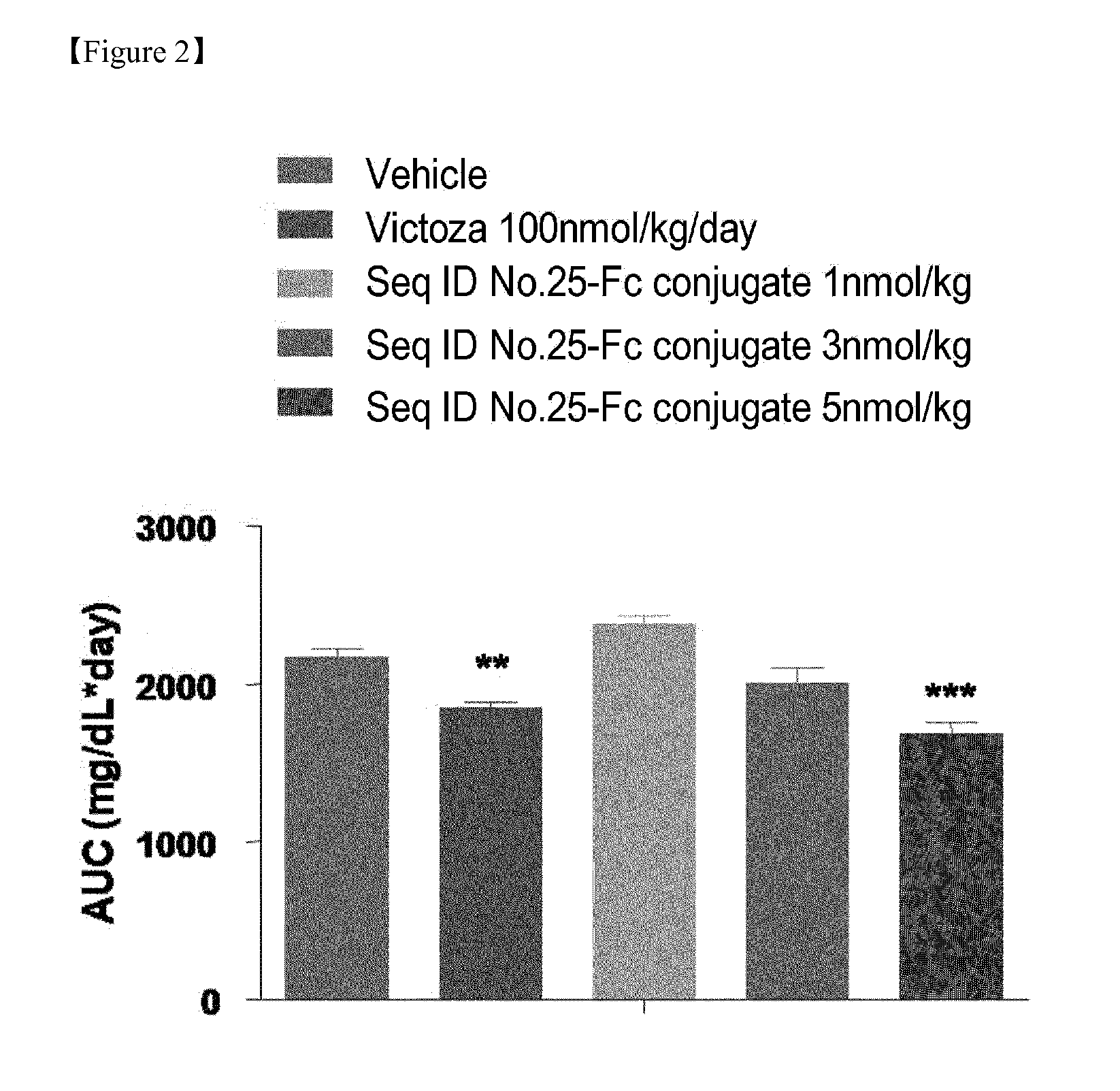
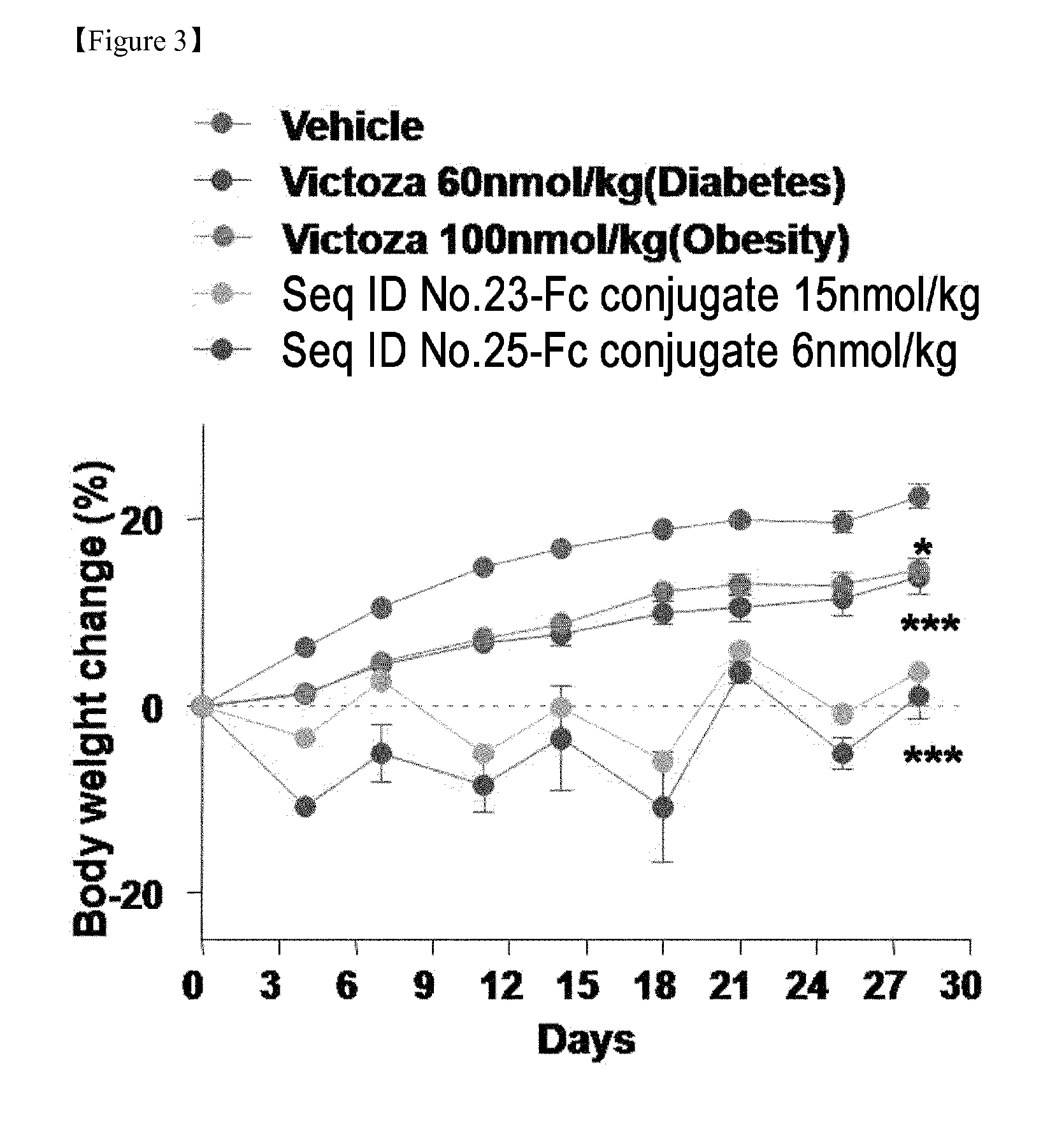
InactiveUS20150299282A1High activityEasy to usePeptide/protein ingredientsMetabolism disorderNormal blood glucoseDiabetic complication
Disclosed are a composition for preventing or treating diabetes, diabesity or diabetic complications, containing an oxyntomodulin analog as an active ingredient and a method for treating diabetes, diabesity or diabetic complications, including administering a pharmaceutically effective amount of an oxyntomodulin analog to a subject. The oxyntomodulin analog shows a greater activity to activate a GLP-1 receptor and a glucagon receptor, than native oxyntomodulin. The oxyntomodulin analog induces an expansion of beta-cells and increases insulin secretion, thereby reducing blood glucose levels that were increased due to a high-calorie and high-fat diet. The oxyntomodulin analog induces decreases in a body weight and appetite to improve insulin sensitivity and is useful in maintaining normal blood glucose levels.
Owner:HANMI PHARMA
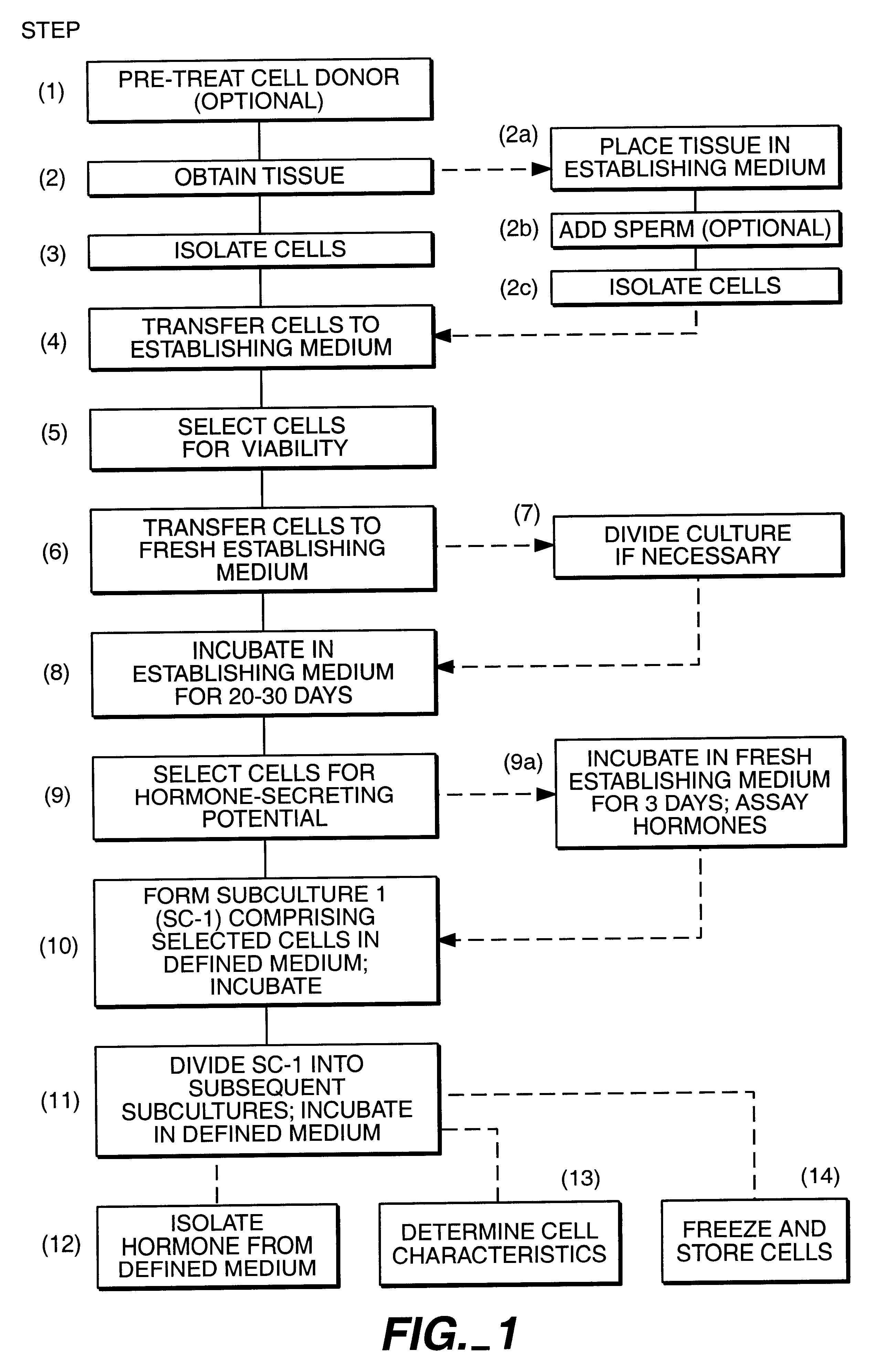
Hormone-secreting cells maintained in long-term culture
InactiveUS6372493B1Increased insulin secretionHigh glucose concentrationPeptide/protein ingredientsDiagnosticsCulture mediumsHuman chorionic gonadotropin
Methods are provided for the establishment and maintenance in long term culture of hormone secreting cells. Cells are derived from tumorous or non-tumorous animal or human tissues, including ovary, endometrium, trophoblast, pituitary, thyroid, and pancreas. The cells secrete into the culture medium hormones such as estrogens, progestins, follicle-stimulating hormone, luteinizing hormone, human chorionic gonadotrophin, thyroxin, glucagon, and insulin, depending on the tissue of origin of individual cell cultures. Contact with an appropriate secretogogue causes the cells to respond with increased hormone secretion. For instance, ovarian follicular cells respond to follicle-stimulating hormone with increased estrogen and progesterone secretion. Pancreatic cells respond to elevated glucose with increased insulin secretion. The cells proliferate in in vitro for up to one year or longer, during which time they retain their hormone-secretion profile. The cells may be frozen for storage, and retain their hormone-secretion profile after thawing. The cell cultures are useful for the production of human hormones, for the bio-assay of drugs such as therapeutic gonadotrophin, for the testing of drug efficacy and design, and for toxicity testing of drugs and chemicals. The cells may also be implanted in an individual to replace deficient hormone secretion. For instance, insulin secreting pancreatic cells may be implanted in a diabetic individual as an adjunct or replacement therapy for exogenously administered insulin.
Owner:PACIFIC BIOMEDICAL RES INC
Drug containing chymase inhibitor as the active ingredient
InactiveUS20070032466A1Improve glucose intoleranceBiocideSenses disorderDiabetic retinopathyDepressant
The present invention provides drugs containing chymase inhibitors as active ingredients for improving glucose intolerance or preventing and / or treating diseases caused by glucose intolerance. The diseases caused by glucose intolerance are diabetes and / or diabetes complications, wherein the diabetes complications include diabetic nephropathy, diabetic retinopathy, diabetic peripheral neuropathy, hyperinsulinism, insulin resistance syndrome, arteriosclerosis, acute coronary syndrome, arteriosclerosis obliterans, angitis, stroke, hypertension, renal insufficiency, nephropathy, nephritis, renal artery aneurysm, renal infarction, obesity and the like.
Owner:TEIJIN PHARMA CO LTD
Metabolic Syndrome-Improving Agent and Medicine, Supplement, Functional Food and Food Additive Containing the Same
ActiveUS20080119417A1Promote secretionAvoid it happening againBiocideCosmetic preparationsDiseaseFood additive
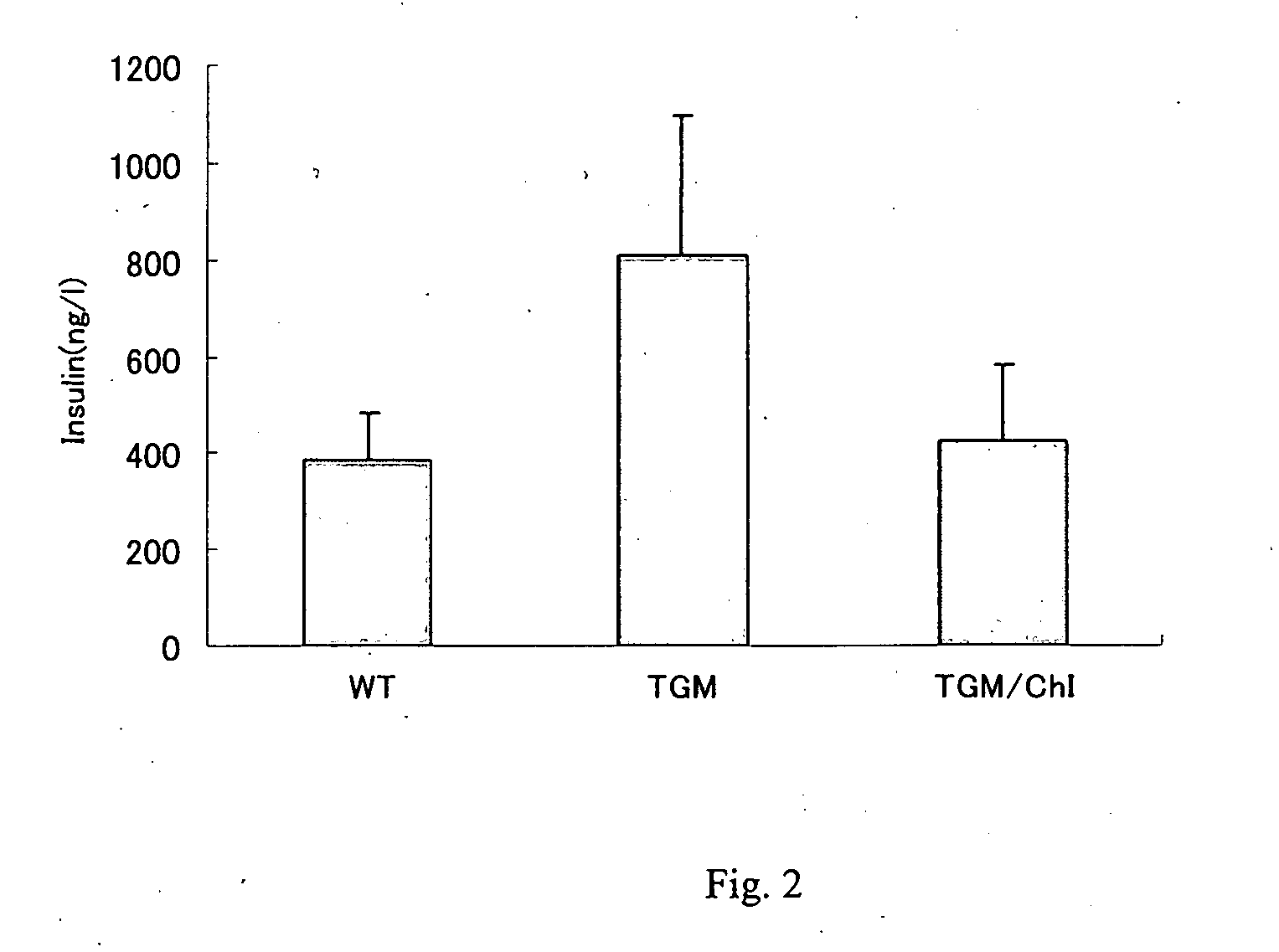
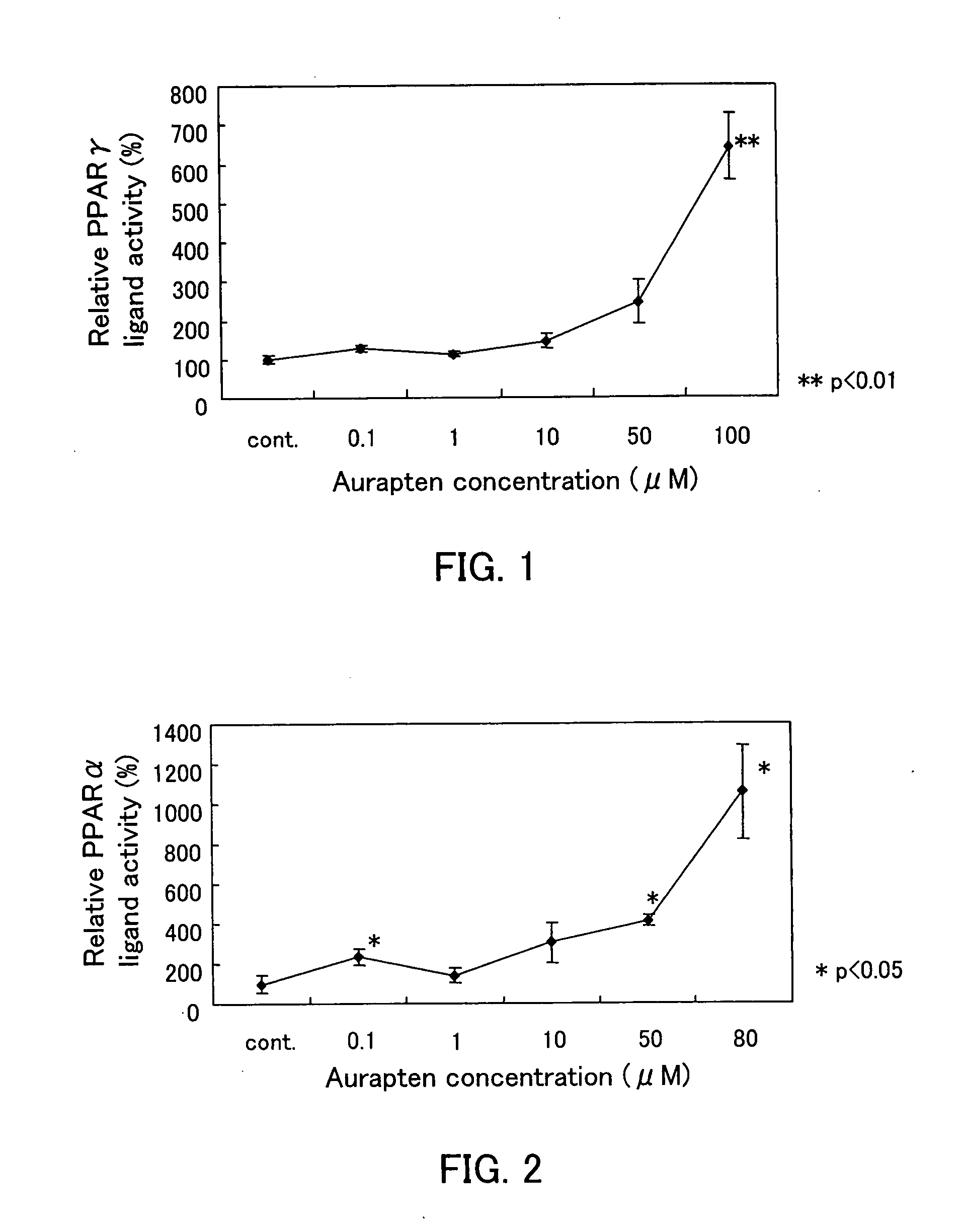
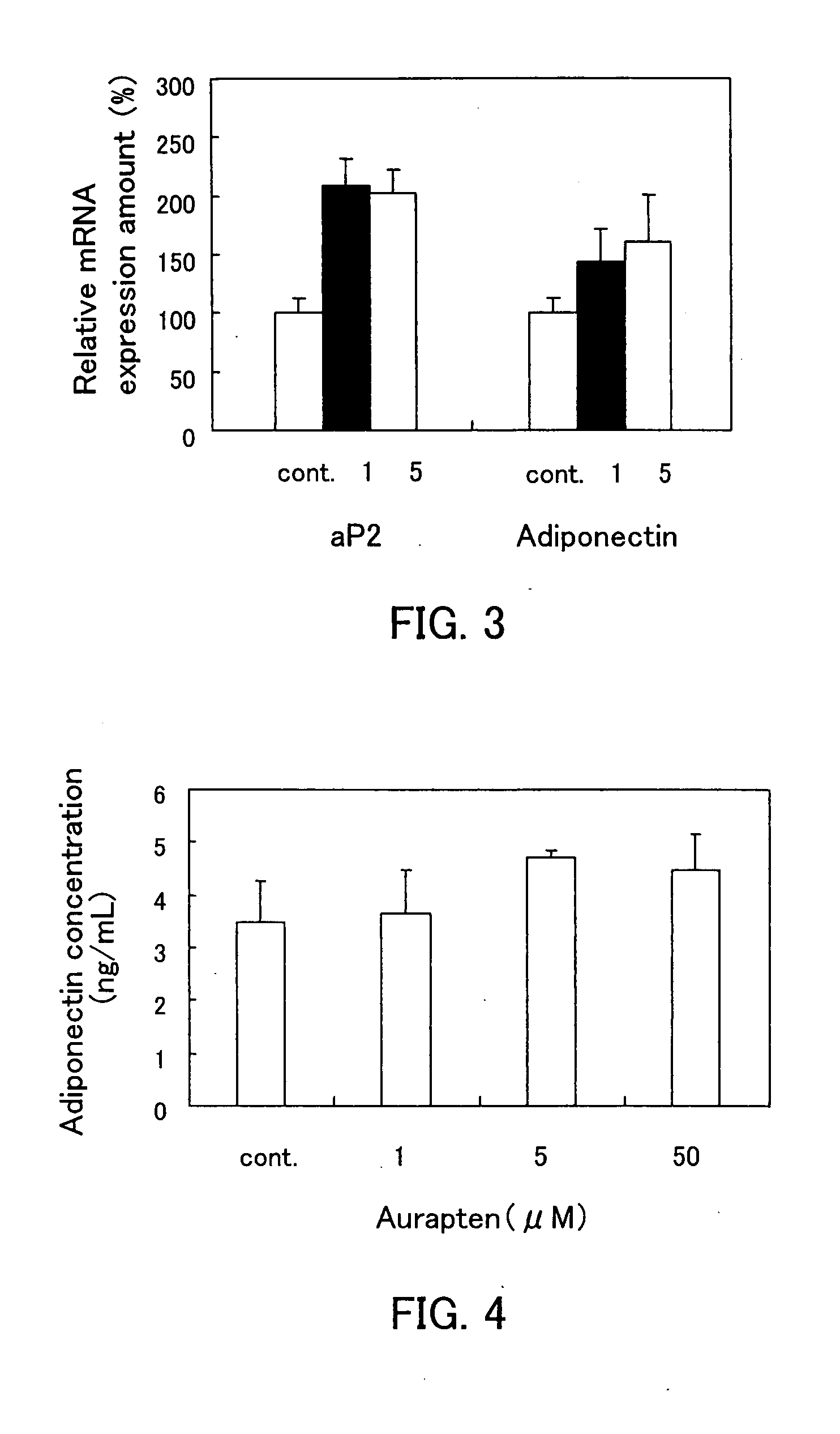
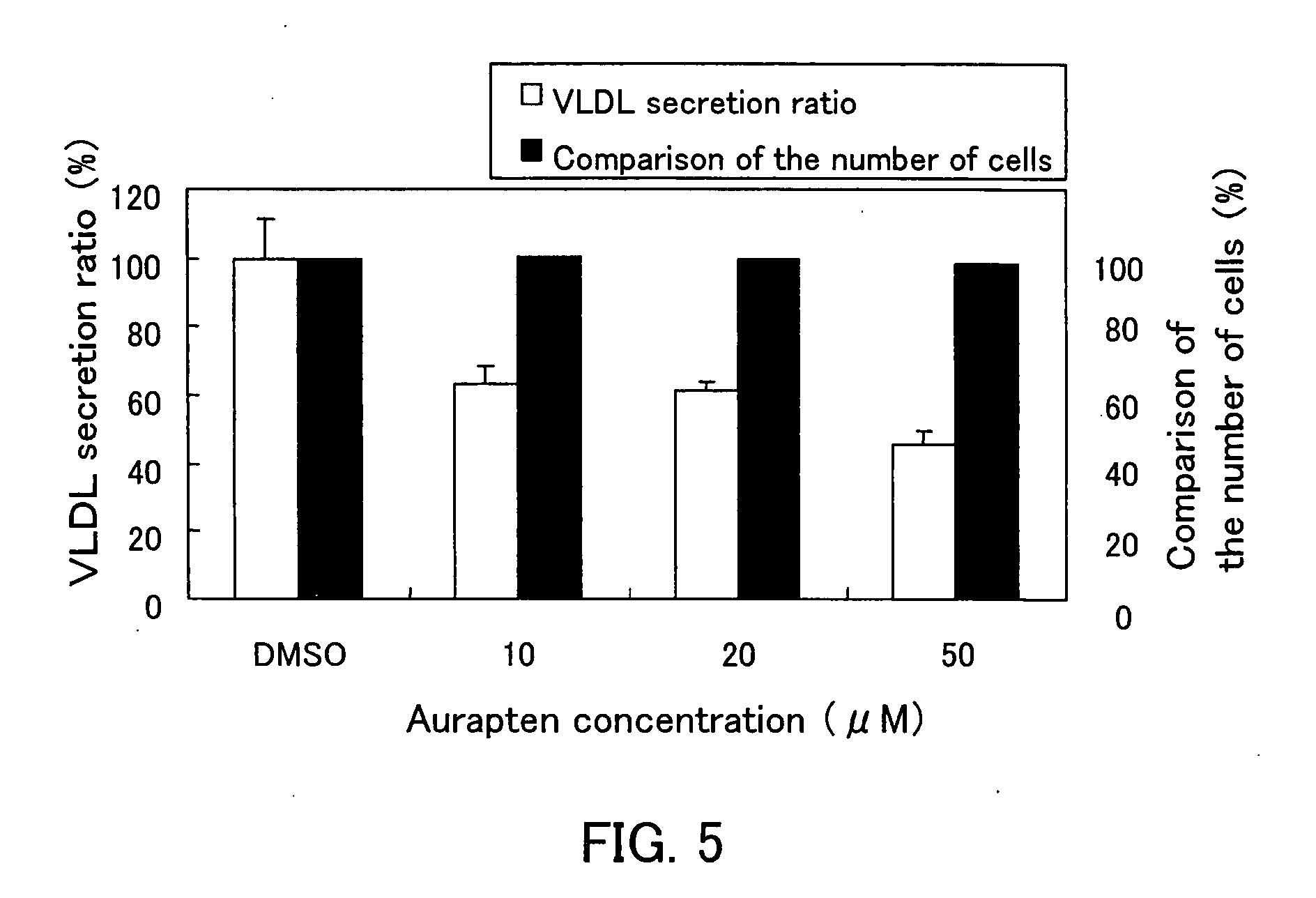
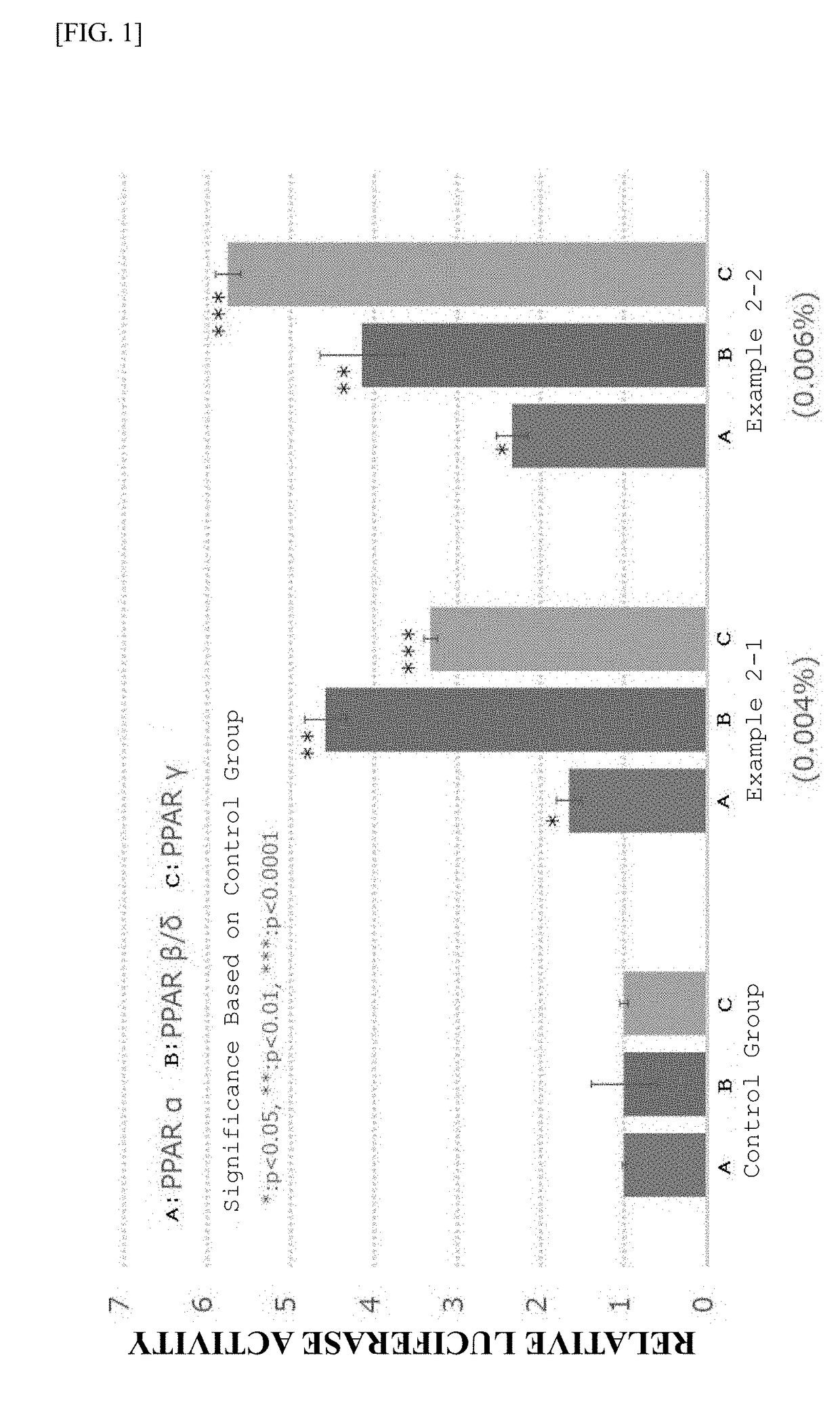
A metabolic syndrome relieving agent that is free from a problem of side effects and can be taken for a long term is provided. Aurapten is used as an agent for relieving a metabolic syndrome. Since aurapten has functions of activating PPARα and PPARγ, promoting the secretion of adiponectin in adipocytes and inhibiting the generation of VLDLs in hepatic cells, it is possible to prevent or treat diseases such as insulin resistance, hyperinsulinism, type 2 diabetes, obesity, visceral fat obesity, hypertension, hyperlipemia, arteriosclerosis and the like and thus prevent or treat the metabolic syndrome. Also, as understood from the fact that citrus fruits such as a hassaku orange, a sweet summer orange or the like containing aurapten have been eaten for many years, they have no problems in terms of safety and have a low calorie content, and therefore, they can be taken for a long term. Further, since aurapten is tasteless and odorless, it does not impair the unique taste of a food when added to this food, so that it can be added to foods and taken.
Owner:ARKRAY INC
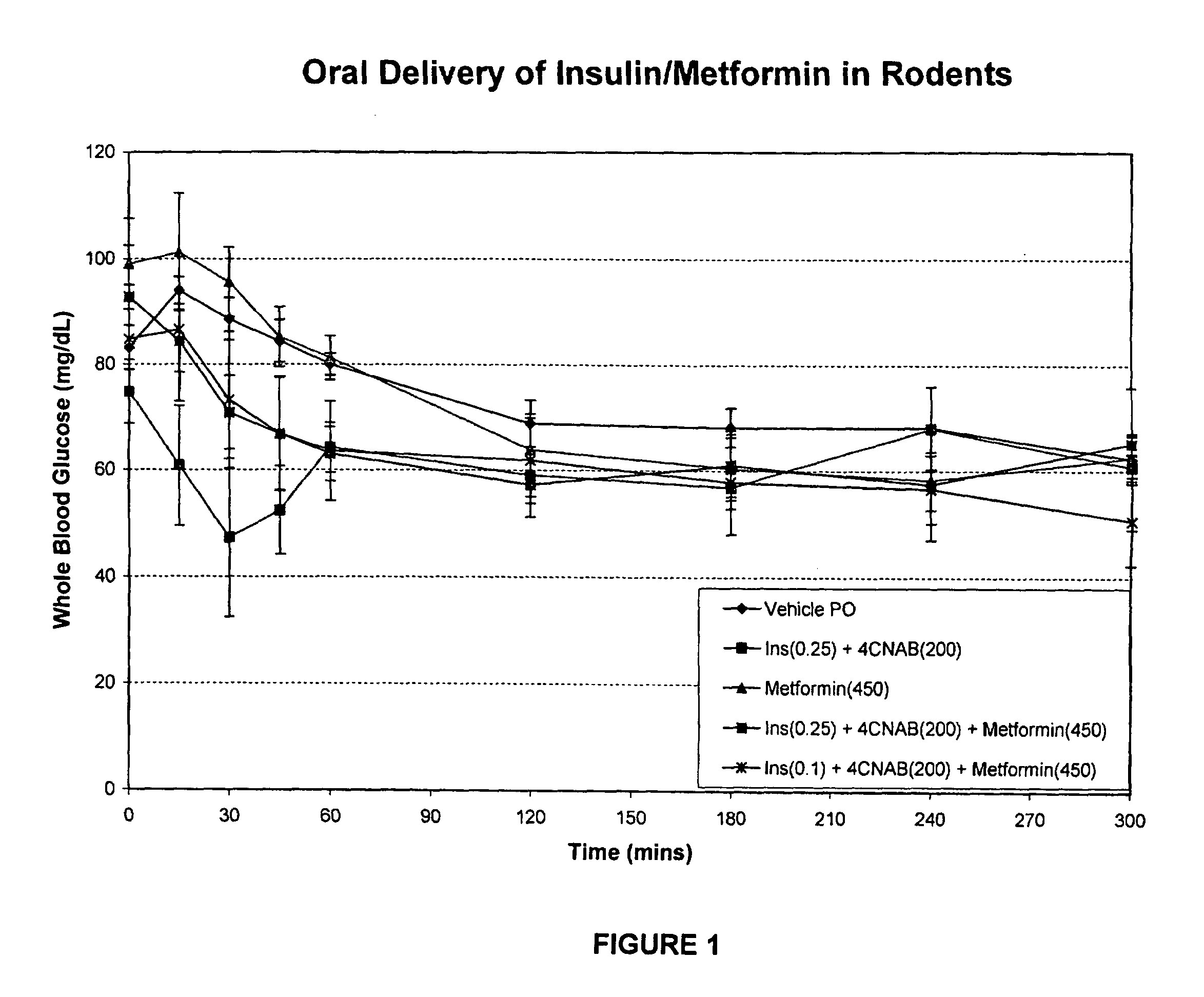
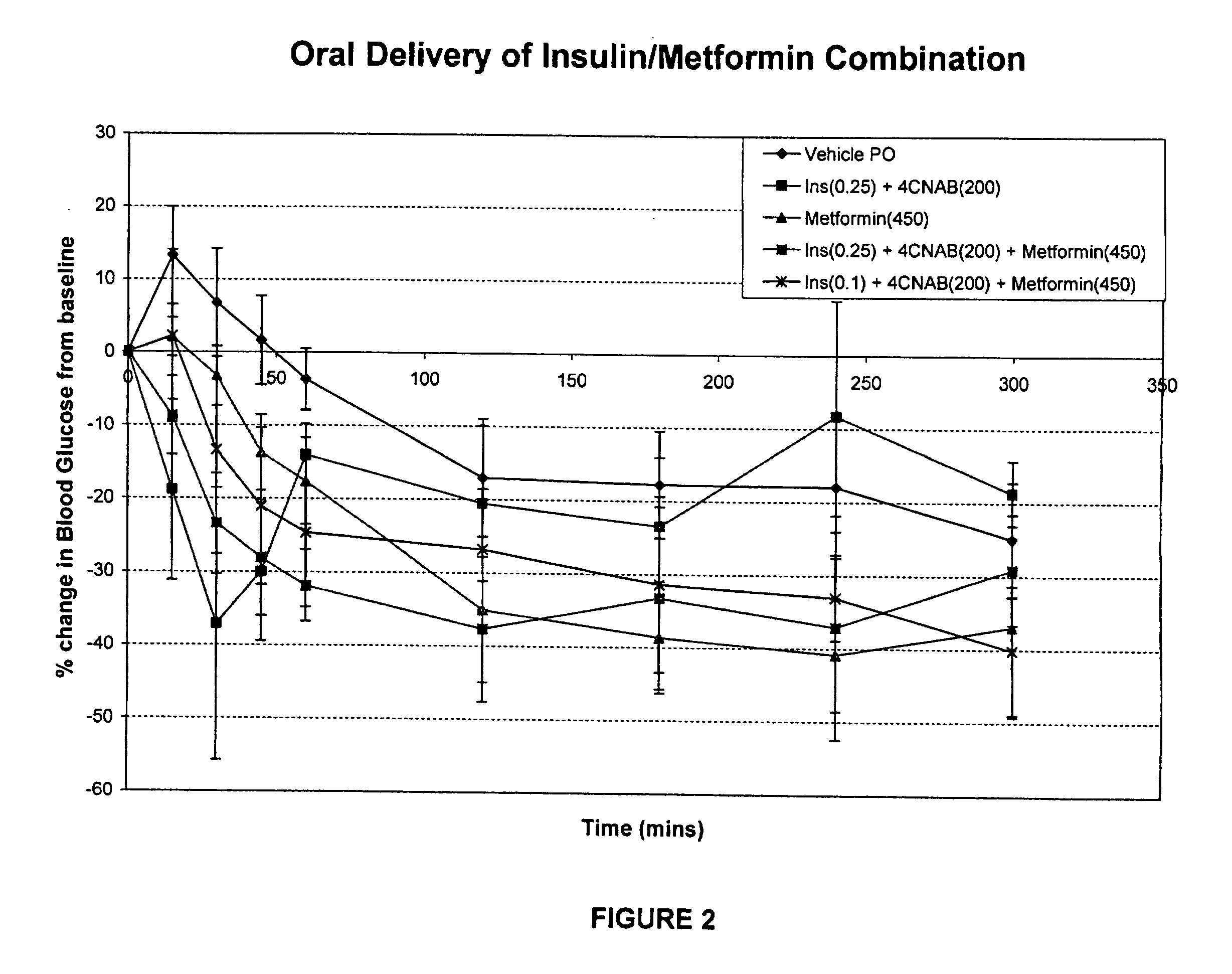
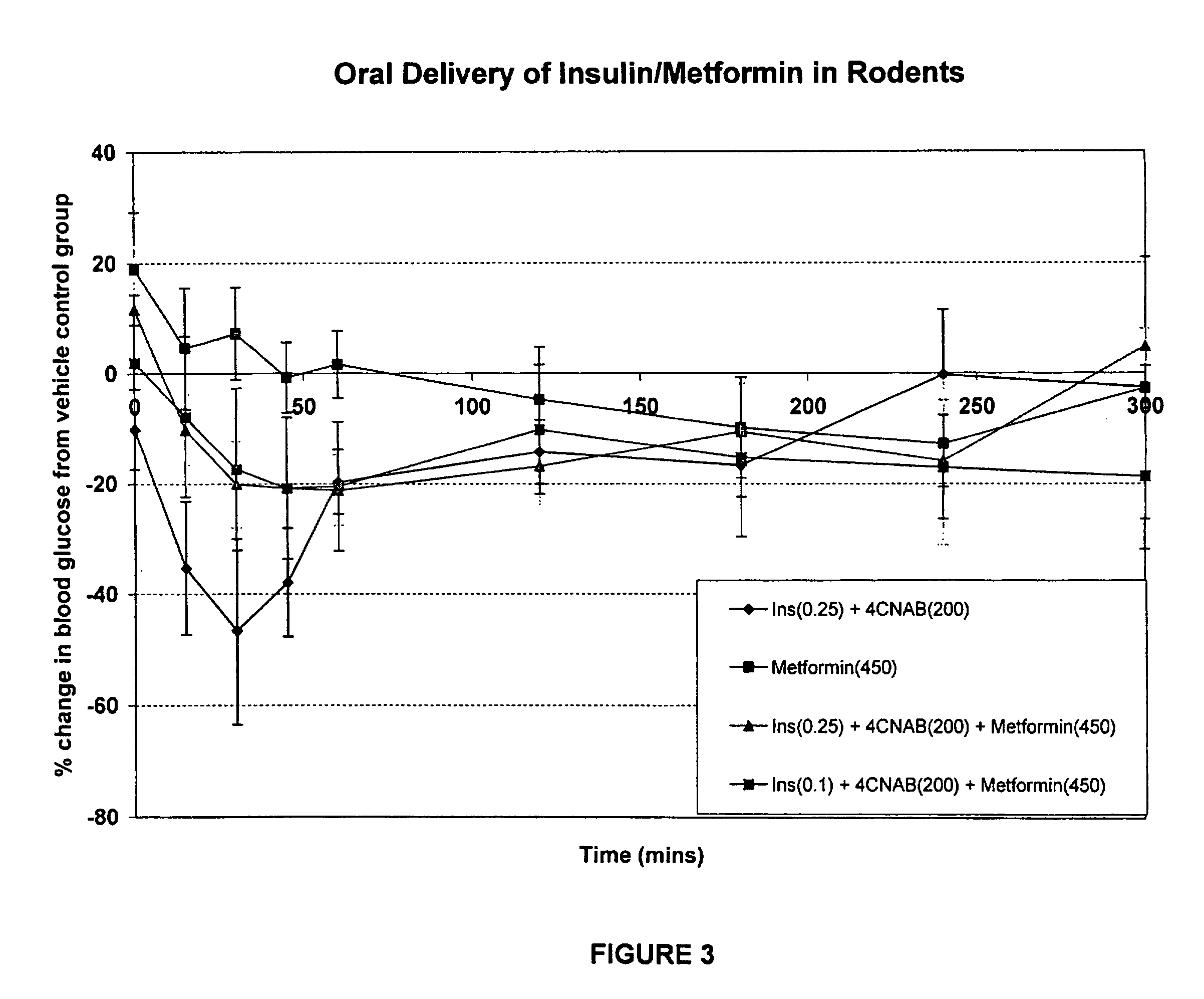
Antidiabetic oral insulin-biguanide combination
InactiveUS20100048454A1Facilitates insulin transportLasting effectOrganic active ingredientsPeptide/protein ingredientsLow glucoseInsulin dependent diabetes
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
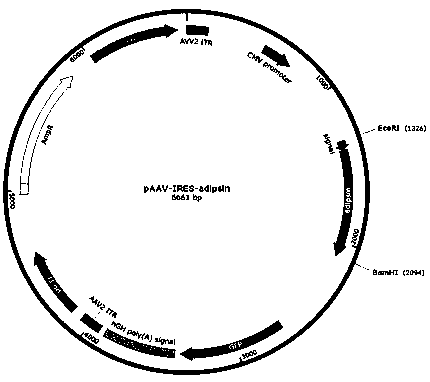
Adipsin gene modified adipose-derived stem cells as well as preparation method and application thereof
ActiveCN111269888AIncrease vitalityDouble the proliferative capacityPancreatic cellsSkeletal/connective tissue cellsPancreatic hormoneBiochemistry
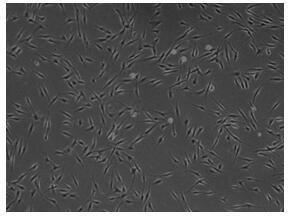
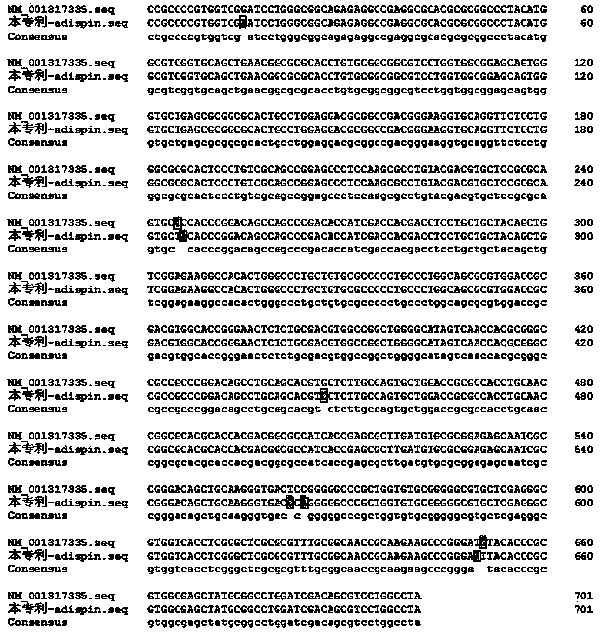
The invention provides adipsin gene modified adipose-derived stem cells, the adipsin gene modified adipose-derived stem cells comprise an adipsin recombinant gene, and the adipsin recombinant gene expresses an adipsin protein; the invention further provides a preparation method and application of the adipose-derived stem cells. The adipsin gene modified adipose-derived stem cells are adopted, theactivity of pancreatic islet cells can be improved, and after the adipsin gene modified adipose-derived stem cells and the pancreatic islet cells are co-cultured, the activity of the pancreatic isletcells is improved by one time compared with common co-culture of the adipose-derived stem cells and the pancreatic islet cells. The adipsin gene modified adipose-derived stem cells are adopted, various growth factors are secreted by utilizing the paracrine effect and the regeneration and repair effect of the adipose-derived stem cells, so that the multiplication capacity of pancreatic beta cells is doubled, and meanwhile, the secreted adipsin proteins can increase insulin secretion of the pancreatic beta cells.
Owner:SHANDONG XINRUI BIOTECH CO LTD
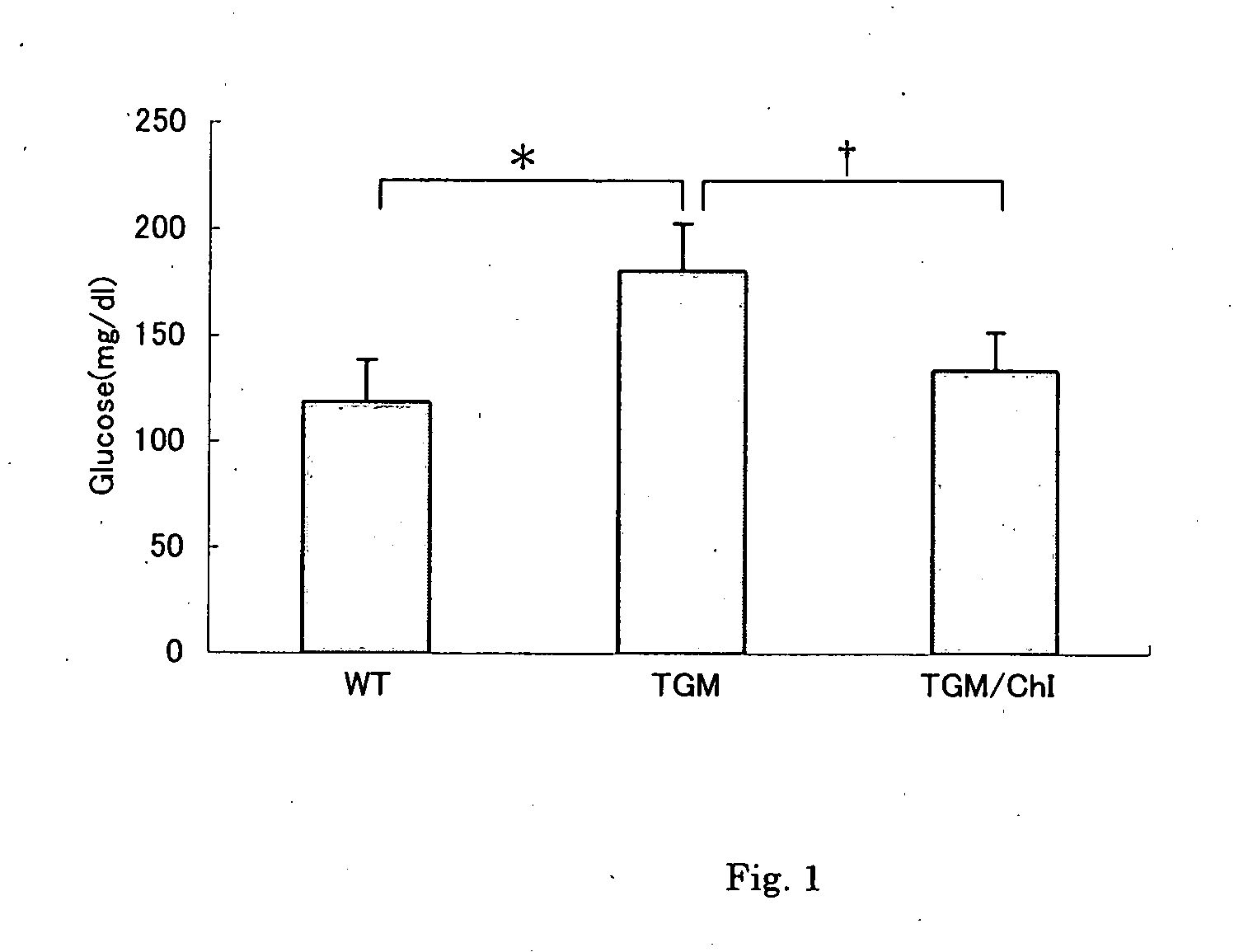
Diagnosis of hyperinsulinemia and type ii diabetes and protection against same based on proteins differentially expressed in serum
InactiveUS20100028326A1Sufficient productionMaintain biological activityOrganic active ingredientsPeptide/protein ingredientsAntagonistIncreased Insulin Secretion
Mouse proteins differentially expressed in serum, in comparisons of normal vs. hyperinsulinemic, hyperinsulinemic vs. type 2 diabetic, and normal vs. type 2 diabetic white adipose tissue have been identified, as have corresponding human proteins. The human molecules, or antagonists thereof, may be used for protection against hyperinsulinemia or type 2 diabetes, or their sequalae.
Owner:OHIO UNIV
Moringa extract
ActiveUS20180318367A1Useful physiological functionImprove securityOrganic active ingredientsSenses disorderDiseaseSide effect
A moringa extract containing a benzyl glucosinolate in a content of 6% by mass or more, calculated as a dry solid content of the extract, wherein the extract does not substantially contain an alkaloid. The moringa extract of the present invention for solving a first aspect is useful in the field of foodstuff or the like. Also, the PPAR activator of the present invention for solving a second aspect has excellent PPAR activation action, and has no disadvantages in side effects, so that it can be ingested for long term, which can be preferably used in foodstuff and the like. Therefore, the PPAR activator of the present invention for solving a second aspect can be expected to be used as a food, a supplement or a medicament not only for prevention of disease such as insulin resistance, hyperinsulinism, Type 2 diabetes, hypertension, hyperlipidemia, arterial sclerosis and obesity, but also for fatigue recovery or endurance improvement by improving basal metabolism. In addition, a benzyl glucosinolate-containing composition for solving a third aspect is useful in the field of foodstuff or the like.
Owner:TAIYO KAGAKU CO LTD
Somatostatin agonists
InactiveUS20050164922A1Improve scalabilityImprove survivalBiocideAntiviralsDecreased body weightVIPoma
The present invention is directed to cyclic peptides of formula (I): X-A1-cyclo(D-Cys-A3-A4-Lys-A6-A7)-A8-Y, or a pharmaceutically acceptable salt thereof. The peptides bind selectively to the somatostatin subtype receptor type-5 and elicit an agonist effect from the somatostatin subtype receptors. The peptides are useful for treating a variety of diseases, including Cushings Syndrome, gonadotropinoma, hyperparathyroidism, Paget's disease, VIPoma, nesidioblastosis, hyperinsulinism, gastrinoma, Zollinger-Ellison Syndrome, hypersecretory diarrhea related to AIDS and other conditions, irritable bowel syndrome, pancreatitis, Crohn's Disease, systemic sclerosis, thyroid cancer, psoriasis, hypotension, panic attacks, sclerodoma, small bowel obstruction, gastroesophageal reflux, duodenogastric reflux, Graves' Disease, polycystic ovary disease, upper gastrointestinal bleeding, pancreatic pseudocysts, pancreatic ascites, leukemia, meningioma, cancer cachexia, acromegaly, restenosis, hepatoma, lung cancer, melanoma, inhibiting the accelerated growth of a solid tumor, decreasing body weight, treating insulin resistance, Syndrome X, prolonging the survival of pancreatic cells, fibrosis, hyperlipidemia, hyperamylinemia, hyperprolactinemia and prolactinemia.
Owner:SOC DE CONSEILS DE RECH
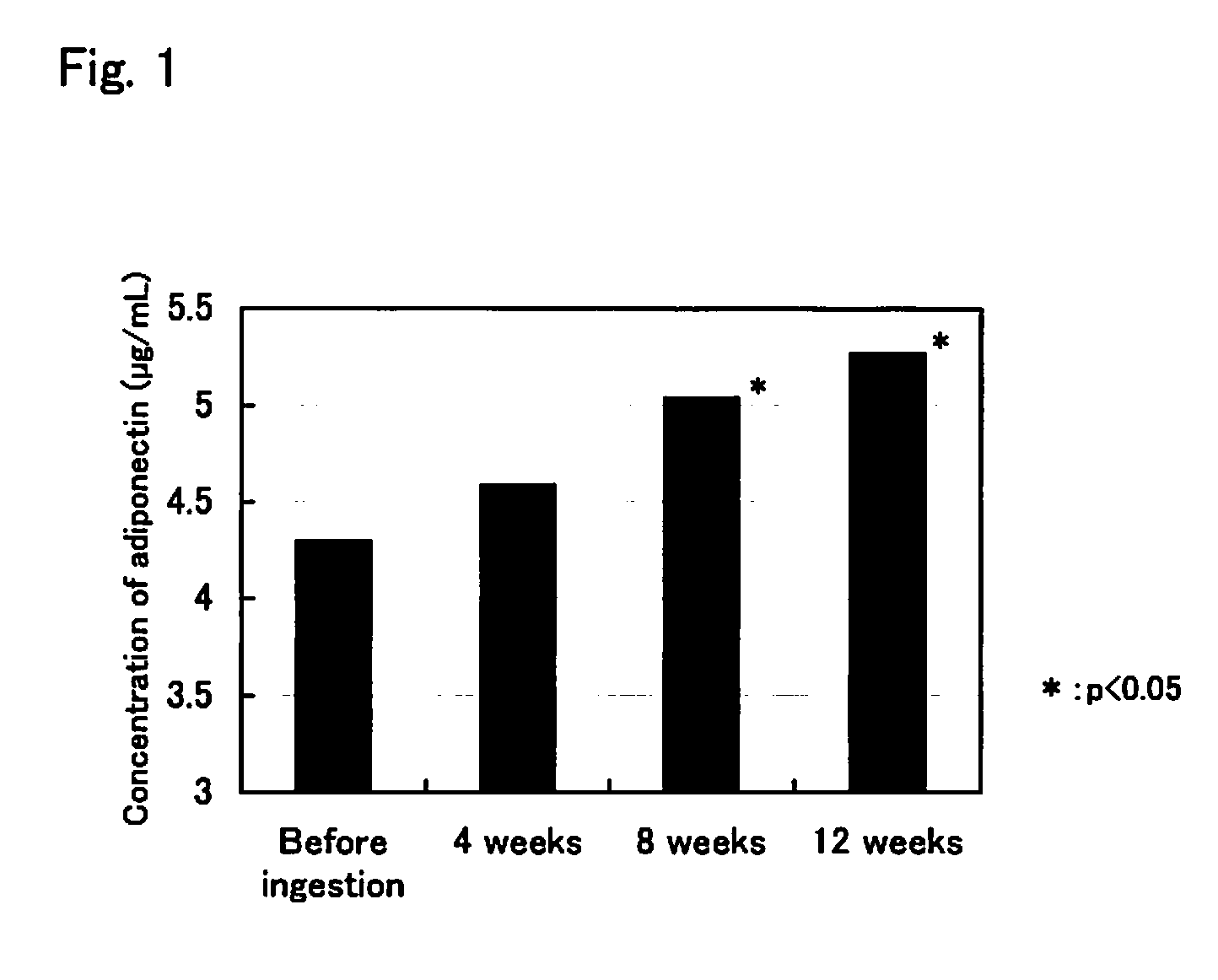
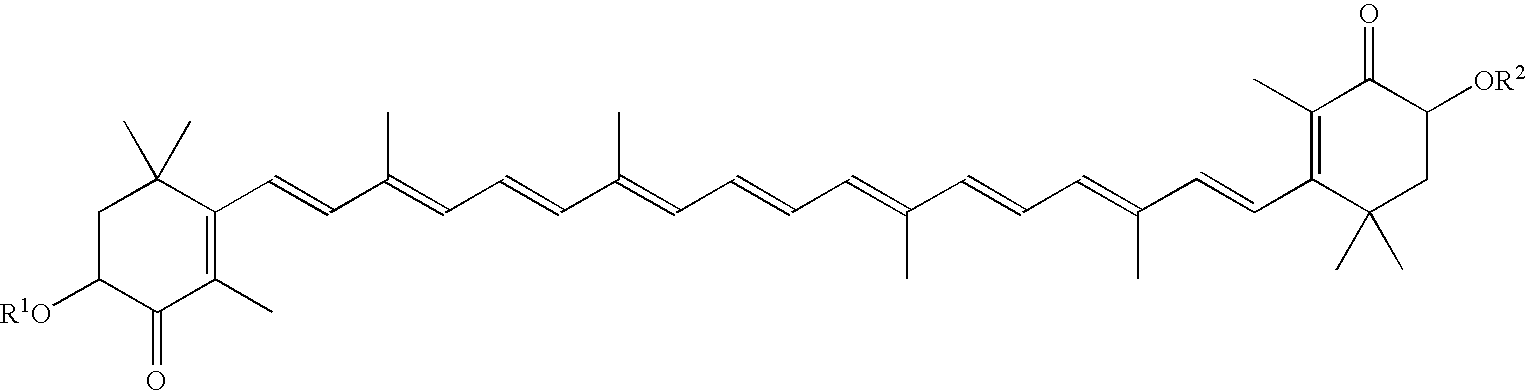
Agent for increasing adiponectin in blood
InactiveUS20080161413A1Increases the amount of adiponectin in bloodHighly effectiveBiocideMetabolism disorderHyperinsulinemiaActive component
Novel, safe, and highly effective agents for increasing adiponectin in blood that contain astaxanthin and / or an ester thereof as an active component are provided. Due to an increase in the amount of adiponectin in blood, the action of adipocytokine such as TNFα can be decreased, or the action of biologically active substances such as insulin can be protected from the adverse action of adipocytokine. It is expected that, as a result, insulin resistance and hyperinsulinemia caused by adipocytokine, and metabolic syndrome are prevented or suppressed. Furthermore, it is expected that progress of arteriosclerosis, which can lead to cardiovascular diseases and cerebrovascular diseases, is inhibited by ingestion of the agent for increasing adiponectin in blood of the present invention, because arteriosclerosis progresses due to a decrease in adiponectin.
Owner:YAMAHA MOTOR CO LTD
Glucokinase activators
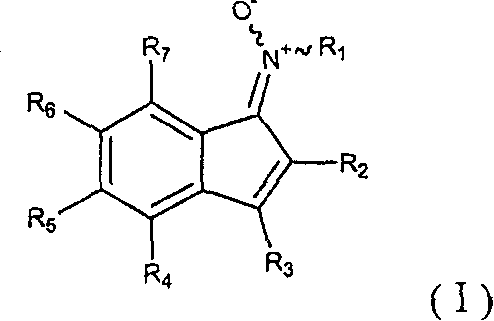
InactiveCN1151140COrganic chemistryOrganic compound preparationGlucokinase activatorIncreased Insulin Secretion
The present invention relates to compounds of formula (I) wherein R<1>, R<2>, R<3> and R<4> are as defined in claim 1 and pharmaceutically acceptable salts thereof. The compounds are glucokinase activators which increase insulin secretion in the treatment of type II diabetes.
Owner:F HOFFMANN LA ROCHE & CO AG
Mentsh analogs as therapeutics for diabetes, obesity, and their associated diseases and complications
ActiveUS20190194275A1Maximum resultImprove the increase effectSenses disorderPeptide/protein ingredientsDiseaseDiabetes Therapy
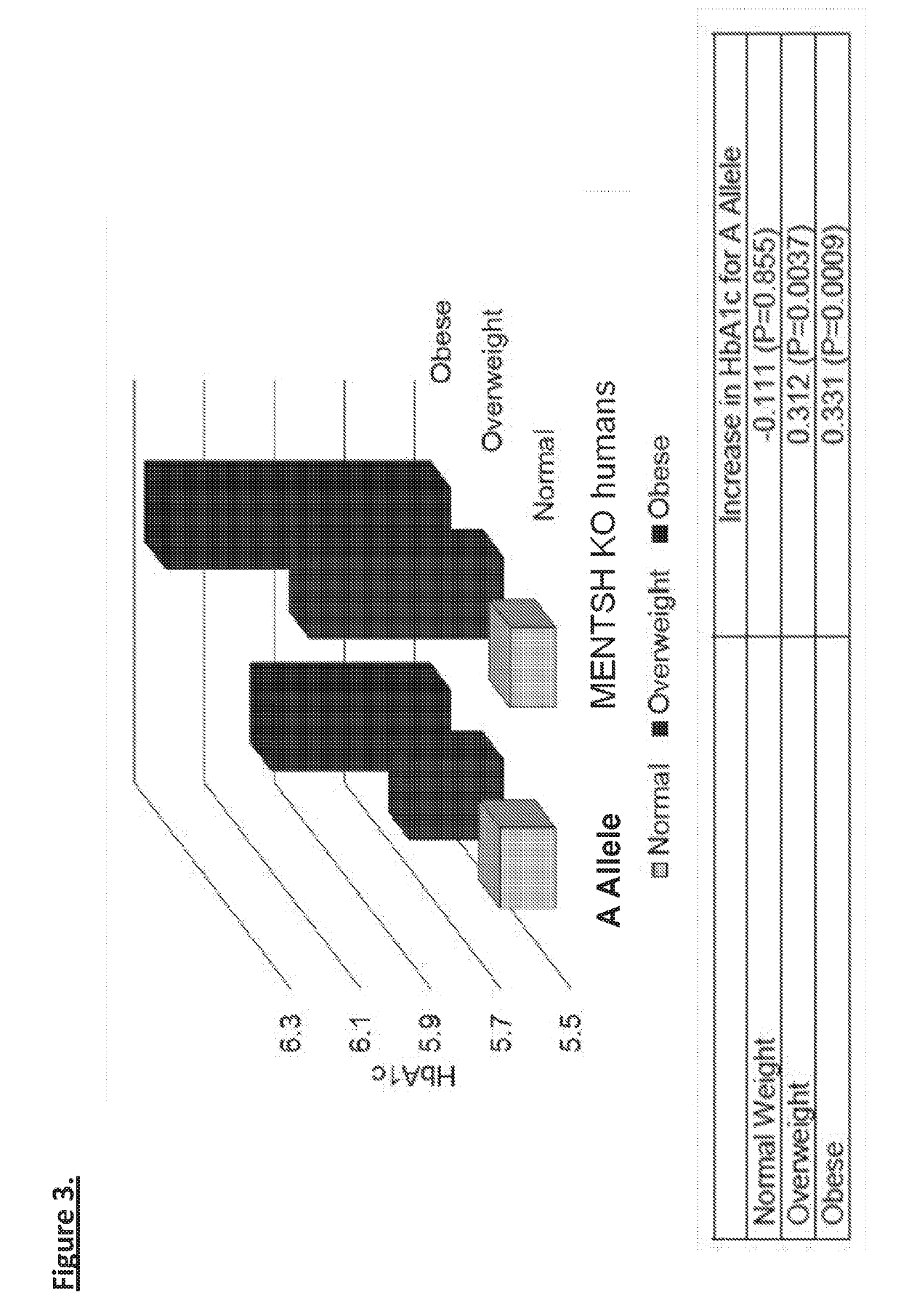
Described herein is a novel, mitochondrial encoded, open reading frame, that leads to the production of a new mitochondrial peptide. Residing within the ND-Two subunit, a specific small nucleotide polymorphism disrupts expression of this mitochondrial peptide, and is correlated with an increase in obesity and diabetes, particularly in certain ethnic populations. In vitro administration of the peptide increases insulin secretion, decreases fat accumulation and improves glucose uptake in muscle cell. Antibodies generated against the peptide can be used for detecting peptide deficiency, in addition to SNP detection, supporting diagnostic approaches. In vivo studies further revealed that administration of the peptide improves glucose tolerance, thereby providing a new therapeutic avenue for a novel diabetes therapy and decreases bodyweight, thus serving as a novel obesity therapy. Generation of synthetic analogs further enhance or abrogated activity relative to the natural peptide.
Owner:UNIV OF SOUTHERN CALIFORNIA
Indene derivatives and process for the preparation thereof
InactiveCN1942434ASelective modulation of activityDoes not cause adverse side effectsOrganic chemistryAntipyreticSide effectCirrhosis
The inventive indene derivatives of formula (I) are capable of selectively modulating the activities of peroxisome proliferator activated receptors (PPARs), causing no adverse side effects, and thus, they are useful for the treatment and prevention of disorders modulated by PPARs, i.e., metabolic syndromes such as diabetes, obesity, arteriosclerosis, hyperlipidemia, hyperinsulinism and hypertension, inflammatory diseases such as osteoporosis, liver cirrhosis and asthma, and cancer.
Owner:KOREA RES INST OF CHEM TECH +3
Application of acridinedione compound in preparation of anti-diabetic drug
ActiveCN111303030APromote secretionImprove the immunityOrganic active ingredientsOrganic chemistryAcridineReceptor
The invention provides an application of an acridinedione compound in preparation of an anti-diabetic medicine, and belongs to the technical field of biological medicines. According to the invention,the results prove that the acridinedione compound can participate in a GPR40-PPAR gamma-PI3K / Akt-GLUT4 signal path by activating and up-regulating GPR40 protein expression so as to promote insulin secretion, increase glucose consumption of liver and muscle tissues and improve insulin resistance; the action target of the acridinedione compound is a GPR40 receptor, the insulin secretion promoting effect of the acridinedione compound has glucose dependence, and when the peripheral blood glucose is lower than a certain degree, the blood glucose reducing effect of the acridinedione compound disappears; and the acridinedione compound is prepared into the anti-diabetic medicine, and brand-new selection and strategy are provided for treatment of diabetes mellitus.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Somatostatin agonists
InactiveUS6864234B1Improve scalabilityLose weightPeptide/protein ingredientsMetabolism disorderVIPomaPercent Diameter Stenosis
The present invention is directed to cyclic peptides of formula (I): X-A1-cyclo(D-Cys-A3-A4-Lys-A6-A7)-A8-Y, or a pharmaceutically acceptable salt thereof. The peptides bind selectively to the somatostatin subtype receptor type-5 and elicit an agonist effect from the somatostatin subtype receptors. The peptides are useful for treating a variety of diseases, including Cushings Syndrome, gonadotropinoma, hyperparathyroidism, Paget's disease, VIPoma, nesidioblastosis, hyperinsulinism, gastrinoma, Zollinger-Ellison Syndrome, hypersecretory diarrhea related to AIDS and other conditions, irritable bowel syndrome, pancreatitis, Crohn's Disease, systemic sclerosis, thyroid cancer, psoriasis, hypotension, panic attacks, sclerodoma, small bowel obstruction, gastroesophageal reflux, duodenogastric reflux, Graves' Disease, polycystic ovary disease, upper gastrointestinal bleeding, pancreatic pseudocysts, pancreatic ascites, leukemia, meningioma, cancer cachexia, acromegaly, restenosis, hepatoma, lung cancer, melanoma, inhibiting the accelerated growth of a solid tumor, decreasing body weight, treating insulin resistance, Syndrome X, prolonging the survival of pancreatic cells, fibrosis, hyperlipidemia, hyperamylinemia, hyperprolactinemia and prolactinemia.
Owner:IPSEN PHARMA SAS
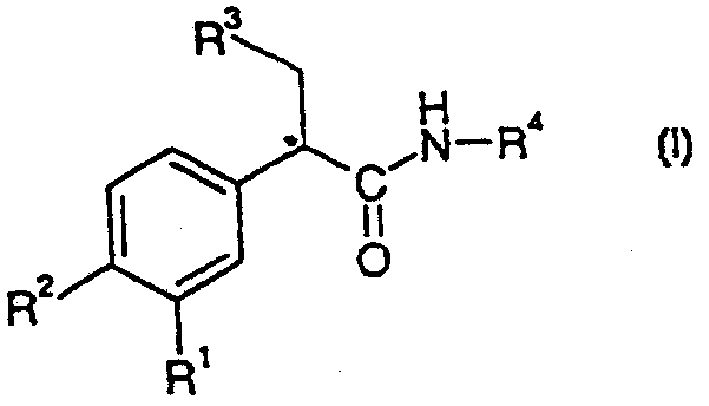
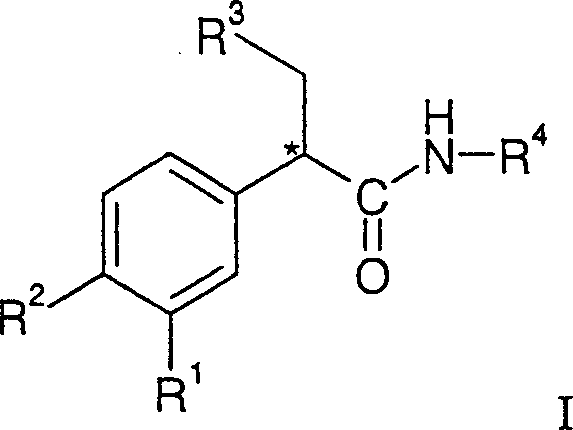
Substituted benzamide derivatives as glucokinase (GK) activators
The present invention relates to substituted benzamide derivatives of the general Formula I and their pharmaceutically acceptable salts, pharmaceutically acceptable solvates, enantiomers, diastereomers, prodrugs, metabolites, and polymorphs and can be useful for treating disease states mediated by glucokinase. Compounds disclosed herein can be used for reducing blood glucose levels and for increasing insulin secretion for treating type II diabetes. The invention also relates to processes for the preparation of the compounds of invention, pharmaceutical compositions containing the compounds, and their use.
Owner:CADILA HEALTHCARE LTD
Chitosan composition for preventing and treating diabetes and preparation method of chitosan composition
InactiveCN103989938AShort process stepsReduce manufacturing costMetabolism disorderPharmaceutical non-active ingredientsThirstBitter gourd
The invention discloses a chitosan composition for preventing and treating diabetes and a preparation method of the chitosan composition. The preparation method comprises the following steps: extracting puerarin, radix rehmanniae recen, bitter gourd, astragalus membranaceus, polygonatum odoratum and ganoderma lucidum to prepare dry paste powder, mixing the dry paste powder with sieved chitosan and starch, granulating, filling capsules, polishing and packaging to prepare the chitosan composition for preventing and treating diabetes. In the formula of the chitosan composition disclosed by the invention, the chitosan can promote internal secretion by regulating organ functions to regulate the pancreatic function, improve the activity of islet cells, so as to promote increased insulin secretion; such traditional Chinese medicinal materials in the formula as the puerarin, the radix rehmanniae recen, the bitter gourd, the astragalus membranaceus and the ganoderma lucidum are dialectically used to ensure functions of nourishing yin, engendering liquid and allaying thirst, moistening dryness and tonifying qi, and cooperatively condition human body organ functions with the chitosan so as to effectively prevent and treat diabetes and complication.
Owner:高益槐 +1
Method for Evaluating Obesity Controller
InactiveUS20100022014A1Decrease and increase insulin secretionCompound screeningApoptosis detectionBlood insulinObesity
A method for efficiently evaluating or selecting an obesity controlling substance, a blood insulin regulating substance or a blood sugar regulating substance, is provided. A method for evaluating or screening an obesity controller, the method including administering a carbohydrate and a lipid to an animal, and evaluating or selecting a substance which decreases or increases insulin secretion, is also provided.
Owner:KAO CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![PYRIDO[4,3-b]INDOLE AND PYRIDO[3,4-b]INDOLE DERIVATIVES AND METHODS OF USE PYRIDO[4,3-b]INDOLE AND PYRIDO[3,4-b]INDOLE DERIVATIVES AND METHODS OF USE](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5c48022f-f95e-4041-9689-c914419c69e0/US20140206711A1-20140724-C00001.png)
![PYRIDO[4,3-b]INDOLE AND PYRIDO[3,4-b]INDOLE DERIVATIVES AND METHODS OF USE PYRIDO[4,3-b]INDOLE AND PYRIDO[3,4-b]INDOLE DERIVATIVES AND METHODS OF USE](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5c48022f-f95e-4041-9689-c914419c69e0/US20140206711A1-20140724-C00002.png)
![PYRIDO[4,3-b]INDOLE AND PYRIDO[3,4-b]INDOLE DERIVATIVES AND METHODS OF USE PYRIDO[4,3-b]INDOLE AND PYRIDO[3,4-b]INDOLE DERIVATIVES AND METHODS OF USE](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5c48022f-f95e-4041-9689-c914419c69e0/US20140206711A1-20140724-C00003.png)
![Pyrido[4,3-b]indole and pyrido[3,4-b]indole derivatives and methods of use Pyrido[4,3-b]indole and pyrido[3,4-b]indole derivatives and methods of use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ce510259-389f-4cba-8a19-665510975d46/US09035056-20150519-C00001.PNG)
![Pyrido[4,3-b]indole and pyrido[3,4-b]indole derivatives and methods of use Pyrido[4,3-b]indole and pyrido[3,4-b]indole derivatives and methods of use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ce510259-389f-4cba-8a19-665510975d46/US09035056-20150519-C00002.PNG)
![Pyrido[4,3-b]indole and pyrido[3,4-b]indole derivatives and methods of use Pyrido[4,3-b]indole and pyrido[3,4-b]indole derivatives and methods of use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ce510259-389f-4cba-8a19-665510975d46/US09035056-20150519-C00003.PNG)